Abstract
Opioid growth factor (OGF) is an endogenous opioid peptide ([Met5]enkephalin) that interacts with the OGF receptor (OGFr) and serves as a tonically active negative growth factor in cell proliferation of normal cells. To clarify the mechanism by which OGF inhibits cell replication in normal cells, we investigated the effect of the OGF–OGFr axis on cell cycle activity in human umbilical vein endothelial cells (HUVECs) and human epidermal keratinocytes (NHEKs). OGF markedly depressed cell proliferation of both cell lines by up to 40% of sterile water controls. Peptide treatment induced cyclin-dependent kinase inhibitor (CKI) p16INK4a protein expression and p21WAF1/CIP1 protein expression in HUVECs and NHEKs, but had no effect on p15, p18, p19, or p27 protein expression in either cell type. Inhibition of either p16INK4a or p21WAF1/CIP1 activation by specific siRNAs blocked OGF inhibitory action. Human dermal fibroblasts and mesenchymal stem cells also showed a similar dependence of OGF action on p16INK4a and p21WAF1/CIP1. Collectively, these results indicate that both p16INK4a and p21WAF1/CIP1 are required for the OGF–OGFr axis to inhibit cell proliferation in normal cells.
INTRODUCTION
The opioid growth factor (OGF), also known as [Met5]enkephalin, is an endogenous opioid peptide that is an important regulator of growth (Zagon et al., 2002). OGF is a constitutively expressed native opioid that is autocrine produced and secreted to inhibit the growth of both normal cells (Zagon and McLaughlin, 1987, 1991; Hauser et al., 1990; Stiene-Martin and Hauser, 1990; Hauser and Stiene-Martin, 1991; Isayama et al., 1991; Villiger and Lotz, 1992; Zagon et al., 1994, 1995a,b, 1996a, 1997a,b, 1998, 1999b, 2000b; McLaughlin, 1996; Vertes et al., 1996; McLaughlin and Wu, 1998; Blebea et al., 2000; Wilson et al., 2000; Kornyei et al., 2003; Robertson and Andrew, 2003; Malendowicz et al., 2005) and cancer cells (Zagon et al., 1996c; Cheng et al., 2007, 2008). The action of OGF is tonic, stereospecific, reversible, noncytotoxic, and nonapoptotic inducing, is not associated with differentiative, migratory, invasive, or adhesive processes, is independent of serum, anchorage-independent and occurs at physiologically relevant concentrations (Zagon et al., 2002, 2007a; Zagon and McLaughlin, 2003, 2004, 2005).
The action of OGF is mediated by interaction with the OGF receptor (OGFr). The gene for human OGFr is at least 9 kb in length, consists of seven exons and six introns, and encodes a 677-amino acid protein that includes 7 imperfect repeats of 20 amino acids each and at least one bipartite nuclear localization signal (Zagon et al., 2002). OGFr has an apparent mass of 62 kDa. The chromosomal location of the human OGFr is 20q13.3 (Zagon et al., 2002). Although OGFr has characteristics of a classical opioid receptor (recognizes opioids, naloxone reversibility, stereospecificity), there is no homology of OGFr with classical opioid receptors at the nucleotide or amino acid levels (Zagon et al., 2002). Antisense experiments with OGFr and continuous blockade of opioid receptors by the potent opioid antagonist naltrexone (NTX) support that the OGF–OGFr axis is a tonically active inhibitory system targeted to cell replication and homeostasis and is ligand-dependent for function (Zagon et al., 2002). Immunoelectron and confocal microscopy have shown that OGFr is localized to the outer nuclear envelope, nucleus, and perinuclear cytoplasm (Zagon et al., 2003, 2005).
The action of the OGF–OGFr axis in normal and cancer cells is targeted to DNA synthesis (Zagon and McLaughlin, 1987, 1991; Isayama et al., 1991; Zagon et al., 1994, 1995b, 2000a; McLaughlin, 1996; McLaughlin and Wu, 1998; McLaughlin et al., 1999; Wilson et al., 2000; Blebea et al., 2002). In squamous cell carcinoma of the head and neck, OGF activity has been shown to be dependent on one CKI, p16INK4a (Cheng et al., 2007), whereas in pancreatic cancer, which often has a mutation/deletion of p16INK4a, another CKI, p21WAF1/CIP1, is the target of OGF with respect to modulating the cell cycle (Cheng et al., 2008). Because OGF depresses DNA synthesis and subsequent cell/tissue growth in a wide variety of normal and developing cells in humans and animals, including ectodermal, mesodermal, and endodermal derivatives (Hauser et al., 1990; Hauser and Stiene-Martin, 1991; Isayama et al., 1991; Zagon and McLaughlin, 1991; Zagon et al., 1994, 1995b, 1996a,b, 1997, 1999b; McLaughlin, 1996; Vertes et al., 1996; McLaughlin and Wu, 1998; Blebea et al., 2000, 2002; Wilson et al., 2000; Kornyei et al., 2003), the question arises as to the mechanism of peptide action on the cell cycle in these cells. The present investigation examined the specific target(s) in the cell cycle for the OGF–OGFr axis in cells derived from four normal human tissues: umbilical vein endothelial cells (HUVECs), epidermal keratinocytes (NHEKs), dermal fibroblasts (NHDFs), and mesenchymal stem cells (hMSCs). In contrast to the cancer cells we have examined, our data reveal that the OGF's inhibitory action on cell proliferation in normal human cells is dependent on both the p16INK4a and p21WAF1/CIP1 signaling pathways.
MATERIALS AND METHODS
Cell Culture and OGF Treatment
HUVECs, NHEKs, and NHDFs were obtained from Clonetics (San Diego, CA). hMSCs were a gift from Dr. H. Donahue (Penn State University). Media for HUVECs, NHEKs, and NHDFs was purchased from Clonetics. HUVECs were grown in endothelial cell growth medium as follows: basal medium supplemented with 2% fetal bovine serum (FBS), 4 μl/ml bovine brain extract (BBE), 1 μl/ml hydrocortisone, 1 μl/ml human endothelial growth factor (hEGF), and 1 μl/ml gentamicin-amphotericin mix (GA-1000). NHEKs were grown in keratinocyte growth medium: basal medium with 4 μl/ml bovine pituitary extract (BPE), 1 μl/ml hEGF, 1 μl/ml insulin, 1 μl/ml hydrocortisone, and 1 μl/ml GA-1000. NHDFs were grown in fibroblast cell medium: basal medium supplemented with 1 μl/ml human fibroblast growth factor, 1 μl/ml insulin, 2% FBS, and 1 μl/ml GA-1000. hMSCs were grown in low glucose DMEM (GIBCO, Carlsbad, CA), supplemented with 10% FBS, and 5000 U/ml penicillin, 5 μg/ml streptomycin, and 10 μg/ml neomycin. All cells were cultured at 37°C in a 5% CO2/95% air environment.
OGF and NTX were purchased from Sigma-Aldrich (St. Louis, MO), dissolved in sterile water, and used at a final concentration of 10−6 M.
Cell Growth and Flow Cytometry
For growth curves, cells were seeded in six-well plates at an initial density of ∼2 × 105 cells/well. All cultures were treated in duplicate. Fresh media and OGF were added 24 h after initial seeding, and media and OGF were replaced daily. At appropriate times, the cells were washed with PBS, trypsinized with 0.25% trypsin-EDTA (Mediatech, Herndon, VA), and viable cell numbers were counted by trypan blue exclusion using a hemacytometer.
For flow cytometry, cells were treated with 10−6 M OGF for the indicated hours; this dosage of OGF was selected because it significantly inhibits cell proliferation of human cancer cell lines (McLaughlin et al., 1999) and does not induce apoptosis or necrosis (Zagon and McLaughlin, 2003), differentiation (Zagon and McLaughlin, 2005), or migration, invasion, adhesion, or chemotaxis (Zagon et al., 2007a). Cells were harvested and fixed with 70% ethanol at −20°C for up to 7 d before DNA analysis. DNA content was obtained by incubating cells in PBS containing propidium iodide (0.1 mg/ml) and RNase A (0.02 mg/ml) for 15 min at 22°C. Fluorescence was measured and analyzed using a Becton-Dickinson Biosciences FACScan flow cytometer (San Diego, CA) and Modfit software (Verity Software House, Topsham, ME).
Small Interfering RNA Knockdown of OGFr
The OGFr-targeted small interfering RNAs (siRNAs; antisense: 5′-uagaaacucagguuuggcg-3′; sense: 5′-cgccaaaccugaguuucua-3′) were designed and obtained as ready-annealed, purified duplex probes from Ambion (Austin, TX). Before experimentation with siRNAs, transfection efficiency was determined by treating cells with a commercially available fluorescein-labeled negative control siRNA (Ambion). Cells were transfected for 24 h at a final concentration of 20 nM and counterstained with Hoechst stain for 5 min. Transfection efficiency was calculated as the percentage of fluorescently labeled cells. Transfection efficiencies ranged from 63 to 75%.
For transfection with OGFr-siRNA, 2 × 105 cells per well were seeded in six-well plates with 1 ml of serum-containing media without antibiotics. In each well, 20 nM OGFr-siRNA or control siRNA (Ambion) solutions in serum-free media were added. Cells were incubated for 4 h at 37°C before the addition of OGF. Cultures were incubated an additional 20 h, and then 1 ml fresh complete media either lacking or containing OGF was added. At 72 h cells were collected for computing growth. Two independent experiments were conducted.
Western Blot Analysis
Cells (∼2 × 106) from each treatment were solubilized in 200 μl RIPA buffer (1× PBS, 10 μM IGEPAL, and 1 mg/ml SDS), containing a cocktail of protease inhibitors. Total protein concentrations were measured using the DC protein assay kit (Bio-Rad Laboratories, Hercules, CA). Equal amounts of protein (40 μg) were subjected to 10% SDS-PAGE followed by transfer of proteins on to polyvinylidene difluoride (Millipore, Billerica, MA) using standard protocols. The following antibodies were utilized: p15, p16INK4a, p18, and p19 (Santa Cruz Biotechnology, Santa Cruz, CA); p21WAF1/CIP1 and p27 (BD PharMingen, San Diego, CA); β-actin (Clone AC-15, Sigma-Aldrich). Membranes were probed with secondary anti-rabbit or anti-mouse horseradish peroxidase–conjugated antibodies (GE Healthcare-Amersham Biosciences, Piscataway, NJ), and developed using a chemiluminescence Western blotting detection system.
To determine equal loading of total protein samples, blots were reprobed with mAb against β-actin at a dilution of 1:2000. If necessary, membranes were processed in stripping buffer (62.5 mM Tris-HCl and 100 mM β-mercaptoethanol/2% SDS, pH 6.7) at 50°C before being reprobed.
Quantitation of Western Blots
To quantify expression levels, the optical density of each band was determined by densitometry and analyzed by QuickOne (Bio-Rad Laboratories). Each value was normalized to β-actin from the same blot. To report the changes due to OGF treatment, we calculated the fold increase at each time point by dividing the normalized value from the OGF-treated or sterile water–treated samples by the normalized value of 0-h control samples. Means and SE were determined from three or more independent experiments.
SiRNA Knockdown of p16INK4 and p21WAF1/CIP1
The p16INK4a-targeted siRNAs (antisense: 5′-acaccgcttctgccttttctt-3′; sense: 5′-gaaaaggcagaagcggtgttt-3′) were obtained as ready-annealed, purified duplex probes (Invitrogen, Carlsbad, CA). The p21WAF1/CIP1-targeted siRNAs were obtained from Santa Cruz Biotechnology, and negative control siRNAs were purchased from Ambion. For transfection, 2 × 105 cells per well were seeded in six-well plates containing 1 ml of serum-free medium without antibiotics. In each well, 20 nM of p16INK4a-siRNA, p21WAF1/CIP1-siRNA, both p16INK4a- and p21WAF1/CIP1-siRNA, or control siRNA solutions in serum-free media were added. Cells were incubated for 4 h at 37°C before the addition of OGF. Twenty hours later, 1 ml of fresh complete media with or without OGF was added to the cultures; media and OGF were replaced daily. At the indicated times, cells were collected for growth curves. Three independent experiments were conducted.
Statistical Analysis
Values were assessed by one-way analysis of variance (ANOVA) and Newman Keul's post-multiple comparison tests.
RESULTS
OGF–OGFr Axis Inhibits Cell Proliferation and Retards Progression through the Cell Cycle
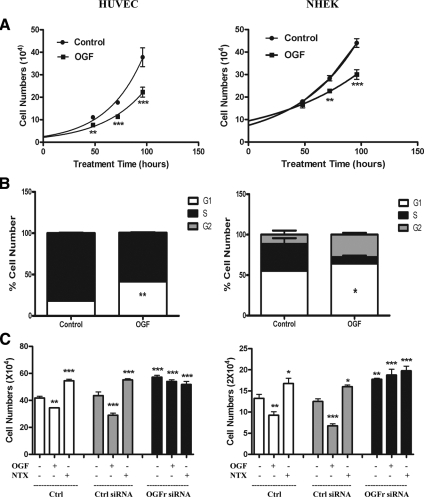
Continuous exposure to exogenous OGF inhibited the growth of HUVECs and NHEKs (Figure 1A). The number of OGF-treated HUVECs was 65 and 60% of control levels at 72 and 96 h, respectively. Cell number in the OGF-treated wells of NHEKs was 79 and 68% of control values at 72 and 96 h, respectively.
Figure 1.
The OGF–OGFr axis inhibits HUVECs and NHEKs (A–C) growth by arresting cells in G1. (A) HUVECs or NHEKs were grown in the presence of 10−6 M OGF and counted at 48, 72, and 96 h of treatment. Both cell types treated with OGF had fewer cells than control cultures receiving sterile water. Significantly different from controls treated with sterile water at ** p < 0.01 or *** p < 0.001. (B) Flow cytometry of HUVECs subjected to sterile water (Control) or OGF for 15 h, or NHEKs treated with OGF for 12 h as determined by FACS analysis. Data are expressed as the percentage of cells in G1, S, and G2 phases. The percentage of cells in G1 in OGF-treated cultures was significantly elevated (* p < 0.05, ** p < 0.01) from that of control cells. (C) OGFr is required for OGF action on growth. HUVECs or NHEKs were transfected with OGFr siRNAs or control siRNAs for 72 h in the presence of 10−6 M OGF, 10−6 M NTX, or sterile water. Cells were harvested at 72 h and counted with a hemacytometer. Significantly different from controls treated with sterile water at *p < 0.05, ** p < 0.01, or *** p < 0.001. Data represent means ± SEM for two or three independent experiments.
Because of the growth inhibition by addition of OGF, the effect of this peptide on the cell cycle was examined by flow cytometry (Figure 1B). The percentage of OGF-treated cells in the G0/G1 phase in HUVECs after 15 h was 41% compared with 18% of the control cells (p < 0.01). The percentage of OGF-treated cells in the G0/G1 phase in these nonsynchronized NHEKs after 12 h was 64% compared with 55% of the control levels (p < 0.05).
To examine the specificity of OGFr, knockdown experiments with OGFr-siRNA were conducted with HUVECs (Figure 1C). Exposure to 10−6 M OGF depressed the growth of sterile water– and control siRNA–treated HUVECs by 17 and 33%, respectively, whereas 10−6 M NTX increased the number of sterile water– and control siRNA–exposed HUVECs by 31 and 27%, respectively. HUVECs subjected to OGFr-siRNA had ∼31% more cells than control siRNA–treated cultures, as well as sterile water–treated cultures. In contrast to cells expressing OGFr (i.e., control siRNA), exposure to 10−6 M OGF or NTX had no further effects on the OGFr-siRNA–treated cultures.
To assess the specificity of OGF action by OGFr mediation in NHEKs, knockdown experiments with OGFr-siRNA were conducted (Figure 1C). Exposure to 10−6 M OGF depressed the growth of sterile water– and control siRNA-treated NHEKs by 30 and 46%, respectively, whereas 10−6 M NTX increased the number of sterile water– and control siRNA–exposed NHEKs by 26 and 28%, respectively. NHEKs subjected to OGFr-siRNA had ∼42% more cells than control siRNA–treated cultures, as well as sterile water–treated cultures. In contrast to cells expressing OGFr (i.e., control siRNA), exposure to 10−6 M OGF or NTX had no further effects on the OGFr-siRNA–treated cultures.
CDK Inhibitors p16INK4a and p21WAF1/CIP1 Expression Are Up-Regulated by OGF
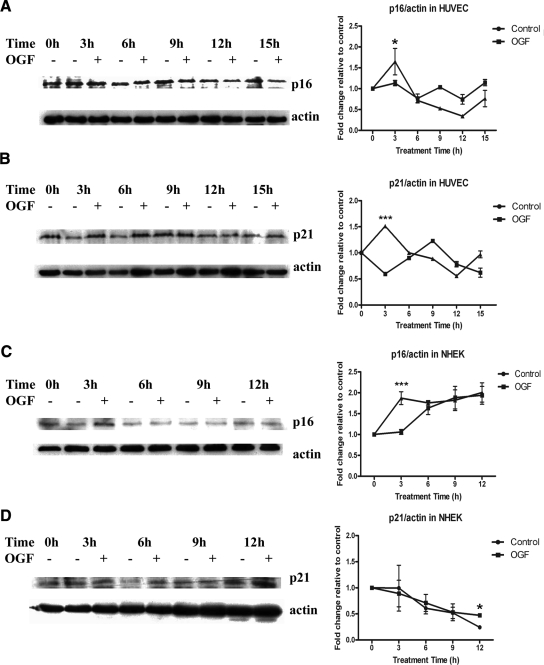
Cell cycle progression depends on both positive and negative regulators. Expression of p16INK4a and p21WAF1/CIP1 were evaluated in nonsynchronized HUVECs after 3, 6, 9, 12, and 15 h of OGF exposure. Both p16INK4a and p21WAF1/CIP1 were significantly (p < 0.05) up-regulated only at 3 h of treatment with OGF relative to control levels (Figures 2, A and B). Expression of p16INK4a and p21WAF1/CIP1 were evaluated in NHEKs after 3, 6, 9 and 12 h of OGF exposure. p16INK4a was significantly up-regulated (p < 0.05) in OGF-treated cells relative to control cultures only at 3 h (Figure 2C). p21WAF1/CIP1 was significantly up-regulated (p < 0.05) in OGF-treated cells relative to control levels only at 12 h (Figure 2D). Variation in p21WAF1/CIP1 up-regulation needs to be taken within the context of the doubling times. Analysis (nonlinear fit) of growth curves of treated and nontreated NHEKs and HUVECs revealed that OGF extended the doubling time from 24 to 28 h, whereas OGF-treated NHEKs extended the doubling time from 37 to 57 h. p16INK4a is known as a tumor suppressor gene, functioning as a cell cycle inhibitor by forming heterotrimeric complexes with cyclin-dependent kinases (Cdks) and cyclins. p21WAF1/CIP1 can also inhibit forming heterotrimeric complex with Cdks and cyclins. Therefore, these data suggest that under the effect of OGF, p16INK4a and p21WAF1/CIP1 protein levels were up-regulated and mediated the cell cycle block.
Figure 2.
OGF induced p16INK4a (A and C) and p21WAF1/CIP1 (B and D) expression in HUVECs and NHEKs. (A and C) HUVECs and NHEKs were treated with 10−6 M OGF or sterile water for 3, 6, 9, 12, or 15 h. Total proteins were resolved by SDS-PAGE, and blotted with p16INK4a-specific antibodies. Densitometric analysis of the Western blots was performed, and p16INK4a expression for OGF-treated cells is expressed relative to controls (0 h). The p16INK4a levels for HUVECs and NHEKs were significantly (* p < 0.05 and *** p < 0.001, respectively) elevated from the control group at 3 h. (B and D) HUVECs and NHEKs were treated with 10−6 M OGF or sterile water for 3, 6, 9, 12, or 15 h. Total proteins were resolved by SDS-PAGE and blotted with p21WAF1/CIP1-specific antibodies. Densitometric analysis of the Western blots was performed, and p21WAF1/CIP1 expression for OGF-treated cells is expressed relative to controls at 0 h. The p21WAF1/CIP1 level for HUVECs was significantly (*** p < 0.001) elevated from the control group at 3 h, whereas p21WAF1/CIP1 levels for NHEKs were significantly (p < 0.05) elevated from the control group at 12 h. Data represent means ± SEM for two or three independent experiments.
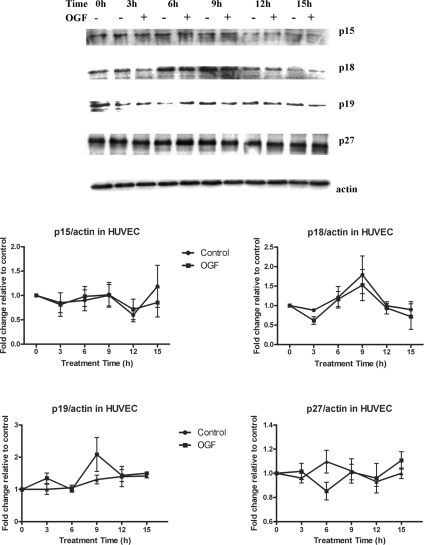
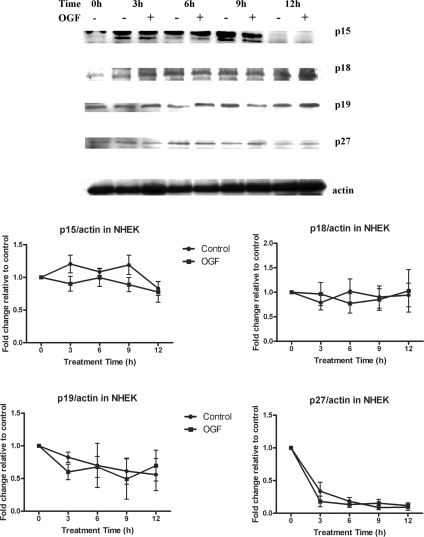
Expression of CDK Inhibitors p15, p18, p19, and p27 Are Not Altered by OGF
Analysis of the expression of cell cycle inhibitors p15, p18, p19, and p27 revealed that OGF treatment had no significant effect on these CKIs in both HUVECs (Figure 3) and NHEKs (Figure 4). Thus, OGF treatment results in the induction of only two CKIs in HUVECs and NHEKs: p16INK4a and p21WAF1/CIP1.
Figure 3.
OGF has no effect on CKIs p15, p18, p19, and p27 expression in HUVECs. HUVECs were treated with 10−6 M OGF for 3, 6, 9, 12, or 15 h. Total protein lysates were resolved by SDS-PAGE and subjected to antibodies specific to each CKI. Densitometric analysis revealed no significant differences between OGF and sterile water–treated values. Data represent means ± SEM for two or three independent experiments.
Figure 4.
OGF has no effect on CKIs p15, p18, p19, and p27 expression in NHEKs. NHEKs were treated with 10−6 M OGF for 3, 6, 9, or 12 h. Total protein lysates were resolved by SDS-PAGE and subjected to antibodies specific to each CKI. Western blots represent one experiment, whereas graphs represent the means of two to three experiments. Densitometric analysis revealed no significant differences between OGF- and sterile water–treated values. Data represent means ± SEM for two or three independent experiments.
siRNA Directed Against p16INK4a or p21WAF1/CIP1 Blocked OGF Inhibitory Action
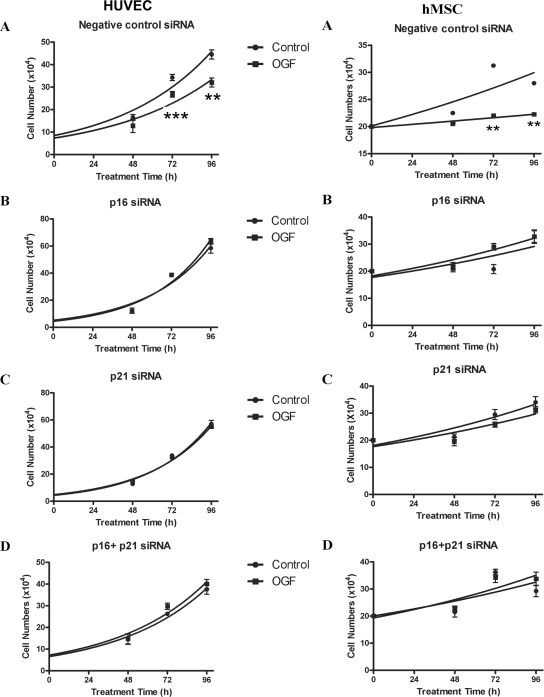
To test the role and specificity of p16INK4a and p21WAF1/CIP1 in OGF-induced inhibitory action on HUVEC and NHEK cell growth, siRNA knockdown experiments were utilized. HUVECs or NHEKs were treated with siRNAs for p16INK4a, p21WAF1/CIP1, both p16INK4a and p21WAF1/CIP1, or negative control. HUVECs transfected with negative control siRNA and exposed to OGF had reductions in growth of 35 and 40% at 72 h and 96 h, respectively (Figure 5). NHEKs transfected with negative control siRNA and treated with OGF had reductions in cell number of 30 and 34% at 72 and 96 h, respectively (data not shown). Growth analysis of HUVECs or NHEKs transfected with p16INK4a siRNA, p21WAF1/CIP1 siRNA, or both p16INK4a and p21WAF1/CIP1 siRNA and subsequently exposed to OGF for 96 h showed that OGF had no inhibitory effect on cell growth (Figure 5).
Figure 5.
p16INK4a and p21WAF1/CIP1 are required for OGF-induced growth inhibition of HUVECs and hMSCs. HUVECs and hMSCs were transfected with control siRNAs (A), p16INK4a siRNA (B), p21WAF1/CIP1 siRNA (C), or both p16INK4a siRNA and p21WAF1/CIP1 siRNA (D) and treated with sterile water (control) or 10−6 M OGF. Cells were harvested at 48, 72, and 96 h after treatment and counted with a hemacytometer. Data represent means ± SEM for three independent experiments. Significantly different from sterile water control cultures at ** p < 0.01 or *** p < 0.001.
The Ubiquity of p16INK4a and p21WAF1/CIP1 Regulation of OGF Inhibitory Action
To assess ubiquity of the role and specificity of p16INK4a and p21WAF1/CIP1 on OGF-induced inhibitory action, two other human normal cell lines (hMSCs and NHDFs) were studied using siRNA knockdown experiments. hMSCs and NHDFs were treated with siRNAs for p16INK4a, p21WAF1/CIP1, both p16INK4a and p21WAF1/CIP1, or negative control. hMSCs transfected with negative control siRNA and exposed to OGF had reductions in growth of 30 and 21% at 72 and 96 h, respectively (Figure 5). NHDFs transfected with negative control siRNA and treated with OGF had reductions in cell number of 17 and 16% at 72 and 96 h, respectively (data not shown). Growth analysis of hMSCs and NHDFs transfected with p16INK4a siRNA, p21WAF1/CIP1 siRNA, or both p16INK4a and p21WAF1/CIP1 siRNA and subsequently exposed to OGF for 96 h showed that OGF had no inhibitory effect on cell growth (Figure 5).
DISCUSSION
The OGF–OGFr axis has been documented by structural, pharmacological, and biochemical evidence to be present and to function as a regulatory system for growth in normal human and animal cells (Zagon et al., 2002). However, these previous studies do not directly demonstrate that OGF, which also recognizes other classical opioid receptors (Leslie et al., 1980), may elicit opioid agonist–sensitive antiproliferative signaling through these receptors instead of, or in addition, to OGFr. The logical extension of this query is that the effects of OGF on the cell cycle may be related to OGFr and/or other opioid receptors. Using OGFr knockdown experiments, the present report now shows that the specific and singular receptor for OGF action on the replication of HUVECs and NHEKs is OGFr. Cells with silenced OGFr are not altered in their growth properties by addition of OGF. Moreover, these cells with a knockdown of OGFr are not increased in cell proliferation by NTX, documenting that up-regulation of cell proliferation by this opioid antagonist is at the OGF–OGFr level of interfacing and that other opioid receptors are not involved with the effects of this general opioid receptor antagonist. These data are consistent with previous in vitro and in vivo studies reporting that treatment of normal cells with antisense RNA to OGFr (Zagon et al., 1999a, 2006) eliminates the inhibitory effects of OGF exposure. Thus, the elucidation of the target of OGF in this study is directly—and solely—related to OGFr.
This study shows for the first time that the target of the negative growth regulator, OGF, in normal human cells consists of both CKIs p16INK4a and p21WAF1/CIP1. Using HUVECs and NHEKs that exhibited growth inhibition after exposure to OGF, and flow cytometry observations documenting that OGF impeded cells exiting G1, we now conclude that peptide action targets key regulators of the G1-to-S phase transition. Confirmation that both p16INK4a and p21WAF1/CIP1 were indeed the target of OGF was validated in siRNA studies whereby HUVECs or NHEKs exposed to p16INK4a siRNA or p21WAF1/CIP1 siRNA exhibited no change in growth after exposure to OGF. Thus, our study makes the novel finding that OGF action is directed to CKIs p16INK4a and p21WAF1/CIP1 of the cell cycle in normal cells.
This investigation also showed that the requirement of p16INK4a and p21WAF1/CIP1 pathways for OGF action extends beyond the two cell lines, HUVECs and NHEKs, initially investigated. Two other human cell types, a dermal fibroblast and mesenchymal stem cell, also utilize p16INK4a and p21WAF1/CIP1 as discovered with siRNA technology. Therefore, a total of four different cell types: fibroblast, keratinocyte, endothelial, and mesenchymal, representing ectodermal and mesodermal derivatives, have a similar signaling pathway for OGF action. Given this diversity of cells and although further study is required, p16INK4a and p21WAF1/CIP1 may serve as a common denominator of OGF's inhibition of cell proliferation in all normal human and animal cells.
p16INK4a and p21WAF1/CIP1 have been shown to be both necessary and sufficient to inhibit cyclin/Cdk activity, and play critical roles in the negative control of cell growth (Sherr and Roberts, 1999). Our results with OGF and normal human cells are consistent in finding that up-regulating p16INK4a and p21WAF1/CIP1 expression has a negative effect on growth. It is important to note that both p16INK4a and p21WAF1/CIP1 are required for OGF's inhibitory activity in these normal cells and that attenuation of either p16INK4a or p21WAF1/CIP1 is not compensated by the other CKIs. Although p16INK4a and p21WAF1/CIP1 also have been found to be utilized by cancer cells in regard to peptide activity, in neoplastic cells the up-regulation of either p16INK4a or p21WAF1/CIP1 was found to be sufficient for OGF action on the cell cycle (Cheng et al., 2007, 2008). Why both CKIs are required in normal cells but only one CKI is sufficient in cancer cells is unclear. In the case of normal cells that are dependent on both p16INK4a and p21WAF1/CIP1 for OGF action, it may be conjectured that the hypophosphorylation of retinoblastoma protein necessitates Ser807/811 by Cdk4-cyclin D, and Thr821 by Cdk2, and that only one CKI is not sufficient to prevent phosphorylation of Rb. Alternatively, p16INK4a and p21WAF1/CIP1 are known to interact in some situations (Han et al., 2005) and that the loss of either p16INK4a or p21WAF1/CIP1 in the knockdown experiments with normal cells disturbed this signaling pathway leading to hypophosphorylation of pRb and repression of transcription of E2F that would lead to cell cycle arrest in G1. In cancer cells, deficiencies in CKIs are well known (Gartel et al., 1996; Sherr, 2004), particularly p16INK4a and p21WAF1/CIP1, and therefore we would postulate that cellular pathways regulating the cell cycle related to OGF action have adapted by having a dependency on only one CKI.
In conclusion, our results support the notion that both p16INK4a and p21WAF1/CIP1 act as suppressors to mediate the growth inhibitory function of the OGF–OGFr axis in normal cells. The clinical ramifications of our findings merit further discussion. For example, drugs used to attenuate p16INK4a or p21WAF1/CIP1 and to decrease OGF–OGFr interfacing would have the net effect of increasing cell proliferation and accelerating processes dependent on cell production (e.g., wound healing). Indeed, topical and systemic application of the opioid antagonist naltrexone stimulates wound repair of ocular surface epithelium in normal and diabetic rats (Zagon et al., 2007b). Increasing both p16INK4a and p21WAF1/CIP1 would be predicted to have an additive effect in concert with the OGF–OGFr axis to slow down cell proliferation. This could have an impact in situations wherein retardation in the generation of cell number would be desirable. For example, in cases of hyperplasia such as endometrial hyperplasia or benign prostatic hyperplasia, cell proliferation would be decreased by increasing p16INK4a and p21WAF1/CIP1 in concert with activating the OGF–OGFr system.
Abbreviations used:
- CKI
cyclin-dependent kinase inhibitor
- NTX
naltrexone hydrochloride
- OGF
opioid growth factor
- OGFr
opioid growth factor receptor
- Rb
retinoblastoma
- siRNA
small interfering RNA.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E08-07-0681) on October 15, 2008.
REFERENCES
- Blebea J., Mazo J. E., Kihara T. K., Vu J. H., McLaughlin P. J., Atnip R. G., Zagon I. S. Opioid growth factor modulates angiogenesis. J. Vasc. Surg. 2000;32:364–373. doi: 10.1067/mva.2000.107763b. [DOI] [PubMed] [Google Scholar]
- Blebea J., Vu J. H., Assadnia S., McLaughlin P. J., Atnip R. G., Zagon I. S. Differential effects of vascular growth factors on arterial and venous angiogenesis. J. Vasc. Surg. 2002;35:532–538. doi: 10.1067/mva.2002.120042. [DOI] [PubMed] [Google Scholar]
- Cheng F., McLaughlin P. J., Verderame M. F., Zagon I. S. The OGF–OGFr axis utilizes the p21 pathway to restrict progression of human pancreatic cancer. Mol. Cancer. 2008;7:5. doi: 10.1186/1476-4598-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng F., Zagon I. S., Verderame M. F., McLaughlin P. J. The opioid growth factor (OGF)–OGF receptor axis uses the p16 pathway to inhibit head and neck cancer. Cancer Res. 2007;67:10511–10518. doi: 10.1158/0008-5472.CAN-07-1922. [DOI] [PubMed] [Google Scholar]
- Gartel A. L., Serfas M. S., Tyner A. L. p21—negative regulator of the cell cycle. Proc. Soc. Exp. Biol. Med. 1996;213:138–149. doi: 10.3181/00379727-213-44046. [DOI] [PubMed] [Google Scholar]
- Han J., Tsukada Y., Hara E., Kitamura N., Tanaka T. Hepatocyte growth factor induces redistribution of p21(CIP1) and p27(KIP1) through ERK-dependent p16(INK4a) up-regulation, leading to cell cycle arrest at G1 in HepG2 hepatoma cells. J. Biol. Chem. 2005;280:31548–31556. doi: 10.1074/jbc.M503431200. [DOI] [PubMed] [Google Scholar]
- Hauser K. F., Osborne J. G., Stiene-Martin A., Melner M. H. Cellular localization of proenkephalin mRNA and enkephalin peptide products in cultured astrocytes. Brain Res. 1990;522:347–353. doi: 10.1016/0006-8993(90)91482-v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser K. F., Stiene-Martin A. Characterization of opioid-dependent glial development in dissociated and organotypic cultures of mouse CNS: critical periods and target specificity. Dev. Brain Res. 1991;62:245–255. doi: 10.1016/0165-3806(91)90172-f. [DOI] [PubMed] [Google Scholar]
- Isayama T., McLaughlin P. J., Zagon I. S. Endogenous opioids regulate cell proliferation in the retina of developing rat. Brain Res. 1991;544:79–85. doi: 10.1016/0006-8993(91)90887-2. [DOI] [PubMed] [Google Scholar]
- Kornyei J. L., Vertes Z., Kovacs K. A., Gocze P. M., Vertes M. Developmental changes in the inhibition of cultured rat uterine cell proliferation by opioid peptides. Cell Prolif. 2003;36:151–163. doi: 10.1046/j.1365-2184.2003.00263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie F. M., Chavkin C., Cox B. M. Opioid binding properties of brain and peripheral tissues: evidence for heterogeneity in opioid ligand binding sites. J. Pharmacol. Exp. Ther. 1980;214:395–402. [PubMed] [Google Scholar]
- Malendowicz L. K., Rebuffat P., Tortorella C., Nussdorfer G. G., Ziolkowska A., Hochol A. Effects of met-enkephalin on cell proliferation in different models of adrenocortical-cell growth. Int. J. Mol. Med. 2005;15:841–845. [PubMed] [Google Scholar]
- McLaughlin P. J. Regulation of DNA synthesis of myocardial and epicardial cells in developing rat heart by [Met5]enkephalin. Am. J. Physiol. 1996;271:R122–R129. doi: 10.1152/ajpregu.1996.271.1.R122. [DOI] [PubMed] [Google Scholar]
- McLaughlin P. J., Levin R. J., Zagon I. S. Regulation of human head and neck squamous cell carcinoma growth in tissue culture by opioid growth factor. Int. J. Oncol. 1999;14:991–998. doi: 10.3892/ijo.14.5.991. [DOI] [PubMed] [Google Scholar]
- McLaughlin P. J., Wu Y. Opioid gene expression in the developing and adult rat heart. Dev. Dyn. 1998;211:153–163. doi: 10.1002/(SICI)1097-0177(199802)211:2<153::AID-AJA4>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Robertson S. A., Andrew S. E. Presence of opioid growth factor and its receptor in the normal dog, cat and horse cornea. Vet. Ophthalmol. 2003;6:131–134. doi: 10.1046/j.1463-5224.2003.00279.x. [DOI] [PubMed] [Google Scholar]
- Sherr C. J. Principles of tumor suppression. Cell. 2004;116:235–246. doi: 10.1016/s0092-8674(03)01075-4. [DOI] [PubMed] [Google Scholar]
- Sherr C. J., Roberts J. M. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- Stiene-Martin A., Hauser K. F. Opioid-dependent growth of glial cultures: suppression of astrocyte DNA synthesis by met-enkephalin. Life Sci. 1990;46:91–98. doi: 10.1016/0024-3205(90)90041-o. [DOI] [PubMed] [Google Scholar]
- Vertes Z., Kornyei J. L., Kovacs S., Vertes M. Opioids regulate cell proliferation in the developing rat uterus: effects during the period of sexual maturation. J. Steroid Biochem. Mol. Biol. 1996;59:173–178. doi: 10.1016/s0960-0760(96)00101-x. [DOI] [PubMed] [Google Scholar]
- Villiger P. M., Lotz M. Expression of prepro-enkephalin in human articular chondrocytes is linked to cell proliferation. EMBO J. 1992;11:135–143. doi: 10.1002/j.1460-2075.1992.tb05036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R. P., McLaughlin P. J., Lang C. M., Zagon I. S. The opioid growth factor, [Met5]-enkephalin, inhibits DNA synthesis during recornification of mouse tail skin. Cell Prolif. 2000;33:63–73. doi: 10.1046/j.1365-2184.2000.00161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagon I. S., Hytrek S. D., McLaughlin P. J. Opioid growth factor tonically inhibits human colon cancer cell proliferation in tissue culture. Am. J. Physiol. 1996c;271:R511–R518. doi: 10.1152/ajpregu.1996.271.3.R511. [DOI] [PubMed] [Google Scholar]
- Zagon I. S., McLaughlin P. J. Endogenous opioid systems regulate cell proliferation in the developing rat brain. Brain Res. 1987;412:68–72. doi: 10.1016/0006-8993(87)91440-5. [DOI] [PubMed] [Google Scholar]
- Zagon I. S., McLaughlin P. J. Identification of opioid peptides regulating proliferation of neurons and glia in the developing nervous system. Brain Res. 1991;542:318–323. doi: 10.1016/0006-8993(91)91585-o. [DOI] [PubMed] [Google Scholar]
- Zagon I. S., McLaughlin P. J. Opioids and the apoptotic pathway in human cancer cells. Neuropeptides. 2003;37:79–88. doi: 10.1016/s0143-4179(03)00007-6. [DOI] [PubMed] [Google Scholar]
- Zagon I. S., McLaughlin P. J. Opioid growth factor (OGF) inhibits anchorage-independent growth in human cancer cells. Int. J. Oncol. 2004;24:1443–1448. [PubMed] [Google Scholar]
- Zagon I. S., McLaughlin P. J. Opioids and differentiation in human cancer cells. Neuropeptides. 2005;39:495–505. doi: 10.1016/j.npep.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Zagon I. S., Rahn K. A., McLaughlin P. J. Opioids and migration, chemotaxis, invasion, and adhesion of human cancer cells. Neuropeptides. 2007a;41:441–452. doi: 10.1016/j.npep.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Zagon I. S., Roesener C. D., Verderame M. F., Ohlsson-Wilhelm B. M., Levin R. J., McLaughlin P. J. Opioid growth factor regulates the cell cycle of human neoplasias. Int. J. Oncol. 2000a;17:1053–1061. doi: 10.3892/ijo.17.5.1053. [DOI] [PubMed] [Google Scholar]
- Zagon I. S., Ruth T. B., Leure-duPree A. E., Sassani J. W., McLaughlin P. J. Immunoelectron microscopic localization of the opioid growth factor receptor (OGFr) and OGF in the cornea. Brain Res. 2003;967:37–47. doi: 10.1016/s0006-8993(02)04172-0. [DOI] [PubMed] [Google Scholar]
- Zagon I. S., Ruth T. B., McLaughlin P. J. Nucleocytoplasmic distribution of opioid growth factor and its receptor in tongue epithelium. Anat. Rec. A. Discov. Mol. Cell Evol. Biol. 2005;282:24–37. doi: 10.1002/ar.a.20161. [DOI] [PubMed] [Google Scholar]
- Zagon I. S., Sassani J. W., Allison G., McLaughlin P. J. Conserved expression of the opioid growth factor, [Met5]enkephalin, and the zeta (zeta) opioid receptor in vertebrate cornea. Brain Res. 1995a;671:105–111. doi: 10.1016/0006-8993(94)01314-8. [DOI] [PubMed] [Google Scholar]
- Zagon I. S., Sassani J. W., Kane E. R., McLaughlin P. J. Homeostasis of ocular surface epithelium in the rat is regulated by opioid growth factor. Brain Res. 1997a;759:92–102. doi: 10.1016/s0006-8993(97)00238-2. [DOI] [PubMed] [Google Scholar]
- Zagon I. S., Sassani J. W., Malefyt K. J., McLaughlin P. J. Regulation of corneal repair by particle-mediated gene transfer of opioid growth factor receptor cDNA. Arch. Ophthalmol. 2006;124:1620–1624. doi: 10.1001/archopht.124.11.1620. [DOI] [PubMed] [Google Scholar]
- Zagon I. S., Sassani J. W., McLaughlin P. J. Opioid growth factor modulates corneal epithelial outgrowth in tissue culture. Am. J. Physiol. 1995b;268:R942–R950. doi: 10.1152/ajpregu.1995.268.4.R942. [DOI] [PubMed] [Google Scholar]
- Zagon I. S., Sassani J. W., McLaughlin P. J. Reepithelialization of the rabbit cornea is regulated by opioid growth factor. Brain Res. 1998;803:61–68. doi: 10.1016/s0006-8993(98)00610-6. [DOI] [PubMed] [Google Scholar]
- Zagon I. S., Sassani J. W., McLaughlin P. J. Reepithelialization of the human cornea is regulated by endogenous opioids. Invest. Ophthalmol. Vis. Sci. 2000b;41:73–81. [PubMed] [Google Scholar]
- Zagon I. S., Sassani J. W., Myers R. L., McLaughlin P. J. Naltrexone accelerates healing without compromise of adhesion complexes in normal and diabetic corneal epithelium. Brain Res. Bull. 2007b;72:18–24. doi: 10.1016/j.brainresbull.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Zagon I. S., Verderame M. F., Allen S. S., McLaughlin P. J. Cloning, sequencing, expression and function of a cDNA encoding a receptor for the opioid growth factor, [Met5]enkephalin. Brain Res. 1999a;849:147–154. doi: 10.1016/s0006-8993(99)02046-6. [DOI] [PubMed] [Google Scholar]
- Zagon I. S., Verderame M. F., McLaughlin P. J. The biology of the opioid growth factor receptor (OGFr) Brain Res. Brain Res. Rev. 2002;38:351–376. doi: 10.1016/s0165-0173(01)00160-6. [DOI] [PubMed] [Google Scholar]
- Zagon I. S., Wu Y., McLaughlin P. J. Opioid growth factor inhibits DNA synthesis in mouse tongue epithelium in a circadian rhythm-dependent manner. Am. J. Physiol. 1994;267:R645–R652. doi: 10.1152/ajpregu.1994.267.3.R645. [DOI] [PubMed] [Google Scholar]
- Zagon I. S., Wu Y., McLaughlin P. J. Opioid growth factor-dependent DNA synthesis in the neonatal rat aorta. Am. J. Physiol. 1996a;270:R22–R32. doi: 10.1152/ajpregu.1996.270.1.R22. [DOI] [PubMed] [Google Scholar]
- Zagon I. S., Wu Y., McLaughlin P. J. The opioid growth factor, [Met5]-enkephalin, and the zeta opioid receptor are present in human and mouse skin and tonically act to inhibit DNA synthesis in the epidermis. J. Invest. Dermatol. 1996b;106:490–497. doi: 10.1111/1523-1747.ep12343712. [DOI] [PubMed] [Google Scholar]
- Zagon I. S., Wu Y., McLaughlin P. J. Opioid growth factor is present in human and mouse gastrointestinal tract and inhibits DNA synthesis. Am. J. Physiol. 1997b;272:R1094–R1104. doi: 10.1152/ajpregu.1997.272.4.R1094. [DOI] [PubMed] [Google Scholar]
- Zagon I. S., Wu Y., McLaughlin P. J. Opioid growth factor and organ development in rat and human embryos. Brain Res. 1999b;839:313–322. doi: 10.1016/s0006-8993(99)01753-9. [DOI] [PubMed] [Google Scholar]