Abstract
We have previously shown that mice with near-complete absence of 5-HT neurons (Lmx1bf/f/p) display a blunted hypercapnic ventilatory response (HCVR) and impaired cold-induced thermogenesis, but have normal baseline ventilation (V̇E), core body temperature (TCore) and hypoxic ventilatory responses (HVR) at warm ambient temperatures (TAmb; 30°C). These results suggest that 5-HT neurons are an important site for integration of ventilatory, metabolic and temperature control. To better define this integrative role, we now determine how a moderate cold stress (TAmb of 25°C) influences ventilatory control in adult Lmx1bf/f/p mice. During whole animal plethysmographic recordings at 25°C, baseline V̇E, metabolic rate (VO2), and TCore of Lmx1bf/f/p mice were reduced (P<0.001) compared to wild type (WT) mice. Additionally, the HCVR was reduced in Lmx1bf/f/p mice during normoxic (−33.1%) and hyperoxic (−40.9%) hypercapnia. However, V̇E in Lmx1bf/f/p mice was equal to that in WT mice while breathing 10% CO2, indicating that non-5-HT neurons may play a dominant role during extreme hypercapnia. Additionally, ventilation was decreased during hypoxia in Lmx1bf/f/p mice compared to WT mice at 25°C due to decreased TCore. These data suggest that a moderate cold stress in Lmx1bf/f/p mice leads to further dysfunction in ventilatory control resulting from failure to adequately maintain TCore. We conclude that 5-HT neurons contribute to the hypercapnic ventilatory response under physiologic, more than during extreme levels of CO2, and that mild cold stress further compromises ventilatory control in Lmx1bf/f/p mice as a result of defective thermogenesis.
Keywords: hypercapnia, hypoxia, control of breathing, serotonin
1. Introduction
The primary goal of the respiratory control system is to establish a rate of alveolar gas exchange to match metabolic demand, and as a result ventilation (V̇E) is closely linked to metabolic rate (V̇O2). However, changing environmental conditions such as ambient temperature (TAmb), O2 availability, or inspired CO2 leads to a shift in homeostatic strategies in an attempt to maintain core body temperature (TCore) and/or blood gases. V̇E and V̇O2 are close to their minimum when TAmb is near or within the thermoneutral zone (~30–32°C in mice (Gordon, C. J., 1985)). Lowering TAmb initiates mechanisms aimed at heat conservation and generation, which increases V̇O2 and consequently V̇E. These shifts in ventilation and metabolism alter the response strategy to respiratory challenges. For example, in rodents the primary response to hypoxia (and hypercapnia) under warm ambient conditions is a large increase in V̇E, whereas V̇O2 is minimally affected (Saiki, C. et al., 1996). In contrast, under cool ambient conditions the predominant effect of hypoxia is to lower V̇O2 with little or no change in V̇E (Mortola, J. P. et al., 1995). The hypoxia-induced reduction in V̇O2 results in a decrease in TCore, which can independently lower the sensitivity of the ventilatory control system depending upon the magnitude of the temperature drop (Maskrey, M., 1990). For example, severe hypothermia (decreasing TCore to ~28°C) lowers both the hypoxic and HCVRs in dogs (although interpretation of these data is complicated by the use of anesthesia) (Natsui, T., 1969). Smaller decreases in abdominal temperature (from 37°C to 35°C) in conscious rats using an abdominal heat exchanger leads to little or no change in the response to hypercapnia, whereas increasing TCore augments CO2 sensitivity (Maskrey, M., 1990). In contrast, hypoxia reduces ventilation in animals under these conditions (Maskrey, M., 1990), suggesting that modest hypothermia has greater effects on ventilatory responses to hypoxia than hypercapnia.
The integration of respiratory, metabolic and thermoregulatory demands is critical for proper blood gas, metabolic and temperature homeostasis, and the hypothalamus and raphé 5-HT system may both represent sites for such integration (Waldrop, T. G. et al., 1986; Hinrichsen, C. F. et al., 1998; Hodges, M. R. et al., 2008b). The preoptic anterior hypothalamus (POAH) contains warm- and cold-sensitive neurons (Griffin, J. D. et al., 1996), and receives afferent inputs from peripheral thermoreceptors (Boulant, J. A. et al., 1974). Additionally, warm-sensitive POAH neurons and lateral hypothalamic hypocretin-producing neurons are also CO2 sensitive, and lesioning orexin neurons blunts the HCVR (Deng, B. S. et al., 2007; Williams, R. H. et al., 2007; Wright, C. L. et al., 2007). Similarly, raphé 5-HT neurons respond to central (Nason, M. W., Jr. et al., 2006) and peripheral (Martin-Cora, F. J. et al., 2000) cooling and augment cold-induced thermogenesis. 5-HT neurons also contribute to central respiratory chemoreception, and facilitate respiratory rhythm generation and respiratory motor output (Al-Zubaidy, Z. A. et al., 1996; Pena, F. et al., 2002; Richerson, G. B., 2004; Hodges, M. R. et al., 2008a).
We have previously examined ventilatory and thermoregulatory control in Lmx1bf/f/p mice, in which Lmx1b (LIM homeobox transcription factor 1β) is deleted selectively in neurons that express Pet1 (plasmacytoma expressed transcript 1). This leads to complete and specific 5-HT neuron loss and central 5-HT depletion, without affecting other monoamine systems (Zhao, Z. Q. et al., 2006; Zhao, Z. Q. et al., 2007). Lmx1bf/f/p mice exhibit a severely blunted HCVR and cold-induced thermogenesis despite normal baseline V̇E and V̇O2, and a normal HVR (Hodges, M. R. et al., 2008b). Those ventilatory measurements were performed at TAmb of 30.0 – 30.8°C (thermoneutral) to prevent confounding effects of changes in TCore. However, the temperature of most animal facilities is maintained near 25°C, which is below thermoneutral temperature for a mouse. Previous measurements of TCore in Lmx1bf/f/p mice revealed a failure to maintain TCore at TAmb of 4°C and 12°C, but 24-hour TCore measurements in their home cages at an TAmb of 25°C revealed no differences between genotypes (Hodges, M. R. et al., 2008b). Here we examine ventilation at rest, during hypoxia, and during normoxic and hyperoxic hypercapnia in WT and Lmx1bf/f/p mice at an TAmb of 25°C using flow-through plethysmography and show that the defects in thermoregulatory and respiratory control induced by 5-HT system dysfunction combine to cause greater deleterious effects on ventilatory control during hypoxia. In addition, we challenged WT and Lmx1bf/f/p mice to pathologically high (10%) levels of CO2 to determine how important 5-HT neurons are for the HCVR under extreme conditions. All data collected at an TAmb of 30°C have been reported previously (Hodges, M. R. et al., 2008b), but are included here for direct comparison to those obtained at 25°C.
2. Methods
2.1 Animal model
The generation of Lmx1bf/f/p mice has previously been described (Zhao, Z. Q. et al., 2006). 22 female age-matched (6–12 months old, see also Table 1) WT (n = 12) and Lmx1bf/f/p (n = 10) mice were used in this study. WT and Lmx1bf/f/p littermates were paired during testing when possible.
Table 1.
Baseline parameters in WT and Lmx1bf/f/p mice
| Parameter | Wild Type | Lmx1bf/f/p | |
|---|---|---|---|
| Age (days) | 263.6 ± 9.9 | 264.6 ± 17.5 | |
| Weight (gm) | 33.0 ± 1.6 | 28.5 ± 1.1 | * |
| TCore (°C) | 37.9 ± 0.2 | 36.4 ± 0.2 | ** |
| V̇E (ml · min−1 gm−1) | 1.32 ± 0.06 | 1.04 ± 0.06 | ** |
| fR (breaths · min−1) | 172.9 ± 6.9 | 143.6 ± 4.7 | ** |
| VT (μl · breath−1 · gm−1) | 7.71 ± 0.37 | 7.24 ± 0.33 | |
| TI(sec−1) | 0.1 ± 0.004 | 0.175 ± 0.007 | ** |
| TE (sec−1) | 0.255 ± 0.012 | 0.255 ± 0.009 | |
| VT/TI(ml · breath−1·sec−1) | 2.55 ± 0.14 | 1.20 ± 0.08 | ** |
| V̇O2 (ml O2 min−1·gm−1) | 0.066 ± 0.003 | 0.056 ± 0.003 | * |
| V̇E/V̇O2 | 20.8 ± 1.3 | 19.0 ± 0.7 |
All values are mean (± SEM); Age (at the time of study), core temperature (TCore), minute ventilation (V̇E), frequency (fR), tidal volume (VT), inspiratory time (TI), expiratory time (TE), ventilatory drive (VT/TI), oxygen consumption (V̇O2), convection requirement (V̇E/V̇O2). All measurements were obtained while breathing room air at an ambient temperature of 25°C. Comparisons were made with an unpaired t-test.
denotes P<0.05,
denotes P<0.005.
2.2 Plethysmography
Ventilation and oxygen consumption were measured using standard flow-through plethysmographic techniques (Drorbaugh, J. E. et al., 1955), as described previously (Hodges, M. R. et al., 2008b). Compressed gas mixtures contained: 21% O2 with 0, 3, 5, 7, or 10% CO2 (normoxic hypercapnia; balance N2), 50% O2 with 0, 3, 5, 7, or 10% CO2 (hyperoxic hypercapnia; balance N2), or 10% O2 (hypoxia; balance N2). Hypercapnia studies consisted of >20 minutes of baseline, followed by 10-minute exposures of 3, 5, 7, and 10% CO2, each interrupted by 10-minute baseline measurements. Similarly, hypoxia studies consisted of >20 minutes of baseline followed by 10 minutes of 10% O2. The plethysmograph was set on top of a telemeter energizer/receiver (Model ER-4000, Mini Mitter, Bend, OR) for continuous measurement of core body temperature. Air temperature (25–26 °C) and humidity (Omega HX-93AV, Omega Engineering Inc., Stamford, CT), animal temperature, breathing-induced pressure oscillations (DC002NDR5, Honeywell International, Morristown, NJ), and O2 and CO2 concentrations (Models CD-3A (CO2) and S3A/I (O2), AEI Technologies Inc., Naperville, IL) were measured continuously, sampled at 100 Hz, digitized using an A/D converter (PCI-6221, National Instruments, Austin, TX) and monitored/stored on disk using a custom-written data acquisition program (Matlab, The MathWorks, Natick, MA). The outflow gases sampled for O2 and CO2 concentrations were dried using a dessication column and measured with continuous flow (200 ml/min) through the analyzers. Oxygen consumption was calculated by subtracting the outflow fraction of O2 from the inflow fraction of O2, and multiplying the difference by the chamber flow rate (700 ml/min) measured using a flow meter.
2.3 Telemetry Probe Implantation
The methods for implantation of telemetric temperature probes have been published (Hodges, M. R. et al., 2008b). Briefly, mice were given pre-operative analgesia (meloxicam (1.0 mg/kg I.P.) or buprenorphine (0.1 mg/kg I.P.)) prior to induction and maintenance of anesthesia with 20% (v/v) isoflurane mixed with polyethylene glycol. Telemetric temperature probes (Emitter G2, Minimitter, Bend, OR) were implanted into the abdomen using a ventral midline incision, and the wound was sutured. Mice received 1.7 μg/ml Meloxicam for 2 days, and studied > 7 days post-op.
2.4 Data analysis
All data were analyzed off-line using custom-written software by an individual blind to the animal genotypes. All samples of continuous ventilatory data segments of 6–10 second duration that did not contain sighs, coughs, sniffing or movement artifacts were selected for analysis during the last five-minute period of exposure to each gas mixture for the normoxic and hyperoxic hypercapnia studies. The average number of 6–10 second segments analyzed under control conditions was 35.0 ± 3.3 and 34.9 ± 2.3 for WT and (Lmx1bf/f/p) mice, respectively, roughly corresponding to 600–1000 breaths analyzed per animal during the control period. Hypoxia data were analyzed during minutes 2–10 of the 10-minute exposure, and divided into 2-minute segments to evaluate the time-course of the response. Inspiratory time (TI, seconds), expiratory time (TE, seconds), inter-breath interval (IBI, seconds, used to calculate respiratory frequency (fR), breaths·minute−1), standard deviation of IBI (seconds), tidal volume (VT, μl), oxygen consumption (V̇O2, ml·min−1) and minute ventilation (V̇E, ml·min−1, which is the product of VT and fR), were calculated for all animals under all conditions, with the exception of V̇O2 during hyperoxia due to an inability to accurately measure high (>48%) O2 concentrations. VT, V̇E and V̇O2 were normalized to animal weight.
2.5 Statistics
All data are presented as mean ± SEM. Comparisons were made using a two-way ANOVA (SYSTAT 11, Systat Software, Inc., San Jose, CA) and valid pair-wise comparisons using either a paired t-test or t-test assuming unequal variances (Excel, Microsoft Corp.), when appropriate. The threshold for significance was P < 0.05.
All animals were housed and maintained in the Yale Animal Resource Center and all protocols approved by the Yale Animal Care and Use Committee.
3. Results
3.1 Baseline ventilation and metabolic rates are reduced in Lmx1bf/f/p mice
Lmx1bf/f/p mice had significantly reduced mass compared to their WT littermates at the ages studied (Table 1). Therefore, all variables that would be affected by weight differences, such as minute ventilation (V̇E), tidal volume (VT), and oxygen consumption (V̇O2) were normalized to body weight. In contrast to previous studies where TAmb was held between 30.0 – 30.8°C, in the current study we made all measurements at an TAmb of 25°C. Under these conditions, VT was equal in WT and Lmx1bf/f/p mice, but V̇E was reduced in Lmx1bf/f/p mice due to a lower breathing frequency at rest (Table 1, Fig. 1A & C). The lower frequency was due to an increased inspiratory time (TI), with expiratory time (TE) equal between genotypes. As a result of the longer TI, ventilatory drive (VT/TI) was significantly reduced in Lmx1bf/f/p mice. In addition, both V̇O2 and TCore were significantly lower in Lmx1bf/f/p mice relative to WT mice at rest. Therefore, the decrease in V̇E was largely due to the decreased metabolic demand. The V̇E/V̇O2 ratio was equal at baseline in WT and Lmx1bf/f/p mice (P = 0.18).
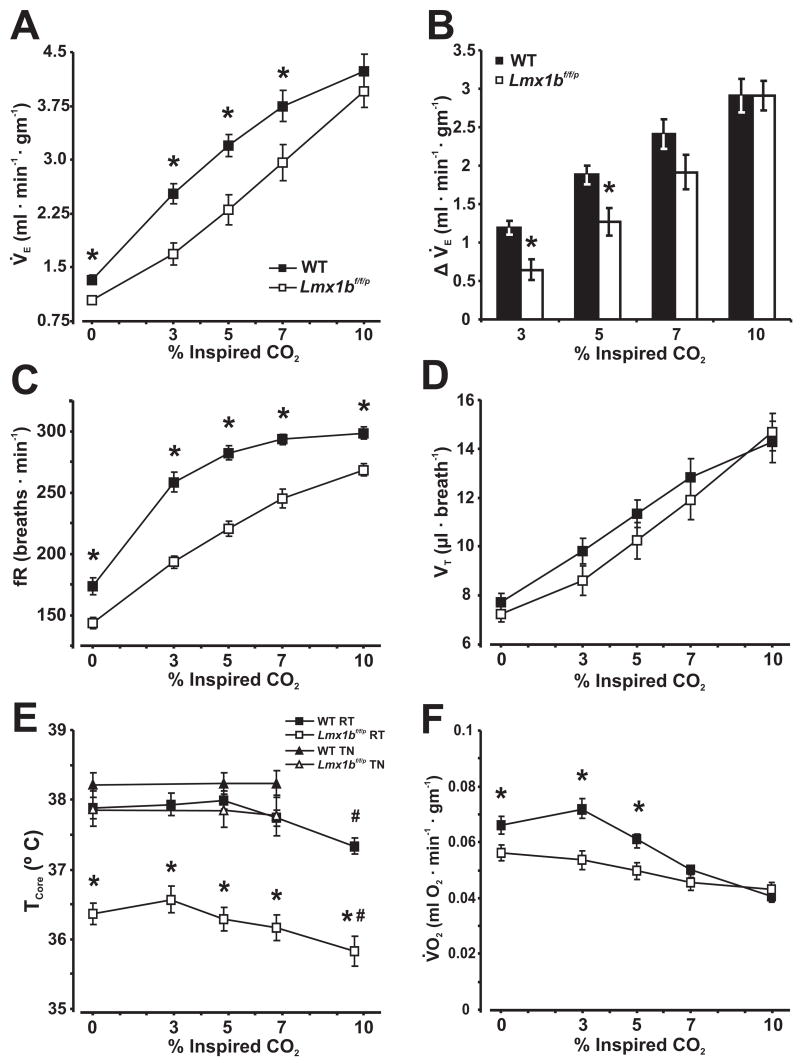
Figure 1. The normoxic hypercapnic ventilatory response and body temperature are reduced in Lmx1bf/f/p mice at 25°C.
A, Minute ventilation (V̇E), B, change in ventilation from baseline, C, respiratory frequency (fR), D, tidal volume (VT), E, core temperature (TCore) and F, oxygen consumption (V̇O2) at rest and breathing 0%, 3%, 5%, 7% and 10% inspired CO2 in normoxia (FIO2 = 0.21) at room temperature (RT: 25°C; A–D, F) or thermoneutral (TN: 30°C (Hodges, M. R. et al., 2008b)) in WT (n=12) and Lmx1bf/f/p mice (n=10). Two-way ANOVA (genotype and condition, or ambient temperature as factors) or unpaired t-test, * denotes P<0.05 for WT versus Lmx1bf/f/p, # denotes P<0.05 for 10% CO2 versus baseline. Data are mean ± SEM.
3.2 Ventilatory responses to hypoxia and hypercapnia are blunted in Lmx1bf/f/p mice
We challenged WT and Lmx1bf/f/p mice to hypoxia and both normoxic (FIO2= 0.21) and hyperoxic (FIO2 = 0.50) hypercapnia at TAmb of 25°C to determine the effects of a moderate cold stress on the response to these challenges.
3.2.1 Normoxic hypercapnia
In normoxia, we found significant effects of both genotype (P ≤ 0.001) and condition (P ≤ 0.001) on V̇E and body temperature, and condition, fR, TI, TE, VT/TI effects on VT (P < 0.0001; two-way ANOVA; Fig. 1). Specifically, V̇E was reduced in Lmx1bf/f/p mice compared to WT mice when breathing room air, 3, 5 and 7% CO2 (Fig. 1A). However, there was no difference in V̇E between genotypes breathing 10% CO2 in normoxia (P = 0.39). We also compared the increase in V̇E at each level of inspired CO2 relative to the baseline (Fig. 1B), and found that the increase in V̇E was significantly reduced breathing 3% and 5% CO2 in Lmx1bf/f/p compared to WT mice. The blunted HCVR was due solely to a smaller frequency response in Lmx1bf/f/p mice (Fig. 1C), with VT increasing equally in WT and Lmx1bf/f/p mice (Fig. 1D). There was also a longer TI in Lmx1bf/f/p mice (P < 0.002), and consequently a smaller VT/TI during all CO2 levels in normoxia (P ≤ 0.002; data not shown). TCore was significantly reduced in Lmx1bf/f/p (36.2 ± 0.2°C) relative to WT (37.7 ± 0.1°C) mice at baseline during normoxia, and remained unchanged from baseline in both genotypes while breathing 3, 5 & 7% CO2 (Fig. 2E). However, TCore dropped significantly relative to baseline in both genotypes when breathing 10% CO2, likely due to increased evaporative and convective heat loss due to the hyperpnea. Unlike WT mice, TCore in Lmx1bf/f/p mice was significantly lower at an TAmb of 25°C relative to 30°C during plethysmographic recordings (Fig. 1E). The V̇E/V̇O2 ratio in Lmx1bf/f/p mice was equal to WT mice while breathing 3% (P = 0.09), 5% (P = 0.1) and 7% (P = 0.085) CO2 due to reductions in both V̇E and V̇O2 (Fig. 1F). However, it is important to note that this measure was significantly reduced in Lmx1bf/f/p mice relative to WT mice when measured at an TAmb of 30°C (Hodges, M. R. et al., 2008b).
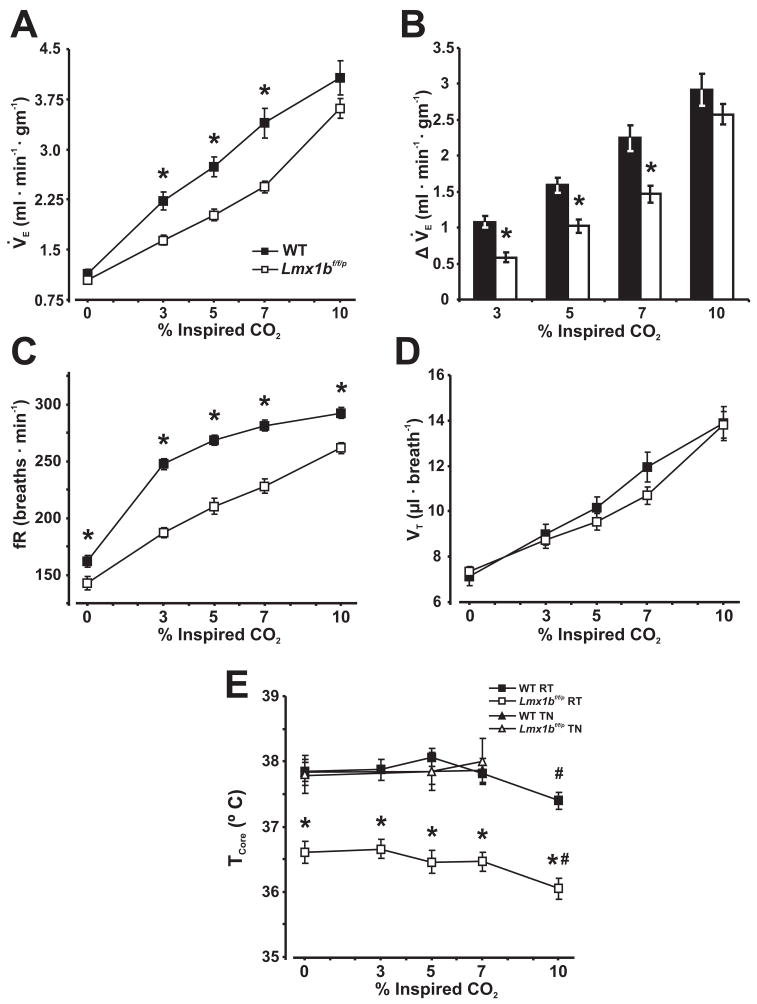
Figure 2. The hyperoxic hypercapnic ventilatory response and body temperature are reduced in Lmx1bf/f/p mice at 25°C.
A, Minute ventilation (V̇E), B, change in ventilation from baseline, C, respiratory frequency (fR), D, tidal volume (VT), and E, core temperature (TCore) at rest and breathing 0%, 3%, 5%, 7% and 10% inspired CO2 in hyperoxia (FIO2 = 0.5) at room temperature (RT: 25°C) or thermoneutral (TN: 30°C (Hodges, M. R. et al., 2008b)) in WT (n=12) and Lmx1bf/f/p mice (n=10). Two-way ANOVA (genotype and condition, or ambient temperature as factors) or unpaired t-test, * denotes P<0.05 for WT versus Lmx1bf/f/p, # denotes P<0.05 for 10% CO2 versus baseline. Data are mean ± SEM.
3.2.2 Hyperoxic hypercapnia
During hyperoxia, there were significant effects of both genotype (P < 0.01) and condition (P ≤ 0.03) on V̇E, fR, TI, TE, VT/TI and body temperature, and condition effects on VT (P < 0.0001; two-way ANOVA; Fig. 2). Hyperoxia decreased baseline V̇E in WT mice from 1.32 ± 0.06 ml · min−1 · g−1 (normoxia) to 1.15 ± 0.06 ml · min−1 · g−1 (hyperoxia: P = 0.015), but not in Lmx1bf/f/p mice, where V̇E in normoxia was 1.04 ± 0.06 ml · min−1 · g−1 and 1.05 ± 0.04 ml · min−1 · g−1 in hyperoxia (P = 0.907). However, hyperoxia per se had no effect on V̇E during hypercapnia relative to normoxia in both WT and Lmx1bf/f/p mice (P > 0.05, two-way ANOVA). Lmx1bf/f/p mice had a lower V̇E. than WT mice while breathing 3, 5, and 7% CO2, but V̇E was not different when breathing room air or 10% CO2 (Fig. 2A). The change in V̇E relative to baseline was also reduced in Lmx1bf/f/p mice breathing 3, 5, and 7% CO2, but not 10% CO2 (Fig. 2B). Lmx1bf/f/p mice also had a lower breathing frequency than WT mice under all conditions in hyperoxia, with no differences in VT (Fig. 2C & D). Additionally, TI was greater (P ≤ 0.02) and VT/TI less (P ≤ 0.0006) in Lmx1bf/f/p mice relative to WT mice at rest and at all CO2 levels tested in hyperoxia (data not shown). Similar to results in normoxia, TCore was significantly lower in Lmx1bf/f/p mice (36.4 ± 0.1°C) relative to WT (37.8 ± 0.1°C) mice at baseline during hyperoxia (Fig. 2E). TCore also dropped significantly from baseline when breathing 10% CO2 in both genotypes. However, there were no differences in TCore between genotypes at an TAmb of 30°C (Fig. 2E).
3.2.3 Hypoxia
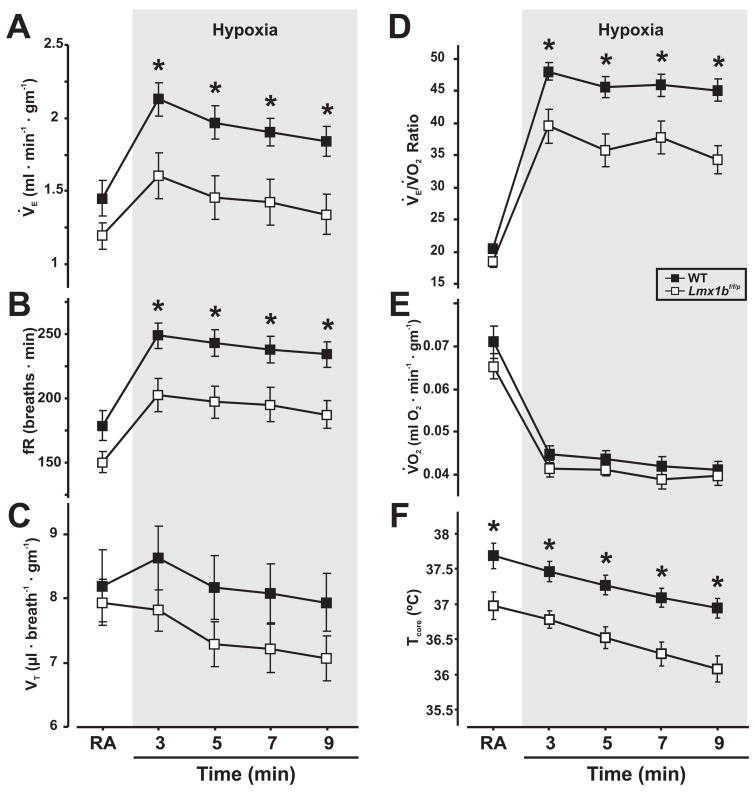
In contrast to our previous experiments in which TAmb was 30°C (Hodges, M. R. et al., 2008b), there were several differences in the response of Lmx1bf/f/p and WT mice to hypoxia when TAmb was 25°C (Fig. 3). There were significant effects of both genotype and condition on V̇E, fR, TI, TE, and body temperature, and there were genotype effects on VT/TI (P < 0.05; ANOVA). Specifically, V̇E (Fig. 3A), fR (Fig. 3B), V̇E/V̇O2 (Fig. 3D) and VT/TI were reduced throughout minutes 2 – 10 of the hypoxic challenge in Lmx1bf/f/p mice, with no effects on VT (Fig. 3C). Interestingly, we did not observe a significant decrease in V̇E or V̇O2 in Lmx1bf/f/p mice relative to WT mice during the control period before the hypoxia challenges as seen prior to the hypercapnia challenges, despite a similar difference in TCore. This may be related to variability in the time required for some animals to shift from an exploratory, active behavior to quiet wakefulness. However, both WT and Lmx1bf/f/p mice significantly decreased V̇O2 during hypoxia, with no difference between genotypes (Fig. 3E). This result indicates that the lower V̇E/V̇O2 during hypoxia was due to a larger reduction in V̇E than in V̇O2. TCore in both WT and Lmx1bf/f/p mice significantly decreased over time during hypoxia, but the initial temperature was lower in Lmx1bf/f/p mice compared to WT mice (Fig. 3F).
Figure 3. Lmx1bf/f/p mice have a blunted hypoxic ventilatory response at 25°C.
A, Minute ventilation (V̇E), B, respiratory frequency (fR), C, tidal volume (VT), D, V̇E/V̇O2 ratio, E, V̇O2, and F, core temperature (TCore) breathing room air (RA) and during minutes 2–10 of a 10-minute hypoxia challenge (FIO2 = 0.1) in WT (n=9) and Lmx1bf/f/p mice (n=7). Two-way ANOVA (genotype and time as factors) and unpaired t-test, * denotes P<0.05 for WT versus Lmx1bf/f/p mice. Data are mean ± SEM.
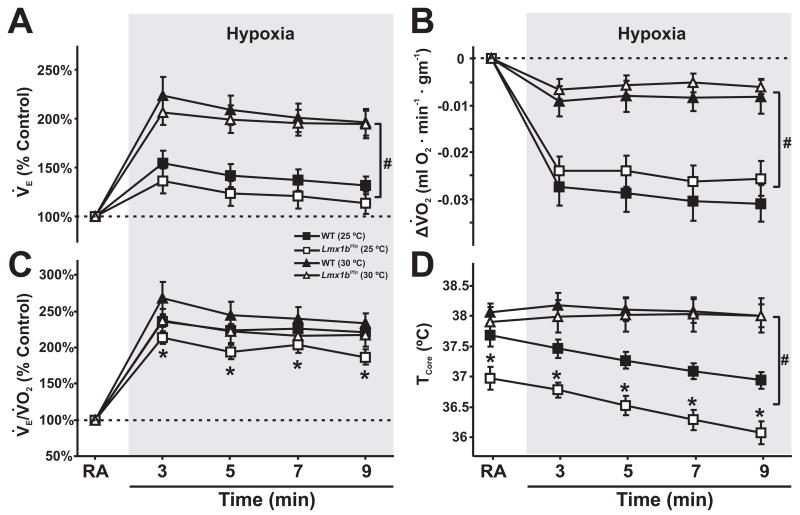
We then compared these data to those obtained from WT and Lmx1bf/f/p mice studied at an TAmb of 30°C (Hodges, M. R. et al., 2008b). For comparison, we normalized the data obtained during hypoxia at both ambient temperatures to the control period, and expressed it as % of control. We found no differences in V̇E (% control; Fig. 4A) or Δ V̇O2 (relative to room air breathing; Fig. 4B) between WT and Lmx1bf/f/p mice during hypoxia studied at an TAmb of either 25 or 30°C. However, TAmb had significant effects on the HVR. At 25°C both genotypes responded to hypoxia with a small increase in V̇E and large decrease in V̇O2. In contrast, at 30°C they responded to hypoxia with a large increase in V̇E and small decrease in V̇ O2. We also found no difference in V̇E/V̇O2 (% control) between WT and Lmx1bf/f/p mice during hypoxia at 30°C, but V̇E/V̇O2 was lower in Lmx1bf/f/p mice compared to WT mice during hypoxia at 25°C due to the smaller increase in V̇E(Fig. 4C). TCore was not different between WT and Lmx1bf/f/p mice studied at 30°C while breathing room air and during hypoxia, and TCore did not change in either genotype in response to hypoxia (Fig. 4D). In contrast, WT and Lmx1bf/f/p mice studied at an TAmb of 25°C both had a lower TCore compared to an TAmb of 30°C, and at the lower TAmb there was a significant decrease in TCore over time in response to hypoxia.
Figure 4. The ventilation to oxygen consumption ratio is reduced at 25°C due to a reduced body temperature in Lmx1bf/f/p mice.
A, Minute ventilation (VE; % control), B, V̇O2, C, V̇E/VO2 ratio (% control), and D, core temperature (TCore) in WT (solid symbols) and Lmx1bf/f/p (open symbols) mice at TAmb of 25°C (squares) and 30°C (triangles (Hodges, M. R. et al., 2008b)). Note that both genotypes shift ventilatory and metabolic strategies during hypoxia at different TAmb, and that the V̇E/VO2 ratio is reduced under cool conditions. Two-way ANOVA (genotype and time, or TAmb as factors) and unpaired t-test, * denotes P<0.05 for WT versus Lmx1bf/f/p mice, # denotes P<0.05 for 25°C versus 30°C. Data are mean ± SEM.
4. Discussion
Mice in which CNS 5-HT neurons have been genetically deleted (Lmx1bf/f/p: (Zhao, Z. Q. et al., 2006) have previously been characterized, and have severe deficits in the HCVR and cold-induced thermogenesis (Hodges, M. R. et al., 2008b). When studied at an TAmb of 30°C, these mice have normal baseline V̇E, V̇O2, TCore and HVR. We show here that when studied under mild cold stress (TAmb of 25°C combined with convective heat loss due to airflow in the plethysmograph), Lmx1bf/f/p mice continue to display an attenuated HCVR, but now also have decreased baseline V̇E, V̇O2, TCore, and ventilatory response to hypoxia. These results are consistent with the conclusion that the primary defects in Lmx1bf/f/p mice are a reduced HCVR and thermogenic capabilities, and that the decreased baseline ventilation and HVR are secondary to dysfunctional thermogenesis. Thus, a mild cold stress further compromises ventilatory control as a consequence of the thermoregulatory deficit in mice with 5-HT system dysfunction.
4.1 Methodological considerations
Mammals respond to a decreased TAmb by initiating mechanisms that drive heat conservation and generation, which increases V̇O2 and consequently ventilation (Mortola, J. P., 2005). Indeed, both WT and Lmx1bf/f/p mice had increased baseline ventilation and V̇O2 under conditions of mild cold stress relative to measurements at 30°C, but V̇O2 and TCore were lower in Lmx1bf/f/p mice compared to WT mice in these cooler conditions. This indicates that 25°C is below the lower critical temperature for these mice, and is consistent with our previous observations of thermoregulatory dysfunction (Hodges, M. R. et al., 2008b). However, we previously found no difference in TCore in WT and Lmx1bf/f/p mice measured during 24-hour recordings in their home cages at 25°C. This indicates that a mildly decreased TAmb is not sufficient alone to decrease TCore in Lmx1bf/f/p mice. It is therefore important to consider the design of the plethysmographic measurements.
We used a flow through plethysmographic chamber with a flow rate of 700 ml·min−1 to ensure rapid gas changes while retaining a large enough drop in O2 concentration to allow accurate V̇O2 measurements. This high flow rate would be expected to cause substantial convective heat loss, and a greater thermal challenge than would occur by decreasing TAmb alone. The attenuated thermogenic response to cold would lead to an exaggerated drop in TCore in Lmx1bf/f/p mice. In addition, the small dimensions of the plethysmograph chamber allowed for free, but somewhat restricted movement, which itself can affect TCore and V̇O2 (Cinelli, P. et al., 2007). This could influence TCore in a variety of ways, including increasing heat generation due to stress-induced activation of the hypothalamic-pituitary-adrenal axis and catecholamine release (Harris, R. B. et al., 2002), while decreasing heat generation due to reduced motor activity. The convective airflow combined with lower TAmb, restricted movement, and the known thermogenic defects in Lmx1bf/f/p mice likely contributed to a significantly lower TCore.
4.2 Primary and secondary effects of an absence of 5-HT neurons
In our previous work (Hodges, M. R. et al., 2008b) we found that the primary effect of near-complete absence of 5-HT neurons was a blunted hypercapnic response and impaired cold-induced thermogenesis. In contrast, there was no effect on baseline V̇E, V̇O2 and TCore. Here, we show that under cool conditions Lmx1bf/f/p mice display reduced V̇E, V̇O2 and TCore at baseline, and decreased V̇E/V̇O2 ratio during hypoxia. We conclude that the lower ventilation at rest and during hypoxia under conditions of moderate cold stress is due to the decreased TCore in Lmx1bf/f/p mice, which is secondary to a primary defect in heat generation (Hodges, M. R. et al., 2008b). While the concept of decreased body temperature blunting the ventilatory response to hypoxia is not novel, these data suggest that when the 5-HT system is not working normally a modest thermal challenge has secondary deleterious effects on ventilatory control as a result of the primary defect in thermogenesis.
Proper coupling of ventilation and metabolism is particularly important during a hypoxic challenge, where the integrated response normally includes both hyperpnea and hypometabolism. At an TAmb near the thermoneutral range, WT and Lmx1bf/f/p mice respond to hypoxia with a robust hyperpnea and only a small decrease in metabolism, whereas when TAmb is cooler both genotypes exhibited only a small increase in ventilation and a large decrease in V̇O2. In addition, both genotypes maintained a constant TCore during hypoxia at 30°C, but TCore decreased significantly in both genotypes during hypoxia at 25°C. This suggests that Lmx1bf/f/p mice retain the ability to shift strategies in changing ambient conditions, but are clearly less effective in maintaining TCore. In addition, the data suggest that 5-HT neurons do not contribute directly to the HVR or hypoxia-induced hypothermia per se, suggesting that the mechanism by which hypoxia inhibits V̇O2 (and subsequently decreases TCore) is independent of raphé 5-HT neurons.
4.3 Effects on the hypercapnic ventilatory response
At an TAmb of 30°C, the HCVR of Lmx1bf/f/p mice is reduced by 42.2% in normoxia and by 51.6% in hyperoxia (Hodges, M. R. et al., 2008b). Under those conditions, there were no differences between Lmx1bf/f/p and WT mice in V̇O2 or TCore at baseline or during hypercapnia, indicating that the deficit in the HCVR is a direct result of the absence of 5-HT and/or 5-HT neurons, and not an indirect effect from altered thermoregulation. Here we found that ventilation while breathing 3, 5 and 7% inspired CO2 was reduced by 33.1% in normoxia and by 40.9% in hyperoxia at an TAmb of 25°C, slightly less but similar to our previous findings. This result is similar to previous reports showing a greater effect of mild hypothermia on the HVR compared to the HCVR (Maskrey, M., 1990).
We previously found that relative to wild type mice, Lmx1bf/f/p mice have a significantly smaller increase in ventilation when challenged with 5% and 7% CO2, but there was no difference in ventilation at baseline or in response to 3% CO2 at 30°C (Hodges, M. R. et al., 2008b). Here we found that the ventilatory response to 3%, 5% and 7% CO2 was less in Lmx1bf/f/p than WT mice at 25°C. However, there was no difference in ventilation between genotypes breathing 10% CO2 in both normoxia and hyperoxia at 25°C. These data support the hypothesis that 5-HT neurons make their greatest contribution to central chemoreception at low levels of CO2 that would occur under physiological conditions, consistent with their high degree of intrinsic chemosensitivity to small changes in pH in vitro (Richerson, G. B., 1995; Wang, W. et al., 2001; Bradley, S. R. et al., 2002; Wang, W. et al., 2002; Richerson, G. B., 2004). The data are also consistent with the hypothesis that non-5-HT neurons make their greatest contributions under conditions of severe hypercapnia (Nattie, E., 1999).
In contrast to our previous findings at a TAmb of 30°C, the decreased ventilation during hypercapnia was not accompanied by a significant reduction in the V̇E/V̇O2 ratio, indicating that under moderate cold stress Lmx1bf/f/p mice retain the ability to generate hyperpnea proportional to metabolism during hypercapnia. The reason that different results were obtained at the two temperatures is unclear, but there are several considerations. One possibility is that a subset of non-5-HT chemoreceptors (including peripheral chemoreceptors) play a greater role at colder temperature as a result of an interaction between temperature regulation and chemoreception. Another possibility is that cold exposure leads to an increase in tonic respiratory drive from non-5-HT sources, which could mask the established primary deficit in V̇E relative to metabolism (Hodges, M. R. et al., 2008b). A third is that 5-HT neurons mediate a decrease in oxygen consumption in response to hypercapnia during cold exposure but not under thermoneutral conditions. Finally, it is important to note that it is difficult to accurately measure O2 consumption in a small species such as a mouse, and even small errors in measurement of V̇O2 could lead to inability to detect real differences. The p values comparing these data were close to the threshold for significance, preventing us from ruling out a small, but real deficit in the V̇E/V̇O2 ratio of Lmx1bf/f/p mice. However, the existing data suggest that there may be a greater degree of compensation for the loss of 5-HT neurons under thermal stress relative to thermoneutral conditions. The source of this compensation is unclear, but it may include an increase in the contribution of peripheral or other central chemoreceptors.
Consistent with developmental compensation, hyperoxia decreased resting ventilation in WT mice relative to normoxia, but had no effect on resting ventilation in Lmx1bf/f/p mice. This suggests that there may be altered carotid body function or central processing of carotid body input in response to the loss of 5-HT neurons. If there is compensation by peripheral chemoreceptors (or possibly by non-5-HT central chemoreceptors) this would lead to an underestimation of the role of 5-HT neurons in the HCVR. As discussed previously (Hodges et al, 2008), this role of 5-HT neurons in the HCVR likely includes both a direct role in sensing CO2 and an indirect role in enhancing chemosensitivity of non-5-HT neurons.
4.4 Summary and Conclusions
Ventilation and TCore can be regulated normally in Lmx1bf/f/p mice under conditions of minimal environmental stress, but both become abnormal when challenged. This is consistent with the idea that 5-HT neurons play a major role in respiratory and thermoregulatory homeostasis under conditions of environmental stress. Abnormalities in the 5-HT system have been identified in sudden infant death syndrome (SIDS) (Paterson, D. S. et al., 2006), which is thought to be due to the inability of a vulnerable infant to maintain homeostasis when challenged by an exogenous stressor (Kinney, H. C., 2005). Based on these new, and previous (Richerson, G. B., 2004; Hodges, M. R. et al., 2008b) data, we conclude that: 1) 5-HT neurons contribute significantly to the HCVR and cold-induced thermogenesis; 2) 5-HT neurons participate in the integration of ventilatory and metabolic demands, and; 3) A mild thermal stress can lead to worsening of the deficits in ventilatory control that are caused by an abnormal 5-HT system.
Acknowledgments
We thank Drs. E.E. Nattie, R.A. Darnall, H.C. Kinney, J.C. Leiter, D. Bartlett, J.A. Daubenspeck and S.M. Dymecki for their insights and thoughtful discussions. We also thank Joe Murillo, Yin Jun, Elisa Yin and Dr. Zhou-Feng Chen for animal genotyping, protocols and primers. This work was supported by the Parker B. Francis Foundation (M.R.H.), NIH P01HD36379, NIH HD052772, and the VAMC (G.B.R.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Al-Zubaidy ZA, Erickson RL, Greer JJ. Serotonergic and noradrenergic effects on respiratory neural discharge in the medullary slice preparation of neonatal rats. Pflugers Arch. 1996;431:942–949. doi: 10.1007/s004240050089. [DOI] [PubMed] [Google Scholar]
- Boulant JA, Hardy JD. The effect of spinal and skin temperatures on the firing rate and thermosensitivity of preoptic neurones. J Physiol. 1974;240:639–660. doi: 10.1113/jphysiol.1974.sp010627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley SR, Pieribone VA, Wang W, Severson CA, Jacobs RA, Richerson GB. Chemosensitive serotonergic neurons are closely associated with large medullary arteries. Nat Neurosci. 2002;5:401–402. doi: 10.1038/nn848. [DOI] [PubMed] [Google Scholar]
- Cinelli P, Rettich A, Seifert B, Burki K, Arras M. Comparative analysis and physiological impact of different tissue biopsy methodologies used for the genotyping of laboratory mice. Lab Anim. 2007;41:174–184. doi: 10.1258/002367707780378113. [DOI] [PubMed] [Google Scholar]
- Deng BS, Nakamura A, Zhang W, Yanagisawa M, Fukuda Y, Kuwaki T. Contribution of orexin in hypercapnic chemoreflex: Evidence from genetic and pharmacological disruption and supplementation studies in mice. J Appl Physiol. 2007 doi: 10.1152/japplphysiol.00075.2007. [DOI] [PubMed] [Google Scholar]
- Drorbaugh JE, FENN WO. A barometric method for measuring ventilation in newborn infants. Pediatrics. 1955;16:81–87. [PubMed] [Google Scholar]
- Gordon CJ. Relationship between autonomic and behavioral thermoregulation in the mouse. Physiol Behav. 1985;34:687–690. doi: 10.1016/0031-9384(85)90365-8. [DOI] [PubMed] [Google Scholar]
- Griffin JD, Kaple ML, Chow AR, Boulant JA. Cellular mechanisms for neuronal thermosensitivity in the rat hypothalamus. J Physiol. 1996;492(Pt 1):231–242. doi: 10.1113/jphysiol.1996.sp021304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris RB, Mitchell TD, Simpson J, Redmann SM, Jr, Youngblood BD, Ryan DH. Weight loss in rats exposed to repeated acute restraint stress is independent of energy or leptin status. Am J Physiol Regul Integr Comp Physiol. 2002;282:R77–R88. doi: 10.1152/ajpregu.2002.282.1.R77. [DOI] [PubMed] [Google Scholar]
- Hinrichsen CF, Maskrey M, Mortola JP. Ventilatory and metabolic responses to cold and hypoxia in conscious rats with discrete hypothalamic lesions. Respir Physiol. 1998;111:247–256. doi: 10.1016/s0034-5687(98)00002-4. [DOI] [PubMed] [Google Scholar]
- Hodges MR, Richerson GB. Contributions of 5-HT neurons to respiratory control: Neuromodulatory and trophic effects. Respir Physiol Neurobiol. 2008a doi: 10.1016/j.resp.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges MR, Tattersall G, Harris MB, McEvoy S, Richerson D, Deneris ES, Johnson RL, Chen ZF, Richerson GB. Defects in breathing and thermoregulation in mice with near-complete absence of central serotonin neurons. J Neurosci. 2008b;28:2495–2505. doi: 10.1523/JNEUROSCI.4729-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney HC. Abnormalities of the brainstem serotonergic system in the sudden infant death syndrome: a review. Pediatr Dev Pathol. 2005;8:507–524. doi: 10.1007/s10024-005-0067-y. [DOI] [PubMed] [Google Scholar]
- Martin-Cora FJ, Fornal CA, Metzler CW, Jacobs BL. Single-unit responses of serotonergic medullary and pontine raphe neurons to environmental cooling in freely moving cats. Neuroscience. 2000;98:301–309. doi: 10.1016/s0306-4522(00)00133-0. [DOI] [PubMed] [Google Scholar]
- Maskrey M. Body temperature effects on hypoxic and hypercapnic responses in awake rats. Am J Physiol. 1990;259:R492–R498. doi: 10.1152/ajpregu.1990.259.3.R492. [DOI] [PubMed] [Google Scholar]
- Mortola JP. Influence of temperature on metabolism and breathing during mammalian ontogenesis. Respir Physiol Neurobiol. 2005;149:155–164. doi: 10.1016/j.resp.2005.01.012. [DOI] [PubMed] [Google Scholar]
- Mortola JP, Gautier H. Interaction between metabolism and ventilation: effects of respiratory gases and temperature. In: Dempsey JA, Pack AI, editors. Regulation of Breathing. Marcel Dekker; New York: 1995. pp. 1011–1064. [Google Scholar]
- Nason MW, Jr, Mason P. Medullary raphe neurons facilitate brown adipose tissue activation. J Neurosci. 2006;26:1190–1198. doi: 10.1523/JNEUROSCI.4707-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natsui T. Respiratory response to hypoxia with hypocapnia or normocapnia and to CO2 in hypothermic dogs. Respir Physiol. 1969;7:188–202. doi: 10.1016/0034-5687(69)90005-x. [DOI] [PubMed] [Google Scholar]
- Nattie E. CO2, brainstem chemoreceptors and breathing. Prog Neurobiol. 1999;59:299–331. doi: 10.1016/s0301-0082(99)00008-8. [DOI] [PubMed] [Google Scholar]
- Paterson DS, Trachtenberg FL, Thompson EG, Belliveau RA, Beggs AH, Darnall R, Chadwick AE, Krous HF, Kinney HC. Multiple serotonergic brainstem abnormalities in sudden infant death syndrome. JAMA. 2006;296:2124–2132. doi: 10.1001/jama.296.17.2124. [DOI] [PubMed] [Google Scholar]
- Pena F, Ramirez JM. Endogenous activation of serotonin-2A receptors is required for respiratory rhythm generation in vitro. J Neurosci. 2002;22:11055–11064. doi: 10.1523/JNEUROSCI.22-24-11055.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richerson GB. Serotonergic neurons as carbon dioxide sensors that maintain pH homeostasis. Nat Rev Neurosci. 2004;5:449–461. doi: 10.1038/nrn1409. [DOI] [PubMed] [Google Scholar]
- Richerson GB. Response to CO2 of neurons in the rostral ventral medulla in vitro. J Neurophysiol. 1995;73:933–944. doi: 10.1152/jn.1995.73.3.933. [DOI] [PubMed] [Google Scholar]
- Saiki C, Mortola JP. Effect of CO2 on the metabolic and ventilatory responses to ambient temperature in conscious adult and newborn rats. J Physiol. 1996;491(Pt 1):261–269. doi: 10.1113/jphysiol.1996.sp021213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldrop TG, Mullins DC, Millhorn DE. Control of respiration by the hypothalamus and by feedback from contracting muscles in cats. Respir Physiol. 1986;64:317–328. doi: 10.1016/0034-5687(86)90125-8. [DOI] [PubMed] [Google Scholar]
- Wang W, Bradley SR, Richerson GB. Quantification of the response of rat medullary raphe neurones to independent changes in pH(o) and P(CO2) J Physiol. 2002;540:951–970. doi: 10.1113/jphysiol.2001.013443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Tiwari JK, Bradley SR, Zaykin RV, Richerson GB. Acidosis-stimulated neurons of the medullary raphe are serotonergic. J Neurophysiol. 2001;85:2224–2235. doi: 10.1152/jn.2001.85.5.2224. [DOI] [PubMed] [Google Scholar]
- Williams RH, Jensen LT, Verkhratsky A, Fugger L, Burdakov D. Control of hypothalamic orexin neurons by acid and CO2. Proc Natl Acad Sci U S A. 2007;104:10685–10690. doi: 10.1073/pnas.0702676104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CL, Boulant JA. Carbon dioxide and pH effects on temperature-sensitive and -insensitive hypothalamic neurons. J Appl Physiol. 2007;102:1357–1366. doi: 10.1152/japplphysiol.00303.2006. [DOI] [PubMed] [Google Scholar]
- Zhao ZQ, Chiechio S, Sun YG, Zhang KH, Zhao CS, Scott M, Johnson RL, Deneris ES, Renner KJ, Gereau RW, Chen ZF. Mice lacking central serotonergic neurons show enhanced inflammatory pain and an impaired analgesic response to antidepressant drugs. J Neurosci. 2007;27:6045–6053. doi: 10.1523/JNEUROSCI.1623-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao ZQ, Scott M, Chiechio S, Wang JS, Renner KJ, Gereau RW, Johnson RL, Deneris ES, Chen ZF. Lmx1b is required for maintenance of central serotonergic neurons and mice lacking central serotonergic system exhibit normal locomotor activity. J Neurosci. 2006;26:12781–12788. doi: 10.1523/JNEUROSCI.4143-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]