Abstract
Cardiovascular sequelae including diabetic cardiomyopathy constitute the major cause of death in diabetic patients. Although several factors may contribute to the development of this cardiomyopathy, the underlying molecular/cellular mechanisms leading to cardiac dysfunction are still partially understood. Recently, a novel paradigm for the role of the adipocytokine resistin in diabetes has emerged. Resistin has been proposed to be a link between obesity, insulin resistance and diabetes. Using microarray analysis, we have recently found that cardiomyocytes isolated from type 2 diabetic hearts express high levels of resistin. However, the function of resistin with respect to cardiac function is unknown. In this study we show that resistin is not only expressed in the heart, but also promotes cardiac hypertrophy. Adenovirus-mediated overexpression of resistin in cultured neonatal rat ventricular myocytes (NRVM) significantly increased sarcomere organization and cell size, increased protein synthesis and increased the expression of atrial natriuretic factor and β-myosin heavy chain. Overexpression of resistin in NRVM was also associated with activation of the mitogen-activated protein (MAP) kinases, ERK1/2 and p38, as well as increased Ser-636 phosphorylation of insulin receptor substrate-1 (IRS-1), indicating that IRS-1/MAPK pathway may be involved in the observed hypertrophic response. Overexpression of resistin in adult cultured cardiomyocytes significantly altered myocyte mechanics by depressing cell contractility as well as contraction and relaxation velocities. Intracellular Ca2+ measurements showed slower Ca2+ transients decay in resistin-transduced myocytes compared to controls, suggesting impaired cytoplasmic Ca2+ clearing or alterations in myofilament activation. We conclude that resistin overexpression alters cardiac contractility, confers to primary cardiomyocytes all the features of the hypertrophic phenotype and promotes cardiac hypertrophy possibly via the IRS-1/MAPK pathway.
Keywords: Resistin, cardiac hypertrophy, insulin resistance, diabetes, MAP kinases, IRS-1, contractility, Ca2+ transients
Introduction
The prevalence of diabetes mellitus and obesity has increased dramatically during the past decade and is reaching epidemic proportions worldwide [1], [2]. Diabetes and obesity are major risk factors for the development of cardiovascular complications and premature death [3]. A significant number of diabetic patients die of heart failure or stroke. With this rise in obesity, interest has been extended to the biology of adipose tissue which releases a large number of cytokines and bioactive mediators (adipokines). These adipokines have been shown to affect several aspects in the pathogenesis of diabetes and cardiovascular diseases.
Resistin, a novel cysteine-rich hormone also known as adipocyte-secreted factor, is secreted by rodent fat cells and is implicated in obesity and type 2 diabetes and insulin resistance [4]. Recombinant resistin protein was found to impair insulin action in normal mice and cultured adipocytes and immunoneutralization of resistin improved insulin action in mice with diet-induced obesity [4]. Plasma resistin levels were increased in genetic murine models of diabetes (db/db), obesity (ob/ob) and diet-induced obese mice, while resistin mRNA levels in obese rodents were often found to be decreased [5],[6]. Resistin is also believed to be a thiazolidinedione (TZD)-regulated protein, a new class of insulin sensitizing drugs. TZD treatment suppresses resistin expression in 3T3-L1 adipocytes and in white adipose tissues of mice fed with a high fat diet. However, the pathophysiological role of resistin in humans has been questioned because the human homologue of resistin is only 59% identical to mouse resistin at the amino acid level and the source of resistin appears to differ between humans and mice [7]. Unlike mice, resistin in humans is undetectable in adipocytes but highly expressed in macrophages. Nevertheless, despite the species differences, survivors of myocardial infarction displayed elevated levels of resistin [8] and increased plasma resistin level was observed in the serum of obese [8] and type 2 diabetic patients [9]. TZD treatment resulted in decreased plasma resistin levels in patients with type 2 diabetes [10], suggesting resistin plays an important role in the etiology of insulin resistance and diabetes; however, others have failed to show this association [11],[12]. Recently, resistin was shown to impair glucose transport in isolated cardiomyocytes [13] and to be up-regulated by cyclic stretch and aorta-caval shut [14], suggesting resistin may affect cardiac function in animal models. However, the underlying mechanism by which resistin impairs cardiac function is unknown.
We identified and sequenced resistin in rat hearts and found that type 1 and 2 diabetic hearts express high levels of resistin. In this study we show for the first time that resistin directly induces cardiac hypertrophy in neonatal cardiomyocytes and induces contractile abnormalities in adult cardiomyocytes. This observation may be important in providing a link between diabetes and hypertrophy.
Materials and Methods
Animal Care, Animal Models of Diabetes
All animal procedures were performed with the approval of IACUC at Mount Sinai School of Medicine and in accordance with the National Institute of Health’s Guide for the Care and Use of Laboratory Animals. For animal models of diabetes we used the streptozotocin (STZ)-induced for type 1 diabetes [15] and the Otsuka Long-Evans Tokushima Fatty (OLETF) (and its control Long-Evans Tokushima Otsuka (LETO)) rat for type 2 diabetes [16].
Production of Recombinant Adenovirus Ad.Retn
The AdEasy Adenoviral vector system (Stratagene, USA) was used to generate recombinant adenoviruses. Full length resistin (Retn) cDNA was isolated from rat heart cDNA library and subcloned into the pShuttle vector (containing the cDNA for enhanced GFP) under the control of CMV promoter. Viral titers were determined by the plaque assay and the absence of replication-competent adenovirus was confirmed by polymerase chain reaction (PCR) to assess for the wild type E1 region.
Real time PCR
The expression of Retn, ANF, β-MHC and 18S rRNA genes was quantified using real time PCR analysis (7500 real-time PCR system, Applied Biosystems). The primers used for amplifying the genes are as follows: Retn forward primer 5’-ATGAGCCACAGCCAGAGCCACAG-3’, Retn reverse primer 5’-TCCTGCCCCCTGCGCTCTC-3’, ANF forward primer 5’-ATCTGATGGATTTCAAGAACC-3’ and ANF reverse primer 5’-CTCTGAGACGGGTTGACTTC-3’, β-MHC forward primer 5’-TTGGCACGGACTGCGTCATC-3’ and β-MHC reverse primer 5’-GAGCCTCCAGAGTTTGCTGAAGGA-3’, 18s rRNA forward primer 5’-TCAAGAA CGAAAGTCGGAGG-3’ and 18s rRNA reverse primer 5’-GGACAT CTAAGGGCAT CAC-3’. Fold changes in gene expression were determined using the relative comparison method with normalization to 18s rRNA.
Isolation and Culture of Neonatal Cardiomyocytes
Spontaneously beating cardiomyocytes were prepared from 1-to 2- day-old Sprague-Dawley rat pups using the Worthington neonatal cardiomyocyte isolation system (Worthington Biochemical Corp.) as described previously [17]
Preparation of protein extracts and Immunoblotting Analysis
Protein samples were prepared from isolated cardiomyocytes using a lysis buffer containing protease inhibitors (Roche). Cell lysates were matched for protein concentration and then separated by SDS-PAGE and transferred onto polyvinlylidene difluoride (PVDF) membranes (Bio-Rad). The membranes were blocked in 5% nonfat milk and incubated with anti-resistin (Axxora), phospho-specific antibodies to ERK1/2, p38, Ser636-IRS-1 (Cell signaling technology), and p54SAPK and p46SAPK (Promega) or total antibodies (Santa Cruz) overnight at 4°C. The membranes were incubated with appropriate secondary antibodies conjugated to horseradish peroxidase (Pierce) and signal intensities were visualized by Chemiluminescence (Pierce). Films from at least four independent experiments were scanned and densities of the immunoreactive bands were evaluated using NIH Image software. The MAPKs activities were verified by the determination of the phosphorylation level of each kinase in cell lysates using specific phosphor-antibodies. Total protein contents of the corresponding MAP kinases were also determined after stripping the phospho-blots in order to verify for protein loading.
Protein Synthesis Rate Measurements
Protein synthesis rates in neonatal cardiomyocytes were determined using [3H]-Leucine incorporation as described previously [17]. [3H]-Leucine incorporation was measured by scintillation counting (MicroBeta Trilux, PerkinElmer). [3H]-Leucine uptakes were measured by stimulating the myocytes with Ad.Retn (MOI 50) or conditioned media containing secreted resistin. Conditioned media were obtained by infecting either HEK 293 cells or cardiomyocytes with Ad.Retn in serum-free medium for 3 hours, the medium was then replaced to remove the adenovirus and the cells were serum-free cultured for an additional 40 hours. The supernatants were then collected and filtered through 0.2 µm filters before use.
Actin Staining and Cell Size Measurements
Cardiomyocytes grown on collagen-coated chamber slides were infected with Ad.Retn for 48 hours and then fixed in 4% paraformaldehyde for 15 minutes, permeabilized with 0.2% Triton X-100, 0.2 M glycine in PBS for 10 minutes and blocked by incubation in 5% BSA solution for 1 hour at room temperature. Cardiomyocytes were stained with Alexa 594 conjugated phalloidin (Invitrogen) and F-actin was analyzed by confocal laser microscopy (Leica TCS-SP confocal microscopy). The surface areas were measured using NIH image software. At least 100 individualized cells were analyzed per each experiment.
Preparation of Adult Cardiomyocytes
Ventricular myocytes were isolated from Sprague-Dawley male rats (body weight 280–300 g) via enzymatic dissociation as described previously [18]. Briefly, the heart was retrogradely perfused for 5 minutes with a Tyrode buffer (137 mM NaCl, 5.4 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 10 mM Glucose, 0.5 mM taurine and 10 mM HEPES (pH 7.4)) then switched to an enzyme solution containing collagenase II (Worthington) and hyaluronidase II (Sigma) for additional 10 to 15 minutes. Ventricular tissues were finely minced and shaken gently in enzyme solution for 20 to 30 minutes. Myocytes were filtered through a nylon mesh, collected, and made calcium tolerant over a period of 15 minutes. They were then resuspended in DMEM supplemented with 5% FBS, 100U/ml penicillin/ streptomycine, 5 mM taurine, 5 mM carnitine and 5 mM creatine and plated at a density of 2×104 cells/ml onto laminin-coated coverslips.
Cell Contractility Measurements
Mechanical properties of ventricular myocytes were assessed using a video-based edge detection system (IonOptix). In brief, isolated control and infected myocytes cultured under identical conditions for 24 hours were attached to coverslips, placed in a chamber mounted on the stage of an inverted microscope (Nikon Eclips TE-100 F) and superfused with a Tyrode buffer (137 mM NaCl, 5.4 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 10 mM Glucose, 0.5 mM Taurine and 10 mM HEPES (pH 7.4)). The cells were field-stimulated at a frequency of 1 Hz, 30V using STIM-AT stimulator/thermostat placed on HLD-CS culture chamber/stim holder (Cell MicroControls). The stimulated myocyte is displayed on a computer monitor using an IonOptix MyoCam camera, which rapidly scans the image area every 8.3 ms such that the amplitude and velocity of shortening or relengthening are recorded with good fidelity. Changes in cell length during shortening and relengthening were captured and analyzed using soft edge software (IonOptix).
Intracellular Ca2+ transient measurements
Adult ventricular myocytes were loaded with 0.5 µM fura2-AM (Invitrogen) for 15 minutes at 25°C and fluorescence measurements were recorded with dual-excitation single-emission fluorescence photomultiplier system (IonOptix). The Myocytes were placed on an inverted microscope and imaged through an Olympus Fluor X40 oil objective, and were exposed to light emitted by a 75-W halogen lamp through either a 360- or 380-nm filter while being stimulated to contract at 1 Hz. Fluorescence emissions were detected between 480 and 520 nm by a photomultiplier tube after initial illumination at 360 nm for 0.5 s and then at 380 nm for the duration of the recording protocol. The 360 nm excitation scan was repeated at the end of the protocol and qualitative changes in the intracellular Ca2+ concentration were inferred from the ratio of the fura-2 fluorescence intensity at both wavelengths.
Statistics
Where appropriate, the data were expressed as mean ± SD. Comparisons of the group means were made with a Student’s t test. P<0.05 was considered statistically significant.
Results
Resistin is expressed in the heart
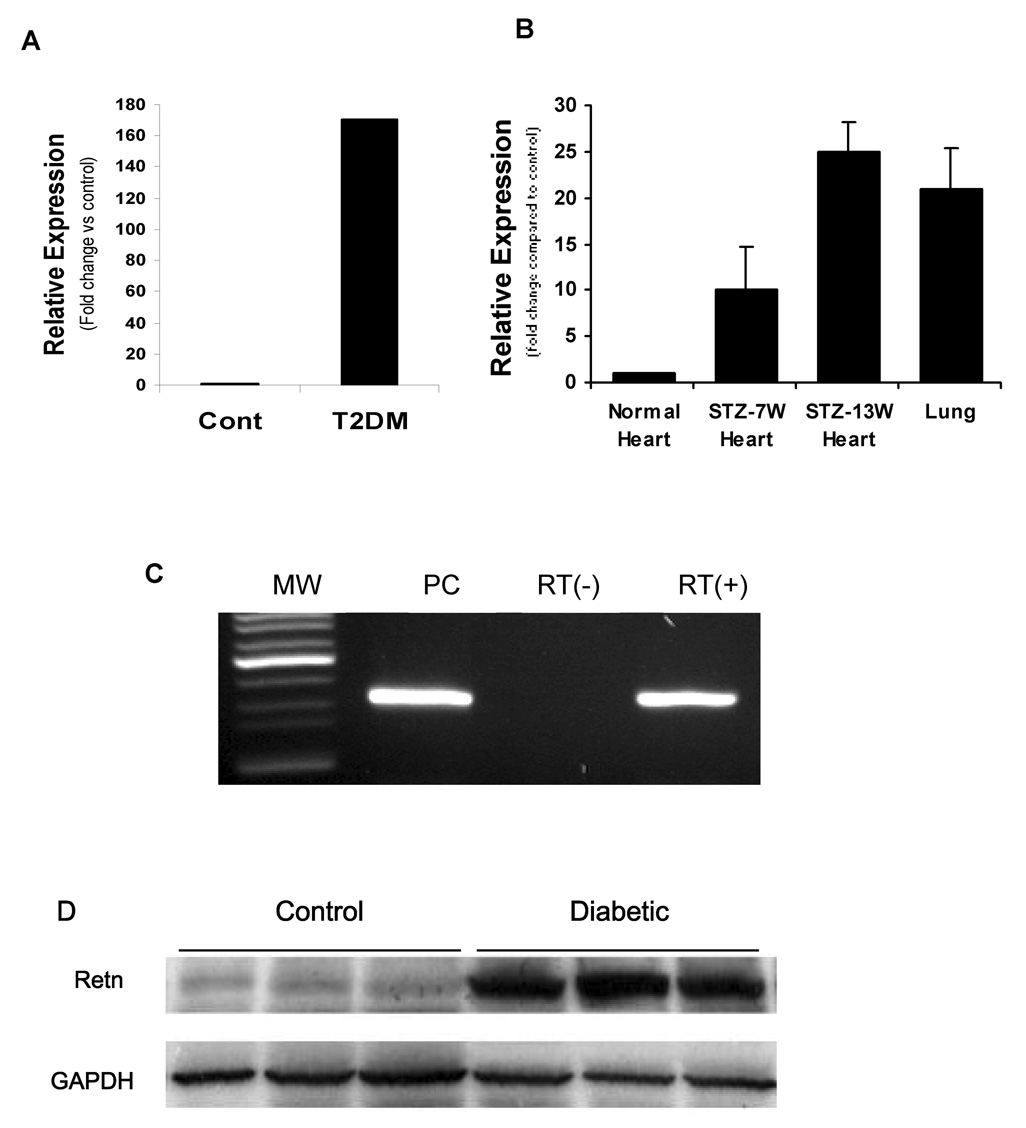
It has been well documented that resistin is highly expressed in fat and lung tissues and in the plasma from diabetic animals. Our observation that heart samples from type 2 diabetic rats showed remarkably elevated expression of resistin mRNA (170.2 fold vs. control) (Figure 1 A) led us to examine whether resistin is also expressed in hearts from normal as well as type 1 diabetic rats. Using qRT-PCR, resistin mRNA was also found to be significantly expressed in type 1 diabetic hearts (25 fold vs. control at 13 weeks post STZ) (Figure 1B) as well as heart and lung tissues from normal rats (Figure 1C). Intriguingly, resistin mRNA expression is remarkably much greater in type 2 than in type 1 diabetic hearts. Immunoblotting analysis shows that resistin is highly expressed in diabetic hearts (figure 1D; type 2 is shown) compared to control hearts which express very low levels. To further confirm resistin expression in the heart, we have isolated and sequenced a full-length resistin cDNA from a rat heart cDNA library. The resistin sequence from the heart was identical to that from the adipose tissue (data not shown).
Figure 1. Detection of resistin mRNA in the heart.
Quantitative RT-PCR showing upregulation of resistin mRNA in cardiomyocytes from type 2 (T2DM) (A) or type 1 (B) diabetic rats compared with normal heart or lung tissue (B). Type 1 diabetic rats were generated by injection of streptozotocin (STZ) for 7 and 13 weeks. Also the expression level was greater in 13 weeks than in 7 weeks post treatment. C, resistin mRNA is detected in the heart (RT+) as well as lung tissue (Positive Control, PC) from normal rats but is not detected in hearts (RT−) without reverse transcriptase during RT-PCR reaction. D. Representative western blot of resistin expression in T2DM hearts. GAPDH is also shown to verify protein loading. MW: Molecular weight standards.
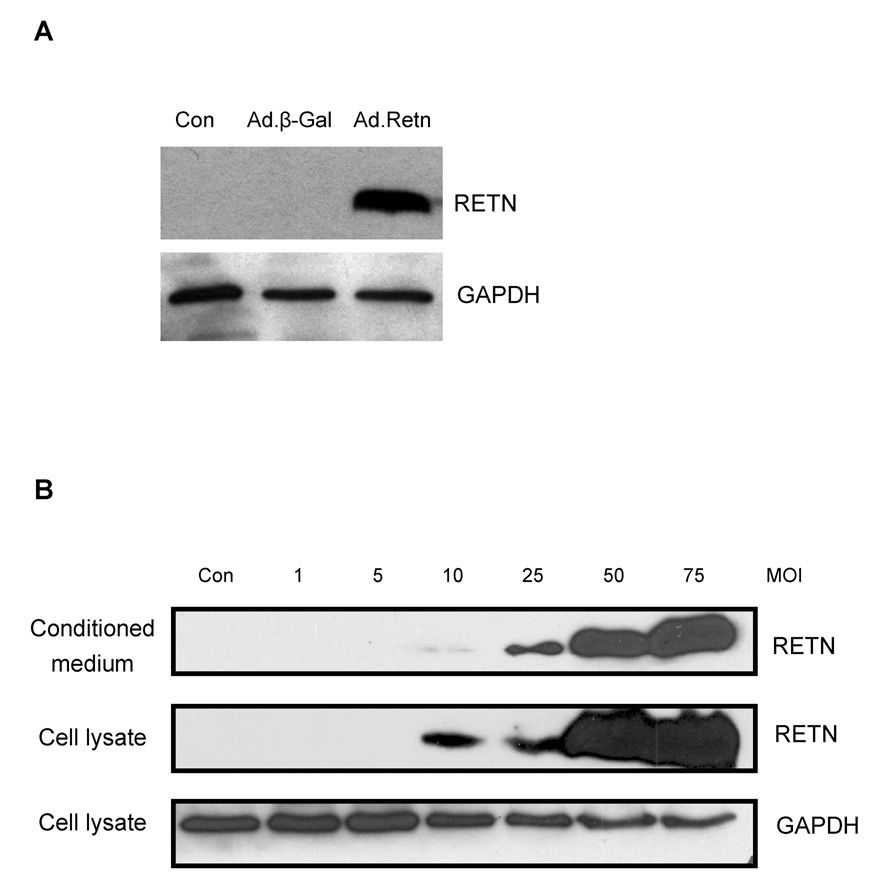
In order to further characterize the function of resistin in the heart, we generated a resistin-expressing recombinant adenovirus, Ad.Retn and β-galactosidase-expressing recombinant adenovirus, Ad.β-Gal. As shown in Figure 2A, cultured neonatal rat ventricular myocytes (NRVM) transduced with Ad.Retn recombinant adenovirus produced a protein band corresponding to resistin as determined by western blot analysis using a rat specific antibody. Since one of the properties of resistin is being a secreted factor, we sought to determine if the cultured myocytes not only expression resistin but they also secrete it into the culture medium. Figure 2B shows that NRVM infected with different multiplicity of infection (MOI) of Ad.Retn express and release into the medium significant amounts of resistin. An MOI of 50 was used in all subsequent experiments.
Figure 2. Overexpression of resistin in neonatal cardiomyocytes.
A, Neonatal cardiomyocytes were infected with adenoviruses encoding β-Gal or Retn. Cardiomyocyte lysates were probed with anti-RETN antibody. B, Neonatal cardiomyocytes were infected with Ad.Retn recombinant adenovirus for 48 hours at the indicated multiplicity of infection (MOI). Resistin protein was detected in both the Ad.Retn-infected cardiomyocytes and conditioned medium at MOI of 10 and up. GAPDH was also immunoblotted to show protein loading.
Hypertrophic Response to Resistin
Since resistin has not been associated with a heart failure phenotype and high expression of resistin in diabetic hearts has been observed for the first time (Fig.1), we sought to investigate whether resistin could induce any phenotypic changes characteristic of the hypertrophic response in cultured NRVM. These include enhanced protein synthesis, increased cell size, enhanced sarcomere organization, and induction of genes including those for several sarcomeric proteins (β-myosin heavy chain, and myosin light chain-2) and for atrial natriuretic proteins.
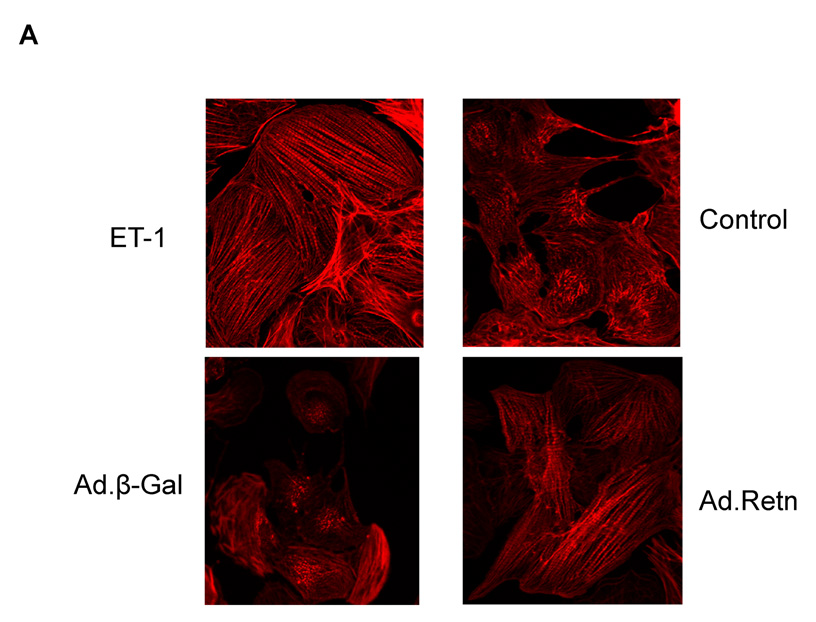
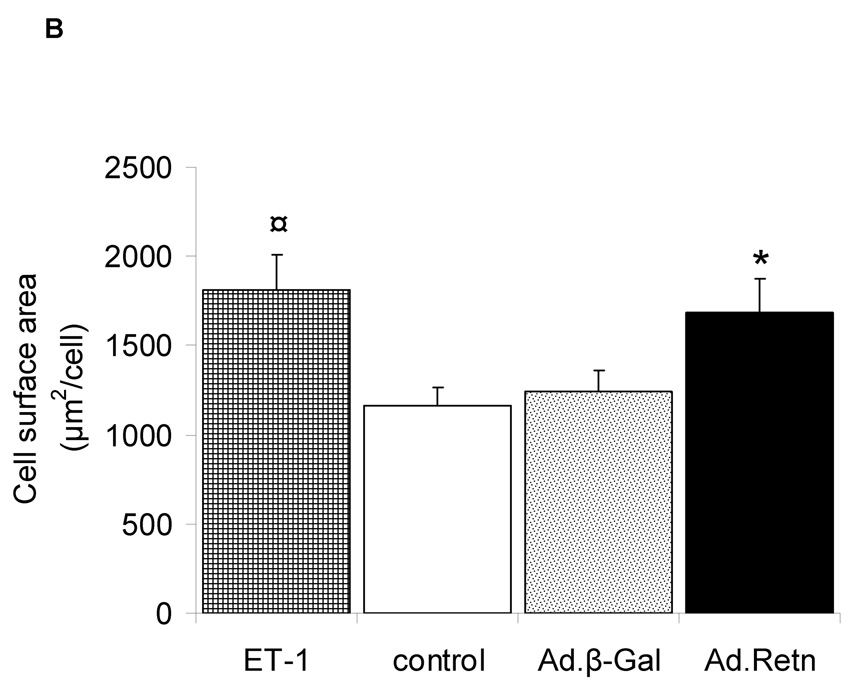
1- Resistin increases Sarcomere Organization and Cell Size
In order to determine whether resistin overexpression induces sarcomere reorganization, we infected cultured NRVM with Ad.Retn or Ad.β-Gal.for 48 hours. Ad.Retn treatment, like ET-1, induced a significant increase in the percentage of cells with highly organized sarcomeres compared with Ad.β-Gal treatment (Figure 3A). Additionally, Ad.Retn significantly increased cell size by about 26% compared to Ad.β-Gal infected cardiomyocytes, as assessed by measuring the surface area of infected cardiomyocytes (Figure 3B). This increase in cell surface area produced by resistin was similar to that induced by ET-1 stimulation (Figure 3B).
Figure 3. Effect of resistin on sarcomere organization and cell surface area.
A, Uninfected neonatal cardiomyocytes (control) or myocytes infected with Ad.β-Gal or Ad.Retn were incubated for 48 hours before staining with Alexa 594-conjugated phallodin. Endothelin-1 (ET-1), a hypertrophic agonist used as a positive control, induced highly organized sarcomere structures. Likewise, Ad.Retn-infected myocytes exhibit a large number of cells with highly organized sarcomeres compared with control or Ad.β-Gal-infected cells. At least 200 cells per condition were scored for the presence of highly organized sarcomeres. B, Cell surface areas of more than 100 individualized cells per condition from 3 independent experiments were measured using Image J software. ¤P<0.01 ET vs control; *P<0.01 Ad.Retn vs Ad.β-Gal. The mean values± SD are shown.
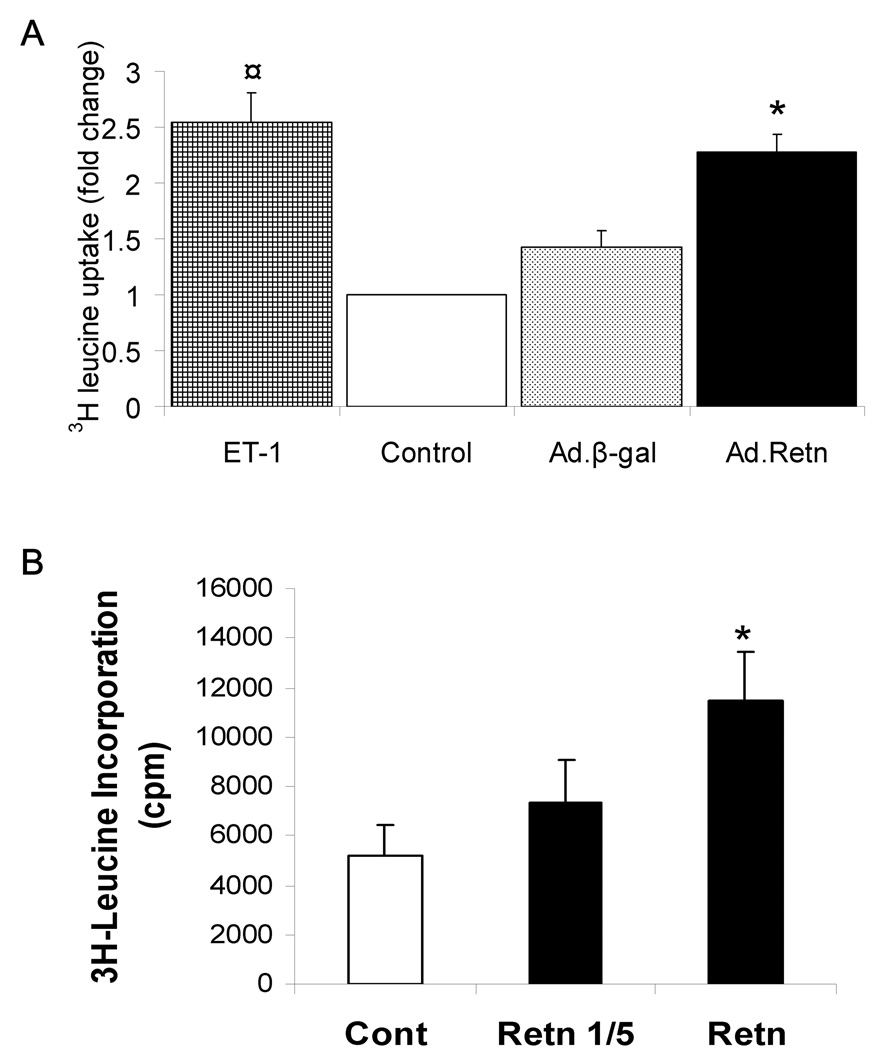
2- Resistin increases 3H-Leucine Incorporation
We examined protein synthesis since it is central to the hypertrophic response. We determined protein synthesis rate by measuring 3H-leucine incorporation in NRVM infected with recombinant adenoviruses. As expected, we found that ET-1 induced a marked increase in [3H]-leucine incorporation (2.55-fold, compared to control; P<0.001). Similarly, cells infected with recombinant adenovirus encoding resistin (figure 4 A) or stimulated with conditioned media containing secreted resistin (figure 4 B) showed a significant increase in [3H]-leucine uptake (2.25- and 2.21-fold increase, respectively, compared to control, P<0.001). Such an effect was not due to infection with the virus alone since infection with Ad.β-Gal did not produce the same enhancement in [3H]-leucine incorporation (P=0.2 versus control) (Figure 4A).
Figure 4. Effect of resistin on protein synthesis.
3H-leucine incorporation was measured in uninfected neonatal cardiomyocytes (control) or myocytes infected with Ad.β-Gal or Ad.Retn (A) and myocytes stimulated with conditioned medium containing secreted resistin (B). Undiluted conditioned medium (Retn) or diluted 1 in 5 (Retn 1/5) was used (B). ¤P<0.001 ET vs control; *P<0.001 Ad.Retn vs Ad.β-Gal. The mean values ±SD are shown.
To further examine the specificity of resistin’s effect on protein synthesis, we performed [3H]-leucine incorporation experiments as above with different MOI (not shown). Infection with a control construct, Ad.β-Gal, resulted in relatively the same [3H]-leucine uptake levels. However, [3H]-leucine uptake in cells infected with Ad.Retn is increased with increasing MOI (not shown). These data indicate that the effect of resistin on protein synthesis is specific to resistin transgene and not due to infection with the virus alone.
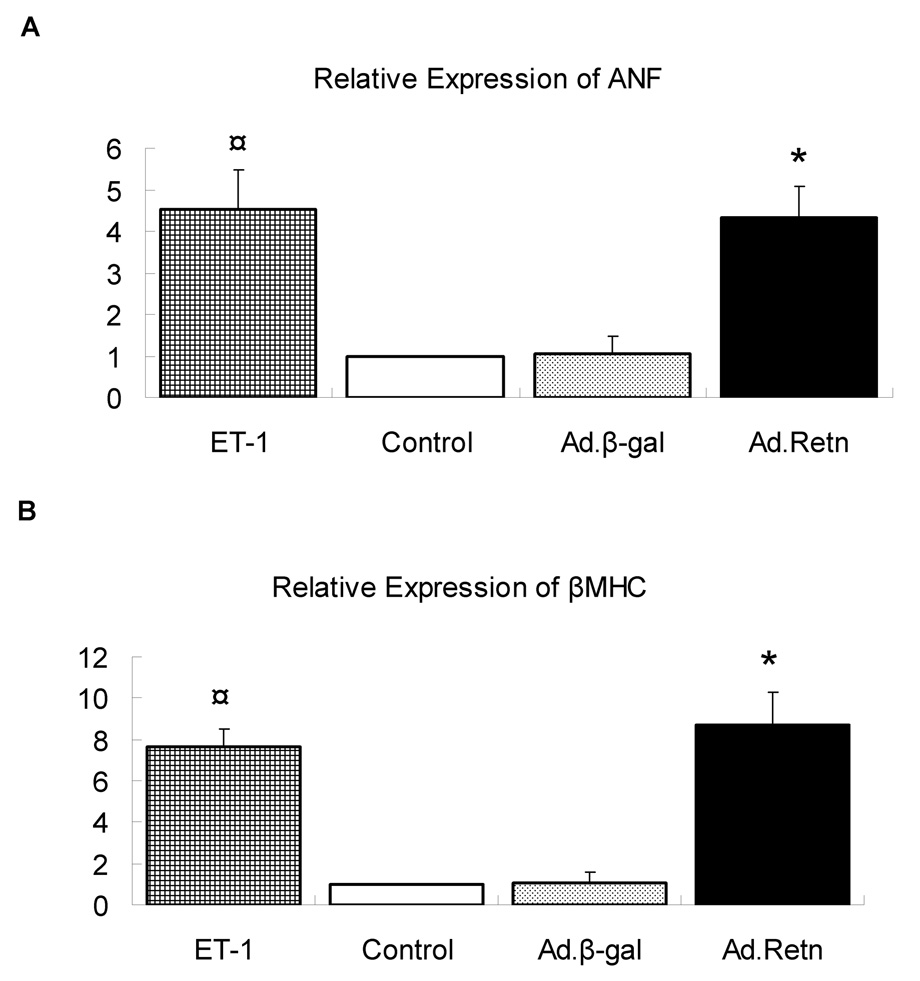
3- Resistin increases ANF and β-MHC Expression
We also examined the role of resistin in modulating the induction of molecular markers such as atrial natriuretic factor (ANF) and β-myosin heavy chain (β-MHC) mRNAs expression. ET-1 induced a significant increase in ANF (4.5-fold, P<0.01; Figure 5A) and β-MHC (7.5-fold, P<0.01; Figure 5B) mRNA levels as determined by quantitative real-time PCR. Interestingly, Ad.Retn overexpression in NRVM also increased significantly the expression of ANF (4.3-fold, P<0.01; Figure 5A) and β-MHC (8.2-fold, P<0.01; Figure 5B) mRNAs compared to either control non-infected or cells infected with Ad.β-Gal. These data further strengthen our hypothesis that resistin overexpression promotes cardiac hypertrophy in NRVM.
Figure 5. Induction of ANF and β-MHC by resistin.
Uninfected neonatal cardiomyocytes (control) or myocytes infected with Ad.β-Gal or Ad.Retn were incubated for 48 hours in serum-free media. The real time PCR for ANF and β-MHC was performed using primers specific to rat genes. The expression was normalized to 18S rRNA. Data normalized against control and expressed as fold change. ¤P<0.01 ET vs control; *P<0.01 Ad.Retn vs Ad.β-Gal‥
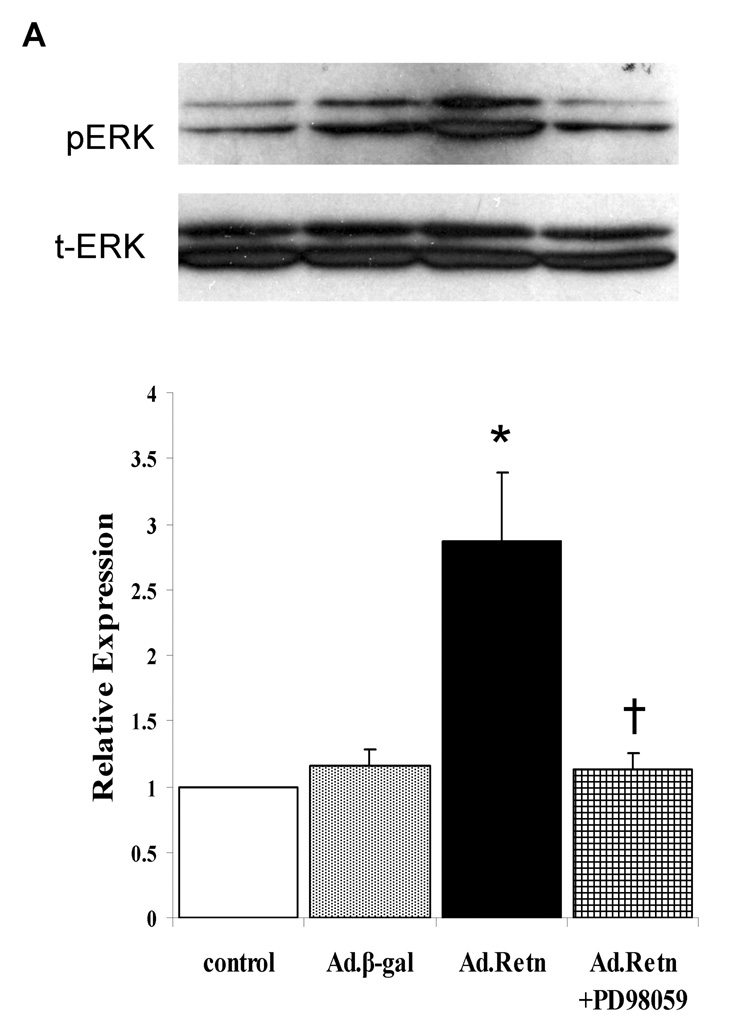
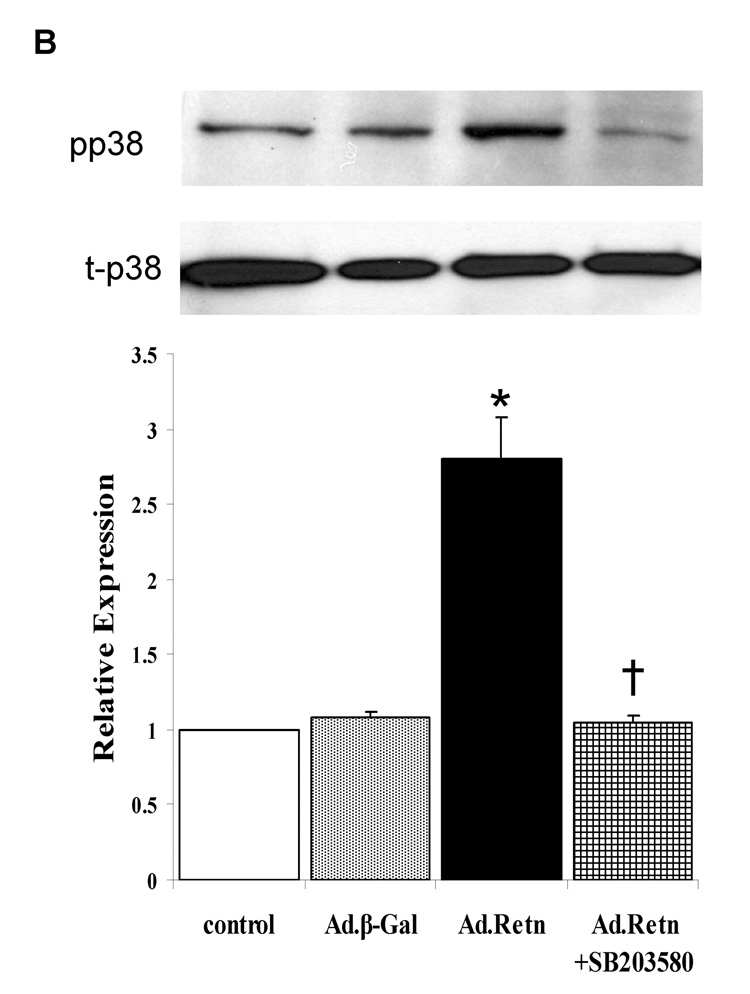
4- Resistin activates MAPKs
Since MAPKinases have been shown to play an important role in cardiac hypertrophy [19], [20], we sought to determine whether resistin potentially mediates its observed effects on cardiac hypertrophic markers through the activation of MAPK signaling pathways (namely, the extracellular signal-regulated protein kinase 1 and 2 (ERK1/2), stress activated protein kinase (SAPK) or c-Jun NH2-terminal kinase (JNK) and p38). Cultured NRVM were infected for 24 hours with Ad.Retn or Ad.β-Gal recombinant adenoviruses and MAPK activity (i.e. phosphorylation) was assessed by western blot analysis using corresponding phospho-specific antibodies. Quantitative analysis of five to six separate phospho-MAPK western blotting experiments is depicted in Figure 6. The data show that resistin overexpression increased significantly the phosphorylation levels of ERKs (2.78-fold, P<0.05; Figure 6A) and p38 (2.75-fold, P<0.05; Figure 6B) compared with controls. This observed resistin-induced ERK1/2 and p38 activation was significantly abolished by the ERK inhibitor PD98059 (Fig. 6A) and the p38 inhibitor SB203580 (Fig. 6B) and certainly was not due to adenoviral infection alone since infection of cells with a virus encoding Ad.β-Gal had no effect on the activities of these kinases (Figure 6A & B). Interestingly, we were not able to detect any significant changes in the phosphorylation level of JNK (not shown). Western blot analysis confirmed the equivalent expression of each of the total kinases in control myocytes and myocytes infected with Ad.β-Gal and Ad.Retn (Figure 6 A & B, blots labeled as total).
Figure 6. ERK and p38 MAP kinases are activated by resistin.
A and B, Uninfected neonatal cardiomyocyte (control) or myocytes infected with Ad.β-Gal or Ad.Retn were incubated for 24 hours in serum-free media. Cell lysates were matched for protein concentration and blotted onto PVDF membranes. The Blots were probed with phospho-specific antibodies against ERK and p38 and then reprobed for the corresponding total protein that confirmed equivalent loading of proteins. C and D, Quantitation of MAPKs activities. The intensity of each chemiluminescent band was quantified by densitometric scanning, and the activity of each MAPK was normalized against its corresponding total protein. Data are from at least three experiments. *P<0.05 Ad.Retn vs Ad.β-Gal; †P<0.05 Ad.Retn vs Ad.Retn + MAPK inhibitors.
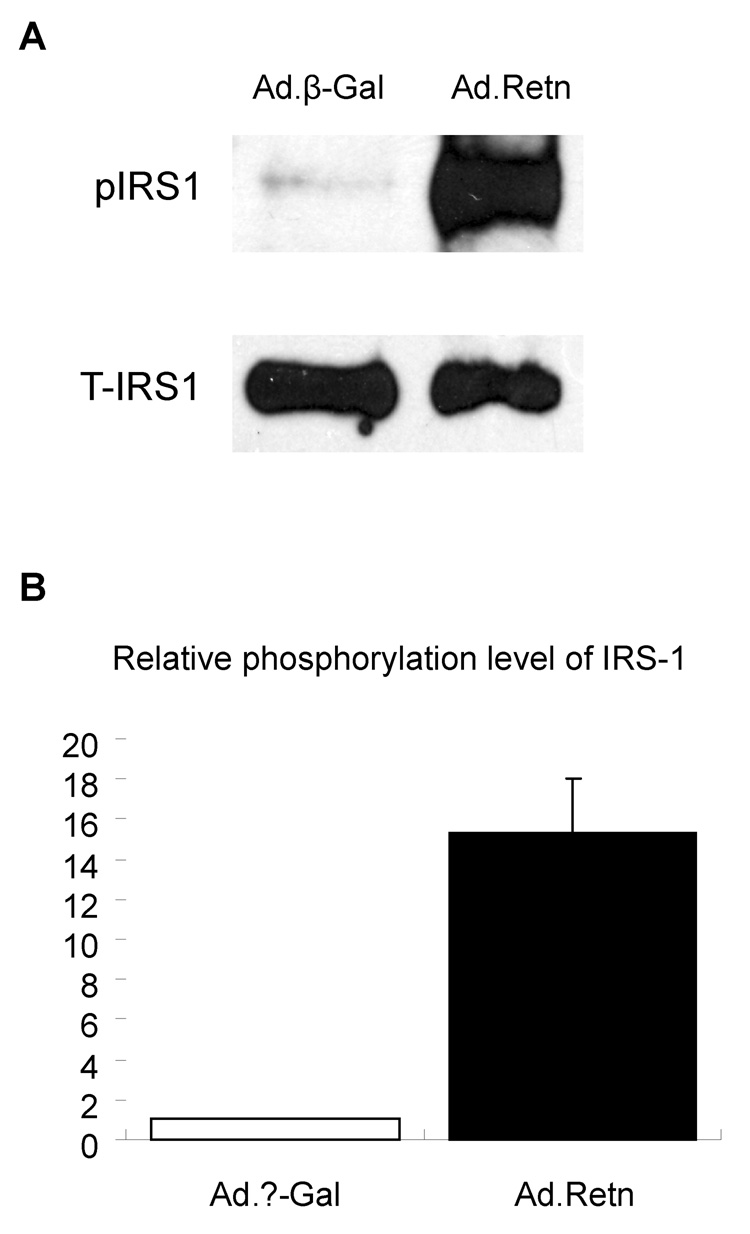
Resistin increases Ser-636 Residue Phosphorylation of IRS-1
Several studies have suggested that serine phosphorylation of insulin receptor substrate (IRS-1) is a potential mechanism for insulin resistance in some models of obesity. We observed that resistin overexpression in rat ventricular myocytes significantly increased the phosphorylation of Ser636/639 residues of IRS-1 compared with Ad.β-Gal infected cardiomyocytes (Figure 7A). Data quantitation and normalization show that resistin induced more than 15-fold increase in IRS-1 phosphorylation compared with β-Gal (P<0.01) (Figure 7B).
Figure 7. Resistin increases serine phosphorylation of IRS-1.
A, Neonatal cardiomyocytes infected with Ad.β-Gal or Ad.Retn were incubated for 24 hours in serum-free media. Western blotting was prepared as described in Materials and Methods. The blots were probed with serine-636/IRS-1 phospho antibody (p-IRS-1) and reprobed with antibody against total IRS-1 protein (T-IRS-1). B. Quantification of serine phosphorylation of IRS-1. Data are from at least three experiments. *P<0.01 Ad.Retn vs Ad. β-Gal.
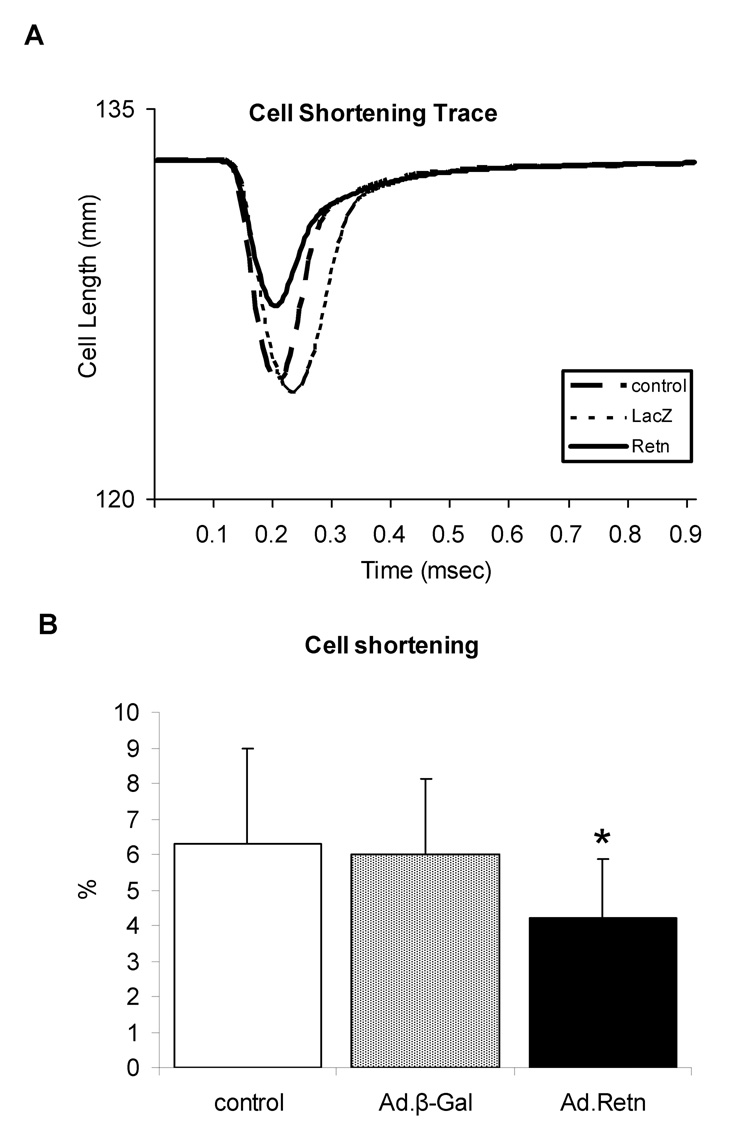
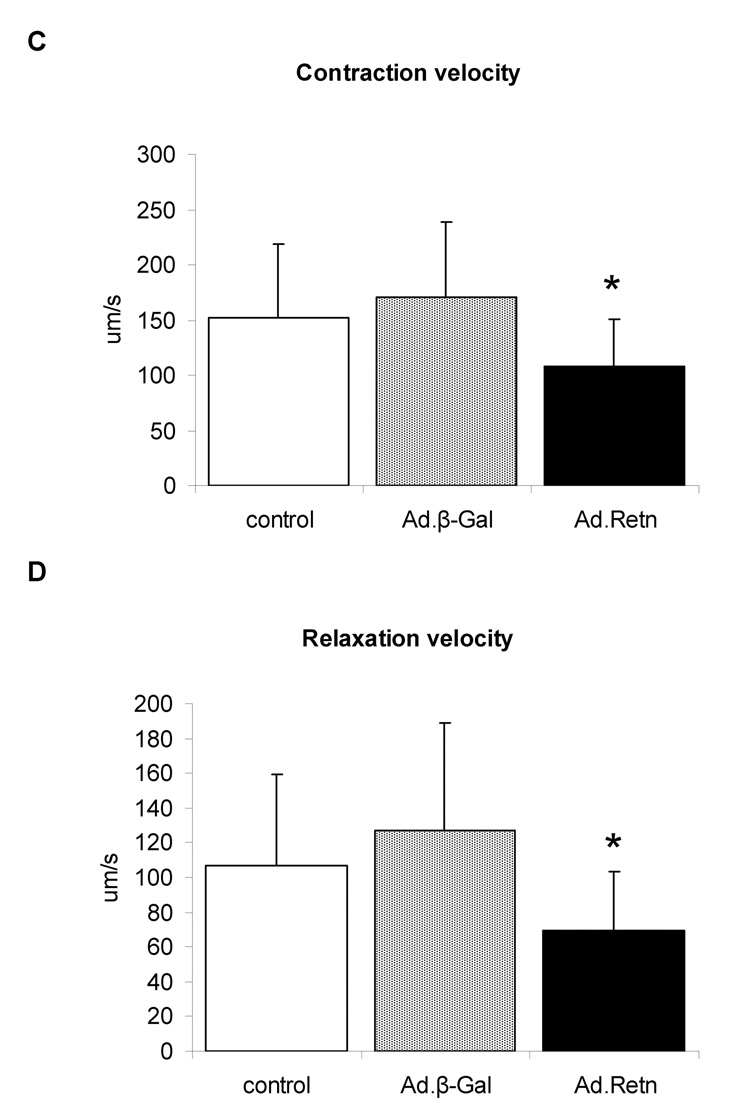
Resistin alters cardiomyocytes contractility
Since impaired contractility has been associated with cardiac hypertrophy and heart failure, we decided to examine whether resistin has any effect on cardiomyocyte contractility and Ca2+ handling. Isolated adult ventricular cardiomyocytes were infected with Ad.Retn or Ad.β-Gal recombinant adenoviruses and then cultured for 24 hours. Myocyte contractility and calcium transients were then determined in the infected cardiomyocytes as described in the Methods section. Figure 8A shows recordings from representative control ventricular myocytes and myocytes infected with Ad.β-Gal or Ad.Retn. Interestingly, Ad.Retn overexpression induced significant decreases in cell shortening (4.2 ± 1.69 %, n=30 vs. 6.0 ± 2.13 %, n=30; P<0.01), in maximal rate of contraction (−dL/dt) (107 ± 43.1 µms, n=30 vs. 170 ± 67.9 µms, n=30; P<0.01) and in maximal rate of relaxation (+dL/dt) (69.6 ± 33.5 µms, n=30 vs. 127.4 ± 61.2 µms, n=30; P<0.01) compared with Ad.β-Gal infected cardiomyocytes (Figure 8B–D). These myocyte mechanics abnormalities are consistent with what has been observed in cardiomyocytes isolated from diabetic rats [21].
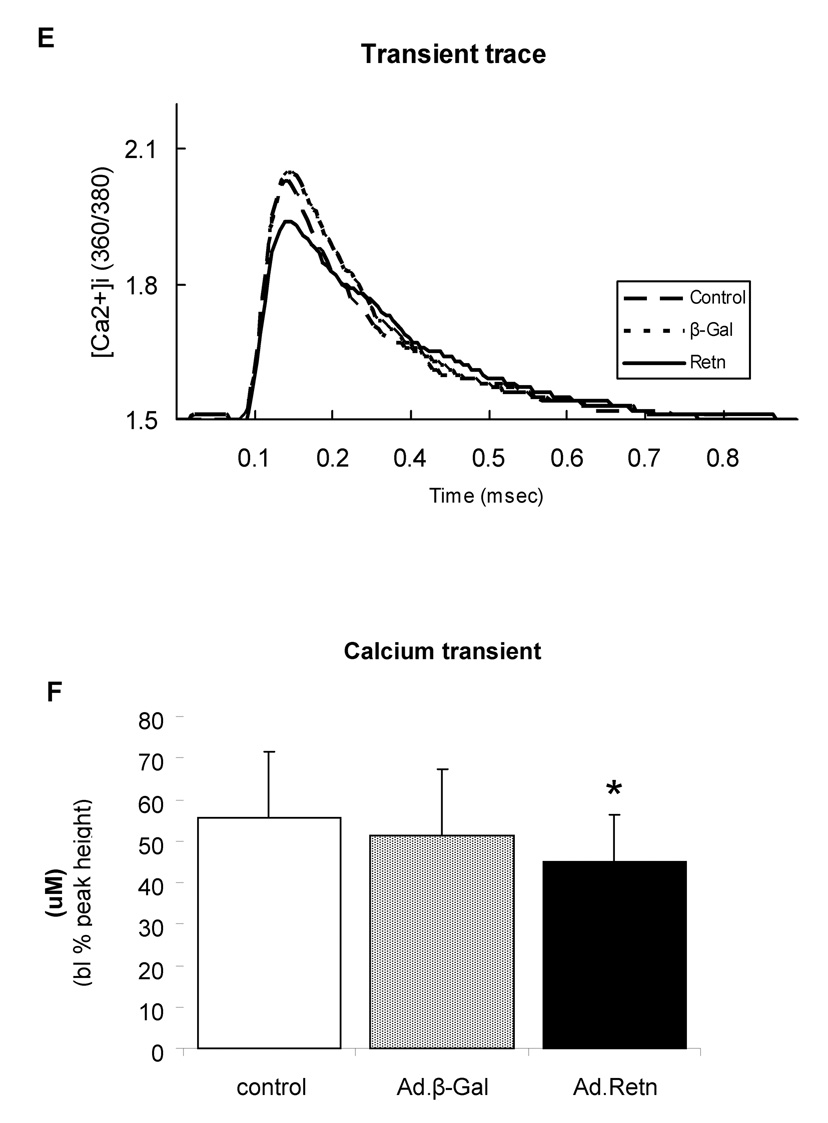
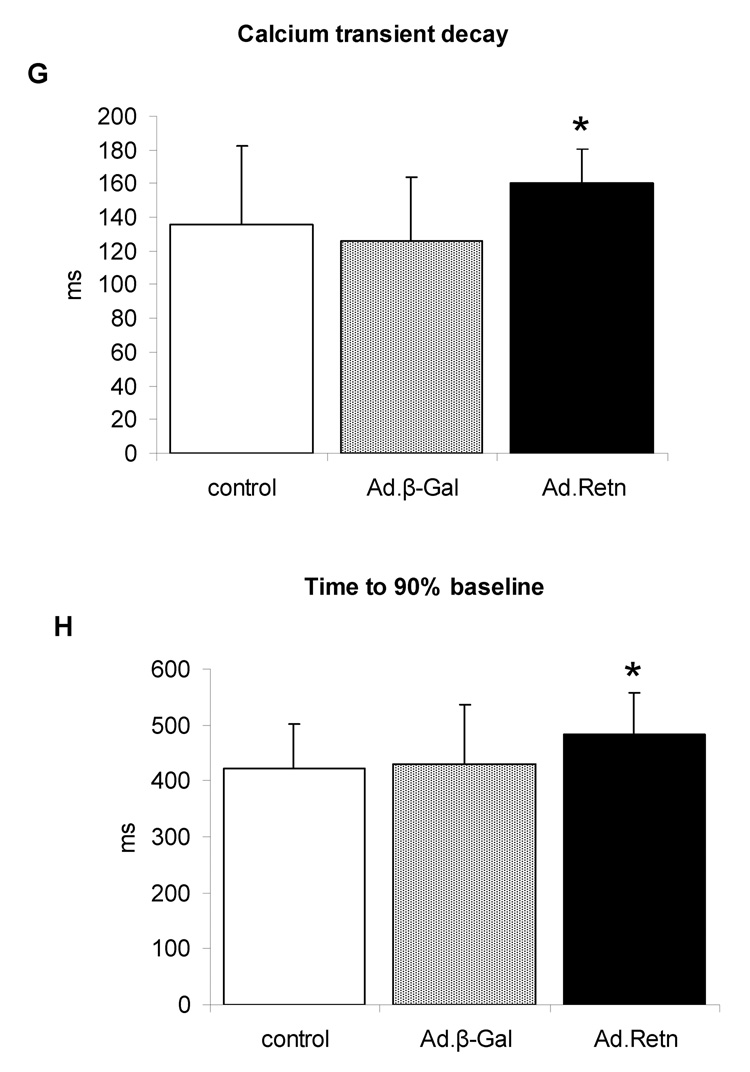
Figure 8. Effects of resistin on cardiomyocyte contractility and Ca2+ transients.
A, Representative recordings of cell shortening from control myocytes or myocytes infected with Ad.β-Gal or Ad.Retn. B. Cell length (%); C. contraction velocity; D. relaxation velocity; E. representative Ca2+ transient traces; F. Ca2+ transients; G. calcium transients decay, and H. time to 90% baseline measured in adult ventricular control myocytes or myocytes infected with Ad.β-Gal or Ad.Retn (cultured under the same conditions) . Average parameters of cardiomyocyte contraction were determined (Ad.β-Gal, n=30; Ad:Retn, n=30); *P<0.01 Ad.Retn vs Ad. β-Gal
To evaluate whether the observed effect of resistin on myocytes contractility are due to its effect on intracellular Ca2+ handling, cytoplasmic Ca2+ transients were determined in fura-2 loaded ventricular myocytes. Ca2+ transients following resistin overexpression were shorter (45 ± 11.4 µM, n=25) compared to either control (55.5 ± 16.1 µM, n=25; P<0.01) or Ad.β-Gal-infected myocytes (51.2 ± 16.1 µM, n=25; P<0.01) (Figure 8 E). In addition, time course of the calcium transients decay was prolonged by resistin (160 ± 21 ms, n=30 vs. β-Gal 126 ± 38 ms, n=30; P<0.01) (Figure 8F). Time to 90% relaxation was 484 ± 74 ms and 423 ± 80 ms, P=0.05 (n=30) for resistin-infected and control myocytes, respectively (Figure 8G). Interestingly, similar changes in Ca2+ transients were observed in myocytes isolated from diabetic animals [21].
Discussion
Obesity is associated with several conditions, the most devastating of which may be diabetes mellitus. According to the International Diabetes Federation [22], the number of people between the ages of 20–79 diagnosed with diabetes in 2007 was 246 million, and it is projected to reach 380 million by 2025 worldwide. Both obesity and diabetes are associated with an increased risk of cardiovascular disease and premature death. Based on this epidemic, the American Heart Association has recently reclassified obesity as a “major, modifiable risk factor” for coronary heart disease [23]. In addition, heart failure caused by ventricular dysfunction, is the major cause of death in diabetic patients.
Although the underlying mechanism linking obesity to cardiovascular-related diseases including cardiac hypertrophy is unknown, recent studies have revealed that the adipose tissue synthesizes and secretes a large number of biologically active factors, including resistin, which may have profound actions in the pathogenesis of diabetes and cardiovascular diseases. Whether resistin is involved in the development of cardiac dysfunction is currently unknown. We therefore studied the effect of resistin as a potential hypertrophic modulator in cultured neonatal rat cardiomyocytes (NRVM) and sought to identify potential mechanisms underlying this effect. In the present study, we show for the first time that resistin is directly involved in the regulation of cardiac hypertrophy. We also show that resistin, known to be secreted mainly by adipose tissue, is expressed in normal rat hearts and in elevated levels in diabetic hearts, suggesting that resistin may have both an autocrine and a paracrine effect on the heart. The abundant expression of resistin in the diabetic heart, particularly in type 2 diabetic subjects, indicates it may be involved in cardiac pathophysiological processes.
Resistin promotion of hypertrophy, in this study, is documented by the observed increases in cell surface area, in percentage of highly organized sarcomeres, in protein synthesis rates and fetal gene expression such as ANF and β-MHC and activation of known hypertrophic signal transduction processes, namely MAPKs. All together, these results show that resistin overexpression confers to primary cardiomyocytes all the features of the hypertrophic phenotype. Our data suggest that the increased levels of resistin observed in diabetic subjects (rodents as well as humans) may contribute to the development of cardiac hypertrophy which is generally associated with diabetes. Diabetic animals present with characteristic phenotypic abnormalities of their heart muscle that result in part from a recapitulation of the fetal gene expression (e.g., ANF, α-skeletal actin and myosin isozyme shifts, with higher β-MHC and lower α-MHC, [24], [25]) similar to what is observed in pressure-overload and heart failure models. These genetic changes lead to myocardial dysfunction in diabetic animal models, resulting in cardiac hypertrophy. Thus heart failure and diabetes appear to induce the same “fetal reprogramming,” which may explain the link between diabetes and cardiac hypertrophy and heart failure.
Hypertrophy involves several intracellular signaling pathways including MAPKs (ERKs, JNKs and p38) activation [26]. We report the sustained activation of the ERK and p38, but not JNK, MAPK signaling pathways following resistin overexpression in neonatal cardiomyocytes. In our hands, the activation of the ERK pathways is observed to occur early followed by p38 activation. This response to resistin was strictly ERK- and p38-dependent since respective inhibitors of both kinases were able to fully inhibit the effect of resistin on cardiomyocytes. This suggests that the induced hypertrophic remodeling observed in cardiomyocytes could result from the upregulation of the ERK as well as p38 pathways following resistin overexpression.
Diabetes is associated with increased levels of resistin which is thought to be responsible for insulin sensitivity impairment in several rat and mouse models [27]. Insulin resistance results in a variety of metabolic defects including hyperglycemia, hyperlipidemia, and hyperinsulinemia. It is generally accepted that the role of insulin may be related to signaling pathways activated by the binding of insulin to its receptor (IR) and the subsequent tyrosine phosphorylation of insulin receptor substrate (IRS) [28], [29], [30]. Insulin actions are generally transmitted through two distinct pathways: 1) phosphorylation of IRS with activation of phosphatidylinositol (PI) 3-kinase that mediates metabolic actions of insulin, and 2) the Ras/MAPK pathway that mediates non-metabolic mitogenic and growth effects of insulin [28], [29], [30, 31], [32].
Concomitant with the observed increase in MAPK pathway in our present study, resistin has greatly enhanced the Ser-636 phosphorylation of IRS-1. This finding is very interesting because phosphorylation of Ser-636 of IRS-1 has recently been shown to interrupt the interaction between IRS-1 and PI3K, leading to reduced PI3K activity and its mediated downstream signaling [33]. IRS-1 hyperphosphorylation at this site was observed in the skeletal muscle of type 2 diabetes subjects [34]. Hence, increased serine phosphorylation of IRS has been proposed as a major cause of insulin resistance induced by a variety of factors including hyperinsulinaemia [35], [36]. Altogether, these data indicate that resistin promotes cardiac hypertrophy probably via IRS-1/MAPK pathway. Several previous studies have established that the activation of the MAPKs leads to down-regulation of IRSs and to the suppression of insulin-induced PI3-Kinase activation through the phosphorylation of the serine residue in IRS-1. Recently, RELMβ (resistin-like molecule beta) was reported to increase the activity of ERK, p38 and, slightly, JNK in primary cultured hepatocytes [37] while resistin was reported to phosphorylate and activate ERK and p38 in smooth muscle cells [38].
Data from our present study reveal that ventricular myocytes transduced with resistin exhibited depressed peak shortening and reduced maximal velocities of shortening and relengthening. Abnormal intracellular Ca2+ regulation, shown here as depressed Ca2+ transients and delayed intracellular Ca2+ clearance, may appear to contribute to these mechanical abnormalities of myocytes overexpressing resistin. These data are consistent with data reported in isolated ventricular myocytes from diabetic models [21]. Depressed rates of Ca2+ removal would result in the inability of the myocyte to adequately maintain low diastolic Ca2+, leading to impairment in myocyte relaxation.
The current observation that resistin may exert a negative effect on cardiac function is further supported by a number of other studies. Resistin has been reported to contribute to the worsening of ischemia/reperfusion injury in isolated rat heart preparations [39] and to be associated with high risk in patients with congestive heart failure [40]. In addition, elevated resistin levels correlated with endothelial dysfunction [41] and with the development of atherosclerosis in mice and in patients with premature coronary artery disease [42]. Our results, therefore, further strengthen the hypothesis that resistin is an important modulator of cardiovascular function.
Traditionally, adipose tissue has been perceived as an inert organ whose function is mainly to store excess energy in the form of triglycerides. However, recent studies have determined that adipose tissue synthesizes and secretes a number of bioactive molecules that have the ability not only to contribute to the pathogenesis of insulin resistance but also to modulate cardiac remodeling. In addition to resistin, as shown in the present study, leptin has also been reported to promote cardiac hypertrophy [43],[44] through a mechanism involving MAPKs, similar to resistin. In contrast, adiponectin was shown to inhibit cardiac hypertrophy possibly through the activation of AMPK (AMP-activated protein kinase) signaling [45]. Interestingly, we found that resistin overexpression in cardiomyocytes leads to the inhibition of AMPK activity (Kim MJ and Lebeche D, unpublished data). Hence, resistin and adiponectin appear to have contrasting effects on cardiac function. Further differences in the action of both peptides have also been reported. In contract to resistin, adiponectin levels correlated negatively with obesity [46], dyslipidemia [47], coronary artery disease [48], insulin resistance [49], [50] and inflammation. Since hypertrophy is one of the earliest manifestations of cardiac dysfunction, such effects further support a role of adipokines in the development of cardiomyopathy. The ability of adipokines to directly affect cardiac function may represent an important mechanistic basis of cardiovascular diseases in subjects with diabetes and obesity.
The present study shows that resistin is expressed in the heart and can modulate cardiac remodeling. Hypertrophy and diastolic dysfunction are frequently observed in diabetes and other obesity-related disorders. The findings reported here suggest that hyper-resistinemia may contribute to the development of pathologic cardiac hypertrophy. Measures to decrease resistin levels could potentially be beneficial for the prevention of cardiac remodeling in pathophysiological disorders such as diabetes and obesity.
Limitations of This Study
This study has demonstrated that overexpression of resistin promotes a hypertrophic response in the short term. It remains to be determined whether long-term infection with Ad.Retn would significantly induce a similar phenotype. Our preliminary data show that chronic expression of resistin is associated with significant increase in left ventricular (LV) wall thickness and LV end-diastolic pressure. It is unknown whether these results may be transferable to the diseased human myocardium. There is a concern related to a potential non-physiological effect of resistin produced by adenoviral infection (with Ad.Retn) of cardiomyocytes. However, as we showed in this study, diabetic hearts express high levels of resistin which we found to be comparable to levels produced by adenoviral infection, suggesting that resistin plays a central role in the maladaptive cardiac phenotype seen in diabetes. Resistin on the other hand is expressed at low levels in normal hearts and may have no effect on cardiac function in normal subjects. Another concern relates to the relevance of resistin upregulation in type 1 diabetes. Although resistin was found to be expressed in both type 1 and type 2 diabetes, it is by far more upregulated in type 2, suggesting that clinically it may be associated with type 2 diabetes. This supports the postulated view of resistin as being a link between obesity, insulin resistance and diabetes. However, this does not exclude the possibility that the molecule is associated with diabetes regardless of its etiology.
Acknowledgments
This study was supported in part by grants from the National Institutes of Health: R01 HL078691, HL057263, HL071763, HL080498, & HL083156, and a Leducq Transatlantic Network (R.J.H.), HL-080498-01 (RJH, WJP), HL-078731 (RJH, DL), and K01 HL076659 (D.L.). WJP was supported by the Global Research Laboratory Program (M6-0605-00-0001) of the Korean Ministry of Science and Technology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.James PT, Rigby N, Leach R. The obesity epidemic, metabolic syndrome and future prevention strategies. Eur J Cardiovasc Prev Rehabil. 2004 Feb;11(1):3–8. doi: 10.1097/01.hjr.0000114707.27531.48. [DOI] [PubMed] [Google Scholar]
- 2.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004 May;27(5):1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 3.Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, Larson MG, et al. Obesity and the risk of heart failure. N Engl J Med. 2002 Aug 1;347(5):305–313. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 4.Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, et al. The hormone resistin links obesity to diabetes. Nature. 2001 Jan 18;409(6818):307–312. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- 5.Rajala MW, Lin Y, Ranalletta M, Yang XM, Qian H, Gingerich R, et al. Cell type-specific expression and coregulation of murine resistin and resistin-like molecule-alpha in adipose tissue. Mol Endocrinol. 2002 Aug;16(8):1920–1930. doi: 10.1210/me.2002-0048. [DOI] [PubMed] [Google Scholar]
- 6.Steppan CM, Lazar MA. Resistin and obesity-associated insulin resistance. Trends Endocrinol Metab. 2002 Jan–Feb;13(1):18–23. doi: 10.1016/s1043-2760(01)00522-7. [DOI] [PubMed] [Google Scholar]
- 7.Ghosh S, Singh AK, Aruna B, Mukhopadhyay S, Ehtesham NZ. The genomic organization of mouse resistin reveals major differences from the human resistin: functional implications. Gene. 2003 Feb 13;305(1):27–34. doi: 10.1016/s0378-1119(02)01213-1. [DOI] [PubMed] [Google Scholar]
- 8.Burnett MS, Devaney JM, Adenika RJ, Lindsay R, Howard BV. Cross-sectional associations of resistin, coronary heart disease, and insulin resistance. J Clin Endocrinol Metab. 2006 Jan;91(1):64–68. doi: 10.1210/jc.2005-1653. [DOI] [PubMed] [Google Scholar]
- 9.McTernan PG, Fisher FM, Valsamakis G, Chetty R, Harte A, McTernan CL, et al. Resistin and type 2 diabetes: regulation of resistin expression by insulin and rosiglitazone and the effects of recombinant resistin on lipid and glucose metabolism in human differentiated adipocytes. J Clin Endocrinol Metab. 2003 Dec;88(12):6098–6106. doi: 10.1210/jc.2003-030898. [DOI] [PubMed] [Google Scholar]
- 10.Bajaj M, Suraamornkul S, Hardies LJ, Pratipanawatr T, DeFronzo RA. Plasma resistin concentration, hepatic fat content, and hepatic and peripheral insulin resistance in pioglitazone-treated type II diabetic patients. Int J Obes Relat Metab Disord. 2004 Jun;28(6):783–789. doi: 10.1038/sj.ijo.0802625. [DOI] [PubMed] [Google Scholar]
- 11.Lee JH, Chan JL, Yiannakouris N, Kontogianni M, Estrada E, Seip R, et al. Circulating resistin levels are not associated with obesity or insulin resistance in humans and are not regulated by fasting or leptin administration: cross-sectional and interventional studies in normal, insulin-resistant, and diabetic subjects. J Clin Endocrinol Metab. 2003 Oct;88(10):4848–4856. doi: 10.1210/jc.2003-030519. [DOI] [PubMed] [Google Scholar]
- 12.Heilbronn LK, Rood J, Janderova L, Albu JB, Kelley DE, Ravussin E, et al. Relationship between serum resistin concentrations and insulin resistance in nonobese, obese, and obese diabetic subjects. J Clin Endocrinol Metab. 2004 Apr;89(4):1844–1848. doi: 10.1210/jc.2003-031410. [DOI] [PubMed] [Google Scholar]
- 13.Graveleau C, Zaha VG, Mohajer A, Banerjee RR, Dudley-Rucker N, Steppan CM, et al. Mouse and human resistins impair glucose transport in primary mouse cardiomyocytes, and oligomerization is required for this biological action. J Biol Chem. 2005 Sep 9;280(36):31679–31685. doi: 10.1074/jbc.M504008200. [DOI] [PubMed] [Google Scholar]
- 14.Wang BW, Hung HF, Chang H, Kuan P, Shyu KG. Mechanical stretch enhances the expression of resistin gene in cultured cardiomyocytes via tumor necrosis factor-alpha. American journal of physiology. 2007 Oct;293(4):H2305–H2312. doi: 10.1152/ajpheart.00361.2007. [DOI] [PubMed] [Google Scholar]
- 15.Rodrigues B, Cam MC, Kong J, Goyal RK, McNeill JH. Strain differences in susceptibility to streptozotocin-induced diabetes: effects on hypertriglyceridemia and cardiomyopathy. Cardiovasc Res. 1997 Apr;34(1):199–205. doi: 10.1016/s0008-6363(97)00045-x. [DOI] [PubMed] [Google Scholar]
- 16.Kawano K, Hirashima T, Mori S, Saitoh Y, Kurosumi M, Natori T. Spontaneous long-term hyperglycemic rat with diabetic complications. Otsuka Long-Evans Tokushima Fatty (OLETF) strain. Diabetes. 1992 Nov;41(11):1422–1428. doi: 10.2337/diab.41.11.1422. [DOI] [PubMed] [Google Scholar]
- 17.Lebeche D, Kang ZB, Hajjar R. Candesartan abrogates G protein-coupled receptors agonist-induced MAPK activation and cardiac myocyte hypertrophy. J Renin-Angio-Aldo S. 2001;2 supplement 1:S154–S161. doi: 10.1177/14703203010020012701. [DOI] [PubMed] [Google Scholar]
- 18.Jeong D, Cha H, Kim E, Kang M, Yang DK, Kim JM, et al. PICOT inhibits cardiac hypertrophy and enhances ventricular function and cardiomyocyte contractility. Circ Res. 2006 Aug 4;99(3):307–314. doi: 10.1161/01.RES.0000234780.06115.2c. [DOI] [PubMed] [Google Scholar]
- 19.Sadoshima J, Izumo S. The cellular and molecular response of cardiac myocytes to mechanical stress. Annu Rev Physiol. 1997;59:551–571. doi: 10.1146/annurev.physiol.59.1.551. [DOI] [PubMed] [Google Scholar]
- 20.Sugden PH, Clerk A. Regulation of mitogen-activated protein kinase cascades in the heart. Adv Enzyme Regul. 1998;38:87–98. doi: 10.1016/s0065-2571(97)00010-1. [DOI] [PubMed] [Google Scholar]
- 21.Ren J, Davidoff AJ. Diabetes rapidly induces contractile dysfunctions in isolated ventricular myocytes. Am J Physiol. 1997 Jan;272(1 Pt 2):H148–H158. doi: 10.1152/ajpheart.1997.272.1.H148. [DOI] [PubMed] [Google Scholar]
- 22.Diabetes Atlas. International Diabetes Federation 2007; 3 rd ed. http://www.eatlas.idf.org/media/ [PubMed]
- 23.Eckel RH, Kahn R, Robertson RM, Rizza RA. Preventing cardiovascular disease and diabetes: a call to action from the American Diabetes Association and the American Heart Association. Circulation. 2006 Jun 27;113(25):2943–2946. doi: 10.1161/CIRCULATIONAHA.106.176583. [DOI] [PubMed] [Google Scholar]
- 24.Depre C, Young ME, Ying J, Ahuja HS, Han Q, Garza N, et al. Streptozotocin-induced changes in cardiac gene expression in the absence of severe contractile dysfunction. J Mol Cell Cardiol. 2000 Jun;32(6):985–996. doi: 10.1006/jmcc.2000.1139. [DOI] [PubMed] [Google Scholar]
- 25.Dillmann WH. Diabetes mellitus induces changes in cardiac myosin of the rat. Diabetes. 1980 Jul;29(7):579–582. doi: 10.2337/diab.29.7.579. [DOI] [PubMed] [Google Scholar]
- 26.Molkentin JD, Dorn IG., 2nd Cytoplasmic signaling pathways that regulate cardiac hypertrophy. Annu Rev Physiol. 2001;63:391–426. doi: 10.1146/annurev.physiol.63.1.391. [DOI] [PubMed] [Google Scholar]
- 27.Lazar MA. Resistin- and Obesity-associated metabolic diseases. Horm Metab Res. 2007 Oct;39(10):710–716. doi: 10.1055/s-2007-985897. [DOI] [PubMed] [Google Scholar]
- 28.Kolter T, Uphues I, Eckel J. Molecular analysis of insulin resistance in isolated ventricular cardiomyocytes of obese Zucker rats. Am J Physiol. 1997 Jul;273(1 Pt 1):E59–E67. doi: 10.1152/ajpendo.1997.273.1.E59. [DOI] [PubMed] [Google Scholar]
- 29.Shaw LM. Identification of insulin receptor substrate 1 (IRS-1) and IRS-2 as signaling intermediates in the alpha6beta4 integrin-dependent activation of phosphoinositide 3-OH kinase and promotion of invasion. Mol Cell Biol. 2001 Aug;21(15):5082–5093. doi: 10.1128/MCB.21.15.5082-5093.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ouwens DM, Diamant M. Myocardial insulin action and the contribution of insulin resistance to the pathogenesis of diabetic cardiomyopathy. Arch Physiol Biochem. 2007 Apr;113(2):76–86. doi: 10.1080/13813450701422633. [DOI] [PubMed] [Google Scholar]
- 31.Withers DJ, White M. Perspective: The insulin signaling system--a common link in the pathogenesis of type 2 diabetes. Endocrinology. 2000 Jun;141(6):1917–1921. doi: 10.1210/endo.141.6.7584. [DOI] [PubMed] [Google Scholar]
- 32.Gual P, Le Marchand-Brustel Y, Tanti JF. Positive and negative regulation of insulin signaling through IRS-1 phosphorylation. Biochimie. 2005 Jan;87(1):99–109. doi: 10.1016/j.biochi.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 33.Tzatsos A, Kandror KV. Nutrients suppress phosphatidylinositol 3-kinase/Akt signaling via raptor-dependent mTOR-mediated insulin receptor substrate 1 phosphorylation. Mol Cell Biol. 2006 Jan;26(1):63–76. doi: 10.1128/MCB.26.1.63-76.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bouzakri K, Roques M, Gual P, Espinosa S, Guebre-Egziabher F, Riou JP, et al. Reduced activation of phosphatidylinositol-3 kinase and increased serine 636 phosphorylation of insulin receptor substrate-1 in primary culture of skeletal muscle cells from patients with type 2 diabetes. Diabetes. 2003 Jun;52(6):1319–1325. doi: 10.2337/diabetes.52.6.1319. [DOI] [PubMed] [Google Scholar]
- 35.Tanti JF, Gremeaux T, van Obberghen E, Le Marchand-Brustel Y. Serine/threonine phosphorylation of insulin receptor substrate 1 modulates insulin receptor signaling. J Biol Chem. 1994 Feb 25;269(8):6051–6057. [PubMed] [Google Scholar]
- 36.Ueno M, Carvalheira JB, Tambascia RC, Bezerra RM, Amaral ME, Carneiro EM, et al. Regulation of insulin signalling by hyperinsulinaemia: role of IRS-1/2 serine phosphorylation and the mTOR/p70 S6K pathway. Diabetologia. 2005 Mar;48(3):506–518. doi: 10.1007/s00125-004-1662-6. [DOI] [PubMed] [Google Scholar]
- 37.Kushiyama A, Shojima N, Ogihara T, Inukai K, Sakoda H, Fujishiro M, et al. Resistin-like molecule beta activates MAPKs, suppresses insulin signaling in hepatocytes, and induces diabetes, hyperlipidemia, and fatty liver in transgenic mice on a high fat diet. J Biol Chem. 2005 Dec 23;280(51):42016–42025. doi: 10.1074/jbc.M503065200. [DOI] [PubMed] [Google Scholar]
- 38.Calabro P, Samudio I, Willerson JT, Yeh ET. Resistin promotes smooth muscle cell proliferation through activation of extracellular signal-regulated kinase 1/2 and phosphatidylinositol 3-kinase pathways. Circulation. 2004 Nov 23;110(21):3335–3340. doi: 10.1161/01.CIR.0000147825.97879.E7. [DOI] [PubMed] [Google Scholar]
- 39.Rothwell SE, Richards AM, Pemberton CJ. Resistin worsens cardiac ischaemia-reperfusion injury. Biochem Biophys Res Commun. 2006 Oct 13;349(1):400–407. doi: 10.1016/j.bbrc.2006.08.052. [DOI] [PubMed] [Google Scholar]
- 40.Takeishi Y, Niizeki T, Arimoto T, Nozaki N, Hirono O, Nitobe J, et al. Serum resistin is associated with high risk in patients with congestive heart failure--a novel link between metabolic signals and heart failure. Circ J. 2007 Apr;71(4):460–464. doi: 10.1253/circj.71.460. [DOI] [PubMed] [Google Scholar]
- 41.Lupattelli G, Marchesi S, Ronti T, Lombardini R, Bruscoli S, Bianchini R, et al. Endothelial dysfunction in vivo is related to monocyte resistin mRNA expression. J Clin Pharm Ther. 2007 Aug;32(4):373–379. doi: 10.1111/j.1365-2710.2007.00832.x. [DOI] [PubMed] [Google Scholar]
- 42.Burnett MS, Lee CW, Kinnaird TD, Stabile E, Durrani S, Dullum MK, et al. The potential role of resistin in atherogenesis. Atherosclerosis. 2005 Oct;182(2):241–248. doi: 10.1016/j.atherosclerosis.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 43.Rajapurohitam V, Gan XT, Kirshenbaum LA, Karmazyn M. The obesity-associated peptide leptin induces hypertrophy in neonatal rat ventricular myocytes. Circ Res. 2003 Aug 22;93(4):277–279. doi: 10.1161/01.RES.0000089255.37804.72. [DOI] [PubMed] [Google Scholar]
- 44.Xu FP, Chen MS, Wang YZ, Yi Q, Lin SB, Chen AF, et al. Leptin induces hypertrophy via endothelin-1-reactive oxygen species pathway in cultured neonatal rat cardiomyocytes. Circulation. 2004 Sep 7;110(10):1269–1275. doi: 10.1161/01.CIR.0000140766.52771.6D. [DOI] [PubMed] [Google Scholar]
- 45.Shibata R, Ouchi N, Ito M, Kihara S, Shiojima I, Pimentel DR, et al. Adiponectin-mediated modulation of hypertrophic signals in the heart. Nat Med. 2004 Dec;10(12):1384–1389. doi: 10.1038/nm1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999 Apr 2;257(1):79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 47.Matsubara M, Maruoka S, Katayose S. Decreased plasma adiponectin concentrations in women with dyslipidemia. J Clin Endocrinol Metab. 2002 Jun;87(6):2764–2769. doi: 10.1210/jcem.87.6.8550. [DOI] [PubMed] [Google Scholar]
- 48.Hotta K, Funahashi T, Arita Y, Takahashi M, Matsuda M, Okamoto Y, et al. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol. 2000 Jun;20(6):1595–1599. doi: 10.1161/01.atv.20.6.1595. [DOI] [PubMed] [Google Scholar]
- 49.Pellme F, Smith U, Funahashi T, Matsuzawa Y, Brekke H, Wiklund O, et al. Circulating adiponectin levels are reduced in nonobese but insulin-resistant first-degree relatives of type 2 diabetic patients. Diabetes. 2003 May;52(5):1182–1186. doi: 10.2337/diabetes.52.5.1182. [DOI] [PubMed] [Google Scholar]
- 50.Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, et al. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001 May;86(5):1930–1935. doi: 10.1210/jcem.86.5.7463. [DOI] [PubMed] [Google Scholar]