Abstract
Rapid membrane-mediated estradiol signaling regulating sexual receptivity requires the interaction of the estrogen receptor (ER)-α and the metabotropic glutamate receptor 1a (mGluR1a). A cell signaling antibody microarray revealed that estradiol activated 42 proteins in the arcuate nucleus of the hypothalamus (ARH). To begin an analysis of various signaling pathways, protein kinase A and protein kinase C (PKC)-θ, whose signaling pathways have been implicated in the estradiol regulation of sexual receptivity, were examined. In the ARH sample, the increase in phospho-protein kinase A could not be confirmed by Western blotting, in either cytosolic or membrane fractions. However, the increase in phosphorylated PKCθ seen with the pathway array was verified by Western blotting. To study whether rapid estradiol activation of PKC regulates the ARH-medial preoptic nucleus pathway regulating lordosis, μ-opioid receptor (MOR) internalization and lordosis reflex were tested. Blocking PKC in ARH with 2-[1-(3-dimethylaminopropyl)-1H-indol-3-yl]3-(1H-indol-3-yl) maleimide significantly attenuated estradiol-induced MOR internalization. Furthermore, disruption of PKC signaling within the ARH at the time of estradiol treatment significantly diminished the lordosis reflex. Moreover, blocking PKC prevented MOR internalization when the circuit was activated by the mGluR1a agonist, (RS)-3,5-dihydroxyphenylglycine. Activation of PKC with phorbol 12, 13-dibutyrate induced MOR internalization, indicating that PKC was a critical step for membrane ERα-initiated mGluR1a-mediated cell signaling and phorbol 12, 13-dibutyrate significantly facilitated the lordosis reflex. Together these findings indicate that rapid membrane ERα-mGluR1a interactions activate PKCθ cell signaling, which regulates female sexual receptivity.
CLASSICAL ESTRADIOL ACTIONS are mediated by the nuclear estrogen receptor (ER) and involve the transcription of target genes. These effects often occur in hours to days compared with rapid nongenomic actions of estradiol, which occur in seconds to minutes, and are initiated through ERs associated with the cell plasma membrane rather than intracellular receptors. Although the classical actions of estradiol have a well-established role in the regulation of female sexual receptivity, recent data have suggested that estradiol acting rapidly at the cell membrane also has a significant role in regulating sex behavior (1,2). Classical ERs targeted to the plasma membrane associate with caveolin and regulate phosphorylation of proteins in the MAPK and ERK signaling pathways (3,4,5,6,7,8,9,10,11). In terms of sexual receptivity, blocking kinase activity attenuates lordosis behavior in rodents, which emphasizes the role of second messengers in addition to transcriptional effects (12,13).
A novel signaling pathway involving membrane ERα and metabotropic glutamate receptor 1a (mGluR1a) mediates rapid estradiol-induced activation of μ-opioid receptors (MORs) in the medial preoptic nucleus, leading to the facilitation of female sexual receptivity (2). Both protein kinase C (PKC) and protein kinase A (PKA) have been implicated in the regulation of lordosis (1,14,15), but their relationship to ERα/mGluR1a signaling has not been elucidated. A likely pathway is the ERα/mGluR1a activation of phospholipase C that increases free cytoplasmic calcium flux via an inositol trisphosphate receptor-mediated release of intracellular calcium (Kuo, J., submitted for publication) and the increase of diacyl glycerol (DAG) levels, which can activate PKC (8). Preliminary data indicate that estradiol does not increase calcium flux in arcuate nucleus of the hypothalamus (ARH) neurons (Karakossian M., and J. Ogi, unpublished observations), suggesting that downstream signaling may be more dependent on DAG-initiated protein kinase activity than calcium.
The present experiments were performed to determine which protein kinase is regulated by estradiol in the ARH and to delineate a role for PKA and/or PKC in the regulation of sexual receptivity. Both lordosis behavior and synaptic events that are correlated with the behavior were tested. Lordosis behavior is regulated by a sexually dimorphic limbic-hypothalamic circuit that involves the β-endorphin (β-END) projection from the ARH to the medial preoptic nucleus (MPN) (16). Estradiol acts in the ARH stimulating β-END neurons that project to the MPN activating MOR which controls lordosis behavior (17,18). Without MOR activation, estradiol does not induce maximal sexual receptivity (18,19,20). Disruption of estradiol membrane signaling in the ARH, either by blocking ER or mGluR1a, prevents MOR internalization and lordosis behavior, suggesting that estradiol signaling is initiated in the ARH (2). Examination of both MOR internalization and the lordosis reflex allows us to monitor rapid estradiol actions and their longer-term behavioral consequences. We studied estradiol downstream signaling in the ARH on MOR internalization and lordosis behavior by activating and antagonizing PKC.
Materials and Methods
Animals
Male and ovariectomized (ovx; by the supplier) female (200–250 g) Long-Evans rats were purchased (Charles River, Portage, MI). Upon arrival, rats were housed in a climate-controlled room, two per cage in a 12-h light,12-h dark cycle room (lights on at 0600 h) and provided food and water ad libitum. All experimental procedures were approved by the Chancellor’s Animal Research Committee at the University of California, Los Angeles and the Institutional Animal Protocol and Care Committee of Baylor College of Medicine.
Steroid priming
17β-Estradiol benzoate (EB) dissolved in safflower oil was injected sc in a total volume of 0.1 ml per rat. For experiments, that examined inhibition of lordosis behavior, 5 μg EB were used to elicit sexual receptivity. For experiments in which facilitation was expected, 2 μg EB were used. The low dose of EB provided a background on which facilitation of the behavior or MOR internalization was studied. Hormonal priming began 2–3 wk after ovariectomy. Females were injected with EB every fourth day between 0900 and 1000 h.
Coimmunoprecipitation
Cell lysate and membrane fractions from the hypothalamic tissue were obtained with a MEM-PER membrane protein extraction kit (Pierce Biotechnology, Rockford, IL). To optimize the protein extraction 1% n-dodecyl-β-d-maltopyranoside (DDM) was added to the cell lysates and membrane fraction samples. Protein concentrations were detected by the Bradford assay (Bio-Rad, Hercules, CA). Equal concentrations of proteins (1.5 mg/ml, sample volume ∼250 μl) from cell lysate and membrane fractions were used for the coimmunoprecipitation. Endogenous IgGs were removed by incubating samples with Protein-G Sepharose 4 Fast Flow (50 μl; GE Healthcare, Piscataway, NJ) at 4 C for 30 min. The suspension was centrifuged (5 min at 850 × g), the supernatant retained and the beads with the bound endogenous IgGs were discarded. For each sample, 2.5 μl rabbit polyclonal antibody against mGluR1a (1:100; Upstate Biotechnology, Inc., Lake Placid, NY) were added and rotated (1 h at 4 C). The antibody-protein complex was absorbed overnight using Protein-G Sepharose 4 Fast Flow with agitation at 4 C (according to the manufacturer protocol). In the morning, the suspension was centrifuged (5 min at 850 × g), the pellet of the beads with the bound proteins was washed 10 times with sodium phosphate (20 mm; pH 7.0) containing 0.2% DDM. The absorbed proteins were eluted with 0.1 m glycine buffer (pH 3.0 containing 0.2% DDM) and the pH of the eluate was increased to pH 7.5 with Tris · HCl buffer (1 m pH 8.0).
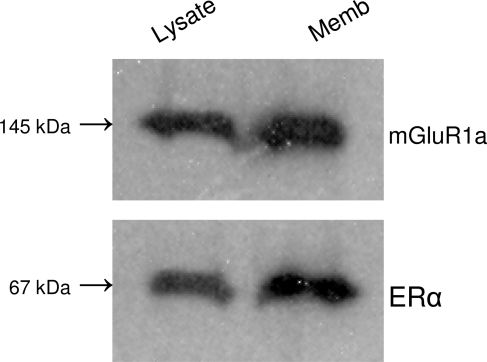
Samples were run on a 10% polyacrylamide gradient SDS-PAGE (Ready Gels; Bio-Rad). Proteins were electrotransferred onto polyvinyl difluoride (PVDF) membrane (GE Healthcare), and probed for 1 h with ERα (1:1000; Upstate Biotechnology, Inc., Temecula, CA). Donkey antirabbit IgG secondary antibodies (1:5000; Jackson ImmunoResearch, West Grove, PA) were incubated with PVDF membranes for 1.5 h. Bands were visualized using an enhanced chemiluminescence (ECL) kit and ECL Hyperfilm (GE Healthcare) with 0.5- to 2-min exposures. Nonimmune rabbit serum (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) was used as a negative control. The procedure was repeated with ERα antibodies in the pull-down phase and mGluR1 antibodies for visualization (Fig. 1).
Figure 1.
Coimmunoprecipitation of ERα and mGluR1a from the ARH region of ovx female rats. Top blot, Antibodies against ERα were used in the pull-down phase, and the complex was detected with anti-mGluR1a sera. Bottom plot, The procedure was reversed. Antibodies against mGluR1a were used in the pull-down phase, and the complex was detected with anti-ERα sera. Lysate, Cell lysate fraction without membranes; Memb, plasma membrane fraction.
Phospho-antibody screening
Ovx rats were primed with 5 μg EB or oil (control group) every 4 d for three cycles. One hour after the last EB injection, animals were killed and the ARH region was dissected out. The tissue was rinsed with PBS and homogenized in lysis buffer [20 mm 3[N-morpholino]propanesulfonic acid (pH 7.0), 2 mm EGTA, 5 mm EDTA, 30 mm sodium fluoride, 60 mm β-glycerophosphate, 20 mm sodium pyrophosphate, 1 mm sodium orthovanadate, 1 mm phenylmethylsulfonylfluoride, 3 mm benzamidine, 5 μm pepstatin A, 10 μm leupeptin, 0.5% Triton X-100, and 1 mm dithiothreitol] using a glass Dounce and sonicated four times for 10 sec on ice each time to shear nuclear DNA. Homogenates were centrifuged (50,000 rpm for 30 min at 4 C). Protein concentration of the supernatant fraction was determined (BCA protein assay; Pierce). EB- and vehicle-treated protein samples (100 μg) were sent to Kinexus Bioinformatics Corp. (Vancouver, Canada) for screening using their Kinex antibody microarray, consisting of more than 600 antibodies targeted to total or phosphorylated forms of signaling proteins, including more than 240 protein kinases, 28 phosphatases, and 90 other cell signaling proteins that regulate cell proliferation, stress and apoptosis.
Immunoblotting
Proteins were extracted from the hypothalamus of estradiol- or oil-treated rats. To detect the total PKC and PKA protein concentrations tissue samples were homogenized in a buffer of 10 mm Tris-HCl, 1.5 mm Na2EDTA, 10% glycerol, and 12 mm monothioglycerol (pH 7.4), containing the following protease inhibitors: 0.5 mm leupeptin, 0.5 mm pepstatin, 1 mm aprotinin, 1 mm bacitracin, 5 mm phenylmethylsulfonyl fluoride, and 1 mm sodium orthovanadate (Sigma, St. Louis, MO) using a glass Dounce. Homogenates were clarified by centrifugation (48,000 × g for 30 min at 4 C) and used to detect protein kinases.
To detect phospho- (p) PKC and pPKA, rats were anesthetized and killed by focused microwave irradiation for 2 sec (Muromachi microwave applicator; Muromachi Kikai Co., Tokyo, Japan) at Baylor College of Medicine (Houston, TX). The brains were removed and the ARH region of the mediobasal hypothalamus was microdissected, frozen at −80 C and shipped overnight to UCLA on dry ice. The microwave-fixed samples were defrosted by boiling in 1% sodium dodecyl sulfate, homogenized by sonication, centrifuged, and the pellet discarded. Protein content of the supernatant was determined with the Bradford Assay (Bio-Rad). Cell lysate and membrane fractions containing 75 μg of protein were combined with sample buffer, boiled, separated by 10% sodium dodecyl sulfate polyacrylamide gel, and then transferred to PVDF membranes (Amersham Biosciences, Piscataway, NJ). To determine the molecular weight of the proteins, samples were run alongside a biotinylated protein ladder (Cell Signaling, Danvers, MA). The membranes were then incubated with primary rabbit polyclonal antibodies: anti-PKCα, -β, -γ (Upstate); pPKCθ (Thr538), PKA C-α, or pPKA Cα (Thr197) (Cell Signaling). Primary antibodies were diluted 1:1000 in either 3% skim milk in PBS or 5% BSA in Tris-buffered saline-Tween 20 [0.1% (vol/vol)] according to the suppliers’ recommendations. β-Tubulin was used as a loading control and detected by an antibody (1:5000; Abcam, Cambridge, MA). Membranes were incubated with primary antibodies overnight on an orbital shaker at 4 C. Secondary antibodies, horseradish peroxidase-linked antirabbit, and horseradish peroxidase-conjugated antibiotin antibodies were used as recommended (Cell Signaling). Antibody binding was visualized with an ECL kit and Hyperfilm (Amersham Biosciences). Blots were exposed to film for 0.5, 1, and 1.5 min.
Guide cannulae implantation surgery
Bilateral guide cannulae (24 gauge; Plastics One Inc., Roanoke, VA) directed at the ARH (coordinates from bregma: anterior −2.0 mm, lateral 0.8 mm, ventral −6.9 mm from dura; tooth bar: −3.3 mm) were implanted using standard stereotaxic procedures while female rats were anesthetized with isoflurane (2–3% in equal parts oxygen and nitrous oxide). Cannulae were secured to the skull with dental acrylic and stainless steel bone screws. Stylets were placed in the guide cannulae, which protruded less than 0.5 mm beyond the opening of the guide cannulae. Animals were individually housed after surgery, received oral antibiotics (trimethoprim and sulfamethoxazole, 0.4 mg/ml; Hi-Tech Pharmacal, Amityville, NY) in the drinking water, and allowed to recover 7 d before behavioral testing.
Microinjection
Fifty nanomoles PKC antagonist, 2-[1-(3-dimethylaminopropyl)-1H-indol-3-yl]3-(1H-indol-3-yl)maleimide (BIS; Biomol International LP, Plymouth Meeting, PA), 25 nmol PKC agonist, phorbol 12,13-dibutyrate (PDBu; Sigma), 50 nmol mGluR1a antagonist, LY367385 (Tocris, Ellisville, MO), and 200 nmol of a mGluR1a agonist, 3,4-dihydroxyphenylglycol (DHPG; Tocris) were dissolved in an artificial cerebrospinal fluid (aCSF) vehicle. Microinjections were performed with an infusion pump (Harvard Apparatus, Holliston, MA) at a rate of 1.0 μl/min. Microinjection needles (28 gauge) protruded 2 mm beyond the opening of the cannula and were allowed to remain in place for 1 min after injection to allow for diffusion of drug treatment or aCSF control vehicle from the injector. After microinjection, the obturators were reinserted into the guide cannulae and animals returned to their home cage until testing.
Behavioral testing
For all experiments, behavioral testing began 30 h after EB injection, 3 h into the dark phase (18). Female rats were tested for sexual receptivity during the second steroid treatment cycle after surgery to confirm responsiveness to steroids. To study the effect of blocking PKC on lordosis behavior, females were first microinjected with a PKC antagonist, BIS, followed 30 min later by 5 μg EB, an estradiol dose designed to produce sexually receptive rats. To activate PKC, an agonist, PDBu was microinjected into the ARH followed 30 min later by a subbehavioral dose of estradiol, 2 μg EB.
Sexual receptivity was measured by placing each female rat in a Plexiglas testing arena with a stud male. Sexually experienced males were acclimatized to the arenas for at least 30 min before testing. Males were allowed to vigorously mount females 10 times. The number of times the female displayed lordosis (lifting of the head, arching of the back, movement of the tail to one side) when mounted by a male was recorded. For each female, the sexual receptivity was quantified as a lordosis quotient (LQ), the number of lordosis displays/number of mounts × 100.
Confirmation of guide cannulae placement
Animals were anesthetized after the behavioral series of experiments with sodium pentobarbital (100 mg/kg) and transcardially perfused with chilled 0.9% saline, followed with a fixative, 4% paraformaldehyde dissolved in 0.2 m Sorenson’s phosphate buffer (pH 7.4). Brains were removed and placed in fixative overnight at 4 C and then replaced with 20% sucrose in phosphate buffer to cryoprotect the tissue. Brains were blocked, sectioned (20 μm) on a cryostat (Zeiss Microm, Thornwood, NY), and collected into chambers filled with PBS. Sections were mounted onto SuperFrost/Plus slides (Fisher, Pittsburgh, PA), stained with thionin, dehydrated, and coverslipped with Permount (Fisher). Injection sites were mapped and verified with bright-field illumination. Rats with cannulae that were not positioned in the ARH (i.e. located above, lateral to the ARH, or where microinjections had compromised the wall of the third ventricle) were excluded from the study.
Immunohistochemistry
Animals were perfused, as described above, 30 min after estradiol injection and immediately processed for immunohistochemistry. For MOR internalization, rabbit primary antibodies directed against MOR (1:24,000; Neuromics, Minneapolis, MN) were used. Sections processed for fluorescence were incubated in blocking buffer (tyramide signal amplification kit; NEN Life Science Products, Boston, MA) and then in biotin conjugated goat antirabbit IgG (Vector Laboratories, Burlingame, CA; 1:200) for 1 h. Tissue was then washed in Tris-buffered saline and incubated in streptavidin-horseradish peroxidase (NEN Life Science Products; 1:100) for 30 min, washed, and incubated for 5 min in fluorescein conjugated tyramide (1:50; tyramide signal amplification kit; NEN Life Science Products). Sections were washed again in 0.1 m Tris buffer and mounted on SuperFrost/Plus slides. Mounted sections were air dried and coverslipped using Aqua Polymount mounting medium (Polysciences, Inc., Warrington, PA).
Image analysis
All immunohistochemically labeled sections were examined using a Zeiss Axioskop 2 equipped with epiflourescent illumination, Axiocam digital camera, and AxioVision digital image analysis system (Carl Zeiss North America, Thornwood, NY) to determine internalizations. Fluorescein isothiocyanate was imaged with a 488-nm excitation and 550-nm emission filters. Images were adjusted for brightness and contrast using the Zeiss LSM-PC and PhotoShop (version 6.0; Adobe, San Jose, CA) programs.
To determine relative internalization, the area of MOR immunoreactivity in the dorsal aspect of the medial preoptic nucleus in every fourth section was estimated using the National Institutes of Health (Bethesda, MD) Image J software (version 1.32j). Images taken at a magnification of ×360 were converted to gray scale and adjusted for brightness and contrast in Adobe Photoshop. Image J was set to the Pixel Inverter function and calibrated. In each picture, a 60-μm-diameter circle was placed on the medial preoptic nucleus and then on an area negative for MOR immunoreactivity. OD measurements were taken in the medial preoptic nucleus, and background staining was subtracted to determine the MOR immunostaining intensity. Studies in our laboratory indicate that internalized MOR immunostaining can be visualized as an increase in MOR immunofluorescence (16). This has been correlated with our previous method of examining changes in density of MOR-positive processes and has been used to determine the time course and intensity of estradiol-induced activation of MOR in the limbic-hypothalamic circuit (16,21).
Statistical analysis
For the pathway microarray, the percentage change from control was used as a measure of the change in normalized signal intensity averages between the estradiol-treated sample and the control sample. An increase of at least 25% above control values was used as a threshold.
Internalization and behavioral data are expressed as the mean ± sem. Mean differences between groups were determined using one- or two-way ANOVAs followed by Student-Newman-Keuls post hoc analysis in which the main effect or interaction was significant at P < 0.05. Statistical analysis was conducted using SigmaStat (version 3.5) software. The number of animals used in each experiment is specified in Results.
Results
ERα-mGluR1a interaction in the ARH
To demonstrate the potential interaction of membrane-associated ERα, coimmunoprecipitation experiments were done with tissue dissected from the ARH. Membrane fractions were used with both ERα and mGluR1a in the pull-down phase of the experiments, and the blots were then probed with antibodies raised against the opposite protein (Fig. 1). Coimmunoprecipitation from cell lysates minus the membrane fraction showed a lower level of interaction of ERα and mGluR1 compared with the membrane fraction. The coimmunoprecipitation of ERα and mGluR1 strongly suggests an interaction between these receptor proteins that confirmed studies done with transected neurons (2).
Estradiol increases levels of phosphoproteins in ARH
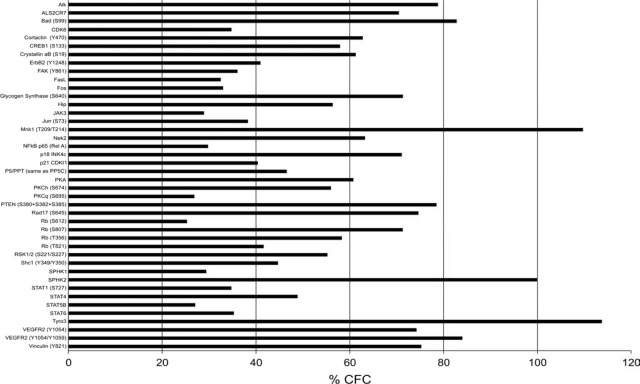
To begin studying changes in cell signaling after EB treatment, a Kinex pathway microarray was used to identify target proteins for future study. The Kinex microarray allowed a comparison of two different tissue lysates, from ovx and ovx+EB rats simultaneously on one array. This minimized systematic errors and ensured that each sample was subject to comparable experimental conditions. EB treatment increased the levels of 42 phospho-proteins in the ARH compared with lysates from the ARH of ovx-treated with oil (control). Among these proteins were tyrosine-protein kinase receptor 3, MAPK interacting protein-serine kinase 1, sphingosine kinase 2, pPKCθ, and pPKA (Fig. 2). We concentrated on PKA and PKC, two proteins implicated in the control of lordosis behavior. As described by the manufacturers, the nature of methodology of the Kinex antibody microarray may produce false positives and negatives. False positives can arise because of the cross-reactivity of antibodies or the capture of dye-labeled proteins on the microarray chips. False negatives can happen when the target epitope recognized by the capture antibody is masked within a protein complex. To verify the results for pPKA and pPKCθ, Western blots were used, as suggested by the manufacturer. An elevated level of pPKCθ was verified, but the apparent increase in pPKA was not verified with Western blotting (Fig. 3). Further experiments were then done to define the role of PKC-mediated signaling in MOR internalization and lordosis behavior.
Figure 2.
Estradiol increases levels of phospho-proteins in the ARH. Kinex antibody pathway microarray was used to detect changes in cell signaling pathways after estradiol treatment of ovx rats. These results are reported as percentage change from control (% CFC) of all phospho-proteins detected by the array that increase more than 25%, an empirically verified limit. Only the apparent pPKCθ and pPKA increases were tested with Western blots (Fig. 3).
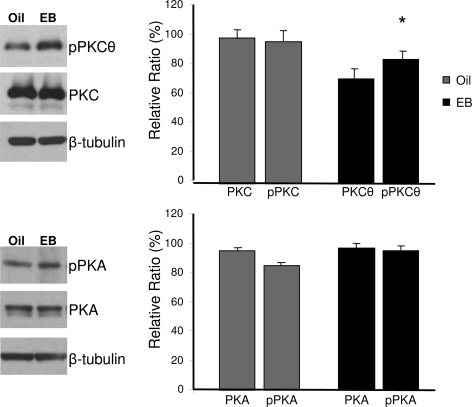
Figure 3.
Western blot analysis of EB induction of pPKCθ in the ARH region of ovx rats. The Western blots confirmed the results of the Kinex antibody array and demonstrated that estradiol (E2) treatment increased pPKCθ levels. Total PKC levels were not altered by steroid treatment. The PKC and pPKCθ levels were normalized to β-tubulin and levels detected by OD measurements. Values are the means ± sem of four trials. *, P < 0.05, as determined by Student’s t test.
PKC induces MOR internalization in the MPN
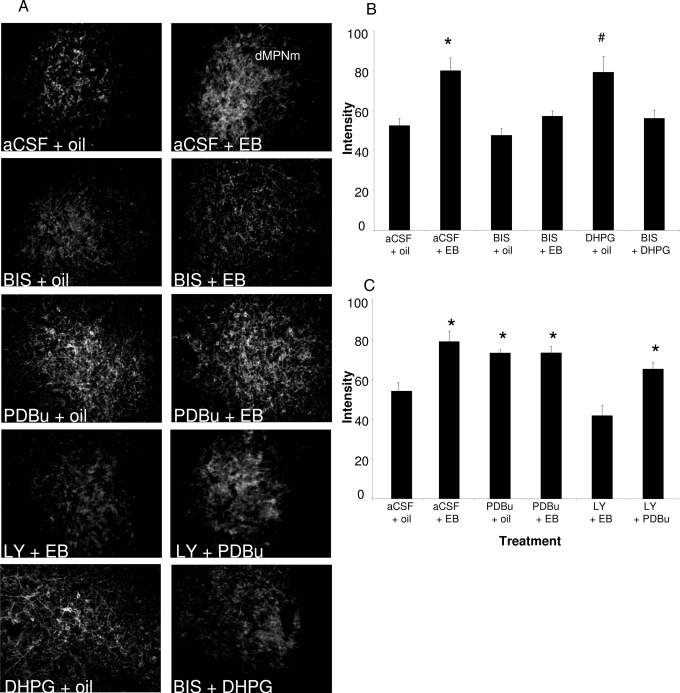
To determine whether estradiol-induced MOR internalization in the MPN is dependent on PKC signaling in the ARH, BIS, a general inhibitor of PKC, was used to pharmacologically block the actions of PKC. Infusions of BIS directly into the ARH 30 min before EB treatment (5 μg per 0.1 ml) significantly attenuated MOR internalization in the MPN [Fig. 4; two-way ANOVA: drug, P = 0.004, F (1,16) = 11.64; steroid, P < 0.001, F (1,16) = 20.94; drug × steroid interaction, P = 0.042, F (1,19) = 4.90; Student-Newman-Keuls, P = 0.002; n = 4–6 animals/group]. BIS by itself did not affect MOR internalization. Further evidence that PKC was critical for signaling in the ARH was obtained by activating PKC in the ARH and monitoring MOR internalization in the MPN. Infusing the PKC agonist, PDBu, into the ARH induced MOR internalization in the MPN [Fig. 4; two-way ANOVA: drug, P = 0.108, F (1,17) = 2.88; steroid, P = 0.007, F (1,17) = 9.43; drug × steroid interaction, P = 0.007, F (1,20) = 9.271; Student-Newman-Keuls, P = 0.005; n = 5–6 animals/group]. Infusing PDBu into the ARH, 30 min before the EB injection (2 μg per 0.1 ml) did not increase the level of internalization above that obtained with either EB or PDBu. Activating PKC with PDBu, in the absence of EB produced levels of internalization similar to those obtained with EB alone, suggesting that estradiol and PDBu act on through the same signaling pathway.
Figure 4.
Estradiol induced MOR internalization in the dorsal part of the medial preoptic nucleus; medial (dMPNm) is dependent on PKC. EB- or oil-treated rats were infused with PKC inhibitor, BIS (50 nmol); PKC activator, PDBu (25 nmol); or aCSF into the ARH. A, Images of the MOR immunohistochemistry of the dMPNm after indicated treatment. B, Animals killed 30 min after EB (or oil) injection had significant MOR internalization, which was attenuated by BIS. C, PDBu induced MOR internalization in the absence of EB treatment. *, Statistical significance at the P < 0.05 level compared with aCSF + oil group as described.
Rapid estradiol-induced cell signaling in the ARH requires an ERα interaction with mGluR1a (2). To test whether PKC was part of this pathway, mGluR1a was activated or inhibited, whereas PKC activity was manipulated. When mGluR1a was stimulated with DHPG in the presence of BIS, to inhibit PKC activity, MOR internalization was blocked [Fig. 4; one-way ANOVA: P = 0.039, F (7) = 6.947; Student-Newman-Keuls, P < 0.05; n = 4 animals/group]. Alternatively, the mGluR1a antagonist LY367385 blocked EB-induced MOR internalization (Fig. 3B). To determine whether PKC activation was downstream of ERα/mGluR1a signaling, the mGluR1a was blocked and PKC activated. PDBu overcame the LY367385 inhibition of the mGluR1a. MOR internalization was observed when LY367385, and 30 min later PDBu was infused into the ARH [Fig. 4; one-way ANOVA: P = 0.003, F (10) = 16.06; Student-Newman-Keuls, P < 0.05; n = 5–6 animals/group]. Together these data strongly indicate that ER/mGluR1a signaling is required for MOR activation/internalization and involves downstream signaling through PKC.
Antagonizing PKC signaling attenuates sexual receptivity
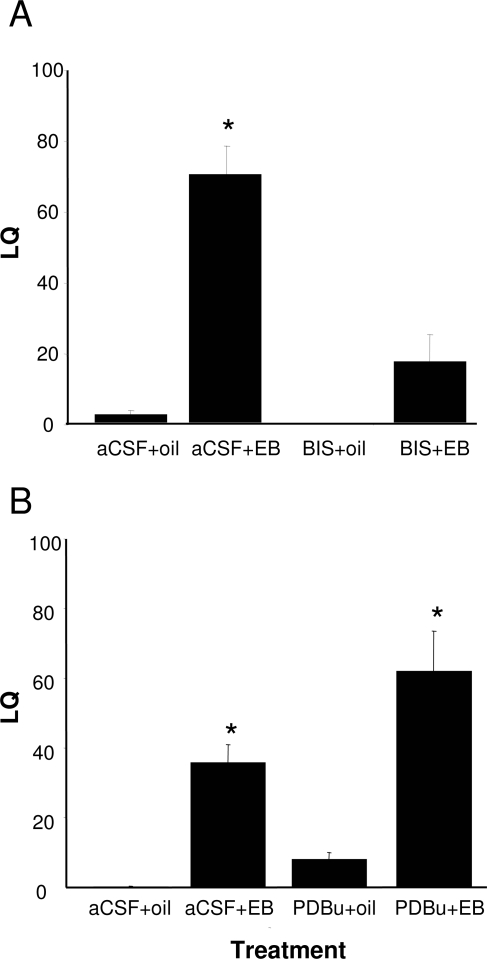
The rapid activation of the ARH-MPN projection by estradiol is essential for the full display of sexual receptivity (16,18,19,20). To determine whether activating PKC signaling was necessary for the lordosis reflex, we antagonized PKC in the ARH. BIS was microinjected into the ARH 30 min before EB injection, and the animals were tested 30 h later to determine their LQ, a quantifiable, estradiol-dependent measure of sexual receptivity. The PKC antagonist, BIS, significantly attenuated the LQ in animals treated with a dose of EB that induces lordosis [Fig. 5A; two-way ANOVA: drug, P = 0.005, F (1,21) = 10.06; steroid, P < 0.001, F (1,21) = 24.02; drug × steroid interaction, P = 0.009, F (1,24) = 8.31; Student-Newman-Keuls, P < 0.001; n = 4–8]. Furthermore, when the PKC agonist PDBu was infused into the ARH, lordosis was facilitated [Fig. 5B; two-way ANOVA: drug, P = 0.015, F (1,18) = 7.25; steroid, P < 0.001, F (1,18) = 49.61; drug × steroid interaction, P = 0.168, F (1,21) = 2.06; Student-Newman-Keuls, P = 0.007; n = 5–7], as predicted if activation of PKC is important for the display of lordosis. These results are consistent with the hypothesis that activation of PKC in the ARH is required and critical during the initial phase of estradiol signaling that regulates sexual receptivity.
Figure 5.
The estradiol-induced lordosis reflex in the medial preoptic nucleus is dependent on PKC. EB- or oil-treated rats were infused with PKC inhibitor, BIS (50 nmol); PKC activator, PDBu (25 nmol); or aCSF into the ARH. A, Rats treated with BIS before EB treatment were less receptive than aCSF-treated, EB-primed rats. B, PDBu did not induce lordosis behavior in animals that were not treated with a subbehavioral dose of EB (2 μg). *, Statistical significance at the P < 0.05 level compared with aCSF + oil group as determined by two-way ANOVA and post hoc analysis.
Discussion
Rapid, membrane initiated signaling has been described in the hypothalamus for the past 30 yr (for review see Ref. 22). Activation of the membrane-associated ER can rapidly alter intracellular signaling cascades including PKC and PKA (1,15,23,24,25,26,27,28,29,30), phosphatidylinositol 3-kinase (31), MAPK pathways (32,33,34), signal-regulated kinase-2 (35), leading to regulation of calcium channels (36,37,38) and potassium channels (38,39). Moreover, membrane ERs have also been shown to signal to the nucleus to modulate transcription through phosphorylation of cAMP response element binding protein (2,7,8,10,40,41). The activation of these pathways suggested that membrane ER was a G protein-coupled receptor (GPCR) (3).
Despite the membrane localization of a population of ERs and the ability to stimulate intracellular pathways associated with GPCRs by various membrane impermeant constructs (1,2,42), there is otherwise little evidence that membrane ER is directly coupled to a G protein. Moreover, membrane ER does not have the consensus seven membrane-pass configuration of a typical GPCR (43). Clearly mER can activate G protein signaling in cells, but the question was how. A seminal observation by Boulware et al. (8) indicated that estradiol signaling requires membrane ERs to interact with mGluR to initiate cell signaling. This hypothesis was supported by coimmunoprecipitation of ERα with mGluR1a in transfected hippocampal cells (2) and in the present experiments with tissue from the ARH region (Fig. 1). Such results are strong evidence for a direct interaction between these receptors. Indeed, rapid actions of estradiol that influence sexual receptivity are dependent on the interaction of membrane ERα with mGluR1a (2). Following on those results, the present study examined intracellular signaling in a portion of the lordosis regulating circuitry of the limbic system and hypothalamus, the ARH to MPN projection, which is dependent on rapid, membrane-initiated signaling. The mGluR1a is primarily coupled to members of the Gq family resulting in activation of phospholipase C and DAG, (1,44,45,46), suggesting the activation of a PKC.
Both PKC and PKA have been implicated in estradiol’s rapid stimulation of the lordosis reflex (1,15,47). Whereas the mechanisms responsible for mERα-mGluR1a mediated activation of lordosis behavior are multifaceted, an important role for PKC signaling has emerged (14). PKCs are a family of serine/threonine kinases that are critical for intracellular signaling to maintain homeostasis, induce migration, proliferation, and apoptosis and modulate ion channels. Three groups of PKC isoforms have been characterized: the conventional forms activated by DAG that are calcium dependent; the novel forms activated by DAG but are not calcium dependent; and the atypical forms that respond to neither DAG nor calcium (48). Interestingly, estradiol had been shown to elevate PKC catalytic activity but not levels of the PKCα, -β, or -γ isoforms in the hypothalamus (49), suggesting that a specific isoform may be involved. Using a signal transduction array with antibodies against phospho- and nonphosphorylated proteins, we detected an increase of the novel, calcium-independent pPKCθ, which was also verified by Western analysis. This PKC isoform is expressed in nervous tissue, the pituitary, skeletal muscle, and tissues with hematopoietic and lymphopoietic potential (50,51,52,53). To our knowledge, this is the first report of estradiol activation of PKCθ in the brain. The estradiol increase in pPKCθ is interesting in light of our preliminary observations that estradiol treatment did not increase intracellular Ca2+ in ARH tissue slices and dissociated neuronal cultures (Karakossian, M., and J. Ogi, unpublished observations).
The rapid ERα/mGluR1a mediated signaling induces the release of β-END in the MPN activating MOR and regulating lordosis (2,16). In the present experiments, blocking PKC in the ARH before estradiol treatment prevented MOR activation/internalization. To demonstrate that this action was dependent on the ERα/mGluR1a interaction, we antagonized mGluR1a with LY367385 and prevented both the estradiol-induced MOR activation/internalization and the lordosis reflex. Similarly, blocking PKC prevented MOR internalization induced by DHPG, a mGluR1a agonist. To demonstrate that the PKC activation was downstream of the mGluR1a, stimulation of PKC by PDBu increased MOR activation/internalization mimicking the effect of estradiol. PDBu also facilitated lordosis when the rats were treated with a subthreshold dose of estradiol. This result is consistent with previous studies that demonstrate that the rapid actions of estradiol facilitate activation of intracellular ERs (1,2).
Our studies demonstrate that PKC is important for the ARH-MPN limb of the lordosis regulating circuit, but other proteins and/or pathways may also be involved, acting either independently or synergistically with PKC. One of these is the interaction of ER and IGF-I receptor, which has been implicated in the regulation of lordosis (54). IGF-I receptor signaling involves PKCδ but apparently not PKCθ (55,56). PKCδ, is an isoform part of the novel or calcium-independent class of PKCs that appears to be upstream from PKA (57). In our experiments, PKA was not implicated in modulating lordosis behavior that originated in the ARH-MPN part of the limbic-hypothalamic lordosis regulating circuit. Another study, however, demonstrated that estradiol can increase the number of PKCδ expressing cells, suggesting a role for this isoforms of PKC (58). Again, we did not observe this estradiol-dependent increase in PKCδ with our pathway array. Rather we observed an increase in the levels of pPKCθ, also an isoform from the same class. Subsequent experiments used nonselective blockers of PKC and established that this family of protein kinases is important in the signaling pathway initiated by the ERα/mGluR1a interaction. This cell signaling pathway rapidly potentiated the lordosis regulating circuit. It is obviously likely that more than one PKC isoform is involved in the rapid estradiol signaling in hypothalamic neurons because these isoforms serve separate yet distinct functions (59) and may be involved in other parts of the behavioral circuit not studied in the present experiments.
It has been speculated that the activation of PKC is upstream from PKA. We tested whether blocking PKA activation with [N-[2-(p-bromocinnamylamino)ethyl]5-isoquinoline sulfonamide would prevent estradiol-induced lordosis. Even at a very high (200 nmol) dose, [N-[2-(p-bromocinnamylamino)ethyl]5-isoquinoline sulfonamide was not effective, strongly suggesting that PKA is not involved in the regulation of lordosis in the ARH-MPN limb of the lordosis regulating circuit. However, these results do not rule out a function of PKA in other parts of the central nervous system that regulate lordosis, such as the periaqueductal gray or ventromedial nucleus of the hypothalamus (60) or in other estradiol activated reproductive functions in the hypothalamus (1,24,26,40,61,62,63).
The activation of lordosis behavior requires both the rapid actions of estradiol shown here and transcriptional events, with the estradiol-induced progesterone receptor as the primary example. Transcription can be initiated through activation of nuclear receptors or through membrane to nuclear signaling (2,8). Membrane mediated estradiol actions potentiate classical transcriptional events in regulating lordosis but are not sufficient to stimulate lordosis on their own (2,15). Regardless, the full display of lordosis behavior requires the activation/internalization of MOR in the MPN, which is dependent on mER-mGluR1a interactions (2,18,19) and as the present results indicate the activation of PKC. Although lordosis behavior is measured 30 h after estradiol treatment, blockade of mGluR1a or PKC only at the time of estradiol treatment attenuates the lordosis behavior, indicating that these are rapid actions of estradiol. Moreover, estradiol-induced MOR internalization is mimicked by the membrane constrained construct E-6-biotin (2).
In summary, these experiments demonstrate that estradiol activation of MOR internalization, which requires activation of mERα and interaction with mGluR1a to initiate intracellular signaling, is dependent on the phosphorylation of PKCθ. Activation of PKCθ suggests a nodal point through which estradiol can rapidly signal to neurons and potentiate transcriptional events. Both rapid and classical effects are critical for sexual receptivity. Interestingly, in this circuit, a role for PKA, which has been suggested as another lordosis-activator, could not be established, indicating a selective activation of intracellular signaling in circuits regulating this reproductive behavior.
Acknowledgments
Dr. Shaila Mani’s assistance with detecting phosphorylated proteins is greatly appreciated.
Footnotes
This work was supported by National Institutes of Health Grant DA013185.
Disclosure Statement: The authors have nothing to disclose.
See editorial p. 5932
First Published Online July 24, 2008
Abbreviations: aCSF, Artificial cerebrospinal fluid; ARH, arcuate nucleus of the hypothalamus; BIS, 2-[1-(3-dimethylaminopropyl)-1H-indol-3-yl]3-(1H-indol-3-yl)maleimide; DAG, diacyl glycerol; DDM, n-dodecyl-β-d-maltopyranoside; DHPG, 3,4-dihydroxyphenylglycol; EB, 17β-estradiol benzoate; ECL, enhanced chemiluminescence; β-END, β-endorphin; ER, estrogen receptor; GPCR, G protein-coupled receptor; LQ, lordosis quotient; mGluR1a, metabotropic glutamate receptor 1a; MOR, μ-opioid receptor; MPN, medial preoptic nucleus; ovx, ovariectomized; p, phospho; PDBu, phorbol 12,13-dibutyrate; PKA, protein kinase A; PKC, protein kinase C; PVDF, polyvinyl difluoride.
References
- Kow LM, Pfaff DW 2004 The membrane actions of estrogens can potentiate their lordosis behavior-facilitating genomic actions. Proc Natl Acad Sci USA 101:12354–12357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewing P, Boulware MI, Sinchak K, Christensen A, Mermelstein PG, Micevych P 2007 Membrane estrogen receptor-α interactions with metabotropic glutamate receptor 1a modulate female sexual receptivity in rats. J Neurosci 27:9294–9300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razandi M, Pedram A, Greene G, Levin E 1999 Cell membrane and nuclear estrogen receptors (ERs) originate from a single transcript: studies of ERα and ERβ expressed in Chinese hamster ovary cells. Mol Endocrinol 13:307–319 [DOI] [PubMed] [Google Scholar]
- Razandi M, Oh P, Pedram A, Schnitzer J, Levin ER 2002 ERs associate with and regulate the production of caveolin: implications for signaling and cellular actions. Mol Endocrinol 16:100–115 [DOI] [PubMed] [Google Scholar]
- Boulware MI, Kordasiewicz H, Mermelstein PG 2007 Caveolin proteins are essential for distinct effects of membrane estrogen receptors in neurons. J Neurosci 27:9941–9950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade CB, Dorsa DM 2003 Estrogen activation of cyclic adenosine 5′-monophosphate response element-mediated transcription requires the extracellularly regulated kinase/mitogen-activated protein kinase pathway. Endocrinology 144:832–838 [DOI] [PubMed] [Google Scholar]
- Zhou Y, Watters JJ, Dorsa DM 1996 Estrogen rapidly induces the phosphorylation of the cAMP response element binding protein in rat brain. Endocrinology 137:2163–2166 [DOI] [PubMed] [Google Scholar]
- Boulware MI, Weick JP, Becklund BR, Kuo SP, Groth RD, Mermelstein PG 2005 Estradiol activates group I and II metabotropic glutamate receptor signaling, leading to opposing influences on cAMP response element-binding protein. J Neurosci 25:5066–5078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham IM, Todman MG, Korach KS, Herbison AE 2004 Critical in vivo roles for classical estrogen receptors in rapid estrogen actions on intracellular signaling in mouse brain. Endocrinology 145:3055–3061 [DOI] [PubMed] [Google Scholar]
- Lee SJ, Campomanes CR, Sikat PT, Greenfield AT, Allen PB, McEwen BS 2004 Estrogen induces phosphorylation of cyclic AMP response element binding (pCREB) in primary hippocampal cells in a time-dependent manner. Neuroscience 124:549–560 [DOI] [PubMed] [Google Scholar]
- Pedram A, Razandi M, Sainson RC, Kim JK, Hughes CC, Levin ER 2007 A conserved mechanism for steroid receptor translocation to the plasma membrane. J Biol Chem 282:22278–22288 [DOI] [PubMed] [Google Scholar]
- Etgen AM, Acosta-Martinez M 2003 Participation of growth factor signal transduction pathways in estradiol facilitation of female reproductive behavior. Endocrinology 144:3828–3835 [DOI] [PubMed] [Google Scholar]
- Mobbs CV, Rothfeld JM, Saluja R, Pfaff DW 1989 Phorbol esters and forskolin infused into midbrain central gray facilitate lordosis. Pharmacol Biochem Behav 34:665–667 [DOI] [PubMed] [Google Scholar]
- Kow LM, Pfaff DW 1998 Mapping of neural and signal transduction pathways for lordosis in the search for estrogen actions on the central nervous system. Behav Brain Res 92:169–180 [DOI] [PubMed] [Google Scholar]
- Vasudevan N, Kow LM, Pfaff D 2005 Integration of steroid hormone initiated membrane action to genomic function in the brain. Steroids 70:388–396 [DOI] [PubMed] [Google Scholar]
- Mills RH, Sohn RK, Micevych PE 2004 Estrogen-induced μ-opioid receptor internalization in the medial preoptic nucleus is mediated via neuropeptide Y-Y1 receptor activation in the arcuate nucleus of female rats. J Neurosci 24:947–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckersell CB, Popper P, Micevych PE 1998 Estrogen-induced alteration of μ-opioid receptor immunoreactivity in the medial preoptic nucleus and medial amygdala. J Neurosci. 18:3967–3976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinchak K, Micevych PE 2001 Progesterone blockade of estrogen activation of μ-opioid receptors regulates reproductive behavior. J Neurosci 21:5723–5729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinchak K, Shahedi K, Dewing P, Micevych P 2005 Sexual receptivity is reduced in the female μ-opioid receptor knockout mouse. Neuroreport 16:1697–1700 [DOI] [PubMed] [Google Scholar]
- Torii M, Kubo K, Sasaki T 1996 Influence of opioid peptides on the priming action of estrogen on lordosis in ovariectomized rats. Neurosci Lett 212:68–70 [DOI] [PubMed] [Google Scholar]
- Sinchak K, Micevych P 2003 Visualizing activation of opioid circuits by internalization of G protein-coupled receptors. Mol Neurobiol 27:197–222 [DOI] [PubMed] [Google Scholar]
- Ronnekleiv OK, Malyala A, Kelly MJ 2007 Membrane-initiated signaling of estrogen in the brain. Semin Reprod Med 25:165–177 [DOI] [PubMed] [Google Scholar]
- Abraham IM, Herbison AE 2005 Major sex differences in non-genomic estrogen actions on intracellular signaling in mouse brain in vivo. Neuroscience 131:945–951 [DOI] [PubMed] [Google Scholar]
- Watters JJ, Dorsa DM 1998 Transcriptional effects of estrogen on neuronal neurotensin gene expression involve cAMP/protein kinase A-dependent signaling mechanisms. J Neurosci 18:6672–6680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer C, Karolczak M 2000 Estrogenic stimulation of neurite growth in midbrain dopaminergic neurons depends on cAMP/protein kinase A signalling. J Neurosci Res 59:107–116 [PubMed] [Google Scholar]
- Shingo AS, Kito S 2005 Estradiol induces PKA activation through the putative membrane receptor in the living hippocampal neuron. J Neural Transm 112:1469–1473 [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Lagrange AH, Wagner EJ, Ronnekleiv OK 1999 Rapid effects of estrogen to modulate G protein-coupled receptors via activation of protein kinase A and protein kinase C pathways. Steroids 64:64–75 [DOI] [PubMed] [Google Scholar]
- Boyan BD, Sylvia VL, Frambach T, Lohmann CH, Dietl J, Dean DD, Schwartz Z 2003 Estrogen-dependent rapid activation of protein kinase C in estrogen receptor-positive MCF-7 breast cancer cells and estrogen receptor-negative HCC38 cells is membrane-mediated and inhibited by tamoxifen. Endocrinology 144:1812–1824 [DOI] [PubMed] [Google Scholar]
- Sylvia VL, Walton J, Lopez D, Dean DD, Boyan BD, Schwartz Z 2001 17β-Estradiol-BSA conjugates and 17β-estradiol regulate growth plate chondrocytes by common membrane associated mechanisms involving PKC dependent and independent signal transduction. J Cell Biochem 81:413–429 [DOI] [PubMed] [Google Scholar]
- Belcher SM, Le HH, Spurling L, Wong JK 2005 Rapid estrogenic regulation of extracellular signal-regulated kinase 1/2 signaling in cerebellar granule cells involves a G protein- and protein kinase A-dependent mechanism and intracellular activation of protein phosphatase 2A. Endocrinology 146:5397–5406 [DOI] [PubMed] [Google Scholar]
- Znamensky V, Akama KT, McEwen BS, Milner TA 2003 Estrogen levels regulate the subcellular distribution of phosphorylated Akt in hippocampal CA1 dendrites. J Neurosci 23:2340–2347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M, Setalo Jr G, Guan X, Warren M, Toran-Allerand CD 1999 Estrogen-induced activation of mitogen-activated protein kinase in cerebral cortical explants: convergence of estrogen and neurotrophin signaling pathways. J Neurosci 19:1179–1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi R, Foy MR, Vouimba RM, Thompson RF, Baudry M 2001 Cyclic changes in estradiol regulate synaptic plasticity through the MAP kinase pathway. Proc Natl Acad Sci USA 98:13391–13395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez P, Azcoitia I, Garcia-Segura LM 2005 Interdependence of oestrogen and insulin-like growth factor-I in the brain: potential for analysing neuroprotective mechanisms. J Endocrinol 185:11–17 [DOI] [PubMed] [Google Scholar]
- Bryant DN, Bosch MA, Ronnekleiv OK, Dorsa DM 2005 17-β Estradiol rapidly enhances extracellular signal-regulated kinase 2 phosphorylation in the rat brain. Neuroscience 133:343–352 [DOI] [PubMed] [Google Scholar]
- Chaban VV, Mayer EA, Ennes HS, Micevych PE 2003 Estradiol inhibits ATP-induced intracellular calcium concentration increase in dorsal root ganglia neurons. Neuroscience 118:941–948 [DOI] [PubMed] [Google Scholar]
- Mermelstein P, Becker J, Surmeister D 1996 Estradiol reduces calcium currents in rat neostriatal neurons via a membrane receptor. J Neurosci 16:595–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly MJ, Qiu J, Wagner EJ, Ronnekleiv OK 2002 Rapid effects of estrogen on G protein-coupled receptor activation of potassium channels in the central nervous system (CNS). J Steroid Biochem Mol Biol 83:187–193 [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Ronnekleiv OK, Ibrahim N, Lagrange AH, Wagner EJ 2002 Estrogen modulation of K(+) channel activity in hypothalamic neurons involved in the control of the reproductive axis. Steroids 67:447–456 [DOI] [PubMed] [Google Scholar]
- Aronica SM, Kraus WL, Katzenellenbogen BS 1994 Estrogen action via the cAMP signaling pathway: stimulation of adenylate cyclase and cAMP-regulated gene transcription. Proc Natl Acad Sci USA 91:8517–8521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Q, Moss RL 1996 17β-Estradiol potentiates kainate-induced currents via activation of the cAMP cascade. J Neurosci 16:3620–3629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micevych PE, Chaban V, Ogi J, Dewing P, Lu JK, Sinchak K 2007 Estradiol stimulates progesterone synthesis in hypothalamic astrocyte cultures. Endocrinology 148:782–789 [DOI] [PubMed] [Google Scholar]
- Rasmussen LM, Zaveri NT, Stenvang J, Peters RH, Lykkesfeldt AE 2007 A novel dual-target steroid sulfatase inhibitor and antiestrogen: SR 16157, a promising agent for the therapy of breast cancer. Breast Cancer Res Treat 106:191–203 [DOI] [PubMed] [Google Scholar]
- Kow LM, Mobbs CV, Pfaff DW 1994 Roles of second-messenger systems and neuronal activity in the regulation of lordosis by neurotransmitters, neuropeptides, and estrogen: a review. Neurosci Biobehav Rev 18:251–268 [DOI] [PubMed] [Google Scholar]
- Doolan CM, Harvey BJ 1996 Modulation of cytosolic protein kinase C and calcium ion activity by steroid hormones in rat distal colon. J Biol Chem 271:8763–8767 [DOI] [PubMed] [Google Scholar]
- Harvey SC, Koster A, Yu H, Skolnick P, Baumbarger P, Nisenbaum ES 2001 AMPA receptor function is altered in GLUR2-deficient mice. J Mol Neurosci 17:35–43 [DOI] [PubMed] [Google Scholar]
- Kow LM, Brown HE, Pfaff DW 1994 Activation of protein kinase C in the hypothalamic ventromedial nucleus or the midbrain central gray facilitates lordosis. Brain Res 660:241–248 [DOI] [PubMed] [Google Scholar]
- Mellor H, Parker PJ 1998 The extended protein kinase C superfamily. Biochem J 332(Pt 2):281–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansonoff MA, Etgen AM 1998 Estradiol elevates protein kinase C catalytic activity in the preoptic area of female rats. Endocrinology 139:3050–3056 [DOI] [PubMed] [Google Scholar]
- Baier G, Telford D, Giampa L, Coggeshall KM, Baier-Bitterlich G, Isakov N, Altman A 1993 Molecular cloning and characterization of PKCθ, a novel member of the protein kinase C (PKC) gene family expressed predominantly in hematopoietic cells. J Biol Chem 268:4997–5004 [PubMed] [Google Scholar]
- MacEwan DJ, Johnson MS, Mitchell R 1999 Protein kinase C isoforms in pituitary cells displaying differential sensitivity to phorbol ester. Mol Cell Biochem 202:85–90 [DOI] [PubMed] [Google Scholar]
- Minami H, Owada Y, Suzuki R, Handa Y, Kondo H 2000 Localization of mRNAs for novel, atypical as well as conventional protein kinase C (PKC) isoforms in the brain of developing and mature rats. J Mol Neurosci 15:121–135 [DOI] [PubMed] [Google Scholar]
- Wilda M, Ghaffari-Tabrizi N, Reisert I, Utermann G, Baier G, Hameister H 2001 Protein kinase C isoenzyme: selective expression pattern of protein kinase Cθ during mouse development. Mech Dev 103:197–200 [DOI] [PubMed] [Google Scholar]
- Quesada A, Etgen AM 2002 Functional interactions between estrogen and insulin-like growth factor-i in the regulation of α(1B)-adrenoceptors and female reproductive function. J Neurosci 22:2401–2408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Z, Liu LZ, Dixon DA, Zheng JZ, Chandran B, Jiang BH 2007 Insulin-like growth factor-I induces cyclooxygenase-2 expression via PI3K, MAPK and PKC signaling pathways in human ovarian cancer cells. Cell Signal 19:1542–1553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ila R, Solem M 2006 Chronic-alcohol exposure alters IGF1 signaling in H9c2 cells via changes in PKCΔ. Alcohol 39:169–178 [DOI] [PubMed] [Google Scholar]
- Qiu J, Wang CG, Huang XY, Chen YZ 2003 Nongenomic mechanism of glucocorticoid inhibition of bradykinin-induced calcium influx in PC12 cells: possible involvement of protein kinase C. Life Sci 72:2533–2542 [DOI] [PubMed] [Google Scholar]
- Devidze N, Mong JA, Jasnow AM, Kow LM, Pfaff DW 2005 Sex and estrogenic effects on coexpression of mRNAs in single ventromedial hypothalamic neurons. Proc Natl Acad Sci USA 102:14446–14451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaken S 1996 Protein kinase C isozymes and substrates. Curr Opin Cell Biol 8:168–173 [DOI] [PubMed] [Google Scholar]
- Uphouse L, Maswood S, Jackson A 2000 Factors elevating cAMP attenuate the effects of 8-OH-DPAT on lordosis behavior. Pharmacol Biochem Behav 66:383–388 [DOI] [PubMed] [Google Scholar]
- Minami T, Oomura Y, Nabekura J, Fukuda A 1990 17β-Estradiol depolarization of hypothalamic neurons is mediated by cyclic AMP. Brain Res 519:301–307 [DOI] [PubMed] [Google Scholar]
- Nabekura J, Oomura Y, Minami T, Mizuno Y, Fukuda A 1986 Mechanism of the rapid effect of 17β-estradiol on medial amygdala neurons. Science 233:226–228 [DOI] [PubMed] [Google Scholar]
- Schreihofer DA, Resnick EM, Lin VY, Shupnik MA 2001 Ligand-independent activation of pituitary ER: dependence on PKA-stimulated pathways. Endocrinology 142:3361–3368 [DOI] [PubMed] [Google Scholar]