Abstract
High levels of maternal estrogens are likely to gain access to the fetal brain, yet little is known regarding the role of the steroid hormone 17β-estradiol in neuronal differentiation and maturation of primate neurons. Previous research documented the presence of estrogen receptors during development in the hippocampus and cortex of the primate brain, but the functional significance of steroid exposure has not been widely investigated. Using both an in vitro preparation of primary hippocampal and frontal cortex neurons and Western blot analysis of fetal hippocampal and frontal cortex tissue, we documented the effects of in utero and acute in vitro exposure to 17β-estradiol on the development of neuronal responsiveness to the amino acid transmitters γ-aminobutyric acid (GABA) and glutamate in fetal baboon, Papio anubis, hippocampal, and cortical neurons. We found that in utero 17β-estradiol exposure enhanced the excitatory action of the GABAergic system on immature cortical and hippocampal neurons, as manifest by increases in intracellular calcium after transient muscimol application and changes in the relevant ion cotransporters. Acute exposure to 17β-estradiol in vitro had limited effect on GABAergic responses in cultured hippocampal and frontal cortex neurons. Moreover, there was limited effect of both prolonged in utero and acute estradiol on the response to glutamatergic system activation, consistent with previous findings in the rat. Along with documenting a prominent role for 17β-estradiol in maturation of the GABAergic system, these findings increase our understanding of neuronal differentiation and maturation in the fetal primate brain.
NEURONAL MATURATION AND synapse formation begins in the mid- to late gestational period in the human and nonhuman primate brain (1,2,3). During this time period, neurotransmitter release and alterations in intracellular calcium initiate the formation and maturation of synapses, and provide the foundation for the mature patterns of innervation. Exuberant connections are eliminated, with synaptic competition and coincidence of input helping to establish patterns of connectivity (4,5,6). Whereas the importance of the glutamatergic system in synaptic patterning is well documented (7,8,9), recent work has indicated an equally important role for the secretion of γ-aminobutyric acid (GABA) system (10,11,12,13,14).
γ-Aminobutyric acid (GABA) is the predominant inhibitory neurotransmitter in the adult brain but acts as a principle source of excitatory drive in immature neurons. During rodent brain development, GABAA receptor activation results in chloride efflux and membrane depolarization sufficient to open voltage-sensitive calcium channels and allow for calcium entry into cells (15,16,17,18). GABAA receptor-mediated increases in intracellular calcium may act as an indirect indicator of GABA-mediated excitation. The resultant increase in intracellular calcium subsequent to GABAA receptor activation confers trophic effects on the developing brain (19,20,21). To date, relatively few studies have documented the effects of GABAA receptor activation in the developing primate brain (3,22).
The mid- to late gestational period in the human and nonhuman primate fetus is characterized by elevated circulating levels of testosterone and estradiol (23,24,25,26). Testosterone, from fetal (23) or placental origin (24), may play critical roles in sex-specific brain and behavioral development (27,28,29,30,31). The steroid binding globulin, α-fetoprotein, has relatively little affinity for estradiol in the primate (32), and thus fetal brain would be predicted to be exposed to high levels of this steroid. Estradiol is a potent modulator of neuronal and glial differentiation and maturation, synaptogenesis, and naturally occurring cell death in the rodent brain (33) but has been relatively unexplored in the primate brain. Previous work by our laboratory demonstrates that 17β-estradiol prolongs the time period and enhances the magnitude of GABA-mediated excitation (34,35,36) and dampens the response to excitatory glutamate (37) in the developing rodent brain.
In the present study, we investigated the response of embryonic neurons from the hippocampus and frontal cortex of fetal baboons to GABAergic and glutamatergic receptor stimulation and the effect of manipulating 17β-estradiol levels on that response. We found that acute exposure to 17β-estradiol in vitro had limited effect, but prolonged in utero 17β-estradiol exposure led to significant enhancement in the magnitude of the excitatory response to GABAA receptor activation, as manifest by increased intracellular calcium and alterations in chloride cotransporter protein levels. In contrast, there was limited effect of both prolonged in utero and acute estradiol in vitro on the glutamatergic system.
Materials and Methods
Female baboons (Papio anubis) were housed in primate cages in air-conditioned rooms under a 12-h light, 12-h dark cycle. Baboons had free access to high-protein monkey chow and water along with fresh fruit twice daily and vitamins once daily. Females were paired with male baboons for a period of 5 d during the ovulatory phase of the menstrual cycle, and pregnancy was determined by palpation. Animals were cared for and used strictly in accordance with U.S. Department of Agriculture regulations and the Guide for the Care and Use of Laboratory Animals (National Research Council, National Academy Press, 1996). The experimental protocol used in the present study was approved by the Institutional Animal Care and Use Committees of the University of Maryland School of Medicine. Brains were obtained from fetuses generated as part of a long-term research project on the hormonal regulation of the fetal-placental unit in the baboon under the direction of one of the authors (E.D.A.) (38,39) and would have been otherwise discarded. To make meaningful comparisons between cohorts of animals, we restricted our analyses to two different stages in gestation, midgestation (gestation d 102–104) and late gestation (gestation d 167–175). Term pregnancy in the baboon is 184 d. We compared responses after prolonged in utero estradiol exposure and acute in-the-dish estradiol exposure to vehicle treated controls.
In utero estradiol exposure of fetuses
Table 1 lists all animals and their treatment. Distinct treatment paradigms were used at the two different gestational ages for purposes of asking distinct experimental questions relevant to the research program of the investigator (E.D.A.). Fetuses collected at midgestation were either untreated or treated with estradiol early in gestation to study the impact on spiral artery invasion of the placenta. Fetuses collected in late gestation were from females were either untreated or treated with the aromatase inhibitor CGS 20267 (Letrozole) and replaced with estradiol benzoate to study development of the fetal adrenal. Letrozole does not cross the blood-brain barrier and so would not be expected to negatively impact on brain estradiol levels, which would be elevated due to exogenous treatment with steroid. Fetuses were delivered by cesarean section from timed pregnant females. We obtained the brains and used them for calcium imaging, immunocytochemistry, and Western blot analysis. A total of eight fetal baboon brains (three females and one male midgestation, and three males and one female late gestation) were obtained over a time period of 12 months (see Table 1). Of the four midgestation pregnant females, two were injected sc with 350 μg estradiol benzoate daily from gestational d 22 or 25 to d 60. This treatment paradigm prematurely elevates maternal circulating estradiol. Two midgestation females were untreated controls. Of the four late gestation pregnant females, three were injected sc daily with 115 μg/kg estradiol benzoate and Letrozole beginning on d 100 and continuing until or just before delivery. This paradigm results in maternal estradiol levels 1.5–2.0 times higher than normal. Because Letrozole does not cross the blood brain barrier, estradiol levels would be expected to be even higher in the fetal brain due to the exogenous estradiol benzoate treatment. One late gestation male was an untreated control. In all cases, halothane-nitrous oxide anesthesia was used during performance of cesarean section. The fetuses were immediately killed with an overdose of sodium pentobarbital and weighed.
Table 1.
Fetuses used in the current study
| Animal | GD | Treatment | Sex | Placental weight (g) | Fetal weight (g) | Maternal weight (kg) |
|---|---|---|---|---|---|---|
| 12986 | 175 | CGS 20267 + estradiol, GD 100–172 | M | 164.0 | 740.0 | 13.9 |
| 13809 | 167 | CGS 20267 + estradiol, GD 100–167 | F | 165.0 | 767.5 | 13.6 |
| 10180 | 171 | Control | M | 170.0 | 851.0 | 17.9 |
| 13361 | 169 | CGS 20267 + estradiol, GD 100–168 | M | 228.0 | 964.6 | 15.4 |
| 13161 | 102 | 350 μg estradiol, GD 25–60 | M | 94.5 | 202.0 | 14.5 |
| 13787 | 102 | Control | F | 77.4 | 185.3 | 13.7 |
| 10668 | 104 | Control | F | 80.0 | 194.7 | 16.2 |
| 13809 | 102 | 350 μg estradiol, GD 22–60 | F | 80.3 | 174.0 | 11.7 |
Animals from gestational days (GD) 167–175 were considered late gestation, whereas those from 102–104 were considered midgestation.
CGS 20267, Letrozole.
Preparation of primary neuron cultures
Upon obtaining a deep and irreversible anesthesia, fetal brains were removed and hemisected. One hemisphere was placed on ice for Western blot analysis (see below), and the hippocampus and cortex were dissected from the remaining hemisphere to be used for primary cell culture. Only one fetus was available at a time, so the primary cell cultures on a given day were derived from one animal. Each individual primary cell culture is treated as an independent subject for the statistical analyses. Both frontal cortex and hippocampal tissue were placed into sterile 15-ml vials containing Hanks’ balanced salt solution (HBSS+) [88 ml sterile H2O, 10 ml HBSS (Ca2+ and Mg2+ free) 10 times, 1 ml HEPES buffer, 1.0 m (pH 7.3), 1 ml antibiotic/antimycotic 100× liquid]. HBSS+ was added to the tube to a volume of 4.5 ml with 0.5 ml Trypsin (2.5%). Cells were incubated in a 37 C water bath for 15 min. The supernatant was removed and washed with HBSS+. Cells were dissociated by pipetting up and down using a Pasteur pipette, and cell number and viability were determined by Trypan blue exclusion. Cells were plated on 18 mm poly-l-lysine-coated coverslips at a density of 300,000 cells per coverslip and placed in 60-mm dishes containing 4 ml plating medium [86 ml MEM, 10 ml horse serum, 3 ml glucose (filter sterilized, 20%) 1 ml pyruvic acid, 100 mm[ and allowed 4 h to adhere to the coverslips in a 37 C-5% CO2 incubator. Plating density was similar across all groups.
The coverslips were removed from the plating dishes and placed into 60-mm dishes filled with Neurobasal+ [1 ml B-27 supplement, 1 ml antibiotic/antimycotic 100× liquid, 125 μl l-glutamine and filled to 50 ml with Neurobasal (phenol red free)].
Acute estradiol exposure of cultured neurons
Cultures were treated on days in vitro (DIV) 0 and 2 with either 1 nm 17β-estradiol dissolved in dimethylsulfoxide (DMSO) or vehicle (DMSO). In all applications, the final concentration of DMSO is less than 0.1%.
RIA of estradiol in medium
To assess levels of estradiol after acute treatments, a 240-μl sample of media was collected from a subset of the culture on DIV 1, 2, 3, 5, and 7. Media were housed in a −80 C freezer until processing for quantification of estradiol by RIA. Samples were shipped on dry ice and underwent RIA for estradiol at the Center for Cellular and Molecular Studies (University of Virginia, Charlottesville, VA). The commercially available third-generation estradiol RIA kit (Diagnostic Systems Laboratories, Webster, TX) was used. The maximum sensitivity of this assay is 1.5 pg/ml. The estradiol assay had an intraassay variability of less than 5.7% and an interassay variability of less than 7.2%.
Calcium imaging
Cultured neurons underwent calcium imaging using the cell permeant fluorescent indicator fura-2-AM on DIV 3. The time course of estradiol treatment and imaging was based on that used to detect significant effects in rat hippocampal neuron primary cultures (34,35,36). On the day of calcium imaging, baboon hippocampal and cortical neurons were incubated with the cell permeant fluorescent indicator fura-2-AM (3 μm) (Molecular Probes, Eugene, OR) in DMSO (<0.5%) at room temperature for 30 min before the coverslips were transferred to a tissue chamber mounted on a microscope stage and superfused with physiological salt solution [PSS: 140 mm NaCl, 5 mm KCl, 1.2 mm Na2PO4, 1.4 mm MgCl2, 1.8 mm CaCl2, 11.5 mm glucose, 10 mm HEPES (pH 7.4)] at 32–34 C to remove extracellular dye and allow for esterification of fura-2-AM. All drugs were administered in PSS. The imaging system uses a Zeiss Axiovert 100 inverted microscope, with illumination provided by a Til Photonics Polychrome II monochromator (Applied Scientific Instrumentation, Eugene, OR). Fluorescent images were obtained using a Hamamatsu charge-coupled device video camera and image intensifier. Image acquisition was performed with the Metafluor 5.0 imaging system (Universal Imaging Corp., Downingtown, PA). Cells in the field of view were characterized morphologically using a ×60 objective, making a distinction between neurons and glia. Individual cells were chosen for analyses by the investigator and traced using the Metafluor program.
Numerous criteria were used to distinguish neurons from glia, but the most important was shape: for hippocampal neurons, the somas of pyramidal neurons are triangular in appearance with rounded and clearly distinct edges. Neurons usually possess at least two primary processes. For cortical neurons, only pyramidal neurons were analyzed. In contrast, glial cells are amorphous in shape, with flat and nondistinct edges and no distinct processes. In the current experiments, only data obtained from neurons was analyzed. In utero and acute 17β-estradiol treatment had no effect on neuronal or glial cell survival in culture. Baseline measurements of resting calcium concentrations for individual cells were obtained over a 5-min period, whereas the cells were superfused with PSS. This was followed by a 50-sec pulse of the test solution (10 μm muscimol or 10 μm glutamate), with data acquired for a further 5 min (allowing for reestablishment of baseline calcium levels). The following parameters were documented during calcium imaging: 1) baseline (resting) intracellular calcium concentration, 2) percentage of cells that responded with muscimol or glutamate-induced calcium transients [the criteria for response (either yes or no) was performed on a cell-to-cell basis, with yes indicated as an increase in intracellular calcium concentration to at least 10% above baseline for that individual cell], and 3) peak intracellular calcium concentration of each responding cell (value of the highest response collected over the sampling period). Across all studies performed by the authors, the se of the baseline intracellular calcium concentration is approximately ± 4%. Therefore, a 10% alteration in the baseline intracellular calcium concentration is 2 times greater than the se and well outside the inherent variability associated with the measurement. Intracellular calcium concentration was calculated from the ratio of the background corrected fura-2emission (520 nm) at two excitation wavelengths (340/380 nm) by in situ solution calibration (40), performed using a calcium calibration buffer concentration kit (Molecular Probes). There were a total of 60–100 neurons analyzed for each treatment group from cells cultured at midgestation and full term.
Immunocytochemistry of cultured tissue
A subset of coverslips containing hippocampal and frontal cortex cells (that did not undergo calcium imaging) were used for immunocytochemistry. Culture medium was replaced with warm fixative (4% paraformaldehyde, 5% sucrose in 0.1 m PBS) for 10 min, followed by rinsing in 0.1 m PBS and 60 min in chilled 50% ethanol. Cells were rinsed and then blocking solution (10% normal goat serum, 0.1% Triton X-100 in 0.1 m PBS) at room temperature for 30 min, and one of the following primary antibodies were used: 1) rabbit R5 monoclonal antibody generated against the phosphorylated form of the mouse Na+-K+-2Cl− cotransporter NKCC1 protein (1:1000; generous gift of Dr. Biff Forbush, Yale University, New Haven, CT), or 2) rabbit polyclonal antibody generated against the rat K+-Cl− cotransporter KCC2 protein (1:1000; Upstate, New York, NY). All antibodies were diluted in 10% normal goat serum in 0.1 m PBS, with cultures incubated overnight at 4 C. On the next day, tissue was rinsed before exposure to the secondary antibody (Vector, Burlingame, CA), followed by rinses and addition of Vectastain Elite ABC reagents (Vector). The cultured cells were visualized via addition of nickel-enhanced diaminobenzidine in sodium acetate. After the reaction, the coverslips containing cells were rinsed, dehydrated, and coverslipped.
Analysis of immunolabeling
Cells immunoreactive for phosphorylated state of NKCC1 (pNKCC1) or KCC2 were counted using the StereoInvestigator program (MicrobrightField version 2.01; MicrobrightField Co., Colchester, VT). An unbiased dissector frame (100 × 100 μm) was randomly placed throughout each coverslip, with a total of 75 fields sampled with a × 20 objective to obtain an estimate (coefficient of error < 0.1) of immunoreactive cell density (cells per square micrometer) from each coverslip. A total of four coverslips were sampled per treatment group.
Western blot analysis
Hippocampal and frontal cortex tissue was removed from mid gestation baboon fetuses. Tissue was microdissected on a chilled surface, flash frozen using isopentane, and stored at −80 C until homogenization with ice-cold lysis buffer containing 50 mm Tris-HCl, 1% Na-deoxycholate, 0.25% Nonidet P-40, 150 mm NaCl, 1 mm EDTA, and protease inhibitors (1 μg/ml of aprotinin, leupeptin, and pepstatin; 1 mm phenylmethylsulfonyl fluoride). After tissue homogenization, samples were centrifuged at 3000 × g for 30 min at −10 C. The supernatant fraction was collected and the protein concentration determined by Bradford assay. Twenty micrograms of total protein from each animal were electrophoresed on a sodium dodecyl sulfate-polyacrylamide gel (8–16% Tris glycine) and transferred to a polyvinylidenedifluoride membrane. Membranes were washed with 0.1 m Tris-buffered saline (TBS) and blocked for 1 h at room temperature in 0.1 m TBS containing 5% nonfat dry milk. Membranes were then incubated with one of the following: 1) rabbit R5 monoclonal antibody generated against the phosphorylated form of the mouse Na+K+2Cl− cotransporter NKCC1 protein (1:2500; generous gift of Dr. Biff Forbush, Yale University, New Haven, CT); 2) rabbit polyclonal antibody generated against the rat K+Cl− cotransporter KCC2 protein (1:2500; Upstate); or 3) mouse monoclonal antibody generated against the housekeeping gene, glyceraldehyde-3-phosphate dehydrogenase (GAPDH; 1:10,000; Chemicon, Temecula, CA).
All antibodies were diluted in TBS containing 0.05% Tween 20 and incubated for 3 h at room temperature. Membranes were incubated in goat antirabbit or rabbit antimouse horseradish peroxidase-linked secondary antibody (1:3000; Cell Signaling Technology, Beverly, MA) for 30 min at room temperature and then washed with TBS containing 0.05% Tween 20. Immunoreactive bands were detected using an enhanced chemiluminescence kit (ECL kit; New England Biolabs, Beverly, MA), and membranes were exposed to film (Hyperfilm-ECL; Amersham Pharmacia Biotech, Arlington Heights, IL). The proteins were detected as bands of specific molecular masses (pNKCC1 = 162 kDa; KCC2 = 140 kDa; GAPDH = 37 kDa), and the integrative gray-scale pixel area-density was captured with a charge-coupled device camera and analysis performed on a Macintosh computer using the public domain NIH Image program (developed at the National Institutes of Health and available at http://rsb.info.nih.gov/nih-image/). To control for gel loading, integrative gray-scale pixel area-density values for each protein was divided by GAPDH. A total of two samples (animals) were analyzed per group for all Western blot analyses.
Statistical analysis
Because of the small number of animals and periodic availability of subjects, it was not feasible to consider the animal as the n for statistical analyses. Multiple culture dishes were generated from each animal, and then half were treated acutely in the dish with estradiol and half with vehicle. When analyzing the level of estradiol in the medium, the culture dish was taken as the unit of analyses. For resting intracellular calcium, an individual cell was considered independent of all others. There was no systematic variation in the resting level of calcium across dishes generated from the same or different animals within one treatment paradigm. For the peak in intracellular calcium, each cell was again considered independent, but only cells with a greater than 10% increase above background were included (see above for rational of this criteria). Two-way analyses of variance were conducted to assess the effect of acute in-the-dish and in utero estradiol treatment on baseline intracellular calcium concentration, in vitro estradiol levels, and peak intracellular calcium concentration. Tissue from mid- vs. late gestation was analyzed separately. One-way analyses of variance were also performed for the effect of in utero estradiol treatment on immunocytochemical cell density and densitometry of Western blots. The nonparametric χ2 test was performed on data for the percentage of cells that responded with calcium influx and was calculated inclusive of all dishes used in calcium imaging. For all ANOVAs, after detection of a main effect, the post hoc Tukey test was used to identify significant differences between groups, with a level of P < 0.05 required to obtain statistical significance.
Results
Hormone levels in cultured cells
RIA was used to assess the physiological relevance of our acute estradiol treatment paradigm. Frontal cortex and hippocampal cultures were treated with 1 nm 17β-estradiol or DMSO (vehicle) on DIV 0 and 2, with culture medium collected on DIV 1, 2, 3, 5, and 7. On DIV 2, medium was collected just before 17β-estradiol treatment. In both the hippocampus and frontal cortex, acute estradiol treatment led to increased levels of estradiol (ANOVA: F4,19 = 131.517, P < 0.0001), between 135 and 161 pg/ml on DIV 1, peaking between 434 and 562 pg/ml on DIV 3, and then falling to 320–419 pg/ml by DIV 7 (Table 2). Estradiol was not detectable in the control and in utero estradiol-treated cultures. Of interest is that although the acute 17β-estradiol treatment doses were the same, estradiol levels were significantly higher in hippocampal cultures than frontal cortex cultures at all ages examined except DIV 1 (ANOVA: F1,19 = 77.20 1, P < 0.0001), suggesting a difference in the rate of metabolism of the steroid.
Table 2.
Estradiol levels in primary cultures of the fetal baboon hippocampus and frontal cortex as assessed by RIA
| DIV | 1 | 2 | 3 | 5 | 7 |
|---|---|---|---|---|---|
| Hippocampus | |||||
| Control | ND | ND | ND | ND | ND |
| Acute estradiol | 161.5 ± 3.7a | 231.9 ± 18.0a,b | 527.8 ± 36.0a,b | 562.9 ± 7.0a,b | 418.9 ± 1.0a,b |
| In utero estradiol | ND | ND | ND | ND | ND |
| Frontal cortex | |||||
| Control | ND | ND | ND | ND | ND |
| Acute estradiol | 145.2 ± 2.0a | 138.1 ± 6.0a | 434.4 ± 39.0a | 345.8 ± 15.0a | 319.8 ± 6.5a |
| In utero estradiol | ND | ND | ND | ND | ND |
Units are picograms per milliliter. ND, Estradiol levels were not detectable in these cultures.
Significant difference from control cultures of the same region of the brain (Tukey; P < 0.05).
Significant difference from acute estradiol-treated frontal cortex cultures of the same DIV (Tukey; P < 0.05).
Intracellular calcium responses in cultured cells
Baseline (resting) intracellular calcium concentration.
At rest, cells maintain a baseline, homeostatic intracellular calcium concentration. By using calcium imaging, we were able to document the effect of estradiol treatment, both acute and in utero, on this parameter in both hippocampal and frontal cortex neurons. There was no difference in mean resting calcium levels between mid- and late gestation neurons for either the hippocampus or cortex.
Frontal cortex.
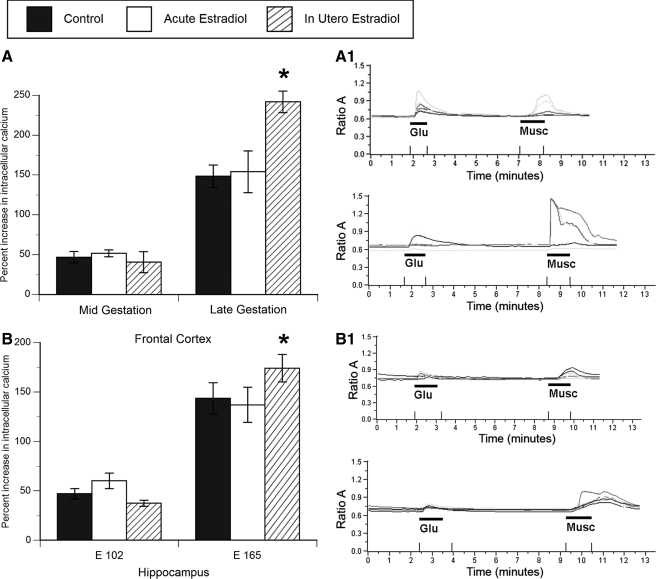
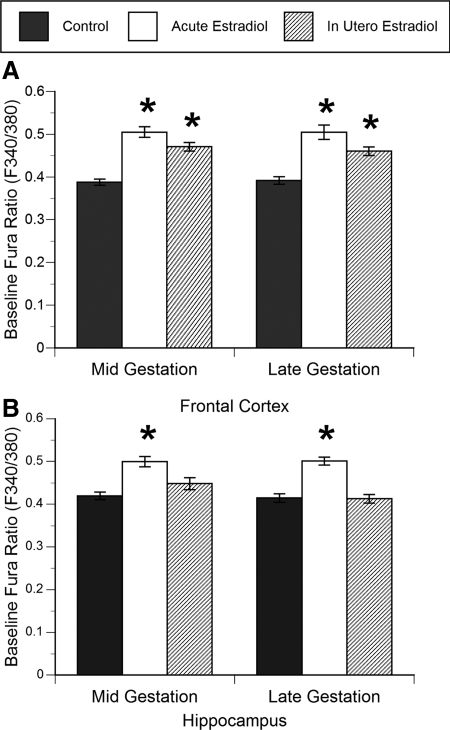
The resting intracellular calcium concentration was significantly increased by both acute in-the-dish and in utero estradiol treatment in both midgestation (acute: F2,98 = 21.7, P < 0.0001; in utero: F2,98 = 18.4, P < 0.0001) and late gestation cultured frontal cortex neurons (acute: F2,75 = 22.8, P < 0.0001; in utero: F2,75 = 12.3, P < 0.0001; Fig. 1A). Whereas in utero estradiol treatment led to a 15–18% increase in baseline intracellular calcium in frontal cortex neurons, this increase was 22–23% after acute in-the-dish estradiol treatment.
Figure 1.
Effect of acute in-the-dish and in utero estradiol treatment on resting (baseline) intracellular calcium levels in cultured primary frontal cortex (A) and hippocampal neurons (B) from the fetal baboon. Both acute (DIV 0 and 1) and in utero (in vivo over gestational d 22–65 or 100–170) estradiol administration significantly elevated resting (baseline) intracellular calcium concentration relative to controls in frontal cortex neurons at both ages (ANOVAs, *, P < 0.05 compared with control), whereas only acute estradiol administration elevated resting intracellular calcium concentration in hippocampal neurons (ANOVA, *, P < 0.05 compared with control; n = 73–98). Data are expressed as the baseline fura ratio (F340:380) sampled over a 5-min period (mean ± sem value).
Hippocampus.
Acute estradiol treatment significantly increased by 16–17% the baseline intracellular calcium concentration in midgestation (acute: F2,88 = 15.4, P < 0.0001) and late gestation cultured hippocampal neurons (acute: F2,73 = 13.8, P < 0.0001; Fig. 1B) over the level in controls, but unlike the cortex, in utero estradiol treatment had no effect on the resting intracellular calcium concentration in hippocampal neurons.
Peak intracellular calcium concentration in response to muscimol
The effect of in-the-dish and in utero estradiol treatment on GABAA receptor-mediated calcium transients was examined in cultured hippocampal and cortical neurons.
Frontal cortex.
There was a significant effect of in utero estradiol treatment but only in late gestation cultured frontal cortex neurons (in utero: F2,49 = 21.9, P < 0.0001; Fig. 2A). Muscimol-induced calcium transients in the in utero estradiol-treated neurons were 36–39% greater than those in all other groups in the frontal cortex. There was no significant effect of either acute in-the-dish or in utero estradiol treatment on frontal cortex neurons cultured from midgestation fetuses. With an increase in gestational age, regardless of treatment, there was an approximately 80% increase in the magnitude of muscimol-induced calcium transients in frontal cortex neurons. In utero estradiol treatment from mid- to late gestation enhanced the response of muscimol-responsive frontal cortex neurons to muscimol-induced calcium transients, but this effect did not occur after acute in-the-dish estradiol treatment.
Figure 2.
The magnitude (percent increase) of muscimol-induced calcium transients after in utero and acute estradiol treatment in frontal cortex (A) and hippocampal neurons (B) cultured from mid- and late gestation fetal baboon. Across both regions and treatment groups, muscimol application induced a significant influx of calcium. The magnitude of the calcium transient was further increased in both cortical and hippocampal neurons by in utero estradiol treatment of late gestation fetuses (ANOVA, *, P < 0.05 compared with control, n = 48–52). Data are expressed as the maximal increase in the fura ratio (F340:380) sampled over a 1-min period of muscimol administration (mean ± sem value). Representative traces from control and in utero estradiol-treated frontal cortex (A1) and hippocampal neurons (B1) after muscimol and glutamate administration. Each line represents the response of an individual cell.
Hippocampus.
Consistent with the frontal cortex, there was a significant effect of in utero estradiol treatment on the maximum amplitude of the calcium transient induced in response to the GABAA receptor agonist, muscimol, but only in late gestation cultures (in utero: F2,52 = 14.6, P < 0.0001; Fig. 2B). Muscimol-induced calcium transients in the in utero estradiol-treated neurons were 17–21% greater than those in all other groups in the hippocampus; thus, the magnitude of the effect of estradiol was considerably less than that observed for cortical neurons. There was no significant effect of estradiol treatment on midgestation hippocampal neurons. Also consistent with the frontal cortex, in hippocampal neurons there was an age-related increase in the peak magnitude of muscimol-induced calcium transients of 56–68%.
Peak intracellular calcium concentration in response to glutamate
Glutamate receptor activation can lead to direct calcium influx via the N-methyl-d-aspartic acid receptor and calcium-permeable 2-amino-3-hydroxy-5-methyl-4-isoxazol propionic acid receptors or indirect via voltage gated calcium channels. Using calcium imaging, we documented the effect of estradiol treatment (acute and in utero) on the peak magnitude of glutamate-induced calcium transients of fetal baboon hippocampal and cortical neurons (Table 3). Consistent with the increase in calcium transients seen in response to GABAA receptor activation, there was an age-related increase in the magnitude of glutamate-induced calcium transients in both regions. However, unlike the response to muscimol, there was no significant effect of either form of estradiol treatment (in utero or acute in the dish) at either time point on the magnitude of glutamate-induced calcium transients.
Table 3.
Effect of acute and in utero estradiol treatment on peak percent increase over baseline in glutamate-induced calcium responses in fetal baboon neurons
| E102 | E165 | |
|---|---|---|
| Frontal cortex | ||
| Control | 90.19 ± 6 | 205.54 ± 26 |
| In utero estradiol | 81.44 ± 25 | 194.4 ± 34 |
| Acute estradiol | 116.42 ± 15 | 209.0 ± 14 |
| Hippocampus | ||
| Control | 80.09 ± 9 | 169.39 ± 12 |
| In utero estradiol | 75.0 ± 23 | 144.37 ± 14 |
| Acute estradiol | 100.0 ± 23 | 146.44 ± 10 |
Units of percent are increase over baseline. E, Embryonic day.
Percent of frontal cortex cells responding to muscimol with calcium transients
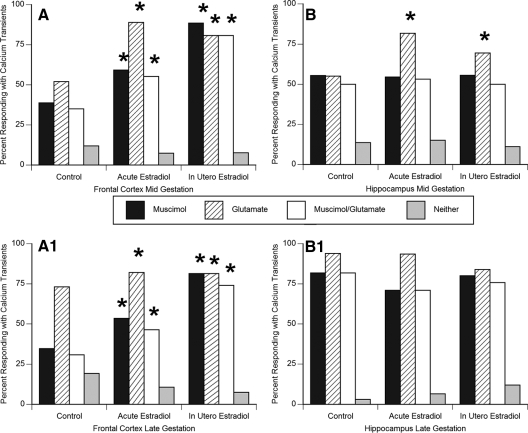
We used the nonparametric χ2 test to document the effect of estradiol treatment (both acute and in utero) on the percent of cells responding to muscimol, glutamate, both muscimol and glutamate, or neither (Fig. 3, A and B). There was a significant effect of both forms of estradiol treatment on the percentage of cells responding to muscimol application with calcium transients (acute: χ2 = 8.11, df = 2, P = 0.008; in utero: χ2 = 12.43, df = 2, P = 0.0009; Fig. 3, A and B). The effect was evident in both mid- and late gestation frontal cortex neurons.
Figure 3.
Either acute in-the-dish or in utero estradiol treatment increases the percent of midgestation (A) and late gestation (B) frontal cortex neurons responding to glutamate, muscimol, or both (not combined, same cell responding sequentially) with a calcium transients (χ2; *, P < 0.05 compared with control). In hippocampal neurons cultured from midgestation fetuses (C), there was an increase in the percent of neurons responding to glutamate with a calcium transient after acute or midgestational in utero estradiol exposure (χ2; *, P < 0.05 compared with control), but there was no change in the number responding to muscimol. In late gestation hippocampal neurons (D), there was no effect of estradiol treatment on percent responding to either muscimol or glutamate compared with control. This may be due to the already high level of response in controls. Data are expressed as the percentage of cells that respond to muscimol and glutamate administration with an increase in intracellular calcium concentration at least 10% above baseline.
Percent of frontal cortex cells responding to glutamate with calcium transients
There was a significant effect of both forms of estradiol treatment on the percent of cells responding to glutamate with increased intracellular calcium (acute: χ2 = 7.98, df = 2, P = 0.01; in utero: χ2 = 11.17, df = 2, P = 0.001; Fig. 3, A and B), and again the effect was evident in both mid- and late gestation frontal cortex neurons. As would be expected, the percent of cells responding to both muscimol and glutamate was also increased by acute or in utero estradiol treatment. The effect was evident in both mid- and late gestation frontal cortex neurons.
Percent of hippocampal cells responding to muscimol with calcium transients
In contrast to cortical neurons, there was no effect of estradiol treatment (either acute in the dish or in utero) on the percentage of cells responding to muscimol with increased intracellular calcium in either mid- or late gestational hippocampal neurons (Fig. 3, C and D).
Percent of hippocampal cells responding with increased intracellular calcium after glutamate administration
Consistent with neurons in the frontal cortex, there was a significant effect of both acute in-the-dish and in utero estradiol treatment on the percentage of cells responding to glutamate with increased intracellular calcium (acute: χ2 = 8.36, df = 2, P = 0.007; in utero: χ2 = 4.01, df = 2, P = 0.05; Fig. 3, C and D). This effect was present only in midgestation hippocampal neurons. By late gestation, estradiol treatment (both acute and in utero) was without effect on glutamate-induced calcium responses. This may be due to the already high level of responding by control neurons.
Immunocytochemical analysis of cultured cells
pNKCC1.
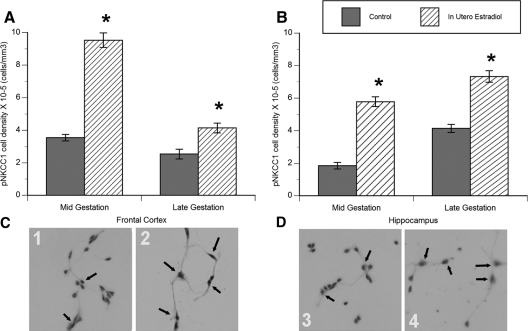
During development, GABA-mediated excitation is determined by the balance of chloride cotransporter expression, in particular NKCC1 and KCC2 (41,42,43,44). NKCC1 promotes chloride, sodium, and potassium transport into cells and high activity facilitates membrane depolarization after GABAA receptor activation. Conversely, KCC2 promotes chloride and potassium exit from the cell, and its expression is low during the period of GABA-mediated excitation. As development progresses, there is a gradual shift from high levels of NKCC1 and low levels of KCC2, to low levels of NKCC1 and high levels of KCC2. The developmental shift in KCC2 and NKCC1 is directly causally related to whether GABAA receptor activation results in depolarization and influx of calcium via voltage-sensitive Ca2+ channels or hyperpolarization and synaptic inhibition (42,43,45,46,47,48,49,50). The pNKCC1 is associated with increased activity of the cotransporter. We observed a significant effect of in utero estradiol treatment on the number of pNKCC1-immunoreactive cells in both the hippocampus and frontal cortex primary cell cultures (hippocampus: treatment − F2,24 = 11.2, P = 0.006; frontal cortex: treatment − F2,20 = 7.0, P = 0.009; Fig. 4, A and B). As with the percent of cells responding with muscimol-induced calcium transients, in utero estradiol treatment increased the number of cells that were pNKCC1 immunoreactive in primary cultures obtained from the hippocampus and frontal cortex at both ages. However, the magnitude of the estradiol-induced increase was not as great in the late gestation neurons as in the younger, midgestation neurons. The age- and treatment-related changes in the number of pNKCC1-immunoreactive cells is not likely due to effects on cell viability because we saw no evidence for differences in cell death between cultures. Moreover, labeling of cultured hippocampal and frontal cortex neurons with both the neuronal marker microtubule-associated protein-2, and the glial marker glial fibrillary acidic protein, revealed no age or treatment effect on the number or percentage of neurons or glia (data not shown).
Figure 4.
In utero estradiol increased the number of neurons expressing the phosphorylated form of the Na+K+2Cl− cotransporter pNKCC1 cultured from the frontal cortex (A) and hippocampus (B) at mid- or late gestation (ANOVAs, *, P < 0.05 for all measures compared with control). Data are expressed as density of pNKCC1-positive cells per cubic millimeter (means ± sem value). Representative photomicrographs of midgestation cultured neurons from: 1) control frontal cortex, 2) in utero estradiol-treated frontal cortex, 3) control hippocampus, and 4) in utero estradiol-treated hippocampus. All cultures are labeled with the pNKCC1 antibody (brown immunoreactive product), with a Nissl counterstain. Arrows indicate pNKCC1-positive neurons. Con, Control; E2, estradiol.
KCC2.
In contrast to pNKCC1, there was no significant effect of estradiol treatment on the number of cultured cells positive for KCC2 immunoreactivity (Table 4). The relatively low levels of cells positive for KCC2 immunoreactivity at both gestation ages, in combination with the higher levels of pNKCC1, is consistent with GABAA receptor-mediated excitation and muscimol-induced calcium influx at both ages.
Table 4.
Effect of age and in utero estradiol treatment on density of KCC2-immunopositive cells in cultured fetal baboon tissue
| E102 | E165 | |
|---|---|---|
| Frontal cortex | ||
| Control | 2.05 ± 0.14 | 3.59 ± 0.38a |
| In utero estradiol | 1.48 ± 0.40 | 3.18 ± 0.29a |
| Hippocampus | ||
| Control | 2.49 ± 0.52 | 2.89 ± 0.32a |
| In utero estradiol | 2.87 ± 0.46 | 3.01 ± 0.35a |
Units are 105 cells/mm3.
Significant difference from E102 cultures of the same treatment groups from the same region of the brain (Tukey; P < 0.05).
Western blot analysis of chloride cotransporters
pNKCC1.
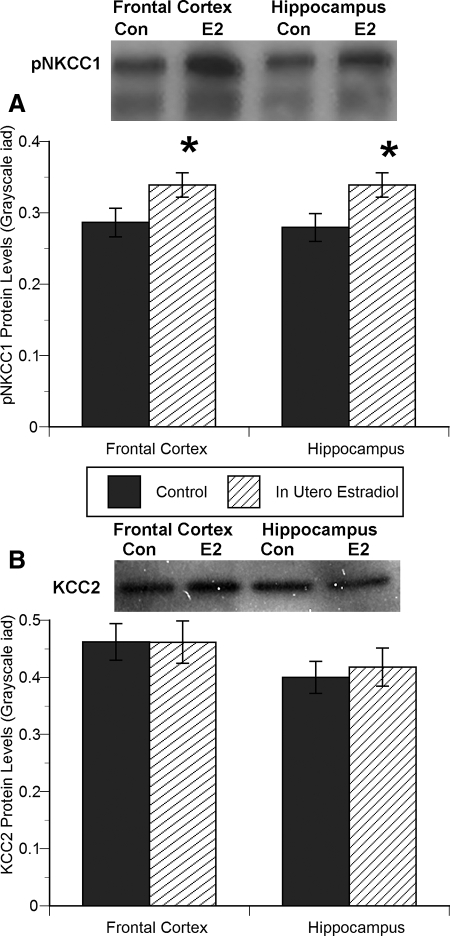
In utero estradiol treatment significantly affected pNKCC1 protein levels in both hippocampal and frontal cortex tissue from late gestation baboon fetuses (hippocampus: in utero − F1,3 = 5.83, P = 0.04; frontal cortex: in utero − F1,3 = 6.19, P = 0.03; Fig. 5A). Consistent with the increased number of pNKCC1-positive cells in the frontal and hippocampal cultures, estradiol treatment enhanced pNKCC1 protein levels by 17–21% over control.
Figure 5.
In utero estradiol treatment increased the protein levels of pNKCC1 in late gestation frontal cortex and hippocampal neurons (A), with no effect on KCC2 protein levels (B) (ANOVA, *, P < 0.05 compared with control). Data are expressed as gray-scale integrative area density, with pNKCC1 or KCC2 protein levels divided by GAPDH (mean ± sem value). Representative photomicrographs above the histograms represent one sample from each group.
KCC2.
There was no effect of estradiol treatment on KCC2 protein levels in tissue from the late gestation baboon frontal cortex or hippocampus (Fig. 5B).
Discussion
Baboons are from the same order as humans (primates) and from a closely related family (Old World monkeys). Baboons share numerous traits in common with humans such as a prolonged gestational period (184 d in baboons, 280 d in humans), fundamentally precocial infants at birth and a life span of greater than 20 yr (3). Given these similarities, investigation of the developmental events in nonhuman primate brain provides much needed insight into human brain development. In the current study, we report effects of exogenous estradiol treatment (administered both acutely to cultured neurons in the dish and in utero) on GABAergic and glutamatergic responses in hippocampal and frontal cortex neurons from fetal baboons. The hypothesis tested was the estradiol alters the responsiveness of the developing neurons. Further work is needed to test whether estradiol also affects the neuronal population (e.g. phenotype, neurogenesis, and neuronal survival).
Estradiol is naturally elevated in the late gestational period in humans and primates (51,52,53,54), and the fetal brain of both males and females is presumed to be exposed to high levels of the steroid. In the current study, estradiol was administered early in gestation, before when it would normally rise, and late in gestation but in the presence of the aromatase inhibitor, Letrozole. The nonsteroidal aromatase inhibitor, Letrozole, inhibits estrogen production by reversibly binding to cytochrome P450. Letrozole is also known to interact with the estrogen receptor, an additional mechanism of inhibiting the actions of estradiol. Letrozole does not affect the production of mineralocorticoids or glucocorticoids (55,56). Whereas peripheral effects of Letrozole are plausible and even likely, there is no immediately apparent mechanism for how this would impact on the development of GABAergic and glutamatergic calcium response in hippocampal and cortical neurons. Because Letrozole does not cross the blood-brain barrier, levels of estradiol in the fetal brain would be elevated by the exogenous hormone treatment, as is also the case for the maternal circulation. In both situations, early and late estradiol exposure, we found that estradiol augmented the magnitude of the intracellular calcium response and increased the percentage of neurons responding to the GABA agonist muscimol with intracellular calcium transients, indicative of depolarizing GABA.
Exogenous administration of estradiol to neurons in the dish elevated the baseline intracellular calcium concentration in hippocampal and cortical neurons, consistent with enhanced cellular excitability. We hypothesized that estradiol prolonged the duration and percentage of cells displaying GABA-mediated increases in intracellular calcium in baboon hippocampal and frontal cortex neurons by increasing the phosphorylation and hence activity of NKCC1, the sodium-chloride cotransporter that elevates intracellular chloride in immature neurons. Our observation of increased levels of pNKCC1 after estradiol treatment is consistent with findings from rodent hippocampal neurons (35,36). Estradiol treatment also increased the percentage of hippocampal and cortical neurons responding to glutamate with calcium influx. Taken together, these findings suggest that estradiol is a naturally occurring modulator of cellular excitation in the developing baboon brain.
It is important to note that not all developing neurons respond to GABAA or glutamate receptor activation administration with an increase in intracellular calcium. This may be due to one of a variety of variables: 1) GABAA and/or glutamate receptors may not be present in those cells, and thus, there is no functional means for a response; 2) GABAA and/or glutamate receptors are present, but the concentration of agonist applied is not sufficient to elicit a response; or 3) the maturational state of the cell precludes a response, too immature, as may be the case of the glutamate receptor, or receptor activation no longer leads to an increase in intracellular calcium, as may be the case for the GABAA receptor.
In utero estradiol treatment (daily treatment from gestational d 100–170) significantly enhanced the magnitude of muscimol-induced calcium transients in cultured hippocampal and cortical neurons generated from late gestation fetuses and increased the percent of cortical cells displaying muscimol-induced calcium transients (the percent of hippocampal cells responding was already close to maximal). There was no effect of in utero estradiol treatment of midgestation fetuses on the magnitude of the muscimol-induced calcium transient. It is critical to note that the treatment of midgestation fetuses was largely during the early gestational period and was terminated approximately 1 month before delivery, whereas late gestation fetuses were treated up to or just before delivery by cesarean section. However, the difference between the two age groups cannot be explained entirely by the timing of estradiol exposure because surprisingly, there was no effect of acute estradiol treatment (defined as estradiol administered in the dish on days in vitro 0 and 2, on the magnitude of muscimol-induced calcium transients. This would suggest that a prolonged period of estradiol exposure is necessary (longer than the two day in vitro exposure), although the in vitro concentration of estradiol (1 nm) may not have been sufficient to induce a response. This seems unlikely because low nanomolar levels of estradiol are well within the physiological range. Moreover, given that the acute estradiol treatment paradigm significantly affected the baseline level of intracellular calcium and the percentage of cells responding to muscimol with increased intracellular calcium, it seems unlikely the in vitro estradiol concentration was insufficient. Alternatively, there may be a critical period during which estradiol must be present to enhance the magnitude of the GABAA-mediated calcium transients. Regardless, from these data we can conclude that the ability of estradiol to enhance the excitatory response to GABAA receptor activation is conserved across rodents and nonhuman primates.
The in utero estradiol treatment-induced increase in the magnitude of muscimol-induced calcium transients is likely due to an increase in the density of cells displaying pNKCC1 immunoreactivity, also reflected as an increase in the protein levels of pNKCC1 detected by Western blot in neurons cultured from estradiol-treated fetuses. The relative levels of the chloride cotransporters KCC2 and NKCC1 are critical components in the control of the intracellular chloride concentration, which thereby regulates the response to GABAA receptor activation. In the newborn rat hippocampus, NKCC1 (Na+K+2Cl− cotransporter promoting chloride entry into the cell) levels are high, whereas KCC2 (K+Cl− cotransporter promoting chloride extrusion) expression is low. It is during this period that GABAA receptor activation leads to chloride efflux and membrane depolarization. By the second postnatal week, the pattern reverses such that KCC2 expression is elevated, and NKCC1 protein levels are decreased (22,41,42,43,49). Consistent with the abundance of the two chloride cotransporters during the second postnatal week, GABAA receptor activation in rat hippocampal neurons shifts from inducing membrane depolarization to membrane hyperpolarization. The estradiol-mediated enhancement in pNKCC1 immunoreactivity and pNKCC1 protein levels observed in the present study may be indicative of the enhanced sensitivity of the cells to GABAA receptor-mediated excitation, thereby increasing the number of cells that respond to muscimol, as observed in frontal cortex neurons, along with an enhanced magnitude of calcium transients.
An unexpected observation from the current studies was the difference in estradiol content in hippocampal vs. cortical cultures after acute administration. The consistently higher levels found in medium from hippocampal neuronal cultures after the same dose of estradiol suggests a slower rate of metabolism, which could prolong or enhance the effect of the steroid during development.
In the rodent, brain sexual differentiation is largely mediated by local neuronal aromatization of testicularly derived androgens to estrogens, and thus, estradiol is considered the dominant masculinizing hormone that mediates the establishment of sex differences in brain and behavior (see Ref. 33 for review). In primates, however, there is little evidence that estradiol exerts a masculinizing effect on the brain and instead direct actions of androgens determine sex differences in brain and behavior (see Ref. 30 for review). This conclusion is supported both empirically in nonhuman primate studies and indirectly by naturally occurring variations in humans with null mutations in the androgen receptor or estrogen receptor-α genes (see Ref. 57 for review). Moreover, the daughters of mothers treated with diethylstilbestrol during pregnancy show essentially normal childhood play and adult gender roles (58). The fact that estradiol does not appear to determine sex differences in the primate brain does not mean that it is not important to primate brain development. The steroid binding globulin, α-fetoprotein, which binds estradiol with high affinity in rodents and thereby sequesters maternal estradiol in the fetal blood stream and deprives the brain (59), does not have the same high affinity for estradiol in primates (32). Thus, it can be assumed that the developing primate brain is exposed to high levels of this potent steroid. The data presented here indicate that estradiol administration in utero over a prolonged period of time enhanced GABA and glutamate-mediated calcium responses in cultured fetal baboon hippocampal and frontal cortex neurons, consistent with a generalized increase in excitation. The survival of individual neurons and the formation of synaptic circuits are hugely impacted by the relative excitation experienced by specific cells during critical periods of development. The excitation conferred by depolarizing GABA is emerging as a critical determinant of neuronal maturation in both the developing and adult brain (60). Variables that impact on the magnitude, frequency, or duration of depolarizing GABA can be expected to have important influence on brain development. Estradiol is clearly one of those variables because it exerts potent enhancing effects on both depolarizing GABA and excitatory glutamate in the primate brain. The future challenge is to determine the functional impact of this modulation in the developing primate brain.
Acknowledgments
We thank Jesse Alt for his expert assistance with culturing hippocampal and cortical neurons.
Footnotes
This work was supported by National Institutes of Health Grant MH 52716 (to M.M.M.), National Institutes of Health Grant HD 13294 (to E.D.A.), and National Institute of Mental Health Grant MH 68347 (to J.L.N.).
Disclosure Statement: J.L.N., G.W.A., E.D.A., and M.M.M. have nothing to declare.
First Published Online August 14, 2008
Abbreviations: DIV, Days in vitro; DMSO, dimethylsulfoxide; GABA, γ-aminobutyric acid; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; HBSS+, Hanks’ balanced salt solution; KCC, K+-Cl−; NKCC, Na+-K+-2Cl−; pNKCC1, phosphorylated state of NKCC1; PSS, physiological salt solution; TBS, Tris-buffered saline.
References
- Michel AE, Garey LJ 1984 The development of dendritic spines in the human visual cortex. Hum Neurobiol 3:223–227 [PubMed] [Google Scholar]
- Huttenlocher PR, de Courten C 1987 The development of synapses in striate cortex of man. Hum Neurobiol 6:1–9 [PubMed] [Google Scholar]
- Khazipov R, Esclapez M, Caillard O, Bernard C, Khalilov I, Tyzio R, Hirsch J, Dzhala V, Berger B, Ben-Ari Y 2001 Early development of neuronal activity in the primate hippocampus in utero. J Neurosci 21:9770–9781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher PR, de Courten C, Garey LJ, Van der Loos H 1982 Synaptogenesis in human visual cortex—evidence for synapse elimination during normal development. Neurosci Lett 33:247–252 [DOI] [PubMed] [Google Scholar]
- Rabinowicz T, de Courten-Myers GM, Petetot JM, Xi G, de los Reyes E 1996 Human cortex development: estimates of neuronal numbers indicate major loss late during gestation. J Neuropathol Exp Neurol 55:320–328 [PubMed] [Google Scholar]
- Shankle WR, Romney AK, Landing BH, Hara J 1998 Developmental patterns in the cytoarchitecture of the human cerebral cortex from birth to 6 years examined by correspondence analysis. Proc Natl Acad Sci USA 95:4023–4028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline HT, Debski EA, Constantine-Paton M 1987 N-methyl-d-aspartate receptor antagonist desegregates eye-specific stripes. Proc Natl Acad Sci USA 84:4342–4345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinschmidt A, Bear MF, Singer W 1987 Blockade of “NMDA” receptors disrupts experience-dependent plasticity of kitten striate cortex. Science 238:355–358 [DOI] [PubMed] [Google Scholar]
- Miller KD, Chapman B, Stryker MP 1989 Visual responses in adult cat visual cortex depend on N-methyl-d-aspartate receptors. Proc Natl Acad Sci USA 86:5183–5187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y 2002 Excitatory actions of GABA during development: the nature of the nurture. Nat Rev Neurosci 3:728–739 [DOI] [PubMed] [Google Scholar]
- Owens DF, Kriegstein AR 2002 Is there more to GABA than synaptic inhibition? Nat Rev Neurosci 3:715–727 [DOI] [PubMed] [Google Scholar]
- Gulledge AT, Stuart GJ 2003 Excitatory actions of GABA in the cortex. Neuron 37:299–309 [DOI] [PubMed] [Google Scholar]
- Stein V, Nicoll RA 2003 GABA generates excitement. Neuron 37:375–378 [DOI] [PubMed] [Google Scholar]
- Represa A, Ben-Ari Y 2005 Trophic actions of GABA on neuronal development. Trends Neurosci 28:278–283 [DOI] [PubMed] [Google Scholar]
- Cherubini E, Gaiarsa JL, Ben-Ari Y 1991 GABA: an excitatory transmitter in early postnatal life. Trends Neurosci 14:515–519 [DOI] [PubMed] [Google Scholar]
- Obrietan K, van den Pol AN 1995 GABA neurotransmission in the hypothalamus: developmental reversal from Ca2+ elevating to depressing. J Neurosci 15:5065–5077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens DF, Boyce LH, Davis MB, Kriegstein AR 1996 Excitatory GABA responses in embryonic and neonatal cortical slices demonstrated by gramicidin perforated-patch recordings and calcium imaging. J Neurosci 16:6414–6423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinekugel X, Khalilov I, McLean H, Caillard O, Gaiarsa JL, Ben-Ari Y, Khazipov R 1999 GABA is the principal fast-acting excitatory transmitter in the neonatal brain. Adv Neurol 79:189–201 [PubMed] [Google Scholar]
- Ben-Ari Y, Tseeb V, Raggozzino D, Khazipov R, Gaiarsa JL 1994 γ-Aminobutyric acid (GABA): a fast excitatory transmitter which may regulate the development of hippocampal neurones in early postnatal life. Prog Brain Res 102:261–273 [DOI] [PubMed] [Google Scholar]
- Obata K 1997 Excitatory and trophic action of GABA and related substances in newborn mice and organotypic cerebellar culture. Dev Neurosci 19:117–119 [DOI] [PubMed] [Google Scholar]
- Fiszman ML, Schousboe A 2004 Role of calcium and kinases on the neurotrophic effect induced by γ-aminobutyric acid. J Neurosci Res 76:435–441 [DOI] [PubMed] [Google Scholar]
- Dzhala VI, Talos DM, Sdrulla DA, Brumback AC, Mathews GC, Benke TA, Delpire E, Jensen FE, Staley KJ 2005 NKCC1 transporter facilitates seizures in the developing brain. Nat Med 11:1205–1213 [DOI] [PubMed] [Google Scholar]
- Resko JA, Malley A, Begley D, Hess DL 1973 Radioimmunoassay of testosterone during fetal development of the rhesus monkey. Endocrinology 93:156–161 [DOI] [PubMed] [Google Scholar]
- Resko JA, Pleom JG, Stadelman HL 1975 Estrogens in fetal and maternal plasma of the rhesus monkey. Endocrinology 97:425–430 [DOI] [PubMed] [Google Scholar]
- Forest MG 1979 Plasma androgens (testosterone and 4-androstenedione) and 17-hydroxyprogesterone in the neonatal, prepubertal and peripubertal periods in the human and the rat: differences between species. J Steroid Biochem 11:543–548 [DOI] [PubMed] [Google Scholar]
- Corbier P, Edwards DA, Roffi J 1992 The neonatal testosterone surge: a comparative study. Arch Int Physiol Biochim Biophys 100:127–131 [DOI] [PubMed] [Google Scholar]
- MacLusky NJ, Naftolin F 1981 Sexual differentiation of the central nervous system. Science 211:1294–1302 [DOI] [PubMed] [Google Scholar]
- McEwen BS 1981 Neural gonadal steroid actions. Science 211:1303–1311 [DOI] [PubMed] [Google Scholar]
- Arnold AP, Gorski RA 1984 Gonadal steroid induction of structural sex differences in the central nervous system. Annu Rev Neurosci 7:413–442 [DOI] [PubMed] [Google Scholar]
- Wallen K 2005 Hormonal influences on sexually differentiated behavior in nonhuman primates. Front Neuroendocrinol 26:7–26 [DOI] [PubMed] [Google Scholar]
- Herman RA, Wallen K 2007 Cognitive performance in rhesus monkeys varies by sex and prenatal androgen exposure. Horm Behav 51:496–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz SK, Soloff MS 1974 The lack of estrogen binding by human α-fetoprotein. J Clin Endocrinol Metab 39:589–591 [DOI] [PubMed] [Google Scholar]
- McCarthy MM 2008 Estradiol and the developing brain. Physiol Rev 88:91–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrot-Sinal TS, Davis AM, Gregerson KA, Kao JP, McCarthy MM 2001 Estradiol enhances excitatory γ-aminobutyric [corrected] acid-mediated calcium signaling in neonatal hypothalamic neurons. Endocrinology 142:2238–2243 [DOI] [PubMed] [Google Scholar]
- Nunez JL, Bambrick LL, Krueger BK, McCarthy MM 2005 Prolongation and enhancement of γ-aminobutyric acid receptor mediated excitation by chronic treatment with estradiol in developing rat hippocampal neurons. Eur J Neurosci 21:3251–3261 [DOI] [PubMed] [Google Scholar]
- Nunez JL, McCarthy MM 2007 Evidence for an extended duration of GABA-mediated excitation in the developing male versus female hippocampus. Dev Neurobiol 67:1879–1890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilton GD, Nunez JL, Bambrick L, Thompson SM, McCarthy MM 2006 Glutamate-mediated excitotoxicity in neonatal hippocampal neurons is mediated by mGluR and prevented by estradiol. Eur J Neurosci 24:3008–3016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht ED, Aberdeen GW, Pepe GJ 2005 Estrogen elicits cortical zone-specific effects on development of the primate fetal adrenal gland. Endocrinology 146:1737–1744 [DOI] [PubMed] [Google Scholar]
- Albrecht ED, Bonagura T, Burleigh D, Enders AC, Aberdeen GW, Pepe GJ 2006 Suppression of extravillous trophoblast invasion of uterine spiral arteries by estrogen during baboon pregnancy. Placenta 27:483–490 [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY 1985 A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260:3440–3450 [PubMed] [Google Scholar]
- Delpire E 2000 Cation-chloride cotransporters in neuronal communication. News Physiol Sci 15:309–312 [DOI] [PubMed] [Google Scholar]
- Lu J, Karadsheh M, Delpire E 1999 Developmental regulation of the neuronal-specific isoform of K-Cl cotransporter KCC2 in postnatal rat brains. J Neurobiol 39:558–568 [PubMed] [Google Scholar]
- Plotkin MD, Snyder EY, Hebert SC, Delpire E 1997 Expression of the Na-K-2Cl cotransporter is developmentally regulated in postnatal rat brains: a possible mechanism underlying GABA’s excitatory role in immature brain. J Neurobiol 33:781–795 [DOI] [PubMed] [Google Scholar]
- Schwartz-Bloom RD, Sah R 2001 γ-Aminobutyric acid(A) neurotransmission and cerebral ischemia. J Neurochem 77:353–371 [DOI] [PubMed] [Google Scholar]
- Payne JA, Rivera C, Voipio J, Kaila K 2003 Cation-chloride cotransporters in neuronal communication, development and trauma. Trends Neurosci 26:199–206 [DOI] [PubMed] [Google Scholar]
- Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, Lamsa K, Pirvola U, Saarma M, Kaila K 1999 The K+/Cl− co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature 397:251–255 [DOI] [PubMed] [Google Scholar]
- Rivera C, Voipio J, Thomas-Crusells J, Li H, Emri Z, Sipila S, Payne JA, Minichiello L, Saarma M, Kaila K 2004 Mechanism of activity-dependent downregulation of the neuron-specific K-Cl cotransporter KCC2. J Neurosci 24:4683–4691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schomberg SL, Bauer J, Kintner DB, Su G, Flemmer A, Forbush B, Sun D 2003 Cross talk between the GABA(A) receptor and the Na-K-Cl cotransporter is mediated by intracellular Cl. J Neurophysiol 89:159–167 [DOI] [PubMed] [Google Scholar]
- Stein V, Hermans-Borgmeyer I, Jentsch TJ, Hubner CA 2004 Expression of the KCl cotransporter KCC2 parallels neuronal maturation and the emergence of low intracellular chloride. J Comp Neurol 468:57–64 [DOI] [PubMed] [Google Scholar]
- Sun D, Murali SG 1999 Na+-K+-2Cl− cotransporter in immature cortical neurons: a role in intracellular Cl− regulation. J Neurophysiol 81:1939–1948 [DOI] [PubMed] [Google Scholar]
- Warne GL, Faiman C, Reyes FI, Winter JS 1977 Studies on human sexual development. V. Concentrations of testosterone, 17-hydroxyprogesterone and progesterone in human amniotic fluid throughout gestation. J Clin Endocrinol Metab 44:934–938 [DOI] [PubMed] [Google Scholar]
- Pomerantz SM, Fox TO, Sholl SA, Vito CC, Goy RW 1985 Androgen and estrogen receptors in fetal rhesus monkey brain and anterior pituitary. Endocrinology 116:83–89 [DOI] [PubMed] [Google Scholar]
- Sholl SA, Goy RW, Kim KL 1989 5α-Reductase, aromatase, and androgen receptor levels in the monkey brain during fetal development. Endocrinology 124:627–634 [DOI] [PubMed] [Google Scholar]
- Sholl SA, Kim KL 1990 Aromatase, 5-α-reductase, and androgen receptor levels in the fetal monkey brain during early development. Neuroendocrinology 52:94–98 [DOI] [PubMed] [Google Scholar]
- Lipton A, Demers LM, Harvey HA, Kambic KB, Grossberg H, Brady C, Adlercreutz H, Trunet PF, Santen RJ 1995 Letrozole (CGS 20267). A phase I study of a new potent oral aromatase inhibitor of breast cancer. Cancer 75:2132–2138 [DOI] [PubMed] [Google Scholar]
- Buzdar AU 2002 New generation aromatase inhibitors—from the advanced to adjuvant setting. Breat Cancer Res Treat 75:13–17 [DOI] [PubMed] [Google Scholar]
- McCarthy MM, De Vries GJ, Forger NG 2008 Sexual differentiation of the brain: mode, mechanisms and meaning. In: Pfaff D, Arnold A, Etgen A, Fahrbach S, Rubin R eds. Hormones, brain and behavior. 2nd ed. San Diego: Academic Press [Google Scholar]
- Lish JD, Meyer-Bahlburg HF, Ehrhardt AA, Travis BG, Verdiano NP 1992 Prenatal exposure to diethylstilbestrol (DES): childhood play behavior and adult gender-role behavior in women. Arch Sex Behav 21:423–441 [DOI] [PubMed] [Google Scholar]
- Bakker J, De Mees C, Douhard Q, Balthazart J, Gabant P, Szpirer J, Szpirer C 2006 α-Fetoprotein protects the developing female mouse brain from masculinization and defeminization by estrogens. Nat Neurosci 9:220–226 [DOI] [PubMed] [Google Scholar]
- Le Van Quyen M, Khalilov I, Ben-Ari Y 2006 The dark side of high-frequency oscillations in the developing brain. Trends Neurosci 29:419–427 [DOI] [PubMed] [Google Scholar]