Abstract
Neonatal exposure of CD-1 mice to diethylstilbestrol (DES) or genistein (GEN) induces uterine adenocarcinoma in aging animals. Uterine carcinogenesis in this model is ovarian dependent because its evolution is blocked by prepubertal ovariectomy. This study seeks to discover novel uterine genes whose expression is altered by such early endocrine disruption via an epigenetic mechanism. Neonatal mice were treated with 1 or 1000 μg/kg DES, 50 mg/kg GEN, or oil (control) on d 1–5. One group of treated mice was killed before puberty on d 19. Others were ovariectomized or left intact, and killed at 6 and 18 months of age. Methylation-sensitive restriction fingerprinting was performed to identify differentially methylated sequences associated with neonatal exposure to DES/GEN. Among 14 candidates, nucleosomal binding protein 1 (Nsbp1), the gene for a nucleosome-core-particle binding protein, was selected for further study because of its central role in chromatin remodeling. In uteri of immature control mice, Nsbp1 promoter CpG island (CGI) was minimally methylated. Once control mice reached puberty, the Nsbp1 CGI became hypermethylated, and gene expression declined further. In contrast, in neonatal DES/GEN-treated mice, the Nsbp1 CGI stayed anomalously hypomethylated, and the gene exhibited persistent overexpression throughout life. However, if neonatal DES/GEN-treated mice were ovariectomized before puberty, the CGI remained minimally to moderately methylated, and gene expression was subdued except in the group treated with 1000 μg/kg DES. Thus, the life reprogramming of uterine Nsbp1 expression by neonatal DES/GEN exposure appears to be mediated by an epigenetic mechanism that interacts with ovarian hormones in adulthood.
IT HAS BECOME APPARENT that both endogenous and exogenous influences during early life contribute significantly to adult disease outcomes (1,2). In this regard, exposure to estrogenic chemicals during critical stages of development exerts long-lasting effects on male and female reproductive organs (3,4,5,6,7,8). In humans, the best-studied example is exposure to diethylstilbestrol (DES). Between 1947 and 1971, approximately 3 million American women were treated with DES during pregnancy to prevent miscarriages (9,10,11). A landmark report linking in utero DES exposure to an increased risk for clear cell adenocarcinoma of the vagina and cervix in female offspring (DES daughters) finally led to the discontinuation of DES use in pregnant women (9). Other adverse transgenerational effects of DES include malformation of the uterus, frequent miscarriages, and an increased incidence of breast cancer in DES daughters (9,11,12,13,14,15), and an increased risk for epididymal cysts, autoimmune disorders, hypospadias, and penile urethra abnormalities in DES sons (16,17,18).
In contrast to in utero exposure to xenoestrogens, peripubertal human exposures to dietary phytoestrogens such as genistein (GEN) appear to reduce later-life breast cancer risk (19). Yet, questions related to the safety of early-life exposure to phytoestrogens remain unanswered (20). Early-life exposure of rats to GEN confers protection against breast cancer (21,22). However, prenatal exposure of rats to GEN increases the incidence of carcinogen-induced mammary tumors (23). Likewise, neonatal exposure of mice to GEN (24,25) or coumestrol (26) adversely affects the reproductive tract. Furthermore, in utero exposure to these phytoestrogens causes significant deficits in the sexual behavior of male offspring (27). Finally, human infants exposed to high levels of dietary phytoestrogens have an increased risk of developing type 1 diabetes (28).
Today, DES is no longer prescribed for use during pregnancy, but thousands of women remain on vegetarian diets, many rich in soy, during pregnancy. Furthermore, nearly 4 million newborns in the United States are fed soy-based formula in the attempt to avoid allergies to cow’s milk protein (29). Thus, the long-term health effects of early-life exposure to soy-derived GEN ought to be a public health concern (20,30). The neonatal DES/GEN-induced mouse uterine adenocarcinoma (UCa) model (31) provides an excellent prototype system for mechanistic investigations (2) addressing these historical and/or contemporary concerns. In this model, CD-1 mice treated during the first 5 d of life with DES (30), GEN (24), or other estrogenic compounds develop, in a dose-dependent manner, UCa that resembles the most prevalent type of human endometrial cancer type I (32) as the animals age. Intriguingly, the evolution of UCa is dependent on postpubertal ovarian steroids because this malignancy does not develop in ovariectomized (OVX) animals (31).
McLachlan et al. (3) were the first to propose that epigenetic modification of gene expression could be a key mediator of developmental influences imposed by estrogens. They reported concordant demethylation of a single CG site (−464) in the promoter of lactoferrin and persistent overexpression of the gene in mature mouse uteri after neonatal, but not adult, treatment with DES (33). However, their follow-up studies on c-fos (34) and two Hox genes (35) were less conclusive. Using methylation-sensitive restriction fingerprinting (MSRF), an unbiased methylation profiling protocol, we recently identified phosphodiesterase 4 variant 4 as a gene susceptible to epigenetic reprogramming in the rat prostate by neonatal exposures to estradiol or bisphenol A (36). Coincidentally, these treatments also increased the risk of cancer in the adult gland. In contrast, evidence supporting epigenetic imprinting due to early-life exposures to GEN or other soy isoflavones is virtually nonexistent, and investigations are needed to fill this data gap.
The objective of this study has been 2-fold: 1) to determine whether neonatal exposure to DES/GEN can alter gene expression in the mouse uterus in later life via an epigenetic mechanism such as DNA methylation, and 2) to unveil any interaction between adult ovarian hormones and neonatal DES/GEN reprogramming. Therefore, we treated neonatal mice with DES or GEN on d 1–5 and used MSRF to identify genes whose methylation status was altered in the treated animals throughout adulthood (6–18 months of age). The studies were conducted in both intact mice and animals OVX before puberty to reveal the contribution of ovarian hormones. Of the candidate genes we identified, we chose to conduct a detailed study on nucleosome binding protein 1 (Nsbp1), correlating the methylation status of its promoter region to the expression of the gene in the various treatment groups. We chose Nsbp1 because its protein product is centrally linked to the process of chromatin remodeling.
Materials and Methods
Animals and neonatal treatment
Adult CD-1 [Crl:CD-1 (ICR) BR] mice were obtained from Charles River Breeding Laboratories (Raleigh, NC) and bred to male mice of the same strain in the breeding facility at the National Institute of Environmental Health Sciences (NIEHS) (Research Triangle Park, NC). The day of detection of a vaginal plug was considered d 0 of pregnancy. Pregnant mice were housed under controlled lighting (12 h light and 12 h dark) and temperature (21–22 C) conditions. Mice were housed in polysulfone-ventilated cages (Technoplast, Exton, PA), and provided with National Institutes of Health (NIH)-31 laboratory mouse chow (NIH, Bethesda, MD) and fresh water ad libitum. Mouse chow was tested for estrogenic activity (37) and found to be negative. All animals were handled according to NIEHS/NIH guidelines and in compliance with an approved NIEHS/NIH animal care protocol.
At birth, pups were separated according to sex and then randomly standardized to eight female pups per litter. Female pups were treated on d 1–5 with 1 (DES 1) or 1000 (DES 1K) μg/kg · d DES (Sigma-Aldrich, St. Louis, MO) or 50 mg/kg·d (GEN 50K) GEN (Sigma-Aldrich) dissolved in corn oil (Sigma-Aldrich), or with corn oil alone (oil-treated controls) by sc injection (n = 42 per treatment group). For each treatment group, 18 pups were killed by CO2 asphyxiation at 19 d of age before puberty. Three uteri were pooled to generate a total of six d-19 samples. The remaining 24 pups were divided into two groups: 12 were OVX before puberty and the other 12 left intact. After the animals reached maturity, six OVX and six intact mice from each treatment group were killed at 6 or 18 months of age. Uteri were collected from individual adult animals and snap frozen in liquid nitrogen for DNA and RNA isolation.
MSRF
MSRF was done as described (36,38,39), with minor modifications. In brief, 1 μg genomic DNA extracted from tissues with the DNeasy Tissue kit (QIAGEN, Inc., Valencia, CA) was digested with MseI alone or double digested with BstUI and MseI (New England Biolabs, Beverly, MA). Digested DNA was amplified by PCR with 2 μCi [α-32P]deoxycytidine triphosphate (3000 Ci/mmol; DuPont New England Nuclear Life Science Products, Boston, MA) with various combinations of paired arbitrary primers chosen from the following: Bs 7, 5′-GAGGTGCGCG-3′; Bs 11, 5′-GAGAGGCGCG-3′; Bs 13, 5′-CGGGGCGCGA-3′; and Bs 17, 5′-GGGGACGCGA-3′. PCR products were separated on 6% nondenaturing polyacrylamide gels, which were dried and exposed to Kodak MS film (Kodak, New Haven, CT) to visualize the labeled bands. Candidate bands displaying differential methylation status among the majority of DES- or GEN-treated samples as compared with the oil-treated controls were cut, reamplified, and cloned directly into pCR2.1 vector (Invitrogen Corp., Carlsbad, CA) for sequencing (Macrogen Inc., Seoul, Korea). The sequence obtained was aligned with the database from GenBank and Reference Sequence (RefSeq) using Basic Alignment Search Tool, expressed sequence tag homology (National Center for Biotechnology Information), and BLAT search (University of California Genome Research, Santa Cruz, CA).
Real-time RT-PCR
Total RNA (2 μg) was isolated from tissues with the TRIzol reagent (Invitrogen) and reverse transcribed with SuperScript III (Invitrogen). The levels of Nsbp1 transcript in various samples were determined by SYBR Green-based real-time PCR method in a 7500 Fast Real-Time System using 2× Power SYBR Green reagent (Applied Biosystems, Foster City, CA). RPL19 transcript levels were used as the internal control. Primers for Nsbp1 and RPL19 were designed in an exon/exon-spanning region and shown as follows: Nsbp1 (NM_016710.1), 5′-AGCCAGGAGCCAAAGAGAA-3′ (forward) and 5′-AATGGGTGCCTCCATAATCTT-3′ (reverse); and RPL19 (NM_009078.1), 5′-GGTGACCT GGATGAGAAGGA-3′ (forward) and 5′-TTCAGCTTGTGGATGTGCTC-3′ (reverse). The 2−ΔΔCt method was used to calculate the relative expression level of the transcripts. Real-time RT-PCR was performed in triplicate for each sample.
Bisulfite genomic sequencing
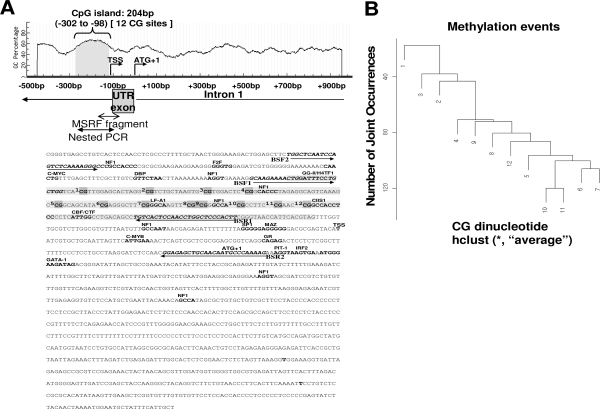
Genomic DNA (200 ng) from tissue samples was modified with sodium bisulfite with the EZ DNA Methylation Gold kit (Zymo Research, Orange, CA) with controls to ensure complete conversion. In silico analyses and detailed database searches were used to predict the CpG island [CGI(s)] in the 5′ flanking region of Nsbp1, to establish its relationship with the original MSRF-identified sequence, and to construct the organizational map for this region (Fig. 1). A 194-bp fragment encompassing the Nsbp1 CGI was obtained by nested PCR with primers designed with Primer3 (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi) and MethPrimer (http://www.urogene. org/methprimer/index.html). The first PCR was performed with the 5′-TGGTTTAATTTAGTTTTAAAAGGGTT-3′ and 5′-CTTTTAAACATTATTACAACTCTCC-3′ as the forward and reverse primer, respectively. The nested PCR primers were 5′-CCAACAAAAAAAT CCAATTTTCTTAC-3′ as the forward– and 5′-TGTTATTTTAATTTGGTTTTTATTT-3′ as the reverse primer. Both PCRs were performed under the following conditions: 94 C for 10 min, 40 cycles of denaturing (94 C for 30 sec), annealing (56 C for 1 min), and extension (72 C for 1 min), followed by a 12-min final extension. The PCR product was gel purified and cloned into pCR2.1 vector. Three clones were picked from each sample for sequencing (Macrogen), and six sets of samples from each group were used. The DNA methylation data from sequencing were analyzed with the BiQ analyzer (40).
Figure 1.
Schematic diagram of CpG content (%) in the 5′ flanking region of the Nsbp1 gene. A, A CGI of 204 bp (gray) was found between −302 and −98 bp upstream of the TSS and translation start site (ATG). There are 12 CG sites encompassing the entire CGI, and an individual CG site is marked as gray in the genomic DNA sequence. The 194-bp nested PCR-amplified region is enclosed by arrows, and the methylation status of this region was determined by bisulfite genomic sequencing. Potential transcription factor binding sites are in bold type. The primers (BSF1, BSR1, BSF2, and BSR2) for nested PCRs are in dark italic bold type. B, Hierarchical cluster analyses of methylation to establish clusters of CG sites based on the entire sequencing data set. The results were summarized from the status of 4320 CG sites from a total of 360 alleles in the entire set of 120 samples. CG dinucleotides are grouped on the basis of the number of joint methylation events of their pairs. The average linkage was used for hierarchical clustering (sites 1–12), e.g. 10 and 11 co-occurred in most clones (120 times). UTR, Untranslated region.
Statistical analysis and hierarchical cluster analyses
Data on relative gene expression were expressed as the mean ± sd. All data groups were analyzed by one-way ANOVA, followed by post hoc Bonferroni tests with two-tailed distribution, and significant differences between groups were accepted at P < 0.05.
Unsupervised two-way hierarchical clustering of methylation profiles was performed on the basis of the extent of methylation at each of the CG sites in the Nsbp1 CGI. The methylation matrix was formed by counting the number of clones with methylated CG sites for each mouse and each site separately. Rows of the matrix represented methylation profiles of individual mice and columns of the matrix methylation profiles of individual CG sites. Mouse methylation profiles (rows) and CG site methylation profiles (columns) were clustered independently with the use of Euclidian distance-based average linkage hierarchical clustering (41). The hierarchical tree based on clustering mouse methylation profiles was “cut” to create two clusters of mice with similar methylation patterns. The statistical significance of the association between the membership in the “high degree of methylation (hypermethylation)” cluster and the down-regulation of the Nsbp1 gene as compared with the respective oil-treated control was assessed with Fisher’s exact test for the 2-by-2 table (42).
Results
Neonatal exposure to DES or GEN induced permanent alterations in DNA methylation status of specific genes in mouse uteri
We first sought to determine whether neonatal treatment of mice with DES or GEN induced long-term alterations in DNA methylation of specific genes. To identify differentially methylated CG-rich sequences between oil-treated controls vs. the various DES/GEN treatment groups in an unbiased manner, we performed MSRF on DNA samples obtained from 6-month-old OVX animals. We chose this group for sequence discovery for two reasons: 1) we were interested in the identification of candidates with permanent but early-onset methylation changes that could potentially be used as future predictive biomarkers of neonatal DES/GEN-induced UCa, and 2) we wanted to avoid the identification of sequences whose methylation changes were induced by adult ovarian steroid per se and not related to the “imprinting effects” of neonatal DES/GEN. Nevertheless, once we identified a target gene, we confirmed its methylation status in all samples among the various treatment and age groups.
After an initial screening for arbitrary primers for those that produced the best signals and maximal coverage, we selected four to conduct six unique MSRFs, at least twice for each, on the sample set. More than 60 DNA sequences (distinct bands in the sequencing gels) were identified as potential leads, with repeatable methylation alterations across multiple samples and treatment groups. These bands were cut, reamplified, cloned, sequenced, and aligned with GenBank and RefSeq databases using Basic Alignment Search Tool, expressed sequence tag homology (National Center for Biotechnology Information), and BLAT (University of California Southern California, Los Angeles, CA). After redundant sequences and those aligned to expressed sequence tags were eliminated, the remaining sequences were mapped to 14 unique known genes with more than 95% homology (Table 1). These genes were known to be involved in the regulation of gene transcription (Nsbp1, Id3, and Supt4h1), cell-cycle progression and DNA repair (Gadd45 g and Rad9B), signal transduction (Pip5k2a, Ptprr, Dusp16, and Kit), fatty acid metabolism (Acox1), protein folding and ubiquitination (Ube4b), protein binding (Cep97), cell adhesion (Nrp2), and vacuole transport (Vps29). Among the 14 candidates, the MSRF-identified sequences of eight were harbored in a CGI located in their proximal promoter region, exon 1 and/or intron 1 (Table 1). Because CGIs located in 5′ flanking region are more likely than to be involved in transcription regulation (6), we decided to choose a candidate among the eight genes with 5′ CGI for further study. On the basis of known function, we selected Nsbp1 because its gene product binds to nucleosome core element and may play critical roles in chromatin remodeling (49,50,51).
Table 1.
Differentially methylated candidate genes identified with MSRF
| Clone | Primer 1 | Primer 2 | Methylation statusa | Chromosome, location | Gene homology | Region matchedb | Related pathway (s) | Refs. c |
|---|---|---|---|---|---|---|---|---|
| 1c p7p11 | 7 | 11 | Hypo in DES0.01,1,1K and GEN50K | 2, 2 A3 | Pip5k2a | Intron 1 | Phosphatidylinositol signaling | |
| 2a p7p11 | 7 | 11 | Hypo in DES0.01,1,1K and GEN50K | 10, 10 A2 | Ptprr | Intron 10 | Protein tyrosine phosphatase-MAPK signaling | |
| 3 p7p11 | 7 | 11 | Hypo in DES0.01,1,1K and GEN50K | 11, 11 E2 | Acox1 | Intron 2 | Fatty acid metabolism | |
| 4 p7p11 | 7 | 11 | Hypo in DES 1K | X, X D | Nsbp1 | 5′end/UTRexon | Regulation of transcription | |
| 5 p7p11 | 7 | 11 | Hypo in DES0.01,1,1K and GEN50K | 6, 6 G1; 6 62.0 cM | Dusp16 | Intron 7 | MAPK signaling | |
| 2 p11p13 | 11 | 13 | Hypo in DES1, GEN50K | 16, 16 C1.1 | Cep97 | Intron1/exon1 | Protein binding | |
| 1 p7p17 | 7 | 17 | Hyper in DES 1, GEN50K | 1, 1 C2 | Nrp2 | Intron6 | Cell adhesion; cell differentiation and development | |
| 3 p7p17 | 7 | 17 | Hyper in DES 1 | 4, 4 D3; 4 66.0 cM | Id3 | Intron1 | Regulation of transcription | 43 |
| 4 p7p17 | 7 | 17 | Hyper in DES 1 | 4, 4 E2; 4 76.4 cM | Ube4b | Intron 6 | Protein folding and ubiquitination | |
| 6 p7p17 | 7 | 17 | Hyper in DES 1, GEN50K | 5, 5 C3.3; 5 42.0 cM | Kit | Intron 14 | Protein kinase signaling | 44,45,46,47,48 |
| 5 p11p17 | 11 | 17 | Hyper in DES0.01,1,GEN50K | 13, 13 A5-B | Gadd45g | Intron 1 | Growth arrest and DNA damage | |
| 3 p11p17 | 11 | 17 | Hyper in DES0.01,1,GEN50K | 5, 5 F | Rad9B | 5′ end/intron1/exon1 | DNA repair and cell cycle arrest | |
| 6 p11p17 | 11 | 17 | Hyper in DES1K | 11, 11 C; 11 49.0 cM | Supt4h1 | Intron 1/exon2/intron2 | Chromatin remodeling; regulation of transcription | |
| 4 p11p17 | 11 | 17 | Hyper in DES0.01, 1,GEN50K | 5, 5 F | Vps29 | 5′end/exon1 | Golgi to vacuole transport |
Fragments were identified based on SWISS-PROT, Translated European Molecular Biology Laboratory, mRNA, and RefSeq search. Hyper, Hypermethylation; Hypo, hypomethylation; UTR, untranslated region.
Methylation statuses of neonatal DES-/GEN-treated mice were compared with those in oil-treated OVX controls at 6 month of age. The differentially methylation changes were found at least in one group of neonatal treatments.
Region that fragments identified from MSRF matched to the gene.
Published studies in uterus development/endometrial cancer.
In the absence of adult ovarian steroids, neonatal treatment with DES/GEN exerted differential effects on alterations in gene expression/methylation status of Nsbp1 in an age-dependent manner
From in silico analysis, we deciphered the overall organization of the 5′ flanking region of Nsbp1 (Fig. 1). A 153-bp CGI (−302 to −98) with 12 CG sites was found immediately upstream of the transcription start site (TSS). Multiple transcriptional factor binding sites were computationally identified in this CGI; they include NF1, LF-A1, and CIIS1 (Fig. 1A). Clustering analysis of the methylation status of 4320 CG from a total of 360 alleles in the entire set of 120 samples representing the treatment by age groups was performed to determine the frequencies of methylation and their joint occurrences (Fig. 1B), an approach used previously to identify methylation hot spots in the promoter CGI of ESR2 (52). In the promoter CGI of Nsbp1, comethylation of CG 10 and 11 was the most frequent methylation event, followed by comethylation of CG 6 and CG 7. With the exception of CG 1 to CG 3, all other sites (four, five, seven through nine) exhibited moderate propensity for comethylation with the highest ranking sites. Thus, no distinctive methylation hot spot was identified within the promoter CGI of Nsbp1, but methylation tended to be more frequent toward its 3′ end.
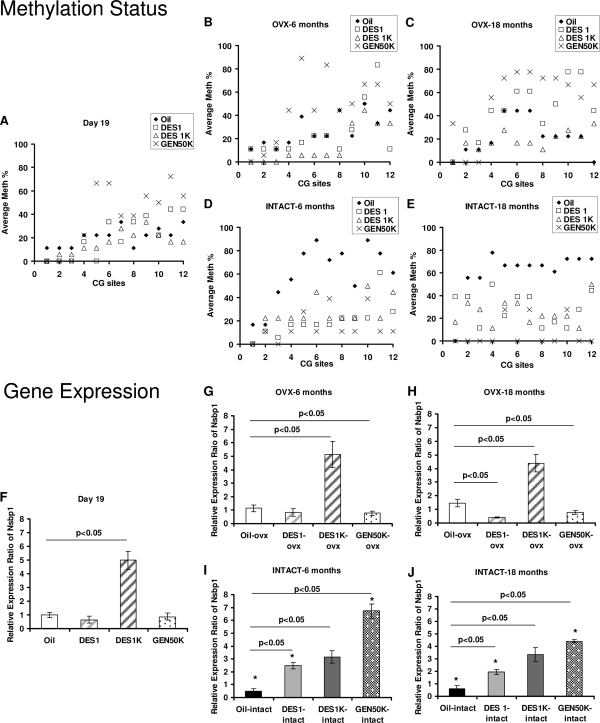
We next examined the methylation frequencies of individual CG sites among the different neonatally treated groups over time and compared those OVX before puberty with those left intact (Fig. 2). Visual inspection of Fig. 2A at d 19 of age found no significant differences between the oil-treated controls and the two DES-treatment groups at d 19 of age. All exhibited minimal to moderate methylation in their uterine Nsbp1 promoter CGI. However, animals treated with GEN 50K showed distinct hypermethylation in this Nsbp1 promoter CGI as compared with the other three groups. In OVX mice the neonatal GEN effect persisted, and methylation increased progressively throughout life (Fig. 2B compared with Fig. 2C). In the oil-treated controls, no drastic changes in the extent of methylation in this CGI were found throughout life. However, the mice treated with DES 1 exhibited a moderate increase in Nsbp1 promoter CGI methylation as they aged (6 vs. 18 months of age), whereas the animals treated with DES 1K consistently displayed the lowest degree of methylation in this CGI at both 6 and 18 months of age (Fig. 2, B and C).
Figure 2.
Promoter hypomethylation and overexpression of Nsbp1 in intact uterus of DES- or GEN-treated mice but gene silencing of Nsbp1 in oil-treated mice. Comparison of Nsbp1 promoter methylation and mRNA transcript levels of samples at d 19, 6 months, and 18 months as determined by bisulfite genomic sequencing and real-time RT-PCR, respectively. A–E, Methylation status at each CG site of Nsbp1 promoter was indicated as average methylation (%) from all clones of each sample. Solid diamond, oil control; open square, DES 1 μg/kg · d (DES 1); open triangle, DES 1 mg/kg · d (DES 1K); and cross, GEN 50 mg/kg · d (GEN 50K). F–J, Relative gene expression of Nsbp1 of d-19 samples from oil-treated mice was set to one. Columns, mean; bars, sd; open bar, mice were OVX before puberty; gradient shading bars, OVX mice treated with DES; dot bar, OVX mice treated with GEN; solid bar, mice without ovariectomization (INTACT). Dark, oil control; light gray, DES 1; dark gray, DES 1K; and dot and solid bar, GEN 50K. All data groups were analyzed by ANOVA, followed by post hoc Bonferroni tests with two-tailed distribution, and significant differences between groups were accepted at P < 0.05. *, P < 0.05 when compared with the OVX samples.
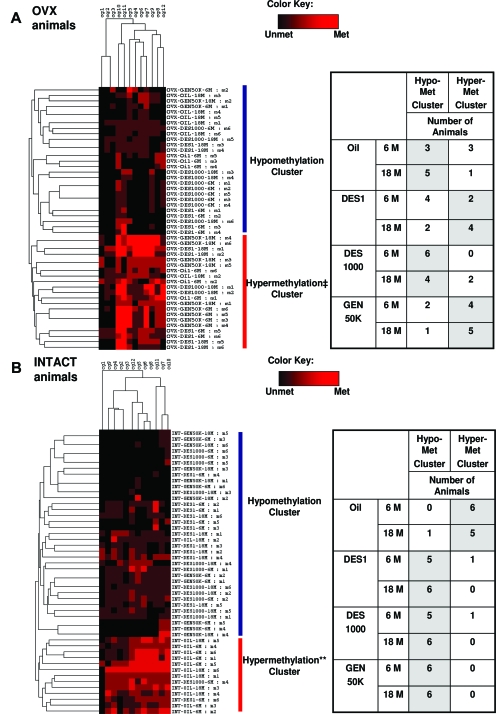
The impact of neonatal DES/GEN exposure-induced life reprogramming of the methylation patterns over time was confirmed and better illustrated when the bisulfite sequencing data were analyzed by a two-way hierarchical clustering analysis on the 6- and 18-month-old OVX animals (Fig. 3A). Individual animals were classified into “hypomethylated” and “hypermethylated” groups on the basis of their methylation profiles consisting of the frequency of methylation at each CG within their uterine Nsbp1 promoter CGI. At 6 months of age, the oil-treated controls were partitioned half (3) and half (3) into the two groups, which acts as a reference for comparison. At 18 months of age, five of the oil-treated controls were in the hypomethylated group, and only one was left in the hypermethylated group, suggesting an age-dependent hypomethylation of the promoter CGI in the control mice. This aging-dependent portioning pattern was reversed in the neonatal DES 1-treated group, with an increase in the number of animals (from two to four) partitioned into the hypermethylated group with old age. A similar “treatment by age” partitioning pattern was observed in the neonatal GEN 50K-treated mice, with more animals (from four to five) classified as hypermethylated as animals aged. However, neonatal DES 1K-treated animals displayed a distinctly opposite partitioning pattern, with all six mice in the hypomethylated group at 6 months, and with four remaining in the hypomethylated group at 18 months of age.
Figure 3.
Two-way hierarchical cluster analyses of the relative methylation of the 12 CG dinucleotides (rows) in the Nsbp1 promoter region measured on 48 tissue samples from eight OVX (A) or eight without ovariectomization (INTACT) (B) animal groups (column). 6 M and 18 M represent animals killed at 6 and 18 months of age, respectively. The number of clones with methylation events at each CG dinucleotide was counted for each mouse. The maximum number of events at each dinucleotide is three. Mice were then clustered on the basis of their methylation (Met) counts using the Euclidian distance measures between each pair of mice and the average linkage hierarchical clustering. Each mouse is represented by a row in the heat map, with three shades of red indicating the counts of one, two, and three for each dinucleotide, and black denoting no methylations (Unmet). The hierarchical clustering tree was “cut” to create two clusters of mice with similar “methylation profiles” (red and blue). The correlation between the membership in the “high degree of methylation (hypermethylation)” cluster (red) and the gene down-regulation of Nsbp1 gene when compared with the respective oil-treated control was assessed using the Fisher’s exact test for the 2-by-2 table. ‡, P value of 0.036 for data obtained from OVX animal groups (A). **, P <10−7 for data obtained from the animal groups without ovariectomization (B). These P values imply a statistically significant enrichment of mice with elevated Nsbp1 in the “low degree of methylation (hypomethylation)” cluster and concentration of mice without elevated Nsbp1 in the “high degree of methylation (hypermethylation)” cluster. The tables in A and B show the number of animals of each group in each cluster.
The life-time changes in methylation status induced by neonatal treatment with DES/GEN found in the promoter CGI of Nsbp1 correlated well with data on relative gene expression (Fig. 2, G and H). In 18-month-old animals, a small but significant increase in Nsbp1 expression was observed in the oil-treated animals (Fig. 2H), whereas a modest decline in gene expression was seen in DES 1- and GEN 50K-treated mice. The most dramatic effect of neonatal treatment was observed in the DES 1K group, which displayed chronic overexpression (>4-fold) of transcript levels of Nsbp1 throughout life (Fig. 2, F–H).
In the presence of adult ovarian steroids, neonatal treatment with DES/GEN exerted similar effects on the alteration of gene expression/methylation status of Nsbp1 in a manner independent of the neonatal treatment regimen
In intact mice, in stark contrast to OVX animals, the life reprogramming of gene expression and methylation changes of the Nsbp1 promoter by neonatal DES- or GEN treatments are similar among all treatment groups. Visual examination (Fig. 2D) showed marked hypermethylation of the uterine Nsbp1 promoter CGI in the oil-treated mice when they reached maturity, and that remained throughout life (Fig. 2E). Neonatal treatment with DES (both high and low dose) and GEN produced similar life reprogramming events. First, the uterine Nsbp1 promoter CGI was unable to undergo developmentally programmed hypermethylation and remained minimally to moderately methylated throughout life (Fig. 2, D and E). Second, Nsbp1 was aberrantly overexpressed in all animals neonatally treated with DES or GEN (Fig. 2, I and J).
Two-way clustering analysis provided statistically significant confirmation of data on the epigenetic changes induced by neonatal treatment with DES/GEN in the Nsbp1 promoter CGI (Fig. 3B). All oil-treated mice were in the “hypermethylated” group at 6 months of age, and five remained in this group at 18 months of age. In contrast, neonatal treatment with DES/GEN caused all but two of 36 animals to fall into the hypomethylation group.
A tight association between gene silencing and gene methylation, and the reverse, was observed. Oil-treated intact controls showed a slight but progressive decline in Nsbp1 expression. An aberrant overexpression of Nsbp1 was observed in all intact animals treated neonatally with DES or GEN (Fig. 2, I and J). A trend toward dose dependency was observed in the two DES groups at 18 months of age. The level of overexpression of Nsbp1 observed in the intact neonatal GEN 50K-treated mice was dramatic. The level of aberrant overexpression had subsided somewhat when intact mice had reached 18 months of age.
Discussion
In this study, using an unbiased methylation profiling method, we first identified 14 candidate genes whose methylation status in the mature mouse uterus was altered by neonatal exposure of the pups to DES and/or GEN. None of these genes except c-kit (53) has previously been characterized in the rodent uterus. It is noteworthy that eight of 14 of these genes have a CGI located in their 5′ flanking region, suggesting that DNA methylation may play a role in their regulation. Knowledge-based analysis revealed that these genes encode proteins involved in signal transduction, receptor activation, tumor angiogenesis/metastasis, cell proliferation, apoptosis, intracellular trafficking, DNA repair, and chromatin remodeling. The discovery of these new candidate genes has strengthened McLachlan et al.’s (33) claim of an epigenetic basis for the developmental effects of DES, and has provided much needed initial evidence that a phytoestrogen such as GEN may possess a similar potential.
Two-way hierarchical clustering analyses were used in this study to partition individual animals based on the methylation status of individual CG sites on the Nsbp1 promoter. This approach has proven to be a creative and useful data analysis tool because it provides the needed statistical power and easy readout of complex patterns. Together with the result from real-time RT-PCR, it revealed that uterine Nsbp1, which is expressed at a steady level throughout life in mice without ovariectomization, undergoes promoter hypermethylation with the advancement of age. This is in agreement to epigenetic changes brought on by aging in the phosphodiesterase 4 variant 4 promoter of the normal rat prostate (36) and the estrogen receptor-α (ESR1) promoter of the normal colorectal mucosa (54), and normal prostate (55); both become hypermethylated with aging. Thus, Nsbp1 expression undergoes an aging-dependent, epigenetic regulation via methylation/demethylation of gene promoters.
In the absence of influences from the adult ovaries, the developmental effects of GEN 50K were distinctly different from that induced by DES 1K, but not by low-dose DES (DES 1). Neonatal treatment with GEN 50K clearly caused hypermethylation of the Nsbp1 promoter as compared with that of oil-treated controls in the mature mouse uteri, and the hypermethylation became more severe with age. The increase in methylation of the Nsbp1 promoter correlated with a modest decline in uterine gene expression in the 18-month-old animals. In contrast, neonatal treatment with DES 1K caused an early and persistent hypomethylation in the Nsbp1 promoter and marked increases (>4-fold) in gene expression in both adult and d-19 animals. Although the adverse effects of widespread usage of DES over three decades have been clearly established in both male and female offspring (3,14), questions about the benefits and health problems related to high GEN exposure through dietary soy consumption or as nutritional supplements during pregnancy or of soy formula by infants (20), have not been resolved. Our finding of differential developmental effects of DES vs. GEN in the epigenetic reprogramming of gene expression may establish a foundation for future investigations addressing these public health concerns.
The stark contrasts between neonatal high-dose DES and GEN treatment were erased or “homogenized” by the strong influences of adult ovarian steroids. Under their influences, the Nsbp1 promoter in the mature uteri of neonatal oil-treated controls underwent developmental hypermethylation and a subduing of gene expression. However, animals exposed to both doses of DES as well as those exposed to GEN early in life exhibited aberrant overexpression of Nsbp1 and a marked hypomethylation of its promoter. The divergence in the methylation of Nsbp1 promoter at maturation, i.e. marked hypermethylation in oil-treated controls and distinct hypomethylation in the neonatal DES/GEN-treated mice, is a remarkable testimony to the powerful impact of epigenetic reprogramming that becomes apparent only when the animals reach maturity. However, this divergence also strongly accentuates the dependency of developmental estrogen reprogramming on adult ovarian steroids to bring out the preprogrammed phenotypes. These data offer an important paradigm for understanding early-life reprogramming of adult diseases. Principally, a two-step process is required, with the early-life exposure establishing the “ground rules” or courses of development but the phenotypes being realized only at maturation, due directly or indirectly to the presence of adult ovarian steroids. Figure 4 is a schematic depiction of the complex interplay between neonatal epigenetic reprogramming and expression of the phenotypes during adulthood. As an extrapolation, if adult ovarian steroids play the proposed roles, the decline in the influence of adult ovarian steroids with age might explain a possible “relaxation of epigenetic regulation.” This latter theory will have to await substantiation by data from future experiments.
Figure 4.
Schematic diagram of our hypothesis about how neonatal DES or GEN exposure interacts with adult ovarian steroids [primarily estradiol (E2)] to alter Nsbp1 transcription in mice uteri. Normally, Nsbp1 is actively transcribed at a moderate to low level before puberty except in the GEN-treated mice. After puberty, in the presence of adult ovarian hormones (estradiol), Nsbp1 is silenced with aging. A group of enzymes, including histone deacetylases (HDACs), DNA methyltransferases (DNMTs), and methylated DNA binding protein (MeCP2), induces compaction of the chromatin around the gene promoter region, leading to gene silencing. Nevertheless, after neonatal exposure to DES or GEN, Nsbp1 fails to “shut-off” in the presence of ovarian hormones (estradiol) and is actively transcribed throughout life. A possible mechanism underlying this gene dysregulation could be related to the association of the remodeled chromatin with histone acetyltransferase (HAT), demethylases, transcription factors (TFs), and other unknown factors (?). These molecules likely prevent the binding of histone deacetylases, DNA methyltransferases, and methylated DNA binding protein, which normally are responsible for the gene silencing. Persistent elevation of Nsbp1 expression is associated with the increase in incidence of uterine cancer later in life. The height and the shape of the rectangle of hexagon under each cartoon depict the level and time line of expression.
Mouse Nsbp1 is a nucleosome core element binding protein. It is structurally similar to the conserved functional domain of the high-mobility-group (HMG) proteins, HMG-14/-17 (51). The interactions of HMGs with nucleosomes induce transient destabilization of the chromatin, increase the access to nucleosomal DNA, and enhance transcriptional activity in the targeted regions (56). The mouse gene is localized on chromosome X, and shows strong expression in liver, kidney, trabecular bone, and bone marrow stromal cells (57). The human homolog, NSBP1, is located in Xq13.3. Very little is known about how this gene is regulated, and no information is available on its involvement in uterine carcinogenesis. However, the purported functions of Nsbp1 in chromatin remodeling and transcriptional activation, together with our current finding that the expression of Nsbp1 is under estrogen-mediated epigenetic regulation, have led us to speculate that Nsbp1 may participate in the tumorigenesis of the mouse uterus after neonatal exposure to DES/GEN.
In summary, we have provided evidence that early-life exposure of the mice to the xenoestrogen DES or the phytoestrogen GEN induces life reprogramming of the mouse uterine epigenome. Specific genes with no previously documented associations with the uterus were identified by an unbiased methylation profiling methodology. These genes encode proteins involved in a wide-range of cellular functions. Detailed studies were conducted on one of the reprogrammable genes, Nsbp1, which is a nucleosome binding and transcriptional activation element. Our data support the paradigm that manifestation of early-life epigenetic reprogrammed gene expression in the mouse uterus is dependent on adult ovarian steroids and changes over the course of natural aging of the animal. The complex interplay among the type of estrogen, timing of exposure, reproductive status, and aging time line all significantly contribute to the phenotypical outcome of the epigenetic reprogramming in this model system.
Acknowledgments
We thank Ping Zhou, Jennifer Barker, Monica Summe, and Carol Szeto for sample preparation.
Footnotes
This research was supported by the Intramural Research Program of the National Institutes of Health (NIH)-National Institute of Environmental Health Sciences (to R.N. and W.J.), NIH Grants ES013071, ES006096, ES015584, ES015905, CA015776, and CA112532 (to S.-M.H.), and HG003749 (to M.M.), and the Department of Defense Prostate Cancer Program Grant W81XWH-06-1-0373 (to W.-Y.T.).
Present address for K.M.: Department of Cancer Biology, University of Massachusetts Medical School, 364 Plantation Street, LRB Room 426, Worcester, Massachusetts 01605.
Present address for R.Y.S.C.: National Cancer Institute at Frederick, National Institutes of Health, Building 528, Room 141, P.O. Box B, Frederick, Maryland 21702.
Disclosure Statement: The authors have nothing to disclose.
See editorial p. 5919
First Published Online July 31, 2008
Abbreviations: CGI, CpG island; DES, diethylstilbestrol; GEN, genistein; HMG, high-mobility-group; MSRF, methylation-sensitive restriction fingerprinting; Nsbp1, nucleosomal binding protein 1; OVX, ovariectomized; RefSeq, Reference Sequence; TSS, transcription start site; UCa, uterine adenocarcinoma.
References
- Sinclair KD, Lea RG, Rees WD, Young LE 2007 The developmental origins of health and disease: current theories and epigenetic mechanisms. Soc Reprod Fertil Suppl 64:425–443 [DOI] [PubMed] [Google Scholar]
- Tang WY, Ho SM 2007 Epigenetic reprogramming and imprinting in origins of disease. Rev Endocr Metab Disord 8:173–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan JA, Simpson E, Martin M 2006 Endocrine disrupters and female reproductive health. Best Pract Res Clin Endocrinol Metab 20:63–75 [DOI] [PubMed] [Google Scholar]
- Prins GS, Huang L, Birch L, Pu Y 2006 The role of estrogens in normal and abnormal development of the prostate gland. Ann NY Acad Sci 1089:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keri RA, Ho SM, Hunt PA, Knudsen KE, Soto AM, Prins GS 2007 An evaluation of evidence for the carcinogenic activity of bisphenol A. Reprod Toxicol 24:240–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho SM, Tang WY 2007 Techniques used in studies of epigenome dysregulation due to aberrant DNA methylation: an emphasis on fetal-based adult diseases. Reprod Toxicol 23:267–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilakivi-Clarke L 2007 Nutritional modulation of terminal end buds: its relevance to breast cancer prevention. Curr Cancer Drug Targets 7:465–474 [DOI] [PubMed] [Google Scholar]
- Woodruff TK, Walker CL 2008 Fetal and early postnatal environmental exposures and reproductive health effects in the female. Fertil Steril 89(Suppl):e47–e51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbst AL, Ulfelder H, Poskanzer DC 1971 Adenocarcinoma of the vagina. Association of maternal stilbestrol therapy with tumor appearance in young women. N Engl J Med 284:878–881 [DOI] [PubMed] [Google Scholar]
- Herbst AL, Kurman RJ, Scully RE 1972 Vaginal and cervical abnormalities after exposure to stilbestrol in utero. Obstet Gynecol 40:287–298 [PubMed] [Google Scholar]
- Herbst AL, Bern HA 1981 Developmental effects of diethylstilbestrol (DES) in pregnancy. New York: Thieme-Stratton [Google Scholar]
- Hammes B, Laitman CJ 2003 Diethylstilbestrol (DES) update: recommendations for the identification and management of DES-exposed individuals. J Midwifery Womens Health 48:19–29 [DOI] [PubMed] [Google Scholar]
- NIH 1999 DES Research Update No. 00-4722. Bethesda, MD: NIH Publication [Google Scholar]
- Giusti RM, Iwamoto K, Hatch EE 1995 Diethylstilbestrol revisited: a review of the long-term health effects. Ann Intern Med 122:778–788 [DOI] [PubMed] [Google Scholar]
- Palmer JR, Hatch EE, Rosenberg CL, Hartge P, Kaufman RH, Titus-Ernstoff L, Noller KL, Herbst AL, Rao RS, Troisi R, Colton T, Hoover RN 2002 Risk of breast cancer in women exposed to diethylstilbestrol in utero: preliminary results (United States). Cancer Causes Control 13:753–758 [DOI] [PubMed] [Google Scholar]
- Henderson BE, Benton B, Cosgrove M, Baptista J, Aldrich J, Townsend D, Hart W, Mack TM 1976 Urogenital tract abnormalities in sons of women treated with diethylstilbestrol. Pediatrics 58:505–507 [PubMed] [Google Scholar]
- Klip H, Verloop J, van Gool JD, Koster ME, Burger CW, van Leeuwen FE 2002 Hypospadias in sons of women exposed to diethylstilbestrol in utero: a cohort study. Lancet 359:1102–1107 [DOI] [PubMed] [Google Scholar]
- Brouwers MM, Feitz WF, Roelofs LA, Kiemeney LA, de Gier RP, Roeleveld N 2007 Risk factors for hypospadias. Eur J Pediatr 166:671–678 [DOI] [PubMed] [Google Scholar]
- Warri A, Saarinen NM, Makela S, Hilakivi-Clarke L 2008 The role of early life genistein exposures in modifying breast cancer risk. Br J Cancer 98:1485–1493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badger TM, Ronis MJ, Hakkak R, Rowlands JC, Korourian S 2002 The health consequences of early soy consumption. J Nutr 132:559S–565S [DOI] [PubMed] [Google Scholar]
- Messina M, Barnes S, Setchell KD 1997 Phyto-oestrogens and breast cancer. Lancet 350:971–972 [DOI] [PubMed] [Google Scholar]
- Lamartiniere CA, Cotroneo MS, Fritz WA, Wang J, Mentor-Marcel R, Elgavish A 2002 Genistein chemoprevention: timing and mechanisms of action in murine mammary and prostate. J Nutr 132:552S–558S [DOI] [PubMed] [Google Scholar]
- Hilakivi-Clarke L, Cho E, Onojafe I, Raygada M, Clarke R 1999 Maternal exposure to genistein during pregnancy increases carcinogen-induced mammary tumorigenesis in female rat offspring. Oncol Rep 6:1089–1095 [DOI] [PubMed] [Google Scholar]
- Newbold RR, Banks EP, Bullock B, Jefferson WN 2001 Uterine adenocarcinoma in mice treated neonatally with genistein. Cancer Res 61:4325–4328 [PubMed] [Google Scholar]
- Jefferson WN, Padilla-Banks E, Newbold RR 2007 Disruption of the female reproductive system by the phytoestrogen genistein. Reprod Toxicol 23:308–316 [DOI] [PubMed] [Google Scholar]
- Burroughs CD, Mills KT, Bern HA 1990 Reproductive abnormalities in female mice exposed neonatally to various doses of coumestrol. J Toxicol Environ Health 30:105–122 [DOI] [PubMed] [Google Scholar]
- Whitten PL, Lewis C, Russell E, Naftolin F 1995 Potential adverse effects of phytoestrogens. J Nutr 125(Suppl):771S–776S [DOI] [PubMed] [Google Scholar]
- 1994 Infant feeding practices and their possible relationship to the etiology of diabetes mellitus. American Academy of Pediatrics Work Group on Cow’s Milk Protein and Diabetes Mellitus. Pediatrics 94:752–754 [PubMed] [Google Scholar]
- American Academy of Pediatrics, Committee on Nutrition 1998 American Academy of Pediatrics. Committee on Nutrition. Soy protein-based formulas: recommendations for use in infant feeding. Pediatrics 101(1 Pt 1):148–53 [PubMed] [Google Scholar]
- Turck D 2007 Soy protein for infant feeding: what do we know? Curr Opin Clin Nutr Metab Care 10:360–365 [DOI] [PubMed] [Google Scholar]
- Newbold RR, Bullock BC, McLachlan JA 1990 Uterine adenocarcinoma in mice following developmental treatment with estrogens: a model for hormonal carcinogenesis. Cancer Res 50:7677–7681 [PubMed] [Google Scholar]
- Deligdisch L, Holinka CF 1987 Endometrial carcinoma: two diseases? Cancer Detect Prev 10:237–246 [PubMed] [Google Scholar]
- Li S, Washburn KA, Moore R, Uno T, Teng C, Newbold RR, McLachlan JA, Negishi M 1997 Developmental exposure to diethylstilbestrol elicits demethylation of estrogen-responsive lactoferrin gene in mouse uterus. Cancer Res 57:4356–4359 [PubMed] [Google Scholar]
- Li S, Hansman R, Newbold R, Davis B, McLachlan JA, Barrett JC 2003 Neonatal diethylstilbestrol exposure induces persistent elevation of c-fos expression and hypomethylation in its exon-4 in mouse uterus. Mol Carcinog 38:78–84 [DOI] [PubMed] [Google Scholar]
- Li S, Ma L, Chiang T, Burow M, Newbold RR, Negishi M, Barrett JC, McLachlan JA 2001 Promoter CpG methylation of Hox-a10 and Hox-a11 in mouse uterus not altered upon neonatal diethylstilbestrol exposure. Mol Carcinog 32:213–219 [DOI] [PubMed] [Google Scholar]
- Ho SM, Tang WY, Belmonte de Frausto J, Prins GS 2006 Developmental exposure to estradiol and bisphenol A increases susceptibility to prostate carcinogenesis and epigenetically regulates phosphodiesterase type 4 variant 4. Cancer Res 66:5624–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thigpen JE, Setchell KD, Ahlmark KB, Locklear J, Spahr T, Caviness GF, Goelz MF, Haseman JK, Newbold RR, Forsythe DB 1999 Phytoestrogen content of purified, open- and closed-formula laboratory animal diets. Lab Anim Sci 49:530–536 [PubMed] [Google Scholar]
- Huang TH, Laux DE, Hamlin BC, Tran P, Tran H, Lubahn DB 1997 Identification of DNA methylation markers for human breast carcinomas using the methylation-sensitive restriction fingerprinting technique. Cancer Res 57:1030–1034 [PubMed] [Google Scholar]
- Wu M, Ho SM 2004 PMP24, a gene identified by MSRF, undergoes DNA hypermethylation-associated gene silencing during cancer progression in an LNCaP model. Oncogene 23:250–259 [DOI] [PubMed] [Google Scholar]
- Bock C, Reither S, Mikeska T, Paulsen M, Walter J, Lengauer T 2005 BiQ Analyzer: visualization and quality control for DNA methylation data from bisulfite sequencing. Bioinformatics 21:4067–4068 [DOI] [PubMed] [Google Scholar]
- Everitt BS 1993 Cluster analysis. London: Edward Arnold [Google Scholar]
- Daniel WW 1999 Biostatistics: a foundation for analysis in the health sciences. 7th ed. New York: Wiley [Google Scholar]
- Janatpour MJ, McMaster MT, Genbacev O, Zhou Y, Dong J, Cross JC, Israel MA, Fisher SJ 2000 Id-2 regulates critical aspects of human cytotrophoblast differentiation, invasion and migration. Development 127:549–558 [DOI] [PubMed] [Google Scholar]
- Salvatierra A, Tarrats A, Gomez C, Sastre JM, Balana C 2006 A case of c-kit positive high-grade stromal endometrial sarcoma responding to imatinib mesylate. Gynecol Oncol 101:545–547 [DOI] [PubMed] [Google Scholar]
- Menczer J, Levy T, Piura B, Chetrit A, Altaras M, Meirovitz M, Glezerman M, Fishman A 2005 A comparison between different postoperative treatment modalities of uterine carcinosarcoma. Gynecol Oncol 97:166–170 [DOI] [PubMed] [Google Scholar]
- Geller MA, Argenta P, Bradley W, Dusenbery KE, Brooker D, Downs Jr LS, Judson PL, Carson LF, Boente MP 2004 Treatment and recurrence patterns in endometrial stromal sarcomas and the relation to c-kit expression. Gynecol Oncol 95:632–636 [DOI] [PubMed] [Google Scholar]
- Leath III CA, Straughn Jr JM, Conner MG, Barnes III MN, Alvarez RD, Partridge EE, Huh WK 2004 Immunohistochemical evaluation of the c-kit proto-oncogene in sarcomas of the uterus: a case series. J Reprod Med 49:71–75 [PubMed] [Google Scholar]
- Elmore LW, Domson K, Moore JR, Kornstein M, Burks RT 2001 Expression of c-kit (CD117) in benign and malignant human endometrial epithelium. Arch Pathol Lab Med 125:146–151 [DOI] [PubMed] [Google Scholar]
- Bustin M, Reeves R 1996 High-mobility-group chromosomal proteins: architectural components that facilitate chromatin function. Prog Nucleic Acid Res Mol Biol 54:35–100 [DOI] [PubMed] [Google Scholar]
- Ding HF, Bustin M, Hansen U 1997 Alleviation of histone H1-mediated transcriptional repression and chromatin compaction by the acidic activation region in chromosomal protein HMG-14. Mol Cell Biol 17:5843–5855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirakawa H, Herrera JE, Bustin M, Postnikov Y 2000 Targeting of high mobility group-14/-17 proteins in chromatin is independent of DNA sequence. J Biol Chem 275:37937–37944 [DOI] [PubMed] [Google Scholar]
- Zhu X, Leav I, Leung YK, Wu M, Liu Q, Gao Y, McNeal JE, Ho SM 2004 Dynamic regulation of estrogen receptor-β expression by DNA methylation during prostate cancer development and metastasis. Am J Pathol 164:2003–2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdogan G, Bassorgun CI, Pestereli HE, Simsek T, Karaveli S 2007 C-kit protein expression in uterine and ovarian mesenchymal tumours. APMIS 115:204–209 [DOI] [PubMed] [Google Scholar]
- Fujii S, Katsumata D, Fujimori T 2008 Limits of diagnosis and molecular markers for early detection of ulcerative colitis-associated colorectal neoplasia. Digestion 77(Suppl 1):2–12 [DOI] [PubMed] [Google Scholar]
- Kwabi-Addo B, Chung W, Shen L, Ittmann M, Wheeler T, Jelinek J, Issa JP 2007 Age-related DNA methylation changes in normal human prostate tissues. Clin Cancer Res 13:3796–3802 [DOI] [PubMed] [Google Scholar]
- Catez F, Lim JH, Hock R, Postnikov YV, Bustin M 2003 HMGN dynamics and chromatin function. Biochem Cell Biol 81:113–122 [DOI] [PubMed] [Google Scholar]
- King LM, Francomano CA 2001 Characterization of a human gene encoding nucleosomal binding protein NSBP1. Genomics 71:163–173 [DOI] [PubMed] [Google Scholar]