Abstract
Cell migration is involved in diverse physiological processes including embryogenesis, immunity, and diseases such as cancer and chronic inflammatory disease. The movement of many cell types is directed by extracellular gradients of diffusible chemicals. This phenomenon, referred to as "chemotaxis", was first described in 1888 by Leber who observed the movement of leukocytes toward sites of inflammation. We now know that a large family of small proteins, chemokines, serves as the extracellular signals and a family of G-protein-coupled receptors (GPCRs), chemokine receptors, detects gradients of chemokines and guides cell movement in vivo. Currently, we still know little about the molecular machineries that control chemokine gradient sensing and migration of immune cells. Fortunately, the molecular mechanisms that control these fundamental aspects of chemotaxis appear to be evolutionarily conserved, and studies in lower eukaryotic model systems allowed us to form concepts, uncover molecular components, develop new techniques, and test models of chemotaxis. These studies have helped our current understanding of this complicated cell behavior. In this review, we wish to mention landmark discoveries in the chemotaxis research field that shaped our current understanding of this fundamental cell behavior and lay out key questions that remain to be addressed in the future.
Keywords: chemotaxis, chemokine, GPCR, actin, inflammation
1. Introduction
Chemotaxis is the phenomenon in which the direction of a cell's locomotion is determined by an extracellular gradient of chemicals. The first recorded observations of chemotaxis were made in 1888 by Leber, who found that leukocytes converge from all directions along straight paths to sites of rabbit corneal irritation [1,2]. This observation gave rise to the hypothesis that diffusible substances generated at sites of injury establish gradients that guide leukocyte migration. However, the extracellular signals responsible for guiding leukocytes to sites of inflammation would not be identified for decades to come. In 1947, Bonner discovered that the eukaryotic soil amoeba, Dictyostelium discoideum, also migrates robustly toward the source of diffusible chemical signals [3]. Since these early discoveries, tremendous progress has been made in understanding the fundamental biological process of eukaryotic cell chemotaxis [4]. Today, it is well known that chemotaxis plays a critical role in many diverse physiological processes, including the recruitment of leukocytes to sites of infection, trafficking of lymphocytes throughout the human body, and patterning of neuronal cells in the developing nervous system. Because of the essential roles of chemotaxis in development and physiology, improperly guided cell movements can cause diverse pathological conditions, including tumor growth, cancer metastasis and inflammatory diseases, such as asthma, arthritis and atherosclerosis [5,6,7].
Many molecular components involved in chemotaxis of eukaryotic cells have been discovered. In mammals, the extracellular signals that guide leukocyte migration are mainly a set of short peptides, called chemokines (chemoattractant cytokines), which exert their effects by binding to members of the large family of G-protein-coupled receptors (GPCRs). Chemokine GPCRs signal through heterotrimeric G-proteins consisting of Gαi and Gβγ subunits, which in turn regulate a diversity of signal transduction pathways involved in chemotaxis (Fig. 1A). In general, cell migration is driven by the dynamic assemblies of the actin cytoskeleton. In chemotaxis, chemokine gradients strongly bias this actin assembly to the cell's leading edge and, hence, the direction of cell movement. In this article, we will review the landmark discoveries that have advanced our understanding of chemokines and their receptors, look back at the evolution of major concepts about the mechanism of chemotaxis, and provide examples of human diseases that are caused by mutations in chemokine receptors and inappropriate cell migrations. Finally, we will discuss the main challenges confronting the field of eukaryotic cell chemotaxis.
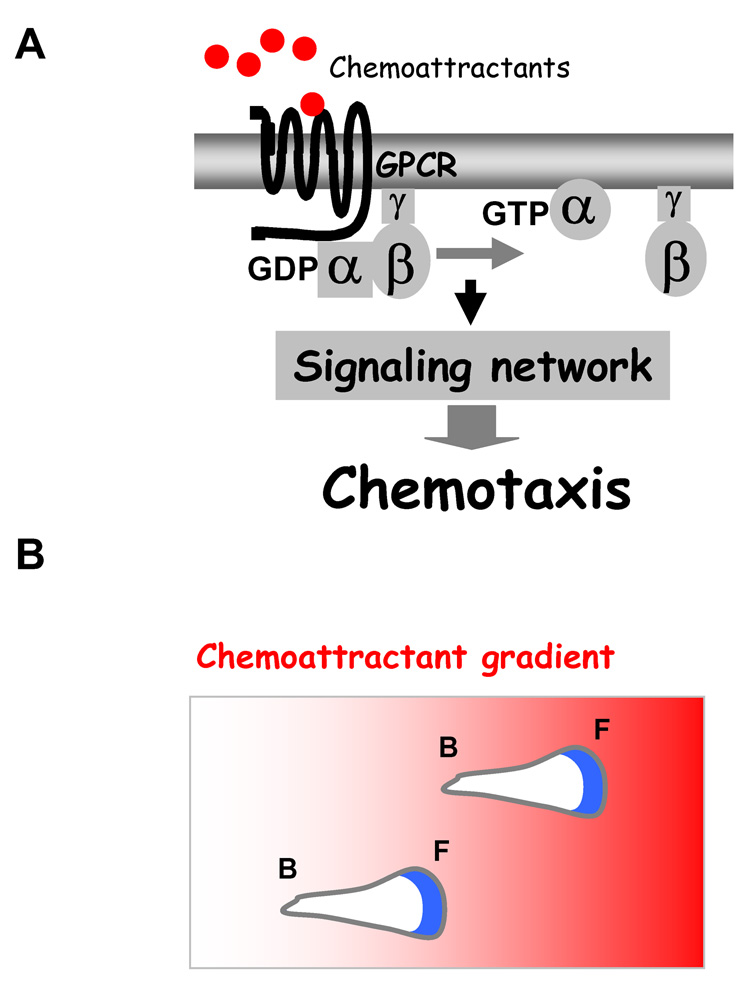
Figure 1.
(A) A model of a chemokine GPCR-mediated signaling network. (B) In a chemoattractant gradient, the polarized cells migrate toward higher chemoattractant concentration (indicated by the intensity of red color). The actin cytoskeleton, which drives extension of leading pseudopods, is indicated by green. F, front; B, back.
2. Chemoattractants, chemokines and their receptors
The idea that gradients established by diffusion of chemical substances can attract eukaryotic cells first took root in the early 1900s as discussed above. One of the first chemoattractants for eukaryotic cells to be reported was 3’,5’-cyclic adenosine monophosphate (cAMP) for D. discoideum cells. It was demonstrated that cells of D. discoideum chemotax robustly along experimentally established gradients of cAMP [8]. Twenty years later, the first leukocyte chemokine, interleukin (IL)-8, was identified [9,10]. This landmark discovery stimulated a search for additional chemokines, the number of which now exceeds fifty. Typical chemokines can be found in genome sequences with relative ease due to their small size (~70–90 amino acids) and conserved N-terminal, cysteine motifs. Based on the number and spacing of cysteines in these motifs, chemokines have been classified into C, CC, CXC and CX3C subfamilies [11]. Most chemokines are produced under pathological conditions by infiltrating leukocytes or cells endogenous to the affected tissue. In addition, some chemokines are involved in normal housekeeping functions, including the maturation of leukocytes in the bone marrow, the trafficking and homing of lymphocytes, and the regeneration of circulating leukocytes. Reflecting these varied physiological roles, most chemokines can be alternatively classified as either “inflammatory” or “homeostatic” [12]. Inflammatory chemokines are specialized for the recruitment of immune cells to the inflamed regions, while homeostatic chemokines are present in various microenvironments in lymphoid or non-lymphoid tissues and support trafficking and positioning of cells belonging to the adaptive immune system [12,13].
The molecular identities of chemoattractant receptors for eukaryotic cells began to be revealed in late 1980s. The first breakthrough was the discovery of a D. discoideum chemoattractant receptor for cAMP and the realization that it belongs to the family of seven-transmembrane G-protein-coupled receptors (GPCRs) [14]. A few years later, the human N-formylpeptide receptor and the IL-8 receptor of human leukocytes were discovered and also found to be GPCRs [15,16]. These landmark findings revealed that chemokine receptors represent a new subfamily of GPCRs. Binding of chemoattractants to the receptors activates heterotrimeric G-proteins, which in turn activate a signaling network that we are just beginning to understand, leading to chemotaxis (Fig. 1A). Currently, 18 chemokine receptors mediate the effects of the more than 50 known chemokines [11]. One interesting notion is that chemokines and their receptors, unlike other types of GPCRs, have overlapping specificities for each other. In addition, chemokine receptors are expressed in different leukocyte subtypes. Many chemokine-receptor-mediated signals appear to be redundant while some chemokine receptors are essential for life. For example, humans who lack functional CCR5 receptors are typically healthy [17,18], but CXCR4 knockout mice are embryonic lethal [19,20]. Given the fact that chemokine receptors are expressed in almost all cell types and have central roles in many biological processes, ranging from immunosurveillance to inflammatory responses, it is essential to understand their significance in human diseases.
3. Chemokine receptors and human disease
The field of chemokine receptors and HIV virology surprisingly intersected in 1996 when CXCR4 and CCR5 were identified as co-receptors that together with CD4 provide docking sites for HIV viruses on the surface of host cells [21,22]. It is currently believed that 90% HIV strains use CCR5 (macrophage tropic) for initial infections, and the infected viruses undergo mutations that allow them to use CXCR4 (T cell tropic) as the infection progresses to AIDS. Entry of HIV into host cells requires the formation of an entry complex including the viral envelop glycoprotein gp120, CD4 and either CCR5 or CXCR4 receptors [23]. Extraordinary progress has been made in solving structures of gp120 and CD4 that lead to current understanding of the formation of the entry complex. The gp120 protein interacts with CD4 and the N-terminus of CCR5 or CXCR4 and these sequential interactions induce structural changes that lead the exposure of gp41 virus proteins that presumably form pores for the entry of HIV into host cells [17,24]. It is not clear whether CCR5- or CXCR4-mediated signal transduction is also involved in the entry of HIV in addition of being the structural components of the HIV entry complex. Earlier reports indicated that signaling by either chemokine receptors is not essential for HIV infection. Blocking chemokine receptor signaling through Gαi by pertussis toxin did not affect HIV infection [25]. However, in order for the HIV large viral nuclear capsid to get through the membrane into the cytoplasm of the host cells, the membrane-associated actin cytoskeletons need to be rearranged. It is possible that the receptor-mediated signaling events contribute to the dynamic changes of the actin cytoskeleton during HIV infection just as they do in chemotaxis. The exact roles of chemokine receptor signaling in HIV infection require further investigation.
Chemokines and their receptors normally guide leukocytes to sites of infection and inflammation. However, excessive recruitment of these cells results in tissue damage and chronic inflammatory diseases. For example, chronic obstruction pulmonary disease (COPD) is an inflammatory disease characterized by irreversible progressive airflow limitation. The primary causes of COPD are significant inflammation and tissue destruction throughout the lung. Smokers who develop COPD have a chronic bronchopulmonary inflammation, characterized by an elevated recruitment of macrophages and CD8 T lymphocytes in the airway wall and of neutrophils in the airway lumen [26]. It has been shown that T cells infiltrating the airways of smokers with COPD display an increased expression of the chemokine receptor CXCR3 and its ligand CXCL10, suggesting that CXCL10-CXCR3 may be involved in the elevated recruitment of T cells in the inflammatory process underlying COPD [27]. An immunohistological study of patients with severe exacerbations of COPD demonstrated that the increase of neutrophil recruitment is associated with up-regulation of the chemokine CXCL5 and its receptor CXCR2, indicating the roles of CXCL5-CXCR2 for initiating and maintaining the increased neutrophilia in acute exacerbations of COPD [28]. Chemokines and their receptors are also involved in the pathogenesis of several neurological diseases, including multiple sclerosis (MS) [29]. MS, the major cause of neurological disability among young adults, is an inflammatory disease of the central nervous system (CNS) white matter characterized by focal myelin destruction, axonal pathology and progressive neurological dysfunction. In typical cases of MS, a prominent histological feature of the lesions that develop in brain and spinal cord is perivascular infiltration of T cells, B cells and monocyte/macrophages. In demyelinating brain lesions of MS patients, chemokines, MIP-1α and IP-10, were found to be expressed, and at the same time, CCR5+, (MIP-1α receptor) and CXCR3+ (IP-10 receptor) T cells, a selective subgroup, were increased in the progressive MS patients, suggesting roles for MIP-1α, acting on CCR5, and IP-10, acting on CXCR3, in the activation and recruitment of T cells to the CNS in MS [30]. In addition, chemokines and their receptors have also been implicated in many other inflammatory diseases, including atherosclerosis [6], inflammatory bowel disease [31], and endocrine autoimmune diseases [32].
Cancer cell migration, or metastasis, displays similarities to leukocyte trafficking, and recent results have shown that chemokines and chemokine receptors also play significant roles in cancer metastasis [5]. Metastasis is the transmission of cancerous cells from an original site to other selective organs elsewhere in the body. For example, in breast-cancer patients, secondary tumors often are found in the lungs and bone marrow, but rarely in the kidneys. A seminal finding by Muller et al. [33] indicated that chemokines and their receptors are important for this bias in breast cancer metastasis. In this study, the authors found that chemokine receptors CXCR4 was highly expressed in human breast cancer cells, and the respective ligand SDF-1α was highly expressed in many other organs, such as lymph nodes, bone marrow and lungs, where breast cancer metastases are often seen. They also showed in vitro that SDF-1α stimulated breast cancer cells to undergo directional migration and to successfully penetrate extracellular matrix for invasion. Additionally, in a mouse model, they successfully blocked metastasis of human breast cancer cells to SDF-1α-rich lung tissue by treating the animals with an antibody that neutralizes CXCR4 activity. The study clearly establishes an association between chemokines, their receptors and cancer metastasis, and also suggests that antagonists of chemokine receptors may be useful in treating cancer patients in the future.
Given the crucial roles of chemokines and their receptors in a wide array of physiological functions and their association with many pathological conditions, the receptors have become important targets for treatment of the various human diseases. However, to successfully develop therapeutic strategies for modulating chemokine action, we need to gain a detailed understanding of the molecular mechanisms of chemotaxis mediated by chemokines and their receptors.
4. Chemotaxis of leukocytes and the social amoebas D. discoideum
Since the early 1900s, it was known that leukocytes migrate from the blood across the wall of micro-vessels and accumulate in inflamed tissues [34]. The mobility of leukocytes closely resembles that of the amoeboid cells of D. discoideum. In the 1970s, microscopic methods were developed to directly observe the behaviors of leukocytes and D. discoideum cells placed in a chemoattractant gradient [35,36]. Using time-lapse video-microscopy, it was observed that these chemotaxing cells have three characteristics: cell polarity, cell motility and the ability to detect and respond to gradients of chemoattractants [37]. In response to uniform stimulation with chemoattractants, the cells become elongated and polarized, with clear leading and trailing ends (Fig. 1B), and their random motility increases, a behavior termed chemokinesis. When confronted with a chemoattractant gradient, the cells undergo chemotaxis, migrating toward higher concentrations of chemoattractants (Fig. 1B). There are two key features of chemoattractant-induced responses. First, responses to sustained, uniformly applied chemoattractants are rapidly terminated, a process called “adaptation”. Second, cells have the ability to detect and translate shallow, extracellular chemoattractant gradients into highly polarized intracellular responses, a process called “amplification” [38,39]. These features allow the cells to detect and respond to a wide range of concentrations of chemoattractants and concentration differences across the cell's length as small as 2% [37]. Dynamic assembly of actin at the leading edge drives the cell forward (Fig. 1B). The central questions are (i) how a cell adapts to the increasing concentration of a chemoattractants when it moves up to the gradient and (ii) how a cell amplifies small differences of extracellular stimuli into highly directional intracellular responses.
5. A GPCR-regulated signaling network generates directional responses
Over the years, many fundamental questions have been raised and concepts have been developed in the studies of chemotaxis using the model system D. discoideum. For example, to explain how a cell can persistently migrate in a gradient, one early hypothesis was that enrichment of chemokine receptors at the leading edge of cells is required for directional migration [40]. However, chemoattractant receptors were shown to be uniformly distributed in the membrane of chemotaxing D. discoideum cells [41], and this was subsequently demonstrated to be the case for chemokine receptors of human neutrophils [42]. Further studies demonstrated that even when receptors and their linked G-proteins are uniformly distributed on the membrane, the cells are still able to interpret gradients of chemoattractants to generate polarized responses [43]. Therefore, other signaling mechanisms instead of the simple localization of the receptors must play a critical role. The current view is that chemotaxing eukaryotic cells utilize an evolutionarily conserved GPCR-mediated signaling network that serves as a molecular "compass", allowing the cells to sense and respond to small concentration differences over their lengths and thereby generate highly polarized responses.
Chemotaxing cells are morphologically polarized with a leading pseudopod and a trailing end (Fig. 2A). Many proteins and lipids involved in chemotactic responses are asymmetrically distributed in chemotaxing D. discoideum cells and neutrophils [44]. As a consequence, the front of a polarized cell shows greater responsiveness to chemoattractant than do the sides and the trailing end. Chemotaxis is a complicated cell behavior that conceptually consists of three inter-connected cellular processes: cell polarity, cell motility and the ability to detect chemoattractant gradients. It was discovered that the gradient sensing machinery (or molecular compass) could be uncoupled from cell polarity and motility by treatment of D. discoideum cells with Latrunculin, an inhibitor of actin polymerization [43,45]. When chemotaxing cells were treated with Latrunculin, they lost their polarity and became immobile but retained their ability to produce polarized biochemical responses to chemoattractant gradients (Fig. 2A and 2B). This approach allowed us to simplify the cell behavior to that of gradient sensing and thus focus specifically on understanding the chemoattractant gradient sensing machinery.
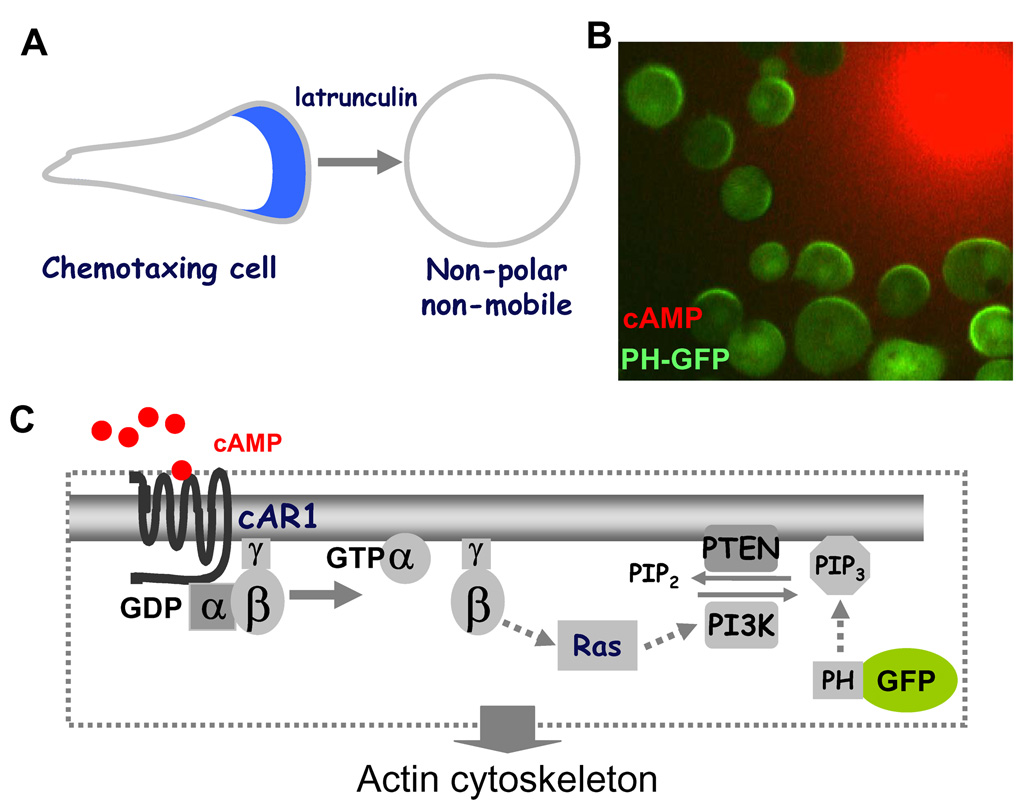
Figure 2.
(A) The gradient sensing machinery can be studied in the absence of cell polarity and motility. When treated with latrunculin, a chemotaxing cell becomes non-polar and non-mobile. (B) The latrunculin-treated cells generate a directional biochemical response by forming PH-GFP (green) crescents toward the cAMP gradient (red). (C) A model of the cAMP receptor-mediated gradient sensing machinery that controls PIP3 levels on the cell membrane.
Many of the core components that constitute the molecular “compass” have been elucidated in D. discoideum. Chemoattractant, cAMP, GPCR-regulated signaling network carries out chemoattractant gradient sensing (Fig. 2C). The binding of chemoattractant to GPCR induces the dissociation of heterotrimeric G-proteins into Gα and Gβγ subunits. Free Gβγ, activates the small G-protein Ras predominantly at the cell's leading edge. Ras in turn directly activates PI3K, promoting conversion of the membrane phospholipid PIP2 into PIP3 [4,46,47]. Once generated, PIP3 mediates intracellular polarization by recruiting proteins with Pleckstrin Homology (PH) domains to the plasma membrane at the leading edge. Among these proteins are CRAC (cytosolic regulator of adenylyl cyclase) and Akt/PKB [42,45,48], which play roles in regulating actin polymerization. Activation of receptors also regulates the membrane association of phosphatase PTEN, which dephosphorylates PIP3 to PIP2 [49,50,51]. Remarkably, the molecular compass of gradient sensing is evolutionarily conserved in human neutrophils. Activation of several GPCRs for chemokines, such as formylated peptides (fMLP), C5a and IL-8, stimulates class IB PI3K (known as PI3Kγ) through actions of Gβγ and Ras, resulting in the production of PIP3 in the plasma membrane [52]. Free Gβγ subunits generated as a consequence of interaction between the chemokine receptor and Gαi subunits lead to activation of Ras. Activated Ras subsequencely binds to PI3Kγ, yielding a complex that begins the rapid conversion PIP2 to PIP3 in the plasma membrane [53]. PIP3 then recruits PH-domain containing proteins such as Akt/PKB as well as small GTPase Rac and Cdc42 to the leading edge of the membrane [54,55]. Activated Rac and Cdc42 interact with the WASP/SCAR complex leading to actin polarization [54]. Activation of chemokine receptors also regulates PTEN localization and activity by small GTPase RhoA and Cdc42 to modulate PIP3 levels around the cell membrane [56]. However, the conserved network appears to predominantly act at the front of migrating neutrophils, and another pathway appears to be opporational at the back. It has been shown that the fMLP receptor activates trimeric G proteins G12 and G13, instead of Gi, at the back. G12 and G13 recruit the RhoA to the membrane to activate Rho-dependent kinase (ROCK) and myosin II, resulting in formation of contracitle actomyosin complexes [57,58].
In order to comprehend how these components work together as a system to allow a cell to adapt to a uniformly applied stimulus and to sustain highly polarized biochemical responses when facing a gradient, it is essential to determine the spatiotemporal dynamics of localization and activation of these components in live cells and in real time.
6. Spatiotemporal dynamics of gradient sensing revealed by live cell imaging
Advances in live cell fluorescence microscopy permit direct visualization and quantitation of many signaling events in live cells that undergo adaptation or amplification. The first breakthrough was the discovery that spatiotemporal changes of PIP3 levels around the cell membrane can be directly visualized by monitoring a green-fluorescent-protein (GFP) fused to CRAC (cytosolic regulator of adenylyl cyclase) or PKB protein kinase B in live D. discoideum cells [45,48]. Following this breakthrough, researchers have developed and applied state-of-the-art live cell imaging techniques to systematically measure spatiotemporal changes of the signaling components at various points along the GPCR-PIP3 pathway in live cells in real time. In well controlled experiments, the spatiotemporal changes of chemoattractant concentrations around the cell surface can be measured by fluorescent dyes [59,60]. The levels of heterotrimeric G-protein activation in various regions of the cell membrane have been monitored by fluorescence resonance energy transfer (FRET) methods [60,61]. In addition, spatiotemporal activation of a small G protein Ras has been observed by tracing the membrane translocation of a fluorescence probe, RBD-GFP (active Ras-Binding Domain fused to GFP) [47]. Furthermore, the dynamic translocation of GFP-tagged versions of PI3K and PTEN to the plasma membrane has also been measured [47,49,50]. For the first time these experiments have allowed us to directly observe various spatiotemporal changes within the signaling network in response to defined perturbations of chemoattractant stimuli and, as a result, have provided quantitative insights into how dynamic molecular interactions within a GPCR-mediated signaling network give rise to cell behaviors [60].
Over the last three decades, models have been constructed to mathematically describe chemotactic behaviors, such as adaptation and amplification [46,62]. Evolution of these conceptual and computational models has been closely linked with an increasing understanding of the molecular mechanisms involved in the signaling network [51]. The rapid developments in live cell imaging experiments and modeling have significantly increased our understanding of the underlying relationships among individual molecular mechanisms in a signaling network. Recently, a spatially resolved computational model of chemosensing has been reported [63]. This model, unlike previous ones that lack the detailed molecular components, is based on interactions of the known components and takes into account the concentrations of each component, numbers of binding sites, diffusion constants, and available kinetic constants [63]. The advantage of the model is the ability to simulate dynamic behaviors of each component in the processes leading to adaptation and amplification. Furthermore, these computer simulations can generate quantitative predictions, which can be experimentally tested in real cells with continuously improving tools [51]. An ongoing interplay between model refinement and experimental verification should ultimately lead to a deeper understanding of how components of the molecular compass work together as a system to produce complex cell behaviors.
7. Chemotaxis of lymphocytes in vivo
Chemokine receptor-mediated chemotaxis has been long implicated in the homing of leukocytes during immune surveillance and in the immune response. Until recently, migratory behaviors of individual lymphocytes had not been observed and it was previously thought that B and T cells were not as motile as neutrophils. In 2002, the application of laser-scanning microscopy, especially two-photon microscopy, enabled visualization of the motility of fluorescently labeled lymphocytes in tissues and lymph nodes in vivo [64]. More recently, the migrations of B cells and T cells in an intact lymph node were imaged in real time using two-photon microscopy 65]. A gradient of chemokine CCL21 was detected within 100 µm of the follicle edge within the lymph nodes. Furthermore, migration of antigen-engaged B cells toward the edge of these follicles was observed and these movements depended on CCR7, the CCL21 receptor [66]. These findings represent the first observation of lymphocyte chemotaxis in vivo. In a separate in vivo dynamic imaging study, Castellino et al. [67] demonstrated the CCR5-mediated movement of T cells toward dendritic cells, a critical prerequisite for the cell-cell interactions involved in antigen presentation and T cell activation. In vivo dynamic imaging of immune cells in tissues has only been developed in last several years. In this short period, imaging approaches have revealed that lymphocytes migrate vigorously in vivo. In tissues, migrating B cells and T cells displayed amoeboid chemotaxis behaviors that are similar to those observed in neutrophils and D. discoideum cells. B and T lymphocytes migrate through different tissues compartments during their development and differentiation. Although much is known about the paths taken by these cells and the chemokines and receptors they use, we are still at an early stage in the process toward of understanding how this complicated cellular trafficking is regulated.
8. Actin cytoskeleton in chemotaxing cells
Spatiotemporal organization of the actin cytoskeleton is essential for cells to undergo polarization and migration. One important function of the actin cytoskeleton is to drive locomotion by the extension of pseudopods. The force required for pseudopod extension is generated by the regulated growth of a branched network of actin filaments that underlie plasma membrane at the cell's leading edge (Fig. 3). In last several decades, actin cytoskeleton-driven cell motility has been extensively studied in model systems, especially Acanthamoeba, fish epidermal keratocytes and D. discoideum amoebae [68]. These cells undergo rapid, constitutive motility and assemble a large actin-filament network at the leading edge, and each of them is ideal for biochemical analysis, light and electron microscopy and (or) genetic manipulations. From extensive studies, the dendritic-nucleation/array-treadmilling model was proposed to explain how growing actin filaments push membranes forward [69,70]. Activation of cell surface receptors generates active Rho-family GTPases and PIP2. These signaling molecules in turn activate WASp/Scar proteins, which together with the Arp2/3 complex catalyze the formation of actin filaments that branch from preexisting ones [71]. Rapid growth of the new actin branch pushes the membrane forward. The formation of actin network at the leading edge is mediated by other proteins that include CARML and myosin I [72], which in concert coordinate extension of the actin network.
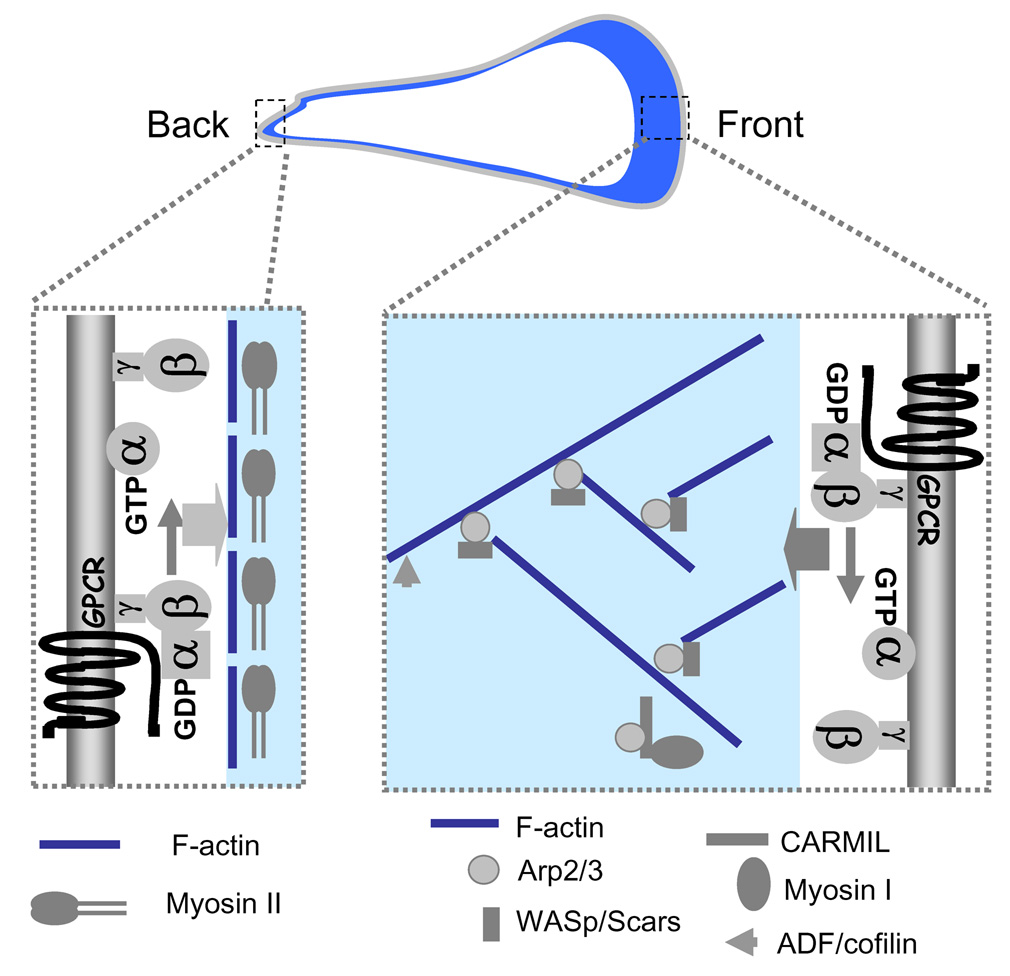
Figure 3.
Actin and myosin assemblies at the leading and trailing ends of a chemotaxing cell. At the front, chemokine receptor coupled with heterotrimeric G-protein controls an actin network that includes Arp2/3, WASp/Scars, CARMIL, myosin I and ADF/cofilin. At the back and sides, the receptor and G-proteins regulate actin/myosin II complexes.
Cell migration requires not only formation of dominant leading front but also suppression of errant pseudopod extension at the sides and retraction of the trailing end. Studies in D. discoideum and fish epithelial keratocytes indicated that myosin II interacts with actin filaments to form cell cortex to enhance cell cortical rigidity at the sides and rear of the cell to prevent lateral pseudopod extension, permit retraction of the trailing end, and ensure cell polarity for effective cell migration [73,74]. It appears that activation of GPCR sends different signals in regulating actin network in the front and actin/myosin filaments elsewhere in the cell [39,75]. For example in neutrophils, a chemokine receptor, the fMLP receptor, activates Gi heterotrimeric G-proteins, leading to Rac activation and actin network assembly in the front, while the same receptor activates G12/G13 heterotrimeric G-proteins, resulting in activation of Rho-dependent kinases and myosin II [58]. However, the pathways from chemoattractant GPCR to the actin network at the leading edge and actin/myosin II assemblies elsewhere in the cell have not been fully mapped.
9. Pathways leading to cell migration
How a GPCR chemosensing network (or molecular compass) regulates assembly of the actin cytoskeleton required for directional force generation is not clear yet. Evidence indicates that local membrane PIP3 levels provide intracellular cues for formation of the actin cytoskeleton. It is clear that PIP3 accumulates at the leading front where actin filament polymerization leads to cell migration. In addition, cells lacking PTEN, which hydrolyzes PIP3 to PIP2, are unable to sharply restrict PIP3 to certain regions and, fittingly, extend actin-filled pseudopodia in all directions [50]. However, a recent study showed that D. discoideum cells lacking five PI3K genes were still able to chemotaxis in steep gradients of cAMP, indicating that PIP3 signaling is not an essential link between the molecular compass and actin cytoskeleton in chemotaxis [76]. Two reports suggested that, in addition to the PIP3 pathway, a parallel phopholipase A2 (PLA2) pathway also links the compass to actin-driven cell migration of D. discoideum cells [77,78]. When either the PIP3 or PLA2 pathway was inactivated, cells were still capable of chemotaxis, while inactivation of both pathways blocked chemotaxis. Furthermore, a more recent study suggested that a soluble guanylyl cyclase provides additional signals to ensure chemotaxis [79]. While the role of PIP3 in gradient sensing and cell polarization has been demonstrated in human neutrophils and fibroblasts [80,81], the functions of PLA2 and guanylyl cyclase in the chemotaxis of these cells remain to be established. In addition, members of a protein family, Elmo (engulfment and cell motility), have been shown to regulate actin cytoskeleton and formation of membrane protrusions during cell migration in C. elegans. Together with Dock180, Elmo serves as an evolutionarily conserved mechanism for Rac activation leading to cell migration or neuronal outgrowth in C. elegans, Drosophila and human lymphocytes [82,83,84,85]. How Elmo proteins function in highly motile neutrophils or D. discoideum cell is still not clear.
How do these signaling pathways regulate actin assembly and pseudopod extension in the cell's front, the suppression of errant pseudopods elsewhere, and the myosin II-mediated retraction of the rear? Recent findings suggest that various small GTPases, some of them are mentioned previously, are involved in regulating these events. The superfamily of small GTPases comprises more than 150 human members with evolutionarily conserved orthologs in all eukaryotes including D. discoideum [86]. The superfamily can be divided into the following five subfamilies on the basis of sequence and functional and similarities: Ras, Rho, Rab, Ran and Arf. Small GTPases functions as GDP/GTP-regulated molecular switches, cycling between active GTP-bound and inactive GDP-bound forms. Two classes of proteins control the cycle, namely guanine-nucleotide-exchange factors (GEFs), which induce the exchange of GTP for GDP, and GTPase-activating proteins (GAPs), which promote the formation of the inactive GDP-bound forms [86]. Accumulating data demonstrate that many small GTPases plays essential roles in chemotaxis. Rho subfamily GTPases, including Rho, Rac and Cdc42, are key regulators of actin reorganization. In the front of neutrophils, fMLP receptor-activated Rac1 and Rac2 regulate the generation of free barbed ends of actin filaments, which drive actin filament elongation and membrane protrusion. Rac1 and Rac2 work through two different pathways. While Rac1 induces generation of free barbed ends through uncapping of the existing actin filaments, Rac2 acts on cofilin activation and on the Arp2/3 complex [87]. At the sides and back, RhoA stimulates assembly of actomyosin contractile complexes [57]. In D. discoideum, several Rac proteins, such as Rac1A, Rac1B, Rac1C and RacG, have been implicated in regulating actin polymerization during chemotaxis [88]. Recently, RasGEF Q, an exchange factor for RasB, was shown to regulate processes requiring myosin II [84]. It has been proposed that active RasB regulates myosin II activity that ensures proper retraction for cell migration. The reader is referred to an excellent review article [88] on the roles of small GTPases in chemoattractant sensing, cell polarization and cell motility. Further studies will be required to dissect the functions of different small GTPases in the parallel signaling pathways that control chemotaxis.
10. Challenges in the research of eukaryotic cell chemotaxis
Despite the enormous progress we have made toward understanding the mechanisms of chemotaxis, many mysteries still remain to be solved. One of the most important challenges in the field is to define the molecular mechanisms that link chemokine receptor signaling to the reorganization of the actin cytoskeleton that drives chemotaxis. Recent progress in the field has resulted from the combined application of biochemical, genetic, cell biology, state-of-the-art microscopy and computational approaches and has permitted generation of dynamic models for the molecular mechanisms of chemosensing and pseudopod protrusion [46,62,63,71]. While, we have yet to understand the linkages between chemoattractant sensing machinery and actin-dependent cell polarization and motility, we expect to gather additional clues through the continued study of the model systems, which have so far shaped our understanding of all fundamental aspects of eukaryotic cell chemotaxis. We are beginning to apply the knowledge gained from these model systems to understand the role of chemotaxis in the in vivo trafficking of immune cells, including lymphocytes and neutrophils, as well as cancer cells undergoing metastasis. However, a number of technical hurdles stand in the way of acquiring an in-depth understanding of immune cell chemotaxis in vivo. For example, it is apparent that spatiotemporal patterns of diffusible chemokines throughout the body direct these immune cells to different target sites. Therefore, quantitative information about the spatial and temporal distribution of chemokines will be essential for understanding how they guide cell movement. However, direct visualization of chemokine gradients is still in its early stages, largely due to the technical difficulties in detecting extracellular diffusible molecules. In addition to understanding the complex biology of chemokines and immune cells, it will also be important to develop a more complete understanding of the molecular mechanisms of chemotaxis inherent in these cells so that we may exploit this knowledge for the treatment of immune system disorders. In this regard, full advantage should be taken of the mechanistic insights that have been and will continue to be gained from the study of model systems such as D. discoideum. Basic research in these areas will provide the foundation of knowledge needed to rationally select the targets for medical interventions and open therapeutic opportunities for inflammatory diseases, autoimmune diseases and immune deficiencies.
Acknowledgements
We thank X. Xiang for helpful comments. T. Jin is supported by NIAID, NIH intramural funding and a grant from NIH intramural AIDS target antiviral program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Leber T. Über die Entstehung der Entzündung und die Wirkung der entzündungserregenden Schädlichkeiten. Fortschr Med. 1888;6:460–464. [Google Scholar]
- 2.McCutcheon M. Chemotaxis in leukocytes. Physiological Reviews. 1946;26:319–410. doi: 10.1152/physrev.1946.26.3.319. [DOI] [PubMed] [Google Scholar]
- 3.Bonner JT. Evidence for the formation of cell aggregates by chemotaxis in the development of the slime mold Dictyostelium discoideum. J Exp Zool. 1947;106:1–26. doi: 10.1002/jez.1401060102. [DOI] [PubMed] [Google Scholar]
- 4.Jin T, Hereld D. Moving toward understanding eukaryotic chemotaxis. Eur J Cell Biol. 2006;85:905–913. doi: 10.1016/j.ejcb.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 5.Koizumi K, Hojo S, Akashi T, Yasumoto K, Saiki I. Chemokine receptors in cancer metastasis and cancer cell-derived chemokines in host immune response. Cancer Sci. 2007;98:1652–1658. doi: 10.1111/j.1349-7006.2007.00606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liehn EA, Zernecke A, Postea O, Weber C. Chemokines: inflammatory mediators of atherosclerosis. Arch Physiol Biochem. 2006;112:229–238. doi: 10.1080/13813450601093583. [DOI] [PubMed] [Google Scholar]
- 7.Silva TA, Garlet GP, Fukada SY, Silva JS, Cunha FQ. Chemokines in oral inflammatory diseases: apical periodontitis and periodontal disease. J Dent Res. 2007;86:306–319. doi: 10.1177/154405910708600403. [DOI] [PubMed] [Google Scholar]
- 8.Konijn TM, Van De Meene JG, Bonner JT, Barkley DS. The acrasin activity of adenosine-3',5'-cyclic phosphate. Proc Natl Acad Sci USA. 1967;58:1152–1154. doi: 10.1073/pnas.58.3.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoshimura T, Matsushima K, Tanaka S, Robinson EA, Appella E, Oppenheim JJ, Leonard EJ. Purification of a human monocyte-derived neutrophil chemotactic factor that has peptide sequence similarity to other host defense cytokines. Proc Natl Acad Sci USA. 1987;84:9233–9237. doi: 10.1073/pnas.84.24.9233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walz A, Peveri P, Aschauer H, Baggiolini M. Purification and amino acid sequencing of NAF, a novel neutrophil-activating factor produced by monocytes. Biochem Biophys Res Commun. 1987;149:755–761. doi: 10.1016/0006-291x(87)90432-3. [DOI] [PubMed] [Google Scholar]
- 11.Murphy PM. International Union of Pharmacology. XXX. Update on chemokine receptor nomenclature. Pharmacol Rev. 2002;54:227–229. doi: 10.1124/pr.54.2.227. [DOI] [PubMed] [Google Scholar]
- 12.Moser B, Loetscher P. Lymphocyte traffic control by chemokines. Nat Immunol. 2001;2:123–128. doi: 10.1038/84219. [DOI] [PubMed] [Google Scholar]
- 13.Pals ST, de Gorter DJ, Spaargaren M. Lymphoma dissemination: the other face of lymphocyte homing. Blood. 2007;110:3102–3111. doi: 10.1182/blood-2007-05-075176. [DOI] [PubMed] [Google Scholar]
- 14.Klein PS, Sun TJ, Saxe CL, 3rd, Kimmel AR, Johnson RL, Devreotes PN. A chemoattractant receptor controls development in Dictyostelium discoideum. Science. 1988;241:1467–1472. doi: 10.1126/science.3047871. [DOI] [PubMed] [Google Scholar]
- 15.Boulay F, Tardif M, Brouchon L, Vignais P. The human N-formylpeptide receptor. Characterization of two cDNA isolates and evidence for a new subfamily of G-protein-coupled receptors. Biochemistry. 1990;29:11123–11133. doi: 10.1021/bi00502a016. [DOI] [PubMed] [Google Scholar]
- 16.Murphy PM, Tiffany HL. Cloning of complementary DNA encoding a functional human interleukin-8 receptor. Science. 1991;253:1280–1283. [PubMed] [Google Scholar]
- 17.Huang Y, Paxton WA, Wolinsky SM, Neumann AU, Zhang L, He T, et al. The role of a mutant CCR5 allele in HIV-1 transmission and disease progression. Nat Med. 1996;2:1240–1243. doi: 10.1038/nm1196-1240. [DOI] [PubMed] [Google Scholar]
- 18.Liu R, Paxton WA, Choe S, Ceradini D, Martin SR, Horuk R, et al. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell. 1996;86:367–377. doi: 10.1016/s0092-8674(00)80110-5. [DOI] [PubMed] [Google Scholar]
- 19.Ma Q, Jones D, Borghesani PR, Segal RA, Nagasawa T, Kishimoto T, et al. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proc Natl Acad Sci USA. 1998;95:9448–9453. doi: 10.1073/pnas.95.16.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393:595–599. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]
- 21.Alkhatib G, Combadiere C, Broder CC, Feng Y, Kennedy PE, Murphy PM, Berger EA. CC CKR5: a RANTES, MIP-1α, MIP-1β receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 22.Feng Y, Broder CC, Kennedy PE, Berger EA. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 23.Berger EA, Murphy PM, Farber JM. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu Rev Immunol. 1999;17:657–700. doi: 10.1146/annurev.immunol.17.1.657. [DOI] [PubMed] [Google Scholar]
- 24.Kwong PD, Wyatt R, Robinson J, Sweet RW, Sodroski J, Hendrickson WA. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature. 1998;393:648–659. doi: 10.1038/31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amara A, Gall SL, Schwartz O, Salamero J, Montes M, Loetscher P, et al. HIV coreceptor downregulation as antiviral principle: SDF-1α-dependent internalization of the chemokine receptor CXCR4 contributes to inhibition of HIV replication. J Exp Med. 1997;186:139–146. doi: 10.1084/jem.186.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saetta M, Baraldo S, Zuin R. Neutrophil chemokines in severe exacerbations of chronic obstructive pulmonary disease: fatal chemo-attraction? Am J Respir Crit Care Med. 2003;168:911–913. doi: 10.1164/rccm.2308002. [DOI] [PubMed] [Google Scholar]
- 27.Saetta M, Mariani M, Panina-Bordignon P, Turato G, Buonsanti C, Baraldo S, et al. Increased expression of the chemokine receptor CXCR3 and its ligand CXCL10 in peripheral airways of smokers with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;165:1404–1409. doi: 10.1164/rccm.2107139. [DOI] [PubMed] [Google Scholar]
- 28.Qiu Y, Zhu J, Bandi V, Guntupalli KK, Jeffery PK. Bronchial mucosal inflammation and upregulation of CXC chemoattractants and receptors in severe exacerbations of asthma. Thorax. 2007;62:475–482. doi: 10.1136/thx.2006.066670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cartier L, Hartley O, Dubois-Dauphin M, Krause KH. Chemokine receptors in the central nervous system: role in brain inflammation and neurodegenerative diseases. Brain Res Brain Res Rev. 2005;48:16–42. doi: 10.1016/j.brainresrev.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 30.Balashov KE, Rottman JB, Weiner HL, Hancock WW. CCR5+ and CXCR3+ T cells are increased in multiple sclerosis and their ligands MIP-1a and IP-10 are expressed in demyelinating brain lesions. Proc Natl Acad Sci USA. 1999;96:6873–6878. doi: 10.1073/pnas.96.12.6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Danese S, Gasbarrini A. Chemokines in inflammatory bowel disease. J Clin Pathol. 2005;58:1025–1027. doi: 10.1136/jcp.2005.030916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rotondi M, Chiovato L, Romagnani S, Serio M, Romagnani P. Role of chemokines in endocrine autoimmune diseases. Endocr Rev. 2007;28:492–520. doi: 10.1210/er.2006-0044. [DOI] [PubMed] [Google Scholar]
- 33.Müller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 34.Metchnikoff E. L'Immunité dans les Maladies Infestieuses. Paris: Masson and Cia; 1901. [Google Scholar]
- 35.Zigmond SH. Ability of polymorphonuclear leukocytes to orient in gradients of chemotactic factors. J Cell Biol. 1977;75:606–616. doi: 10.1083/jcb.75.2.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gerisch G. Chemotaxis in Dictyostelium. Annu Rev Physiol. 1982;44:535–552. doi: 10.1146/annurev.ph.44.030182.002535. [DOI] [PubMed] [Google Scholar]
- 37.Devreotes PN, Zigmond SH. Chemotaxis in eukaryotic cells: a focus on leukocytes and Dictyostelium. Annu Rev Cell Biol. 1988;4:649–686. doi: 10.1146/annurev.cb.04.110188.003245. [DOI] [PubMed] [Google Scholar]
- 38.Parent CA, Devreotes PN. A cell's sense of direction. Science. 1999;284:765–770. doi: 10.1126/science.284.5415.765. [DOI] [PubMed] [Google Scholar]
- 39.Chung CY, Funamoto S, Firtel RA. Signaling pathways controlling cell polarity and chemotaxis. Trends Biochem Sci. 2001;26:557–566. doi: 10.1016/s0968-0004(01)01934-x. [DOI] [PubMed] [Google Scholar]
- 40.Nieto M, Frade JM, Sancho D, Mellado M, Martinez-A C, Sánchez-Madrid F. Polarization of chemokine receptors to the leading edge during lymphocyte chemotaxis. J Exp Med. 1997;186:153–158. doi: 10.1084/jem.186.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xiao Z, Zhang N, Murphy DB, Devreotes PN. Dynamic distribution of chemoattractant receptors in living cells during chemotaxis and persistent stimulation. J Cell Biol. 1997;139:365–374. doi: 10.1083/jcb.139.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Servant G, Weiner OD, Herzmark P, Balla T, Sedat JW, Bourne HR. Polarization of chemoattractant receptor signaling during neutrophil chemotaxis. Science. 2000;287:1037–1040. doi: 10.1126/science.287.5455.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jin T, Zhang N, Long Y, Parent CA, Devreotes PN. Localization of the G protein βγ complex in living cells during chemotaxis. Science. 2000;287:1034–1036. doi: 10.1126/science.287.5455.1034. [DOI] [PubMed] [Google Scholar]
- 44.Comer FI, Parent CA. PI 3-kinases and PTEN: how opposites chemoattract. Cell. 2002;109:541–544. doi: 10.1016/s0092-8674(02)00765-1. [DOI] [PubMed] [Google Scholar]
- 45.Parent CA, Blacklock BJ, Froehlich WM, Murphy DB, Devreotes PN. G protein signaling events are activated at the leading edge of chemotactic cells. Cell. 1998;95:81–91. doi: 10.1016/s0092-8674(00)81784-5. [DOI] [PubMed] [Google Scholar]
- 46.Iijima M, Huang YE, Devreotes P. Temporal and spatial regulation of chemotaxis. Dev Cell. 2002;3:469–478. doi: 10.1016/s1534-5807(02)00292-7. [DOI] [PubMed] [Google Scholar]
- 47.Sasaki AT, Chun C, Takeda K, Firtel RA. Localized Ras signaling at the leading edge regulates PI3K, cell polarity, and directional cell movement. J Cell Biol. 2004;167:505–518. doi: 10.1083/jcb.200406177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meili R, Ellsworth C, Lee S, Reddy TB, Ma H, Firtel RA. Chemoattractant-mediated transient activation and membrane localization of Akt/PKB is required for efficient chemotaxis to cAMP in Dictyostelium. EMBO J. 1999;18:2092–2105. doi: 10.1093/emboj/18.8.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Funamoto S, Meili R, Lee S, Parry L, Firtel RA. Spatial and temporal regulation of 3-phosphoinositides by PI 3-kinase and PTEN mediates chemotaxis. Cell. 2002;109:611–623. doi: 10.1016/s0092-8674(02)00755-9. [DOI] [PubMed] [Google Scholar]
- 50.Iijima M, Devreotes P. Tumor suppressor PTEN mediates sensing of chemoattractant gradients. Cell. 2002;109:599–610. doi: 10.1016/s0092-8674(02)00745-6. [DOI] [PubMed] [Google Scholar]
- 51.Xu X, Meier-Schellersheim M, Yan J, Jin T. Locally controlled inhibitory mechanisms are involved in eukaryotic GPCR-mediated chemosensing. J Cell Biol. 2007;178:141–153. doi: 10.1083/jcb.200611096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Andrews S, Stephens LR, Hawkins PT. PI3K class IB pathway in neutrophils. Sci STKE. 2007;2007:cm3. doi: 10.1126/stke.4072007cm3. [DOI] [PubMed] [Google Scholar]
- 53.Bourne HR, Weiner O. A chemical compass. Nature. 2002;419:21. doi: 10.1038/419021a. [DOI] [PubMed] [Google Scholar]
- 54.Stradal TE, Rottner K, Disanza A, Confalonieri S, Innocenti M, Scita G. Regulation of actin dynamics by WASP and WAVE family proteins. Trends Cell Biol. 2004;14:303–311. doi: 10.1016/j.tcb.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 55.Weiner OD. Rac activation: P-Rex1 - a convergence point for PIP3 and Gβγ? Curr Biol. 2002;12:R429–R431. doi: 10.1016/s0960-9822(02)00917-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li Z, Hannigan M, Mo Z, Liu B, Lu W, Wu Y, et al. Directional sensing requires Gβγ-mediated PAK1 and PIXα-dependent activation of Cdc42. Cell. 2003;114:215–227. doi: 10.1016/s0092-8674(03)00559-2. [DOI] [PubMed] [Google Scholar]
- 57.Wong K, Van Keymeulen A, Bourne HR. PDZRhoGEF and myosin II localize RhoA activity to the back of polarizing neutrophil-like cells. J Cell Biol. 2007;179:1141–1148. doi: 10.1083/jcb.200706167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu J, Wang F, Van Keymeulen A, Herzmark P, Straight A, Kelly K, et al. Divergent signals and cytoskeletal assemblies regulate self-organizing polarity in neutrophils. Cell. 2003;114:201–214. doi: 10.1016/s0092-8674(03)00555-5. [DOI] [PubMed] [Google Scholar]
- 59.Ueda M, Sako Y, Tanaka T, Devreotes P, Yanagida T. Single-molecule analysis of chemotactic signaling in Dictyostelium cells. Science. 2001;294:864–867. doi: 10.1126/science.1063951. [DOI] [PubMed] [Google Scholar]
- 60.Xu X, Meier-Schellersheim M, Jiao X, Nelson LE, Jin T. Quantitative imaging of single live cells reveals spatiotemporal dynamics of multistep signaling events of chemoattractant gradient sensing in Dictyostelium. Mol Biol Cell. 2005;16:676–688. doi: 10.1091/mbc.E04-07-0544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Janetopoulos C, Jin T, Devreotes P. Receptor-mediated activation of heterotrimeric G-proteins in living cells. Science. 2001;291:2408–2411. doi: 10.1126/science.1055835. [DOI] [PubMed] [Google Scholar]
- 62.Iglesias PA, Devreotes PN. Navigating through models of chemotaxis. Curr Opin Cell Biol. 2008;20:35–40. doi: 10.1016/j.ceb.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 63.Meier-Schellersheim M, Xu X, Angermann B, Kunkel EJ, Jin T, Germain RN. Key role of local regulation in chemosensing revealed by a new molecular interaction-based modeling method. PLoS Comput Biol. 2006;2:e82. doi: 10.1371/journal.pcbi.0020082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Germain RN, Miller MJ, Dustin ML, Nussenzweig MC. Dynamic imaging of the immune system: progress, pitfalls and promise. Nat Rev Immunol. 2006;6:497–507. doi: 10.1038/nri1884. [DOI] [PubMed] [Google Scholar]
- 65.Okada T, Miller MJ, Parker I, Krummel MF, Neighbors M, Hartley SB, et al. Antigen-engaged B cells undergo chemotaxis toward the T zone and form motile conjugates with helper T cells. PLoS Biol. 2005;3:e150. doi: 10.1371/journal.pbio.0030150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Allen CD, Okada T, Cyster JG. Germinal-center organization and cellular dynamics. Immunity. 2007;27:190–202. doi: 10.1016/j.immuni.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Castellino F, Huang AY, Altan-Bonnet G, Stoll S, Scheinecker C, Germain RN. Chemokines enhance immunity by guiding naive CD8+ T cells to sites of CD4+ T cell-dendritic cell interaction. Nature. 2006;440:890–895. doi: 10.1038/nature04651. [DOI] [PubMed] [Google Scholar]
- 68.Pollard TD, Blanchoin L, Mullins RD. Molecular mechanisms controlling actin filament dynamics in nonmuscle cells. Annu Rev Biophys Biomol Struct. 2000;29:545–576. doi: 10.1146/annurev.biophys.29.1.545. [DOI] [PubMed] [Google Scholar]
- 69.Maly IV, Borisy GG. Self-organization of a propulsive actin network as an evolutionary process. Proc Natl Acad Sci USA. 2001;98:11324–11329. doi: 10.1073/pnas.181338798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schaus TE, Taylor EW, Borisy GG. Self-organization of actin filament orientation in the dendritic-nucleation/array-treadmilling model. Proc Natl Acad Sci USA. 2007;104:7086–7091. doi: 10.1073/pnas.0701943104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112:453–465. doi: 10.1016/s0092-8674(03)00120-x. [DOI] [PubMed] [Google Scholar]
- 72.Jung G, Remmert K, Wu X, Volosky JM, Hammer JA., 3rd The Dictyostelium CARMIL protein links capping protein and the Arp2/3 complex to type I myosins through their SH3 domains. J Cell Biol. 2001;153:1479–1497. doi: 10.1083/jcb.153.7.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bosgraaf L, van Haastert PJ. The regulation of myosin II in Dictyostelium. Eur J Cell Biol. 2006;85:969–979. doi: 10.1016/j.ejcb.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 74.Yam PT, Wilson CA, Ji L, Hebert B, Barnhart EL, Dye NA, et al. Actin-myosin network reorganization breaks symmetry at the cell rear to spontaneously initiate polarized cell motility. J Cell Biol. 2007;178:1207–1221. doi: 10.1083/jcb.200706012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li Z, Dong X, Wang Z, Liu W, Deng N, Ding Y, et al. Regulation of PTEN by Rho small GTPases. Nat Cell Biol. 2005;7:399–404. doi: 10.1038/ncb1236. [DOI] [PubMed] [Google Scholar]
- 76.Hoeller O, Kay RR. Chemotaxis in the absence of PIP3 gradients. Curr Biol. 2007;17:813–817. doi: 10.1016/j.cub.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 77.Chen L, Iijima M, Tang M, Landree MA, Huang YE, Xiong Y, et al. PLA2 and PI3K/PTEN pathways act in parallel to mediate chemotaxis. Dev Cell. 2007;12:603–614. doi: 10.1016/j.devcel.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.van Haastert PJ, Keizer-Gunnink I, Kortholt A. Essential role of PI3-kinase and phospholipase A2 in Dictyostelium discoideum chemotaxis. J Cell Biol. 2007;177:809–816. doi: 10.1083/jcb.200701134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Veltman DM, Keizer-Gunnik I, Van Haastert PJ. Four key signaling pathways mediating chemotaxis in Dictyostelium discoideum. J Cell Biol. 2008;180:747–753. doi: 10.1083/jcb.200709180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Haugh JM, Codazzi F, Teruel M, Meyer T. Spatial sensing in fibroblasts mediated by 3' phosphoinositides. J Cell Biol. 2000;151:1269–1280. doi: 10.1083/jcb.151.6.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang F, Herzmark P, Weiner OD, Srinivasan S, Servant G, Bourne HR. Lipid products of PI(3)Ks maintain persistent cell polarity and directed motility in neutrophils. Nat Cell Biol. 2002;4:513–518. doi: 10.1038/ncb810. [DOI] [PubMed] [Google Scholar]
- 82.Bianco A, Poukkula M, Cliffe A, Mathieu J, Luque CM, Fulga TA, Rørth P. Two distinct modes of guidance signalling during collective migration of border cells. Nature. 2007;448:362–365. doi: 10.1038/nature05965. [DOI] [PubMed] [Google Scholar]
- 83.Nombela-Arrieta C, Lacalle RA, Montoya MC, Kunisaki Y, Megías D, Marqués M, et al. Differential requirements for DOCK2 and phosphoinositide-3-kinase γ during T and B lymphocyte homing. Immunity. 2004;21:429–441. doi: 10.1016/j.immuni.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 84.Ravichandran KS, Lorenz U. Engulfment of apoptotic cells: signals for a good meal. Nat Rev Immunol. 2007;7:964–974. doi: 10.1038/nri2214. [DOI] [PubMed] [Google Scholar]
- 85.Reddien PW, Horvitz HR. The engulfment process of programmed cell death in Caenorhabditis elegans. Annu Rev Cell Dev Biol. 2004;20:193–221. doi: 10.1146/annurev.cellbio.20.022003.114619. [DOI] [PubMed] [Google Scholar]
- 86.Wennerberg K, Rossman KL, Der CJ. The Ras superfamily at a glance. J Cell Sci. 2005;118:843–846. doi: 10.1242/jcs.01660. [DOI] [PubMed] [Google Scholar]
- 87.Sun CX, Magalhães MA, Glogauer M. Rac1 and Rac2 differentially regulate actin free barbed end formation downstream of the fMLP receptor. J Cell Biol. 2007;179:239–245. doi: 10.1083/jcb.200705122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Charest PG, Firtel RA. Big roles for small GTPases in the control of directed cell movement. Biochem J. 2007;401:377–390. doi: 10.1042/BJ20061432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mondal S, Bakthavatsalam D, Steimle P, Gassen B, Rivero F, Noegel AA. Linking Ras to myosin function: RasGEF Q, a Dictyostelium exchange factor for RasB, affects myosin II functions. J Cell Biol. 2008;181:747–760. doi: 10.1083/jcb.200710111. [DOI] [PMC free article] [PubMed] [Google Scholar]