Abstract
When cells cease migrating through the vasculature, adhere to extracellular matrix, and begin to spread, they exhibit rapid changes in contraction and relaxation at peripheral regions newly contacting the underlying substrata. We describe here a requirement in this process for myosin II disassembly at the cell cortex via the action of myosin phosphatase (MP), which in turn is regulated by a plasma membrane signaling lipid. Cells in suspension exhibit high levels of activity of the signaling enzyme phospholipase D2 (PLD2), elevating production of the lipid second messenger phosphatidic acid (PA) at the plasma membrane, which in turn recruits MP and stores it there in a presumed inactive state. On cell attachment, down-regulation of PLD2 activity decreases PA production, leading to MP release, myosin dephosphorylation, and actomyosin disassembly. This novel model for recruitment and restraint of MP provides a means to effect a rapid cytoskeletal reorganization at the cell cortex upon demand.
INTRODUCTION
Cell adhesion and spreading play central roles in inflammation and metastasis (Sastry and Burridge, 2000). Cell spreading is a highly dynamic process that involves rebalancing the forces that contract cells into compact, spherical shapes versus those that flatten the cells and extend their outer boundaries. The best characterized part of the cell spreading pathway is analogous to the forward extension of lamellipodia and filopodia exhibited by migrating cells. This extension is driven by actin polymerization promoted by Ras-triggered activation of the Rho-family GTPases Cdc42 and Rac1 (Price et al., 1998; Berrier et al., 2000; Small et al., 2002) and contractile force provided by myosin II (Wakatsuki et al., 2003; Giannone et al., 2004), which becomes assembled in association with the newly formed Rho-generated stress fibers and focal adhesions at the cell center/bottom. Myosin II activation is stimulated by phosphorylation of the myosin regulatory light chain (MLC) and terminated via dephosphorylation. The phosphorylation is performed in this setting by Rho-activated Rho kinase (ROCK), and the dephosphorylation by myosin phosphatase (MP; Hirano et al., 2003; Somlyo and Somlyo, 2003). In addition to activating myosin II by phosphorylating MLC, ROCK also suppresses MP action by phosphorylating it as well (Ito et al., 2004).
Before these well-described events, though, it is necessary to diminish contractile activity at the cell cortex (Ren et al., 1999, 2000; Arthur et al., 2000; Arthur and Burridge, 2001). This step likely proceeds independent of the Rho-pathway activities, because the cells at this time are nearly devoid of stress fibers, having instead extensive F-actin–rich ruffles and lamellipodia at the cell periphery (Arthur and Burridge, 2001). Myosin II activity at the cell periphery regulates subsequent membrane dynamics during cell migration, and this activity is regulated by a signaling pathway other than Rho-ROCK (Totsukawa et al., 2000, 2004; Giannone et al., 2004). However, it has not been investigated yet whether control of myosin II activity is key early in cell spreading.
Taken together, these reports raise the issue of whether myosin inactivation triggers the spreading observed early after attachment, and if so, what signaling pathway regulates this event. We show here that myosin regulates early stages of cell spreading and provide evidence for a novel regulatory mechanism based on integrin-stimulated changes in lipid signaling that result in dynamic release and activation of locally stored, quiescent myosin phosphatase. Taken together with recent reports on phospholipase D2 (PLD2) regulation of signaling-stimulated Ras activation at the plasma membrane in T-cells and transformed cells (Mor et al., 2007; Zhao et al., 2007), this regulatory pathway suggests an unexpected means through which myosin fibril reorganization is coordinated and synergizes with Ras-mediated changes in the actin cytoskeleton during cell spreading.
MATERIALS AND METHODS
General Reagents
Y-27632, ML-7, blebbistatin, and human fibronectin were obtained from Calbiochem (La Jolla, CA); cell culture media (DMEM, F-12, and Opti-MEM-I) and LipofectAmine Plus from Invitrogen (Carlsbad, CA); [3H]palmitic acid from American Radiolabeled Chemicals (St. Louis, MO); and thin layer chromatography (TLC) plates from Fisher (Pittsburgh, PA). Reagents were of analytical grade unless otherwise specified. The plasmid expressing a constitutively active MLC mutant, MLC-DD, in which MLC phosphorylation is mimicked by replacing the two major phosphorylation sites Thr18 and Ser19 with aspartic acid, was generously provided by Hiroshi Hosoya (Hiroshima University, Higashi-Hiroshima 739-8526, Japan).
Antibodies
Mouse monoclonal anti-pMLCS19, anti-cofilinS3, and anti-AKTT308 were obtained from Cell Signaling Technology (Beverly, MA). Antibodies against FAKY397, anti-protein phosphatase 1 c δ (PP1c δ isoform) polyclonal antibodies, and a Rac1 mAb were from Upstate Biotechnology (Lake Placid, NY). Focal adhesion kinase (FAK), protien kinase B (AKT) (clone 55), and cofilin antibodies were also obtained from BD PharMingen (San Jose, CA). Rabbit anti-myosin binding subunit (MBS) was purchased from Covance Research Products (Berkeley, CA). Anti-MAPK and anti-phospho-MAPK (clone MAPK-YT) were purchased from Sigma (St. Louis, MO). Rat monoclonal anti-HA (3F10) was obtained from Roche (Indianapolis, IN). Rabbit anti-HA and goat anti-mouse and anti-rabbit IgG conjugated with IRDye 800 were from Rockland Immunochemicals (Gilbertsville, PA). Rhodamine- and Alexa 647–conjugated phalloidin, goat anti-mouse, and anti-rabbit IgG conjugated with Alexa 488, Alexa 647, and Alexa 680, To-Pro3, and DAPI were from Invitrogen.
Cell Culture and Transfection
Tetracycline (Tet)-inducible Chinese hamster ovary (CHO) stable cells expressing wild-type and inactive alleles of PLD1 and PLD2 were generated using the T-Rex system from Invitrogen (Du et al., 2004; Su et al., 2006) and were grown in F-12 medium containing 10% Tet-free fetal bovine serum (FBS) from Clontech (Palo Alto, CA). The expression of recombinant proteins was induced for 24 h by addition of 1 μg/ml doxycycline (Dox, a Tet analogue). Although very little expression of the recombinant PLD was observed in the absence of Dox in the stable cell lines (Du et al., 2004; Su et al., 2006), we chose to use the parental CHO T-Rex cells instead of uninduced cells as the control cell line, to avoid even a small amount of potential leaky expression with the Tet expression system. The T-Rex cells were incubated with Dox under identical conditions to the PLD-inducible cell lines.
To generate retroviruses carrying short hairpin RNAs (shRNAs), PLD2 (target sequence: GACTGGACATTATGCTCAA), and luciferase (target sequence: GATTTCGAGTCGTCTTAAT) shRNAs were cloned into the pSuper.Retro.Bla vector. The packaging cell line GP2-293 (Clontech), seeded on 100 × 20-mm tissue culture dishes, was cotransfected with 5 μg each of retroviral vector and the envelope glycoprotein expression vector pVSV-G using Lipofectamine Plus (Invitrogen). Transfection medium was replaced with 10 ml growth medium 4 h later, and the cells were incubated for 48 h. Retrovirus-containing supernatant was then harvested, passed through a 0.45-μm cellulose acetate filter, and used to infect the CHO cells, which, 2 d later, were selected with 10 μg/ml blasticidin for 3 d.
Cell Spreading Assay
Plating surfaces (coverslips or tissue culture plates) were coated with 10 μg/ml fibronectin (Calbiochem) at room temperature for 1 h, followed by blocking for 1 h with 1% heat-inactivated fatty acid-free bovine serum albumin (BSA) from Serological Richmond Institute (Richmond, CA). Cells were plated in the 10% FBS F12 2 d before the experiment, and changed to the medium in the presence of 1 μg/ml Dox the day before the experiment. The cells were then suspended by trypsinization, to which an equal volume of trypsin inhibitor (Sigma) was immediately added. The cells were then spun down, resuspended in F12 containing 0.1% fatty acid-free BSA, and rested in the incubator for 2 h. Before plating, the medium was changed to prewarmed F12 containing 10% FBS. The cells were plated on fibronectin-coated surface for the indicated times. Cells in suspension were fixed in 4% paraformaldehyde, centrifuged to adhere them to poly-l-lysine–coated coverslips and then were processed as below for immunofluorescent staining.
Quantification was followed using previously described criteria (Randazzo et al., 2000). Multiple fields from at least three independent experiments were imaged from each condition, and cells were scored blindly as either “not spreading” if the outer diameter of F-actin staining was less than twice the diameter of the nuclei, as “spreading” if the outer diameter of F-actin staining was greater than twice the diameter of the nuclei, or as “accelerated spreading” if the outer diameter of F-actin staining was greater than three times the diameter of the nuclei. All data shown were obtained from experiments repeated at least three times. Data shown are mean ± SD.
Immunofluorescence Staining
Cells were fixed with 2% paraformaldehyde for 10 min followed by permeabilization with 0.1% Triton X-100. The hemagglutinin (HA)-tagged PLD2 proteins were detected by a rabbit polyclonal anti-HA tag antibody (Y-11) or an mAb (3F10), followed by secondary antibodies conjugated with Alexa 488 or Alexa 568. F-actin was visualized by phalloidin conjugated with Rhodamine or Alexa 647. Nuclei were stained with To-Pro3 or DAPI. Other proteins were stained with primary antibodies followed by a secondary antibody conjugated with Alexa 488, Alexa 568, or Alexa 647. After overlaying the coverslips, the slides were imaged using an Olympus fluorescent microscope (Melville, NY) or a Leica TCS SP2 confocal microscope (Deerfield, NY). Images were processed using Adobe Photoshop 8.0 (San Jose, CA) or the simulator program from Leica. All experiments were performed at least four times with similar results.
PLD Activity Assays
For in vivo PLD assays, the transfection mixtures were replaced with complete DMEM containing 2 μCi of [3H]palmitic acid, and the cells were incubated for 24 h. PLD activity assays were carried out using the in vivo transphosphatidylation assay as previously described (Du et al., 2000, 2002).
Rac GTPase Activity Assays
Rac activity was measured as reported (del Pozo et al., 2000). In brief, CHO cells were detached and kept in suspension in F-12 medium with 0.1% BSA for 2 h. The cells were then plated on dishes coated with 10 μg/ml fibronectin in the presence of 10% FBS. Fifteen minutes after plating, the cells were chilled on ice, washed with ice-cold phosphate-buffered saline (PBS), and lysed in buffer containing 0.5% NP-40, 50 mM Tris, pH 7.4, 150 mM NaCl, 10% glycerol, 1× protease inhibitor cocktail (Roche, Indianapolis, IN), and 20 μg recombinant glutathione S-transferase (GST)-PBD. The lysates were then incubated with glutathione-agarose beads (Amersham Pharmacia, Piscataway, NJ) for 30 min at 4°C, washed three times with lysis buffer, and eluted with SDS sample buffer. Bound Rac and whole cell lysates were analyzed by Western blotting using a monoclonal anti-Rac antibody.
RESULTS
Activated Myosin II Exhibits Dynamic Subcellular Compartmentalization and Regulation during Cell Spreading
The contractile force generated by nonmuscle myosin II is critical late in cell spreading in conjunction with stress fibers and focal adhesion formation and is regulated via phosphorylation of myosin by the Rho–ROCK pathway (Ren et al., 1999; Totsukawa et al., 2000; Khyrul et al., 2004). However, whether myosin II has a role in cell spreading at earlier stages is not clear.
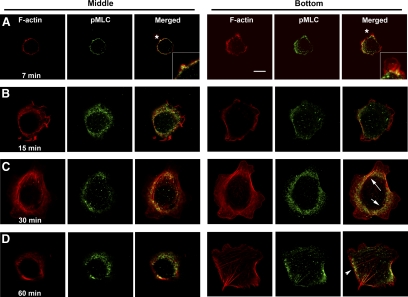
We thus examined the spatiotemporal activation of myosin II during cell spreading, using Ser19 MLC phosphorylation as a means to visualize activated myosin in CHO cells plated onto fibronectin-coated coverslips (Figure 1). Confocal microscopy revealed that phospho-MLC is always present, but localizes to different subcellular compartments at different time points during the spreading process. As the spherical cells attach, the bottom (ventral) surface in contact with the coverslip begins to flatten and spread (Figure 1A). In the upper, spherical region (middle section), phospho-MLC is found at the plasma membrane in association with peripheral (cortical) F-actin. At the bottom, however, the phospho-MLC has begun to retreat from the cell periphery, as defined by separation from the F-actin (asterisk, inset), and to appear in more central regions. By 15 min (Figure 1B), the cells are more flattened, and very little peripheral phospho-MLC is observed at both the middle and bottom sections. By 30 min (Figure 1C), central phospho-MLC is predominant. Stress fibers start to form at the cell bottom at this time (arrows) and weak, discrete phospho-MLC staining is seen along them. By 60 min (Figure 1D), phospho-MLC coincides with actin stress fibers and focal adhesions (arrowhead). By 2 h, most of the phospho-MLC aligns along the stress fibers (not shown).
Figure 1.
MLC phosphorylation relocates from the cell cortex to the cell bottom and center during cell spreading. CHO cells (T-Rex parental cells) were trypsinized, washed, suspended in medium containing 0.1% BSA for 2 h, plated on coverslips precoated with 10 μg/ml fibronectin for the indicated times (A, 7 min; B, 15 min; C, 30 min; D, 60 min), and fixed. Phospho-MLC was detected using an mAb that recognizes MLCpSer19 in combination with Alexa 488–conjugated goat anti-mouse secondary antibody. F-actin was visualized using rhodamine-phalloidin. The images shown were captured by a Leica TCS2 confocal microscope with the imaging processing software provided. Right panels (Bottom) indicate the optical section of the cell closest to the coverslip. Representative cells are shown from one of multiple experiments. Asterisks indicate location shown in higher magnification in inset. Arrows, actin stress fibers; arrowhead, focal adhesion. Scale bar, 10 μm.
Thus, spatiotemporal regulation of myosin activity is evident during the early stages of spreading, and the pattern of localization correlates decreased MLC phosphorylation and presumed activity at the cell cortex with peripheral spreading. The initial absence of stress fibers suggests a lack of Rho–ROCK pathway activity early in spreading. In contrast, the subsequent appearance of central phospho-MLC in association with stress fibers and focal adhesions suggests that the generation of this phosphomyosin pool should be under the control of Rho-ROCK, consistent with published reports (Ren et al., 1999; Totsukawa et al., 2000; Khyrul et al., 2004).
Myosin II Activity Controls the Rate of Early Cell Spreading through a ROCK-independent Pathway
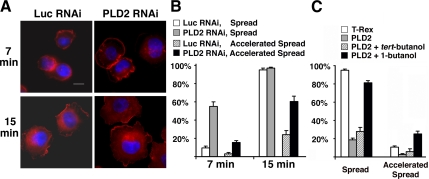
We next examined functional roles for myosin and Rho-ROCK in the early stages of cell spreading. As shown with a representative cell in the left panel and tabulated in the right panel in Figure 2A, most of the control cells had spread by 15 min after plating (with spreading defined as the outer diameter of F-actin staining being greater than twice the diameter of the nuclei). However, exposure of the cells to blebbistatin, an inhibitor of myosin-II ATPase activity, accelerated the rate at which the cells flattened and spread (with accelerated spreading defined as the outer diameter of F-actin staining being greater than three times the diameter of the nuclei). Conversely, expression of a constitutively active MLC allele, MLC-DD, but not the wild-type MLC allele, slowed the rate of cell spreading (Figure 2B), again suggesting that myosin II activity controls this process. In contrast, ablation of ROCK activity using its specific inhibitor, Y-27632, did not have significant effect on the speed of cell spreading (Figure 2C), although it did block subsequent stress fiber formation (data not shown). These results indicate that relaxation of myosin contractility is required for the initial stage of cell spreading and that it proceeds via a pathway unconnected to ROCK activity.
Figure 2.
Myosin II activity regulates the initial cell spreading and is independent of ROCK activity. (A) Inhibiting myosin II activity accelerates cell spreading. Blebbistatin (100 μM, final concentration) or vehicle (DMSO) was added to CHO cells in suspension 20 min before plating. (B) Increasing myosin activity by overexpressing MLC-DD-GFP, a constitutively active MLC allele, inhibits cell spreading. The wild-type MLC-GFP is used as a negative control. (C) Cell spreading early in cell adhesion is independent of ROCK activity. Y-27632, a ROCK-specific inhibitor, was added (50 μM, final concentration) to cells in suspension 20 min before plating. (A–C) In all experiments, the suspended cells were plated on coverslips coated with 10 μg/ml fibronectin and cultured for 15 min before being fixed for imaging. F-actin (red channel) was visualized using rhodamine-conjugated phalloidin, and the nuclei (blue) using DAPI. GFP (as an reporter of MLC-DD expression) is presented as green fluorescence. Representative cells are shown at the left and quantification of cell spreading at the right; see Materials and Methods for details. Scale bar, 10 μm.
Attachment-triggered Suppression of PLD2 Production of Phosphatidic Acid Regulates the Rate of Early Cell Spreading
ROCK promotes myosin assembly in the cell center by phosphorylating MLC and by preventing its dephosphorylation through inhibition of MP. In the absence of ROCK activity at the cell periphery, there exist other kinases that could phosphorylate MLC. However, alternate cellular pathways that might inhibit MP have not been well characterized. One hint for such a mechanism was suggested by biochemical studies showing that both MBS and the catalytic subunit PP1c of MP bind with specificity to the phospholipid phosphatidic acid (PA) and that MP enzymatic activity is inhibited by the interaction with PA (Ito et al., 1997; Kishikawa et al., 1999; Jones and Hannun, 2002). We thus examined whether dynamic changes in PA affect the early rate of cell spreading. PA can be generated by several types of enzymes responsive to signaling events including members of the PLD family (Jenkins and Frohman, 2005). To test the potential role of PLD in this process, we took advantage of previously established Tet-inducible stable CHO cell lines capable of expressing wild-type and dominant-negative alleles of PLD1 and PLD2, the two classical PLD isoforms (Du et al., 2004; Su et al., 2006). PLD1 is tightly regulated by signaling events initiated by G-protein–coupled receptors and receptor tyrosine kinases that trigger activation of ARF and Rho small GTPase families and protein kinase C (PKC; reviewed in Jenkins and Frohman, 2005). Cells expressing even very high levels of PLD1 do not exhibit elevated levels of activity until stimulated by agonists. PLD1 is found in intracellular vesicles in most cell types but traffics to the plasma membrane during stimulatory events. PLD2, in contrast, exhibits significant activity when overexpressed in resting cells and increases its level of activity upon G-protein–coupled receptors and receptor tyrosine kinase cell stimulation, although not through pathways involving Rho family small GTPases. PLD2 localizes to the plasma membrane and is thought to be regulated by incompletely understood inhibitory mechanisms rather than stimulatory ones.
As described earlier and in Figure 3A, most control T-Rex CHO cells have spread by 15 min after plating. In contrast, PLD2 overexpression dramatically slowed the rate of spread (Figure 3, A and B), and the cells remained relatively spherical. Conversely, the dominant-negative allele PLD2-K758R accelerated spreading. PLD1 and its dominant-negative allele K898R were without effect, suggesting a lack of involvement for this family member. PLD2 and PLD2-K758R did not alter the numbers of cells attached regardless of washing conditions (data not shown), suggesting a lack of effect on the net levels or binding avidity of cell surface integrin-mediated cell attachment to fibronectin, a finding consistent with reports that inhibition of Rho family members, while perturbing cell spreading, has no effect on cell adhesion (Clark et al., 1998).
Figure 3.
Levels of PLD2 activity regulate the rate of cell spreading. (A) Induced expression of PLD2 but not PLD1 affects cell spreading. T-Rex CHO PLD-inducible cell lines were induced using Dox for 24 h as described in Materials and Methods. The cells were then suspended, replated as above for 15 min, and stained with Alexa 488-phalloidin to visualize F-actin and DAPI to indicate nuclei. Representative cells are shown. (B) Quantification of cell spreading in A. (C) The PLD activity observed is attributable to PLD2 and is down-regulated by cell attachment. In vivo PLD activity assay measurements were performed on control and PLD-induced CHO cells that were in suspension or that had been allowed attach to fibronectin-coated tissue culture plates and to spread for 15 min. PLD activity is presented as the % of total 3H-palmitate–labeled phospholipids recovered as phosphatidylbutanol from TLC analysis. T-Rex, parental CHO cell line used to generate stable PLD-inducible cell lines. (D) Spatiotemporal change of PA in the control CHO cells and the PLD2-expressing cells. PA was visualized by a EGFP-conjugated PA-binding domain (PABD) described previously (Zeniou-Meyer et al., 2007). One day before the experiment, the cells were transfected with EGFP-PABD or an EGFP-PABD mutant allele that lacks PA-binding capacity. Four hours after transfection, the T-Rex CHO PLD2-inducible cell lines were induced using Dox. Cells were either directly fixed in suspension and then spun to poly-l-lysine–coated coverslips (0 min) or fixed 7 or 15 min after replating onto fibronectin-coated coverslips. F-actin was imaged using rhodamine-phalloidin. Scale bar, 10 μm. Asterisks indicate location shown in higher magnification in inset.
The observation that altering the level of PA production via overexpression of PLD2 or its dominant negative allele changes the rate of cell spreading thus demonstrates that this could be a relevant mechanism if the endogenous levels of PLD2 activity change with cell attachment. We thus measured PLD activity before and after attachment using an in vivo PLD assay previously described (Du et al., 2000, 2002). We found that control cells in suspension exhibit relatively high levels of PLD activity, which decline by more than 50% when the cells attach (Figure 3C). Expression of PLD1 and PLD1-K898R had no effect on PLD activity, suggesting that the PLD activity changing in this process is not attributable to PLD1, and consistent with the result above that forced expression of the PLD1 alleles did not affect cell spreading. In contrast, PLD2 overexpression increased the level of PLD activity observed for cells in suspension, and this elevated level of activity declined with attachment, although remaining above that seen for wild-type cells in suspension. Conversely, expression of PLD2-K758R inhibited endogenous PLD activity in both settings, lowering the initial level of PLD activity to near that observed for wild-type cells after attachment. Taken together, these results suggest that PLD2 is active in cells in suspension but undergoes down-regulation when the cells attach, consistent with the hypothesis that decreases in PLD2 activity would lower the production of PA and promote cell spreading by decreasing MP inhibition, allowing MP to dephosphorylate MLC and decrease cortical contractile force.
To support our biochemical data that PLD (PLD2) activity undergoes spatiotemporal changes during cell spreading, we used a PA sensor (GFP-PABD) developed in our laboratory (Zeniou-Meyer et al., 2007) to follow dynamic changes in PA using an imaging approach (Figure 3D). In control CHO cells in suspension (0 min), although the majority of the GFP-PABD sensor is found in the nucleus (similar to what is observed for untagged GFP; data not shown), the sensor also clearly decorates the plasma membrane (see inset). Within a short time after attachment (7 and 15 min), plasma membrane-localized GFP-PABD becomes undetectable. For CHO cells overexpressing PLD2 in suspension, even higher levels of GFP-PABD are observed on the plasma membrane, and in contrast to the wild-type CHO cells, the GFP-PABD sensor remains detectable on the plasma membrane at both 7 and 15 min after plating. As an imaging control, the PA-binding deficient mutant, PABD mutant, does not specifically localize to the plasma membrane regardless of the level of expression of PLD2 or the state of the cells (Figure 3D, bottom panel). These findings indicate that cells in suspension have a detectable level of plasma membrane-localized PA that decreases upon attachment and that overexpression of PLD2 both increases the initial levels of plasma membrane PA and causes it to persist there at levels higher than that seen in wild-type cells in suspension even after the PLD2-expressing cells have attached.
To confirm the involvement of endogenous PLD2 in cell spreading, we used an RNA interference (RNAi) approach previously described (Du et al., 2004; Zhao et al., 2007). Cells expressing PLD2 shRNA spread much faster and to a greater extent than control cells expressing luciferase shRNA (Figure 4, A and B), confirming the result generated by expression of the dominant-negative allele PLD2-K758R.
Figure 4.
Endogenous PLD2 inhibits cell spreading. (A) Cells infected with retrovirus expressing luciferase or PLD2 shRNAs were suspended and then replated on fibronectin-coated coverslips for 7 or 15 min. The cells were fixed, stained with rhodamine-conjugated phalloidin and DAPI, and examined by fluorescent microscopy. The images shown are representative of each cell population. Scale bar, 10 μm. (B) Quantification of results in A. (C) Transient inhibition of cell spreading by 1-butanol reverses the cell-spreading delay caused by PLD2 overexpresion. T-Rex CHO PLD2-inducible cell lines were induced using Dox for 24 h as described in Materials and Methods. The cells were suspended for 2 h and then incubated with 0.1% 1-butanol or tert-butanol for 15 min before being replated as above for 15 min. Rhodamine-phalloidin and DAPI were used to visualize F-actin and nuclei. Cell spreading was quantitated as described in Materials and Methods.
Altering PLD2 expression or activity by overexpressing PLD2 alleles or shRNA for multiple days could have other long-term cellular effects, complicating the assessment of whether PLD2 activity is directly involved in cell spreading as proposed above. To address this concern, we made use of 1-butanol as a short-term pharmacological agent to inhibit PLD generation of PA (Jenkins and Frohman, 2005). As shown previously in Figure 3, almost all wild-type CHO cells spread within 15 min of attaching, and ∼10–15% exhibited accelerated spreading. In contrast, overexpression of PLD2 greatly diminished both the spreading and the accelerated spreading. Acute pretreatment of the PLD2-overexpressing cells with 0.1% 1-butanol for 15 min before the start of the experiment rescued the cell spreading delay induced by PLD2 (Figure 4C), whereas the control agent tert-butanol, which does not inhibit PLD production of PA, had no affect on the PLD2-mediated inhibition of cell spreading. In fact, the 0.1% butanol treatment of the PLD2-overexpressing cells resulted in a frequency of accelerated cell spreading that substantially exceeded that of the wild-type CHO cells. Taken together, these findings both demonstrate that PA generation on the plasma membrane acutely blocks cell spreading and validate the PLD2 overexpression and PLD2 dominant-negative and shRNA approaches and findings.
PLD2 Regulates Myosin II Activity during Early Cell Spreading
PLD2 has been proposed to mediate effects in other signaling pathways that could alter cell morphology (Jenkins and Frohman, 2005), so we next examined whether there was a causal relationship between the action of PLD2 on cell spreading and the contractile force contributed by myosin activity.
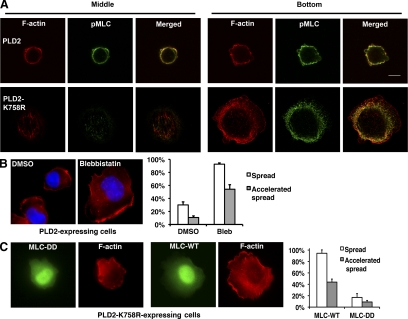
At 7 min after attachment, phospho-MLC is in retreat from the cell cortex at the bottom of cell and is beginning to appear in central regions (Figure 1A). By 15 min, phospho-MLC is largely excluded from the cell periphery (Figure 1B). In contrast, at 15 min after attachment in cells overexpressing PLD2, phospho-MLC was found exclusively at the cell periphery (Figure 5A), which resembled the nonflattened regions of wild-type cells at the earliest stages of cell spreading examined (Figure 1A). Conversely, expression of PLD2-K758R led to the loss of phospho-MLC staining at the cell periphery and accelerated MLC phosphorylation in central regions of the cell bottom (Figure 5A). A similar result was obtained for cells expressing PLD2 shRNA (data not shown). These results show that altering PA levels affects the spatiotemporal termination of MLC phosphorylation at the cell cortex early in the spreading process.
Figure 5.
PLD2 lies upstream of myosin II in the cell-spreading signaling pathway. (A) PLD2 expression increases the accumulation of phosphorylated MLC at the cell periphery. Phospho-MLC was visualized using an mAb against Ser19, and F-actin using rhodamine-phalloidin. Images were processed as in Figure 1 for cells induced to expressed PLD2 or its dominant-negative allele. Cross-sections at the cell bottom and the middle level are shown for representative cells. (B) A myosin inhibitor rescues the block of cell spreading caused by PLD2 overexpression. Cells induced to overexpress PLD2 were treated with blebbistatin as in Figure 2A. (C) The constitutively active myosin mutant, MLC-DD, inhibits the accelerated spreading observed in cells induced to express PLD2-K758R. Cells were fixed at 15 min after plating. The images shown at the left panels in B and C are representative of each cell population, and the diagram at the right panel shows quantification of three or more experiments performed. Scale bar, 10 μm.
To establish functional linkage, we ablated the contractile force contributed by myosin II in cells overexpressing PLD2. Blebbistatin reversed the delay of cell spreading caused by PLD2 overexpression (Figure 5, B and C), leading to the cells becoming fully spread by 15 min after attachment. Conversely, expressing the constitutively active MLC allele, MLC-DD (Fumoto et al., 2003) inhibited the exaggerated cell spreading caused by PLD2-K758R (Figure 5D). Taken together, these findings demonstrate that the effects caused by manipulation of PA generation by PLD2 are mediated by regulation of myosin phosphorylation and activation at the cell cortex.
PLD2 Recruits Myosin Phosphatase to the Plasma Membrane
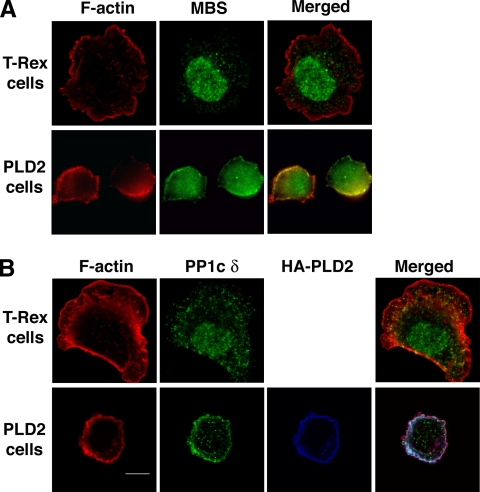
We next examined whether altering the production of PA affects the subcellular localization of MP as a means to restrict or focus its function. MP consists of a catalytic subunit known as PP1c δ, a large subunit termed the myosin phosphatase-targeting subunit (MYPT, also called the MBS or M130) and a 20-kDa small subunit (Ito et al., 2004). MBS binds phosphorylated myosin II, thus targeting the catalytic subunit, PP1c δ, to it. Endogenous MBS and PP1c δ localized to the cytoplasm and nucleus in control cells (Figure 6) and on occasion to a minor extent to the plasma membrane, consistent with prior reports (Hirano et al., 1999; Eto et al., 2005; Lontay et al., 2005). On PLD2 overexpression, however, MBS and PP1c δ translocated in part to the plasma membrane of attached cells (Figure 6) and cells in suspension (not shown), where PLD2 resides (Colley et al., 1997; Du et al., 2004). Recruitment of active MP to the plasma membrane should result in MLC dephosphorylation rather than the coaccumulation of phospho-MLC, suggesting that the presence of PA on the plasma membrane both recruits MP and simultaneously sequesters it in an inactive state.
Figure 6.
PLD2 overexpression leads to accumulation of myosin phosphatase at the cell periphery. (A and B) Accumulation of the MP subunits MBS and PP1c δ at the cell periphery in PLD2-overexpressing but not control (T-Rex) cells. Scale bar, 10 μm.
Lack of Evidence for Regulation of Other Signaling Pathways by PLD2 during Early Stages of Cell Spreading
Cell attachment to the extracellular matrix triggers a variety of signaling cascades that might work cooperatively with myosin II to mediate PLD2-controlled cell spreading. We thus examined whether changes PLD2 activity affect any of these other pathways in the early stages of cell spreading.
No change was observed in phosphorylation of cofilin (Supplementary Information, Supplementary Figure S1), a downstream Rho effector (Maekawa et al., 1999), and the ROCK inhibitor Y-27632 did not alter the effects caused by PLD2 and PLD2 K758R expression (Supplementary Information, Supplementary Figure S2), confirming that PLD2-regulated cell spreading is independent of RhoA-mediated signaling, Similarly, no changes were observed for activation of Rac1 or PI3 kinase (phosphoinositide-3 kinase; Figure 7, A and C), which are important for later stages of cell spreading (Price et al., 1998; Berrier et al., 2000; Small et al., 2002), and expression of Rac1V12, a constitutively active Rac1 mutant, had no effect on the delay in cell spreading caused by PLD2 (Figure 7B). Wortmanin (a PI3 kinase inhibitor), was also without effect (data not shown), and altering PLD2 activity did not affect the activation (Figure 7, D and E) or spatial distribution (data not shown) of FAK and ERK (extracellular signal–regulated kinase), which control the formation and turnover of focal adhesions and focal complexes (Webb et al., 2002, 2004).
Figure 7.
PLD2-regulated myosin II activity is independent of Rac, PI3 kinase, FAK, and MAPK pathways. (A) Lack of effect of PLD2 overexpression on the activation of Rac1. Levels of active GTP-bound Rac1 at 15 min after plating in the indicated lines were determined by incubating cell lysates with GST fusion proteins containing the PAK1 RBD. Affinity precipitates and samples of the whole cell lysates were analyzed by SDS-PAGE and Western blotting with Rac1 antibody. (B) Failure to rescue PLD2-inhibited cell spreading by expression of RAC1-V12, a constitutively active mutant of Rac1. Rac1-V12–transfected cells were identified by immunofluorescent staining of the T7 tag at the Rac1 N-terminus. Actin was visualized using rhodamine-phalloidin staining. (C) PLD2 doe not affect the activation of PI3 kinase. (D) FAK activation is not affected by changes in PLD2 expression. (E) MAPK activation is the same in control cells and cells expressing PLD2 or PLD2 K758R. Control and PLD2-expressing cells were plated on tissue culture plates coated with 10 μg/ml fibronectin and collected at indicated times for the assays shown (C–E). Western blotting was performed using an Odyssey imaging system. All results shown are representative of at least three experiments.
PLD2 has also been proposed to play roles in actin cytoskeletal reorganization (Colley et al., 1997; Honda et al., 1999) via PA stimulation of PI4P5-kinase to generate PI(4,5)P2 (phosphatidylinositol 4,5-bisphosphate) However, we did not observe alterations in plasma membrane targeting of a PI(4,5)P2 sensor (GFP-PLC1δ-PH) in PLD2-overexpressing cells, nor did overexpression of the sensor to sequester PI(4,5)P2 affect cell spreading in control cells or cells expressing PLD2 or PLD2-K758R (data not shown).
Taken together, these results indicate that altering levels of PLD2 expression and PA generation do not affect the general signaling pathways ensuing from integrin activation as cells attach to the substratum.
DISCUSSION
Integration of a spherical motile cell into a solid tissue via attachment and spreading requires dynamically balancing the different types of forces cells exert on themselves and their surrounding environment. On first impact, cells spread to maximize surface contact. Once spread, they form structures including stress fibers and focal adhesions that solidly anchor them. Thus, the balance of extension versus contraction needs to be actively regulated so that different functions can be performed in different physiological contexts.
One way to balance such forces is through the spatially restricted control of myosin II activity. The understanding that myosin II undertakes different roles at the periphery and bottom of migrating cells (Totsukawa et al., 2000, 2004; Giannone et al., 2004) or at the cell leading and rear edges (Kolega, 2003; Lo et al., 2004) is a topic that has only recently come into focus and one that has not been examined in the early phases of cell spreading. Late phases of cell spreading at the bottom of the cell involve PI3 kinase, Rac-Rho kinase, and Cdc42-MRC kinase, but relatively little is known about the early phases. We propose that myosin II dephosphorylation at the cell periphery triggers the initial stage of cell spreading via a regulatory mechanism different from down-regulation of Rho–ROCK signaling and describe here a role for a lipid signal in controlling this pathway. Myosin activation is required at the cell bottom at later stages of cell spreading to collaborate with stress fibers and focal adhesions to anchor cells. We propose here that this is preceded by myosin deactivation at the cell cortex to allow peripheral spreading to occur.
A number of lines of evidence generated from our model system readily suggested and confirmed a role for PLD in myosin dynamics via inhibition of myosin phosphatase. PA has been well defined as an inhibitor of MP activity in biochemical studies. In vitro assays have suggested that PA binds to the myosin targeting subunit (MBS) of MP in a manner that inhibit MP's binding to phosphorylated myosin (Ito et al., 1997); PA has also been reported to specifically bind to and inhibit the α and γ isoforms of MP's catalytic subunit, PP1c (Kishikawa et al., 1999; Jones and Hannun, 2002), at a proposed high-affinity PA-binding site that is found identically in PP1c δ, the isoform under investigation in this study.
There are multiple ways to generate PA in cells, including via the action of the classical isoforms PLD1 and PLD2, DAG kinases, or LysoPA acetyltransferases. We present evidence here that overexpression of PLD1 does not alter levels of PLD activity during cell spreading, nor does overexpressing it or a dominant negative allele alter cell spreading dynamics. In contrast, overexpression of PLD2, which is found on the plasma membrane, alters spreading dynamics, PLD activity during spreading, and levels of PA on the plasma membrane. Moreover, PLD2 dominant negative and RNAi approaches have the converse effect on spreading. Taken together, these findings make PLD2 the lead candidate as regulator of PA during cell spreading, although we cannot entirely rule out contributions from other enzymatic sources at present.
We propose the following model: Cells in suspension exhibit high levels of PLD2 activity on the plasma membrane. The PA generated recruits nearby MP and sequesters it at the plasma membrane in a presumed inactive state. The creation of a peripheral zone deficient in MP activity tips the local balance in factor of MLC phosphorylation and stabilizes the assembly of actomyosin filaments that generate a substantial cortical contractile force, resulting in spherically shaped cells. On attachment, integrin or other signaling down-regulates the PLD2 activity, leading to a bolus of MP release from the plasma membrane in an active state and rapid dephosphorylation of cortical MLC, resulting ultimately in relaxation and extension of the cell periphery. Subsequent activation of Rho–ROCK signaling at the cell bottom leads to stress fiber formation with myosin integration there, as promoted by ROCK-mediated MLC phosphorylation and suppression of MP activity.
One way to view this model would be that PLD2 and MP collaborate to “charge” the membrane with latent MP that is ready to be released and act much more rapidly than could be achieved via diffusion. This kinetic model would be the equivalent of a phosphorylation capacitor that stores MP while the cells are in suspension and PLD2 activity levels are high and then discharges it once the signaling environment switches to favoring attachment. Transducing such signals directly from the plasma membrane might be an efficient way to control cortical forces because the plasma membrane is both close to the site in which the cortical contractile force is generated and is capable of responding rapidly and in a spatially restricted manner to a changing external environment. An even more intriguing and efficient possibility would be that MP is recruited to the membrane through interaction with both PA and phospho-MLC and thus is positioned on its substrate in an inactive form, waiting only for the down-regulation of PA to become active and immediately dephosphorylate MLC.
Current challenges include demonstrating that MP is inhibited at the plasma membrane while bound to PA, determining how efficiently endogenous levels of PA recruit MP to the plasma membrane, and what the regulatory mechanism is that down-regulates PLD2 activity upon attachment.
A number of proteins and lipids have been reported to inhibit PLD2 activity (reviewed in McDermott et al., 2004), but it is not known which one(s) initiates the changes seen early in cell spreading. We also do not know which kinase phosphorylates MLC at the cell cortex and thus promotes contractile force for cells in suspension; an interesting possibility might be ZIP kinase (Murata-Hori et al., 1999). Finally, PLD2-generated PA has recently been shown to play key roles in the activation of Ras (Mor et al., 2007; Zhao et al., 2007). Ras regulates actin dynamics, suggesting that PLD2 may serve as an integrator of myosin- and actin-reorganizing pathways during cytoskeletal reorganization by coordinating the regulation of MP and Ras in parallel. PLD2 polymorphisms and altered expression and activity have been associated with several types of cancer (reviewed in Huang and Frohman, 2007). PLD2's role in regulating myosin- and Ras-signaling pathways in metastasizing cells homing to new locations may underlie this linkage.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Dr. Hiroshi Hosoya for the enhanced GFP-MLC constructs. The authors also thank the members in Frohman lab, H. Crawford, N. Takizawa, and E. Luna for suggestions and discussions on the manuscript. This work was supported by a Scientist Development Grant from the American Heart Association to G.D. (0430096 N) and research grants from National Institutes of Health to G.D. (GM071475) and M.A.F. (DK64166 and GM71520).
Abbreviations used:
- MP
myosin phosphatase
- MYPT (also called myosin-binding subunit, MBS, or M130)
myosin phosphatase–targeting subunit
- MLC
myosin regulatory light chain
- PA
phosphatidic acid
- PLD2
phospholipase D2
- PP1c δ
protein phosphatase 1 δ isoform
- ROCK
Rho-activated Rho kinase.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E08-06-0555) on October 22, 2008.
REFERENCES
- Arthur W. T., Burridge K. RhoA inactivation by p190RhoGAP regulates cell spreading and migration by promoting membrane protrusion and polarity. Mol. Biol. Cell. 2001;12:2711–2720. doi: 10.1091/mbc.12.9.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur W. T., Petch L. A., Burridge K. Integrin engagement suppresses RhoA activity via a c-Src-dependent mechanism. Curr. Biol. 2000;10:719–722. doi: 10.1016/s0960-9822(00)00537-6. [DOI] [PubMed] [Google Scholar]
- Berrier A. L., Mastrangelo A. M., Downward J., Ginsberg M., LaFlamme S. E. Activated R-ras, Rac1, PI 3-kinase and PKCepsilon can each restore cell spreading inhibited by isolated integrin beta1 cytoplasmic domains. J. Cell Biol. 2000;151:1549–1560. doi: 10.1083/jcb.151.7.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark E. A., King W. G., Brugge J. S., Symons M., Hynes R. O. Integrin-mediated signals regulated by members of the rho family of GTPases. J. Cell Biol. 1998;142:573–586. doi: 10.1083/jcb.142.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colley W., Sung T. C., Roll R., Hammond S. M., Altshuller Y. M., Bar-Sagi D., Morris A. J., Frohman M. A. Phospholipase D2, a distinct phospholipase D isoform with novel regulatory properties that provokes cytoskeletal reorganization. Curr. Biol. 1997;7:191–201. doi: 10.1016/s0960-9822(97)70090-3. [DOI] [PubMed] [Google Scholar]
- del Pozo M. A., Price L. S., Alderson N. B., Ren X. D., Schwartz M. A. Adhesion to the extracellular matrix regulates the coupling of the small GTPase Rac to its effector PAK. EMBO J. 2000;19:2008–2014. doi: 10.1093/emboj/19.9.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du G., Altshuller Y. M., Kim Y., Han J. M., Ryu S. H., Morris A. J., Frohman M. A. Dual requirement for Rho and protein kinase C in phospholipase D1 activation through G-protein coupled receptor signaling. Mol. Biol. Cell. 2000;11:4359–4368. doi: 10.1091/mbc.11.12.4359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du G., Huang P., Liang B. T., Frohman M. A. Phospholipase D2 localizes to the plasma membrane and regulates angiotensin II receptor endocytosis. Mol. Biol. Cell. 2004;15:1024–1030. doi: 10.1091/mbc.E03-09-0673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du G., Morris A. J., Sciorra V. A., Frohman M. A. G-protein-coupled receptor regulation of phospholipase D. Methods Enzymol. 2002;345:265–274. doi: 10.1016/s0076-6879(02)45022-7. [DOI] [PubMed] [Google Scholar]
- Eto M., Kirkbride J. A., Brautigan D. L. Assembly of MYPT1 with protein phosphatase-1 in fibroblasts redirects localization and reorganizes the actin cytoskeleton. Cell Motil. Cytoskelet. 2005;62:100–109. doi: 10.1002/cm.20088. [DOI] [PubMed] [Google Scholar]
- Fumoto K., Uchimura T., Iwasaki T., Ueda K., Hosoya H. Phosphorylation of myosin II regulatory light chain is necessary for migration of HeLa cells but not for localization of myosin II at the leading edge. Biochem. J. 2003;370:551–556. doi: 10.1042/BJ20021559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannone G., Dubin-Thaler B. J., Dobereiner H. G., Kieffer N., Bresnick A. R., Sheetz M. P. Periodic lamellipodial contractions correlate with rearward actin waves. Cell. 2004;116:431–443. doi: 10.1016/s0092-8674(04)00058-3. [DOI] [PubMed] [Google Scholar]
- Hirano K., Derkach D. N., Hirano M., Nishimura J., Kanaide H. Protein kinase network in the regulation of phosphorylation and dephosphorylation of smooth muscle myosin light chain. Mol. Cell Biochem. 2003;248:105–114. doi: 10.1023/a:1024180101032. [DOI] [PubMed] [Google Scholar]
- Hirano M., Niiro N., Hirano K., Nishimura J., Hartshorne D. J., Kanaide H. Expression, subcellular localization, and cloning of the 130-kDa regulatory subunit of myosin phosphatase in porcine aortic endothelial cells. Biochem. Biophys. Res. Comm. 1999;254:490–496. doi: 10.1006/bbrc.1998.9973. [DOI] [PubMed] [Google Scholar]
- Honda A., et al. Phosphatidylinositol 4-phosphate 5-kinaseα is a downstream effector of the small G protein ARF6 in membrane ruffle formation. Cell. 1999;99:521–532. doi: 10.1016/s0092-8674(00)81540-8. [DOI] [PubMed] [Google Scholar]
- Huang P., Frohman M. A. The potential for phospholipase D as a new therapeutic target. Expert Opin. Ther. Targets. 2007;11:707–716. doi: 10.1517/14728222.11.5.707. [DOI] [PubMed] [Google Scholar]
- Ito M., Feng J., Tsujino S., Inagaki N., Inagaki M., Tanaka J., Ichikawa K., Hartshorne D. J., Nakano T. Interaction of smooth muscle myosin phosphatase with phospholipids. Biochemistry. 1997;36:7607–7614. doi: 10.1021/bi9702647. [DOI] [PubMed] [Google Scholar]
- Ito M., Nakano T., Erdodi F., Hartshorne D. J. Myosin phosphatase: structure, regulation and function. Mol. Cell Biochem. 2004;259:197–209. doi: 10.1023/b:mcbi.0000021373.14288.00. [DOI] [PubMed] [Google Scholar]
- Jenkins G. M., Frohman M. A. Phospholipase D: a lipid centric review. Cell. Mol. Life. Sci. 2005;62:2305–2316. doi: 10.1007/s00018-005-5195-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J. A., Hannun Y. A. Tight binding inhibition of protein phosphatase-1 by phosphatidic acid. Specificity of inhibition by the phospholipid. J. Biol. Chem. 2002;277:15530–15538. doi: 10.1074/jbc.M111555200. [DOI] [PubMed] [Google Scholar]
- Khyrul W. A., LaLonde D. P., Brown M. C., Levinson H., Turner C. E. The integrin-linked kinase regulates cell morphology and motility in a rho-associated kinase-dependent manner. J. Biol. Chem. 2004;279:54131–54139. doi: 10.1074/jbc.M410051200. [DOI] [PubMed] [Google Scholar]
- Kishikawa K., Chalfant C. E., Perry D. K., Bielawska A., Hannun Y. A. Phosphatidic acid is a potent and selective inhibitor of protein phosphatase 1 and an inhibitor of ceramide-mediated responses. J. Biol. Chem. 1999;274:21335–21341. doi: 10.1074/jbc.274.30.21335. [DOI] [PubMed] [Google Scholar]
- Kolega J. Asymmetric distribution of myosin IIB in migrating endothelial cells is regulated by a rho-dependent kinase and contributes to tail retraction. Mol. Biol. Cell. 2003;14:4745–4757. doi: 10.1091/mbc.E03-04-0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo C. M., Buxton D. B., Chua G. C., Dembo M., Adelstein R. S., Wang Y. L. Nonmuscle myosin IIb is involved in the guidance of fibroblast migration. Mol. Biol. Cell. 2004;15:982–989. doi: 10.1091/mbc.E03-06-0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lontay B., Kiss A., Gergely P., Hartshorne D. J., Erdodi F. Okadaic acid induces phosphorylation and translocation of myosin phosphatase target subunit 1 influencing myosin phosphorylation, stress fiber assembly and cell migration in HepG2 cells. Cell Signal. 2005;17:1265–1275. doi: 10.1016/j.cellsig.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Maekawa M., Ishizaki T., Boku S., Watanabe N., Fujita A., Iwamatsu A., Obinata T., Ohashi K., Mizuno K., Narumiya S. Signaling from Rho to the actin cytoskeleton through protein kinases ROCK and LIM-kinase. Science. 1999;285:895–898. doi: 10.1126/science.285.5429.895. [DOI] [PubMed] [Google Scholar]
- McDermott M., Wakelam M. J., Morris A. J. Phospholipase D. Biochem. Cell Biol. 2004;82:225–253. doi: 10.1139/o03-079. [DOI] [PubMed] [Google Scholar]
- Mor A., Campi G., Du G., Zheng Y., Foster D. A., Dustin M. L., Philip M. R. The lymphocyte function-associated antigen-1 receptor costimulates plasma membrane Ras via phospholipase D2. Nat. Cell. Biol. 2007;9:712–719. doi: 10.1038/ncb1592. [DOI] [PubMed] [Google Scholar]
- Murata-Hori M., Suizu F., Iwasaki T., Kikuchi A., Hosoya H. ZIP kinase identified as a novel myosin regulatory light chain kinase in HeLa cells. FEBS Lett. 1999;451:81–84. doi: 10.1016/s0014-5793(99)00550-5. [DOI] [PubMed] [Google Scholar]
- Price L. S., Leng J., Schwartz M. A., Bokoch G. M. Activation of Rac and Cdc42 by integrins mediates cell spreading. Mol. Biol. Cell. 1998;9:1863–1871. doi: 10.1091/mbc.9.7.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randazzo P. A., Andrade J., Miura K., Brown M. T., Long Y. Q., Stauffer S., Roller P., Cooper J. A. The Arf GTPase-activating protein ASAP1 regulates the actin cytoskeleton. Proc. Nat. Acad. Sci. USA. 2000;97:4011–4016. doi: 10.1073/pnas.070552297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X. D., Kiosses W. B., Schwartz M. A. Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO J. 1999;18:578–585. doi: 10.1093/emboj/18.3.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X. D., Kiosses W. B., Sieg D. J., Otey C. A., Schlaepfer D. D., Schwartz M. A. Focal adhesion kinase suppresses Rho activity to promote focal adhesion turnover. J. Cell Sci. 2000;113(Pt 20):3673–3678. doi: 10.1242/jcs.113.20.3673. [DOI] [PubMed] [Google Scholar]
- Sastry S. K., Burridge K. Focal adhesions: a nexus for intracellular signaling and cytoskeletal dynamics. Exp. Cell. Res. 2000;261:25–36. doi: 10.1006/excr.2000.5043. [DOI] [PubMed] [Google Scholar]
- Small J. V., Stradal T., Vignal E., Rottner K. The lamellipodium: where motility begins. Trends Cell Biol. 2002;12:112–120. doi: 10.1016/s0962-8924(01)02237-1. [DOI] [PubMed] [Google Scholar]
- Somlyo A. P., Somlyo A. V. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol. Rev. 2003;83:1325–1358. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- Su W., Chardin P., Yamazaki M., Kanaho Y., Du G. RhoA-mediated Phospholipase D1 signaling is not required for the formation of stress fibers and focal adhesions. Cell Signal. 2006;18:469–478. doi: 10.1016/j.cellsig.2005.05.027. [DOI] [PubMed] [Google Scholar]
- Totsukawa G., Wu Y., Sasaki Y., Hartshorne D. J., Yamakita Y., Yamashiro S., Matsumura F. Distinct roles of MLCK and ROCK in the regulation of membrane protrusions and focal adhesion dynamics during cell migration of fibroblasts. J. Cell Biol. 2004;164:427–439. doi: 10.1083/jcb.200306172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totsukawa G., Yamakita Y., Yamashiro S., Hartshorne D. J., Sasaki Y., Matsumura F. Distinct roles of ROCK (Rho-kinase) and MLCK in spatial regulation of MLC phosphorylation for assembly of stress fibers and focal adhesions in 3T3 fibroblasts. J. Cell Biol. 2000;150:797–806. doi: 10.1083/jcb.150.4.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakatsuki T., Wysolmerski R. B., Elson E. L. Mechanics of cell spreading: role of myosin II. J. Cell Sci. 2003;116:1617–1625. doi: 10.1242/jcs.00340. [DOI] [PubMed] [Google Scholar]
- Webb D. J., Donais K., Whitmore L. A., Thomas S. M., Turner C. E., Parsons J. T., Horwitz A. F. FAK-Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nat. Cell. Biol. 2004;6:154–161. doi: 10.1038/ncb1094. [DOI] [PubMed] [Google Scholar]
- Webb D. J., Parsons J. T., Horwitz A. F. Adhesion assembly, disassembly and turnover in migrating cells–over and over and over again. Nat. Cell. Biol. 2002;4:E97–E100. doi: 10.1038/ncb0402-e97. [DOI] [PubMed] [Google Scholar]
- Zeniou-Meyer M., et al. Phospholipase D1 production of phosphatidic acid at the plasma membrane promotes exocytosis of large dense-core granules at a late stage. J. Biol. Chem. 2007;282:21746–21757. doi: 10.1074/jbc.M702968200. [DOI] [PubMed] [Google Scholar]
- Zhao C., Du G., Skowronek K., Frohman M. A., Bar-Sagi D. Phospholipase D2-generated PA couples EGFR stimulation to Ras activation by Sos. Nat. Cell Biol. 2007;9:707–712. doi: 10.1038/ncb1594. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.