Abstract
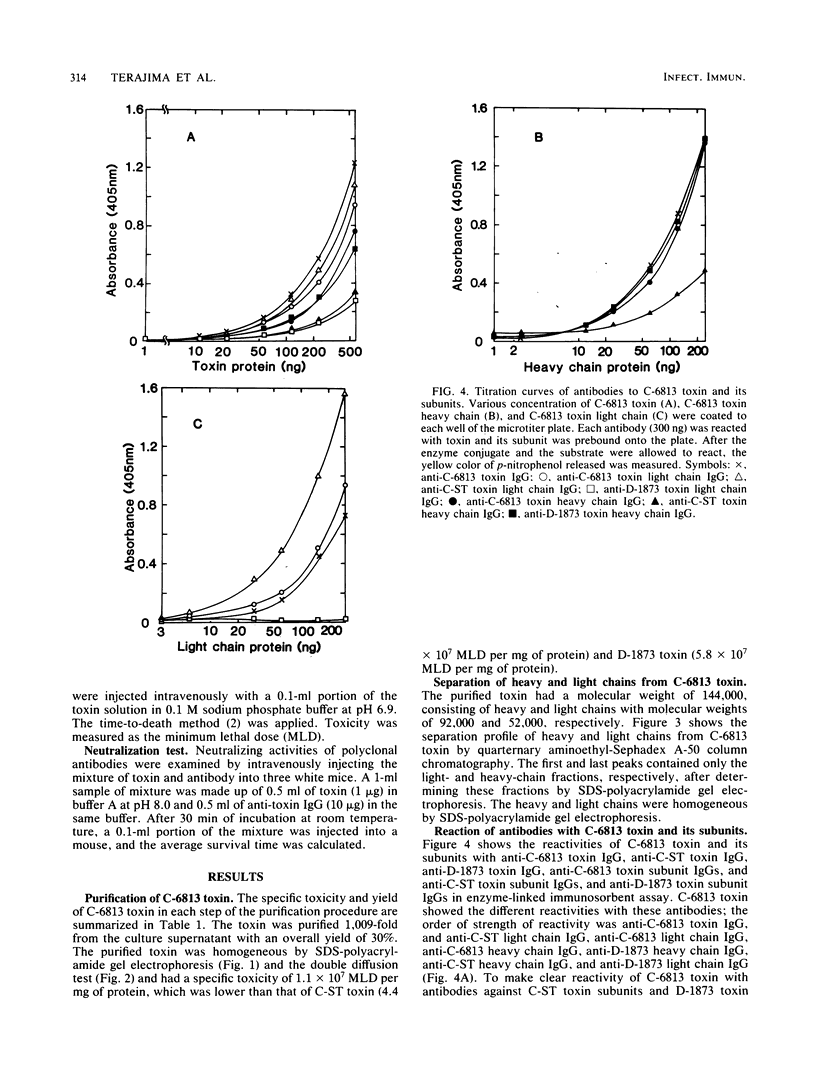
The toxin produced by Clostridium botulinum type C 6813 (C-6813) was purified 1,009-fold from the culture supernatant in an overall yield of 30%. The specific toxicity was 1.1 X 10(7) mouse minimum lethal doses per mg of protein. The toxin had a molecular weight of 144,000, composed of the light and heavy chains with molecular weights of 52,000 and 92,000, respectively, linked by one or two disulfide bond(s). The purified C-6813 toxin heavy and light chains reacted strongly with anti-type D heavy chain immunoglobulin G and anti-type C1 light chain immunoglobulin G, respectively. The amino acid compositions of C-6813 toxin heavy and light chains were more similar to those of type D heavy chain and type C1 light chain than to those of type C1 heavy chain and type D light chain, respectively. These results suggest that in the toxin produced by the type C strain at least two subtypes exist.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baillie R. D., Horowitz P. M. Denaturation-induced disulfide formation in the enzyme rhodanese. Biochim Biophys Acta. 1976 Apr 8;429(2):383–390. doi: 10.1016/0005-2744(76)90286-2. [DOI] [PubMed] [Google Scholar]
- Boroff D. A., Fleck U. Statistical analysis of a rapid in vivo method for the titration of the toxin of Clostridium botulinum. J Bacteriol. 1966 Nov;92(5):1580–1581. doi: 10.1128/jb.92.5.1580-1581.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Goodwin T. W., Morton R. A. The spectrophotometric determination of tyrosine and tryptophan in proteins. Biochem J. 1946;40(5-6):628–632. doi: 10.1042/bj0400628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIRS C. H. The oxidation of ribonuclease with performic acid. J Biol Chem. 1956 Apr;219(2):611–621. [PubMed] [Google Scholar]
- Inoue K., Iida H. Phage-conversion of toxigenicity in Clostridium botulinum types C and D. Jpn J Med Sci Biol. 1971 Feb;24(1):53–56. [PubMed] [Google Scholar]
- Iwasaki M., Ohishi I., Sakaguchi G. Evidence that botulinum C2 toxin has two dissimilar components. Infect Immun. 1980 Aug;29(2):390–394. doi: 10.1128/iai.29.2.390-394.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen B. C. The toxic antigenic factors produced by Clostridium botulinum types C and D. Onderstepoort J Vet Res. 1971 Jun;38(2):93–98. [PubMed] [Google Scholar]
- Jensen W. I., Duncan R. M. The susceptibility of the mallard duck (Anas platyrhynchos) to Clostridium botulinum C2 toxin. Jpn J Med Sci Biol. 1980 Apr;33(2):81–86. doi: 10.7883/yoken1952.33.81. [DOI] [PubMed] [Google Scholar]
- Kahn R., Rubin R. W. Quantitation of submicrogram amounts of protein using coomassie brilliant blue R on sodium dodecyl sulfate-polyacrylamide slab-gels. Anal Biochem. 1975 Jul;67(1):347–352. doi: 10.1016/0003-2697(75)90305-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Murayama S., Syuto B., Oguma K., Iida H., Kubo S. Comparison of Clostridium botulinum toxins type D and C1 in molecular property, antigenicity and binding ability to rat-brain synaptosomes. Eur J Biochem. 1984 Aug 1;142(3):487–492. doi: 10.1111/j.1432-1033.1984.tb08312.x. [DOI] [PubMed] [Google Scholar]
- Ochanda J. O., Syuto B., Oguma K., Iida H., Kubo S. Comparison of antigenicity of toxins produced by Clostridium botulinum type C and D strains. Appl Environ Microbiol. 1984 Jun;47(6):1319–1322. doi: 10.1128/aem.47.6.1319-1322.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oguma K., Agui T., Syuto B., Kimura K., Iida H., Kubo S. Four different monoclonal antibodies against type C1 toxin of Clostridium botulinum. Infect Immun. 1982 Oct;38(1):14–20. doi: 10.1128/iai.38.1.14-20.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oguma K., Murayama S., Syuto B., Iida H., Kubo S. Analysis of antigenicity of Clostridium botulinum type C1 and D toxins by polyclonal and monoclonal antibodies. Infect Immun. 1984 Feb;43(2):584–588. doi: 10.1128/iai.43.2.584-588.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oguma K., Syuto B., Agui T., Iida H., Kubo S. Homogeneity and heterogeneity of toxins produced by Clostridium botulinum type C and D strains. Infect Immun. 1981 Nov;34(2):382–388. doi: 10.1128/iai.34.2.382-388.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oguma K., Syuto B., Iida H., Kubo S. Antigenic similarity of toxins produced by Clostridium botulinum type C and D strains. Infect Immun. 1980 Dec;30(3):656–660. doi: 10.1128/iai.30.3.656-660.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohishi I., Iwasaki M., Sakaguchi G. Purification and characterization of two components of botulinum C2 toxin. Infect Immun. 1980 Dec;30(3):668–673. doi: 10.1128/iai.30.3.668-673.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohishi I., Iwasaki M., Sakaguchi G. Vascular permeability activity of botulinum C2 toxin elicited by cooperation of two dissimilar protein components. Infect Immun. 1981 Mar;31(3):890–895. doi: 10.1128/iai.31.3.890-895.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama H. Clostridium botulinum neurotoxin. Microbiol Rev. 1980 Sep;44(3):419–448. doi: 10.1128/mr.44.3.419-448.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syuto B., Kubo S. Isolation and molecular size of Clostridium botulinum type C toxin. Appl Environ Microbiol. 1977 Feb;33(2):400–405. doi: 10.1128/aem.33.2.400-405.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syuto B., Kubo S. Separation and characterization of heavy and light chains from Clostridium botulinum type C toxin and their reconstitution. J Biol Chem. 1981 Apr 25;256(8):3712–3717. [PubMed] [Google Scholar]
- Weber K., Pringle J. R., Osborn M. Measurement of molecular weights by electrophoresis on SDS-acrylamide gel. Methods Enzymol. 1972;26:3–27. doi: 10.1016/s0076-6879(72)26003-7. [DOI] [PubMed] [Google Scholar]




