Abstract
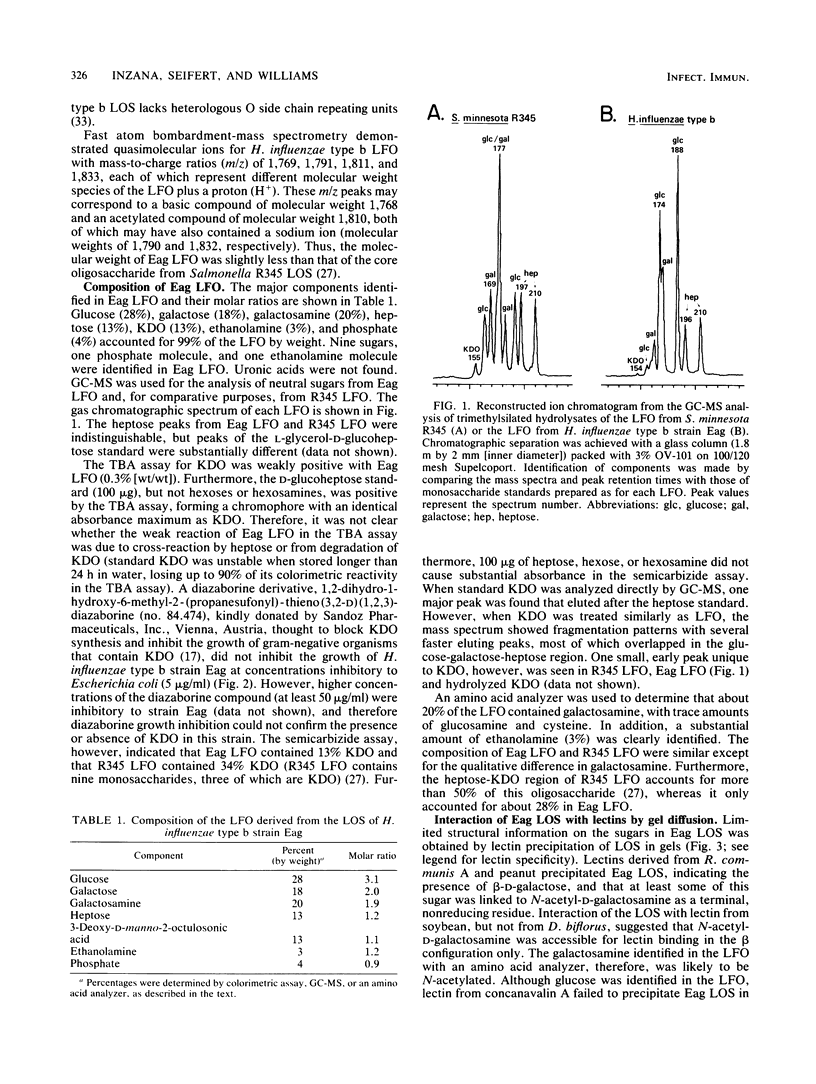
The oligosaccharide moiety of the lipooligosaccharide of Haemophilus influenzae type b strain Eag was isolated from the lipid component by mild acid hydrolysis and purified by gel filtration. Fast atom bombardment-mass spectrometry indicated that the lipid-free oligosaccharide had a basic molecular weight of 1,768; polysaccharides comparable to high-molecular-weight O side chains were not found. Glucose, galactose, galactosamine, heptose, 3-deoxy-D-manno-2-octulosonic acid (KDO), ethanolamine, and phosphate were identified in the lipid-free oligosaccharide by colorimetric assays, gas chromatography-mass spectrometry, or an amino acid analyzer. The presence of KDO was not clearly established by a thiobarbituric acid assay or by growth inhibition by a diazaborine derivative thought to block KDO synthesis. However, the semicarbizide assay and gas chromatography-mass spectrometry confirmed the presence of KDO. Lectin precipitation by Eag lipooligosaccharide in gels indicated that beta-D-galactose was present and that some of this monosaccharide was a terminal, nonreducing residue linked to N-acetyl-D-galactosamine. The lipid-free oligosaccharide was antigenic and completely inhibited lipooligosaccharide antibody (predominantly immunoglobulin G [IgG] and IgM) in an enzyme-linked immunosorbent assay, whereas the solubilized lipid A moiety did not. H. influenzae type b lipid-free oligosaccharide differed from core oligosaccharide of Salmonella lipooligosaccharide by the presence of galactosamine and a smaller percentage of heptose and KDO.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahamed N. M., Radziejewska-Lebrecht J., Widemann C., Mayer H. Reactivity of isolated lipopolysaccharides of enterobacterial R mutants with complete or incomplete core structures with lectins. Zentralbl Bakteriol A. 1980;247(4):468–482. [PubMed] [Google Scholar]
- Allen P. Z., Connelly M. C., Apicella M. A. Interaction of lectins with Neisseria gonorrhoeae. Can J Microbiol. 1980 Apr;26(4):468–474. doi: 10.1139/m80-078. [DOI] [PubMed] [Google Scholar]
- Anderson P., Flesher A., Shaw S., Harding A. L., Smith D. H. Phenotypic and genetic variation in the susceptibility of Haemophilus influenzae type b to antibodies to somatic antigens. J Clin Invest. 1980 Apr;65(4):885–891. doi: 10.1172/JCI109741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson P., Johnston R. B., Jr, Smith D. H. Human serum activities against Hemophilus influenzae, type b. J Clin Invest. 1972 Jan;51(1):31–38. doi: 10.1172/JCI106793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banoub J. H., Shaw D. H., Michon F. Hydrolytic release, and identification by g.l.c.-m.s., of 3-deoxy-D-manno-2-octulosonic acid in the lipopolysaccharides isolated from bacteria of the Vibrionaceae. Carbohydr Res. 1983 Nov 11;123(1):117–122. doi: 10.1016/0008-6215(83)88386-4. [DOI] [PubMed] [Google Scholar]
- Bennett G. M., Seaver A., Calcott P. H. Effect of defined lipopolysaccharide core defects on resistance of Salmonella typhimurium to freezing and thawing and other stresses. Appl Environ Microbiol. 1981 Nov;42(5):843–849. doi: 10.1128/aem.42.5.843-849.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornson A. B., Bjornson H. S. Activation of complement by opportunist pathogens and chemotypes of Salmonella minnesota. Infect Immun. 1977 Jun;16(3):748–753. doi: 10.1128/iai.16.3.748-753.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costerton J. W., Ingram J. M., Cheng K. J. Structure and function of the cell envelope of gram-negative bacteria. Bacteriol Rev. 1974 Mar;38(1):87–110. doi: 10.1128/br.38.1.87-110.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flesher A. R., Insel R. A. Characterization of lipopolysaccharide of Haemophilus influenzae. J Infect Dis. 1978 Dec;138(6):719–730. doi: 10.1093/infdis/138.6.719. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. Preparation and properties of antisera against the lipid-A component of bacterial lipopolysaccharides. Eur J Biochem. 1971 Dec 22;24(1):116–122. doi: 10.1111/j.1432-1033.1971.tb19661.x. [DOI] [PubMed] [Google Scholar]
- Goldstein I. J., Hayes C. E. The lectins: carbohydrate-binding proteins of plants and animals. Adv Carbohydr Chem Biochem. 1978;35:127–340. doi: 10.1016/s0065-2318(08)60220-6. [DOI] [PubMed] [Google Scholar]
- Högenauer G., Woisetschläger M. A diazaborine derivative inhibits lipopolysaccharide biosynthesis. Nature. 1981 Oct 22;293(5834):662–664. doi: 10.1038/293662a0. [DOI] [PubMed] [Google Scholar]
- Insel R. A., Anderson P., Loeb M. R., Smith D. H. A polysaccharide-protein complex from Haemophilus influenzae type b. II. Human antibodies to its somatic components. J Infect Dis. 1981 Dec;144(6):521–529. doi: 10.1093/infdis/144.6.521. [DOI] [PubMed] [Google Scholar]
- Inzana T. J. Electrophoretic heterogeneity and interstrain variation of the lipopolysaccharide of Haemophilus influenzae. J Infect Dis. 1983 Sep;148(3):492–499. doi: 10.1093/infdis/148.3.492. [DOI] [PubMed] [Google Scholar]
- Jantzen E., Bryn K., Bovre K. Cellular monosaccharide patterns of Neisseriaceae. Acta Pathol Microbiol Scand B. 1976 Aug;84(4):177–188. doi: 10.1111/j.1699-0463.1976.tb01923.x. [DOI] [PubMed] [Google Scholar]
- Johns M. A., Bruins S. C., McCabe W. R. Immunization with R mutants of Salmonella minnesota. II. Serological response to lipid A and the lipopolysaccharide of Re mutants. Infect Immun. 1977 Jul;17(1):9–15. doi: 10.1128/iai.17.1.9-15.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. G., Perry M. B. Improved techniques for the preparation of bacterial lipopolysaccharides. Can J Microbiol. 1976 Jan;22(1):29–34. doi: 10.1139/m76-004. [DOI] [PubMed] [Google Scholar]
- Karkhanis Y. D., Zeltner J. Y., Jackson J. J., Carlo D. J. A new and improved microassay to determine 2-keto-3-deoxyoctonate in lipopolysaccharide of Gram-negative bacteria. Anal Biochem. 1978 Apr;85(2):595–601. doi: 10.1016/0003-2697(78)90260-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MACGEE J., DOUDOROFF M. A new phosphorylated intermediate in glucose oxidation. J Biol Chem. 1954 Oct;210(2):617–626. [PubMed] [Google Scholar]
- Marks M. I., Ziegler E. J., Douglas H., Corbeil L. B., Braude A. I. Induction of immunity against lethal Haemophilus influenzae type b infection by Escherichia coli core lipopolysaccharide. J Clin Invest. 1982 Apr;69(4):742–749. doi: 10.1172/JCI110512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan W. T., Elson L. A. A colorimetric method for the determination of N-acetylglucosamine and N-acetylchrondrosamine. Biochem J. 1934;28(3):988–995. doi: 10.1042/bj0280988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. C., Kline L. F. Activation of the classical and properdin pathways of complement by bacterial lipopolysaccharides (LPS). J Immunol. 1977 Jan;118(1):362–368. [PubMed] [Google Scholar]
- Munson R. S., Jr, Shenep J. L., Barenkamp S. J., Granoff D. M. Purification and comparison of outer membrane protein P2 from Haemophilus influenzae type b isolates. J Clin Invest. 1983 Aug;72(2):677–684. doi: 10.1172/JCI111017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Seitz E., Jann B., Jann K. Degradation studies on the lipopolysaccharide from E. coli 071:K?:H12. Separation and investigation of O-specific and core polysaccharides. FEBS Lett. 1968 Oct;1(5):311–314. doi: 10.1016/0014-5793(68)80141-3. [DOI] [PubMed] [Google Scholar]
- O'Reilly R. J., Anderson P., Ingram D. L., Peter G., Smith D. H. Circulating polyribophosphate in Hemophilus influenzae, type b meningitis. Correlation with clinical course and antibody response. J Clin Invest. 1975 Oct;56(4):1012–1022. doi: 10.1172/JCI108148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr T. R., Jr, Bryan L. E. Lipopolysaccharide composition of three strains of Haemophilus influenzae. Can J Microbiol. 1984 Sep;30(9):1184–1187. doi: 10.1139/m84-185. [DOI] [PubMed] [Google Scholar]
- Raichvarg D., Brossard C., Agneray J. Chemical composition and biological activities of a phenol-water extract from Haemophilus influenzae type a. Infect Immun. 1979 Nov;26(2):415–421. doi: 10.1128/iai.26.2.415-421.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenep J. L., Munson R. S., Jr, Barenkamp S. J., Granoff D. M. Further studies of the role of noncapsular antibody in protection against experimental Haemophilus influenzae type b bacteremia. Infect Immun. 1983 Oct;42(1):257–263. doi: 10.1128/iai.42.1.257-263.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenep J. L., Munson R. S., Jr, Granoff D. M. Human antibody responses to lipopolysaccharide after meningitis due to Haemophilus influenzae type b. J Infect Dis. 1982 Feb;145(2):181–190. doi: 10.1093/infdis/145.2.181. [DOI] [PubMed] [Google Scholar]
- Strain S. M., Fesik S. W., Armitage I. M. Characterization of lipopolysaccharide from a heptoseless mutant of Escherichia coli by carbon 13 nuclear magnetic resonance. J Biol Chem. 1983 Mar 10;258(5):2906–2910. [PubMed] [Google Scholar]
- Strittmatter W., Weckesser J., Salimath P. V., Galanos C. Nontoxic lipopolysaccharide from Rhodopseudomonas sphaeroides ATCC 17023. J Bacteriol. 1983 Jul;155(1):153–158. doi: 10.1128/jb.155.1.153-158.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaki S., Sato T., Matsuhashi M. Role of lipopolysaccharides in antibiotic resistance and bacteriophage adsorption of Escherichia coli K-12. J Bacteriol. 1971 Mar;105(3):968–975. doi: 10.1128/jb.105.3.968-975.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright B. G., Rebers P. A. Procedure for determining heptose and hexose in lipopolysaccharides. Modification of the cysteine-sulfuric acid method. Anal Biochem. 1972 Oct;49(2):307–319. doi: 10.1016/0003-2697(72)90433-2. [DOI] [PubMed] [Google Scholar]
- Ziegler E. J., McCutchan J. A., Fierer J., Glauser M. P., Sadoff J. C., Douglas H., Braude A. I. Treatment of gram-negative bacteremia and shock with human antiserum to a mutant Escherichia coli. N Engl J Med. 1982 Nov 11;307(20):1225–1230. doi: 10.1056/NEJM198211113072001. [DOI] [PubMed] [Google Scholar]
- Zoon K. C., Scocca J. J. Constitution of the cell envelope of Haemophilus influenzae in relation to competence for genetic transformation. J Bacteriol. 1975 Aug;123(2):666–677. doi: 10.1128/jb.123.2.666-677.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwahlen A., Winkelstein J. A., Moxon E. R. Surface determinants of Haemophilus influenzae pathogenicity: comparative virulence of capsular transformants in normal and complement-depleted rats. J Infect Dis. 1983 Sep;148(3):385–394. doi: 10.1093/infdis/148.3.385. [DOI] [PubMed] [Google Scholar]



