Abstract
The estrogen receptor-α (ERα) acts through multiple pathways, including estrogen response element (ERE)-dependent (classical) and ERE-independent (nonclassical) mechanisms. We previously created a mouse model harboring a two-amino-acid mutation of the DNA-binding domain (E207A, G208A) that precludes direct binding of ERα to an ERE. After crossing heterozygous mutant mice with an ERα knockout (ERKO) line, it was possible to assess the degree of physiological rescue by the isolated ERα nonclassical allele (−/AA; AA) when compared with ERKO mice (−/−) and to wild type (+/+; WT). In male ERKO mice up to 8 months of age, testosterone levels were high, although LH levels were similar to WT. Testosterone was normal in the AA mice, indicating that the AA allele rescues the enhanced testosterone biosynthesis in ERKO mice. Male ERKO mice exhibited distention of the seminiferous tubules as early as 2–3 months of age as a consequence of decreased water resorption in the efferent ducts. By 3–4 months of age, ERKO mice had impaired spermatogenesis in approximately 40% of their tubules, and sperm counts and motility declined in association with the histological changes. In the AA mice, histological defects were greatly reduced or absent, and sperm counts and motility were rescued. Levels of aquaporins 1 and 9, which contribute to water uptake in the efferent ducts, were reduced in ERKO mice and partially or fully rescued in AA mice, whereas another water transporter, sodium-hydrogen exchanger-3, was decreased in both ERKO and AA mice. We conclude that non-ERE-dependent estrogen pathways are sufficient to rescue the defective spermatogenesis observed in ERKO mice and play a prominent role in ERα action in the testis, including pathways that regulate water resorption and androgen biosynthesis.
ALTHOUGH INITIALLY identified as a female reproductive hormone, estrogen is now recognized to alter the function of the male reproductive system as well as other physiological systems including the skeleton, the cardiovascular system, and the brain (1). Estrogen is also involved in the pathogenesis of a number of diseases including breast cancer, cardiovascular disease, and osteoporosis. For these reasons, it is important to understand the cellular pathways mediating estrogen action.
Most estrogen actions occur via the two estrogen receptors, ERα (2) and ERβ (3,4). Initial studies of ER action demonstrated that the receptor binds to a consensus estrogen response element (ERE), and it was anticipated that this mechanism, referred to as classical ER signaling, would explain the majority of ER action. However, more recent work has shown that ER signals through multiple pathways that do not involve EREs (5). These pathways involve tethering to other transcription factors bound to DNA (6) as well as non-DNA-dependent mechanisms such as signaling initiated by a cell-membrane-bound complex that includes the ERα or other ER-binding proteins (7). For those pathways that involve DNA binding, many EREs and other ER binding sites have been shown to reside within introns or at sites distant from coding sequences that may be under their control (8,9).
Murine knockouts (KOs) of the ERα and the ERβ have been created and extensively characterized, providing insight into their physiological functions (10,11,12). Although ERβ is expressed in the male reproductive system (13,14,15), male ERβ KO mice have phenotypically normal testes, and four of the five published ERβ KO models are fertile (11). In one recent publication, a null mutant of ERβ was shown to be infertile despite normal sperm production (16), consistent with a role for ERβ in male reproductive behavior. By contrast, ERα KO males exhibit defects in water resorption in the efferent ducts, excess androgen biosynthesis (17), and infertility (18).
Previously, we created a murine model that distinguishes classical estrogen signaling from the other pathways described above, referred to collectively as nonclassical (19). This model was generated by inserting two point mutations into the first zinc finger of the DNA-binding domain of the ERα, replacing the glutamic acid at position 207 and the glycine at position 208 with alanines (AA allele). These changes effectively prevent the ERα from binding to or signaling through an ERE in vitro. However, ERα-AA can still regulate gene transcription by tethering to activator protein-1 proteins, and ERα-cofactor interactions remain intact (20).
The AA allele has also been knocked into the ERα locus of mice. Heterozygous female mice (+/AA) are infertile due to anovulation and other reproductive deficits (19). However, heterozygous male mice are fertile, as are heterozygous ERKO mice of both sexes (10). By crossing +/AA males with −/+ females, we generated −/AA males that have isolated nonclassical ERα signaling (−/AA; AA). In this report, structure and function of the testis in AA males was compared with ERKO (−/−) and wild-type (+/+; WT) males to determine whether nonclassical signaling is sufficient to rescue the defects observed in the total absence of ERα signaling. We find that many of the structural and functional abnormalities observed in ERKO testes are restored after replacement of nonclassical ERα signaling.
Materials and Methods
Animal husbandry
ERKO mice were provided by Dr. Ken Korach (10). AA mice were developed in our laboratory (19). All animals were bred in-house on a C57/B6 background (>10 generations) and fed standard Harlan Teklad chow from weaning. Experimental males were group housed with littermate males and maintained on a 14-h light, 10-h dark cycle. All experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Animals, and all studies were approved by the Northwestern University Animal Care and Use Committee.
Serum collection and assays
Blood was allowed to coagulate for 2 h at 4 C and centrifuged at 2000 × g for 15 min. Serum was transferred to a fresh tube and stored at −20 C. Sera were randomized and assayed using a mouse LH sandwich immunoradiometric assay, testosterone RIA, and FSH RIA at the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core.
Histological analysis
Bouin’s fixed, paraffin-embedded testes were cut in cross and longitudinal sections and stained with hematoxylin and eosin for qualitative assessment or with periodic acid Schiff-hematoxylin for quantitative analysis. Longitudinal periodic acid Schiff-hematoxylin testis sections were scored to best capture regional damage observed in ERKO testes. Each tubule of the section was scored on a five-point scale from A (best) to E (worst) based on the cell types present, taking into account the tubule’s stage in the cycle of the seminiferous epithelium. Categories were as follows: A, normal spermatogenesis; B, missing more than 75% of elongated spermatids; C, missing more than 75% of all spermatids; D, missing more than 75% of spermatocytes; E, missing more than 75% of spermatogonia. Data were calculated as percentage of tubules with each score. One full section from each animal was scored, and three to nine animals were scored for each age/genotype group.
Sperm counts and motility
Males were killed between 3 and 12 wk of age and epididymides and vas deferens were collected immediately. A single cauda epididymis and vas deferens was placed in a microfuge tube containing 1 ml Quinn’s sperm wash medium at 37 C. Using a sterile scissors, four to five cuts were made in the tissue to allow sperm to exit the epididymis. After incubation at 37 C for 10 min, the microfuge tube was slowly inverted several times, and the tissue was allowed to settle to the bottom of the tube. Using a Rainin wide-orifice 250-μl pipette tip, a 100-μl aliquot of sperm was transferred to a microfuge tube containing 900 μl fresh Quinn’s at 37 C. The tube was inverted several times, and a 20-μl aliquot was taken using wide-orifice pipette tips to load a standard hemocytometer. Three separate fields in a nine-square grid were counted for total sperm and motility. Counts were averaged for three fields and corrected for dilution factors to yield a total sperm concentration per milliliter for the contents of one cauda epididymis.
Immunohistochemistry
For immunohistochemical staining, tissues were fixed in 4% paraformaldehyde solution overnight at 4 C, paraffin embedded, and sectioned at 5 μm. Tissue sections were stained for aquaporin 1 (Aq1) (SC20810; Santa Cruz Biotechnology, Santa Cruz, CA), Aq9 (AQP91-A; Alpha Diagnostics, San Antonio, TX), and sodium-hydrogen exchanger-3 (NHE3) (AB3085; Chemicon, Temecula, CA). Each antibody was used at a 1:500 dilution, and a published protocol was followed (21). Liquid DAB+ Substrate Chromogen System (DakoCytomation K3468; Dako, Carpinteria, CA) was used to detect the peroxidase-conjugated secondary antibody. These sections were counterstained with hematoxylin.
Western blot analysis
Efferent duct tissues were homogenized at 4 C in radioimmunoprecipitation assay buffer (50 mm Tris buffer containing 150 mm NaCl, 1% Nonidet P-40, 0.1% sodium dodecyl sulfate, 0.5% deoxycholic acid, 1 mm EDTA, pH 8.0) in the presence of a protease inhibitor cocktail (Roche, Indianapolis, IN). The homogenate was centrifuged at 15,000 × g for 2 min, and the resulting supernatant was used for Western blot analysis (50 μg protein per lane). Concentrations of the protein samples were determined by the Bradford method using a protein assay kit (Pierce Chemical, Rockford, IL). Samples were electrophoresed on 10% SDS-PAGE gels and blotted onto nitrocellulose membranes (0.45 μm; Bio-Rad Laboratories, Inc., Hercules, CA). The membranes were probed with an antibody against Aq1 (Santa Cruz) and reprobed with β-actin antibody (Sigma Chemical Co., St. Louis, MO) to confirm equal protein loading. Western blot analysis was performed with the ECL Western blot detection system (Amersham Pharmacia Biotech, Piscataway, NJ).
Statistical analysis
Age-grouped animals were compared across genotypes using ANOVA, and differences within groups were determined using the Newman-Keuls or Bonferroni post hoc tests at a threshold of P < 0.05. In cases where statistical differences were not detected by ANOVA and the data were not normally distributed, analysis was repeated using the nonparametric Kruskal-Wallis test at a threshold of P < 0.05.
Results
Serum hormone levels
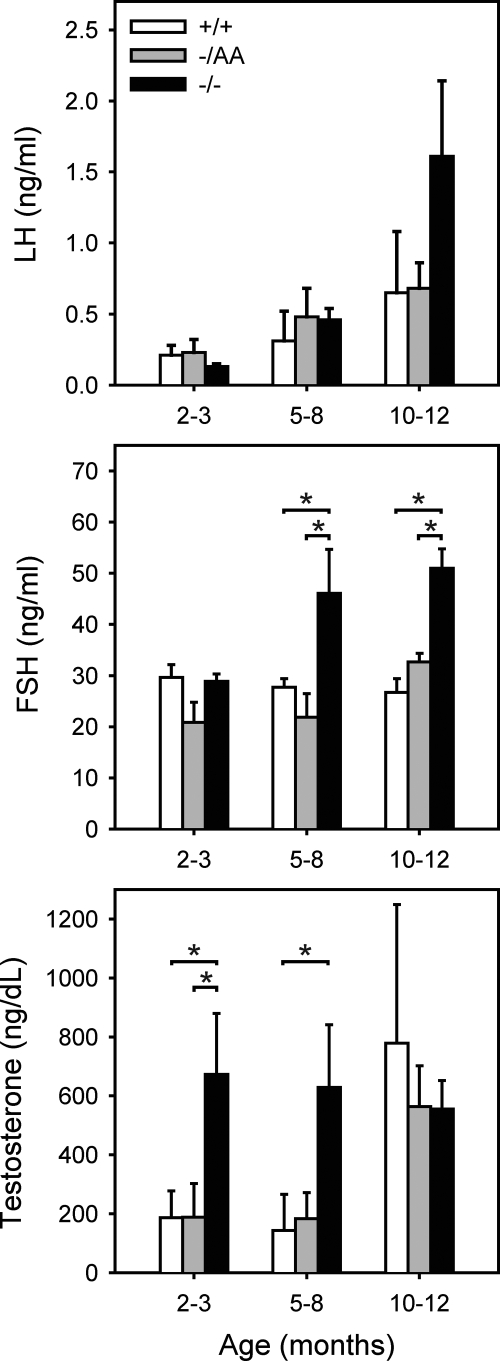
Serum hormones were measured in WT, ERKO, and AA mice to assess the relative roles of classical and nonclassical signaling on the reproductive axis. Levels of LH were not different between genotypes before 10–12 months of age, when LH began to rise in the ERKO mice, although this difference was not statistically significant (Fig. 1). FSH was elevated in the ERKO mice at 5–8 months of age and remained elevated at 10–12 months compared with WT and AA levels. Testosterone was elevated in ERKO but not AA mice at 2–3 and 5–8 months of age. By 10–12 months, testosterone levels in WT animals were elevated relative to younger animals, and no differences were seen between genotypes. These findings suggest that the AA allele is sufficient to rescue the enhanced androgen synthesis seen in ERKO mice as well as feedback inhibition of FSH production.
Figure 1.
Serum hormone levels. Bars represent mean ± sem of three to 17 animals. *, Statistical difference at P < 0.05.
Whole testis morphology
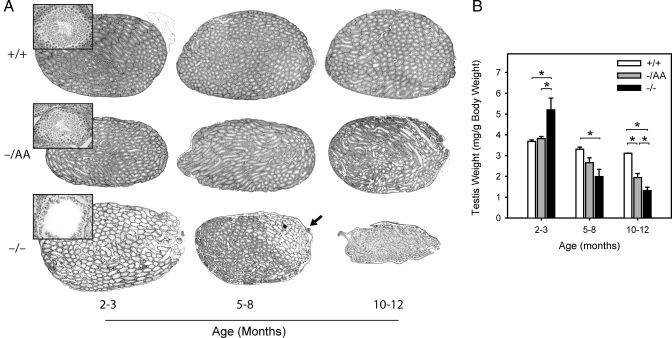
For each animal, testes were weighed and then sectioned for histological analysis. A previous study reported graded damage along the ERKO testis, with more severe histological changes nearer the rete testis (22). Therefore, longitudinal sections are presented for a qualitative assessment of morphology. At 2–3 months, tubules in ERKO testes were dilated compared with WT or AA mice (compare whole testes in Fig. 2A). The seminiferous epithelium was thinner in ERKO mice and spermatogenesis was decreased (insets). At 5–8 months, the dilation was less apparent in ERKO mice, although in some sections, the pathology was more evident at one pole (Fig. 2A, arrow), consistent with the previous report (22). By 10–12 months, ERKO testes exhibited widespread damage and were noticeably smaller than WT. Dilated tubules were occasionally observed in AA testes; however, these testes more closely resembled WT at all ages.
Figure 2.
Histological and morphological examination of the testis. For each animal, testes were weighed individually and prepared for histological examination. A, Representative longitudinal sections for each age and genotype, with insets showing a representative tubule from each section; B, testis weights (mean weight of both testes per animal). Bars represent mean ± sem of four to 12 animals. *, Statistical difference at P < 0.05.
Testis weights were consistent with these observations. At 2–3 months, ERKO testes were heavier than WT and AA (140% of WT), likely reflecting fluid retention (Fig. 2B) (18). By 5–8 months, however, the ERKO testes were only 60% of WT, and this difference became greater at 10–12 months (40% of WT). The AA allele was partially protective against this loss. AA mice did not exhibit the initial increase in testis weight at 2–3 months and remained intermediate between the WT and ERKO testis weights at 5–8 months (80% of WT) and 10–12 months (62% of WT).
Seminiferous tubule histology
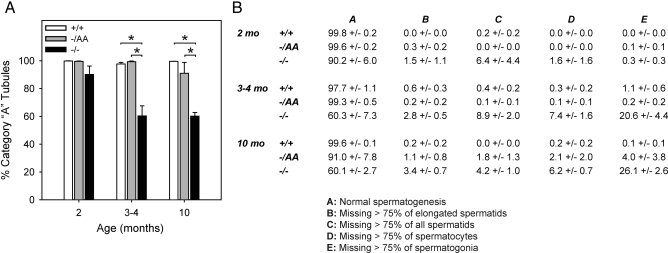
Seminiferous tubule damage was quantitated in each of the genotypes from 2–10 months of age (Fig. 3). For each animal, every tubule in one longitudinal section was scored for progression through spermatogenesis. A five-point scale was used, as follows: A, all appropriate cell types expected for the tubule’s stage are present, and at least 25% the expected amount of each cell type is observed, relative to the total number of cells in the tubule section; B, missing more than 75% of elongated spermatids (only for stage I–VIII tubules in which two populations of spermatids are expected); C, missing more than 75% of all spermatids; D, missing more than 75% of spermatocytes; and E, missing more than 75% of spermatogonia.
Figure 3.
Quantitative assessment of germ cell loss. A, Percentage of tubules in one longitudinal section that exhibit normal spermatogenesis (category A tubules); B, numerical data showing percentage of tubules in each category (A–E). Bars and data are presented as mean ± sem of three to nine animals. *, Statistical difference at P < 0.05.
Differences between genotypes were noted in some of the youngest animals examined, and the age groupings were therefore adjusted relative to Fig. 2 to highlight these differences. The bar graph illustrates the percentage of tubules that exhibited no histological damage (category A) for each genotype and time point. The table contains numerical data for all five categories.
At 2 months of age, no statistical difference was observed between genotypes (Fig. 3A). However, by 3–4 months, 40% of ERKO tubules exhibited some degree of germ cell loss, and among these tubules approximately half (20.6%) displayed a loss of more than 75% of all germ cells (category E, Fig. 3B). The overall number of damaged tubules did not increase in ERKO animals between 4 and 10 months of age, although the percentage of tubules in category E increased to 26.1%. By contrast, animals carrying the AA allele had normal histology and were not distinguishable from WT at any time point.
Sperm production and motility
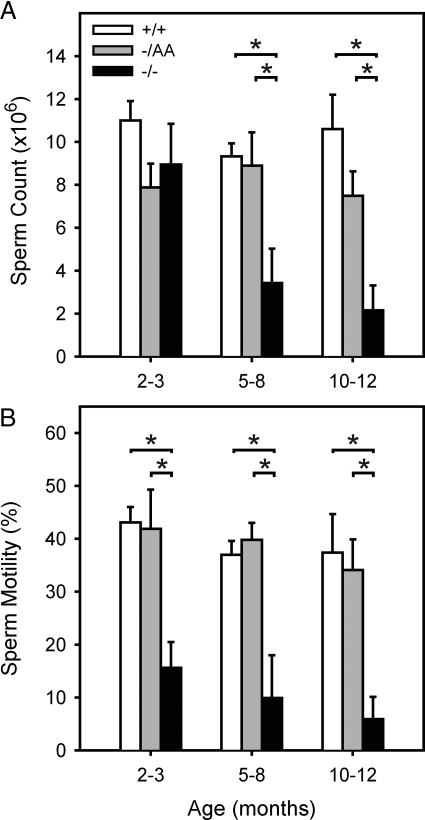
The functional consequences of the damage to the seminiferous epithelium were assessed by measuring sperm count and motility for each animal (Fig. 4). When compared with WT animals, sperm counts were preserved in 2- to 3-month-old ERKO mice. However, sperm motility was decreased at this time point. Sperm counts and motility were both decreased in the 5- to 8- and 10- to 12-month-old ERKO age groups, consistent with their histological profile. Notably, AA mice exhibited complete preservation of sperm count and motility at each time point studied.
Figure 4.
Sperm counts and motility. Sperm counts and motility were determined as a quantitative measure of damage to the seminiferous epithelium. A, Sperm counts are presented as total sperm from one cauda epididymis; B, sperm motility is presented as percent of total. Bars represent the mean ± sem of four to 17 animals. *, Statistical difference at P < 0.05.
Water channel expression
It has been demonstrated that fluid resorption in the efferent ducts is decreased in ERKO mice (18). Other work has shown that multiple aquaporins and the NHE3 are expressed in the efferent ducts of WT mice (21,23) and that expression of Aq1 and Aq9 are estrogen dependent in the efferent ducts in rats (24).
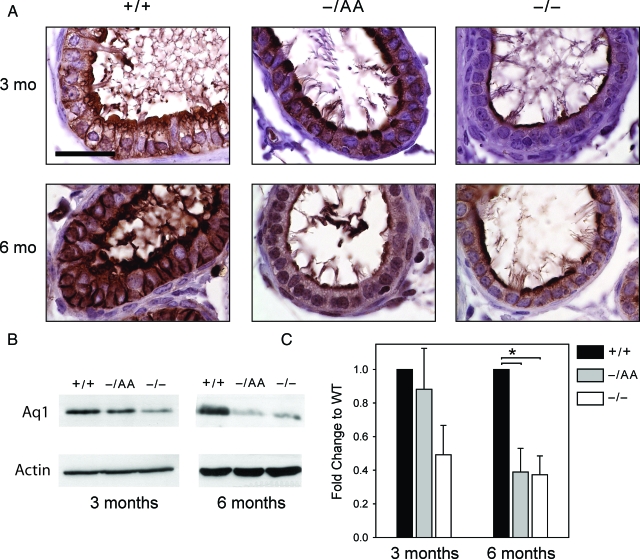
Immunohistochemistry in 3-month-old WT animals demonstrated that Aq1 is expressed at the apical borders of nonciliated cells and at the lateral borders of all cells lining the efferent ducts (Fig. 5A, left), as seen in previous reports (21,23). A similar pattern was observed in AA animals, although the apical staining was more condensed than in WT. In ERKO animals, Aq1 staining was present at 3 months but was diminished at the apical border and absent from the lateral borders. Comparing animals at 6 months of age, decreased staining persisted in ERKO animals compared with WT. However, staining in AA animals was more similar to ERKO than WT at this later time point, suggesting that nonclassical ERα signaling can delay but not prevent the loss of Aq1 expression.
Figure 5.
Aq1 expression. A, Immunohistochemical staining for Aq1 in the efferent ducts. Scale bar, 30 μm. B, Representative Western blots. Each lane contains protein pooled from the efferent ducts of three to five animals. C, Western blot data from all animals. Each bar is the mean ± sem of three experiments, with each experiment representing a pool of three to five animals.
This observation was supported by Western blots of whole efferent ducts for Aq1. Representative blots are shown in Fig. 5B, and data for three experiments are summarized in Fig. 5C. In 3-month-old animals, Aq1 in ERKO mice was half the level of that in WT, and expression was rescued in the AA mice. By 6 months of age, however, Aq1 levels in ERKO and AA mice were similar at approximately 40% of WT levels.
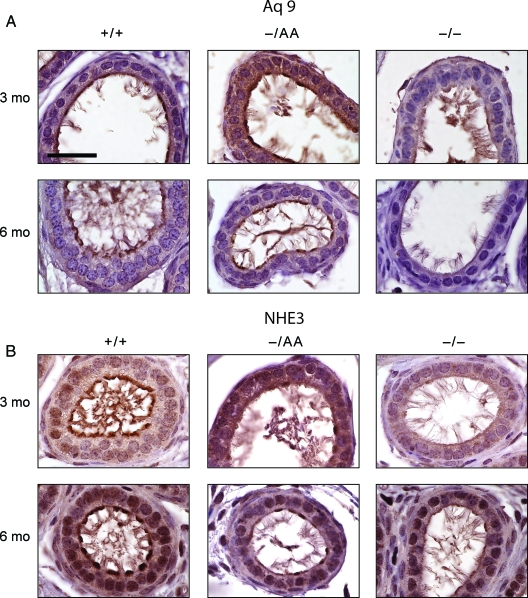
By immunohistochemistry, Aq9 was also detected at the apical borders of nonciliated cells in 3-month-old animals (Fig. 6A). Expression was maintained at the apical borders and increased in the cytoplasm in AA animals but was greatly diminished in ERKO mice. Expression of Aq9 was less consistent than that for Aq1, with some areas exhibiting more staining than others. At 6 months of age, Aq9 expression remained similar in WT and AA mice, although somewhat lower than at 3 months, and expression was virtually absent in ERKO mice. Thus, the rescue of Aq9 by nonclassical signaling appears to be more complete than for Aq1.
Figure 6.
Aq9 and NHE3 expression. A, Immunohistochemical staining for Aq9 in the efferent ducts; B, immunohistochemical staining for NHE3 in the efferent ducts. Scale bar, 30 μm.
Immunohistochemistry for NHE3 revealed a different pattern when compared with the aquaporins. NHE3 was expressed at the apical border in WT animals (Fig. 6B), but apical expression was lower in AA and ERKO animals at both 3 and 6 months of age.
Discussion
Initially envisioned as a single receptor and DNA response element, our knowledge of estrogen signaling has evolved into a complex network of receptors, responsive DNA sequences, and pathway interactions (25). Consequently, although the ERKO mouse was a great stride forward (26) and remains a powerful tool for investigating ER action, more refined genetic models will be necessary to dissect the pathways in greater detail.
The development of a mouse model (AA allele on an ERα-null background) with isolated nonclassical ERα signaling has proven useful for discriminating estrogen action through ERE vs. non-ERE pathways. Previous studies have used this model to investigate the roles of nonclassical ERα signaling in the female reproductive tissues (19,27), breast cancer cells (28), the female neuroendocrine axis (29), male steroid production (30), and the skeleton (31,32). These studies have underscored the importance of nonclassical signaling for estrogen-mediated feedback of pituitary LH secretion (29) and estrogen-stimulated cell proliferation in the uterus (27) as examples of the physiological role of the nonclassical pathway.
In the current study, we compared the reproductive phenotype of WT and ERKO males to mice expressing the AA allele. Our data confirm previous reports demonstrating that ERKO males have dilated seminiferous tubules, a gradient of germ cell loss depending on position relative to the rete testis, and decreased sperm counts and motility (22), defects that have been attributed to failed water resorption (18). This syndrome of pressure atrophy is also observed in other models of reduced tubular flow (33), suggesting that increased luminal pressure is sufficient to damage the seminiferous epithelium. However, an additional direct role for estrogen in the testes of ERKO mice is not precluded. Importantly, each of the defects in male ERKO mice was delayed, diminished, or reversed in the AA males, suggesting that nonclassical ERα signaling is responsible in large part for estrogen-dependent actions in the testes and associated ducts.
The fluid exiting the testis is concentrated over 25-fold during its passage through the efferent ducts (34), which express ERα at high levels. Previous studies have demonstrated that water resorption in the efferent ducts is estrogen dependent (18,35) and that resorption occurs through the activity of multiple water transport molecules, including Aq1 and Aq9 (24), carbonic anhydrase II (CAII), and NHE3 (21).
Aquaporins are channels that play a prominent role in water transport in a number of tissues. Studies in rodent efferent ducts have demonstrated that the levels of these water transporters are regulated directly in response to estrogen and indirectly as a result of changes in epithelial architecture.
Aq1 is expressed at high levels in the cells lining the efferent ducts in WT mice and rats (21,23). It is not clear whether levels of Aq1 change directly or indirectly in response to estrogen. In rats, Aq1 is less sensitive to the estrogen antagonist ICI 182,780 when compared with Aq9 (24), suggesting that direct estrogen actions may not be the primary mechanism for changes in Aq1 levels. On the other hand, immunohistochemical studies showed a decrease in overall Aq1 staining in ERKO efferent ducts, with some epithelial cells losing expression entirely and the remainder exhibiting a more limited pattern of expression (23). In the current study, we observed by immunohistochemistry and confirmed by Western blot that Aq1 levels are decreased in ERKO mice and that this loss is substantially delayed in AA mice. These data suggest that changes in Aq1 contribute to the male testis phenotype in ERKO mice and that levels of Aq1 are regulated, at least in part, through ERE-independent mechanisms. The eventual loss of Aq1 expression may reflect cumulative damage to the efferent ducts. Nonetheless, the loss of Aq1 expression at 6 months despite the persistent rescue of testis histology and sperm production suggests that other transporters or pathways are also involved.
In rats, Aq9 is expressed in the efferent ducts and the initial segment of the epididymis, and expression in the efferent ducts is selectively suppressed by ICI 182,780 (24). In mice, Aq9 staining is present in the efferent ducts of both WT and ERKO animals, although some change in the pattern of expression was observed (23). Notably, expression was maintained in AA mice in our study, whereas levels were consistently lower in ERKO mice. This rescue was observed at both 3 and 6 months of age. Thus, more so than for Aq1, Aq9 appears to be under the control of nonclassical ERα pathways. This observation is also consistent with the greater estrogen sensitivity of Aq9 when compared with Aq1 (24).
NHE3 has been demonstrated to be a critical water transporter in efferent ductules (21). In those studies, levels of NHE3 were decreased in ERKO mice and genetic disruption of the NHE3 gene led to faulty water resorption and infertility. Our experiments confirmed lower NHE3 expression in ERKO mice. However, in contrast to the aquaporins, NHE3 was not substantially rescued in the AA mice. As noted above, the histological changes seen in ERKO mice were rescued by the AA allele, suggesting that residual NHE3 expression, in combination with the aquaporins, is sufficient to support spermatogenesis.
We also measured serum levels of the pituitary gonadotropins LH and FSH and testosterone. There is consensus in the literature that testosterone is elevated in ERKO males and in a previous study, we demonstrated that the elevated testosterone is rescued in AA mice (30). The current study confirms and extends those results by examining testosterone levels at different developmental stages.
In contrast to testosterone, there is not agreement in the literature as to whether LH is elevated in ERKO males. In an initial report, LH levels were similar in WT and ERKO mice (22), whereas a subsequent study reported elevated LH levels in ERKO mice (17). In the current study and in our previous work (30), LH levels in ERKO mice were not different from WT mice. By contrast, testosterone levels were elevated 3-fold in 2- to 3-month-old ERKO mice and remained elevated through 8 months of age.
These data suggest that the elevated testosterone observed in ERKO mice is not entirely the result of increased LH stimulation. Rather, it seems likely that an intrinsic alteration in the testis enhances LH sensitivity and/or androgen synthesis pathways. This conclusion is consistent with data demonstrating higher androgen synthesis in cultured Leydig cells from ERKO mice compared with WT, although those authors did observe higher LH in the serum of ERKO animals and concluded that ER may influence testosterone both directly and through alterations in pituitary secretion (17). As noted, elevated testosterone levels were reversed in the AA mouse. It is thus possible that the histological rescue is related to the rescue in androgen synthesis, although our experiments do not address this point directly.
For both androgen synthesis and germ cell loss, it is worth considering whether the damage observed in the adult animal might reflect a developmental defect. Both androgen synthesis (36) and expression of Insl3, which is necessary for the first stage of testicular descent, are sensitive to estrogen in the embryo (37). Of note, cryptorchidism was not observed in any of the genotypes. ERKO mouse testes appear normal at postnatal d 10 (22), by which time the fetal Leydig cells present during development have been replaced by the adult Leydig cells. The absence of ERα does not impact the number of Sertoli cells or gonocytes in neonates (38). We also did not observe histological changes until after 2 months of age, making it less likely that a developmental abnormality is the major cause of tubular dilatation and germ cell loss.
In summary, we have demonstrated that the loss of nonclassical ERα signaling pathways is responsible for most of the reproductive tract defects observed in male ERKO mice. Our data do not, however, distinguish between the various nonclassical pathways (e.g. tethering vs. membrane signaling). These pathways are now being studied in several laboratories and comparison of our model with other genetic models should shed additional light on the mechanisms of ER action.
Footnotes
This work was supported by National Institutes of Health/National Institute of Child Health and Human Development (NICHD) Grant PO1 HD21921, Training Grant T32 GM008061, NICHD (SCCPRR) Grant U54-HD28934 (University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core), and the Division of Intramural Research of the National Institute of Environmental Health Sciences.
Disclosure Summary: The authors have nothing to disclose.
First Published Online August 21, 2008
Abbreviations: Aq1, Aquaporin 1; ER, estrogen receptor; ERE, estrogen response element; KO, knockout; NHE3, sodium-hydrogen exchanger-3; WT, wild type.
References
- Turgeon JL, Carr MC, Maki PM, Mendelsohn ME, Wise PM 2006 Complex actions of sex steroids in adipose tissue, the cardiovascular system, and brain: Insights from basic science and clinical studies. Endocr Rev 27:575–605 [DOI] [PubMed] [Google Scholar]
- Green S, Walter P, Greene G, Krust A, Goffin C, Jensen E, Scrace G, Waterfield M, Chambon P 1986 Cloning of the human oestrogen receptor cDNA. J Steroid Biochem 24:77–83 [DOI] [PubMed] [Google Scholar]
- Mosselman S, Polman J, Dijkema R 1996 ERβ: identification and characterization of a novel human estrogen receptor. FEBS Lett 392:49–53 [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA 1996 Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci USA 93:5925–5930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JS, Brown M 2006 Estrogen receptor target gene: an evolving concept. Mol Endocrinol 20:1707–1714 [DOI] [PubMed] [Google Scholar]
- Paech K, Webb P, Kuiper GG, Nilsson S, Gustafsson J, Kushner PJ, Scanlan TS 1997 Differential ligand activation of estrogen receptors ERα and ERβ at AP1 sites. Science 277:1508–1510 [DOI] [PubMed] [Google Scholar]
- Hammes SR, Levin ER 2007 Extranuclear steroid receptors: nature and actions. Endocr Rev 28:726–741 [DOI] [PubMed] [Google Scholar]
- Carroll JS, Meyer CA, Song J, Li W, Geistlinger TR, Eeckhoute J, Brodsky AS, Keeton EK, Fertuck KC, Hall GF, Wang Q, Bekiranov S, Sementchenko V, Fox EA, Silver PA, Gingeras TR, Liu XS, Brown M 2006 Genome-wide analysis of estrogen receptor binding sites. Nat Genet 38:1289–1297 [DOI] [PubMed] [Google Scholar]
- Lin Z, Reierstad S, Huang CC, Bulun SE 2007 Novel Estrogen receptor-α binding sites and estradiol target genes identified by chromatin immunoprecipitation cloning in breast cancer. Cancer Res 67:5017–5024 [DOI] [PubMed] [Google Scholar]
- Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O 1993 Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci USA 90:11162–11166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, Sar M, Korach KS, Gustafsson JA, Smithies O 1998 Generation and reproductive phenotypes of mice lacking estrogen receptor β. Proc Natl Acad Sci USA 95:15677–15682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, Mark M 2000 Effect of single and compound knockouts of estrogen receptors α (ERα) and β (ERβ) on mouse reproductive phenotypes. Development 127:4277–4291 [DOI] [PubMed] [Google Scholar]
- Hess RA, Gist DH, Bunick D, Lubahn DB, Farrell A, Bahr J, Cooke PS, Greene GL 1997 Estrogen receptor (α and β) expression in the excurrent ducts of the adult male rat reproductive tract. J Androl 18:602–611 [PubMed] [Google Scholar]
- Kuiper GG, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, Gustafsson JA 1997 Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors α and β. Endocrinology 138:863–870 [DOI] [PubMed] [Google Scholar]
- Saunders PT, Maguire SM, Gaughan J, Millar MR 1997 Expression of oestrogen receptor β (ERβ) in multiple rat tissues visualised by immunohistochemistry. J Endocrinol 154:R13–R16 [DOI] [PubMed] [Google Scholar]
- Antal MC, Krust A, Chambon P, Mark M 2008 Sterility and absence of histopathological defects in nonreproductive organs of a mouse ERβ-null mutant. Proc Natl Acad Sci USA 105:2433–2438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akingbemi BT, Ge R, Rosenfeld CS, Newton LG, Hardy DO, Catterall JF, Lubahn DB, Korach KS, Hardy MP 2003 Estrogen receptor-α gene deficiency enhances androgen biosynthesis in the mouse Leydig cell. Endocrinology 144:84–93 [DOI] [PubMed] [Google Scholar]
- Hess RA, Bunick D, Lee KH, Bahr J, Taylor JA, Korach KS, Lubahn DB 1997 A role for oestrogens in the male reproductive system. Nature 390:509–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakacka M, Ito M, Martinson F, Ishikawa T, Lee EJ, Jameson JL 2002 An estrogen receptor (ER)α deoxyribonucleic acid-binding domain knock-in mutation provides evidence for nonclassical ER pathway signaling in vivo. Mol Endocrinol 16:2188–2201 [DOI] [PubMed] [Google Scholar]
- Jakacka M, Ito M, Weiss J, Chien PY, Gehm BD, Jameson JL 2001 Estrogen receptor binding to DNA is not required for its activity through the nonclassical AP1 pathway. J Biol Chem 276:13615–13621 [DOI] [PubMed] [Google Scholar]
- Zhou Q, Clarke L, Nie R, Carnes K, Lai LW, Lien YH, Verkman A, Lubahn D, Fisher JS, Katzenellenbogen BS, Hess RA 2001 Estrogen action and male fertility: roles of the sodium/hydrogen exchanger-3 and fluid reabsorption in reproductive tract function. Proc Natl Acad Sci USA 98:14132–14137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddy EM, Washburn TF, Bunch DO, Goulding EH, Gladen BC, Lubahn DB, Korach KS 1996 Targeted disruption of the estrogen receptor gene in male mice causes alteration of spermatogenesis and infertility. Endocrinology 137:4796–4805 [DOI] [PubMed] [Google Scholar]
- Ruz R, Gregory M, Smith CE, Cyr DG, Lubahn DB, Hess RA, Hermo L 2006 Expression of aquaporins in the efferent ductules, sperm counts, and sperm motility in estrogen receptor-α deficient mice fed lab chow versus casein. Mol Reprod Dev 73:226–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira CA, Carnes K, Franca LR, Hermo L, Hess RA 2005 Aquaporin-1 and -9 are differentially regulated by oestrogen in the efferent ductule epithelium and initial segment of the epididymis. Biol Cell 97:385–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JM, Couse JF, Korach KS 2001 The multifaceted mechanisms of estradiol and estrogen receptor signaling. J Biol Chem 276:36869–36872 [DOI] [PubMed] [Google Scholar]
- Couse JF, Korach KS 1999 Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev 20:358–417 [DOI] [PubMed] [Google Scholar]
- O'Brien JE, Peterson TJ, Tong MH, Lee EJ, Pfaff LE, Hewitt SC, Korach KS, Weiss J, Jameson JL 2006 Estrogen-induced proliferation of uterine epithelial cells is independent of estrogen receptor α binding to classical estrogen response elements. J Biol Chem 281:26683–26692 [DOI] [PubMed] [Google Scholar]
- Glidewell-Kenney C, Weiss J, Lee EJ, Pillai S, Ishikawa T, Ariazi EA, Jameson JL 2005 ERE-independent ERα target genes differentially expressed in human breast tumors. Mol Cell Endocrinol 245:53–59 [DOI] [PubMed] [Google Scholar]
- Glidewell-Kenney C, Hurley LA, Pfaff L, Weiss J, Levine JE, Jameson JL 2007 Nonclassical estrogen receptor α signaling mediates negative feedback in the female mouse reproductive axis. Proc Natl Acad Sci USA 104:8173–8177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDevitt MA, Glidewell-Kenney C, Weiss J, Chambon P, Jameson JL, Levine JE 2007 Estrogen response element-independent estrogen receptor (ER)-α signaling does not rescue sexual behavior but restores normal testosterone secretion in male ERα knockout mice. Endocrinology 148:5288–5294 [DOI] [PubMed] [Google Scholar]
- Syed FA, Fraser DG, Spelsberg TC, Rosen CJ, Krust A, Chambon P, Jameson JL, Khosla S 2007 Effects of loss of classical estrogen response element signaling on bone in male mice. Endocrinology 148:1902–1910 [DOI] [PubMed] [Google Scholar]
- Syed FA, Modder UI, Fraser DG, Spelsberg TC, Rosen CJ, Krust A, Chambon P, Jameson JL, Khosla S 2005 Skeletal effects of estrogen are mediated by opposing actions of classical and nonclassical estrogen receptor pathways. J Bone Miner Res 20:1992–2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffs B, Meeks JJ, Ito M, Martinson FA, Matzuk MM, Jameson JL, Russell LD 2001 Blockage of the rete testis and efferent ductules by ectopic Sertoli and Leydig cells causes infertility in Dax1-deficient male mice. Endocrinology 142:4486–4495 [DOI] [PubMed] [Google Scholar]
- Clulow J, Jones RC, Hansen LA 1994 Micropuncture and cannulation studies of fluid composition and transport in the ductuli efferentes testis of the rat: comparisons with the homologous metanephric proximal tubule. Exp Physiol 79:915–928 [DOI] [PubMed] [Google Scholar]
- Clulow J, Jones RC, Hansen LA, Man SY 1998 Fluid and electrolyte reabsorption in the ductuli efferentes testis. J Reprod Fertil Suppl 53:1–14 [PubMed] [Google Scholar]
- Delbes G, Levacher C, Duquenne C, Racine C, Pakarinen P, Habert R 2005 Endogenous estrogens inhibit mouse fetal Leydig cell development via estrogen receptor α. Endocrinology 146:2454–2461 [DOI] [PubMed] [Google Scholar]
- Nef S, Shipman T, Parada LF 2000 A molecular basis for estrogen-induced cryptorchidism. Dev Biol 224:354–361 [DOI] [PubMed] [Google Scholar]
- Delbes G, Levacher C, Pairault C, Racine C, Duquenne C, Krust A, Habert R 2004 Estrogen receptor β-mediated inhibition of male germ cell line development in mice by endogenous estrogens during perinatal life. Endocrinology 145:3395–3403 [DOI] [PubMed] [Google Scholar]