Abstract
Axis specification during Drosophila embryonic development requires transfer of maternal components during oogenesis from nurse cells (NCs) into the oocyte through cytoplasmic bridges. We found that the asymmetrical distribution of Golgi, between nurse cells and the oocyte, is sustained by an active transport process. We have characterized actin basket structures that asymmetrically cap the NC side of Ring canals (RCs) connecting the oocyte. Our results suggest that these actin baskets structurally support transport mechanisms of RC transit. In addition, our tracking analysis indicates that Golgi are actively transported to the oocyte rather than diffusing. We observed that RC transit is microtubule-based and mediated at least by dynein. Finally, we show that actin networks may be involved in RC crossing through a myosin II step process, as well as in dispatching Golgi units inside the oocyte subcompartments.
INTRODUCTION
Throughout the animal kingdom, germ cells commonly develop as part of syncytia in which cytoplasmic canals connect sister cells. Intercellular bridges represent specific types of cellular contacts that allow the flow of cytoplasm to be shared among sister cells in order to synchronize their division, differentiation, or hormone release (see for review Robinson and Cooley, 1996). These intercellular communications between adjacent cells are commonly found in the developing male and female germline of diverse species: in mammal ovaries, in insects and mammalians spermatocytes as well as in plants, Caenorhabditis elegans, or mammalsomatic lineages (see for review Robinson and Cooley, 1996). The intercellular bridges in Drosophila oogenesis are better characterized and have a slightly different role than in the male germline: it allows the transfer of maternal components, including key mRNAs that encode axis-determining proteins, into the oocyte to support the subsequent development of the embryo. Additionally, oocyte growth also requires a supply of plasma membrane that is provided by the secretion pathway (Janusckhe et al., 2007). Thus, we were interested in understanding how membrane trafficking occurs and how it is regulated during Drosophila oogenesis.
A Drosophila egg chamber is composed of 16 germline cells encapsulated by somatically derived follicle cells (Brown and King, 1964). This cyst of 16 germ cells is formed when cystocytes undergo four rounds of cell division without fully completing cytokinesis, giving rise to cells interconnected by cytoplasmic bridges. As development proceeds, these cytoplasmic connections are modified into stable ones, ring canals (RCs). A RC is made up of two parts: a layer of circumferentially oriented F-actin–rich filaments that form the inner rim and a thickening of the plasma membrane, originally derived from the arrested cleavage furrow forming the outer rim (Hime et al., 1996).
Among these 16 cells, only one differentiates into a mature oocyte, whereas the other 15 become polyploïd cells called nurse cells (NCs). The Drosophila oocyte is transcriptionally inactive throughout much of oogenesis, therefore the majority of nutrients (mRNA, proteins, and organelles) required for its development are synthesized in the NC and transported into the oocyte through the RC in a slow process of cytoplasmic transfer (Clark et al., 2007; Theurkauf and Hazelrigg, 1998). A key question concerns the mechanism by which this cytoplasmic transport is achieved. There is growing evidence for the importance of microtubule (MT) cytoskeleton and its associated motor proteins, kinesin (Brendza et al., 2002; Januschke et al., 2002) and dynein (Cha et al., 2002; Clark et al., 2007, Januschke et al., 2002) in active transport of organelles (Januschke et al., 2007). Less is known concerning the role of the actin cytoskeleton and its associated proteins during this process. However, studies have shown the involvement of myosins in the transport of vesicles/organelles in vertebrates neurons (DePina and Langford, 1999) and the transient association of MyoVI with mitochondria during their RC transit in the Drosophila egg chamber (Bohrmann and Schill, 1997), suggesting a role for the actin network in organelle transport.
The mechanism of cellular organelle transport through RCs remains poorly understood. It is not known for instance how the selection of what gets into the oocyte occurs or whether the secretion pathway sustains it by means of specific motors and cytoskeletal tracks. To address this issue, we focused on the regulation of transport of Golgi units from NCs to the oocyte. In mammalian cells, Golgi is a discrete organelle that contains dozens of stacked cisternae linked together by tubules which form a single large structure capping the nucleus (Mellman and Warren, 2000). In Drosophila, the Golgi apparatus does not always exhibit a morphology of stacked cisternae. But, when stacks are present, they do not form a single copy organelle. Instead, they remain scattered throughout the cytoplasm (Kondylis and Rabouille, 2003; Herpers and Rabouille, 2004), an organization that is similar to that in yeast (Rossanese et al., 1999). Whatever the morphology of the Golgi apparatus, they are in proximity to tER sites (trans-endoplasmic reticulum). The resulting structure (one tERsite and one Golgi complex) is called tER-Golgi unit (Kondylis and Rabouille, 2003).
To understand the regulation of cytoplasmic transport of Golgi to the oocyte through RCs, we have analyzed the movement of particles expressing a Golgi marker, in living Drosophila egg chambers. We show that they are actively transported to the RCs, where they accumulate before a subset transits through the cytoplasmic bridges at a much slower speed. Mechanisms of transport through RCs seem to be structurally sustained by the presence of an asymmetric basket-like actin structure capping the NC side of RCs. In addition, we show that MTs are required for the integrity of these baskets and that the transport toward and through RCs is dynein- and MyoII-dependant.
MATERIALS AND METHODS
Fly Stocks
w1118 was used as wild type, GalT (A. Debec, Institut Jacques Monod, Paris, France) as a Golgi marker (Januschke et al., 2007), Rab6-GFP (Januschke et al., 2002), Ph-GFP (L. Gervais Institut Jacques Monod) to visualize plasma membrane and tub-GFP-Dmn/CyO to visualize MTs. Overexpression of dynamitin was performed as described previously in Januschke et al. (2002), with the following stocks: tub-gal4/UAS-hDmn. Germline clones were generated by FRT/FLP-mediated recombination (Chou and Perrimon, 1992) by crossing y w sqh1 sn3 FRT101/FM7 females to ovoD1, FRT101/Y; hs-flipF38 males. Twenty-four-hour pulses of egg were allowed to develop to second or early third instar larvae before a 2-h heat shock at 37°C.
Immunostaining of Whole Mount Egg Chambers
Egg chambers were dissected in phosphate-buffered saline (PBS)–0.,1% Triton X-100 and fixed in 4% paraformaldehyde (PFA) in PBS. Primary antibody staining was performed overnight at 4°C. To visualize the actin basket, egg chambers were fixed as described above and incubated 1 h 30 min in a 1/20 rhodamine-conjugated phalloïdin (Molecular Probes, Eugene, OR) dilution and subsequently imaged on a spinning-disk confocal microscope. Green fluorescent protein (GFP)-expressing strains were fixed 5 min in 4% PFA if no additional antibody staining was required and imaged either on a spinning-disk confocal microscope or an Apotome system. Image J (http://rsbweb.nih.gov/ij/) and Photoshop (Adobe Photosystems, San Jose, CA) were used to process images.
Time-Lapse Imaging
To keep egg chambers alive during several hours, we used a POC chamber system (Leica, Deerfield, IL). Living egg chambers were separated form each other directly on a coverslip in a 15-μl drop of M3 medium supplemented with 2% fetal bovine serum (FBS, Sigma, St. Louis, MO), 0.24 μl insulin (Sigma), 0.01 μg/ml juvenile hormone (Sigma), and 50 μg/ml penicillin/streptomycin solution (Sigma). To prevent evaporation, samples were covered by a special FoilCover (Leica), which is gas permeable CultFoil. Time-lapse videomicroscopy was performed with an inverted spinning-disk confocal microscope CSU 10 (Perkin Elmer-Cetus, Norwalk, CT) connected to a Coolsnap HQL camera (Photometrics, Tucson, AZ) with a 40×/1.25 NA or a 63×/1.4 NA objective lens. To follow particles that moved along the Z-axis, three Z-positions were selected at each time point, with a step size of 0.5 μm. Given that 4D visualization is not possible yet, trails of particles were generated by a Z-projection. Speed of particles is shown as average speed ± SE.
Particle Tracking in a Noisy Biological Environment
A dedicated program was developed to detect and track fluorescently labeled Golgi particles visualized in 3D with a spinning disk microscope. Spot detection was performed automatically by a multiresolution algorithm based on wavelet decomposition of the image, correlation analysis ofwavelet coefficients, and thresholding the output binary masks for each detected object (Olivo-Marin, 2002). Once particles have been detected in the image sequences, a Bayesian tracking algorithm is used to link them. Associations between existing tracks and detections at a given time were selected on the basis of the maximization of their kinetic likelihood. This likelihood was computed using the Interacting Multiple Model (Genovesio et al., 2006) estimator that makes an adaptive weighted mixture of three models of motion: Brownian motion, directed motion, and curvilinear motion. Because the weights of these models are automatically updated, changes in type of motion are automatically taken into account. Once all the tracks were determined, motions of particles in the ovocyte and the ring canal were characterized by their mean SDs and velocities.
Inhibitor Treatment
Colchicine.
Flies were fed with colchicines as described in Januschke et al. (2002).
Latrunculin B.
Directly after dissection in PBS + 0.1% Triton X-100, egg chambers were incubated for 5 min in 150 mM or 300 mM latrunculin B (LatB; Sigma) and fixed 20 min in 4% PFA. Stainings were subsequently performed as described in the Immunostaining of Whole Mount Egg Chambers. For live imaging, the drug was diluted in M3 medium +hormones (see Time Lapse Imaging) and washed away after 5 min of incubation. For live imaging, we worked at a 150 mM concentration to prevent chambers from blowing up as soon as the permeable membrane was in contact with them.
RESULTS
Golgi Units Are Asymmetrically Distributed within the Drosophila Egg Chamber
Unlike mammalian cells in which Golgi units are found close to the nucleus, Drosophila egg chambers present Golgi dispersed both throughout the NC and the oocyte cytoplasm (Herpers and Rabouille, 2004). Its correct distribution is essential for the proper development of the oocyte (Januschke et al., 2007; Coutelis and Ephrussi, 2007), but the process controlling the transport of Golgi to the oocyte has not been addressed. To investigate the way the subcellular distribution of Golgi is controlled, we took advantage of a GFP-trap strain, GalT (Morin et al., 2001), in which a GFP-tag is inserted into a gene encoding UDP galactosyl:beta-N-acetylglucosamine beta-1,3-galactosyltransferase, a resident enzyme of Golgi stacks in mammals (Elhammer and Kornfeld 1984). In Drosophila, this protein copurifies with Golgi fractions and colocalizes with Golgi markers (Janushcke et al., 2007). We chose to focus our analysis on stages 7-10A, before cytoplasmic streaming occurs (Gutzeit, 1986). During these developmental steps, transport of proteins and lipids to the plasma membrane are required for subsequent oocyte growth, presumably involving Golgi stacks and secretion vesicles to ensure the process.
We observed that, during the early phases of development, even though dispersed throughout NCs and oocyte cytoplasm, Golgi units were present with a much higher density in the oocyte as shown in Figure 1, B–D. Indeed, we observed that GalT-containing dots gradually accumulated in the oocyte as development progressed. First, at stage 6, the Golgi was found evenly distributed between NCs and the oocyte (data not shown), but as soon as the nucleus migrated to the anteroposterior region of the oocyte (stage 7), the Golgi started to accumulate in the oocyte (Figure 1B). At stage 8 it was distributed throughout the entire oocyte, although less abundantly at the anterior margin (Figure 1C). Finally, by stage 10, GalT-containing vesicles were uniformly scattered within the oocyte (Figure 1D). We interpreted the accumulation of the Golgi in the oocyte as an indication of active transport of Golgi into the oocyte against a concentration gradient. In Drosophila egg chamber, the Golgi is observed in close vicinity of ER-exit sites and forms functional units called “tER-Golgi units” (Kondylis and Rabouille, 2003; Herpers and Rabouille, 2004). To discriminate the transport that we were following, either Golgi vesicles or tER-Golgi units, we performed a double labeling with a dCOG5-GFP transgene, which labels ER-exit sites (Herpers and Rabouille, 2004), and a lectin wheat germ agglutinin (WGA), which distribution is similar to GalT (Januschke et al., 2007). We observed that 45% of the WGA signal is associated with the dCOG5 labeling, either at the vicinity or inside RCs (Supplemental Figure S1), indicating that whole tER-Golgi units were transported into the oocyte. The WGA signal that did not colocalize with dCOG5 may correspond to membrane vesicles that have left the tER-Golgi units. In the this article, we will use the term “Golgi units” when referring to tER-Golgi units.
Figure 1.
Asymmetrical distribution of Golgi units between NC and the oocyte. (A) Actin distribution at the plasma membrane cortex and in ring canals (RCs) connecting adjacent nurse cells (NCs) or between NCs and the oocyte (Oo). (B–D) Golgi units labeled with galT-GFP gradually accumulate in the oocyte from stage 7 to stage10 egg chambers. Scale bars, 38 μm.
Golgi Units Are Transported toward and through the RCs in a Three-Step Process
To elucidate the Golgi unit transport mechanism from the NC to the oocyte, we studied their movement at both high magnification and time resolution in living egg chambers using a spinning disk confocal microscope. Speed measure of Golgi units allowed us to demonstrate sequential events and distinct mechanisms of transport during RC approach and crossing. We used a computer program for automated 4D tracking of fluorescent particles, which allows the quantification of intracellular movements of vesicles relative to the mass center of the cell nucleus (see Materials and Methods). First, in the NC cytoplasm, we observed two categories of movements (Figure 2A): 1) “random” movements consisting of sequences of discrete zigzag steps, previously described as Brownian movement (Doob, 1957); 2) linear and rapid movements interrupted from time to time by short stops or backward shifts, trajectories that would be compatible with directed transport along cytoskeleton tracks (Figure 2A, yellow arrow). Among these linear paths, we focused on a subset heading toward the oocyte and more specifically to the RC (Figure 2, B, yellow arrow, E, left part of the panel, and E′, AT; Supplemental Movie S1). We managed to track Golgi units moving toward RCs and calculated an average velocity of 0.190 ± 0.024 μm/s during RC approach.
Figure 2.
Golgi units move in a direct path toward and across the oocyte. (A–D) Trails of Golgi units were obtained by superimposing time-lapse snapshots taken every 5 s, during approximately 2 min. (A and C) and 9 min (D). (A) In NCs, Golgi units exhibited either Brownian movements (labeled as B) or directional trajectories (yellow arrow). (B) Trail of a Golgi unit approaching the RC (yellow arrow). Others linger at the RC front (red line). Blue stars point to the RC rims. (C) Transit of Golgi units across the RCs. (D) Golgi units leaving the RCs. Scale bar, 9 μm. n = number of egg chambers; p = number of Golgi units. (E-E″) Tracks of Golgi units while approaching and transiting across an RC were calculated by a computer program (see Material and methods). (E) Trajectories of Golgi units were recorded during 15 min. Snapshots were taken at 6-s intervals. Arrival, pausing, and crossing are shown together with a different color for each tracked particle. GalT cortical accumulation (delineated in green) highlights the RC rims. NC, nurse cell; Oo, oocyte. (E′) A close- up of the RC entrance showing four trajectories selected in E. AT, Golgi units actively transported toward the RC; B, Golgi units pausing at the RC entrance. They exhibited short back-and-forth movements, presumably Brownian movements. (E″) Close-up at the RC crossing. Time-point calculation and trajectory analysis suggest that Golgi units are either actively transported AT or transit to the oocyte by successive Brownian and directed movements AT+B. (F) Graph of Golgi unit velocity during RC transit. Time point 0 corresponds to the RC entrance. Time point 10 corresponds to the RC exit. According to their location within the RC, Golgi units exhibited speed differences during RC transit. In the center (blue line) they crossed the RC with a constant speed, and when close to the rim (pink line), they exhibited acceleration and deceleration phases.
In front of the RCs, no subsequent direct translocation into the oocyte was observed; instead, Golgi units paused and clustered at the RC entrance (Figure 2B, red bracket). This second step was characterized by short Brownian movements (Figure 2E′, B label). Then in a third step, a subset of Golgi units detached from lingering clusters and translocated through RC into the oocyte (Figure 2, C and E″), suggesting that the transit through RCs might be selective. Indeed, we scored only 17% of the Golgi units arriving at the RCs transiting through them (see Supplemental Movie S1: eight particles crossed out of 47 that were lingering outside the RC). Interestingly, speed calculation revealed a switch from fast motion during the approach (0.190 ± 0.024 μm/s) to slower movement during RC transit (0.110 ± 0.026 μm/s), suggesting that different molecular motors might be required for RC approach and transit. To further characterize RC crossing, trajectories and speed parameters for each Golgi units were calculated at each time point (Figure 2, E–E″; Supplemental Movie S2). It enabled us to distinguish two types of directional trajectories during RC transit depending on where Golgi units crossed (Figure 2E″). Transit in the middle of the RC exhibited straight trajectories (Figure 2E″, AT label) and a continuous motion (Figure 2F, blue line), which are features of active transport. On the other hand Golgi units crossing close to the canal rim displayed more complex trajectories of acceleration and short stops together (Figure 2F, pink line), with slight deflections from linear (Figure 2E″, AT+B label), suggesting sequences of successive Brownian movements and active motion. Finally, Golgi, units that managed to get into the oocyte left the RC vicinity with a slow motion along straight trajectories (Figure 2D).
Altogether these data suggest that Golgi units are transported in a three-step process into the oocyte through RCs, characterized by a switch from fast motion during approach, to a slow movement during RC transit, interrupted in between by a pause at the RC entrance. In addition, we pointed out a selective process that sort out Golgi units in front of RC.
A Basket-like Actin Structure Associated with RCs
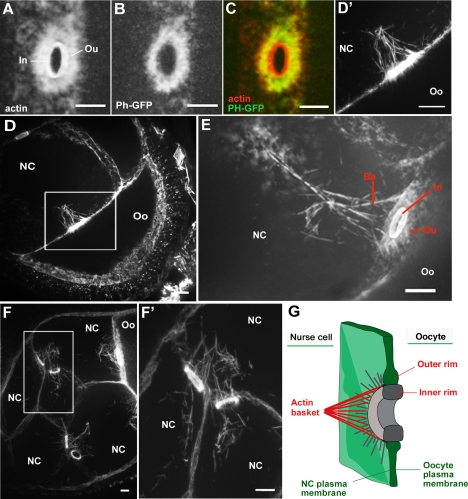
To gain insight into the regulation of cytoplasmic transport from NCs to the oocyte, we analyzed the detailed structure of RCs themselves. These bridges, interconnecting either NCs or linking NCs with the oocyte (Figure 1A), are derived from the contractile rings of the incomplete cytokinesis that characterizes cystocyte mitosis (Riparbelli and Callaini, 1995). To label RCs, we used rhodamine-conjugated phalloidin, which mainly stains the inner rim (Tilney et al., 1996). These canals consist of a layer of circumferentially oriented actin filaments, the inner rim and an outer region containing subcortical actin filaments forming a crown radiating from the inner rim (Figure 3A). This outer domain is attached to a thickening of the plasma membrane (Tilney et al., 1996; see review Robinson and Cooley, 1996), as shown by a double labeling with actin and pleckstrin homology domain of the phospholipase C fused to GFP (Ph-GFP) that specifically labels the plasma membrane (Varnai and Balla, 1998; Figure 3, B and C). Under the standard staining protocol, cytoplasmic actin filament bundles are weakly stained. However, we were able to detect actin cytoplasmic structures composed of bundles of filaments that extended in the NC cytoplasm from the external margin of the outer rim. They had an overall shape like a conical basket (Figure 3, D and E) asymmetrically distributed on the NC side of the RCs, connecting the oocyte with its four neighboring NCs. Such actin baskets were also observed on RCs connecting adjacent NCs (Figure 3, F and F′), with the difference that they were present on both sides of the RCs. The asymmetrical actin baskets were detected from stages 6–7, and gradually increased in size until stage 9–10, in term of length and number of filaments. By stage 10B, its identification was made difficult by the appearance of cytoplasmic actin filament bundles radiating from the plasma membrane, which prevented the nucleus to physically block RCs during the dumping process (Guild et al., 1997). The identification of this actin basket between NCs and the oocyte raised the possibility that this asymmetrical structure might have a function at the RC entrance.
Figure 3.
An actin-based structure capping RCs. (A–C) RCs are composed of an inner rim (In), which corresponds to bundles of actin filaments organized into a ring, and an outer rim (Ou) containing actin filaments that radiate from the inner rim. (C) This crown of actin filaments is embedded in a thickening of the plasma membrane as shown by the double-labeling with a membrane marker, PH-GFP. Although these actin baskets are asymmetrically present on the NC side of the four RC connecting the oocyte (D and E), they cap both sides of RC that connect neighboring NC (F and F′). Ba, actin basket; In, inner rim; Ou, outer rim. Scale bar, 8 μm. (G) Diagram of an actin basket–like structure capping a RC.
Actin Network and MyoII Are Involved in the RC Crossing Efficiency and the Golgi Redistribution within the Egg Chamber
In the Drosophila egg chamber, there are at least three sets of actin filaments in the NCs: a subcortical layer associated with the cortical region of NC membrane, actin filaments lining the RCs and a network of cytoplasmic actin bundles that extend in the cytoplasm (Guild et al., 1997). Because actin filaments are involved in the maintenance of the Golgi structure (see for review Egea et al., 2006), we asked whether the actin network could also be involved specifically in the transport of Golgi units from the NCs to the oocyte in Drosophila egg chambers. We chose to focus on myosins, because they have been found to be involved in the transport and steady-state localization of cytoplasmic particles or organelles in several systems (Mermall et al., 1994, Bohrmann and Schill, 1997). At first, we observed the expression of a sqh-GFP construct in the RCs (Figure 4A). The spaghetti squash (sqh) gene encodes the regulatory light chain of nonmuscle myosin II (MyoII) without which myosin is nonfunctional (Karess et al., 1991). Then, to assess the requirement for MyoII in the Golgi units cytoplasmic transport, we induced homozygous germline clones of the hypomorphic mutation sqh1. In 14% (n = 65) of sqh1 egg chambers, movements were detected: Brownian motion in 7% of the chambers and directional trajectories in the other 7% (see Table 2). However present, rectilinear trajectories toward RCs were slowed down by a factor 4 in a sqh1 mutant background (0.052 ± 0.010 μm/s) compared with wild type (wt; 0.190 ± 0.024 μm/s; Table 1), suggesting a role of MyoII in the Golgi unit transport to RCs. Interestingly, at the RC vicinity, we noticed an abnormal lingering of the Golgi units along filaments of the actin baskets. Indeed, the transient pause observed in wt egg chambers lasted much longer in sqh mutants (data not shown). This observation gave us a first hint of the MyoII requirement during RC transit. Consistently, we also noticed an unusual accumulation of Golgi units clusters either in front of the RCs (23%; Figure 4B) or inside the RCs (18%; Figure 4C; Table 2), which reinforced the hypothesis for which MyoII may be required during RC transit. However, transport of Golgi units to the oocyte did not seem to be completely abolished in the absence of MyoII. Indeed, the Golgi gradient was still present in sqh mutants (Figure 4B). In fact, speed calculation pointed out a fourfold slower motion than in a wt context (0.023 ± 0.002 vs. 0.110 ± 0.026 μm/s in wt). This remaining activity favors the hypothesis for which it is a complex of molecular motors that account for the transit process, rather than a single partner. Finally, we noticed as previously reported by Jordan and Karess (1997), a delay in oocyte growth (Figure 4, D compared with E, wt). In sqh background, the size of the oocyte was reduced in stage 10A egg chamber (borders cells contact the anterior margin, Figure 4, D and E, yellow arrowhead). This phenotype may reflect a defect in membrane trafficking across RC. Altogether, these sets of data suggest that actin filaments network may contribute to RC transit through MyoII activity.
Figure 4.
Actin network is involved in RC crossing and in Golgi unit redistribution within the oocyte. (A) MyoII is present in the RC inner rim. (B and C) Snapshots of sqh ovoD living egg chambers. Golgi units cluster at the RC entrance (B) or are clogged inside the RC (C). (D and E) The development of the oocyte is frequently delayed in sqh mutants (D), compared with wt (E): the oocyte is much smaller in sqh mutant egg chambers, whereas the border cells have already reached the anterior margin (white arrowhead). n, number of egg chambers; p, number of Golgi units. Scale bars, (A)6 μm; (B, C, and D–G′) 38 μm. (F–G′) Snapshots of one time point of wt (F and F′) or LatB-treated (G and G′) living egg chambers. (G–G′, arrowhead) Upon actin filaments depolymerization, Golgi units cluster at the RC exit. Scale bars, 6 μm.
Table 2.
Quantification of defects in control, UAS-Dmn–overexpressing, and sqh living egg chambers
| Phenotype | Wild type (n = 20) | UAS-Dmn (n = 20) | sqh clone (n = 65) | |
|---|---|---|---|---|
| In NCsa | Rectilinear trajectories + Brownian motion | 20/20 (100) | 3/20 (15) | 5/65 (7) |
| Only Brownian motion | 7/20 (35) | 5/65 (7) | ||
| No movement | 10/20 (50) | 55/65 (86) | ||
| At RC entranceb | Clusters at the RC entrance | 0/20 | 5/20 (25) | 15/65 (23) |
| Clog inside the RC | 0/20 | 2/20 (10) | 12/65 (18) |
Percentage of defects are calculated from movies of living egg chambers of each genotype, indicated in the column heads. n, number of egg chambers scored.
a The number of chambers with defects per total number of egg chambers scored in which rectilinear trajectories vs. only Brownian motion are detected in NCs; values in parentheses are percentages.
b The number of chambers with defects per total number of egg chambers scored in which RC transit is slow down or does not occur, either because of Golgi unit clustered at the entrance, or blocked inside the canal; values in parentheses are percentages.
Table 1.
Velocity of Golgi unit transport in control, UAS-Dmn–overexpressing, and sqh living egg chambers
| Wild type |
UAS-Dmn |
sqh clones |
|||
|---|---|---|---|---|---|
| Velocity (μm/s) | Velocity (μm/s) | Phenotype | Velocity (μm/s) | Phenotype | |
| Approach | 0.190 ± 0.024 (n = 14; p = 35) | 0.114 ± 0.033 (n = 2; p = 2) | Reduced | 0.052 ± 0.010 (n = 4; p = 12) | Slow |
| Pause | Short | Longer | Longer | ||
| Transit | 0.110 ± 0.026 (n = 14; p = 27) | 0.025 ± 0.007 (n = 1; p = 5) | Slow | 0.023 ± 0.002 (n = 2; p = 3) | Slow |
| Gradient | Yes | Yes | Yes | ||
Rates are calculated from movies of living egg chambers of each genotype indicated at the top of the table. n, number of egg chambers scored; p, number of Golgi units scored.
Next, we analyzed Golgi units trajectories in the presence of LatB, an inhibitor of actin filament assembly (Spector et al., 1983). In extreme cases, in which no cortical actin remained, treatment with LatB eliminated all particle movements (data not shown). In less affected egg chambers with partial disassembly of the actin scaffold, some Golgi units still managed to reach the RC vicinity. However, they did not accumulate in front of the RCs (Figure 4, G and G′), which correlates well with the disappearance of the actin basket in 100% of the chambers (data not shown) and its putative role as a selective barrier. On the other hand, we observed in four of six LatB-treated egg chambers a large cluster of Golgi units at the RC exit (Figure 4, G and G′, arrowhead) compared with wt (Figure 4, F and F′). These observations suggest that an actin-dependent process might be involved in dispatching Golgi units to the oocyte subcellular compartments where they are required. This is also consistent with data from other systems implicating actin networks in aspects of vesicle sorting and distribution (Lantz et al., 1998; see for review Buss et al., 2002; Rogers and Gelfand, 2000).
Microtubules Inhibitors Prevent Golgi Transportation to the Oocyte and Disassemble the RC Actin Baskets
Given that MTs are required for subcellular localization of several mRNAs and Rab6 transport (Theurkauf and Hazelrigg 1998; Clark et al., 2007; Januschke et al., 2007), we next checked whether they could also be involved in the cytoplasmic transport of Golgi units. MTs are enriched at the RC entrance (Grieder and Hazelrigg, 2000; Moon and Hazelrigg, 2004; Clark et al., 2007; Mische et al., 2007). They concentrate and converge toward the cytoplasmic bridges (Figure 5, A and B), and some extend through them (Figure 5B), suggesting that MTs might serve as tracks along which Golgi units could be towed.
Figure 5.
Alteration of Golgi unit transport toward and through RCs upon MTs depletion. (A) MTs are visualized with a αtub84-GFP or (B) a Dmn-GFP construct (green). Phalloidin (red) labels actin at the RCs and at the plasma membrane cortex. MTs converge toward RCs. Some seem to extend through it. Arrowheads point to the RC hedges (A). Scale bar, 40 μm. (C and D) MT depletion upon colchicine treatment (D) induces the redistribution of Golgi units between NCs and the oocyte as well as their clustering in big aggregates compared with untreated (C). Scale bar, 40 μm. (E–H′) Successive snapshots taken at different time intervals in colchicine-treated living egg chambers. (E–H) Entrance: in the absence of MTs, Golgi units lying at RC vicinity enter the oocyte with a straight trajectory and at a constant and slow velocity, suggesting a switch from active transport to diffusion between NC and the oocyte. (E′–F′) Exit. Unidirectional transport is abolished in absence of MTs network. Golgi units can now leave the oocyte. n, number of chambers; p, number of particles. Scale bar, 6 μm. (I–J) Fixed preparations of wt and colchicine-treated egg chambers. When the MTs network is impaired, actin baskets are not present anymore. Scale bar, 6 μm.
To address this question, we first depolymerized MTs by a colchicine treatment (Janusckhe et al., 2002). We particularly focused on chambers in which the nucleus was mislocalized in the oocyte central region, a consequence of MTs depolymerization (Koch and Spitzer, 1982). This displacement served as an internal control (Figure 5D). In these egg chambers, Golgi units formed clusters that were scattered throughout NCs and the oocyte cytoplasm (Figure 5, D compared with C, the untreated egg chamber). In addition, colchicine treatment abolished Golgi units accumulation at the RC entrance as well as fast directional movements, indicating that transport of Golgi units toward RCs along straight paths is MT dependent. Moreover, in colchicine-treated egg chambers, the gradient of Golgi units was not present anymore; instead, Golgi units were equally distributed between the NCs and the oocyte (Figure 5D), suggesting that MTs may also be required for active transport of Golgi units through RCs. Even though no direct transport toward the RCs occurred, we observed that as soon as a cluster of Golgi units happened to be close to the RCs, it was able to get into the oocyte with a different trajectory and velocity than in untreated conditions (Figure 5, E–H; 0.160 ± 0.020 vs. 0.194 ± 0.024 μm/s in wt; Supplemental Movie S4). No pause or selection was observed. Instead, trajectories were straight, with a constant velocity as if clusters of Golgi units were dumped in the oocyte. Importantly, we also observed clusters leaving the oocyte back toward the NCs (Figure 5E′–H′; 0.080 ± 0.018 μm/s (n = 7); Supplemental Movie S5). They moved more slowly than those entering the oocyte, suggesting that an MT-independent transport mechanism toward the oocyte may still remain. Altogether, these records suggest that active transport was switched to free exchanges between both compartments, which would then imply that nothing maintained the gradient at the RCs anymore. Indeed, a close look at the RCs after colchicine treatment confirmed that the actin baskets were no longer present at the RC entrance (Figure 5, J compared with I), suggesting that actin baskets may also play the role of physical barriers to prevent any outflow from the oocyte.
Dynein Is Required for Golgi Unit Transport from the NCs to the Oocyte
To obtain further evidence for MTs involvement in RC transit, we sought to interfere with the MT function by blocking dynein, a minus-end–directed MT motor involved in the mRNA transport from the NC to the oocyte (Januschke et al., 2002; Tekotte and Davis, 2002; Duncan and Warrior, 2002; Bullock et al., 2006; Clark et al., 2007). Because null alleles of dynein heavy-chain (dhc) mutation compromise oocyte development (McGrail et al., 1995, 1997), we chose to disrupt dynein activity indirectly by overexpressing the dynactin subunit, dynamitin (Dmn; Burkhardt et al., 1997; Duncan and Warrior, 2002; Januschke et al., 2002). In these egg chambers, the Golgi units organized into big clusters (Figure 6, B and C, white arrow) compared with wt chambers (Figure 6A), which is consistent with a role for dynein in Golgi subcellular organization. First, we observed that the gradient of Golgi units between the NCs and the oocyte was always conserved when dynein activity was impaired (n = 20), as well as the presence of the actin basket (Supplemental Figure S2). Second, in chambers in which movements were not totally abolished (10 of 20 scored), we mainly observed Golgi units manifesting Brownian movements. Directional trajectories were detected in 15% of the chambers (Table 2), but the Golgi unit's velocity was substantially reduced, (0.114 ± 0.033 vs. 0.190 ± 0.024 μm/s in wt; Table 1). These results suggest that dynein actively transports Golgi units toward RC.
Figure 6.
Dynein is required for RC crossing of Golgi units. Snapshot at one time point of wt (A) and UAS-Dmn–overexpressing (mimicking dynein loss-of-function) living egg chambers. (A′–C′) Close-ups of the above pictures. (B and B′) When dynein function is impaired, Golgi units cluster (white arrowhead) and accumulate in front of RCs. Most of them do not managed to cross. (C–C′) However, in extreme cases, some do cross as long stretches. Those stretches manage to get to the RC exit, but with a much slower motion than in wt chambers. Scale bars, 10 μm. n, number of chambers; p, number of particles. (D–E) Distribution of MTs at the vicinity of the RC-capping actin baskets. MTs are visualized with a Dmn-GFP construct (green) and actin by a phalloidin labeling (red). A subpopulation of MTs run parallel to the actin basket filaments (D–D″, white arrowhead), whereas others connect directly to the inner rim (E–E″) or to actin filaments of the basket (D–D″, red arrowhead).
Next, we had a closer look at RC transit in egg chambers in which Golgi units still exhibited directional movements (Figure 6, C and C′; Supplemental Movie S6). In the absence of functional dynein, translocation through RCs was about fourfold slower than in control chambers (0.025 ± 0.007 vs. 0.110 ± 0.026 μm/s in wt). In extreme cases, either clusters accumulated in front of the RCs (25%; Table 2) without crossing or did not managed to disassemble as soon as RC transit started (10%; Table 2; Figure 6, C and C′). Instead, they formed some sort of filaments that stretched out from one side of the canal to the other, before they reached the oocyte and retracted. These results suggest that dynein participates in the active translocation of Golgi units through RCs along MTs.
To visualize MTs in the vicinity of actin baskets, we took advantage of a transgenic strain expressing moderately a Dmn-GFP fusion protein that decorates the MTs without perturbing the transport (Januschke et al., 2002). Labeling of both MTs and actin networks revealed that MTs come very close to the actin baskets (Figure 6, D–E″) and could be divided into at least three populations: 1) MTs running parallel to the actin filaments of the basket (see white arrowheads in Figure 6, D–D″); 2) MTs connecting either the filaments of actin baskets (see red arrowheads in Figure 6, D–D″) or the ring itself (Figure 6, E–E″); and 3) MTs coming directly from NC cytoplasm and passing through the RCs as shown in Figure 5B (and mentioned by Grieder et al., 2000). Altogether, these observations suggest that each MT subgroup might be involved specifically in different steps of the Golgi transport from the NCs to the oocyte.
DISCUSSION
In this study, we have described the first live visualization of the transport of Golgi units through RCs into the Drosophila oocyte. We carried out a detailed analysis with high-resolution time-lapse images and functional disruption approaches that provided new aspects required for transport from the NC to the oocyte. We have characterized an asymmetric basket-like actin structure capping the NC side of the four RC that connect the oocyte with its neighboring NC. We have shown that Golgi units are actively transported to the oocyte instead of diffusing. They move in a direct path toward the RCs where they accumulate before a subset transits to the oocyte. We propose that the actin baskets structurally support the Golgi units pause at the RC entrance. We show that partial loss of either dynein or MyoII activity reduces velocity of Golgi units, which is consistent with a MT/actin-dependent transport from the NCs to the oocyte.
Identification of an Asymmetric Actin Basket at the RCs Connecting the NCs with the Oocyte
We have characterized a new actin structure capping every RC present in the Drosophila egg chamber, overall shape of which looks like a conical basket. These actin baskets are present on both sides of the RCs connecting adjacent NCs but are asymmetrically distributed on the NC side of the RCs connecting the oocyte with its four neighboring NCs. We showed that these actin baskets are sensitive to MTs depolymerization and brefeldin A treatment (E. Nicolas, personal communication). It suggests that either MTs may serve as a scaffold that helps maintaining the basket structure or that MTs may sustain the addition of proteins or components that participate in the anchoring or maintenance of the actin baskets at the RC surface.
Golgi Units Transport from NCs to Oocyte Can Be Divided into Three Distinct Steps
Altogether, our observations led us to propose the following working model for the NC to oocyte transport (Figure 7). Panel A shows the RC approach: Golgi units associated with the dynein motors complex and MyoII are actively transported along MTs and actin filaments toward the RCs. Panel B shows the pause: At the RC entrance, Golgi units pause along the filaments of the actin baskets that decorate the NC side of the RCs. We hypothesize that they might dissociate or/and associate with different motors and regulators, allowing them to switch onto a second group of MTs that cross the canal. We propose that this step enables a specific selection of Golgi units that can get into the oocyte to prevent any occasional accidents such as direct crossing of organelles. However, the presence of MTs coming from the NC lumen and entering directly into the RC suggests that some particles might be able to directly transit to the oocyte. These specific particles might be already hooked onto the “crossing” partners. Panel C shows the RC Transit: Once associated with the right partners, Golgi units transit through the RC. Our data show that dynein and MyoII are required for RC crossing. Panel D shows the distribution: Once at the RC exit side, vesicles may be transferred onto a third population of MTs in order to be distributed to the subcompartments where they are required. The presence of large clusters of Golgi units at the RC exit upon LatB treatment supports the actin network role in the redistribution of secretory vesicles within the oocyte, which is consistent with other data showing functional interactions of actin and MT networks (Lantz et al., 1998, Buss et al., 2002).
Figure 7.
Working model for Golgi units transport across RCs. Cross section of an RC is shown, capped by an actin basket (orange). Left (green) is the NC side, and right (light orange) is the oocyte (Oo). (A) Approach. Black lines are MTs and red lines are filaments of the actin network. Green circles, Golgi units; blue circles, dynein motor complex; and pink circles, MyoII. These two motors contribute to Golgi units transport toward RC. (B) Pause. Once at the RC entrance, Golgi units pause in order to associate with the right motors for transit. (C) Transit. Dynein and MyoII contribute to RC crossing, though MyoII to a lesser extend. (D) Distribution. Once in the oocyte, Golgi units redistribution onto new MTs is actin network mediated.
Actin Baskets Participates in the Transport Mechanism through RCs
The presence of actin baskets at the RC entrance led us to propose that active transport toward the oocyte might be structurally supported by these baskets for three reasons. 1) We observed, in living egg chambers, Golgi units organized in transient filament-like structures that colocalize with actin baskets. 2) Upon colchicine or LatB treatment, the absence of actin baskets correlates with no net accumulation of Golgi units in front of the RC and with bidirectional RC transit (colchicine treatment only). These two observations led us to speculate that actin baskets might “force” Golgi units to pause. This stop could provide the opportunity to change motor complex composition in order to modulate parameters of RC transit. Thus, actin baskets may function as platforms upon which specific Golgi units recruit specific motors and partners that ultimately direct them to the oocyte. 3) In sqh clones, Golgi units linger much longer on the actin basket filaments and, in extreme cases, cluster in front of the RCs. 4) Finally, these baskets may also serve as physical barriers preventing outflow from the oocyte, thus assuring the maintenance of the gradient as shown by the observation of Golgi units getting out of the oocyte in colchicine-treated egg chambers.
Approach and RCs Transit Are Two Specific Processes
This study provides the following evidence for different transport mechanisms sustaining RCs approach and RCs transit: 1) Dynamics specificity. Vesicles arrive much more quickly (1.7-fold greater) at the RCs than they do to cross it. The association to different motors and/or regulators could explain this difference (Bullock et al., 2006). 2) Trajectory specificity. Although Golgi units exhibit straight trajectories to reach the RC entrance, transit path characteristics depend on where the crossing occurs inside the RCs. In the center, linear tracks correspond to active transport, whereas on the edges, Golgi units are constantly switching from Brownian motion to active transport. 3) Velocity specificity. Transport in NCs is rapid. In contrast, Golgi units movement is significantly slower once they transit into the RCs. This indicates that motor dependent movement is down-regulated as Golgi units cross the RCs.
Transport of Golgi Units Is Dynein and MyoII mediated
We observed that transport of Golgi units toward and through the RC is colchicine sensitive. It is reduced in NCs and even more dramatically impaired during RC transit when dynein, a minus-end–directed motor, also known to associate with membranes of the trans-Golgi network (Matanis et al., 2002), is absent. These observations indicate an MT-dependent mechanism for Golgi units transport to the oocyte. In addition, we highlighted the presence of at least three different groups of MTs relative to their localization at the actin basket and RC vicinity. We propose that they may be specifically involved in the different steps of RC approach and transit. Indeed, MacDougal et al., 2003 have suggested that accessory factors are required to specify the MTs subset along which dynein-mediated transport occurs. We also identified MyoII as a motor required for transport during RC approach as well as RC transit. Surprisingly, the reduction of Sqh activity seems to have a stronger effect on Golgi unit velocity than on the impairment of dynein activity. One can hypothesize that, in UAS-Dmn–overexpressing egg chambers, the remaining activity of dynein may be higher than what is left of Sqh function in sqh clones. Overall, our results show that both MTs and actin networks contribute to the regulation of Golgi units transport, as we showed that disruption of either MTs or actin motors impaired Golgi units transport. Comparison of motility toward RCs and during RC transit in both mutant backgrounds suggests that at least both dynein and MyoII are required, given that the absence of a single one does not completely stop the traffic. It would be interesting to determine what their contribution is and how it is differentially regulated in order to better understand the cross-talk between actin filaments and MTs. Investigating the role of cytoskeletal linkers such as Short stop, will be interesting, given that shot mutant egg chambers have a similar phenotype to dynein associated proteins Egalitarian and Bicaudal B (Röper and Brown, 2003, 2004). In this study, we show that Golgi units as for mRNA such as grk (Clark et al., 2007), bcd (Mische et al., 2007), or hairy (Bullock et al., 2006), depend on dynein for transport from NCs to the oocyte. Interestingly, the average velocity of mRNA is faster than the transport of Golgi units (1.45 ± 0.087 μm/s (Clark et al., 2007) vs. 0.190 ± 0.024 μm/s, toward RC) and 0.25 ± 0.036 μm/s (Clark et al., 2007) vs. 0.110 ± 0.026 μm/s, through RCs). This discrepancy of a factor 7 and 2, respectively, suggests that although a dynein-based transport is conserved, their motor partners and/or regulators may differ. Indeed, recent observations suggest that 1) dynein may be associated with motors of opposite polarity, like kinesin I that acts as an antagonist of dynein mediated-transport of Exu RNP complexes (Mische et al., 2007); and 2) cooperativity of multiple motors can regulate force and velocity of motor complexes (Kural et al., 2005; Mallik et al., 2005; Levi et al., 2006). Thus, regulation of opposing motors may provide the means to control velocity of Golgi units during the different steps of their journey to the oocyte. Further experiments will be required to characterize these specific partners.
Supplementary Material
ACKNOWLEDGMENTS
We thank T. Piolot and C. Chamot for their technical help for images and time-lapse acquisitions. We thank Jean-René Huynh, Roger Caress, and Véronique Brodu for their comments on the manuscript. This work was supported by ACI “Biologie cellulaire,” “Jeune Chercheur” Grant 035117, and ANR “Blanche” (Cymempol, Grant Blan06-3-139786).
Abbreviations used:
- MT
microtubule
- NC
nurse cell
- RC
ring canals.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E08-04-0360) on November 12, 2008.
REFERENCES
- Bohrmann J., Schill S. Cytoplasmic transport in Drosophila ovarian follicles: the migration of microinjected fluorescent probes through intercellular bridges depends neither on electrical charge nor on external osmolarity. Int. J. Dev. Biol. 1997;41:499–507. [PubMed] [Google Scholar]
- Brendza R. P., Serbus L. R., Saxton W. M., Duffy J. B. Posterior localization of dynein and dorsal-ventral axis formation depend on kinesin in Drosophila oocytes. Curr. Biol. 2002;12:1541–1545. doi: 10.1016/s0960-9822(02)01108-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E. H., King R. C. Studies on the events resulting in the formation of an egg chamber in Drosophila melanogaster. Growth. 1964;28:41–81. [PubMed] [Google Scholar]
- Bullock S. L., Nicol A., Gross S. P., Zicha D. Guidance of bidirectional motor complexes by mRNA cargoes through control of dynein number and activity. Curr. Biol. 2006;16:1447–1452. doi: 10.1016/j.cub.2006.05.055. [DOI] [PubMed] [Google Scholar]
- Burkhardt J. K., Echeverri C. J., Nilsson T., Vallee R. B. Overexpression of the dynamitin (p50) subunit of the dynactin complex disrupts dynein-dependent maintenance of membrane organelle distribution. J. Cell Biol. 1997;139:469–484. doi: 10.1083/jcb.139.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss F., Luzio J. P., Kendrick-Jones J. myosin VI, an actin motor for membrane traffic and cell migration. Traffic. 2002;3:851–858. doi: 10.1034/j.1600-0854.2002.31201.x. [DOI] [PubMed] [Google Scholar]
- Cha B. J., Serbus L. R., Koppetsch B. S., Theurkauf W. E. Kinesin I-dependent cortical exclusion restricts pole plasma to the oocyte posterior. Nat. Cell Biol. 2002;4:592–598. doi: 10.1038/ncb832. [DOI] [PubMed] [Google Scholar]
- Chou T. B., Perrimon N. Use of a yeast site-specific recombinase to produce female germline chimeras in Drosophila. Genetics. 1992;131:643–653. doi: 10.1093/genetics/131.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A., Meignin C., Davis I. A dynein-dependent shortcut rapidly delivers axis determination transcripts into the Drosophila oocyte. Development. 2007;134:1955–1965. doi: 10.1242/dev.02832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooley L., Theurkauf W. E. Cytoskeletal functions during Drosophila oogenesis. Science. 1994;266:590–596. doi: 10.1126/science.7939713. [DOI] [PubMed] [Google Scholar]
- Coutelis J. B., Ephrussi A. Rab6 mediates membrane organization and determinant localization during Drosophila oogenesis. Development. 2007;134:1419–1430. doi: 10.1242/dev.02821. [DOI] [PubMed] [Google Scholar]
- DePina A. S., Langford G. M. Vesicle transport: the role of actin filaments and myosin motors. Microsc. Res. Tech. 1999;47:93–106. doi: 10.1002/(SICI)1097-0029(19991015)47:2<93::AID-JEMT2>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Doob J. L. Conditional brownian motion and the boundary limits of harmonic functions. Bulletin de la Société Mathématique de France. 1957;85:431–458. [Google Scholar]
- Duncan J. E., Warrior R. The cytoplasmic dynein and kinesin motors have interdependent roles in patterning the Drosophila oocyte. Curr. Biol. 2002;12:1982–1991. doi: 10.1016/s0960-9822(02)01303-9. [DOI] [PubMed] [Google Scholar]
- Egea G., Lazaro-Dieguez F., Vilella M. Actin dynamics at the Golgi complex in mammalian cells. Curr. Opin. Cell Biol. 2006;18:168–178. doi: 10.1016/j.ceb.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Elhammer A., Kornfeld S. Two enzymes involved in the synthesis of O-linked oligosaccharides are localized on membranes of different densities in mouse lymphoma BW5147 cells. The J. Cell Biol. 1984;99:327–331. doi: 10.1083/jcb.99.1.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovesio A., Liedl T., Emiliani V., Parak W. J., Coppey-Moisan M., Olivo-Marin J. C. Multiple particle tracking in 3-D+t microscopy: method and application to the tracking of endocytosed quantum dots. IEEE Trans. Image Process. 2006;15:1062–1070. doi: 10.1109/tip.2006.872323. [DOI] [PubMed] [Google Scholar]
- Grieder N. C., de Cuevas M., Spradling A. C. The fusome organizes the microtubule network during oocyte differentiation in Drosophila. Development. 2000;127:4253–4264. doi: 10.1242/dev.127.19.4253. [DOI] [PubMed] [Google Scholar]
- Guild G. M., Connelly P. S., Shaw M. K., Tilney L. G. Actin filament cables in Drosophila nurse cells are composed of modules that slide passively past one another during dumping. J. Cell Biol. 1997;138:783–797. doi: 10.1083/jcb.138.4.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutzeit H. O. The role of microfilaments in cytoplasmic streaming in Drosophila follicles. J. Cell Sci. 1986;80:159–169. doi: 10.1242/jcs.80.1.159. [DOI] [PubMed] [Google Scholar]
- Herpers B., Rabouille C. mRNA localization and ER-based protein sorting mechanisms dictate the use of transitional endoplasmic reticulum-golgi units involved in gurken transport in Drosophila oocytes. Mol. Biol. Cell. 2004;15:5306–5317. doi: 10.1091/mbc.E04-05-0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hime G. R., Brill J. A., Fuller M. T. Assembly of ring canals in the male germ line from structural components of the contractile ring. J. Cell Sci. 1996;109(Pt 12):2779–2788. doi: 10.1242/jcs.109.12.2779. [DOI] [PubMed] [Google Scholar]
- Januschke J., Gervais L., Dass S., Kaltschmidt J. A., Lopez-Schier H., St Johnston D., Brand A. H., Roth S., Guichet A. Polar transport in the Drosophila oocyte requires Dynein and Kinesin I cooperation. Curr. Biol. 2002;12:1971–1981. doi: 10.1016/s0960-9822(02)01302-7. [DOI] [PubMed] [Google Scholar]
- Januschke J., Nicolas E., Compagnon J., Formstecher E., Goud B., Guichet A. Rab6 and the secretory pathway affect oocyte polarity in Drosophila. Development. 2007;134:3419–3425. doi: 10.1242/dev.008078. [DOI] [PubMed] [Google Scholar]
- Jordan P., Karess R. myosin light chain-activating phosphorylation sites are required for oogenesis in Drosophila. J. Cell Biol. 1997;139:1805–1819. doi: 10.1083/jcb.139.7.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karess R. E., Chang X. J., Edwards K. A., Kulkarni S., Aguilera I., Kiehart D. P. The regulatory light chain of nonmuscle myosin is encoded by spaghetti-squash, a gene required for cytokinesis in Drosophila. Cell. 1991;65:1177–1189. doi: 10.1016/0092-8674(91)90013-o. [DOI] [PubMed] [Google Scholar]
- Koch E. A., Spitzer R. H. Autoradiographic studies of protein and polysaccharide synthesis during vitellogenesis in Drosophila. Cell Tissue Res. 1982;224:315–333. doi: 10.1007/BF00216876. [DOI] [PubMed] [Google Scholar]
- Kondylis V., Rabouille C. A novel role for dp115 in the organization of tER sites in Drosophila. J. Cell Biol. 2003;162:185–198. doi: 10.1083/jcb.200301136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kural C., Kim H., Syed S., Goshima G., Gelfand V. I., Selvin P. R. Kinesin and dynein move a peroxisome in vivo: a tug-of-war or coordinated movement? Science. 2005;308:1469–1472. doi: 10.1126/science.1108408. [DOI] [PubMed] [Google Scholar]
- Lantz V. A., Miller K. G. A class VI unconventional myosin is associated with a homologue of a microtubule-binding protein, cytoplasmic linker protein-170, in neurons and at the posterior pole of Drosophila embryos. J. Cell Biol. 1998;140:897–910. doi: 10.1083/jcb.140.4.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi V., Serpinskaya A. S., Gratton E., Gelfand V. Organelle transport along microtubules in Xenopus melanophores: evidence for cooperation between multiple motors. Biophys. J. 2006;90:318–327. doi: 10.1529/biophysj.105.067843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDougall N., Clark A., MacDougall E., Davis I. Drosophila gurken (TGFalpha) mRNA localizes as particles that move within the oocyte in two dynein-dependent steps. Dev. Cell. 2003;4:307–319. doi: 10.1016/s1534-5807(03)00058-3. [DOI] [PubMed] [Google Scholar]
- Mallik R., Petrov D., Lex S. A., King S. J., Gross S. P. Building complexity: an in vitro study of cytoplasmic dynein with in vivo implications. Curr. Biol. 2005;15:2075–2085. doi: 10.1016/j.cub.2005.10.039. [DOI] [PubMed] [Google Scholar]
- Matanis T., et al. Bicaudal-D regulates COPI-independent Golgi-ER transport by recruiting the dynein-dynactin motor complex. Nat. Cell Biol. 2002;4:986–992. doi: 10.1038/ncb891. [DOI] [PubMed] [Google Scholar]
- McGrail M., Gepner J., Silvanovich A., Ludmann S., Serr M., Hays T. S. Regulation of cytoplasmic dynein function in vivo by the Drosophila Glued complex. J. Cell Biol. 1995;131:411–425. doi: 10.1083/jcb.131.2.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrail M., Hays T. S. The microtubule motor cytoplasmic dynein is required for spindle orientation during germline cell divisions and oocyte differentiation in Drosophila. Development. 1997;124:2409–2419. doi: 10.1242/dev.124.12.2409. [DOI] [PubMed] [Google Scholar]
- Mellman I., Warren G. The road taken: past and future foundations of membrane traffic. Cell. 2000;100:99–112. doi: 10.1016/s0092-8674(00)81687-6. [DOI] [PubMed] [Google Scholar]
- Mermall V., McNally J. G., Miller K. G. Transport of cytoplasmic particles catalysed by an unconventional myosin in living Drosophila embryos. Nature. 1994;369:560–562. doi: 10.1038/369560a0. [DOI] [PubMed] [Google Scholar]
- Mische S., Li M., Serr M., Hays T. S. Direct observation of regulated ribonucleoprotein transport across the nurse cell/oocyte boundary. Mol. Biol. Cell. 2007;18:2254–2263. doi: 10.1091/mbc.E06-10-0959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon W., Hazelrigg T. The Drosophila microtubule-associated protein mini spindles is required for cytoplasmic microtubules in oogenesis. Curr. Biol. 2004;14:1957–1961. doi: 10.1016/j.cub.2004.10.023. [DOI] [PubMed] [Google Scholar]
- Morin X., Daneman R., Zavortink M., Chia W. A protein trap strategy to detect GFP-tagged proteins expressed from their endogenous loci in Drosophila. Proc. Natl. Acad. Sci. USA. 2001;98:15050–15055. doi: 10.1073/pnas.261408198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivo-Marin J.-C. Extraction of spots in biological images using multiscale products. Pattern Recognit. 2002;35:1989–1996. [Google Scholar]
- Riparbelli M. G., Callaini G. Cytoskeleton of the Drosophila egg chamber: new observations on microfilament distribution during oocyte growth. Cell Motil. Cytoskelet. 1995;31:298–306. doi: 10.1002/cm.970310406. [DOI] [PubMed] [Google Scholar]
- Robinson D. N., Cooley L. Stable intercellular bridges in development: the cytoskeleton lining the tunnel. Trends Cell Biol. 1996;6:474–479. doi: 10.1016/0962-8924(96)84945-2. [DOI] [PubMed] [Google Scholar]
- Rogers S. L., Gelfand V. I. Membrane trafficking, organelle transport, and the cytoskeleton. Curr. Opin. Cell Biol. 2000;12:57–62. doi: 10.1016/s0955-0674(99)00057-5. [DOI] [PubMed] [Google Scholar]
- Röper K., Brown N. H. Maintaining epithelial integrity: a function for gigantic spectraplakin isoforms in adherens junctions. J. Cell Biol. 2003 Sep 29;162(7):1305–15. doi: 10.1083/jcb.200307089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röper K., Brown N. H. A spectraplakin is enriched on the fusome and organizes microtubules during oocyte specification in Drosophila. Curr. Biol. 2004;14(2):99–110. [PubMed] [Google Scholar]
- Rossanese O. W., Soderholm J., Bevis B. J., Sears I. B., O'Connor J., Williamson E. K., Glick B. S. Golgi structure correlates with transitional endoplasmic reticulum organization in Pichia pastoris and Saccharomyces cerevisiae. J. Cell Biol. 1999;145:69–81. doi: 10.1083/jcb.145.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector I., Shochet N. R., Kashman Y., Groweiss A. Latrunculins: novel marine toxins that disrupt microfilament organization in cultured cells. Science. 1983;219:493–495. doi: 10.1126/science.6681676. [DOI] [PubMed] [Google Scholar]
- Stow J. L., Fath K. R., Burgess D. R. Budding roles for myosin II on the Golgi. Trends Cell Biol. 1998;8:138–141. doi: 10.1016/s0962-8924(98)01238-0. [DOI] [PubMed] [Google Scholar]
- Tekotte H., Davis I. Intracellular mRNA localization: motors move messages. Trends Genet. 2002;18:636–642. doi: 10.1016/s0168-9525(02)02819-6. [DOI] [PubMed] [Google Scholar]
- Theurkauf W. E., Hazelrigg T. I. In vivo analyses of cytoplasmic transport and cytoskeletal organization during Drosophila oogenesis: characterization of a multi-step anterior localization pathway. Development. 1998;125:3655–3666. doi: 10.1242/dev.125.18.3655. [DOI] [PubMed] [Google Scholar]
- Tilney L. G., Tilney M. S., Guild G. M. Formation of actin filament bundles in the ring canals of developing Drosophila follicles. J. Cell Biol. 1996;133:61–74. doi: 10.1083/jcb.133.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Várnai P. B. T. Visualization of phosphoinositides that bind pleckstrin homology domains: calcium- and agonist-induced dynamic changes and relationship to myo-[3H]inositol-labeled phosphoinositide pools. J. Cell Biol. 1998;143(2):501–510. doi: 10.1083/jcb.143.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.