Abstract
Stress-induced shedding of motile cilia (autotomy) has been documented in diverse organisms and likely represents a conserved cellular reaction. However, little is known about whether primary cilia are shed from mammalian epithelial cells and what impact deciliation has on polarized cellular organization. We show that several chemically distinct agents trigger autotomy in epithelial cells. Surprisingly, deciliation is associated with a significant, but reversible increase in transepithelial resistance. This reflects substantial reductions in tight junction proteins associated with “leaky” nephron segments (e.g., claudin-2). At the same time, apical trafficking of gp80/clusterin and gp114/CEACAM becomes randomized, basal-lateral delivery of Na,K-ATPase is reduced, and expression of the nonciliary apical protein gp135/podocalyxin is greatly decreased. However, ciliogenesis-impaired MDCK cells do not undergo continual junction remodeling, and mature cilia are not required for autotomy-associated remodeling events. Deciliation and epithelial remodeling may be mechanistically linked processes, because RNAi-mediated reduction of Exocyst subunit Sec6 inhibits ciliary shedding and specifically blocks deciliation-associated down-regulation of claudin-2 and gp135. We propose that ciliary autotomy represents a signaling pathway that impacts the organization and function of polarized epithelial cells.
INTRODUCTION
Primary cilia mediate various chemo- and mechanosensory functions, and defects in ciliary assembly and signaling activities are linked to numerous human diseases, including cystic diseases of the kidney and liver (Marshall and Nonaka, 2006; Singla and Reiter, 2006). The emerging consensus is that primary cilia are required to maintain a differentiated cell state and that anything disrupting normal ciliary activities (e.g., mutations or environmental stress) upsets cellular homeostasis. However, molecular mechanisms linking aberrant ciliary function with renal cystogenesis are incompletely understood. For example, it is not known whether primary cilia transmit environmental signals to intercellular junctions and the secretory apparatus of renal epithelial cells to achieve optimal surface polarity and transport activities.
Cells may lose their primary cilia by either of two mechanisms: resorption or deciliation (Quarmby, 2004). Resorption is a normal process in which cells gradually retract the cilium. It usually precedes mitosis and, perhaps, reentry of quiescent cells into the cell cycle. It may involve regulating the efficiency of intraflagellar transport (IFT), the bidirectional process responsible for the growth and maintenance of ciliary length (Rosenbaum and Witman, 2002). Ciliary resorption may also be regulated by de-acetylation of axonemal microtubules (Pugacheva et al., 2007). In contrast, deciliation is a rapid shedding of cilia in response to environmental stress (Blum, 1971; Quarmby, 2004). This may include growth factors that stimulate quiescent cells to reenter the cell cycle (Tucker et al., 1979a,b). Also known as autotomy, this is a conserved cellular response that has been best studied in simple eukaryotes but has also been documented in numerous ciliated epithelia, including the oviduct, which undergoes deciliation in response to hormones and microbial infection, and the upper respiratory tract, which deciliates in response to smoke and infection (Quarmby, 2004). Nevertheless, very little is known about whether or how primary cilia are shed from epithelial cells and whether deciliation is solely a response to pathological conditions and environmental stress or represents a normal process that resting cells execute before returning to the cell cycle.
MATERIALS AND METHODS
Chemicals
All common chemicals used were of analytical grade or better and purchased from Sigma-Aldrich (St. Louis, MO) or Fisher Chemical (Fairlawn, NJ). Chloral hydrate, dibucaine, BAPTA-AM, and thapsigargin were purchased from Sigma-Aldrich. Calphostin C and ML-7 were obtained from Calbiochem (La Jolla, CA). [3H]inulin and 35S-EasyTag were purchased from Perkin Elmer-Cetus (Boston, MA). Recombinant human epidermal growth factor (EGF) was purchased from PeproTech (Rocky Hill, NJ). Human transferrin (Tfn) was labeled with 125I using Iodo-Gen reagent (Pierce Chemical, Rockford, IL) as described (Podbilewicz and Mellman, 1990).
Antibodies and Fluorescent Probes
Mouse monoclonal antibodies against acetylated α-tubulin (diluted 1:250 for immunofluorescent labeling) and β-tubulin (diluted 1:400 for immunofluorescent labeling and 1:1000 for Western blotting) were purchased from Sigma-Aldrich. Mouse monoclonal anti-gp114 (cl. Y652; hybridoma supernatant diluted 1:10 for immunofluorescent labeling and Western blotting) and anti-gp135 (cl. 3F2; ascites diluted 1:300 for immunofluorescent labeling and 1:500 for Western blotting) have been described previously (Balcarova-Stander et al., 1984; Ojakian and Schwimmer, 1988). Antibodies to occludin, ZO-1 and -2, and claudins-1, -2, -3, -4, -7, -8, and -10 were purchased from Zymed Laboratories, (South San Francisco, CA) and diluted 1:200 for immunofluorescent labeling and Western blotting. Polyclonal anti-ZO-3 antibodies were obtained from Chemicon (Temecula, CA) and diluted 1:250 for immunofluorescent labeling. Rabbit polyclonal antibodies to actin (diluted 1:500 for Western blotting) and desmoplakin I/II (diluted 1:500 for immunofluorescent labeling) were obtained from Abcam (Cambridge, MA). Rabbit polyclonal antibodies against the conserved cytoplasmic domain of mouse E-cadherin (E2; diluted 1:250 for immunofluorescent labeling and 1:500 for Western blotting) and the α subunit of the sodium, potassium ATPase (NaKα; diluted 1:500 for immunofluorescent labeling and 1:1000 for Western blotting) have been described previously (Marrs et al., 1993). All Texas Red– and FITC-conjugated affinity-purified and minimal cross-reacting goat or donkey anti-mouse and anti-rabbit secondary antibodies were purchased from Jackson Immunoresearch Laboratories (West Grove, PA) and used at 1:200 dilution. FITC-phalloidin was obtained from Sigma and used at a 1:100 dilution.
Cell Culture Methodology
Madin-Darby canine kidney (MDCK) strain I and strain II cells were maintained in low-glucose Dulbecco's modified Eagle's media (LG-DMEM) containing 1 g/l sodium bicarbonate and supplemented with 10% fetal bovine serum (FBS; Atlas Biologicals, Fort Collins, CO), penicillin, streptomycin, and gentamicin (PSG). In experiments examining EGF-induced TJ remodeling, cultures were rinsed thrice in serum-free LG-DMEM containing 0.5% BSA and then incubated in this medium for 24 h to render them quiescent. EGF (100 ng/ml) was then applied in starving medium to cells for 24 h. Pooled clones of MDCK II cells expressing ∼90% reduced levels of Sec6 (MDCK shSec6 cells) were generated by stable integration of a short hairpin RNA (shRNA) targeting canine Sec6 (sense: 5′-GCTGCTCAGATAAGTGAAGAT-3′), delivered via transduction with a recombinant lentiviral vector that was pseudotyped with vesicular stomatitis virus G protein. Cells were selected and maintained in media containing 5 μg/ml puromycin. As a negative control, MDCK II cells were transduced with lentiviral vectors encoding a nontargeting shRNA (sense: 5′-CCCAAGAATTGGAAGGAGAAA-3′) and selected in puromycin, as above. MDCK II sh14-3-3η cells were generously provided by Ben Margolis (University of Michigan Medical School; Fan et al., 2004). MDCK II shPolaris cells were generated by transfecting cells with pSuper plasmids encoding shRNA targeting canine Polaris/IFT88 (sense: 5′- GAGCTAGCAAATGATCTGG-3′) and selecting stable clones after selection in 500 μg/ml G418. MDCK cells stably expressing the human transferrin receptor in pCB6 (MDCKT) were previously described (Sheff et al., 1999). IMCD-3 cells were maintained in Ham's F12 medium supplemented with 10% FBS (Hyclone, Logan, UT), PSG, MEM-nonessential amino acids, and sodium pyruvate. LLC-PK1 cells were maintained in α-MEM medium supplemented with 10% FBS (Hyclone) and PSG. Caco-2 and human bronchial epithelial cells (HBECs) were maintained in high-glucose DMEM (HG-DMEM) supplemented with 10% FBS (Hyclone) and PSG.
Measurement of TER
Cultures of MDCK II, IMCD-3, LLC-PK1, and HBE cells were plated on Transwell 0.45-μm polycarbonate filters at confluence (8 × 105 cells/12-mm filter and 3.2 × 106 cells/24-mm filter) and cultured for a minimum of 4–5 d to polarize and grow primary cilia. Caco-2 cells were maintained on filters for 2–3 wk. Thereafter, triplicate filters of cells were cultured in the absence or presence of various agents, which were added to media in both apical and basal-lateral chambers, as indicated in figures. Two Transwell chambers were left blank to determine the intrinsic resistance of the membrane. A minimum of three independent transepithelial electrical resistance (TER) readings were collected for each filter at various time points after treatment using a Millicell electrical resistance device (Millipore, Bedford, MA). Final values were obtained by subtracting the averaged blank value from each averaged sample reading and then multiplying that value by the surface area of the filter. These final results are expressed as Ohms·cm2. The presence of functional tight junctions (TJs) was verified by measuring the rate of diffusion of [3H]inulin across the monolayer.
Measurement of [Ca2+]i
Cells were imaged and labeled in LG-DMEM. MDCKs were incubated for 30 min with 2 μM of the AM form of fura-2 at room temperature (22°C) and then washed three times in LG-DMEM. Cells were placed in a flow-through chamber mounted on the stage of an inverted IX-71 microscope (Olympus, Melville, NY) and washed for 2 min before the experiment. Fluorescence was alternately excited at 340 (12-nm bandpass) and 380 (12-nm bandpass) using the Polychrome IV monochromator (TILL Photonics, Martinsried, Germany), via a 20× objective (NA 0.75; Olympus). Emitted fluorescence was collected at 510 (80) nm using an IMAGO CCD camera (TILL Photonics). Pairs of 340/380-nm images were sampled at 0.2 Hz. Fluorescence was corrected for background, as determined in an area that did not contain cells. Data were processed using TILLvisION 4.0.1.2 (TILL Photonics) and Origin 7.0 (Microcal Software, Northhampton, MA) software.
Immunofluorescence Staining
Cells were fixed in 4% paraformaldehyde for 30 min and then permeabilized by incubation at 0°C for 10 min with 1% Triton X-100 in buffer containing 10 mM Pipes, pH 6.8, 50 mM NaCl, 300 mM sucrose, 3 mM MgCl2, and protease inhibitors (1 mM pefabloc and 10 μg/ml each of aprotinin, antipain, leupeptin, and pepstatin A; CSK buffer). Antibodies were diluted in blocking buffer (Ringer's saline: 154 mM NaCl, 1.8 mM Ca2+, 7.2 mM KCl, and 10 mM HEPES, pH 7.4) containing 0.2% fish skin gelatin and 100 mM NH4Cl) and applied to cells for 2 h at 4°C. After five washes in blocking buffer, FITC and Texas Red–conjugated secondary antibodies were applied for 1 h at 4°C. Coverslips and filters were washed five times and mounted in VectaShield containing DAPI (Vector Laboratories, Burlingame, CA) or in Elvanol-PPD. Samples were viewed with either a Nikon Microphot-FX microscope (Melville, NY; 63× or 100× objectives), an Olympus BX-51 microscope (40× or 60× objectives) or a Zeiss 510 laser scanning confocal microscope (Thornwood, NY; 63× objective) using krypton/argon laser with 488 nm (FITC) and 543 nm (Texas Red) laser lines, as noted in figure legends. Digital images of data collected from the Nikon Microphot-FX microscope were obtained with a Kodak DCS 760 digital camera (Eastman Kodak, Rochester, NY).
Assay of gp80 Secretion
For metabolic radiolabeling, polarized MDCK II cells on Transwell filters were preincubated in DMEM/FBS in the absence of methionine/cysteine for 60 min and then in the presence of medium containing 2 mCi/ml (25 μCi/filter) [35S]methionine/cysteine (Amersham Pharmacia, Piscataway, NJ) for 15 min. Cells were then rinsed three times in prewarmed DMEM/FBS containing 5× methionine/cysteine and then incubated in that medium for different time points, at which time an aliquot was removed from both the apical and basal-lateral compartments of triplicate filters to assess gp80 secretion. Protein samples were incubated in SDS sample buffer for 10 min at 65°C before separation by SDS-PAGE. gp80 is the predominant labeled secretory protein observed in MDCK II cells and under reducing conditions migrates as two bands at 35 and 45 kDa (Urban et al., 1987). Fixed and stained gels were prepared for fluorography by soaking in Amplify solution (Amersham) for 30 min, dried under vacuum, and exposed to phosphorimager screens (Molecular Dynamics, Sunnyvale, CA). The amount of labeled protein in both the 35- and 45-kDa bands was determined directly using a phosphorimager (Typhoon, Molecular Dynamics) and ImageQuant software (ver. 1.2, Molecular Dynamics).
Transferrin Recycling Assay
Polarized MDCK-T cells on Transwell filters were incubated in the absence or presence of chloral hydrate (CH) for 48 h, as indicated in the figure. Before experiments, cells were induced with 5 mM butyrate overnight and then placed in butyrate-free medium for 4 h before analysis. Binding and recycling of Tfn was performed as described in detail previously (Sheff et al., 2002). Briefly, 125I-labeled Tfn was selectively bound to the basal-lateral surface of the cells on ice for 45 min. After cells were washed on ice, the attached Tfn was chased into the cells with media containing 0.1 mg/ml unlabeled Tfn for up to 1 h. Internalization rates were derived from the clearance of acid-labile, labeled Tfn from the cell surface. Recycling and transcytosis data were determined from counts released into the media at various times.
Cell Surface Biotinylation
MDCK cells were biotinylated as previously described (Le Bivic et al., 1990). Briefly, cells cultured on 24-mm Transwell filters were rinsed three times with Ringer's saline. Sulfo-NHS-SS-Biotin (Pierce; 0.5 mg/ml in Ringer's saline) was applied to either apical or basal-lateral surfaces (0.67 ml apical/1.33 ml basal-lateral), and the cells were incubated twice for 20 min each at 0°C. The biotinylation reaction was quenched by washing cells in five changes of TBS (120 mM NaCl, 10 mM Tris, pH 7.4) containing 50 mM NH4Cl and 0.2% BSA (quenching buffer) at 4°C.
Cells were lysed for 30 min in 1 ml/filter CSK buffer. Lysates were centrifuged at 15,000 × g for 10 min, and supernatant fractions were transferred to clean tubes. 100 μl of lysate was removed and mixed with SDS-PAGE sample buffer for quantitation of total protein expression. The remaining lysate (900 μl) was combined with 50 μl streptavidin-agarose (Pierce), incubated for 2 h at 4°C on a tube rotator, and then washed under stringent conditions and prepared for SDS-PAGE as described previously (Pasdar and Nelson, 1988).
Gel Electrophoresis and Immunoblotting
Protein samples were incubated in SDS-PAGE sample buffer for 10 min at 65°C before separation in 7.5, 10, or 12.5% SDS polyacrylamide gels. Proteins were electrophoretically transferred from gels to Immobilon PVDF membrane (Millipore). Blots were blocked in Blotto (5% nonfat dry milk, 0.5% normal goat serum, and 0.1% sodium azide in TBS) overnight at 4°C. Primary antibodies were incubated with blots at room temperature for 1 h. After five washes, 10 min each, in TBS containing 0.1% Tween-20, the blots were incubated with 125I-labeled goat anti-mouse or goat anti-rabbit secondary antibody (Amersham) for 1 h at room temperature. Blots were washed as above, then twice in TBS and exposed to phosphorimager screens. The amount of labeled antibody bound to the blots was determined directly using a Phosphorimager, as described above.
RESULTS
Epithelial Cells Are Reversibly Deciliated by Diverse Agents
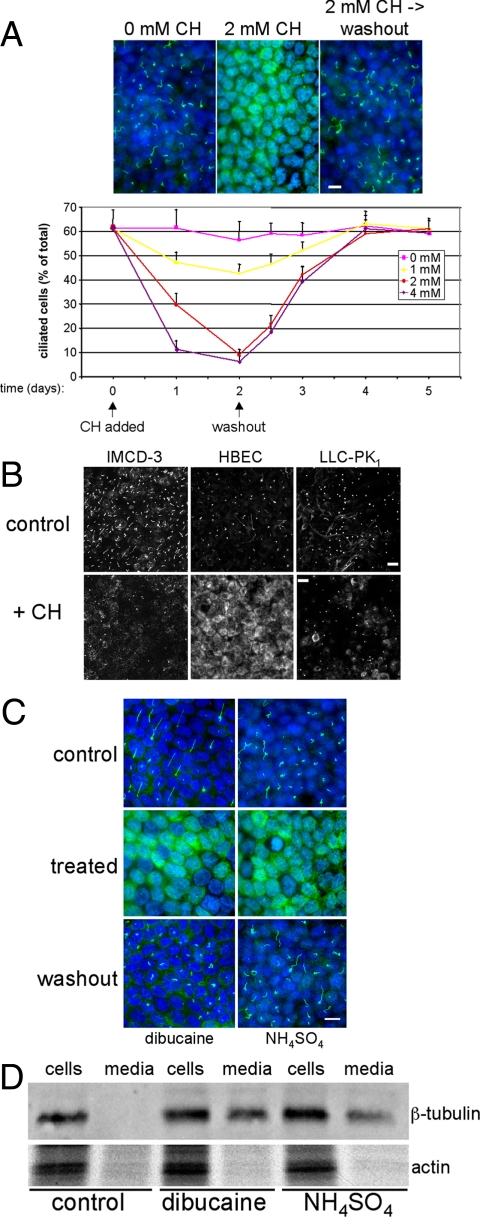
As an initial effort to determine the consequences of stress-induced deciliation on epithelial cell function, methods for reversibly removing primary cilia from polarized epithelial cells were developed. In previous studies, it was reported that treatment of polarized MDCK cells (MDCK II) with 4 mM CH for 3 d resulted in complete removal of primary cilia and that functional cilia reemerged 5 d after CH washout (Praetorius and Spring, 2003). This analysis has been refined and shows that treatment of cells with 4 mM CH for 24 h or 2 mM CH for 48 h is sufficient to achieve nearly complete deciliation without disrupting epithelial monolayers or causing gross morphological changes to cells (Figures 1A and Supplemental Figure S1A). Deciliation is reversible; primary cilia began to reemerge within 12 h of CH washout, and monolayers were ciliated to control levels by 24–48 h (Figure 1A).
Figure 1.
Deciliation of polarized epithelial cells. (A) MDCK II cells were cultured in the absence or presence of indicated concentrations of CH for the durations specified. To examine the reversibility of observed effects, CH was removed at the indicated time (“washout”), and cells were cultured for an additional 3 d. At various time points, cultures were fixed and labeled with anti-acetylated tubulin antibodies (to mark primary cilia) and DAPI (to label nuclei). Bar, 10 μM. The percentage of ciliated cells was quantified by dividing the total number of cilia by the number of nuclei counted in 10 randomly selected fields from duplicate filters. Error bars, SEM (B) Polarized cultures of the indicated cell lines were incubated in the absence (control) or presence (+ CH) of either 2 mM (IMCD-3 cells and HBECs) or 6 mM (LLC-PK1 cells) CH for 3 d and then fixed and labeled with anti-acetylated tubulin antibodies. Bars, 20 μM. (C) Polarized MDCK II cells were cultured in the absence (control) or presence (treated) of either 0.75 mM dibucaine for 30 min or 30 mM NH4SO4 for 3 h. For washout, cultures were incubated an additional 48 h in the absence of deciliating agents. (D) Polarized MDCK II cells were cultured as in C, but in serum-free culture medium. Cell extracts and culture media samples, normalized for total protein content, were resolved by SDS-PAGE, and Western blots were probed for β-tubulin and actin. Note that tubulin, but not actin, is present in the culture medium after treatment of cells with dibucaine or NH4SO4, indicating the presence of shed cilia in the media.
Mouse inner medullary collecting duct cells (IMCD-3) and HBECs were also induced to shed their cilia (Figure 1B). This indicates that ciliary autotomy is a feature of other epithelial cell types and is not a peculiarity of MDCK II cells. However, we note that porcine kidney epithelial (LLC-PK1) cells were not induced to deciliate by CH even at concentrations that were much higher than those that induced complete deciliation of MDCK II cells (Figure 1). Therefore, LLC-PK1 cells appear to lack component(s) required for stress-induced deciliation.
Two other chemicals, dibucaine and ammonium sulfate, also triggered deciliation, indicating that agents unrelated to CH, including potentially physiologically relevant metabolites, promote ciliary autotomy in kidney epithelial cells (Figures 1C and Supplemental Figure S2). In contrast to CH, which promoted ciliary shedding relatively slowly and asynchronously over a 24-h time course, dibucaine- and NH4-mediated autotomy occurred within 30 min or 3 h of treatment, respectively. However, dibucaine is more cytotoxic than CH. Consequently, there is a rather narrow concentration range (0.5–1.5 mM) and duration (30–60 min) in which dibucaine-induced deciliation can be studied before cell viability is compromised. Importantly, in all cases the mechanism of ciliary loss is shedding, and not resorption, because components of the severed cilia were recovered in the culture medium after stress-induced deciliation (Figure 1D).
After stress-induced deciliation, a striking increase in cytoplasmic staining of acetylated tubulin was observed, particularly in MDCK II and HBECs (Figure 1). This finding was unexpected, because axonemal tubulin was recovered in the culture medium after deciliation (Figure 1D). However, quantitative immunoblot analysis showed that amounts of acetylated tubulin, when normalized to total tubulin amounts, was unchanged in deciliated cells compared with control cultures (Supplemental Figure S1B). This result indicates that cells continue to acetylate α-tubulin molecules, even during stress-induced deciliation, but that this pool of acetylated tubulin remains cytosolic because there is no ciliary axoneme to incorporate it. It is possible that antibodies to acetylated tubulin have greater access to epitopes on the cytosolic pool than the axonemal pool. As a consequence, cytoplasmic staining of acetylated tubulin in deciliated cells appears much brighter than ciliary staining in control cells, even though the actual amount of protein is unchanged.
Deciliation Is Accompanied by Changes in Intercellular Junctions
Deciliation of MDCK II cells was accompanied by a reversible approximately fourfold increase in TER, indicating that TJ barrier function was better, not worse, after CH treatment of cells (Figure 2A). Transepithelial diffusion of inulin was similar in control and deciliated monolayers (Supplemental Figure S3A). Changes in TER were temporally correlated with deciliation and cilia regrowth. During the first 24 h of treatment, a dramatic rise in TER was observed as cilia were shed from cells (Figure 2A). TER reached a maximum when deciliation was complete and then returned to baseline during a 1–2-d period after CH washout, concurrent with cilial reemergence. The effects of CH on primary cilia and TJs required that cells were continuously exposed to the drug. Transient exposure to CH was not sufficient to induce either deciliation or TJ remodeling (Supplemental Figure S3B). An increase in TER was also observed in HBECs and IMCD-3 cells during CH-induced deciliation, indicating that the observed effect was not restricted to MDCK II cells (Figure 2B). However, changes in TER were not observed in LLC-PK1 cells, which did not shed their cilia, nor in Caco-2 cells, which lack primary cilia (Figure 2A). Finally, an increase in TER similar in magnitude to that after CH treatment was also observed when cells were deciliated by exposure to NH4, suggesting that changes in TJ function are not simply a side effect of CH treatment, but may instead be coupled to the deciliation event itself (Figure 2C).
Figure 2.
TJ remodeling accompanies deciliation. (A) Polarized cultures of MDCK II, LLC-PK1, and Caco-2 cells were incubated in the absence (control) or presence (+ CH) of 4 mM CH for indicated times, at which TER was measured. (B) Polarized cultures of HBECs were incubated in the absence or presence of 4 mM CH for indicated times, whereupon TER was measured. IMCD-3 cells were incubated without or with 2 mM CH for 3 d. (C) Polarized MDCK II cells were incubated in the absence or presence of 30 mM NH4SO4 for 24 h, followed by an additional 48 h in the absence of NH4SO4 for washout. Note that TER increase after NH4SO4-induced deciliation is of a similar magnitude as that after CH-induced deciliation (A). In all panels, n = 3 filters per condition; error bars, SEM.
The increase in TER after deciliation of MDCK II cells was accompanied by a striking reduction in expression of TJ components associated with “leaky” nephron segments (e.g., claudin-2; Figures 3, A and C, and 9B). In contrast, expression of claudins that are associated with “tight” nephron segments (e.g., claudins-3, -4, -7 and -8; Kiuchi-Saishin et al., 2002), were either slightly increased or not significantly altered (Figure 3, A and C). Interestingly, claudin-1 expression was also reduced after deciliation, although how this change contributes to altered TJ barrier function is not presently understood (Figure 3, A and C). After washout of CH, the repertoire of claudin expression returned to that of nontreated cells (Figure 3C). Expression and localization of other TJ components, including occludin and ZO-1, were not affected (Figure 3B). Intriguingly, both ZO-2 and -3 were expressed in deciliated MDCK II cells, but were to different extents redistributed from the plasma membrane to the cytoplasm (Figure 3B). Claudin-2 loss after deciliation of MDCK II cells was likely the major factor contributing to increased TER, because CH-triggered deciliation of MDCK strain I cells, which do not express claudin-2, had no effect on TER (Figure 3D; Furuse et al., 2001).
Figure 3.
TJ composition changes after epithelial deciliation. (A) Polarized MDCK II cells were treated without (control) or with (+ CH) 4 mM CH for 2 d and then fixed and labeled with antibodies to indicated claudin proteins. Confocal microscopic images were collected at identical settings for control and experimental samples. Note that intensity of staining of claudins-1 and -2 are decreased and that of claudin-4 is increased after deciliation. (B) Cultures, treated as in A were labeled with antibodies to indicated TJ proteins. Note that ZO-2 and -3 distributions appear to shift toward cytosolic distributions after deciliation. (C) Duplicate cultures, treated as in A or with indicated CH concentrations or treated and cultured an additional 2 d in the absence of CH (washout), were lysed and subjected to SDS-PAGE and immunoblotting with indicated anti-claudin antibodies. Note that expression of claudins-1 and -2 are significantly decreased in deciliated cultures, whereas that of other claudins is not substantially altered. (D) Polarized cultures of MDCK I cells were incubated in the presence of 4 mM CH for indicated times, at which TER was measured. n = 3 filters; error bars, SEM (E) Linearity of TJ segments from control or CH-treated cultures was measured using NIH ImageJ software. A straight line was defined as having a linearity = 1. n = 50 TJ segments per condition; error bars, SEM.
Figure 9.
Inhibition of ciliary shedding prevents epithelial remodeling. (A) Blocking exocyst function prevents stress-induced deciliation. Polarized MDCK II cells or MDCK II cells with ∼90% reduced levels of Sec6 (MDCK II shSec6; top right) were incubated in the absence or presence of either 2 mM CH for 2 d or 30 mM NH4SO4 for 24 h. Cultures were fixed and labeled with anti-acetylated tubulin antibodies and DAPI (left), and the fraction of ciliated cells in the population was determined as described in Figure 1 (bottom right). “Control MDCK II” cells were transduced with lentiviral vectors encoding a nontargeting shRNA and were selected in puromycin, as described in Materials and Methods. (B) In cultures in which ciliary shedding is inhibited, TJs do not undergo remodeling and gp135/podocalyxin expression is maintained. Cells, cultured in the absence or presence of either 2 mM CH for 2 d, 30 mM NH4SO4 for 24 h, or 100 ng/ml EGF for 24 h, were monitored for TER at the time of harvest (left). In addition, cells were lysed and lysate was subjected to SDS-PAGE and immunoblotting with antibodies to claudin-2 and β-tubulin (top right) or gp135 (bottom right). n = 3 filters; error bars, SEM.
We also note that TJs of deciliated cells had a subtly altered morphology. TJs of untreated cells typically had a wavy appearance, whereas those of deciliated cells had a more linear appearance (Figure 3, A and B). Quantitative image analysis confirmed that the TJ of deciliated cells were on average ∼21% straighter than those of control cells (Figure 3E). The straighter TJ of deciliated cells would be expected to have a lower conduction because of a reduced length, and this likely contributed to the increased TER observed. Together, these results showed that deciliation was associated with specific changes in the composition, appearance, and function of TJs.
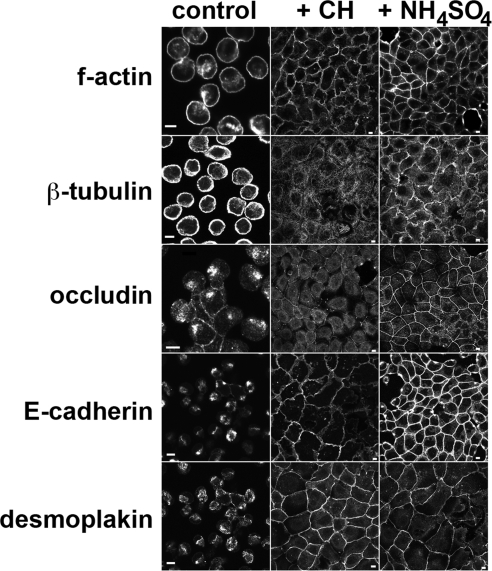
We also observed changes in adherens junctions and desmosomes in deciliated cells. The function of each junction type is regulated by dynamic turnover of components in endosomes (Burdett, 1993; Le et al., 1999; Morimoto et al., 2005), and removing calcium from the extracellular environment experimentally induces internalization of intercellular junctions. When MDCK II cells were incubated in calcium-free buffer, they detached from one another and internalized junction components (Figure 4). In contrast, cells that had been deciliated by exposure to either CH or NH4 retained these components at the plasma membrane, maintained organized actin and microtubule cytoskeletons, and remained tightly adherent to one another in calcium-free buffer (Figure 4). We infer from these data that stress-induced deciliation affects cellular mechanisms that regulate composition, trafficking, and function of intercellular junctions and cytoskeleton organization.
Figure 4.
Monolayer dissolution assay. Confluent cultures of MDCK II cells were incubated in the absence (control) or presence of 2 mM CH for 3 d or of 30 mM NH4SO4 for 24 h. Media was aspirated, and cultures were incubated in calcium/magnesium-free Hanks' buffered saline solution at 37°C for 30 min before being fixed and labeled with antibodies to indicated proteins or FITC-phalloidin to label f-actin. Note the absence of intercellular contacts and accumulation of junction proteins in internal compartments in control cultures, but the retention of junctions in deciliated cultures. Bars, 5 μm.
Deciliation Is Accompanied by Changes in Cell Surface Polarity
Trafficking and steady-state localizations of several endogenous apical (gp80/clusterin, gp114/CEACAM, gp135/podocalyxin) and basal-lateral (E-cadherin, Na,K-ATPase, transferrin receptor) proteins were examined after cilia removal, and a consistent and significant impact on the organization and effectiveness of post-Golgi sorting was observed. For example, gp80/clusterin, a protein that is normally secreted from the apical pole of MDCK II cells (Urban et al., 1987), became randomly secreted when cilia were removed (Figure 5A). Because deciliated cells have tighter junctions than control cells, the increased accumulation of gp80/clusterin in the basal-lateral medium likely reflects decreased apical sorting fidelity in the trans-Golgi network (TGN). This effect was reversible. After CH washout and cilia regrowth, gp80/clusterin was once again vectorially secreted into the apical medium (Figure 5A).
Figure 5.
Alteration of apical protein expression and sorting accompanies deciliation. (A) Polarized cultures of MDCK II cells were treated as described in Figure 3, and secretion of gp80/clusterin was measured as described in Materials and Methods. Note that apical secretion of this protein (control) becomes randomized after deciliation (+ CH), but then returns to normal after regrowth of cilia (washout). n = 4 filters per condition; error bars, SEM. (B) Left, polarized MDCK II cells were fixed and labeled with antibodies to gp114/CEACAM. En face (top) and orthogonal (bottom) images are shown. Bar, 5 μm. Right, triplicate cultures of polarized MDCK II cells were biotinylated apically or basolaterally. Biotinylated proteins were precipitated on streptavidin agarose, resolved by SDS-PAGE, and immunoblotted for gp114/CEACAM. Levels of protein recovered were quantified by phosphorimaging, and results are presented as % of total surface-labeled gp114/CEACAM. Note that apical polarity of gp114/CEACAM is lost in deciliated cultures. n = 3; error bars, SEM. (C) Left, polarized MDCK II cells were fixed and labeled with antibodies to gp135/podocalyxin. En face (top) and orthogonal (bottom) images are shown. Bar, 10 μm. Right, surface biotinylation assay was performed as in B. Note that expression level of gp135/podocalyxin is greatly decreased in deciliated cultures.
Similarly, the apical plasma membrane glycoprotein gp114/CEACAM (Balcarova-Stander et al., 1984; Fullekrug et al., 2006) was significantly missorted to basal-lateral membranes after cilia removal (Figure 5B). This protein is excluded from primary cilia of untreated cells and is normally expressed in a discrete region of the apical surface above TJs (Figure 5B). After deciliation, gp114/CEACAM became evenly distributed across both apical and basal-lateral surfaces. Most strikingly, gp135/podocalyxin (Ojakian and Schwimmer, 1988), another apical protein, but one that is not expressed in the ciliary membrane, was almost completely (>95%) absent in deciliated cells (Figure 5C). Because gp135/podocalyxin has been proposed to function in establishing apical-basal polarity (Meder et al., 2005) and in organizing subdomains within the apical plasma membrane (Takeda et al., 2001; Li et al., 2002), it is noteworthy that ciliary function appears to be required to maintain its expression in renal epithelial cells.
Although E-cadherin expression and localization were largely unaffected after deciliation (Figure 6A), Na,K-ATPase (α subunit) expression at the basal-lateral surface was dramatically reduced (Figure 6B). The α subunit was expressed at normal levels, as determined by immunoblotting, but a large fraction accumulated internally. The undelivered α subunit was sequestered in a perinuclear compartment in deciliated cells (Figure 6B). Collectively, these results suggested that deciliation was accompanied by major changes in either TGN/endosomal sorting fidelity or trafficking efficiency of cargo-laden transport vesicles to apical and basal-lateral membrane domains. Importantly, these effects appeared to be selective for secretory traffic, because endosomal transferrin receptor recycling was not significantly altered after deciliation (Supplemental Figure S4).
Figure 6.
Deciliation is accompanied by selective effects on basal-lateral trafficking. (A) Left, polarized MDCK II cells were fixed and labeled with antibodies to E-cadherin. En face (top) and orthogonal (bottom) images are shown. Bar, 5 μm. Right, surface biotinylation assay. Note that expression level and polarized distribution of E-cadherin is not substantially altered after deciliation. (B) Top, polarized MDCK II cells were fixed and labeled with antibodies to N, K-ATPase α subunit. (Bottom) Quadruplicate cultures of polarized MDCK II cells were incubated in the presence of indicated concentrations of CH for 3 d, and then duplicate cultures were biotinylated either apically or basolaterally. Labeled proteins were recovered by precipitation with streptavidin agarose, resolved by SDS-PAGE, and immunoblotted with anti-N,K-ATPase α antibodies. Note that this protein is inefficiently retained at the basal-lateral membrane in deciliated cultures and instead accumulates within perinuclear endosomes.
Calcium-dependent and -independent Pathways Trigger Deciliation and TJ Remodeling
Both CH and dibucaine induce deflagellation of lower eukaryotes by a mechanism that requires elevating intracellular free calcium concentration ([Ca2+]i; Quarmby and Hartzell, 1994; Quarmby, 2004). Although the cellular target of CH is not known, CH is a potent inhibitor of Ca2+/Mg2+-ATPases in vitro and increased [Ca2+]i up to 20-fold in PtK1 kangaroo rat kidney epithelial cells (Bergesse et al., 1983; Lee et al., 1987). Quantitative calcium imaging revealed a rapid approximately fivefold increase and a sustained elevation of about twofold in [Ca2+]i after treatment of MDCK II cells with CH (Figure 7A). When CH was applied to cells together with either BAPTA-AM or EGTA-AM, deciliation was largely prevented and TJ remodeling did not occur (Figure 7B), indicating that elevated [Ca2+]i was required for both events.
Figure 7.
Calcium signaling is involved in deciliation and TJ remodeling. (A) Ca2+ concentration was determined by digitized fluorescence microscopy of Fura-2–stained MDCK II cells incubated without or with 4 mM CH or NH4SO4 for indicated durations. n = 5 cells per condition; error bars, SEM. (B) Cells were preloaded with either 25 μM EGTA-AM or 25 μM BAPTA-AM before addition of 4 mM CH or 30 mM NH4SO4 or were left untreated (control). Twenty-four hours later, TER was measured and cultures were fixed and labeled with anti-acetylated tubulin antibodies. Note that preloading cells with calcium chelators largely prevented deciliation and blocked the associated rise in TER induced by CH, but had no effect on NH4SO4-induced deciliation and remodeling. Cilia were counted in five randomly selected fields per filter. n = 3 filters per condition; error bars, SEM. (C) Polarized MDCK II cells were cultured in HG-DMEM in the absence or presence of 1 μM thapsigargin for 24 h, at which time TER was measured and cultures were fixed and labeled with anti-acetylated tubulin antibodies. n = 3 filters per condition; error bars, SEM.
Thapsigargin treatment resulted in elevated [Ca2+]i, quantitatively similar to what has been previously reported (Lien et al., 1995) and that which we observed after CH treatment (not shown). This treatment promoted both deciliation and TJ remodeling, although the magnitude of these effects was somewhat less than that observed after CH treatment (Figure 7C). We infer from these results that CH-induced deciliation and TJ remodeling were dependent on elevated [Ca2+]i.
Pharmacological inhibition of two specific protein kinase families that are activated by calcium and are known to be involved in TJ remodeling (Balda et al., 1991; Stuart and Nigam, 1995; Turner et al., 1997) failed to block either deciliation or TJ remodeling. Calphostin C, a potent and specific inhibitor of conventional protein kinase C isoforms (IC50 = 50 nM) failed to inhibit both CH-induced deciliation and the associated rise in TER (Figure S5). Likewise, ML-7, which blocks calcium-activated myosin light-chain kinase activity (IC50 = 300 nM), also had no affect on CH-induced deciliation and TJ remodeling (Supplemental Figure S5).
In contrast to the calcium-dependent mechanism by which CH induced deciliation and TJ remodeling, NH4 triggered these processes by a mechanism that did not appear to require increased [Ca2+]i. Although a transient increase in [Ca2+]i was observed after treatment with NH4, this response was much smaller in amplitude than that which was observed in response to CH (Figure 7A). In addition, a sustained elevation in [Ca2+]i during NH4-induced deciliation was not observed. Moreover, BAPTA-AM treatment blocked the small transient increase in [Ca2+]i after NH4 treatment, but did not inhibit either deciliation or TJ remodeling (Figure 7B). These results show that stress-induced deciliation of renal epithelial cells may occur by either a Ca2+-dependent pathway (in the case of CH) or a Ca2+-independent pathway (in the case of NH4). Because TJ remodeling accompanied deciliation induced by either protocol, it is likely that deciliation caused the remodeling.
Deciliation and TJ Remodeling May Be Mechanistically Coupled Events
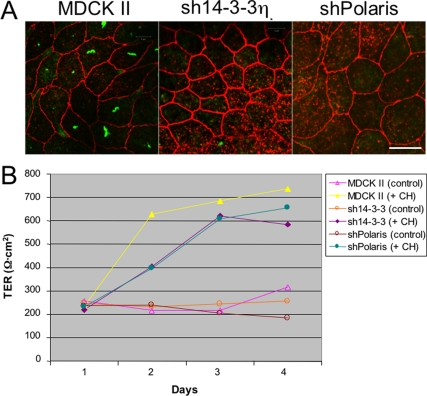
Considering that Caco-2 cells, which had no detectable primary cilia, did not remodel TJs when exposed to autotomy-inducing stimuli (Figure 2A), we wanted to determine whether elongated, mature primary cilia need be present for autotomy-associated epithelial remodeling to occur. 14-3-3η and polaris/IFT88 are both components required for ciliogenesis (Pazour et al., 2002; Fan et al., 2004). Cells lacking either of these proteins have very short primary cilia, in which microtubules fail to extend beyond the transition zone. However, RNAi-mediated reduction of either 14-3-3η or polaris/IFT88 did not induce the remodeling events observed during stress-induced deciliation. Both sh14-3-3η and shPolaris cell lines expressed claudin-2 (Figure 8A) and had a TER identical to that of control MDCK II cells (Figure 8B). However, treatment of either sh14-3-3η or shPolaris cells with CH nonetheless induced epithelial remodeling. In each case, cells down-regulated gp135 and claudin-2 expression (not shown) and showed an elevated TER, similar to parental MDCK II cells when CH was added (Figure 8B). These results suggested that full-length, mature primary cilia need not be present in order to activate the stress-induced deciliation pathway. Instead, it may be sufficient that cells have nucleated nascent primary cilia, or “procilia,” for autotomy-associated remodeling to occur.
Figure 8.
Mature primary cilia are not required for autotomy-associated epithelial remodeling. (A) Polarized MDCK II cells, or cultures with reduced expression of 14-3-3η or polaris/IFT88, were fixed and labeled with antibodies to acetylated tubulin (green) and claudin-2 (red). Note that mature primary cilia are absent in sh14-3-3η and shPolaris cells, but that these cells retain claudin-2 expression. Confocal images were collected at the focal plane of TJs to show claudin-2. Procilia do not label with anti-acetylated tubulin antibodies. Bar, 10 μm. (B) Polarized MDCK II, sh14-3-3η or shPolaris cells were cultured in the absence or presence of 4 mM CH, and TER was measured at indicated times. Note that sh14-3-3η or shPolaris cells tighten their junctions in response to deciliation-inducing signals, but that their response is somewhat slower than that of parental MDCK II cells. n = 3 filters per condition.
Deciliation is a multistep process involving both axonemal severing (Quarmby, 2004) and Exocyst-dependent delivery of vesicles to the site of autotomy to drive fission and release of cilia (Spiczka and Yeaman, unpublished results). If deciliation was causally connected to epithelial remodeling, we reasoned that inhibiting ciliary shedding would prevent associated changes in intercellular junctions and surface composition. RNAi-mediated knockdown of Sec6, a component of the Exocyst, protected MDCK cells against both CH- and NH4-induced deciliation (Figure 9A). In addition, shSec6 cells expressed normal levels of claudin-2 and gp135 in the presence of autotomy-inducing agents and maintained a TER identical to that of untreated cells (Figure 9B). We note that Sec6 knockdown did not inhibit a CH-induced elevation of [Ca2+]i (not shown), indicating that shSec6 cells were able to sense autotomy-inducing insults, but were unable to complete the deciliation process.
Because the Exocyst is required for numerous trafficking events in MDCK cells (Grindstaff et al., 1998; Oztan et al., 2007), it was important to determine whether Sec6 knockdown prevented epithelial remodeling specifically because deciliation was inhibited and not because some other process, unrelated to deciliation, was blocked. For example, Sec6 knockdown might prevent TJ remodeling by inhibiting endocytosis and trafficking of claudin-2. Therefore, we examined TJ remodeling induced by signals that are not associated with deciliation. Treatment of quiescent MDCK II cells with EGF caused claudin-2 down-regulation and increased TER (Singh and Harris, 2004; Figure 9B). This response was unaffected by Sec6 knockdown (Figure 9B). Therefore, obstructing Exocyst activity specifically inhibited deciliation-associated TJ remodeling. Together, these results suggested that stress-induced deciliation was associated with remodeling of epithelial junctions and plasma membrane domains, and that inhibition of ciliary shedding prevented associated changes in epithelial cell organization.
DISCUSSION
When considered together, these data suggest that a specific connection exists between stress-induced deciliation and changes in intercellular junctions, post-Golgi sorting mechanisms, and polarized membrane domains. The observed effects were reversible: upon washout of the deciliating insult, cilia reemerged and membrane domains and junctions returned to normal, and all of these processes occurred with similar kinetics. Importantly, changes in junctions and membrane domains appeared to be associated with the autotomy event itself and did not occur because of resorption or simply because mature primary cilia were not present. We suggest that activation of a deciliation signaling pathway is the driving force behind epithelial remodeling.
Why do cells that cannot generate a full-length, mature primary cilium (such as those lacking either 14-3-3η or polaris/IFT88) nonetheless respond to agents that promote stress-induced deciliation? Is it because these agents have unintended side effects that are unrelated to ciliary autotomy, or is it that the deciliation mechanism can be engaged even when mature cilia are absent? Five pieces of data encourage us to favor the latter possibility. First, ciliary shedding and TJ remodeling were cotriggered by more than one type of stimulus, indicating that these effects were independent of the specific agent used to induce deciliation. Second, LLC-PK1 cells, which did not deciliate after CH treatment, and Caco-2 cells, which had no cilium to shed, also did not remodel their TJs when exposed to CH. Third, ciliary autotomy and TJ remodeling shared a requirement for intracellular calcium signaling when CH was the insulting agent and a lack of such requirement when NH4 was used. Fourth, both CH-induced deciliation and TJ remodeling were similarly resistant to inhibitors that target two common signaling components downstream of calcium. Fifth, and perhaps most compelling, when ciliary shedding was inhibited, TJ remodeling also did not occur, despite the fact that autotomy-inducing agents were present in the culture medium. Although the formal possibility remains that the panoply of effects observed after exposure of cells to deciliating agents reflects divergent cellular mechanisms, these various lines of evidence suggest that ciliary shedding and epithelial remodeling share at least a common upstream signal, and perhaps even a close functional association.
Why would epithelial cells have a mechanism to rapidly shed their cilia, and why would activation of this mechanism lead to remodeling of junctions and surface domains? Primary cilia are susceptible to insult, by virtue of the fact that they extend far away from the cell body and into an external environment that may contain harmful or noxious agents. Indeed, kidney cells may be exposed to certain metabolites in the filtrate, such as ammonium ions, that have previously been shown to mediate the deciliating activity of pathogenic Ureaplasma infection of the oviduct (Stalheim and Gallagher, 1977). Mycoplasma infection of the upper respiratory tract also causes deciliation (Stadtlander, 2006), but the consequences of urinary tract infections on renal epithelial cilia have not been reported. Perhaps ciliary shedding provides a protective signal to the epithelium, informing cells to “hunker down” by tightening their junctions and regulating their endocytic and exocytic trafficking pathways.
An alternative idea is that primary cilia must be severed and released by cells in order to facilitate rapid reentry of quiescent cells into the cell cycle. The earliest articles describing primary cilia put forth the suggestion that ciliogenesis forced cells to become quiescent by removing the centriole from the mitotic cycle (Blum, 1971; Quarmby, 2004). Nearly a century later, quiescent fibroblasts were observed to undergo a biphasic loss of primary cilia when stimulated with growth factors (Tucker et al., 1979a,b). Cells in G0 abruptly lost their primary cilia and then transiently regrew them during G1, only to lose them permanently during S phase. Perhaps agents that induce ciliary autotomy mimic the effects of growth factors that promote deciliation. In principle, this could be associated with a tightening of junctions and an altering of endocytic and exocytic trafficking, if similar events occur during mitosis in epithelial cells.
Can we learn anything about the pathogenesis of cystic kidney diseases by studying stress-induced deciliation? One reasonable hypothesis is that renal epithelia have evolved a mechanism to protect primary cilia against stress-induced autotomy and that mutations in genes whose protein products function in this mechanism render cells more susceptible to deciliation, and consequently to altered transepithelial fluid transport and mis-regulated proliferation, hallmarks of polycystic kidney diseases. Alternatively, ciliary autotomy might represent an important decision that quiescent cells make before returning to the cell cycle, and mutations in genes responsible for regulating this mechanism could cause changes in renal cell differentiation and abnormal growth control, thereby leading to cystogenesis and disease.
These two ideas are not mutually exclusive. Considering that nearly all differentiated cells in the body have primary cilia (Marshall and Nonaka, 2006; Singla and Reiter, 2006), it is possible that deciliation represents an important decision that all cells must make before reentering the cell cycle. It is logical to suggest that ciliary shedding is subject to regulation and that mutations that compromise the fidelity of this regulation will affect cell growth and differentiation. At the same time, it seems likely that cells have mechanisms in place to ensure that ciliary shedding happens only at the correct time and place, thus protecting cells from aberrant deciliation in response to environmental stress, such as bacterial infection or chemicals. Loss-of-function mutations in genes responsible for protecting cells from stress-induced deciliation could render cells more sensitive to such agents, leading to improper autotomy and loss of cellular differentiation and growth control.
Supplementary Material
ACKNOWLEDGMENTS
Yuriy Usachev and ManSu Kim are gratefully acknowledged for their technical assistance in performing calcium imaging studies. We also thank Ben Margolis for MDCK II sh14-3-3η cells and Vann Bennett (Duke University Medical Center, Durham, NC) for HBE cells. This work was supported by grants from the National Institutes of Health (GM067002 and DK052617) and the PKD Foundation.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E08-07-0741) on November 12, 2008.
REFERENCES
- Balcarova-Stander J., Pfeiffer S. E., Fuller S. D., Simons K. Development of cell surface polarity in the epithelial Madin-Darby canine kidney (MDCK) cell line. EMBO J. 1984;3:2687–2694. doi: 10.1002/j.1460-2075.1984.tb02194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balda M. S., Gonzalez-Mariscal L., Contreras R. G., Macias-Silva M., Torres-Marquez M. E., Garcia-Sainz J. A., Cereijido M. Assembly and sealing of tight junctions: possible participation of G-proteins, phospholipase C, protein kinase C and calmodulin. J. Membr. Biol. 1991;122:193–202. doi: 10.1007/BF01871420. [DOI] [PubMed] [Google Scholar]
- Bergesse J. R., Domenech C. E., Balegno H. F. Chloral hydrate inhibition in vitro of ATPase in membrane of rat erythrocytes and in microsomes of dog kidney external medulla. Biochem. Pharmacol. 1983;32:3221–3225. doi: 10.1016/0006-2952(83)90207-1. [DOI] [PubMed] [Google Scholar]
- Blum J. J. Existence of a breaking point in cilia and flagella. J. Theor. Biol. 1971;33:257–263. doi: 10.1016/0022-5193(71)90065-8. [DOI] [PubMed] [Google Scholar]
- Burdett I.D. Internalisation of desmosomes and their entry into the endocytic pathway via late endosomes in MDCK cells. Possible mechanisms for the modulation of cell adhesion by desmosomes during development. J. Cell Sci. 1993;106(Pt 4):1115–1130. doi: 10.1242/jcs.106.4.1115. [DOI] [PubMed] [Google Scholar]
- Fan S., Hurd T. W., Liu C. J., Straight S. W., Weimbs T., Hurd E. A., Domino S. E., Margolis B. Polarity proteins control ciliogenesis via kinesin motor interactions. Curr. Biol. 2004;14:1451–1461. doi: 10.1016/j.cub.2004.08.025. [DOI] [PubMed] [Google Scholar]
- Fullekrug J., Shevchenko A., Simons K. Identification of glycosylated marker proteins of epithelial polarity in MDCK cells by homology driven proteomics. BMC Biochem. 2006;7:8. doi: 10.1186/1471-2091-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M., Furuse K., Sasaki H., Tsukita S. Conversion of zonulae occludentes from tight to leaky strand type by introducing claudin-2 into Madin-Darby canine kidney I cells. J. Cell Biol. 2001;153:263–272. doi: 10.1083/jcb.153.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grindstaff K. K., Yeaman C., Anandasabapathy N., Hsu S. C., Rodriguez-Boulan E., Scheller R. H., Nelson W. J. Sec6/8 complex is recruited to cell-cell contacts and specifies transport vesicle delivery to the basal-lateral membrane in epithelial cells. Cell. 1998;93:731–740. doi: 10.1016/s0092-8674(00)81435-x. [DOI] [PubMed] [Google Scholar]
- Kiuchi-Saishin Y., Gotoh S., Furuse M., Takasuga A., Tano Y., Tsukita S. Differential expression patterns of claudins, tight junction membrane proteins, in mouse nephron segments. J. Am. Soc. Nephrol. 2002;13:875–886. doi: 10.1681/ASN.V134875. [DOI] [PubMed] [Google Scholar]
- Le Bivic A., Sambuy Y., Mostov K., Rodriguez-Boulan E. Vectorial targeting of an endogenous apical membrane sialoglycoprotein and uvomorulin in MDCK cells. J. Cell Biol. 1990;110:1533–1539. doi: 10.1083/jcb.110.5.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le T. L., Yap A. S., Stow J. L. Recycling of E-cadherin: a potential mechanism for regulating cadherin dynamics. J. Cell Biol. 1999;146:219–232. [PMC free article] [PubMed] [Google Scholar]
- Lee G. M., Diguiseppi J., Gawdi G. M., Herman B. Chloral hydrate disrupts mitosis by increasing intracellular free calcium. J. Cell Sci. 1987;88(Pt 5):603–612. doi: 10.1242/jcs.88.5.603. [DOI] [PubMed] [Google Scholar]
- Li Y., Li J., Straight S. W., Kershaw D. B. PDZ domain-mediated interaction of rabbit podocalyxin and Na(+)/H(+) exchange regulatory factor-2. Am. J. Physiol. Renal Physiol. 2002;282:F1129–F1139. doi: 10.1152/ajprenal.00131.2001. [DOI] [PubMed] [Google Scholar]
- Lien Y. H., Wang X., Gillies R. J., Martinez-Zaguilan R. Modulation of intracellular Ca2+ by glucose in MDCK cells: role of endoplasmic reticulum Ca(2+)-ATPase. Am. J. Physiol. 1995;268:F671–F679. doi: 10.1152/ajprenal.1995.268.4.F671. [DOI] [PubMed] [Google Scholar]
- Marrs J. A., Napolitano E. W., Murphy E. C., Mays R. W., Reichardt L. F., Nelson W. J. Distinguishing roles of the membrane-cytoskeleton and cadherin mediated cell-cell adhesion in generating different Na(+),K(+)-ATPase distributions in polarized epithelia. J. Cell Biol. 1993;123:149–164. doi: 10.1083/jcb.123.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall W. F., Nonaka S. Cilia: tuning in to the cell's antenna. Curr. Biol. 2006;16:R604–R614. doi: 10.1016/j.cub.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Meder D., Shevchenko A., Simons K., Fullekrug J. Gp135/podocalyxin and NHERF-2 participate in the formation of a preapical domain during polarization of MDCK cells. J. Cell Biol. 2005;168:303–313. doi: 10.1083/jcb.200407072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto S., Nishimura N., Terai T., Manabe S., Yamamoto Y., Shinahara W., Miyake H., Tashiro S., Shimada M., Sasaki T. Rab13 mediates the continuous endocytic recycling of occludin to the cell surface. J. Biol. Chem. 2005;280:2220–2228. doi: 10.1074/jbc.M406906200. [DOI] [PubMed] [Google Scholar]
- Ojakian G. K., Schwimmer R. The polarized distribution of an apical cell surface glycoprotein is maintained by interactions with the cytoskeleton of Madin-Darby canine kidney cells. J. Cell Biol. 1988;107:2377–2387. doi: 10.1083/jcb.107.6.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oztan A., Silvis M., Weisz O. A., Bradbury N. A., Hsu S. C., Goldenring J. R., Yeaman C., Apodaca G. Exocyst requirement for endocytic traffic directed toward the apical and basolateral poles of polarized MDCK cells. Mol. Biol. Cell. 2007;18:3978–3992. doi: 10.1091/mbc.E07-02-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasdar M., Nelson W. J. Kinetics of desmosome assembly in Madin-Darby canine kidney epithelial cells: temporal and spatial regulation of desmoplakin organization and stabilization upon cell-cell contact. I. Biochemical analysis. J. Cell Biol. 1988;106:677–685. doi: 10.1083/jcb.106.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour G. J., Baker S. A., Deane J. A., Cole D. G., Dickert B. L., Rosenbaum J. L., Witman G. B., Besharse J. C. The intraflagellar transport protein, IFT88, is essential for vertebrate photoreceptor assembly and maintenance. J. Cell Biol. 2002;157:103–113. doi: 10.1083/jcb.200107108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podbilewicz B., Mellman I. ATP and cytosol requirements for transferrin recycling in intact and disrupted MDCK cells. EMBO J. 1990;9:3477–3487. doi: 10.1002/j.1460-2075.1990.tb07556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praetorius H. A., Spring K. R. Removal of the MDCK cell primary cilium abolishes flow sensing. J. Membr. Biol. 2003;191:69–76. doi: 10.1007/s00232-002-1042-4. [DOI] [PubMed] [Google Scholar]
- Pugacheva E. N., Jablonski S. A., Hartman T. R., Henske E. P., Golemis E. A. HEF1-dependent Aurora A activation induces disassembly of the primary cilium. Cell. 2007;129:1351–1363. doi: 10.1016/j.cell.2007.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quarmby L. M. Cellular deflagellation. Int. Rev. Cytol. 2004;233:47–91. doi: 10.1016/S0074-7696(04)33002-0. [DOI] [PubMed] [Google Scholar]
- Quarmby L. M., Hartzell H. C. Two distinct, calcium-mediated, signal transduction pathways can trigger deflagellation in Chlamydomonas reinhardtii. J. Cell Biol. 1994;124:807–815. doi: 10.1083/jcb.124.5.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum J. L., Witman G. B. Intraflagellar transport. Nat. Rev. Mol. Cell Biol. 2002;3:813–825. doi: 10.1038/nrm952. [DOI] [PubMed] [Google Scholar]
- Sheff D. R., Daro E. A., Hull M., Mellman I. The receptor recycling pathway contains two distinct populations of early endosomes with different sorting functions. J. Cell Biol. 1999;145:123–139. doi: 10.1083/jcb.145.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheff D. R., Kroschewski R., Mellman I. Actin dependence of polarized receptor recycling in Madin-Darby canine kidney cell endosomes. Mol. Biol. Cell. 2002;13:262–275. doi: 10.1091/mbc.01-07-0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A. B., Harris R. C. Epidermal growth factor receptor activation differentially regulates claudin expression and enhances transepithelial resistance in Madin-Darby canine kidney cells. J. Biol. Chem. 2004;279:3543–3552. doi: 10.1074/jbc.M308682200. [DOI] [PubMed] [Google Scholar]
- Singla V., Reiter J. F. The primary cilium as the cell's antenna: signaling at a sensory organelle. Science. 2006;313:629–633. doi: 10.1126/science.1124534. [DOI] [PubMed] [Google Scholar]
- Stadtlander C. T. A model of the deciliation process caused by Mycoplasma fermentans strain incognitus on respiratory epithelium. Scanning. 2006;28:212–218. doi: 10.1002/sca.4950280403. [DOI] [PubMed] [Google Scholar]
- Stalheim O. H., Gallagher J. E. Ureaplasmal epithelial lesions related to ammonia. Infect. Immun. 1977;15:995–996. doi: 10.1128/iai.15.3.995-996.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart R. O., Nigam S. K. Regulated assembly of tight junctions by protein kinase C. Proc. Natl. Acad. Sci. USA. 1995;92:6072–6076. doi: 10.1073/pnas.92.13.6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda T., McQuistan T., Orlando R. A., Farquhar M. G. Loss of glomerular foot processes is associated with uncoupling of podocalyxin from the actin cytoskeleton. J. Clin. Invest. 2001;108:289–301. doi: 10.1172/JCI12539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker R. W., Pardee A. B., Fujiwara K. Centriole ciliation is related to quiescence and DNA synthesis in 3T3 cells. Cell. 1979a;17:527–535. doi: 10.1016/0092-8674(79)90261-7. [DOI] [PubMed] [Google Scholar]
- Tucker R. W., Scher C. D., Stiles C. D. Centriole deciliation associated with the early response of 3T3 cells to growth factors but not to SV40. Cell. 1979b;18:1065–1072. doi: 10.1016/0092-8674(79)90219-8. [DOI] [PubMed] [Google Scholar]
- Turner J. R., Rill B. K., Carlson S. L., Carnes D., Kerner R., Mrsny R. J., Madara J. L. Physiological regulation of epithelial tight junctions is associated with myosin light-chain phosphorylation. Am. J. Physiol. 1997;273:C1378–C1385. doi: 10.1152/ajpcell.1997.273.4.C1378. [DOI] [PubMed] [Google Scholar]
- Urban J., Parczyk K., Leutz A., Kayne M., Kondor-Koch C. Constitutive apical secretion of an 80-kD sulfated glycoprotein complex in the polarized epithelial Madin-Darby canine kidney cell line. J. Cell Biol. 1987;105:2735–2743. doi: 10.1083/jcb.105.6.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.