Abstract
Circulating angiotensin II (ANGII) elicits water intake and activates the hypothalamic-pituitary-adrenal (HPA) axis by stimulating angiotensin type 1 receptors (AT1Rs) within circumventricular organs. The subfornical organ (SFO) and the organum vasculosum of the lamina terminalis (OVLT) are circumventricular organs that express AT1Rs that bind blood-borne ANGII and stimulate integrative and effector regions of the brain. The goal of these studies was to determine the contribution of AT1Rs within the SFO and OVLT to the water intake and HPA response to increased circulating ANGII. Antisense oligonucleotides directed against the AT1R [AT1R antisense (AT1R AS)] were administered into the OVLT or SFO. Quantitative receptor autoradiography confirmed that AT1R AS decreased ANGII binding in the SFO and OVLT compared with the scrambled sequence control but did not affect AT1R binding in other nuclei. Subsequently, water intake, ACTH, and corticosterone (CORT) were assessed after administration of isoproterenol, a β-adrenergic agonist that decreases blood pressure and elevates circulating ANGII. Delivery of AT1R AS into the SFO attenuated water intake, ACTH, and CORT after isoproterenol, whereas similar treatment in the OVLT had no effect. To determine the specificity of this blunted drinking and HPA response, the same parameters were measured after treatment with hypertonic saline, a stimulus that induces drinking independently of ANGII. Delivery of AT1R AS into the SFO or OVLT had no effect on water intake, ACTH, or CORT after hypertonic saline. The results imply that AT1R within the SFO mediate drinking and HPA responses to stimuli that increase circulating ANGII.
CHALLENGES TO HOMEOSTASIS are met with activation of neural and endocrine systems aimed at alleviating perturbations in the internal environment. One such system, the renin-angiotensin system (RAS), is critically involved in the maintenance of blood pressure and hydromineral balance. Blood loss, hypotension, or sodium depletion activates the RAS and increases circulating levels of angiotensin II (ANGII). ANGII in turn mediates compensatory responses to these perturbations by stimulating angiotensin type 1 receptors (AT1Rs) in the periphery and brain. In the periphery, ANGII elicits vasoconstriction and renal sodium reabsorption by binding to AT1R on blood vessels. In the brain, ANGII binds to AT1R to activate the sympathetic nervous system and hypothalamic-pituitary-adrenal (HPA) axis, and to increase water and sodium consumption (1,2).
Although much is known about the effects of ANGII on vasculature reactivity and renal sodium handling, the central pathways governing behavioral and endocrine responses to ANGII remain unclear. Central AT1Rs mediate the water intake and the ACTH secretion resulting from increased circulating ANGII (3,4), but the specific brain areas contributing to these responses are undefined. In this regard, recent studies suggest that central AT1Rs are capable of eliciting different behavioral responses by stimulating distinct second messenger systems (5), and it is possible that these receptor populations are anatomically segregated. That is, brain regions expressing AT1Rs may make differential contributions to responses initiated by generalized activation of the RAS. Thus, the role of individual brain regions in the mediation of water intake and HPA activation after increased circulating ANGII has not been unequivocally discerned.
Although AT1Rs are present in several brain regions (6), it is believed that circulating ANGII elicits water intake and HPA activation by stimulating AT1Rs within forebrain circumventricular organs (CVOs) such as the subfornical organ (SFO) and the organum vasculosum of the lamina terminalis (OVLT). Each expresses AT1Rs that bind blood-borne ANGII and stimulate integrative and effector regions of the brain (7). The goal of the present experiments was to determine the contribution of AT1Rs within the SFO or OVLT to the water intake and HPA response that are elicited by increased circulating ANGII. To accomplish this goal, antisense oligonucleotides directed against the AT1Rs were administered into the SFO or OVLT, and specific inhibition of AT1R expression was confirmed by quantitative receptor autoradiography. Subsequently, water intake, ACTH, and corticosterone (CORT) were measured after administration of isoproterenol, a β-adrenergic agonist that decreases blood pressure and greatly elevates circulating ANGII (8,9,10). To determine the specificity of any observed effects, the same parameters were assessed after treatment with hypertonic saline, a stimulus that elicits water intake and HPA activation independent of ANGII. The results indicate that AT1Rs within the SFO contribute to the drinking and ACTH/CORT release that follows systemic administration of isoproterenol.
Materials and Methods
Animals
Adult male Sprague Dawley rats (Harlan, Indianapolis, IN) weighing 250–300 g at the beginning of the study were used. Rats arrived at least 2 wks before the onset of the experiment and were individually housed on a 12-h light, 12-h dark cycle (0600–1800 h) with ad libitum access to pelleted rat chow (LM-485; Harlan Teklad, Madison, WI) and water unless noted otherwise. All procedures were approved by the University of Cincinnati Internal Animal Care and Use Committee. Body weights were monitored throughout the course of the study.
Oligonucleotides
The AT1R antisense (AT1R AS) was constructed based on the 5′-initiation region of the AT1R gene; the sequence was 5′-AGAAGAGTTAAGGGCCAT-3′. We have found that this sequence selectively reduces AT1R expression in the brain as well as ANGII-induced water intake (11,12). In addition, those previous studies established that administration of AT1R AS does not affect daily fluid intake and that receptor function returns after cessation of antisense treatment. The control oligonucleotide probe was constructed based on a random sequence (5′-CCCTTTGAAGGTTCCGAA-3′). Oligonucleotide probes were obtained from Oligos Etc., Inc. (Wilsonville, OR).
Stereotaxic surgery
For all experiments rats were anesthetized under ketamine HCl (100 mg/kg body wt, ip; Bristol Laboratories, Syracuse, NY) and acepromazine (1.37 mg/kg body wt, ip; Ayerst Laboratories, Inc., New York, NY); subsequently, microinjection cannulae (Plastics One Inc., Roanoke, VA) were stereotaxically implanted in the SFO (26 gauge) or OVLT (22 gauge). The stereotaxic coordinates from bregma, using a 10° lateral angle from the vertical plane, were as follows: SFO, anterior −1.2 mm, lateral 1.0 mm, ventral −5.6 mm; and OVLT, anterior 0.7 mm, lateral 1.0 mm, and ventral −7.8 mm. One week of recovery was allowed for body weight to return to presurgical levels before testing was initiated.
Confirmation of cannula placement
Behavioral verification.
Rats were pulse injected with 1 μl of 10 ng ANGII or 0.5 m NaCl into the SFO or OVLT, respectively. Subsequently, water intake was measured for 1 h. Rats that consumed more than 5 ml water in 1 h were considered hits and included in further studies.
Histological verification.
At the time of being killed, all rats were pulse injected with 1 μl Chicago blue dye, brains were removed and sectioned, and cannula placement was evaluated. If the tract of the guide cannula was not adjacent to the SFO or OVLT and/or caused damage to these or surrounding nuclei, then the subject was excluded from the study.
Biotinylated AT1R AS.
A subset of animals (n = 8) was administered (0.25 μl/min over 4 min) in the SFO or OVLT with 500 ng/μl biotinylated AT1R AS each day for 3 consecutive days to determine the localization of the injection. Three hours after the final injection, rats were anesthetized with an overdose of sodium pentobarbital and perfused with 200 ml 0.9% NaCl that included 200 U sodium heparin at room temperature, followed by 500 ml cold 4% paraformaldehyde in 0.1 m phosphate buffer (pH 7.4). The brains were removed, blocked, and postfixed overnight in the same fixative. Brains were cut into 40 μm coronal sections on a cryostat and frost mounted onto microscope slides. Subsequent immunohistochemical processing involved washing the sections three times for 5 min in the 0.1 m Tris-buffered saline (TBS) (pH 7.5). They were then incubated for 30 min with 2% normal goat serum and 0.2% Triton X-100 in 0.1 TBS. The sections were washed again in TBS for 5 min then incubated in Strep-avidin-horseradish peroxidase (1:3000) diluted in 2% normal goat serum-TBS for 2 h. The sections were then washed in TBS, and the final staining of the sections was achieved with 50 mg 3.3′ diaminobenzidine tetrahydrochloride in 100 ml TBS with 20 μl 30% H2O2 for 5 min, followed by three TBS rinses. Sections were finally dried, dehydrated, and coverslipped. Subsequently, the distribution of the injection and the integrity of the nuclei were qualitatively examined.
Receptor autoradiography
One week after behavioral testing of cannula placement, rats were injected (0.25 μl/min over 4 min) in the SFO or OVLT with 500 ng/μl AT1R AS or the scrambled sequence control (scram) once daily in the morning for 3 consecutive days. Our previous studies have found this concentration of AT1R AS to maximally decrease ANGII-induced water intake when administered into the third ventricle (12). Three hours after the final injection, rats were killed, brains were removed, frozen in dry ice, sectioned at 20 μm, and stored at −80 C. Receptor autoradiography was performed as previously described (13). Briefly, sections were dried and preincubated in 50 mm Tris (pH 7.4), 150 mm 0.15 NaCl, 10 mm MgCl2, and 0.2% BSA for 30 min at room temperature. Slides then were immersed for 1 h in fresh buffer to which 0.1 trypsin inhibitor unit/ml aprotinin, 0.1 mg/ml 1, 10-phenanthroline, and 0.5 nm [125I]AngII (2200 Ci/mmol) had been added. To terminate the assay, slides were washed three times for 5 min in ice-cold buffer [50 mm Tris (pH 7.4), 150 mm 0.15 NaCl, and 10 mm MgCl2], followed by a dip in ice-cold distilled water. Specific binding to the AT1Rs and angiotensin type 2 receptors and nonspecific binding were determined by incubating adjacent sections with PD 123319 (1 μm), losartan (1 μm), and unlabeled AngII (1 μm), respectively. Slides were allowed to dry completely before exposing them to Biomax MR Film (Kodak Industrie, Cedex, France) along with [125I]autoradiographic microscales (Amersham Biosciences, Pittsburgh, PA).
Image analysis
Films were quantified using computer-assisted densitometry (Scion Image 1.62; Scion Corp., Frederick, MD). Tissue background readings were subtracted from gray scale values from the region of interest. Gray scale values were converted into OD and averaged, providing one mean value per region per animal. To prevent sampling errors, OD measurements were averaged over at least three consecutive sections from each nucleus. Values were converted from OD and expressed as fmol/mg protein (14).
Blood sampling for analysis of plasma-renin activity (PRA)
A separate cohort of rats was used to determine the effect of AT1R AS, isoproterenol, and 2 m NaCl on PRA, an indicator of circulating levels of ANGII. Rats were injected in the SFO or OVLT with 500 ng/μl (0.25 μl/min over 4 min) AT1R AS or scram once daily in the morning for 3 consecutive days. Three hours after the last injection, rats were given isoproterenol (30 μg/kg sc) or 2 m NaCl (1 ml sc), and water bottles were removed. Fifteen minutes later, rats were killed, and trunk blood samples were collected in chilled tubes containing EDTA. Sampling began at 1000 h during the light phase, and samples were centrifuged at 4 C at 3500 rpm. Subsequently, plasma was stored at −80 C until analysis of PRA via RIA.
Water intake
Rats were injected (0.25 μl/min over 4 min) in the SFO or OVLT with 500 ng/μl AT1R AS or scram once daily in the morning for 3 consecutive days. Three hours after the last injection, rats were given isoproterenol (30 μg/kg sc). Rats then were given access to water, and intakes were recorded after 2 h. Experiments examining isoproterenol-induced water intake used a counterbalanced within-subjects design with 10 d between trials. Specifically, administration of AT1R AS and scram were counterbalanced. To determine the specificity of any observed effects, a separate cohort of rats was run through a similar experiment, but a between-subjects design was used, and water intake was elicited by injection of 2 m NaCl (1 ml sc).
Blood sampling for analysis of HPA response
A separate cohort of rats was used to determine the role of central AT1R in the HPA response to isoproterenol or 2 m NaCl, and these studies used a between-subjects design. Rats were injected in the SFO or OVLT with 500 ng/μl (0.25 μl/min over 4 min) AT1R AS or scram once daily in the morning for 3 consecutive days. Three hours after the last injection, rats were given isoproterenol (30 μg/kg sc) or 2 m NaCl (1 ml sc), and water bottles were removed. Tail blood samples (250–300 μl) were gently taken at 0, 15, 30, 60, and 120 min. Specifically, the distal 0.5 mm of the tail was removed using a sterile scalpel blade, and blood was allowed to drain directly into a chilled microcentrifuge tube containing EDTA. Using this method, adequate samples can be obtained in approximately 1–2 min, and serial samples are taken by gently removing the clot at the tip of the tail with a sterile piece of gauze. Previous studies have found that blood sampling using this method does not confound ACTH and CORT measurements during acute stress studies, and produces results similar to those obtained from venous catheter sampling (15). Blood sampling began at 1000 h during the light phase, and samples were centrifuged at 4 C at 3500 rpm.
RIA
Plasma ACTH was measured by RIA using an antiserum donated by Dr. William Engeland [University of Minnesota, Minneapolis, MN (16)] using 125I ACTH (Amersham Biosciences, Piscataway, NJ) as tracer. PRA was measured by RIA using a 125I kit from DiaSorin (Vercelli, Italy). Plasma CORT was measured by RIA using a 125I kit from MP Biomedicals (Orangeburg, NY).
Statistical analyses
All data are expressed as means ± sem. Data were analyzed using Statistica (StatSoft, Inc., Tulsa, OK). ANGII binding, 2 m NaCl-induced water intake, and area under the curve (AUC) were analyzed using a one-factor ANOVA with treatment (AS or scram) as the factor. Isoproterenol-induced water intake was assessed using a repeated measures ANOVA with treatment (AS or scram) as the factor. PRA was assessed using a factorial ANOVA with region (SFO or OVLT), treatment (AT1R AS or scram), and condition (isoproterenol or 2 m NaCl) as the factors. Plasma levels of CORT and ACTH were assessed using a two-factor repeated measures ANOVA with treatment (AS or scram) and time as the factors. Main effects or interactions (P < 0.05) were assessed with Student-Newman-Keuls tests.
Results
Biotinylated AT1R AS
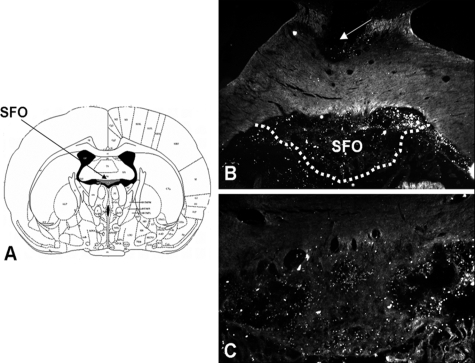
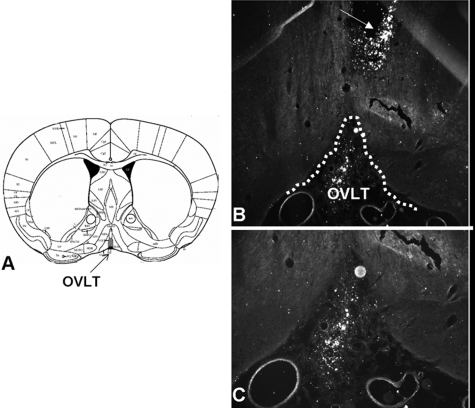
Figures 1 and 2 depict the labeling observed after three daily injections of biotinylated AT1R AS in the SFO or OVLT, respectively. As can be seen, despite the close apposition of the guide cannula to the regions of interest, these nuclei remain intact. Qualitative analysis found that labeling of AT1R AS was most concentrated in the SFO or OVLT but also slightly diffused into surrounding brain regions. Accordingly, quantitative receptor autoradiography was performed to determine the effect of AT1R AS on ANGII binding in the SFO or OVLT, relative to other forebrain regions expressing angiotensin receptors.
Figure 1.
Coronal sections through the SFO. A, Atlas section depicting the SFO. B, Low-power (×5) dark-field image of brain section from a rat implanted with cannula terminating in the SFO; arrow denotes cannula tract. C, High-power (×10) dark-field image of brain section from a rat administered biotinylated AT1R AS into the SFO. White dots are positive label for biotinylated AT1R AS.
Figure 2.
Coronal sections through the OVLT. A, Atlas section depicting the OVLT. B, Low-power (×5) dark-field image of brain section from a rat implanted with cannula terminating in the OVLT; arrow denotes cannula tract. C, High-power (×10) dark-field image of brain section from a rat administered biotinylated AT1R AS into the OVLT. White dots are positive label for biotinylated AT1R AS.
ANGII receptor binding
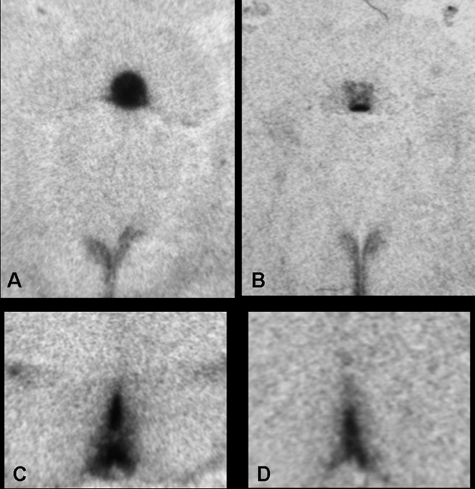
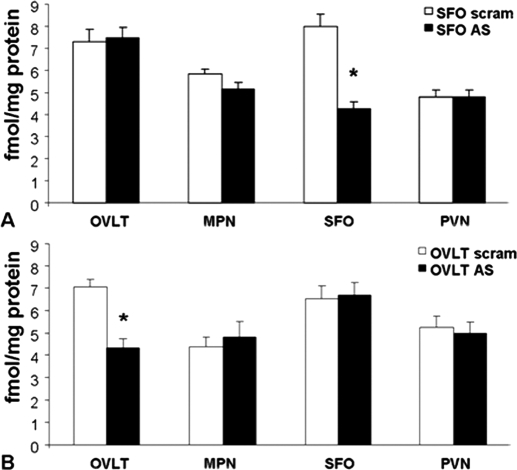
Delivery of AT1R AS into the SFO (n = 9) significantly reduced [F (1, 16) = 34.4; P < 0.0001] AT1R binding in this nucleus when compared with that of rats given the scrambled sequence control (n = 9; Fig. 3, A and B). However, as seen in Fig. 4A, injection of AT1R AS into the SFO had no effect on ANGII binding in other forebrain nuclei expressing AT1R. Similarly, administration of AT1R AS into the OVLT (n = 7) significantly reduced [F (1, 15) = 24.0; P < 0.001] AT1R binding in this nucleus when compared with that of rats given the scrambled sequence control (n = 10; Fig. 3, B and C), and injection of AT1R AS into the OVLT had no effect on ANGII binding in other forebrain nuclei (Fig. 4B). As we have previously found (13), angiotensin type 2 receptor binding was not detected in the nuclei examined.
Figure 3.
Autoradiographs of angiotensin receptor binding in rats injected with scram or AT1R AS into the SFO (top) or OVLT (bottom). A, ANGII binding in the SFO and PVN is unaffected by scram. B, ANGII binding in the SFO is decreased by AT1R AS; however, the PVN is unaffected. C, ANGII binding in the OVLT is unaffected by scram. D, ANGII binding in the OVLT is decreased by AT1R AS.
Figure 4.
Receptor autoradiography indicative of ANGII binding in forebrain nuclei containing angiotensin receptors. A, Rats were injected with scram or AT1R AS into the SFO. *, Significantly different from scram (P < 0.05). B, Rats were injected with scram or AT1R AS into the OVLT. *, Significantly different from scram. MPN, Median preoptic nucleus.
PRA after isoproterenol or 2 m NaCl
Figure 5 shows PRA after administration of isoproterenol or 2 m NaCl in rats given AT1R AS or scram in the SFO or OVLT. As can be seen, there was an effect of condition with isoproterenol significantly increasing (P < 0.01) PRA when compared with 2 m NaCl. There was no effect of region or treatment with rats given AT1R AS or scram in the SFO or OVLT having similar PRA.
Figure 5.
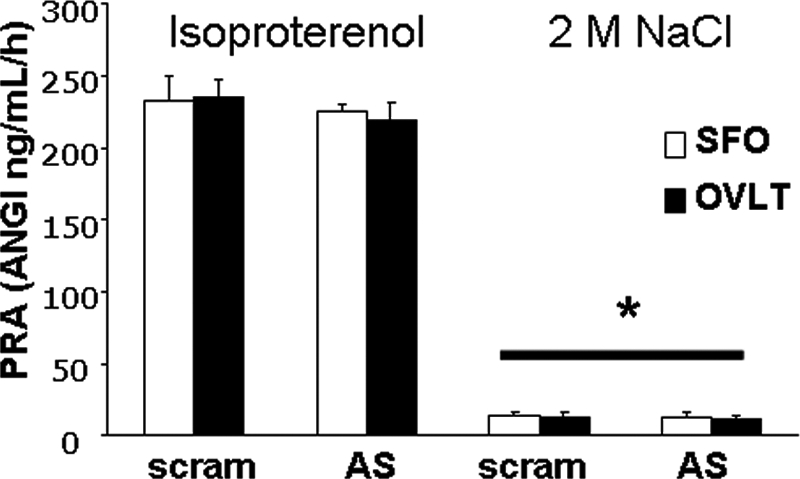
PRA in rats given scram or AT1R AS in the SFO or OVLT and then treated with isoproterenol or 2 m NaCl. *, Significantly different from isoproterenol (P < 0.001). ANGI, Angiotensin I, all groups n = 3.
Water intake elicited by isoproterenol or 2 m NaCl
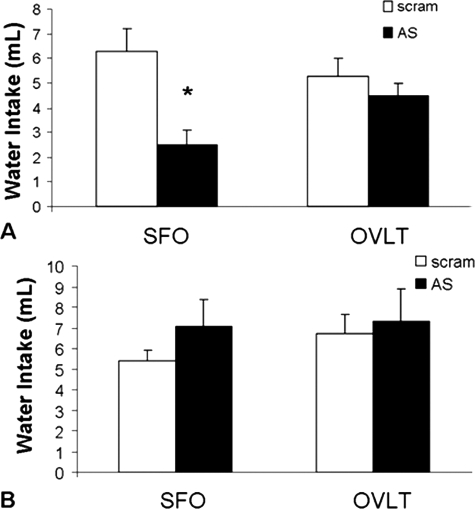
Figure 6A depicts isoproterenol-induced water intake of rats given AT1R AS or scram into the SFO or OVLT. Administration of AT1R AS in the SFO significantly decreased water intake after isoproterenol [F (1, 7) = 17.0; P < 0.01] when compared with scram-treated controls. However, similar administration into the OVLT had no such effect [F (1, 9) = 1.29; P = 0.28], with rats given AT1R AS and scram consuming similar amounts of water after isoproterenol. It should be noted that this experiment used a mixed within-subjects design and demonstrates that AT1R function returns 10 d after antisense administration. Thus, the decreased water intake observed after antisense administration is the result of selective inhibition of AT1Rs and is not the consequence of damage to these nuclei or the surrounding neuropil. To determine the specificity of the blunted drinking response, we examined water intake elicited by 2 m NaCl. Delivery of AT1R AS into the SFO or OVLT had no effect on water intake induced by 2 m NaCl (Fig. 6B).
Figure 6.
Water intake after injection of isoproterenol (top) or 2 m NaCl (bottom). A, AT1R AS given into the SFO significantly attenuates 2 h isoproterenol-elicited water intake. *, Significantly different from scram (P < 0.05). B, AT1R AS given into the SFO or OVLT has no effect on amount of water consumed 2 h after injection of 2 m NaCl.
HPA response to isoproterenol or 2 m NaCl
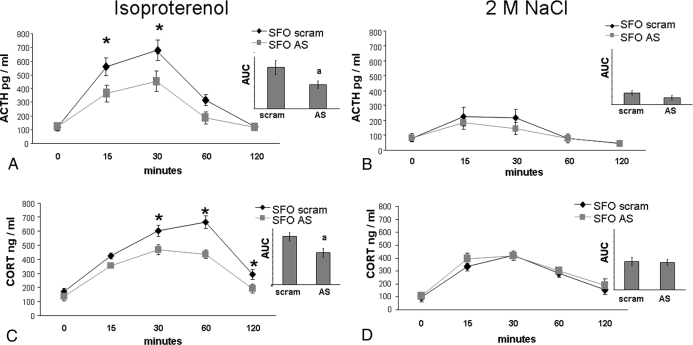
Figure 7A depicts the ACTH response to isoproterenol in rats given AT1R AS (n = 6) or scram (n = 6) into the SFO. There was an effect of time [F (4, 40) = 36.43; P < 0.001] on this response, with isoproterenol significantly elevating ACTH. Specifically, isoproterenol significantly (P < 0.05) increased ACTH above baseline at 15, 30, and 60 min in rats given AT1R AS or scram into the SFO. There was also an effect of treatment [F (1, 10) = 8.423; P < 0.5] on the ACTH response to isoproterenol. Post hoc analysis revealed that AT1R AS delivered into the SFO significantly decreased ACTH at 15 and 30 min (P < 0.05) when compared with ACTH levels of rats administered scram. In addition, rats treated with AT1R AS in the SFO had a significantly smaller AUC when compared with their scram-treated counterparts [F (1, 10) = 5.86; P < 0.05); Fig. 7A, inset]. To determine the specificity of this blunted response, ACTH was assessed after administration of 2 m NaCl in rats given AT1R AS (n = 10) or scram (n = 10) into the SFO. As expected, there was an effect of time [F (4, 72) = 16.41; P < 0.001] with 2 m NaCl significantly increasing ACTH above baseline at 15 and 30 min (P < 0.001; Fig. 7B). However, similar to what occurred with water intake, AT1R AS in the SFO had no effect on the ACTH response to 2 m NaCl. When AUC was examined, there were also no differences (Fig. 7B, inset).
Figure 7.
HPA response to isoproterenol (left) or 2 m NaCl (right). A, AT1R AS delivered into the SFO significantly decreases the ACTH response to isoproterenol. *, Significantly different from AT1R AS (P < 0.05); a, significantly different from scram (P < 0.05). B, AT1R AS in the SFO has no effect on the ACTH response to 2 m NaCl. C, AT1R AS delivered into the SFO significantly decreases the CORT response to isoproterenol. *, Significantly different from AT1R AS (P < 0.05); a, significantly different from scram (P < 0.05). D, AT1R AS in the SFO has no effect on the CORT response to 2 m NaCl.
To determine the influence of AT1R within the SFO on another level of the HPA axis, CORT was measured after isoproterenol. As can be seen in Fig. 7C, isoproterenol treatment produced an effect of time [F (4, 64) = 66.65; P < 0.001] on CORT. Specifically, isoproterenol significantly increased (P < 0.001) CORT above baseline at 15, 30, and 60 min in rats given AT1R AS into the SFO, whereas levels in rats given scram were significantly increased (P < 0.001) above basal levels at 15, 30, 60, and 120 min. There also was an effect of treatment [F (1, 16) = 33.61; P < 0.001] on the CORT response to isoproterenol. Post hoc analysis revealed that AT1R AS delivered into the SFO significantly decreased CORT at 30, 60, and 120 min (P < 0.05) when compared with that of rats administered scram. In addition, rats treated with AT1R AS into the SFO had a significantly smaller AUC when compared with their scram-treated counterparts [F (1, 18) = 6.82; P < 0.05; Fig. 7C, inset]. Once again, to determine the specificity of these effects, we examined CORT after administration of 2 m NaCl. As expected, there was an effect of time [F (4, 76) = 33.7, P < 0.001] on the CORT response to 2 m NaCl, with significant increases (P < 0.05) from baseline occurring at 15, 30, 60, and 120 min. However, there was no effect of treatment with rats given AT1R AS or scram into the SFO, having similar increases in CORT after 2 m NaCl (Fig. 7D). There were also no differences in the AUC (Fig. 7D, inset).
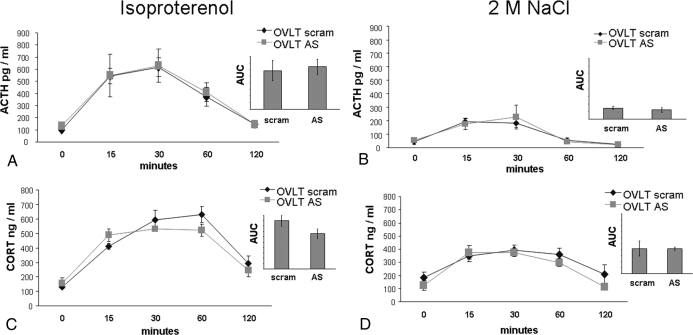
We also evaluated the contribution of AT1R within the OVLT to the HPA response to isoproterenol or 2 m NaCl. Administration of isoproterenol produced an effect [F (4, 36) = 21.2; P < 0.001] of time on ACTH, with significant increases (P < 0.05) above basal levels occurring at 15, 30, and 60 min (Fig. 8A). The increase of ACTH was similar in rats given AT1R AS (n = 4) or scram (n = 7) into the OVLT. There were no differences in the AUC (Fig. 8A, inset). Administration of 2 m NaCl produced an effect of time on ACTH levels [F (4, 64) = 17.1; P < 0.001], with 2 m NaCl significantly increasing (P < 0.05) ACTH above baseline at 15 and 30 min. There was no effect of treatment, with rats given AT1R AS (n = 9) or scram (n = 8) into the OVLT having similar increases in ACTH after 2 m NaCl. There were also no differences in the AUC (Fig. 8B, inset).
Figure 8.
HPA response to isoproterenol (left) or 2 m NaCl (right). A, AT1R AS delivered into the OVLT had no effect on the ACTH response to isoproterenol. B, AT1R AS in the OVLT has no effect on the ACTH response to 2 m NaCl. C, AT1R AS in the OVLT has no effect on the CORT response to isoproterenol. D, AT1R AS in the OVLT has no effect on the CORT response to 2 m NaCl.
To determine the influence of AT1R within the OVLT on another level of the HPA axis, CORT was measured after isoproterenol or 2 m NaCl. Figure 8C depicts the CORT response to isoproterenol in rats given AT1R AS (n = 9) or scram (n = 11) into the OVLT. There was an effect of time [F (4, 64) = 38.46, P < 0.05], with isoproterenol significantly increasing (P < 0.05) CORT above baseline at 0, 15, 30, 60, and 120 min. There was no effect of treatment, with rats given AT1R AS or scram into the OVLT having similar increases in CORT after isoproterenol. There were also no differences in the AUC (Fig. 8C, inset). Figure 8D depicts the CORT response to 2 m NaCl in rats given AT1R AS (n = 9) or scram (n = 9) into the OVLT. There was an effect of time [F (4, 64) = 15.7; P < 0.001], with 2 m NaCl significantly increasing (P < 0.05) CORT above baseline at 15, 30, and 60 min. There was no effect of treatment, with rats given AT1R AS or scram into the OVLT having similar increases in CORT after 2 m NaCl. There were also no differences in the AUC (Fig. 8D, inset).
Discussion
The goal of these experiments was to evaluate the role of central AT1Rs in the drinking and HPA responses to increased circulating ANGII. Antisense oligonucleotides directed against the AT1R administered into either the SFO or the OVLT significantly decreased ANGII binding in each nucleus. However, only inhibition of AT1Rs in the SFO blunted the drinking and HPA responses in paradigms where circulating ANGII was increased, whereas similar inhibition of binding of these receptors in the OVLT had no such effect. Moreover, knockdown of AT1Rs in these CVOs did not affect the drinking or the HPA response to administration of 2 m NaCl. Together, these results strongly imply that the AT1Rs in the SFO mediate both the drinking and HPA response that accompany elevated circulating ANGII. To our knowledge, this study demonstrates for the first time that endogenously generated ANGII specifically acts on AT1Rs within the SFO to elicit activation of the HPA axis.
ANGII is a major player in body fluid homeostasis, and administration of the β-adrenergic agonist, isoproterenol, greatly increases endogenous levels of this peptide (8,9,10). Although the drinking that follows extreme hypotension may be mediated, in part, by baroreceptor activation (17), drinking elicited by isoproterenol is believed to be dependent on increased circulating ANGII because interference with the biosynthesis of ANGII markedly reduces the elicited water intake. Specifically, isoproterenol-induced water intake is abolished by nephrectomy, which eliminates RAS activation during hypotension (18,19). Moreover, pharmacological inhibition of the biosynthesis of ANGII or angiotensin receptors greatly reduces β-adrenergic drinking (4,20,21,22,23). Finally, a more recent study demonstrates that hydralazine administration, which causes hypotension but disrupts the formation of circulating ANGII, does not produce a significant water intake (8). Thus, several studies from different laboratories strongly suggest that the drinking response accompanying administration of isoproterenol is dependent on increased circulating ANGII binding to AT1Rs.
Isoproterenol-elicited Fos induction in the SFO and OVLT can be significantly reduced by peripheral pretreatment with AT1R antagonists (24), and these findings suggest that increased circulating ANGII stimulates AT1Rs in the SFO or OVLT, which in turn, activates the central pathways mediating water intake. In the present study, administration of AT1R AS significantly reduced ANGII binding in the SFO and OVLT but did not affect binding in other forebrain nuclei expressing angiotensin receptors. In this regard, inhibition of AT1Rs in SFO, but not in OVLT, significantly attenuated isoproterenol-induced water intake. To determine the specificity of this blunted response, we examined drinking after 2 m NaCl, which elicits a water intake comparable to isoproterenol, but does so independently of circulating ANGII. Interestingly, water intake after 2 m NaCl was unaffected by AT1R AS, thereby demonstrating that the blunted intake is specific to ANGII-induced thirst. These results are consistent with those of previous research that used lesion and pharmacological approaches to determine the site of action for isoproterenol-induced drinking (19,25).
Thus, we demonstrate that AT1R AS can be used to inhibit ANGII binding in specific nuclei and produce results that are consistent with the existing literature, thereby allowing the use of this technique to determine the role of central AT1Rs in the HPA response to injection of isoproterenol or 2 m NaCl.
Hypotension and dehydration are physiological stressors that elicit activation of the HPA axis (26,27). Briefly, physiological and psychological stressors trigger activation of the medial parvocellular neurons within the hypothalamic paraventricular nucleus (PVN). These neurons project to the median eminence and secrete CRH into the portal vascular system to stimulate the synthesis of proopiomelanocortin, which is subsequently processed into ACTH. ACTH is released into the blood from the anterior pituitary and causes the adrenal gland to secrete CORT into the systemic circulation where it gains access to and binds to glucocorticoid receptors in various tissues. Alternatively, sympathetic innervation of the adrenal may influence the release of CORT by altering sensitivity to ACTH or by modulating glucocorticoid secretion directly through a neural mechanism (28,29).
Anatomical, pharmacological, and electrophysiological studies have implicated the SFO as a contributor to HPA axis activation. The SFO has numerous projections to the subdivisions of the PVN, including the medial parvocellular neurons that govern HPA activation (30,31). The connectivity of the SFO, considered in conjunction with its incomplete blood-brain barrier, suggests that circulating hormones act on receptors in this nucleus to elicit secretion of CRH and ACTH. In this regard, iv injection of ANGII produced dose-dependent elevations in ACTH release and CRH content of the median eminence; however, these increases were reduced or abolished by peripheral administration of the angiotensin receptor antagonist, saralasin, which inhibits ANGII binding in CVOs (3). Subsequent electrophysiological studies demonstrated that stimulation of the SFO by ANGII elicited activation of medial parvocellular neurons in the PVN and increased circulating ACTH (32,33). Together, these studies suggest that circulating ANGII acts on the SFO to contribute to activation of the HPA axis, although whether this actually occurs after stressors that activate the RAS remains to be discerned.
In the present study, AT1R AS delivered into the SFO significantly reduced ACTH and CORT after isoproterenol, and this is consistent with the hypothesized role of the SFO in the mediation of the HPA response to stressors that activate the RAS. Although central AT1Rs have been implicated in the HPA response to a variety of stressors (34), our results suggest that separate populations of these receptors differentially contribute to this response. Inhibition of AT1Rs in the SFO had no effect on the HPA response to 2 m NaCl, indicating that these receptors specifically contribute to the increase in ACTH and CORT that follows increased circulating ANGII. Reduced AT1R binding in the OVLT did not affect HPA activation after isoproterenol or 2 m NaCl, suggesting that this degree of inhibition does not influence the HPA response. Rather, the AT1Rs in the OVLT have been implicated in the salt intake and vasopressin secretion that follows increased circulating ANGII (35,36). It should be noted that AT1Rs have been localized to parvocellular neurons in the PVN with identified projections to the median eminence, and such neurons often contain CRH (37). Although the AT1Rs in the PVN can be activated by angiotensinergic projections from the SFO (38), it is unlikely that these receptors bind blood-borne ANGII. Therefore, it is likely that the AT1Rs in the PVN contribute to the release of ACTH after a variety of other kinds of stressors, whereas those within the SFO specifically mediate responses to increased circulating ANGII.
In addition to eliciting drinking and stimulating the HPA axis, ANGII is a potent activator of the sympathetic nervous system. Given that stressors that activate the sympathetic nervous system can directly influence the secretion of CORT by acting on the adrenal, it is possible that inhibition of central AT1Rs blunt the release of CORT by dampening the influence of ANGII on sympathetic outflow. However, it is unlikely that the attenuated CORT that we observed in animals given AT1R AS in the SFO was solely the result of dampened sympathetic nerve activity because ACTH release, which also drives CORT secretion, was attenuated as well. That is, the blunted HPA activation that occurred after isoproterenol is likely due, in part, to the decreased ANGII-induced stimulation of CRH containing neurons in the PVN.
Although isoproterenol and 2 m NaCl were comparable elicitors of water intake, isoproterenol was a more potent activator of the HPA axis. Specifically, administration of each stimulus significantly increased ACTH and CORT above basal levels, but isoproterenol was approximately three times more effective at doing so. It is possible that the contribution of AT1Rs in the SFO is only revealed when stressors that potently activate the HPA axis are used. However, such stressors increase renal sympathetic nerve activity (39,40), which in turn, activates the RAS (41). Thus, elevated circulating ANGII likely accompanies robust activation of the HPA axis, thereby making it difficult to delineate these effects.
In contrast to the water intake that follows isoproterenol or 2 m NaCl, the HPA response associated with administration of these stimuli cannot be solely attributed to increased circulating ANGII or osmotic dehydration, respectively. Hypotension elicits activation of brainstem neurons with identified projections to the PVN (42), and it is generally accepted that this pathway has an excitatory influence on the HPA axis. Similarly, it is unclear whether osmotic dehydration is solely responsible for the HPA response that follows 2 m NaCl injection. Although osmotic dehydration increases ACTH secretion in horses (43), increased plasma osmolarity suppresses activation of the HPA axis in rats (44). Therefore, it is likely that the HPA response that we observed after administration of hypertonic saline is attributed, in part, to the pain of the injection, consistent with another study using this paradigm (45). However, regardless of the complexity of the stimuli that we used, our results clearly demonstrate that inhibition of AT1Rs in the SFO blunted the HPA response to a stressor that greatly increases circulating ANGII (i.e. isoproterenol) but had no effect on ACTH and CORT release after acute hypernatremia (i.e. 2 m NaCl), which suppresses activation of the RAS (46). Thus, the blunted ACTH and CORT that accompanied administration of AT1R AS into the SFO is specific to a stressor that increased circulating ANGII.
In summary, inhibition of AT1Rs in the SFO reduced the drinking and HPA responses to increased circulating ANGII. This effect was specific to AT1Rs in the SFO because similar inhibition of AT1Rs in the OVLT had no such effect. Moreover, water intake and HPA activation induced by 2 m NaCl was unaffected by AT1R AS, suggesting that AT1Rs in the SFO selectively influence responses to increased circulating ANGII. Collectively, our results indicate that the AT1Rs in the SFO mediate compensatory behavioral and endocrine responses to stressors that produce activation of the RAS.
Acknowledgments
We thank James P. Herman, Mary Nguyen, Yvonne Ulrich-Lai, Ben Packard, and Clinton Elfers for their valuable advice and assistance.
Footnotes
This work was supported by National Institutes of Health DK79710 (to E.G.K.), DK66223 (to S.C.B.), DK68273 (to R.R.S.), and DK66596 (to R.R.S.).
Disclosure Statement: EG.K., S.J.M., J.F.D., K.A.S., L.Y.M., A.D.d.K., S.C.B., and R.R.S. have nothing to declare. S.C.W. consults for Sanofi-Aventis and McNeil Nutritionals and has received lecture fees from Merck.
First Published Online August 7, 2008
Abbreviations: ANGII, Angiotensin II; AT1R, angiotensin type 1 receptor; AT1R AS, angiotensin type 1 receptor antisense; AUC, area under the curve; CORT, corticosterone; CVO, circumventricular organ; HPA, hypothalamic-pituitary-adrenal; OVLT, organum vasculosum of the lamina terminalis; PRA, plasma-renin activity; PVN, paraventricular nucleus; RAS, renin-angiotensin system; SFO, subfornical organ; TBS, Tris-buffered saline.
References
- Fitzsimons JT 1998 Angiotensin, thirst, and sodium appetite. Physiol Rev 78:583–686 [DOI] [PubMed] [Google Scholar]
- Saavedra JM, Benicky J 2007 Brain and peripheral angiotensin II play a major role in stress. Stress 10:185–193 [DOI] [PubMed] [Google Scholar]
- Murakami K, Ganong WF 1987 Site at which angiotensin II acts to stimulate ACTH secretion in vivo. Neuroendocrinology 46:231–235 [DOI] [PubMed] [Google Scholar]
- Dourish CT, Duggan JA, Banks RJ 1992 Drinking induced by subcutaneous injection of angiotensin II in the rat is blocked by the selective AT1 receptor antagonist DuP 753 but not by the selective AT2 receptor antagonist WL 19. Eur J Pharmacol 211:113–116 [DOI] [PubMed] [Google Scholar]
- Daniels D, Yee DK, Faulconbridge LF, Fluharty SJ 2005 Divergent behavioral roles of angiotensin receptor intracellular signaling cascades. Endocrinology 146:5552–5560 [DOI] [PubMed] [Google Scholar]
- Lenkei Z, Palkovits M, Corvol P, Llorens-Cortes C 1998 Distribution of angiotensin type-1 receptor messenger RNA expression in the adult rat brain. Neuroscience 82:827–841 [DOI] [PubMed] [Google Scholar]
- McKinley MJ, Davern P, Giles ME, Penschow J, Sunn N, Uschakov A, Oldfield BJ 2003 The sensory circumventricular organs of the mammalian brain. Adv Anat Embryol Cell Biol 172:3–12 [DOI] [PubMed] [Google Scholar]
- Stocker SD, Sved AF, Stricker EM 2000 Role of renin-angiotensin system in hypotension evoked thirst: studies with hydralazine. Am J Physiol Regul Integr Comp Physiol 279:R576–R585 [DOI] [PubMed] [Google Scholar]
- Krause EG, Curtis KS, Davis LM, Stowe JR, Contreras RJ 2003 Estrogen influences stimulated water intake by ovariectomized female rats. Physiol Behav 79:267–274 [DOI] [PubMed] [Google Scholar]
- Krause EG, Curtis KS, Stincic TL, Markle JP, Contreras RJ 2006 Oestrogen and weight loss decrease isoproterenol-induced Fos immunoreactivity and angiotensin type 1 mRNA in the subfornical organ of female rats. J Physiol 573(Pt 1):251–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai RR, He PF, Yang XD, Ma LY, Guo YF, Reilly JJ, Moga CN, Fluharty SJ 1994 Intracerebroventricular administration of AT1 receptor antisense oligonucleotides inhibits the behavioral actions of angiotensin II. J Neurochem 62:2053–2056 [DOI] [PubMed] [Google Scholar]
- Sakai RR, Ma LY, He PF, Fluharty SJ 1995 Intracerebroventricular administration of angiotensin type 1 (AT1) receptor antisense oligonucleotides attenuate thirst in the rat. Regul Pept 59:183–192 [DOI] [PubMed] [Google Scholar]
- Kisley LR, Sakai RR, Fluharty SJ 1999 Estrogen decreases hypothalamic angiotensin II AT1 receptor binding and mRNA in the female rat. Brain Res 844:34–42 [DOI] [PubMed] [Google Scholar]
- Artymyshyn R, Smith A, Wolfe BB 1990 The use of 3H standards in 125I autoradiography. J Neurosci Methods 32:185–192 [DOI] [PubMed] [Google Scholar]
- Vahl TP, Ulrich-Lai YM, Ostrander MM, Dolgas CM, Elfers EE, Seeley RJ, D‘Alessio DA, Herman JP 2005 Comparative analysis of ACTH and corticosterone sampling methods in rats. Am J Physiol Endocrinol Metab 289:E823–E828 [DOI] [PubMed] [Google Scholar]
- Jasper MS, Engeland WC 1991 Synchronous ultradian rhythms in adrenocortical secretion detected by microdialysis in awake rats. Am J Physiol 261(5 Pt 2):R1257–R1268 [DOI] [PubMed] [Google Scholar]
- Rettig R, Ganten D, Johnson AK 1981 Isoproterenol-induced thirst: renal and extrarenal mechanisms. Am J Physiol 241:R152–R157 [DOI] [PubMed] [Google Scholar]
- Houpt KA, Epstein AN 1971 The complete dependence of β-adrenergic drinking on the renal dipsogen. Physiol Behav 7:897–902 [DOI] [PubMed] [Google Scholar]
- Gutman Y, Benzakein F, Livneh P 1971 Polydipsia induced by isoprenaline and by lithium: relation to kidneys and renin. Eur J Pharmacol 16:380–384 [DOI] [PubMed] [Google Scholar]
- Simpson JB, Epstein AN, Camardo JS 1978 Localization of receptors for the dipsogenic action of angiotensin II in the subfornical organ of rat. J Comp Physiol Psychol 92:581–601 [DOI] [PubMed] [Google Scholar]
- Katovich MJ, Barney CC, Fregly MJ, McCaa RE 1979 Effect of an angiotensin converting enzyme inhibitor (SQ 14,225) on β-adrenergic and angiotensin-induced thirsts. Eur J Pharmacol 56:123–130 [DOI] [PubMed] [Google Scholar]
- Evered MD, Robinson MM 1981 The renin-angiotensin system in drinking and cardiovascular responses to isoprenaline in the rat. J Physiol 316:357–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evered MD 1990 Relationship between thirst and diazoxide-induced hypotension in rats. Am J Physiol 259(2 Pt 2):R362–R370 [DOI] [PubMed] [Google Scholar]
- Oldfield BJ, McKinley MJ 1994 Distribution of Fos in rat brain resulting from endogenously-generated angiotensin II. Kidney Int 46:1567–1569 [DOI] [PubMed] [Google Scholar]
- Fitts DA 1994 Angiotensin II receptors in SFO but not in OVLT mediate isoproterenol-induced thirst. Am J Physiol 267(1 Pt 2):R7–R15 [DOI] [PubMed] [Google Scholar]
- Pralong FP, Corder R, Gaillard RC 1991 Responses of the rat pituitary-adrenal axis to hypotensive infusions of corticotropin-releasing factor, vasoactive intestinal peptide and other depressor agents. Regul Pept 32:217–226 [DOI] [PubMed] [Google Scholar]
- Windle RJ, Forsling ML, Smith CP, Balment RJ 1993 Patterns of neurohypophysial hormone release during dehydration in the rat. J Endocrinol 137:311–320 [DOI] [PubMed] [Google Scholar]
- Wilkinson CW, Shinsako J, Dallman MF 1982 Rapid decreases in adrenal and plasma corticosterone concentrations after drinking are not mediated by changes in plasma adrenocorticotropin concentration. Endocrinology 110:1599–1606 [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Engeland WC 2002 Adrenal splanchnic innervation modulates adrenal cortical responses to dehydration stress in rats. Neuroendocrinology 76:79–92 [DOI] [PubMed] [Google Scholar]
- Miselis RR 1981 The efferent projections of the subfornical organ of the rat: a circumventricular organ within a neural network subserving water balance. Brain Res 230:1–23 [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Swanson LW 1983 The organization of forebrain afferents to the paraventricular and supraoptic nuclei of the rat. J Comp Neurol 218:121–144 [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Sutton SW, Bruhn TO, Ferguson AV 1988 Analysis of the role of angiotensin II in mediation of adrenocorticotropin secretion. Endocrinology 122:538–545 [DOI] [PubMed] [Google Scholar]
- Ferguson AV 1988 Systemic angiotensin acts at the subfornical organ to control the activity of paraventricular nucleus neurons with identified projections to the median eminence. Neuroendocrinology 47:489–497 [DOI] [PubMed] [Google Scholar]
- Saavedra JM, Ando H, Armando I, Baiardi G, Bregonzio C, Juorio A, Macova M 2005 Anti-stress and anti-anxiety effects of centrally acting angiotensin II AT1 receptor antagonists. Regul Pept 128:227–238 [DOI] [PubMed] [Google Scholar]
- Fitts DA, Tjepkes DS, Bright RO 1990 Salt appetite and lesions of the ventral part of the ventral median preoptic nucleus. Behav Neurosci 104:818–827 [DOI] [PubMed] [Google Scholar]
- McKinley MJ, Mathai ML, McAllen RM, McClear RC, Miselis RR, Pennington GL, Vivas L, Wade JD, Oldfield BJ 2004 Vasopressin secretion: osmotic and hormonal regulation by the lamina terminalis. J Neuroendocrinol 16:340–347 [DOI] [PubMed] [Google Scholar]
- Oldfield BJ, Davern PJ, Giles ME, Allen AM, Badoer E, McKinley MJ 2001 Efferent neural projections of angiotensin receptor (AT1) expressing neurones in the hypothalamic paraventricular nucleus of the rat. J Neuroendocrinol 13:139–146 [DOI] [PubMed] [Google Scholar]
- Li Z, Ferguson AV 1993 Subfornical organ efferents to paraventricular nucleus utilize angiotensin as a neurotransmitter. Am J Physiol 265(2 Pt 2):R302–R309 [DOI] [PubMed] [Google Scholar]
- Randall DC, Brown DR, Brown LV, Kilgore JM 1994 Sympathetic nervous activity and arterial blood pressure control in conscious rat during rest and behavioral stress. Am J Physiol 267(5 Pt 2):R1241–R1249 [DOI] [PubMed] [Google Scholar]
- Kanbar R, Orea V, Barres C, Julien C 2007 Baroreflex control of renal sympathetic nerve activity during air-jet stress in rats. Am J Physiol Regul Integr Comp Physiol 292:R362–R367 [DOI] [PubMed] [Google Scholar]
- Kopp UC, DiBona GF 1993 Neural regulation of renin secretion. Semin Nephrol 13:543–551 [PubMed] [Google Scholar]
- Krukoff TL, MacTavish D, Harris KH, Jhamandas JH 1995 Changes in blood volume and pressure induce c-fos expression in brainstem neurons that project to the paraventricular nucleus of the hypothalamus. Brain Res Mol Brain Res 34:99–108 [DOI] [PubMed] [Google Scholar]
- Irvine CH, Alexander SL, Donald RA 1989 Effect of an osmotic stimulus on the secretion of arginine vasopressin and adrenocorticotropin in the horse. Endocrinology 124:3102–3108 [DOI] [PubMed] [Google Scholar]
- Jessop DS, Chowdrey HS, Lightman SL 1990 Inhibition of rat corticotropin-releasing factor and adrenocorticotropin secretion by an osmotic stimulus. Brain Res 523:1–4 [DOI] [PubMed] [Google Scholar]
- Kiss A, Aguilera G 1993 Regulation of the hypothalamic pituitary adrenal axis during chronic stress: responses to repeated intraperitoneal hypertonic saline injection. Brain Res 630:262–270 [DOI] [PubMed] [Google Scholar]
- Husain A, DeSilva P, Speth RC, Bumpus FM 1987 Regulation of angiotensin II in rat adrenal gland. Circ Res 60:640–648 [DOI] [PubMed] [Google Scholar]