Abstract
The transcription factors Runx2 and estrogen receptor-α (ERα) are involved in numerous normal and disease processes, including postmenopausal osteoporosis and breast cancer. Using indirect immunofluorescence microscopy and pull-down techniques, we found them to colocalize and form complexes in a ligand-dependent manner. Estradiol-bound ERα strongly interacted with Runx2 directly through its DNA-binding domain and only indirectly through its N-terminal and ligand-binding domains. Runx2’s amino acids 417–514, encompassing activation domain 3 and the nuclear matrix targeting sequence, were sufficient for interaction with ERα’s DNA-binding domain. As a consequence of the interaction, Runx2’s transcriptional activation activity was strongly repressed, as shown by reporter assays in COS7 cells, breast cancer cells, and late-stage MC3T3-E1 osteoblast cultures. Metaanalysis of gene expression in 779 breast cancer biopsies indicated negative correlation between the expression of ERα and Runx2 target genes. Selective ER modulators (SERM) induced ERα-Runx2 interactions but led to various functional outcomes. The regulation of Runx2 by ERα may play key roles in osteoblast and breast epithelial cell growth and differentiation; hence, modulation of Runx2 by native and synthetic ERα ligands offers new avenues in selective ER modulator evaluation and development.
THE MAMMALIAN RUNT-RELATED gene family encodes three transcription factors that play pivotal roles in lineage-specific cell growth and differentiation. Whereas Runx1 is implicated in definitive hematopoiesis and Runx3 in gut and nervous system development (1), Runx2 is a master transcription factor controlling osteoblast differentiation (2). Runx2-null mice lack osteoblasts and fail to form bone (3,4). Low bone mass has been observed in Runx2 heterozygous mice (5), mice missing one of two Runx2 isoforms (6), as well as transgenic mice whose osteoblasts express a dominant-negative (DN) form of Runx2 under the control of the osteocalcin (OC) promoter (7). Runx2 has also been shown to act downstream of pro-skeletal agents, including bone morphogenetic proteins, PTH, and estrogens (8,9,10). Surprisingly, however, transgenic mice whose osteoblasts overexpress Runx2 under the control of the α1(I) collagen promoter also have low bone mass, which leads to spontaneous fractures (11,12); and conversely, transgenic mice expressing a Runx2-DN under the control of the same promoter have high bone mass and are protected against ovariectomy-induced bone loss (9). Conceivably, therefore, pro-skeletal agents may inhibit Runx2 under some conditions to prevent bone loss.
The runt-related proteins also play important roles in cancer. Depending on the context, they can function as either tumor suppressors or promoters (13). Runx2 deficiency has been recently shown to promote immortalization and tumorigenesis (14). This is consistent with earlier studies of Runx1 and Runx3, in which inactivation of the former (via chromosomal translocation) and epigenetic silencing of the latter (via DNA methylation) were found associated with hematological and gastric cancers, respectively (15). On the other hand, Runx2 has been implicated in cancer progression (13), including breast cancer bone metastasis (16). Possibly, transient inactivation of Runx2 promotes tumorigenesis, and its subsequent reactivation supports the metastatic phenotype.
Like Runx2, estrogens play critical roles in bone metabolism and carcinogenesis. Loss of estrogen function, at menopause, or during antiestrogen therapy for the management of hormone-driven malignancy increases the risk for osteoporotic fractures, cardiovascular disease, and dementia (17,18,19). On the other hand, estrogens have been associated with carcinogenesis (20), because hormone replacement therapy for postmenopausal women increases the risk for breast, endometrial, and ovarian cancer (21). Molecular mechanisms of estrogens’ carcinogenic activity are not well understood.
The pro-skeletal property of estrogens is multifaceted. Estrogens increase cell proliferation and survival in the osteoblast lineage, resulting in a bone anabolic effect (22,23,24). Most importantly, however, estrogens attenuate bone resorption and turnover, and this occurs via at least two mechanisms. First, estrogens directly promote osteoclast apoptosis (24), at least in part by stimulating FASL expression (25). Second, estrogens limit osteoblastogenesis from precursor cells, thereby attenuating bone turnover (26), a process that is skewed away from formation and toward resorption in most adults. Upon estrogen loss, increased osteoblast numbers fuel excessive bone turnover (23), and in the absence of estrogens, each of these osteoblasts expresses higher levels of osteoclastogenic factors (24). Molecular mechanisms by which estrogens attenuate osteoblastogenesis and osteoblast-driven osteoclastogenesis remain to be elucidated.
In our pursuit of interactions between sex steroids and Runx2, we made a novel observation that estrogens strongly inhibit Runx2 in COS7 cells, breast cancer cells, and late-stage MC3T3-E1 osteoblast cultures. We dissect this inhibitory activity in molecular terms and discuss how it may contribute to two well-established activities of estrogens: the restraining of bone turnover and the promotion of breast cancer.
Materials and Methods
Materials
Flag antibodies were obtained from Sigma-Aldrich (St. Louis, MO). The other primary antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA), including sc-10758 for Runx2 and sc-787 (or sc-7207 when indicated) for estrogen receptor-α (ERα). Fluorochrome-conjugated antibodies for confocal microscopy were obtained from Pierce Biotechnology (Rockford, IL). Estradiol (E2), ICI 182780, and hydroxytamoxifen (OHT) were from Sigma-Aldrich. Expression vectors encoding human ERα, ERβ, deletion constructs lacking the ligand-binding domain (LBD) or the N-terminal domain (NTD), and ERα-GST-LBD as well as ERE-luc were described previously (27,28,29,30). Human Runx1 and Runx2 expression vectors were obtained from Dr. Westendorf (Mayo Clinic, Rochester, MN). Mouse Runx2 expression vector and the 6XOSE2-luc Runx reporter were from Dr. Gerard Karsenty (Columbia University, New York, NY). All other expression plasmids were prepared using standard cloning procedures and confirmed by sequencing.
Cell culture
COS7 cells were maintained in DMEM and 5% fetal bovine serum (FBS). The mouse osteoblastic cell line MC3T3-E1 was maintained in α-MEM and 10% FBS. Runx-reporter osteoblasts were generated by stable transfection of MC3T3-E1 cells with the 6XOSE2-luc reporter construct using the calcium phosphate coprecipitation method and hygromycin (100 ng/ml) as the selection drug. Resistance to hygromycin was conferred by cotransfection of the pCEP plasmid (1:15 molar ratio). To support development of the osteoblast phenotype, cells were treated with 50 μg/ml ascorbic acid and 10 nm β-glycerophosphate commencing at confluence. The human breast cancer cell lines MDA-MB-231, T47D, and MCF7 were maintained in DMEM and 5% FBS.
Transient transfections
Cells were seeded at a density of 10,000 per well in 96-well plates and grown in 5% charcoal-stripped serum (CSS)-containing phenol red-free medium for 24 h. The indicated plasmids (50 ng reporter, 52 ng total) were transfected using LTX and Plus reagent according to the manufacturer’s specification (Invitrogen, Carlsbad, CA). Equimolar amounts of the empty vectors pCDNA 3.1 and pSG5 were used as controls, and the pCAT-basic promoterless plasmid was used as filler DNA. Under these conditions, the total amounts of promoter equivalents (molar) and DNA mass (nanograms) were the same in all wells of a particular experiment. As an internal control, 0.01 ng tk-Renilla-luc or CMV-renilla-luc (Promega, Madison, WI) was used for correction of transfection efficiency. Cells were subsequently treated with vehicle (ethanol, 0.01%) or the indicated ligands for 24 h. Luciferase activity was determined using the dual-assay luciferase kit (Promega).
Coimmunoprecipitation (co-IP)
Cells were seeded at a density of 300,000 per well in six-well plates and grown in 5% CSS-containing phenol red-free medium for 24 h. Cells were transfected when indicated, and 24 h later, they were lysed in a 50 mm Tris-HCl buffer (pH 7.4) containing 150 mm NaCl, 1 mm EDTA, 1% Triton X-100, and fresh protease inhibitors cocktail (1%; Sigma). After homogenization by passing 10 times through a 1-ml microfine insulin syringe, lysates were cleared by centrifugation at 14,000 rpm for 5 min in a bench-top microfuge, and 15% of the lysate solution was set aside and used as input. The remaining lysate was immunoprecipitated with approximately 3 μg of the specified antibody and 30 μl protein G-beads (Amersham Biosciences, Freiburg, Germany) and washed three times for 5 min each with the same buffer, followed by centrifugation at 4000 rpm for 1 min. Thirty microliters of the immunoprecipitate/bead suspension were mixed with 20 μl 2.5 × sample buffer [50% glycerol, 10% sodium dodecyl sulfate, 1 m Tris-HCl (pH 6.8), 25% β-mercaptoethanol, 0.5% bromophenol blue] and boiled for 5 min. Input and immunoprecipitates were analyzed by Western blot analysis.
Glutathione S-transferase (GST) pull-down interaction assay
Expression constructs for GST pull-down bait proteins were generated using pGEX-4T-1 (Amersham Biosciences) by in-frame fusions of DNA fragments encoding ERα’s amino acids 1–180, 181–265, and 266–595, representing the NTD, DNA-binding domain (DBD), and LBD, respectively. Bait proteins were purified with the GST purification module (Amersham Biosciences) according to the manufacturer’s protocol. The preys, 35S-labeled full-length and fragments of Runx2, were prepared using TNT T7 Quick Kit from Promega according to the manufacturer’s protocol. Ten micrograms of each bait, bound to glutathione-Sepharose, was incubated with the preys in 20 mm Tris-HCl (pH 7.5), 150 mm NaCl, 5 mm MgCl2, and 0.01% Nonidet P-40, supplemented with Complete protease inhibitor mix (Roche Diagnostics, Indianapolis, IN). Bound proteins were analyzed by SDS-PAGE and autoradiography.
Real-time RT-PCR
Total RNA was isolated using Aurum Total RNA kit (Bio-Rad, Hercules, CA) following the manufacturer’s recommendations. One microgram of total RNA was reverse transcribed (Invitrogen), and the cDNA was subjected to real-time PCR amplification (quantitative RT-PCR) using IQ SYBR Green (Bio-Rad). PCR primers were designed using the Primer3 program (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi) and are listed in supplemental Table 1, published as supplemental data on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org).
Immunofluorescence
COS7 cells were grown on 18-mm2 coverslips in six-well plates for 24 h using 5% CSS-containing phenol red-free DMEM. Cells were transfected with 100 ng each of ERα- and Runx2-expressing plasmids and treated with vehicle or 10 nm E2 for 24 h. Cells were then fixed with 95% methanol for 15 min and permeabilized with 1% saponin. ERα and Runx2 were visualized with the respective primary antibodies and secondary antibodies conjugated to either rhodamine or a fluorescein, respectively. Cells were mounted using Vectashield Hard Set mounting medium with 4′,6-diamidino-2-phenylindole (Burlingame, CA). Cells were viewed using an LSM 510 Zeiss confocal microscope at × 60 magnification. Images were processed using the default settings on Image J for colocalization finder, surface plot diagrams, and RG color picker. Using the default settings on the colocalization finder, we quantitated the percent colocalization where both red and green pixels overlapped.
Microarray data mining
Normalized expression data were obtained from Oncomine (31). Runx2 target genes from the literature (supplemental Table 2) were subjected to an unsupervised two-dimensional hierarchical cluster analysis using the software Jmp version 5.0 (http://www.jmp.com). ERα target genes were mapped using Heatmap version 1.0 (http://quertermous.stanford.edu/heatmap.htm) to the corresponding Runx2 cluster.
Results
ERα, not ERβ, inhibits Runx2 activity in a ligand-specific manner
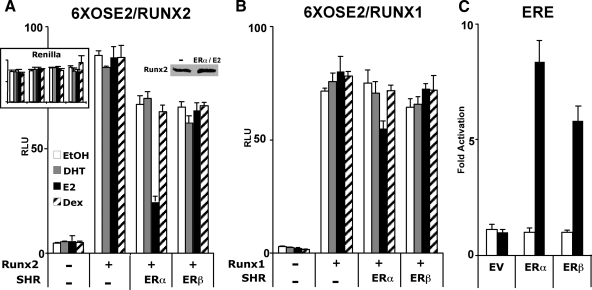
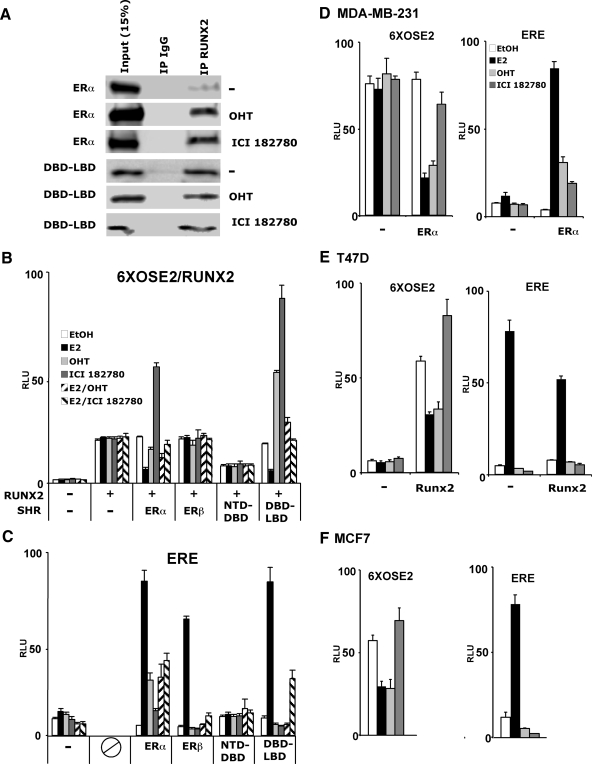
We initially investigated the influence of E2 and its receptors ERα and ERβ on Runx2 after transfecting COS7 cells with the respective expression vectors along with the Runx2 reporter 6XOSE2-luc (32). As shown in Fig. 1A, E2 inhibited the activity of Runx2 by 76% in the presence of ERα. The inhibition ranged from 43–78% in eight independent experiments. E2 did not inhibit Runx2 activity in the presence of ERβ, although as shown in Fig. 1C, the latter was almost as potent as ERα in stimulating a luciferase reporter driven by classical estrogen response elements (ERE). In the absence of ligand, both ERα and ERβ inhibited Runx2 only minimally (Fig. 1A). The strong inhibitory activity of ERα was elicited by E2 but not by dihydrotestosterone or dexamethasone (Fig. 1A). Similar to human Runx2 (Fig. 1), the mouse homolog was also inhibited by E2-bound ERα (supplemental Fig. 1). Interestingly, E2-activated ERα only slightly inhibited transcription from 6XOSE2-luc when the reporter was stimulated by Runx1 rather than Runx2 (Fig. 1B). These results indicate a strong and specific inhibitory mechanism, whereby E2-bound ERα suppresses Runx2’s transactivation activity. In a complementary experiment, Runx2 did not affect ERα-mediated activation of an ERE-containing reporter (supplemental Fig. 2).
Figure 1.
ERα inhibits Runx2. A and B, COS7 cells were transiently transfected with the Runx reporter 6XOSE2-luc (firefly) along with expression vectors encoding human Runx2 (A), Runx1 (B), ERα, and/or ERβ as indicated. Cells were treated with dihydrotestosterone (DHT), (E2) Estradiol, dexamethasone (Dex) (10 nm each), or ethanol (EtOH) vehicle (0.01%) for 24 h and subjected to luciferase assay as described in Materials and Methods. C, COS7 cells were transiently transfected with an ER reporter (ERE-luc) along with an expression vector encoding either ERα or ERβ and treated for 24 h with E2, followed by the luciferase assay. In each experiment, the firefly luciferase results were corrected for the expression of a cotransfected CMV-renilla luciferase construct (internal control), except for A, where the renilla luciferase values are shown in the inset. The immunoblot in A shows that Runx2 expression was not inhibited in the presence of ERα and its ligand. All data are presented as mean values ± sem, with n = 4 dish replicates of a representative experiment, repeated at least three times. EV, Empty vector; RLU, relative light units; SHR, steroid hormone receptor.
Functional mapping of ERα domains responsible for Runx2 repression indicates independence of ERα’s transactivation activity
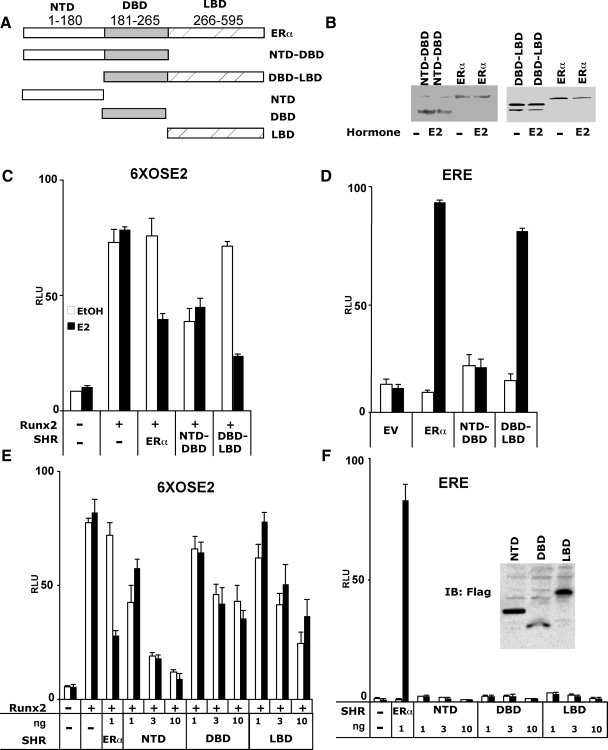
To functionally map the domains of ERα responsible for Runx2 repression, and to test whether repression was dependent on ER-mediated stimulation of ERE-containing promoters, we measured the influence of the ERα deletion constructs illustrated in Fig. 2A on Runx2 activity. Stimulation of ERE-luc was assessed in parallel. Most importantly, deletion of the ERα-LBD, which was associated with near complete loss of ERE-luc activity in COS7 cells (Fig. 2D), resulted in constitutive full repression of Runx2 activity (Fig. 2C). Further suggesting dissociation between ERα’s transcriptional activation activity from Runx2 repression, each of the ERα-NTD, -DBD, and -LBD inhibited Runx2-mediated activation of 6XOSE2-luc (Fig. 2E) while leaving ERE-luc (as well as CMV-renilla-luc and tk-renilla-luc) essentially unaffected (Fig. 2F). The ERα DBD-LBD also fully repressed Runx2 activity (Fig. 2C), and just like full-length ERα, repression by the DBD-LBD was ligand dependent. These results suggest that ERα represses Runx2 independent of the activation of ERE-containing target genes and that the repression mechanism employs multiple domains of ERα.
Figure 2.
Functional mapping of ERα substructures and the dissociation between ERα-mediated transcriptional activation and Runx2 repression. A, Schematic illustration showing full-length and ERα fragments that were transiently expressed in COS7 cells. The NTD, DBD, and LBD fragments were FLAG-tagged to facilitate immunoblot detection shown in the inset of F. B, Immunoblot analysis of cells transfected with the indicated constructs using antibodies against ERα NTD (sc-7207, left blot) or ERα LBD (sc-787, right blot). C–F, COS7 cells were transiently transfected with either the 6XOSE2-luc (C and E) or the ERE-luc reporter (D and F), along with the indicated expression plasmids. Luciferase activity was measured 24 h after treatment with either ethanol (0.01%) or E2 (10 nm). All data are presented as mean values ± sem, with n = 4 dish replicates of a representative experiment, repeated at least three times.
ERα physically interacts with Runx2 in a ligand-dependent manner
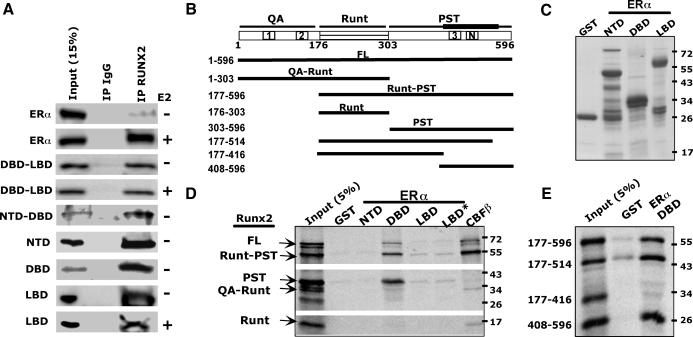
Because repression of Runx2 is independent of ERα’s transactivation activity, it could occur via physical interaction between the two proteins. Indeed, ERα was present in Runx2 immunoprecipitates; the ERα/Runx2 interaction was weak in untreated cells and increased dramatically upon E2 treatment (Fig. 3A). A series of ERα deletion constructs, including the DBD-LBD and NTD-DBD as well as the individual domains, were also employed in the co-IP assay, and each of them was associated with Runx2 (Fig. 3A). However, unlike the full-length receptor, the receptor fragments tested did not require E2 to form complexes with Runx2 (Fig. 3A). These data suggest that ERα interacts with Runx2 through multiple surfaces and that these surfaces are exposed only after the full-length protein has assumed a ligand-induced permissive conformation.
Figure 3.
Interaction between ERα and Runx2 domains in Co-IP and GST pull-down assays. A, COS7 cells were transiently transfected with plasmids encoding each of the specified ERα fragments and Runx2, followed by 24 h treatment with either 10 nm E2 or vehicle. The ERα and its fragments were detected in either whole-cell extracts as input or IgG or Runx2 immunoprecipitates. B, Schematic diagram of full-length (FL) Runx2 and fragments transcribed and translated in vitro. The scheme at the top depicts the three Runx2 domains. Boxes with 1, 2, 3, or N mark the positions of the respective activation domains and the nuclear matrix targeting sequences. The thick line above the PST domain represents the surface interacting with ERα. C, Coomassie-stained SDS-PAGE of the bacterially expressed and purified GST or GST fusion proteins used as baits in the pull-down assays. D and E, A mixture of the indicated radiolabeled Runx2 fragments was incubated with the depicted GST-fusion proteins used as baits. The positive control GST-CBFβ was used as a bait for the Runt domain. The autoradiograph shows the fragments pulled down by the indicated baits. Asterisk in D denotes presence of 10 nm E2.
To test whether the physical interactions demonstrated by co-IP are direct, we asked whether immobilized ERα domains (Fig. 3C) could pull down in vitro transcribed and translated Runx2. In these GST pull-down assays, the ERα-DBD strongly interacted with Runx2, and this strong interaction was recapitulated with Runx2’s proline-serine-threonine (PST) domain (alone or with the Runt domain), but not with the QA-Runt or Runt domains (Fig. 3D). The PST domain continued to interact with ERα’s DBD after deletion of the 515–596 but not the 417–596 amino acid sequence (Fig. 3E). Positive pull-down was also observed after deletion of the PST’s N-terminal 303–407 amino acid sequence (Fig. 3E), thereby mapping the interaction surface to Runx2’s amino acids 417–514, which contain activation domain 3 (AD3) and the nuclear matrix targeting sequences. In contrast to ERα’s DBD, the NTD did not interact with full-length Runx2 or fragments of Runx2 (Fig. 3D). The ERα-LBD displayed a weak interaction with Runx2, which, like the ERα-DBD, mapped to Runx2’s PST domain (Fig. 3D). This interaction was not influenced by physiological estrogen concentrations (Fig. 3D) but was increased in the presence of 1 μm E2 (supplemental Fig. 3). Thus, ligand-dependent ERα/Runx2 interaction is mediated primarily by a contact between Runx2’s amino acids 417–514 within the PST domain and ERα’s DBD, a potential secondary direct contact of Runx2 with the ERα-LBD (via the PST domain), and an indirect contact with the NTD.
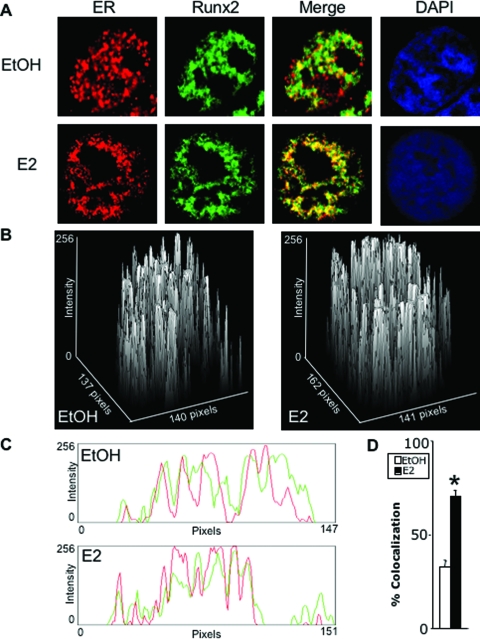
Indirect confocal fluorescence microscopy was then performed to test whether ERα colocalized with Runx2 in living cells (Fig. 4). Both transcription factors were concentrated in the nucleus regardless of E2 treatment and occupied distinct domains. Quantitative analysis of 10 randomly selected untreated cells indicated 34% colocalization (Fig. 4D), where ERα and Runx2 could interact in vivo. Upon treatment with E2, there was a decrease in areas occupied by either protein alone, with a concomitant increase in areas containing both proteins together (Fig. 4, A–C). Quantitative analysis of 10 randomly selected E2-treated cells indicated a highly significant (P < 8.3 × 10−8) 2.1-fold increase in colocalization compared with the 10 randomly selected untreated cells (Fig. 4D), likely reflecting the E2-induced physical interaction between the two transcription factors (see Fig. 3).
Figure 4.
Immunofluorescence of ERα and Runx2. COS7 cells were transiently transfected with ERα and Runx2 and treated for 24 h with ethanol vehicle (EtOH) or E2 (10 nm). A–C, ERα (red) and Runx2 (green) were visualized using confocal microscopy as described in Materials and Methods. Colocalization (yellow in A) is demonstrated by surface plots (B) and by red/green profiles (C). D, Ten cells were randomly selected from each of a set of four untreated and four treated cultures, and colocalization was quantified and plotted as mean ± sem. *, P = 8.3 × 10−8. DAPI, 4′,6-Diamidino-2-indole.
Inhibition of Runx2 by estradiol in late-stage MC3T3-E1 osteoblast cultures
To address the ERα-Runx2 interaction in osteoblasts, we first confirmed by co-IP assay the physical association between endogenous ERα and endogenous Runx2 in E2-treated MC3T3-E1 osteoblasts (Fig. 5A). The influence of E2 on Runx2 was then examined during the development of the osteoblast phenotype in cultures of MC3T3-E1 cells that had been stably transfected with the 6XOSE2-luc Runx2 reporter construct (32). We measured the expression of the mRNAs for OC, a classical Runx2 target (33,34); MMP9, also a Runx2 target (35); RANKL, which may be regulated by Runx2 (36); and luciferase (driven by the six Runx2 binding sites). Whereas OC and MMP9 were at best weakly stimulated on d 4, all mRNAs were suppressed on d 18 (Fig. 5, B–E). Thus, although in early cultures, Runx2 may be stimulated by E2, the results demonstrate E2-mediated repression in late MC3T3-E1 cultures, which is similar to the inhibition observed in COS7 cells (Fig. 1) and breast cancer cells (see below). Although E2 did not affect mineralization in the MC3T3-E1 cultures (data not shown), inhibition of RANKL and MMP9 may attenuate their osteoclastogenic activity.
Figure 5.
Developmental stage-specific inhibition of Runx2 by E2 in osteoblasts. A, MC3T3-E1 cells were treated with ethanol or E2 (10 nm) and the presence of ERα in Runx2 immunocomplexes was examined as described in Fig. 3A. B–E, MC3T3-E1 cells stably transfected with the 6XOSE2-luc Runx2 reporter were subjected to differentiation conditions and treated with ethanol (white bars) or E2 (10 nm; black bars) commencing at confluence. Levels of the indicated mRNAs were measured on d 4, 11, and 18 by quantitative RT-PCR as described in Materials and Methods. Data were corrected for the expression of ribosomal protein L10A, which itself did not significantly change during culture progression or in response to E2. RANKL was not expressed on d 4, and the low expression of OC and luciferase on this day are shown in the respective insets (mean ± sd; n = 3).
Synthetic ER ligands inhibit or stimulate Runx2 in a compound- and cell type-specific manner
Selective ER modulators (SERM) are widely used for the management of breast cancer (37). A variety of SERM are available, with different levels of partial agonism or antagonism with respect to the native ligand. Different SERM induce in the receptor a variety of conformational changes, reflected for example by increased sensitivity of helix 12 to trypsin digestion in the presence of ICI compounds compared with E2 and even higher sensitivity in the presence of OHT (38). We first asked whether these SERM mimicked E2 in inducing interaction with Runx2. As demonstrated by co-IP assays, OHT and ICI 182780 each strongly induced the formation of ERα/Runx2 complexes (Fig. 6A). Remarkably, however, the functional consequences with regard to Runx2 activity were different compared with E2. Although OHT slightly inhibited Runx2 activity, ICI 182780 had the opposite effect, stimulating Runx2 activity by 3.1-fold (Fig. 6B). This contrasted with the activity of these SERM on an ERE-containing template, where ICI 182780 and OHT had weak and stronger partial agonist effects, respectively (Fig. 6C). Interestingly, OHT also had a stimulatory effect on Runx2 when bound to the ERα DBD-LBD (Fig. 6B), raising the possibility that different domains of OHT-bound ERα have opposing effects on Runx2.
Figure 6.
Various effects of SERM-bound ERα on Runx2. A, Co-IP assays were performed as in Fig. 3A after treatment of COS7 cells with 100 nm OHT or 100 nm ICI 182780. B and C, COS7 cells were transfected with Runx2 and its 6XOSE2-luc reporter (B) or with ERE-luc (C) along with expression vectors coding for the indicated ER isoforms or fragments. Cells were treated for 24 h with ethanol, E2 (10 nm), OHT (100 nm), ICI 182780 (100 nm), or combinations thereof and then subjected to luciferase assays. D–F, Three breast cancer cell lines, MDA-MB-231 (D), T47D (E), and MCF7 (F), were transfected with the 6XOSE2-luc (left) or the ERE-luc (right) reporter, along with expression vectors for ERα (D), Runx2 (E), or empty vector control and treated with 10 nm E2, 100 nm OHT, or 100 nm ICI 182780 as indicated. All data are presented as mean values ± sem, with n = 4 dish replicates of a representative experiment, repeated at least three times.
We subsequently examined the effects of SERM on Runx2 in breast cancer cells. When ERα was expressed in the ER-negative MDA-MB-231 cell line, E2 strongly inhibited the cotransfected 6XOSE2 Runx2 reporter (Fig. 6D). Unlike COS7 cells, OHT inhibited Runx2 in MDA-MB-231 cells as strongly as E2 (Fig. 6D). ICI 182780, which stimulated Runx2 in COS7 cells (Fig. 6B), had a slight if any inhibitory effect on Runx2 in MDA-MB-231 cells (Fig. 6D). In T47D and MCF7 breast cancer cells, ICI 182780 slightly stimulated Runx2 (Fig. 6, E and F), whereas the effect of OHT was uniformly inhibitory across all three breast cancer cell lines (Fig. 6D-F). Thus, OHT mimics the strong E2-mediated inhibition of Runx2 specifically in breast cancer cells, and ICI 182780, which stimulates Runx2 in COS7 cells, only mildly regulates Runx2 activity in breast cancer cells, either upward or downward. The various effects of SERM-bound ERα on Runx2 activity may be relevant to their selective effects in different tissues in vivo.
Negative correlation between ERα and Runx2 target genes in breast cancer tissues
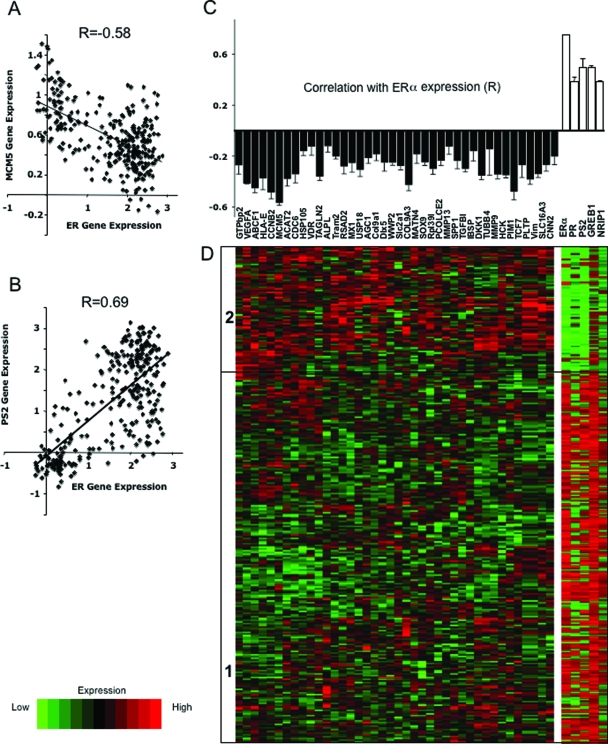
If ERα inhibits Runx2 in breast cancer, then one could expect to see an inverse relationship between the expression of ERα and Runx2 target genes in breast cancer biopsies. We therefore mined three datasets from Oncomine (31) for the expression of 40 known Runx2 target genes. The datasets represented comprehensive gene expression analyses of three cohorts of 286, 295, and 198 breast cancer biopsies (39,40,41). The 40 Runx2 target genes, listed in supplemental Table 2, were the top hits from each of three unbiased studies designed to discover Runx2 targets (42,43,44). Remarkably, expression of each of the Runx2 target genes tested was negatively correlated with the expression of ERα across each of the three cohorts. For example, in the series of 286 biopsies, the correlation coefficient between ERα expression and that of the Runx2 target gene MCM5 (43) was −0.58 (Fig. 7A). This correlation was close to the positive correlation between the expression of ERα and its classical target pS2 (r = 0.69; Fig. 7B). Similar observations were made in the other two series (supplemental Figs. 4, A and B, and 5, A and B, respectively). Metaanalysis of the three studies altogether revealed a statistically significant negative correlation between ERα expression and each of the 40 Runx2 target genes (Fig. 7C). The average correlation coefficient was −0.29, with a se of 0.05 (n = 40), compared with 0.59 ± 0.05 (n = 4) for classical ERα targets (Fig. 7C). A significant negative correlation (P = 0.03) was also observed between the expression of ERα and OC (not shown). To visually demonstrate the correlation between the expression of ERα and Runx2 target genes, we subjected the 40 Runx2 target genes to unsupervised clustering and examined the expression of ERα and ERα target genes in each cluster. In each of the three studies, we observed clusters that indicated an inverse relationship between genes regulated by Runx2 vs. ERα and its targets. For example, the cohort of 286 biopsies was clustered into two main branches, one with low expression of Runx2 target genes and high expression of ERα and its targets (Fig. 7D, cluster 1) and the other displaying a mirror image (Fig. 7D, cluster 2). Similar relationships were observed with the other two cohorts (supplemental Figs. 4C and 5C). The negative correlation between expression of ERα and Runx2 target genes is consistent with the idea that inhibition of Runx2 by ERα occurs not only in breast cancer cell lines (Fig. 6) but also in human breast epithelial cells in vivo.
Figure 7.
Metaanalysis of the correlation between expression of ERα and Runx2 target genes in breast cancer biopsies. A and B, Scatter plots of the expression of MCM5 (A) or pS2 (B) vs. ERα in 286 beast cancer biopsies previously subjected to comprehensive gene expression analysis (39). C, Correlation coefficients between the expression of ERα and each of 40 Runx2 target genes based on metaanalysis of 779 breast cancer biopsies described in three published databases (42,43,44). Each of the correlation coefficients was significant with P < 10−4. D, Expression levels of the Runx2 target genes in one cohort of 286 breast cancer biopsies (39) was subjected to an unsupervised cluster analysis, resulting in two major branches of tumor samples designated in the heat map as 1 and 2. The expression levels of ERα and four ERα target genes in each of the 286 biopsies are represented as a heat map on the right.
Discussion
Two ERs, α and β, mediate the various actions of estrogens. In addition to their classical action on ERE-containing promoters, the ligand-activated receptors form complexes with and modulate the activity of other regulatory proteins, including signal transducers (45) and transcription factors. For example, estrogens have been shown to activate activation protein-1 and specificity protein-1, resulting in increased expression of cyclin D1 (46). Here we show that ERα interacts with and inhibits Runx2 in osteoblasts and breast cancer cells. Transfection experiments comparing ERα and ERβ in COS7 cells show that the inhibition is specific for ERα, the same receptor that also mediates most of the physiological effects of estrogens in bone (47,48) and breast (49).
E2 was required for ERα/Runx2 interaction in both the co-IP and the functional inhibition assays. Ligand is also required for ER interaction with transcriptional coactivators, which results in stimulation of ERE-containing promoters. However, Runx2 repression by ERα does not require classical activation through EREs, because 1) the NTD-DBD as well as individual domains of ERα each suppressed Runx2 activity without activating EREs, 2) the transcriptionally competent ERβ did not inhibit Runx2, and 3) the partial agonist activity of ICI 182780 with regard to classical ERE activation was associated with stimulation rather than inhibition of Runx2. Furthermore, dose-response curves constructed in parallel for the two activities of ERα showed that E2 represses Runx2-mediated activation of 6XOSE2-luc at concentrations lower than those necessary for activation of ERE-luc (data not shown). Thus, Runx2 repression is dissociable from ERα’s canonical transcriptional activation activity and appears to occur via direct protein-protein interaction. Among possible mechanisms leading to inhibition of Runx2 by ERα, the two proteins could be present together at OSE2-like sites and form platforms for the further recruitment of corepressors.
According to our co-IP data, ERα’s NTD, DBD, and LBD each interact with Runx2, although strong direct interaction was observed in the GST pull-down assay only with the DBD. These results are consistent with those of McCarthy et al. (10), who used a two-hybrid assay to demonstrate that each of the ERα domains, and mostly the DBD, interacted with Runx2. In this study, however, the reciprocal two-hybrid analysis mapped the interacting surfaces in Runx2 to both the QA and the PST domains, whereas our GST pull-down assay indicated interaction only at the PST domain. Be that as it may, the fine mapping of the interaction surface to Runx2’s amino acids 417–514 within the PST domain raises the interesting possibility that ERα inhibits Runx2 by masking its C-terminal activation domain and/or its nuclear matrix targeting sequence (2).
The suggested inhibition of Runx2 by ERα in breast cancer cells in vivo could be either pro- or antioncogenic depending on the presiding function of Runx2. Initially, Runx2 likely plays a tumor-suppressive role, and thus the E2-mediated inhibition at this stage would constitute a mechanism of hormonal carcinogenesis. However, Runx2 activity in later stages of cancer progression may promote expression of the metastatic phenotype (16). Therefore, in advanced breast cancer, inhibition of Runx2 by ERα may become beneficial. This could possibly explain why, despite the well-established oncogenic activity of ER signaling in breast epithelial cells (50), breast cancers that maintain ERα expression during tumor progression are generally less aggressive than ERα-negative tumors; in these tumors, Runx2 would promote metastasis without ERα opposition.
In osteoblasts, estrogens influence Runx2 in a developmental stage-specific manner. Inhibition of Runx2 and its target genes OC and MMP9 is observed in late MC3T3-E1 cultures, when Runx2 activity and OC expression are maximal. However, data presented here and elsewhere (10,51) show that in early MC3T3-E1 and in primary osteoblast cultures, estrogens do not inhibit and may even stimulate Runx2 activity and OC expression. Both the stimulation and inhibition of Runx2 by estrogens could contribute to their pro-skeletal properties. During specific differentiation stages, stimulation of Runx2 may promote osteoblast function and bone formation. During other stages, inhibition of Runx2 may benefit the skeleton by 1) attenuating osteoblastogenesis thereby restraining bone turnover (23,26,52) and 2) decreasing the expression of osteoclastogenic genes, possibly RANKL and MMP9, which were down-regulated along with OC in our late MC3T3-E1 cultures. The skeletal benefits of keeping Runx2 in check is suggested by the excessive endosteal bone resorption (reminiscent of postmenopausal bone loss) and spontaneous fractures observed in transgenic mice overexpressing Runx2 (11,12) as well as the resistance of Runx2-DN transgenic mice to ovariectomy-induced bone loss (9).
Inhibition of Runx2 is a potential novel mechanism of action of estrogen in breast and bone and may contribute to drug discovery. Specifically, if the inhibition were indeed important for bone and breast epithelial cell growth and differentiation, then SERM would behave most naturally if they mimicked estrogens not only in stimulating ERE-driven transcription but also in restraining Runx2. Furthermore, indications for SERM therapy for breast cancer should possibly take into account the potential consequences on Runx2 activity.
In conclusion, we documented and dissected strong physical interaction and inhibition of Runx2 by ERα. Suppression of Runx2 in response to E2, and the various responses to synthetic ligands, may mediate some of their effects on bone, breast, and other organ systems. In addition, Runx2 inhibition assays may prove useful in SERM evaluation and development.
Supplementary Material
Acknowledgments
We thank Dr. Gerard Karsenty (Columbia University, New York, NY) and Dr. Jennifer Westendorf (Mayo Clinic, Rochester, MN) for reagents.
Footnotes
This work was supported by Grants DK071122 (to B.F.) and CA109147 (to G.A.C.) from the National Institutes of Health (NIH); by W81XWH-05-1-0025 to B.F., W81XWH-04-1-0823, and W81XWH-07-1-0067 to G.A.C. and W81XWH-07-1-0067 predoctoral grant to O.K. from the Department of Defense; and by awards from the Prostate Cancer Foundation to G.A.C. B.F. is the holder of the J. Harold and Edna L. LaBriola Chair in Genetic Orthopaedic Research at the University of Southern California. The experiments were conducted in facilities supported by P30 CA 014089-30 from the NIH/National Cancer Institute and constructed with support from Research Facilities Improvement Program Grant No. C06 (RR10600-01, CA62528-01, and RR14514-01) from the NIH/National Center for Research Resources. Y.G. was partially supported by a Meyer Young Investigator Fellowship from the Arthritis Foundation Southern California Chapter. S.K.B. was partially supported by California Community Foundation Grant from the Arthritis Foundation Southern California Chapter.
Disclosure Statement: The authors have nothing to disclose.
First Published Online August 28, 2008
Abbreviations: CSS, Charcoal-stripped serum; DBD, DNA-binding domain; DN, dominant negative; E2, estradiol; ERα, estrogen receptor-α; ERE, estrogen response element; FBS, fetal bovine serum; IP, immunoprecipitation; LBD, ligand-binding domain; NTD, N-terminal domain; OC, osteocalcin; OHT, hydroxytamoxifen; PST, proline-serine-threonine; SERM, selective ER modulator.
References
- Coffman JA 2003 Runx transcription factors and the developmental balance between cell proliferation and differentiation. Cell Biol Int 27:315–324 [DOI] [PubMed] [Google Scholar]
- Schroeder TM, Jensen ED, Westendorf JJ 2005 Runx2: a master organizer of gene transcription in developing and maturing osteoblasts. Birth Defects Res C Embryo Today 75:213–225 [DOI] [PubMed] [Google Scholar]
- Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, Shimizu Y, Bronson RT, Gao YH, Inada M, Sato M, Okamoto R, Kitamura Y, Yoshiki S, Kishimoto T 1997 Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell 89:755–764 [DOI] [PubMed] [Google Scholar]
- Otto F, Thornell AP, Crompton T, Denzel A, Gilmour KC, Rosewell IR, Stamp GW, Beddington RS, Mundlos S, Olsen BR, Selby PB, Owen MJ 1997 Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell 89:765–771 [DOI] [PubMed] [Google Scholar]
- Salingcarnboriboon R, Tsuji K, Komori T, Nakashima K, Ezura Y, Noda M 2006 Runx2 is a target of mechanical unloading to alter osteoblastic activity and bone formation in vivo. Endocrinology 147:2296–2305 [DOI] [PubMed] [Google Scholar]
- Xiao Z, Awad HA, Liu S, Mahlios J, Zhang S, Guilak F, Mayo MS, Quarles LD 2005 Selective Runx2-II deficiency leads to low-turnover osteopenia in adult mice. Dev Biol 283:345–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducy P, Starbuck M, Priemel M, Shen J, Pinero G, Geoffroy V, Amling M, Karsenty G 1999 A Cbfa1-dependent genetic pathway controls bone formation beyond embryonic development. Genes Dev 13:1025–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MH, Kim YJ, Kim HJ, Park HD, Kang AR, Kyung HM, Sung JH, Wozney JM, Kim HJ, Ryoo HM 2003 BMP-2-induced Runx2 expression is mediated by Dlx5, and TGF-β1 opposes the BMP-2-induced osteoblast differentiation by suppression of Dlx5 expression. J Biol Chem 278:34387–34394 [DOI] [PubMed] [Google Scholar]
- Maruyama Z, Yoshida CA, Furuichi T, Amizuka N, Ito M, Fukuyama R, Miyazaki T, Kitaura H, Nakamura K, Fujita T, Kanatani N, Moriishi T, Yamana K, Liu W, Kawaguchi H, Nakamura K, Komori T 2007 Runx2 determines bone maturity and turnover rate in postnatal bone development and is involved in bone loss in estrogen deficiency. Dev Dyn 236:1876–1890 [DOI] [PubMed] [Google Scholar]
- McCarthy TL, Chang WZ, Liu Y, Centrella M 2003 Runx2 integrates estrogen activity in osteoblasts. J Biol Chem 278:43121–43129 [DOI] [PubMed] [Google Scholar]
- Liu W, Toyosawa S, Furuichi T, Kanatani N, Yoshida C, Liu Y, Himeno M, Narai S, Yamaguchi A, Komori T 2001 Overexpression of Cbfa1 in osteoblasts inhibits osteoblast maturation and causes osteopenia with multiple fractures. J Cell Biol 155:157–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geoffroy V, Kneissel M, Fournier B, Boyde A, Matthias P 2002 High bone resorption in adult aging transgenic mice overexpressing cbfa1/runx2 in cells of the osteoblastic lineage. Mol Cell Biol 22:6222–6233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blyth K, Cameron ER, Neil JC 2005 The RUNX genes: gain or loss of function in cancer. Nat Rev 5:376–387 [DOI] [PubMed] [Google Scholar]
- Zaidi SK, Pande S, Pratap J, Gaur T, Grigoriu S, Ali SA, Stein JL, Lian JB, van Wijnen AJ, Stein GS 2007 Runx2 deficiency and defective subnuclear targeting bypass senescence to promote immortalization and tumorigenic potential. Proc Natl Acad Sci USA 104:19861–19866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y 2004 Oncogenic potential of the RUNX gene family: ‘overview.’ Oncogene 23:4198–4208 [DOI] [PubMed] [Google Scholar]
- Barnes GL, Hebert KE, Kamal M, Javed A, Einhorn TA, Lian JB, Stein GS, Gerstenfeld LC 2004 Fidelity of Runx2 activity in breast cancer cells is required for the generation of metastases-associated osteolytic disease. Cancer Res 64:4506–4513 [DOI] [PubMed] [Google Scholar]
- Lindsay R 1991 Estrogens, bone mass, and osteoporotic fracture. Am J Med 91:10S–13S [DOI] [PubMed] [Google Scholar]
- Mendelsohn ME, Karas RH 1999 The protective effects of estrogen on the cardiovascular system. N Engl J Med 340:1801–1811 [DOI] [PubMed] [Google Scholar]
- Yaffe K 2001 Estrogens, selective estrogen receptor modulators, and dementia: what is the evidence? Ann NY Acad Sci 949:215–222 [DOI] [PubMed] [Google Scholar]
- Platet N, Cathiard AM, Gleizes M, Garcia M 2004 Estrogens and their receptors in breast cancer progression: a dual role in cancer proliferation and invasion. Crit Rev Oncol Hematol 51:55–67 [DOI] [PubMed] [Google Scholar]
- Persson I 2000 Estrogens in the causation of breast, endometrial and ovarian cancers: evidence and hypotheses from epidemiological findings. J Steroid Biochem Mol Biol 74:357–364 [DOI] [PubMed] [Google Scholar]
- Riggs BL, Khosla S, Melton 3rd LJ 2002 Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev 23:279–302 [DOI] [PubMed] [Google Scholar]
- Manolagas SC, Kousteni S, Jilka RL 2002 Sex steroids and bone. Recent Prog Horm Res 57:385–409 [DOI] [PubMed] [Google Scholar]
- Syed F, Khosla S 2005 Mechanisms of sex steroid effects on bone. Biochem Biophys Res Commun 328:688–696 [DOI] [PubMed] [Google Scholar]
- Nakamura T, Imai Y, Matsumoto T, Sato S, Takeuchi K, Igarashi K, Harada Y, Azuma Y, Krust A, Yamamoto Y, Nishina H, Takeda S, Takayanagi H, Metzger D, Kanno J, Takaoka K, Martin TJ, Chambon P, Kato S 2007 Estrogen prevents bone loss via estrogen receptor α and induction of Fas ligand in osteoclasts. Cell 130:811–823 [DOI] [PubMed] [Google Scholar]
- Di Gregorio GB, Yamamoto M, Ali AA, Abe E, Roberson P, Manolagas SC, Jilka RL 2001 Attenuation of the self-renewal of transit-amplifying osteoblast progenitors in the murine bone marrow by 17β-estradiol. J Clin Invest 107:803–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding XF, Anderson CM, Ma H, Hong H, Uht RM, Kushner PJ, Stallcup MR 1998 Nuclear receptor-binding sites of coactivators glucocorticoid receptor interacting protein 1 (GRIP1) and steroid receptor coactivator 1 (SRC-1): multiple motifs with different binding specificities. Mol Endocrinol 12:302–313 [DOI] [PubMed] [Google Scholar]
- Webb P, Nguyen P, Shinsako J, Anderson C, Feng W, Nguyen MP, Chen D, Huang SM, Subramanian S, McKinerney E, Katzenellenbogen BS, Stallcup MR, Kushner PJ 1998 Estrogen receptor activation function 1 works by binding p160 coactivator proteins. Mol Endocrinol 12:1605–1618 [DOI] [PubMed] [Google Scholar]
- Norris JD, Fan D, Stallcup MR, McDonnell DP 1998 Enhancement of estrogen receptor transcriptional activity by the coactivator GRIP-1 highlights the role of activation function 2 in determining estrogen receptor pharmacology. J Biol Chem 273:6679–6688 [DOI] [PubMed] [Google Scholar]
- Kim JH, Yang CK, Stallcup MR 2006 Downstream signaling mechanism of the C-terminal activation domain of transcriptional coactivator CoCoA. Nucleic Acids Res 34:2736–2750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes DR, Kalyana-Sundaram S, Mahavisno V, Varambally R, Yu J, Briggs BB, Barrette TR, Anstet MJ, Kincead-Beal C, Kulkarni P, Varambally S, Ghosh D, Chinnaiyan AM 2007 Oncomine 3.0: genes, pathways, and networks in a collection of 18,000 cancer gene expression profiles. Neoplasia 9:166–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducy P, Karsenty G 1995 Two distinct osteoblast-specific cis-acting elements control expression of a mouse osteocalcin gene. Mol Cell Biol 15:1858–1869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G 1997 Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell 89:747–754 [DOI] [PubMed] [Google Scholar]
- Banerjee C, McCabe LR, Choi JY, Hiebert SW, Stein JL, Stein GS, Lian JB 1997 Runt homology domain proteins in osteoblast differentiation: AML3/CBFA1 is a major component of a bone-specific complex. J Cell Biochem 66:1–8 [DOI] [PubMed] [Google Scholar]
- Pratap J, Javed A, Languino LR, van Wijnen AJ, Stein JL, Stein GS, Lian JB 2005 The Runx2 osteogenic transcription factor regulates matrix metalloproteinase 9 in bone metastatic cancer cells and controls cell invasion. Mol Cell Biol 25:8581–8591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Q, Manolagas SC, O'Brien CA 2006 Parathyroid hormone controls receptor activator of NF-κB ligand gene expression via a distant transcriptional enhancer. Mol Cell Biol 26:6453–6468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan VC, O'Malley BW 2007 Selective estrogen-receptor modulators and antihormonal resistance in breast cancer. J Clin Oncol 25:5815–5824 [DOI] [PubMed] [Google Scholar]
- Tamrazi A, Carlson KE, Katzenellenbogen JA 2003 Molecular sensors of estrogen receptor conformations and dynamics. Mol Endocrinol 17:2593–2602 [DOI] [PubMed] [Google Scholar]
- Wang Y, Klijn JG, Zhang Y, Sieuwerts AM, Look MP, Yang F, Talantov D, Timmermans M, Meijer-van Gelder ME, Yu J, Jatkoe T, Berns EM, Atkins D, Foekens JA 2005 Gene-expression profiles to predict distant metastasis of lymph-node-negative primary breast cancer. Lancet 365:671–679 [DOI] [PubMed] [Google Scholar]
- Desmedt C, Sotiriou C 2006 Proliferation: the most prominent predictor of clinical outcome in breast cancer. Cell Cycle 5:2198–2202 [DOI] [PubMed] [Google Scholar]
- van de Vijver MJ, He YD, van't Veer LJ, Dai H, Hart AA, Voskuil DW, Schreiber GJ, Peterse JL, Roberts C, Marton MJ, Parrish M, Atsma D, Witteveen A, Glas A, Delahaye L, van der Velde T, Bartelink H, Rodenhuis S, Rutgers ET, Friend SH, Bernards R 2002 A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med 347:1999–2009 [DOI] [PubMed] [Google Scholar]
- Hecht J, Seitz V, Urban M, Wagner F, Robinson PN, Stiege A, Dieterich C, Kornak U, Wilkening U, Brieske N, Zwingman C, Kidess A, Stricker S, Mundlos S 2007 Detection of novel skeletogenesis target genes by comprehensive analysis of a Runx2−/− mouse model. Gene Expr Patterns 7:102–112 [DOI] [PubMed] [Google Scholar]
- Young DW, Hassan MQ, Yang XQ, Galindo M, Javed A, Zaidi SK, Furcinitti P, Lapointe D, Montecino M, Lian JB, Stein JL, van Wijnen AJ, Stein GS 2007 Mitotic retention of gene expression patterns by the cell fate-determining transcription factor Runx2. Proc Natl Acad Sci USA 104:3189–3194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaes BL, Ducy P, Sijbers AM, Hendriks JM, van Someren EP, de Jong NG, van den Heuvel ER, Olijve W, van Zoelen EJ, Dechering KJ 2006 Microarray analysis on Runx2-deficient mouse embryos reveals novel Runx2 functions and target genes during intramembranous and endochondral bone formation. Bone 39:724–738 [DOI] [PubMed] [Google Scholar]
- Kousteni S, Bellido T, Plotkin LI, O'Brien CA, Bodenner DL, Han L, Han K, DiGregorio GB, Katzenellenbogen JA, Katzenellenbogen BS, Roberson PK, Weinstein RS, Jilka RL, Manolagas SC 2001 Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: dissociation from transcriptional activity. Cell 104:719–730 [PubMed] [Google Scholar]
- Castro-Rivera E, Samudio I, Safe S 2001 Estrogen regulation of cyclin D1 gene expression in ZR-75 breast cancer cells involves multiple enhancer elements. J Biol Chem 276:30853–30861 [DOI] [PubMed] [Google Scholar]
- Sims NA, Clement-Lacroix P, Minet D, Fraslon-Vanhulle C, Gaillard-Kelly M, Resche-Rigon M, Baron R 2003 A functional androgen receptor is not sufficient to allow estradiol to protect bone after gonadectomy in estradiol receptor-deficient mice. J Clin Invest 111:1319–1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims NA, Dupont S, Krust A, Clement-Lacroix P, Minet D, Resche-Rigon M, Gaillard-Kelly M, Baron R 2002 Deletion of estrogen receptors reveals a regulatory role for estrogen receptors-β in bone remodeling in females but not in males. Bone 30:18–25 [DOI] [PubMed] [Google Scholar]
- Pearce ST, Jordan VC 2004 The biological role of estrogen receptors α and β in cancer. Crit Rev Oncol Hematol 50:3–22 [DOI] [PubMed] [Google Scholar]
- Duss S, Andre S, Nicoulaz AL, Fiche M, Bonnefoi H, Brisken C, Iggo RD 2007 An oestrogen-dependent model of breast cancer created by transformation of normal human mammary epithelial cells. Breast Cancer Res 9:R38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawate H, Wu Y, Ohnaka K, Takayanagi R 2007 Mutual transactivational repression of Runx2 and the androgen receptor by an impairment of their normal compartmentalization. J Steroid Biochem Mol Biol 105:46–56 [DOI] [PubMed] [Google Scholar]
- Jilka RL, Takahashi K, Munshi M, Williams DC, Roberson PK, Manolagas SC 1998 Loss of estrogen up-regulates osteoblastogenesis in the murine bone marrow. Evidence for autonomy from factors released during bone resorption. J Clin Invest 101:1942–1950 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.