Abstract
G protein-activated inwardly rectifying K+ (GIRK) channels regulate neuronal excitability by mediating inhibitory effects of G protein-coupled receptors for neurotransmitters and neuromodulators. Notwithstanding many studies reporting modulation of GIRK channel function, whether neuronal activity regulates GIRK channel trafficking remains an open question. Here we report that NMDA receptor activation in cultured dissociated hippocampal neurons elevates surface expression of the GIRK channel subunits GIRK1 and GIRK2 in the soma, dendrites, and dendritic spines within 15 min. This activity-induced increase in GIRK surface expression requires protein phosphatase-1-mediated dephosphorylation of a serine residue (Ser-9) preceding the GIRK2 Val-13/Leu-14 (VL) internalization motif, thereby promoting channel recycling. Because activation of GIRK channels hyperpolarizes neuronal membranes, the NMDA receptor-induced regulation of GIRK channel trafficking may represent a dynamic adjustment of neuronal excitability in response to inhibitory neurotransmitters and/or neuromodulators.
Keywords: GIRK, NMDA receptor, trafficking, protein phosphatase-1, dendrites
G protein-activated inwardly rectifying K+ (GIRK) channels belong to the Kir3.x subfamily of inwardly rectifying potassium channels, with each subunit containing 2 transmembrane segments and cytoplasmic N- and C-terminal domains (1). They regulate neuronal excitability in response to neurotransmitters and neuromodulators that activate G protein-coupled receptors (GPCRs) coupled to pertussis toxin-sensitive Gi/o proteins, inducing the exchange of GDP for GTP on the Gα and dissociation of Gα-GTP from Gβγ (1). GIRK channel activation by direct binding of Gβγ causes hyperpolarization, thus reducing neuronal excitability (1). GIRK channels are also modulated by intracellular Na+, Mg2+, phosphatidylinositol 4,5-bisphosphate, Gαi, and regulators of G protein signaling (2).
Neuronal GIRK channels in the central nervous system are mostly heterotetramers of GIRK1 and GIRK2 subunits, whereas midbrain dopaminergic neurons express homomeric GIRK2 channels (1). GIRK channels reside predominantly in the soma and dendrites of pyramidal neurons (1), where their current (3, 4) dampens the effects of excitatory synaptic input (5, 6). They also mediate slow inhibitory postsynaptic current upon GABAB receptor activation and account for the hyperpolarization induced by adenosine and serotonin receptors (7). Underscoring the physiologic importance of GIRK channels, mice lacking GIRK2 display sporadic seizures, increased susceptibility to convulsant agents, and hyperactivity, as well as abnormality in cocaine self-administration, pain threshold, response to analgesics including opioids, and sensitivity to ethanol's motivational effects (8).
Dynamic regulation of GIRK channel number affords a powerful way to modulate neuronal activity. Because GIRK1 (9) and GIRK2 (10) reside in the dendrites and dendritic spines that harbor the majority of the excitatory synapses, we wondered whether activation of glutamate receptors would regulate GIRK channel density. In this study we found that activation of NMDA receptors (NMDAR) in cultured hippocampal neurons increased surface expression of GIRK1 and GIRK2 subunits in the soma, dendrites, and some spines by stimulating protein phosphatase-1 (PP1)-dependent dephosphorylation of Ser-9 preceding the GIRK2 Val-13/Leu-14 (VL) internalization signal, thereby enhancing channel delivery from recycling endosomes. Our findings of activity-dependent phosphorylation and trafficking of GIRK channels reveal a novel mechanism for dynamic modulation of neuronal excitability.
Results
Neuronal Activity Increases Surface Expression of Endogenous GIRK Channels.
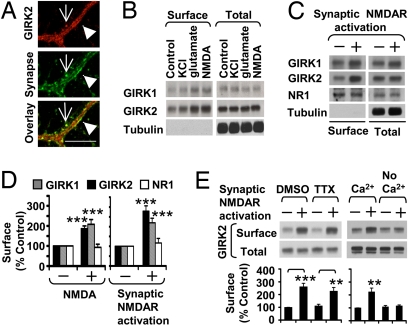
The proximity of GIRK channels to the synapses (9, 10) (Fig. 1A) raised the question of whether neuronal activity might regulate GIRK channel density. We therefore quantified endogenous GIRK channels on the surface of cultured hippocampal neurons [17 days in vitro (DIV)] by performing surface biotinylation [Fig. S1]. At steady state, a fraction of the endogenous GIRK1 (7.2% ± 4.3% of total GIRK1, n = 4) and GIRK2 (18.7% ± 4.2% of total GIRK2, n = 4) is on the cell membrane, consistent with the observation that the majority of GIRK1 and GIRK2 proteins remains inside the cell (11). When neuronal activity was induced by elevating extracellular KCl to cause depolarization or bath application of 100 μM glutamate, surface but not total protein levels of endogenous GIRK1 and GIRK2 were increased within 20 min (Fig. 1B). Activation of the NMDA subtype of glutamate receptors by treatment with 100 μM NMDA and 1 μM glycine also elevated surface expression of GIRK1 and GIRK2 by ≈2-fold (P < 0.001) but not that of the NMDAR subunit NR1, within 20 min (Fig. 1 B and D).
Fig. 1.
Neuronal activity increases surface expression of endogenous GIRK channels in cultured hippocampal neurons. (A) Immunostaining of hippocampal neurons (21 DIV) with anti-GIRK2 C-terminal (red) and anti-synaptophysin antibodies (green). Arrows point to excitatory synapses on dendritic spines, whereas arrowheads point to inhibitory synapses on dendritic shafts. (Scale bar, 2 μm.) (B) Surface biotinylation of hippocampal neurons (17 DIV) treated with 0.1% (vol/vol) H2O (Control), 50 mM KCl for 20 min, 100 μM glutamate, or 100 μM NMDA and 1 μM glycine (NMDA) for 1 min, respectively, followed by incubation in ACSF for 20 min. Endogenous tubulin is shown as cytoplasmic control. (C) Surface biotinylation of hippocampal neurons (14–17 DIV, pretreated with 200 μM APV for 3 days) incubated in media with 200 μM APV (control) or without APV for 15 min (synaptic NMDAR activation). Endogenous tubulin is shown as cytoplasmic control. (D) Quantification of surface GIRK1, GIRK2, and NMDAR subunit NR1 proteins after NMDA/glycine bath application (n = 13, 14, and 5, respectively) or synaptic NMDAR activation (n = 18, 25, and 5, respectively). (E) NMDAR-induced GIRK2 surface expression was not affected by the voltage-gated Na+ channel blocker tetrodotoxin (TTX) (1 μM) (n = 7) but was abolished by omitting extracellular Ca2+ (n = 7). **P < 0.01; ***P < 0.001.
Because functional extrasynaptic as well as synaptic NMDARs are present in hippocampal neurons (12), bath application of NMDA is expected to activate both. In an alternative approach for NMDAR activation, we treated neurons that had formed mature synapses (11–14 DIV) (13) with the NMDAR antagonist DL-2-amino-5-phosphonovaleric acid (APV) (200 μM) for 3 to 4 days and then removed APV for 15 min, thereby allowing glutamate released from presynaptic nerve terminals to activate synaptic NMDARs (14); it seems unlikely that significant amounts of glutamate could spill over from the synaptic cleft to activate extrasynaptic NMDARs without being diluted to an insignificant concentration in the bathing media. Compared with control neurons maintained in APV for an additional 15 min, removal of APV for 15 min significantly increased surface expression of endogenous GIRK1 and GIRK2 (P < 0.001) but not NR1 proteins (Fig. 1 C and D). This NMDAR-induced GIRK surface expression required Ca2+ but not action potential generation (Fig. 1E).
NMDAR-Induced Surface Expression of GIRK Channels Requires Activation of PP1 but Not PP2B.
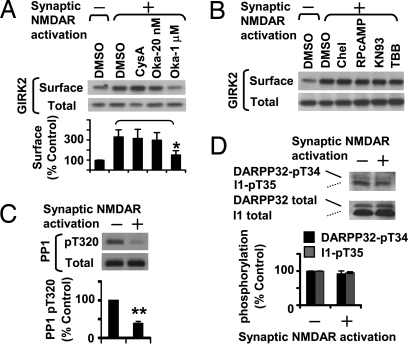
NMDAR signaling may involve kinases or phosphatases (15). To test whether phosphorylation or dephosphorylation events are involved in NMDAR-induced GIRK surface expression, we treated neurons with 20 nM okadaic acid to inhibit protein phosphatase 2A (PP2A), 1 μM okadaic acid to inhibit PP1 and PP2A, or 10 μM cyclosporine A to inhibit protein phosphatase 2B (PP2B)/calcineurin (Fig. 2A). Whereas cyclosporine A, 20 nM okadaic acid, and several kinase inhibitors had no effect (Fig. 2 A and B), 1 μM okadaic acid treatment significantly decreased NMDAR-induced surface expression of endogenous GIRK2 (P < 0.05; Fig. 2A), indicating that activity of PP2A or PP2B alone was not sufficient, whereas PP1, possibly acting together with PP2A, was required for NMDAR-induced GIRK surface expression.
Fig. 2.
NMDAR-induced surface expression of GIRK channels requires activation of PP1 via PP2B-independent signaling pathway. (A) Surface biotinylation of hippocampal neurons (DIV 17) treated with 0.1% (vol/vol) DMSO, 10 μM cyclosporine A (CysA, n = 7), 20 nM or 1 μM okadaic acid (Oka-20, Oka-1, n = 6 and n = 8, respectively). (B) Surface biotinylation of hippocampal neurons (DIV 17) treated with 0.1% (vol/vol) DMSO or kinase inhibitors, including 5 μM chelerythrine (Chel), 10 μM RP-8-Br-cAMP (RPcAMP), 10 μM KN93, or 20 μM 4,5,6,7-tetrabromobenzotriazole (TBB). (C) Synaptic NMDAR activation induces dephosphorylation of PP1 at Thr-320 (n = 3), as determined by immunoblot analysis with phosphorylation site-specific anti-PP1-pThr320 and phosphorylation-independent anti-PP1 antibodies. (D) Synaptic NMDAR activation did not cause PP2B-dependent dephosphorylation of DARPP32/I1 at Thr-34/Thr-35 (n = 4), as determined by immunoblot analysis with phosphorylation site-specific anti-DARPP32-pThr34 and phosphorylation-independent anti-DARPP32 antibodies. *P < 0.05; **P < 0.01.
We next investigated how NMDAR activation stimulates PP1 activity. Although PP2B is known to mediate NMDAR activation of PP1 by dephosphorylating the Thr-34/Thr-35 residues of dopamine- and cAMP-regulated phosphoprotein, 32-kDa (DARPP32)/Inhibitor-1 (I1), thereby relieving PP1 inhibition (16), we found that NMDAR activation did not induce dephosphorylation of DARPP32/I1 at Thr-34/Thr-35 (Fig. 2D and Fig. S2 A and B). Instead, NMDAR activation caused dephosphorylation of PP1 at Thr-320 (P < 0.01; Fig. 2C). Given that Cdk5 phosphorylation of PP1 at Thr-320 causes PP1 inactivation (17), the observed dephosphorylation of PP1 at Thr-320 as well as AMPA receptor subunit GluR1 at Ser-831 (Fig. S2C), a known substrate of PP1 (15), demonstrates PP1 stimulation by NMDAR activity.
NMDAR-Induced Surface Expression of GIRK Channels Requires the VL Internalization Motif of GIRK2.
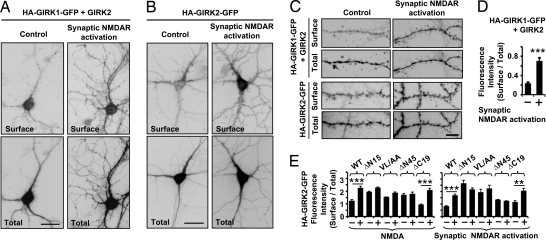
Given the presence of GIRK1 and GIRK2 in dendritic shafts and spines (9, 10) (Fig. 1A), it was important to examine surface distribution of GIRK channels in neurons after NMDAR activation. Because there are no antibodies against extracellular epitope of endogenous GIRK1 or GIRK2, we transfected neurons with recombinant GIRK1 or GIRK2 with extracellular HA and C-terminal GFP tag (HA-GIRK-GFP) (11, 18). Surface immunostaining of neurons transfected with HA-GIRK1-GFP and GIRK2A or HA-GIRK2A-GFP alone showed that synaptic NMDAR activation increased surface expression of recombinant GIRK1 and GIRK2 proteins in soma, dendritic shafts, and some dendritic spines (Fig. 3A-C), recapitulating the somatodentritic distribution of endogenous GIRK1 and GIRK2 proteins (9, 10) and functional GIRK channels (3, 4). The ratio of mean fluorescence intensity (surface/total) revealed that NMDAR activation increased dendritic surface expression of HA-GIRK1-GFP/GIRK2 proteins by ≈3-fold (P < 0.001; Fig. 3D) and HA-GIRK2A-GFP by ≈2-fold (P < 0.001; Fig. 3E).
Fig. 3.
NMDAR activation increases GIRK surface expression in neuronal soma, dendrites, and some spines. (A and C) Surface expression of GIRK1 with extracellular HA and C-terminal GFP tags (HA-GIRK1-GFP) cotransfected with GIRK2A in hippocampal neurons (14 DIV) after synaptic NMDAR activation for 15 min. Surface HA-GIRK1-GFP was labeled using anti-HA antibody without permeabilization (Surface), whereas total proteins were visualized with GFP fluorescence (Total). (B and C) Surface staining of HA-GIRK2-GFP after synaptic NMDAR activation. (Scale bars, 10 μm in A and B, 2 μm in C.) (D) Quantification of surface HA-GIRK1-GFP (cotransfected with GIRK2A) in dendrites after synaptic NMDAR activation (n = 21) or APV control solution change (n = 16). (E) Quantification of surface expression of HA-GIRK2-GFP wild type (WT), deletion mutants (ΔN15, ΔN45, ΔC19), and a VL/AA mutant (in which the VL internalization motif was mutated to AA) after NMDA/glycine bath application, or synaptic NMDAR activation. **P < 0.01; ***P < 0.001.
Surface expression of HA-GIRK1-GFP depends on its coassembly with GIRK2 to form heteromeric GIRK1/2 channels, because GIRK2 but not GIRK1 contains forward trafficking motifs (11). We therefore tested whether NMDAR activation increases surface expression of GIRK2-containing channels by regulating forward or endocytic trafficking of GIRK2 (11). Indeed, deletion or mutation of VL internalization motif (ΔN15, ΔN45, VL/AA) increased basal surface expression of GIRK2 in dendrites (P < 0.001) and occluded the NMDAR-induced increase of surface expression (Fig. 3E), suggesting that deletion or mutation of VL motif increased basal surface expression of GIRK2 to the saturated level, which could no longer be regulated by NMDAR activation. In contrast, NMDAR activation increased dendritic surface expression of wild-type GIRK2 or mutant GIRK2 with deletion of its C-terminal endoplasmic reticulum export motif (ΔC19) by ≈2-fold (Fig. 3E).
NMDAR-Induced GIRK Surface Expression Requires Rme1-Dependent Recycling of GIRK Channels to Plasma Membrane.
Because NMDAR-induced surface expression of GIRK2 requires the VL internalization motif (Fig. 3E), it is likely that NMDAR activation regulates endocytic trafficking of GIRK channels, including internalization and subsequent sorting to late endosomes for degradation or recycling endosomes, and channel delivery from recycling endosomes. In support of this notion, NMDAR-induced GIRK surface expression persisted in the presence of cycloheximide, which inhibits protein synthesis, or brefeldin A, which blocks endoplasmic reticulum to Golgi transport (Fig. S3). Moreover, the half-life of surface GIRK2 proteins in cultured hippocampal neurons was 34 h (Fig. S4)—too long for a change of degradation rate to double GIRK surface density within 15 min of NMDAR activation. These observations suggest that NMDAR activation increases GIRK surface expression by reducing internalization of surface channels, or increasing insertion of the channels from recycling endosomes into the plasma membrane, or both.
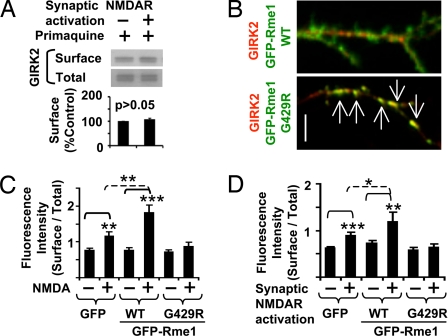
To test whether the regulation involves channel trafficking from recycling endosomes, we examined NMDAR-induced GIRK surface expression in the presence of primaquine, which acutely and selectively inhibits recycling but not endocytosis (19). Treatment with 60 μM primaquine not only blocked recycling of endogenous transferrin receptors (Fig. S5) but also abolished NMDAR-induced surface expression of endogenous GIRK2 proteins (Fig. 4A). Moreover, a mutant form of Eps15-homology domain protein EHD1/Rme1 (Rme1-G429R), which selectively impairs traffic from recycling endosomes (20), trapped GIRK2 proteins to recycling endosomes in dendrites (Fig. 4B) and abolished NMDAR-induced GIRK2 surface expression (Fig. 4 C and D). In contrast, wild-type Rme1 further enhanced NMDAR-induced GIRK2 surface expression, as compared with the control experiments with GFP (Fig. 4 C and D). Because wild-type Rme1 or Rme1-G429R had no effect on basal surface expression of GIRK2 (Fig. 4C), NMDAR-induced surface expression but not constitutive expression of GIRK channels seems to depend on Rme1-mediated traffic from recycling endosomes.
Fig. 4.
NMDAR-induced GIRK surface expression requires Rme1-dependent trafficking of GIRK channels from recycling endosomes to plasma membrane. (A) NMDAR-induced surface expression of endogenous GIRK2 was abolished by pretreating neurons with primaquine (60 μM, n = 4). (B) Coexpression of dominant negative Rme1 G429R traps GIRK2 in recycling endosomes in dendrites. Arrows indicate GIRK2 proteins that colocalized with dominant negative Rme1 G429R (Bottom). (Scale bar, 2 μm.) (C and D) Quantification of dendritic surface expression of HA-GIRK2 after NMDA/glycine bath application (C) or synaptic NMDAR activation (D) in hippocampal neurons transfected with HA-GIRK2 and GFP, GFP-Rme1 or dominant negative GFP-Rme1 G429R, which blocks recycling. *P < 0.05; **P < 0.01; ***P < 0.001.
NMDAR Activation Induces PP1-Dependent Dephosphorylation of GIRK2 Ser-9 Near VL Internalization Motif.
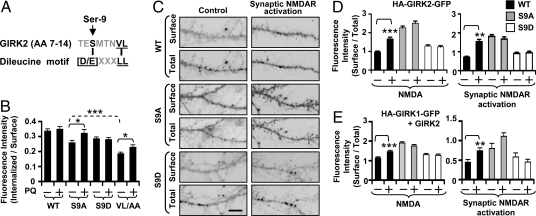
How might NMDAR-induced GIRK surface expression depend on channel delivery from recycling endosomes, VL internalization motif of GIRK2, and PP1 activity? Compared with the canonical dileucine internalization signal [D/E]xxxL[L/I] that mediates rapid internalization via the dileucine motif L[L/I] and targeting to late endosomes and lysosomes via an acidic amino acid residue [D/E] at the −4 position (21), the VL internalization signal of GIRK2 (SMTNVL) has a serine at the −4 position (Fig. 6A). Given that phosphorylation of a residue at the −4 position can modulate dileucine-mediated internalization (21), we wondered whether the phosphorylation state of GIRK2 Ser-9 could be altered by neuronal activity to regulate GIRK channel recycling and hence its surface expression.
Fig. 6.
NMDAR-induced GIRK surface expression requires dephosphorylation of Ser-9 of GIRK2, which promotes channel delivery from recycling endosomes. (A) Comparison between a typical dileucine internalization motif ([D/E]xxxL[L/I]) and GIRK2 N-terminal sequence (1–15) containing serine (Ser-9) instead of [D/E] at −4 position from VL. (B) Channel endocytosis assay in COS7 cells with or without 60 μM primaquine (PQ) treatment. COS7 cells were transfected with HA-GIRK2 wild-type (WT) or mutants (S9A, S9D, and VL/AA). Mutation of Ser-9 to alanine (S9A) and aspartate (S9D) mimics dephosphorylated and phosphorylated Ser-9, respectively. The mean fluorescence intensity of surface HA-GIRK2 proteins that have been internalized and remained inside the cells at 80 min was divided by the mean fluorescence intensity of the surface GIRK2 proteins at 0 min (Fig. S8 A, B, and D) to compute the relative level of internalization. (C) Surface staining of HA-GIRK2-GFP WT, S9A, or S9D in a dendritic process after APV control solution change (Control) or synaptic NMDAR activation. (Scale bar, 2 μm.) (D) Quantification of dendritic surface expression of HA-GIRK2-GFP WT, S9A, or S9D after NMDA/glycine bath application or synaptic NMDAR activation. (E) Quantification of dendritic surface expression of HA-GIRK1-GFP coexpressed with GIRK2 WT, S9A, or S9D after NMDA/glycine bath application or synaptic NMDAR activation. **P < 0.01; ***P < 0.001.
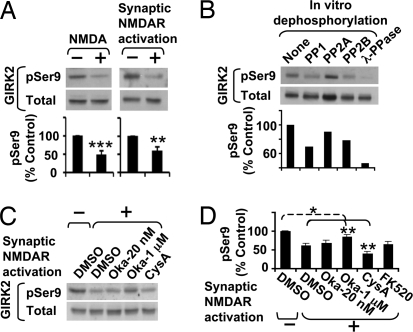
To test whether GIRK2 Ser-9 is phosphorylated in vivo, we generated a phosphorylation site-specific antibody against a peptide containing the first 17 aa of GIRK2, including a phosphorylated Ser-9 (Fig. S6A), and demonstrated its specificity (Fig. S6B). The resulting antibody detected a protein of 48 kDa in rat brain membrane homogenates, which comigrated with the GIRK2 proteins detected by anti-GIRK2 N-terminal antibody (Fig. S6C) and immunoprecipitated 9.9% ± 3.9% of total GIRK2 proteins from cultured hippocampal neurons (n = 4). Moreover, phosphorylation of GIRK2 Ser-9 was reduced to half by bath application of NMDA (P < 0.001) and synaptic NMDAR activation (P < 0.01; Fig. 5A), the same treatments that increased surface density of endogenous GIRK channels (Fig. 1).
Fig. 5.
NMDAR activation induces dephosphorylation of GIRK2 Ser-9. (A) NMDA/glycine treatment (n = 8) and synaptic NMDAR activation (n = 6) significantly decreased Ser-9 phosphorylation of GIRK2 in hippocampal neurons, as determined by immunoblot analysis with phosphorylation site-specific anti-GIRK2-pSer9 antibody (pSer9) and phosphorylation-independent anti-GIRK2 N terminus antibody (Total). (B) In vitro dephosphorylation reaction of immunoprecipitated GIRK2 proteins with purified PP1 (2.5 U), PP2A (0.1 U), PP2B (50 U), or λ-phosphatase (400 U). (C and D) Quantitative immunoblot analysis of GIRK2 Ser-9-phosphorylation in neurons treated with 0.1% (vol/vol) DMSO (n = 8), 10 μM cyclosporine A (CysA, n = 5), 20 nM or 1 μM okadaic acid (Oka-20, Oka-1, n = 3 and n = 5, respectively), or 5 μM FK520 (n = 4). *P < 0.05; **P < 0.01; ***P < 0.001.
Although both PP1 and PP2B can dephosphorylate GIRK2 Ser-9 in vitro (Fig. 5B), NMDAR-induced dephosphorylation of Ser-9 was partially blocked by 1 μM okadaic acid but not by 20 nM okadaic acid, cyclosporine A, or FK520 (Fig. 5 C and D). These findings indicate that PP1 but not PP2B mediates dephosphorylation of Ser-9 upon NMDAR activation, analogous to the requirement of PP1 but not PP2B for NMDAR-induced GIRK surface expression (Fig. 2). Interestingly, cyclosporine A but not FK520 enhanced NMDAR-induced dephosphorylation of Ser-9 (Fig. 5 C and D), suggesting that regulation of Ser-9 phosphorylation may use a complex signaling mechanism in which PP1 and PP2B are oppositely involved.
NMDAR-Induced GIRK Surface Expression Requires Dephosphorylation of GIRK2 Ser-9, Which Promotes Channel Delivery from Recycling Endosomes.
To test whether the phosphorylation state of Ser-9 near VL internalization motif affects GIRK surface expression, we mutated Ser-9 of GIRK2 to alanine (S9A) or aspartate (S9D) to mimic dephosphorylated or phosphorylated Ser-9, respectively. Whereas the single channel conductance was comparable between wild-type and Ser-9 mutant channels (Fig. S7 A and B), S9A mutant channels yielded ≈3-fold higher surface expression than wild-type and S9D mutant channels in HEK293T cells (P < 0.001; Fig. S7C). To test whether the phosphorylation state of Ser-9 modulates GIRK recycling, we performed channel endocytosis assay (22) (Fig. S8A) to follow the internalization of surface GIRK2 proteins (Fig. S8B) to endocytic compartments with or without prior treatment of primaquine to block recycling (19) (Fig. S8C) and quantified the amount of surface GIRK2 proteins that had been internalized and remained inside the cell over a period of 80 min (Fig. 6B). Under control condition, VL/AA and S9A mutations reduced the net amount of internalized GIRK2 proteins (P < 0.001; Fig. 6B). Whereas primaquine treatment increased the amount of S9A proteins and VL/AA proteins that remain internalized, it only restored the internalized GIRK2 bearing the S9A mutation close to the wild-type level (Fig. 6B), indicating that S9A mutation—and presumably Ser-9 dephosphorylation—facilitates GIRK2 recycling to plasma membrane, whereas the VL motif mediates internalization. In contrast, primaquine application did not affect the lysosomal localization or the net amount of S9D proteins that remained internalized (Figs. 6B and S8C), consistent with the notion that S9D mutation—and presumably Ser-9 phosphorylation—targets GIRK2 to lysosomes, thus reducing the chance for recycling. Similar traffic regulation by Ser-9 phosphorylation was also observed in GIRK1/GIRK2 heterotetramers (Fig. S8 E and F).
In cultured hippocampal neurons, both wild-type GIRK2 and S9D mutant proteins showed punctate distribution in soma and dendrites, whereas S9A mutant proteins displayed more diffuse distribution in both proximal and distal dendrites as well as spines, consistent with its elevated basal surface expression (Fig. 6D and Fig. S9). As expected, NMDAR activation increased dendritic surface expression of HA-GIRK2-GFP (Fig. 6 C and D) and HA-GIRK1-GFP coexpressed with wild-type GIRK2 (Fig. 6E). In contrast, NMDAR-induced surface expression of GIRK1 and GIRK2 was blocked by S9D mutation that mimics phosphorylated GIRK2 but is resistant to dephosphorylation, whereas S9A mutation of GIRK2 increased basal surface expression and occluded the NMDAR effect (Fig. 6 C-E). These results indicate that NMDAR-induced surface expression of GIRK1 and GIRK2 requires dephosphorylation of GIRK2 Ser-9.
Discussion
Activity-Dependent Dephosphorylation and Trafficking of GIRK Channels.
In this study we demonstrate that NMDAR activation in cultured hippocampal neurons increases surface expression of GIRK channels by stimulating PP1-dependent dephosphorylation of Ser-9 near the VL internalization motif of GIRK2. Ser-9 phosphorylation targets GIRK2-containing channels to lysosomes, whereas dephosphorylation of Ser-9 promotes Rme1-dependent traffic of these channels from recycling endosomes to plasma membrane, similar to the phosphorylation-dependent endocytic traffic regulation of T cell antigen receptors and CD4 coreceptor proteins (21). This Ser-9-containing recycling motif is unique to GIRK2 and is not found in other GIRK subunits. The level of basal phosphorylation at Ser-9 (≈10% of total GIRK2 proteins) is large enough to allow a 2-fold reduction of Ser-9 phosphorylation induced by NMDAR activation to increase surface expression of GIRK2 (from ≈18% to ≈36% total GIRK2 proteins) and GIRK1 (from ≈7% to ≈14% total GIRK1 proteins) if Ser-9 dephosphorylation of one GIRK2 subunit promotes recycling of GIRK2 homotetramers and GIRK1/2 heterotetramers to the plasma membrane. Given that newly synthesized membrane proteins, such as transferrin receptors and E-cadherin, have been shown to move from Golgi directly to recycling endosomes, which serve as a reserve pool for subsequent plasma membrane insertion (23, 24), it is possible that a similar reserve pool of GIRK2-containing channels in recycling endosomes could be subject to traffic regulation by NMDAR and PP1.
We also discovered that NMDAR activation stimulates PP1 by decreasing phosphorylation of PP1 at Thr-320, a site for Cdk5 phosphorylation to inhibit PP1 (17), rather than PP2B-mediated dephosphorylation of PP1 inhibitor proteins DARPP32/I1, implicating PP1 but not PP2B in the signaling pathway. We speculate that, as reported in previous studies, NMDAR activation may induce calpain-mediated cleavage of the Cdk5 activator p35 to p25 (25), causing dissociation of Cdk5 from membrane (26). This could remove Cdk5 from the proximity of membrane-associated PP1 (16), resulting in reduction of Thr-320 phosphorylation and activation of PP1. In light of recent findings that the sorting nexin SNX27 regulates trafficking of GIRK subunits to the early endosome by associating with the C terminus of GIRK2C and GIRK3 (27), it will be important to elucidate how phosphorylation state of Ser-9 affects GIRK endocytic trafficking by identifying proteins that interact with GIRK2 in a Ser-9 phosphorylation-dependent manner.
Physiologic Implications of NMDAR-Induced Increase in GIRK Surface Density.
Activity-dependent K+ channel trafficking could influence neuronal excitability. For example, glutamate-induced neuronal activity in hippocampal neurons redistributes Kv2.1 on the somatic membrane and modifies the delayed rectifier Kv current (28). Neuronal activity also causes Kv4.2 internalization in spines and dendrites to reduce the A-type Kv current and regulate synaptic integration (29). Here we demonstrate that NMDAR activation increases surface density of GIRK channels in soma, dendrites, and some spines of hippocampal neurons within 15 min. Considering the distribution of adenosine A1 receptors (30, 31) and GABAB receptors (10, 32) in the spines and dendritic shafts of hippocampal neurons and the functional coupling of GIRK channels to these GPCRs (7), as well as basal activities of GIRK channels on dendrites (4), NMDAR-induced GIRK surface expression could rapidly reduce membrane excitability in dendrites and spines, shunt the excitatory synaptic inputs, regulate synaptic integration of excitatory inputs, and alter the neuron's response to inhibitory transmitters or modulators (5, 6, 33). Thus, NMDAR-induced GIRK surface expression may also modulate activity-dependent changes in synaptic strength (synaptic plasticity), such as long-term potentiation, a cellular correlate of learning and memory (15). Given the expected difference in traffic patterns of GIRK channels with different subunit composition (11), it is of interest to note that GIRK channels containing GIRK1 and GIRK4 move from thyroid-stimulating hormone-containing dense core vesicles to the plasma membrane upon vesicle fusion, likely providing feedback regulation of hormone secretion in the anterior pituitary lobe (34).
Materials and Methods
Details of materials and experimental methods are in SI Materials. The use and care of animals in this study follows the guideline of the Institutional Animal Care and Use Committee at University of California, San Francisco.
Induction of Neuronal Activity in Primary Hippocampal Culture.
Primary hippocampal cultures from 18-day embryonic rats were prepared as described previously (35). Neurons (14–17 DIV) were incubated with artificial cerebrospinal fluid (ACSF, pH 7.4, osmolarity 300; 10 mM Hepes-free acid, 145 mM NaCl, 2.5 mM KCl, 10 mM glucose, 1 mM MgCl2, 2 mM CaCl2, and 0.1 mM picrotoxin) for 20 min (control) or with modified ACSF containing 50 mM KCl for 20 min, or with ACSF containing 100 μM glutamate, or 100 μM NMDA/1 μM glycine/5 μM strychnine for 1 min at 37 °C, followed by ACSF for 20 min. Removal of APV to activate synaptic NMDARs was performed as described previously (14).
Biotinylation Assay.
Surface biotinylation and degradation were performed on cultured hippocampal neurons as described elsewhere (13). Neurons were treated with the indicated inhibitors 1 h before and during NMDAR activation. The surface/total density ratio of test samples was normalized to the ratio of control samples to obtain percentage control.
Immunocytochemistry.
Surface immunostaining of the transfected neurons with anti-HA antibody (Covance), fluorescence image acquisition, representation, and quantification was perform as described elsewhere (35). Channel endocytosis assay was performed as described elsewhere (22).
Regulation of Ser-9 Phosphorylation of GIRK2 in Hippocampal Neurons.
Anti-GIRK2-pSer9 antibody was raised against the synthetic peptide corresponding to amino acids 1–17 of GIRK2 with phosphoserine included at the Ser-9 (Covance) and affinity purified from the sera (Pierce). Immunoblot analysis with anti-GIRK2-pSer9 and N-terminal antibodies, anti-DARPP32 and pThr34 antibodies, or anti-PP1α and pThr320 antibodies (Cell Signaling) was performed on lysates of cultured hippocampal neurons. The extent of phosphorylation was quantified by calculating the phosphorylation/total protein density ratio of test samples and was normalized to the ratio of control samples to obtain percentage control.
Statistical Analysis.
All data are reported as mean ± SEM. Sample size n refers to the number of dishes analyzed in biotinylation and phosphorylation experiments, or the number of transfected cells processed in surface immunostaining, or channel endocytosis. ANOVA and post-ANOVA Tukey's multiple comparison tests were performed to test the statistical difference between the groups of 3 or more, whereas Student's t test or paired t test was performed for groups of 2 using Prism4 (GraphPad Software).
Supplementary Material
Acknowledgments.
This work was supported a by National Institutes of Health National Research Service Award postdoctoral fellowship (to H.J.C.) and by National Institute of Mental Health Grant MH65334. L.Y.J. and Y.N.J. are Howard Hughes Medical Institute investigators.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0811615106/DCSupplemental.
References
- 1.Yamada M, Inanobe A, Kurachi Y. G protein regulation of potassium ion channels. Pharmacol Rev. 1998;50:723–760. [PubMed] [Google Scholar]
- 2.Mark MD, Herlitze S. G-protein mediated gating of inward-rectifier K+ channels. Eur J Biochem. 2000;267:5830–5836. doi: 10.1046/j.1432-1327.2000.01670.x. [DOI] [PubMed] [Google Scholar]
- 3.Takigawa T, Alzheimer C. G protein-activated inwardly rectifying K+ (GIRK) currents in dendrites of rat neocortical pyramidal cells. J Physiol. 1999;517(Pt 2):385–390. doi: 10.1111/j.1469-7793.1999.0385t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen X, Johnston D. Constitutively active G-protein-gated inwardly rectifying K+ channels in dendrites of hippocampal CA1 pyramidal neurons. J Neurosci. 2005;25:3787–3792. doi: 10.1523/JNEUROSCI.5312-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takigawa T, Alzheimer C. Phasic and tonic attenuation of EPSPs by inward rectifier K+ channels in rat hippocampal pyramidal cells. J Physiol. 2002;539(Pt 1):67–75. doi: 10.1113/jphysiol.2001.012883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takigawa T, Alzheimer C. Interplay between activation of GIRK current and deactivation of Ih modifies temporal integration of excitatory input in CA1 pyramidal cells. J Neurophysiol. 2003;89:2238–2244. doi: 10.1152/jn.00957.2002. [DOI] [PubMed] [Google Scholar]
- 7.Luscher C, Jan LY, Stoffel M, Malenka RC, Nicoll RA. G protein-coupled inwardly rectifying K+ channels (GIRKs) mediate postsynaptic but not presynaptic transmitter actions in hippocampal neurons. Neuron. 1997;19:687–695. doi: 10.1016/s0896-6273(00)80381-5. [DOI] [PubMed] [Google Scholar]
- 8.Kobayashi T, Ikeda K. G protein-activated inwardly rectifying potassium channels as potential therapeutic targets. Curr Pharm Des. 2006;12:4513–4523. doi: 10.2174/138161206779010468. [DOI] [PubMed] [Google Scholar]
- 9.Drake CT, Bausch SB, Milner TA, Chavkin C. GIRK1 immunoreactivity is present predominantly in dendrites, dendritic spines, and somata in the CA1 region of the hippocampus. Proc Natl Acad Sci USA. 1997;94:1007–1012. doi: 10.1073/pnas.94.3.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kulik A, et al. Compartment-dependent colocalization of Kir3.2-containing K+ channels and GABAB receptors in hippocampal pyramidal cells. J Neurosci. 2006;26:4289–4297. doi: 10.1523/JNEUROSCI.4178-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma D, et al. Diverse trafficking patterns due to multiple traffic motifs in G protein-activated inwardly rectifying potassium channels from brain and heart. Neuron. 2002;33:715–729. doi: 10.1016/s0896-6273(02)00614-1. [DOI] [PubMed] [Google Scholar]
- 12.Rosenmund C, Feltz A, Westbrook GL. Synaptic NMDA receptor channels have a low open probability. J Neurosci. 1995;15:2788–2795. doi: 10.1523/JNEUROSCI.15-04-02788.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mammen AL, Huganir RL, O'Brien RJ. Redistribution and stabilization of cell surface glutamate receptors during synapse formation. J Neurosci. 1997;17:7351–7358. doi: 10.1523/JNEUROSCI.17-19-07351.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liao D, Zhang X, O'Brien R, Ehlers MD, Huganir RL. Regulation of morphological postsynaptic silent synapses in developing hippocampal neurons. Nat Neurosci. 1999;2:37–43. doi: 10.1038/4540. [DOI] [PubMed] [Google Scholar]
- 15.Lee HK. Synaptic plasticity and phosphorylation. Pharmacol Ther. 2006;112:810–832. doi: 10.1016/j.pharmthera.2006.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Munton RP, Vizi S, Mansuy IM. The role of protein phosphatase-1 in the modulation of synaptic and structural plasticity. FEBS Lett. 2004;567:121–128. doi: 10.1016/j.febslet.2004.03.121. [DOI] [PubMed] [Google Scholar]
- 17.Dohadwala M, et al. Phosphorylation and inactivation of protein phosphatase 1 by cyclin-dependent kinases. Proc Natl Acad Sci USA. 1994;91:6408–6412. doi: 10.1073/pnas.91.14.6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen L, et al. A glutamate residue at the C terminus regulates activity of inward rectifier K+ channels: Implication for Andersen's syndrome. Proc Natl Acad Sci USA. 2002;99:8430–8435. doi: 10.1073/pnas.122682899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Weert AW, Geuze HJ, Groothuis B, Stoorvogel W. Primaquine interferes with membrane recycling from endosomes to the plasma membrane through a direct interaction with endosomes which does not involve neutralisation of endosomal pH nor osmotic swelling of endosomes. Eur J Cell Biol. 2000;79:394–399. doi: 10.1078/0171-9335-00062. [DOI] [PubMed] [Google Scholar]
- 20.Lin SX, Grant B, Hirsh D, Maxfield FR. Rme-1 regulates the distribution and function of the endocytic recycling compartment in mammalian cells. Nat Cell Biol. 2001;3:567–572. doi: 10.1038/35078543. [DOI] [PubMed] [Google Scholar]
- 21.Bonifacino JS, Traub LM. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu Rev Biochem. 2003;72:395–447. doi: 10.1146/annurev.biochem.72.121801.161800. [DOI] [PubMed] [Google Scholar]
- 22.Hu K, Huang CS, Jan YN, Jan LY. ATP-sensitive potassium channel traffic regulation by adenosine and protein kinase C. Neuron. 2003;38:417–432. doi: 10.1016/s0896-6273(03)00256-3. [DOI] [PubMed] [Google Scholar]
- 23.Futter CE, Connolly CN, Cutler DF, Hopkins CR. Newly synthesized transferrin receptors can be detected in the endosome before they appear on the cell surface. J Biol Chem. 1995;270:10999–11003. doi: 10.1074/jbc.270.18.10999. [DOI] [PubMed] [Google Scholar]
- 24.Lock JG, Stow JL. Rab11 in recycling endosomes regulates the sorting and basolateral transport of E-cadherin. Mol Biol Cell. 2005;16:1744–1755. doi: 10.1091/mbc.E04-10-0867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kerokoski P, Suuronen T, Salminen A, Soininen H, Pirttila T. Both N-methyl-D-aspartate (NMDA) and non-NMDA receptors mediate glutamate-induced cleavage of the cyclin-dependent kinase 5 (cdk5) activator p35 in cultured rat hippocampal neurons. Neurosci Lett. 2004;368:181–185. doi: 10.1016/j.neulet.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 26.Patrick GN, et al. Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration. Nature. 1999;402:615–622. doi: 10.1038/45159. [DOI] [PubMed] [Google Scholar]
- 27.Lunn ML, et al. A unique sorting nexin regulates trafficking of potassium channels via a PDZ domain interaction. Nat Neurosci. 2007;10:1249–1259. doi: 10.1038/nn1953. [DOI] [PubMed] [Google Scholar]
- 28.Misonou H, et al. Regulation of ion channel localization and phosphorylation by neuronal activity. Nat Neurosci. 2004;7:711–718. doi: 10.1038/nn1260. [DOI] [PubMed] [Google Scholar]
- 29.Kim J, Jung SC, Clemens AM, Petralia RS, Hoffman DA. Regulation of dendritic excitability by activity-dependent trafficking of the A-type K+ channel subunit Kv4.2 in hippocampal neurons. Neuron. 2007;54:933–947. doi: 10.1016/j.neuron.2007.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ochiishi T, et al. Cellular localization of adenosine A1 receptors in rat forebrain: Immunohistochemical analysis using adenosine A1 receptor-specific monoclonal antibody. J Comp Neurol. 1999;411:301–316. [PubMed] [Google Scholar]
- 31.Rebola N, Pinheiro PC, Oliveira CR, Malva JO, Cunha RA. Subcellular localization of adenosine A(1) receptors in nerve terminals and synapses of the rat hippocampus. Brain Res. 2003;987:49–58. doi: 10.1016/s0006-8993(03)03247-5. [DOI] [PubMed] [Google Scholar]
- 32.Kulik A, et al. Subcellular localization of metabotropic GABA(B) receptor subunits GABA(B1a/b) and GABA(B2) in the rat hippocampus. J Neurosci. 2003;23:11026–11035. doi: 10.1523/JNEUROSCI.23-35-11026.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ehrengruber MU, et al. Activation of heteromeric G protein-gated inward rectifier K+ channels overexpressed by adenovirus gene transfer inhibits the excitability of hippocampal neurons. Proc Natl Acad Sci USA. 1997;94:7070–7075. doi: 10.1073/pnas.94.13.7070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morishige K, et al. Secretagogue-induced exocytosis recruits G protein-gated K+ channels to plasma membrane in endocrine cells. J Biol Chem. 1999;274:7969–7974. doi: 10.1074/jbc.274.12.7969. [DOI] [PubMed] [Google Scholar]
- 35.Chung HJ, Jan YN, Jan LY. Polarized axonal surface expression of neuronal KCNQ channels is mediated by multiple signals in the KCNQ2 and KCNQ3 C-terminal domains. Proc Natl Acad Sci USA. 2006;103:8870–8875. doi: 10.1073/pnas.0603376103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.