Abstract
Purpose
Glutamate receptor activation-induced excitotoxicity has been hypothesized to cause retinal ganglion cell (RGC) death in glaucoma and to link mitochondrial dysfunction in both acute and chronic neurodegenerative disorders. However, the relationships among elevated intraocular pressure (IOP), glutamate receptor-mediated excitotoxicity, and mitochondrial dysfunction in glaucoma remains unknown. The goal of this study was to determine whether the N- methyl D-aspartate (NMDA) glutamate receptor antagonist MK801 can block optic atrophy 1 (OPA1) release and subsequent apoptotic cell death, as well as whether acute IOP elevation triggers OPA1 release and alters OPA1 gene and protein expression in the rat retina after ischemia.
Methods
Sprague Dawley rats received injections of MK801 (10 mg/kg) or vehicle and then transient retinal ischemia was induced by acute IOP elevation. Following subcellular fractionation, changes in cytoplasmic and mitochondrial OPA1 were assessed by western blot analysis. Also, the expression of OPA1 mRNA was measured by Taqman qPCR, the distribution of OPA1 protein was assessed by immunohistochemistry, and apoptotic cell death was assessed by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining.
Results
The ~65 and 90 kDa isoforms of OPA1 were increased in the cytosol in the rat retina at 6 h and at 12 h, but only the 90 kDa isoform of OPA1 was decreased at 12 h after ischemia induced by acute IOP elevation. This suggests that ischemic insult induced OPA1 release from the mitochondria in retinas. Pretreatment with MK801 blocked this effect and significantly increased OPA1 immunoreactivity in the inner retinal layers, as well as OPA1 gene expression and total protein expression in retinas at 12 h after ischemia. Further, pretreatment with MK801 prevented apoptotic cell death in retinas at 12 h after ischemia. Following acute IOP elevation, Bcl-2 mRNA expression in retinas was decreased at 3 h and 6 h but increased at 12 h and 24 h. In contrast, Bax mRNA expression in these retinas was increased in the first 12 h and then plateaued. Moreover, pretreatment with MK801 increased Bcl-2 mRNA expression, but did not alter the course of Bax mRNA expression.
Conclusions
These results indicate that OPA1 release from mitochondria triggered by acute IOP elevation is inhibited by blockade of glutamate receptor activation. Because this effect was accompanied by increases of Bcl-2 expression, no changes of Bax expression, and blockade of apoptosis, these findings indicate that glutamate receptor activation following acute IOP elevation may lead to a distinct mitochondria-mediated cell death pathway in ischemic retina. These results support further studies to determine whether ischemia-induced OPA1 release may be an important component of the biochemical cascade leading to pressure-related ischemic damage in glaucomatous retina.
Introduction
Elevated intraocular pressure (IOP) is an important risk factor for optic nerve damage in glaucoma [1]. However, the precise pathophysiological relationship between elevated IOP and retinal ganglion cell (RGC) death remains poorly understood. It has been hypothesized that glutamate receptor activation may contribute to RGC death in glaucoma [2]. In addition, glutamate receptor activation-induced excitotoxicity has been linked to mitochondrial dysfunction in both acute and chronic neurodegenerative disorders [3-6]. However, the relationship among elevated IOP, glutamate excitotoxicity, and mitochondrial dysfunction in glaucoma remains unknown. Recently, we reported that moderately elevated hydrostatic pressure could induce abnormal cristae depletion, cytochrome C release, cellular ATP reduction, and translocation of dynamin-related protein-1 (Drp-1) in differentiated RGC-5 cells [7]. Further, we also found that elevated hydrostatic pressure triggers release of optic atrophy type 1 protein (OPA1) and cytochrome C, and induces subsequent apoptotic cell death in differentiated RGC-5 cells [8]. These observations raise the possibility that pressure-induced mitochondrial dysfunction may contribute to RGC death in glaucoma.
In healthy cells, mitochondria are autonomous and morphologically dynamic organelles that structurally reflect a precise balance of ongoing fission and fusion within a cell [9-11]. This balance is regulated by a family of dynamin-related GTPases that exert opposing effects. Drp-1 regulates mitochondrial fission, while OPA1, the human ortholog of Mgm1p/Msp1p, and the mitofusins are required for mitochondria fusion [10,12]. OPA1 mRNA is transcribed from nuclear DNA and new OPA1 protein is then translocated to the inner membrane of mitochondria [13,14]. Of particular interest, mutations in OPA1 are linked with neurodegenerative diseases in human and can cause autosomal dominant optic atrophy (ADOA), the most common form of hereditary optic neuropathy [15,16]. Retinal OPA1 is expressed in the soma and axons of the RGCs as well as in horizontal cells [17-19]. Although the specific functional role of OPA1 in these cells remains unknown, it has been shown that down-regulation of OPA1 causes mitochondrial fission, leading to cytochrome C release and apoptosis in HeLa cells, as well as induces aggregation of the mitochondrial network in purified RGCs [20-23]. Proteolytic processing of OPA1 has been observed during mitochondrial fission, although its significance remains poorly investigated [24-27]. Also, OPA1 release during mitochondrial fission contributes to apoptotic cell death [22,26]. Nevertheless, it is unknown whether acute IOP elevation can directly alter OPA1 expression and distribution in the mammalian retina.
Thus, we evaluated whether the N- methyl D-aspartate (NMDA) glutamate receptor antagonist MK801 can block OPA1 release and subsequent apoptotic cell death as well as whether acute IOP elevation triggers OPA1 release and alters OPA1 gene and protein expression in the rat retina after ischemia. Also, we compared the progression of these changes with changes in Bcl-2 and Bax expression.
Methods
Transient retinal ischemia
All procedures were in compliance with the National Institute of Health Guide for the Care and Use of Laboratory Animals and the ARVO Statement on the Use of Animals in Ophthalmic Research. Sprague-Dawley female rats, 3 months of age (200–250 g in weight) were anesthetized with ketamine (100 mg/kg) and xylazine (20 mg/kg, i.p. injection). A cannula was inserted into the anterior chamber that was connected by flexible tubing to a reservoir. By raising the reservoir, IOP was elevated above systolic blood pressure (100–120 mmHg) for 60 min. Animals were allowed to recover for 3 to 24 h.
Pharmacological treatment
Two groups of rats were studied following unilateral transient retinal ischemia: a group treated with vehicle (0.9% saline, i.p. injection) and a group treated with MK801 (10 mg/kg in 0.9% saline; i.p. injection; n=5 animals/group; Sigma, St. Louis, MO) given 30 min before ischemia.
Tissue preparations
The light-adapted rats were anesthetized with isoflurane followed by an i.p. injection of ketamine/xylazine as above. Both eyes were enucleated and then the rats were killed by CO2 inhalation. The retinas were dissected from the choroid and fixed in the 4% paraformaldehyde in 0.1 M phosphate buffer (PB, pH 7.4) for 2 h at 4 °C. Retinas were dehydrated through graded ethanols and then embedded in polyester wax [18].
Western blot analysis
The retinas were homogenized in a glass-teflon Potter homogenizer in lysis buffer (20 mM Hepes, pH 7.5, 10 mM KCl, 1.5 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, 0.5% CHAPS, complete protease inhibitors; Roche Biochemicals, Indianapolis, IN). Each sample (10 µg) was separated by PAGE and electro-transferred to Polyvinylidene Fluoride (PVDF) membranes. The membrane was blocked with 5% nonfat dry milk and 0.05% in Tween-20 in phosphate buffer saline (PBS), incubated with monoclonal mouse anti-OPA1 antibody (H-300/1:1000; BD Transduction Laboratories, San Diego, CA) or monoclonal mouse anti-actin antibody (Ab-1/1:3,000; Calbiochem, La Jolla, CA), rinsed with 0.05% Tween-20 in PBS, incubated with peroxidase-conjugated goat anti-mouse IgG (1:2,000; Bio-Rad, Hercules, CA), or goat anti-rabbit IgM (1:5,000; Calbiochem), and developed using chemiluminescence detection (ECL Plus; GE Healthcare Bio-Sciences, Picataway, NJ). Images were analyzed by digital fluorescence imager (Storm 860; GE Healthcare Bio-Sciences) and band densities were normalized using actin as cytosolic fraction calibrator and Voltage-dependent Anion Channel (VDAC) as mitochondrial fraction calibrator with ImageQuant TL (GE Healthcare Bio-Sciences).
To assess the subcellular distribution of OPA1, the cytosolic and mitochondrial fractions were isolated from retinas by differential centrifugation (Mitochondrial Isolation Kit; Pierce, Rockford, IL) and western blot analysis was performed as above. Equal loading was confirmed by reprobing with actin as above or with polyclonal rabbit anti-VDAC antibody (Ab-5/1:1,000, Calbiochem).
Taqman quantitative polymerase chain reaction
Total RNA from retinas was extracted with Trizol (Invitrogen, Carlsbad, CA), purified on RNeasy mini columns (Qiagen, Valencia, CA), and treated with RNase-free DNase I (Qiagen). OPA1, Bax, and Bcl-2 gene expression were measured by Taqman quantitative PCR (MX3000P, Stratagene, La Jolla, CA) using 50 ng of cDNA from retinas and 2X Taqman universal PCR master mix (Applied Biosystems, Foster City, CA) with a one-step program (95 °C for 10 min, 95 °C for 30 s, and 60 °C for 1 min for 50 cycles). Primers for OPA1, Bax, Bcl-2, and GAPDH, as well as Taqman probe for GAPDH were designed using Primer Express 2.0 software (Applied Biosystems), obtained from Roche Diagnostics (Mannheim, Germany; Table 1), and the optimal concentrations for probe and primers were determined using heart tissue. Duplicate samples without cDNA (no-template control) showed no contaminating DNA. Standard curves were constructed using nine twofold dilutions (50 ng-0.195 ng) for both the targets (OPA1, Bax and Bcl-2) and the endogenous reference (GAPDH). The samples were run in triplicates for target and endogenous GAPDH control.
Table 1. Primer and probe sequences for OPA1, Bax, Bcl-2, and GAPDH for Taqman quantitative PCRa.
| Gene (GenBank) | Type | Sequences (5′–3′) |
|---|---|---|
|
OPA1 rat (NM_133585) |
Forward |
CAGCTGGCAGAAGATCTCAAG |
| |
Reverse |
CATGAGCAGGATTTTGACACC |
| |
Probe |
Universal Probe Library probe #2 |
| |
|
Cat. # 04684982001 |
|
Bax rat (NM_017059) |
Forward |
GTGAGCGGCTGCTTGTCT |
| |
Reverse |
GTGAGCGGCTGC-TTGTCT |
| |
Probe |
Universal Probe Library probe #83 |
| |
|
Cat. # 04689062001 |
|
Bcl-2 rat (NM_016993) |
Forward |
GGGATGCCTTTGTGGAACT |
| |
Reverse |
CTGAGCAGCGTCTTCAGAGA |
| |
Probe |
Universal Probe Library probe #2 |
| |
|
Cat. # 04684982001 |
|
GAPDH rat (X02231) |
Forward |
GAACATCATCCCTGCATCCA |
| |
Reverse |
CCAGTGAGCTTCCCGTTCA |
| Probe | CTTGCCCACAGCCTTGGCAGC |
aTaqman probe containing 5′ reporter FAM and 3′ quencher BHQ-1 dyes.
Immunohistochemical analysis
Immunohistochemical staining 7 μm wax section of full thickness retina was done by the Tyramide Signal Amplication Kit (Molecular Probes, Eugene, OR) as described below [18]. To increase the sensitivity of OPA1 immunohistochemistry, sections exposed to primary and secondary antibodies were incubated with solutions from the Tyramide Signal Amplification Kit (Molecular Probes). Tissues were permeabilized with 0.1% Triton X-100 in PBS, incubated with quenching buffer (Amplication buffer and 0.0015% H2O2) for 1 h at room temperature, blocked with 1% BSA/PBS, and then incubated with antibody against OPA1 (provided by Drs. Misaka and Kubo, University of Tokyo, 1:100) for 16 h at 4 °C. After several washing, tissues were incubated with peroxidase-conjugated goat anti-rabbit IgG for 1 h at room temperature, washed, and incubated with tyramide working solution for 10 min at room temperature.
TUNEL staining
Sections were incubated with proteinase K (10 µg/ml, 10 mM Tris, pH 7.4–8.0) for 10 min at 37 °C. After rinsing in PBS, the sections were incubated with terminal deoxynucleotidyl transferase plus nucleotide mixture in reaction buffer for 60 min at 37 °C (In situ Cell Death Detection kit, Roche Diagnostics).
Statistical analysis
Experiments presented were repeated at least three times with triplicate samples. The data are presented as the mean±SD. Comparison of two experimental conditions was evaluated using the unpaired Student’s t-test. A p<0.05 was considered to be statistically significant.
Results
Acute intraocular pressure elevation induces OPA1 release from mitochondria in the rat retina
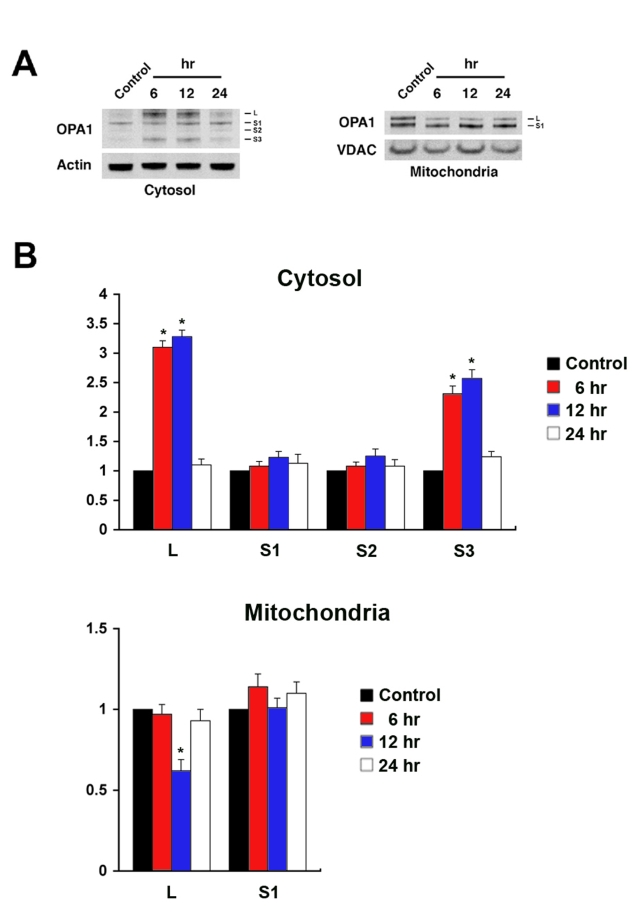
The OPA1 antibody recognized a major band (80 kDa) in the cytosolic fraction and two major bands (90 and 80 kDa) in the mitochondrial fraction of the normal rat retina. After ischemia, at least 4 major isoforms of OPA1 (90 kDa:L, 80 kDa:S1, ~75 kDa:S2, and ~65 kDa:S3) were observed in the cytosolic fraction. In contrast, only 2 major isoforms of OPA1 (L and S1) were observed in the mitochondrial fraction (Figure 1A). Increased L and S3 isoforms were prominent in the cytosolic fraction at 6 h and at 12 h after ischemia but there were no changes in the S1 and S2 isoforms. In the mitochondrial fraction from ischemic retina, L isoform OPA1 was significantly decreased at 12 h but there was no significant change in S1 isoform OPA1 (Figure 1B).
Figure 1.
Acute IOP elevation induces OPA1 release in ischemic rat retina. A: The OPA1 protein bands observed at 80 kDa in cytosolic fraction and at 80 and 90 kDa forms in mitochondrial fraction of normal retina. Following ischemia, there were at least four major isoforms of OPA1 protein bands (90:L, 80:S1, ~75:S2, and ~65 kDa:S3) in the cytosolic fraction. B: Relative intensity of chemiluminescence for each protein band was normalized using actin as cytosolic fraction calibrator and VDAC as mitochondrial fraction calibrator. The isoforms of OPA1 protein (L and S3) were significantly increased in the cytosolic fraction. However, the isoform of OPA1 protein (L) was significantly decreased at 12 h but the isoform of OPA1 protein (S1) was not changed in the mitochondrial fraction at 6–24 h after ischemia. Error bars represent the standard deviation (*p<0.05 by Student’s t-test, n=3).
Blockade of glutamate receptor activation increases OPA1 gene and protein expression in the rat retina following transient ischemia
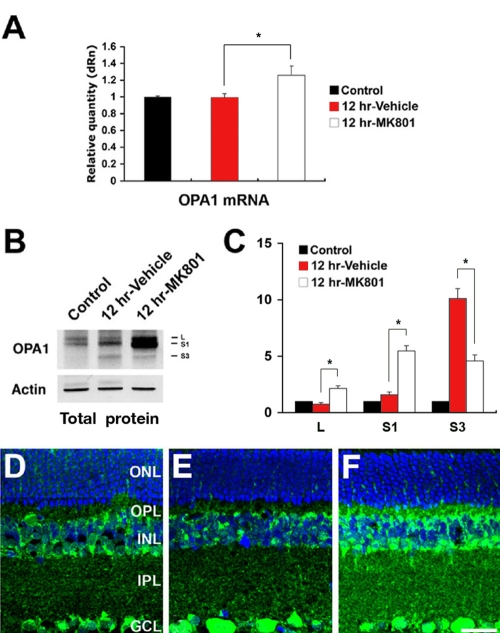
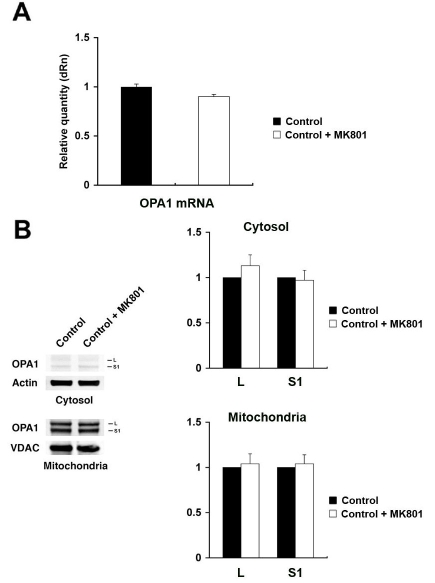
OPA1 mRNA expression did not differ in vehicle-pretreated retina after ischemia, compared to normal retina. However, pretreatment with MK801 significantly increased OPA1 mRNA expression by 26.0±1.1% in retina after ischemia (Figure 2A). Interestingly, pretreatment with MK801 significantly increased total L and S1 isoforms of OPA1 protein at 12 h of ischemic retina but significantly decreased total S3 isoform of OPA1 protein (Figure 2B,C). OPA1 immunoreactivity was localized in the ganglion cell layer (GCL), inner plexiform layer (IPL), inner nuclear layer (INL), and outer plexiform layer (OPL) in the normal rat retina as previously reported (Figure 2D) [18]. In ischemic rat retina pretreated with vehicle, OPA1 immunoreactivity in the GCL, IPL, INL, and OPL did not show significant changes at 12 h (Figure 2E). In contrast, pretreatment with MK801 significantly increased OPA1 immunoreactivity in the IPL, INL, and OPL at 12 h after ischemia (Figure 2F). In addition, we found that there were no significant changes of both OPA1 mRNA and protein expression level between two control conditions (non-pressurized retina and non-pressurized retina plus MK801; Figure 3).
Figure 2.
Blockade of glutamate receptor activation increases OPA1 gene and protein expression in ischemic rat retina. A: OPA1 gene expression was not changed in vehicle-pretreated ischemic retinas but increased in MK801-pretreated ischemic retinas at 12 h. B: The total isoformes of OPA1 protein bands (80–90 kDa) in the normal rat retina were increased after ischemia, and had a small released fragment of OPA1 (~65 kDa:S3). Pretreatment with MK801 induced a larger increase of total L and S1 isoforms of OPA1 protein bands but decrease of total S3 isoform of OPA1 protein band. C: Relative intensity of chemiluminescence for each protein band was normalized using actin as cytosolic fraction calibrator. Error bars represent the standard deviation (*p<0.05 by Student’s t-test, n=3). D-F: OPA1 immunoreactivity in normal retina (D), retina of vehicle pre-treated rats at 12 h after ischemia (E), and retina of MK801 pre-treated rats at 12 h after ischemia (F). Abbreviations: ONL represents outer nuclear layer; OPL represents outer plexiform layer; INL represents inner nuclear layer; IPL represents inner plexiform layer; GCL represents ganglion cell layer. The scale bar represents 20 μm (D-F).
Figure 3.
The effect of MK801 in the normal rat retina. A: OPA1 gene expression was not changed in MK801-pretreated normal control retinas compared to non-treated normal control retinas. B: The OPA1 protein bands observed at 80 and 90 kDa forms in cytosolic and mitochondrial fractions in both non-treated and MK801-treated normal control retina. Relative intensity of chemiluminescence for each protein band was normalized using actin as cytosolic fraction calibrator and VDAC as mitochondrial fraction calibrator. Note that there were no significant changes between non-treated and MK801-treated normal control retina. Error bars represent the standard deviation.
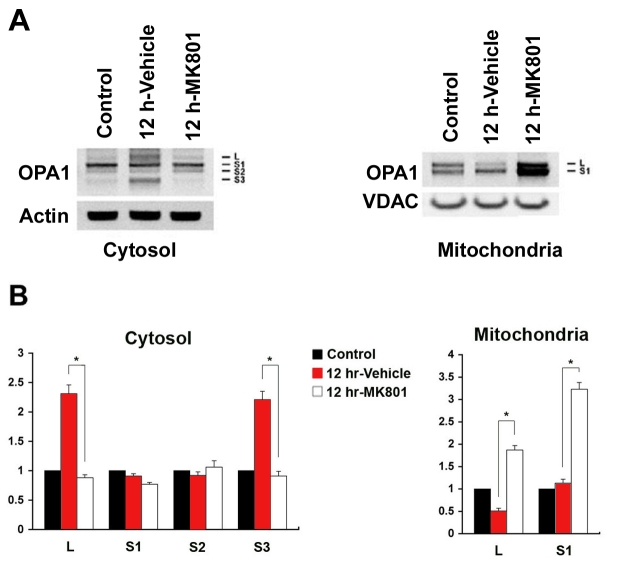
Blockade of glutamate receptor activation significantly prevents OPA1 release in the rat retina following transient ischemia
In ischemic retina pretreated with vehicle, the L and S3 isoforms of OPA1 were significantly increased in the cytosolic fraction (Figure 4A,B). In contrast, the L isoform was significantly decreased in the mitochondrial fraction (Figure 4A,B). Pretreatment with MK801 blocked all of these ischemia-induced changes in cytosolic OPA1. Moreover, pretreatment with MK801 significantly increased both L and S1 isoforms OPA1 in the mitochondrial fraction (Figure 4A,B).
Figure 4.
Blockade of glutamate receptor activation blocks OPA1 release in ischemic rat retina. A: The OPA1 protein bands observed at 80 kDa in cytosolic fraction and at 80 and 90 kDa forms in mitochondrial fraction of normal retina. Following ischemia, there were at least 4 major isoforms of OPA1 protein bands (90:L, 80:S1, ~75:S2, and ~65 kDa:S3) in the cytosolic fraction. B: Relative intensity of chemiluminescence for each protein band was normalized using actin as cytosolic fraction calibrator and VDAC as mitochondrial fraction calibrator. The isoforms of OPA1 protein (L and S3) were significantly increased in the cytosolic fraction and decreased in the mitochondrial fraction at 12 h after ischemia. Pretreatment with MK801 significantly blocks OPA1 release to the cytosol and increased OPA1 protein in the mitochondria at 12 h following ischemia. Error bars represent the standard deviation (*p<0.05 by Student’s t-test, n=3).
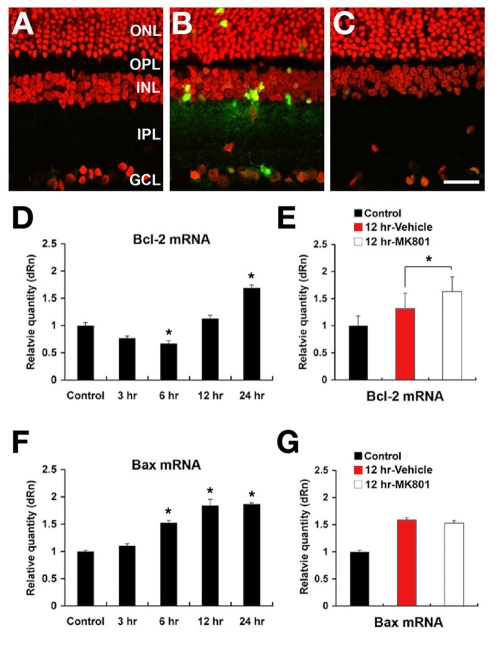
Blockade of glutamate receptor activation inhibits apoptotic cell death, and increased Bcl-2 expression but did not alter Bax expression
In normal retina, there were no TUNEL-positive cells (Figure 5A). Ischemic insult by acute IOP elevation induced apoptotic cell death in the GCL, INL, and ONL at 12 h (Figure 5B). In contrast, pretreatment with MK801 blocked this apoptosis in all layers following ischemia (Figure 5C). Bcl-2 mRNA was decreased in early phase (3 h and 6 h) but increased in delayed phase (12 h and 24 h) in vehicle-pretreated ischemic retina, compared to normal retina (Figure 5D). In addition, pretreatment with MK801 significantly increased Bcl-2 mRNA expression by 31.0±2.7% at 12 h (Figure 5E), compared to vehicle-pretreated ischemic retina. Bax mRNA expression was increased earlier than Bcl-2 and plateaued by 12 h in vehicle-pretreated ischemic retina (Figure 5F). Pretreatment with MK801 did not significantly change Bax mRNA expression at 12 h (Figure 5G), compared to vehicle-pretreated ischemic retina.
Figure 5.
Blockade of glutamate receptor activation blocks apoptotic cell death and induces increase of Bcl-2 expression in ischemic rat retina. A: There were no TUNEL-positive cells in the normal retina. B: In ischemic retina of rats receiving vehicle, apoptotic cell death was present in the ONL, INL,and GCL. C: MK801 treatment before ischemia blocked apoptotic cell death in all layers. D: Bcl-2 mRNA significantly decreased at 6 h but increased at 24 h, compared to normal retinas. E: Bcl-2 mRNA following ischemia was significantly increase at 12 h by pretreatment with MK801, compared to the vehicle-pretreated retinas. F: Bax mRNA was significantly increased from 6 to 12 h, compared to normal retina. G: No Bax expression following ischemia was shown at 12 h by pretreatment with MK801, compared to the vehicle-pretreated retinas. Error bars represent the standard deviation (*p<0.05 by Student’s t-test, n=3). Abbreviations: ONL represents outer nuclear layer; OPL represents outer plexiform layer; INL represents inner nuclear layer; IPL represents inner plexiform layer; GCL represents ganglion cell layer. The scale bar represents 20 μm (A-C).
Discussion
Our results demonstrate that (1) ischemic insult by acute IOP elevation triggers OPA1 release in the early neurodegenerative events (within 12 h), (2) blockade of glutamate receptor activation prevents OPA1 release and apoptotic cell death as well as increases OPA1 gene and total protein expression at 12 h after ischemia, and (3) blockade of glutamate receptor activation induces increases of Bcl-2 expression but no changes of Bax expression at 12 h after ischemia. These findings indicate that glutamate receptor activation following acute IOP elevation may lead to a distinct mitochondria-mediated cell death pathway in ischemic retina. Further, this ischemia-induced OPA1 release may be an important component of the biochemical cascade leading to pressure-related ischemic damage in glaucomatous retina.
Emerging evidence indicates that mitochondrial morphology and dynamics play an important role in cell and animal physiology. An imbalance in the control of mitochondrial fusion and fission dramatically alters overall mitochondrial morphology [11,12]. This balance is regulated by a family of dynamin-related GTPases that exert opposing effects. In mammals, OPA1 and mitofusins are required for mitochondria fusion. In contrast, Drp-1 regulates mitochondrial fission [10,12]. Recent evidence indicates that OPA1 release participates in the rapid and complete release of cytochrome C in apoptotic cell death as well as controls apoptotic cristae remodeling [22,26]. In the present study, we found a small isoform of OPA1 (80 kDa) in the cytosolic fraction and two isoforms of OPA1 (90 and 80 kDa) in the mitochondrial fraction of the normal rat retina. In contrast, ischemic rat retina showed at least four isoforms of OPA1 in the cytosolic fraction at 6 h and 12 h. Interestingly, while both 90 and ~65 kDa isoforms of OPA1 were significantly increased in the cytosolic fraction, the large isoform of OPA1 (90 kDa) was significantly decreased in the mitochondrial fraction. These results indicate that ischemic damage following acute IOP elevation directly induces OPA1 release from mitochondria to the cytoplasm in the retina. Activation of rhomboid intramembrane protease (PARL) may explain the cleavage of OPA1 into truncated forms observed in the present study following ischemia [28]. OPA1 may be crucial for the anti-apoptotic effects of PARL because it maintains the bottleneck configuration of cristae and the comparmententalization of cytochrome C [25,28]. Thus, it is likely that the unexpected smaller molecular weight of OPA1 fragments presently observed might include the truncated forms of OPA1 that localize to in the intermembrane space or possibly one of the degradation products. Although OPA1 consists of at least five isoforms in other types of cells including HeLa cells [29-31], the precise functional role of the OPA1 isoforms that are released from mitochondria remains unknown. Thus, the potential proteolytic processing of OPA1 as well as the functional role of each OPA1 isoform in the cytosolic and mitochondrial fractions of the ischemic retina need to be further explored.
OPA1 is ubiquitously expressed in several tissues but is most abundant in the retina [15,17,18]. Pesch et al. [17] reported that the OPA1 gene is expressed and its protein is present in RGCs and displaced amacrine cells in the normal rat retina. Further, we also reported that OPA1 protein is present in the amacrine cells and RGCs as well as horizontal cells of the normal rat retina [18]. Like OPA1, glutamate receptor subunits are present in horizontal cells, bipolar cells, amacrine cells, displaced amacrine cells, or RGCs in the rat retina [32-38]. It has been reported that many amacrine cells in the INL were rapidly and dramatically injured or killed by glutamate or NMDA and that displaced amacrine cells in the GCL also became swollen [38]. Thus, it is possible that glutamate receptor activation-mediated excitotoxicity may directly or indirectly contribute to neuronal cell death in the inner and outer retinal layer. Moreover, glutamate neurotoxicity is partly responsible for ischemic retinal damage [39-43]. Hence, it is possible that OPA1-positive cells in the rat retina may also have glutamate receptors.
In the present study, blockade of glutamate receptor activation prevented OPA1 release and apoptotic cell death as well as increased OPA1 gene and total protein expression in ischemic rat retina. These results are good agreement with previous studies that down-regulation of OPA1 causes mitochondrial fission, leading to cytochrome C release and apoptosis in HeLa cells, as well as induces aggregation of the mitochondrial network in purified RGCs [20-23] and that increased OPA1 expression protects cells from apoptosis by preventing cytochrome C release and by stabilizing the shape of mitochondrial cristae [22,23]. Moreover, OPA1 deficiency in mouse models of ADOA impairs mitochondrial morphology, optic nerve structure, and visual function [44,45]. Together with these findings, our observations suggest that alteration of OPA1 expression and distribution by glutamate receptor activation may signal in the neurodegenerative events in ischemic insult-related glaucomatous optic neuropathy.
Bax is a proapoptotic member of the Bcl-2 family that is essential for many pathways of apoptosis [46]. Bax directly interacts with the components forming the mitochondrial permeability transition pore (MPTP) that allow proteins to escape from the mitochondria into the cytosol to initiate apoptosis [47-49]. In the present study, we found that ischemia induced significant increases of Bcl-2 and Bax mRNA expression in the retina at 12 h showing apoptotic cell death. Interestingly, blockade of glutamate receptor activation did not alter the increase of Bax expression but significantly increased Bcl-2 expression at 12 h. Perhaps, this increased Bcl-2 may block the Bax-mediated MPTP formation that may cause OPA1 release from mitochondria in ischemic retina. These observations suggest that increased OPA1 expression by blockade of glutamate receptor activation may contribute to retinal neuron survival in the presence of ischemic stress. Further studies will be needed to determine the mechanism of this effect and to clarify the relationships among glutamate receptor activation, OPA1 release, and increases of Bcl-2 and Bax following ischemic damage.
In summary, our findings demonstrated that glutamate receptor activation triggers OPA1 release in the early neurodegenerative events and that blockade of glutamate receptor activation prevents OPA1 release and apoptotic cell death as well as increases OPA1 expression and total protein expression in the ischemic rat retina. This suggests that defined OPA1 distribution changes may be a factor in the apoptosis induced by glutamate receptor activation in ischemic retina. Moreover, Bcl-2 and Bax alterations may also contribute to these events though further study will be needed to clarify their relationship. Together, these results raise the possibility that OPA1 release induced by glutamate receptor activation contributes to mitochondrial dysfunction in the pathophysiology of pressure-related ischemic damage in glaucomatous retina.
Acknowledgments
We thank Drs. Takumi Misaka (The University of Tokyo, Japan) and Yoshihiro Kubo (National Institute for Physiology, Japan) for providing antibody against mOPA1. This work was supported by National Institute Health grants EY01466 (J.D.L.) and EY105990 (R.N.W.).
References
- 1.Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet. 2004;363:1711–20. doi: 10.1016/S0140-6736(04)16257-0. [DOI] [PubMed] [Google Scholar]
- 2.Lipton SA. Possible role for memantine in protecting retinal ganglion cells from glaucomatous damage. Surv Ophthalmol. 2003;48(Suppl 1):S38–46. doi: 10.1016/s0039-6257(03)00008-0. [DOI] [PubMed] [Google Scholar]
- 3.Beal MF. Aging, energy, and oxidative stress in neurodegenerative diseases. Ann Neurol. 1995;38:357–66. doi: 10.1002/ana.410380304. [DOI] [PubMed] [Google Scholar]
- 4.Mattson MP, Pedersen WA, Duan W, Culmsee C, Camandola S. Cellular and molecular mechanisms underlying perturbed energy metabolism and neuronal degeneration in Alzheimer's and Parkinson's diseases. Ann N Y Acad Sci. 1999;893:154–75. doi: 10.1111/j.1749-6632.1999.tb07824.x. [DOI] [PubMed] [Google Scholar]
- 5.Nicholls DG. Mitochondrial dysfunction and glutamate excitotoxicity studied in primary neuronal cultures. Curr Mol Med. 2004;4:149–77. doi: 10.2174/1566524043479239. [DOI] [PubMed] [Google Scholar]
- 6.Fan MM, Raymond LA. N-methyl-D-aspartate (NMDA) receptor function and excitotoxicity in Huntington’s diseases. Prog Neurobiol. 2007;81:272–93. doi: 10.1016/j.pneurobio.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 7.Ju WK, Liu Q, Kim KY, Crowston JG, Lindsey JD, Agarwal N, Ellisman MH, Perkins GA, Weinreb RN. Elevated Hydrostatic pressure triggers mitochondrial fission and decreases cellular ATP in differentiated RGC-5 cells. Invest Ophthalmol Vis Sci. 2007;48:2145–51. doi: 10.1167/iovs.06-0573. [DOI] [PubMed] [Google Scholar]
- 8.Ju WK, Kim KY, Lindsey JD, Angert M, Patel A, Scott RT, Liu Q, Crowston JG, Ellisman MH, Perkins GA, Weinreb RN.Elevated hydrostatic pressure triggers release of OPA1 and cytochrome c, and induces apoptotic cell death in differentiated RGC-5 cells. Mol Vis 2009. in press [PMC free article] [PubMed] [Google Scholar]
- 9.Nunnari J, Marshall WF, Straight A, Murray A, Sedat JW, Walter P. Mitochondrial transmission during mating in Saccharomyces cerevisiae is determined by mitochondrial fusion and fission and the intramitochondrial segregation of mitochondrial DNA. Mol Biol Cell. 1997;8:1233–42. doi: 10.1091/mbc.8.7.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karbowski M, Youle RJ. Dynamics of mitochondrial morphology in healthy cells and during apoptosis. Cell Death Differ. 2003;10:870–80. doi: 10.1038/sj.cdd.4401260. [DOI] [PubMed] [Google Scholar]
- 11.Okamoto K, Shaw JM. Mitochondrial morphology and dynamics in yeast and multicellular eukaryotes. Annu Rev Genet. 2005;39:503–36. doi: 10.1146/annurev.genet.38.072902.093019. [DOI] [PubMed] [Google Scholar]
- 12.Chen H, Chan DC. Emerging functions of mammalian mitochondrial fusion and fission. Hum Mol Genet. 2005;14:R283–9. doi: 10.1093/hmg/ddi270. [DOI] [PubMed] [Google Scholar]
- 13.Eiberg H, Kjer B, Kjer P, Rosenberg T. Dominant optic atrophy (OPA1) mapped to chromosome 3q region. I. Linkage analysis. Hum Mol Genet. 1994;3:977–80. doi: 10.1093/hmg/3.6.977. [DOI] [PubMed] [Google Scholar]
- 14.Delettre C, Lenaers G, Belenguer P, Hamel CP. Gene structure and chromosomal localization of mouse Opa1: its exclusion from the Bst locus. BMC Genet. 2003;4:8. doi: 10.1186/1471-2156-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alexander C, Votruba M, Pesch UE, Thiselton DL, Mayer S, Moore A, Rodriguez M, Kellner U, Leo-Kottler B, Auburger G, Bhattacharya SS, Wissinger B. OPA1, encoding a dynamin-related GTPase, is mutated in autosomal dominant optic atrophy linked to chromosome 3q28. Nat Genet. 2000;26:211–5. doi: 10.1038/79944. [DOI] [PubMed] [Google Scholar]
- 16.Delettre C, Lenaers G, Griffoin JM, Gigarel N, Lorenzo C, Belenguer P, Pelloquin L, Grosgeorge J, Turc-Carel C, Perret E, Astarie-Dequeker C, Lasquellec L, Arnaud B, Ducommun B, Kaplan J, Hamel CP. Nuclear gene OPA1, encoding a mitochondrial dynamin-related protein, is mutated in dominant optic atrophy. Nat Genet. 2000;26:207–10. doi: 10.1038/79936. [DOI] [PubMed] [Google Scholar]
- 17.Pesch UE, Fries JE, Bette S, Kalbacher H, Wissinger B, Alexander C, Kohler K. OPA1, the disease gene for autosomal dominant optic atrophy, is specifically expressed in ganglion cells and intrinsic neurons of the retina. Invest Ophthalmol Vis Sci. 2004;45:4217–25. doi: 10.1167/iovs.03-1261. [DOI] [PubMed] [Google Scholar]
- 18.Ju WK, Misaka T, Kushnareva Y, Nakagomi S, Agarwal N, Kubo Y, Lipton SA, Bossy-Wetzel E. OPA1 expression in the normal rat retina and optic nerve. J Comp Neurol. 2005;488:1–10. doi: 10.1002/cne.20586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang AG, Fann MJ, Yu HY, Yen MY. OPA1 expression in the human retina and optic nerve. Exp Eye Res. 2006;83:1171–8. doi: 10.1016/j.exer.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 20.Olichon A, Baricault L, Gas N, Guillou E, Valette A, Belenguer P, Lenaers G. Loss of OPA1 perturbates the mitochondrial inner membrane structure and integrity, leading to cytochrome C release and apoptosis. J Biol Chem. 2003;278:7743–6. doi: 10.1074/jbc.C200677200. [DOI] [PubMed] [Google Scholar]
- 21.Lee YJ, Jeong SY, Karbowski M, Smith CL, Youle RJ. Roles of the mammalian mitochondrial fission and fusion mediators Fis1, Drp1, and Opa1 in apoptosis. Mol Biol Cell. 2004;15:5001–11. doi: 10.1091/mbc.E04-04-0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arnoult D, Grodet A, Lee YJ, Estaquier J, Blackstone C. Release of OPA1 during apoptosis participates in the rapid and complete release of cytochrome C and subsequent mitochondrial fragmentation. J Biol Chem. 2005;280:35742–50. doi: 10.1074/jbc.M505970200. [DOI] [PubMed] [Google Scholar]
- 23.Kamei S, Chen-Kuo-Chang M, Cazevieille C, Lenaers G, Olichon A, Belenguer P, Roussignol G, Renard N, Eybalin M, Michelin A, Delettre C, Brabet P, Hamel CP. Expression of the Opa1 mitochondrial protein in retinal ganglion cells: its downregulation causes aggregation of the mitochondrial network. Invest Ophthalmol Vis Sci. 2005;46:4288–94. doi: 10.1167/iovs.03-1407. [DOI] [PubMed] [Google Scholar]
- 24.van der Bliek AM, Koehler CM. A mitochondrial rhomboid protease. Dev Cell. 2003;4:769–70. doi: 10.1016/s1534-5807(03)00167-9. [DOI] [PubMed] [Google Scholar]
- 25.Cipolat S, Rudka T, Hartmann D, Costa V, Serneels L, Craessaerts K, Metzger K, Frezza C, Annaert W, D'Adamio L, Derks C, Dejaegere T, Pellegrini L, D'Hooge R, Scorrano L, De Strooper B. Mitochondrial rhomboid PARL regulates cytochrome C release during apoptosis via OPA1-dependent cristae remodeling. Cell. 2006;126:163–75. doi: 10.1016/j.cell.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 26.Frezza C, Cipolat S, Martins de Brito O, Micaroni M, Beznoussenko GV, Rudka T, Bartoli D, Polishuck RS, Danial NN, De Strooper B, Scorrano L. OPA1 controls apoptotic cristae remodeling independently from mitochondrial fusion. Cell. 2006;126:177–89. doi: 10.1016/j.cell.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 27.Griparic L, Kanazawa T, van der Bliek AM. Regulation of the mitochondrial dynamin-like protein Opa1 by proteolytic cleavage. J Cell Biol. 2007;178:757–64. doi: 10.1083/jcb.200704112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gottlieb E. OPA1 and PARL keep a lid on apoptosis. Cell. 2006;126:27–9. doi: 10.1016/j.cell.2006.06.030. [DOI] [PubMed] [Google Scholar]
- 29.Duvezin-Caubet S, Jagasia R, Wagener J, Hofmann S, Trifunovic A, Hansson A, Chomyn A, Bauer MF, Attardi G, Larsson NG, Neupert W, Reichert AS. Proteolytic processing of OPA1 links mitochondrial dysfunction to alterations in mitochondrial morphology. J Biol Chem. 2006;281:37972–9. doi: 10.1074/jbc.M606059200. [DOI] [PubMed] [Google Scholar]
- 30.Ishihara N, Fujita Y, Oka T, Mihara K. Regulation of mitochondrial morphology through proteolytic cleavage of OPA1. EMBO J. 2006;25:2966–77. doi: 10.1038/sj.emboj.7601184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olichon A, Elachouri G, Baricault L, Delettre C, Belenguer P, Lenaers G. OPA1 alternate splicing uncouples an evolutionary conserved function in mitochondrial fusion from a vertebrate restricted function in apoptosis. Cell Death Differ. 2007;14:682–92. doi: 10.1038/sj.cdd.4402048. [DOI] [PubMed] [Google Scholar]
- 32.Brandstätter JH, Hartveit E, Sassoè-Pognetto M, Wässle H. Expression of NMDA and high-affinity kainate receptor subunit mRNAs in the adult rat retina. Eur J Neurosci. 1994;6:1100–12. doi: 10.1111/j.1460-9568.1994.tb00607.x. [DOI] [PubMed] [Google Scholar]
- 33.Hartveit E, Veruki ML. AII amacrine cells express functional NMDA receptors. Neuroreport. 1997;8:1219–23. doi: 10.1097/00001756-199703240-00032. [DOI] [PubMed] [Google Scholar]
- 34.Fletcher EL, Hack I, Brandstätter JH, Wässle H. Synaptic localization of NMDA receptor subunits in the rat retina. J Comp Neurol. 2000;420:98–112. [PubMed] [Google Scholar]
- 35.Araki CM, Hamassaki-Britto DE. Calretinin co-localizes with the NMDA receptor subunit NR1 in cholinergic amacrine cells of the rat retina. Brain Res. 2000;869:220–4. doi: 10.1016/s0006-8993(00)02364-7. [DOI] [PubMed] [Google Scholar]
- 36.Gründer T, Kohler K, Kaletta A, Guenther E. The distribution and developmental regulation of NMDA receptor subunit proteins in the outer and inner retina of the rat. J Neurobiol. 2000;44:333–42. [PubMed] [Google Scholar]
- 37.Gründer T, Kohler K, Guenther E. Alterations in NMDA receptor expression during retinal degeneration in the RCS rat. Vis Neurosci. 2001;18:781–7. doi: 10.1017/s0952523801185111. [DOI] [PubMed] [Google Scholar]
- 38.Ullian EM, Barkis WB, Chen S, Diamond JS, Barres BA. Invulnerability of retinal ganglion cells to NMDA excitotoxicity. Mol Cell Neurosci. 2004;26:544–57. doi: 10.1016/j.mcn.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 39.Lombardi G, Moroni F, Moroni F. Glutamate receptor antagonists protect against ischemia-induced retinal damage. Eur J Pharmacol. 1994;271:489–95. doi: 10.1016/0014-2999(94)90810-9. [DOI] [PubMed] [Google Scholar]
- 40.Adachi K, Kashii S, Masai H, Ueda M, Morizane C, Kaneda K, Kume T, Akaike A, Honda Y. Mechanism of the pathogenesis of glutamate neurotoxicity in retinal ischemia. Graefes Arch Clin Exp Ophthalmol. 1998;236:766–74. doi: 10.1007/s004170050156. [DOI] [PubMed] [Google Scholar]
- 41.Joo CK. Necrosis and apoptosis after retinal ischemia: involvement of NMDA-mediated excitotoxicity and p53. Invest Ophthalmol Vis Sci. 1999;40:713–20. [PubMed] [Google Scholar]
- 42.Goto W, Ota T, Morikawa N, Otori Y, Hara H, Kawazu K, Miyawaki N, Tano Y. Protective effects of timolol against the neuronal damage induced by glutamate and ischemia in the rat retina. Brain Res 2002 58:10–9. [DOI] [PubMed] [Google Scholar]
- 43.Osborne NN, Wood JP, Cupido A, Melena J, Chidlow G. Topical flunarizine reduces IOP and protects the retina against ischemia-excitotoxicity. Invest Ophthalmol Vis Sci. 2002;43:1456–64. [PubMed] [Google Scholar]
- 44.Alavi MV, Bette S, Schimpf S, Schuettauf F, Schraermeyer U, Wehrl HF, Ruttiger L, Beck SC, Tonagel F, Pichler BJ, Knipper M, Peters T, Laufs J, Wissinger B. A splice site mutation in the murine Opa1 gene features pathology of autosomal dominant optic atrophy. Brain. 2007;130:1029–42. doi: 10.1093/brain/awm005. [DOI] [PubMed] [Google Scholar]
- 45.Davies VJ, Hollins AJ, Piechota MJ, Yip W, Davies JR, White KE, Nicols PP, Boulton ME, Votruba M. Opa1 deficiency in a mouse model of autosomal dominant optic atrophy impairs mitochondrial morphology, optic nerve structure and visual function. Hum Mol Genet. 2007;16:1307–18. doi: 10.1093/hmg/ddm079. [DOI] [PubMed] [Google Scholar]
- 46.Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB, Korsmeyer SJ. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–30. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Antonsson B, Conti F, Ciavatta A, Montessuit S, Lewis S, Martinou I, Bernasconi L, Bernard A, Mermod JJ, Mazzei G, Maundrell K, Gambale F, Sadoul R, Martinou JC. Inhibition of Bax channel-forming activity by Bcl-2. Science. 1997;277:370–2. doi: 10.1126/science.277.5324.370. [DOI] [PubMed] [Google Scholar]
- 48.Gross A, Yin XM, Yamamoto K, Saito M, Waksman G, Korsmeyer SJ. Comparison of the ion channel characteristics of proapoptotic BAX and antiapoptotic BCL-2. Proc Natl Acad Sci USA. 1997;94:11357–62. doi: 10.1073/pnas.94.21.11357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Desagher S, Martinou JC. Mitochondria as the central control point of apoptosis. Trends Cell Biol. 2000;10:369–77. doi: 10.1016/s0962-8924(00)01803-1. [DOI] [PubMed] [Google Scholar]