Abstract
The composition of the β-cell exocytic machinery is very similar to that of neuronal synapses, and the developmental pathway of β-cells and neurons substantially overlap. β-Cells secrete γ-aminobutyric acid and express proteins that, in the brain, are specific markers of inhibitory synapses. Recently, neuronal coculture experiments have identified three families of synaptic cell-surface molecules (neurexins, neuroligins, and SynCAM) that drive synapse formation in vitro and that control the differentiation of nascent synapses into either excitatory or inhibitory fully mature nerve terminals. The inhibitory synapse-like character of the β-cells led us to hypothesize that members of these families of synapse-inducing adhesion molecules would be expressed in β-cells and that the pattern of expression would resemble that associated with neuronal inhibitory synaptogenesis. Here, we describe β-cell expression of the neuroligins, neurexins, and SynCAM, and show that neuroligin expression affects insulin secretion in INS-1 β-cells and rat islet cells. Our findings demonstrate that neuroligins and neurexins are expressed outside the central nervous system and help confer an inhibitory synaptic-like phenotype onto the β-cell surface. Analogous to their role in synaptic neurotransmission, neurexin-neuroligin interactions may play a role in the formation of the submembrane insulin secretory apparatus.
INSULIN-SECRETING, pancreatic islet β-cells appear to have evolved from a neuronal precursor, and to have retained exocytic mechanisms that, in neurons, are important for neurotransmitter release. Evidence linking the β-cells to a neuronal ancestor includes the finding that in some nonvertebrate species, such as Drosophila, insulin is exclusively released from neurons in the brain, and also the observation that neural progenitor cells can be differentiated into glucose-responsive, insulin-secreting cells (1,2). β-Cells also express many of the scaffolding and synaptic vesicle proteins important for neurotransmitter secretion, and these seem to be key components of the insulin secretory machinery (3,4,5,6,7,8,9). Consistent with their resemblance to neurons, β-cells express high levels of the major inhibitory neurotransmitter γ-aminobutyric acid (GABA) and all of the machinery necessary for GABA synthesis, secretion, and signaling (10,11,12). The β-cell GABAergic signaling machinery most likely functions to regulate islet hormone secretion via an autocrine or paracrine signaling mechanism (12).
In the central nervous system (CNS), the differentiation and maturation of synapses to either a glutamatergic (excitatory) or GABAergic (inhibitory) phenotype are regulated, at least in part, by the synaptic adhesion molecules neurexin and neuroligin (13). The neurexins are encoded by three distinct genes, are subject to a high degree of alternative splicing, and each can be transcribed from two promoters, generating longer α and shorter β-forms (14). Transcription of α and β-variants of each of the three neurexin genes results in the expression of six different neurexin transcripts: 1α, 1β, 2α, etc. Their expression is restricted to axons (presynaptic), and they interact across the synaptic cleft with the neuroligins, which are localized to dendrites (postsynaptic). The neuroligins are encoded by five genes in humans and four in rodents, and are also subject to alternative splicing (15,16). SynCAM 1, a member of a family of adhesion molecules encoded by four distinct genes, also helps drive the functional differentiation of synapses (17).
Coculture experiments using nonneuronal cell lines expressing members of the neurexin and neuroligin gene families revealed that neurexins specifically induce postsynaptic differentiation, and neuroligins specifically induce presynaptic differentiation at points of contact between the nonneuronal cells expressing these proteins and hippocampal neurons (18,19,20,21). SynCAM induces both presynaptic and postsynaptic differentiation in contacting neurons (22). Neuroligin-1 and specific β-neurexin splice variants are important for the formation of glutamatergic synapses, whereas the α-neurexins and neuroligin-2 drive the formation of GABAergic synapses (23,24). These results are in accord with differential expression patterns of neuroligins-1 and -2, where neuroligin-1 is detected primarily at excitatory synapses (25) and neuroligin-2 exclusively at inhibitory synapses (26), and with overexpression studies demonstrating that neuroligin-1 exclusively affects excitatory neurotransmission, and neuroligin-2 exclusively affects inhibitory neurotransmission (23). More recent in vivo studies with mice lacking the neuroligin genes confirm that theses genes are important for determining whether a maturing synaptic connection will be excitatory or inhibitory (23).
Previously, we described the expression of the vesicular GABA transporter in islet β-cells (11,27). Because β-cells express the protein machinery necessary for GABAergic neurotransmission and because neuroligin-2 is important for driving the functional differentiation of GABAergic (inhibitory) synapses, we hypothesized that neuroligin-2 and the interacting neurexins are expressed in β-cells. There, they may play a role in the maturation of autocrine or paracrine GABAergic signaling mechanisms. Furthermore, because neuroligin-neurexin interactions induce assembly of the synaptic exocytic machinery in tissue culture and are necessary for its proper functioning in vivo (18,21,28,29), we postulated that neurexins and neuroligins may be important for the normal maturation and/or functioning of the highly similar insulin secretory apparatus.
Here, we demonstrate expression of neuroligin and neurexin family members in β-cells, as well as expression of intracellular, inhibitory synapse-associated neuroligin and neurexin binding partners, and provide evidence that neuroligin family members play a role in the insulin secretory mechanism. Our findings suggest that the functional maturation of the β-cell may share common mechanisms with that of the inhibitory synapse.
Materials and Methods
Antibodies
The following primary antibodies were obtained commercially: mouse anti-gephyrin, mouse anti-postsynaptic density protein 95 (PSD-95), and mouse anti-calcium/calmodulin-dependent serine protein kinase (CASK) (BD Biosciences, San Diego, CA); goat anti-neurexin 1 (P-15) and goat anti-neuroligin 2 (D-15) (Santa Cruz Biotechnology, Inc., Santa Cruz, CA); rabbit anti-neuroligin-3 (Synaptic Systems, Goettingen, Germany); mouse anti-insulin, mouse anti-glucagon, and mouse anti-syntaxin-1 (Sigma-Aldrich Corp., St. Louis, MO); and chicken anti-SynCAM (Medical & Biological Laboratories Co., Woburn, MA). Rabbit antibodies against neuroligin-2 were made by Alpha Diagnostics International Inc. (San Antonio, TX) after injection of the extracellular domain, which was generated in human embryonic kidney 293 (HEK293) cells as previously described (30). Antiserum was validated for affinity to neuroligin-2 by ELISA and Western blotting.
Plasmid constructs
cDNAs encoding rat neuroligin-1, -2, and -3 and human neuroligin-4 were subcloned into a vector encoding the FLAG epitope tag (Sigma-Aldrich) downstream of the amino-terminal cleaved signal sequence of preprotrypsin (31,32). Neuroligin-4 cDNA was a kind gift of Dr. Sergio Gloor (Swiss Federal Institute of Technology, Zurich, Switzerland). A previously described splice variant of acetylcholinesterase that lacks a transmembrane domain but that is anchored to the membrane via a glycosylphosphatidylinositol linkage was used in transfection experiments (32).
To generate reference templates for real-time quantitative PCR (qPCR) analysis, regions of the neurexin-2 and neurexin-3 genes flanking the real-time PCR amplicons were amplified by PCR of rat brain cDNA. The following primers were used: neurexin-2 (5′-gcactgttggggtgattttt-3′ and 5′-tggctgttgaagatggtcag-3′) and neurexin-3 (5′-agcggtggtctcatcctcta-3′ and 5′-tcgttgacaggggttctctc-3′). These PCR products were ligated into the TOPO TA vector (Invitrogen Corp., Carlsbad, CA), transformed into TOP10 cells (Invitrogen), isolated, and then quantified.
Quantitative RT-PCR
Total RNA was isolated from INS-1 cells and rat brain using TRIzol (Invitrogen), and from human and rat islets using the GenElute Total Mammalian RNA miniprep kit (Sigma-Aldrich). It was then treated with Turbo deoxyribonuclease (Ambion, Inc., Austin, TX) to remove any contaminating genomic DNA. RNA was reverse transcribed with Superscript II reverse transcriptase (Invitrogen) and random hexamers. Using gene-specific primers and TaqMan probes, qPCR analysis was performed with the cDNA product from 50 ng RNA per well on an ABI Prism 7700 (Applied Biosystems, Foster City, CA). Primers and TaqMan probes are listed in supplemental Tables 1–4, which are published as supplemental data on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org. Each sample was analyzed in duplicate along with a corresponding sample to which no reverse transcriptase was added (no reverse transcriptase control). To generate standard curves, real-time PCR analysis was performed with 101 to 106 copies of plasmid DNA encoding either the full-length neuroligin/neurexin genes or the portions of the neurexin genes flanking the qPCR amplicons. The standard curves allowed for calculation of the number of copies of each gene present in the cDNA samples. For analysis of RNA interference studies, neuroligin gene expression was normalized to the expression of 18s rRNA. RNA knockdown was analyzed using the formula: 2−ΔΔCT where ΔΔCT is calculated as follows: [(CT neuroligin-2 in cells treated with siRNA - CT 18s rRNA in same cells)] - [CT neuroligin-2 in cells treated with control siRNA - CT 18s rRNA in same cells)] (68).
Cell culture and transfection
INS-1 cells were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum, 50 μm β-mercaptoethanol, 1 mm sodium pyruvate, 2 mm l-glutamine, 100 U/ml penicillin, and 100 mg/ml streptomycin in a humidified 37 C incubator with 5% CO2. NIT-1 cells (69) were cultured in DMEM supplemented with 10% fetal bovine serum, 2 mm l-glutamine, 100 U/ml penicillin, and 100 mg/ml streptomycin. Rat islets were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum, 2 mm l-glutamine, 100 U/ml penicillin, and 100 mg/ml streptomycin. Twenty-four hours before transfection, rat islets were dispersed using 0.05% trypsin-EDTA and plated onto 24-well plates coated with poly-d-lysine.
For transfections, INS-1 cells were plated onto 12-well plates and allowed to grow to 50% confluency in antibiotic-free media. The cells were then transfected with the gene of interest using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s protocol. Cells were incubated in the transfection cocktail for 48 h before conducting insulin secretion experiments and confirmation of gene expression by Western blot analysis.
RNA interference
A pool of small interfering RNA (siRNA) duplexes targeted to neuroligin-2 was purchased from Dharmacon Research (Thermo Fisher Scientific Inc., Waltham, MA). The siRNA duplexes lacked significant homology to other rat genes as confirmed by a BLAST search. The sequences of each of the duplexes are listed in supplemental Table 5. The pool of siRNA duplexes was transfected into INS-1 cells or dissociated rat islets with Lipofectamine 2000 according to the manufacturer’s protocol. As a control, a pool of nontargeting siRNA duplexes was used. Insulin secretion assays were performed 48 h after transfection, and knockdown was analyzed via qPCR analysis. For rat islet experiments, 12 wells were treated with nontargeting siRNA, and 12 wells were treated with neuroligin-2 siRNA. Half of each of these samples was used to isolate RNA for analysis of knockdown, and half was used to isolate protein for analysis of total insulin content.
Insulin secretion assays
Cells were washed twice with Hanks’ balanced salt solution and then incubated in Krebs-Ringer bicarbonate buffer containing 0.2% BSA and 2.75 mm glucose. After 30 min incubation, this solution was removed and replaced with Krebs-Ringer containing 2.5 mm glucose for 1 h. The solution was collected and replaced with Krebs-Ringer containing 15 mm glucose for INS-1 cells or 25 mm glucose for rat islets for an additional hour. The solution was collected, and insulin content was measured using a rat insulin RIA (Linco/Millipore, Billerica, MA).
Immunofluorescence
Cells were seeded on four-well chamber slides (BD Biosciences) coated with poly-d-lysine and allowed to grow to confluency. Before immunostaining, they were fixed in a 4% paraformaldehyde solution for 10 min, washed twice in 1× PBS, and permeabilized with 0.2% Triton X-100 in PBS for 20 min.
Sections (16-μm) of frozen rat pancreas were prepared by the University of California, San Diego histology core. Before staining, frozen sections were fixed in a 1:1 acetone-methanol solution at −20 C for 20 min. For paraffin sections, the tissue was fixed for 24 h in Pen-Fix (Thermo Fisher Scientific), embedded in paraffin, and sections were cut at 6 μm. Immunostaining experiments were performed as previously described (11,33). Images were captured using either a Nikon-TE-200 deconvolution microscope (Nikon Corp., Tokyo, Japan) or an Olympus FV1000 spectral confocal microscope (Olympus, Hamburg, Germany) configured with an Argon/Krypton laser (488- and 568-nm excitation lines) and either 40 or 60× oil immersion lenses. Controls included tissue stained with either secondary antibody and no primary antibody or preimmune serum followed by secondary antibody.
Islet isolation
Rat islets were provided by the University of Washington Diabetes and Endocrinology Research Center Islet Cell and Functional Analysis Core (11). Briefly, islets were isolated from the pancreata of Sprague Dawley rats by digestion with Liberase (Roche Molecular Biochemicals, Indianapolis, IN) followed by Optiprep (Nycomed, Zurich, Switzerland) gradient purification as previously described (34). Isolated islets were handpicked in Hanks’ balanced salt solution (Invitrogen).
Western blot analysis
Cell extract was made by homogenizing the tissue or cells of interest in radioimmunoprecipitation assay buffer (PBS, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 45 μg/ml aprotinin, 100 μg/ml phenylmethylsulfonylfluoride, and 1 mm sodium orthovanadate). Cell extract was quantified using the Bio-Rad Dc Protein Assay (Bio-Rad Laboratories, Inc., Hercules, CA). Protein diluted 1:4 with reducing sample buffer was electrophoresed on either 4–12% Bis-Tris NuPage gel or 3–8% Tris-Acetate NuPage Gels (Invitrogen). The protein was then transferred to polyvinylidene fluoride membrane and analyzed by immunoblot analysis as previously described (11,27).
Statistical analysis
Data are presented as means ± se. Differences between quantitative data sets were analyzed by the t test. P < 0.05 was considered significant.
Results
Expression of neurexin and neuroligin transcripts in islets and β-cell lines at levels comparable to brain
Initial PCR studies revealed the expression of neuroligin-1, -2, and -3, and the neurexin family members 1α, 1β, 2α 2β, and 3β in human islets (supplemental Fig. 1A). Expression was also observed in the rat-derived INS-1 and mouse-derived NIT β-cell lines, and in the developing human pancreas (supplemental Fig. 1, B–D).
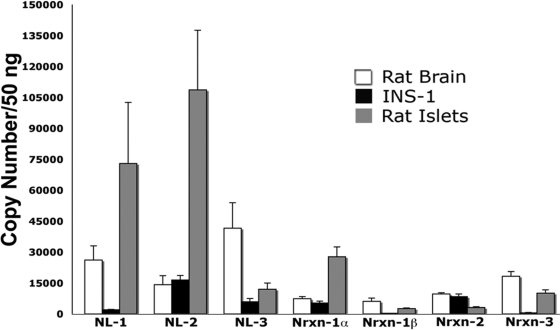
To compare the expression levels of individual neuroligin and neurexin family members in islets and to determine how these levels compare with those observed in the brain, absolute real-time qPCR analysis was performed using cDNA derived from rat islets, INS-1 cells, and rat brain. Rat islets were found to express high levels of neuroligin-1, neuroligin-2, neurexin-1α, and neurexin-3 (Fig. 1). Transcript copy numbers were comparable to or greater than those observed in the brain. Relatively lower levels of neuroligin-3, neurexin-1β, and neurexin-2 are detected in rat islets (Fig. 1). The total number of copies observed for neurexin-2 and neurexin-3 represents the sum of both the α and β-isoforms. The restricted pattern of expression observed in rat islets is distinct from that observed in the brain where neuroligin and neurexin family members are expressed at relatively similar levels. The pattern of expression in human islets is similar to that observed in rat islets (supplemental Fig. 2).
Figure 1.
Abundance of neuroligin and neurexin transcripts in INS-1 β-cells and rat islets. Absolute real-time qPCR analysis was used to compare the expression levels of individual neuroligin (NL) and neurexin (Nrxn) family members within rat-derived INS-1 β-cells, rat islets, and rat brain. Standard curves were generated using plasmids encoding either whole or partial neuroligin/neurexin sequences, and these were used to calculate the number of copies of each transcript per cDNA sample. Samples were prepared in parallel, though rat islet RNA was prepared separately because of the need to use an alternative method. Each cDNA sample represents an equal amount (50 ng) of input total RNA. In INS-1 cells, neuroligin-2 (16,489 ± 1,055 copies/50 ng RNA), neurexin-1α (5,296 ± 927 copies/50 ng RNA), and neurexin-2 (8,370 ± 1,302 copies/50 ng RNA) are expressed at the highest levels relative to other neuroligin and neurexin family members. (The neurexin-2 and -3 primer sets could not distinguish between the α and β-forms.) In rat islets, neuroligin-1 (72,914 ± 14,836 copies), neuroligin-2 (108,683 ± 14,413 copies), neurexin-1α (27,668 ± 2,417 copies), and neurexin-3 (10,115 ± 769 copies) are expressed at the highest levels relative to other neuroligin and neurexin family members. In rat brain, expression of neuroligins and neurexin mRNA is more evenly distributed. Values obtained were: neuroligin-1 (15,058 ± 3,987 copies); neuroligin-2 (9,260 ± 2888 copies); neuroligin-3 (26,156 ± 8,018 copies); neurexin-1α (4,412 ± 1,077 copies); neurexin-1β (3,464 ± 883 copies); neurexin-2 (6,451 ± 1,552 copies); and neurexin-3 (11,239 ± 2,727 copies). Equal loading of input RNA was verified by parallel qPCR analysis of 18s rRNA. 18s cycle threshold values (CT) in INS-1 cells and rat brain were consistently identical. Rat islet 18s cycle threshold values were higher (slightly less than two CT values), likely the result of differences in the technique used for RNA isolation. (n of 3 separately prepared RNA/cDNA samples per experiment).
In the rat β-cell line INS-1, neuroligin-2 was the most abundant transcript detected (Fig. 1). Its level of expression was 8-fold greater than neuroligin-1 and 3-fold greater than neuroligin-3. Of the neurexin family members, neurexin-1α and neurexin-2 were the most abundant transcripts detected. Both of these transcripts were expressed at levels that were at least 10-fold greater than neurexin-1β and neurexin-3. The levels of neuroligin-2, neurexin-1α, and neurexin-2 observed in INS-1 cells did not differ statistically from those observed in the brain (supplemental Fig. 3).
Expression of alternatively spliced neuroligin exons in human islets and INS-1 cells
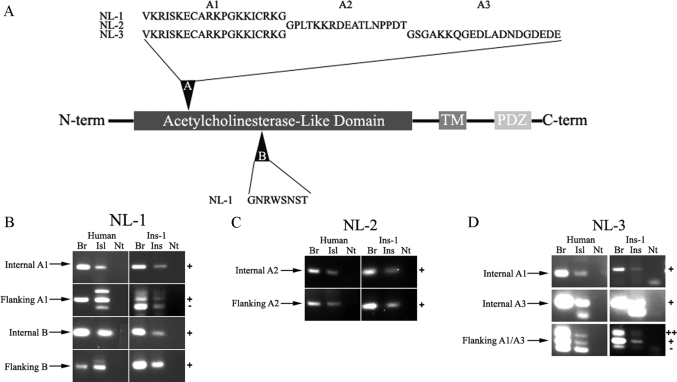
Alternative splicing within the extracellular domain occurs in all neuroligin family members (16,35). For neuroligin-1, two alternative exons, A and B, have been described (Fig. 2A) (36). The other neuroligin family members undergo alternative splicing at site A, but not site B (35). This alternative splicing helps to determine synapse type (excitatory or inhibitory) and to determine the neurexin isoforms to which neuroligin molecules can bind (16,37). To compare alternative splicing of neuroligin transcripts in β-cells to splicing in the brain, primers that either flank or sit within each of the potential inserts (supplemental Table 4) were used to amplify neuroligin regions from human islet and INS-1 cDNA. In parallel, cDNA samples prepared from rat and human brain RNA were also amplified.
Figure 2.
Characterization of neuroligin splice variants in pancreatic β-cells. A, Schematic representation of alternative splice inserts in neuroligin (NL) gene family members. Splice site A is present in all neuroligin family members, whereas splice site B is only present in neuroligin-1. Different alternative exons (A1–A3) can be included at site A. B–D, To determine which neuroligin splice variants are expressed in islets (Isl), primers were designed to either flank or sit on each of the known neuroligin splice inserts. PCR was performed using cDNA from either human islets or INS-1 (Ins) cells and from either human or rat brain (Br). Each of the known splice inserts in neuroligin-1 (B), neuroligin-2 (C), and neuroligin-3 (D) was detected in both human islets and INS-1 cells. Transcripts lacking inserts at splice site A in neuroligin-1 were detected in human islets, rat brain, and INS-1 cells, but not in human brain (B, row 2). Neuroligin-1 transcripts lacking inserts at splice site B and neuroligin-2 inserts lacking site A were not detected in the brain, INS-1 cells, or human islets (bottom two panels of B and C). Neuroligin-3 transcripts lacking an insert at splice site A were detected in all tissues tested (D, bottom two panels). −, Absence of insert; Nt, no template; ++, presence of both inserts; +, presence of insert.
Amplification using primers internal to the alternatively spliced exons yielded products of the expected size in both human islets and INS-1 cells for each of the alternatively spliced exons (Fig. 2, B–D). This indicates that neuroligin mRNA processing in the β-cells can yield the same pattern of alternative splicing as observed in the brain. Sequencing of neuroligin transcripts from human islet and INS-1 cells confirmed that the alternatively spliced exons were identical in islet and brain (data not shown).
In human brain, only primers flanking neuroligin-3 site A yielded a PCR product consistent with the absence of the alternatively spliced exon (Fig. 2D). The absence of PCR products lacking inserts at splice sites A and B for neuroligin-1 (Fig. 2B) and splice site A for neuroligin-2 (Fig. 2C) suggests that, in human brain, neuroligin-1 and -2 transcripts that contain the alternatively spliced exons predominate. In human islets, PCR products consistent with the absence of inserts at splice site A in neuroligin-1 and neuroligin-3 were detected (Fig. 2, B and D). PCR products lacking splice inserts at splice site B in neuroligin-1 and at splice site A in neuroligin-2 were not detected (Fig. 2, B and C). Findings concerning the presence or absence of splice variants are summarized in Table 1.
Table 1.
Neuroligin and neurexin splice variants detected
| Human
|
Rat
|
|||
|---|---|---|---|---|
| Brain | Islet | Brain | INS-1 β-cells | |
| NL-1, site A | [+] | [+, −] | +, − | +, − |
| NL-1, site B | + | + | + | + |
| NL-2, site A | + | + | + | + |
| NL-3, site A | ++, +, − | ++, +, − | ++, +, − | ++, +, − |
| Nx-1, site 4 | + | + | [+, −] | [+] |
Differences between brain and islet/INS-1 tissue are in brackets. At site A in neuroligin (NL)-3, the alternative exon used in neuroligin-1 and an exon specific to neuroligin-3 can either be incorporated in tandem (++), or only one of the alternative exons may be spliced in (+). Nx, Neurexin.
Rat brain cDNA, unlike human, yielded a neuroligin-1 PCR product lacking an insert at splice site A (Fig. 2B). This transcript appears to be the predominant form. As with human brain, amplification of neuroligin-2 from rat brain cDNA yielded only a PCR product containing a spliced-in exon at site A (Fig. 2C). These data are consistent with previous studies of neuroligin-1 and -2 splicing in rat brain (16). Alternative splicing at neuroligin-1 site A results in inclusion of a different alternative exon than splicing at neuroligin-2 site A (Fig. 2A). Splice site A in neuroligin-1 is conserved in neuroligin-3. In addition, a distinct exon can be used at this site (A3, Fig. 2A). Unexpectedly, both of these alternatively spliced exons were used at site A in neuroligin-3: neuroligin-3 transcripts containing both alternative exons, one exon, or lacking both were detected (Fig. 2D and Table 1).
Expression of an insert at splice site 4 in neurexin-1 transcripts
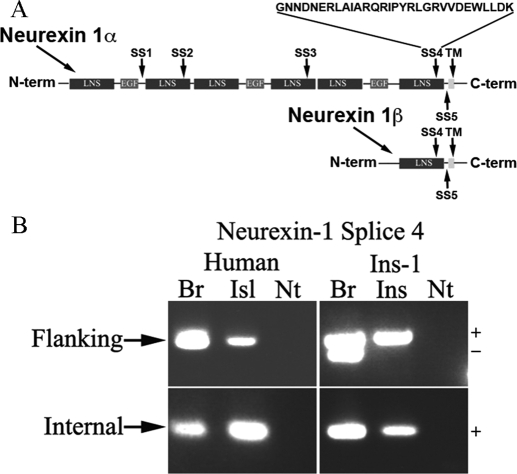
Alternative splicing of α-neurexin can occur at five sites; these are numbered one through five, beginning at the site closest to the amino-terminus of the α-neurexin gene, as depicted in Fig. 3A. Three sites occur exclusively in α-variants, and two (splice sites 4 and 5) are shared by both α and β-variants (14). A splice insert at splice site 4 favors the formation of inhibitory synapses (38). Because we hypothesize that the functional development of the β-cell may parallel that of the inhibitory synapse, we asked whether β-cell neurexin-1 transcripts contain the alternatively spliced insert at site 4.
Figure 3.
Expression of an insert at splice site 4 in neurexin-1 islet transcripts. A, Schematic representation of splice insertions in neurexin gene family members. A total of five alternative exons have been described in the neurexin-1 gene. An insert at splice site 4 is known to promote the formation of inhibitory synapses. The laminin-neurexin-SHBG (LNS) domains are extracellular. B, Τo determine whether neurexin transcripts in human islets (Isl) and rat-derived INS-1 (Ins) β-cells contain an insert in splice site 4, primers were designed to either flank or sit on this insert. PCR was performed using cDNA from either human islets or INS-1 cells and either rat or human brain (Br). Transcripts with an insert at splice site 4, but not ones lacking, were detected in both human islets and INS-1 cells. As expected, neurexin-1 transcripts both with and without an insert at site 4 were detected in rat brain. In contrast, in human brain, human islets and INS-1 β-cells, only transcripts containing an insert at splice site 4 were detected. −, Absence of insert; EGF, epidermal growth factor domain; Nt, no template; +, presence of insert.
As seen in Fig. 3B, the splice insert at site 4 was detected in both human islets and INS-1 cells, as well as in the brain controls. When PCR was performed with primers flanking the splice site, transcripts both containing and lacking the insert were detected in rat brain (Fig. 3B, upper panels). It is unclear why transcripts lacking the insert were not detected in human brain; it is possible that transcripts containing the splice insert represent the predominant form in the human brain. In islets and INS-1 cells, only neurexin transcripts containing the inhibitory synapse-associated insert at site 4 were detected (Fig. 3B and Table 1).
Expression of specific inhibitory and excitatory postsynaptic protein markers in β-cells
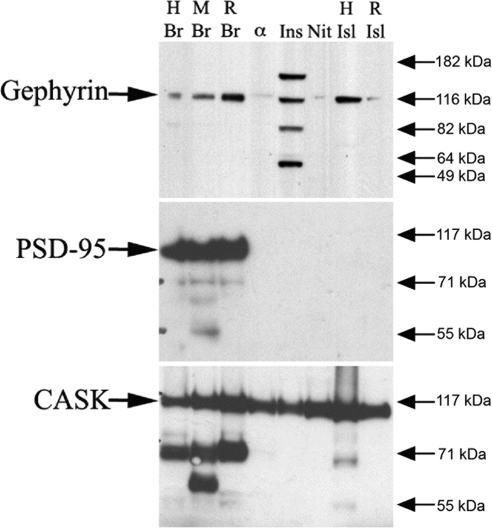
In coculture experiments, neurexin expression on nonneuronal cells induces the clustering of both inhibitory and excitatory postsynaptic markers in the dendrites of cultured hippocampal neurons (19). Gephyrin and PSD-95 are examples of specific markers of the inhibitory and excitatory postsynaptic densities, respectively (39,40). Because β-cells appear to differentiate along a pathway that results in an inhibitory synapse-like phenotype (with β-cell expression of both presynaptic and postsynaptic proteins), we predicted that β-cells would express gephyrin, a cytoplasmic neuroligin binding partner and the central scaffolding protein at the inhibitory synapse, but not the scaffolding protein PSD-95, a central constituent of the excitatory postsynaptic density (41,42). Immunoblot analysis revealed that rat and human islets, as well as the β-cell lines INS-1 and NIT, express gephyrin, but not PSD-95 (Fig. 4). As expected, both gephyrin and PSD-95 were detected in extracts from human, mouse, and rat brain.
Figure 4.
Expression of gephyrin and CASK but not PSD-95 in islets and β-cell lines. Western blot analysis was performed on islet and β-cell line extracts using antibodies to gephyrin, PSD-95, and CASK. Brain (Br) lysates were used as positive controls. As expected, gephyrin, PSD-95, and CASK were detected in human (H), mouse (M), and rat (R) brain near their expected molecular masses of 93, 95, and 120 kDa, respectively. Gephyrin was also detected in human islets, rat islets, INS-1 cells, NIT-1 cells, and αTC-6 cells (with the latter two extracts yielding a weaker band). α-TC6 is a partially dedifferentiated islet α-cell line. PSD-95 was not detected in human islets, rat islets, or any of the islet cell lines tested. CASK was detected in human islets, rat islets, and all islet cell lines. α, αtc-6 cell lysate; Isl, islet tissue lysate; Ins, INS-1 cell lysate; Nit, NIT-1 cell lysate.
The cytoplasmic protein CASK, along with munc-18-interacting protein (Mint), directly interacts with neurexins to help initiate the assembly of the presynaptic secretory machinery (43). Previously, Mint was shown to be expressed in β-cells (9). If, as in axons, assembly of the β-cell secretory machinery is dependent on neurexin clustering, we hypothesized that CASK would also be expressed in β-cells. As shown in Fig. 4, CASK was detected in all islet and β-cell extracts tested by immunoblot analysis.
Expression of neuroligin-2 protein in pancreatic islets
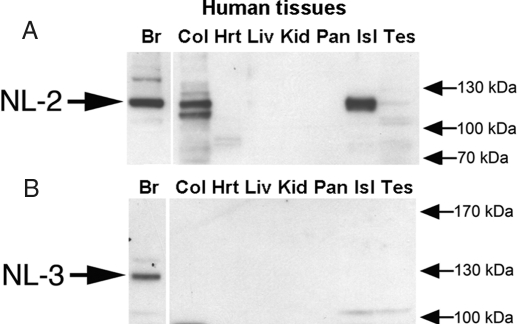
Neuroligin-2 was detected by Western blot analysis in human brain, human colon, and human islets but was not detected in human heart, liver, kidney, or whole pancreas (Fig. 5A). Due to sequence homology between the neuroligins, it is possible that this antibody, which was raised to the extracellular domain of neuroligin-2, has some level of affinity for other neuroligin proteins. Identification of the neuroligin band detected in human islets rests on the observations that: 1) the band comigrates with neuroligin-2, and not neuroligin-1; and 2) neuroligin-3 is not detected in human islets using an antibody that is specific for this protein and that detects neuroligin-3 in brain (Fig. 5B). We were unable to identify a neuroligin-1 antibody that detects the human variant of this protein (data not shown).
Figure 5.
Expression of neuroligin (NL)-2 in islets. To determine whether neuroligin-2 and neuroligin-3 proteins are expressed in islets, Western blot analysis was performed using rabbit polyclonal antibodies to neuroligin-2 (A) and neuroligin-3 (B). A, Neuroligin-2 was detected in human brain, human colon, and human islet cell lysate. Possible low-level expression was also observed in the testes cell lysate. B, Neuroligin-3 was only detected in human brain. Br, Brain extract; Col, colon extract; Hrt, heart extract; Isl, islet extract; Kid, kidney extract, Liv, liver extract; Pan, pancreas extract; Tes, testes extract.
Localization of the synaptogenic adhesion molecules neurexin, neuroligin, and SynCAM in pancreatic islets
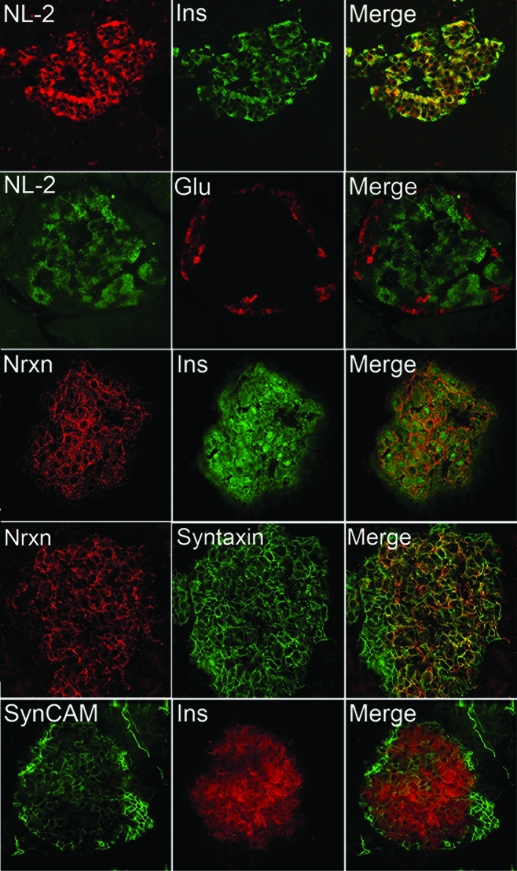
To determine the localization of neuroligin and neurexin proteins, immunohistochemistry was performed on rat pancreas sections using antibodies to neurexin and neuroligin. Both neurexin-1 and neuroligin-2 were detected exclusively in cells expressing insulin (β-cells) (Fig. 6, rows 1 and 3), but not in cells expressing glucagon (α-cells) (Fig 6, row 2, and supplemental Fig. 4). Neuroligin-2 staining was not cell-surface specific, indicating that there is a significant cytoplasmic content. This lack of plasma membrane-specific localization of neuroligin is consistent with the pattern of immunostaining previously observed by others in neurons and nonneuronal cell lines: exclusive cell-surface localization of neuroligin proteins is only observed when neuroligins are overexpressed in nonneuronal cell lines such as HEK293 or COS (21,25,44). Neurexin-1 was expressed in a punctate pattern on the surface of the β-cells and did not colocalize with insulin (Fig. 6, row 3). Neurexin-1 colocalized with syntaxin-1, a soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) protein expressed on the cytoplasmic face of the β-cell plasma membrane (Fig. 6, row 4) (45).
Figure 6.
Localization of neurexin 1, neuroligin-2 (NL-2), and SynCAM in rat pancreas. To determine the localization of neurexin, neuroligin, and SynCAM in rat pancreas sections, immunohistochemical localization was performed using antibodies to neurexin-1 (Nrxn), neuroligin-2, and SynCAM. Tissues were costained for insulin (Ins), glucagon (Glu), or syntaxin-1. Images were captured under either a confocal or deconvolution microscope equipped with a ×60 lens. Both neuroligin (row 1) and neurexin (row 3) were exclusively detected in cells that expressed insulin and absent in cells that expressed glucagon (row 2 and supplemental Fig. 4), indicating a β-cell specific pattern of expression. Neuroligin-2 displayed some intracellular colocalization with insulin (row 1, yellow in merged image). Neurexin was primarily expressed on the cell surface and colocalized in β-cells with the plasma-membrane associated SNARE protein syntaxin-1 (row 4). SynCAM was detected both in the islet mantle (the periphery, which primarily consists of non-β-endocrine cells: mostly α-cells) and in the insulin positive β-cells (row 5). Staining for SynCAM was consistently stronger in the islet mantle region.
SynCAM, like the neuroligins and neurexins, induces synaptic differentiation in adjacent neurons in coculture experiments (17). SynCAM’s synaptogenic activity is dependent on homophilic SynCAM1 binding interactions or on heterophilic SynCAM1-SynCAM2 binding across the synaptic cleft (17,22). If the pattern of expression of synapse-inducing adhesion molecules on the β-cell surface resembles that in the synaptic cleft, we reasoned that the β-cells would express SynCAM. Immunostaining revealed that SynCAM is expressed on the cell surface of both the α and β-cells, with higher expression evident in the α-cells and evident cell-surface localization (Fig. 6, row 5). The observed staining pattern was reminiscent of that previously reported for the neural cell adhesion molecule (NCAM) (46). Overall, islet-specific expression of members of each of the three families of synapse-inducing synaptic adhesion molecules, i.e. the neuroligins, neurexins, and SynCAMs, was observed.
Overexpression of neuroligin family members increases insulin secretion
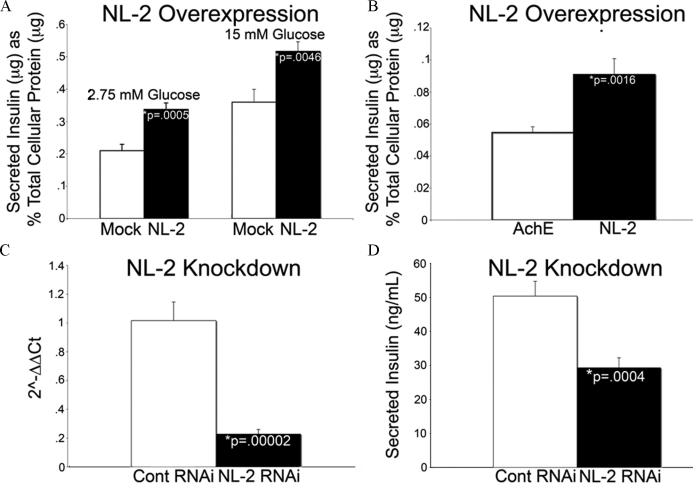
Because the neuronal neurotransmitter and β-cell insulin secretory apparatuses are highly similar to one another, we asked whether neuroligins are important for insulin secretion (47,48). To test this hypothesis, INS-1 cells were transiently transfected with neuroligin-2, and secreted insulin was measured 48 h after transfection. Controls included either cells treated with Lipofectamine alone, cells transfected with empty vector, or cells transfected with acetylcholinesterase, an α-β-hydrolase-fold protein that shares significant sequence identity with the extracellular domain of the neuroligins (49).
At basal levels of glucose, no significant difference in insulin secretion was observed between mock-transfected cells and cells transfected with acetylcholinesterase. Overexpression of neuroligin-2 resulted in 61% (Fig. 7A) and 66% (Fig. 7B) increases in secreted insulin relative to mock-transfected cells and cells overexpressing acetylcholinesterase. When insulin secretion was measured in cells transfected with neuroligin-2 at stimulating levels of glucose (15 mm), a 44% increase in insulin secretion was observed relative to mock-transfected controls (Fig. 7A). Transfection of the other three major neuroligins into INS-1 cells also increased insulin secretion at basal glucose levels (supplemental Fig. 5).
Figure 7.
Overexpression and knockdown of neuroligin-2 (NL-2) alters insulin secretion in INS-1 cells. INS-1 cells were transfected with a construct encoding full-length neuroligin-2, cultured for 48 h and then conditioned for 1 h in a solution containing 2.75 mm glucose. After conditioning, secreted insulin was measured by RIA after sequential treatment with low (2.75 mm) and high (15 mm) glucose for 1 h each. Secreted insulin was normalized to total cellular protein content. A, Overexpression of neuroligin-2 increased secreted insulin levels by 61% (n = 6; P = 0.0005) and 44% (n = 6; P = 0.0046) relative to mock-transfected cells when cultured under conditions of low and high glucose. B, As a control, INS-1 cells were transfected with acetylcholinesterase (AchE), a protein that shares significant homology with the extracellular domain of neuroligin. Secreted insulin levels were measured at basal glucose levels and compared with cells transfected in parallel with neuroligin-2. A 66% increase in secreted insulin was observed in neuroligin-2-transfected cells relative to acetylcholinesterase-transfected cells (n = 12; P = 0.0016). Overexpression of neuroligins-1, -3, and -4 similarly increased basal insulin secretion (supplemental Fig. 5). C and D, INS-1 cells were transfected with either a pool of nontargeting siRNAs or a pool of siRNAs targeted to neuroligin-2, cultured for 48 h and then conditioned for 1 h in a solution containing 2.75 mm glucose. After conditioning, secreted insulin was measured by RIA after treatment with 15 mm glucose for 1 h. RNA was isolated, reverse transcribed to cDNA, and knockdown was analyzed by real-time qPCR analysis. C, Neuroligin-2 mRNA was decreased by 77% in cells treated with neuroligin-2 siRNAs relative to those treated with nontargeting siRNAs (n = 10; P = 0.00002). D, A 42% decrease in secreted insulin was observed in neuroligin-2 siRNA treated cells relative to nontargeting siRNA treated cells (n = 10; P = 0.0004).
Neuroligin knockdown reduces insulin secretion in INS-1 cells and rat islet cells
INS-1 cells were transiently transfected with either a pool of neuroligin-2 siRNAs or a pool of nontargeting, control siRNAs, and secreted insulin was measured 48 h after transfection. Knockdown was verified by real-time PCR analysis. Neuroligin-2 RNA was decreased by 77% in cells treated with neuroligin-2 siRNAs relative to those treated with nontargeting siRNAs (Fig. 7C). After treatment with 15 mm glucose, a 42% decrease in insulin secretion was observed relative to nontargeting siRNA treated control cells (Fig. 7D).
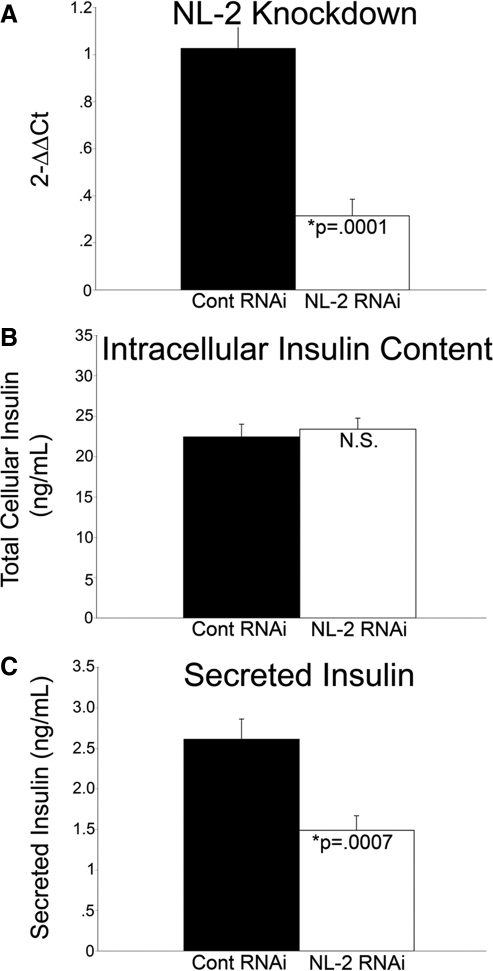
Dissociated rat islets were also transfected with either a pool of nontargeting siRNAs or a pool of siRNAs targeted to neuroligin-2, and secreted insulin was measured 48 h after transfection. Neuroligin-2 RNA was decreased by 69% in islet cells treated with neuroligin-2 siRNAs relative to those treated with nontargeting siRNAs (Fig. 8A). Total intracellular insulin was virtually identical between cells treated with control siRNAs and neuroligin-2 siRNAs (Fig. 8B). Under high glucose conditions (25 mm), a 43% decrease in secreted insulin was observed in neuroligin-2 siRNA treated dispersed islet cells relative to nontargeting siRNA treated dispersed islet cells. Dissociated islet cells treated with high glucose (25 mm) and nontargeting siRNA secreted 40% more insulin than those treated with low glucose (2.75 mm) and nontargeting siRNA (n = 6; P = 0.01; supplemental Fig. 6). Consistent with previous studies performed on dissociated rat islet cells, robust glucose-stimulated insulin secretion was not observed in these studies (50). Under low glucose conditions, a significant difference in secreted insulin was not observed between dispersed islet cells treated with neuroligin-2 siRNA and cells treated with nontargeting siRNA.
Figure 8.
Knockdown of neuroligin-2 (NL-2) in dissociated rat islets inhibits insulin secretion. Dissociated rat islets were transfected with a pool of control (Cont), nontargeting siRNAs, or a pool of siRNAs targeted to neuroligin-2, cultured for 48 h, and then conditioned for 1 h in a solution containing 2.75 mm glucose. After conditioning, secreted insulin was measured by RIA after treatment with high (25 mm) glucose for 1 h. A, RNA was isolated from half of the samples, reverse transcribed to cDNA, and knockdown was analyzed by real-time qPCR analysis. Neuroligin-2 was decreased by 69% in dispersed islet cells treated with neuroligin-2 siRNAs relative to those treated with control siRNAs (n = 6; P = 0.0001). B, Protein was isolated from the other half of the samples, and total cellular insulin content was measured in the cell lysate. No significant difference (N.S.) in insulin content was detected between cells treated with control siRNAs and neuroligin-2 siRNAs. An equal amount of lysis buffer was used to prepare each sample. C, Under high glucose conditions (25 mm), a 43% decrease in secreted insulin was observed in neuroligin-2 siRNA treated dispersed islet cells relative to control siRNA treated cells (n = 12; P = 0.0007). At 2.75 mm glucose, there was no significant change in insulin secretion between cells treated with control siRNA and those treated with neuroligin-2 siRNA (not shown).
Discussion
The great resemblance at the molecular level between the β-cells of the endocrine pancreas and inhibitory (GABAergic) synapses suggests that key insights into the final functional maturation of β-cells can be derived from knowledge of synapse formation and differentiation (11,12,51,52). A similar resemblance has been observed between the α-cells of the endocrine pancreas and excitatory (glutamatergic) synapses (53). During synaptogenesis the synaptogenic adhesion molecules neurexin and neuroligin appear to be important for instructing a developing synaptic density to become either glutamatergic (excitatory) or GABAergic (inhibitory) (13,23,24). Here, we demonstrate that islets express several neurexin and neuroligin family members. and reveal a pattern of expression in β-cells that resembles the pattern observed in inhibitory synapses. Overall, these results indicate that knowledge of the pathways that guide inhibitory synapse formation will aid in our understanding of β-cell development and functional differentiation.
Neuroligin and neurexin gene expression in rat and human islets and INS-1 β-cells
In the CNS, neuroligin-2 is specifically expressed by and important for the differentiation of inhibitory synaptic densities (23,26), and neuroligin-1 is primarily expressed by and implicated in the differentiation of excitatory synapses (23,25). α-Neurexins, like neuroligin-2, specifically promote the formation of inhibitory synaptic densities in coculture studies (24). Rat and human islets and the β-cell line INS-1 all express high levels of both neuroligin-2 and neurexin-1α, a finding consistent with published microarray results identifying these genes as having high-level expression in human islets (54). In marked contrast to the brain, in which neurexin-1α and neurxin-1β are expressed at similar levels, islet and INS-1 neurexin-1 expression consists almost exclusively of the α-form. Neuroligin-1 is also expressed at high levels by rat and human islets but is only detected at low levels in INS-1 cells. The expression of high levels of neuroligin-2 and neurexin-1α in these cells, and relatively low levels of neuroligin-1 in INS-1 β cells, along with immunolocalization studies demonstrating that expression of neuroligin-2 and neurexin-1 is restricted to β-cells are consistent with the idea that the functional differentiation of the β-cells parallels that of the inhibitory synapse.
Expression by β-cells of the inhibitory synaptic marker gephyrin, but not the excitatory synaptic marker PSD-95, is further evidence of their parallel development. Interestingly, neuroligin-1 promotes the formation of inhibitory synapses in the absence of PSD-95 (44). Although an effect on inhibitory synapses is not observed in the CNS of mice lacking the neuroligin-1 gene (23), the high-level expression of neuroligin-1 in islets along with the absence of PSD-95 might favor the development of an inhibitory phenotype by β-cells. Alternatively, neuroligin-1 might, as it does in the CNS, promote the development of the excitatory phenotype observed in α-cells.
Alternative splicing of neurexin and neuroligin in islets
Because alternative splicing of neuroligin and neurexin transcripts helps to determine the ability of specific pairs of neurexin and neuroligin proteins to interact, the pattern of expression of alternative splicing contributes toward the specification of nascent synapses as either excitatory or inhibitory (16,24,37,38). We found that human islet and INS-1 β-cell neurexin-1 transcripts uniformly contain an insert at splice site 4. This clearly differentiates the pattern of INS-1 β-cell neurexin-1 splicing from that observed in the rat brain, in which both insert-positive and insert-negative forms are expressed (24). This splicing pattern, along with the predominance of the α-form of neurexin-1, would be expected to favor strongly the development of an inhibitory synaptic phenotype (24,38).
Neuroligin-1 transcripts with an insert at splice site B (neuroligin-1B+) are predominant in islets, matching the pattern observed in the brain (16). The splice insert at site B introduces a site of N-glycosylation that impedes the binding of neuroligin-1B+ to splice site 4-positive neurexin-1β molecules. In neurons, inclusion of the insert at neuroligin-1 site B favors excitatory synapse formation. In islets, neuroligin-1B+ molecules might be important for promoting the development of glutamatergic signaling within α-cells, an autocrine signaling mechanism important in the regulation of glucagon secretion from these cells (53). As in the brain, β-cell neuroligin-1 and -2 transcripts containing an insert at splice site A are predominant. A report by Chih et al. (16) suggests an association between inclusion of the alternatively spliced exon at this site and the formation of GABAergic synapses.
In neuroligin-3, two splice inserts can be alternatively spliced into splice site A. Neuroligin-3 has been less well studied than neuroligins-1 and -2, and the effects of alternative splicing on interactions with neurexins are unknown. We found that in both islets and the brain, neuroligin-3 transcripts are produced containing none, one, or both of the alternative site A inserts. Further work will be required to unravel the function of neuroligin-3 and the role of alternative splicing at neuroligin-3 site A in both neurons and β-cells.
Expression of gephyrin, but not PSD-95, in β-cells
Gephyrin is a postsynaptic cytoplasmic protein that clusters near GABAA receptors and binds the intracellular domain of neuroligin-2 (39). β-Cell expression of gephyrin is consistent with an inhibitory synapse-like character. The presence of GABAA receptors on the surface of human β-cells further enhances this similarity (55). In contrast, PSD-95, a protein that clusters exclusively at excitatory synapses, was not detected in islets or in β-cell lines (56).
β-Cell-specific expression of neurexin and neuroligin
Immunolocalization studies demonstrated that β-cells express members of each of the three synaptic adhesion protein families that are capable of inducing synaptogenesis in cultured neurons: the neuroligins, neurexins, and SynCAMs. The ability of these proteins to initiate assembly of the presynaptic exocytic machinery points to their potential importance in β-cell secretory function. The expression of these proteins suggests that the β-cell surface displays synaptic adhesion proteins capable of heterophilic or (in the case of SynCAM) homophilic extracellular binding interactions (17,22,57). Because β-cells lack an equivalent of the axon/dendrite polarization of synaptic protein expression in neurons, neuroligin-neurexin interactions can potentially occur in trans (between molecules on different β-cells) and/or cis (between molecules on the surface of the same β-cell). Structural characterization of neurexin-neuroligin interactions in neurons indicates that a trans interaction is likely (30,37,58). The possibility of trans-cellular interactions between heterophilic binding partners coexpressed on the β-cell surface is exemplified by Ephrin A and EphA, which were recently shown to be coexpressed on the β-cell surface, to localize to different microdomains and to engage in trans interactions that help regulate insulin secretion (59).
It has been observed that mature nerve terminals develop in neuronal processes at sites of contact with cultured islet endocrine cells (60). The presence on the β-cell surface of neuroligins, neurexins, and SynCAM available for interaction with their neuronal binding partners would readily explain how cultured islet cells induce synaptic differentiation in neurons.
Unlike neuroligin-2 or neurexin, SynCAM is present on both the α and β-cell surface, and SynCAM binding interactions could potentially bridge the two different cell types. The neural adhesion protein NCAM shares with SynCAM a pattern of α-cell predominant islet expression (46,61). NCAM is neither synapse specific nor synapse inducing, so it is unclear whether, despite their similar intraislet distribution, NCAM’s function in mediating islet cell organization provides clues as to the role of SynCAM.
Involvement of neurexin and neuroligins in exocytosis, and conclusion
During synaptogenesis, transcellular neurexin-neuroligin interactions initiate the assembly of a complex presynaptic scaffolding assembly around Mint and CASK proteins bound to neurexin PDZ domains (62). Mint is expressed in β-cells (9). Consistent with the idea that β-cell and synaptic secretory function develops along parallel pathways, we have found that CASK is also expressed. It is possible that neuroligin-neurexin interactions similarly enable GABA secretion by β-cells. However, it is also conceivable that the GABAergic and insulin secretory assemblies are separate, perhaps being formed at sites seeded by a distinct combination of neurexin-neuroligin interactions.
There is a very high degree of overlap between the proteins known to mediate neurotransmitter secretion and those known to mediate insulin secretion, and it is highly likely that assembly of the apparatus necessary for insulin release is, like assembly of the presynaptic active site, dependent on neuroligin-neurexin interactions. Our finding that neuroligin overexpression increases insulin secretion and that neuroligin knockdown decreases insulin secretion in both INS-1 cells and rat islet cells supports the hypothesis that the neuroligins are involved in insulin secretion. This is consistent with the recent observation that the neuroligin-1 gene is one of two genetic loci linked to fasting insulin levels (63). Preliminary evidence that neurexin knockout mice exhibit impaired insulin secretion and hyperglycemia also implicates the neurexins as participating in the insulin secretory mechanism (64). The involvement of transcellular neurexin-neuroligin interactions in insulin exocytosis may, like Ephrin-EphA interactions, contribute to the decreased glucose-stimulated insulin secretion by individual β-cells that is observed after islet dissociation (50,65,66,67). Extracellular interactions involving SynCAM may similarly function to help establish normal β-cell secretory function.
In the future, it will be important to identify the specific roles in β-cell functional maturation and exocytic function of each of the individual neuroligins, neurexins, and SynCAMs expressed within the islet. This may lead to new methods by which to coax β-cells generated in vitro into behaving more like true β-cells, e.g. by inducing expression of inhibitory synapse-associated proteins, and to new therapies for modulating islet function and thereby treating diabetes.
Supplementary Material
Acknowledgments
Islet isolations were performed by the University of Washington Diabetes and Endocrinology Research Center Islet Cell and Functional Analysis Core (DK17047). We thank Ian Sweet and Ben Reed for their assistance, and Mark Huising for additional assistance with islet isolation and culture techniques. The Islet Cell Resource Basic Science Islet Distribution Program provided human islets for these studies. We acknowledge the University of California San Diego, Center for AIDS Research Genomics Core Laboratory (Director, Dr. Christopher Woelk), the San Diego Veterans Medical Research Foundation, and the University of California, San Diego Neuroscience Microscopy Shared Facility.
Footnotes
This work was supported by a national type 1 diabetes pilot and feasibility award from the National Institute of Diabetes and Digestive and Kidney Diseases (DK063491-4S1) (to S.D.C.). A.T.S. is supported by a graduate research fellowship from the National Science Foundation. Support to the UCSD Genomics Core and Neuroscience Microscopy Shared Facility was provided by the National Institutes of Health (Grants 5P30 AI36214 and NINDS P30 NS047101, respectively).
Disclosure Statement: D.C., M.A.W., M.M., S.E., and P.T. have nothing to declare. A.T.S. and S.D.C. are inventors on United States patent application 60/706,133. S.D.C. has previously received research funding from Pfizer.
First Published Online August 28, 2008
Abbreviations: CASK, Calcium/calmodulin-dependent serine protein kinase; CNS, central nervous system; GABA, γ-aminobutyric acid; HEK293, human embryonic kidney 293; Mint, munc-18-interacting protein; NCAM, neural cell adhesion molecule; qPCR, quantitative PCR; siRNA, small interfering RNA; PSD-95, postsynaptic density protein 95; SNARE, soluble N-ethylmaleimide-sensitive factor attachment protein receptor.
References
- Hori Y, Gu X, Xie X, Kim SK 2005 Differentiation of insulin-producing cells from human neural progenitor cells. PLoS Med 2:e103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rulifson EJ, Kim SK, Nusse R 2002 Ablation of insulin-producing neurons in flies: growth and diabetic phenotypes. Science 296:1118–1120 [DOI] [PubMed] [Google Scholar]
- Dresbach T, Qualmann B, Kessels MM, Garner CC, Gundelfinger ED 2001 The presynaptic cytomatrix of brain synapses. Cell Mol Life Sci 58:94–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto K, Shibasaki T, Yokoi N, Kashima Y, Matsumoto M, Sasaki T, Tajima N, Iwanaga T, Seino S 2002 Piccolo, a Ca2+ sensor in pancreatic β-cells. Involvement of cAMP-GEFII.Rim2.Piccolo complex in cAMP-dependent exocytosis. J Biol Chem 277:50497–50502 [DOI] [PubMed] [Google Scholar]
- Ohara-Imaizumi M, Ohtsuka T, Matsushima S, Akimoto Y, Nishiwaki C, Nakamichi Y, Kikuta T, Nagai S, Kawakami H, Watanabe T, Nagamatsu S 2005 ELKS, a protein structurally related to the active zone-associated protein CAST, is expressed in pancreatic β cells and functions in insulin exocytosis: interaction of ELKS with exocytotic machinery analyzed by total internal reflection fluorescence microscopy. Mol Biol Cell 16:3289–3300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regazzi R, Wollheim CB, Lang J, Theler JM, Rossetto O, Montecucco C, Sadoul K, Weller U, Palmer M, Thorens B 1995 VAMP-2 and cellubrevin are expressed in pancreatic β-cells and are essential for Ca(2+)-but not for GTP γ S-induced insulin secretion. EMBO J 14:2723–2730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadoul K, Lang J, Montecucco C, Weller U, Regazzi R, Catsicas S, Wollheim CB, Halban PA 1995 SNAP-25 is expressed in islets of Langerhans and is involved in insulin release. J Cell Biol 128:1019–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Efanov A, Yang SN, Fried G, Kolare S, Brown H, Zaitsev S, Berggren PO, Meister B 2000 Munc-18 associates with syntaxin and serves as a negative regulator of exocytosis in the pancreatic β-cell. J Biol Chem 275:41521–41527 [DOI] [PubMed] [Google Scholar]
- Zhang W, Lilja L, Bark C, Berggren PO, Meister B 2004 Mint1, a Munc-18-interacting protein, is expressed in insulin-secreting β-cells. Biochem Biophys Res Commun 320:717–721 [DOI] [PubMed] [Google Scholar]
- Baekkeskov S, Aanstoot HJ, Christgau S, Reetz A, Solimena M, Cascalho M, Folli F, Richter-Olesen H, De Camilli P 1990 Identification of the 64K autoantigen in insulin-dependent diabetes as the GABA-synthesizing enzyme glutamic acid decarboxylase. Nature 347:151–156 [DOI] [PubMed] [Google Scholar]
- Suckow AT, Sweet IR, Van Yserloo B, Rutledge EA, Hall TR, Waldrop M, Chessler SD 2006 Identification and characterization of a novel isoform of the vesicular γ-aminobutyric acid transporter with glucose-regulated expression in rat islets. J Mol Endocrinol 36:187–199 [DOI] [PubMed] [Google Scholar]
- Franklin IK, Wollheim CB 2004 GABA in the endocrine pancreas: its putative role as an islet cell paracrine-signalling molecule. J Gen Physiol 123:185–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AM, Kang Y 2007 Neurexin-neuroligin signaling in synapse development. Curr Opin Neurobiol 17:43–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missler M, Sudhof TC 1998 Neurexins: three genes and 1001 products. Trends Genet 14:20–26 [DOI] [PubMed] [Google Scholar]
- Bolliger MF, Pei J, Maxeiner S, Boucard AA, Grishin NV, Sudhof TC 2008 Unusually rapid evolution of Neuroligin-4 in mice. Proc Natl Acad Sci USA 105:6421–6426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chih B, Gollan L, Scheiffele P 2006 Alternative splicing controls selective trans-synaptic interactions of the neuroligin-neurexin complex. Neuron 51:171–178 [DOI] [PubMed] [Google Scholar]
- Biederer T, Sara Y, Mozhayeva M, Atasoy D, Liu X, Kavalali ET, Sudhof TC 2002 SynCAM, a synaptic adhesion molecule that drives synapse assembly. Science 297:1525–1531 [DOI] [PubMed] [Google Scholar]
- Dean C, Scholl FG, Choih J, DeMaria S, Berger J, Isacoff E, Scheiffele P 2003 Neurexin mediates the assembly of presynaptic terminals. Nat Neurosci 6:708–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf ER, Zhang X, Jin SX, Linhoff MW, Craig AM 2004 Neurexins induce differentiation of GABA and glutamate postsynaptic specializations via neuroligins. Cell 119:1013–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sara Y, Biederer T, Atasoy D, Chubykin A, Mozhayeva MG, Sudhof TC, Kavalali ET 2005 Selective capability of SynCAM and neuroligin for functional synapse assembly. J Neurosci 25:260–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiffele P, Fan J, Choih J, Fetter R, Serafini T 2000 Neuroligin expressed in nonneuronal cells triggers presynaptic development in contacting axons. Cell 101:657–669 [DOI] [PubMed] [Google Scholar]
- Fogel AI, Akins MR, Krupp AJ, Stagi M, Stein V, Biederer T 2007 SynCAMs organize synapses through heterophilic adhesion. J Neurosci 27:12516–12530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chubykin AA, Atasoy D, Etherton MR, Brose N, Kavalali ET, Gibson JR, Sudhof TC 2007 Activity-dependent validation of excitatory versus inhibitory synapses by neuroligin-1 versus neuroligin-2. Neuron 54:919–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y, Zhang X, Dobie F, Wu H, Craig AM 2008 Induction of GABAergic postsynaptic differentiation by α-neurexins. J Biol Chem 283:2323–2334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JY, Ichtchenko K, Sudhof TC, Brose N 1999 Neuroligin 1 is a postsynaptic cell-adhesion molecule of excitatory synapses. Proc Natl Acad Sci USA 96:1100–1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varoqueaux F, Jamain S, Brose N 2004 Neuroligin 2 is exclusively localized to inhibitory synapses. Eur J Cell Biol 83:449–456 [DOI] [PubMed] [Google Scholar]
- Chessler SD, Simonson WT, Sweet IR, Hammerle LP 2002 Expression of the vesicular inhibitory amino acid transporter in pancreatic islet cells: distribution of the transporter within rat islets. Diabetes 51:1763–1771 [DOI] [PubMed] [Google Scholar]
- Missler M, Zhang W, Rohlmann A, Kattenstroth G, Hammer RE, Gottmann K, Sudhof TC 2003 α-neurexins couple Ca2+ channels to synaptic vesicle exocytosis. Nature 423:939–948 [DOI] [PubMed] [Google Scholar]
- Varoqueaux F, Aramuni G, Rawson RL, Mohrmann R, Missler M, Gottmann K, Zhang W, Sudhof TC, Brose N 2006 Neuroligins determine synapse maturation and function. Neuron 51:741–754 [DOI] [PubMed] [Google Scholar]
- Comoletti D, Flynn RE, Boucard AA, Demeler B, Schirf V, Shi J, Jennings LL, Newlin HR, Sudhof TC, Taylor P 2006 Gene selection, alternative splicing, and post-translational processing regulate neuroligin selectivity for β-neurexins. Biochemistry 45:12816–12827 [DOI] [PubMed] [Google Scholar]
- Comoletti D, De Jaco A, Jennings LL, Flynn RE, Gaietta G, Tsigelny I, Ellisman MH, Taylor P 2004 The Arg451Cys-neuroligin-3 mutation associated with autism reveals a defect in protein processing. J Neurosci 24:4889–4893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comoletti D, Flynn R, Jennings LL, Chubykin A, Matsumura T, Hasegawa H, Sudhof TC, Taylor P 2003 Characterization of the interaction of a recombinant soluble neuroligin-1 with neurexin-1β. J Biol Chem 278:50497–50505 [DOI] [PubMed] [Google Scholar]
- Reed NA, Oh DJ, Czymmek KJ, Duncan MK 2001 An immunohistochemical method for the detection of proteins in the vertebrate lens. J Immunol Methods 253:243–252 [DOI] [PubMed] [Google Scholar]
- Sweet IR, Cook DL, DeJulio E, Wallen AR, Khalil G, Callis J, Reems J 2004 Regulation of ATP/ADP in pancreatic islets. Diabetes 53:401–409 [DOI] [PubMed] [Google Scholar]
- Ichtchenko K, Nguyen T, Sudhof TC 1996 Structures, alternative splicing, and neurexin binding of multiple neuroligins. J Biol Chem 271:2676–2682 [DOI] [PubMed] [Google Scholar]
- Ichtchenko K, Hata Y, Nguyen T, Ullrich B, Missler M, Moomaw C, Sudhof TC 1995 Neuroligin 1: a splice site-specific ligand for β-neurexins. Cell 81:435–443 [DOI] [PubMed] [Google Scholar]
- Boucard AA, Chubykin AA, Comoletti D, Taylor P, Sudhof TC 2005 A splice code for trans-synaptic cell adhesion mediated by binding of neuroligin 1 to α- and β-neurexins. Neuron 48:229–236 [DOI] [PubMed] [Google Scholar]
- Graf ER, Kang Y, Hauner AM, Craig AM 2006 Structure function and splice site analysis of the synaptogenic activity of the neurexin-1 β LNS domain. J Neurosci 26:4256–4265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassoe-Pognetto M, Fritschy JM 2000 Mini-review: gephyrin, a major postsynaptic protein of GABAergic synapses. Eur J Neurosci 12:2205–2210 [DOI] [PubMed] [Google Scholar]
- O'Brien RJ, Lau LF, Huganir RL 1998 Molecular mechanisms of glutamate receptor clustering at excitatory synapses. Curr Opin Neurobiol 8:364–369 [DOI] [PubMed] [Google Scholar]
- Irie M, Hata Y, Takeuchi M, Ichtchenko K, Toyoda A, Hirao K, Takai Y, Rosahl TW, Sudhof TC 1997 Binding of neuroligins to PSD-95. Science 277:1511–1515 [DOI] [PubMed] [Google Scholar]
- Poulopoulos A, Meyer G, Soykan T, Craig AM, Brose N, Varoqueaux F, Interaction of neuroligins with gephyrin is a critical link in inhibitory postsynaptic organization. Society for Neuroscience Meeting Planner, San Diego, CA, 2007, online:35.37/E16; available at www.abstractsonline.com/viewer/viewAbstractPrintFriendly.asp?CKey={BDE0A39C-4622-401C-B16A-7A0B 0F903C19}&SKey={330F04B6-5567-486C-A696-B29C71969451}&MKey={FF8B70E5-B7F9-4D07-A58A-C1068FDE9D25}&AKey={3A7DC0B9-D787-44AA-BD08-FA7BB2FE9004} [Google Scholar]
- Tabuchi K, Biederer T, Butz S, Sudhof TC 2002 CASK participates in alternative tripartite complexes in which Mint 1 competes for binding with caskin 1, a novel CASK-binding protein. J Neurosci 22:4264–4273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prange O, Wong TP, Gerrow K, Wang YT, El-Husseini A 2004 A balance between excitatory and inhibitory synapses is controlled by PSD-95 and neuroligin. Proc Natl Acad Sci USA 101:13915–13920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohara-Imaizumi M, Nishiwaki C, Nakamichi Y, Kikuta T, Nagai S, Nagamatsu S 2004 Correlation of syntaxin-1 and SNAP-25 clusters with docking and fusion of insulin granules analysed by total internal reflection fluorescence microscopy. Diabetologia 47:2200–2207 [DOI] [PubMed] [Google Scholar]
- Cirulli V, Baetens D, Rutishauser U, Halban PA, Orci L, Rouiller DG 1994 Expression of neural cell adhesion molecule (N-CAM) in rat islets and its role in islet cell type segregation. J Cell Sci 107(Pt 6):1429–1436 [DOI] [PubMed] [Google Scholar]
- Craig AM, Graf ER, Linhoff MW 2006 How to build a central synapse: clues from cell culture. Trends Neurosci 29:8–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easom RA 2000 β-Granule transport and exocytosis. Semin Cell Dev Biol 11:253–266 [DOI] [PubMed] [Google Scholar]
- De Jaco A, Kovarik Z, Comoletti D, Jennings LL, Gaietta G, Ellisman MH, Taylor P 2005 A single mutation near the C-terminus in α/β hydrolase fold protein family causes a defect in protein processing. Chem Biol Interact 157- 158:371–372 [DOI] [PubMed] [Google Scholar]
- Weir GC, Halban PA, Meda P, Wollheim CB, Orci L, Renold AE 1984 Dispersed adult rat pancreatic islet cells in culture: A, B, and D cell function. Metabolism 33:447–453 [DOI] [PubMed] [Google Scholar]
- Chessler SD, Lernmark A 2000 Alternative splicing of GAD67 results in the synthesis of a third form of glutamic-acid decarboxylase in human islets and other non-neural tissues. J Biol Chem 275:5188–5192 [DOI] [PubMed] [Google Scholar]
- Gammelsaeter R, Froyland M, Aragon C, Danbolt NC, Fortin D, Storm-Mathisen J, Davanger S, Gundersen V 2004 Glycine, GABA and their transporters in pancreatic islets of Langerhans: evidence for a paracrine transmitter interplay. J Cell Sci 117:3749–3758 [DOI] [PubMed] [Google Scholar]
- Cabrera O, Jacques-Silva MC, Speier S, Yang SN, Kohler M, Fachado A, Vieira E, Zierath JR, Kibbey R, Berman DM, Kenyon NS, Ricordi C, Caicedo A, Berggren PO 2008 Glutamate is a positive autocrine signal for glucagon release. Cell Metab 7:545–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei A, Liu Z, Witkowski P, Moschella F, Del Pozzo G, Liu E, Herold K, Winchester RJ, Hardy MA, Harris PE 2004 Identification of tissue-restricted transcripts in human islets. Endocrinology 145:4513–4521 [DOI] [PubMed] [Google Scholar]
- von Blankenfeld G, Turner J, Ahnert-Hilger G, John M, Enkvist MO, Stephenson F, Kettenmann H, Wiedenmann B 1995 Expression of functional GABAA receptors in neuroendocrine gastropancreatic cells. Pflugers Arch 430:381–388 [DOI] [PubMed] [Google Scholar]
- Kennedy MB 1997 The postsynaptic density at glutamatergic synapses. Trends Neurosci 20:264–268 [DOI] [PubMed] [Google Scholar]
- Fabrichny IP, Leone P, Sulzenbacher G, Comoletti D, Miller MT, Taylor P, Bourne Y, Marchot P 2007 Structural analysis of the synaptic protein neuroligin and its β-neurexin complex: determinants for folding and cell adhesion. Neuron 56:979–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comoletti D, Grishaev A, Whitten AE, Tsigelny I, Taylor P, Trewhella J 2007 Synaptic arrangement of the neuroligin/β-neurexin complex revealed by X-ray and neutron scattering. Structure 15:693–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstantinova I, Nikolova G, Ohara-Imaizumi M, Meda P, Kucera T, Zarbalis K, Wurst W, Nagamatsu S, Lammert E 2007 EphA-Ephrin-A-mediated β cell communication regulates insulin secretion from pancreatic islets. Cell 129:359–370 [DOI] [PubMed] [Google Scholar]
- Hooghe-Peters EL, Meda P, Orci L 1981 Co-culture of nerve cells and pancreatic islets. Brain Res 227:287–292 [DOI] [PubMed] [Google Scholar]
- Moller CJ, Christgau S, Williamson MR, Madsen OD, Niu ZP, Bock E, Baekkeskov S 1992 Differential expression of neural cell adhesion molecule and cadherins in pancreatic islets, glucagonomas, and insulinomas. Mol Endocrinol 6:1332–1342 [DOI] [PubMed] [Google Scholar]
- Lise MF, El-Husseini A 2006 The neuroligin and neurexin families: from structure to function at the synapse. Cell Mol Life Sci 63:1833–1849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe RC, Chen W-M, Erdos MR, Saxena R, Jackson AU, Lyssenko V, Uda M, Buchanan TA, Schlessingor D, Groop L, Collins FS, Altshuler D, Abecasis G, Boehnke M, Scuteri A, Novel genetic loci for fasting glucose and insulin identified by genome-wide association in Caucasians. Proc 68th American Diabetes Association Annual Meeting, Philadelphia, PA, 2008 (Abstract 137-OR) [Google Scholar]
- Sons MS, Busche N, Strenzke N, Moser T, Ernsberger U, Mooren FC, Zhang W, Ahmad M, Steffens H, Schomburg ED, Plomp JJ, Missler M 2006 α-Neurexins are required for efficient transmitter release and synaptic homeostasis at the mouse neuromuscular junction. Neuroscience 138:433–446 [DOI] [PubMed] [Google Scholar]
- Bosco D, Orci L, Meda P 1989 Homologous but not heterologous contact increases the insulin secretion of individual pancreatic B-cells. Exp Cell Res 184:72–80 [DOI] [PubMed] [Google Scholar]
- Hauge-Evans AC, Squires PE, Persaud SJ, Jones PM 1999 Pancreatic β-cell-to-β-cell interactions are required for integrated responses to nutrient stimuli: enhanced Ca2+ and insulin secretory responses of MIN6 pseudoislets. Diabetes 48:1402–1408 [DOI] [PubMed] [Google Scholar]
- Luther MJ, Hauge-Evans A, Souza KL, Jorns A, Lenzen S, Persaud SJ, Jones PM 2006 MIN6 β-cell-β-cell interactions influence insulin secretory responses to nutrients and non-nutrients. Biochem Biophys Res Commun 343:99–104 [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD 2001 Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- Hamaguchi K, Guskins HR, Leiter EH 1991 NIT-1, a pancreatic β-cell line established from a transgenic NOD/Lt mouse. Diabetes 40:842–849 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.