Abstract
Low-molecular-weight (LMW) antagonists for TSH receptor (TSHR) may have therapeutic potential as orally active drugs to block stimulating antibodies (TsAbs) in Graves’ hyperthyroidism. We describe an approach to identify LMW ligands for TSHR based on Org41841, a LMW partial agonist for the LH/choriogonadotropin receptor and TSHR. We used molecular modeling and functional experiments to guide the chemical modification of Org41841. We identified an antagonist (NIDDK/CEB-52) that selectively inhibits activation of TSHR by both TSH and TsAbs. Whereas initially characterized in cultured cells overexpressing TSHRs, the antagonist was also active under more physiologically relevant conditions in primary cultures of human thyrocytes expressing endogenous TSHRs in which it inhibited TSH- and TsAb-induced up-regulation of mRNA transcripts for thyroperoxidase. Our results establish this LMW compound as a lead for the development of higher potency antagonists and serve as proof of principle that LMW ligands that target TSHR could serve as drugs in patients with Graves’ disease.
TSH RECEPTOR (TSHR), LH/chorionic gonadotropin receptor (LHCGR) and FSH receptor (FSHR) belong to the glycoprotein hormone receptor subfamily of seven transmembrane-spanning receptors (7TMRs), also known as G protein-coupled receptors. The endogenous ligands of glycoprotein hormone receptors are large, heterodimeric glycoprotein hormones of approximately 30 kDa, which bind to the leucine-rich repeat domain of the large extracellular N termini of their receptors (1,2,3,4). These receptors couple preferentially to Gs, resulting in activation of the cAMP-protein kinase A cascade (4).
Low-molecular-weight (LMW) agonists of LHCGR and FSHR have the potential to become oral therapeutics for infertility treatment, whereas antagonists might be used in oral contraception. This led to strong interest in LMW ligands for these receptors and subsequent identification of first-generation LMW ligands for LHCGR and FSHR (5). The progress in identifying LMW ligands for LHCGR and FSHR encouraged us to perform similar studies on ligands for TSHR, and we identified several LMW agonists by high throughput screening (6). The development of LMW compounds that antagonize thyroid-stimulating antibody activation of TSHR could lead to therapeutic agents for treatment of Graves’ disease.
We recently showed that a LMW ligand Org41841, originally identified as a partial agonist for LHCGR was also a partial agonist for TSHR, providing the first report of a LMW ligand for TSHR (7). Here we identify a LMW antagonist for TSHR that was found by rational design using a model of the Org41841/TSHR complex. The LMW antagonist described here may serve as a lead for the development of higher-affinity ligands with therapeutic potential. Moreover, these results serve as proof of principle that LMW ligands that target TSHR could serve as drugs in patients with Graves’ disease.
Materials and Methods
Synthesis of NIDDK/CEB-52 (compound 52)
The synthesis of compound 52 was accomplished from a final step Suzuki coupling from the precursor brominated analog [5-amino-4-(4-bromophenyl)-N-tert-butyl-2(methylthio)thieno[2,3-d]pyrimidine-6-carboxamide], which was synthesized according to methods previously reported (8). Details are provided in supplemental information, published as supplemental data on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org.
Molecular modeling and docking studies
The homology model of the transmembrane region of human TSHR was generated with Sybyl 7.3.5 based on the recently solved x-ray structures of the β2-adrenergic receptor (β2-AR) (9,10) [Protein Data Base (PDB) entry codes: 2RH1; 2R4R]. The reported low root mean square deviation (RMSD) values between backbones of the transmembrane helices of the structures used and the previously solved x-ray structures of bovine rhodopsin [PDB entry codes: 1F88 (11), 2I35 (12), 2J4Y (13)] support the reliability of our TSHR-transmembrane helices (TMH) model. The major structural difference concerns extracellular loop (ECL) 2. Due to the higher similarity in length and amino acid sequence of ECL2 between rhodopsin and TSHR (36.0%) vs. β2-AR and TSHR (27.6%) and the β2-AR-specific additional internal cysteine-bridge, which stabilizes a helical fold in the ECL2 of the β2-AR, we modeled ECL2 of TSHR on the basis of the β-hairpin-like structure and location of ECL2 of rhodopsin. Additionally, this rhodopsin-like ECL2 conformation is consistent with results of diverse studies at other G protein-coupled receptors (14,15,16) and TSHR (17).
However, several TSHR-specific corrections were made, such as regular helix extensions in TMH2 and TMH5 of TSHR instead of structural bulges in the two helices of β2-AR, which are caused specifically by side chains that are not present in TSHR (prolines in TMH2 and TMH5). Loops were refined by best fit and homology to fragments of other proteins from PDB. Gaps of missing residues in the loops of the template structure were closed by the Loop Search tool implemented in Sybyl 7.3.5 (Tripos Inc., St. Louis, MO). Conjugate gradient minimizations were performed until converging at a termination gradient of 0.05 kcal/mol * Å using the AMBER 7.0 force field (18). Quality and stability of the model were validated by checking the geometry by PROCHECK (19) and monitoring the RMSD during a molecular dynamics simulation of 2 nsec (overall backbone RMSD 1.8 Å). For examining the ligand binding site, several tools from the Tripos package, such as site identification and manual and automatic docking (Dock, FlexS, FlexX), were applied preferentially to regions with sequence differences between TSHR and LHCGR. The designation of the amino acids in the transmembrane domain was based on the nomenclature of Ballesteros and Weinstein (20).
Cell culture and transient transfection
HEK-EM 293 cells were grown in DMEM supplemented with 10% fetal bovine serum, 100 U/ml penicillin, and 10 μg/ml streptomycin (Mediatech Inc., Manassas, VA) at 37 C in a humidified 5% CO2 incubator. Cells were transiently transfected with wild-type TSHR and mutant receptors in 24-well plates (7.5 × 104 cells/well) with 0.4 μg DNA/well using FuGENE 6 reagent (Roche, Diagnostics, Indianapolis, IN) according to the manufacturer’s protocol.
Generation of stable cell lines expressing TSHR, LHCGR, or FSHR
The expression vectors for human TSHR and LHCGR are described elsewhere (7). The FSHR cDNA in pcDNA3.1 was obtained from the Missouri S&T cDNA Resource Center (www.cDNA.org) and was subcloned into the pcDNA3.1(−)/hygromycin vector. HEK-EM 293 cells were transfected with the cDNA of TSHR, LHCGR, or FSHR using FuGENE 6 transfection reagent (Roche Diagnostics, Indianapolis, IN). Hygromycin (250 μg/ml) was used as selection marker. Details are provided in supplemental methods.
Site-directed mutagenesis of TSHR
The M9 mutant was described elsewhere (7). The Y7.42A mutant was introduced into hTSHR-pcDNA3.1 via the QuikChange XL site-directed mutagenesis kit (Stratagene, La Jolla, CA). The construct was verified by sequencing (MWG Biotech, High Point, NC).
Determination of intracellular cAMP accumulation and cell surface expression
Transiently transfected cells were cultured for 48 h before the cAMP assay. HEK-EM293 cells stably expressing TSHR, LHCGR, or FSHR were seeded into 24-well plates with a density of 2.2 × 105 cells/well 24 h before the cAMP assay. After removal of growth medium, cells were incubated for 1 h in Hanks’ balanced salt solution (HBSS; Cellgro, Manassas, VA) with 10 mm HEPES (Cellgro) containing 1 mm 3-isobutyl-1-methylxanthine (Sigma, St. Louis, MO) and the ligand of interest in a humidified 5% CO2 incubator at 37 C. The intracellular cAMP content was determined with the cAMP Biotrak enzyme immunoassay system (GE Healthcare, Piscataway, NJ). Data were analyzed using GraphPad Prism 4 for Windows (GraphPad Inc., San Diego, CA). Receptor expression was measured as described previously (7) and detailed in supplemental methods.
Culture of primary human thyrocytes
Thyroid tissue samples were obtained through the National Institutes of Health Clinical Center during surgery for unrelated reasons. Patients provided informed consent on an institutional review board-approved protocol, and materials were received anonymously via approval of research activity through the Office of Human Subjects Research. The specimens were maintained in HBSS on ice, and isolation of cells was initiated within 4 h after surgery. All preparations were performed under sterile conditions. Tissue samples were minced into small pieces by fine surgical forceps and scissors in a 10-cm dish with a small volume of HBSS. Tissue pieces were transferred to a 15-ml tube (Falcon, Oxnard, CA) and washed at least three times with HBSS. Afterward tissue pieces were incubated with HBSS containing 3 mg/ml collagenase type IV (Life Technologies). Enzymatic digestion proceeded for 30 min or longer with constant shaking in a water bath at 37 C until a suspension of isolated cells was obtained. After centrifugation for 5 min at 1000 rpm, the supernatant was removed and cells were resuspended in 10 ml DMEM with 10% fetal bovine serum. Cells were plated in 10-cm tissue culture dishes and incubated at 37 C in a humidified 5% CO2 incubator. After 24 h, the supernatant containing nonadherent cells was removed. The primary cultures of thyroid cells formed a confluent monolayer within 5–7 d. For determination of thyroperoxidase (TPO) mRNA expression, thyrocytes were seeded into 24-well plates at a density of 6 × 104 cells/well 24 h before the experiment.
Quantitative RT-PCR
Total RNA was purified using RNeasy microkits (QIAGEN, Valencia, CA). First-strand cDNA was prepared using a high-capacity cDNA archive kit (Applied Biosystems, Foster City, CA). RT-PCR was performed in 25-μl reactions using cDNA prepared from 100 ng of total RNA and universal PCR master mix (Applied Biosystems). Primers and probes were Assay-on-Demand (Applied Biosystems). Quantitative RT-PCR results were normalized to GAPDH to correct for differences in RNA input.
Results
Rational design of a TSHR antagonist
We showed previously that Org41841 (Fig. 1A), which was originally reported as a LMW agonist for LHCGR, is a partial agonist for TSHR. We identified Org41841 binding pockets within the seven-transmembrane helices of TSHR and LHCGR by a combination of homology modeling and evaluation of biological activity using TSHR-LHCGR chimeras (7). Specifically, we proposed that Org41841 binds in a pocket localized between TMH3, -4, -5, -6, and -7, which is covered by ECL2. Furthermore, the binding pocket of TSHR contains strongly hydrophobic and bulky residues at the junctions between TMH4/ECL2, TMH5/ECL2, and TMH6/ECL3 that form an extracellular cover over the binding cleft, whereas generally less hydrophobic and/or bulky residues are involved in LHCGR. Therefore, the binding pocket for Org41841 in TSHR is structurally narrower than that in the homologous LHCGR.
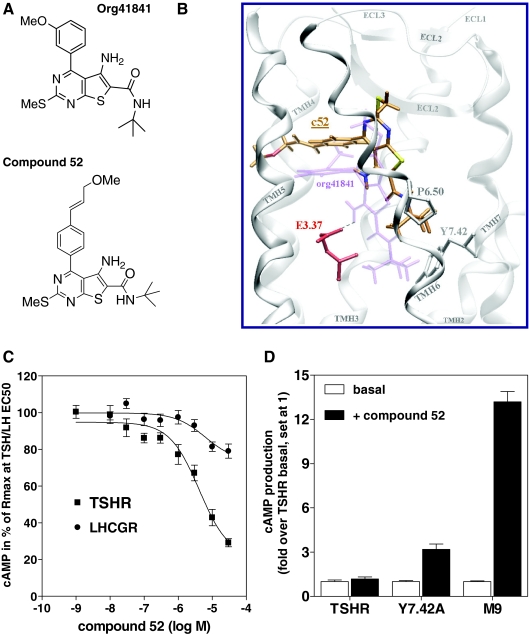
Figure 1.
TSHR antagonist NIDDK/CEB-52 (compound 52; c52): rational design and pharmacology. A, Structures of Org41841 and compound 52. B, Comparison of docking modes of compound 52 (C atoms orange) and Org41841 (light purple) in a model of TSHR. The binding pocket of compound 52 is within the extracellular half of the transmembrane helical bundle between TMH3, -5, -6, and -7 (white) and close to ECL2 (white). In contrast to Org41841, the t-butyl group of compound 52 sits higher in the binding cleft and points toward P6.50. Therefore, movement of TMH6 during TSHR activation is not enforced by compound 52. This structural constraint may cause an antagonistic effect on TSHR signaling (C). Noteworthy, experimental data reveal that in the TSHR single mutant Y7.42A, compound 52 acts as an agonist (D). In Y7.42A, the alanine is less bulky compared with tyrosine; therefore, compound 52 moves downward to TMH6 and TMH7, similar to Org41841, and presses the kinked TMH6 apart below P6.50, which likely leads to movement of the intracellular part of TMH6 and TSHR activation. C, Antagonistic activity of compound 52 at TSHR and LHCGR. Intracellular cAMP accumulation was determined in response to increasing concentrations of compound 52. EC50 concentrations of native ligands were as follows: TSH, 1.8 nm; LH, 0.34 nm. D, Compound 52 activates TSHR mutants Y7.42A and M9 in contrast to TSHR. Intracellular cAMP accumulation was determined without ligands (basal) or in response to 30 μm of compound 52. In M9 (7); TSHR residues within and covering the Org41841 binding cleft were replaced by the corresponding residues of LHCGR: I560V (LHCGR: V505), L570F (LHCGR: F515), P5.34T, A5.36S, L5.37Q, A5.38V, F5.42T, Y6.54F, and I5.59A. The data are presented as mean ± sem of three independent experiments, each performed in duplicate.
Based on this knowledge, we predicted that elongated analogs of Org41841 would be situated differently in the binding pockets of TSHR and LHCGR. Because modifications of the t-butyl moiety of Org41841 (Fig. 1A) led to loss of activity (data not shown), we enlarged the molecule at the opposite end by adding a propylene-methyl-ether group at the paraposition of the aromatic moiety of the 3-methoxyphenyl group to elongate the longitudinal axis. This Org41841 analog was named NIDDK/CEB-52 (compound 52) (Fig. 1A).
Molecular docking studies, performed initially at a bovine rhodopsin-based (11,12) and subsequently at a β2-AR-based homology model of the TSHR (Fig. 1B), suggest that compound 52 binds in a pocket between TMH3, -5, -6, and -7 and ECL2 similar to Org41841. However, the presence of the propylene-methyl-ether substituent on the methoxy phenyl moiety leads to a spatial relocation of compound 52 toward ECL2 compared with Org41841 (Fig. 1B). As a consequence of this shift, the t-butyl group of compound 52 sits higher in the binding cleft than does Org41841, pointing toward the proline at position 6.50 (P6.50) (20). Therefore, movement of TMH6 at the intracellular portion during binding is not predicted to be effected by compound 52, and impairment of TSHR activation was expected. In addition, modeling suggested that compound 52 would not be an effective agonist because its amino group does not interact with the highly conserved negatively charged glutamic acid (E) 3.37 that was found to be essential for TSHR activation by Org41841 (7,8) (Fig. 1B).
Compound 52 is a selective antagonist for TSHR
Indeed, compound 52 was found to be an antagonist for TSHR (Fig. 1C) with no agonist activity (Fig. 1D). The TSH-mediated cAMP response of TSHR was inhibited by a maximum of 70.8 ± 5.5% at 30 μm compound 52. The IC50 of compound 52 for TSHR inhibition is 4.2 μm (95% confidence interval 2.3-7.5 μm). Noteworthy, compound 52 is selective toward TSHR when compared with the closely related LHCGR and FSHR (Fig. 1C). In contrast to TSHR, compound 52 is a partial agonist at LHCGR (17.25 ± 2.52% activity compared with full activation of LHCGR by LH, set at 100%) (data not shown). Compound 52 has no activity at FSHR.
Nonspecific effects by aggregation between other proteins and LMW compounds have been reported (21). To exclude the possibility that compound 52 might be acting independently of TSHR by building aggregates with TSH, thereby inhibiting the TSH-induced response, we tested for a possible aggregation between these two ligands. Compound 52 and TSH were preincubated for up to 15 min before addition to HEK-EM 293 cells stably expressing TSHR. Intracellular cAMP accumulation was determined in response to 30 μm compound 52 in the presence of 1.8 nm TSH (EC50). There was no difference in the antagonistic effect whether TSH and compound 52 were preincubated together or not (data not shown), thereby excluding the possibility that its effect was caused by aggregation with TSH.
Evidence from receptor mutants for interaction of compound 52 with TSHR
Although compound 52 does not activate TSHR, it shows partial agonism at two TSHR mutants, one in which a tyrosine at position 7.42 in TMH7 was substituted by alanine (Y7.42A) and another, M9, in which nine residues in or near the Org41841 binding pocket were substituted by the corresponding residues of the LHCGR (7). These results are consistent with the hypothesis that compound 52 interacts with TSHR in the transmembrane domain. This hypothesis is supported by data that show that compound 52 does not compete with 125I-labeled TSH for binding to TSHR (supplemental data Fig. 1).
The TSHR expresses high basal activity, and therefore, basal activity of mutants or ligand-stimulated activity of TSHR can be expressed as fold stimulation of this basal (constitutive) activity. Compound 52 stimulated cAMP production in HEK-EM 293 cells expressing these mutant receptors by 3.2 ± 0.9- and 13.2 ± 1.7-fold over TSHR basal activity of Y7.42A and M9, respectively (Fig. 1D). Org41841, which is a partial agonist with 23% of TSH activity at TSHR, acts as a full agonist for M9, which can be explained by changes in hydrophobicity and gain of space at the three TMH/ECL junctions (7). Due to enlargement of the binding pocket of the chimeric M9 mutant compared with TSHR, compound 52 is located similarly to Org41841 in M9 and acts as an agonist. It is noteworthy that Y7.42A is constitutively active (1.73 ± 0.38-fold over TSHR basal), even though it exhibits a cell surface expression of 74.90 ± 8.04% compared with TSHR (data not shown). Because Y7.42A is sterically more relaxed than TSHR and the alanine is less bulky than tyrosine, compound 52 can move downward to the intracellular part of TMH6 and TMH7, as does Org41841. In this case the t-butyl group of compound 52 may sterically press apart the kinked TMH6 below P6.50, leading to a distinct TMH6 movement and activation of Y7.42A rather than antagonism observed in TSHR in which compound 52 sits higher in the binding pocket (Fig. 1B).
Inhibition of TSH stimulation of TPO mRNA expression in human thyrocytes by compound 52
We next asked whether compound 52 could act as an antagonist of endogenous TSHR activity in the more physiologically relevant context of human thyrocytes in primary cultures. We received normal thyroid tissue from two donors who underwent total thyroidectomy. Cells were either incubated with TSH or pretreated for 1 h with compound 52 and then incubated with compound 52 in the presence of TSH for 24 h. In thyrocytes from both donors, TSH alone increased TPO mRNA expression, and this increase was inhibited by compound 52, at both 10 and 30 μm compound (Fig. 2B and supplemental data Fig. 2). In summary, compound 52 is an effective antagonist of TSH stimulation of endogenous TSHR activity in primary cultures of thyrocytes.
Figure 2.
Compound 52 (c52) inhibits TSHR activation by TSH and TsAbs of Graves’ disease sera. A, Intracellular cAMP accumulation in HEK-EM 293 cells stably expressing TSHR was determined in response to a 1:50 dilution of sera from patients with Graves’ disease (GD) or the EC50 concentration of bovine TSH (1.8 nm) in the presence or absence of c52. Serum from a patient with multinodular goiter was used as a control. The data are presented as mean ± sem of four independent experiments. B, c52 inhibits TPO mRNA expression in primary cultures of human thyrocytes from donor 2 stimulated by bovine TSH or GD sera. Thyrocytes were incubated with bovine TSH (1.8 nm) or a 1:50 dilution of GD sera and 10 μm of c52 for 24 h. Cells receiving 10 μm c52 were preincubated for 1 h with the same concentration of c52 before the 24-h incubation with bovine TSH. Data are presented as mean ± sem of two independent experiments.
Compound 52 inhibits TSHR activation by thyroid-stimulating antibodies (TsAbs) from patients with Graves’ disease
To assess the therapeutic potential of compound 52 in patients with Graves’ disease, we tested its ability to inhibit TSHR activation by TsAbs. First, four patient sera (GD 5, 19, 29, 30) were used at a dilution of 1:50 to test the effect of compound 52 on TsAb-stimulated cAMP accumulation in HEK-EM 293 cells expressing TSHR. All four sera increased cAMP accumulation but to different extents (Fig. 2A). To assess inhibition, cAMP accumulation was measured in response to TsAbs in the presence of 30 μm compound 52. Indeed, compound 52 reduced TsAb-mediated responses of the different sera (set at 100% for each patient’s serum) by 28–79% (Fig. 2A).
We confirmed the inhibitory effect of compound 52 on TsAb stimulation in primary cultures of human thyrocytes. TsAbs of all four patient’s sera increased expression of TPO mRNA and addition of compound 52 inhibited TsAb-stimulated TPO mRNA expression for all sera tested (Fig. 2B). This is an initial indication of the therapeutic potential of LMW antagonists.
Discussion
This study was undertaken to identify LMW ligands for TSHR with the long-term goal of developing drugs that could be used to treat patients with Graves’ hyperthyroidism. Our approach using molecular modeling in conjunction with rational design identified a compound with specific TSHR antagonist activity.
Structure-based drug design has been successfully applied to drug discovery and lead optimization for proteins whose crystal structures are known (22,23). However, ligand docking and virtual screening using experimental structures continue to reveal limitations (24). The experimentally determined conformation of the receptor is not optimal for docking studies because significant differences may exist between apoprotein and bound forms (25). For a long time, molecular modeling of 7TMRs has been based on the structure of the inactive conformation of rhodopsin (11). Although the activated conformation of a 7TMR still remains experimentally unsolved, several cases of successful ligand design by three-dimensional homology modeling approaches have been reported for 7TMRs using the rhodopsin template (26,27,28,29). The recently published crystal structure of β2-AR complexed with an inverse agonist showed that the conformation of its transmembrane domain resembles that of rhodopsin (9,10), thus supporting the application of homology modeling to the study of 7TMRs.
From a previously reported model of TSHR complexed with the LMW partial agonist Org41841 (7), a number of analogs were designed with the assistance of molecular modeling. Additionally, experimental tests of the models derived from docking experiments (7,8) identified several key amino acids in the receptor that can be targeted as interacting residues for new ligands. Among the synthesized analogs, compound 52 was found to be a moderately potent antagonist with potential for further development. The model explains the pharmacological profile by predicting that the presence of an elongated substituent on the methoxy phenyl group of Org41841 would cause the ligand to sit higher in the binding pocket, nearer the extracellular region, and prevent critical contacts with residues deeper in the TMH that were known to be important for agonist activity. Thus, compound 52 presumably acts as an antagonist by occupying the TMH binding pocket without itself promoting or allowing the structural changes required for receptor activation. It is likely that its interaction within the TMH bundle allows it to inhibit activation by TSH and TsAbs as well.
Compound 52 bears many similarities to Org41841 because it was derived from this molecule. Both Org41841 and compound 52 bind to the same cleft in the transmembrane domain, but in our model they occupy this binding pocket with slightly shifted orientations. For example, one key receptor-ligand interaction for TSHR activation by Org41841 between E3.37 and the amino group of the ligand (7,8) is not established between compound 52 and E3.37 (Fig. 1B), which might contribute to the antagonism of compound 52.
Human thyrocytes were also used to test the effect of compound 52 under more physiological conditions. Although it has only moderate potency, compound 52 had antagonist actions on TSH as well as TsAb-induced TPO mRNA expression in human thyrocytes. Hence, we demonstrated the antagonistic effect of compound 52 on important activators of TSHR with different binding sites. Compound 52 will serve as a lead for rational design of higher-affinity ligands with potential as drugs for treatment of TSHR-mediated hyperthyroidism including Graves’ disease.
Supplementary Material
Acknowledgments
We thank Francesco Celi and Valentina Congedo for providing human thyroid tissue and Elizabeth Geras-Raaka for advice and assistance in preparing thyrocytes. We also thank John C. Morris for providing sera samples from patients with Graves’ disease and Marguerite Lichay and Mudit Kaushal for technical assistance.
Footnotes
This work was supported by the intramural research programs of the National Institute of Diabetes and Digestive and Kidney Diseases and the National Human Genome Research Institute, National Institutes of Health.
Disclosure Statement: The authors have nothing to disclose.
First Published Online July 31, 2008
See editorial p. 5943
Abbreviations: β2-AR, β2-Adrenergic receptor; ECL, extracellular loop; FSHR, FSH receptor; HBSS, Hanks’ balanced salt solution; LHCGR, LH/chorionic gonadotropin receptor; LMW, low-molecular-weight; P6.50, Proline 6.50; PDB, Protein Data Base; RMSD, root mean square deviation; TMH, transmembrane helices; 7TMR, seven transmembrane-spanning receptor; TPO, thyroperoxidase; TsAb, thyroid-stimulating antibody; TSHR, TSH receptor.
References
- Ascoli M, Fanelli F, Segaloff DL 2002 The lutropin/choriogonadotropin receptor, a 2002 perspective. Endocr Rev 23:141–174 [DOI] [PubMed] [Google Scholar]
- Fan QR, Hendrickson WA 2005 Structure of human follicle-stimulating hormone in complex with its receptor. Nature 433:269–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szkudlinski MW, Fremont V, Ronin C, Weintraub BD 2002 Thyroid-stimulating hormone and thyroid-stimulating hormone receptor structure-function relationships. Physiol Rev 82:473–502 [DOI] [PubMed] [Google Scholar]
- Vassart G, Pardo L, Costagliola S 2004 A molecular dissection of the glycoprotein hormone receptors. Trends Biochem Sci 29:119–126 [DOI] [PubMed] [Google Scholar]
- Guo T 2005 Small molecule agonists and antagonists for the LH and FSH receptors. Expert Opin Ther Patents 15:1555–1564 [Google Scholar]
- Titus S, Neumann S, Zheng W, Southall N, Michael S, Klumpp C, Yasgar A, Shinn P, Thomas CJ, Inglese J, Gershengorn MC, Austin CP 2008 Quantitative high throughput screening using a live cell cAMP assay identifies small molecule agonists of the TSH receptor. J Biomol Screen 13:120–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaschke H, Neumann S, Moore S, Thomas CJ, Colson AO, Costanzi S, Kleinau G, Jiang JK, Paschke R, Raaka BM, Krause G, Gershengorn MC 2006 A low molecular weight agonist signals by binding to the transmembrane domain of thyroid-stimulating hormone receptor (TSHR) and luteinizing hormone/chorionic gonadotropin receptor (LHCGR). J Biol Chem 281:9841–9844 [DOI] [PubMed] [Google Scholar]
- Moore S, Jaeschke H, Kleinau G, Neumann S, Costanzi S, Jiang JK, Childress J, Raaka BM, Colson A, Paschke R, Krause G, Thomas CJ, Gershengorn MC 2006 Evaluation of small-molecule modulators of the luteinizing hormone/choriogonadotropin and thyroid stimulating hormone receptors: structure-activity relationships and selective binding patterns. J Med Chem 49:3888–3896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherezov V, Rosenbaum DM, Hanson MA, Rasmussen SG, Thian FS, Kobilka TS, Choi HJ, Kuhn P, Weis WI, Kobilka BK, Stevens RC 2007 High-resolution crystal structure of an engineered human β2-adrenergic G protein-coupled receptor. Science 318:1258–1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen SG, Choi HJ, Rosenbaum DM, Kobilka TS, Thian FS, Edwards PC, Burghammer M, Ratnala VR, Sanishvili R, Fischetti RF, Schertler GF, Weis WI, Kobilka BK 2007 Crystal structure of the human β2 adrenergic G-protein-coupled receptor. Nature 450:383–387 [DOI] [PubMed] [Google Scholar]
- Palczewski K, Kumasaka T, Hori T, Behnke CA, Motoshima H, Fox BA, Le Trong I, Teller DC, Okada T, Stenkamp RE, Yamamoto M, Miyano M 2000 Crystal structure of rhodopsin: a G protein-coupled receptor. Science 289:739–745 [DOI] [PubMed] [Google Scholar]
- Salom D, Lodowski DT, Stenkamp RE, Le Trong I, Golczak M, Jastrzebska B, Harris T, Ballesteros JA, Palczewski K 2006 Crystal structure of a photoactivated deprotonated intermediate of rhodopsin. Proc Natl Acad Sci USA 103:16123–16128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standfuss J, Xie G, Edwards PC, Burghammer M, Oprian DD, Schertler GF 2007 Crystal structure of a thermally stable rhodopsin mutant. J Mol Biol 372:1179–1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aarons EJ, Beddows S, Willingham T, Wu L, Koup RA 2001 Adaptation to blockade of human immunodeficiency virus type 1 entry imposed by the anti-CCR5 monoclonal antibody 2D7. Virology 287:382–390 [DOI] [PubMed] [Google Scholar]
- Dragic T, Trkola A, Lin SW, Nagashima KA, Kajumo F, Zhao L, Olson WC, Wu L, Mackay CR, Allaway GP, Sakmar TP, Moore JP, Maddon PJ 1998 Amino-terminal substitutions in the CCR5 coreceptor impair gp120 binding and human immunodeficiency virus type 1 entry. J Virol 72:279–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B, Sharron M, Blanpain C, Doranz BJ, Vakili J, Setoh P, Berg E, Liu G, Guy HR, Durell SR, Parmentier M, Chang CN, Price K, Tsang M, Doms RW 1999 Epitope mapping of CCR5 reveals multiple conformational states and distinct but overlapping structures involved in chemokine and coreceptor function. J Biol Chem 274:9617–9626 [DOI] [PubMed] [Google Scholar]
- Kleinau G, Claus M, Jaeschke H, Mueller S, Neumann S, Paschke R, Krause G 2007 Contacts between extracellular loop two and transmembrane helix six determine basal activity of the thyroid-stimulating hormone receptor. J Biol Chem 282:518–525 [DOI] [PubMed] [Google Scholar]
- Case DA, Pearlman DA, Caldwell JW, Cheatham III TE, Wang J, Ross WS, Simmerling CL, Darden TA, Merz KM, Stanton RV, Cheng AL, Vincent JJ, Crowley M, Tsui V, Gohlke H, Radmer RJ, Duan Y, Pitera J, Massova I, Seibel GL, Singh UC, Weiner PK, Kollman PA 2002 AMBER 7 computer program. San Francisco: University of California, San Francisco [Google Scholar]
- Laskowski RA, Moss DS, Thornton JM 1993 Main-chain bond lengths and bond angles in protein structures. J Mol Biol 231:1049–1067 [DOI] [PubMed] [Google Scholar]
- Ballesteros JA, Weinstein H 1995 Integrated methods for the construction of three-dimensional models and computational probing of structure-function relationships in G-protein coupled receptors. Methods Neurosci. 25:366–428 [Google Scholar]
- McGovern SL, Caselli E, Grigorieff N, Shoichet BK 2002 A common mechanism underlying promiscuous inhibitors from virtual and high-throughput screening. J Med Chem 45:1712–1722 [DOI] [PubMed] [Google Scholar]
- Cavasotto CN, Orry AJ 2007 Ligand docking and structure-based virtual screening in drug discovery. Curr Top Med Chem 7:1006–1014 [DOI] [PubMed] [Google Scholar]
- Congreve M, Murray CW, Blundell TL 2005 Structural biology and drug discovery. Drug Discov Today 10:895–907 [DOI] [PubMed] [Google Scholar]
- Klebe G 2006 Virtual ligand screening: strategies, perspectives and limitations. Drug Discov Today 11:580–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marco E, Gago F 2007 Overcoming the inadequacies or limitations of experimental structures as drug targets by using computational modeling tools and molecular dynamics simulations. Chem Med Chem 2:1388–1401 [DOI] [PubMed] [Google Scholar]
- Becker OM, Marantz Y, Shacham S, Inbal B, Heifetz A, Kalid O, Bar-Haim S, Warshaviak D, Fichman M, Noiman S 2004 G protein-coupled receptors: in silico drug discovery in 3D. Proc Natl Acad Sci USA 101:11304–11309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klabunde T, Hessler G 2002 Drug design strategies for targeting G-protein-coupled receptors. Chembiochem 3:928–944 [DOI] [PubMed] [Google Scholar]
- Schlegel B, Laggner C, Meier R, Langer T, Schnell D, Seifert R, Stark H, Holtje HD, Sippl W 2007 Generation of a homology model of the human histamine H(3) receptor for ligand docking and pharmacophore-based screening. J Comput Aided Mol Des 21:437–453 [DOI] [PubMed] [Google Scholar]
- Tunaru S, Lattig J, Kero J, Krause G, Offermanns S 2005 Characterization of determinants of ligand binding to the nicotinic acid receptor GPR109A (HM74A/PUMA-G). Mol Pharmacol 68:1271–1280 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.