Abstract
The oocytes of many invertebrate and non-mammalian vertebrate species are not only asymmetrical but also polar in the distribution of organelles, localized RNAs and proteins, and the oocyte polarity dictates the patterning of the future embryo. Polarily located within the oocytes of many species is the Balbiani body (Bb), which in Xenopus is known to be associated with the germinal granules responsible for the determination of germ cell fate. In contrast, in mammals, it is widely believed that the patterning of the embryo does not occur before implantation, and that oocytes are non-polar and symmetrical. Although the oocytes of many mammals, including mice and humans, contain Bbs, it remains unknown how and if the presence of Bbs relates to mouse oocyte and egg polarity. Using three-dimensional reconstruction of mouse neonatal oocytes, we showed that mouse early oocytes are both asymmetrical and transiently polar. In addition, the specifics of polarity in mouse oocytes are highly reminiscent of those in Xenopus early oocytes. Based on these findings, we conclude that the polarity of early oocytes imposed by the position of the centrioles at the cytoplasmic bridges is a fundamental and ancestral feature across the animal kingdom.
Keywords: mouse, oocyte, polarity, Balbiani body, Golgi, centriole, zona pellucida
Introduction
In many vertebrate and invertebrate species, oocytes show a dramatic asymmetry in the distribution of their organelles, and in many cases, this asymmetry is the manifestation of the structural and molecular polarity of the oocyte (1-4). There is profound conceptual difference between cell asymmetry and polarity: the asymmetrical distribution of molecules and/or organelles within a cell does not impose or necessarily indicate the existence of cell polarity. Only when (in the given species) the asymmetrically distributed organelles or molecules have an invariable, non-interchangeable, and irreplaceable position shared by all cells of the same type, is a cell considered polar. In a polar cell, any interference with the polar distribution of cell components leads to a disruption of specific functions, and in oocytes, this disruption may have devastating developmental consequences for the future embryo.
The oocyte of the African clawed frog, Xenopus laevis, is one of the most spectacular examples of ultrastructural and molecular polarity. The Xenopus oocyte has a very well-defined animal vegetal polarity (5-7); its animal hemisphere contains the nucleus and the vegetal hemisphere contains a specific structure called the mitochondrial cloud or Balbiani body (Bb), which is composed of thousands of mitochondria, germinal granules (believed to be a germ cell fate determinant), and a subpopulation of localized RNAs and proteins (8-12).
In Xenopus, as in a majority of the other vertebrate and invertebrate species (including Drosophila), each oogonium undergoes several (usually 4) consecutive oogonial divisions with incomplete cytokinesis, resulting in the formation of a cyst (nest) of 16 oocytes connected by intercellular bridges. In panoistic ovaries (e.g. in Xenopus and mouse) all germ cells of the cluster become the oocytes, whereas in meroistic ovaries (e.g. in Drosophila) only one sibling cell becomes the oocyte and the remaining cells differentiate into nurse cells (3,13-20). Xenopus Bbs form during oogonial division as a result of the aggregation of mitochondria and germinal granule material around the centriole pair that invariably faces the cytoplasmic bridges connecting the oogonia and marks where the vegetal hemisphere of the future oocyte will be (18). Consequently, after oogonial division and stage I oocyte formation, the Bb is invariably positioned within the vegetal hemisphere and the nucleus within the animal hemisphere of every oocyte (18). During oocyte growth, the Bb disperses after delivering its germinal granules and localized RNAs and proteins to the oocyte vegetal cortex – the location indispensable for the proper formation of future germ cells and the patterning of the future embryo (5-9, 12, 21,22).
The oocytes of other vertebrate and invertebrate species also contain Bbs, although the organellar and molecular composition of the structure often differs from that in Xenopus, and in a majority of cases, the function of the Bb remains a mystery (2, 3). Although the oocytes of many mammalian species, including humans, have been known for many years to contain Bbs, it was always believed that the mouse oocyte was an exception (2,3). However, in 2007, Pepling and collaborators described the presence of Bb in neonatal mouse oogonia and in the oocytes of primordial follicles. In Xenopus, the predominant components of Bbs are mitochondria (plus germinal granules and sparse Golgi stacks), whereas the mouse Bb is an aggregate of Golgi stacks (with sparse mitochondria and possibly germinal granule material). However, in both species Bbs are positioned asymmetrically to one side of the nucleus (23).
Since it has been generally accepted that mammalian oocytes are non-polar and symmetrical (24), we were very curious about whether, as in Xenopus, the asymmetrical positioning of mouse Bbs would turn out to be a manifestation of or in some way related to oocyte polarity. The information that can be extracted from examining single sections of oocytes using light, confocal, or electron microscopy is insufficient for both the reconstruction of the spatial distribution of organelles within the single oocyte and the comparison of their distribution in different oocytes. To address this issue, we used a computer programs to create a three-dimensional reconstruction of serial semi-thin and thin sections of mouse neonatal oocytes. This allowed us to visualize the spatial distribution of oocyte organelles in relation to the cytoplasmic bridges connecting early oocytes. On the basis of these data and comparisons of mouse and Xenopus early oocytes, we postulate that the existence of an ancestral interspecific principle may govern the formation of oocyte polarity.
Material and methods
Animals and electron microscopy
We isolated ovaries from P0-, P1-, P3-, and P4-stage neonatal outbred Swiss mice and fixed the ovaries in a mixture of 2% formaldehyde (from electron microscopy–grade 16% stock; Ted Pella, Redding, CA) and 0.5% glutaraldehyde (from electron microscopy–grade 8% stock; Ted Pella) in 1x PBS. After fixation for 1 h at room temperature, samples were incubated at 4°C overnight; dehydrated and embedded in Epon (Electron Microscopy Sciences, Hatfield, PA), and then sectioned, processed, and analyzed as described previously (18). For ultrastructural analysis, the ovaries were sectioned at 70 nm; for the three-dimensional reconstruction, they were sectioned at approximately 180 nm. In addition, nest analysis was also performed using light microscopy on semithin 1-μM sections stained with 1% methylene blue in 1% borax as described previously (18). In total we analyzed 20 ovaries and several hundred oocytes.
Immunoelectron microscopy
For immunogold labeling, the ovaries were fixed in 2% glutaraldehyde and 0.5% glutaraldehyde in PBS for 1 h and processed for embedding in Lowicryl (Electron Microscopy Sciences, Hatfield, PA) according to the manufacturer’s specifications. Ultrathin sections (60- to 80-nm thick) were collected on formvar-coated nickel single-slot grids and blocked with 2% bovine serum albumin (Sigma, St. Louis, MO) in PBS and 0.1% NaN3 for 20 min. Following overnight incubation at 4°C with the primary antibodies (rabbit anti-PCM1, Bethyl Laboratories, Inc., Montgomery, TX) or rabbit anti-PCM1 (H-262); Santa Cruz Biotechnology, Santa Cruz, CA) diluted 1:500-1:1000 in the incubation solution (PBS, 1% BSA, 0.1% NaN3). After being washed several times in PBS, the grids were incubated for 2 h at room temperature with the secondary antibody (goat anti-rabbit conjugated to 18 nm gold particles; Jackson Immuno Research Lab. Inc., West Grove, PA), diluted 1:100-1:200 in the incubation solution, and washed in PBS and then in distilled water. After being dried, the sections were treated with uranyl acetate and lead citrate contrast media and viewed with a JEOL 100SX electron microscope at 80 kV. In control experiments, sections were treated as described above, except that incubation with the primary antibody was omitted.
Three-dimensional reconstruction
Three-dimensional reconstructions were performed as in Kloc et al., (18). In short, a set of serial ultrathin or semithin images were aligned using Image Vision Library, a tool kit for the analysis and processing of two-dimensional images (SGI; http://www.sgi.com/software/imagervision). Annotated TIFF images were further processed using Iris Explorer visualization software (NAG), which uses the empirical data and visualizes it in three dimensions (Toll, Numerical Algorithms Group,) http://www.nag.com). Individual stacks of images were combined into a single three-dimensional dataset that contained the bioinformatics needed to define a biological structure. This integrated dataset with the segmentation information was used to extract individual cellular components using various image processing and interpolation modules, some of which were developed by the National Center for Macromolecular Imaging at Baylor College of Medicine (NCMI–BCM, http://ncmi.bcm.tmc.edu). Extracted cellular components were further processed using a marching cube isosurface module to create suitable three-dimensional Open Inventor geometries (Silicon Graphics; http://www.oss.sgi.com/projects/inventor).
Results
Balbiani body and asymmetry of mouse early oocytes
Previously, Pepling et al. (23) showed that Bbs composed of Golgi stacks are present in the oocytes of newborn mice (P0). We analyzed the distribution and ultrastructure of Bbs in the oocytes taken from the ovaries of embryonic (starting from stage E17.5) and newborn (stage P0-P4) mice. We found that fully developed and easily recognizable Bbs are already present in the oocytes of E17.5 embryos, persist through stages P0-P3 (Fig. 1A), and disperse in the majority of P4 oocytes (Fig. 1B). The ultrastructural and three-dimensional reconstruction analyses clearly showed profound asymmetry of the oocytes. We found that the oocyte nucleus was always located in one hemisphere, and the Bbs and majority of mitochondria were in the opposite hemisphere of the oocyte (Figs.1-3). Electron microscopy analysis of P0 oocytes (Fig.1A) showed that the oocytes were surrounded by somatic cells, and that an asymmetrically positioned Bbs were composed of multiple Golgi stacks arranged around the centriole (Fig. 1A). In these oocytes the mitochondria and cisternae of the rough endoplasmic reticulum were distributed at the Bb periphery. In P4 oocytes the Bb disperses (Fig.1B, dashed line marks the remnants of the Bb). We used the semithin serial sections of the oocyte cluster from stage P0 ovary (Fig.2) for the three-dimesional reconstruction of the distribution of organelles in mouse early oocytes. Sixteen semithin sections of a P0 oocyte were used for the reconstruction Fig. 3 A-C shows three different views of single P0 oocytes showing the asymmetrical distribution of the Bb (yellow), nucleus (red) and mitochondria (black).
Fig. 1. Asymmetric distribution of organelles in mouse early oocytes.
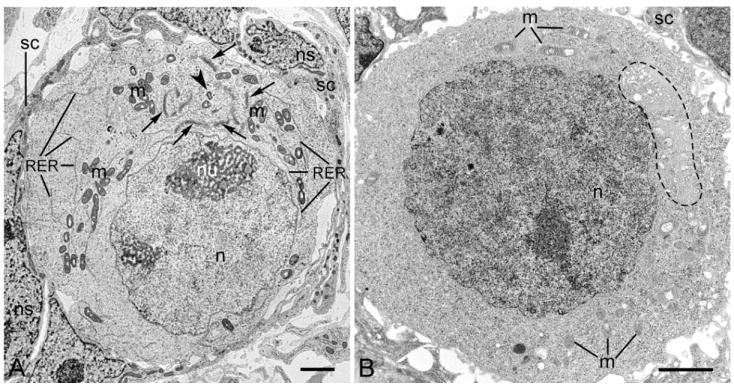
Electron microscopy images of P0 (A) and P4 (B) oocytes. A. An asymmetrically positioned Balbiani body (Bb) is composed of multiple Golgi stacks (arrows) arranged around the centriole (arrowhead). Mitochondria (m) and cisternae of the rough endoplasmic reticulum (RER) are distributed at the Bb periphery; an oocyte nucleus (n) is also marked. The oocyte is surrounded by somatic cells (sc). Somatic cell nucleus (ns), nucleolus (nu). In P4 oocytes, the Bb disperses (dashed line marks the remnants of the Bb). The nucleus (n), m – mitochondria (m), and somatic cell (sc) are also marked. Scale bars are equal to 2 μm.
Fig. 3. Three-dimensional reconstruction of the distribution of organelles in mouse early oocytes.

Sixteen semithin sections of a P0 oocyte were used for the reconstruction (see Fig. 2 and Materials and methods for details). A-C. Three different views of a single P0 oocyte showing the asymmetrical distribution of the Bb (yellow), nucleus (red), and mitochondria (black). A. For clarity, this view shows the nucleus and Bb only. B, C. These views show the nucleus, Bb, and mitochondria (which partially mask the visibility of Bb).
Fig. 2. Semithin sections of oocyte cluster.

Examples of some of the semithin serial sections of the oocyte cluster (2 oocytes are visible) within a mouse ovary (stage P0) used for three-dimesional reconstruction shown in Figs. 3 and 6. The oocyte nucleus is encircled, the Bb (Golgi stacks) is indicated by arrows; and mitochondria are labeled with the letter “m.” Sections were stained with Methylene blue. The scale bar is equal to10 μm.
Balbiani bodies are organized around the centrioles located at the cytoplasmic bridge
We used the electron microscopy images of eighteen ultrathin sections to perform three-dimensional reconstruction of the ultrastructure of Bb in P0 oocytes. Fig. 4 A-D shows four different views of the fragment of P0 oocyte. These images clearly show that Bb is located to one side of the nucleus and is composed of elaborate network of intercalating Golgi stacks arranged around centrioles. The area surrounding the centrioles is enriched with an aggregation of electron-dense pericentriolar material (PCM). The Golgi stacks of the Bb are surrounded by peripherally located mitochondria with some mitochondria penetrating between the Golgi stacks (Fig. 4 A-C). Ultrastructural analysis showed that the pair of centrioles is located in the vicinity (at the rim) of the cytoplasmic bridges (resulting from an incomplete cytokinesis of dividing oogonia) connecting oogonia and early oocytes (Fig. 5A). Immunogold labeling with PCM1 antibody showed that PCM is enriched with PCM1 proteins (Fig. 5C).
Fig. 4. Three-dimensional reconstruction of the ultrastructure of Balbiani body in mouse early oocytes.

Eighteen ultrathin sections of a P0 oocyte were used for the reconstruction (see Materials and methods for details). A-D. These views represent different fragments of the oocyte. The Bb is located to one side of the nucleus (a fragment of the nucleus is colored in red) and contains an elaborate network of Golgi stacks (brown). Golgi and mitochondria (blue) are arranged around the centriole (green; only one centriole of the pair is visible).
Fig. 5. Polarity of mouse early oocytes.

Electron microscopy images of ultrastructure (A, B) and immunogold staining (C) of P0 oocytes. A. An intercellular bridge (circled) connects a P0 oocyte to another oocyte, which is not visitble because it is located on a different plane. Note the characteristic distribution of Bb components: a centriole (arrowhead) at the intercellular bridge is surrounded by Golgi stacks (G); mitochondria (m) are more externally located and are interspersed with the cisternae of the rough endoplasmic reticulum (RER). The synaptonemal complex (open arrow) is visible in the oocyte nucleus (n). The somatic cell (sc) is also labeled. B. In this view, using a higher magnification of the Bb in a P0 oocyte, the centriole (arrowhead), with the adjacent pericentriolar material (PCM; arrows), is surrounded by the Golgi stacks (G). Mitochondrion (m), nucleus (n), and cisternae of the rough endoplasmic reticulum (RER) also also labeled. C. Immunogold labeling with PCM-1 antibody: nanogold particles marking the location of PCM-1 protein are visible within the aggregates of pericentriolar material (arrows) located in the vicinity of the centriole (arrowhead). Scale bar is equal to 1 μm in A, 500 nm in B, and 200 nm in C.
Polarity of the oocyte
Although we established that mouse early oocytes are asymmetrical in the distribution of Bbs, mitochondria, and nuclei, asymmetry does not preclude polarity, as discussed above. Also, because the information that can be obtained from examining single sections of oocytes using light, confocal, or electron microscopy is insufficient for the comparison of the distribution of the organelles between different oocytes, we used a computer-designed three-dimensional reconstruction of serial semithin and thin sections to examine oocyte polarity. Doing so allowed us to visualize the spatial distribution of oocyte organelles in relation to the cytoplasmic bridges connecting early oocytes. Analysis of over 200 oocytes from 20 different ovaries showed that in different oocytes Bbs were invariably located in the same region, i.e., in the vicinity i.e. at the rim of the cytoplasmic bridge (Fig. 6). In addition, Bbs were always organized around the pair of centrioles that faced the cytoplasmic bridge. Electron microscopy analysis of P0 oocytes also showed the presence of the aggregates of vesicles filled with electron-dense material pinching off from the transmembrane face of Golgi complexes (see discussion below). These aggregates were always located polarily at the side of Bb apposing oocyte plasma membrane (Fig. 7). All these findings indicate that early mouse oocytes are both asymmetrical and polar. We believe that the cytoplasmic polarity of mouse early oocyte does not relate to the polarity of the oocyte nucleus. Although some images captured polar localization of synaptonemal complexes in the oocyte nucleus (Fig. 5A), the ultrastructural analysis of tens of nuclei showed nonpolar distribution of the synaptonemal complexes and nucleoli within the oocyte nucleus.
Fig. 6. Polarity of mouse early oocyte; three-dimensional reconstruction of two P0 oocytes connected by intercellular bridge.

A-C. Three different views of two oocytes connected by an intercellular bridge. A, B. For clarity, these views show the nucleus (red) and Bb (yellow) only. Panel B shows the cytoplasmic bridge (black) connecting two oocytes. In panel A, the same bridge, although present, is not shown. C. This view shows the position of the Bb, nucleus (red), mitochondria (gray), and the cytoplasmic bridge (black).
Fig. 7. Polar formation of multivesicular aggregates (MVAs).

Electron microscopy images of a P0 oocyte showing the location of MVAs, which form by budding off from the Golgi stacks. A. Located at one pole of the nucleus (n) is the Bb, with a pair of centrioles (double arrowhead and arrowhead). The centrioles are surrounded by electron-dense pericentriolar material (arrows) and more peripherally by hemispherically distributed Golgi stacks (G). The asymmetry in the distribution of organelles is further shown by the localization of MVAs (v) at one pole of the oocyte, close to the oocyte membrane. The vesicles are filled with electron-dense material. Mitochondria (m), nuclear envelope (ne), and somatic cell (sc) are also labeled. B. The perinuclear cytoplasm bordering the Bb is occupied by mitochondria interspersed with cisternae of the rough endoplasmic reticulum (RER). The nuclear envelope (ne) and nucleus (n) are also labeled. Scale bars are equal to1 μm.
Discussion
By using three-dimensional analysis, we reconstructed the ultrastructure of mouse early oocytes and compared the location of Bbs in different oocytes. We found that Bbs were invariably located in the same region, i.e., in the vicinity of the cytoplasmic bridge. In addition, Bbs were always organized around the pair of centrioles that faced the cytoplasmic bridge. These findings indicate that early mouse oocytes are both asymmetrical and polar.
As is commonly accepted and as has been experimentally confirmed by many independent laboratories, mammalian embryos have a regulative capacity, and the patterning of mammalian embryos does not occur before implantation (25-27). Both of these facts have led many to hold the conviction that mammalian oocytes are non-polar and symmetrical (24). Some recent studies have challenged this notion and instead suggest that blastomeres in mouse early embryos have certain developmental biases (25, 28) and that there is axial polarity (such as GV and spindle positioning and anchoring) during mouse egg maturation (29). However, no studies had yet indicated that mouse early oocytes were polar. In fact, it was commonly believed that mouse oocytes were an exception to those of other animals in their lacking Bbs and being completely symmetrical until Pepling and collaborators published their findings in 2007. Their studies of neonatal mouse oogonia and the oocytes of primordial follicles indicated that Bbs are located asymmetrically, i.e., to one side of the nucleus (23). This finding however, raises the question of whether the location of Bbs to either side of the nucleus is just a manifestation of asymmetry (i.e., that it is random, whether within the oocyte or between different oocytes) or of polarity (i.e., always identical, whether within the oocyte or between different oocytes).
There is astonishing similarity between the polar organization of early oocytes in mice and in Xenopus (Fig. 8; also compare Fig. 5 with Fig.4 in Kloc et al., (18). Although the main components of mouse and Xenopus Bbs are quantitatively different, with mouse Bbs having abundant Golgi stacks with relatively sparse mitochondria and Xenopus Bb having abundant mitochondria with sparse Golgi stacks, in both species, Bbs form around centrioles that face the cytoplasmic bridge. We postulate that the polarity of early oocyte is imposed during oogonial divisions by the position of cytoplasmic bridges and the centrioles, which act as a cytocenters by recruiting in their vicinity various cytoplasmic organelles (which then aggregate into distinct structures described in the literature under the common name of Balbiani body), and that such polarity, imposed by the position of centrioles at the cytoplasmic bridges, is a fundamental and ancestral feature of early oocytes across the animal kingdom (Fig.8)
Fig. 8. The ancestral polarity of animal oocyte.

We believe that it is the positioning of the centriole at the cytoplasmic bridge that imposes the axial polarity on the oocyte, and that the cytocentric role of the centrioles in the aggregation of various cytoplasmic organelles are the features characteristic of developing oocytes across the animal kingdom, i.e., in hypothetical ancestor species. The cytocentric role of the centrioles results in the formation of Bbs that are composed of numerous mitochondria and some Golgi stacks in Xenopus and of numerous Golgi stacks and some mitochondria in mouse early oocytes. In Xenopus, the aggregation of mitochondria around the centrioles probably occurs via mitochondrial movement on the microtubules radiating from the cytocenter (Kloc et al., 2004). Although, at present, it remains unknown if the microtubules participate in the aggregation of the organelles around the centrioles in mouse oocytes, the presence of pericentriolar material (PCM) that is known to be involved in microtubule polymerization strongly suggests such a possibility.
The fact that centrioles act as cytocenters is not surprising. Both in Xenopus and Drosophila, centrioles display microtubule organizing center (MTOC) activity during oogonial divisions and later at the beginning of oocyte differentiation. Also, the position of centrioles is interrelated with the position of the cytoplasmic bridges connecting the oogonia and early oocytes (18, 30, 31). In addition, the Golgi-nucleating function of the centrioles, possibly through the involvement of microtubules (32) and interactions with Golgi-resident proteins TGN38, golgin 97 (33) and formiminotransferase cyclodeaminase (FTCD; 34), has been extensively documented in various cell types (32). In Xenopus early oocytes, the microtubules emanate from the centrioles toward the mitochondria of the assembling Bb and are probably involved in the movement and aggregation of mitochondria around the centrioles (18).
Although we were not able to visualize microtubules in the mouse early oocytes (from P0-P4 neonates), we found abundant pericentriolar material (PCM) close to the centrioles. Since one of the suggested roles of PCM is the nucleation of microtubules (35, 36), the lack of visible microtubules in P0–P4 oocytes might have been a result of either unknown technical issues or an actual absence of the microtubule in P0-P4 oocytes, perhaps because their function is limited to the earlier stages of oocyte development. Thus, it is possible that PCM nucleates microtubules in mouse oocytes, that the microtubules in turn participate in the assembly of Golgi stacks and mitochondria around the centrioles, and that this process occurs during the embryonic stages and is completed before neonatal stage P0, when the Bb is already fully assembled.
In Xenopus and mice, Bbs disperse during oocyte growth (10, 23). In Xenopus, the Bb delivers localized molecules and germinal granules to the vegetal cortex of the oocyte, where they are used for the proper patterning of the embryo (5, 9-12). We still do not know whether the dispersing Bbs in mice polarily deliver any molecules or components to the oocyte cortex or oocyte membrane. In our study, we found that there is very clear polarity in the formation of Golgi vesicles, which form on the trans face of the Golgi stacks in the region of oocyte where the cytoplasmic bridge had previously formed. It is possible that these vesicles polarily deliver or secrete certain components of the oocyte membrane or extracellular matrix, such as the components of the zona pellucida (ZP). The zona pellucida is a glycoprotein extracellular matrix (composed in mice of sulfated glycoproteins ZP1, ZP2 and ZP3) surrounding the plasma membrane of an oocyte. It is essential for fertilization and early development (37). Although the issue of whether the zona pellucida is polar remains controversial (24, 38) showed that the ZP1, ZP2, and ZP3 glycoproteins are asymmetrically distributed in the zona pellucida at various stages of oocyte development. They also showed that distinct multivesicular aggregates (MVAs) were involved in the processing and/or secretion of the ZPs. The origin of MVA (also called vesicular aggregates, VA) has never been traced in details and remains unknown. It is very possible (based on morphology and ultrastructural criteria) that the aggregates of vesicles pinching off from the Golgi complexes observed in our studies, are related to or identical with MVA. If this is the case than the polar distribution of these vesicles may contribute to the initial asymmetry and polarity of the zona pellucida. This in turn would suggest that transient polarity of mouse oocyte translates into polarity of zona pellucida with possible developmental consequences.
In summary, our study clearly shows that early mouse oocytes are asymmetrical and transiently polar. However, it is still unclear whether this structural polarity has a functional role in mouse oocytes or during mouse development or whether it is just a non-functional phylogenetic recapitulation of an ancestral sequence of events characteristic of oocyte development across the animal kingdom.
Acknowledgments
We acknowledge support from National Institutes of Health (NIH) grant HD30284 to Richard Behringer, NIH training grant 5T32HD07324 to L. Nel-Themaat, and National Cancer Institute training grant CA 009299 to M. David Stewart. The National Center for Macromolecular Imaging was supported by National Center for Research Resources/NIH grant P41-RR-02250. Electron microscopy analysis was supported by NIH grant CA 16672 for the M. D. Anderson electron microscopy core facility. The authors thank Ms. Ada Jankowska and Mr. Kenneth Dunner Jr. for superb electron microscopy work and Ms. Elzbieta Kisiel for the preparation of illustrations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gurdon JB. The generation of diversity and pattern in animal development. Cell. 1992;68:185–199. doi: 10.1016/0092-8674(92)90465-o. [DOI] [PubMed] [Google Scholar]
- 2.De Smedt V, Szollosi D, Kloc M. The Balbiani body: Asymmetry in the mammalian oocyte. Genesis. 2000;26:208–212. doi: 10.1002/(sici)1526-968x(200003)26:3<208::aid-gene6>3.3.co;2-e. [DOI] [PubMed] [Google Scholar]
- 3.Kloc M, Bilinski S, Etkin LD. The Balbiani body and germ cell determinants: 150 years later. Curr Topics Dev Biol. 2004b;59:1–36. doi: 10.1016/S0070-2153(04)59001-4. [DOI] [PubMed] [Google Scholar]
- 4.Wodarz A. Establishing cell polarity in development. Nat Cell Biol. 2002;4:E39–44. doi: 10.1038/ncb0202-e39. [DOI] [PubMed] [Google Scholar]
- 5.Heasman J. Patterning the early Xenopus embryo. Development. 2006a;133:1205–17. doi: 10.1242/dev.02304. [DOI] [PubMed] [Google Scholar]
- 6.Heasman J. Maternal determinants of embryonic cell fate. Semin Cell Dev Biol. 2006b;17:93–98. doi: 10.1016/j.semcdb.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 7.White JA, Heasman J. Maternal control of pattern formation in Xenopus laevis. J Exp Zoolog B Mol Dev Evol. 2008;310:73–84. doi: 10.1002/jez.b.21153. [DOI] [PubMed] [Google Scholar]
- 8.Haesman J, Quarmby J, Wylie CC. The mitochondrial cloud of Xenopus oocytes: the source of germinal granule material. Dev Biol. 1984;105:458–469. doi: 10.1016/0012-1606(84)90303-8. [DOI] [PubMed] [Google Scholar]
- 9.Kloc M, Zearfoss R, Etkin LD. Mechanisms of subcellular mRNA localization. Cell. 2002b;108:533–544. doi: 10.1016/s0092-8674(02)00651-7. [DOI] [PubMed] [Google Scholar]
- 10.Kloc M, Etkin LD. Two distinct pathways for the localization of RNAs at the vegetal cortex in Xenopus oocytes. Development. 1995;121:287–297. doi: 10.1242/dev.121.2.287. [DOI] [PubMed] [Google Scholar]
- 11.Kloc M, Etkin LD. RNA localization mechanisms in oocytes. J Cell Sci. 2005;118:269–282. doi: 10.1242/jcs.01637. [DOI] [PubMed] [Google Scholar]
- 12.Zhou Y, King ML. Sending RNAs into the future: RNA localization and germ cell fate. IUBMB Life. 2004;56:19–27. doi: 10.1080/15216540310001658886. [DOI] [PubMed] [Google Scholar]
- 13.Cox RT, Spradling AC. A Balbiani body and the fusome mediate mitochondrial inheritance during Drosophila oogenesis. Development. 2003;130:1579–1590. doi: 10.1242/dev.00365. [DOI] [PubMed] [Google Scholar]
- 14.deCuevas M, Lilly MA, Spradling AC. Germline cyst formation in Drosophila. Annu Rev Genet. 1997;31:405–428. doi: 10.1146/annurev.genet.31.1.405. [DOI] [PubMed] [Google Scholar]
- 15.Gondos B. Intercellular bridges and mammalian germ cell differentiation. Differentiation. 1973;1:177–182. [Google Scholar]
- 16.Gondos B, Zamboni L. Ovarian development: the functional importance of germ cell interconnections. Fert Steril. 1969;20:176–182. doi: 10.1016/s0015-0282(16)36916-3. [DOI] [PubMed] [Google Scholar]
- 17.Huynh JR, St Johnston D. The origin of asymmetry: early polarisation of the Drosophila germline cyst and oocyte. Curr Biol. 2004;14:R438–449. doi: 10.1016/j.cub.2004.05.040. [DOI] [PubMed] [Google Scholar]
- 18.Kloc M, Bilinski S, Dougherty MT, Brey EM, Etkin LD. Formation architecture and polarity of female germline cyst in Xenopus. Dev Biol. 2004a;266:43–61. doi: 10.1016/j.ydbio.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 19.McLaren A. Primordial germ cells in the mouse. Dev Biol. 2003;262:1–15. doi: 10.1016/s0012-1606(03)00214-8. [DOI] [PubMed] [Google Scholar]
- 20.Pepling ME, DeCuevas M, Spradling AC. Germline cysts: a conserved phase of germ cell development. Trends Cell Biol. 1999;9:257–262. doi: 10.1016/s0962-8924(99)01594-9. [DOI] [PubMed] [Google Scholar]
- 21.Kloc M, Dougherty M, Bilinski S, Chan A-P, Brey E, King ML, Patrick P, Etkin LD. Three dimensional ultrastructural analysis of RNA distribution in germinal granules in Xenopus. Dev Biol. 2002a;241:79–93. doi: 10.1006/dbio.2001.0488. [DOI] [PubMed] [Google Scholar]
- 22.Wilk K, Bilinski S, Dougherty MT, Kloc M. Delivery of germinal granules and localized RNAs via the messenger transport organizer pathway to the vegetal cortex of Xenopus oocytes occurs through directional expansion of the mitochondrial cloud. Int J Dev Biol. 2005;49:17–21. doi: 10.1387/ijdb.041906kw. [DOI] [PubMed] [Google Scholar]
- 23.Pepling ME, Wilhelm JE, O’Hara AL, Gephardt GW, Spradling AC. Mouse oocytes within germ cell cysts and primordial follicles contain a Balbiani body. Proc Natl Acad Sci USA. 2007;104:187–192. doi: 10.1073/pnas.0609923104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Motosugi N, Dietrich JE, Polanski Z, Solter D, Hiiragi T. Space asymmetry directs preferential sperm entry in the absence of polarity in the mouse oocyte. PLoS Biol. 2006;4(5):e135. doi: 10.1371/journal.pbio.0040135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Behringer RR. Developmental biology. Dance of the embryo. Science. 2007;316:697–698. doi: 10.1126/science.1142967. [DOI] [PubMed] [Google Scholar]
- 26.Rivera-Perez JA. Axial specification in mice: ten years of advances and controversies. J Cell Physiol. 2007;213:654–660. doi: 10.1002/jcp.21292. [DOI] [PubMed] [Google Scholar]
- 27.Tarkowski AK, Ozdzenski W, Czolowska R. Identical triplets and twins developed from isolated blastomeres of 8- and 16-cell mouse embryos supported with tetraploid blastomeres. Int J Dev Biol. 2005;49:825–832. doi: 10.1387/ijdb.052018at. [DOI] [PubMed] [Google Scholar]
- 28.Zernicka-Goetz M. The first cell-fate decisions in the mouse embryo: destiny is a matter of both chance and choice. Curr Opin Genet Dev. 2006;16:406–412. doi: 10.1016/j.gde.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 29.Albertini DF, Barrett SL. The developmental origins of mammalian oocyte polarity. Semin Cell Dev Biol. 2004;15:599–606. doi: 10.1016/j.semcdb.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 30.Grieder NC, de Cuevas M, Spradling AC. The fusome organizes the microtubule network during oocyte differentiation in Drosophila. Development. 2000;127:4253–4264. doi: 10.1242/dev.127.19.4253. [DOI] [PubMed] [Google Scholar]
- 31.Megraw TL, Kaufman TC. The centrosome in Drosophila oocyte development. Curr Top Dev Biol. 2000;49:385–407. doi: 10.1016/s0070-2153(99)49019-2. [DOI] [PubMed] [Google Scholar]
- 32.Thyberg J, Moskalewski S. Role of microtubules in the organization of the Golgi complex. Exp Cell Res. 1999;246(2):263–279. doi: 10.1006/excr.1998.4326. [DOI] [PubMed] [Google Scholar]
- 33.Takatsuki A, Nakamura M, Kono Y. Possible implication of Golgi-nucleating function for the centrosome. Biochem Biophys Res Commun. 2002;291:494–500. doi: 10.1006/bbrc.2002.6433. [DOI] [PubMed] [Google Scholar]
- 34.Hagiwara H, Tajika Y, Matsuzaki T, Suzuki T, Aoki T, Takata K. Localization of Golgi 58K protein (formiminotransferase cyclodeaminase) to the centrosome. Histochem Cell Biol. 2006;126:251–259. doi: 10.1007/s00418-006-0166-5. [DOI] [PubMed] [Google Scholar]
- 35.Loncarek J, Hergert P, Magidson V, Khodjakov A. Control of daughter centriole formation by the pericentriolar material. Nat Cell Biol. 2008;10:322–328. doi: 10.1038/ncb1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ou Y, Zhang M, Rattner JB. The centrosome: The centriole-PCM coalition. Cell Motil Cytoskeleton. 2004;57:1–7. doi: 10.1002/cm.10154. [DOI] [PubMed] [Google Scholar]
- 37.Hoodbhoy T, Avilés M, Baibakov B, Epifano O, Jiménez-Movilla M, Gauthier L, Dean J. ZP2 and ZP3 traffic independently within oocytes prior to assembly into the extracellular zona pellucida. Mol Cell Biol. 2006;26:7991–7998. doi: 10.1128/MCB.00904-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.El-Mestrah M, Castle PE, Borossa G, Kan FW. Subcellular distribution of ZP1, ZP2, and ZP3 glycoproteins during folliculogenesis and demonstration of their topographical disposition within the zona matrix of mouse ovarian oocytes. Biol Reprod. 2002;66:866–876. doi: 10.1095/biolreprod66.4.866. [DOI] [PubMed] [Google Scholar]


