Abstract
The ability of exercise to decrease fat mass and increase bone mass may occur through mechanical biasing of mesenchymal stem cells (MSCs) away from adipogenesis and toward osteoblastogenesis. C3H10T1/2 MSCs cultured in highly adipogenic medium express peroxisome proliferator-activated receptor γ and adiponectin mRNA and protein, and accumulate intracellular lipid. Mechanical strain applied for 6 h daily inhibited expression of peroxisome proliferator-activated receptor γ and adiponectin mRNA by up to 35 and 50%, respectively, after 5 d. A decrease in active and total β-catenin levels during adipogenic differentiation was entirely prevented by daily application of mechanical strain; furthermore, strain induced β-catenin nuclear translocation. Inhibition of glycogen synthase kinase-3β by lithium chloride or SB415286 also prevented adipogenesis, suggesting that preservation of β-catenin levels was important to strain inhibition of adipogenesis. Indeed, mechanical strain inactivated glycogen synthase kinase-3β, which was preceded by Akt activation, indicating that strain transmits antiadipogenic signals through this pathway. Cells grown under adipogenic conditions showed no increase in osteogenic markers runt-related transcription factor (Runx) 2 and osterix (Osx); subsequent addition of bone morphogenetic protein 2 for 2 d increased Runx2 but not Osx expression in unstrained cultures. When cultures were strained for 5 d before bone morphogenetic protein 2 addition, Runx2 mRNA increased more than in unstrained cultures, and Osx expression more than doubled. As such, mechanical strain enhanced MSC potential to enter the osteoblast lineage despite exposure to adipogenic conditions. Our results indicate that MSC commitment to adipogenesis can be suppressed by mechanical signals, allowing other signals to promote osteoblastogenesis. These data suggest that positive effects of exercise on both fat and bone may occur during mesenchymal lineage selection.
OBESITY, A DISEASE of excess adipose tissue, and osteoporosis, indicated by decreased bone mass, are each suppressed by exercise. Linking these diseases further, adipocytes and osteoblasts occur from a common progenitor, the mesenchymal stem cell (MSC) (1,2,3), and signals that promote bone marrow stem cell differentiation toward one lineage may preclude the formation of the other. For example, there is an inverse relation between bone marrow adiposity and the amount of bone in the axial and appendicular skeleton of young adults (4), whereas in aging individuals, trabecular bone is essentially replaced by fat tissue (5). Conversely, when the Wnt coreceptor LRP5 is constitutively activated, causing an increase in bone mass, there is also decreased fat in the bone marrow (6). Evidence suggests that mechanical factors might have similar effects on fat and bone.
Exercise effectively combats obesity while promoting the formation of bone and muscle (7,8). This reciprocal effect raises the possibility that exercise might influence MSC lineage allocation. Indeed, immobilization leads to a near doubling of marrow fat within 15 wk bed rest (9), and microgravity simulation decreases osteogenesis while increasing adipogenesis (10). Similarly, running decreases marrow fat expression (11), whereas exposure to extremely low-magnitude mechanical signals can alter the cell fate of MSCs in growing mice by inhibiting adipogenesis (12). In vitro, mechanical loading of osteoprogenitor cells promotes the proliferation (13,14) as well as the function of osteoblasts (15,16,17). Mechanical strain induction of osteoblastogenesis is further associated with down-regulation of peroxisome proliferator-activated receptor (PPAR) γ (11), a transcription factor critical for adipogenesis.
Haploinsufficiency of PPARγ is associated with suppressed adipogenesis and augmented osteoblastogenesis (18). Increased PPARγ enhances the proteasomal degradation of β-catenin, a critical regulator of osteoblastogenesis, with the sum effect being retarded osteogenesis (19). This suggests that preventing degradation of β-catenin could bias stem cell differentiation away from formation of adipocytes, leaving MSCs to respond to environmental cues that engage other lineages. Recent work indicates that mechanical signals can activate canonical β-catenin signals in osteoblast cells (20) and promote expression of Wnt/β-catenin target genes (21). Alteration of the canonical β-catenin action, either by a gain of function mutation in LRP5 or by the presence of a glycogen synthase kinase (GSK) 3β inhibitor, enhances the anabolic response of osteoblasts to mechanical loading (22). Together, these data suggest that β-catenin should be a key factor influencing exercise’s reciprocal control of fat and bone.
The work presented here demonstrates that mechanical signals can participate in regulating lineage selection of mesenchymal precursors. Our approach involved attempting to counteract mechanically a strong adipogenic stimulus by subjecting MSCs to a daily regimen of mechanical strain. Daily loading increased the β-catenin signal duration, and suppressed PPARγ induction of adiponectin and lipid droplet accumulation. At the same time, mechanical strain activated the β-catenin pathway through alteration of GSK3β phosphorylation via Akt. Finally, mechanical strain preserved the ability of MSCs to respond to an osteogenic stimulus despite prior exposure to an adipogenic environment.
Materials and Methods
Experimental overview
On d 1 of all experiments, C3H10T1/2 cells were switched to either an adipogenic or multipotential medium (M medium). Mechanical signals were delivered to cultures beginning on the first experimental day, continuing daily until the end of the experiment (except where noted), and compared with unstrained controls.
Reagents
Fetal bovine serum was obtained from Atlanta Biologicals (Atlanta, GA). Culture media, glutamine, trypsin-EDTA reagent, antibiotics, oligofectamine, reverse transcriptase, and Taq polymerase were purchased from Invitrogen Corp. (Carlsbad, CA). Insulin, all trans-retinoic acid, 4′,6-diamidino-2-phenylindole (DAPI), oil red O, l-ascorbic acid 2-phosphate, clostridium histolyticum neutral collagenase, p-nitrophenyl phosphate, SB415286, and lithium chloride (LiCl) were obtained from Sigma-Aldrich Corp. (St. Louis, MO). The RNA isolation kit and deoxyribonuclease I were from QIAGEN, Inc. (Valencia, CA), and random primers were from Ambion, Inc. (Austin, TX).
Culture conditions
C3H10T1/2 cells were maintained in growth medium consisting of α-MEM with 10% fetal bovine serum, 1.25 mm glutamine, and 100 μg/ml penicillin/streptomycin up until passage 24. For experiments, cells were plated at a density of 6,000–10,000 cells per cm2 in BioFlex plates (Flexcell Intl. Corp., Hillsborough, NC) and cultured for 2 d before change to adipogenic or M medium on d 1 of an experiment. For adipogenic “A” medium, 0.1 μm dexamethasone, 5 μg/ml insulin, and 50 μm indomethacin were added to the growth medium. For the M medium, 10 nm dexamethasone, 50 μg/ml ascorbic acid, 1 μm β-glycerol phosphate, 10 nm all trans-retinoic acid, 5 μg/ml insulin, and 0.5 mm 3-isobutyl-1-methylxanthine were added.
Mechanical strain
Uniform biaxial strain was applied to C3H10T1/2 cells plated on six-well BioFlex Collagen-I coated plates using the Flexcell FX-4000 system. A daily regimen of 2% strain was delivered at 10 cycles per min for 3600 total cycles. Strain regimens were initiated at the beginning of each experiment except as noted.
Real-time RT-PCR
Total RNA was isolated by using the RNeasy mini kit (QIAGEN) and treated with deoxyribonuclease I to remove contaminating genomic DNA. Reverse transcription was performed with 1 μg RNA in a total volume of 20 μl per reaction. Real-time PCR was performed on a Bio-Rad iCycler (Bio-Rad Laboratories, Inc., Hercules, CA). Twenty-five-microliter amplification reactions contained primers at 0.5 μm, deoxynucleotide triphosphates (0.2 mm each) in PCR buffer, and 0.03 U Taq polymerase along with SYBR-green (Molecular Probes, Inc., Eugene, OR) at 1:150,000. Aliquots of cDNA were diluted 5- to 5000-fold to generate relative standard curves with which sample cDNA was compared. Wnt1 inducible signaling pathway protein (WISP) 1, cyclin D1, PPARγ, adiponectin, runt-related transcription factor (Runx) 2, osterix (Osx), and 18S primers are shown in Table 1. Standards and samples were run in triplicate. PCR products from all species were normalized for the amount of 18S amplicons in the reverse transcription sample, which was standardized on a dilution curve (23).
Table 1.
Primers for real-time PCR
| Target gene | (mouse) | |
|---|---|---|
| PPARγ | Forward | 5′-GCTTATTTATGATAGGTGTGATC-3′ |
| Reverse | 5′-GCATTGTGAGACATCCCCAC-3′ | |
| Adiponectin | Forward | 5′-GCA GAG ATG GCA CTC CTG GA-3′ |
| Reverse | 5′-CCC TTC AGC TCC TGT CAT TCC-3′ | |
| Cyclin D1 | Forward | 5′-GAGAAATGTACTCTGCTTTGCTGAA-3′ |
| Reverse | 5′-GGGCTGTAGGCACTGAGCAA-3′ | |
| WISP1 | Forward | 5′-CTGGACAGAAAAGGGCATGT-3′ |
| Reverse | 5′-AGGAAGGAGGGGAAATCTCA-3′ | |
| Runx2 | Forward | 5′-GAATGGCAGCACGCTATTAAATCC-3′ |
| Reverse | 5′-GCCGCTAGAATTCAAAACAGTTGG-3′ | |
| Osx | Forward | 5′-CCTCTCGACCCGACTGCAGATC-3′ |
| Reverse | 5′-AGCTGCAAGCTCTCTGTAACCATGAC-3′ | |
| 18S | Forward | 5′-GAACGTCTGCCCTATCAACT-3′ |
| Reverse | 5′-CCAAGATCCAACTACGAGCT-3′ |
Western blotting
Whole cell lysates were prepared with lysis buffer [150 mm NaCl, 50 mm Tris HCl, 1 mm EDTA, 0.24% sodium deoxycholate, and 1% Nonidet P-40 (pH 7.5)] containing 25 mm NaF and 2 mm Na3VO4. Aprotinin, leupeptin, pepstatin, and phenylmethylsulfonylfluoride were added before each lysis. Five to 20 μg protein was loaded onto a 7–10% polyacrylamide gel for chromatography and transferred to polyvinylidene fluoride membrane. After blocking, the membrane was incubated with primary antibodies overnight at 4 C. Antibodies directed against active β-catenin (clone 8E7; Upstate, Temecula, CA), total β-catenin (BD, Bedford, MA), phospho-GSK3β (ser9, clone 2D3; Upstate Biotechnology Inc., Lake Placid, NY), total-GSK3β (CHEMICON International, Inc., Billerica, MA), phospho-Akt (ser 473; Cell Signaling Technology, Inc., Danvers, MA), total Akt (11E7; Cell Signaling Technology), PPARγ, adiponectin, and actin (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) were used. The antibody for active β-catenin was specific for the hypophosphorylated form of β-catenin (24). Secondary antibody conjugated with horseradish peroxidase was used to allow detection by the enhanced chemiluminescence plus kit (Amersham Biosciences Inc., Piscataway, NJ).
Images were acquired with a Hewlett-Packard Scanjet (Hewlett-Packard Co., Palo Alto, CA), and densitometry was determined using National Institutes of Health ImageJ, version 1.37 (Bethesda, MD). To analyze differences in protein phosphorylation, arbitrary scanning units obtained for phospho-protein band intensities were divided by arbitrary scanning units obtained for total protein band intensities for each sample analyzed. The average values obtained from at least three independent experiments for each variable were pooled.
Immunofluorescence
After strain application, strained and control samples were fixed in 4% paraformaldehyde and permeabilized in 0.1% triton/PBS. An antibody to active β-catenin was applied overnight at 4 C. An antimouse fluorescein isothiocyanate-conjugated antibody (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) was applied for 30 min. Samples were mounted in antifade reagent (Molecular Probes) and viewed with a Leica SP2-AOBS confocal microscope (Leica Microsystems GmbH, Wetzlar, Germany).
Histochemical staining
After fixation in 2% formaldehyde, the cultures were then rinsed three times for 5 min in deionized water, and cytoplasmic triglyceride droplets were stained with oil red O (25).
Statistical analysis
Results are expressed as the mean ± sem. Statistical significance was evaluated by one-way ANOVA or t test (GraphPad Prism; GraphPad Software Inc., San Diego, CA). All experiments involving chromatography or confocal imaging were replicated at least once to ensure reproducibility. Densitometry data, where given, were compiled from at least three separate experiments.
Results
Adipogenic medium (A medium) induces rapid acquisition of adipocyte phenotype
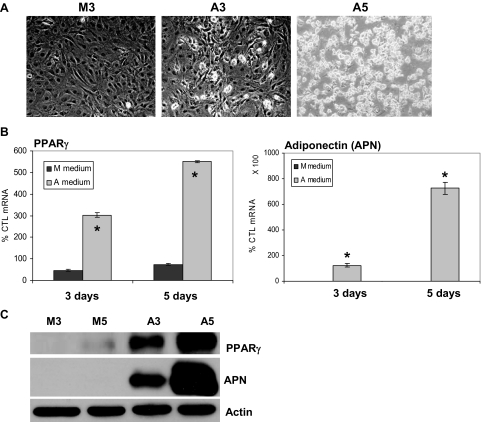
Lipid was appreciable in C3H10T1/2 cells cultured in A medium at 3 d and was present in a majority of cells by 5 d (Fig. 1A); only cells grown in A medium stained for oil red O (See color plate in Fig. 7B). mRNA analysis by semiquantitative RT-PCR showed that PPARγ and adiponectin expression increased at 3 d in cells cultured in A medium, but not in those grown in M medium (Fig. 1B), and continued to increase. As measured by Western blot, PPARγ protein was barely detectable after 5 d in cells exposed to M medium but was strongly evident by 3 d in cells exposed to A medium, and continued to increase (Fig. 1C). Adiponectin protein was not measurable in cells cultured in M medium, whereas in A medium, adiponectin levels also continued to increase at 5 d.
Figure 1.
A medium induces rapid adipogenesis of C3H10T1/2 cells. C3H10T1/2 cells were seeded in six-well BioFlex Collagen-I coated plates (100,000 cells per well) in MEMα and cultured in M or A medium for 3 or 5 d. A, Cytoplasmic triglyceride droplets appeared at 3 d under adipogenic conditions, shown at ×40. B, Real-time RT-PCR was performed for PPARγ and adiponectin. mRNA is represented as a percent mRNA level measured in MEMα at d 0 (100%). *, P < 0.001 difference between M and A media on the same day. C, Immunoblot for PPARγ and adiponectin and actin: lanes refer to cells grown in M or A conditions for 3 or 5 d. CTL, Control.
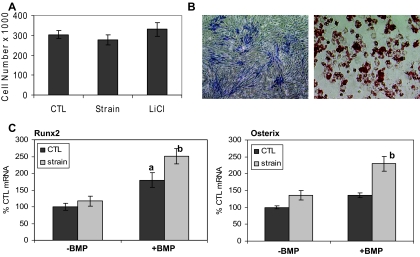
Figure 7.
Strain preserves MSCs in a multipotential state. A, Cell number compiled from three separate experiments showed no differences between culture conditions. B, MSCs in M medium stained for alkaline phosphatase by 14 d. Shown in the right panel are cells cultured in the A medium at 5 d stained for oil red O as expected for cells containing lipid. Both are shown at ×40. C, Real-time RT-PCR was performed on cells cultured in A medium × 5 d ± strain (strained groups are shown in gray) and then switched to M ± BMP-2 (300 ng/ml) × 2 d (ANOVA). a, Difference from control (CTL)unstrained (100%) (P < 0.001); b, difference from all other conditions (P < 0.05).
Mechanical strain inhibits adipogenesis while preventing a decrease in β-catenin
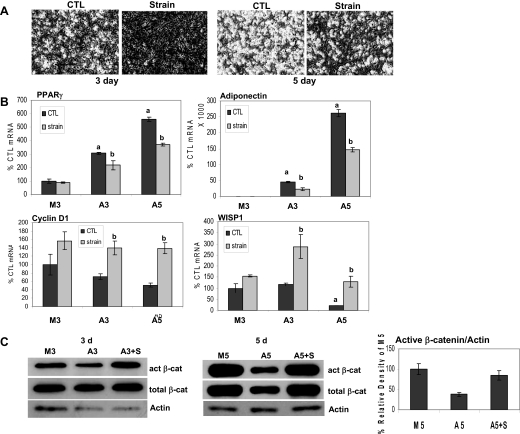
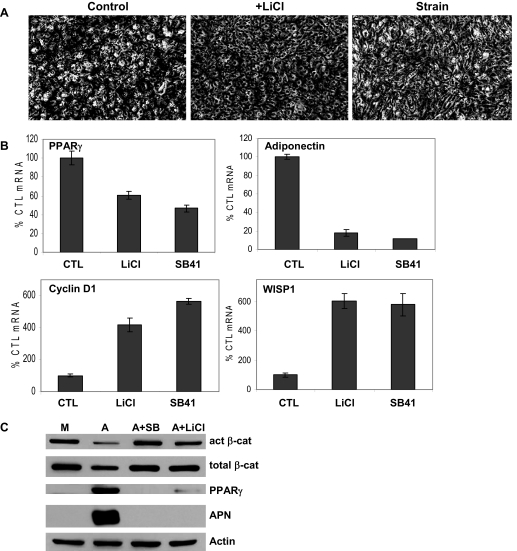
To determine whether MSC adipogenesis could be counteracted by mechanical signals, cultures were subjected to a daily regimen of mechanical strain. Because β-catenin is associated with inhibition of adipogenesis, and mechanical strain can transiently increase β-catenin activity in osteoblasts (20), we hypothesized that mechanical suppression of adipogenesis might involve β-catenin. C3H10T1/2 cells were exposed to A medium, and daily application of the strain regimen was initiated immediately. Shown in Fig. 2A, whereas lipid droplets were evident in adipogenic conditions at 3 d, few if any lipid droplets were present in cultures subjected to strain. At 5 d, a majority of unstrained cells accumulated lipid, whereas less than half of strained cells exhibited lipid droplets.
Figure 2.
Mechanical loading inhibits adipogenesis and prevents a fall in β-catenin (β-cat). Strain was applied to cells in M or A medium for 3 or 5 d. Panel A, Cytoplasmic triglyceride droplets appeared by 3 d in unstrained cells and continued to increase; strain inhibited this accumulation (magnification, ×40). Panel B, Real-time RT-PCRs performed for PPARγ, adiponectin, cyclin D1, and WISP1 on cells ± strain as indicated. ANOVA: a, Significant difference from M3 unstrained control (CTL) (100%) (P < 0.001); b, significant difference between unstrained and strained condition (P < 0.05). Panel C, Cellular proteins extracted from cells ± strain for the indicated times and analyzed by Western blotting for active (act) and total β-catenin, and actin (loading control). Densitometry compiled from three separate 5-d experiments shows that effects on active β-catenin were significant. A3 or A5, A medium with +S referring to the strained condition at either 3 or 5 d; M3, M medium for 3-d culture.
Mechanical strain of C3H10T1/2 cells also modulated the expression of PPARγ and adiponectin (Fig. 2B). Compared with cells grown in the M medium (control mRNA = 100%), PPARγ mRNA expression in A medium increased by nearly 6-fold after 5 d. Daily application of strain decreased PPARγ expression in adipogenic cultures by up to 35% (P < 0.001). Adiponectin mRNA was significantly induced under adipogenic conditions at both days as expected, and was reduced by half by application of the daily strain regimen.
The β-catenin regulated genes cyclin D1 and WISP1 were assayed (Fig. 2B). Cyclin D1 mRNA decreased by half after 5 d under adipogenic conditions. Mechanical challenge increased cyclin D1 mRNA expression by nearly 3-fold at 5 d. WISP1 mRNA responses paralleled those seen for cyclin D1, decreasing significantly at 5 d in A medium; in accord, mechanical strain increased WISP1 expression by nearly 6-fold over unstrained cultures at 5 d.
Both active and total β-catenin levels were reproducibly lower in cells in adipogenic compared with those in M medium (Fig. 2C). This reduction in β-catenin was prevented by the strain regimen, such that active and total β-catenin levels were equivalent to those found in cells grown in the M medium. Densitometry of active β-catenin bands measured after 5 d showed that the decrease of β-catenin in A medium represented a significant change (data compiled from three separate experiments).
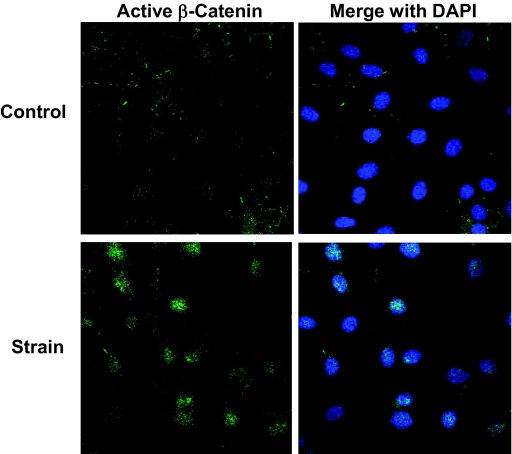
To confirm the increase in β-catenin due to strain, cultures were immunostained for active β-catenin after 3 d daily loading. As shown in Fig. 3, active β-catenin staining increased in cell nuclei of strained cultures, shown by the overlap of DAPI and β-catenin antibodies on confocal microscopy.
Figure 3.
Active β-catenin is increased in strained cultures. Cells ± strain applied daily for 3 d in A medium were immunostained for DAPI and active β-catenin. DAPI nuclear images were merged with active β-catenin in the right of the figure, and showed that strain causes elevation of active β-catenin and its appearance in the nucleus.
Inhibiting GSK3β mimics strain effects
To explore whether strain-mediated preservation of β-catenin levels was involved in the mechanism by which loading inhibits adipogenesis, we used pharmacological methods of maintaining β-catenin. LiCl is a commonly used β-catenin activator with demonstrated effectiveness both in vitro and in vivo (26,27). LiCl (10 mm) was added to C3H10T1/2 cells grown under adipogenic conditions: lipid accumulation was reduced in the presence of LiCl at 3 d (Fig. 4A). The pattern of adipogenic gene expression in the presence of LiCl, as well as the GSK3 inhibitor SB415286 (20 μm), replicated the strain effect to prevent both the increase in PPARγ and adiponectin mRNA (Fig. 4B). As well, both LiCl and SB415286 induced expression of cyclin D1 and WISP1, as did daily application of strain (Fig. 2B). In accordance with mRNA results, both agents also suppressed the accumulation of PPARγ and adiponectin proteins, with the effect on the latter nearly complete (Fig. 4C). As predicted, β-catenin levels under adipogenic conditions were preserved in the presence of both GSK3 inhibitors.
Figure 4.
Inhibition of GSK3β mimics effects of strain. Inhibitors of GSK3 were added to cells cultured in A medium for 3 d. A, Cytoplasmic triglyceride droplets are shown with bright-field microscopy and compared with effect of strain (magnification, ×40). B, Real-time RT-PCR for mRNA species as noted showed effects of 3 d treatment with LiCl (10 mm) or the GSK3 inhibitor SB415286 (SB41, 20 μm). C, Western blotting of total cellular proteins showed that inhibition of GSK3 prevented events associated with adipogenesis. Act, Active; APN, adiponectin; cat, catenin; CTL, control.
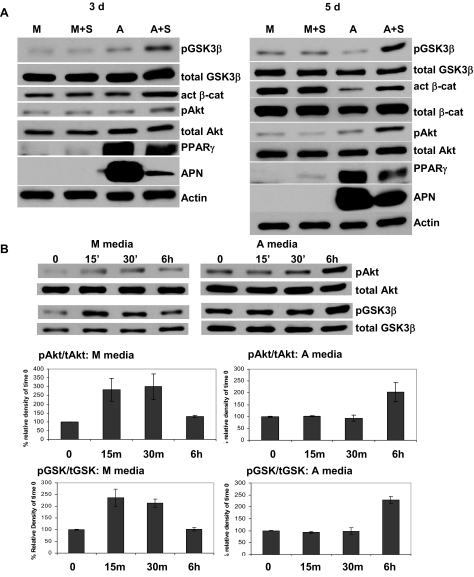
Strain inactivates GSK3β
Our results suggested that strain might maintain β-catenin levels by targeting GSK3β. Indeed, application of strain caused phosphorylation of GSK3β on its Ser9 site (causing inactivation) at both 3 and 5 d, as shown in Fig. 5A. Cultures were assayed for changes in protein at the end of the 6-h strain period on the day specified. Inactivation of GSK3β by strain was accompanied by increased levels of active β-catenin, as well as decreased expression of both PPARγ and adiponectin. Because Akt is known to directly phosphorylate and thereby inactivate GSK3 isoforms (28), we asked whether this mechanism was involved in the durability of β-catenin signaling in strained cultures subject to adipogenic conditions. Mechanical input has activated Akt in many cell types (29,30). Strain caused significant increases in Akt phosphorylation without changing total Akt levels at 3 d, an effect also present at 5 d, as shown in the figure.
Figure 5.
Strain inactivates GSK3β. Cultures in M medium without strain (M) or A medium ± mechanical strain are shown after 3 (left) or 5 d (right). A, Total cellular proteins were blotted with antibodies for phospho-Akt (Ser473, pAkt), phospho-GSK3β (Ser9, pGSK3β), active (act) β-catenin (cat), PPARγ, adiponectin (APN), and actin (loading control). M+S, +strain; likewise A+S. B, On d 3, cells in either M or A medium were strained for 1, 15, and 30 min, or 6 h, and protein was extracted immediately for Western analysis. Below, Graphs show average densitometry from three experiments: both pAkt and pGSK3β increased with strain at 15 and 30 min in M medium but not until 6 h in A medium (P < 0.05).
In contrast, when cultured in M medium, strain had no effect on Akt or GSK3β phosphorylation at either 3 or 5 d after 6 h strain (Fig. 5A). We considered that strain-induced signaling might be more transient in non-A medium, as it has been reported in other cells. Indeed, cells cultured in M medium showed a rapid response to strain with increased phosphorylation of Akt and GSK3β by 15 min, decreasing by 6 h to levels not significantly different from baseline (Fig. 5B). These changes were significant, as shown in averaged densitometries of phospho-proteins from three experiments. This rapid phosphorylation pattern was not seen in cells grown in adipogenic conditions.
Strain actions must occur early and be sustained
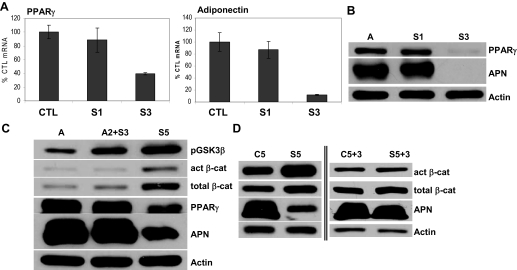
The ability of mechanical signals to prevent adipogenesis was further explored by altering the number of loading days or by altering the day of loading initiation. To determine whether a single day of a mechanical signal was sufficient to counter the strong adipogenic bias to MSC differentiation, the strain regimen was delivered the first day only, followed by 2 d without loading, and was compared with daily loading. As shown in Fig. 6A, mRNA for PPARγ and adiponectin were equivalent in cultures that received a single day of loading and control cultures, in contrast to the reduction in PPARγ and adiponectin expression with daily loading. This was further confirmed by Western blot evidence that neither the increase in PPARγ nor adiponectin could be prevented with a single application of loading (Fig. 6B).
Figure 6.
Mechanical inhibition of adipogenesis requires application early and repetitively. A, Strain was begun on the first day of adipogenic stimulus, and either dosed for 1 (S1) or 3 d (S3); mRNA for PPARγ and adiponectin (APN) was analyzed after 3 d in the A medium. Only the S3 condition inhibited adipocytic mRNA (P < 0.05). B, PPARγ and adiponectin protein from 3-d cultures in A medium: S1, Cells strained first day only; S3, strain each day of culture. C, Strain application was delayed for 2 d (A2+S3) or applied daily (S5) and protein analyzed at 5 d. D, Western blotting of total cellular protein of cells cultured in A medium ± strain for 5 d shows expected strain effects (left side of blot); culture for a further 3 d without strain shows equilibration between proteins studied in the two culture conditions (right side of blot). Act, Active; cat, catenin; CTL, control.
Whether mechanical signals must be applied during the early phase of adipogenesis was then tested by applying strain either immediately upon the switch to A medium, as before, or by delaying load for 2 d. In the delayed strain condition, neither PPARγ nor adiponectin protein levels were influenced by the later application of the mechanical regimen, and failed to prevent entry of MSCs into the adipogenic state. β-Catenin was also not significantly activated when strain was delayed for 2 d. Furthermore, whereas GSK3β was activated by strain in both regimens compared with the no-strain condition, the effect was greatest when strain was delivered for all 5 d (Fig. 6C). These data suggested that once cells had committed to adipogenic differentiation, mechanical signals could not restrain this process.
Finally, cells were cultured in A media in the presence or absence of strain for 5 d and then cultured without strain for 3 further days. In Fig. 6D, cultures strained during the initial 5 d showed higher active and total β-catenin as expected. Three days after stopping the daily application of the strain regimen, β-catenin levels were indistinguishable between the two conditions (±prestrain), and an increase in adiponectin was seen in the previously strained cultures. Thus, removal of the mechanical stimulus allowed β-catenin to decrease and adipogenesis to proceed apace.
Strain preserves the multipotential state of MSCs
The expression of cyclin D1 is directly regulated by β-catenin (31), and cyclin D1 is associated with progression into the proliferative stage of the cell cycle (32). Because strain up-regulated the expression of cyclin D1 in C3H10T1/2 cells, it was important to determine whether proliferation was increased by the mechanical regimen. Three days after switching to adipogenic conditions, cell numbers were not different in unstrained, strained, or cells treated with LiCl (Fig. 7A), showing that in these cells, preserving β-catenin did not induce proliferation.
We next asked whether MSCs exposed to adipogenic conditions would respond to an osteogenic stimulus and whether strain might enhance this response. C3H10T1/2 cells have the ability to differentiate into osteoblasts when grown in M medium, as shown by alkaline phosphatase staining at 14 d, but this is a lengthy process (Fig. 7B). Shown in comparison are cells cultured in the A medium for 5 d and stained for oil red O reflecting intracellular lipid. Application of strain during 5 d of A medium failed to increase expression of osteogenic transcription factors, Runx2, and Osx, as evaluated by RT-PCR, and shown in Fig. 7C. This was not surprising in view of our data showing that C3H10T1/2 cells require longer culture periods to induce osteogenesis as well as that of others (11,33,34). Thus, we elected, after the initial 5 d in culture, to add a strong osteogenic stimulus, bone morphogenetic protein (BMP)-2 (300 ng/ml) for 2 d. Shown in Fig. 7C, BMP-2 was able to cause a significant increase in Runx2 expression in both unstrained and strained cultures. The increase in Runx2 was significantly more in cultures that had been strained before the BMP-2 stimulation compared with unstrained cultures, effecting a 2.5-fold increase compared with 1.8-fold in cells that had not been prestrained (P < 0.05 for a difference).
The effects of prestraining cultures had an even greater influence on the ability of BMP-2 to induce Osx. Indeed, whereas BMP-2 failed to increase Osx expression in unstrained cells, cells subjected to strain showed a nearly 2-fold increase in this osteogenic transcription factor. Similar effects on these osteogenic genes were seen when BMP-2 was added at 100 ng/ml, i.e. BMP-2 induced a small increase in Runx2 but no change in Osx in unstrained cells, but caused significant increases in both mRNA species when added to cultures that were strained during exposure to adipogenic conditions. These data indicate that mechanical signals preserve C3H10T1/2 cells in a state where they can respond robustly to an osteogenic challenge despite an adipogenic environment.
Discussion
The increase in adiposity and the progressive decline of bone mass are the key phenotypical features in obesity and osteoporosis, respectively. Although the majority of treatment strategies have targeted the resident cell population, work presented here indicates that the mechanical biasing of pluripotent MSCs may represent a unique method of mitigating the pathogenesis of these distinct, but related, diseases. Given the accumulating evidence for a reciprocal relationship between the emergence of adipocytic and osteoblastic lineages (3,5,35), it would appear that factors that drive differentiation toward one cell fate will suppress the other. Here, we show that the conversion of MSCs into adipocytes driven by strongly adipogenic conditions can be inhibited by mechanical signals that also allow osteoblast lineage selection. This provides unique insight into a potential mechanism by which exercise may control fat production while conversely improving bone formation.
It is now well accepted that MSC selection of the adipocyte lineage is influenced by the presence of the nuclear receptor PPARγ (36). Culture of MSCs in an A medium here resulted in the rapid up-regulation of PPARγ, followed by an increase in the adipokine, adiponectin. Importantly, adipogenesis was markedly suppressed by daily exposure to mechanical signals. The efficacy of the mechanical influence relies on repeated exposure because a single day of loading was insufficient to restrict the emergence of the adipocytic phenotype.
Accompanying the adipogenic transformation in MSCs, we measured a progressive decrease in both active and total β-catenin. Mechanical signals completely prevented decreases in β-catenin, while simultaneously limiting expression of both PPARγ and adiponectin. If the physical input was delayed by only 2 d, it failed to prevent both the decline in β-catenin levels and the commitment toward fat, suggesting a relationship between these effects.
Mechanical preservation of the cellular β-catenin levels depends at least partially on the ability of strain to inactivate GSK3β. Strain caused sustained phosphorylation of GSK3β at serine 9, which prevents its association with β-catenin. Presumably, in the “disuse,” or unstrained, condition, active GSK3β would be permissive to adipogenesis by targeting β-catenin for degradation. As a corollary, inhibitors of GSK3β maintained β-catenin levels in our cultures and, furthermore, effectively prevented adipogenic conversion in the MSCs. Phosphorylation and deactivation of GSK3β can occur via the action of Akt (37). As mechanical stimulation increased the phosphorylation of both Akt and GSK3β, mechanical promotion of β-catenin level and activity likely involves this regulatory pathway. Interestingly, mechanical strain caused only a transient but rapid increase in this signaling cascade in M medium; the reasons for this significant alteration in signal response are likely to be consequent to other cellular changes that accompany differentiation.
Activation of Wnt/β-catenin signaling suppresses PPARγ expression, shifting mesenchymal cell differentiation toward the osteoblast lineage (38). Importantly, there is increasing evidence that Wnt/β-catenin signaling can be activated by mechanical input (14,20,21), an event likely involved in promoting osteoblastogenesis. Indeed, mechanical signals do increase the number of osteoblasts that emerge from multipotential MSC cultures while simultaneously decreasing expression of PPARγ (11). The experiments we report here show that mechanical strain can suppress PPARγ expression even in a strongly adipogenic environment, and simultaneously prevent induction of adiponectin by PPARγ.
Further confirmation of a significant downstream response to mechanical activation of β-catenin was measured by increases in WISP-1 and cyclin D1, both known to be directly regulated by β-catenin (31). Although cyclin D1 can increase cell proliferation (32) and inhibit transactivating factors, including PPARγ (39), the mechanical regimen used in our studies did not alter cell number. It is possible that the cyclin D1 response had an indirect effect to counteract PPARγ transactivation of adiponectin.
Given that MSCs are common precursors to both adipocytes and osteoblasts, and that the lack of exercise is associated with both increased fat mass and decreased bone mass, it is possible that mechanical signals generated by exercise might bias the precursor pool against fat formation and toward bone formation. We were unable to show that mechanical stimulation caused osteogenic differentiation of MSCs under these strong adipogenic conditions after only 1 wk culture because osteogenic differentiation is known to be a more gradual process (33,34). To overcome this time constraint, we applied a strong osteogenic differentiation factor, BMP-2. MSCs subject to mechanical loading during culture in A medium were able to respond briskly to BMP with increases in the osteogenic markers Runx2 and Osx. These data suggest that if the decrease in β-catenin level that accompanies adipocytic differentiation can be prevented by mechanical input, not only can adipogenesis be inhibited, but MSCs will remain available for alternate cell lineage allocation. In this vein, one critical contribution of a daily input of exercise may be to preserve MSCs in a multipotential state, suppressing the emergence of adipocytes from the MSC pool by stimulating a durable β-catenin signal.
These in vitro experiments cannot model the entirety of the complex mechanical environment in which cells contributing to skeletal architecture and remodeling exist. Osteoprogenitor cells, as well as osteoblasts in varying stages of differentiation, exist in the marrow along trabeculae, as well as on the periosteum, both sites that experience tissue strain along with oscillatory shear, pressure waves, and even electrical fields (40). The levels of strain that these cells experience are controversial: whereas the hard tissue strain ranges from very low up to approximately 2000 microstrain, cell membrane strain can be at least two orders greater (41). Furthermore, the distortion of the bone matrix during loading will cause deformation of the marrow with changes in intramedullary pressure that are also experienced as strain by cells in the marrow (42). In sum, whereas in vitro experiments cannot replicate the multidimensional skeletal environment, they can provide explicit mechanistic data informing our understanding of the environmental milieu.
We believe our data represent further evidence that the functional loading environment of the skeleton is central to defining bone mass and morphology, and includes mechanical influences on the bone marrow stem cell population that simultaneously influence the fat phenotype (12). However, that is not to say that the bone marrow stem cell is the only pathway by which the skeleton recognizes and responds to functional challenges. It is accepted that osteocytes serve an essential role in defining the mechanosensitivity of the skeleton, and the three-dimensional aspect of this syncytium is ideally configured to perceive and amplify biophysical stimuli (43). However, the ability to respond to mechanical factors long precedes the terminal placement of bone cells in this network, and the behavior of both stromal cells and osteoblasts is also known to be regulated directly by biophysical factors (40). Indeed, a direct method of promoting progenitors to skeletal lineage may represent a particularly efficient and proximal method of ensuring an ever-ready population of bone-forming cells. As such, mechanical factors not only regulate bone mass through osteocyte appreciation of skeletal stress and strain but also appear to prevent osteopenia through influencing fundamental stage development of bone cells, increasing their supply.
It is well accepted that exercise in general, and mechanical signals in particular, prevents obesity. However, it has been presumed that exercise limits fat mass expansion through metabolism and calorie expenditure. The work presented here alternatively suggests a “developmental” influence on adipogenesis, showing that the genesis of fat cells from stem cell pools is suppressed by mechanical signals. The mechanism by which the mechanical signal is transduced appears to involve activation of Akt, inactivation of GSK3β, and preservation of β-catenin. Ultimately, our data suggest that particular mechanical signals may be able to inhibit the genesis of fat by promoting alternate pathway decisions for the MSC pool. Indeed, given the role of mechanical signals in preventing both obesity and osteoporosis, it is conceivable that controlling precursor lineage decisions might represent a strategy to curb both diseases simultaneously.
Footnotes
This work was supported by National Institutes of Health Grants AR42360 (to J.R.), AR52014 (to J.R.), and AR43498 (to C.R.).
Disclosure Statement: B.S., Z.X., N.C., M.M., and J.R. have nothing to declare. C.R. has equity interests in Juvent Medical.
First Published Online August 7, 2008
Abbreviations: A medium, Adipogenic medium; BMP, bone morphogenetic protein; DAPI, 4′,6-diamidino-2-phenylindole; GSK, glycogen synthase kinase; LiCl, lithium chloride; M medium, multipotential medium; MSC, mesenchymal stem cell; Osx, osterix; PPAR, peroxisome proliferator-activated receptor; Runx, runt-related transcription factor; WISP, Wnt1 inducible signaling pathway protein.
References
- Beresford JN 1989 Osteogenic stem cells and the stromal system of bone and marrow. Clin Orthop Relat Res 240:270–280 [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti, DW, Craig S, Marshak DR 1999 Multilineage potential of adult human mesenchymal stem cells. Science 284:143–147 [DOI] [PubMed] [Google Scholar]
- Park SR, Oreffo RO, Triffitt JT 1999 Interconversion potential of cloned human marrow adipocytes in vitro. Bone 24:549–554 [DOI] [PubMed] [Google Scholar]
- Di Iorgi N, Rosol M, Mittelman SD, Gilsanz V 2008 Reciprocal relation between marrow adiposity and the amount of bone in the axial and appendicular skeleton of young adults. J Clin Endocrinol Metab 93:2281–2286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier P, Courpron P, Edouard C, Bernard J, Bringuier J, Vignon G 1973 Physiological senile involution and pathological rarefaction of bone. Quantitative and comparative histological data. Clin Endocrinol Metab 2:239–256 [DOI] [PubMed] [Google Scholar]
- Qiu W, Andersen TE, Bollerslev J, Mandrup S, Abdallah BM, Kassem M 2007 Patients with high bone mass phenotype exhibit enhanced osteoblast differentiation and inhibition of adipogenesis of human mesenchymal stem cells. J Bone Miner Res 22:1720–1731 [DOI] [PubMed] [Google Scholar]
- Judex S, Gross TS, Zernicke RF 1997 Strain gradients correlate with sites of exercise-induced bone-forming surfaces in the adult skeleton. J Bone Miner Res 12:1737–1745 [DOI] [PubMed] [Google Scholar]
- Hamrick MW, Samaddar T, Pennington C, McCormick J 2006 Increased muscle mass with myostatin deficiency improves gains in bone strength with exercise. J Bone Miner Res 21:477–483 [DOI] [PubMed] [Google Scholar]
- Minaire P, Edouard C, Arlot M, Meunier PJ 1984 Marrow changes in paraplegic patients. Calcif Tissue Int 36:338–340 [DOI] [PubMed] [Google Scholar]
- Zayzafoon M, Gathings WE, McDonald JM 2004 Modeled microgravity inhibits osteogenic differentiation of human mesenchymal stem cells and increases adipogenesis. Endocrinology 145:2421–2432 [DOI] [PubMed] [Google Scholar]
- David V, Martin A, Lafage-Proust MH, Malaval L, Peyroche S, Jones DB, Vico L, Guignandon A 2007 Mechanical loading down-regulates peroxisome proliferator-activated receptor γ in bone marrow stromal cells and favors osteoblastogenesis at the expense of adipogenesis. Endocrinology 148:2553–2562 [DOI] [PubMed] [Google Scholar]
- Rubin CT, Capilla E, Luu YK, Busa B, Crawford H, Nolan DJ, Mittal V, Rosen CJ, Pessin JE, Judex S 2007 Adipogenesis is inhibited by brief, daily exposure to high-frequency, extremely low-magnitude mechanical signals. Proc Natl Acad Sci USA 104:17879–17884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutahar N, Guignandon A, Vico L, Lafage-Proust MH 2004 Mechanical strain on osteoblasts activates autophosphorylation of focal adhesion kinase and proline-rich tyrosine kinase 2 tyrosine sites involved in ERK activation. J Biol Chem 279:30588–30599 [DOI] [PubMed] [Google Scholar]
- Lau KH, Kapur S, Kesavan C, Baylink DJ 2006 Up-regulation of the Wnt, estrogen receptor, insulin-like growth factor-I, and bone morphogenetic protein pathways in C57BL/6J osteoblasts as opposed to C3H/HeJ osteoblasts in part contributes to the differential anabolic response to fluid shear. J Biol Chem 281:9576–9588 [DOI] [PubMed] [Google Scholar]
- Fan X, Rahnert JA, Murphy TC, Nanes MS, Greenfield EM, Rubin J 2006 Response to mechanical strain in an immortalized pre-osteoblast cell is dependent on ERK1/2. J Cell Physiol 207:454–460 [DOI] [PubMed] [Google Scholar]
- Matziolis G, Tuischer J, Kasper G, Thompson M, Bartmeyer B, Krocker D, Perka C, Duda G 2006 Simulation of cell differentiation in fracture healing: mechanically loaded composite scaffolds in a novel bioreactor system. Tissue Eng 12:201–208 [DOI] [PubMed] [Google Scholar]
- Chen X, Macica CM, Ng KW, Broadus AE 2005 Stretch-induced PTH-related protein gene expression in osteoblasts. J Bone Miner Res 20:1454–1461 [DOI] [PubMed] [Google Scholar]
- Akune T, Ohba S, Kamekura S, Yamaguchi M, Chung UI, Kubota N, Terauchi Y, Harada Y, Azuma Y, Nakamura K, Kadowaki T, Kawaguchi H 2004 PPARγ insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J Clin Invest 113:846–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Wang H, Zuo Y, Farmer SR 2006 Functional interaction between peroxisome proliferator-activated receptor γ and β-catenin. Mol Cell Biol 26:5827–5837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong VJ, Muzylak M, Sunters A, Zaman, G, Saxon LK, Price JS, Lanyon LE 2007 Wnt/β-catenin signaling is a component of osteoblastic bone cells’ early responses to load-bearing, and requires estrogen receptor α. J Biol Chem 282:20715–20727 [DOI] [PubMed] [Google Scholar]
- Robinson JA, Chatterjee-Kishore M, Yaworsky PJ, Cullen DM, Zhao W, Li C, Kharode Y, Sauter L, Babij P, Brown EL, Hill AA, Akhter MP, Johnson ML, Recker RR, Komm BS, Bex FJ 2006 WNT/β-catenin signaling is a normal physiological response to mechanical loading in bone. J Biol Chem 281:31720–31728 [DOI] [PubMed] [Google Scholar]
- Sawakami K, Robling AG, Ai M, Pitner ND, Liu D, Warden SJ, Li J, Maye P, Rowe DW, Duncan RL, Warman ML, Turner CH 2006 The Wnt co-receptor LRP5 is essential for skeletal mechanotransduction but not for the anabolic bone response to parathyroid hormone treatment. J Biol Chem 281:23698–23711 [DOI] [PubMed] [Google Scholar]
- Rubin J, Murphy T, Fan X, Goldschmidt M, Taylor W 2002 Activation of extracellular signal-regulated kinase is involved in mechanical strain inhibition of RANKL expression in bone stromal cells. J Bone Miner Res 17:1452–1460 [DOI] [PubMed] [Google Scholar]
- van Noort M, Meeldijk J, van der Zee R, Destree O, Clevers H 2002 Wnt signaling controls the phosphorylation status of β-catenin. J Biol Chem 277:17901–17905 [DOI] [PubMed] [Google Scholar]
- Novikoff AB, Novikoff PM, Rosen OM, Rubin CS 1980 Organelle relationships in cultured 3T3–L1 preadipocytes. J Cell Biol 87:180–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stambolic V, Ruel L, Woodgett JR 1996 Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signalling in intact cells. Curr Biol [Erratum (1997) 7:196] 6:1664–1668 [DOI] [PubMed] [Google Scholar]
- Hedgepeth CM, Conrad LJ, Zhang J, Huang HC, Lee VM, Klein PS 1997 Activation of the Wnt signaling pathway: a molecular mechanism for lithium action. Dev Biol 185:82–91 [DOI] [PubMed] [Google Scholar]
- Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA 1995 Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 378:785–789 [DOI] [PubMed] [Google Scholar]
- Hasaneen NA, Zucker S, Lin RZ, Vaday GG, Panettieri RA, Foda HD 2007 Angiogenesis is induced by airway smooth muscle strain. Am J Physiol Lung Cell Mol Physiol 293:L1059–L1068 [DOI] [PubMed] [Google Scholar]
- Krepinsky JC, Li Y, Chang Y, Liu L, Peng F, Wu D, Tang D, Scholey J, Ingram AJ 2005 Akt mediates mechanical strain-induced collagen production by mesangial cells. J Am Soc Nephrol 16:1661–1672 [DOI] [PubMed] [Google Scholar]
- Shtutman M, Zhurinsky J, Simcha I, Albanese C, D'Amico M, Pestell R, Ben-Ze'ev A 1999 The cyclin D1 gene is a target of the β-catenin/LEF-1 pathway. Proc Natl Acad Sci USA 96:5522–5527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr CJ 1996 Cancer cell cycles. Science 274:1672–1677 [DOI] [PubMed] [Google Scholar]
- Gordeladze JO, Noel D, Bony C, Apparailly F, Louis-Plence P, Jorgensen C 2008 Transient down-regulation of cbfa1/Runx2 by RNA interference in murine C3H10T1/2 mesenchymal stromal cells delays in vitro and in vivo osteogenesis, but does not overtly affect chondrogenesis. Exp Cell Res 314:1495–1506 [DOI] [PubMed] [Google Scholar]
- Backesjo CM, Li Y, Lindgren U, Haldosen LA 2006 Activation of Sirt1 decreases adipocyte formation during osteoblast differentiation of mesenchymal stem cells. J Bone Miner Res 21:993–1002 [DOI] [PubMed] [Google Scholar]
- Beresford JN, Bennett JH, Devlin C, Leboy PS, Owen ME 1992 Evidence for an inverse relationship between the differentiation of adipocytic and osteogenic cells in rat marrow stromal cell cultures. J Cell Sci 102(Pt 2):341–351 [DOI] [PubMed] [Google Scholar]
- Farmer SR 2006 Transcriptional control of adipocyte formation. Cell Metab 4:263–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frame S, Zheleva D 2006 Targeting glycogen synthase kinase-3 in insulin signalling. Expert Opin Ther Targets 10:429–444 [DOI] [PubMed] [Google Scholar]
- Kang S, Bennett CN, Gerin I, Rapp LA, Hankenson KD, Macdougald OA 2007 Wnt signaling stimulates osteoblastogenesis of mesenchymal precursors by suppressing CCAAT/enhancer-binding protein α and peroxisome proliferator-activated receptor γ. J Biol Chem 282:14515–14524 [DOI] [PubMed] [Google Scholar]
- Wang C, Pattabiraman N, Zhou JN, Fu M, Sakamaki T, Albanese C, Li Z, Wu K, Hulit J, Neumeister P, Novikoff PM, Brownlee M, Scherer PE, Jones JG, Whitney KD, Donehower LA, Harris EL, Rohan T, Johns DC, Pestell RG 2003 Cyclin D1 repression of peroxisome proliferator-activated receptor γ expression and transactivation. Mol Cell Biol 23:6159–6173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin J, Rubin C, Jacobs CR 2006 Molecular pathways mediating mechanical signaling in bone. Gene 367:1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowin SC, Weinbaum S 1998 Strain amplification in the bone mechanosensory system. Am J Med Sci 316:184–188 [DOI] [PubMed] [Google Scholar]
- Qin YX, Lin W, Rubin C 2002 The pathway of bone fluid flow as defined by in vivo intramedullary pressure and streaming potential measurements. Ann Biomed Eng 30:693–702 [DOI] [PubMed] [Google Scholar]
- Han Y, Cowin SC, Schaffler MB, Weinbaum S 2004 Mechanotransduction and strain amplification in osteocyte cell processes. Proc Natl Acad Sci USA 101:16689–16694 [DOI] [PMC free article] [PubMed] [Google Scholar]