Abstract
Cardiac contractility is regulated through the activity of various key Ca2+-handling proteins. The sarco(endo)plasmic reticulum (SR) Ca2+ transport ATPase (SERCA2a) and its inhibitor phospholamban (PLN) control the uptake of Ca2+ by SR membranes during relaxation. Recently, the antiapoptotic HS-1–associated protein X-1 (HAX-1) was identified as a binding partner of PLN, and this interaction was postulated to regulate cell apoptosis. In the current study, we determined that HAX-1 can also bind to SERCA2. Deletion mapping analysis demonstrated that amino acid residues 575–594 of SERCA2's nucleotide binding domain are required for its interaction with the C-terminal domain of HAX-1, containing amino acids 203-245. In transiently cotransfected human embryonic kidney 293 cells, recombinant SERCA2 was specifically targeted to the ER, whereas HAX-1 selectively concentrated at mitochondria. On triple transfections with PLN, however, HAX-1 massively translocated to the ER membranes, where it codistributed with PLN and SERCA2. Overexpression of SERCA2 abrogated the protective effects of HAX-1 on cell survival, after hypoxia/reoxygenation or thapsigargin treatment. Importantly, HAX-1 overexpression was associated with down-regulation of SERCA2 expression levels, resulting in significant reduction of apparent ER Ca2+ levels. These findings suggest that HAX-1 may promote cell survival through modulation of SERCA2 protein levels and thus ER Ca2+ stores.
INTRODUCTION
The sarco(endo)plasmic reticulum (SR) Ca2+ transport ATPase (SERCA2a) is a critical regulator of Ca 2+ homeostasis and contractility in the heart. During muscle relaxation, SERCA2a transports Ca2+ from the cytosol into the SR lumen in an ATP-dependent manner (Asahi et al., 2003). Because SERCA2a activity controls both the rate of cytosolic Ca2+ removal and the degree of SR Ca2+ load, it represents a major determinant of both cardiac relaxation and contraction (Vangheluwe et al., 2005). Hence, the deteriorated cardiac muscle function associated with heart failure has been largely attributed to the decreased levels and activity of SERCA2a (Hasenfuss et al., 1994; Meyer et al., 1995; Hasenfuss and Pieske, 2002).
The structure of the SERCA pump has been determined by a combination of structural and biochemical information, along with x-ray crystallography studies. The protein is composed of 10 transmembrane helices (M1–M10) and a large cytoplasmic head piece, which is linked to the transmembrane domains by a narrow stalk (Toyoshima et al., 1993; MacLennan et al., 1997; Zhang et al., 1998). The cytoplasmic region of SERCA, which forms the bulk of the protein, can be further divided into three distinct domains: the actuator (A), which is involved in regulation of Ca2+ binding and release; the nucleotide binding (N) region; and the phosphorylation (P) domain.
The activity of SERCA2a is regulated by phospholamban (PLN), a 52-amino acid transmembrane phosphoprotein in cardiac SR, which interacts with SERCA and reversibly inhibits its affinity for Ca2+ (Simmerman and Jones, 1998). Detailed cross-linking, site-directed mutagenesis and structural modeling studies have demonstrated that residues in both the cytoplasmic and the transmembrane portions of SERCA2a are involved in direct interaction with PLN (James et al., 1989; Toyofuku et al., 1994a; Kimura et al., 1996; Asahi et al., 1999; Toyoshima et al., 2003). Studies in genetically engineered mouse models with altered PLN expression levels and the identification of PLN mutations in patients with familial dilated cardiomyopathy have demonstrated a critical role of PLN in regulating SR Ca2+ homeostasis and cardiac physiology (Luo et al., 1994; Kadambi et al., 1996; Haghighi et al., 2003, 2006; Schmitt et al., 2003).
Recently, HAX-1, an ∼35-kDa ubiquitously expressed protein with antiapoptotic function, was found to interact with PLN, and this association enhanced the protective effects of HAX-1 on cell survival (Vafiadaki et al., 2007). HAX-1 was originally identified to interact with HS1, a protein specifically expressed in hemopoietic cells with proposed involvement in B cell signal transduction (Suzuki et al., 1997). Subsequent studies have further demonstrated that HAX-1 interacts with a number of cytoskeletal and viral proteins, indicating its involvement in multiple cellular pathways (Vafiadaki et al., 2008). Based on its weak sequence similarity to Nip3 and its homology to Bcl-2 domains BH1 and BH2, HAX-1 was initially proposed to be involved in promoting cell survival. Experimental evidence, however, demonstrated that HAX-1 overexpression provides protection against Fas treatment, gamma-irradiation, serum deprivation, or Bax-induced apoptosis (Suzuki et al., 1997; Sharp et al., 2002). In cardiac myocytes, adenoviral overexpression of HAX-1 was shown to prevent caspase-9 processing and inhibit caspase-3 activation, after hypoxia/reoxygenation-induced cell death (Han et al., 2006), thus indicating that the antiapoptotic effect of HAX-1 is mediated, at least partly, through the mitochondrial apoptotic program.
In the present study, we report that HAX-1 can also bind to SERCA2 independently of PLN. However, overexpression of HAX-1 displaced endogenous SERCA from the membrane fraction and led to SERCA down-regulation in a proteasome-dependent manner, affecting apparent ER Ca2+ levels. These findings reveal a novel role of HAX-1 in cell survival through regulation of SERCA protein levels.
MATERIALS AND METHODS
Glutathione Transferase (GST)-Pull Down Assay
GST-pull down assays were performed using cardiac homogenates prepared from PLN-deficient hearts, as described previously (Kontrogianni-Konstantopoulos et al., 2003; Vafiadaki et al., 2007; Arvanitis et al., 2007). The PLN-knockout (PLN-KO) mouse was generated, using gene-targeting technology, and its cardiac phenotype has been extensively characterized previously (Luo et al., 1994; Chu et al., 1996; Slack et al., 2001). In brief, frozen cardiac muscle was homogenized in 10 mM NaPO4, pH 7.2, 2 mM EDTA, 10 mM NaN3, 120 mM NaCl, and 1% NP-40, supplemented with protease inhibitors (Sigma-Aldrich, Munich, Germany). Equal amounts of recombinant GST and GST-HAX-1 proteins were mixed with 0.5 mg of cardiac homogenates at 4°C for 16 h. The samples were washed three times at 4°C with 10 mM NaPO4, pH 7.2, 10 mM NaN3, 120 mM NaCl, and 0.1% Tween 20, and they were then resuspended in 2× SDS Laemmli sample buffer. After SDS-PAGE analysis and transfer to nitrocellulose membrane (Whatman Schleicher and Schuell, Dassel, Germany), the membranes were incubated with monoclonal antibodies to phospholamban or SERCA2 (Affinity Bioreagents, Golden, CO) and were subsequently washed in 50 mM Tris-HCl, pH 7.5, 150 mM NaCl, and 0.05% Tween 20 before incubating with a peroxidase-conjugated anti-mouse secondary antibody (Sigma-Aldrich). Immunoreactive bands were detected using enhanced chemiluminescence reagents (GE healthcare, Little Chalfont, Buckinghamshire, United Kingdom).
Yeast Two-Hybrid Assay
For the identification of the minimal binding regions of HAX-1 on SERCA2, a series of SERCA2 deletion constructs was generated by PCR amplification, and these were subcloned in the EcoRI/SalI sites of the yeast BD pGBKT7 vector (Matchmaker system; BD Biosciences Clontech, Erembodegem, Belgium). Construct SERCA2-A (amino acids 126–252), which includes the region encoding for the actuator domain (MacLennan et al., 1997; Toyoshima et al., 2000; Dode et al., 2003), was generated using sense primer 1, 5′-CATGGGCAAAGTGTATCGACAG-3′ and antisense primer 1, 5′-TTTTTGCTGAAGGGGTGTTC-3′, whereas construct SERCA2-B (amino acids 338–551), which includes the phosphorylation site at amino acid position 351, was generated with sense primer 2, 5′-TGTGGAAACCCTTGGTTGT-3′) and antisense primer 2, (5′-ACCCCACTCTCGAATGACAG-3′). Construct SERCA2-C (amino acids 525-732), encoding for the nucleotide binding domain (MacLennan et al., 1997; Toyoshima et al., 2000; Dode et al., 2003) was produced using sense primer 3 (5′-ACCCACATTCGAGTTGGAAG-3′) and antisense primer 3 (5′-CCATCTCAGAGGCGGTTTTA-3′). For the generation of deletion constructs within the nucleotide binding domain: 1) sense primer 3 (described above) and antisense primer 4 (5′-CCAACGAAGGTCAGATTGGT-3′) were used for SERCA2-D (amino acids 525–593); and 2) sense primer 4 (5′-ACCAATCTGACCTTCGTTGG-3′) and antisense primer 3 (described above) were used for construct SERCA2-E (amino acids 587–732). For construct SERCA2-F (amino acids 575–593), sense primer 5 (5′-ATGCACCTTGAGGACTCTGC-3′) and antisense primer 4 were used, whereas sense primer 3 and antisense primer 5 (5′-TCTTCTCAGTGGGTTGTC-3′) were used to generate SERCA2-G (amino acids 525–571). Finally, construct SERCA2-H (amino acids 620–732) was generated using sense primer 6 (5′-ATCATGATCACTGGGGACA-3′) and antisense primer 3 (see above).
For the identification of the HAX-1 minimal binding region to PLN or SERCA2, a series of successive HAX-1 deletion constructs were generated by polymerase chain reaction (PCR) amplification and subsequent cloning in the EcoRI/XhoI sites of the yeast AD pACT2 vector (Matchmaker system; BD Biosciences Clontech). Constructs HAX-1-A and HAX-1-B have been described previously (Vafiadaki et al., 2007). HAX-1-C construct (amino acids 203-245) was generated using sense primer A (5′-AGCCCAAATCCTATTTCA-3′) and antisense primer A (5′-GCTTCGTGTCGGGTTACTGT-3′), whereas sense primer A and antisense primer B (5′-TCCTCCACTATCCCATCTGG-3′) were used for construct HAX-1-D (amino acids 203-225). For the generation of HAX-1-E construct (amino acids 222-245), sense primer B (5′-ATAGTGGAGGAGCGCCGGA-3′) and antisense primer A were used, whereas for HAX-1-F construct (amino acids 215–245) sense primer C (5′-AGATCACTAAACCAGATG-3′) and antisense primer A were used. HAX-1-G construct (amino acids 203–232) was generated using sense primer A and antisense primer C (5′-TGTCCGGCCCTCACTGTC-3′), whereas sense primer D (5′-TAGTGGAGGAGCGCCGGA-3′) and antisense primer D (5′-GCTGGAGGTCTTGGTGATTC-3′) were used for HAX-1-H construct (amino acids 222–260). The authenticity of all constructs was confirmed through sequence analysis by Macrogen (Seoul, Korea).
Generation and Purification of Recombinant Proteins
The SERCA2 fragment, containing amino acids 525-732, was cloned in the EcoRI/XhoI sites of pGEX 5x-1 (GE Healthcare) vector, whereas the HAX-1 C-terminal fragment containing amino acids 203-279 was cloned in the pGEX 5x-1 and pET28 (Novagen, Nottingham, United Kingdom) vectors as described previously (Vafiadaki et al., 2007).
Expression of GST-and His-tagged proteins was induced with 0.1 mM isopropyl-β-d-thiogalactopyranoside for 3 h, and proteins were purified by affinity chromatography on glutathione-Sepharose 4B (GE Healthcare) or nickel-nitrilotriacetic acid agarose (QIAGEN, Hilden, Germany) resins following the manufacturer's instructions.
In Vitro Binding Assays
Equivalent amounts of GST-SERCA2and GST-protein bound to glutathione matrices were allowed to interact for 16 h at 4°C with 3 μg of His-HAX-1 recombinant protein in 50 μl of binding buffer containing 50 mM Tris-HCl, pH 7.2, 120 mM NaCl, 10 mM NaN3, 2 mM dithiothreitol, and 0.5% Tween 20. The beads were washed three times at 4°C with 50 mM Tris-HCl, pH 7.2, 120 mM NaCl, 10 mM NaN3, and 0.1% Tween 20 and were resuspended in 2× SDS Laemmli sample buffer. Samples were analyzed by SDS-PAGE, transferred to nitrocellulose membrane (Whatman Schleicher and Schuell) and probed with a poly-histidine antibody (Sigma-Aldrich) and an anti-rabbit peroxidase-conjugated secondary antibody (GE Healthcare).
Titration with Ca2+
To study the effect of alterations in Ca2+ concentration on the interaction between HAX-1 and SERCA2, Ca2+ titration assays were performed as described previously (Asahi et al., 2000; Vafiadaki et al., 2007). In brief, cardiac extracts (0.3 mg) from wild-type or PLN-KO mice were incubated at room temperature for 5 min with 150 μl of reaction buffer (20 mM Tris-HCl, pH 6.8, 100 mM KCl, 5 mM MgCl2, 5 mM ATP, 1 mM EGTA, and 5 mM potassium oxalate) containing 10−8 to 10−5 M CaCl2 concentrations, and subsequent GST-pull down assays were carried out as described above.
Cell Culture, Transfections, and Immunofluorescence Studies
A GST-HAX1 construct containing HAX-1 amino acids 118–260 was generated by PCR by using sense primer 5′-AGACTACGGGAGGGACAGAC-3′ and antisense primer D (described above) and was subsequently cloned in the EcoRI/SalI sites of pGEX 5x-1. GST-HAX1 recombinant protein was expressed in bacteria, as described above, and was then used for antibody generation in rabbit (Covance, Denver, PA). The HAX-1 antibody was affinity-purified on GST and GST-HAX-1 columns, as described previously (Kontrogianni-Konstantopoulos et al., 2003).
Human embryonic kidney (HEK) 293 cells (European Collection of Cell Cultures, Salisbury, United Kingdom) were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum (Invitrogen, Carlsbad, CA). Full-length green fluorescent protein (GFP)-PLN, myc-HAX-1 (Vafiadaki et al., 2007), SERCA1 (a kind gift from D. H. MacLennan, Banting and Best Department of Medical Research, University of Toronto, Toronto, Canada), or SERCA2 (National Institutes of Health Mammalian Gene collection, Clone ID 5503508; Invitrogen) constructs were transiently transfected in HEK 293 cells with Lipofectamine 2000 (Invitrogen), according to the manufacturer's instructions. Forty-eight hours after transfection, cells were fixed for 20 min at 25°C with ice-cold methanol, washed three times with phosphate-buffered saline (1× PBS), and permeabilized for 30 min at 25°C in PBS containing 0.1% Triton X-100. After three washes with PBS, cells were incubated with blocking buffer (1× PBS, 1 mg/ml bovine serum albumin, and 10 mM NaN3) for 1 h at 25°C, and then primary antibodies (rabbit HAX-1 or SERCA2) diluted in blocking buffer were applied to the cells for 1 h at 25°C. The samples were washed three times with PBS and counterstained for 1 h at 25°C with the appropriate secondary antibody (Alexa Fluor anti-rabbit 488, Alexa Fluor anti-mouse 568, or Alexa Fluor anti-mouse 633; Invitrogen) diluted 1:500 in blocking buffer. After further washes with PBS, samples were mounted with Vectashield medium containing 4,6-diamidino-2-phenylindole (DAPI; Vector Laboratories, Burlingame, CA) and analyzed by confocal microscopy. For MitoTracker staining, cultured cells were incubated for 20 min at 37°C with MitoTracker CMXRos Red dye (Invitrogen). After three washes with PBS, cells were fixed and permeabilized as described above.
Cell Viability Assay
Induction of cell death in transfected HEK 293 cells was performed by incubation with 5 mM H2O2 for 15 h or 1 μM thapsigargin (Sigma-Aldrich) for 30 h. After treatment, cell viability was determined by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT) colorimetric assay, whereas DNA laddering was assessed by gel electrophoresis, as described previously (Vafiadaki et al., 2007). ImageJ software (National Institutes of Health, Bethesda, MD) was used for quantitative analysis of the intensity of the 200-base pair band in the DNA samples.
HEK 293 Cell Lysate Preparation for Western Blot Analysis
Protein lysates were prepared by lysis of HEK 293 cells in 10 mM Tris-HCl, pH 7.4, 150 mM NaCl, 5 mM EDTA, and 1% Triton X-100 supplemented with protease inhibitors (Sigma-Aldrich). Lysates were incubated on ice for 10 min and then centrifuged at 13,000 rpm for 5 min to remove cell debris. For Western blot analysis, ∼50 μg of cell lysates were separated by SDS-PAGE, transferred to nitrocellulose membranes, and immunodetected with SERCA2, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), voltage-dependent anion channel 1 (VDAC1) (Abcam, Cambridge, United Kingdom), calreticulin (Affinity Bioreagents), and calnexin (Santa Cruz Biotechnology, Heidelberg, Germany) antibodies. To investigate the mechanism involved in SERCA2 down-regulation, transfected cells were incubated with 50 nM proteasome inhibitor I (PSI) (Calbiochem, Darmstadt, Germany) or dimethyl sulfoxide (DMSO) for 16 h. Samples were harvested and lysed, as described above, and SERCA2 protein levels were evaluated.
Cell Fractionation
Fractionation of HEK 293 cells was performed as described previously (Aga-Mizrachi et al., 2008). Briefly, cells were harvested in buffer A (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 2 mM EDTA, and 1 mM EGTA) supplemented with protease inhibitors (Sigma-Aldrich) and lysed by three freeze-thaw cycles. Samples were centrifuged at 17,000 × g for 20 min at 4°C, and the supernatant was collected as the cytosolic fraction. The pellet was resuspended in buffer A containing 1% Triton X-100, incubated on ice for 30 min, and centrifuged at 17,000 × g for 20 min at 4°C. The supernatant was then collected as the membrane fraction.
Ca2+ Measurements
Transfected HEK 293 cells were loaded with Fura-2 by incubation in 4 μM fura-2 acetoxymethyl ester (Invitrogen) in HEPES-buffered solution (HBS) containing 128 mM NaCl, 6 mM KCl, 1 mM MgCl2, 1 mM CaCl2, 5.5 mM glucose, and 10 mM HEPES, pH 7.4, for 35 min at 37°C. Cells were washed in HBS buffer and maintained in this buffer during imaging. Sequential fluorescence images were obtained by measurement of cytosolic Fura-2 emission intensity (510 nm) under dual excitation at 340- and 380-nm wavelengths using the ImageMaster Imaging system (Photon Technology International, Princeton, NJ). Thapsigargin binds to SERCA with high affinity and with a 1:1 stoichiometry. Previous reports have demonstrated that thapsigargin treatment in the range of 1–5 μM causes maximal release of Ca2+ from endoplasmic/sarcoplasmic reticulum vesicles (Lytton et al., 1991; Sagara and Inesi, 1991; Berman, 2000). Consequently, addition of 12 μM thapsigargin in our studies achieved maximal inhibition of SERCA's activity and induced maximal release of Ca2+ from ER stores. Images were analyzed using the ImageMaster software (Photon Technology International), which enabled background subtraction and measurement of fluorescence intensity for the time course of the experiment. At least 10–20 individual cells were measured for each experiment.
RESULTS
Identification of a Direct Interaction between HAX-1 and SERCA2 and the Effect of Ca2+ Concentration
We had previously shown the presence of SERCA2 in GST-HAX-1 pull down samples from cardiac homogenates (Vafiadaki et al., 2007), which suggested that either PLN can simultaneously interact with both HAX-1 and SERCA2 or that HAX-1 may bind directly to SERCA2. To determine whether HAX-1 may be able to bind to SERCA2 independently of PLN, we performed pull down assays using cardiac homogenates from the PLN-KO mouse and a GST-HAX-1 recombinant construct containing HAX-1 amino acids 203-279 (Figure 1A), which was shown previously to bind PLN. Western blot analysis revealed that GST-HAX-1 but not control GST-protein was able to absorb native SERCA2 (Figure 1A), demonstrating that the interaction between HAX-1 and SERCA2 can occur independently of the presence of PLN.
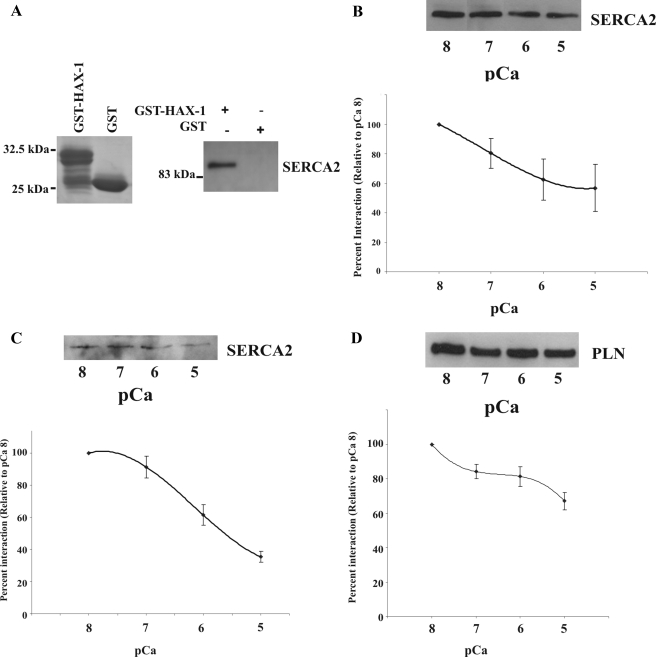
Figure 1.
HAX-1 binds to native SERCA2 in PLN-KO cardiac homogenates and the interaction is diminished by elevation in Ca2+ concentration. (A) Recombinant GST-HAX-1aa 203-279 (∼30 kDa) and GST (∼25 kDa) were separated by SDS-PAGE and visualized by Coomassie Blue staining. Equivalent amounts of GST-HAX-1aa 203-279 and GST-protein bound to glutathione matrices were incubated with PLN-KO cardiac homogenates. GST-HAX-1aa 203-279 but not GST bound native SERCA2, as determined by immunoblot analysis with an anti-SERCA2 antibody. (B) GST-pull down assays, using GST-HAX-1 recombinant protein and PLN-KO cardiac homogenates that had been preincubated with different Ca2+ concentrations, demonstrated a concentration-dependent decrease in SERCA2 binding. Data are expressed as percentage of maximal binding and presented as means ± SE (n = 3). A concentration-dependent decrease in SERCA2/HAX-1 (C) or PLN/HAX-1 (D) binding was detected in wild-type mouse cardiac homogenates (n = 4), indicating that the presence or absence of PLN does not affect the SERCA2/HAX-1 binding.
To examine whether Ca2+ elevation may also regulate the SERCA2 and HAX-1 interaction, we determined the amount of native SERCA2, associated with GST-HAX-1, in PLN-KO cardiac homogenates at various Ca2+ levels. Previous studies have shown that the PLN/SERCA2a interaction (James et al., 1989; Asahi et al., 2000) and the PLN/HAX-1 interaction (Vafiadaki et al., 2007) may be reduced by elevating Ca2+ concentration. Immunoblot analysis using the SERCA2 antibody determined that, similar to the PLN/SERCA2a and PLN/HAX-1 interactions, increases in Ca2+ concentration caused a concentration-dependent decrease in the amount of SERCA2 interacting with HAX-1 (Figure 1B). Quantitative analysis using ImageJ software revealed an ∼44% reduction in the amount of SERCA2 interacting with HAX-1 at pCa 5, compared with the levels observed at pCa 8.
We next performed Ca2+ titration assays, using wild-type mouse cardiac homogenates, to examine any potential effects of PLN on the SERCA2/HAX-1 interaction. Immunoblot analysis, using the SERCA2 antibody, revealed an ∼65% reduction in the amount of SERCA2 that interacted with GST-HAX-1 at pCa 5, relative to the amount bound at pCa 8 (Figure 1C). Notably, the trends of the Ca2+ titration curves of the SERCA2/HAX-1 interaction were similar in the presence or absence of PLN.
Immunoblot analysis using the PLN antibody was also performed on these samples and identified an ∼33% reduction in the amount of PLN interacting with HAX-1 at pCa 5, compared with the amount interacting at pCa 8 (Figure 1D), consistent with our previous findings (Vafiadaki et al., 2007).
Determination of the SERCA2 and HAX-1 Minimal Binding Domains Involved in Their Interaction
Having determined that HAX-1 can bind to SERCA2 in PLN-KO cardiac homogenates, we subsequently investigated the region of SERCA2 required for its interaction with HAX-1. To this end, three SERCA2 constructs were generated and assayed in the yeast two-hybrid system for their ability to bind to HAX-1. Because the GST-HAX-1 recombinant protein was shown previously to bind to the cytosolic domain of PLN, we hypothesized that the cytosolic portion of SERCA2 would be also involved in its interaction with HAX-1, and focused our studies on this part of the protein. We generated SERCA2 constructs containing the actuator domain (construct SERCA2-A, amino acids 126–252), the phosphorylation site (construct SERCA2-B, amino acids 338–551) and the nucleotide binding domain (construct SERCA2-C, amino acids 525-732) of the protein. Only the nucleotide binding domain construct was found to interact with HAX-1 in the yeast two-hybrid system, indicating that this C-terminal fragment of SERCA2 is required for binding to HAX-1 (Figure 2A).
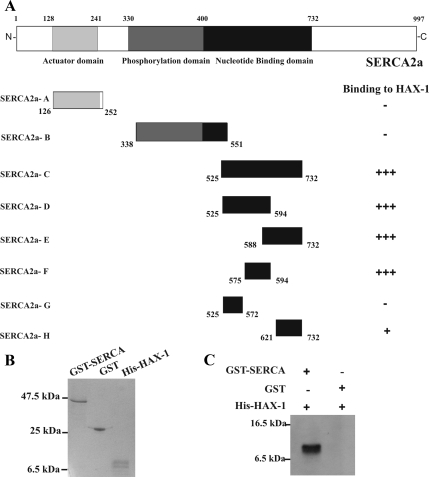
Figure 2.
Amino acids 575–594 within the nucleotide binding domain of SERCA2 are required for binding to HAX-1. (A) To determine the minimal region of SERCA2 required for its association with HAX-1, three constructs (SERCA2-A, SERCA2-B, and SERCA2-C) containing the cytoplasmic regions of SERCA2 were generated and tested for their ability to bind to HAX-1 in yeast. Generation of several overlapping deletion constructs (SERCA2-D-H) for the region contained in construct SERCA2-C mapped the minimal binding domain to SERCA2 amino acids 575–594. (B) Recombinant GST-SERCA2aa 525-732 (∼47 kDa), GST (∼25 kDa) and His-HAX-1aa 203-279 (∼10 kDa) were subjected to SDS-PAGE and stained with Coomassie Blue. (C) Equivalent amounts of GST-SERCA2aa 525-732 and GST recombinant proteins attached to glutathione matrices were incubated with His-HAX-1aa 203-279. Bound His-HAX-1aa 203-279 was detected by Western blot analysis with an anti-Histidine antibody. GST-SERCA2aa 525-732 but not GST-protein bound His-HAX-1aa 203-279, demonstrating that binding of SERCA2 to HAX-1 is direct and specific.
To further define the minimal binding region of SERCA2, several overlapping deletion constructs spanning the nucleotide binding domain were generated, that contained SERCA2 amino acids (aa) 525-732. These allowed us to map the minimal binding region for HAX-1 to SERCA2 between amino acids 575–594 (Figure 2A). Furthermore, in vitro binding assays, using bacterially expressed GST-SERCA2aa 525-732 and the His-HAX-1aa 203-279 (∼10-kDa recombinant protein) described above (Figure 2B), were performed to confirm the direct interaction between SERCA2 and HAX-1. A larger peptide (∼47k Da) than the minimal biding regions of SERCA2 (amino acids 575–593) was used to more readily separate SERCA from the control GST-protein (25 kDa). Equivalent amounts of GST-SERCA2aa 525-732 and control GST proteins, bound to glutathione matrices, were incubated with affinity-purified His-HAX-1aa 203-279 protein. Western blot analysis with a histidine-antibody demonstrated that His-HAX-1 bound specifically to GST-SERCA2aa 525-732, but not to control GST-protein (Figure 2C), confirming that SERCA2 binds directly to HAX-1.
Because the HAX-1 recombinant proteins (GST-HAX-1aa 203-279 and His-HAX-1aa 203-279) that interact with SERCA2 contain the C-terminal region of HAX-1, which was shown previously to bind PLN, we generated several overlapping deletion constructs to further define the HAX-1 amino acids required for its interaction with SERCA2 or PLN. Using the yeast two-hybrid system, HAX-1 amino acids 203-245 were found to bind to either PLN (Vafiadaki et al., 2007) or SERCA2. In contrast, HAX-1 amino acids 203-225 were able to bind to PLN but not to SERCA2 (Figure 3A). None of the other HAX-1 C-terminal deletion constructs (HAX-1-E or HAX-1-H) or the N-terminal construct containing HAX-1's amino acids 1-130 (HAX-1-A) were found to interact with either PLN or SERCA2, indicating that the minimal binding regions of HAX-1 for PLN and SERCA2 include amino acids 203-225, and 203-245, respectively (Vafiadaki et al., 2007; Figure 3A). These findings were also confirmed by pull down assays in cardiac homogenates. Recombinant GST-HAX1aa 203-245 protein was able to retain both native PLN and SERCA2, whereas GST-HAX1aa 203-225 protein bound only PLN (Figure 3, B and C).
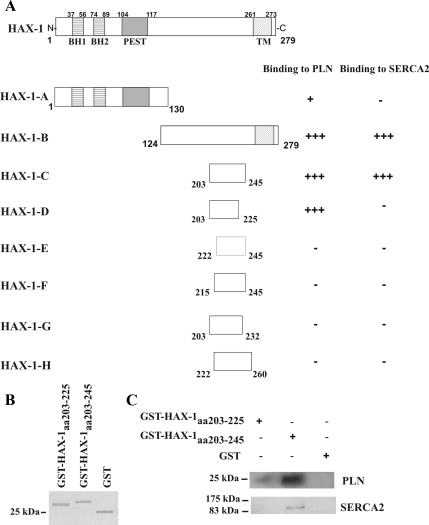
Figure 3.
Determination of HAX-1 minimal binding domain required for PLN or SERCA2 interaction. (A) To delineate the HAX-1 C-terminal region required for binding to either PLN or SERCA2, a series of HAX-1 deletion constructs were generated and tested for their ability to bind to PLN or SERCA2 in yeast. Amino acids 203-225 are required for binding to PLN, whereas a larger region of HAX-1, encompassing amino acids 203-245, is required for its binding to SERCA2. The Bcl-2 homology domains 1 and 2 (BH1 and BH2), PEST sequence and transmembrane domain (TM) of HAX-1 are indicated. (B) Recombinant GST-HAX-1aa 203-225 (∼28 kDa), GST-HAX-1aa 203-245 (∼30 kDa) and GST (∼25 kDa) proteins were subjected to SDS-PAGE and stained with Coomassie Blue. (C) To confirm the findings from the yeast two-hybrid system, GST pull-down assays were performed using equivalent amounts of GST-HAx-1aa 203-225, GST-HAX-1aa 203-245, and GST-protein bound to glutathione matrices and cardiac homogenates. Immunoblot analysis using anti-PLN or anti-SERCA2 antibodies determined that GST-HAX-1aa 203-245 binds to both PLN and SERCA2 native proteins, whereas GST- HAx-1aa 203-225 binds only to PLN.
Analysis of HAX-1 Subcellular Distribution in HEK 293-transfected Cells
HAX-1 has been localized to mitochondria, ER, and nuclear envelope by transient transfections in COS-7, HeLa, and DG75 cells (Suzuki et al., 1997; Gallagher et al., 2000; Dufva et al., 2001; Sharp et al., 2002; Yedavalli et al., 2005; Kasashima et al., 2006). Previous studies from our laboratory have demonstrated that overexpression of myc-HAX-1 in HEK 293 cells resulted in a speckled HAX-1 localization pattern, which represented mitochondrial distribution, as determined by its codistribution with the mitochondrial dye MitoTracker (Vafiadaki et al., 2007). Importantly, GFP-PLN and myc-HAX-1 cotransfection resulted in extensive redistribution of HAX-1, which preferentially targeted and colocalized with PLN at the ER compartment (Vafiadaki et al., 2007). To determine whether SERCA2 may also cause a similar change in HAX-1's localization, we performed transient cotransfections of constructs encoding full-length proteins in HEK 293 cells. Forty-eight hours after transfection, immunofluorescence analysis determined the expected preferential ER distribution for SERCA2, as shown by its colocalization with GFP-PLN (Figure 4A). However, in the presence of SERCA2, HAX-1 did not concentrate at the ER membranes but instead exhibited a speckled distribution (Figure 4B), representing mitochondrial localization, as determined by MitoTracker costaining (Figure 4C). Therefore, contrary to PLN, SERCA2 overexpression does not seems to affect the localization of HAX-1. Triple transfections of HEK 293 cells with GFP-PLN, myc-HAX-1, and SERCA2 revealed a preferential ER distribution for HAX-1, most likely due to the presence of PLN, resulting in colocalization of all three proteins at the ER compartment (Figure 4D). Similar findings on the cellular distribution of HAX-1 were also determined using a SERCA1 overexpression construct (data not shown).
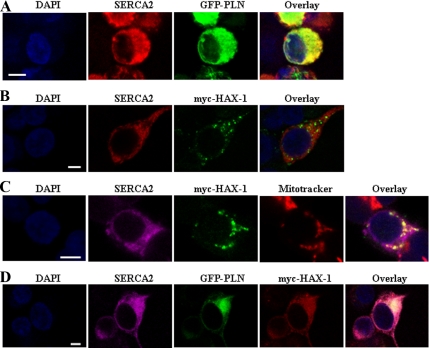
Figure 4.
Immunofluorescence analysis of SERCA2, PLN, and HAX-1 proteins in transiently transfected HEK 293 cells. (A) The SERCA2 protein localizes to the ER, as demonstrated by its colocalization with GFP-PLN. SERCA2 was detected with an anti-SERCA2 mAb and Alexa Fluor anti-mouse 568 secondary antibody. Cell nuclei were visualized with DAPI staining. (B and C) Cotransfection of myc-HAX-1 and SERCA2 determined a mitochondrial localization for HAX-1, as indicated by MitoTracker costaining. HAX-1 was detected with a rabbit anti-HAX-1 antibody and Alexa Fluor anti-rabbit 488 secondary antibody, whereas for SERCA2 immunofluorescence Alexa Fluor anti-mouse 568 or 633 secondary antibodies were used, respectively. (D) To the contrary, triple transfections showed that the presence of PLN causes a significant redistribution of HAX-1, thus resulting in colocalization of the three proteins (GFP-PLN, myc-HAX-1, and SERCA2) at the ER. The localization pattern of HAX-1 was detected using rabbit HAX-1 antibody and Alexa Fluor anti-rabbit 568 secondary antibody, whereas Alexa Fluor anti-mouse 633 secondary antibody was used for SERCA2 immunofluorescence. Bar, 5 μm.
Evaluation of HAX-1's Antiapoptotic Role in the Presence of SERCA2
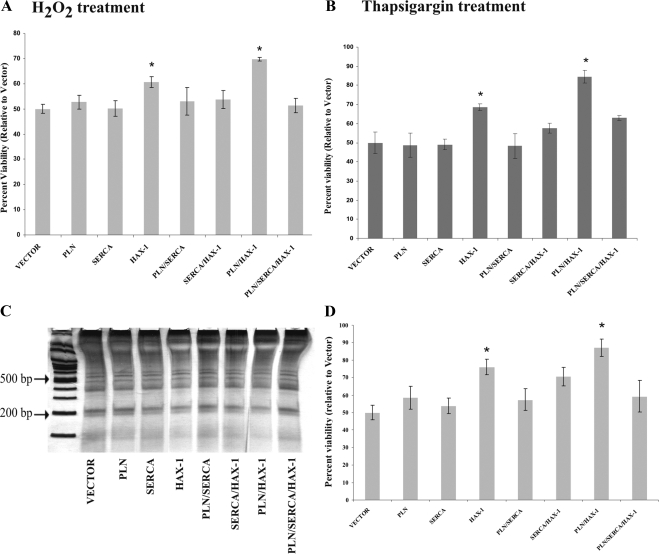
Overexpression of HAX-1 has been shown to provide protection against apoptosis (Suzuki et al., 1997; Sharp et al., 2002; Cilenti et al., 2004; Han et al., 2006) and the presence of PLN enhances its antiapoptotic role in HEK 293 cells, subjected to hypoxia (Vafiadaki et al., 2007). To assess whether SERCA expression may also affect the function of HAX-1, we performed transient transfections in HEK 293 cells and induced cell death by exposure to 5 mM H2O2 for 15 h. Cell viability assays determined that although HAX-1–transfected cells displayed increased viability compared with control cells transfected with empty vector, this antiapoptotic effect of HAX-1 was abolished in the presence of SERCA2 (Figure 5A). Additionally, SERCA2 overexpression prevented the enhanced cellular protection mediated by coexpression of HAX-1 and PLN (Figure 5A). Similar results were obtained from DNA laddering analysis, which further confirmed that overexpression of SERCA reduces the antiapoptotic activity of HAX-1 and HAX-1/PLN (Figure 5, C and D). These findings suggest that PLN enhances the protective role of HAX-1 against cell death (Vafiadaki et al., 2007), but SERCA2 seems to have an opposite effect on the antiapoptotic capacity of HAX-1.
Figure 5.
Overexpression of SERCA inhibits the antiapoptotic effect of HAX-1 in HEK 293 cells. Cells transfected with the indicated constructs were exposed to 5 mM H2O2 (A) or 1 μM thapsigargin (B), and cell viability was determined by the MTT assay. Under both treatments, HAX-1–transfected cells exhibited increased cellular viability, compared with control cells. However, this effect seemed to be abolished upon cotransfection with SERCA. Data are presented as mean ± SE (n = 4; t test, *p < 0.05). (C) DNA laddering analysis of genomic DNA isolated from transfected HEK 293 cells exposed to H2O2, determined that the observed changes in cell viability were due to apoptotic cell death. A representative gel from a single experiment is shown. (D) Quantitative analysis of the intensity of the 200-base pair band in all samples shown in C. Data are presented as means ± SE (n = 3; t test, *p < 0.05).
We next used thapsigargin, a selective inhibitor of SERCA2, that induces apoptosis through ER stress after perturbation of Ca2+ levels (Thastrup et al., 1990; Lytton et al., 1991) to further evaluate the antiapoptotic role of HAX-1. The HAX-1–transfected cells exhibited increased viability (Figure 5B), and cotransfection with PLN resulted in a further increase in cellular survival (Figure 5B). On the contrary, cotransfection with SERCA2 caused a reduction in cell viability in HAX-1 or in PLN and HAX-1–cotransfected cells (Figure 5B). These data suggest that HAX-1 exhibits a protective effect on cellular survival even upon treatment with another apoptosis-inducing agent, such as thapsigargin. Furthermore, our findings indicate that under these experimental conditions, whereas the presence of PLN induces a stronger protective role for HAX-1 in the cell, SERCA2 seems to abolish its antiapoptotic capability.
HAX-1 Overexpression in HEK 293 Cells Results in Down-Regulation of Endogenous SERCA2 Protein Levels and Reduced ER Ca2+ Content
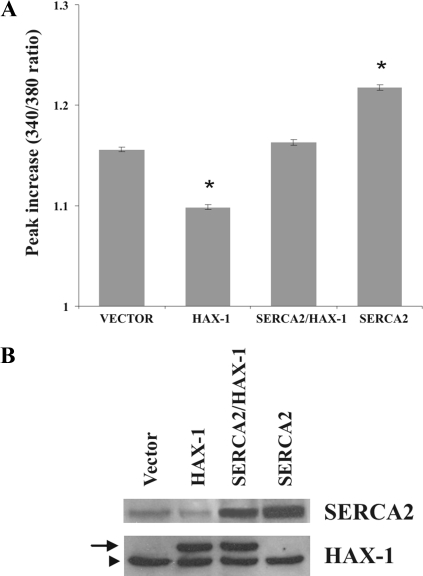
The antiapoptotic proteins of the Bcl-2 family, such Bcl-2 and Bcl-xL, have been previously shown to modulate ER Ca2+ levels (Foyouzi-Youssefi et al., 2000; Pinton et al., 2000; Li et al., 2002; Vanden Abeele et al., 2002). To examine whether the protective role of HAX-1 may be also mediated through regulation of Ca2+ homeostasis, we performed cytosolic Ca2+ measurements in transiently transfected HEK 293 cells, using the Fura-2 fluorescent indicator. Addition of thapsigargin, an irreversible inhibitor of SERCA activity that leads to passive release of Ca2+ from ER stores and subsequent increase in the cytosolic Ca2+ levels (Thastrup et al., 1990; Lytton et al., 1991), was used for indirect determination of ER Ca2+ content. Previous studies have demonstrated that thapsigargin treatment in the range of 1–5 μM results in maximal Ca2+ release from the ER/SR vesicles (Lytton et al., 1991; Sagara and Inesi, 1991; Berman, 2000). In our experimental system, we chose 12 μM thapsigargin to achieve maximal inhibition of SERCA's activity and measure immediate and maximal Ca2+ release from the ER stores. Thapsigargin treatment resulted in ∼37% reduction in the levels of cytosolic Ca2+ in HAX-1–overexpressing cells, compared with vector transfected cells (Figure 6A). On the contrary, cotransfection with SERCA2 abolished this inhibitory effect of HAX-1, resulting in a 9% increase in the levels of cytosolic Ca2+, compared with cells transfected with vector (Figure 6A). Notably, overexpression of SERCA2 by itself resulted in a further increase in cytosolic Ca2+ levels (∼40%), compared with vector-transfected cells (Figure 6A). Western blot analysis verified the expression levels of transfected proteins in these studies (Figure 6B).
Figure 6.
Overexpression of HAX-1 reduces releasable ER Ca2+ levels. (A) HEK 293 cells were transfected with indicated constructs and were loaded with Fura-2. Treatment of control cells with thapsigargin resulted in release of ER Ca2+ and subsequent elevation of cytosolic Ca2+ levels. To the contrary, ER Ca2+ release was significantly reduced after thapsigargin treatment in HAX-1–overexpressing cells, compared with control cells, whereas cotransfection with SERCA2 abolished this effect. Transfection of SERCA2 by itself resulted in further increase in ER Ca2+ release, after thapsigargin treatment, compared with control cells. Data are presented as mean ± SE (n = 3; t test, *p < 0.05). (B) Western blot analysis of HEK 293 cells transfected with the indicated constructs determined the levels of SERCA2 or myc-HAX-1 overexpressing proteins. The HAX-1 antibody detects both endogenous (arrowhead) and transfected myc-HAX1 protein (arrow), which are different in size due to the presence of the myc tag. Similarly, the SERCA2 antibody detects both the endogenous and overexpressed SERCA2 proteins; these, however, have the same size because there is no tag on the exogenous protein.
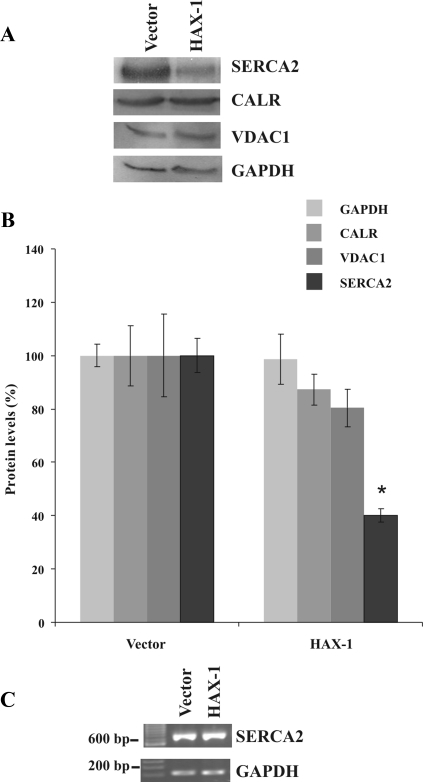
To determine whether the effect of HAX-1 on ER Ca2+ levels may be mediated through alterations in the endogenous SERCA2 protein levels, we performed Western blot analysis on HEK 293 cell lysates. To demonstrate the specificity of any observed effects, we also evaluated the expression levels of appropriate marker proteins. Because SERCA2 is an ER protein, and HAX-1 is primarily localized in the mitochondria, we used calreticulin, an ER Ca2+-binding protein, and the VDAC1, an outer mitochondrial membrane protein, as markers of the respective organelles. HAX-1 overexpression was found to cause a significant down-regulation (∼60%) of the endogenous SERCA2 protein levels, compared with control cells transfected with empty vector (Figure 7, A and B). No significant changes in the endogenous levels of calreticulin, or VDAC1 were observed in these HAX-1–overexpressing cells (Figure 7, A and B). Importantly, RT-PCR analysis did not detect any alterations in SERCA2 mRNA levels, after HAX-1 overexpression (Figure 7C), suggesting that the observed down-regulation of endogenous SERCA2 levels is occurring posttranslationally.
Figure 7.
HAX-1 overexpression down-regulates endogenous SERCA2 protein levels. (A) Western blot analysis of HEK 293 cells transfected with the indicated constructs determined a significant reduction in the SERCA2 protein levels as the result of HAX-1 overexpression. HEK 293 cell lysates were separated by SDS-PAGE and incubated with SERCA2, calreticulin (CALRT), VDAC1, or GAPDH antibodies. A representative Western blot from a single experiment is shown. (B) Quantitative analysis of SERCA2, calreticulin (CALRT), VDAC1, or GAPDH protein levels from three independent experiments determined a statistically significant down-regulation of SERCA2 protein levels in HAX-1 transfected cells, compared with controls. Data are presented as means ± SE (n = 3; t test, *p < 0.05). (C) RT-PCR analysis of HEK 293 cells transfected with the indicated constructs did not identify any changes at SERCA2 mRNA levels.
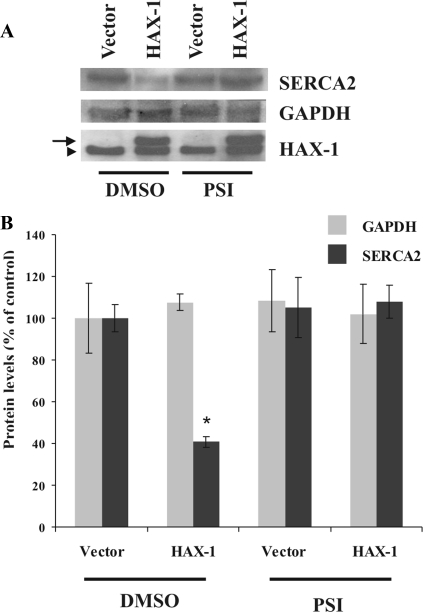
Because previous studies have demonstrated the involvement of the proteasomal pathway in mediating degradation of SERCA2 after calreticulin overexpression in myocardial H9c2 cells (Ahn et al., 2003; Ihara et al., 2005), we examined whether down-regulation of SERCA2 in HAX-1–overexpressing cells may also be occurring in a proteasome-dependent manner. Inhibitors of the proteasomal chymotrypsin-like activity were used previously to demonstrate the proteasomal-mediated degradation of SERCA2 (Ahn et al., 2003; Ihara et al., 2005); consequently, we chose to use such an inhibitor, and in particular PSI, in our studies. As expected, the levels of SERCA2 were reduced by ∼59% in HEK 293 cells transfected with HAX-1 in the absence of PSI; on the contrary, treatment with 50 nM PSI restored SERCA2 expression to levels similar to vector-transfected cells (Figure 8, A and B).
Figure 8.
HAX-1 overexpression promotes proteasome-dependent down-regulation of SERCA2. (A) Immunoblot analysis indicating that the presence of PSI suppressed degradation of SERCA2, promoted by HAX-1, which suggests the involvement of the proteasome pathway in SERCA2 down-regulation. Protein levels of transfected myc-HAX-1 are also shown (arrow), whereas endogenous HAX-1 is indicated by an arrowhead. (B) Quantitative analysis of SERCA2 or GAPDH protein levels determined the occurrence of a statistical significant down-regulation of SERCA2 in HAX-1–transfected cells that had been incubated with DMSO, whereas SERCA2 protein degradation was found to be inhibited in the presence of the proteasome inhibitor PSI. Data are presented as means ± SE (n = 3; t test, *p < 0.05).
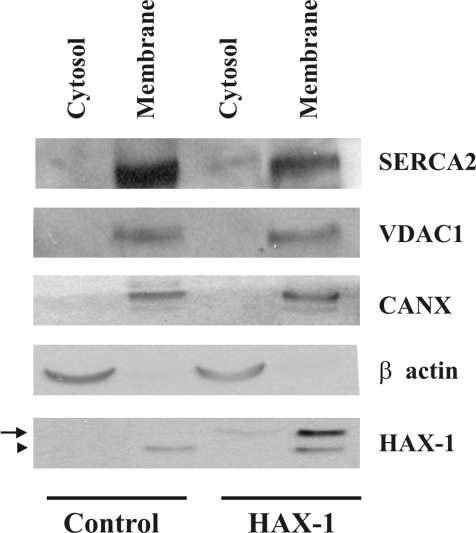
Furthermore, to determine whether HAX-1 overexpression may lead to SERCA2 proteasomal degradation through alterations in its subcellular distribution, we fractionated HEK 293-transfected cells into cytosolic and membrane fractions. To demonstrate the purity of the generated fractions, we examined the presence of representative marker proteins in our samples. Because SERCA2 and HAX-1 are membrane proteins of the ER and mitochondria, respectively, we evaluated the subcellular distribution of additional membrane proteins of these organelles. VDAC1, a mitochondrial outer membrane protein and calnexin (CANX), an ER membrane protein were chosen for this analysis mainly because of their membrane distribution in these organelles and their expression in HEK 293 cells. In control, vector-transfected cells, SERCA2 was detected only in the membrane fraction. HAX-1 overexpression resulted in reduction of SERCA2 protein levels in the membrane fraction and displacement of a portion of the protein to the cytosolic fraction (Figure 9). This subcellular alteration was specific for SERCA2 as no effects on the subcellular distribution of CANX, VDAC1 or the cytosolic protein β-actin were observed. The displacement in the subcellular distribution of the endogenous SERCA2 protein could therefore lead to its subsequent reduction through the proteasome degradation pathway.
Figure 9.
HAX-1 overexpression affects the subcellular distribution of SERCA2. Western blot analysis of cytosolic and membrane fractions determined that a portion of the endogenous SERCA2 protein was present in the cytosolic fraction of HAX-1 overexpressing cells. No alterations were found in the subcellular distribution of VDAC1, an outer mitochondrial membrane protein, CANX, an ER membrane protein, or cytosolic β-actin. The subcellular distribution of transfected myc-HAX-1 is also shown (arrow), with the endogenous HAX-1 protein being indicated by an arrowhead. Similar findings were observed in three independent experiments.
DISCUSSION
This study is the first to report that HAX-1, a primarily mitochondrial protein with antiapoptotic function, can bind to SERCA2. The interaction between HAX-1 and SERCA2 occurs independently of PLN, and it is diminished by increasing Ca2+ concentration, similar to the PLN/SERCA and PLN/HAX-1 interactions (James et al., 1989; Asahi et al., 2000; Vafiadaki et al., 2007). Furthermore, overexpression of HAX-1 seems to regulate the SERCA2 protein levels and ER Ca2+ stores, which may play an important role in the antiapoptotic function of HAX-1.
Deletion mapping determined that a region including amino acids 575–594 within the nucleotide binding domain of SERCA2 is required for its interaction with HAX-1. Because the nucleotide binding region is proposed to play a crucial role in determining the high Ca2+ affinity of SERCA2 (Toyofuku et al., 1992, 1993), it is possible that its interaction with HAX-1 may affect the SERCA activity. Previous cross-linking, site-directed mutagenesis and structural modeling studies have shown that residues Lys397-Val402 within the cytoplasmic region of SERCA2 are involved in binding to PLN (James et al., 1989; Toyofuku et al., 1994b; Toyoshima et al., 2003). Hence, the interaction with PLN occurs at residues close to the phosphorylation site (Asp 351) of SERCA, whereas HAX-1 binding requires residues within the nucleotide binding domain.
Immunofluorescence microscopy studies have localized HAX-1 to the mitochondria, endoplasmic reticulum and the nuclear envelope (Suzuki et al., 1997; Gallagher et al., 2000; Dufva et al., 2001; Sharp et al., 2002; Yedavalli et al., 2005; Han et al., 2006; Kasashima et al., 2006). Our transient transfections in HEK 293 cells have demonstrated that recombinant HAX-1 preferentially localizes to mitochondria, whereas, upon cotransfection with PLN, it undergoes cellular redistribution and colocalizes with PLN at the ER (Vafiadaki et al., 2007). Contrary to the effect of PLN on HAX-1 localization, transient cotransfections with SERCA did not seem to affect HAX-1 subcellular distribution, which was still preferentially targeted to mitochondria. However, as immunofluorescence studies in oligodendrocytes and RBL-2H3 mucosal mast cells have previously determined the close association of SERCA with mitochondria (Simpson and Russell, 1997; Csordas and Hajnoczky, 2001), it is possible that even though there was no direct colocalization of the overexpressed proteins, they may partially colocalize at sites of ER and mitochondrial membrane apposition. Consistent with this, HAX-1 has been shown to be an integral protein of the outer mitochondrial membrane (Kasashima et al., 2006), which may allow it to directly interact with SERCA at sites of close association between mitochondrial and ER membranes. Indeed, high-resolution microscopy approaches have determined the existence of such mitochondrial and ER membrane contact sites, with an estimated 5–20% of mitochondrial surface being in association with ER in HeLa cells (Mannella et al., 1998; Rizzuto et al., 1998). Importantly, the presence of tethering structures that physically link the ER and outer mitochondrial membranes were recently revealed by electron tomography, a finding which further highlights the existence of the ER-mitochondrial contact sites (Csordas et al., 2006). Although the interface area between the two organelles may represent only a small part of the mitochondrial and ER surface, it was proposed that the close apposition of ER and mitochondrial membranes may exert local Ca2+ control and thus insulate mitochondria from moderate increases in Ca2+ levels originated in regions outside the ER–mitochondria junctions (Csordas and Hajnoczky, 2001). Based on its localization and on direct measurements of Ca2+ signals, SERCA was proposed to regulate local Ca2+ microdomains at the ER–mitochondria junctions (Csordas and Hajnoczky, 2001), and the interaction of SERCA with HAX-1 may provide an additional control point.
Accumulating evidence indicates that regulation of ER Ca2+ homeostasis represents a critical determinant of cellular sensitivity to apoptotic stimuli. Overexpression of the antiapoptotic protein Bcl-2 in HeLa and HEK 293 cells resulted in alterations in Ca2+ handling, causing significant reduction of ER Ca2+ levels. This effect was proposed to confer protection against apoptosis by reducing Ca2+ efflux across ER membrane and preventing sustained Ca2+ increase in the cytoplasm and mitochondria that would trigger activation of cell death signaling cascades (Lam et al., 1994; Foyouzi-Youssefi et al., 2000; Pinton et al., 2000; Palmer et al., 2004). In addition to Bcl-2, the proapoptotic proteins BAX and BAK have also been shown to influence cell survival through regulation of ER Ca2+ homeostasis and mitochondrial Ca2+ accumulation (Nutt et al., 2002). Embryonic fibroblast cells from BAX and BAK double knockout mice exhibited reduced resting ER Ca2+ levels, decreased uptake of Ca2+ by mitochondria after ER Ca2+ release and resistance to apoptotic stimuli, that release Ca2+ from intracellular stores (Scorrano et al., 2003). Importantly, overexpression of SERCA in these cells resulted in restoration of ER Ca2+ levels and increased susceptibility to apoptotic agents, demonstrating the crucial role of ER Ca2+ content in determining cell survival (Scorrano et al., 2003).
In accordance with the above studies on Bcl-2–related proteins (Lam et al., 1994; Foyouzi-Youssefi et al., 2000; Pinton et al., 2000; Palmer et al., 2004), our findings indicate that HAX-1 may also promote cell survival through modulation of Ca2+ homeostasis. Indeed, overexpression of HAX-1 in HEK 293 cells resulted in decreased cytosolic Ca2+ levels, after thapsigargin treatment, suggesting a significant reduction in ER Ca2+ content. This effect of HAX-1 was abolished upon cotransfection with SERCA2, which counteracts HAX-1's antiapoptotic activity. In agreement with our observations, previous studies also reported that overexpression of SERCA2b in MCF7 breast cancer epithelial cells transfected with Bcl-2 restored ER Ca2+ content to control levels (Palmer et al., 2004), whereas adenoviral overexpression of SERCA in COS-1 cells resulted in ER Ca2+ overload (Ma et al., 1999). However, it is likely that additional mechanisms may contribute to the apparent alterations of the ER Ca2+ content observed in our study as well as previous studies. In this regard, studies on the effects of SERCA's down-regulation may provide additional insights on the role of HAX-1 in regulating ER Ca2+ stores and cell survival.
The reduced Ca2+ release from ER stores in HAX-1–overexpressing cells is associated with significant down-regulation of SERCA2 expression levels. Similar to our findings, Bcl-2 overexpression also decreased SERCA levels in LNCaP prostate cancer epithelial cells (Vanden Abeele et al., 2002). Conversely, our studies suggest that overexpression of SERCA2 resulted in increases in ER Ca2+ content, diminishing the protective effects of HAX-1. This inhibition of HAX-1's antiapoptotic activity may also be mediated by trapping of HAX-1 by the large size of SERCA2, perhaps preventing its proper localization or folding. Thus, overexpression of HAX-1 or Bcl-2 may promote cell survival by modulation of SERCA levels, resulting in diminished ER Ca2+ content and protection of mitochondria from Ca2+ overload. Previous in vitro studies have also indicated that, through direct binding, Bcl-2 inhibits SERCA activity and causes a conformational unfolding of the protein (Dremina et al., 2004), which may be associated with displacement of the enzyme from caveolae-related domains of the SR (Dremina et al., 2006). We also report here that overexpression of HAX-1 is associated with reduced SERCA2 protein in the membrane fraction, which may potentially lead to its degradation in the cytosol by the proteasomal pathway.
In summary, our findings suggest that HAX-1 interacts with SERCA2 in vitro and HAX-1 may down-regulate the SERCA2 protein levels in HEK 293 cells. Importantly, overexpression of HAX-1 was associated with reduction in ER Ca2+ content and increased cell survival, indicating that the antiapoptotic function of HAX-1 may be partially mediated through regulation of SERCA. However, it is currently unclear whether similar effects occur in striated muscle or whether our findings may be restricted to nonmuscle tissues. Consistent with this, adenoviral overexpression of SERCA2 in COS-1 cells significantly induced apoptosis (Ma et al., 1999), whereas increased SERCA2 expression in transgenic animal hearts or cultured cardiomyocytes did not accelerate apoptosis (Hajjar et al., 1997; He et al., 1997; Baker et al., 1998; Wu et al., 2004). Future studies should be designed to address the role of HAX-1 in ER/SR function in vivo, in muscle and nonmuscle tissues.
ACKNOWLEDGMENTS
We are grateful to the Biological Imaging Unit at the Biomedical Research Foundation for assistance with confocal imaging. This work was supported by research funds from the Biomedical Research Foundation of the Academy of Athens; the John F. Kostopoulos Foundation; the Hellenic Cardiological Society; National Institutes of Health grants HL26057, HL64018, and HL77101; the Leducq Foundation Trans-Antlantic alliance; and the European Union 6th Framework Program for Research and Technological Development, “Life sciences, genomics and biotechnology for health,” VALAPODYN, contract LSHG-CT-2006-037277.
Abbreviations used:
- GFP
green fluorescent protein
- GST
glutathione transferase
- HAX-1
HS-1 associated protein X-1
- HEK
human embryonic kidney
- PBS
phosphate-buffered saline
- PLN
phospholamban
- SERCA2a
sarcoplasmic reticulum Ca2+-ATPase
- SR
sarcoplasmic reticulum.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E08-06-0587) on October 29, 2008.
REFERENCES
- Aga-Mizrachi S., Brutman-Barazani T., Jacob A. I., Bak A., Elson A., Sampson S. R. Cytosolic protein tyrosine phosphatase-{epsilon} is a negative regulator of insulin signaling in skeletal muscle. Endocrinology. 2008;149:605–614. doi: 10.1210/en.2007-0908. [DOI] [PubMed] [Google Scholar]
- Ahn W., Lee M. G., Kim K. H., Muallem S. Multiple effects of SERCA2b mutations associated with Darier's disease. J. Biol. Chem. 2003;278:20795–20801. doi: 10.1074/jbc.M301638200. [DOI] [PubMed] [Google Scholar]
- Arvanitis D. A., Vafiadaki E., Fan G. C., Mitton B. A., Gregory K. N., Del Monte F., Kontrogianni-Konstantopoulos A., Sanoudou D., Kranias E. G. Histidine-rich Ca-binding protein interacts with sarcoplasmic reticulum Ca-ATPase. Am. J. Physiol. Heart Circ. Physiol. 2007;293:H1581–H1589. doi: 10.1152/ajpheart.00278.2007. [DOI] [PubMed] [Google Scholar]
- Asahi M., Kimura Y., Kurzydlowski K., Tada M., MacLennan D. H. Transmembrane helix M6 in sarco(endo)plasmic reticulum Ca(2+)-ATPase forms a functional interaction site with phospholamban. Evidence for physical interactions at other sites. J. Biol. Chem. 1999;274:32855–32862. doi: 10.1074/jbc.274.46.32855. [DOI] [PubMed] [Google Scholar]
- Asahi M., McKenna E., Kurzydlowski K., Tada M., MacLennan D. H. Physical interactions between phospholamban and sarco(endo)plasmic reticulum Ca2+-ATPases are dissociated by elevated Ca2+, but not by phospholamban phosphorylation, vanadate, or thapsigargin, and are enhanced by ATP. J. Biol. Chem. 2000;275:15034–15038. doi: 10.1074/jbc.275.20.15034. [DOI] [PubMed] [Google Scholar]
- Asahi M., Nakayama H., Tada M., Otsu K. Regulation of sarco(endo)plasmic reticulum Ca2+ adenosine triphosphatase by phospholamban and sarcolipin: implication for cardiac hypertrophy and failure. Trends Cardiovasc. Med. 2003;13:152–157. doi: 10.1016/s1050-1738(03)00037-9. [DOI] [PubMed] [Google Scholar]
- Baker D. L., et al. Targeted overexpression of the sarcoplasmic reticulum Ca2+-ATPase increases cardiac contractility in transgenic mouse hearts. Circ. Res. 1998;83:1205–1214. doi: 10.1161/01.res.83.12.1205. [DOI] [PubMed] [Google Scholar]
- Berman M. C. Characterisation of thapsigargin-releasable Ca(2+) from the Ca(2+)-ATPase of sarcoplasmic reticulum at limiting [Ca(2+)] Biochim. Biophys. Acta. 2000;1509:42–54. doi: 10.1016/s0005-2736(00)00280-7. [DOI] [PubMed] [Google Scholar]
- Chu G., et al. Compensatory mechanisms associated with the hyperdynamic function of phospholamban-deficient mouse hearts. Circ. Res. 1996;79:1064–1076. doi: 10.1161/01.res.79.6.1064. [DOI] [PubMed] [Google Scholar]
- Cilenti L., Soundarapandian M. M., Kyriazis G. A., Stratico V., Singh S., Gupta S., Bonventre J. V., Alnemri E. S., Zervos A. S. Regulation of HAX-1 anti-apoptotic protein by Omi/HtrA2 protease during cell death. J. Biol. Chem. 2004;279:50295–50301. doi: 10.1074/jbc.M406006200. [DOI] [PubMed] [Google Scholar]
- Csordas G., Hajnoczky G. Sorting of calcium signals at the junctions of endoplasmic reticulum and mitochondria. Cell Calcium. 2001;29:249–262. doi: 10.1054/ceca.2000.0191. [DOI] [PubMed] [Google Scholar]
- Csordas G., Renken C., Varnai P., Walter L., Weaver D., Buttle K. F., Balla T., Mannella C. A., Hajnoczky G. Structural and functional features and significance of the physical linkage between ER and mitochondria. J. Cell Biol. 2006;174:915–921. doi: 10.1083/jcb.200604016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dode L., Andersen J. P., Leslie N., Dhitavat J., Vilsen B., Hovnanian A. Dissection of the functional differences between sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA) 1 and 2 isoforms and characterization of Darier disease (SERCA2) mutants by steady-state and transient kinetic analyses. J. Biol. Chem. 2003;278:47877–47889. doi: 10.1074/jbc.M306784200. [DOI] [PubMed] [Google Scholar]
- Dremina E. S., Sharov V. S., Kumar K., Zaidi A., Michaelis E. K., Schoneich C. Anti-apoptotic protein Bcl-2 interacts with and destabilizes the sarcoplasmic/endoplasmic reticulum Ca2+-ATPase (SERCA) Biochem. J. 2004;383:361–370. doi: 10.1042/BJ20040187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dremina E. S., Sharov V. S., Schoneich C. Displacement of SERCA from SR lipid caveolae-related domains by Bcl-2, a possible mechanism for SERCA inactivation. Biochemistry. 2006;45:175–184. doi: 10.1021/bi050800s. [DOI] [PubMed] [Google Scholar]
- Dufva M., Olsson M., Rymo L. Epstein-Barr virus nuclear antigen 5 interacts with HAX-1, a possible component of the B-cell receptor signalling pathway. J. Gen. Virol. 2001;82:1581–1587. doi: 10.1099/0022-1317-82-7-1581. [DOI] [PubMed] [Google Scholar]
- Foyouzi-Youssefi R., Arnaudeau S., Borner C., Kelley W. L., Tschopp J., Lew D. P., Demaurex N., Krause K. H. Bcl-2 decreases the free Ca2+ concentration within the endoplasmic reticulum. Proc. Natl. Acad. Sci. USA. 2000;97:5723–5728. doi: 10.1073/pnas.97.11.5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher A. R., Cedzich A., Gretz N., Somlo S., Witzgall R. The polycystic kidney disease protein PKD2 interacts with Hax-1, a protein associated with the actin cytoskeleton. Proc. Natl. Acad. Sci. USA. 2000;97:4017–4022. doi: 10.1073/pnas.97.8.4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haghighi K., et al. A mutation in the human phospholamban gene, deleting arginine 14, results in lethal, hereditary cardiomyopathy. Proc. Natl. Acad. Sci. USA. 2006;103:1388–1393. doi: 10.1073/pnas.0510519103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haghighi K., et al. Human phospholamban null results in lethal dilated cardiomyopathy revealing a critical difference between mouse and human. J. Clin. Invest. 2003;111:869–876. doi: 10.1172/JCI17892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajjar R. J., Kang J. X., Gwathmey J. K., Rosenzweig A. Physiological effects of adenoviral gene transfer of sarcoplasmic reticulum calcium ATPase in isolated rat myocytes. Circulation. 1997;95:423–429. doi: 10.1161/01.cir.95.2.423. [DOI] [PubMed] [Google Scholar]
- Han Y., Chen Y.-S., Liu Z., Bodyak N., Rigor D., Bisping E., Pu W. T., Kang P. M. Overexpression of HAX-1 protects cardiac myocytes from apoptosis through caspase-9 inhibition. Circ. Res. 2006;99:415–423. doi: 10.1161/01.RES.0000237387.05259.a5. [DOI] [PubMed] [Google Scholar]
- Hasenfuss G., Pieske B. Calcium cycling in congestive heart failure. J. Mol. Cell. Cardiol. 2002;34:951–969. doi: 10.1006/jmcc.2002.2037. [DOI] [PubMed] [Google Scholar]
- Hasenfuss G., et al. Relation between myocardial function and expression of sarcoplasmic reticulum Ca(2+)-ATPase in failing and nonfailing human myocardium. Circ. Res. 1994;75:434–442. doi: 10.1161/01.res.75.3.434. [DOI] [PubMed] [Google Scholar]
- He H., et al. Overexpression of the rat sarcoplasmic reticulum Ca2+ ATPase gene in the heart of transgenic mice accelerates calcium transients and cardiac relaxation. J. Clin. Invest. 1997;100:380–389. doi: 10.1172/JCI119544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihara Y., Kageyama K., Kondo T. Overexpression of calreticulin sensitizes SERCA2a to oxidative stress. Biochem. Biophys. Res. Commun. 2005;329:1343–1349. doi: 10.1016/j.bbrc.2005.02.112. [DOI] [PubMed] [Google Scholar]
- James P., Inui M., Tada M., Chiesi M., Carafoli E. Nature and site of phospholamban regulation of the Ca2+ pump of sarcoplasmic reticulum. Nature. 1989;342:90–92. doi: 10.1038/342090a0. [DOI] [PubMed] [Google Scholar]
- Kadambi V. J., Ponniah S., Harrer J. M., Hoit B. D., Dorn G. W., 2nd., Walsh R. A., Kranias E. G. Cardiac-specific overexpression of phospholamban alters calcium kinetics and resultant cardiomyocyte mechanics in transgenic mice. J. Clin. Invest. 1996;97:533–539. doi: 10.1172/JCI118446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasashima K., Ohta E., Kagawa Y., Endo H. The pleiotropic human prohibitin 2, mitochondrial functions and estrogen receptor-dependent nuclear translocation. J. Biol. Chem. 2006;281:36401–36410. doi: 10.1074/jbc.M605260200. [DOI] [PubMed] [Google Scholar]
- Kimura Y., Kurzydlowski K., Tada M., MacLennan D. H. Phospholamban regulates the Ca2+-ATPase through intramembrane interactions. J. Biol. Chem. 1996;271:21726–21731. doi: 10.1074/jbc.271.36.21726. [DOI] [PubMed] [Google Scholar]
- Kontrogianni-Konstantopoulos A., Jones E. M., Van Rossum D. B., Bloch R. J. Obscurin is a ligand for small ankyrin 1 in skeletal muscle. Mol. Biol. Cell. 2003;14:1138–1148. doi: 10.1091/mbc.E02-07-0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam M., Dubyak G., Chen L., Nunez G., Miesfeld R. L., Distelhorst C. W. Evidence that BCL-2 represses apoptosis by regulating endoplasmic reticulum-associated Ca2+ fluxes. Proc. Natl. Acad. Sci. USA. 1994;91:6569–6573. doi: 10.1073/pnas.91.14.6569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Fox C. J., Master S. R., Bindokas V. P., Chodosh L. A., Thompson C. B. Bcl-X(L) affects Ca(2+) homeostasis by altering expression of inositol 1,4,5-trisphosphate receptors. Proc. Natl. Acad. Sci. USA. 2002;99:9830–9835. doi: 10.1073/pnas.152571899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W., Grupp I. L., Harrer J., Ponniah S., Grupp G., Duffy J. J., Doetschman T., Kranias E. G. Targeted ablation of the phospholamban gene is associated with markedly enhanced myocardial contractility and loss of beta-agonist stimulation. Circ. Res. 1994;75:401–409. doi: 10.1161/01.res.75.3.401. [DOI] [PubMed] [Google Scholar]
- Lytton J., Westlin M., Hanley M. R. Thapsigargin inhibits the sarcoplasmic or endoplasmic reticulum Ca-ATPase family of calcium pumps. J. Biol. Chem. 1991;266:17067–17071. [PubMed] [Google Scholar]
- Ma T. S., Mann D. L., Lee J. H., Gallinghouse G. J. SR compartment calcium and cell apoptosis in SERCA overexpression. Cell Calcium. 1999;26:25–36. doi: 10.1054/ceca.1999.0049. [DOI] [PubMed] [Google Scholar]
- MacLennan D. H., Rice W. J., Green N. M. The mechanism of Ca2+ transport by sarco(endo)plasmic reticulum Ca2+-ATPases. J. Biol. Chem. 1997;272:28815–28818. doi: 10.1074/jbc.272.46.28815. [DOI] [PubMed] [Google Scholar]
- Mannella C. A., Buttle K., Rath B. K., Marko M. Electron microscopic tomography of rat-liver mitochondria and their interaction with the endoplasmic reticulum. Biofactors. 1998:225–228. doi: 10.1002/biof.5520080309. [DOI] [PubMed] [Google Scholar]
- Meyer M., et al. Alterations of sarcoplasmic reticulum proteins in failing human dilated cardiomyopathy. Circulation. 1995;92:778–784. doi: 10.1161/01.cir.92.4.778. [DOI] [PubMed] [Google Scholar]
- Nutt L. K., Pataer A., Pahler J., Fang B., Roth J., McConkey D. J., Swisher S. G. Bax and Bak promote apoptosis by modulating endoplasmic reticular and mitochondrial Ca2+ stores. J. Biol. Chem. 2002;277:9219–9225. doi: 10.1074/jbc.M106817200. [DOI] [PubMed] [Google Scholar]
- Palmer A. E., Jin C., Reed J. C., Tsien R. Y. Bcl-2-mediated alterations in endoplasmic reticulum Ca2+ analyzed with an improved genetically encoded fluorescent sensor. Proc. Natl. Acad. Sci. USA. 2004;101:17404–17409. doi: 10.1073/pnas.0408030101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinton P., Ferrari D., Magalhaes P., Schulze-Osthoff K., Di Virgilio F., Pozzan T., Rizzuto R. Reduced loading of intracellular Ca(2+) stores and downregulation of capacitative Ca(2+) influx in Bcl-2-overexpressing cells. J. Cell Biol. 2000;148:857–862. doi: 10.1083/jcb.148.5.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto R., Pinton P., Carrington W., Fay F. S., Fogarty K. E., Lifshitz L. M., Tuft R. A., Pozzan T. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science. 1998;280:1763–1766. doi: 10.1126/science.280.5370.1763. [DOI] [PubMed] [Google Scholar]
- Sagara Y., Inesi G. Inhibition of the sarcoplasmic reticulum Ca2+ transport ATPase by thapsigargin at subnanomolar concentrations. J. Biol. Chem. 1991;266:13503–13506. [PubMed] [Google Scholar]
- Schmitt J. P., et al. Dilated cardiomyopathy and heart failure caused by a mutation in phospholamban. Science. 2003;299:1410–1413. doi: 10.1126/science.1081578. [DOI] [PubMed] [Google Scholar]
- Scorrano L., Oakes S. A., Opferman J. T., Cheng E. H., Sorcinelli M. D., Pozzan T., Korsmeyer S. J. BAX and BAK regulation of endoplasmic reticulum Ca2+: a control point for apoptosis. Science. 2003;300:135–139. doi: 10.1126/science.1081208. [DOI] [PubMed] [Google Scholar]
- Sharp T. V., Wang H. W., Koumi A., Hollyman D., Endo Y., Ye H., Du M. Q., Boshoff C. K15 protein of Kaposi's sarcoma-associated herpesvirus is latently expressed and binds to HAX-1, a protein with antiapoptotic function. J. Virol. 2002;76:802–816. doi: 10.1128/JVI.76.2.802-816.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmerman H. K., Jones L. R. Phospholamban: protein structure, mechanism of action, and role in cardiac function. Physiol. Rev. 1998;78:921–947. doi: 10.1152/physrev.1998.78.4.921. [DOI] [PubMed] [Google Scholar]
- Simpson P. B., Russell J. T. Role of sarcoplasmic/endoplasmic-reticulum Ca2+-ATPases in mediating Ca2+ waves and local Ca2+-release microdomains in cultured glia. Biochem. J. 1997;325:239–247. doi: 10.1042/bj3250239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack J. P., et al. The enhanced contractility of the phospholamban-deficient mouse heart persists with aging. J. Mol. Cell. Cardiol. 2001;33:1031–1040. doi: 10.1006/jmcc.2001.1370. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Demoliere C., Kitamura D., Takeshita H., Deuschle U., Watanabe T. HAX-1, a novel intracellular protein, localized on mitochondria, directly associates with HS1, a substrate of Src family of tyrosine kinases. J. Immunol. 1997;158:2736–2744. [PubMed] [Google Scholar]
- Thastrup O., Cullen P. J., Drobak B. K., Hanley M. R., Dawson A. P. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc. Natl. Acad. Sci. USA. 1990;87:2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyofuku T., Kurzydlowski K., Lytton J., MacLennan D. H. The nucleotide binding/hinge domain plays a crucial role in determining isoform-specific Ca2+ dependence of organellar Ca(2+)-ATPases. J. Biol. Chem. 1992;267:14490–14496. [PubMed] [Google Scholar]
- Toyofuku T., Kurzydlowski K., Tada M., MacLennan D. H. Identification of regions in the Ca(2+)-ATPase of sarcoplasmic reticulum that affect functional association with phospholamban. J. Biol. Chem. 1993;268:2809–2815. [PubMed] [Google Scholar]
- Toyofuku T., Kurzydlowski K., Tada M., MacLennan D. H. Amino acids Glu2 to Ile18 in the cytoplasmic domain of phospholamban are essential for functional association with the Ca(2+)-ATPase of sarcoplasmic reticulum. J. Biol. Chem. 1994a;269:3088–3094. [PubMed] [Google Scholar]
- Toyofuku T., Kurzydlowski K., Tada M., MacLennan D. H. Amino acids Lys-Asp-Asp-Lys-Pro-Val402 in the Ca(2+)-ATPase of cardiac sarcoplasmic reticulum are critical for functional association with phospholamban. J. Biol. Chem. 1994b;269:22929–22932. [PubMed] [Google Scholar]
- Toyoshima C., Asahi M., Sugita Y., Khanna R., Tsuda T., MacLennan D. H. Modeling of the inhibitory interaction of phospholamban with the Ca2+ ATPase. Proc. Natl. Acad. Sci. USA. 2003;100:467–472. doi: 10.1073/pnas.0237326100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoshima C., Nakasako M., Nomura H., Ogawa H. Crystal structure of the calcium pump of sarcoplasmic reticulum at 2.6 A resolution. Nature. 2000;405:647–655. doi: 10.1038/35015017. [DOI] [PubMed] [Google Scholar]
- Toyoshima C., Sasabe H., Stokes D. L. Three-dimensional cryo-electron microscopy of the calcium ion pump in the sarcoplasmic reticulum membrane. Nature. 1993;362:467–471. doi: 10.1038/362469a0. [DOI] [PubMed] [Google Scholar]
- Vafiadaki E., Papalouka V., Arvanitis D. A., Kranias E. G., Sanoudou D. The role of SERCA2a/PLN complex, Ca(2+) homeostasis, and anti-apoptotic proteins in determining cell fate. Pflugers Arch. 2008 doi: 10.1007/s00424-008-0506-5. in press. [DOI] [PubMed] [Google Scholar]
- Vafiadaki E., Sanoudou D., Arvanitis D. A., Catino D. H., Kranias E. G., Kontrogianni-Konstantopoulos A. Phospholamban interacts with HAX-1, a mitochondrial protein with anti-apoptotic function. J. Mol. Biol. 2007;367:65–79. doi: 10.1016/j.jmb.2006.10.057. [DOI] [PubMed] [Google Scholar]
- Vanden Abeele F., Skryma R., Shuba Y., Van Coppenolle F., Slomianny C., Roudbaraki M., Mauroy B., Wuytack F., Prevarskaya N. Bcl-2-dependent modulation of Ca(2+) homeostasis and store-operated channels in prostate cancer cells. Cancer Cell. 2002;1:169–179. doi: 10.1016/s1535-6108(02)00034-x. [DOI] [PubMed] [Google Scholar]
- Vangheluwe P., Raeymaekers L., Dode L., Wuytack F. Modulating sarco(endo)plasmic reticulum Ca2+ ATPase 2 (SERCA2) activity: cell biological implications. Cell Calcium. 2005;38:291–302. doi: 10.1016/j.ceca.2005.06.033. [DOI] [PubMed] [Google Scholar]
- Wu G., Long X., Marin-Garcia J. Adenoviral SERCA1 overexpression triggers an apoptotic response in cultured neonatal but not in adult rat cardiomyocytes. Mol. Cell. Biochem. 2004;267:123–132. doi: 10.1023/b:mcbi.0000049361.89265.2b. [DOI] [PubMed] [Google Scholar]
- Yedavalli V. S., et al. Human immunodeficiency virus type 1 Vpr interacts with antiapoptotic mitochondrial protein HAX-1. J. Virol. 2005;79:13735–13746. doi: 10.1128/JVI.79.21.13735-13746.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P., Toyoshima C., Yonekura K., Green N. M., Stokes D. L. Structure of the calcium pump from sarcoplasmic reticulum at 8-A resolution. Nature. 1998;392:835–839. doi: 10.1038/33959. [DOI] [PubMed] [Google Scholar]