Abstract
The BONEII study is a large two-phase study. The baseline study (Study 1) aims to estimate the prevalence of diminished bone mineral density (BMD) in patients treated for childhood acute lymphoblastic leukemia (ALL) and identify risk factors for BMD deficits. The interventional phase (Study 2) of BONEII has a placebo-controlled double-blind randomized longitudinal design to evaluate the effects of nutritional counseling and calcium and vitamin D supplementation on changes in BMD and serum and urine markers of bone metabolism. The extensive information being collected through this large study will serve as a repository of relational data about BMD and bone turnover and will support further investigations to assess the association of calcium metabolism, bone turnover, nutritional intake, lifestyle factors (such as exercise and the use of alcohol and tobacco), and the specific agents used in ALL therapy in this rapidly increasing population of childhood cancer survivors.
Keywords: bone mineral density, repeated measures analysis, bone loss, acute lymphoblastic leukemia, pediatric leukemia, skeletal toxicities, childhood cancer survivors
Background and Aims
Among late toxicities identified in childhood cancer survivors are deficits in age-matched bone mineral density (BMD), which may predispose childhood cancer survivors to increased prevalence, earlier onset, and more severe osteoporosis than the general population [1–7] and may adversely affect quality of life [1–5, 7–14]. However, the prevalence, severity, and implications of this treatment outcome have not yet been defined clearly and are the subject of ongoing investigations. Also unclear are the relative contributions of genetic predisposition (vitamin D receptor polymorphism), treatment effects (chemotherapy and cranial irradiation), altered nutritional status, and physical activity on diminished trabecular BMD, cortical BMD, and the ratio of cortical to trabecular BMD in this population.
Acute lymphoblastic leukemia (ALL) is the most common curable childhood cancer, with long-term event-free survival rates of approximately 80% [15]. The treatment of ALL involves agents from most or all classes of anticancer drugs (as well as cranial irradiation in some cases). Therefore, studies in the ALL survivor population can potentially disclose information applicable to the management of other childhood cancer survivors. Efficacy of interventions to improve BMD, though studied in healthy children [16–26], have not been studied in the ALL survivor cohort.
This study has two main phases: Study 1, designed as a cross-sectional study, comprises a single visit to obtain information from which to estimate the prevalence of a variety of deficits and disorders and their predictors (BMD, nutrition, lifestyle factors, patient demographics, disease and treatments, etc.) in a very large cohort (n=424). The second primary phase (Study 2) is designed as a double-blind randomized longitudinal (Phase III) prospective study, conducted to reduce BMD loss in the subset of patients (n=279) identified in Study 1 as having BMD deficit.
Thus, this study will fill a void in estimating the prevalence of adverse events and determining the efficacy of interventions to improve BMD in pediatric and young adult survivors of childhood ALL. The data collected through BONEII will provide the backbone for future studies to assess the association of calcium metabolism, bone turnover, nutritional intake, lifestyle factors (such as exercise and the use of alcohol and tobacco), and the specific agents used in ALL therapy in this rapidly increasing population of childhood cancer survivors. This information and associated analyses of BONEII are likely to be applicable to childhood cancer survivorship as a whole.
Participants and Methods
Enrollment Criteria
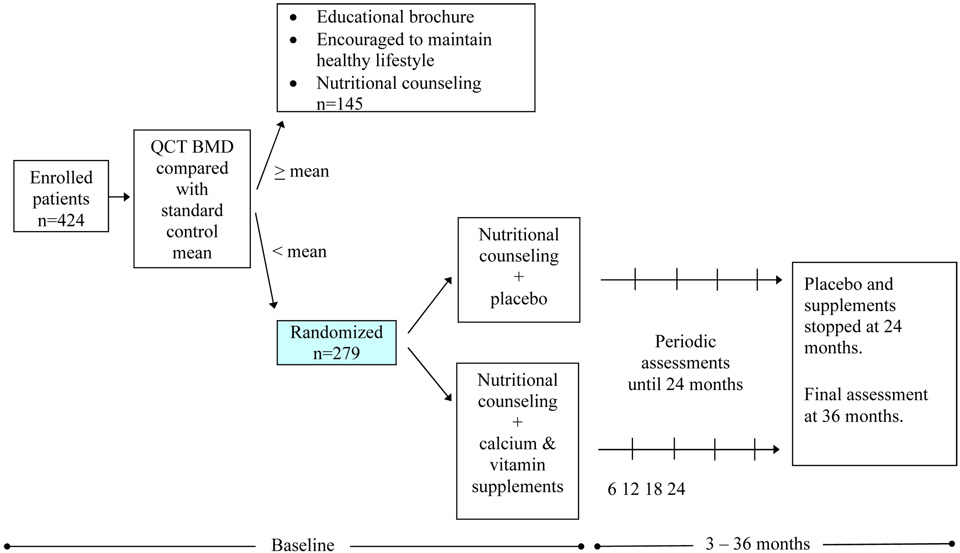
Patient eligibility criteria for participation in BONEII are: first remission and at least 5 years from completion of therapy from one of three consecutive St. Jude Children’s Research Hospital institutional childhood ALL protocols (Total Therapy studies XI, XII, and XIII) using contemporary multiagent chemotherapy with or without cranial irradiation. Ineligible patients are those who have active malignancy, are pregnant or lactating, do not give informed consent, consumed more than 800 mg of supplemental calcium or more than 800 IU of supplemental vitamin D daily in the 3 months preceding enrollment, are anemic, are unable to take pills, or have undergone bone marrow transplantation. Participants enrolled at the baseline visit with initial BMD below the mean for age- and sex-matched controls (i.e., Z-score < 0) are eligible for participation and randomization in the intervention trial, Study 2 [Figure 1]. In this prospective placebo-controlled double-blind randomized interventional trial (Study 2), patients are randomized to receive nutritional counseling in conjunction with vitamin D (vitamin D3, 800 IU/d) and calcium (2500 mg/d calcium carbonate; equal to 1000 mg/d elemental calcium) supplementation or an educational program of nutritional counseling and placebo pills (with the same shape, color, and size).
Figure 1.
BONEII study schema
Study setting
Participants are recruited from 2 sources: 1) active patients who continue with annual onsite evaluations, and 2) former patients who no longer travel annually for off-therapy evaluation but who continue to be monitored by the St. Jude Cancer Registry by postal questionnaires. For active patients, study-directed visits are coordinated with annual examinations in the After Completion of Therapy Clinic at St. Jude to minimize patient travel and limit resource stresses in the clinic that could be caused by additional patient visits.
All participants enrolled on BONEII were treated on previously reported institutional protocols for ALL [30–34], each of which had been approved by the institutional review board at St. Jude. The current study was also approved by the board, is being conducted according to the Health Insurance Portability and Accountability Act of 1996, and is overseen by a data and safety monitoring board.
It would be difficult to complete another study similar to BONEII because of the associated expense and unique access of clinical researchers at St. Jude to a large population of long-term survivors of childhood ALL. Cancer survivors at St. Jude demonstrate a willingness to participate in further clinical trials. Many have expressed a desire to contribute to the body of knowledge that can help in the care of other patients in gratitude for the care they received and the prolongation of life they have experienced.
Selected Measures
Mindful of imposing undue burden on participants and their parents or guardians, we designed BONEII to incorporate numerous and diverse outcome measures. Because bone mineralization results from a complex interaction of genetic programming [27] and environmental and lifestyle factors [28, 29], primary endpoints of BONEII include pharmacogenetic and bone metabolic biomarkers, lifestyle assessment, nutritional intake, and medical history.
The first primary objective (Study 1) of BONEII is to estimate, using quantitative computed tomography (QCT), the prevalence of BMD deficits in patients treated with contemporary protocol-based multiagent chemotherapy (with or without cranial irradiation) for childhood ALL and to investigate possible risk factors for the development of BMD deficits in these patients. Factors examined include patient characteristics (age at the time of treatment, sex, race, body mass index, physical activity and nutritional status, pubertal status, oral contraceptive use, growth hormone therapy, smoking and alcohol intake, birth weight, and fracture history), treatment effects (intensity of treatment with antimetabolites and glucocorticoids and history of cranial irradiation), and genetic predisposition to diminished BMD (vitamin D and CYP3A4 receptor polymorphism and family history of osteoporosis) (Table 1).
Table 1.
Factors investigated
| Baseline | 6 mo | 12 mo | 18 mo | 24 mo | 36 mo | |
|---|---|---|---|---|---|---|
| QCT* | ✓ | ✓ | ✓ | ✓ | ||
| DXA | ✓ | ✓ | ✓ | ✓ | ||
| Bone age (from hand or wrist) | ✓a | ✓b | ||||
| Blood for: | ||||||
| Bone-specific alkaline phosphatase | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Intact parathyroid hormone | ✓ | |||||
| Osteocalcin | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Hemoglobin and hematocrit | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| 25-hydroxyvitamin D | ✓ | |||||
| Calcium and albumin | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Phosphorus | ✓ | ✓ | ✓ | ✓ | ||
| Testosterone or estradiol | ✓ | ✓ | ✓ | ✓ | ||
| Liver function enzymes | ✓ | ✓ | ✓ | ✓ | ||
| Blood urea nitrogen and creatinine | ✓ | ✓ | ✓ | ✓ | ||
| Free T4, T4, thyroid stimulating hormone | ✓ | ✓ | ||||
| Ferritin | ✓ | ✓ | ✓ | ✓ | ||
| β human chorionic gonadotropin | ✓ | ✓ | ✓ | ✓ | ||
| 3A4 receptor genotyping | ✓ | |||||
| Urine for: | ||||||
| Sodium | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Calcium:creatinine ratio | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| N-telopeptide | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Height and weight | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Tanner stage | ✓ | ✓ | ✓ | ✓ | ||
| Dietary intake: | ||||||
| Magnesium | ✓ | ✓ | ✓ | ✓ | ||
| Potassium | ✓ | ✓ | ✓ | ✓ | ||
| Calcium | ✓ | ✓ | ✓ | ✓ | ||
| Protein | ✓ | ✓ | ✓ | ✓ | ||
| Phosphorus | ✓ | ✓ | ✓ | ✓ | ||
Mo=months
QCT=quantitative computed tomography
DXA=dual-energy x-ray absorptiometry
Bone age will not be done on patients older than 25 years.
Bone age will be obtained at 24 months only if the patient is skeletally immature at enrollment.
QCT-determined BMD serves as an eligibility screen for participation in the interventional phase (Study 2)
The second primary objective (Study 2) is to evaluate, in a prospective placebo-controlled double-blind randomized trial in patients with BMD scores below the mean (Z-score <0), the combined effects of vitamin D and calcium supplementation and nutritional counseling versus placebo and nutritional counseling. Repeated measures of bone mineral assessment and related laboratory assessments and other variables at consistent time points (such as baseline and 12, 18, 24, and 36 months; Table 1) are unique features of the protocol.
Minor objective
In addition to the two primary objectives, the interventional phase of the study (Study 2), includes a minor objective that correlates two measures of BMD—QCT and dual-energy x-ray absorptiometry (DXA)—and explores predictors of differences in BMD values.
Bone mineral density measures
QCT of the lumbar spine is used to determine eligibility for participation in Study 2. QCT is performed using a Siemens Somatom-Plus spiral CT scanner (Siemens, Iselin, NY) and Mindways QCT Calibration Phantoms and software (Mindways Software, Inc., South San Francisco, CA.). BMD is determined by obtaining direct axial images of the centers of the first and second lumbar vertebrae (L1 and L2, respectively) as localized from a sagittal scout image, as previously described [35–37]. BMD (mg/cm3) is recorded for the individual vertebral bodies, and the mean value is calculated. Normative values in the manufacturer’s reference database are used to calculate the Z-score, which is generated by the QCT software program.
A Hologic 4500 QDR-A fan beam system (Hologic Inc., Bedford, MA) is used to measure BMD of the lumbar spine in anterior projections of L1–L4 and lateral projections of L2–L4. All BMD values are analyzed with QDR software for Windows (version 12.1). Normative values in the manufacturer’s reference database are used to calculate the Z-score, which is generated by the DXA software program.
The quality-control program for both QCT and DXA comprises weekly determinations of the imaging and quantification parameters recommended by the manufacturers of the equipment and as prescribed in software modules supplied by the manufacturer. If either piece of equipment requires service, a quality-control assessment is repeated before it is used. Evaluation of quality-control measures enables adjustment of bone measurement values in light of drift in sensitivity of the equipment over time.
Biomarkers
Biomarkers of bone metabolism provide a quantitative measure of the relationship between bone deposition and bone resorption. Defining this balance and its relationship to BMD is integral to developing prospective studies designed to minimize and ameliorate BMD deficits.
Biomarkers of bone metabolism that are followed in BONEII include two markers of bone formation—serum osteocalcin and bone-specific alkaline phosphatase—and one marker of bone resorption, urine N-telopeptide. These markers are measured (for osteocalcin and alkaline phosphatase: Quidel Corporation, San Diego, CA; for N-telopeptide: Ostex International, Inc., Seattle, WA) by the enzyme-linked immunosorbent assay of samples collected at baseline and at 6, 12, 24, and 36 months after enrollment. Specimens are collected in the morning after an overnight fast (urine for analysis is the second morning void), and all assays are performed in duplicate by using the same biomarker kit, and the mean value is recorded (Table 1). In our laboratory, the intra-assay coefficients of variation for osteocalcin, alkaline phosphatase, and N-telopeptide are 4.18%, 3.83%, and 1.35%, respectively; the inter-assay coefficients of variation are 6%, 5%–7.6%, and 1.48%, respectively.
Dietary intake measures
A food-frequency questionnaire (Block ’98 FFQ, NutritionQuest, Berkeley, CA, www.nutritionquest.com) is self-administered by the study participants at the start of their enrollment in the study (baseline) and again after 12, 24, and 36 months. This full-length (110 food items) questionnaire is designed to estimate usual and customary intake of calcium, potassium, magnesium, phosphorus, and protein. The food list for this questionnaire was developed from the NHANES III dietary recall data. The nutrient database was developed from the U.S. Department of Agriculture Nutrient Database for Standard Reference [38]. The questionnaire solicits information regarding individual portions for each food and provides pictures to enhance the accuracy of quantification. A registered dietitian reviews submitted questionnaires for completeness, and completed questionnaires are processed by NutritionQuest to yield nutrient and food-item intake over the previous 12 months.
Six and 18 months after randomization, patients provide food recalls for the 24 hours prior to their visit, which offer a measure of compliance with the intervention. All records are obtained by registered dietitians who have received comprehensive training and certification from the Nutrition Coordinating Center at the University of Minnesota. All food recalls are obtained using Nutrition Data System for Research (Nutrition Coordinating Center, University of Minnesota) which is a multiple-pass interview method that allows the respondent several opportunities to recall their intake; 10% of the records are reviewed for completeness and quality assurance.
Intervention
At the time of enrollment, all participants complete the Block ’98 FFQ and receive individual nutritional counseling. This education session includes information on the number of daily servings of dairy products, serving sizes of dairy foods, and nutritional intake of a healthy diet. Group sessions are also available. For participants who are randomized between the interventional arms of Study 2, ongoing educational interactions with clinical nutrition services occur at 3-month intervals for the 24 months of the intervention (Table 2). The research staff also offers suggestions on how to mask the taste and texture of the calcium or placebo tablets. Because patients may sometimes forget to take the supplements, incentives such as pill boxes and calendars are offered to improve compliance. Participation incentives such as T-shirts, Frisbees, erasers, and ice cream and no-cost provision of study supplements and placebo are intended to enhance patient compliance. To assess participant compliance with the study supplements, a manual pill count is performed at each patient visit. Other measures include demographic, lifestyle, disease, and treatment factors. Some of these are measured longitudinally.
Table 2.
Interactive nutrition education and intervention*
| Time point | Intervention |
|---|---|
| Baseline (0 mo) | Block ’98 food-frequency questionnaire completed Nutrition education provided by a nutritionist or a member of clinical nutrition services on the following topics: • Number of servings of dairy foods people need • Serving sizes of various dairy foods Group sessions for nutritional counseling are offered at St. Jude. We have set up a system that will allow group sessions on a daily basis. Patients can be booked through the electronic scheduling system for group sessions. |
| 6-month follow-up | Food diary obtained at St. Jude Nutrition education materials on the following topics mailed to the patient’s home by a member of Clinical Nutritional Services: • Food groups in Food Guide Pyramid [48] • Healthy snacks with high-calcium foods |
| 9-month follow-up | Nutrition education material on the following topics mailed to patient’s home by a member of clinical nutrition services: • Ways to incorporate calcium- and vitamin D-rich foods into school lunches, snacks, and fast-food meals and at restaurants |
| 12-month follow-up | Block ’98 food-frequency questionnaire completed again Nutrition education provided by a nutritionist or a member of clinical nutrition services on the following topics: • Importance of calcium and vitamin D in the diet • Calcium and vitamin D in long-term health • Ways to find other nutrition information and resources in the future Group sessions for nutritional counseling are offered again at St. Jude. |
| 15-month follow-up | Nutrition education materials on the following topics mailed to patient’s home by a member of clinical nutrition services: • Healthy snacks with high-calcium foods • Servings sizes of each food group and recommended amount |
| 18-month follow-up | Food diary obtained at St. Jude Nutrition education materials on the following topics mailed to the patient’s home by a member of clinical nutrition services: • Sources of calcium and vitamin D other than dairy foods • Ways to incorporate dairy foods into meals and snacks |
| 21-month follow-up | Nutrition education materials on the following topics mailed to patient’s home by a member of clinical nutrition services: • Calcium and vitamin D in diet for growth and bone development • Calcium and vitamin D in long-term health (osteoporosis) |
| 24-month follow-up | Block ’98 food-frequency questionnaire completed again Nutrition education provided by a nutritionist or a member of clinical nutrition services on the following topics: • Number of servings of dairy foods people need • Serving sizes of various dairy foods • Sources of calcium and vitamin D other than dairy foods • Ways to incorporate dairy foods into meals and snacks that may help to “disguise” or blend them into the food Group sessions for nutritional counseling are offered again at St. Jude. |
| 36-month follow-up | Block ’98 food-frequency questionnaire completed again |
This timeline is supplemented by educational materials mailed to each participant every 3 months.
Monitoring of Patient Safety
Abnormal laboratory values
The primary physician responsible for a patient’s care is notified of any abnormal laboratory values, and the primary healthcare service directs additional assessment, treatment, and disposition. Participants with abnormal renal function (as shown by an elevated creatinine level for the patient’s age) are evaluated for prevalence and risk factor analysis but are excluded from randomization because of the potential for increased risk of hypercalciuria. Patients with abnormal pituitary function are referred to the endocrinology service for evaluation and are eligible for enrollment and randomization in the BONEII trial.
Renal calculi
All study participants undergo urine collection for creatinine and calcium at baseline and after 6, 12, 18, 24, and 36 months to monitor for possible hypercalciuria related to the study intervention (calcium and vitamin D supplementation). Spot calcium:creatinine ratios exceeding 0.2 are followed by 24-hour urine collection. The patient is evaluated by a nephrologist if the calcium:creatinine ratio of the 24-hour sample remains elevated, the patient has symptoms indicating a possible kidney stone, or a renal stone is identified by diagnostic imaging. Otherwise, the calcium:creatinine ratio is assessed at each visit.
Regardless of their calcium:creatinine ratio, patients with kidney stones at presentation and those who develop a kidney stone while enrolled in the study are referred to the nephrology service and receive a comprehensive stone risk analysis. Whether patients with calculi continue on BONEII to receive additional therapy or require an adjustment of study treatments is determined by a nephrologist. The decision is provided to and verified by the principal investigator of the study, who, in turn, notifies the primary healthcare provider.
Bone Mineral Density Loss
Most patients on BONEII enroll during growth periods of most active bone mineral accretion. If a participant loses more than 5% of baseline BMD, that individual is referred for an endocrinology consultation for assessment of other factors that may contribute to BMD loss. Continued participation in the study is contingent on the results of the endocrine evaluation.
Statistical Considerations
Justification of sample size
The design of BONEII incorporates two features in particular: the collection of baseline measurements on as many survivors as possible (Study1), and the longitudinal study of a selected subset of survivors (Study 2) requiring 196 evaluable patients. St. Jude is committed to enrolling all eligible ALL survivors treated on St. Jude Total Therapy studies XI, XII, and XIII in BONEII. When the study began, 527 patients were potentially eligible, and 156 were likely to become potentially eligible within 3 years. Of these 683 potential participants, we expected that 613 (90%) would be truly eligible (after exclusion of patients who died, were on active treatment for a second malignancy, were pregnant, or could not be contacted). Eligibility can be verified only at the time of assessment; we planned to enroll as many patients as possible.
Because as many as 63% of ALL patients treated on St. Jude Total Therapy Study XI experienced bone loss after completion of therapy [2], we developed the BONEII placebo-controlled double-blind randomized longitudinal study. In this Phase III trial, determination of the required sample size was based on the work of Liu and Liang [39], Liu et al. [35], and Diggle et al. [36]. We assumed an auto-correlated error structure for repeated observations over time within patients [35, 36, 39]. This assumption seems reasonable given the estimated correlation matrix from a study of calcium supplementation in adolescents [37]. In that study, the correlation between adjacent time points exceeded 0.95. In our sample-size calculations, we conservatively assumed a correlation of 0.7 [37].
Using preliminary data [2], we assumed that the mean BMD would be 160.50 mg/ml with a standard deviation (SD) of 17.9 mg/ml for our patient population. We also assumed that the proposed treatment (calcium and vitamin D supplementation) will at least maintain baseline BMD, even if it does not improve it; we therefore plan to test the effectiveness of the treatment by using a 1-sided test. Because the treatment lasts for only 2 years, we will use the BMDs at baseline, 12 months, and 24 months to test treatment effect. Patients also are assessed after 36 months (but without drug or placebo) to evaluate the sustainability of the treatment effect. To maximize the pool of randomized patients, all eligible survivors (those with BMD below the assumed mean) are enrolled in this longitudinal portion of the study.
Given that the institution generally has a high retention rate and low refusal rate for study enrollment and participation, we expect to randomize at least 196 evaluable patients. To detect a difference of 3.3 mg/ml in the mean annual increase of BMD (total difference of 6.6 mg/ml over 2 years) between the treatment and control groups (98 patients per arm), we will have 82% power for a 1-sided test at α = 0.05. The power calculation for the longitudinal study is based on comparing the rate of change in BMD between 2 groups, but the correlation structure used is the autoregressive lag one model, assuming observations farther away are less correlated.
Sample size justification also establishes the effect of a risk factor (e.g., cranial irradiation) on bone loss. In this case, an age- and sex-standardized BMD Z-score of less than –2 SD is considered an event (severe BMD decrement). The percentage of patients receiving prophylactic irradiation changed during each of the three St. Jude Total Therapy studies. Overall, approximately 50% of the survivors of these trials will have received cranial irradiation. With this sample size (424 patients), there will be more than 90% power (α = 0.05, 2-sided exact test) to detect a difference between a prevalence of BMD of 8% in the nonirradiated group and 18% in the irradiated group.
Randomization (Study 2)
This prospective placebo-controlled double-blind randomized trial tests whether vitamin D and calcium supplementation increases bone density in survivors of childhood ALL who have age- and sex-standardized BMD Z-scores below 0. Randomization is stratified by sex, race (white and other), and age- and sex-standardized BMD Z-score (0 to −1 SD, −1 to −2 SD, and below −2 SD) as determined by QCT. The outcome measure is the actual BMD as measured by QCT and DXA (minor objective). Patients are assessed at study entry and annually for 3 years, for 4 repeated measures on most patients. The stratified randomization used in BONEII is based on the system proposed by Zelen [40] and incorporates a blocking factor of 4. Only the St. Jude pharmacy has access to the randomization system, which is maintained by the Department of Biostatistics at St. Jude. Participants whose BMD falls more than 1 SD below the mean at study entry also undergo BMD assessment using DXA (minor objective).
Statistical Analysis
Descriptive measures will be produced for the study population. We will estimate the prevalence of severe bone loss and its 95% confidence interval in the entire sample and in the subsets. Multivariate regression (linear for BMD, cortical BMD, and Z-score and logistic for indicator of severe loss) analyses will be used to explore baseline measures and different prognostic factors (many of these listed in Table 3). In the randomized subset of participants, we will use mixed-model analyses to assess the effect of vitamin D and calcium supplementation. Because we have used stratification in the trial design, we will include all stratification factors (whether significant or not) in the model and, using contrast, will test the effect of supplementation on BMD and other measures.
Table 3.
Patient characteristics at baseline (n = 424)
| QCT group | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| >=0 SD | <0 to −1 SD | <−1 to −2 SD | <−2 SD | Total | ||||||
| n | % | n | % | n | % | n | % | n | % | |
| Age at cancer treatment (years) | ||||||||||
| 0–1 | 17 | 42.5 | 14 | 35.0 | 9 | 22.5 | 0 | 0 | 40 | 9.4 |
| 2–5 | 74 | 33.2 | 79 | 35.4 | 53 | 23.8 | 17 | 7.6 | 223 | 52.6 |
| 6–12 | 32 | 31.1 | 35 | 34.0 | 26 | 25.2 | 10 | 9.7 | 103 | 24.3 |
| >=13 | 22 | 38.6 | 20 | 35.1 | 14 | 24.6 | 2 | 3.5 | 57 | 13.4 |
| Age at BONEII enrollment (years) | ||||||||||
| 9–12 | 27 | 27.6 | 36 | 36.7 | 25 | 25.5 | 10 | 10.2 | 98 | 23.1 |
| 13–18 | 57 | 34.1 | 54 | 32.3 | 43 | 25.7 | 13 | 7.8 | 167 | 39.4 |
| 19–30 | 50 | 37.6 | 49 | 36.8 | 28 | 21.1 | 6 | 4.5 | 133 | 31.4 |
| 31–40 | 11 | 42.3 | 9 | 34.6 | 6 | 23.1 | 0 | 0 | 26 | 6.1 |
| Sex | ||||||||||
| Female | 85 | 41.3 | 71 | 34.5 | 40 | 19.4 | 10 | 4.9 | 206 | 48.6 |
| Male | 60 | 27.5 | 77 | 35.3 | 62 | 28.4 | 19 | 8.7 | 218 | 51.4 |
| Race | ||||||||||
| White | 111 | 30.4 | 137 | 37.5 | 93 | 25.5 | 24 | 6.6 | 365 | 86.1 |
| Black | 32 | 60.4 | 9 | 17.0 | 7 | 13.2 | 5 | 9.4 | 53 | 12.5 |
| Other | 2 | 33.3 | 2 | 33.3 | 2 | 33.3 | 0 | 0 | 6 | 1.4 |
| Body mass index | ||||||||||
| Below Normal (< 19 | 15 | 23.1 | 26 | 40.0 | 18 | 27.7 | 6 | 9.2 | 65 | 15.3 |
| Normal (≥ 19 but < 25) | 53 | 29.8 | 64 | 36.0 | 47 | 26.4 | 14 | 7.9 | 178 | 42.0 |
| Overweight (≥ 25 but < 30) | 31 | 33.3 | 35 | 37.6 | 21 | 22.6 | 6 | 6.5 | 93 | 21.9 |
| Obese (≥ 30) | 46 | 56.8 | 23 | 29.1 | 16 | 20.3 | 3 | 3.8 | 79 | 20.7 |
| Menarchal status | ||||||||||
| Menstruating | 76 | 44.4 | 59 | 34.5 | 31 | 18.1 | 5 | 2.9 | 171 | 40.3 |
| Not menstruating | 7 | 22.6 | 12 | 38.7 | 7 | 22.6 | 5 | 16.1 | 31 | 7.3 |
| Not applicable – male | 62 | 27.9 | 77 | 34.7 | 64 | 28.8 | 19 | 8.6 | 222 | 52.4 |
| Unknown | 2 | 22.2 | 4 | 44.4 | 2 | 22.2 | 1 | 11.1 | 9 | 2.1 |
| Cranial irradiation | ||||||||||
| None | 81 | 29.6 | 101 | 36.9 | 70 | 25.5 | 22 | 8.0 | 274 | 64.6 |
| 1800 cGy | 54 | 47.0 | 37 | 32.2 | 21 | 18.3 | 3 | 2.6 | 115 | 27.1 |
| >2400 cGy | 10 | 28.6 | 10 | 28.6 | 11 | 31.4 | 4 | 11.4 | 35 | 8.3 |
| 6-Mercaptopurine | ||||||||||
| <=17338 mg (median) | 68 | 33.0 | 76 | 36.9 | 48 | 23.3 | 14 | 6.8 | 206 | 48.6 |
| >17338 mg | 77 | 35.3 | 72 | 33.0 | 54 | 24.8 | 15 | 6.9 | 218 | 51.4 |
| Cyclophosphamide | ||||||||||
| <=5868 mg (median) | 83 | 34.6 | 80 | 33.3 | 60 | 25.0 | 17 | 7.1 | 240 | 56.6 |
| >5868 mg | 62 | 33.7 | 68 | 37.0 | 42 | 22.8 | 12 | 6.5 | 184 | 43.4 |
| Methotrexate | ||||||||||
| <=8406 mg (median) | 69 | 33.0 | 70 | 33.5 | 55 | 26.3 | 15 | 7.2 | 209 | 49.3 |
| >8406 mg | 76 | 35.3 | 78 | 36.3 | 47 | 21.9 | 14 | 6.5 | 215 | 50.7 |
| Prednisone | ||||||||||
| <=3735 mg (median) | 79 | 36.2 | 70 | 32.1 | 53 | 24.3 | 16 | 7.3 | 218 | 51.4 |
| >3735 mg | 66 | 32.0 | 78 | 37.9 | 49 | 23.8 | 13 | 6.3 | 206 | 48.6 |
| Total | 145 | 34.2 | 148 | 34.9 | 102 | 24.1 | 29 | 6.8 | 424 | 100 |
The significance level will be set at α = 0.05. Statistical analyses will be performed using SAS statistical software [37]. Because the more traditional Bonferroni adjustment is too conservative, we will use the Bonferroni–Holm adjustment procedure [40, 41], which will maintain the α level (0.05). When statistically significant findings before adjustment become nonsignificant after adjustment, we will insert a caveat when reporting the results.
Missing Observations
We will use the intent-to-treat principle in the analysis. If the overall proportion of missing data is less than the expected attrition rate (which is likely) and if data are missing at random, the following proposed analytic methods can be used without biasing the results. In the event that data on some subjects are missing at some time points, the entire subject history will not be excluded from the analysis. When data are missing at rates higher than the expected attrition rate or the mechanism underlying missing data cannot be discounted, the following steps will be taken: 1) If data regarding independent variables are missing but data for the corresponding dependent variables are present, we will do multiple imputations for the missing values to simplify the analysis. 2) If some data associated with a dependent variable are missing (i.e., follow-up data are available at some time points) and the underlying mechanism is random, the entire subject history will not be excluded (by using the Mixed Procedure in SAS); only the missing observations will be excluded. Power will be reduced when the expected attrition rate is exceeded. 3) If some dependent-variable data are missing and the underlying mechanism is nonrandom, we will estimate group effects according to the methods proposed by Wu and Bailey [42] and Milliken and Johnson [43]. Violations of the missing-at-random assumption will be investigated by following established precedents in pediatric oncology studies [42]. 4) If all dependent variable data are missing (i.e., no follow-up data available at any time point), that participant’s data will be deleted.
Results
Of the 679 patients treated in St. Jude Total Therapy studies XI, XII, and XIII, 83% were white, 13% were African–American, and 4% were of other race. The enrolled study population was divided almost evenly between male (52%) and female (48%) patients. Of the 429 survivors of ALL who were enrolled in BONEII, 5 were found to be ineligible. Of the remaining 424 participants, 279 were randomized for the longitudinal interventional study (Study 2). This large pool of survivors, comprising 63% of all ALL patients and 70% of eligible ALL survivors at St. Jude, will enable us to address many questions pertaining to BMD.
The demographic characteristics of enrolled patients are listed in Table 3. As expected, the largest group of patients began treatment between the ages of 2 and 5 years and enrolled in the current study during their teenage years (n=167; 39%). Nearly equal numbers of male (n=218, 51%) and female (n=206, 49%) patients agreed to participate; most were white (n=365, 86%). Body mass index was normal in 178 (42%) of the participants at enrollment. Treatment regimens captured indicated that most participants (n=274; 65%) received no cranial irradiation. Median doses of chemotherapeutic agents were nearly evenly divided between above versus below the median, except that slightly more than half received a cyclophosphamide dose below the median.
We found that most participants had BMD deficits, as shown in Table 3. Only 34% (n=145) of the study cohort had a BMD Z-score falling at the mean or greater, 35% (n=148) had BMD Z-score between 0 and −1 SD, 24% (n=102) had Z-score between −1 and −2 SD, and 7% (n=29) had a Z-score lower than −2 SD. Further analyses addressing risk factors for BMD deficits will be published in the future.
Discussion
Data on bone-turnover markers in healthy children is limited. Most models suggest that children are in a state of bone modeling in which bone formation predominates over bone resorption [44–46]. The effects of calcium and vitamin D in a subset of children with established low bone mass for their age and sex have not yet been examined. Such a study would be expected to provide valuable insight into the underlying mechanisms of calcium and vitamin D on bone modeling.
The BONEII study is designed primarily to investigate whether calcium and vitamin D supplementation is comparable to placebo in increasing BMD in a large cohort of childhood ALL survivors. However, this study is likely to yield further important contributions, including the following: 1) An extensive data set on the longitudinal measurement of BMD and related laboratory values in children, adolescents, and young adults previously treated for ALL will be gathered. 2) Given that ALL is the most prevalent malignancy in children, the results of BONEII will ultimately affect a vast population in whom amelioration of BMD deficits is warranted but for whom interventional trials have been sparse and have involved only small numbers of participants. 3) Because treatment for ALL includes many chemotherapeutic agents used to treat a wide variety of childhood malignancies, the results of BONEII are likely to be generalizable to the entire childhood cancer population.
We expect that institutional support for nutritional counseling follow-up visits, as well as counseling sessions held on days and during hours convenient for patients, will optimize compliance with both the intervention and study completion. Furthermore, these patients and their families historically have been compliant throughout intensive treatment for ALL and during the follow-up period. Our experience in the first years of the study showed a retention rate of about 84%. Intolerance of study medication is the most common reason for participant withdrawal [47].
Our experience with this study supports initiating an interventional trial comprising patient (and parent/guardian) education, nutritional counseling, and calcium and vitamin D supplementation in a large cohort of childhood cancer survivors at a specialty institution. To accomplish such an undertaking, sufficient resources (e.g., personnel, funds, imaging resources, and clinic space) must be allocated and maintained. As ALL is the most prevalent pediatric cancer and survival now approaches 90% [15] , results of this study are expected to affect future clinical management of the growing survivor cohort worldwide.
St. Jude places no restrictions on care on the basis of sex, race, or socioeconomic status of patients. Patients are accepted regardless of their ability to pay for care. Clinical care costs for uninsured patients are underwritten by the hospital. St. Jude also subsidizes travel and lodging for patients and family members. These benefits to patients and families are extended by the American Lebanese Syrian Associated Charities (ALSAC), the non-profit fundraising foundation that supports St. Jude.
Our preliminary assessment of the baseline data at the time of enrollment indicates that survivors of childhood ALL—and their parents and guardians—generally understand the implications of BMD deficits and are interested in improving skeletal health. Regardless, personnel dedicated to educating participants, coordinating visits, and following up on missed patient visits are integral to successful completion of such a study. The specific findings of BONEII are undergoing completion and analysis and will be reported when finished.
Preliminary review of the baseline participant demographics and treatment histories indicates a distribution of patients among the various categories that should allow adequate statistical analyses of the multiple variables to be explored that contribute to baseline BMD. Such a distribution will also allow for assessment of factors that may influence response to our study interventions and, hence, provide information important for developing future programs to minimize and ameliorate BMD deficits in childhood cancer survivors.
The successful completion of BONEII will answer vital questions relevant to bone metabolism, the effect of a nutritional and educational intervention on bone density, and its likelihood of sustainability in children and young adult survivors of ALL. This clinical trial model may then be extrapolated to other similar cohorts for whom improving health status is a priority.
Acknowledgments
The authors thank Drs. Amy L.B. Frazier, Donald D. Samulack and David Galloway for their editorial assistance with the manuscript and Sandra Gaither for manuscript preparation. We also thank the hundreds of St. Jude families who are participating in this study and the dozens of clinic, laboratory, and imaging personnel who contributed their expertise. Thanks also goes to those who provided promotional incentives (Procter & Gamble, Dippin’ Dots) and to GlaxoSmithKline for supplying Tums and placebo.
Supported in part by grants P30 CA-21765 and P01 CA-20180 from the National Institutes of Health, a Center of Excellence grant from the State of Tennessee, and the American Lebanese Syrian Associated Charities (ALSAC).
Abbreviations
- BMD
bone mineral density
- ALL
acute lymphoblastic leukemia
- QCT
quantitative computed tomography
- DXA
dual-energy x-ray absorptiometry
- SD
standard deviation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Kaste SC, Chesney RW, Hudson MM, et al. Bone mineral status during and after therapy of childhood cancer: an increasing population with multiple risk factors for impaired bone health. J Bone Miner Res. 1999;14:2010–2014. doi: 10.1359/jbmr.1999.14.12.2010. [DOI] [PubMed] [Google Scholar]
- 2.Kaste SC, Jones-Wallace D, Rose SR, et al. Bone mineral decrements in survivors of childhood acute lymphoblastic leukemia: frequency of occurrence and risk factors for their development. Leukemia. 2001;15:728–734. doi: 10.1038/sj.leu.2402078. [DOI] [PubMed] [Google Scholar]
- 3.Kaste SC, Rai SN, Fleming K, et al. Changes in bone mineral density in survivors of childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2006;46:77–87. doi: 10.1002/pbc.20553. [DOI] [PubMed] [Google Scholar]
- 4.Arikoski P, Komulainen J, Riikonen P, et al. Impaired development of bone mineral density during chemotherapy: a prospective analysis of 46 children newly diagnosed with cancer. J Bone Miner Res. 1999;14:2002–2009. doi: 10.1359/jbmr.1999.14.12.2002. [DOI] [PubMed] [Google Scholar]
- 5.Arikoski P, Kroger H, Riikonen P, et al. Disturbance in bone turnover in children with a malignancy at completion of chemotherapy. Med Pediatr Oncol. 1999;33:455–461. doi: 10.1002/(sici)1096-911x(199911)33:5<455::aid-mpo4>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 6.Arikoski P, Komulainen J, Riikonen P, et al. Alterations in bone turnover and impaired development of bone mineral density in newly diagnosed children with cancer: a 1-year prospective study. J Clin Endocrinol Metab. 1999;84:3174–3181. doi: 10.1210/jcem.84.9.5968. [DOI] [PubMed] [Google Scholar]
- 7.Arikoski P, Komulainen J, Voutilainen R, et al. Reduced bone mineral density in long-term survivors of childhood acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 1998;20:234–240. doi: 10.1097/00043426-199805000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Arikoski P, Voutilainen R, Kroger H. Bone mineral density in long-term survivors of childhood cancer. J Pediatr Endocrinol Metab. 2003;16 Suppl 2:343–353. [PubMed] [Google Scholar]
- 9.van der Sluis I, de Muinck Keizer-Schrama SM, van den Heuvel-Eibrink MM. Bone mineral density in childhood acute lymphoblastic leukemia (ALL) during and after treatment. Pediatr Blood Cancer. 2004;43:182–183. doi: 10.1002/pbc.20051. [DOI] [PubMed] [Google Scholar]
- 10.van der Sluis I, van den Heuvel-Eibrink MM, Hahlen K, et al. Altered bone mineral density and body composition, and increased fracture risk in childhood acute lymphoblastic leukemia. J Pediatr. 2002;141:204–210. doi: 10.1067/mpd.2002.125728. [DOI] [PubMed] [Google Scholar]
- 11.van der Sluis I, van den Heuvel-Eibrink MM, Hahlen K, et al. Bone mineral density, body composition, and height in long-term survivors of acute lymphoblastic leukemia in childhood. Med Pediatr Oncol. 2000;35:415–420. doi: 10.1002/1096-911x(20001001)35:4<415::aid-mpo4>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 12.Kaste SC, Ahn H, Liu T, et al. Bone mineral density deficits in pediatric patients treated for sarcoma. Pediatr Blood Cancer. 2007 doi: 10.1002/pbc.21281. [DOI] [PubMed] [Google Scholar]
- 13.Kaste SC. Bone-mineral density deficits from childhood cancer and its therapy. A review of at-risk patient cohorts and available imaging methods. Pediatr Radiol. 2004;34:373–378. doi: 10.1007/s00247-003-1132-1. [DOI] [PubMed] [Google Scholar]
- 14.Kaste SC, Shidler TJ, Tong X, et al. Bone mineral density and osteonecrosis in survivors of childhood allogeneic bone marrow transplantation. Bone Marrow Transplant. 2004;33:435–441. doi: 10.1038/sj.bmt.1704360. [DOI] [PubMed] [Google Scholar]
- 15.Pui CH, Evans WE. Treatment of acute lymphoblastic leukemia. N Engl J Med. 2006;354:166–178. doi: 10.1056/NEJMra052603. [DOI] [PubMed] [Google Scholar]
- 16.Valdimarsson O, Linden C, Johnell O, et al. Daily physical education in the school curriculum in prepubertal girls during 1 year is followed by an increase in bone mineral accrual and bone width--data from the prospective controlled Malmo pediatric osteoporosis prevention study. Calcif Tissue Int. 2006;78:65–71. doi: 10.1007/s00223-005-0096-6. [DOI] [PubMed] [Google Scholar]
- 17.Cheng S, Lyytikainen A, Kroger H, et al. Effects of calcium, dairy product, and vitamin D supplementation on bone mass accrual and body composition in 10-12-y-old girls: a 2-y randomized trial. Am J Clin Nutr. 2005;82:1115–1126. doi: 10.1093/ajcn/82.5.1115. [DOI] [PubMed] [Google Scholar]
- 18.Courteix D, Jaffre C, Lespessailles E, Benhamou L. Cumulative effects of calcium supplementation and physical activity on bone accretion in premenarchal children: a double-blind randomised placebo-controlled trial. Int J Sports Med. 2005;26:332–338. doi: 10.1055/s-2004-821040. [DOI] [PubMed] [Google Scholar]
- 19.Schneider M, Dunton GF, Bassin S, et al. Impact of a school-based physical activity intervention on fitness and bone in adolescent females. J Phys Act Health. 2007;4:17–29. doi: 10.1123/jpah.4.1.17. [DOI] [PubMed] [Google Scholar]
- 20.Ianc D, Serbescu C, Bembea M, et al. Effects of an exercise program and a calcium supplementation on bone in children: a randomized control trial. Int J Sport Nutr Exerc Metab. 2006;16:580–596. doi: 10.1123/ijsnem.16.6.580. [DOI] [PubMed] [Google Scholar]
- 21.Macdonald HM, Kontulainen SA, Khan KM, McKay HA. Is a school-based physical activity intervention effective for increasing tibial bone strength in boys and girls? J Bone Miner Res. 2007;22:434–446. doi: 10.1359/jbmr.061205. [DOI] [PubMed] [Google Scholar]
- 22.Bass SL, Naughton G, Saxon L, et al. Exercise and calcium combined results in a greater osteogenic effect than either factor alone: a blinded randomized placebo-controlled trial in boys. J Bone Miner Res. 2007;22:458–464. doi: 10.1359/jbmr.061201. [DOI] [PubMed] [Google Scholar]
- 23.DeBar LL, Ritenbaugh C, Aickin M, et al. Youth: a health plan-based lifestyle intervention increases bone mineral density in adolescent girls. Arch Pediatr Adolesc Med. 2006;160:1269–1276. doi: 10.1001/archpedi.160.12.1269. [DOI] [PubMed] [Google Scholar]
- 24.Winzenberg T, Shaw K, Fryer J, Jones G. Effects of calcium supplementation on bone density in healthy children: meta-analysis of randomised controlled trials. BMJ. 2006;333:775. doi: 10.1136/bmj.38950.561400.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Winzenberg TM, Shaw K, Fryer J, Jones G. Calcium supplementation for improving bone mineral density in children. Cochrane Database Syst Rev. 2006 doi: 10.1002/14651858.CD005119.pub2. CD005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Borer KT. Physical activity in the prevention and amelioration of osteoporosis in women : interaction of mechanical, hormonal and dietary factors. Sports Med. 2005;35:779–830. doi: 10.2165/00007256-200535090-00004. [DOI] [PubMed] [Google Scholar]
- 27.Ralston SH, de Crombrugghe B. Genetic regulation of bone mass and susceptibility to osteoporosis. Genes Dev. 2006;20:2492–2506. doi: 10.1101/gad.1449506. [DOI] [PubMed] [Google Scholar]
- 28.Janz KF, Gilmore JM, Burns TL, et al. Physical activity augments bone mineral accrual in young children: The Iowa Bone Development study. J Pediatr. 2006;148:793–799. doi: 10.1016/j.jpeds.2006.01.045. [DOI] [PubMed] [Google Scholar]
- 29.Wang X, Kammerer CM, Wheeler VW, et al. Genetic and environmental determinants of volumetric and areal BMD in multi-generational families of African ancestry: the Tobago Family Health Study. J Bone Miner Res. 2007;22:527–536. doi: 10.1359/jbmr.070106. [DOI] [PubMed] [Google Scholar]
- 30.Rivera GK, Raimondi SC, Hancock ML, et al. Improved outcome in childhood acute lymphoblastic leukaemia with reinforced early treatment and rotational combination chemotherapy. Lancet. 1991;337:61–66. doi: 10.1016/0140-6736(91)90733-6. [DOI] [PubMed] [Google Scholar]
- 31.Evans WE, Relling MV, Rodman JH, et al. Conventional compared with individualized chemotherapy for childhood acute lymphoblastic leukemia. N Engl J Med. 1998;338:499–505. doi: 10.1056/NEJM199802193380803. [DOI] [PubMed] [Google Scholar]
- 32.Pui CH, Mahmoud HH, Rivera GK, et al. Early intensification of intrathecal chemotherapy virtually eliminates central nervous system relapse in children with acute lymphoblastic leukemia. Blood. 1998;92:411–415. [PubMed] [Google Scholar]
- 33.Pui CH, Boyett JM, Rivera GK, et al. Long-term results of Total Therapy studies 11, 12 and 13A for childhood acute lymphoblastic leukemia at St Jude Children's Research Hospital. Leukemia. 2000;14:2286–2294. doi: 10.1038/sj.leu.2401938. [DOI] [PubMed] [Google Scholar]
- 34.Pui CH, Sandlund JT, Pei D, et al. Improved outcome for children with acute lymphoblastic leukemia: results of Total Therapy Study XIIIB at St Jude Children's Research Hospital. Blood. 2004;104:2690–2696. doi: 10.1182/blood-2004-04-1616. [DOI] [PubMed] [Google Scholar]
- 35.Liu A, Boyett JM, Xiong X. Sample size calculation for planning group sequential longitudinal trials. Stat Med. 2000;19:205–220. doi: 10.1002/(sici)1097-0258(20000130)19:2<205::aid-sim295>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 36.Diggle P, Heagerty P, Liang KY. Analysis of longitudinal data. Oxford University Press; 2002. [Google Scholar]
- 37.Lloyd T, Andon MB, Rollings N, et al. Calcium supplementation and bone mineral density in adolescent girls. JAMA. 1993;270:841–844. [PubMed] [Google Scholar]
- 38.United States Department of Agriculture. Human Nutrition Information Service. Composition of Foods: Raw, Processed, Prepared. Washington, DC: U.S. GPO; Nutrition Monitoring Division. 1988 1976–1988.
- 39.Liu G, Liang KY. Sample size calculations for studies with correlated observations. Biometrics. 1997;53:937–947. [PubMed] [Google Scholar]
- 40.Zelen M. The randomization and stratification of patients to clinical trials. J Chronic Dis. 1974;27:365–375. doi: 10.1016/0021-9681(74)90015-0. [DOI] [PubMed] [Google Scholar]
- 41.Cary, NC: SAS Institute Inc; The SAS System V9. 2003
- 42.Wu MC, Bailey KR. Estimation and comparison of changes in the presence of informative right censoring: conditional linear model. Biometrics. 1989;45:939–955. [PubMed] [Google Scholar]
- 43.Milliken GA, Johnson DE. Analysis of Messy Data, Volume 1: Designed Experiments. New York: Van Nostrand Reinhold; 1992. [Google Scholar]
- 44.van der Sluis I, de Muinck Keizer-Schrama SM. Osteoporosis in childhood: bone density of children in health and disease. J Pediatr Endocrinol Metab. 2001;14:817–832. doi: 10.1515/jpem.2001.14.7.817. [DOI] [PubMed] [Google Scholar]
- 45.Szulc P, Seeman E, Delmas PD. Biochemical measurements of bone turnover in children and adolescents. Osteoporos Int. 2000;11:281–294. doi: 10.1007/s001980070116. [DOI] [PubMed] [Google Scholar]
- 46.Crofton PM, Kelnar CJ. Bone and collagen markers in paediatric practice. Int J Clin Pract. 1998;52:557–565. [PubMed] [Google Scholar]
- 47.Crom DB, Tyc VL, Rai SN, et al. Retention of survivors of acute lymphoblastic leukemia in a longitudinal study of bone mineral density. J Child Health Care. 2006;10:337–350. doi: 10.1177/1367493506067886. [DOI] [PubMed] [Google Scholar]
- 48.U.S. Department of Agriculture Home and Garden. Food Guide Pyramid Bulletin No. 252. 2005