Abstract
The progesterone derivative allopregnanolone (ALLO) rapidly potentiates γ-aminobutyric acidA (GABAA) receptor mediated inhibition. The present studies determined whether specific manipulation of neurosteroid levels in the hippocampus would alter seizure susceptibility in an animal model genetically susceptible to severe ethanol (EtOH) withdrawal, Withdrawal Seizure-Prone (WSP) mice. Male WSP mice were surgically implanted with bilateral guide cannulae aimed at the CA1 region of the hippocampus one week prior to measuring seizure susceptibility to the convulsant pentylenetetrazol (PTZ), given via timed tail vein infusion. Bilateral intra-hippocampal infusion of ALLO (0.1 g/side) was anticonvulsant, increasing the threshold dose of PTZ for onset to myoclonic twitch and face and forelimb clonus by 2–3 fold. In contrast, infusion of the 5α-reductase inhibitor finasteride (FIN; 2 g/side), which decreases endogenous ALLO levels, exhibited a proconvulsant effect. During withdrawal from chronic EtOH exposure, WSP mice were tolerant to the anticonvulsant effect of intra-hippocampal ALLO infusion, consistent with published results following systemic injection. Finally, administration of intra-hippocampal FIN given only during the development of physical dependence significantly increased EtOH withdrawal severity, measured by handling-induced convulsions. These findings are the first demonstration that bi-directional manipulation of hippocampal ALLO levels produces opposite behavioral consequences that are consistent with alterations in GABAergic inhibitory tone in drug naïve mice. Importantly, EtOH withdrawal rendered WSP mice less sensitive to ALLO’s anticonvulsant effect and more sensitive to FIN’s proconvulsant effect, suggesting an alteration in the sensitivity of hippocampal GABAA receptors in response to fluctuations in GABAergic neurosteroids during ethanol withdrawal.
Keywords: allopregnanolone, GABAA receptors, finasteride, pentylenetetrazol, 5α-reductase, ethanol, withdrawal
1. Introduction
One well-documented nongenomic effect of steroid hormones and their derivatives is the rapid potentiation of γ-aminobutyric acidA (GABAA) receptor function. The progesterone derivative allopregnanolone (ALLO) is the most potent endogenous positive modulator of GABAA receptors yet identified and fluctuations in endogenous levels in vivo occur within the range of concentrations that potentiate GABAergic inhibition in vitro (Belelli and Lambert, 2005). Clinical and preclinical research show that ALLO fluctuations can contribute to diverse disorders such as premenstrual and postpartum dysphoric disorder (Follesa et al., 2004), catamenial epilepsy (Herzog and Frye, 2003), depression (Uzunova et al., 1998) and ethanol (EtOH) withdrawal (Romeo et al., 1996). The inverse relationship between endogenous ALLO levels and anxiety, depression, and seizure susceptibility are consistent with ALLO’s pharmacological profile, since exogenous administration produces anxiolytic, anticonvulsant and antidepressant effects (Gasior et al., 1999; Hirani et al., 2002). This inverse relationship was reported in cohorts of male subjects with depression (Uzunova et al., 1998) or in the early phase of alcoholic withdrawal (Romeo et al., 1996). Treatment with drugs which restored ALLO levels to those found in control subjects significantly reduced the symptoms of anxiety and depression in both the depressed and alcoholic subjects (Romeo et al., 2000; Ströhle et al., 1999). Thus, endogenous ALLO levels may be important in maintaining normal GABAergic brain function.
Recent studies have demonstrated that withdrawal from chronic EtOH exposure significantly decreased plasma ALLO levels in seizure prone mouse genotypes (Finn et al., 2004a) and hippocampal ALLO levels in rats (Cagetti et al., 2004). These decreases in endogenous ALLO levels corresponded to increased anxiety and seizure susceptibility and decreased expression of the biosynthetic enzyme 5α-reductase (Cagetti et al., 2003; Cagetti et al., 2004; Finn et al., 2004a), suggesting that there might be an inverse relationship between GABAergic neurosteroid levels and behavioral changes in excitability during EtOH withdrawal. Given this evidence, it was hypothesized that a sustained reduction in ALLO during EtOH withdrawal to levels below the physiologically relevant range would decrease GABAergic inhibition in vivo and contribute to the manifestation of a more severe withdrawal syndrome.
Results in one genetic animal model of EtOH withdrawal severity, namely the Withdrawal Seizure Prone (WSP) and Resistant (WSR) selected lines, are consistent with the idea that genetic differences in EtOH withdrawal severity are due, in part, to differences in the modulatory effects of GABAergic neurosteroids. The WSP and WSR selected lines were bred in duplicate for their severity of (WSP) or resistance to (WSR) handling-induced convulsions (HICs) following chronic EtOH exposure (Crabbe et al., 1985). EtOH withdrawal convulsions are at least 10-fold higher in the WSP than in the WSR line following an equivalent exposure to 72 hr EtOH vapor (Crabbe et al., 1985). Notably, there is a persistent decrease in endogenous ALLO levels as well as decreased sensitivity to ALLO during EtOH withdrawal in WSP, but not in WSR mice (Beckley et al., 2008; Finn et al., 2006; Finn et al., 2004a). Tolerance to the anticonvulsant effect of ALLO during EtOH withdrawal in WSP mice corresponded to a decrease in functional sensitivity of GABAA receptors to ALLO. Thus, the WSP line is an ideal animal model to examine critical brain regions important for ALLO’s modulatory effects on brain function during EtOH-naive and EtOH withdrawal conditions.
Investigations into the neuroanatomical substrates influenced by withdrawal from chronic EtOH exposure determined that c-fos expression was significantly increased in the hippocampus during withdrawal from chronic EtOH exposure (Dave et al., 1990; Morgan et al., 1992), and that co-administration of the convulsant pentylenetetrazol (PTZ) during EtOH withdrawal produced a further increase in c-fos expression (Putzke et al., 1996). Relevant to GABAergic neurosteroids, several studies documented that the brain sites where ALLO shows the highest potency for modulation of GABAA receptor function across brain regions are: hippocampus > cortex = amygdala (Finn and Gee, 1993; Gee et al., 1988). However, there is heterogeneity within the hippocampus since electrophysiological studies determined that GABAA receptors in the CA1 region of the hippocampus were much more sensitive to the positive modulatory effect of neurosteroids than receptors in the dentate gyrus (Belelli and Herd, 2003; Harney et al., 2003). Additionally, chronic EtOH withdrawal produced significant decreases in the expression and activity of hippocampal 5α-reductase in rats (Cagetti et al., 2004) and in WSP mice (Finn et al., 2004a). Thus, the hippocampus is a brain region relevant for seizure susceptibility during EtOH withdrawal, GABAA receptor sensitivity to ALLO, and changes in the expression and activity of the 5α-reductase during EtOH withdrawal.
The goal of the present set of experiments was to examine the physiological significance of manipulation of GABAergic neurosteroid levels in the hippocampus of WSP mice that were EtOH naïve or undergoing withdrawal. Separate studies determined whether: 1) manipulating ALLO levels in the hippocampus would produce bi-directional effects on seizure susceptibility, measured by the change in sensitivity to PTZ-induced convulsions (i.e., an increase in hippocampal ALLO with ALLO infusions would be anticonvulsant and conversely, a decrease in hippocampal ALLO with finasteride (FIN) infusion would be proconvulsant), 2) withdrawal from chronic EtOH exposure would produce tolerance to the anticonvulsant effect of hippocampal ALLO infusion similar to results following systemic ALLO administration, measured by the change in sensitivity to PTZ-induced convulsions (Finn et al., 2006), and 3) intra-hippocampal infusions of FIN during the development of physical dependence would increase chronic EtOH withdrawal severity, measured by HICs.
2. Results
2.1 Intra-hippocampal infusion revealed an anticonvulsant effect of ALLO and a proconvulsant effect of FIN
Since the hippocampus is an integral component of the limbic seizure circuitry (Gale, 1988), the overall hypothesis of these studies was that enhancing the positive modulation of hippocampal GABAA receptors via ALLO infusion would be anticonvulsant and that reducing the positive modulation of hippocampal GABAA receptors via FIN injection and infusion would be proconvulsant. The CA1 region of the hippocampus was targeted for drug delivery, based on the enhanced sensitivity of GABAA receptors in this region versus the dentate gyrus (Belelli and Herd, 2003; Harney et al., 2003). Seizure susceptibility to PTZ, measured by timed tail vein infusion (5 mg/ml, 0.5 ml/min), was the dependent measure in these studies. By analyzing the PTZ threshold dose for onset to the various convulsant endpoints (see Methods for details), we could determine whether a drug was having an anticonvulsant (i.e., an increase in PTZ seizure threshold) or proconvulsant (i.e., a decrease in PTZ seizure threshold) effect.
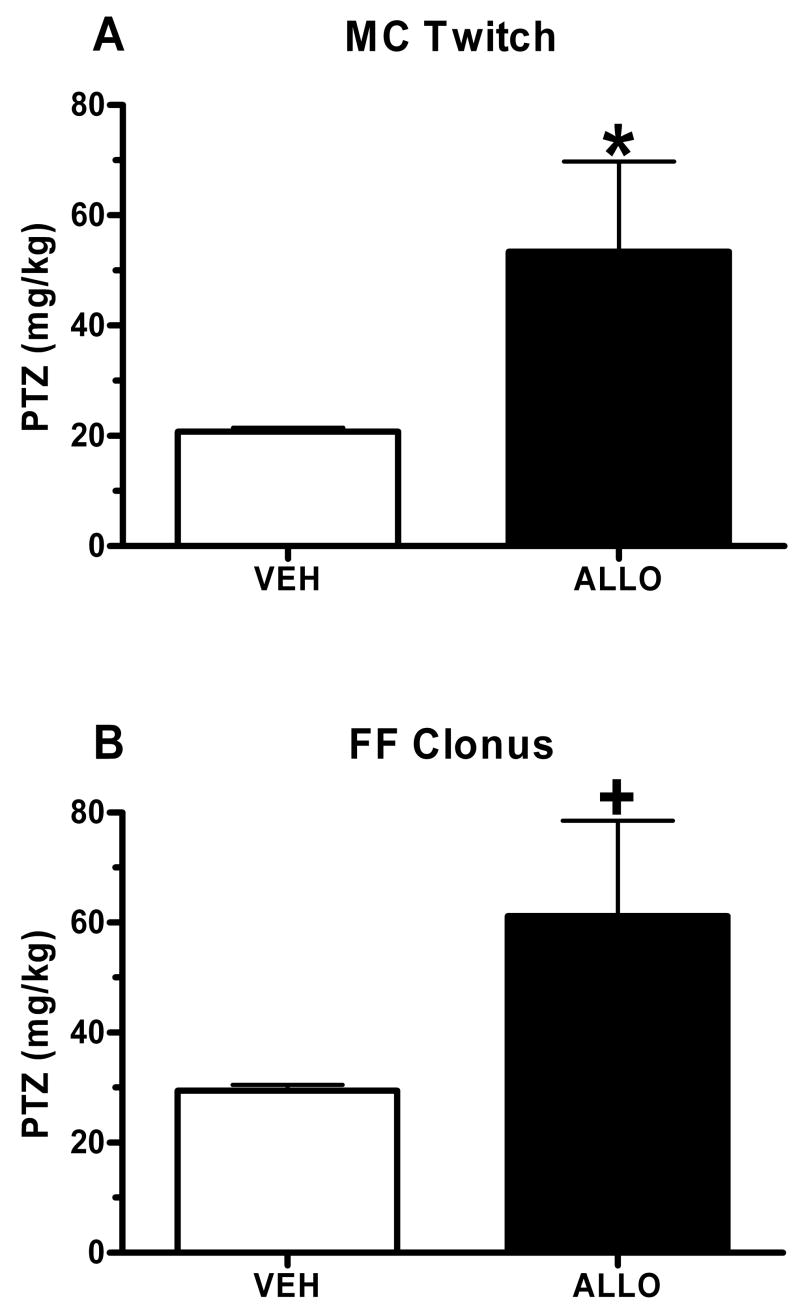
In the 1st study, bilateral intra-hippocampal ALLO (0.1 μg/side) or vehicle (VEH) was infused approximately 5 min prior to administration of PTZ. ALLO infusion produced an anticonvulsant effect, as indicated by the significant increase in the threshold dose for onset to myoclonic twitch (MC twitch; sudden involuntary muscle jerk; Figure 1A; t(13)= 4.59, p= 0.05), with a trend for an increase in face and forelimb clonus (FF clonus; rapid writhing movements of the head and neck and forelimb clonus; Figure 1B; t(14)= 3.352, p= 0.08), when compared with mice receiving an infusion of VEH. There was no effect of intra-hippocampal ALLO administration on running bouncing clonus (RB clonus, whole body clonus, including running and jumps), or tonic hindlimb extension (THE; extreme rigidity, with forelimbs and hindlimbs extended caudally; data not shown).
Figure 1. Anticonvulsant effect of intra-hippocampal ALLO administration in naïve WSP-1 mice.
Bilateral infusions of ALLO (0.1 μg/side) produced a 2–3 fold increase in the PTZ threshold dose for onset to MC Twitch (A) and FF Clonus (B). Bars represent the mean SEM for 7–8 mice per group. *p < 0.05, +p < 0.10 versus respective VEH infusion group.
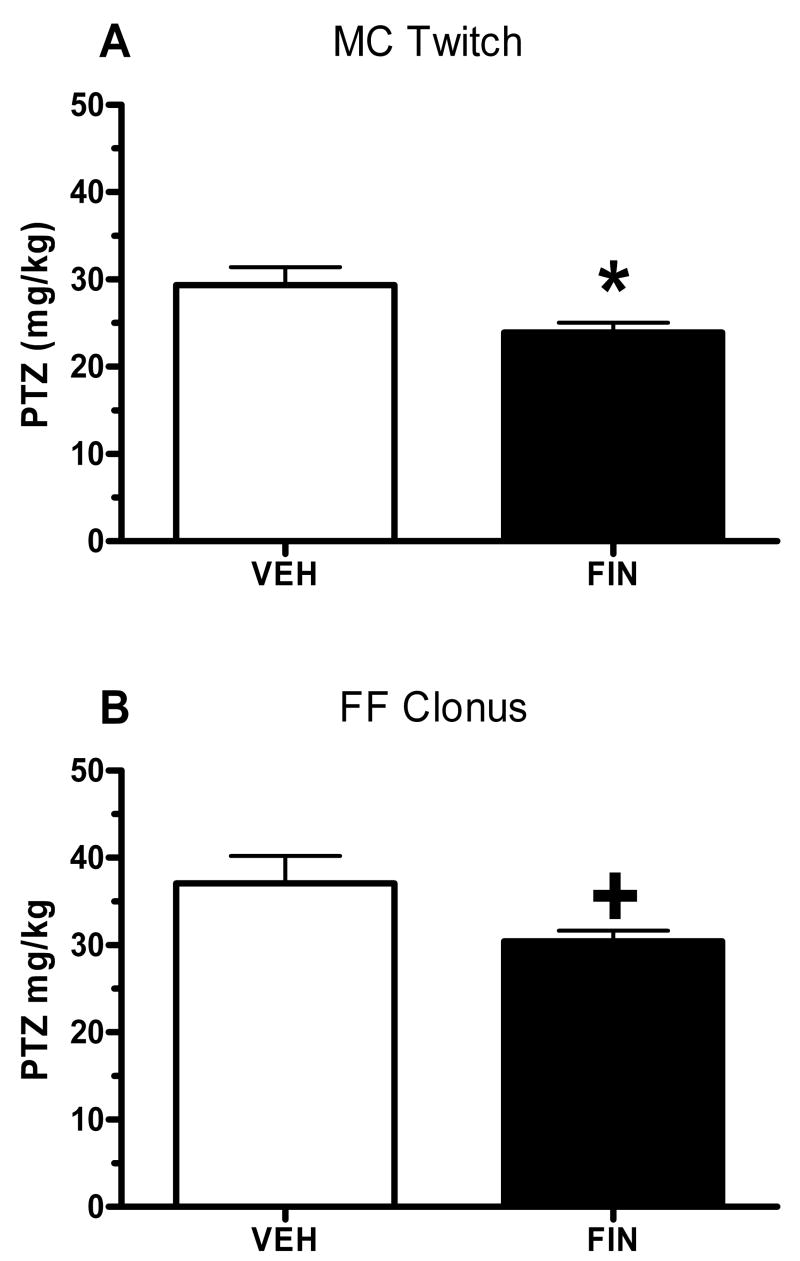
In the 2nd study, FIN pre-treatment (50 mg/kg) or VEH was administered systemically approximately 24 hours prior to the bilateral infusion of FIN (2 μg/side) or VEH into the hippocampus. The infusions occurred 3–4 hours prior to testing for seizure susceptibility to PTZ. As depicted in Figure 2, FIN pretreatment was proconvulsant, as indicated by the significant decrease in the threshold dose for onset to MC Twitch (Figure 2A; t(14)= −2.332, p= 0.035) and the trend for a decrease in FF Clonus (Figure 2B; t(14)= −1.958, p= 0.08) versus values in mice pretreated with VEH. There were no effects of intra-hippocampal FIN infusion on RB clonus or THE (data not shown). It is worth noting that in both studies, infusion of ALLO or FIN had minimal effects on susceptibility to the subsequently occurring brainstem seizure endpoints (RB Clonus and THE), suggesting that manipulation of the neurosteroid environment of the hippocampus was selectively affecting limbic seizure endpoints.
Figure 2. Intra-hippocampal FIN is proconvulsant in drug-naïve WSP-1 mice.
Systemic (50 mg/kg) followed 24 hours later by bilateral infusions (2 μg/side) of FIN produced an 18% and 17% decrease in the PTZ threshold dose for onset to MC Twitch (A) and FF Clonus (B). Bars represent the mean SEM for 8 mice per group. *p < 0.05, +p < 0.10 versus respective VEH infusion group.
A 3rd study was conducted to address the issue of site specificity, with mice receiving a bilateral infusion of ALLO (0.1 μg/side) or VEH at a site 1 mm dorsal to the CA1 region in the dorsal parietal cortex, approximately 5 minutes prior to administration of PTZ. Notably, this anatomical control study determined that infusion of ALLO did not significantly alter PTZ-induced convulsions for any seizure endpoint, when compared with values in mice receiving VEH infusions [ts(8)< 1.872, ps> 0.10, data not shown]. The mean ± SEM threshold dose of PTZ for onset to MC twitch was 25.85± 1.19 mg/kg (VEH, n= 4) and 29.21± 2.95 mg/kg (ALLO, n=6) and for onset to FF clonus was 34.65± 2.65 mg/kg (VEH) and 38.81± 4.35 mg/kg (ALLO).
Finally, a subset of mice from the first two studies (~75% of mice in each study) were microinjected with methylene blue (a dye with a similar molecular weight to that of ALLO) into the CA1 region of the hippocampus to histologically determine the accuracy and diffusion parameters of the intracerebral injections. As depicted in Figure 3, the microinjection of methylene blue did not diffuse beyond the boundaries of the injection target (CA1 region of the hippocampus), confirming that our microinjection studies were localized to the region of interest.
Figure 3. Wet mount photomicrograph of a coronal section showing minimal dye diffusion outside the dorsal CA1 region of the hippocampus.
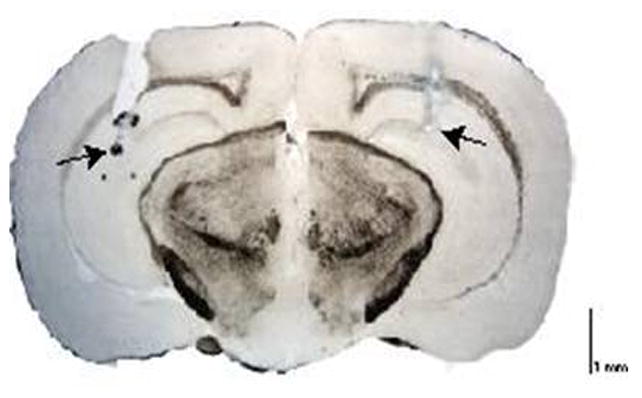
The injectors extended an additional 0.7 mm below the guide cannulae (arrows).
2.2 Intra-hippocampal infusion during EtOH withdrawal revealed tolerance to the anticonvulsant effect of ALLO
Based on the finding that WSP mice exhibited tolerance to the anticonvulsant effect of systemic ALLO during EtOH withdrawal (Beckley et al., 2008; Finn et al., 2006), the goal of this study was to determine whether WSP mice would exhibit tolerance to the anticonvulsant effect of intra-hippocampal ALLO during EtOH withdrawal. Separate groups of WSP mice were exposed to 72 hours of EtOH vapor or air and tested for sensitivity to PTZ, measured by tail vein infusion, during peak withdrawal (hours 5.5–8). PTZ was administered approximately 5 min after the intra-CA1 infusion of ALLO (0.1 μg/side) or VEH.
Daily blood samples taken from a subset of mice during the 72 hours of EtOH vapor exposure documented that there was no significant difference in blood EtOH concentration (BEC) during the development of physical dependence in mice that would subsequently receive ALLO versus VEH infusions during EtOH withdrawal [t(25)= 0.004, p>0.05, data not shown]. The mean ± SEM BEC upon the initiation of withdrawal after 72 hours of vapor exposure was 1.59± 0.17 and 1.59± 0.10 mg/ml in the mice receiving infusions of ALLO and VEH, respectively.
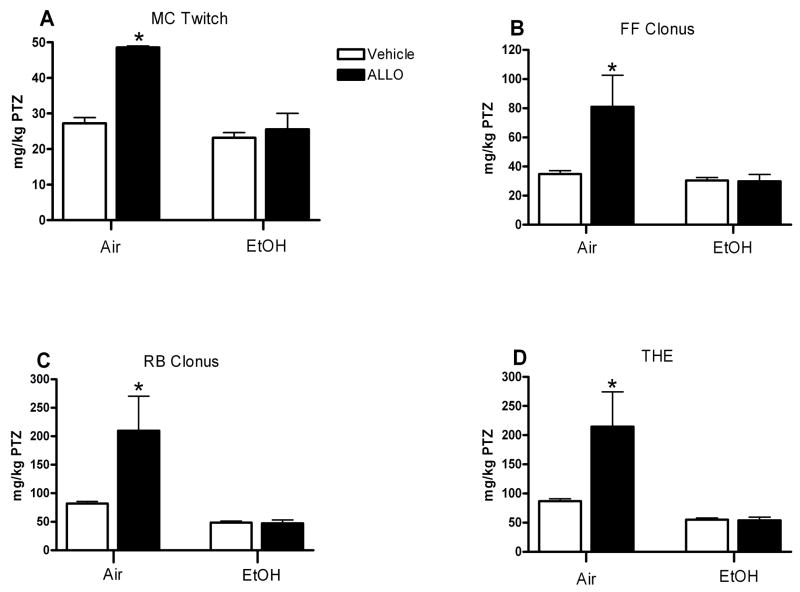
Analysis of the PTZ threshold dose with a two by two ANOVA [treatment (air or EtOH) by drug (ALLO or VEH)] revealed that there was a significant main effect of treatment on all four seizure endpoints [Fs(1,39)> 14.143, ps< 0.001], with EtOH exposed mice requiring lower PTZ doses to reach each endpoint (Figure 4A–D). There also was a significant main effect of ALLO infusion on all four endpoints [Fs(1,39)> 13.262, ps< 0.001] and a significant interaction between treatment and ALLO infusion [Fs (1,39)> 8.461, p< 0.006]. Due to these interactions, subsequent analyses confirmed that the anticonvulsant effect of ALLO was markedly and significantly reduced during EtOH withdrawal. In the air-exposed mice, ALLO infusion significantly increased the PTZ threshold dose for onset to all four convulsion endpoints [ts(10)> 5.118, ps< 0.001]. In contrast, infusion of ALLO during EtOH withdrawal did not significantly increase the PTZ threshold dose for onset to any seizure endpoint. Additional analyses in VEH treated animals indicate that EtOH exposure significantly decreased the PTZ threshold dose for onset to RB clonus and THE [ts(29)> 6.110, ps< 0.001], this finding suggests that the proconvulsant effect of EtOH withdrawal was evident primarily by the change in PTZ sensitivity to the two brainstem convulsion endpoints.
Figure 4. EtOH withdrawal decreases the anticonvulsant efficacy of intra-hippocampal ALLO in WSP-1 mice.
During withdrawal from 72 hr exposure to EtOH vapor or air, separate groups of animals received bilateral infusions of ALLO (0.1 μg/side) or VEH approximately 5 min prior to the timed tail vein infusion of PTZ. ALLO produced a significant anticonvulsant effect only in the air-exposed animals, measured by the significant increase in PTZ threshold dose for onset to MC Twitch (A), FF Clonus (B), RB Clonus (C) and THE (D). Bars represent the mean SEM for 10 mice (Air/VEH), 2 mice (Air/ALLO), 25 mice (EtOH/VEH) and 6 mice (EtOH/ALLO) . *p < 0.05 versus respective VEH infusion group.
2.3 Intra-hippocampal infusions revealed enhanced sensitivity to the pro-convulsant effect of FIN during withdrawal from chronic ethanol exposure
Based on FIN’s proconvulsant effect, the goal of this study was to determine whether intra-hippocampal FIN during the development of physical dependence would significantly increase EtOH withdrawal severity. Because it was not known if this paradigm would affect seizure susceptibility at peak withdrawal or if it would precipitate the onset of withdrawal, we choose to measure hourly HICS as this would provide a better characterization of seizure susceptibility across the entire withdrawal profile. For this study, separate groups of air- and EtOH- exposed mice received a total of 4 daily infusions of FIN (2 μg/side) or VEH (administered 24 hr prior to, and each day of, chamber exposure to EtOH vapor or air).
Daily blood samples taken from a subset of mice during the 72 hours of EtOH vapor exposure documented that there was no significant difference in BEC during the development of physical dependence in mice receiving FIN versus VEH infusions [t(24)= −1.143, p>0.05, data not shown]. The mean ± SEM BEC upon the initiation of withdrawal after 72 hours of vapor exposure was 1.44± 0.12 and 1.61± 0.08 mg/ml in the mice receiving infusions of FIN and VEH, respectively.
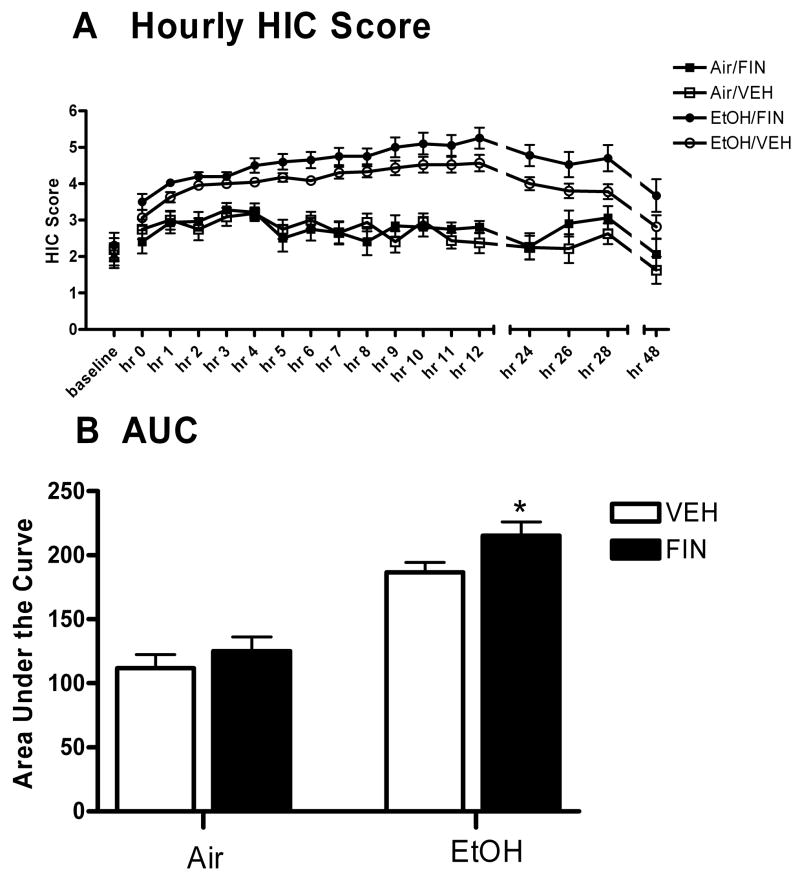
The hourly HIC measurements were compiled to create a withdrawal time course (see Figure 5A). A multifactorial ANOVA revealed a main effect of FIN infusion [F(1,71)= 4.061, p= 0.048 (FIN>VEH)], a main effect of time [F(17,1206)= 16.421, p< 0.001], a main effect of EtOH exposure [F(1,71)= 87.38, p< 0.001 (EtOH>air)] and a significant time by EtOH exposure interaction [F(17,1207)= 8.45, p< 0.001].
Figure 5. EtOH withdrawal enhances the proconvulsant effect of intra-hippocampal FIN in WSP-1.
Animals received 4 daily bilateral infusions of FIN (2 μg/side) or VEH 24 hrs before, and each day of the 72 hours of EtOH vapor or air exposure. Animals were scored for HIC hourly for 12 hours and at 24, 26, 28, and 48 hours. FIN treatment increased EtOH withdrawal severity, measured by HIC scores (A) and the area under the withdrawal curve (AUC) (B). Points represent the mean SEM for 16–20 mice per group. *p < 0.05 versus respective VEH infusion group.
As another index of withdrawal severity, the area under the withdrawal curve (AUC) was calculated by the trapezoidal method and compared with a two way ANOVA (treatment by drug). This analysis revealed significant main effects of EtOH exposure [F(1,66)= 64.42, p< 0.001 (EtOH>air)], a main effect of FIN administration [F(1,66)= 4.352, p= 0.041 (FIN>VEH)] but no significant interaction on AUC [F(1,34)= 0.12, p= 0.73]. Due to our a priori hypotheses that the proconvulsant effect of FIN would be enhanced during EtOH withdrawal, planned comparisons were conducted. FIN administration significantly increased AUC in EtOH-exposed mice [t(36)= 2.144, p= 0.039], while having no effect in air-exposed animals [t(30)= 0.883, p= 0.384] (Figure 5B).
3. Discussion
The present findings provide strong evidence to indicate that the neurosteroid environment in the hippocampus has bi-directional effects on seizure susceptibility in EtOH naïve mice and during EtOH withdrawal. Intra-hippocampal ALLO was anticonvulsant, whereas FIN was proconvulsant, in EtOH naïve mice. During EtOH withdrawal, animals were tolerant to the anticonvulsant effect of ALLO infusion and more sensitive to the pro-convulsant effects of inhibiting 5α-reductase. The present results indicate that GABAA receptor sensitivity in the hippocampus is a dynamic process and that alterations in local endogenous neurosteroid levels can have behavioral consequences. Consistent with the idea that manipulation of local ALLO concentration can produce physiologically relevant effects, Belelli and Herd (2003) demonstrated that the use of inhibitors to block the oxidation of ALLO (thereby increasing ALLO levels) unmasked a GABAergic inhibitory tone in hippocampal dentate gyrus neurons. The present findings represent one of the first demonstrations that bi-directional manipulation of hippocampal ALLO levels produces opposite behavioral consequences that are consistent with alterations in GABAergic inhibition.
The anticonvulsant effect of bilateral infusion of ALLO into the CA1 region of the hippocampus was similar to that seen with systemic injection. The potent anticonvulsant effect of intro-hippocampal ALLO against forebrain (predominantly limbic) convulsant endpoints was documented by the 157% and 107% increases in the PTZ threshold dose for onset to MC Twitch and FF Clonus, respectively. This result is consistent with the involvement of the hippocampus in the limbic seizure circuit (Gale, 1988), and with the contribution of GABAA receptors in the hippocampus to the anticonvulsant effect of ALLO (Rhodes and Frye, 2005a). For comparative purposes, systemic administration of a 5 mg/kg dose of ALLO increased the threshold dose of PTZ for onset to FF Clonus by 2-fold in air-exposed WSP mice (Finn et al., 2006). Taken in conjunction with the present findings, activation of GABAA receptors in the hippocampus following ALLO infusion is sufficient to produce an anticonvulsant effect against limbic convulsion endpoints that is comparable to that seen following systemic injection of ALLO.
Use of FIN to decrease endogenous ALLO levels had a proconvulsant effect in EtOH naïve mice, as evidenced by the 18% and 17 % decrease in the PTZ threshold dose for onset to MC Twitch and FF clonus respectively. This effect of FIN was selective for limbic convulsion endpoints, as RB Clonus and THE endpoints were not significantly affected. Recent work in progesterone-primed female rats also found a potent proconvulsant effect of intra-hippocampal FIN (10 μg/side) against PTZ-induced convulsions (Rhodes and Frye, 2005a). Notably, the increased seizure susceptibility following FIN infusion in the study by Rhodes and Frye (2005a) corresponded to a significant decrease in hippocampal ALLO levels. Taken in conjunction with the present finings, hippocampal ALLO levels can impact PTZ sensitivity.
The change in seizure susceptibility in naïve mice following infusion of FIN was less pronounced than that seen with ALLO. One explanation for this difference could be the manner in which the local neurosteroid environment was manipulated. That is, infusion of FIN would decrease basal endogenous ALLO concentrations whereas infusion of ALLO would increase ALLO levels to supra-physiological concentrations. Although ALLO levels were not measured in the current studies, previous reports indicate that microinjection of a 0.1 μg ALLO dose increased ALLO concentrations 3-fold over basal levels (Frye and Rhodes, 2006) and that infusions of FIN (10 μg) into the ventral tegmental area or hippocampus reduced ALLO levels by approximately 60%–80% respectively (Frye and Vongher, 2001; Rhodes and Frye, 2005a; 2005b).
Another consideration with regard to the FIN data is that inhibition of 5α-reductase could alter the concentrations of other 5α-reduced steroids, some of which are GABAergic (e.g., the deoxycorticosterone metabolite tetrahydrodeoxycorticosterone), or could shift local steroid metabolism to favor the production of steroids with proconvulsant effects such as corticosterone or estrogen Additionally, FIN administration may produce 3α-hydroxypregn-4en-20-on (3α-HP) by inducing the reduction of progesterone by 3α-hydroxysteroid dehydrogenase (Purdy et al., 1990). 3α-HP is a potent agonist at the GABAA receptor, but is not found in high concentrations in rodents (Purdy et al., 1990). While all of these steroids must be taken into consideration, ALLO is the most potent GABAA receptor agonist and is found in physiological ranges in the blood and brain.
One concern with the interpretation of microinjection studies is whether a drug’s effect is due to a specific action at the site of the microinjection or to drug diffusion (primarily, to sites distal to the injection target). The results of our anatomical control studies strongly suggested that the effect of ALLO following microinjection into the CA1 was not due to diffusion up the injector track, but to a localized neurosteroid action at the injection target. Thus, these anatomical and diffusion controls provide evidence that the effects of ALLO and FIN were likely confined to the CA1 of the hippocampus.
EtOH withdrawal significantly increased sensitivity to PTZ in WSP-mice, consistent with an overall proconvulsant effect of EtOH withdrawal. However, the change in PTZ sensitivity was only true for the later two convulsion endpoints, RB clonus and THE. This finding is consistent with previous data -from our lab, whereby seizure prone genotypes (e.g. WSP and DBA/2J) do not exhibit an increased sensitivity to all PTZ convulsion endpoints during EtOH withdrawal (Finn and Crabbe, 1999; Finn et al., 2006; Finn et al., 2000). It is likely that the main effect of EtOH withdrawal for all 4 seizure endpoints was due in part, to the concomitant decrease in sensitivity to ALLO’s anticonvulsant effect during withdrawal.
Notably, EtOH withdrawal was accompanied by decreased sensitivity to the anticonvulsant effect of intra-hippocampal ALLO administration in mice and increased sensitivity to the proconvulsant effects of intra-hippocampal FIN. Our reasoning for using two different measures of sensitivity was based on the following. With regard to ALLO, we had previously shown that the anticonvulsant effect of ALLO against withdrawal-related HICS was very transient (Finn et al., 1995). We subsequently determined that the assessment of ALLO sensitivity during EtOH withdrawal was much more quantitative when the examination was limited to a single time point and that timed tail infusion of PTZ was a highly sensitive measure of sensitivity at a single time point of withdrawal (Finn et al., 2006). Further, we wanted to directly compare the change in sensitivity to ALLO during EtOH withdrawal following systemic versus intra-hippocampal administration, since we had recently found that functional sensitivity of GABAA receptors to ALLO was significantly reduced during EtOH withdrawal (Finn et al., 2006). Specifically, the potency of ALLO to potentiate GABA (10 M) stimulated chloride flux was significantly reduced (rightward shift in the dose-response curve and significant increase in EC50) concomitant with a reduction in efficacy of ALLO in the 60–600 nM concentration range during EtOH withdrawal. Consistent with this decrease in the functional sensitivity of GABAA receptors to ALLO during EtOH withdrawal, our data indicate that the anticonvulsant effect of intra-hippocampal ALLO was significantly reduced during EtOH withdrawal. These results provide further evidence for the critical involvement of the hippocampus in mediating tolerance to the anticonvulsant effect of ALLO during EtOH withdrawal.
We chose a different tactic to assess sensitivity to FIN during EtOH withdrawal. While FIN produced a slight but significant proconvulsant effect in naïve WSP mice when PTZ threshold dose was the dependent measure, we reasoned that quantifying the effect of FIN on EtOH withdrawal severity would be best achieved by an examination of the withdrawal time course (which had never been done). We also were asking a slightly different question, namely, does suppression of endogenous GABAergic neurosteroids during the development of physical dependence alter the expression of EtOH withdrawal? Interestingly, four daily infusions of FIN produced a greater proconvulsant effect during EtOH withdrawal, suggesting that decreasing endogenous ALLO levels during the development of physical dependence produced a greater decrease in GABAergic inhibition and concomitant increase in withdrawal severity. This result contrasts with recent findings in our lab showing that FIN systemically administered during the development of physical dependence decreased withdrawal severity (Finn et al., 2004b; Gorin et al., 2005). However, systemically administered FIN significantly decreased BEC during EtOH exposure and upon the initiation of withdrawal, suggesting that the decrease in withdrawal severity in the earlier work was due to an indirect effect on EtOH pharmacokinetics. Notably, intra-hippocampal FIN did not alter BEC in the current study, suggesting that the increase in withdrawal severity was due to the manipulation of GABAergic neurosteroid tone in the hippocampus.
Decreased sensitivity to ALLO, which we have characterized as tolerance to the anticonvulsant effect of ALLO, was only observed in one other seizure prone genotype during EtOH withdrawal, the DBA/2J inbred mouse strain (Finn et al., 2000). In contrast, rats, C57BL/6J and WSR mice exhibit increased sensitivity to the anticonvulsant effect of ALLO and alphaxalone during EtOH withdrawal (Beckley et al., 2008; Cagetti et al., 2004; Devaud et al., 1996; Finn et al., 2000). Collectively, data to date suggest that the plasticity of GABAA receptors during EtOH withdrawal may differ between seizure prone and resistant genotypes, particularly with regard to ALLO sensitivity.
Evidence indicates that neurosteroid modulation of GABAA receptors can undergo dynamic change. For example, one mechanism implicated in the timed release of oxytocin from hypothalamic neurons involves fluctuations in ALLO levels and a concomitant decrease in sensitivity of GABAA receptors to ALLO (Brussaard et al., 1997; Koksma et al., 2003). This decreased GABAergic inhibition, due in part to a switch in GABAA receptor subunit expression as well as to the activity of constitutive phosphatases and kinases (which would alter the phosphorylation state of GABAA receptors), allows for the timed release of oxytocin. Chronic intermittent EtOH exposure has been shown to alter the responsivity of synaptic and extrasynaptic GABAA receptors (Liang et al., 2004), which also exhibit differential sensitivity to GABAergic neurosteroids (Belelli and Lambert, 2005). Chronic intermittent EtOH exposure also increased the localization of α4 subunits within GABAergic synapses, which would decrease the sensitivity to neurosteroids at synaptic GABAergic receptors (Liang et al., 2006). A number of mechanisms have been suggested to contribute to the changes in GABAA receptor sensitivity to different modulators during EtOH withdrawal, such as alterations in subunit expression and assembly of GABAA receptors, post-translational modifications, alterations in receptor trafficking or changes in the subcellular or synaptic localization of receptors (Kumar et al., 2004). However, the specific mechanism(s) underlying the tolerance to ALLO during EtOH withdrawal in WSP mice remains to be determined.
In conclusion, the present findings are the first demonstration that bi-directional manipulation of hippocampal ALLO levels produces opposite behavioral consequences that are consistent with alterations in GABAergic inhibition. These results have important implications for understanding neurosteroid plasticity as it pertains to disorders such as EtOH withdrawal.
4. Experimental Procedure
4.1 General Procedures
4.1.1 Subjects
Two genetically independent WSP (WSP–1 and –2) and WSR (WSR–1 and 2) lines have been bred from a genetically heterogeneous stock of known composition (i.e., HS/Ibg) using within-family, bi-directional selection with replicate lines (Crabbe et al., 1985). Drug naïve adult male WSP mice from the first genetic replicate (WSP-1) were used in all studies. The mice were bred in the Veterinary Medical Unit at the Veterans Affairs Medical Center (Portland, OR). Mice were maintained in groups of 4 in individually ventilated polycarbonate cages (Thorens) under a 12:12 hr light/dark cycle (lights on at 0600) at 23 ± 1°C. All animals had free access to rodent chow (Labdiet 5001 rodent diet, PMI international) and tap water throughout the experiments. At the time of testing, mice were from selected generation 26 (filial generations 95–100) and were approximately 100 days old. All procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals as adopted and disseminated by the U.S. National Institutes of Health, and were approved by the local Institutional Animal Care and Use Committee.
4.1.2 Surgery and Microinjections
Mice were surgically implanted under isoflurane anesthesia with bilateral 26-gauge guide cannulae aimed at the dorsal CA1 region of the hippocampus (A –3.3 mm, L –2.7 mm, V –1.5 mm), using previously described procedures (Mark and Finn, 2002) a minimum of one-week prior to any behavioral manipulations. Anterior, lateral and ventral coordinates are referenced to bregma, midsaggital sinus and surface of the level skull, respectively (Paxinos and Franklin, 2001). The guide cannulae were kept patent with 220 m o.d. stainless steel wire stylets. During drug injections, the microinjector (made of silica glass tubing; 75 m i.d., 150 m o.d.) extended 0.7 mm beyond the guide shaft so that the ventral position of the injection site was 2.2 mm. Thus, only the silica tubing penetrated the tissue beyond the implanted guide shaft. A separate study utilized anatomical controls that were surgically implanted with bilateral guide shafts aimed 1 mm dorsal to the CA1 (A –3.3 mm, L ± 2.7 mm, V −0.5 mm), so that the ventral position of the injection site was in the overlying cortex (−1.2 mm).
To conduct the microinjection, mice were gently restrained by hand, the stylets were removed, and the microinjectors were lowered to the target site. The distal ends of the injectors were attached to 10 l Hamilton syringes mounted in syringe pumps (Razel Sci. Instr. Inc. Stanford, CN) calibrated to deliver fluid at a rate of 200 nl over 60 sec. The silica microinjectors remained in place for an additional one-minute after injections. The extremely small lumen of the silica injector permits reliable infusions of nanoliter volumes while preventing leakage out of the injector before the pump was activated (Parada et al., 1993).
4.1.3 Drugs
Drugs for microinfusion (ALLO and FIN) were dissolved in a 20% w/v solution of 2-hydroxypropyl-β-cyclodextrin (β-cyclodextrin) and delivered in a total volume of 200 nl/side. ALLO (0.1 g/side) was synthesized by and purchased from Dr. Robert Purdy (VA Research Foundation, San Diego, CA), and FIN (2 g/side) was purchased from Steraloids (Newport, RI). Vehicle (VEH) infusions were the β-cyclodextrin solution delivered in a total volume of 200 nl/side. These doses were chosen to reliably decrease brain concentrations of ALLO (Rhodes and Frye, 2005b) or to increase brain levels of ALLO to physiologically relevant concentrations (Frye and Rhodes, 2006).
PTZ (Sigma, St. Louis, MO) was dissolved in 0.9% saline (Baxter, Deerfield, IL) as a 5-mg/ml solution and administered via timed tail vein infusion (0.5 ml/min) into a lateral vein (Finn and Crabbe, 1999; Finn et al., 2006; Mark and Finn, 2002). The latency to onset of the four convulsion endpoints that characterize PTZ-induced convulsions was recorded in seconds and subsequently converted to threshold dose (mg/kg). This method allowed for the observation and quantitative analysis of four different endpoints that characterize PTZ-induced convulsions and reliably occur in progression: MC twitch (sudden involuntary muscle jerk), FF clonus (rapid writhing movements of the head and neck and forelimb clonus), RB clonus (whole body clonus, including running and jumps), and THE (extreme rigidity, with forelimbs and hindlimbs extended caudally). Upon exhibiting THE, the mice were immediately euthanized.
4.1.4 Ethanol Vapor Inhalation
WSP-1 mice were exposed to continuous EtOH vapor or air for 72 hrs in inhalation chambers, which is a well-documented method in our laboratory to induce physical dependence (Finn and Crabbe, 1999; Finn et al., 2006). Briefly, on the first day of the experiment, mice were divided into one of two groups: EtOH or control. Mice in the EtOH groups were weighed and injected intraperitoneally (IP) with a priming dose of EtOH (1.5 g/kg; Pharmco Products, Brookfield, CT) and exposed to EtOH vapor (7 9 mg EtOH/liter air) inside the inhalation chamber. Mice in the control groups were injected with an equivalent volume of saline and exposed to air in a separate inhalation chamber. All mice were given daily IP injections of pyrazole hydrochloride, an EtOH dehydrogenase inhibitor (68.1 mg/kg, ip), to stabilize blood EtOH concentrations in the EtOH-exposed mice and to control for any nonspecific effects of pyrazole injection in the air-exposed mice. At 24 and 48 hrs, all animals were briefly removed from the chambers, weighed, injected with pyrazole and placed back into their respective chamber. Tail blood samples (20 μl) were taken from a subset of the EtOH-exposed animals to monitor BEC. At 72 hrs, all animals were removed from the chambers, and tail blood samples were taken from the EtOH-exposed animals for subsequent analysis of BEC. Tails were nicked for the air-exposed groups, but no blood was taken. Then, mice were housed in polycarbonate cages with cob bedding and taken to a procedure room for behavioral testing. Depending on the study, mice were either tested for sensitivity to the anticonvulsant effect of ALLO during peak withdrawal (5.5–8 hrs), measured by seizure susceptibility to PTZ or were tested for withdrawal severity, measured by HICs, at select time points.
4.1.5 BEC determination
Blood samples were assessed for EtOH concentration by gas chromatography, using an Agilent 6890N gas chromatograph and a well documented method in the laboratory of Dr. John Crabbe (Finn et al., 2006). Briefly, an Agilent 7683 autosampler and injector were used to inject a 1 μl sample of the supernatant (from the processed blood sample) onto a capillary column (DB-ALC2, J & W Scientific Inc., Folsom, CA). Sample peak area was interpolated from a standard curve derived from 7 pairs of standards (0.25 4.0 mg/ml).
4.1.6 HIC scoring
HICs were scored according to a previously published scale (Crabbe et al., 1991). Briefly, the mouse was quickly observed in the home cage. If no spontaneous convulsions were observed, a mild convulsion could be elicited by gently lifting the mouse by the tail, observing it, and then turning it 180 degrees if necessary. The HIC scoring was on a scale from 0 to 7; a score of 0 indicated no convulsions, a score of 1–3 indicated tonic or clonic convulsions obtained by a gentle turn, a score of 4–6 indicated convulsions elicited by only lifting the mouse by the tail, and a score of 7 indicated a spontaneous convulsion observed in the home cage. Scores ranging from 0–5 were observed.
4.1.7 Data Analysis
Analyses were conducted on data from animals in which cannulae placement was verified to be in the hippocampus (primarily the CA1 region), or 1 mm dorsal (anatomical controls). For the studies measuring sensitivity to PTZ-induced convulsions, either separate t-tests or ANOVAs were used to examine effects of drug, treatment, or both factors on the PTZ threshold dose for onset to each of the 4 convulsion endpoints: MC twitch, FF clonus, RB clonus, and THE. For the studies measuring HICs, repeated measures ANOVAs (with time as a repeated factor) were used to analyze treatment and drug effects on hourly HICs. Withdrawal severity was quantified by calculating the AUC for each animal, using the trapezoidal method, as previously described (Crabbe et al., 1983; Metten and Crabbe, 2005). The AUC was analyzed for treatment and drug effects with ANOVA. In the case of a priori hypotheses, specific groups were analyzed with t-tests. When appropriate, Tukey’s post-hoc test was used. In all cases, significance was set at P ≤ 0.05.
4.2 The effect of intra-hippocampal ALLO or FIN on sensitivity to PTZ
This experiment was carried out in three separate studies. In the first study, animals were administered PTZ approximately 5 min after a hippocampal infusion of ALLO (0.1 μg/side) or VEH. In the second study animals were administered PTZ approximately 3–4 hrs after infusion of FIN (2 μg/side) or VEH. In the FIN study, all mice were also were pretreated with a systemic injection of FIN (50 mg/kg) or VEH at 24 hr prior to the infusion of FIN, as this schedule was found to significantly decrease endogenous ALLO levels in whole brain (Finn, unpublished). A subset of animals in the FIN study had previously been exposed to a 72 hour EtOH paradigm. These animals were equally distributed throughout the groups and had been drug free for a period of 3 weeks. In the third study, animals received an infusion of ALLO (0.1 μg/side) or VEH at a site 1 mm dorsal to the hippocampus as an anatomical control. These animals were also administered PTZ approximately 5 min after infusion. In all three studies, latencies to MC twitch, FF clonus, RB clonus, and THE were recorded in seconds and subsequently converted to threshold dose in mg/kg. Upon exhibiting THE, the mice were immediately euthanized by cervical dislocation and brains were removed for verification of cannulae placement using standard histological techniques. A subset of mice from the first two studies (~75% of mice from each study) was used to measure the site specificity of the microinjection. Following cervical dislocation, animals received bilateral infusions (200 nl/side) of a dye (methylene blue, Sigma, MW = 373.9) with a similar molecular weight to that of ALLO (MW = 318.5) that was solubulized in the β-cyclodextrin vehicle solution. After dye infusion, all brains were removed and analyzed using standard histological techniques.
The advantage of timed tail vein infusion is that qualitatively distinct convulsive endpoints are produced as a function of PTZ dose. As discussed in Gale (1988), there are two qualitatively distinct convulsion components that are mediated by separate and independent anatomical circuits. MC twitch and FF clonus are associated with forebrain (limbic) neural circuits, whereas RB clonus and THE depend on hindbrain circuitry. Thus, interpretations of the present studies discuss MC twitch and FF clonus as similar types of convulsions and similarly group results for RB clonus and THE.
4.3 Sensitivity to the anticonvulsant effect of intra-hippocampal ALLO during ethanol withdrawal
WSP-1 mice were exposed to continuous EtOH vapor or air for 72 hrs in inhalation chambers. Mice were tested for sensitivity to the anticonvulsant effect of intra-hippocampal ALLO during peak withdrawal (5.5–8 hrs), measured by seizure susceptibility to PTZ, similar to that described above. PTZ was administered approximately 5 min after hippocampal infusion of ALLO (0.1 μg/side) or VEH. Latencies to the 4 convulsion endpoints were recorded in seconds and subsequently converted to threshold dose in mg/kg. Upon exhibiting THE, the mice were immediately euthanized by cervical dislocation, and brains were removed for verification of cannulae placement.
4.4 The effect of intra-hippocampal FIN during the development of physical dependence on ethanol withdrawal severity
WSP-1 mice were exposed to continuous EtOH vapor or air for 72 hours in inhalation chambers. For this study, separate groups of air- and EtOH-exposed mice received a total of 4 daily infusions of FIN (2 μg/side) or VEH (administered 24 hr prior to, and each day of, chamber exposure to EtOH vapor or air). Prior to the first FIN or VEH infusion, baseline HICs were measured. Upon removal from the inhalation chambers at 72 hrs, mice were scored for HICs hourly for the initial 12 hrs and then at hrs 24, 26, 28 and 48. Mice were euthanized following the final HIC determination at 48 hrs, and brains were removed for verification of cannulae placement.
Acknowledgments
This research was supported by USPHS grants AA12439 (DAF) and AA10760 and AA13519 (JCC) from the National Institute on Alcohol Abuse and Alcoholism and the Department of Veterans Affairs (DAF, JCC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beckley EH, Fretwell AM, Tanchuck MA, Gililland KR, Crabbe JC, Finn DA. Decreased anticonvulsant efficacy of allopregnanolone during ethanol withdrawal in female Withdrawal Seizure-Prone vs. Withdrawal Seizure-Resistant mice Neuropharmacology. 2008;54:365–374. doi: 10.1016/j.neuropharm.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belelli D, Herd MB. The contraceptive agent Provera enhances GABAA receptor- mediated inhibitory neurotransmission in the rat hippocampus: evidence for endogenous neurosteroids? J Neurosci. 2003;23:10013–10020. doi: 10.1523/JNEUROSCI.23-31-10013.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belelli D, Lambert JJ. Neurosteroids: endogenous regulators of the GABAA receptor. Nat Rev Neurosci. 2005;6:565–575. doi: 10.1038/nrn1703. [DOI] [PubMed] [Google Scholar]
- Brussaard AB, Kits KS, Baker RE, Willems WP, Leyting-Vermeulen JW, Voorn P, Smit AB, Bicknell RJ, Herbison AE. Plasticity in fast synaptic inhibition of adult oxytocin neurons caused by switch in GABAA receptor subunit expression. Neuron. 1997;19:1103–1114. doi: 10.1016/s0896-6273(00)80401-8. [DOI] [PubMed] [Google Scholar]
- Cagetti E, Liang J, Spigelman I, Olsen RW. Withdrawal from chronic intermittent ethanol treatment changes subunit composition, reduces synaptic function, and decreases behavioral responses to positive allosteric modulators of GABAA receptors. Mol Pharmacol. 2003;63:53–64. doi: 10.1124/mol.63.1.53. [DOI] [PubMed] [Google Scholar]
- Cagetti E, Pinna G, Guidotti A, Baicy K, Olsen RW. Chronic intermittent ethanol (CIE) administration in rats decreases levels of neurosteroids in hippocampus, accompanied by altered behavioral responses to neurosteroids and memory function. Neuropharmacology. 2004;46:570–579. doi: 10.1016/j.neuropharm.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Kosobud A, Young ER, Tam BR, McSwigan JD. Bidirectional selection for susceptibility to ethanol withdrawal seizures in Mus musculus. Behav Genet. 1985;15:521–536. doi: 10.1007/BF01065448. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Merrill CD, Belknap JK. Effects of convulsants on handling-induced convulsions in mice selected for ethanol withdrawal severity. Brain Res. 1991;550:1–6. doi: 10.1016/0006-8993(91)90397-e. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Young ER, Kosobud A. Genetic correlations with ethanol withdrawal severity. Pharmacol Biochem Behav. 1983;18(Suppl 1):541–547. doi: 10.1016/0091-3057(83)90233-2. [DOI] [PubMed] [Google Scholar]
- Dave JR, Tabakoff B, Hoffman PL. Ethanol withdrawal seizures produce increased c-fos mRNA in mouse brain. Mol Pharmacol. 1990;37:367–371. [PubMed] [Google Scholar]
- Devaud LL, Purdy RH, Finn DA, Morrow AL. Sensitization of γ-aminobutyric acidA receptors to neuroactive steroids in rats during ethanol withdrawal. J Pharmacol Exp Ther. 1996;278:510–517. [PubMed] [Google Scholar]
- Finn DA, Crabbe JC. Chronic ethanol differentially alters susceptibility to chemically induced convulsions in Withdrawal Seizure-Prone and -Resistant mice. J Pharmacol Exp Ther. 1999;288:782–790. [PubMed] [Google Scholar]
- Finn DA, Douglass AD, Beadles-Bohling AS, Tanchuck MA, Long SL, Crabbe JC. Selected line difference in sensitivity to a GABAergic neurosteroid during ethanol withdrawal. Genes Brain Behav. 2006;5:53–63. doi: 10.1111/j.1601-183X.2005.00137.x. [DOI] [PubMed] [Google Scholar]
- Finn DA, Ford MM, Wiren KM, Roselli CE, Crabbe JC. The role of pregnane neurosteroids in ethanol withdrawal: Behavioral genetic approaches. Pharmacol Ther. 2004a;101:91–112. doi: 10.1016/j.pharmthera.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Finn DA, Gallaher EJ, Crabbe JC. Differential change in neuroactive steroid sensitivity during ethanol withdrawal. J Pharmacol Exp Ther. 2000;292:394–405. [PubMed] [Google Scholar]
- Finn DA, Gee KW. The influence of estrus cycle on neurosteroid potency at the γ-aminobutyric acidA receptor complex. J Pharmacol Exp Ther. 1993;265:1374–1379. [PubMed] [Google Scholar]
- Finn DA, Long SL, Tanchuck MA, Crabbe JC. Interaction of chronic ethanol exposure and finasteride: sex and strain differences. Pharmacol Biochem Behav. 2004b;78:435–443. doi: 10.1016/j.pbb.2004.04.016. [DOI] [PubMed] [Google Scholar]
- Finn DA, Roberts AJ, Crabbe JC. Neuroactive steroid sensitivity in Withdrawal Seizure-Prone and -Resistant mice. Alcohol Clin Exp Res. 1995;19:410–415. [Google Scholar]
- Follesa P, Biggio F, Caria S, Gorini G, Biggio G. Modulation of GABAA receptor gene expression by allopregnanolone and ethanol. Eur J Pharmacol. 2004;500:413–425. doi: 10.1016/j.ejphar.2004.07.041. [DOI] [PubMed] [Google Scholar]
- Frye CA, Rhodes ME. Infusions of 5α-pregnan-3α-ol-20-one (3α,5α-THP) to the ventral tegmental area, but not the substantia nigra, enhance exploratory, anti-anxiety, social and sexual behaviours and concomitantly increase 3α,5α-THP concentrations in the hippocampus, diencephalon and cortex of ovariectomised oestrogen-primed rats. J Neuroendocrinol. 2006;18:960–975. doi: 10.1111/j.1365-2826.2006.01494.x. [DOI] [PubMed] [Google Scholar]
- Frye CA, Vongher JM. Ventral tegmental area infusions of inhibitors of the biosynthesis and metabolism of 3α,5α-THP attenuate lordosis of hormone-primed and behavioural oestrous rats and hamsters. J Neuroendocrinol. 2001;13:1076–1086. doi: 10.1046/j.1365-2826.2001.00731.x. [DOI] [PubMed] [Google Scholar]
- Gale K. Progression and generalization of seizure discharge: Anatomical and neurochemical substrates. Epilepsia. 1988;29(Suppl 2):S15–34. doi: 10.1111/j.1528-1157.1988.tb05795.x. [DOI] [PubMed] [Google Scholar]
- Gasior M, Carter RB, Witkin JM. Neuroactive steroids: potential therapeutic use in neurological and psychiatric disorders. Trends Pharmacol Sci. 1999;20:107–112. [Google Scholar]
- Gee KW, Bolger MB, Brinton RE, Coirini H, McEwen BS. Steroid modulation of the chloride ionophore in rat brain: structure-activity requirements, regional dependence and mechanism of action. J Pharmacol Exp Ther. 1988;246:803–812. [PubMed] [Google Scholar]
- Gorin RE, Crabbe JC, Tanchuck MA, Long SL, Finn DA. Effects of finasteride on chronic and acute ethanol withdrawal severity in the WSP and WSR selected lines. Alcohol Clin Exp Res. 2005;29:939–948. doi: 10.1097/01.alc.0000167742.11566.01. [DOI] [PubMed] [Google Scholar]
- Harney SC, Frenguelli BG, Lambert JJ. Phosphorylation influences neurosteroid modulation of synaptic GABAA receptors in rat CA1 and dentate gyrus neurones. Neuropharmacology. 2003;45:873–883. doi: 10.1016/s0028-3908(03)00251-x. [DOI] [PubMed] [Google Scholar]
- Herzog AG, Frye CA. Seizure exacerbation associated with inhibition of progesterone metabolism. Ann Neurol. 2003;53:390–391. doi: 10.1002/ana.10508. [DOI] [PubMed] [Google Scholar]
- Hirani K, Khisti RT, Chopde CT. Behavioral action of ethanol in Porsolt's forced swim test: modulation by 3α-hydroxy-5α-pregnan-20-one. Neuropharmacology. 2002;43:1339–1350. doi: 10.1016/s0028-3908(02)00330-1. [DOI] [PubMed] [Google Scholar]
- Koksma JJ, van Kesteren RE, Rosahl TW, Zwart R, Smit AB, Luddens H, Brussaard AB. Oxytocin regulates neurosteroid modulation of GABAA receptors in supraoptic nucleus around parturition. J Neurosci. 2003;23:788–797. doi: 10.1523/JNEUROSCI.23-03-00788.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Fleming RL, Morrow AL. Ethanol regulation of γ-aminobutyric acidA receptors: genomic and nongenomic mechanisms. Pharmacol Ther. 2004;101:211–226. doi: 10.1016/j.pharmthera.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Liang J, Cagetti E, Olsen RW, Spigelman I. Altered pharmacology of synaptic and extrasynaptic GABAA receptors on CA1 hippocampal neurons is consistent with subunit changes in a model of alcohol withdrawal and dependence. J Pharmacol Exp Ther. 2004;310:1234–1245. doi: 10.1124/jpet.104.067983. [DOI] [PubMed] [Google Scholar]
- Liang J, Zhang N, Cagetti E, Houser CR, Olsen RW, Spigelman I. Chronic intermittent ethanol-induced switch of ethanol actions from extrasynaptic to synaptic hippocampal GABAA receptors. J Neurosci. 2006;26:1749–1758. doi: 10.1523/JNEUROSCI.4702-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark GP, Finn DA. The relationship between hippocampal acetylcholine release and cholinergic convulsant sensitivity in Withdrawal Seizure-Prone and Withdrawal Seizure-Resistant selected mouse lines. Alcohol Clin Exp Res. 2002;26:1141–1152. doi: 10.1097/01.ALC.0000024125.23287.CA. [DOI] [PubMed] [Google Scholar]
- Metten P, Crabbe JC. Alcohol withdrawal severity in inbred mouse (Mus musculus) strains. Behav Neurosci. 2005;119:911–925. doi: 10.1037/0735-7044.119.4.911. [DOI] [PubMed] [Google Scholar]
- Morgan PF, Nadi NS, Karanian J, Linnoila M. Mapping rat brain structures activated during ethanol withdrawal: role of glutamate and NMDA receptors. Eur J Pharmacol. 1992;225:217–223. doi: 10.1016/0922-4106(92)90023-o. [DOI] [PubMed] [Google Scholar]
- Parada MA, Puig DP, Hoebel BG. A remote insertion technique for intracerebral microinjections in freely moving animals. J Neurosci Methods. 1993;50:237–241. doi: 10.1016/0165-0270(93)90012-g. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin K. The Mouse Brain in Stereotaxic Coordinates. 2. Academic Press; San Diego, CA: 2001. [Google Scholar]
- Purdy RH, Morrow AL, Blinn JR, Paul SM. Synthesis, metabolism, and pharmacological activity of 3α-hydroxy steroids which potentiate GABA-receptor-mediated chloride ion uptake in rat cerebral cortical synaptoneurosomes. J Med Chem. 1990;33:1572–1581. doi: 10.1021/jm00168a008. [DOI] [PubMed] [Google Scholar]
- Putzke J, Spanagel R, Tolle TR, Zieglgansberger W. The anti-craving drug acamprosate reduces c-fos expression in rats undergoing ethanol withdrawal. Eur J Pharmacol. 1996;317:39–48. doi: 10.1016/s0014-2999(96)00696-6. [DOI] [PubMed] [Google Scholar]
- Rhodes ME, Frye CA. Actions at GABAA receptors in the hippocampus may mediate some antiseizure effects of progestins. Epilepsy Behav. 2005a;6:320–327. doi: 10.1016/j.yebeh.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Rhodes ME, Frye CA. Attenuating 5α-pregnane-3α-ol-20-one formation in the hippocampus of female rats increases pentylenetetrazole-induced seizures. Epilepsy Behav. 2005b;6:140–146. doi: 10.1016/j.yebeh.2004.11.019. [DOI] [PubMed] [Google Scholar]
- Romeo E, Brancati A, De Lorenzo A, Fucci P, Furnari C, Pompili E, Sasso GF, Spalletta G, Troisi A, Pasini A. Marked decrease of plasma neuroactive steroids during alcohol withdrawal. Clin Neuropharmacol. 1996;19:366–369. doi: 10.1097/00002826-199619040-00011. [DOI] [PubMed] [Google Scholar]
- Romeo E, Pompili E, di Michele F, Pace M, Rupprecht R, Bernardi G, Pasinib A. Effects of fluoxetine, indomethacine and placebo on 3α, 5α tetrahydroprogesterone (THP) plasma levels in uncomplicated alcohol withdrawal. World J Biol Psychiatry. 2000;1:101–104. doi: 10.3109/15622970009150572. [DOI] [PubMed] [Google Scholar]
- Ströhle A, Romeo E, Hermann B, Pasini A, Spalletta G, di Michele F, Holsboer F, Rupprecht R. Concentrations of 3α-reduced neuroactive steroids and their precursors in plasma of patients with major depression and after clinical recovery. Biol Psychiatry. 1999;45:274–277. doi: 10.1016/s0006-3223(98)00328-x. [DOI] [PubMed] [Google Scholar]
- Uzunova V, Sheline Y, Davis JM, Rasmusson A, Uzunov DP, Costa E, Guidotti A. Increase in the cerebrospinal fluid content of neurosteroids in patients with unipolar major depression who are receiving fluoxetine or fluvoxamine. Proc Natl Acad Sci. 1998;95:3239–3244. doi: 10.1073/pnas.95.6.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]