Abstract
Stem cells are classically defined by their multipotent, long-term proliferation, and self-renewal capabilities. Here, we show that increased antioxidant capacity represents an additional functional characteristic of muscle-derived stem cells (MDSCs). Seeking to understand the superior regenerative capacity of MDSCs compared with myoblasts in cardiac and skeletal muscle transplantation, our group hypothesized that survival of the oxidative and inflammatory stress inherent to transplantation may play an important role. Evidence of increased enzymatic and nonenzymatic antioxidant capacity of MDSCs were observed in terms of higher levels of superoxide dismutase and glutathione, which appears to confer a differentiation and survival advantage. Further when glutathione levels of the MDSCs are lowered to that of myoblasts, the transplantation advantage of MDSCs over myoblasts is lost when transplanted into both skeletal and cardiac muscles. These findings elucidate an important cause for the superior regenerative capacity of MDSCs, and provide functional evidence for the emerging role of antioxidant capacity as a critical property for MDSC survival post-transplantation.
INTRODUCTION
Myogenic cell transplantation has been proposed as a promising therapeutic approach in the treatment of skeletal and cardiac muscle injury. Early studies of myoblast transplantation into dystrophic skeletal muscle yielded limited regeneration of dystrophin-expressing muscle fibers (Beauchamp et al., 1994; Gussoni et al., 1997; Qu-Petersen et al., 2002). Similarly, given the limited regenerative ability of cardiac muscle, cell transplantation has been proposed as an alternative to heart transplantation (Assmus et al., 2006; Lunde et al., 2006; Schachinger et al., 2006).
A major obstacle in both cardiac and skeletal myogenic therapies is the poor rate of engraftment of myogenic cells after transplantation (Taylor et al., 1998; Oshima et al., 2005). In skeletal muscle, numerous groups have observed a rapid inflammatory response that appears to contribute to rapid cell loss and limit therapeutic success (Beauchamp et al., 1994; Gussoni et al., 1997; Beauchamp et al., 1999). Multiple groups have postulated that the small number of injected cells that survive transplantation in both cardiac and skeletal muscle may represent a special subpopulation of stem-like cells (Qu et al., 1998; Beauchamp et al., 1999; Qu-Petersen et al., 2002).
For these reasons, our group attempted to isolate this putative subpopulation of cells using a modified preplate technique. This procedure was initially developed to purify myoblasts from nonmyogenic cells, including fibroblasts, of whole tissue preparations based on the differential adhesion characteristics of the cells to a collagen coated flask (Rando and Blau, 1994). Our group modified this technique to isolate various populations of myogenic cells, including a population of early adhering preplate (EP) cells and a late adhering preplate (LP) cell population from which a subpopulation of long-term proliferating (LTP) cells, also known as muscle derived stem cells (MDSCs), has been isolated (Gharaibeh et al., 2008). Although it should be noted that both myoblasts and MDSCs fuse to one another and with host endogenous myofibers, forming dystrophin(+) myofibers, MDSCs demonstrate a superior ability to regenerate skeletal muscle fibers (Jankowski et al., 2001; Qu-Petersen et al., 2002). Similarly, MDSCs injected into hearts after myocardial infarction improve cardiac function to a greater extent than transplanted myoblasts (Oshima et al., 2005; Payne et al., 2007).
MDSCs differ from myoblasts not only in their cell marker expression but also in their behavior, which includes: long-term proliferation, multipotency, self-renewal ability, and their high regenerative capacity. It should be noted that MDSCs are isolated based on their late adhesion characteristics and not on their cell marker expression. Although their cell marker profile has been characterized subsequent to preplate isolation (Jankowski et al., 2001, 2002; Jankowski and Huard, 2002; Deasy et al., 2005, 2007), their designation as stem cells is multifactorial. It is predicated on their multipotency, their cell marker expression, their self-renewal abilities, and most importantly by their significantly increased regenerative capacity when compared with myoblasts (Qu-Petersen et al., 2002; Oshima et al., 2005). In fact, the least reliable method for their appropriate designation as stem cells has been their maintenance of a stable marker profile. Myoblasts, on the other hand, differ significantly from MDSCs in that they cannot usually be cultured for long periods of time due to their rapid differentiation into myotubes, they express the late myogenic cell marker Pax7 (unlike MDSCs), and they engraft poorly when transplanted into the skeletal muscle of mdx mice.
CD34 and Sca1 cell marker expression in MDSCs has been shown to be influenced by cell culture even after clonal isolation. Although other groups have used cell markers to isolate a multipotent cell fraction from skeletal muscle, the CD34 and Sca-1 marker profiles of MDSCs are heterogeneous (Jankowski et al., 2001, 2002). When MDSCs are sorted using fluorescence-activated cell sorting (FACS) by their CD34 and Sca-1 expression, heterogeneity is reestablished after in vitro culture. Furthermore, we have found using FACS-sorted and clonal populations of MDSCs, that expression of these two cell markers does not exclusively predict the engraftment size of dystrophin-(+) myofibers or regeneration index (RI = number of dystrophin(+) myofibers/number of cells transplanted) in dystrophin deficient, mdx mice (Jankowski et al., 2001; Qu-Petersen et al., 2002; Deasy et al., 2005, 2007).
Several groups have isolated stem cell and early progenitor populations from skeletal muscle based on differential adhesion and cell marker expression (Young et al., 2001; Gharaibeh et al., 2008). More recently, a skeletal muscle precursor population was isolated via FACS sorting CD45−, Sca1, Mac1, CXCR4+, and β1 integrin+ subpopulations of satellite cells (Cerletti et al., 2008), and a myogenic endothelial cell population in skeletal muscle was initially reported based on CD34+ and CD45− expression, which was shown to improve cardiac function after myocardial infarction (Tamaki et al., 2002, 2008a). Tamaki et al. (2007, 2008b) have also clonally isolated so-called skeletal double-negative cells (Sk-DN), which are CD34−/CD45−, and have demonstrated their multipotency in vitro and in vivo. Our group has reported a similar population of so-called myoendothelial cells isolated from human skeletal muscle based on their coexpression of CD56, CD34, and CD144 (Zheng et al., 2007). Furthermore, pericytes isolated from skeletal muscle also display a high regeneration index in skeletal muscle similar to myogenic endothelial cells and MDSCs (Dellavalle et al., 2007; Crisan et al., 2008), which has led to the hypothesis that all of these populations may originate from a blood vessel wall niche in skeletal muscle (Tamaki et al., 2002; Tavian et al., 2005; Peault et al., 2007).
Defined by their properties to differentiate into multiple tissue types, their propensity for self-renewal, and their capacity for long-term proliferation, stem cells promise to pave the way for potential cell therapies in the future; however, oxidative, inflammatory, and other cell stress associated with transplantation can restrict the regenerative capacity of these cells. Emerging evidence of increased antioxidant capacity as a characteristic of stem cells suggests a further justification for the pursuit of stem cell therapies. Stem cells may possess an ability to avoid the oxidative damage to which their more differentiated counterparts, such as myoblasts, are more vulnerable (Dernbach et al., 2004; He et al., 2004).
The presence of inflammation at the site of transplantation in both injured and diseased skeletal and cardiac muscle suggests that inflammatory and oxidative stress may play an important role in the regenerative potential of a given cell population (Beauchamp et al., 1994; Huard et al., 1994; Mendell et al., 1995; Fan et al., 1996; Gussoni et al., 1997; Urish et al., 2005). Inflammation, the associated release of proinflammatory cytokines, and oxidative stress have been associated with multiple types of cell transplantation, including myoblast transplantation into skeletal and cardiac muscle (Suzuki et al., 2004; Urish et al., 2005), transplantation of pancreatic islets (Bottino et al., 2004), and bone marrow transplantation (Blackwell et al., 2000; Evens et al., 2004). The destructive power of oxygen is a major component of inflammation through its ability to strip electrons and form highly reactive oxygen species (ROS). After cell transplantation, inflammation via the secretion of cytokines, recruitment of inflammatory cells, and vascular exudation can induce mechanical perturbation of the microvascular barrier and local ischemia (Gute et al., 1998; Carden and Granger, 2000). Ischemia and the associated reperfusion injury is directly linked to the production of various ROS (Kaminski et al., 2002). In addition to the respiratory burst of inflammatory cells such local ischemia can further induce ROS production via xanthine oxidase conversion from xanthine dehydrogenase.(Chanock et al., 1994; Nishino, 1994; Gute et al., 1998; Makazan et al., 2007).
Multiple groups have shown that stem cells appear to have an increased antioxidant capacity (Dernbach et al., 2004; He et al., 2004), whereas others have demonstrated the deleterious role of inflammation and oxidative stress in cell transplantation (Qu et al., 1998; Suzuki et al., 2000). Our group has shown that MDSCs have lower rates of stress-induced cell death, and we have speculated that the MDSCs' increased regenerative capacity may relate to an increased resistance to oxidative and inflammatory stress (Oshima et al., 2005; Deasy et al., 2007). In this article, we extend this work and hypothesize that the MDSCs' increased antioxidant capacity may be responsible for the increased regeneration capacity of these cells when compared with myoblasts.
MATERIALS AND METHODS
Cell Isolation and Culture
MDSCs were isolated from the skeletal muscle of 3-wk-old C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME) as previously described using a modified version of the preplate technique (Qu-Petersen et al., 2002; Gharaibeh et al., 2008). Myoblasts were isolated from 1–3-wk-old C57BL/6J mice using this same method as previously described (Qu-Petersen et al., 2002; Gharaibeh et al., 2008). All cells were cultured and expanded in high-serum proliferation media (DMEM, 10% fetal bovine serum, 10% horse serum, 1% penicillin/streptomycin, and 0.5% chick embryo extract). Myoblasts were cultured for <8 passages before each experiment. The MDSCs that were used for the transplantation experiments were all ≤20 passages. It is important to note that recent work has shown that MDSCs can be cultured past the Hayflick limit of 200 doublings while preserving their regeneration index and not exhibiting any abnormal neoplastic transformation properties (Lee et al., 2000; Deasy et al., 2002, 2003, 2005, 2007).
Fusion Index
The rate and extent of myoblast and MDSC fusion into syncytial myotubes was monitored after a single 24 h exposure to H2O2 or TNF-α to simulate an oxidative or inflammatory stress challenge. Cells were plated at an initial density of 1250 cells/cm2 and cultured in high-serum proliferation media. After 48 h, the culture medium was replaced with low-serum medium (DMEM, 2% fetal bovine serum, and 1% penicillin/streptomycin) to induce differentiation. At this point H2O2 (10, 25, 50, and 100 μM; Sigma, St. Louis, MO) and TNF-α (1.0, 2.5, and 5.0 ng/ml; R&D Systems, Minneapolis, MN) was added. Media were replaced daily with fresh differentiation media (without H2O2 or TNF-α).
At days 3 and 4, the cells were fixed in cold methanol and evaluated for the presence of skeletal fast myosin heavy chain-positive myotubes (1:400, MY-32 clone, Sigma) and counterstained with DAPI (1:1000, Sigma). Fluorescence microscopy was performed on a Leica DMIRB microscope (Deerfield, IL) with a Retiga 1300 digital camera (QImaging, Burnaby, BC, Canada). All images were acquired with Northern Eclipse software (version 6.0; Empix Imaging, Mississauga, ON, Canada).
These images were used to measure the fusion index (Jankowski et al., 2002), defined as the ratio of the total number of nuclei in myosin-heavy-chain–expressing cells compared with the total number of nuclei of the entire cell population. Each dose- and time-dependent experiment was performed in triplicate using six randomly selected microscope fields for quantification in each experiment.
Additional experiments to test the effects of sustained inflammatory stress (TNF-α) on myogenic differentiation were conducted in an identical manner as described above with the following changes. Fresh differentiation medium with 5.0 ng/ml TNF-α was exchanged daily in each cell population. The fusion index was measured on days 3, 4, and 7.
Cell Death
MDSCs and myoblasts were cultured for 24 h under normal culture conditions and then incubated in H2O2 (100, 250, and 500 μM) at 37°C. After 15 h, the media were collected; cells were washed in PBS, harvested in 0.01% trypsin-EDTA (Invitrogen Laboratories, Carlsbad, CA), and resuspended in proliferation media. The number of apoptotic and necrotic cells was measured by staining the cells with annexin-V and propidium iodide according to manufacturer's directions (BD Bioscience, San Jose, CA) and quantifying their numbers using FACS (FACSAria; FACSDiva Software; BD Bioscience) with standard calibration and one-color control for compensation of fluorochromes. Total cell death was determined as the sum of necrotic and apoptotic cell death fractions normalized to exclude cellular debris.
To monitor the rate of cell death over a continuous time period, a modified live cell microscopy technique was used to measure percentages of annexin-V–positive cells. Cells were seeded at an initial density of 2000 cells/cm2 on collagen Type-I 24-well plates and cultured for 24 h. Cells were loaded with Cell Tracker Red-CMTPX (Molecular Probes, Eugene, OR) according to manufacturer's directions to aid in segmentation of the entire cell population. Cells were then placed in proliferation media containing 15 μg/ml annexin-V FITC (BD Bioscience) and 10 μg/ml propidium iodide. A microscope imaging system (Kairos, Harmarville, PA) was used to acquire light and fluorescent time-lapsed images on a 30-min time interval (Deasy et al., 2002; Bahnson et al., 2005). At each time point, nine images in each plate were collected, resulting in over 45,000 images and 1–3 × 105 total events being recorded. After an initial baseline measurement was collected, cells were incubated with increasing doses of H2O2 (10, 25, 50, 100, 250, and 500 μM; Sigma) and TNF-α (1.0, 2.5, and 5.0 ng/ml; R&D Systems). Images were collected on a 30-min interval over a 24-h period. The percentages of annexin-positive cells in each population at each time period were measured using open source software.
Antioxidant Capacity
The antioxidant capacity of each cell population was assessed by measuring the levels of reduced glutathione (GSH), superoxide dismutase (SOD) activity, and intracellular ROS after H2O2 challenge. Levels of ROS were measured using 5-(and-6)-carboxy-2′,7′-dichlorodihydrofluorescein diacetate (carboxy-H2DCFDA; Molecular Probes). Briefly, cells were plated at 2500 cells/cm2 and after 48 h were loaded with 5 μM carboxy-H2DCFDA for 30 min in proliferation media, washed, and exposed to 500 μM H2O2. At 15-min time intervals, cells were harvested and green fluorescence was immediately measured using flow cytometry (Hempel et al., 1999).
Levels of GSH were monitored using monochlorobimane (MCB; Molecular Probes), a nonfluorescent bimane that reacts with free GSH to form a highly fluorescent derivative. Cells were loaded with 4 μM of MCB for 30 min in proliferation media, harvested, washed, and the fluorescence was measured using FACS with single parameter controls.
Total activity of SOD was measured using a colorimetric assay (Chemicon, Temecula, CA; APT290). Myoblast and MDSC cell samples containing 2 × 106 cells were homogenized using a lysis buffer (10 mM Tris, pH 7.5, 150mN NaCl, 0.1 mM EDTA, and 0.5% Triton X-100) and centrifuged at 12,000 × g for 10 min to collect cell lysate. SOD activity was measured according to manufacturer's directions.
GSH Depletion
To assess the role of GSH in regenerative capacity, MDSCs were depleted of cellular GSH using diethyl maleate (DEM, Sigma), which conjugates directly with GSH and renders it inactive (Plummer et al., 1981). After 48 h in standard culture conditions, cells were treated with 50 μM DEM for 2 h, washed twice in PBS, and then cultured in fresh proliferation media. In some in vitro experiments, other concentrations of DEM were used using the same protocol where noted in the results section.
Cell Transplantation
All animal surgical procedures were approved by The Institutional Animal Care and Use Committee, Children's Hospital of Pittsburgh (Protocol 7/03). MDSCs and myoblasts were injected into Mdx mice as previously described (Lee et al., 2000; Qu-Petersen et al., 2002). Briefly, 2 × 105 viable cells suspended 30 μl of PBS were injected into the gastrocnemius muscle of 4–6-wk-old Mdx mice (C57BL/10ScSn DMDmdx/J, The Jackson Laboratory). The mice used in the experiment were not immunosuppressed, and the injected muscle was not injured nor irradiated before or after cell transplantation. These methods were not used because in our experience immune suppression and muscle injury (via irradiation or cardiotoxin injection) are not required for large engraftments of the MDSCs (Qu-Petersen et al., 2002; Deasy et al., 2007; Cerletti et al., 2008). Because the myoblasts and MDSCs were isolated from inbred mice with identical backgrounds (C57BL/6J) and are injected into inbred host mdx mice, any immunological response elicited by the injected cells should be identical unless the cell type injected is exhibiting an immunologically privileged behavior—a behavior that is, indeed, exhibited by stem cells (Qu-Petersen et al., 2002).
Coronary artery ligation and cell transplantation into infarcted hearts were performed in 14-wk-old C57BL/6J mice (Jackson Laboratory), and physiological function was measured as previously described (Oshima et al., 2005; Payne et al., 2007). Briefly, myocardial infarctions were induced by ligating the left anterior descending coronary artery. Cells (3 × 105) or PBS were injected into the anterior, center, and lateral aspects of the infracted myocardium. Echocardiography (Sequoia C256 system; Siemens, Malvern, PA) was performed at 6 wk to assess the systolic function. Two-dimensional images of the heart were obtained at the midpapillary muscle level. The end-diastolic area (EDA) and end-systolic area (ESA) were measured from short-axis images of the left ventricle. The end-diastolic dimension (EDD) and end-systolic dimension (ESD) were measured from at least six consecutive beats from an M-mode tracing. Systolic function was assessed by measuring the fractional shortening (FS) and fractional area change (FAC), a measure of the change in length and area of the myocardium during systole, respectively, that represents the degree of muscle contraction. FS is defined as [(EDD − ESD)/EDD], and the FAC is defined as [(EDA − ESA)/EDA].
Histological Analysis
Skeletal muscle sections were stained for dystrophin (1:50, Dys-2, Novocastra, Burlingame, CA) using protocols previously described (Deasy et al., 2007). Infarct scar on cardiac sections were stained using Mason's Modified IMEB Trichrome Stain Kit (International Medical Equipment, San Marcos, CA) according to manufacturer's instructions. A mouse anti-fast skeletal myosin heavy-chain (MHC) antibody (1:400, MY-32 clone, Sigma) and a rat anti-mouse CD31 antibody (1:1000, Becton Dickinson Pharmingen, San Diego, CA) was used to immunostain cardiac muscle sections for myofibers and capillaries as previously described (Oshima et al., 2005; Payne et al., 2007). Fluorescence and bright field microscopy was performed using Nikon Eclipse E800 microscope (Melville, NY) equipped with a Retiga Exi digital camera (QImaging). All images were acquired with Northern Eclipse software (version 6.0; Empix Imaging).
The scar tissue ratio was measured from digital images collected at low power (20×) of the entire left ventricle cross section after staining with Mason's trichrome. Scar tissue ratio was measured as the number of pixels in the area of fibrosis to the number of pixels in the entire area of the left ventricle cross section (six sections/animal). Myofiber engraftment size was measured as the total number of pixels in the skeletal myofiber engraftment on the cardiac cross section. The largest engraftment was used for each animal was used for this measurement. We measured the capillary density by counting the number of CD31+ capillary structures per high-power field (200×) within the infarct and MHC+ area (six images/animal).
Image Analysis
Image analysis using computing software was conducted as noted above to measure rates of apoptosis over a continuous time period and to measure the fusion index. All programs, except where noted, were written as open source, freely available code using the Insight Toolkit, an image segmentation and registration C++ code library (Yoo et al., 2002). The area of MHC expression, the scar tissue ratio, and the capillary density in the induced myocardial infarction animal model were measured using ImageJ (version 1.23j, National Institute of Health; http://rsb.info.nih.gov/ij/).
Statistical Analysis
Data are expressed as a mean ± SE, except where noted. Direct comparisons between two cell populations were made using an unpaired, two-tailed Student's t test. Statistical significance was determined if p < 0.05. All statistical tests were completed using R (R Core Development Team, www.r-project.org). Comparisons of single groups were completed using one-way ANOVA. Multiple group comparisons were made using two-way ANOVA. In both cases, significance levels were determined using the Student-Newman-Keuls pairwise comparison.
RESULTS
MDSCs Have Less Oxidative and Inflammatory Stress–induced Cell Death
Given that MDSCs have demonstrated an increased regenerative capacity in both skeletal and myocardial muscle (Qu-Petersen et al., 2002; Oshima et al., 2005), we hypothesized that the inflammatory response observed at the site of transplantation is a major determinant in the differential regenerative capacity of MDSCs and myoblasts. Our primary objective was to determine if a differential resistance to oxidative (H2O2) or inflammatory (TNF-α) stress could be observed in vitro between the two cell populations, in terms of survival, differentiation, and antioxidant capacity.
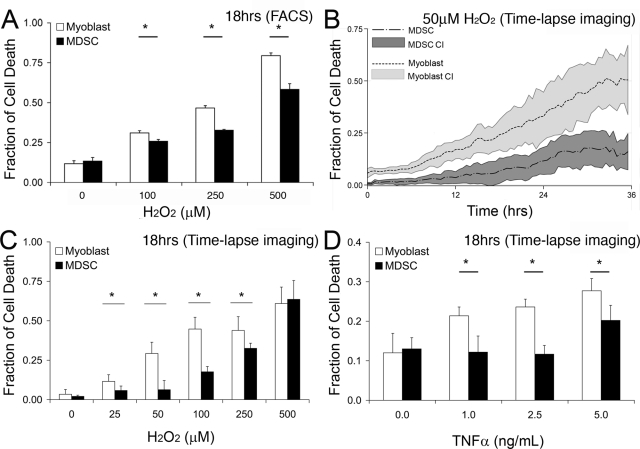
To test this hypothesis in terms of survival, myoblasts and MDSCs were exposed to H2O2 (100, 250, 500 μM) in vitro for a period of 18 h to determine if MDSCs have a survival advantage after oxidative stress. The early apoptotic marker, annexin-V, and the later apoptotic marker, propidium iodide exclusion, were used to measure total cell death using FACS. Myoblasts had significantly higher rates of cell death compared with MDSCs at each dose tested (p < 0.05; Figure 1A), suggesting that MDSCs were more resistant to oxidative stress–induced cell death after 18 h of exposure. A limiting factor in these experiments was the ability to only collect a limited series of measurements at one time point.
Figure 1.
MDSCs have lower rates of cell death after exposure to H2O2 and the inflammatory cytokine TNF-α than myoblasts. (A) Total levels of apoptosis and necrosis were measured at 18 h after exposure to H2O2 using flow cytometry with annexin-V and propidium iodide staining. (B) Levels of cell death were measured over a continuous 36 h period using time-lapse imaging with annexin-V staining after exposure to 50 μM H2O2. Solid lines represent the mean fraction of annexin-V-positive cells, whereas the range-plot represents the 95% confidence interval (CI) of these measurements. (C and D) Using the approach outlined in B, levels of annexin-V staining over a 24 h period were monitored after exposure to increasing doses of H2O2 (C) and TNF-α (D). ANOVA; *p ≤ 0.05; mean values ± CI shown.
This differential resistance to stress was confirmed using a robotic live-cell microscopy system to measure levels of annexin-positive cells continuously for a period of 24 h (Deasy et al., 2003). Using this approach, it was possible to screen a larger number of doses of H2O2 over a continuous time period. MDSCs exhibited superior survival rates compared with myoblasts over a broad range of H2O2 concentrations (p < 0.05; 0, 25, 50, 100, 250 μM H2O2; Figures 1B). Figure 1C is an 18 h time point snapshot of the live-cell microscopy experiment showing agreement with data obtained in Figure 1A. This observed survival advantage of MDSCs is lost at a H2O2 concentration of 500 μM.
A similar MDSC survival advantage was observed when both cell populations were exposed to increasing doses of the inflammatory cytokine TNF-α (1.0, 2.5, 5.0 ng/ml), using the same robotic live-cell microscopy system. A representative summary of these results at 18 h reveals that myoblasts have significantly higher rates of cell death than MDSCs at all doses (p < 0.05; Figure 1D). These results mirror the observations from measuring cell death after exposure to H2O2.
MDSCs Maintain the Ability to Differentiate After Oxidative Stress
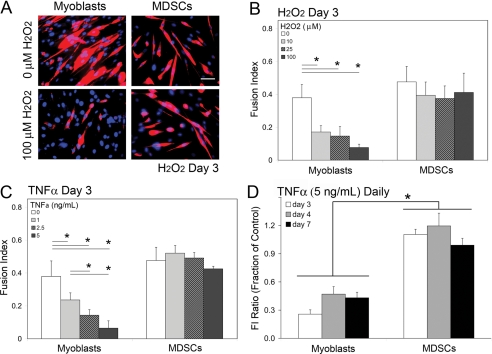
The capacity of a cell population to differentiate is an important measure of how well the cell may engraft to and/or induce regeneration of the host muscle. Therefore, a survival advantage of MDSCs alone may not be sufficient for their superior regenerative capacity in skeletal and cardiac muscle. We hypothesized that MDSCs would maintain their ability to differentiate into myotubes after exposure to oxidative and inflammatory stresses better than myoblasts. Myogenic differentiation of both MDSC and myoblast cell populations were measured after exposure to both H2O2 and TNF-α. After high-density culture in low serum media to induce differentiation, cells were exposed to either H2O2 (10, 25, 100 μM) or TNF-α (1.0, 2.5, 5.0 ng/ml) for 24 h. After this 24 h stress challenge, the low-serum media were exchanged on a daily basis. (Note: H2O2 and TNF-α were not included in the media after the stress challenge). Differentiation was quantified at 3 and 4 d after treatment using the fusion index, which is defined as the ratio of the total number of nuclei in fast MHC-expressing cells, a late differentiation myogenic protein, to the total number of nuclei.
Large differences between the ability of MDSCs and myoblasts to form myotubes after exposure to oxidative and inflammatory stresses were observed at day 3. Representative images of MDSCs and myoblasts exposed to 100 μM H2O2, and untreated controls show that MDSCs maintained similar rates of myogenic differentiation after H2O2 exposure, whereas myoblasts had a substantial decrease in myogenic differentiation (Figure 2A). Although the fusion index of MDSCs remained constant at increasing doses of H2O2, myoblasts had a significant and progressive dose-modulated decrease in their fusion index (p < 0.05; Figure 2B). A similar response was measured when both cell populations were exposed to TNF-α (p < 0.05; Figure 2C). In both cases, the dose response behavior was temporary, given that by day 4, no difference in the rate of differentiation of myoblasts or MDSCs was observed (Supplemental Figure S1).
Figure 2.
Oxidative and inflammatory stress delays differentiation in myoblasts but not in MDSCs. (A) Representative images of the MHC expression (red) of MDSCs and myoblasts at day 3 after a 24 h exposure to H2O2 (nuclei stained with dapi in blue) are shown. (A) Bar, 50 μm. (B) At day 3, MDSCs exposed to H2O2 displayed similar rates of differentiation as untreated MDSC controls. Myoblasts displayed less differentiation than myoblast controls. Results were similar with exposure to TNF-α. (C) At day 3, MDSCs exposed to TNF-α displayed similar rates of differentiation as untreated MDSC controls. Myoblasts displayed less differentiation than myoblast controls. (D) Myoblast differentiation inhibited on daily exposure to TNF-α (5 ng/ml), whereas MDSC differentiation is unaffected. Data are expressed as an FI ratio of stressed and unstressed cell groups. (B–D) ANOVA; *p ≤ 0.05; mean values ± SEM.
These experiments were conducted using only an initial temporary stress insult. In general, H2O2 added to cell culture systems is rapidly neutralized by catalase. Further, medium was changed on a daily basis, limiting the effects of TNF-α, H2O2, and any additionally generated redox or inflammatory species. To extend the time period of exposure to oxidative and inflammatory stresses, cells undergoing myogenic differentiation were exposed to a daily, repeated exposure of TNF-α (5 ng/ml) for 1 wk. That is, the low-serum-differentiation media were supplemented with TNF-α each day it was replaced. This is approximately the concentration of TNF-α seen in different pathological conditions (Vreugdenhil et al., 1992; Chen et al., 2004). For clarity, data are expressed as a ratio of the fusion index of the stressed cell group to the unstressed cell group, termed fusion index (FI) ratio. For example, an FI ratio of 0.5 indicates that the stressed cell population had 50% of the myogenic differentiation compared with the unstressed cell group at the same time point. Similar to the previous experiments, the rate of MDSC myogenic differentiation was unaffected by the exposure. The average fusion index ratio was approximately 1 at all three time points, indicating that the rate of myogenic differentiation of stressed MDSCs was nearly identical to the unstressed MDSCs. In contrast, myoblast differentiation remained suppressed. The average FI ratio was 0.39 ± 0.07 (mean ± SEM), indicating that stressed myoblasts had ∼40% of the differentiation as that of unstressed myoblasts (Figure 2D).
MDSCs Exhibit Lower Levels of Intracellular ROS
Given that MDSCs had lower rates of cell death and maintained their ability to differentiate compared with myoblasts after exposure to oxidative stress, we hypothesized that these differences in survival would correlate with the cells' sustaining differential oxidative damage. We monitored the levels of intracellular ROS in MDSCs and myoblasts after exposure to a high dose of H2O2 (500 μM) using FACS with the fluorescent indicator carboxy-H2DCFDA over a 2 h time period. Our results indicate that myoblasts had a significant peak in intracellular ROS concentration 30 min after H2O2 exposure that was sevenfold higher than that of MDSCs for the same time point (p < 0.05; Figure 3A).
Figure 3.
MDSCs have lower degrees of oxidative damage and a higher antioxidant capacity than myoblasts. (A) Measured intracellular ROS levels after exposure to 500 μM H2O2 for 2 h. MDSCs had little increase in ROS, whereas myoblasts had a large increase. (B) MDSCs have higher levels of GSH as shown by the mean fluorescence after MCB staining. (C) MDSCs had a significantly higher total SOD activity than myoblasts. (B and C) Student's t test; *p ≤ 0.05; mean values ± SEM.
MDSCs Have Increased Protection from ROS
Decreased levels of intracellular ROS suggest that MDSCs have increased antioxidant activity to defend against redox imbalances. A cell has a vast array of enzymes and compounds to defend against these oxidative stresses including GSH, SOD, peroxiredoxins, glutathione peroxidase (GPx), catalase, and others. The dominant forms in terms of activity are GSH and the multiple isoforms of SOD.
As an initial study of the sources of MDSCs' increased antioxidant capacity, we sought to investigate the activity of GSH and SOD. Basal concentrations of GSH were measured using FACS after staining with monochlorobimane (MCB). MDSCs had a 2.5-fold increase in mean fluorescence, revealing that the differences in GSH levels are significant (p < 0.05; Figure 3B). Total SOD activity was measured using a colorimetric assay. MDSCs had a 0.5-fold higher level of total basal SOD activity when compared with myoblasts (p < 0.05; Figure 3C). These experiments combined with MDSCs having lower levels of ROS after exposure with H2O2 suggest that MDSCs have an increased antioxidant capacity when compared with myoblasts.
Decreasing the GSH Levels of MDSCs Decreases the Regeneration Capacity of MDSCs in Skeletal Muscle
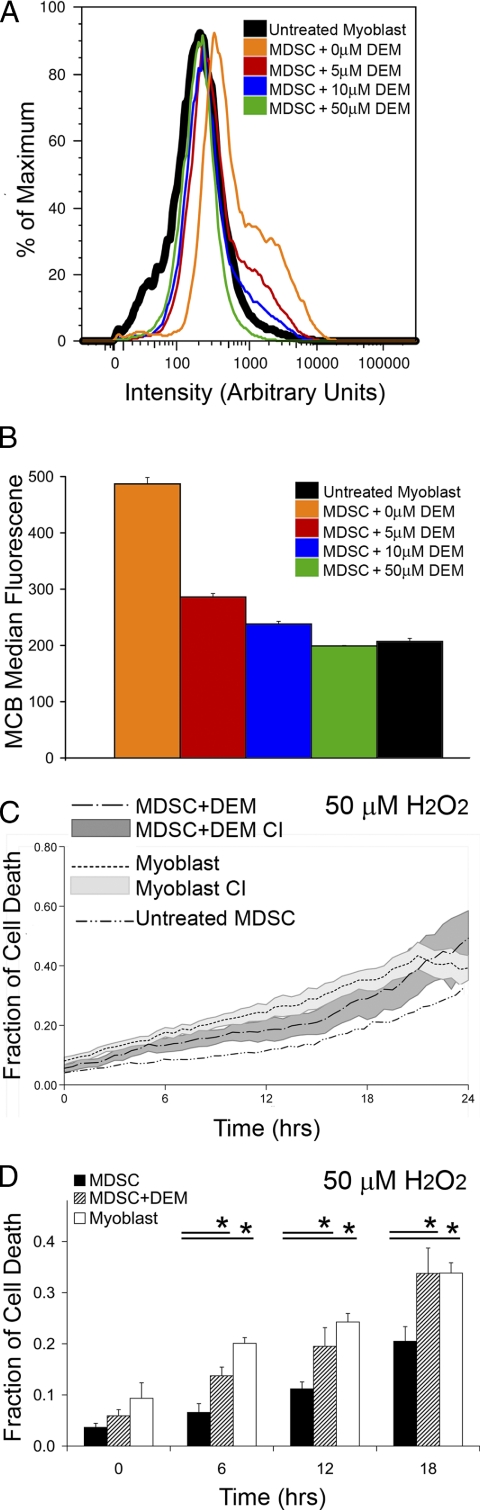
To assess the role antioxidant capacity plays after cell transplantation in vivo, we decreased the MDSC GSH levels to that similar to myoblasts and measured the regenerative capacity of the cells after injection into the gastrocnemius muscles of Mdx mice. For this purpose, cells were exposed to DEM, a specific, nonprotein thiol-depleting agent that selectively inhibits glutathione activity (Plummer et al., 1981). After exposure to increasing doses of DEM, the basal levels of GSH were measured by MCB fluorescence using flow cytometry. DEM concentrations of 10 or 50 μM were found to reduce the level of GSH in MDSCs to levels comparable to those in myoblasts (Figure 4, A and B). To ensure that the overall antioxidant capacity of MDSCs had been reduced to that comparable to myoblasts, oxidative stress experiments using the live cell microscopy system were repeated using DEM-treated (50 μM) MDSCs and myoblasts both under conditions of H2O2 stress (100 μM). Indeed the DEM-treated MDSCs had similar rates of cell death as the myoblast population across multiple time points (Figure 4, C and D). Thus, 50 μM of DEM was selected to inhibit MDSCs' resistance to oxidative stress and make the MDSCs comparable to myoblasts in terms of antioxidant capacity for the remainder of the in vivo experiments.
Figure 4.
Decreasing the levels of GSH in MDSCs, by treatment with DEM, decreases the MDSCs' resistance to stress. (A) Histogram plots of GSH levels of MDSCs treated with increasing doses of DEM (0, 5, 10, 50 μM) compared with untreated myoblasts. (B) The median fluorescence of the groups in A is shown for clarity. (C) Rates of cell death for MDSCs treated with 50 μM DEM, untreated MDSCs, and untreated myoblasts after exposure to 100 μM H2O2. The area associated with each line represents the 95% CI of the respective mean. Untreated MDSCs were included for reference; the CI of this group is not included to increase visual clarity. (D) Six-hour interval snapshots of C indicate that MDSCs treated with DEM have rates of oxidative stress–induced cell death comparable to that of myoblasts, and both groups have higher rates of cell death compared with MDSCs. ANOVA; *p ≤ 0.05; mean values ± CI are shown, except in B where the median value ± SEM is shown.
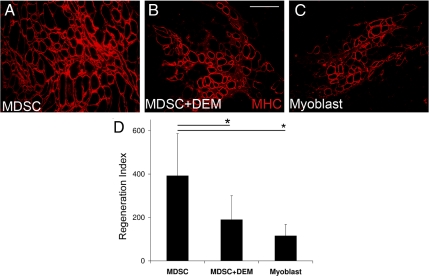
To clarify the role oxidative stress plays in the regenerative capacity of MDSCs, an equal number of myoblasts, untreated MDSCs, and DEM-treated MDSCs were injected into the gastrocnemius of Mdx mice. After 2 wk, the regeneration index was measured. MDSCs regenerated a significantly greater number of dystrophin-positive myofibers than the DEM-treated MDSCs (Figure 5, A–C). Furthermore, there was no statistically significant difference between the regeneration index of the DEM-treated MDSCs and myoblasts. Yet both of these groups had significantly less dystrophin-positive myofibers than untreated MDSCs (p < 0.05; Figure 5D). These results suggest that when the antioxidant advantage of the MDSC is lost, the superior ability to regenerate dystrophin-positive myofibers is also lost.
Figure 5.
Decreasing the antioxidant capacity of MDSCs decreases the ability of MDSCs to regenerate dystrophin-positive myofibers. MDSCs treated and untreated with 50 μM of DEM and myoblasts were injected into the skeletal muscle of Mdx mice that were sacrificed at 2 wk, and the regeneration index (RI) was measured. (A–C) Representative images of the dystrophin-positive engraftments of MDSCs, skeletal muscles treated with MDSCs, and myoblasts, respectively, are shown. (B) Bar, 50 μm. (D) MDSCs treated with 50 μM of DEM had regeneration indices similar to that of myoblasts and statistically significant lower regeneration indices than untreated MDSCs. ANOVA; *p ≤ 0.05; n = 8 in each group; mean values ± SEM.
Decreasing the GSH Levels of MDSCs Decreases the Regeneration Capacity of MDSCs in Cardiac Muscle
We proceeded to determine whether decreased antioxidant capacity would also decrease the regenerative capacity of MDSCs in other tissues such as cardiac muscle. Induced myocardial infarctions are a valuable injury model because functional outcomes after cell transplantation can be measured using echocardiography. The goal of these experiments was to measure the changes in MDSCs' ability to improve cardiac function after their antioxidant capacity had been reduced.
Myocardial infarctions were induced in adult wild-type C57 mice by ligating their left anterior descending artery and which was followed by immediately injecting both cell populations as previously reported (Oshima et al., 2005; Payne et al., 2007). Functional analysis was performed using echocardiography at 6 wk and histological analysis was performed at 8 wk to measure the amount of fibrosis, the degree of regeneration, and the capillary density at the injection site.
Cross sections of hearts were stained with Mason trichrome to measure the degree and area of fibrosis. On microscopic observation, infarcted hearts injected with DEM-treated MDSCs and PBS controls had comparable degrees of fibrosis, whereas both groups had more fibrosis than untreated MDSCs (Figure 6A, 1–3). These results were quantified by calculating the scar tissue ratio, defined as the ratio of the area of fibrosis compared with the total cross-sectional area of the heart. Infarcted hearts injected with MDSCs treated with DEM and PBS displayed large and comparable scar tissue ratios. In contrast, untreated MDSCs had a significantly smaller scar tissue ratio than both of these groups (p < 0.05; Figure 6A, 4). These results suggest that decreasing the antioxidant capacity of MDSCs also deteriorates the ability of MDSCs to improve cardiac wound healing and prevent adverse cardiac remodeling, such as scar tissue.
Figure 6.
Decreasing the antioxidant capacity of MDSCs decreases the ability of MDSCs to mitigate adverse remodeling after an induced myocardial infarction. MDSCs, DEM-treated MDSCs, or PBS was injected into the infarction site. Animals were sacrificed at 8 wk for histological analysis. (A) Mason trichrome staining was performed to measure fibrosis. Representative images of infarcted hearts injected with MDSCs (A1), MDSCs treated with DEM (A2), and PBS (A3) are shown. Arrowheads indicate the main areas of infarcted myocardium. Note the thin fibrotic walls in A2 and A3 compared with the small area of fibrosis in A1. Infarcted hearts injected with MDSCs had significantly less fibrosis than hearts injected with MDSCs treated with DEM or PBS as measured by the scar tissue ratio (A4). (A2) Bar, 1000 μm. (B) The vascularity of the infarcted area was measured by staining the sections for CD31, an endothelial cell marker. Representative images of the capillary density of the infarcted area of hearts injected with MDSCs (B1), MDSCs treated with DEM (B2), and PBS (B3) are shown. Arrowheads outline the myocardial wall. Note the reduced capillary density in the myocardium in B2 and B3 when compared with B1. The capillary density was measured by counting the number of capillaries per pixel area. The infarcted area of hearts injected with MDSCs had significantly higher capillary densities than hearts injected with MDSCs treated with DEM and PBS (B4). (B2) Bar, 50 μm. (A4 and B4) MDSCs + DEM, n = 6; MDSCs, n = 6; PBS control, n = 5. ANOVA; *p ≤ 0.05; mean values ± SEM.
Previously, we have shown that the vast majority of MDSCs and myoblasts injected into the heart differentiate into skeletal myocytes expressing MHC (Oshima et al., 2005; Payne et al., 2007). Interestingly, skeletal muscle engraftment size in cardiac cell therapy does not correlate with functional improvement after myocardial infarction. Instead, we have shown that MDSC transplantation mitigates adverse remodeling of cardiac muscle after an infarction possibly by promoting angiogenesis (Oshima et al., 2005; Payne et al., 2007). As a result, we stained remodeling heart cross sections after cell transplantation for CD31, an endothelial cell marker, to measure possible differences in vascularity. On microscopic observation, the vascularity of the infarction sites injected with MDSCs treated with DEM and the PBS control were comparable. Both of these groups appeared to have lower numbers of CD31+ cells than the infarction site of hearts injected with MDSCs (Figure 6B, 1–3). The capillary density was measured by counting the number of capillaries per area of the infarcted myocardium in the image field. Infarcted hearts injected with MDSCs treated with DEM and PBS have statistically comparable capillary densities, and both of these groups' capillary densities are significantly lower than hearts injected with MDSCs (p < 0.05; Figure 6B, 4). These results suggest that decreasing the antioxidant capacity of MDSCs impairs the cell population's ability to promote angiogenesis and mitigate adverse remodeling or scar formation.
Echocardiography was performed to compare left ventricle function 6 wk after infarction. Two-dimensional images were obtained at diastole and systole to observe the contraction of the myocardium. M-mode tracings were used to measure the EDD and ESD as previously described (Figure 7A; Oshima et al., 2005; Payne et al., 2007). No differences in left ventricular cavity size as measured by the area of the myocardium during diastole were observed among all three groups as assessed by EDA (Figure 7B), a result commonly observed in this type of experiments (Oshima et al., 2005; Payne et al., 2005). As assessed by FS and FAC, infarcted hearts injected with MDSCs had significantly better systolic function relative to the infarcted hearts injected with both MDSCs treated with DEM and PBS (p < 0.05). Measuring FS, infarcted hearts injected with MDSCs treated with DEM had statistically increased systolic function compared with hearts injected with PBS (p < 0.05). Measuring FAC, infarcted hearts injected with MDSCs treated with DEM had systolic function comparable to that of hearts injected with PBS (Figure 7, C and D). Together, the functional experiments suggest that when the antioxidant capacity of MDSCs is decreased, MDSCs have a limited ability to repair cardiac function.
Figure 7.
Decreasing the antioxidant capacity of MDSCs decreases the ability of MDSCs to improve cardiac function after an induced myocardial infarction. After myocardial infarctions were induced, functional analysis was performed at 6 wk using echocardiography. (A) Representative echocardiograph images of one of the MDSC groups are shown to demonstrate functional measurements. Two-dimensional echocardiograph images of the left ventricle at diastole and systole used to calculate FAC are shown. The myocardium is outlined to demonstrate the change in area during contraction between diastole and systole. The widest diameter of the ventricle during diastole in the two-dimensional echocardiograph image, as indicated by the dashed line, was followed for a period of time over consecutive heartbeats as shown in the M-mode tracing. EDD and ESD were measured to calculate FS. (B) Infarcted hearts injected with MDSCs, MDSCs treated with DEM, and PBS all have similar values of EDA indicating these groups have comparable left ventricular cavity size after infarction. (C) Hearts injected with MDSCs have a significantly increased FS compared with MDSCs treated with DEM and the PBS control, indicating improved cardiac function. Infarcted hearts injected with MDSCs treated with DEM and PBS have comparable FS, indicating similar cardiac function after infarction. (D) Hearts injected with MDSCs have a significantly increased FAC compared with MDSCs treated with DEM and the PBS control indicating improved contractile function. Both groups of MDSCs have an increased FAC over the PBS control. (B–D) MDSCs+DEM, n = 8; MDSCs, n = 7; PBS control, n = 8. ANOVA; *p ≤ 0.05; mean values ± SEM.
DISCUSSION
Multiple cell populations have been investigated in clinical and preclinical trials for dystrophic and cardiac muscle cell therapies (Tremblay et al., 1993; Gussoni et al., 1997; Assmus et al., 2006; Hagege et al., 2006; Lunde et al., 2006). However, a mechanism that drives the variable regenerative capacities between different cell populations in cell therapy has yet to be fully elucidated. We investigated the possible role of MDSCs' antioxidant capacity in its superior regenerative capacity compared with myoblasts. Previous reports have shown that MDSCs have an increased ability to regenerate dystrophin-positive myofibers in dystrophic skeletal muscle compared with myoblasts (Qu-Petersen et al., 2002). Although the increased regenerative capacity of MDSCs has often been attributed to their long-term proliferation and multipotent behavior, we demonstrate here that a resistance to stress plays an important role in this process as well. MDSCs display a high resistance to oxidative stress both in terms of superior rates of differentiation and survival over that of myoblasts. Furthermore the antioxidant capacity appears to play a major role in the regenerative potential of MDSCs in skeletal and cardiac muscles. Other groups have also demonstrated that stem cell populations have increased resistance to stress when compared with their more differentiated counterparts (Dernbach et al., 2004; He et al., 2004). Expanding on this work, we demonstrate that this increased resistance to stress is a major determinant in the cell population's improved regenerative capacity in skeletal muscle and in cardiac muscle at a functional level.
It was noted in this study that MDSCs were maintained at a higher passage number than myoblasts. MDSCs can be maintained in culture through 200 population doublings with no evidence of neoplastic transformation, chromosome abnormalities, or decrease in regenerative capacity (Deasy et al., 2005). There is no evidence that MDSCs obtain increased antioxidant capacity with increasing passage number; indeed, just the opposite has been reported in multiple groups that have demonstrated that low passage cell populations have an increased resistance to stress, antioxidant capacity, and ability to withstand adverse environmental conditions compared with higher passaged cell populations (Luce and Cristofalo, 1992; Yuan et al., 1996; Kaneko et al., 2001; Gurjala et al., 2005; Dowling et al., 2006).
Inflammation clearly is one of the most important hurdles to overcome in order to increase the efficacy of cellular therapies. A major source of the destructive power of inflammation is the direct and indirect generation of ROS and free radicals after the inflammatory cytokine response (Dhalla et al., 1999). In myogenic cell transplantation, a large percentage of myoblasts die within 24–48 h after transplantation into both skeletal and myocardial muscles, which correlates with the time frame of an acute inflammatory response that is observed at the site of injection (Beauchamp et al., 1999; Gussoni et al., 1999; Oshima et al., 2005; Urish et al., 2005). Furthermore and relevant to the cardiac studies presented herein, the pathological hallmark after a myocardial infarction is a rapid and strong inflammatory process (Dhalla et al., 1999; Kaminski et al., 2002; Nian et al., 2004).
We postulated that one of the mechanisms behind the MDSCs' superior regenerative capacity may involve a resistance to these stresses that occurs during cell transplantation. MDSCs consistently had lower rates of cell death and increased rates of differentiation and fusion after exposure to both H2O2 and TNF-α when compared with myoblasts. These results demonstrate that MDSCs possess different and perhaps enhanced mechanisms of handling oxidative stress and inflammation compared with myoblasts.
Different groups have reported that oxidative stress can either induce a “differentiation checkpoint” (Puri et al., 2002) or permanently force a cell into a state of senescence (Chen et al., 2004). A differentiation checkpoint is similar to a cell cycle check-point in that differentiation is arrested until the damage has been repaired and the stress has dissipated. After exposure to inflammatory cytokines such as TNF-α or IL-1, myogenic differentiation is inhibited through activation of the nuclear factor kappa B (NF-κB) pathway (Guttridge et al., 1999; Langen et al., 2004). Inhibiting the activation of NF-κB in Mdx mice, a mouse model of Duchenne muscular dystrophy, improves the ability of muscle progenitor cells to proliferate, form myotubes, and repair damaged muscle fibers (Kumar and Boriek, 2003; Acharyya et al., 2007). Further, direct exposure to oxidative stress inhibits myoblast differentiation through the same mechanism of NF-κB activation (Catani et al., 2004).
Given that the NF-κB pathways may be activated by both oxidative and inflammatory stress, our results support these observations. MDSCs demonstrated no change in their ability to fuse and form myotubes after in vitro exposure to oxidative stress in the form of H2O2 and TNF-α. Myoblasts exposed to increased levels of oxidative stress and an inflammatory cytokine had a temporary decrease in the ability to differentiate, and when this stress was removed, myoblasts resumed differentiation. If myogenic differentiation is inhibited via the NF-κB pathway, this implies that MDSCs may maintain their rate of differentiation after they are exposed to inflammatory and oxidative stresses by mitigating NF-κB activation.
The phenotypic differences in survival and differentiation between MDSCs and myoblasts could be explained by the antioxidant capacity of each population. When the GSH level of MDSCs was reduced to levels similar to myoblasts to decrease antioxidant capacity, MDSCs' capacity to regenerate both skeletal and cardiac muscles was decreased to that of myoblasts. We also showed that these differences were manifested at a functional level in the myocardial infarction animal model. The suggestion that stem cells have an increased resistance to oxidative stress compared with their more differentiated progeny has been described previously (Dernbach et al., 2004; He et al., 2004). Other studies focused on the importance of stress in the transplantation process by genetically engineering the transplanted cell population to resist the effects of inflammation and stress, most notably with heat-shock proteins (Suzuki et al., 2000; Zhang et al., 2001). In this study we extend these findings by showing that the MDSCs' superior antioxidant capacity not only improves its regenerative capacity in skeletal and cardiac muscle but enhances the host tissue's ability to mitigate adverse remodeling in the case of infracted myocardium.
The term “stemness” has been used to describe the properties that define a stem cell and its molecular signature (Ivanova et al., 2002; Ramalho-Santos et al., 2002; Dernbach et al., 2004). These include the upregulation of genes responsible for self-renewal, long-term proliferation, and multipotent differentiation. An emerging stem cell property is its antioxidant capacity and corresponding response to oxidative and inflammatory stresses. After an injury, a toxic environment can be created from inflammation, ischemia, reperfusion, and the ensuing inflammatory cytokine storm. A stem cell's ability to function as a regenerative building block depends on its capacity to withstand and perhaps respond to this noxious environment.
In this study we have observed an increased antioxidant capacity in MDSCs that we believe is a critical if not necessary feature of their superior regeneration capacity in skeletal and cardiac muscles. This feature not only suggests that the MDSCs may have a higher stress capacity than myoblasts, but it also lends credence to our emerging understanding of how an enhanced resistance to oxidative and inflammatory stress pertains to the concept of cellular “stemness.”
Supplementary Material
ACKNOWLEDGMENTS
The authors thank A. Logar for technical assistance, and Dr. Bruno Péault (Pediatrics, University of Pittsburgh) for insightful discussions. This work was supported by the Jesse's Journey Foundation, the Muscular Dystrophy Association, the National Institutes of Health (NIH) Grants R01 AR49684 and R01 AR47973, the William F. and Jean W. Donaldson Chair at Children's Hospital of Pittsburgh, and the Henry J. Mankin Chair at the University of Pittsburgh.
Abbreviations used:
- DEM
diethyl maleate
- EDA
end-diastolic area
- EDD
end-diastolic dimension
- ESD
end-systolic dimension
- MHC
skeletal fast myosin heavy chain
- FAC
fractional area change
- FS
fractional shortening
- GSH
glutathione
- MCB
monochlorobimane
- MDSC
muscle-derived stem cell
- NF-κB
nuclear factor kappa B
- ROS
reactive oxygen species
- SOD
superoxide dismutase.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E08-03-0274) on November 12, 2008.
REFERENCES
- Acharyya S., et al. Interplay of IKK/NF-kappaB signaling in macrophages and myofibers promotes muscle degeneration in Duchenne muscular dystrophy. J. Clin. Invest. 2007;117:889–901. doi: 10.1172/JCI30556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assmus B., et al. Transcoronary transplantation of progenitor cells after myocardial infarction. N. Engl. J. Med. 2006;355:1222–1232. doi: 10.1056/NEJMoa051779. [DOI] [PubMed] [Google Scholar]
- Bahnson A. L., et al. Automated measurement of cell motility and proliferation. BMC Cell Biol. 2005;6:19. doi: 10.1186/1471-2121-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp J. R., Morgan J. E., Pagel C. N., Partridge T. A. Quantitative studies of efficacy of myoblast transplantation. Muscle Nerve. 1994;18(Suppl.):261. [Google Scholar]
- Beauchamp J. R., Morgan J. E., Pagel C. N., Partridge T. A. Dynamics of myoblast transplantation reveal a discrete minority of precursors with stem cell-like properties as the myogenic source. J. Cell Biol. 1999;144:1113–1122. doi: 10.1083/jcb.144.6.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell T. S., Christman J. W., Hagan T., Price P., Edens T., Morris P. E., Wolff S. N., Goodman S. A., Christman B. W. Oxidative stress and NF-kappaB activation: correlation in patients following allogeneic bone marrow transplantation. Antioxid. Redox. Signal. 2000;2:93–102. doi: 10.1089/ars.2000.2.1-93. [DOI] [PubMed] [Google Scholar]
- Bottino R., Balamurugan A. N., Tse H., Thirunavukkarasu C., Ge X., Profozich J., Milton M., Ziegenfuss A., Trucco M., Piganelli J. D. Response of human islets to isolation stress and the effect of antioxidant treatment. Diabetes. 2004;53:2559–2568. doi: 10.2337/diabetes.53.10.2559. [DOI] [PubMed] [Google Scholar]
- Carden D. L., Granger D. N. Pathophysiology of ischaemia-reperfusion injury. J. Pathol. 2000;190:255–266. doi: 10.1002/(SICI)1096-9896(200002)190:3<255::AID-PATH526>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Catani M. V., Savini I., Duranti G., Caporossi D., Ceci R., Sabatini S., Avigliano L. Nuclear factor kappaB and activating protein 1 are involved in differentiation-related resistance to oxidative stress in skeletal muscle cells. Free Radic, Biol. Med. 2004;37:1024–1036. doi: 10.1016/j.freeradbiomed.2004.06.021. [DOI] [PubMed] [Google Scholar]
- Cerletti M., Jurga S., Witczak C. A., Hirshman M. F., Shadrach J. L., Goodyear L. J., Wagers A. J. Highly efficient, functional engraftment of skeletal muscle stem cells in dystrophic muscles. Cell. 2008;134:37–47. doi: 10.1016/j.cell.2008.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanock S. J., el Benna J., Smith R. M., Babior B. M. The respiratory burst oxidase. J. Biol. Chem. 1994;269:24519–24522. [PubMed] [Google Scholar]
- Chen J. H., Stoeber K., Kingsbury S., Ozanne S. E., Williams G. H., Hales C. N. Loss of proliferative capacity and induction of senescence in oxidatively stressed human fibroblasts. J. Biol. Chem. 2004;279:49439–49446. doi: 10.1074/jbc.M409153200. [DOI] [PubMed] [Google Scholar]
- Crisan M., Deasy B., Gavina M., Zheng B., Lazzari L., Peault B. Purification and long-term culture of multipotent progenitor cells affiliated with the walls of human blood vessels: myoendothelial cells and pericytes. Methods Cell Biol. 2008;86:295–309. doi: 10.1016/S0091-679X(08)00013-7. [DOI] [PubMed] [Google Scholar]
- Deasy B. M., Gharaibeh B. M., Pollett J. B., Jones M. M., Lucas M. A., Kanda Y., Huard J. Long-term self-renewal of postnatal muscle-derived stem cells. Mol. Biol. Cell. 2005;16:3323–3333. doi: 10.1091/mbc.E05-02-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deasy B. M., Jankowski R. J., Payne T. R., Cao B., Goff J. P., Greenberger J. S., Huard J. Modeling stem cell population growth: incorporating terms for proliferative heterogeneity. Stem Cells. 2003;21:536–545. doi: 10.1634/stemcells.21-5-536. [DOI] [PubMed] [Google Scholar]
- Deasy B. M., et al. A role for cell sex in stem cell-mediated skeletal muscle regeneration: female cells have higher muscle regeneration efficiency. J. Cell Biol. 2007;177:73–86. doi: 10.1083/jcb.200612094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deasy B. M., Qu-Peterson Z., Greenberger J. S., Huard J. Mechanisms of muscle stem cell expansion with cytokines. Stem Cells. 2002;20:50–60. doi: 10.1634/stemcells.20-1-50. [DOI] [PubMed] [Google Scholar]
- Dellavalle A., et al. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat. Cell Biol. 2007;9:255–267. doi: 10.1038/ncb1542. [DOI] [PubMed] [Google Scholar]
- Dernbach E., Urbich C., Brandes R. P., Hofmann W. K., Zeiher A. M., Dimmeler S. Antioxidative stress-associated genes in circulating progenitor cells: evidence for enhanced resistance against oxidative stress. Blood. 2004;104:3591–3597. doi: 10.1182/blood-2003-12-4103. [DOI] [PubMed] [Google Scholar]
- Dhalla N. S., Golfman L., Takeda S., Takeda N., Nagano M. Evidence for the role of oxidative stress in acute ischemic heart disease: a brief review. Can. J. Cardiol. 1999;15:587–593. [PubMed] [Google Scholar]
- Dowling P., O'Driscoll L., O'Sullivan F., Dowd A., Henry M., Jeppesen P. B., Meleady P., Clynes M. Proteomic screening of glucose-responsive and glucose non-responsive MIN-6 beta cells reveals differential expression of proteins involved in protein folding, secretion and oxidative stress. Proteomics. 2006;6:6578–6587. doi: 10.1002/pmic.200600298. [DOI] [PubMed] [Google Scholar]
- Evens A. M., Mehta J., Gordon L. I. Rust and corrosion in hematopoietic stem cell transplantation: the problem of iron and oxidative stress. Bone Marrow Transplant. 2004;34:561–571. doi: 10.1038/sj.bmt.1704591. [DOI] [PubMed] [Google Scholar]
- Fan Y., Maley M., Beilharz M., Grounds M. Rapid death of injected myoblasts in myoblast transfer therapy. Muscle Nerve. 1996;19:853–860. doi: 10.1002/(SICI)1097-4598(199607)19:7<853::AID-MUS7>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Gharaibeh B., Lu A., Tebbets J. C., Zheng B., Feduska J. M., Crisan M., Peault B., Cummins J., Huard J. Isolation of a slowly adhering cell fraction containing stems cells from murine skeletal muscle by the preplate technique. Nat. Protocol. 2008;3:1501–1509. doi: 10.1038/nprot.2008.142. [DOI] [PubMed] [Google Scholar]
- Gurjala A. N., Liu W. R., Mogford J. E., Procaccini P. S., Mustoe T. A. Age-dependent response of primary human dermal fibroblasts to oxidative stress: cell survival, pro-survival kinases, and entrance into cellular senescence. Wound Repair Regen. 2005;13:565–575. doi: 10.1111/j.1524-475X.2005.00079.x. [DOI] [PubMed] [Google Scholar]
- Gussoni E., Blau H. M., Kunkel L. M. The fate of individual myoblasts after transplantation into muscles of DMD patients. Nat. Med. 1997;3:970–977. doi: 10.1038/nm0997-970. [DOI] [PubMed] [Google Scholar]
- Gussoni E., Soneoka Y., Strickland C. D., Buzney E. A., Khan M. K., Flint A. F., Kunkel L. M., Mulligan R. C. Dystrophin expression in the mdx mouse restored by stem cell transplantation. Nature. 1999;401:390–394. doi: 10.1038/43919. [DOI] [PubMed] [Google Scholar]
- Gute D. C., Ishida T., Yarimizu K., Korthuis R. J. Inflammatory responses to ischemia and reperfusion in skeletal muscle. Mol. Cell Biochem. 1998;179:169–187. doi: 10.1023/a:1006832207864. [DOI] [PubMed] [Google Scholar]
- Guttridge D. C., Albanese C., Reuther J. Y., Pestell R. G., Baldwin A. S., Jr. NF-kappaB controls cell growth and differentiation through transcriptional regulation of cyclin D1. Mol. Cell. Biol. 1999;19:5785–5799. doi: 10.1128/mcb.19.8.5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagege A. A., et al. Skeletal myoblast transplantation in ischemic heart failure: long-term follow-up of the first phase I cohort of patients. Circulation. 2006;114:I108–113. doi: 10.1161/CIRCULATIONAHA.105.000521. [DOI] [PubMed] [Google Scholar]
- He T., Peterson T. E., Holmuhamedov E. L., Terzic A., Caplice N. M., Oberley L. W., Katusic Z. S. Human endothelial progenitor cells tolerate oxidative stress due to intrinsically high expression of manganese superoxide dismutase. Arterioscler Thromb. Vasc. Biol. 2004;24:2021–2027. doi: 10.1161/01.ATV.0000142810.27849.8f. [DOI] [PubMed] [Google Scholar]
- Hempel S. L., Buettner G. R., O'Malley Y. Q., Wessels D. A., Flaherty D. M. Dihydrofluorescein diacetate is superior for detecting intracellular oxidants: comparison with 2′,7′-dichlorodihydrofluorescein diacetate, 5(and 6)-carboxy-2′,7′-dichlorodihydrofluorescein diacetate, and dihydrorhodamine 123. Free Radic Biol. Med. 1999;27:146–159. doi: 10.1016/s0891-5849(99)00061-1. [DOI] [PubMed] [Google Scholar]
- Huard J., Roy R., Guerette B., Verreault S., Tremblay G., Tremblay J. P. Human myoblast transplantation in immunodeficient and immunosuppressed mice: evidence of rejection. Muscle Nerve. 1994;17:224–234. doi: 10.1002/mus.880170214. [DOI] [PubMed] [Google Scholar]
- Ivanova N. B., Dimos J. T., Schaniel C., Hackney J. A., Moore K. A., Lemischka I. R. A stem cell molecular signature. Science. 2002;298:601–604. doi: 10.1126/science.1073823. [DOI] [PubMed] [Google Scholar]
- Jankowski R. J., Deasy B. M., Cao B., Gates C., Huard J. The role of CD34 expression and cellular fusion in the regeneration capacity of myogenic progenitor cells. J. Cell Sci. 2002;115:4361–4374. doi: 10.1242/jcs.00110. [DOI] [PubMed] [Google Scholar]
- Jankowski R. J., Haluszczak C., Trucco M., Huard J. Flow cytometric characterization of myogenic cell populations obtained via the preplate technique: potential for rapid isolation of muscle-derived stem cells. Hum. Gene Ther. 2001;12:619–628. doi: 10.1089/104303401300057306. [DOI] [PubMed] [Google Scholar]
- Jankowski R. J., Huard J. Myogenic cellular transplantation and regeneratioin: sorting through progenitor heterogeneity. Panminerva Medica. 2002;46:81–91. [PubMed] [Google Scholar]
- Kaminski K. A., Bonda T. A., Korecki J., Musial W. J. Oxidative stress and neutrophil activation—the two keystones of ischemia/reperfusion injury. Int. J. Cardiol. 2002;86:41–59. doi: 10.1016/s0167-5273(02)00189-4. [DOI] [PubMed] [Google Scholar]
- Kaneko T., Tahara S., Taguchi T., Kondo H. Accumulation of oxidative DNA damage, 8-oxo-2′-deoxyguanosine, and change of repair systems during in vitro cellular aging of cultured human skin fibroblasts. Mutat. Res. 2001;487:19–30. doi: 10.1016/s0921-8777(01)00100-8. [DOI] [PubMed] [Google Scholar]
- Kumar A., Boriek A. M. Mechanical stress activates the nuclear factor-kappaB pathway in skeletal muscle fibers: a possible role in Duchenne muscular dystrophy. FASEB J. 2003;17:386–396. doi: 10.1096/fj.02-0542com. [DOI] [PubMed] [Google Scholar]
- Langen R. C., Van Der Velden J. L., Schols A. M., Kelders M. C., Wouters E. F., Janssen-Heininger Y. M. Tumor necrosis factor-alpha inhibits myogenic differentiation through MyoD protein destabilization. FASEB J. 2004;18:227–237. doi: 10.1096/fj.03-0251com. [DOI] [PubMed] [Google Scholar]
- Lee J. Y., et al. Clonal isolation of muscle-derived cells capable of enhancing muscle regeneration and bone healing. J. Cell Biol. 2000;150:1085–1100. doi: 10.1083/jcb.150.5.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luce M. C., Cristofalo V. J. Reduction in heat shock gene expression correlates with increased thermosensitivity in senescent human fibroblasts. Exp. Cell Res. 1992;202:9–16. doi: 10.1016/0014-4827(92)90398-r. [DOI] [PubMed] [Google Scholar]
- Lunde K., et al. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N. Engl. J. Med. 2006;355:1199–1209. doi: 10.1056/NEJMoa055706. [DOI] [PubMed] [Google Scholar]
- Makazan Z., Saini H. K., Dhalla N. S. Role of oxidative stress in alterations of mitochondrial function in ischemic-reperfused hearts. Am. J. Physiol. Heart Circ. Physiol. 2007;292:H1986–H1994. doi: 10.1152/ajpheart.01214.2006. [DOI] [PubMed] [Google Scholar]
- Mendell J. R., et al. Myoblast transfer in the treatment of Duchenne's muscular dystrophy. N. Engl. J. Med. 1995;333:832–838. doi: 10.1056/NEJM199509283331303. [DOI] [PubMed] [Google Scholar]
- Nian M., Lee P., Khaper N., Liu P. Inflammatory cytokines and postmyocardial infarction remodeling. Circ. Res. 2004;94:1543–1553. doi: 10.1161/01.RES.0000130526.20854.fa. [DOI] [PubMed] [Google Scholar]
- Nishino T. The conversion of xanthine dehydrogenase to xanthine oxidase and the role of the enzyme in reperfusion injury. J. Biochem. 1994;116:1–6. doi: 10.1093/oxfordjournals.jbchem.a124480. [DOI] [PubMed] [Google Scholar]
- Oshima H., Payne T. R., Urish K. L., Sakai T., Ling Y., Gharaibeh B., Tobita K., Keller B. B., Cummins J. H., Huard J. Differential myocardial infarct repair with muscle stem cells compared to myoblasts. Mol. Ther. 2005;12:1130–1141. doi: 10.1016/j.ymthe.2005.07.686. [DOI] [PubMed] [Google Scholar]
- Payne T. R., Oshima H., Okada M., Momoi N., Tobita K., Keller B. B., Peng H., Huard J. A relationship between vascular endothelial growth factor, angiogenesis, and cardiac repair after muscle stem cell transplantation into ischemic hearts. J. Am. Coll. Cardiol. 2007;50:1677–1684. doi: 10.1016/j.jacc.2007.04.100. [DOI] [PubMed] [Google Scholar]
- Payne T. R., Oshima H., Sakai T., Ling Y., Gharaibeh B., Cummins J., Huard J. Regeneration of dystrophin-expressing myocytes in the mdx heart by skeletal muscle stem cells. Gene Ther. 2005;12:1264–1274. doi: 10.1038/sj.gt.3302521. [DOI] [PubMed] [Google Scholar]
- Peault B., Rudnicki M., Torrente Y., Cossu G., Tremblay J. P., Partridge T., Gussoni E., Kunkel L. M., Huard J. Stem and progenitor cells in skeletal muscle development, maintenance, and therapy. Mol. Ther. 2007;15:867–877. doi: 10.1038/mt.sj.6300145. [DOI] [PubMed] [Google Scholar]
- Plummer J. L., Smith B. R., Sies H., Bend J. R. Chemical depletion of glutathione in vivo. Methods Enzymol. 1981;77:50–59. doi: 10.1016/s0076-6879(81)77010-1. [DOI] [PubMed] [Google Scholar]
- Puri P. L., Bhakta K., Wood L. D., Costanzo A., Zhu J., Wang J. Y. A myogenic differentiation checkpoint activated by genotoxic stress. Nat. Genet. 2002;32:585–593. doi: 10.1038/ng1023. [DOI] [PubMed] [Google Scholar]
- Qu Z., Balkir L., van Deutekom J. C., Robbins P. D., Pruchnic R., Huard J. Development of approaches to improve cell survival in myoblast transfer therapy. J. Cell Biol. 1998;142:1257–1267. doi: 10.1083/jcb.142.5.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu-Petersen Z., Deasy B., Jankowski R., Ikezawa M., Cummins J., Pruchnic R., Mytinger J., Cao B., Gates C., Wernig A., Huard J. Identification of a novel population of muscle stem cells in mice: potential for muscle regeneration. J. Cell Biol. 2002;157:851–864. doi: 10.1083/jcb.200108150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramalho-Santos M., Yoon S., Matsuzaki Y., Mulligan R. C., Melton D. A. “Stemness”: transcriptional profiling of embryonic and adult stem cells. Science. 2002;298:597–600. doi: 10.1126/science.1072530. [DOI] [PubMed] [Google Scholar]
- Rando T. A., Blau H. M. Primary mouse myoblast purification, characterization, and transplantation for cell-mediated gene therapy. J. Cell Biol. 1994;125:1275–1287. doi: 10.1083/jcb.125.6.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachinger V., et al. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N. Engl. J. Med. 2006;355:1210–1221. doi: 10.1056/NEJMoa060186. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Murtuza B., Beauchamp J. R., Smolenski R. T., Varela-Carver A., Fukushima S., Coppen S. R., Partridge T. A., Yacoub M. H. Dynamics and mediators of acute graft attrition after myoblast transplantation to the heart. FASEB J. 2004;18:1153–1155. doi: 10.1096/fj.03-1308fje. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Smolenski R. T., Jayakumar J., Murtuza B., Brand N. J., Yacoub M. H. Heat shock treatment enhances graft cell survival in skeletal myoblast transplantation to the heart. Circulation. 2000;102:III216–221. doi: 10.1161/01.cir.102.suppl_3.iii-216. [DOI] [PubMed] [Google Scholar]
- Tamaki T., Akatsuka A., Ando K., Nakamura Y., Matsuzawa H., Hotta T., Roy R. R., Edgerton V. R. Identification of myogenic-endothelial progenitor cells in the interstitial spaces of skeletal muscle. J. Cell Biol. 2002;157:571–577. doi: 10.1083/jcb.200112106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaki T., Akatsuka A., Okada Y., Uchiyama Y., Tono K., Wada M., Hoshi A., Iwaguro H., Iwasaki H., Oyamada A., Asahara T. Cardiomyocyte formation by skeletal muscle-derived multi-myogenic stem cells after transplantation into infarcted myocardium. PLoS ONE. 2008a;3:e1789. doi: 10.1371/journal.pone.0001789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaki T., Okada Y., Uchiyama Y., Tono K., Masuda M., Nitta M., Hoshi A., Akatsuka A. Skeletal muscle-derived CD34(+)/45(−) and CD34(-)/45(-) stem cells are situated hierarchically upstream of Pax7(+) cells. Stem Cells Dev. 2008b;17:653–667. doi: 10.1089/scd.2008.0070. [DOI] [PubMed] [Google Scholar]
- Tamaki T., Okada Y., Uchiyama Y., Tono K., Masuda M., Wada M., Hoshi A., Ishikawa T., Akatsuka A. Clonal multipotency of skeletal muscle-derived stem cells between mesodermal and ectodermal lineage. Stem Cells. 2007;25:2283–2290. doi: 10.1634/stemcells.2006-0746. [DOI] [PubMed] [Google Scholar]
- Tavian M., Zheng B., Oberlin E., Crisan M., Sun B., Huard J., Peault B. The vascular wall as a source of stem cells. Ann. NY Acad. Sci. 2005;1044:41–50. doi: 10.1196/annals.1349.006. [DOI] [PubMed] [Google Scholar]
- Taylor D. A., Atkins B. Z., Hungspreugs P., Jones T. R., Reedy M. C., Hutcheson K. A., Glower D. D., Kraus W. E. Regenerating functional myocardium: improved performance after skeletal myoblast transplantation. Nat. Med. 1998;4:929–933. doi: 10.1038/nm0898-929. [DOI] [PubMed] [Google Scholar]
- Tremblay J. P., Malouin F., Roy R., Huard J., Bouchard J. P., Satoh A., Richards C. L. Results of a triple blind clinical study of myoblast transplantations without immunosuppressive treatment in young boys with Duchenne muscular dystrophy. Cell Transplant. 1993;2:99–112. doi: 10.1177/096368979300200203. [DOI] [PubMed] [Google Scholar]
- Urish K. L., Kanda Y., Huard J. Initial failure in myoblast transplantation therapy has led the way toward the isolation of muscle stem cells: potential for tissue regeneration. Curr. Top. Dev. Biol. 2005;68:263–280. doi: 10.1016/S0070-2153(05)68009-X. [DOI] [PubMed] [Google Scholar]
- Vreugdenhil G., Lowenberg B., Van Eijk H. G., Swaak A. J. Tumor necrosis factor alpha is associated with disease activity and the degree of anemia in patients with rheumatoid arthritis. Eur. J. Clin. Invest. 1992;22:488–493. doi: 10.1111/j.1365-2362.1992.tb01495.x. [DOI] [PubMed] [Google Scholar]
- Yoo T. S., Ackerman M. J., Lorensen W. E., Schroeder W., Chalana V., Aylward S., Metaxes D., Whitaker R. Amsterdam: IOS Press; 2002. Engineering and Algorithm Design for an Image Processing API: A Technical Report on ITK—The Insight Toolkit. [PubMed] [Google Scholar]
- Young H. E., et al. Clonogenic analysis reveals reserve stem cells in postnatal mammals: I. Pluripotent mesenchymal stem cells. Anat. Rec. 2001;263:350–360. doi: 10.1002/ar.1112. [DOI] [PubMed] [Google Scholar]
- Yuan H., Kaneko T., Matsuo M. Increased susceptibility of late passage human diploid fibroblasts to oxidative stress. Exp. Gerontol. 1996;31:465–474. doi: 10.1016/0531-5565(96)00001-0. [DOI] [PubMed] [Google Scholar]
- Zhang M., Methot D., Poppa V., Fujio Y., Walsh K., Murry C. E. Cardiomyocyte grafting for cardiac repair: graft cell death and anti-death strategies. J. Mol. Cell Cardiol. 2001;33:907–921. doi: 10.1006/jmcc.2001.1367. [DOI] [PubMed] [Google Scholar]
- Zheng B., et al. Prospective identification of myogenic endothelial cells in human skeletal muscle. Nat. Biotechnol. 2007;25:1025–1034. doi: 10.1038/nbt1334. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.