Abstract
It was earlier shown that expression of kinesin superfamily-associated protein 3 (KAP3), involved in the neuronal anterograde, microtubule-dependent transport of membrane organelles, increases in the hypothalamus of female rats during the juvenile phase of sexual development. KAP3 mRNA is abundant in the hypothalamus, suggesting that it might be expressed in broadly disseminated neuronal systems controlling neuroendocrine function. The present study identifies one of these systems and provides evidence for an involvement of KAP3 in the excitatory control of female puberty. In situ hybridization and immunohistofluorescence studies revealed that the KAP3 gene is expressed in glutamatergic neurons but not in GABAergic or GnRH neurons. Hypothalamic KAP3 mRNA levels increase during the juvenile period of female prepubertal development, remaining elevated throughout puberty. These changes appear to be, at least in part, estradiol dependent because ovariectomy decreases and estradiol increases KAP3 mRNA abundance. Lowering hypothalamic KAP3 protein levels via intraventricular administration of an antisense oligodeoxynucleotide resulted in reduced release of both glutamate and GnRH from the median eminence and delayed the onset of puberty. The median eminence content of vesicular glutamate transporter 2, a glutamate neuron-selective synaptic protein, and synaptophysin, a synaptic vesicle marker, were also reduced, suggesting that the loss of KAP3 diminishes the anterograde transport of these proteins. Altogether, these results support the view that decreased KAP3 synthesis diminishes GnRH output and delays female sexual development by compromising hypothalamic release of glutamate.
THE ONSET OF PUBERTY depends upon an increased secretion of the neuropeptide GnRH from hypothalamic GnRH-secreting neurons. Although the primary mechanism responsible for this change has not been identified, it appears now clear that the pubertal activation of GnRH release requires coordinated changes in transsynaptic communication and glial activity (reviewed in Refs. 1 and 2). The neuronal networks controlling GnRH secretion are multiple (3,4,5) and subject to the modulatory influence of gonadal steroids (6). The most important excitatory components of this transsynaptic system are provided by glutamatergic neurons and the newly discovered kisspeptin-producing neurons (reviewed in Refs. 1 and 7). The inhibitory counterpart is mostly supplied by γ-aminobutyric acid (GABA), but also by opioid peptides (8). Although GABA may inhibit GnRH secretion mainly by acting on neuronal subsets connected to the GnRH neuronal network (1,8), it also exerts direct excitatory effects on GnRH neurons (9).
At puberty, there appears to be a synchronized increase in glutamatergic/kisspeptin stimulation of GnRH neurons and a decrease in GABA inhibition (reviewed in Refs. 1 and 10). Notwithstanding the newly discovered importance of kisspeptin neurons in the control of GnRH secretion (7), it is clear that glutamatergic neurons provide a major excitatory input to GnRH neurons and cells associated with the GnRH neuronal network (11,12). Although much is known about synaptic glutamate release, the molecules involved in the process by which glutamate becomes available for release remain incompletely characterized (13).
The discovery of vesicular glutamate transporters (14,15) as integral components of the mechanism used by neurons to transport glutamate to presynaptic terminals suggests that additional proteins involved in intracellular trafficking may contribute to maintaining an adequate level of neuronal glutamate output. Using low-density cDNA arrays coupled to gene differential display, it was earlier shown (16) that the hypothalamic content of an mRNA encoding kinesin superfamily-associated protein 3 (KAP3), a protein involved in microtubule-dependent cargo transport (17,18), increases in young adult female rats sterilized by neonatal administration of 17β-estradiol 3-benzoate (EB). This study also showed that KAP3 mRNA levels increase in the hypothalamus of untreated rats during the first 30 d of postnatal development, suggesting that changes in KAP3 gene expression may be relevant to sexual maturation and to the attainment of female reproductive capacity. Because KAP3 is predominantly expressed in neurons (17), the aforementioned changes in KAP3 expression are likely to occur mostly in neurons instead of glial cells.
KAP3 functions in association with kinesin superfamily proteins (KIFs). KIFs and cytoplasmic dyneins serve as motors that move along microtubules carrying cargoes such as membranous organelles, protein complexes, and mRNAs (19,20,21). About 45 genes encoding KIFs have been identified in mouse and human genomes (19,22,23). Different KIFs serve as anterograde transporters in different cellular systems (24,25,26,27,28,29). The KIF3 motor is expressed most abundantly in neurons compared with other cells and is composed of KIF3A/KIF3B/KAP3 subunits that together serve as a microtubule plus-end-directed translocator of membrane organelles (30,31,32). KAP3 was identified in mouse brain and testis as a nonmotor subunit of this heterotrimeric complex that appears to regulate the association of the KIF3 motor with its cargo (17,18). Because of this feature, KAP3 may play an important role in regulating the capacity of KIF3A/KIF3B heterodimers to transport membrane organelles from their site of synthesis to their site of action in synaptic terminals.
We now report that KAP3 is selectively expressed in glutamatergic neurons of the neuroendocrine brain and that inhibition of KAP3 synthesis via intraventricular administration of an antisense (AS) oligodeoxynucleotide (ODN) results in decreased median eminence (ME) content of two synaptic proteins that are transported anterogradely: vesicular glutamate transporter 2 (VGLUT2), a protein specifically expressed in glutamatergic neurons, and synaptophysin (Syp), a structural component of synaptic vesicles. AS ODN-treated animals had delayed puberty, and the MEs of these animals exhibited a reduced capacity to release glutamate and GnRH, suggesting that KAP3 is a component of presynaptic trafficking required for the pubertal augmentation of neuronal glutamate release.
Materials and Methods
Animals
Immature female Sprague Dawley rats from either the Daehan Animal Breeding Center (Eumsung, Chungbuk, Korea) or from Harlan Sprague Dawley (Indianapolis, IN) were used in these studies. Animal experiments were conducted as required by the University of Ulsan Regulations, and the Oregon National Primate Research Center Animal Care and Use Committee, in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Rats used for molecular analysis (Northern and Western blots) and for in vitro evaluation of GnRH release were euthanized by decapitation; animals used for in situ hybridization and immunohistochemical analysis were anesthetized with tribromoethanol (1 ml/100 g body weight of a 2.5 g/ml solution administered ip) before perfusion with the appropriate fixative (see below). For experiments involving ovariectomy and estrogen treatment, the animals were ovariectomized (OVX) at postnatal d 21 (P21) under ether anesthesia. Thereafter, some animals were injected with sesame oil and others with EB (10 μg/rat in sesame oil; Sigma Chemical Co., St. Louis, MO) or vehicle (V) twice (at 1000 h on P27 and P29), and were euthanized six h after the second injection for tissue collection.
Probes
A KAP3 cDNA was generated by PCR amplification of KAP3 mRNA extracted from rat hypothalamus, using a sense primer (5′-TGT TGG TGA AGG CTC TTG ATC G-3′) corresponding to 22 nucleotides (nt) (1129–1150) in KAP3 mRNA (NM_001105964), and an antisense primer (5′-TGC AGT GAG CTT CGG GAG GAG T-3′) complementary to nt 1364–1385. The resulting 257-nt cDNA fragment was cloned into pGEM-T Easy vector (Promega, Madison, WI) and was used to generate a cDNA probe for Northern blot hybridization. cDNAs corresponding to segments in the coding region of vesicular glutamate transporter 2 (VGLUT2) and glutamic acid decarboxylase 67 (GAD67) mRNA were used as templates for the preparation of cRNA probes for in situ hybridization, as described earlier (15,33).
Hybridization histochemistry
To determine the cellular sites of KAP3 mRNA expression, the brains of late juvenile (28- to 30-d-old) female rats were fixed by transcardiac perfusion of 4% paraformaldehyde-borate buffer (pH 9.5), as recommended (34). Thereafter, the brains were postfixed for 1800–2000 h at 4 C in the same fixative containing 10% sucrose, blocked coronally, frozen on dry ice, and stored at −85 C until use. Thirty-micrometer sections were then prepared with a freezing sliding microtome and processed for hybridization as previously reported (35). The hybridization procedure employed was that described by Simmons et al. (34), with minor modifications (35), using a [35S]UTP-labeled KAP3 cRNA probe. The probe was transcribed from the linearized KAP3 cDNA template described above using T7 RNA polymerase (Promega) in the presence of [35S]UTP (Amersham Biosciences, Buckinghamshire, UK) and was purified using a Nick column (Amersham Biosciences). A double-label in situ hybridization procedure, described in detail elsewhere (35), combining the [35S]UTP-labeled KAP3 probe with digoxigenin-labeled VGLUT2 or GAD67 cRNAs was used to determine whether KAP3 mRNA is expressed in glutamatergic or GABAergic neurons, respectively. These cRNAs were prepared using the cDNAs mentioned above as templates and either SP6 (VGLUT2) or T7 (GAD67) RNA polymerase to drive the transcription reaction (35). Sense probes were prepared using the same templates but transcribing them from the opposite direction. After an overnight hybridization at 55–56 C, the slides were washed and processed for digoxigenin detection of VGLUT2 and GAD67 as reported (35). After dehydration, the slides were dipped in Ilford K5 emulsion (without defatting) instead of the NTB-2 emulsion used for isotopic hybridization and were exposed to the emulsion for 3 wk at 4 C. At this time, the slides were developed, quickly dehydrated, dried, and coverslipped for microscopic examination.
Immunohistofluorescence
The brains of 28-d-old female rats were fixed by transcardiac perfusion with Zamboni’s fixative and 30-μm frozen sections prepared with a sliding microtome were subjected to double immunohistofluorescence-confocal microscopy, as previously described (36,37). KAP3 was detected with a rabbit polyclonal antibody (18), used at a 1:2000 dilution. The reaction was developed to a green color with fluorescein-conjugated streptavidin (1:1000; Jackson ImmunoResearch Laboratories, West Grove, PA) using the biotyramine enhancement method and biotinylated goat antirabbit γ-globulin (1:5000; Jackson ImmunoResearch) as the secondary antibodies. GnRH neurons were detected with monoclonal antibody HU4H3 (1:3000), which has conformational specificity and only recognizes the intact decapeptide molecule (38). The GnRH immunoreaction was developed to a red color with Texas Red-conjugated horse antimouse IgG (1:250; Jackson ImmunoResearch). After completion of the immunohistofluorescence procedure, cell nuclei were stained with Hoescht 33258 (1:10,000) for 1 min at room temperature. Confocal images were acquired using a Leica TCS NT confocal system, as described (37) using the 488- and 568-nm lines of argon and krypton gas lasers to detect the fluorescein isothiocyanate and Texas Red fluorochromes used to visualize each immunoreaction. Colors were merged, and sections were projected into a single plain using MetaMorph (Universal Imaging, West Chester, PA). Images were further processed using Photoshop 7.0 (Adobe Systems, San Jose, CA).
For both in situ hybridization and immunohistofluorescent images, processing involved only adjustment of contrast and brightness to 1) more faithfully represent the image captured by the microscope and 2) to merge bright- and dark-field images.
Northern blot hybridization
KAP3 cDNA was labeled with [32P]dCTP (Amersham Biosciences) using a random primer labeling kit (Amersham Biosciences). The labeled probe was separated from free isotope with a Nick column (Amersham Biosciences). Northern blot hybridizations, carried out to determine changes in the hypothalamic KAP3 mRNA level, were performed as previously described (16). Total RNA samples (20 μg) extracted from medial basal hypothalamic (MBH) and preoptic area (POA) fragments were separated on a 1.2% formaldehyde-agarose gel and transferred onto a nylon membrane (Amersham Biosciences). Membranes were hybridized with radiolabeled probe in hybridization solution at a specific activity of 1 × 106 cpm/ml. Final posthybridization washes were performed in 0.1× standard saline citrate at 60 C. We used two different methods to obtain autoradiographic images: x-ray film and a phosphoimager (Optic Quant Phosphorimage Analysis System; Packard Bioscience, Meriden, CT). The signals derived from x-ray films were normalized using 28S and 18S rRNA bands stained with ethidium bromide as the normalizing unit. Densities from the phosphoimager were normalized using GAPDH mRNA, and the values obtained were expressed as a percentage of control values.
Targeted disruption of KAP3 synthesis
To examine the involvement of KAP3 in glutamate and GnRH release, as well as the initiation of puberty in female rats, an AS ODN was delivered to the lateral ventricle of the brain via a stereotaxically implanted cannula. AS ODN (5′-CTC GCC TTG CAT GGC GGC GGC-3′) was directed against a 21-nt sequence of KAP3 mRNA that includes the translation initiation site (18). An ODN containing the same nucleotide composition with AS ODN, but in a scrambled (SCR) order (5′-TCC GTG GGT CCC GGA GCG CCT-3′) was used as a control. This scrambled sequence does not bear similarity with any sequence thus far deposited in the NCBI GenBank.
For the intracerebroventricular (icv) injection, the ODNs were diluted in saline at a final concentration of 0.5 mm. Under pentobarbital (97.5 mg/kg body weight) and ketamine hydrochloride (25 mg/kg body weight) anesthesia, a polyethylene guide cannula (outer diameter 1.05 mm, inner diameter 0.35 mm) with an inner stylet (27 gauge) was stereotaxically implanted into a lateral ventricle (1.1 mm rostral to bregma, 1.7 mm lateral from the midline, and 4.2 mm vertical from the surface of the skull) of P21 female rats and fixed in place with anchor screws and dental cement. At the end of a recovery period of 6 d, the inner stylet was removed and the ODNs (2 nmol/rat) were icv injected at 1000 h on 3 consecutive days (P27–P29) using a 10-μl Hamilton syringe. Six hours later, the MBH and ME were collected separately for Western blot analysis of KAP3, Syp, and VGLUT2 content (MBH and ME) and in vitro measurement of glutamate and GnRH release (ME).
Western blots
The hypothalamic tissues from ODN-injected rats were homogenized in lysis buffer (T-PER tissue protein extract reagent; Pierce Chemical Co., Rockford, IL) containing a protease inhibitor cocktail (1 mm phenylmethylsulfonyl fluoride, leupeptin at 10 μg/ml, 3 mm aprotinin) and 1 mm sodium orthovanadate (pH 6.8). The extracted proteins were resuspended in sample buffer containing 62 mm Tris-HCl (pH 6.8), 1 mm EDTA, 10% glycerol, 5% sodium dodecyl sulfate (SDS), and 50 mm dithiothreitol and separated by SDS-PAGE on 10% gels. Proteins on the gels were transferred onto nitrocellulose membranes (Amersham Biosciences) using an electrophoretic transfer cell (Bio-Rad Laboratories, Hercules, CA). The membranes were incubated overnight with KAP3 antibody (1:500; Santa Cruz Biotechnology Inc., Santa Cruz, CA), VGLUT2 antibody (1:2000; Chemicon, Temecula, CA), or Syp antibody (1:5000; Sigma). The immunoreactions were detected with an enhanced chemiluminescence (ECL) detection kit (Amersham Biosciences) according to the protocol provided by the manufacturer.
Determination of glutamate and GnRH release from ME fragments incubated in vitro
MEs were dissected from 29-d-old immature female rats that had received ODNs icv for 3 consecutive days. The tissues were collected under a dissecting microscope according to a previously described procedure (39) and were placed into 24-well plates (two fragments per well) containing 300 μl Krebs Ringer bicarbonate (KRB) solution. After a 30-min preincubation at 37 C under an atmosphere of 95% air/5% CO2, the medium was replaced with fresh KRB buffer, and the incubation was continued for 180 min, collecting the medium every 30 min. The tissues were weighed after the incubation. GnRH released from each well was detected by RIA, as previously described (40), using 125I-labeled GnRH and a rabbit polyclonal antiserum (HU60) that recognizes the fully processed, mature decapeptide (41) at a 1:25,000 dilution. The sensitivity of this assay is 0.4 pg/tube. All samples from a given experiment were measured in the same assay.
Glutamate release was measured using the Amplex Red glutamic acid assay kit (Molecular Probes, Eugene, OR). To inhibit reuptake of released glutamate, a glutamate reuptake inhibitor, l-trans-pyrrolidine-2,4-dicarboxylic acid (Sigma) was added to the KRB buffer at a final concentration of 100 nm. Concentration of all reagents was that reported to be optimal for glutamate release (42), i.e. 0.1 m Tris-HCl (pH 7.5), 100 μm Amplex Red, 0.25 U/ml horseradish peroxidase, 0.08 U/ml l-glutamate oxidase, 0.5 U/ml l-glutamate-pyruvate transaminase, and 200 μm l-alanine. Released glutamate was measured by mixing 50 μl of the reaction mixture with 50 μl of each sample. Samples were subsequently incubated for 30 min at 37 C and analyzed with a plate reader (Wallac Victor 1420 multilabel counter; EG&G Wallac, Turku, Finland). The excitation wavelength was 563 nm, and the fluorescence emission was monitored at 587 nm with 5-nm slit widths.
Phases of puberty
To determine the changes in hypothalamic KAP3 mRNA abundance that may occur during prepubertal and peripubertal development, groups of animals were euthanized in the morning and afternoon (between 0900–1000 h and 1530–1630 h, respectively) at different times of juvenile development (22, 24, 26, 30–32, and 32–38 d of age). According to criteria previously established (43), 22-d-old animals are considered to be in the early juvenile phase of prepubertal development, whereas 30- to 32-d-old rats are classified as being in the late juvenile (LJ) stage. At this time, the vagina is not yet patent, and the uterine weight is 60 mg or less, with no accumulation of intrauterine fluid. A few days later (range, d 32–38), animals enter puberty. Rats with enlarged uteri and detectable intrauterine fluid are considered to be initiating puberty; as such, they are classified as being in early proestrus (EP). Animals showing a uterus ballooned with fluid and a uterine weight of at least 200 mg are in late proestrus (LP), the phase of puberty when the first preovulatory surge of GnRH and gonadotropins take place. The first ovulation occurs the following day, on the first estrus (E). At this time, the vagina becomes patent exhibiting a cytology of cornified cells, and the ovaries contain fresh corpora lutea.
Data analysis
The differences between several groups were analyzed by ANOVA followed by the Student-Newman-Keuls’ multiple comparison test for unequal replications. The Student’s t test was used to compare two groups.
Results
KAP3 mRNA is widely expressed throughout the brain
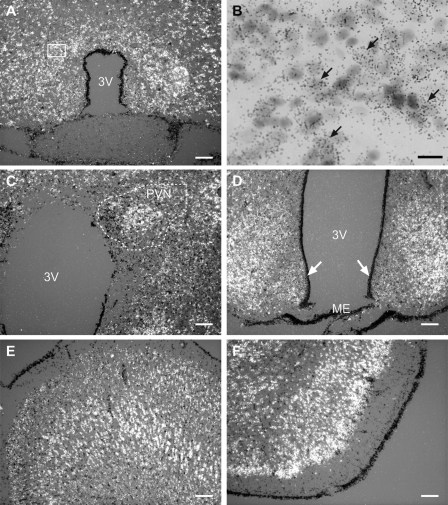
We used in situ hybridization to determine the cellular sites of KAP3 expression in the rat brain. Because of our interest in the neuroendocrine control of female sexual development, we employed prepubertal (28- to 30-d-old) female rats in these studies. Abundant KAP3 mRNA transcripts were seen throughout the brain. Within the POA region, cells containing KAP3 mRNA transcripts were particularly abundant in the anteroventral periventricular nucleus (AVPV, Fig. 1A). A higher magnification view of the AVPV revealed that KAP3 mRNA-positive cells had large, pale nuclei (Fig. 1B, examples denoted by arrows) and, thus, are likely to be neurons. A similar cellular localization was observed in all other brain regions examined (not shown). In the hypothalamus, KAP3 mRNA-containing cells were prominent in several nuclei, including the paraventricular nucleus (PVN, Fig. 1C), and the ventromedial nuclei (VMH, Fig. 1D). No hybridization was observed in either tanycytes lining the third ventricle or the ME (Fig. 1D, arrows). Elsewhere in the brain, KAP3-containing cells were also abundant in the cerebral cortex (CTX, Fig. 1E) and piriform cortex (PIR, Fig. 1F), in addition to the thalamus and hippocampus (not shown). Altogether, this distribution was remarkably similar to that of neuron-specific epidermal growth factor-like protein 2 (NELL2) selectively expressed in glutamatergic neurons (44).
Figure 1.
KAP3 mRNA is expressed throughout the rat brain, as assessed by in situ hybridization using a [35S]UTP-labeled rat KAP3 cRNA probe and brain sections from 28- to 30-d-old late juvenile female rats. Selected regions are shown. A and B, AVPV of the POA; B, high-magnification view of A, depicting the presence of silver grain surrounding large, pale nuclei, characteristic of neurons, with examples denoted by arrows; C, periventricular nucleus (PVN) of the hypothalamus; D, VMH and ARC of the MBH; E, CTX; F, piriform cortex. Arrows point to tanycytes of the third ventricle (3V). Scale bars, 100 μm; bar in B, 20 μm.
KAP3 mRNA expression in the hypothalamus changes during female sexual development
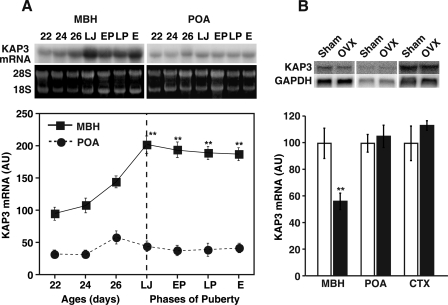
To determine whether normal sexual development is associated with changes in hypothalamic KAP3 expression, we examined the changes in KAP3 mRNA abundance that occur in the MBH and POA during pre- and peripubertal development. As shown in Fig. 2A, the content of KAP3 mRNA increased in the MBH (but not the POA) during early juvenile development (P22–P26), reaching maximal value just before the initiation of puberty, i.e. during the late juvenile phase (postnatal days 30–32). Thereafter, KAP3 mRNA content remained elevated throughout the early proestrous, late proestrous, and first estrous phases of puberty, both in the morning (0900–1000 h; Fig. 2A) and afternoon (1530–1630 h; data not shown). This pattern of expression with KAP3 mRNA prevalence increasing during prepubertal, but not peripubertal, development is again similar to that reported for NELL2 (44), except that in this case, NELL2 mRNA levels decline through puberty after reaching maximal values at the end of juvenile development.
Figure 2.
Changes in KAP3 mRNA levels in the MBH and POA during juvenile and pubertal development of the female rat, as assessed by Northern blot analysis using a 32P-labeled KAP3 cDNA probe. A, Changes in KAP3 mRNA contents measured at 0900–1000 h at each phase of pre- and peripubertal period. Upper panel, Representative autoradiograms showing KAP3 mRNA contents and 28S and 18S rRNA bands used for normalization of KAP3 mRNA signals; lower panel, quantitation of the changes shown in upper panel. Band densities were calculated and represented as fold change from control values (22-d-old group) considered as 100%. E, First estrus; EP, early proestrous phase of puberty; LJ, late juvenile phase; LP, late proestrus. Each point represents the mean of six independent observations per group, and each observation is derived from tissues pooled from three rats. AU, Arbitrary units. **, P < 0.01 vs. 22, 24, and 26 d of age. B, Effect of OVX on KAP3 mRNA level at the time of puberty. Female rats were OVX at 26 d of age; 7 d later (at 1600 h), they were killed, and total RNA from the MBH, POA, and CTX was extracted for Northern blot analysis. Band densities were calculated and represented as fold change from each sham-operated control value. Each bar represents the mean of five independent observations. **, P < 0.01.
To determine whether these changes in KAP3 gene expression are influenced by ovarian steroids, we OVX rats on P26 and measured KAP3 mRNA levels in brain tissues collected 7 d later. As shown in Fig. 2B, OVX significantly decreased KAP3 mRNA content in the MBH but not POA or CTX, indicating that prepubertal increase in KAP3 mRNA abundance and the maintenance of these high levels in the MBH during puberty are events due at least in part to ovarian steroids.
GnRH neurons do not express the KAP3 gene
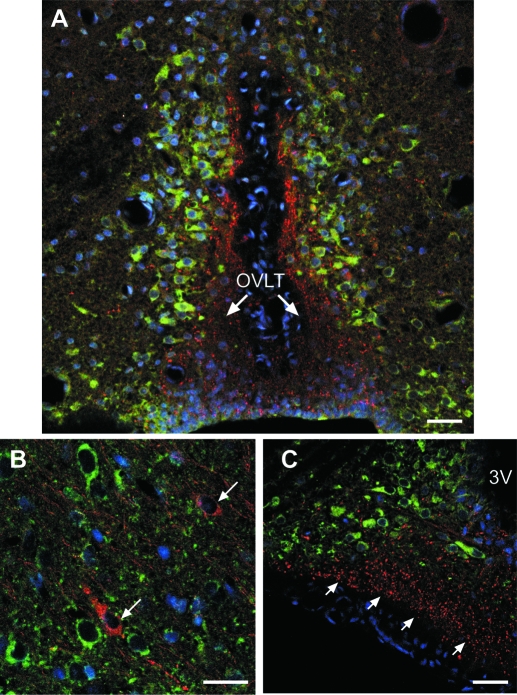
Because the aforementioned experiments suggested a relationship between the pattern of KAP3 mRNA expression in the MBH and the progression of prepubertal female sexual development, we sought to determine whether GnRH neurons contain KAP3. Double immunohistofluorescence showed that although GnRH neurons are devoid of KAP3-immunoreactive materials, they are surrounded by KAP3-positive cells (Fig. 3). For instance, at the level of the preoptic region, KAP3 can be seen in neurons adjacent to GnRH nerve terminals projecting to the organum vasculosum of the lamina terminalis (OVLT, Fig. 3A) and in neurons adjacent to GnRH neuronal perikarya (Fig. 3B). At the level of the arcuate nucleus (ARC)-ME, KAP3 is abundant in GnRH-negative neurons of the ARC (Fig. 3C). Although KAP3 is thought to be involved in the KIF3A/KIF3B motor-dependent anterograde transport of membrane organelles from their site of synthesis to their site of action in synaptic terminals, no KAP3 immunoreactivity was detected in neuronal processes, (Fig. 3, A and C). This lack of staining is likely due to unavailability of the antigenic sites to antibody binding due to association of KAP3 to the microtubule-based motor complex transporting organelles to the synaptic region (16,17). Supporting this view is the ability of Western blots to detect abundant levels of KAP3 in the ME (see below).
Figure 3.
KAP3 immunoreactivity is detected in cells near GnRH neurons but not in GnRH neurons themselves. The immunohistochemical reaction was performed on brain sections derived from two 28-d-old female rats. A, KAP3 immunoreactive neurons (green) adjacent to GnRH nerve terminals (red) projecting to the organum vasculosum of the lamina terminalis (OVLT) in the preoptic region of a 28-d-old female rat. B, KAP3 immunoreactive neurons (green) near GnRH neurons (red, denoted by arrows) lacking KAP3. C, KAP3 immunoreactivity is absent in GnRH nerve terminals of the ME (red) but is abundant in GnRH-negative neurons of the ARC (green). Cell nuclei stained with Hoescht are seen in blue. Scale bars, 10 μm.
The lack of KAP3 in GnRH neurons suggest that if KAP3 plays a role in regulation of neuronal subsets involved in the control of puberty, it must do so by acting within neurons synaptically connected to GnRH neurons and not on GnRH neurons themselves. Because the pattern of KAP3 expression in the hypothalamus corresponds very closely to that of VGLUT2, a glutamate transporter selectively expressed in glutamatergic neurons (15,45), and NELL2, which is expressed in VGLUT2-containing neurons (44), we performed double in situ hybridization experiments to determine whether KAP3 mRNA is expressed in glutamatergic neurons of the hypothalamus.
KAP3 mRNA is abundant in glutamatergic neurons but not in GABAergic neurons of the neuroendocrine brain
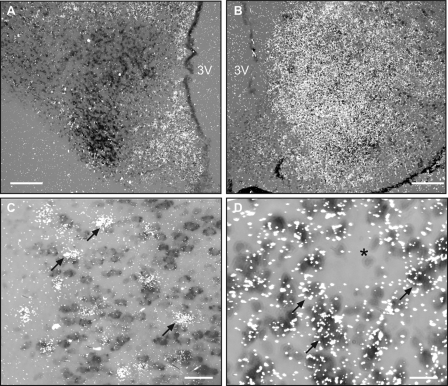
Double in situ hybridization experiments showed that throughout the brain, KAP3 mRNA is expressed in neurons containing VGLUT2 mRNA transcripts but not in GAD67 mRNA-positive cells. Figure 4, A and B, illustrates this cellular localization, using the VMH of the hypothalamus as an example. Figure 4A shows that the VMH is rich in KAP3 mRNA-containing cells (brown staining) but contains few, if any, GAD67 mRNA-positive cells (white grains). Instead, GAD67 mRNA transcripts are seen in cells of the ARC and the region dorsal to the VMH, which also contain KAP3 mRNA, but apparently in different cells. Figure 4B shows that the VMH is rich in both KAP3 and VGLUT2 mRNA transcripts and suggest that they are expressed on the same cells. To define these sites of KAP3 expression more precisely, we used two different brain regions. The CTX was selected (Fig. 4C) because it displays a discrete number of GAD67 mRNA-positive cells (white grains) amid an abundance of cells containing KAP3 mRNA transcripts (brown staining). The VMH, on the other hand, contains an abundance of KAP3 mRNA-positive cells (Fig. 4D), the majority of which also contain VGLUT2 mRNA (white grains, examples denoted by arrows). Cells lacking VGLUT2 mRNA transcripts were also devoid of detectable KAP3 mRNA (examples denoted by asterisks). A similar pattern of KAP3 mRNA expression was observed in all other brain regions examined (not shown), suggesting that KAP3 is preferentially expressed in glutamatergic neurons of the brain.
Figure 4.
KAP3 mRNA is expressed in glutamatergic but not GABAergic neurons of the brain. The in situ hybridization reaction was performed on brain sections derived from three 28- to 30-d-old female rats. The VMH of the hypothalamus and the CTX are used to illustrate this cellular localization. A, The VMH is rich in cells containing KAP3 mRNA (identified with a digoxigenin-labeled cRNA probe, brown staining) but lacks GAD67 mRNA-positive cells (identified with a [35S]UTP-labeled probe, white grains). B, VMH cells contain an abundance of both VGLUT2 and KAP3 mRNA transcripts. C, High-magnification view showing that GAD67 mRNA-containing cells (examples denoted by arrows) in the CTX are different from cells expressing KAP3 mRNA. D, In the VMH, all cells containing VGLUT2 mRNA (white grains) are also KAP3 mRNA positive (brown staining). Cells that are negative for VGLUT2 mRNA also lack KAP3 mRNA (asterisks). Bars, 200 μm (A and B) and 20 μm (C).
In vivo inhibition of KAP3 synthesis decreases GnRH release from the ME and delays the initiation of puberty
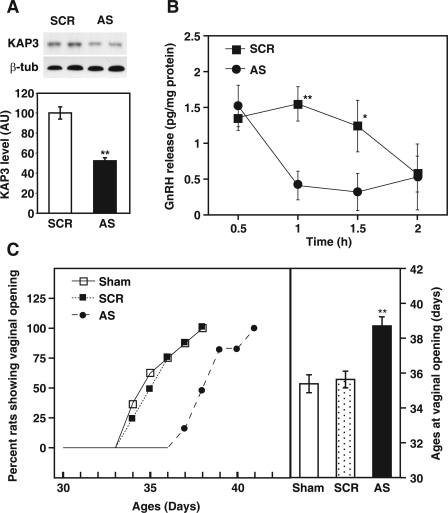
To determine whether KAP3 is required for GnRH secretion and, hence, for the timely initiation of the pubertal process, we subjected juvenile 27-d-old female rats to three consecutive single icv administration of an AS ODN targeting the translation initiation site of KAP3 (see Materials and Methods for details). As shown in Fig. 5A, AS ODN significantly decreased the MBH content of KAP3 protein detected by Western blot 6 h after the last injection. To determine whether this reduction in hypothalamic KAP3 affects GnRH release from the hypothalamus, ME fragments collected from SCR, and AS ODN-injected rats were incubated in vitro 6 h after the third injection, and their basal ability to release GnRH was determined (Fig. 5B). No difference in GnRH levels was detected in medium collected after the initial 30 min incubation, likely reflecting the nonspecific discharge of GnRH typically observed when MBH fragments are studied in this in vitro system (39,46). Thereafter, basal GnRH release was significantly lower (P < 0.05–0.01) than that of ME fragments derived from SCR-injected controls, but this difference was no longer apparent after 2 h incubation when GnRH release from SCR-exposed explants had spontaneously diminished to very low levels.
Figure 5.
In vivo inhibition of KAP3 synthesis via icv administration of an AS ODN against KAP3 mRNA reduces basal GnRH release from the ME in vitro and delays the onset of puberty. A, Upper panel, Western blot showing that KAP3 AS ODN decreased KAP3 protein content in the MBH; middle panel, Western blot showing the lack of changes in β-tubulin (β-tub) content; this housekeeping protein was used as an internal control for normalization purposes; lower panel, quantitation of the changes shown in the upper panels. Each bar represents the mean of three Western blots, each using a pool of three MBH fragments. Vertical lines are sem. **, P < 0.01. AU, Arbitrary units. B, Effect of in vivo KAP3 AS ODN-mediated inhibition of KAP3 synthesis on the in vitro ability of the ME to release GnRH. Each point represents the mean ± sem of five to six independent observations. *, P < 0.05; **, P < 0.01 vs. AS ODN-treated group. C, Delay of female puberty caused by the disruption of KAP3 synthesis. The left panel depicts the percentage of animals in each group showing vaginal opening at different peripubertal ages; the right panel compares the mean age at vaginal opening in AS ODN- and SCR ODN-injected rats and sham-operated rats (n = 20 rats per group). Vertical lines are sem. **, P < 0.01 vs. the two control groups.
To determine whether inhibition of KAP3 synthesis disrupts the timing of puberty we evaluated the effect of icv administration of KAP3 AS ODN on the age at vaginal opening. Consistent with the reduction in GnRH release observed in vitro after the in vivo inhibition of KAP3 synthesis, the age at vaginal opening was significantly (P < 0.01) delayed in KAP3 AS ODN-treated rats (Fig. 5C). These results suggest that KAP3 is involved in the neuroendocrine process underlying the activation of GnRH release at puberty.
Estradiol increases KAP3 protein content in the MBH and ME, but the suppression of KAP3 levels by AS ODN is estradiol independent
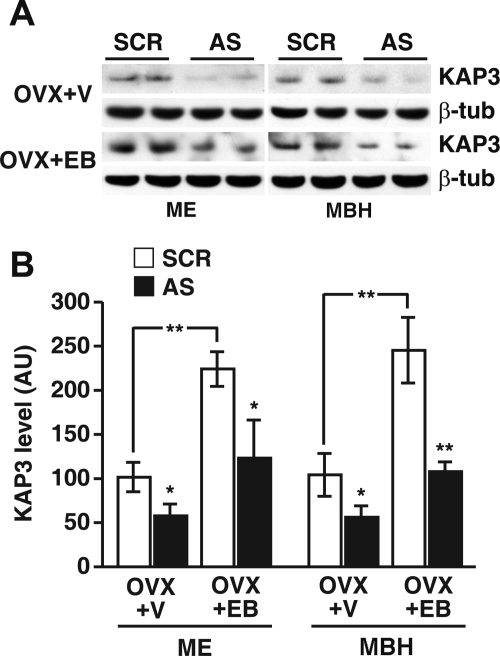
Because KAP3 mRNA content in the MBH was reduced by OVX (Fig. 2), we sought to determine whether estradiol increases KAP3 protein levels in the MBH and/or influences the suppressive effect of KAP3 AS ODN on KAP3 protein abundance. The ME was examined separately because it constitutes a terminal field for glutamatergic neurons controlling neuroendocrine function. Estradiol treatment of rats OVX on d 21, using a regime that results in preovulatory levels of the steroid (two injections, one on P27, the other on P29, killing the animals 6 h after the last injection), increased (P < 0.01) KAP3 protein content in both the MBH and ME (blots in Fig. 6A, white bars in B). The icv injection of KAP3 AS ODN was effective (P < 0.05–0.01) in reducing KAP3 levels in the MBH and ME in both OVX rats and OVX rats treated with EB (blots in Fig. 6A, black bars in B), suggesting that the prevalence of KAP3 protein in the hypothalamus is not regulated by estrogen-dependent posttranslational mechanisms.
Figure 6.
Stimulatory effect of EB on the MBH and ME content of KAP3 protein and suppressive effect of in vivo inhibition of KAP3 synthesis via administration of an AS ODN on the content of KAP3 in the MBH and ME of OVX rats and OVX rats treated with EB as determined by Western blot analysis. A, Representative Western blots; B, quantification of the changes observed in A. Band densities were normalized using the densities of β-tubulin (β-tub) as the normalizing unit and were calculated as percentage of the values observed in samples from OVX+V (vehicle, sesame oil) animals treated with SCR ODN. Each value represents the mean of five to seven independent observations, and vertical lines are sem. Notice that treatment of OVX animals with EB induced a significant increase in KAP3 contents both in the ME and MBH. AU, Arbitrary units. *, P < 0.05; **, P < 0.01 vs. SCR ODN (SCR)-injected controls.
Estradiol affects neither VGLUT2 nor Syp prevalence in the MBH and ME, but suppression of KAP3 synthesis reduces the content of both VGLUT2 and Syp in the ME
Because KAP3 has been shown to be involved in anchoring membranous organelles to the motor protein KIF3A/3B for anterograde transport (17,18), and VGLUT2 is a protein present in synaptic vesicles, we measured the content of VGLUT2 protein in MBH and ME after EB treatment and after inhibition of KAP3 synthesis. As shown in Fig. 7, A and B, EB failed to increase VGLUT2 levels significantly. However, the KAP3 AS ODN was as effective (P < 0.01) in OVX as in OVX rats treated with EB in reducing VGLUT2 protein abundance. These results suggested that loss of KAP3 may also affect other proteins associated with synaptic vesicles. To address this possibility, we determined whether KAP3 deficiency in the presence or absence of estradiol depletes the cellular content of Syp, an integral protein component of synaptic vesicles (47,48). The results showed that, like in the case of VGLUT2, estrogen treatment did not affect Syp levels in either MBH or ME (Fig. 7, C and D). However, suppression of KAP3 synthesis with AS ODN resulted in a selective decrease in Syp abundance in the ME (Fig. 7, C and D).
Figure 7.
Absence of an estrogen effect on the MBH and ME content of VGLUT2 and Syp proteins and suppressive effect of in vivo inhibition of KAP3 synthesis via administration of an AS ODN on the content of VGLUT2 and Syp in the ME of OVX rats and OVX rats treated with EB as determined by Western blot analysis. A, Representative Western blots showing that inhibition of KAP3 synthesis decreases VGLUT2 protein content in the MBH and ME. β-Tubulin (β-tub) was used as an internal control. B, Quantification of the changes shown in A. C, Representative Western blots showing that inhibition of KAP3 synthesis decreases the Syp content in the ME but not the MBH. D, Quantification of the changes illustrated in C. Band densities were normalized using the densities of β-tubulin as the normalizing unit and were calculated as percentage of the values observed in samples from OVX+V animals treated with SCR ODN. B and D, Each bar represents the mean of six Western blots. Vertical lines are sem. AU, Arbitrary units. *, P < 0.05; **, P < 0.01 vs. SCR ODN (SCR)-injected controls.
Inhibiting KAP3 synthesis reduces glutamate release from the ME
If the decrease in VGLUT2 protein content observed in the MBH after inhibition of KAP3 synthesis affects the functional capacity of glutamatergic neurons, a reduction in glutamate release from the ME would be expected. To evaluate this premise, we measured basal glutamate release from ME fragments derived from OVX+V− and OVX+EB-treated rats 6 h after the third icv injection of KAP3 AS ODN or its SCR ODN control. The in vitro glutamate release from the ME was significantly reduced by the inhibition of KAP3 synthesis in both OVX+V and OVX+EB groups at several collection times (Fig. 8, A and B). However, both groups responded similarly to KCl-induced depolarization compared with the control SCR ODN-injected groups (Fig. 8, A and B), suggesting that the reduction in glutamate release is not due to an overall defect in neuronal function. The total amount of the glutamate released from the ME during the entire incubation period of 3 h was significantly lower in KAP3 AS ODN-treated groups as compared with control, SCR ODN-injected animals (Fig. 8C). However, the KAP3 AS ODN treatment appeared to be more effective (P < 0.05) in decreasing glutamate release in OVX+V-treated animals than in the OVX+EB group (Fig. 8C), perhaps due to the stimulatory effect of estrogen on KAP3 content noticed earlier.
Figure 8.
In vivo inhibition of KAP3 synthesis via icv administration of an AS ODN against KAP3 mRNA reduces glutamate release from the ME in vitro equally well in both OVX rats and OVX rats treated with EB. A and B, ME fragments were incubated as indicated in Materials and Methods, and medium samples were collected at 30-min intervals. Each point represents the mean ± sem of six independent observations. C, Total amount of glutamate released from the ME during a 3-h period. *, P < 0.05; **, P < 0.01 vs. SCR ODN (SCR)-treated groups.
Discussion
The present results demonstrate that KAP3, a component of the KIF3A/KIF3B amino-terminal motor domain type of microtubule-associated motors (17,18), is selectively expressed in glutamatergic neurons. Although the examination of several sections throughout the brain did not reveal the existence of glutamatergic neurons devoid of KAP3 or the presence of KAP3 mRNA in some GABAergic neurons, we cannot rule out the existence of such subpopulations, because we did not perform a systematic, quantitative examination of the entire brain. Moreover, it is still possible that discrete subsets of neurons that use neurotransmitters other than glutamate or GABA may express KAP3.
Our results show that the icv administration of an AS ODN targeting KAP3 mRNA decreased hypothalamic expression of KAP3, reduced glutamate and GnRH output from the ME of the hypothalamus, and resulted in delayed puberty. Because of the route used for AS ODN administration, it seems likely that the function of glutamatergic neurons not involved in neuroendocrine regulation, but located near the ventricular system, was also diminished. Further studies are necessary to identify the physiological systems affected by this deficiency.
The absence of detectable KAP3 mRNA and/or protein in GABAergic and GnRH neurons of the rat hypothalamus suggests that the alterations in GnRH secretion and the consequent delay in puberty that follow inhibition of KAP3 synthesis are largely due to a selective loss of KAP3 function in glutamatergic neurons. This conclusion is supported by the decreased content of VGLUT2, a specific vesicular glutamate transporter (15), detected in the MBH-ME of AS ODN-treated rats.
KAP3 is a globular protein that binds to the tail domain of the KIF3A/3B complex but has no effect on the motor activity of KIF3A/3B (18). Instead, it is believed that KAP3 functions to regulate binding of KIF3A/3B to its cargo. By showing that AS ODN-mediated decrease of KAP3 synthesis resulted in decreased hypothalamic glutamate release, and a reduced content of Syp and VGLUT2 in the ME, our results imply that KAP3-mediated recognition of synaptic vesicles by the heterotrimeric KIF3A/3B/KAP3 motor complex is necessary for the anterograde transport of these vesicles and the release of glutamate. This, however, may not be the case because the KIF3A/KIF3B complex transports membrane-bound organelles of 90–160 nm in diameter (31,32), a size different from that of synaptic vesicle precursors, and from vesicles carried by other well-characterized motors such as KIF1, KIF2, and kinesin (17). In fact, the KIF3A/KIF3B motor has been shown not to transport the synaptic vesicle markers Syp, synaptotagmin, and Rab 3A (49). An alternative explanation is that blockade of KAP3 synthesis may affect glutamatergic neuron function by changing the availability of a releasable pool of synaptic vesicles and/or by reducing activity-dependent release of glutamate. This interpretation is supported by recent findings showing that the KIF3 motor is involved in the transport of certain membranous organelles and protein complexes such as fordrin vesicles (50), Kv1 channel-containing vesicles (51), and protein complex attached to tau mRNA (52), which are known to be important for the modulation of axonal outgrowth, neuronal excitability, and microtubule assembly, respectively. It is also possible that the KIF3A/KIF3B/KAP3 complex is required for the transport of membrane organelles (such as precursors of plasma membrane) that are indirectly required for the availability of glutamate-containing vesicles to the synapse (32). Further studies are necessary to distinguish between these possibilities.
Glutamate is the most abundant excitatory neurotransmitter in the nervous system, accounting for nearly 99% of the total brain excitatory transsynaptic communication (53). Glutamate is also the predominant excitatory neurotransmitter involved in neuroendocrine regulation (54). One of the physiological processes under glutamate control within the neuroendocrine brain is sexual maturation (2). Glutamate facilitates this process by stimulating GnRH release into the portal system (55), which conveys neurosecretory signals from the hypothalamus to the anterior pituitary gland. The icv administration of glutamate antagonists diminished both pulse and surge mode of LH release (56,57) and in vitro GnRH release from the MBH (58). Glutamate can facilitate GnRH release by a direct action on receptors located on GnRH neuronal perikarya and processes (12,59) but also via neuronal and glial subsets structurally and/or functionally connected to GnRH neurons (1,60,61). The ME, a region containing numerous GnRH nerve terminals (62), is considered as a bona fide site of glutamate action (11,63,64). In keeping with these reports, our results show that disruption of KAP3 synthesis in vivo results not only in decreased content of VGLUT2 in the MBH and ME but also in a reduced capability of the isolated ME to release glutamate and GnRH in vitro. The absence of changes in Syp content in the MBH after knocking down KAP3 synthesis, in the face of decreased Syp levels in the ME, is consistent with this view, because Syp is a synaptic protein generally expressed in neurons, and as such it would not be directly affected throughout the MBH by the loss of KAP3. Altogether, these results suggest that KAP3 contributes to the excitatory control of female puberty by sustaining, at an intracellular level, a level of glutamatergic output able to excite GnRH neurons at appropriate times during sexual maturation.
Although our study does not demonstrate an involvement of KAP3 in the transport of VGLUT2, the decrease in VGLUT2 seen in the ME and MBH of AS ODN-treated animals suggests that KAP3 is required, at least partially, to maintain this vesicular transporter at normal levels in neuroendocrine glutamatergic axonal terminals. Although VGLUT1 is present in glutamatergic neurons of telencephalic regions such as the cerebral cortex, hippocampus, and cerebellum, VGLUT2 has mostly a diencephalic localization with expression in glutamatergic neurons of the thalamus, hypothalamus, and brainstem (15,45). Previous reports, showing that glutamate release was greatly reduced by the allosteric inhibition of VGLUT binding activity (65,66) indicated that VGLUTs are required for neuronal glutamate release. Our results are consistent with these observations.
KAP3 mRNA abundance increases in the hypothalamus during pre- and peripubertal development, as also after estrogen administration to OVX rats, implying the KAP3 gene expression may be, at least in part, under estrogenic control. That this control may be exerted directly at the level of gene transcription is suggested by the presence of a well conserved estrogen receptor binding domain (www.cbil. upenn.edu/tess/) in the mouse KAP3 promoter (NCBI accession number 047390.2) (the rat KAP3 promoter sequence is currently unavailable). The responsiveness of the KAP3 gene to estrogen is consistent with earlier observations showing that hypothalamic glutamate release is enhanced by estradiol (11) and further supports the idea that the cellular mechanism by which KAP3 influences GnRH secretion and the timing of the pubertal process may involve intraneuronal regulation of glutamate release. In contrast to its effect on KAP3 prevalence, estradiol was ineffective in altering VGLUT2 and Syp levels in the prepubertal hypothalamus. This outcome is at odds with the reported effectiveness of estradiol to enhance VGLUT levels in the adult brain (67) and with the loss of VGLUT2 and Syp observed in the hypothalamus of rats in which KAP3 expression was decreased by the administration of AS ODN. A potential explanation for these discrepancies is that neither VGLUT2 nor Syp are directly targeted by estradiol, so that their content can be modified only by either a larger dose of estradiol or after an interval after administration of the steroid longer than the 6 h we employed in this study. Both interpretations appear to be supported by the tendency of VGLUT2 and Syp abundance to increase in the MBH after estradiol treatment.
Another interesting finding is the lack of effect of EB on glutamate release, despite the effectiveness of AS ODN to reduce release of the amino acid from the ME in both OVX and OVX animals treated with EB. It is possible that estradiol not only stimulates glutamate release but may also increase the effectiveness of glutamate transporters that actively reduce the availability of glutamate for release from the explants to the culture medium. This interpretation is supported by results published elsewhere (68) showing that in vitro blockade of glutamate transport by l-trans-pyrrolide-2,4-dicarboxylic acid, a wide-spectrum inhibitor of excitatory amino acid transporters, results in more glutamate release from the hypothalamus of prepubertal female rats treated with pregnant mare serum gonadotropin to increase estrogen production than from the hypothalamus of untreated animals.
Taken altogether, our results support the idea that blocking KAP3 synthesis in the neuroendocrine brain results in decreased glutamate release, which in turn leads to diminished GnRH output and to a delayed initiation of puberty.
Footnotes
This study was supported by a grant from the Basic Science Research Fund (KRF-2002-015-CS0045) from the Korea Research Foundation. J.C., C.M.H., and E.J.C. were supported by a grant from the Neurobiological Research Fund (B020203), and the Korea Research Foundation Grant (MOEHRD, Basic Research Promotion Fund, KRF-2007-412-J00301), and BK 21 program. J.-H.B. was supported by Research Grants (M103KV010020-03K2201-02030 and M103KV010020-05K2201-02030) from the Brain Research Center of the 21st Century Frontier Research Program. S.R.O. was supported by National Institutes of Health (NIH) Grants HD25123, the Eunice Kennedy Shriver National Institute of Child Health and Human Development/NIH through cooperative agreement (U54 HD18185) as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research, and RR00163 for the operation of the Oregon National Primate Research Center.
Disclosure Statement: The authors have nothing to disclose.
First Published Online August 14, 2008
Abbreviations: ARC, Arcuate nucleus; AS, antisense; AVPV, anteroventral periventricular nucleus; CTX, cerebral cortex; EB, estradiol benzoate; GABA, γ-aminobutyric acid; GAD67, glutamic acid decarboxylase 67; icv, intracerebroventricular; KAP3, kinesin superfamily-associated protein 3; KIF, kinesin superfamily protein; KRB, Krebs Ringer bicarbonate; MBH, medial basal hypothalamus; ME, median eminence; NELL2, neuron-specific epidermal growth factor-like protein 2; nt, nucleotide; ODN, oligodeoxynucleotide; SCR, scrambled; OVX, ovariectomized; P21, postnatal d 21; POA, preoptic area; Syp, synaptophysin; V, vehicle; VGLUT2, vesicular glutamate transporter 2; VMH, ventromedial nuclei.
References
- Ojeda SR, Skinner MK 2006 Puberty in the rat. In: Neill JD, ed. The physiology of reproduction. 3rd ed. San Diego: Academic Press/Elsevier; 2061–2126 [Google Scholar]
- Ojeda SR, Terasawa E 2002 Neuroendocrine regulation of puberty. In: Pfaff D, Arnold A, Etgen A, Fahrbach S, Moss R, Rubin R, eds. Hormones, brain and behavior. Vol 4. New York: Elsevier; 589–659 [Google Scholar]
- Kalra SP, Crowley WR 1992 Neuropeptide Y: a novel neuroendocrine peptide in the control of pituitary hormone secretion, and its relation to luteinizing hormone. Front Neuroendocrinol 13:1–46 [PubMed] [Google Scholar]
- Levine JE, Bauer-Dantoin AC, Besecke LM, Conaghan LA, Legan SJ, Meredith JM, Strobl FJ, Urban JH, Vogelsong KM, Wolfe AM 1991 Neuroendocrine regulation of the luteinizing hormone-releasing hormone pulse generator in the rat. Recent Prog Horm Res 47:97–151; discussion 151–153 [DOI] [PubMed] [Google Scholar]
- Ojeda SR 1994 The neurobiology of mammalian puberty: has the contribution of glial cells been underestimated? J NIH Res 6:51–56 [Google Scholar]
- Herbison AE 1998 Multimodal influence of estrogen upon gonadotropin-releasing hormone neurons. Endocr Rev 19:302–330 [DOI] [PubMed] [Google Scholar]
- Seminara SB 2005 Metastin and its G protein-coupled receptor, GPR54: critical pathway modulating GnRH secretion. Front Neuroendocrinol 26:131–138 [DOI] [PubMed] [Google Scholar]
- Terasawa E, Fernandez DL 2001 Neurobiological mechanisms of the onset of puberty in primates. Endocr Rev 22:111–151 [DOI] [PubMed] [Google Scholar]
- DeFazio RA, Heger S, Ojeda SR, Moenter SM 2002 Activation of A-type γ-aminobutyric acid receptors excites gonadotropin-releasing hormone neurons. Mol Endocrinol 16:2872–2891 [DOI] [PubMed] [Google Scholar]
- Dungan HM, Clifton DK, Steiner RA 2006 Kisspeptin neurons as central processors in the regulation of gonadotropin-releasing hormone secretion. Endocrinology 147:1154–1158 [DOI] [PubMed] [Google Scholar]
- Brann DW 1995 Glutamate: a major excitatory transmitter in neuroendocrine regulation. Neuroendocrinology 61:213–225 [DOI] [PubMed] [Google Scholar]
- Jennes L, Lin W, Lakhlani S 2002 Glutamatergic regulation of gonadotropin-releasing hormone neurons. Prog Brain Res 141:183–192 [DOI] [PubMed] [Google Scholar]
- Südhof TC 2008 Neurotransmitter release. Handb Exp Pharmacol 184:1–21 [DOI] [PubMed] [Google Scholar]
- Takamori S, Rhee JS, Rosenmund C, John R 2000 Identification of a vesicular glutamate transporter that defines a glutamatergic phenotype in neurons. Nature 407:189–194 [DOI] [PubMed] [Google Scholar]
- Fremeau Jr RT, Troyer MD, Pahner I, Nygaard GO, Tran CH, Reimer RJ, Bellocchio EE, Fortin D, Storm-Mathisen J, Edwards RH 2001 The expression of vesicular glutamate transporters defines two classes of excitatory synapse. Neuron 31:247–260 [DOI] [PubMed] [Google Scholar]
- Choi EJ, Ha CM, Choi J, Kang SS, Choi WS, Park SK, Kim K, Lee BJ 2001 Low-density cDNA array-coupled to PCR differential display identifies new estrogen-responsive genes during the postnatal differentiation of the rat hypothalamus. Mol Brain Res 97:115–128 [DOI] [PubMed] [Google Scholar]
- Hirokawa N 2000 Stirring up development with the heterotrimeric kinesin KIF3. Traffic 1:29–34 [DOI] [PubMed] [Google Scholar]
- Yamazaki H, Nakata T, Okada Y, Hirokawa N 1996 Cloning and characterization of KAP3: a novel kinesin superfamily-associated protein of KIF3A/3B. Proc Natl Acad Sci USA 93:8443–8448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa N 1996 Organelle transport along microtubules: the role of KIFs. Trends Cell Biol 6:135–141 [DOI] [PubMed] [Google Scholar]
- Hirokawa N 1998 Kinesin and dynein superfamily proteins and the mechanism of organelle transport. Science 279:519–526 [DOI] [PubMed] [Google Scholar]
- Brendza RP, Serbus LR, Duffy JB, Saxton WM 2000 A function for kinesin I in the posterior transport of oskar mRNA and Staufen protein. Science 289:2120–2122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navone F, Niclas J, Hom-Booher N, Sparks L, Bernstein HD, McCaffrey G, Vale RD 1992 Cloning and expression of a human kinesin heavy chain gene: interaction of the COOH-terminal domain with cytoplasmic microtubules in transfected CV-1 cells. J Cell Biol 117:1263–1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niclas J, Navone F, Hom-Booher N, Vale RD 1994 Cloning and localization of a conventional kinesin motor expressed exclusively in neurons. Neuron 12:1059–1072 [DOI] [PubMed] [Google Scholar]
- Vale RD, Reese TS, Sheetz MP 1985 Identification of a novel force-generating protein, kinesin, involved in microtubule-based motility. Cell 42:39–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale RD, Schnapp BJ, Reese TS, Sheetz MP 1985 Organelle, bead, and microtubule translocations promoted by soluble factors from the squid giant axon. Cell 40:559–569 [DOI] [PubMed] [Google Scholar]
- Brady ST 1985 A novel brain ATPase with properties expected for the fast axonal transport motor. Nature 317:73–75 [DOI] [PubMed] [Google Scholar]
- Hirokawa N 1993 Axonal transport and the cytoskeleton. Curr Opin Neurobiol 3:724–731 [DOI] [PubMed] [Google Scholar]
- Hirokawa N, Sato-Yoshitake R, Kobayashi N, Pfister KK, Bloom GS, Brady ST 1991 Kinesin associates with anterogradely transported membranous organelles in vivo. J Cell Biol 114:295–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata T, Hirokawa N 1995 Point mutation of adenosine triphosphate-binding motif generated rigor kinesin that selectively blocks anterograde lysosome membrane transport. J Cell Biol 131:1039–1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizawa H, Sekine Y, Takemura R, Zhang Z, Nangaku M, Hirokawa N 1992 Kinesin family in murine central nervous system. J Cell Biol 119:1287–1296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo S, Sato-Yoshitake R, Noda Y, Aizawa H, Nakata T, Matsuura Y, Hirokawa N 1994 KIF3A is a new microtubule-based anterograde motor in the nerve axon. J Cell Biol 125:1095–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki H, Nakata T, Okada Y, Hirokawa N 1995 KIF3A/B: a heterodimeric kinesin superfamily protein that works as a microtubule plus end-directed motor for membrane organelle transport. J Cell Biol 130:1387–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilger M, Heger S, Brann DW, Paredes A, Ojeda SR 2001 A conditional tetracycline-regulated increase in γ-amino butyric acid production near luteinizing hormone-releasing hormone nerve terminals disrupts estrous cyclicity in the rat. Endocrinology 142:2102–2114 [DOI] [PubMed] [Google Scholar]
- Simmons DM, Arriza JL, Swanson LW 1989 A complete protocol for in situ hybridization of messenger RNAs in brain and other tissues with radiolabeled single-stranded RNA probes. J Histotechnol 12:169–181 [Google Scholar]
- Berg-von der Emde K, Dees WL, Hiney JK, Hill DF, Dissen GA, Costa ME, Moholt-Siebert M, Ojeda SR 1995 Neurotrophins and the neuroendocrine brain: different neurotrophins sustain anatomically and functionally segregated subsets of hypothalamic dopaminergic neurons. J Neurosci 15:4223–4237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung H, Shannon EM, Fritschy JM, Ojeda SR 1998 Several GABAA receptor subunits are expressed in LHRH neurons of juvenile female rats. Brain Res 780:218–229 [DOI] [PubMed] [Google Scholar]
- Lomniczi A, Cornea A, Costa ME, Ojeda SR 2006 Hypothalamic tumor necrosis factor-α converting enzyme mediates excitatory amino acid-dependent neuron-to-glia signaling in the neuroendocrine brain. J Neurosci 26:51–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanski HF 1991 Monoclonal antibodies to luteinizing hormone-releasing hormone: production, characterization, and immunocytochemical application. Biol Reprod 44:681–686 [DOI] [PubMed] [Google Scholar]
- Ojeda SR, Negro-Vilar A, McCann SM 1979 Release of prostaglandin Es by hypothalamic tissue: evidence for their involvement in catecholamine-induced luteinizing hormone-releasing hormone release. Endocrinology 104:617–624 [DOI] [PubMed] [Google Scholar]
- Ojeda SR, Urbanski HF, Katz KH, Costa ME 1986 Activation of estradiol-positive feedback at puberty: estradiol sensitizes the LHRH-releasing system at two different biochemical steps. Neuroendocrinology 43:259–265 [DOI] [PubMed] [Google Scholar]
- Urbanski HF, Kim SO, Connolly ML 1990 Influence of photoperiod and 6-methoxybenzoxazolinone on the reproductive axis of inbred LSH/Ss Lak male hamsters. J Reprod Fertil 90:157–163 [DOI] [PubMed] [Google Scholar]
- Chapman J, Zhou MJ 1999 Microplate-based fluorometric methods for the enzymatic determination of l-glutamate: application in measuring l-glutamate in food samples. Anal Chim Acta 402:47–52 [Google Scholar]
- Ojeda SR, Urbanski HF 1994 Puberty in the rat. In: Knobil E, Neill JD, eds. The physiology of reproduction. 2nd ed. New York: Raven Press; 363–409 [Google Scholar]
- Ha CM, Choi J, Choi EJ, Costa ME, Lee BJ, Ojeda SR 2008 NELL2, a neuron-specific EGF-like protein, is selectively expressed in glutamatergic neurons and contributes to the glutamatergic control of GnRH neurons at puberty. Neuroendocrinology 88:199–211 [DOI] [PubMed] [Google Scholar]
- Hisano S, Hoshi K, Ikeda Y, Maruyama D, Kanemoto M, Ichijo H, Kojima I, Takeda J, Nogami H 2000 Regional expression of a gene encoding a neuron-specific Na+-dependent inorganic phosphate cotransporter (DNPI) in the rat forebrain. Brain Res 83:34–43 [DOI] [PubMed] [Google Scholar]
- Negro-Vilar A, Ojeda SR, McCann SM 1979 Catecholaminergic modulation of luteinizing hormone-releasing hormone release by median eminence terminals in vitro. Endocrinology 104:1749–1757 [DOI] [PubMed] [Google Scholar]
- Greengard P, Valtorta F, Czernik AJ, Benfenati F 1993 Synaptic vesicle phosphoproteins and regulation of synaptic function. Science 259:780–785 [DOI] [PubMed] [Google Scholar]
- Murphy DD, Segal M 1996 Regulation of dendritic spine density in cultured rat hippocampal neurons by steroid hormones. J Neurosci 16:4059–4068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa N 1997 The mechanisms of fast and slow transport in neurons: identification and characterization of the new kinesin superfamily motors. Curr Opin Neurobiol 7:605–614 [DOI] [PubMed] [Google Scholar]
- Takeda S, Yamazaki H, Seog DH, Kanai Y, Terada S, Hirokawa N 2000 Kinesin superfamily protein 3 (KIF3) motor transports fodrin-associating vesicles important for neurite building. J Cell Biol 148:1255–1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu C, Zhou W, Puthenveedu MA, Xu M, Jan YN, Jan LY 2006 The microtubule plus-end tracking protein EB1 is required for Kv1 voltage-gated K+ channel axonal targeting. Neuron 52:803–816 [DOI] [PubMed] [Google Scholar]
- Aronov S, Aranda G, Behar L, Ginzburg I 2002 Visualization of translated tau protein in the axons of neuronal P19 cells and characterization of tau RNP granules. J Cell Sci 115:3817–3827 [DOI] [PubMed] [Google Scholar]
- Erecinska M, Silver IA 1990 Metabolism and role of glutamate in mammalian brain. Prog Neurobiol 35:245–296 [DOI] [PubMed] [Google Scholar]
- van den Pol AN, Wuarin JP, Dudek FE 1990 Glutamate, the dominant excitatory transmitter in neuroendocrine regulation. Science 250:1276–1278 [DOI] [PubMed] [Google Scholar]
- Brann DW, Mahesh VB 1997 Excitatory amino acids: evidence for a role in the control of reproduction and anterior pituitary hormone secretion. Endocr Rev 18:678–700 [DOI] [PubMed] [Google Scholar]
- Lopez FJ, Donoso AO, Negro-Vilar A 1990 Endogenous excitatory amino acid neurotransmission regulates the estradiol-induced LH surge in ovariectomized rats. Endocrinology 126:1771–1773 [DOI] [PubMed] [Google Scholar]
- Ping L, Mahesh VB, Brann DW 1995 Effect of NMDA and non-NMDA receptor antagonists on pulsatile luteinizing hormone secretion in the adult male rat. Neuroendocrinology 61:226–234 [DOI] [PubMed] [Google Scholar]
- Bourguignon JP, Gerard A, Mathieu J, Simons J, Franchimont P 1989 Pulsatile release of gonadotropin-releasing hormone from hypothalamic explants is restrained by blockade of N-methyl-d,l-aspartate receptors. Endocrinology 125:1090–1096 [DOI] [PubMed] [Google Scholar]
- Kuehl-Kovarik MC, Pouliot WA, Halterman GL, Handa RJ, Dudek FE, Partin KM 2002 Episodic bursting activity and response to excitatory amino acids in acutely dissociated gonadotropin-releasing hormone neurons genetically targeted with green fluorescent protein. J Neurosci 22:2313–2322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore AC 2001 Gonadotropin-releasing hormone neurons, NMDA receptors, and their regulation by steroid hormones across the reproductive life cycle. Brain Res Brain Res Rev 37:235–248 [DOI] [PubMed] [Google Scholar]
- Pabst O, Herbrand H, Arnold HH 1998 Nkx2–9 is a novel homeobox transcription factor which demarcates ventral domains in the developing mouse CNS. Mech Dev 73:85–93 [DOI] [PubMed] [Google Scholar]
- Kawano H, Daikoku S 1981 Immunohistochemical demonstration of LHRH neurons and their pathways in the rat hypothalamus. Neuroendocrinology 32:179–186 [DOI] [PubMed] [Google Scholar]
- Donoso AO, Lopez FJ, Negro-Vilar A 1990 Glutamate receptors of the non-N-methyl-d-aspartic acid type mediate the increase in luteinizing hormone-releasing hormone release by excitatory amino acids in vitro. Endocrinology 126:414–420 [DOI] [PubMed] [Google Scholar]
- Lopez FJ, Donoso AO, Negro-Vilar A 1992 Endogenous excitatory amino acids and glutamate receptor subtypes involved in the control of hypothalamic luteinizing hormone-releasing hormone secretion. Endocrinology 130:1986–1992 [DOI] [PubMed] [Google Scholar]
- Ogita K, Hirata K, Bole DG, Yoshida S, Tamura Y, Leckenby AM, Ueda T 2001 Inhibition of vesicular glutamate storage and exocytotic release by Rose Bengal. J Neurochem 77:34–42 [DOI] [PubMed] [Google Scholar]
- Montana V, Ni Y, Sunjara V, Hua X, Parpura V 2004 Vesicular glutamate transporter-dependent glutamate release from astrocytes. J Neurosci 24:2633–2642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrabovszky E, Kallo I, Turi GF, May K, Wittmann G, Fekete C, Liposits Z 2006 Expression of vesicular glutamate transporter-2 in gonadotrope and thyrotrope cells of the rat pituitary. Regulation by estrogen and thyroid hormone status. Endocrinology 147:3818–3825 [DOI] [PubMed] [Google Scholar]
- Roth CL, McCormack AL, Lomniczi A, Mungenast AE, Ojeda SR 2006 Quantitative proteomics identifies a major change in glial glutamate metabolism at the time of female puberty. Mol Cell Endocrinol 254–255:51–59 [DOI] [PubMed] [Google Scholar]