Separase, an endopeptidase, plays a pivotal role in chromosomal segregation by separating sister chromatids through its proteolytic cleavage of cohesin protein Rad21 during the metaphase to anaphase transition (reviewed by Uhlmann, 2001). During DNA replication, sister chromatid cohesion maintains chromosomal fidelity by establishing accurate chromosomal segregation. The failure in the formation and maintenance of the cohesin complex will result in the premature segregation of chromatids and aneuploidy.
In a recently published study in the Proceedings of the National Academy of Sciences Zhang et al. has demonstrated that that Separase, when overexpressed can also act as an oncogene. Using a tetracycline-inducible system the authors have shown that conditional overexpression of Separase in mouse mammary gland not only induces aneuploidy (aberrant chromosome number) but also mammary tumorigenesis. Overexpression of Separase results in premature separation of chromatids (PCS), lagging chromosomes and anaphase bridges. Up to 30% of PCS and formation of anaphase bridges from lagging chromosomes was observed in Separase induced cells. These findings suggest that hyperactive Separase results in prematurely segregated chromosomes and lagging chromosomes at anaphase. Increased PCS and formation of anaphase bridges is an indicator of chromosomal instability and development of aneuploidy. Induction of Separase resulted in trisomies for chromosome 8, 15 and 17, monosomy for chromosome 10, and amplification of the distal region of chromosomes 8 and 11. This study is also relevant to human breast cancer as Separase protein is found to be significantly overexpressed in >60% of human breast tumors compared to the matched normal tissue.
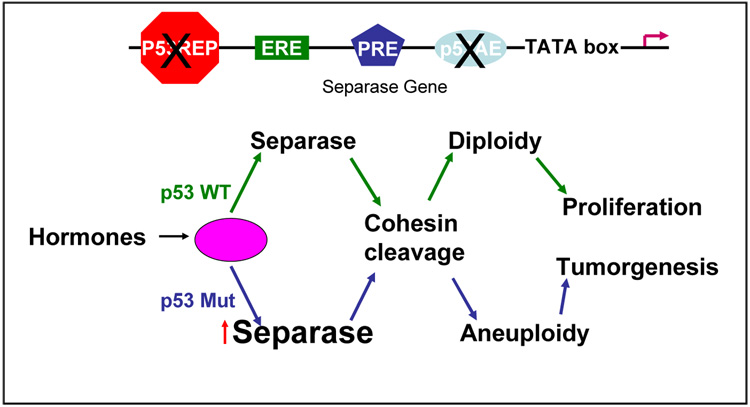
Until now, the role of chromosomal cohesion and separation protein in the development of aneuploidy and tumorigenesis was not tested in vivo. Previous studies from this group reported that hormonal (progesterone and estrogen) stimulation of p53-null mice mammary glands not only induces aneuploidy, but also results in the overexpression of the Separase gene (Pati et al., 2004). FSK3 mouse mammary epithelial cells used in the present studies have both p53 WT and mutant (Ser233–234) alleles. Since overexpression of Separase either constitutionally or conditionally can cause aneuploidy independent of hormonal stimulation, Separase induction is therefore likely to be a key mechanism of hormone-induced aneuploidy in p53 null mammary epithelium. It can be noted that Separase gene contains a number of putative steroid hormone and p53 regulatory elements (Pati et al., 2004).
The casual link between aneuploidy and cancer development has not been fully substantiated. The present study uncovers a direct link from the regulation of cell cycle progression to genomic instability, implying that aneuploidy may be an early event in cancer that exerts strong pressure for tumor development. Identification of Separase protein as an aneuploidy promoter provides a new paradigm in our understanding of the molecular basis of chromosomal instability and tumorigenesis. It is possible that there is a set of regulatory proteins (termed PRAN, Promoter of Aneuploidy) whose failed regulation causes chromosomal instability, resulting in loss or gain of whole or part of one or more chromosomes. It is also probable that a set of PRAN proteins interactively regulated by steroid hormones and tumor suppressor p53. In particular, hormones may regulate the expression of Separase, a key player in chromosomal segregation directly and/or in collaboration with p53 (Fig. 1). Dysregulation of Separase leads to chromosomal instability and tumor formation. Identification of Separase as a PRAN provides a novel class of proteins involved in chromosomal stability and breast cancer progression, and Separase misexpression provides a new experimental model to study the molecular mechanisms of aneuploidy. In summary, these results support hypothesis that Separase when overexpressed act as an oncogene and plays an important role in the development of aneuploidy and tumorigenesis, and inhibition of Separase activity may constitute a novel strategy for treating aneuploid cancers.
Fig. 1.
Hypothetical model showing the interactions of steroids, p53 and Separase in the development of aneuploidy and tumorigenesis
References
- 1.Uhlmann F. Secured cutting: controlling separase at the metaphase to anaphase transition. EMBO Rep. 2001;2(6):487–492. doi: 10.1093/embo-reports/kve113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang N, Ge G, Meyer R, Basu D, Sethi S, Pradhan S, Zhao Yi-Jue, Li X-N, Cai WW, El-Naggar AK, Baladandayuthapani V, Kittrell FS, Rao P, Medina D, Pati D. Overexpression of Separase induces aneuploidy and mammary tumorigenesis. PNAS. 2008 doi: 10.1073/pnas.0801610105. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pati D, Haddad BR, Haegele A, Thompson H, Kittrell FS, Shepard A, Montagna C, Zhang N, Ge G, Otta K, McCarthy M, Ullrich RL, Medina D. Hormone-induced chromosomal instability in p53-null mammary epithelium. Cancer Res. 2004;64:5608–5616. doi: 10.1158/0008-5472.CAN-03-0629. [DOI] [PubMed] [Google Scholar]