Abstract
Silymarin, a flavonolignan from milk thistle (Silybum marianum) plant, is used for the protection against various liver conditions in both clinical settings and experimental models. In this review, we summarize the recent investigations and mechanistic studies regarding possible molecular targets of silymarin for cancer prevention. Number of studies has established the cancer chemopreventive role of silymarin in both in vivo and in vitro models. Silymarin modulates imbalance between cell survival and apoptosis through interference with the expressions of cell cycle regulators and proteins involved in apoptosis. In addition, silymarin also showed anti-inflammatory as well as anti-metastatic activity. Further, the protective effects of silymarin and its major active constituent, silibinin, studied in various tissues, suggest a clinical application in cancer patients as an adjunct to estabilished therapies, to prevent or reduce chemotherapy as well as radiotherapy-induced toxicity. This review focuses on the chemistry and analogues of silymarin, multiple possible molecular mechanisms, in vitro as well as in vivo anticancer activities, and studies on human clinical trials.
Keywords: Silymarin, silibinin, chemoprevention, carcinogenesis, cell cycle, cancer
Introduction
Research over the last three decades has provided convincing evidence to support that the diets rich in fruits and vegetables may be protective against the risk of different types of cancers. Of late, several medicinal herbs from plant origin have also received great attention due to their wide range of pharmacological effects. All these dietary agents, medicinal plants and herbs have been tested for their cancer chemopreventive activity. The reduced cancer risk and lack of toxicity associated with high intake of natural products suggest that specific concentrations of phytochemicals from these plant sources may produce cancer chemopreventive effects without causing significant levels of toxicity. Natural agents are believed to suppress the inflammatory process that lead to neoplastic transformation, hyperproliferation, promotion and progression of carcinogenic process and angiogenesis. It is estimated that nearly one-third of all cancer deaths in the United States could be prevented through appropriate dietary modification. Accumulating research evidence suggests that many dietary agents/medicinal plants may be used alone or in combination with traditional chemotherapeutic agents to prevent the occurrence of cancer, their metastatic spread, or even to treat cancer [1,2].
Silymarin has been used for more than 2000 years as a natural remedy for treating hepatitis and cirrhosis and to protect liver from toxic substances. Silymarin acts by antioxidative, anti-lipid peroxidative, antifibrotic, anti-inflammatory, membrane stabilizing, immunomodulatory and liver regenerating mechanisms in experimental liver diseases. Furthermore, silymarin has been extensively studied, both in vivo and in vitro, for its cancer chemopreventive potential against various cancers [3]. This article reviews the current studies regarding various aspects of silymarin as they relate to its efficacy against cancer and associated molecular mechanisms.
Silymarin - Chemistry and Analogues
Silymarin is an active extract from the seeds of the plant milk thistle (Silybum marianum (L.) Gaertn. (Asterceae), and contains approximately 65-80% silymarin flavonolignans (silymarin complex) with small amounts of flavonoids and approximately 20-35% fatty acids and other polyphenolic compounds. The major component of the silymarin complex is silybin that is synonymous with silibinin (Figure 1), together with other flavonolignans namely isosilybin, silychristin, silydianin, and flavonoid taxifolin [4]. Studies have also reported that silybin and isosilybin are the mixture of two diastereoisomers namely silybin A and silybin B and isosilybin A and isosilybin B; the later two are regioisomers of silybin A and silybin B, respectively, from the silymarin mixture [5]. White-flowering varieties of S. marianum contain additional compounds such as 3-deoxyflavanolignans silandrin, silymonin, silyhermin and neosilyhermin A and B [6]. Recently, Mackinnon et al [7] have isolated a new flavonolignan silyamandin from the tincture preparations of the milk thistle fruit. The most commonly utilized silymarin and silibinin products used in clinical trials are Legalon, Thisilyn, Siliphos and Silipide.
Figure 1.
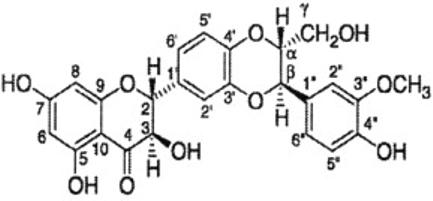
Structure of silibinin.
Silibinin is the most active antihepatotoxic agent in silymarin mixture and contains 1,4-dioxane ring in addition to flavonoid moiety. Ahmed et al [8] have prepared some flavones and coumarins containing the 1,4-dioxane ring system and evaluated them for antihepatotoxic activity against carbon tetrachloride induced hepatotoxicity in albino rats. The compounds 3′, 4′(1″,4″-dioxino) flavone and 3′,4′(2-hydroxy methyl, 1″,4″-dioxino) flavone exhibited a significant activity comparable to standard drug silymarin (silybon-70). Structure activity relationship (SAR) studies revealed that flavonoid analogues containing a hydroxy methyl group at position-2″ in the dioxane ring exhibited superior antihepatotoxic activity in comparison to coumarin derivatives. Varga et al [9] have synthesized structural analogues (flavanone: 2-4 and flavone: 5 and 6) of silybin and tested for inhibitory activity on superoxide anion and protein kinase C (PKC) translocation in phorbol myristate acetate (PMA)-stimulated neutrophils as well as xanthine oxidase activity to identify the molecular structures responsible for the antioxidant property of silybin. It has been shown that different moieties of silybin are involved in the inhibition of overproduction of superoxide anion in stimulated neutrophils, xanthine oxidase activity, and for prevention of hem-mediated oxidative modification of low density lipoproteins (LDL). Gazak et al [10] have prepared carboxylic acid derivatives of silybin and 2,3-dehydrosilybin with improved hydrophilicity. The presence of 2, 3-double bond in the C-ring of flavonoid improved the scavenging/antioxidant potency of the compound. 2,3-dehydrosilybinic acid is a fairly soluble derivative with anti-lipid peroxidation and antiradical activities better than that of silybin.
Regarding anti-cancer activity, Dzubak et al [11] have prepared a series of O-alkyl derivatives (methyl and benzyl) of silybin and 2,3-dehydrosilybin and tested for cytotoxicity in multidrug resistant cell lines and the ability to inhibit P-glycoprotein mediated efflux activity. The 3, 7, 20-Tri-O-methyl-2,3-dehydrosilybin was found to be the best inhibitor with relatively low cytotoxicity comparable with that of parent 2,3-dehydrosilybin. More recently, Davis-Searles et al [12] identified seven distinct flavonolignan compound and a flavonoid from commercial silymarin extracts. Among these, four compounds namely silybin A, silybin B, isosilybin A and isosilybin B, showed most consistent anti-proliferative effects in three different human prostate carcinoma LNCaP, DU145 and PC3 cell lines. In further expanding these preliminary observations, recently we have shown that isosilybin B and isosilybin A exert growth inhibition and cell death together with a strong G1 arrest and apoptosis in human prostate carcinoma LNCaP and 22Rv1 cell [13]. Overall, completed studies suggest that the preparations enriched of these two compounds may be preferable for future studies in prostate cancer.
Silymarin - Molecular Targets for Anti-cancer Efficacy
Carcinogenesis is a multistep process that is activated by altered expression of transcriptional factors and proteins involved in proliferation, cell cycle regulation, differentiation, apoptosis, angiogenesis, invasion and metastasis. Deregulated cell cycle progression and apoptosis together with increased angiogenic potential, invasion and metastasis have been described as hallmarks of cancer. Accordingly, the agents that could target one or more of these processes should be effective and ideal cancer chemopreventive agents. Silymarin and/or silibinin modulate imbalance between cell survival and apoptosis through interference with the expressions of cell cycle regulators and proteins involved in apoptosis. In addition, silymarin also showed anti-inflammatory as well as anti-metastatic activity by modulating specific proteins [14]. Figure 2 shows the different molecular targets of silymarin.
Figure 2.
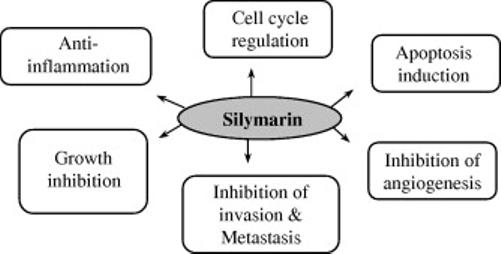
Possible anti-cancer mechanisms of silymarin.
Both silymarin and silibinin are particularly effective in inhibiting epidermal growth factor receptor (EGFR) signaling with suppression of cyclin-dependent kinase (CDK) expression and up-regulation of the CDK-inhibitors p21CIP1 and p27KIP1, with concomitant increase in their binding to CDKs. Silymarin induces growth arrest at the G1 and G2 checkpoints. Silymarin, in lower doses induces the growth arrest through extracellular signal-regulated kinases (ERK1/2) inhibition and in higher doses leads to apoptosis through mitogen activated protein kinase (MAPK)/ c-Jun N-terminal kinase (JNK) pathway [14-16]. Our studies have shown that silymarin inhibits both constitutively active and transforming growth factor (TGF)-α mediated tyrosine phosphorylation of EGFR in advanced human prostate cancer DU145 cells [17]. Studies have shown that silymarin and silibinin down-regulate EGFR signaling via the inhibition in the expression and secretion of growth factors, and by inhibiting growth factor binding to and activation of EGFR and subsequent impairment of downstream mitogenic events causing anticancer efficacy in tumor cell lines [18].
Anti-inflammatory effects of silymarin
Anti-inflammatory effects of silymarin are related to inhibition of the transcription factor nuclear factor-κB (NF-κB), which regulates and coordinates the expression of various genes involved in inflammation, cell survival, differentiation and growth. In particular, NF-κB contributes to the production of interleukin (IL)-1 and -6, tumor necrosis factor (TNF)-α, lymphotoxin, granulocyte macrophage colony-stimulating factor (GM-CSF) and interferon (IFN)-γ. In most of the resting cells, NF-κB is sequestered in the cytoplasm by binding to the inhibitory-κB (IκB)-proteins which block the nuclear localization sequences of NF-κB. Studies have demonstrated that silymarin is a potent inhibitor of NF-κB activation in response to TNFα. This effect was mediated through the inhibition of phosphorylation and degradation of IκB [18]. It also decreases the p65 subunit nuclear translocation and NF-κB dependent reporter gene transcription. Silymarin also blocked NF-κB activation induced by phorbol ester, lipopolysaccharide, okadaic acid and ceramide, whereas H2O2-induced NF-κB activation was not significantly affected. Manna et al [19] studied the effect of silymarin on NF-κB activation induced by various inflammatory agents. Silymarin also inhibited TNF-α induced activation of MAPK and JNK, TNF-induced cytotoxicity and caspase activation. Silymarin in combination with tetrandrine attenuates NF-κB activated pathways and the induction of metallothionein gene transcription in the liver of dimethylnitrosamine (DMN) administered rats [20]. In human mesangial cells, silymarin showed dose dependent inhibition of TNF-α- and IL-1β-induced NF-κB activation, and TNF-α-induced intracellular calcium and MCP-1 expression [21]. Silymarin has a protective effect against endotoxin-induced sepsis and also have the inhibitory effect on the production of IL-1β and prostaglandin (PG)-E2 [22]. Silymarin dose-dependently inhibits both cytokine-induced nitric oxide (NO) production and cell death in RINm5F cells, and prevents IL-1β and IFN-γ-induced NO production and β-cell dysfunction in human pancreatic islets. [23].
Modulation of cell cycle progression by silymarin
Disruption of the normal regulation of cell cycle progression and division is an important event in malignant transformation. The regulation of the cell cycle is controlled by a family of cyclins, CDKs and CDK inhibitors (CDKIs). Silymarin has been reported to suppress the proliferation of tumor cells in various cancers including prostate [15-17], ovarian [25], breast [26], lung [27], skin [18] and bladder [28,29]. Numerous reports indicate that silymarin inhibits proliferation of cells by inhibiting cell cycle progression at different stages of the cell cycle. Studies from our laboratory have demonstrated that silymarin induces G1 arrest and/or G2-M arrest in human prostate cancer LNCaP, PC3 and DU145 cells. Silymarin caused an induction of the CDK inhibitors Cip1/p21 and Kip1/p27, and a decrease in CDK2 and CDK4 and associated kinase activities that led to G1 arrest [18]. Silibinin treatment showed dose- and time-dependent growth inhibition together with a G1 arrest in bladder transitional cell carcinoma (TCC) cells, T-24 (high-grade tumor) and TCC-SUP (high-grade invasive tumor). Furthermore, silibinin at high concentration induced G2/M arrest in TCC-SUP cells that was associated with a decrease in pCdc25c (Ser216), Cdc25c, pCdc2 (Tyr15), Cdc2 and cyclin B1 protein levels [29]. Silymarin treatment has been shown to inhibit the growth of androgen dependent (LNCaP) and androgen independent (PC3 and DU145) prostate cancer cells. [12]. Silymarin also induces G1 arrest through an increase in Cip1/p21 and a decrease in the kinase activity of CDK and associated cyclins in human breast cancer MDA-MB 468 cells [26]. Silymarin treatment induced binding of Cip1/p21 with CDK2 and CDK6 paralleled a significant decrease in CDK2-, CDK6-, cyclin D1-, and cyclin E-associated kinase activities, along with a decrease in cyclins D1 and E. Our studies have also shown that silymarin and silibinin modulate G1 phase cyclins-CDKs-CDKIs for G1 arrest, and the Chk2-Cdc25C-Cdc2/cyclin B1 pathway for G2-M arrest, together with an altered subcellular localization of critical cell cycle regulators [18]. Recently, we have shown that silibinin inhibits UVB-caused increase in cell proliferation and microvessel density and down-regulation of inflammatory and angiogenic responses in SKH-1 hairless mice [30]. In other studies, silibinin significantly up-regulated p21/CDK4 and p27/CDK4 complexes and down-regulated Rb-phosphorylation and E2F1/DP1 complex thereby inhibiting human hepatoma HuH7 cell growth [31]. We have also demonstrated the anticancer activity of two pure compounds isosilybin B and isosilybin A, isolated from silymarin, in human prostate carcinoma LNCaP and 22Rv1 cells that is mediated via cell cycle arrest and apoptosis induction [13]. These studies suggested that regulation of cell cycle is one of the mechanisms of action of silymarin in the prevention and therapeutic intervention of cancer.
Induction of apoptosis by silymarin
Apoptosis or programmed cell death that occurs in various physiological and pathological conditions is one of the hallmarks of cancer. Several phytochemicals that are known to inhibit NF-kB and AP-1 activation can suppress cell proliferation and sensitize cells to apoptosis induction. Studies have reported that silymarin exerts its anticancer effects by causing cell cycle arrest and inducing apoptosis in different type of cancers. Li et al [32] have shown that silymarin induces apoptotic cell death in CH11-treated human malignant melanoma A375-S2 cells by increasing the expression of Fas-associated proteins with death domain (FADD), a downstream molecule of the death receptor pathway followed by cleavage of procaspase-8 that induces apoptosis. In UV-irradiated human malignant melanoma A375-S2 cells, silymarin activated SIRT1, a cell survival protein, down-regulated Bax and poly(ADP-ribose) polymerase (PARP) expression with decreased release of cytochrome c and induced G2-M arrest [33]. Recently, our studies have shown that diastereoisomers from silymarin induce apoptosis by causing the cleavage of PARP, caspase 9 and caspase 3 and decreasing survivin levels in human prostate cancer LNCaP and 22Rv1 cells [13]. Similar results have also been seen in DU145 cells with inhibition of active Stat3. Silibinin synergies the growth-inhibitory effect of mitoxantrone, a topoisomerase II inhibitor in prostate carcinoma PC-3 cells by reducing cell viability with increased apoptosis [34]. Silymarin causes apoptosis in human K562 leukemia cells by inhibiting Akt activity associated with activation of caspases-9 and -3 as well as PARP cleavage [35]. We have also shown apoptosis induction by silibinin involving p53-caspase 2 activation and caspase-mediated cleavage of Cip1/p21 in bladder transitional-cell papilloma RT4 cells [36]. Silibinin also promotes apoptosis of human hepatoma HuH7 cells by down-regulating survivin and up-regulating activated caspase-3 and -9 [31]. It has been shown that dietary supplementation with silymarin inhibits 3,2′-dimethyl-4-aminobiphenyl-induced prostate carcinogenesis in male F344 rats by increasing apoptosis and modification of cell proliferation [37].
Anti-angiogenic activity of silymarin
Anti-angiogenic activity is one of the fundamental ways of the cancer treatment. The anti-angiogenic potential of silymarin has been demonstrated in various cancers. We have demonstrated that silymarin inhibits the growth and survival of human umbilical vein endothelial cells (HUVECs) by inhibiting capillary tube formation, and induction of cell cycle arrest and apoptosis together with a reduction in invasion and migration. The molecular events associated with these effects include an up regulation of Kip1/p27, Cip1/p21 and p53; mitochondrial apoptosis and caspase activation; down regulation of survivin and inhibition of Akt and NF-kB signaling; and matrix metalloproteinase (MMP)-2 secretion [14, 15, 18, 38]. Other studies have also demonstrated that silymarin decreases the secreted vascular endothelial growth factor (VEGF) levels in prostate DU145 and breast MCF and MDA-MB-468 cancer cells [26, 38]. Yang et al [39] have shown that silymarin/silibinin treatment up-regulates VEGF receptor (VEGFR-1(Flt-1)) gene expression but not kinase insert domain containing receptor (KDR) in EA.hy 926 cells. They also reported that silymarin/silibinin causes a dose-dependent decrease in the vascular density index in LoVo cells. Gallo et al [40] have shown that administration of silybin-phosphatidylcholine complex, IdB 1016, down-regulates VEGFR- 3 and up-regulates angiopoietin-2 in female nude mice bearing human ovarian cancer xenografts. Furthermore, our in vivo work has shown that silibinin inhibits microvessel density and inhibits VEGF secretion in prostate and lung tumors [14, 41]. The anti-angiogenic effects of silibinin have also been shown in terms of down-regulation of MMP2 and CD34 in human hepatoma cell lines. [31]. Together, these findings clearly suggest an anti-angiogenic efficacy of silymarin and silibinin in different cancers, which could be an additional important mechanism of their chemopreventive efficacy.
Anti-metastatic activity of silymarin
Cancer metastasis, a primary cause of cancer death and which may complicate the clinical management, depends on the motility and invasiveness of cancer cells. MMPs play an important role in the invasion and metastasis of cancer cells. Silibinin at 100μM concentration inhibited invasion and motility of SCC-4 tongue cancer as well as A459 lung cancer cells by down-regulating MMP-2 and urokinase-type plasminogen activator (u-PA) and up-regulating tissue inhibitor of metalloproteinase-2 (TIMP-2) and PAI-1 expressions [42, 43]. Moreover, in A549 lung cancer cells, silibinin inhibited MMP-2 and u-PA expression through reducing ERK1/2 and Akt phosphorylation, which in turn led to the reduced invasiveness of the cancer cells [43]. In human osteosarcoma MG-63 cells, silibinin inhibited u-PA and MMP-2 expressions, IL-6-induced ERK 1/2 and c-Jun phosphorylation, and cell invasiveness [44].
Activated protein-1 (AP-1), a complex consisting of homo- or heterodimers of the members of jun and fos family of proteins, regulates the expression of several genes involved in malignant transformation. In particular, AP-1 is known to promote epithelial to mesenchymal transition of tumor cells that is considered as a key step in cancer metastasis. Our previous studies have shown that silibinin suppresses UVB-induced AP-1 and NF-kB activation in mouse skin models [18]. Recently, Lee et al [45] have reported that silibinin reduces PMA-induced invasion of MCF-7 cells through the specific inhibition of AP-1-dependent MMP-9 gene expression. These findings suggest that by suppressing the cancer cells invasion through the specific inhibition of AP-1-dependent MMP-9 gene expression, silibinin represents a potential anti-metastatic agent. Together, the anti-invasive as well as anti- metastatic potential of silibinin could be of great value in the development of a potential cancer therapy.
Antioxidant activity of silymarin
Silymarin and silibinin exert antioxidant activity and support redox homeostasis in several in vitro and in vivo models. Kiruthiga et al [46] have shown that administration of silymarin increases the activities of antioxidant enzymes like superoxide dismutase (SOD), catalase, glutathione peroxidase (GPx), glutathione reductase (GR) and glutathione-s-transferase (GST) together with a decrease in the levels of malondialdehyde (MDA), a marker for lipid peroxidation, in erythrocytes exposed to H2O2. Silymarin application extensively reduces GSH depletion and ROS production as well as lipid peroxidation in UVA irradiation-induced damage in human keratinocytes. Formation of UVA-induced DNA single strand breaks and caspase-3 activity was also significantly decreased by silymarin [47]. Our studies have shown that silymarin inhibits MDA formation in epidermal microsomes in a dose-dependent manner, and also inhibits TPA-and benzoyl peroxide (BPO)-caused lipid peroxidation in mouse skin epidermis, which shows its strong in vivo antioxidant activity [14]. Other studies have shown that silymarin significantly inhibits UVB-induced production of H2O2. With the notion that the free radical scavenging and antioxidant properties of silymarin could prevent or reduce the onset and progression of chemotherapy-induced toxicity, in the patients with acute lymphoblastic leukemia, oral administration of Siliphos at a dose of 5.1mg/Kg/day has exerted protective effect on chemotherapy-induced hepatotoxicity [48].
Anticancer Activity of Silymarin: In Vitro Studies
Anticancer activity of silymarin has been demonstrated in human breast cancer, skin cancer, androgen-dependent and —independent prostate cancer, cervical cancer, colon cancer, ovarian cancer, hepatocellular carcinoma, bladder cancer and lung cancer cells [14-16]. Active compounds of silymarin, isosilybin B and isosilybin A, treatment has been shown to result in growth inhibition and cell death together with a strong G1 arrest and apoptotic death in human prostate carcinoma LNCaP and 22Rv1 cells [13]. Our in vitro studies have also shown that silibinin inhibits constitutively active Stat3 and induces apoptosis in DU145 cells, and thus might have potential significance in therapeutic intervention. In other studies, we have shown that silibinin synergizes human prostate carcinoma DU145 cells to doxorubicin, cisplatin and carboplatin induced growth inhibition and apoptotic death [18]. Similar synergistic effects of silibinin with doxorubicin and cisplatin have also been reported in breast and ovarian cancer cell lines [25]. The other anticancer effects of silymarin and silibinin in cell lines and their associated mechanisms have been extensively summarized in the earlier sections.
Anticancer Activity of Silymarin: In Vivo Studies
The efficacy of silymarin has been shown against chemically induced carcinogenesis, growth of tumor xenograft, as well as in various transgenic models. We were the first one to demonstrate the activity of silymarin against 12-O-tetradecanoyl-phorbol-13-acetate (TPA) induced tumor promotion by inhibiting the activity and expression of epidermal ornithine decarboxylase [49]. Further studies suggested the important role of silymarin in inhibiting the chemical- and UV-induced skin carcinogenesis [18]. Recently, Gu et al [30] have shown that topical or dietary silibinin treatment causes a strong protection against UVB-induced photocarcinogenesis by inhibiting cell proliferation, inflammation and angiogenesis in SKH-1 hairless mice. We have also reported that dietary feeding of silibinin prevents UVB radiation-induced skin damages including thymidine dimer-positive cells, proliferating cell nuclear antigen (PCNA) expression and apoptotic sunburn cells. Studies from our laboratory have demonstrated that silibinin can also inhibit the member of MAPKs family (ERK1/2, JNK and p38) and Akt activation induced by either acute or chronic UVB exposure of SKH-1 mouse skin [18].
With regard to prostate cancer, it has been shown that dietary administration of silymarin significantly decreased the incidence of 3,2-dimethyl-4-aminobiphenyl (DMBA)-induced prostatic adenocarcinoma in male F344 rats [37]. Studies from our laboratory have shown that dietary administration of silibinin inhibits the advanced human prostate tumor xenograft growth in athymic nude mice by exhibiting antiproliferative, proapoptotic and antiangiogenic efficacy against prostate tumor [14, 15]. Recently, we have also demonstrated that dietary silibinin inhibits prostate tumor growth and progression in transgenic adenocarcinoma of the mouse prostate (TRAMP) mice by modulating the expression of CDKs, CDKIs, and insulin like growth factor (IGF)-1 and IGF binding protein (IGFBP)-3 [50]. In other studies, we have demonstrated that administration of silibinin significantly inhibits N-butyl-N-(4-hydroxybutyl) nitrosamine induced urinary bladder carcinogenesis in male ICR mice by causing cell cycle arrest and induction of apoptosis [28]. We also found that silibinin inhibits the growth of human bladder tumor xenograft in athymic nude mice by down-regulating survivin and an increase in p53 expression together with enhanced apoptosis [51]. Vinh et al [52] have shown that administration of silymarin reduces the labeling index for BrdU and the cyclin D1-positive cell ratio in various bladder lesions.
Chemopreventive efficacy of silibinin against lung cancer has been extensively studied by our group in both in vitro and in vivo systems. Studies from our laboratory have reported that silibinin suppresses the growth of human non-small-cell lung carcinoma A549 xenograft growth in athymic BALB/c nu/nu mice. Silibinin also reduces systemic toxicity of doxorubicin with an enhanced therapeutic efficacy by modulating NF kappa B mediated signaling pathway in this model [53]. Further studies have carried out to assess the chemopreventive efficacy of silibinin in urethane-induced lung carcinogenesis in A/J mice. Dietary silibinin supplementation significantly inhibited the urethane-induced lung tumorigenesis and tumor size by modulating proteins involved in cell proliferation, inflammation and angiogenesis [41].
Anticancer activity of silymarin has also been demonstrated in both in vivo and in vitro models of colon cancer. Volate et al [54] have shown that silymarin significantly decreases the number of aberrant crypt foci (ACF) in an azoxymethane (AOM) induced rat colon cancer model. Kohno et al [55] have observed that dietary administration of silymarin (100, 500 and 1,000 ppm in diet), either during or after carcinogen exposure (AOM) for 4 weeks, causes significant reduction in the frequency of colonic ACF in a dose-dependent manner. In a long-term experiment, dietary feeding of silymarin (100 and 500 ppm) during the initiation or post-initiation phase of AOM-induced colon carcinogenesis reduced the incidence and multiplicity of colonic adenocarcinoma. They also found that silymarin reduces the PCNA labeling index and increases the number of apoptotic cells associated with decreased levels of beta-glucuronidase activity, PGE2 level and polyamine content in colonic mucosa.
Different laboratories have also investigated the potential of silymarin against breast cancer and there are conflicting reports regarding the chemopreventive efficacy of silymarin/silibinin in mammary carcinogenesis [14, 25, 56]. Dietary supplementation of silymarin increased the number of mammary tumors in 1-methyl-1- nitrosourea (MNU)-induced mammary carcinogenesis and also increased incidence and multiplicity of mammary tumors in MMTV-neu/HER2 transgenic mice [56]. On the contrary, silibinin treatment was shown to strongly inhibit development of mammary tumors as well as lung metastasis in HER-2/neu transgenic mice [57]. Further studies are needed to clearly understand the effect of silymarin and its active constituents against mammary carcinogenesis.
Anticancer effects of silymarin have also been reported in ovarian cancer xenograft models. Gallo et al [40] have found that administration of Silipide by oral gavage to nude mice bearing tumor xenograft of human ovarian cancer cell line A2780 produces significant tumor inhibition, and the downregulation of VEGF receptor 3 and upregulationof angiopoietin-2 was the possible mechanisms for the antiangiogenic activity. Giacomelli et al [58] have shown that Silipide was able to potentiate the cytotoxicity of anticancer drug cisplatin (CDDP) under in vitro conditions, whereas under in vivo conditions, administration of Silipide significantly enhanced the anti-tumor activity of CDDP in mice along with alleviating the toxicity associated with CDDP.
Anticancer effects of silibinin have been reported in renal cell carcinoma where oral administration of silibinin was found to suppress the growth of local and metastatic tumors in xenograft model of renal cell carcinoma by increasing the plasma levels of IGFBP-3, a binding protein for IGF-1 [59]. Yanaida et al [60] have demonstrated that dietary administration of silymarin suppresses 4-nitroquinoline 1-oxide-induced tongue carcinogenesis in male F344 rats by inhibiting cell proliferation and increased apoptotic index. They also found that silymarin decreases the polyamine content and PGE2 level, and that it modifies phase II enzymes’ activity. Overall, these studies strongly suggest the cancer chemopreventive efficacy of silymarin and/or silibinin, and provide a strong rationale for their use in the clinical trials.
Silymarin in Clinical Trials
Human clinical studies have demonstrated that milk thistle extract has significant hepatoprotective, antidiabetic, and cardioprotective effects. The efficacy of silymarin is being evaluated in cancer patients either alone or in combination with other chemotherapeutic agents. Several doses of silymarin have been tested both alone and in conjunction with other drugs in several populations. Silipide, a silibinin formulation, was given orally to patients with colorectal adenocarcinoma at doses of 360, 720, or 1440 mg daily for 7 days and high levels of silibinin were achieved in colorectal mucosa of the patients [61]. Our group has also recently completed a phase-I clinical trial with silibinin in prostate cancer patients. Silibinin phytosome (SiliphosR), a commercial preparation of silibinin, at a dose of 13g, divided in 3 daily doses, appears to be well tolerated in patients with advanced prostate cancer. We also found over 100μM of plasma levels of free silibinin in patients, though this level was not sustained [62]. Now, we are in the process of starting a pilot Phase II clinical trial to assess the effect of silibinin administration on prostate cancer progression using surrogate biomarkers as endpoints. Silymarin was used (along with soy, lycopene and antioxidants) in a phase III clinical trials to delay prostate specific antigen progression after prostatectomy and radiotherapy in prostate cancer patients [63].
Chemotherapeutic agents provide significant protective effects against many cancers; however it has been shown that hepatotoxicity is a frequent even caused by these drugs. Studies have shown that milk thistle has been used in treatment of chemotherapy induced hepatotoxicity and protecting the liver during chemotherapy. Invernizzi et al [64] have shown the use of silymarin in a 34-year-old woman with promyelocytic leukemia. The investigators administered 800 mg/d silymarin during the patient’s methotrexate and 6-mercaptopurine chemotherapy. During the 4 months of treatment with silymarin, the patient had normal liver transaminase levels and there was no further interruption of therapy. Milk thistle supplementation in children with acute lymphopblastic leukemia (ALL) and higher hepatic toxicity has been associated with decrease in liver transaminases level and greater than 50% reduction in total bilirubin. Studies have demonstrated that silymarin may play a role in adjuvant cancer therapy. In vitro studies have shown that silymarin increased daunomycin accumulation, potentiated doxorubicin toxicity and inhibited efflux of these drugs from cancer cells. In a nonrandomized study, patients with brain metastases receiving stereotactic radiotherapy with omega 3 fatty acids and silymarin had longer survival times and a decreased number of radionecroses [65]. Future clinical studies are warranted to examine silymarin activity in multifatorial mechanisms of action, well-designed clinical trials and clarification of adverse effects.
Pharmacology and Metabolism of Silymarin
Preclinical and clinical studies have examined the pharmacokinetics, pharmacodyanamics and metabolism of silymarin. The in vivo effectiveness of silymarin flavonolignans depends on bioavailability and achieving therapeutics concentrations in the organs of interest. The components of the silymarin are poorly soluble in water and studies in both preclinical and clinical have shown only ng/ml in plasma following oral administration of powdered extracts. However, pharmacokinetics studies have shown that bioavailability of silybin has been increased when combines with phosphaditylcholine as silipide, Siliphos or IdB 1016. Barzaghi et al [66] have reported on a trial in nine healthy volunteers receiving IdB 1016 (equivalent to 120 mg silybin) for 8 consecutive days, and showed 340ng/ml and 183 ng/ml silybin plasma levels from day 1 to day 8. Most of the silybin present in the systemic circulation was in conjugated form. Less than 3% of the administered dose was accounted for by urinary recovery of total silybin. To increase the bioavailability of silymarin, Wu et al [67] developed lipid-based self-microemulsifying drug delivery system (SMEDDS). In this study, Wu et al [67] compared the bioavailability of silymarin SMEDDS with that in solution and suspension. After intragastric administration to rabbits, the bioavailability of silymarin SMEDDS was 1.88-and 48.82-fold that of silymarin solution and suspension. Kim et al [68] examined the comparative bioavailability of a Liverman capsule to a Legalon capsule and a silymarin tablet in 24 healthy Korean male volunteers, who received a silybin dose of 120 mg in a 3 × 3 crossover study. They found that oral bioavailability of siibinin after liverman capsule was significantly faster and greater than that after legalon capsule and silymarin tablet. IdB 1016 (silipide), a silibinin phosphoditylcholine formulation showed improved oral availability compared with silymarin, achieving peak plasma levels in healthy volunteers. In another study, patients with colorectal carcinoma received IdB 1016 at dosages of 360, 720, or 1440mg of silibinin daily for 7 days. The achieved levels of silibinin were 0.3 to 4μmol/L in the plasma, 0.3 to 2.5 nmol/g in the liver, and 20 to 141 nmol/g in colorectal tissue [61].
Conclusions
This mini review briefly summarizes multi-targeted chemopreventive and interventive targets and mechanisms of silymarin/silibinin in various in vitro and in vivo cancer models. All these results validate pharmacological safety of silymarin, which is needed for effective chemopreventive as well as chemotherapeutic agent. Silymarin exerts its anticancer effects by multiple molecular mechanisms that could block all stages of carcinogenesis, initiation, promotion and progression. In particular, anti-invasive and anti-metastatic effects of silymarin authenticate its possible usefulness as preventive and therapeutic agent in the treatment of more advanced and aggressive forms of cancer. Clinical studies have shown that silymarin treatment in combination with chemotherapeutic agents reduces the toxicity associated with chemotherapy. Additional clinical research is warranted to evaluate further the chemopreventive as well as chemotherapeutic effects of silymarin and its analogues against various human cancers.
Acknowledgements
Original studies are supported in part by the NCI RO1 grants CA64514, CA102514, CA104286, CA112304, CA113876 and CA116636.
Abbreviations
- CDK
Cyclin-dependent kinase
- EGFR
Epidermal growth factor receptor
- GM-CSF
Granulocyte macrophage colony-stimulating factor
- GSH
Glutathione
- GSHPx
Glutathione peroxidase
- GST
Glutathione S-transferase
- HUVEC
Human umbilical vein endothelial cells
- IFN
Interferon
- IL
Interleukin
- JNK
c-Jun N-terminal kinase
- MAPK
Mitogen activated protein kinase
- MMPs
Matrix metalloproteinases
- iNOS
Inducible nitric oxide synthase
- ROS
Reactive oxygen species
- SAR
Structure activity relationship
- TGF
Transforming growth factor
- TPA
12-O-tetradecanoyl phorbol-13-acetate
- TNF
Tumor necrosis factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Prasain JK, Barnes S. Metabolism and Bioavailability of Flavonoids in Chemoprevention: Current Analytical Strategies and Future Prospectus. Mol. Pharm. 2007;4:846–864. doi: 10.1021/mp700116u. [DOI] [PubMed] [Google Scholar]
- 2.Nishino H, Satomi Y, Tokuda H, Masuda M. Cancer control by phytochemicals. Curr. Pharm. Des. 2007;13:3394–3399. [PubMed] [Google Scholar]
- 3.Post-White J, Ladas EJ, Kelly KM. Advances in the use of milk thistle (Silybum marianum) Integr. Cancer Ther. 2007;6:104–109. doi: 10.1177/1534735407301632. [DOI] [PubMed] [Google Scholar]
- 4.Kroll DJ, Shaw HS, Oberlies NH. Milk thistle nomenclature: why it matters in cancer research and pharmacokinetic studies. Integr. Cancer Ther. 2007;6:110–119. doi: 10.1177/1534735407301825. [DOI] [PubMed] [Google Scholar]
- 5.Lee DY, Liu Y. Molecular structure and stereochemistry of silybin A, silybin B, isosilybin A, and isosilybin B, Isolated from Silybum marianum (milk thistle) J. Nat. Prod. 2003;66:1171–1174. doi: 10.1021/np030163b. [DOI] [PubMed] [Google Scholar]
- 6.Szilági I, Tétényi P, Antus S, Seligmann O, Chari VM, Seitz M, Wagner H. Structure of silandrin and silymonin, two new flavanolignans from a White Blooming silybum marianum variety. Planta Med. 1981;43:121–127. doi: 10.1055/s-2007-971488. [DOI] [PubMed] [Google Scholar]
- 7.MacKinnon SL, Hodder M, Craft C, Simmons-Boyce J. Silyamandin, a new flavonolignan isolated from milk thistle tinctures. Planta Med. 2007;73:1214–1216. doi: 10.1055/s-2007-981595. [DOI] [PubMed] [Google Scholar]
- 8.Ahmed B, Khan SA, Alam T. Synthesis and antihepatotoxic activity of some heterocyclic compounds containing the 1,4-dioxane ring system. Pharmazie. 2003;58:173–176. [PubMed] [Google Scholar]
- 9.Varga Z, Ujhelyi L, Kiss A, Balla J, Czompa A, Antus S. Effect of silybin on phorbol myristate actetate-induced protein kinase C translocation, NADPH oxidase activity and apoptosis in human neutrophils. Phytomed. 2004;11:206–212. doi: 10.1078/0944-7113-00358. [DOI] [PubMed] [Google Scholar]
- 10.Gazak R, Svobodova A, Psotova J, Sedmera P, Prikrylova V, Walterova D, Kren V. Oxidised derivatives of silybin and their antiradical and antioxidant activity. Bioorg. Med. Chem. 2004;12:5677–5687. doi: 10.1016/j.bmc.2004.07.064. [DOI] [PubMed] [Google Scholar]
- 11.Dzubák P, Hajdúch M, Gazák R, Svobodová A, Psotová J, Walterová D, Sedmera P, Kren V. New derivatives of silybin and 2,3-dehydrosilybin and their cytotoxic and P-glycoprotein modulatory activity. Bioorg. Med. Chem. 2006;14:3793–810. doi: 10.1016/j.bmc.2006.01.035. [DOI] [PubMed] [Google Scholar]
- 12.Davis-Searles PR, Nakanishi Y, Kim NC, Graf TN, Oberlies NH, Wani MC, Wall ME, Agarwal R, Kroll DJ. Milk thistle and prostate cancer: differential effects of pure flavonolignans from Silybum marianum on antiproliferative end points in human prostate carcinoma cells. Cancer Res. 2005;65:4448–4457. doi: 10.1158/0008-5472.CAN-04-4662. [DOI] [PubMed] [Google Scholar]
- 13.Deep G, Oberlies NH, Kroll DJ, Agarwal R. Isosilybin B and isosilybin A inhibit growth, induce G1 arrest and cause apoptosis in human prostate cancer LNCaP and 22Rv1 cells. Carcinogenesis. 2007;28:1533–1542. doi: 10.1093/carcin/bgm069. [DOI] [PubMed] [Google Scholar]
- 14.Agarwal R, Agarwal C, Ichikawa H, Singh RP, Aggarwal BB. Anticancer potential of silymarin: from bench to bed side. Anticancer Res. 2006;26:4457–4498. [PubMed] [Google Scholar]
- 15.Singh RP, Agarwal R. Prostate cancer chemoprevention by silibinin: bench to bedside. Mol. Carcinog. 2006;45:436–442. doi: 10.1002/mc.20223. [DOI] [PubMed] [Google Scholar]
- 16.Singh RP, Agarwal R. Cancer chemopreventive agent silibinin, targets mitogenic and survival signaling in prostate cancer. Mutat. Res. 2004;555:21–32. doi: 10.1016/j.mrfmmm.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 17.Zi X, Grasso AW, Kung HJ, Agarwal R. A flavonoid antioxidant, silymarin, inhibits activation of erbB1 signaling and induces cyclin-dependent kinase inhibitors, G1 arrest, and anticarcinogenic effects in human prostate carcinoma DU145 cells. Cancer Res. 1998;58:1920–1929. [PubMed] [Google Scholar]
- 18.Deep G, Agarwal R. Chemopreventive efficacy of silymarin in skin and prostate cancer. Integr. Cancer Ther. 2007;6:130–145. doi: 10.1177/1534735407301441. [DOI] [PubMed] [Google Scholar]
- 19.Manna SK, Mukhopadhyay A, Van NT, Aggarwal BB. Silymarin suppresses TNF-induced activation of NF-kappa B, c- Jun N-terminal kinase, and apoptosis. J. Immunol. 1999;163:6800–6809. [PubMed] [Google Scholar]
- 20.Hsu YC, Chiu YT, Cheng CC, Wu CF, Lin YL, Huang YT. Antifibrotic effects of tetrandrine on hepatic stellate cells and rats with liver fibrosis. J. Gastroenterol. Hepatol. 2007;22:99–111. doi: 10.1111/j.1440-1746.2006.04361.x. [DOI] [PubMed] [Google Scholar]
- 21.Chang JW, Kim CS, Kim SB, Park SK, Park JS, Lee SK. Proinflammatory cytokine-induced NF-kappaB activation in human mesangial cells is mediated through intracellular calcium but not ROS: effects of silymarin. Nephron Exp. Nephrol. 2006;103:156–165. doi: 10.1159/000092906. [DOI] [PubMed] [Google Scholar]
- 22.Kang JS, Jeon YJ, Park SK, Yang KH, Kim HM. Protection against lipopolysaccharide-induced sepsis and inhibition of interleukin-1beta and prostaglandin E2 synthesis by silymarin. Biochem. Pharmacol. 2004;67:175–181. doi: 10.1016/j.bcp.2003.08.032. [DOI] [PubMed] [Google Scholar]
- 23.Matsuda T, Ferreri K, Todorov I, Kuroda Y, Smith CV, Kandeel F, Mullen Y. Silymarin protects pancreatic beta-cells against cytokine-mediated toxicity: implication of c-Jun NH2-terminal kinase and janus kinase/signal transducer and activator of transcription pathways. Endocrinology. 2005;146:175–185. doi: 10.1210/en.2004-0850. [DOI] [PubMed] [Google Scholar]
- 24.Tyagi A, Bhatia N, Condon MS, Bosland MC, Agarwal C, Agarwal R. Antiproliferative and apoptotic effects of silibinin in rat prostate cancer cells. Prostate. 2002;53:211–217. doi: 10.1002/pros.10146. [DOI] [PubMed] [Google Scholar]
- 25.Scambia G, De Vincenzo R, Ranelletti FO, Panici PB, Ferrandina G, D’Agostino G, Fattorossi A, Bombardelli E, Mancuso S. Antiproliferative effect of silybin on gynaecological malignancies: synergism with cisplatin and doxorubicin. Eur. J. Cancer. 1996;32:877–882. doi: 10.1016/0959-8049(96)00011-1. [DOI] [PubMed] [Google Scholar]
- 26.Zi X, Feyes DK, Agarwal R. Anticarcinogenic effect of a flavonoid antioxidant, silymarin, in human breast cancer cells MDA-MB 468: induction of G1 arrest through an increase in Cip1/p21 concomitant with a decrease in kinase activity of cyclin-dependent kinases and associated cyclins. Clin. Cancer Res. 1998;4:1055–1064. [PubMed] [Google Scholar]
- 27.Sharma G, Singh RP, Chan DC, Agarwal R. Silibinin induces growth inhibition and apoptotic cell death in human lung carcinoma cells. Anticancer Res. 2003;23:2649–2655. [PubMed] [Google Scholar]
- 28.Tyagi A, Raina K, Singh RP, Gu M, Agarwal C, Harrison G, Glode LM, Agarwal R. Chemopreventive effects of silymarin and silibinin on N-butyl-N-(4-hydroxybutyl) nitrosamine induced urinary bladder carcinogenesis in male ICR mice. Mol. Cancer Ther. 2007;6:3248–3255. doi: 10.1158/1535-7163.MCT-07-2006. [DOI] [PubMed] [Google Scholar]
- 29.Tyagi A, Agarwal C, Harrison G, Glode LM, Agarwal R. Silibinin causes cell cycle arrest and apoptosis in human bladder transitional cell carcinoma cells by regulating CDKI-CDK-cyclin cascade, and caspase 3 and PARP cleavages. Carcinogenesis. 2004;25:1711–1720. doi: 10.1093/carcin/bgh180. [DOI] [PubMed] [Google Scholar]
- 30.Gu M, Singh RP, Dhanalakshmi S, Agarwal C, Agarwal R. Silibinin inhibits inflammatory and angiogenic attributes in photocarcinogenesis in SKH-1 hairless mice. Cancer Res. 2007;67:3483–3491. doi: 10.1158/0008-5472.CAN-06-3955. [DOI] [PubMed] [Google Scholar]
- 31.Lah JJ, Cui W, Hu KO. Effects and mechanisms of silibinin on human hepatoma cell lines. World J. Gastroenterol. 2007;13:5299–5305. doi: 10.3748/wjg.v13.i40.5299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li LH, Wu LJ, Jiang YY, Tashiro S, Onodera S, Uchiumi F, Ikejima T. Silymarin enhanced cytotoxic effect of anti-Fas agonistic antibody CH11 on A375-S2 cells. J. Asian Nat. Prod. Res. 2007;9:593–602. doi: 10.1080/10286020600882502. [DOI] [PubMed] [Google Scholar]
- 33.Li LH, Wu LJ, Tashiro SI, Onodera S, Uchiumi F, Ikejima TT. Activation of the SIRT1 pathway and modulation of the cell cycle were involved in silymarin’s protection against UV-induced A375-S2 cell apoptosis. J. Asian Nat. Prod. Res. 2007;9:245–252. doi: 10.1080/10286020600604260. [DOI] [PubMed] [Google Scholar]
- 34.Flaig TW, Su LJ, Harrison G, Agarwal R, Glodé LM. Silibinin synergizes with mitoxantrone to inhibit cell growth and induce apoptosis in human prostate cancer cells. Int. J. Cancer. 2007;120:2028–2033. doi: 10.1002/ijc.22465. [DOI] [PubMed] [Google Scholar]
- 35.Zhong X, Zhu Y, Lu Q, Zhang J, Ge Z, Zheng S. Silymarin causes caspases activation and apoptosis in K562 leukemia cells through inactivation of Akt pathway. Toxicology. 2006;227:211–216. doi: 10.1016/j.tox.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 36.Tyagi A, Singh RP, Agarwal C, Agarwal R. Silibinin activates p53-caspase 2 pathway and causes caspase-mediated cleavage of Cip1/p21 in apoptosis induction in bladder transitional-cell papilloma RT4 cells: evidence for a regulatory loop between p53 and caspase 2. Carcinogenesis. 2006;27:2269–2280. doi: 10.1093/carcin/bgl098. [DOI] [PubMed] [Google Scholar]
- 37.Kohno H, Suzuki R, Sugie S, Tsuda H, Tanaka T. Dietary supplementation with silymarin inhibits 3,2′-dimethyl-4-aminobiphenyl-induced prostate carcinogenesis in male F344 rats. Clin. Cancer Res. 2005;11:4962–4967. doi: 10.1158/1078-0432.CCR-05-0137. [DOI] [PubMed] [Google Scholar]
- 38.Jiang C, Agarwal R, Lu J. Anti-angiogenic potential of a cancer chemopreventive flavonoid antioxidant, silymarin: inhibition of key attributes of vascular endothelial cells and angiogenic cytokine secretion by cancer epithelial cells. Biochem. Biophys. Res. Commun. 2000;276:371–378. doi: 10.1006/bbrc.2000.3474. [DOI] [PubMed] [Google Scholar]
- 39.Yang SH, Lin JK, Chen WS, Chiu JH. Anti-angiogenic effect of silymarin on colon cancer LoVo cell line. J. Surg. Res. 2003;113:133–138. doi: 10.1016/s0022-4804(03)00229-4. [DOI] [PubMed] [Google Scholar]
- 40.Gallo D, Giacomelli S, Ferlini C, Raspaglio G, Apollonio P, Prislei S, Riva A, Morazzoni P, Bombardelli E, Scambia G. Antitumour activity of the silybinphosphatidylcholine complex, IdB 1016, against human ovarian cancer. Eur J Cancer. 2003;39:2403–2410. doi: 10.1016/s0959-8049(03)00624-5. [DOI] [PubMed] [Google Scholar]
- 41.Singh RP, Deep G, Chittezhath M, Kaur M, Dwyer-Nield LD, Malkinson AM, Agarwal R. Effect of silibinin on the growth and progression of primary lung tumors in mice. J. Natl. Cancer Inst. 2006;98:846–855. doi: 10.1093/jnci/djj231. [DOI] [PubMed] [Google Scholar]
- 42.Chen PN, Hsieh YS, Chiang CL, Chiou HL, Yang SF, Chu SC. Silibinin inhibits invasion of oral cancer cells by suppressing the MAPK pathway. J. Dent. Res. 2006;85:220–225. doi: 10.1177/154405910608500303. [DOI] [PubMed] [Google Scholar]
- 43.Chu SC, Chiou HL, Chen PN, Yang SF, Hsieh YS. Silibinin inhibits the invasion of human lung cancer cells via decreased productions of urokinase-plasminogen activator and matrix metalloproteinase-2. Mol. Carcinog. 2004;40:143–149. doi: 10.1002/mc.20018. [DOI] [PubMed] [Google Scholar]
- 44.Hsieh YS, Chu SC, Yang SF, Chen PN, Liu YC, Lu KH. Silibinin suppresses human osteosarcoma MG-63 cell invasion by inhibiting the ERK-dependent c-Jun/AP-1 induction of MMP-2. Carcinogenesis. 2007;28:977–987. doi: 10.1093/carcin/bgl221. [DOI] [PubMed] [Google Scholar]
- 45.Lee SO, Jeong YJ, Im HG, Kim CH, Chang YC, Lee IS. Silibinin suppresses PMA-induced MMP-9 expression by blocking the AP-1 activation via MAPK signaling pathways in MCF-7 human breast carcinoma cells. Biochem. Biophys. Res. Commun. 2007;354:165–171. doi: 10.1016/j.bbrc.2006.12.181. [DOI] [PubMed] [Google Scholar]
- 46.Kiruthiga PV, Shafreen RB, Pandian SK, Devi KP. Silymarin protection against major reactive oxygen species released by environmental toxins: exogenous H2O2 exposure in erythrocytes. Basic Clin. Pharmacol. Toxicol. 2007;100:414–419. doi: 10.1111/j.1742-7843.2007.00069.x. [DOI] [PubMed] [Google Scholar]
- 47.Svobodová A, Zdarilová A, Malisková J, Mikulková H, Walterová D, Vostalová J. Attenuation of UVA-induced damage to human keratinocytes by silymarin. J. Dermatol. Sci. 2007;46:21–30. doi: 10.1016/j.jdermsci.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 48.Kidd P, Head K. A review of the bioavailability and clinical efficacy of milk thistle phytosome: a silybin-phosphatidylcholine complex (Siliphos) Altern. Med. Rev. 2005;10:193–203. [PubMed] [Google Scholar]
- 49.Agarwal R, Katiyar SK, Lundgren DW, Mukhtar H. Inhibitory effect of silymarin, an anti-hepatotoxic flavonoid, on 12-O-tetradecanoylphorbol-13-acetate-induced epidermal ornithine decarboxylase activity and mRNA in SENCAR mice. Carcinogenesis. 1994;15:1099–1103. doi: 10.1093/carcin/15.6.1099. [DOI] [PubMed] [Google Scholar]
- 50.Raina K, Blouin MJ, Singh RP, Majeed N, Deep G, Varghese L, Glodé LM, Greenberg NM, Hwang D, Cohen P, Pollak MN, Agarwal R. Dietary feeding of silibinin inhibits prostate tumor growth and progression in transgenic adenocarcinoma of the mouse prostate model. Cancer Res. 2007;67:11083–11091. doi: 10.1158/0008-5472.CAN-07-2222. [DOI] [PubMed] [Google Scholar]
- 51.Singh RP, Tyagi A, Sharma G, Mohan S, Agarwal R. Oral silibinin inhibits in vivo human bladder tumor xenograft growth involving down-regulation of survivin. Clin. Cancer Res. 2008;14:300–308. doi: 10.1158/1078-0432.CCR-07-1565. [DOI] [PubMed] [Google Scholar]
- 52.Vinh PQ, Sugie S, Tanaka T, Hara A, Yamada Y, Katayama M, Deguchi T, Mori H. Chemopreventive effects of a flavonoid antioxidant silymarin on N-butyl-N-(4-hydroxybutyl)nitrosamine-induced urinary bladder carcinogenesis in male ICR mice. Jpn. J. Cancer Res. 2002;93:42–49. doi: 10.1111/j.1349-7006.2002.tb01199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Singh RP, Mallikarjuna GU, Sharma G, Dhanalakshmi S, Tyagi AK, Chan DC, Agarwal C, Agarwal R. Oral silibinin inhibits lung tumor growth in athymic nude mice and forms a novel chemocombination with doxorubicin targeting nuclear factor kappaB-mediated inducible chemoresistance. Clin. Cancer Res. 2004;10:8641–8647. doi: 10.1158/1078-0432.CCR-04-1435. [DOI] [PubMed] [Google Scholar]
- 54.Volate SR, Davenport DM, Muga SJ, Wargovich MJ. Modulation of aberrant crypt foci and apoptosis by dietary herbal supplements (quercetin, curcumin, silymarin, ginseng and rutin) Carcinogenesis. 2005;26:1450–1456. doi: 10.1093/carcin/bgi089. [DOI] [PubMed] [Google Scholar]
- 55.Kohno H, Tanaka T, Kawabata K, Hirose Y, Sugie S, Tsuda H, Mori H. Silymarin, a naturally occurring polyphenolic antioxidant flavonoid, inhibits azoxymethane-induced colon carcinogenesis in male F344 rats. Int. J. Cancer. 2002;101:461–468. doi: 10.1002/ijc.10625. [DOI] [PubMed] [Google Scholar]
- 56.Malewicz B, Wang Z, Jiang C, Guo J, Cleary MP, Grande JP, Lü J. Enhancement of mammary carcinogenesis in two rodent models by silymarin dietary supplements. Carcinogenesis. 2006;27:1739–1747. doi: 10.1093/carcin/bgl032. [DOI] [PubMed] [Google Scholar]
- 57.Provinciali M, Papalini F, Orlando F, Pierpaoli S, Donnini A, Morazzoni P, Riva A, Smorlesi A. Effect of the silybin-phosphatidylcholine complex (IdB 1016) on the development of mammary tumors in HER-2/neu transgenic mice. Cancer Res. 2007;67:2022–2029. doi: 10.1158/0008-5472.CAN-06-2601. [DOI] [PubMed] [Google Scholar]
- 58.Giacomelli S, Gallo D, Apollonio P, Ferlini C, Distefano M, Morazzoni P, Riva A, Bombardelli E, Mancuso S, Scambia G. Silybin and its bioavailable phospholipid complex (IdB 1016) potentiate in vitro and in vivo the activity of cisplatin. Life Sci. 2002;70:1447–1459. doi: 10.1016/s0024-3205(01)01511-9. [DOI] [PubMed] [Google Scholar]
- 59.Cheung CW, Taylor PJ, Kirkpatrick CM, Vesey DA, Gobe GC, Winterford C, Nicol DL, Johnson DW. Therapeutic value of orally administered silibinin in renal cell carcinoma: manipulation of insulin-like growth factor binding protein-3 levels. BJU Int. 2007;100:438–444. doi: 10.1111/j.1464-410X.2007.07012.x. [DOI] [PubMed] [Google Scholar]
- 60.Yanaida Y, Kohno H, Yoshida K, Hirose Y, Yamada Y, Mori H, Tanaka T. Dietary silymarin suppresses 4-nitroquinoline 1-oxide-induced tongue carcinogenesis in male F344 rats. Carcinogenesis. 2002;23:787–794. doi: 10.1093/carcin/23.5.787. [DOI] [PubMed] [Google Scholar]
- 61.Hoh C, Boocock D, Marczylo T, Singh R, Berry DP, Dennison AR, Hemingway D, Miller A, West K, Euden S, Garcea G, Farmer PB, Steward WP, Gescher AJ. Pilot study of oral silibinin, a putative chemopreventive agent, in colorectal cancer patients: silibinin levels in plasma, colorectum, and liver and their pharmacodynamic consequences. Clin. Cancer Res. 2006;12:2944–2950. doi: 10.1158/1078-0432.CCR-05-2724. [DOI] [PubMed] [Google Scholar]
- 62.Flaig TW, Gustafson DL, Su LJ, Zirrolli JA, Crighton F, Harrison GS, Pierson AS, Agarwal R, Glodé LM. A phase I and pharmacokinetic study of silybin-phytosome in prostate cancer patients. Invest. New Drugs. 2007;25:139–146. doi: 10.1007/s10637-006-9019-2. [DOI] [PubMed] [Google Scholar]
- 63.Schröder FH, Roobol MJ, Boevé ER, de Mutsert R, Zuijdgeest-van Leeuwen SD, Kersten I, Wildhagen MF, van Helvoort A. Randomized, double-blind, placebo-controlled crossover study in men with prostate cancer and rising PSA: effectiveness of a dietary supplement. Eur. Urol. 2005;48:922–930. doi: 10.1016/j.eururo.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 64.Invernizzi R, Bernuzzi S, Ciani D, Ascari E. Silymarine during maintenance therapy of acute promyelocytic leukemia. Haematologica. 1993;78:340–341. [PubMed] [Google Scholar]
- 65.Greenlee H, Abascal K, Yarnell E, Ladas E. Clinical applications of Silybum marianum in oncology. Integr. Cancer Ther. 2007;6:158–165. doi: 10.1177/1534735407301727. [DOI] [PubMed] [Google Scholar]
- 66.Barzaghi N, Crema F, Gatti G, Pifferi G, Perucca E. Pharmacokinetic studies on IdB 1016, a silybinphosphatidylcholine complex, in healthy human subjects. Eur. J. Drug Metab. Pharmacokinet. 1990;15:333–338. doi: 10.1007/BF03190223. [DOI] [PubMed] [Google Scholar]
- 67.Wu W, Wang Y, Que L. Enhanced bioavailability of silymarin by self-microemulsifying drug delivery system. Eur. J. Pharm. Biopharm. 2006;63:288–294. doi: 10.1016/j.ejpb.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 68.Kim YC, Kim EJ, Lee ED, Kim JH, Jang SW, Kim YG, Kwon JW, Kim WB, Lee MG. Comparative bioavailability of silibinin in healthy male volunteers. Int. J. Clin. Pharmacol. Ther. 2003;41:593–596. doi: 10.5414/cpp41593. [DOI] [PubMed] [Google Scholar]


