Abstract
β-Catenin plays an important role in development and tumorigenesis. However, the effect of a key acetyltransferase p300/CBP-associated factor (PCAF) on β-catenin signaling is largely unknown. In this study, we found PCAF could increase the β-catenin transcriptional activity, induce its nuclear translocation, and up-regulate its protein level by inhibiting its ubiquitination and improving its stability. Further studies showed that PCAF directly binds to and acetylates β-catenin. The key ubiquitination sites Lys-19 and Lys-49 of β-catenin were shown as the critical residues for PCAF-induced acetylation and stabilization. Knockdown of PCAF in colon cancer cells markedly reduced the protein level, transcriptional activity, and acetylation level of β-catenin; promoted cell differentiation; inhibited cell migration; and repressed xenografted tumorigenesis and tumor growth in nude mice. All these data demonstrate that PCAF acetylates β-catenin and regulates its stability, and they raise the prospect that therapies targeting PCAF may be of clinical use in β-catenin–driven diseases, such as colon cancer.
INTRODUCTION
The Wnt signaling pathway has important roles in a variety of developmental processes (Logan and Nusse, 2004; Clevers, 2006). The key output of this pathway is the stabilization and nuclear translocation of β-catenin, which determines the activation of β-catenin–responsive genes. Aberrant activation of Wnt signaling is often associated with carcinogenesis. Colorectal tumors are among most common human neoplasms, and >90% of colorectal cancers have a mutation that activates Wnt signaling (Giles et al., 2003; Doucas et al., 2005). Inactivating adenomatous polyposis coli (APC) mutations has been demonstrated in ∼85% of sporadic colorectal tumors. Among the 15% colon carcinomas without APC mutations, β-catenin or the scaffolding protein Axin2 is mutant (Morin et al., 1997; Liu et al., 2000). Accumulating studies strongly suggest that disruption of Wnt signaling is a promising strategy for the prevention of colon cancers, and how to regulate the stability of β-catenin is the key point (Clapper et al., 2004; Dihlmann and von Knebel Doeber, 2005; Dvory-Sobol et al., 2006).
It is well known that Wnt signaling is regulated by phosphorylation. Besides phosphorylation, ubiquitination and acetylation have also been reported to be involved in Wnt signaling (Aberle et al., 1997; Sun et al., 2000; Takemaru and Moon, 2000; Winer et al., 2006). Lys-19 and Lys-49 of β-catenin were reported as important ubiquitination sites (Winer et al., 2006), and Lys-49 of β-catenin was also reported to be acetylated by CBP (Wolf et al., 2002). Whether there is some cross-talk between the ubiquitination and acetylation of β-catenin is yet to be elucidated. Some acetyltransferases such as CREB-binding protein (CBP)/p300 are related to colon cancer causation and progression (Muraoka et al., 1996; Ionov et al., 2004), and CBP/p300 can interact with β-catenin and synergistically activate β-catenin/T cell factor (TCF) transcription (Sun et al., 2000; Takemaru and Moon, 2000). Recent studies have shown that acetylation by CBP/p300 enhances TCF/LEF homologue POP-1 nuclear localization during early embryogenesis in Caenorhabditis elegans (Gay et al., 2003). Furthermore, β-catenin was shown to be acetylated by p300 at Lys-345, and this acetylation increased the affinity of β-catenin to Tcf4 (Levy et al., 2004), suggesting that acetylation should be involved in regulating the β-catenin transcriptional activity. However, the role of the well-known acetyltransferase p300/CBP-associated factor (PCAF) in Wnt signaling is still largely unknown.
PCAF is a transcription cofactor, which has intrinsic histone acetyltransferase (HAT) activity (Yang et al., 1996). PCAF can also acetylate many other proteins, including the chromatin proteins HMG17 and HMG I(Y), human immunodeficiency virus Tat, transcription factor MyoD, and general transcription factors TFIIE and TFIIF (Sterner and Berger, 2000). PCAF plays a dual role in regulation of cell viability. It has been suggested that PCAF is a potential tumor suppressor to decrease cell viability (Schiltz and Nakatani, 2000). PCAF was reported to acetylate and activate tumor suppressor p53 in response to DNA damage (Liu et al., 1999), to be a coactivator for p73-mediated transactivation (Zhao et al., 2003), and to promote Bax-mediated apoptosis by acetylation of the C terminus of Ku70 (Cohen et al., 2004a). But, PCAF may also act as a tumor activator to increase cell viability. The acetylation of oncoprotein c-Myc by PCAF increased its protein stability (Patel et al., 2004), and acetylation of tumor suppressor PTEN by PCAF led to reduced function of PTEN (Okumura et al., 2006). Furthermore, the inhibitors for both PCAF and p300 including CCT077791 and CCT077792 decreased cellular acetylation and inhibited the growth of colon cancer cell line HCT116 and HT29 (Stimson et al., 2005). However, the direct evidence for the role of PCAF in colon cancer and the underlying mechanism is yet to be elucidated.
In this study, we investigated the effect of PCAF on β-catenin. We found that PCAF acetylates β-catenin and improves its stability by inhibiting ubiquitin-dependent degradation, and PCAF might be considered as a potential target for β-catenin–related diseases.
MATERIALS AND METHODS
Plasmids
The reporter plasmid Super8×TOPFlash and XBC40-β-catenin-Myc were kindly provided by Dr. Randall Moon (University of Washington, Seattle, WA). The plasmid β-catenin-FLAG, T41A-β-catenin-FLAG, and Wnt1 constructed in pCS2+ were generous gifts from Dr. Chunming Liu (University of Texas Medical Branch, Galveston, TX) as described previously (Liu et al., 2002). The plasmid T41A-β-catenin-Myc in pCMV was provided by Dr. Christine Neuveut (Institut Pasteur, Paris, France; Levy et al., 2004). The plasmid β-catenin-hemagglutinin (HA) was kindly provided by Dr. Lin Li (Shanghai Institutes for Biological Sciences, Shanghai, China; Pan et al., 2005). The wild-type (WT)-β-catenin, K19R-β-catenin, K49R-β-catenin, K19,49R-β-catenin cDNAs kindly provided by Dr. Eric R. Fearon (University of Michigan, Ann Arbor, MI; Winer et al., 2006) were subcloned into pCMV-Tag3B vector and pCX-PCAF-FLAG expression FLAG-tagged PCAF and pCX-ΔHAT2-PCAF-FLAG expressing PCAF with deletion of amino acids 608–623 were provided by Dr. Xiang-Jiao Yang (McGill University, Montreal, Canada) as described previously (Yang et al., 1996; Zhao et al., 2003). pCMV-PCAF-Myc and pCMV-ΔHAT2-PCAF-Myc expressing Myc-tagged PCAF or ΔHAT2-PCAF were obtained by insertion of PCAF cDNA fragment cut out with KpnI (blunted with Klenow fragment) and EcoRI from pCX-PCAF-FLAG or pCX-ΔHAT2-PCAF-FLAG into pCMV-Tag 3A (Stratagene, La Jolla, CA) at EcoRV and EcoRI sites. pCMV-ΔHAT1,2-PCAF-Myc expressing Myc-tagged ΔHAT1,2- PCAF with deletion of amino acids 556–623 was obtained by insertion of ΔHAT2-PCAF-Myc cut out from pCMV-ΔHAT2-PCAF-Myc with SacI and XhoI into pGL3-basic (Promega, Madison, WI), deletion of HAT1 in the new construct with NdeI and PacI and insertion with a linker annealed from TAAAGATGGCCGTGTTATT and TAAATAACACGGCCATCTTTAAT, and finally insertion of ΔHAT1,2-PCAF-Myc cut out with SacI and XhoI from the new construct from the last step into pCMV-Tag 3A. Lentiviral-mediated RNA interference were done largely as reported previously (Abbas-Terki et al., 2002). Briefly, the human H1 promoter was amplified by polymerase chain reaction (PCR) from pSilencer 3.1-H1 hygro (Ambion, Austin, TX) by using GCTCTAGAGGGTTTTCCCAGTCACGA and ccgctcgaggtgagcgcaacgcaatta as primers and then inserted into XbaI and XhoI sites of the pLentiLox 3.7 plasmid (kind gift from C. Dillon, Massachusetts Institute of Technology). Renilla luciferase-targeting oligonucleotide templates are GATCCGTAGCGCGGTGTATTATACTTCAAGAGAGTATATACACCCGCGCTACTTTTTTGGAAG and TCGACTTCCAAAAAAGTAGCGCGGTGTATTATACTCTCTTGAAGTATATACACCGCGCTACG. PCAF-targeting oligonucleotide templates are GATCCGTCGCCGTGAAGAAAGCGCATTCAAGAGATGCGCTTTCTTCACGGCGATTTTTTGGAAA and TCGATTTCCAAAAAATCGCCGTGAAGAAAGCGCATCTCTTGAATGCGCTTTCTTCACGGCGACG (Zhao et al., 2003). β-Catenin-targeting oligonucleotide templates are GATCCGTGGGTGGTATAGAGGCTCTTCAAGAGAGAGCCTCTATACCACCCACTTTTTTGGAAA and AGCTTTTCCAAAAAAGTGGGTGGTATAGAGGCTCTCTCTTGAAGAGCCTCTATACCACCCACG. These oligonucleotides were annealed and ligated into the BamHI and XhoI sites of pLentiLox 3.7 with H1 promoter to create the pLentiLox 3.7-H1-luc-RNAi, pLentiLox 3.7-H1-PCAF-RNAi, and pLentiLox 3.7-H1-β-catenin-RNAi.
Cell Culture, Transfection, and Luciferase Assay
Human embryonic kidney (HEK)293T, HEK293, and HeLa cells were maintained in DMEM with 10% calf serum. LoVo cells were grown in F-12 medium with 10% fetal bovine serum. Cells were transfected with Lipofectamine 2000 (Invitrogen) or polyethylenimine (Polysciences, Warrington, PA) according to the manufacturer's instruction. For luciferase assay, HEK293T cells were cultured in 24-well plates. Total amounts of transfected plasmids were balanced with empty vector. Excepted indicated, after transfection with Super8×TOPFlash (0.1 μg/well), pSV40-β-gal (0.1 μg/well), β-catenin, or its mutant (0.3 μg/well), and PCAF or its deletion form (0.6 μg/well) for 40 h, cells were harvested and measured with a luciferase assay kit (Promega) and normalized to β-galactosidase activity determined as described previously (Wolf et al., 2002). For RNA interference (RNAi)-related luciferase assay, after transfection with 0.1 μg of β-catenin-FLAG, T41A-β-catenin-FLAG, or Wnt1, and 0.9 μg luc-RNAi or PCAF-RNAi in 24-well plates for 36 h, HEK293 cells were retransfected with 0.1 μg of Super8×TOPFlash and 0.1 μg of pSV40-β-gal for another 36 h. Then, the cells were harvested for luciferase assay and normalized to β-galactosidase activity.
RNA Isolation and Reverse Transcription (RT)-PCR
Total RNA was prepared from HEK293T cells transfected with PCAF-FLAG or ΔHAT2-PCAF-FLAG (2 μg) in 35-mm dish for 40 h by TRIzol reagent (Invitrogen, Carlsbad, CA). RT-PCR was performed with the primers GATTTGATGGAGTTGGACATGG and TGTTCTTGAGTGAAGGACTGAG for β-catenin, CCTACCCTCTCAACGACAGC and GTTGTGTGTTCGCCTCTTGA for c-Myc, GGATGCTGGAGGTCTGCGAGGAAC and GAGAGGAAGCGTGTGAGGCGGTAG for cyclinD1, GAGAGTGAGCGGCAGAGCAA and GCTTGGATTGGAGAAGGGTG for axin2, and TGCTGTCCCTCTACGCCTCTG and GCCGCAAGATTCCATACCC for actin. Quantification was carried out by Quantity One software (Bio-Rad, Hercules, CA), and results were normalized to actin.
Immunofluorescence
Immunofluorescence was performed as described previously (Jia et al., 2007), with rabbit anti-Myc antibody (Santa Cruz Biotechnology, Santa Cruz, CA) or mouse anti-FLAG mAb (Sigma-Aldrich, St. Louis, MO), followed by incubation with Cy2-conjugated anti-rabbit IgG (Jackson ImmunoResearch Laboratories, West Grove, PA) or Cy3-conjugated anti-mouse immunoglobulin G (IgG) (Jackson ImmunoResearch Laboratories). Then, the cells were stained with 0.5 μg/ml 4,6-diamidino-2-phenylindole (DAPI) (Sigma-Aldrich) and observed under a fluorescence microscope (IX71; Olympus, Tokyo, Japan).
Immunoprecipitation and Pull-Down Assay
Cells cultured in a 60-mm dish were harvested in 300 μl of lysis buffer (20 mM Tris, pH 7.5, 100 mM KCl, 0.1% Nonidet P-40, 1 mM EDTA, 10% glycerol, 50 mM NaF, and 10 mM sodium pyrophosphate) containing 10 μg/ml pepstatin, 5 μg/ml leupeptin, 20 μg/ml aprotinin, 1 mM phenylmethylsulfonyl fluoride [PMSF], and 50 nM trichostatin A and passed through a 26-gauge needle 10 times. Cell lysates were incubated on ice for 30 min. For pull-down assay, proteins are incubated in lysis buffer with 1% bovine serum albumin for 30 min at room temperature on a rotating platform. The supernatant was collected by centrifugation and incubated overnight at 4°C with 3 μl indicated primary antibody with gentle shaking. Then, 30 μl of protein A/G PLUS-Agarose beads (Santa Cruz Biotechnology) was added and gently shaken at 4°C for 4 h. After being washed four times with 20 mM Tris, pH 7.5, 150 mM KCl, 0.5% Nonidet P-40, 1 mM EDTA, 10% glycerol, 50 mM NaF, and 10 mM sodium pyrophosphate, the beads were boiled in SDS-PAGE loading buffer for Western blot. The immunoprecipitated proteins were balanced to detect its acetylation or ubiquitination level, or the relative level of coimmunoprecipitated proteins. Wnt3a conditioned medium and the control medium were prepared as described previously (Shibamoto et al., 1998).
Western Blot
Cells were directly harvested in SDS-polyacrylamide gel electrophoresis (PAGE) loading buffer as the whole cell lysates. Anti-β-catenin antibody was purchased from BD Biosciences Transduction Laboratories (Lexington, KY). Mouse anti-myc (9E10), anti-PCAF (E-8), anti-Ub (P4D1), and rabbit anti-myc (A-14) antibodies were obtained from Santa Cruz Biotechnology. Anti-FLAG and anti-tubulin antibodies were from Sigma-Aldrich. Anti-villin antibody was from BD Biosciences (San Jose, CA). Monoclonal anti-acetyl-lysine antibody (Ac-k-103) and polyclonal anti-acetyl-lysine were from Cell Signaling Technology (Danvers, MA). Secondary antibodies linked to horseradish peroxidase, including anti-rabbit IgG and anti-mouse IgG, were from Jackson ImmunoResearch Laboratories.
In Vitro Acetylation Assay
Glutathione transferase (GST) fusion β-catenin and PCAF containing the catalytic domain were purchased from Millipore (Billerica, MA). Then, 500 ng of GST-PCAF and 2.5 μg of GST-β-catenin were incubated in 50 μl of acetyltransferase assay buffer (50 mM Tris, pH 8, 10% glycerol, 10 mM butyric acid, 0.1 mM EDTA, 1 mM dithiothreitol, and 1 mM PMSF) with or without 10 μM acetyl CoA (Sigma-Aldrich) at 30°C for 45 min on a rotating platform. Subsequently, SDS-PAGE loading buffer was added to stop the reaction and used for Western blot with anti-β-catenin, anti-PCAF, or polyclonal anti-acetyl-lysine antibodies.
Preparation of Recombinant Lentivirus
Recombinant lentiviruses were produced in HEK293T cells by cotransfecting the construct containing luciferase RNAi (luc-RNAi) or PCAF-RNAi with the packaging vector Δ8.9 and vesicular stomatitis virus G. The resulting lentiviruses expressing luc-RNAi or PCAF-RNAi were concentrated and then titered on HEK293T cells.
Alkaline Phosphatase Activity Assay
After wash with phosphate-buffered saline (PBS), LoVo cells were stained with 5-bromo-4-chloro-3-indolyl phosphate (BCIP)/nitro blue tetrazolium (NBT) solution (0.02% BCIP, 0.03% NBT, 100 mM Tris, pH 9.5, 100 mM NaCl, 5 mM MgCl2, and 0.05% Tween 20) in the dark for 10 to 20 min. After staining, the green fluorescent protein (GFP)-positive cells with blue color in randomly selected field were counted under microscope, and they were considered as differentiated cells. The percentage of differentiated GFP-positive cells in the total GFP-positive cells is defined as the percentage of differentiation level of cells transfected with the indicated RNAi constructs.
Wound Healing Assay
The wound healing assays were performed as described previously (Qian et al., 2003). Wounds were created in confluent LoVo cells in six-well plates by using a sterile pipette tips. Three days later, wound healing was observed and photographed with an IX71 microscope (Olympus).
Animals and Tumor Model
All animals were maintained and used in accordance with the guidelines of the Institutional Animal Care and Use Committee of the Institute for Nutritional Sciences. Four-week-old BALB/c nu/nu male mice were obtained from Slaccas (Shanghai, China). LoVo cells (5 × 106) infected with lentiviruses expressing luciferase RNAi or PCAF RNAi for 7 d were injected subcutaneously into both flanks of total 22 nude mice. The mice were killed two months later. Tumor size was evaluated by caliper measurements, and tumor volume was calculated as length × width2 × 0.5.
Statistics
Data are expressed as mean ± SD of at least three independent experiments. Except where specifically indicated, statistical significance was assessed by Student's t test. Values were considered statistically significant when p < 0.05.
RESULTS
PCAF Regulates β-Catenin Transcriptional Activity, Intracellular Localization, and Protein Level
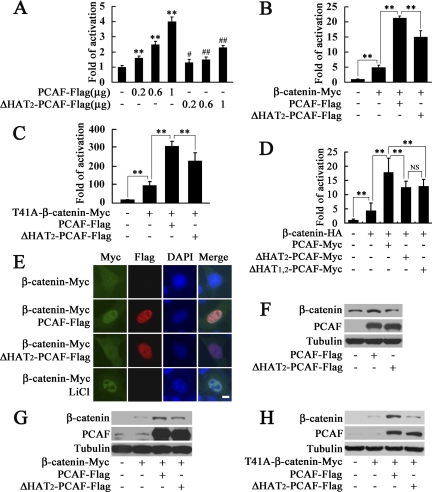
To test whether PCAF regulates β-catenin transcriptional activity, luciferase activity assay based on Super8×TOPFlash was performed. As shown in Figure 1A, PCAF activated β-catenin transcriptional activity in a dose-dependent manner, and deletion of one acetyltransferase domain HAT2 in PCAF inhibited this effect. In addition, PCAF synergized with exogenous β-catenin or T41A-β-catenin, a stable dominant form of β-catenin, to activate Super8×TOPFlash, and this effect was also significantly dependent on its acetyltransferase activity (Figure 1, B and C). PCAF with both HAT domains deleted had the similar effect on β-catenin transcriptional activity as PCAF with HAT2 domain deleted (Figure 1D), which is consistent with previous reports that that deletion of only one HAT domain in PCAF will almost abrogate its histone acetylase activity (Blanco et al., 1998; Jiang et al., 1999). These results demonstrated that PCAF could enhance β-catenin transcriptional activity significantly dependent on its acetyltransferase activity.
Figure 1.
PCAF enhances β-catenin transcriptional activity, induces its nuclear translocation and up-regulates its protein level. (A) PCAF activated β-catenin transcriptional activity in a dose-dependent manner. HEK293T cells were transfected with Super8×TOPFlash and the indicated amount of PCAF-FLAG or ΔHAT2-PCAF-FLAG for luciferase assay. **p < 0.01, versus the cells transfected with empty vector; #p < 0.05, ##p < 0.01, versus the cells transfected with equal amount of PCAF-FLAG. In this and all other figures, error bars represent SD (B–D) PCAF synergized with β-catenin or T41A-β-catenin to activate Super8×TOPFlash. **p < 0.01; NS, no significant difference. (E) PCAF induced nuclear translocation of β-catenin. HeLa cells were transfected with indicated constructs. After transfection for 28 h, the cells shown (bottom) were treated with 20 mM LiCl. After transfection for 40 h, cells were fixed and immunostained with anti-Myc or anti-FLAG antibody. Nuclei were visualized by DAPI staining. Bar, 10 μm. (F) Up-regulation of endogenous β-catenin by increased expression of PCAF. After transfection with PCAF-FLAG or ΔHAT2-PCAF-FLAG in HEK293T cells for 40 h, total β-catenin, PCAF, and tubulin level in the extract were detected by Western blot. (G and H) PCAF increased β-catenin protein level when cotransfected with β-catenin-Myc or T41A-β-catenin-Myc. After transfection with indicated constructs in HEK293T cells for 40 h, β-catenin, PCAF, and tubulin in cell lysates were detected with anti-Myc, anti-PCAF, or anti-tubulin antibody.
Increased β-catenin transcriptional activity could be attributed to the accumulation of β-catenin in the nucleus. To address this possibility, immunostaining was performed to detect the intracellular localization of β-catenin. As shown in Figure 1E, HeLa cells transfected with β-catenin-Myc alone showed a very weak Myc staining distributed in both cytoplasm and nucleus. When HeLa cells were cotransfected with β-catenin-Myc and PCAF-FLAG, β-catenin mainly colocalized with PCAF in nucleus. Deletion of the HAT2 domain in PCAF significantly impaired the colocalization of β-catenin and PCAF in the nucleus. LiCl treatment was applied as a positive control to confirm the nuclear translocation of β-catenin (Feyt et al., 2005). These findings demonstrated that PCAF can induce nuclear translocation of β-catenin.
Increased β-catenin transcriptional activity and accumulation of β-catenin in the nucleus could be attributed to the up-regulated β-catenin protein level. As expected, increased expression of PCAF significantly up-regulated the endogenous β-catenin protein level, and the HAT2 domain was required for this effect (Figure 1F). In addition, when cotransfected with β-catenin-Myc or T41A-β-catenin-Myc, PCAF markedly increased the exogenous β-catenin protein level, and this effect was also dependent on its acetyltransferase activity (Figure 1, G and H). These results showed that PCAF could up-regulate β-catenin protein level at different conditions. The improved stability of T41A-β-catenin by PCAF suggested that the effect of PCAF on the stability of β-catenin might be at least partially independent of the glycogen synthase kinase (GSK)3β-mediated phosphorylation pathway as described previously (Liu et al., 2001; Matsuzawa and Reed, 2001).
PCAF Improves the Stability of β-Catenin by Inhibiting Its Ubiquitination
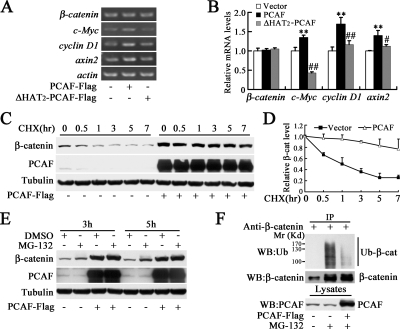
To test whether PCAF regulates β-catenin at transcriptional level, the mRNA level of β-catenin was analyzed by RT-PCR. As shown in Figure 2, A and B, PCAF or ΔHAT2-PCAF did not affect the mRNA level of β-catenin, but the mRNA levels of β-catenin target gene c-Myc, cyclinD1 and axin2 were increased by PCAF dependent on its acetyltransferase activity. These data suggested that PCAF might regulate β-catenin protein level at the posttranscriptional level. To investigate whether PCAF affects β-catenin stability, we measured β-catenin protein level in the presence of cycloheximide, an inhibitor of protein biosynthesis. As shown in Figure 2, C and D, PCAF significantly attenuated the degradation of β-catenin. Usually, β-catenin is degraded by ubiquitin–proteasome system, so the proteasome inhibitor MG-132 was applied to examine whether PCAF affect β-catenin stability through the proteasome pathway. As shown in Figure 2E, MG-132 failed to up-regulate β-catenin protein level in the presence of overexpressed PCAF, although MG-132 significantly up-regulated β-catenin protein level in the absence of exogenous PCAF. In addition, strong ubiquitination of β-catenin was detected in the absence of exogenous PCAF, but ubiquitination of β-catenin was significantly blocked in the presence of exogenous PCAF (Figure 2F). These data showed that PCAF improves the stability of β-catenin by inhibiting its ubiquitination-dependent degradation.
Figure 2.
PCAF improves the stability of β-catenin. (A) PCAF did not affect β-catenin mRNA level. After transfected with indicated constructs for 40 h, cells were harvested for RT-PCR. (B) Quantification of mRNA levels showed in (A). **p < 0.01 versus vector; #p < 0.05, ##p < 0.01 versus PCAF. (C) PCAF significantly inhibited degradation of β-catenin. HEK293T cells were transfected with PCAF-FLAG or empty vector. Thirty hours later, the cells were treated with 100 μg/ml cycloheximide (CHX) for indicated times and then harvested for Western blot. (D) Quantification of endogenous β-catenin level in (C). (E) MG-132, a proteasome inhibitor, failed to up-regulate β-catenin protein level in the presence of overexpressed exogenous PCAF. After transfection with PCAF-FLAG or empty vector for 30 h, HEK293T cells were treated with or without 25 μM MG-132 for 3 or 5 h. (F) PCAF inhibited the ubiquitination of endogenous β-catenin. After transfection with PCAF-FLAG for 30 h, HEK293T cells were treated with 10 μM MG-132 for 8 h and then harvested for immunoprecipitation. The immunoprecipitated protein level was balanced to detect its ubiquitination level.
PCAF Interacts with β-catenin, and This Interaction Can Be Enhanced by Activation of Wnt Signaling
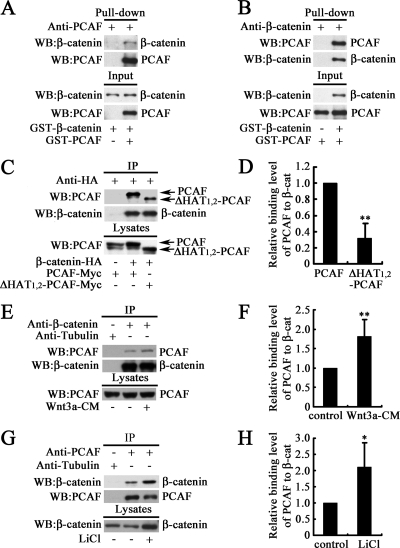
The functional synergism and colocalization of PCAF and β-catenin imply that they might have direct interaction. To address this implication, pull-down assay was performed to examine the interaction between PCAF and β-catenin in vitro. As shown in Figure 3, A and B, GST-PCAF and GST-β-catenin directly interacted in vitro. To examine whether PCAF and β-catenin could interact in vivo, immunoprecipitation was performed. As shown in Figure 3C, Myc-tagged PCAF was coimmunoprecipitated with exogenous β-catenin with HA tag. This result indicated that PCAF could interact with β-catenin. PCAF with deletion of the HAT1 and HAT2 domains was also coimmunoprecipitated with exogenous β-catenin, whereas the ΔHAT1,2–PCAF protein level coimmunoprecipitated with the same amount of β-catenin was lower than PCAF (Figure 3, C and D). These results demonstrated that the HAT1 and HAT2 domains in PCAF were not essential for the interaction, but deletion of HAT domains could attenuate this interaction. To test the endogenous interaction between PCAF and β-catenin, cell lysates were immunoprecipitated with anti-β-catenin antibody. As shown in Figure 3C, endogenous PCAF was coimmunoprecipitated with endogenous β-catenin. In addition, when treated with Wnt3a to activate Wnt signaling, the interaction between PCAF and β-catenin was enhanced (Figure 3, E and F). To further confirm the endogenous interaction between PCAF and β-catenin, cell lysates were immunoprecipitated with anti-PCAF antibody. As expected, endogenous β-catenin was coimmunoprecipitated with endogenous PCAF (Figure 3G). When treated with LiCl, a GSK3β inhibitor, to mimic the activation of Wnt signaling (Stambolic et al., 1996), the interaction between PCAF and β-catenin was also significantly enhanced (Figure 3, G and H). These findings demonstrated that PCAF and β-catenin could interact at endogenous level, and this effect was enhanced by activation of Wnt signaling.
Figure 3.
PCAF interacts with β-catenin, and this interaction is enhanced by activation of Wnt signaling. (A and B) PCAF interacts with β-catenin in vitro. Purified GST-PCAF and GST-β-catenin was mixed, and then immunoprecipitation was carried out with an anti-PCAF antibody (A) or anti-β-catenin antibody (B). (C) PCAF was coimmunoprecipitated with β-catenin. After transfection with indicated constructs for 30 h, cells were harvested for immunoprecipitation with anti-HA antibody. Western blots were performed with the indicated antibodies. (D) Quantification of coimmunoprecipitated PCAF and ΔHAT1,2-PCAF level in (C). **p < 0.01. (E) Endogenous PCAF was coimmunoprecipitated with anti-β-catenin antibody, and this effect was enhanced by Wnt3a treatment. After treated with Wnt3a conditioned medium (Wnt3a-CM) or the control medium for 12 h, HEK293T cells were harvested for immunoprecipitation. (F) Quantification of coimmunoprecipitated PCAF level in (E). **p < 0.01. (G) Endogenous β-catenin was coimmunoprecipitated by anti-PCAF antibody, and this effect was enhanced by LiCl treatment. After treated with or without 20 mM LiCl for 12 h. HEK293T cells were harvested for immunoprecipitation. (H) Quantification of coimmunoprecipitated β-catenin level in (G). *p < 0.05. The immunoprecipitated protein level was balanced to detect the relative level of coimmunoprecipitated proteins.
β-Catenin Is Acetylated by PCAF
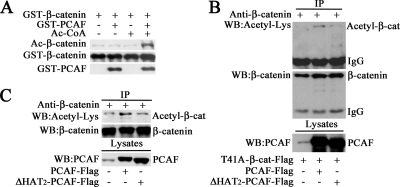
The interaction and functional synergism between PCAF and β-catenin imply that PCAF might acetylate β-catenin to regulate its stability. As shown in Figure 4A, with purified PCAF and β-catenin, the acetylation level of β-catenin was dramatically increased in the presence of both PCAF and Ac-CoA in an in vitro assay system. Furthermore, we tested whether PCAF acetylated β-catenin in vivo. As shown in Figure 4B, when T41A-β-catenin-FLAG was cotransfected with PCAF-FLAG, the protein level of acetylated β-catenin was significantly increased. Deletion one of the acetyltransferase domain HAT2 in PCAF markedly inhibited this effect. These data demonstrated that PCAF acetylates β-catenin depending on its acetyltransferase activity. To further confirm this result, the acetylation of endogenous β-catenin was examined. As shown in Figure 4C, increased expression of PCAF significantly up-regulated the acetylation level of endogenous β-catenin. The acetyltransferase activity was also required for this effect. Together, these data showed that PCAF was capable of acetylating β-catenin to regulate its stability.
Figure 4.
PCAF acetylates β-catenin in vitro and in vivo. (A) PCAF acetylated β-catenin in vitro. The acetylation of GST-β-catenin by PCAF was measured by in vitro acetylation assay and finally detected by Western blot. (B) PCAF acetylated exogenous β-catenin. After transfected with T41A-β-catenin-FLAG and PCAF-FLAG or ΔHAT2-PCAF-FLAG for 40 h, HEK293T cells were harvested for immunoprecipitation. The same membrane was stripped and reblotted with anti-β-catenin antibody. (C) PCAF acetylated endogenous β-catenin. After transfected with PCAF-FLAG or ΔHAT2-PCAF-FLAG for 40 h, HEK293T cells were harvested for immunoprecipitation with anti-β-catenin antibody. Western blots were performed with the indicated antibodies. The immunoprecipitated protein level was balanced to detect its acetylation level.
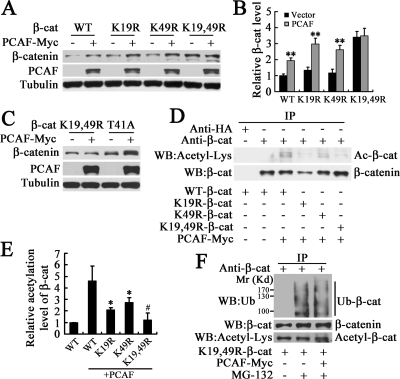
K19 and K49 of β-Catenin Are Important for Its Acetylation and Stabilization Induced by PCAF
It has been shown that the K19/K49 double mutant form of β-catenin is stabilized as a result of reduced βTrCP-dependent ubiquitination (Winer et al., 2006). According to the above-mentioned results, it is possible that K19 and K49 of β-catenin are involved in regulating its stability by PCAF. As showed in Figure 5, A and B, PCAF up-regulated the protein level of WT, K19R, K49R-β-catenin, but not that of K19,49R-β-catenin. To exclude the possibility that it is simply due to the high protein level or high stability of K19,49R-β-catenin, we showed that T41A-β-catenin with high protein level and high stability was still able to be up-regulated (Figure 5C). These data implied that Lys-19 and Lys-49 of β-catenin might be the acetylation sites of PCAF. To confirm this hypothesis, we transfected cells with PCAF, WT, K19R, K49R, and/or K19,49R-β-catenin, and then β-catenin was immunoprecipitated to detect its acetylation level. We found that the acetylation level of K19,49R-β-catenin was significantly decreased compared with that of WT, K19R, or K49R-β-catenin, and the acetylation levels of K19R-β-catenin and K49R-β-catenin were also decreased compared with WT-β-catenin (Figure 5, D and E). Acetylation of Lys-19 and Lys-49 in β-catenin implies that the ubiquitination at the same sites will be blocked. As expected, PCAF could not increase the acetylation of K19,49R-β-catenin and inhibit its ubiquitination anymore (Figure 5F). These data demonstrated that the Lys-19 and Lys-49 are the critical residues for the acetylation and stabilization of β-catenin induced by PCAF.
Figure 5.
K19 and K49 of β-catenin are critical residues for its acetylation and stabilization induced by PCAF. (A) PCAF up-regulated the protein level of WT, K19R, or K49R-β-catenin but not that of K19,49R-β-catenin. After transfection with WT-β-catenin or its mutants and/or PCAF-Myc to HEK293T cells for 40 h, total β-catenin, PCAF, and tubulin level were detected by Western blot. (B) Quantification of β-catenin level in (A). **p < 0.01, versus the vector in the same group. (C) PCAF up-regulated the protein level of T41A-β-catenin but not that of K19,49R-β-catenin. (D) PCAF could not induce the acetylation of K19,49R-β-catenin. After transfection with WT-β-catenin or its mutants and/or PCAF-Myc for 40 h, HEK293T cells were harvested for immunoprecipitation. (E) Quantification of acetylation level of β-catenin in (D). *p < 0.05, versus WT-β-catenin with PCAF. #p < 0.05 versus K19R or K49R-β-catenin with PCAF. (F) PCAF could not inhibit the ubiquitination of K19,49R-β-catenin. HEK293T cells were transfected with K19,49R-β-catenin and/or PCAF-Myc for 30 h and then treated with 10 μM MG-132 for 8 h and harvested for immunoprecipitation. The immunoprecipitated protein level was balanced to detect its acetylation or ubiquitination level.
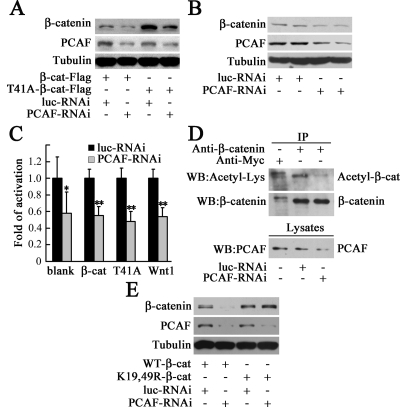
PCAF Is Essential for Maintaining the Protein Level, Transcriptional Activity, and Acetylation Level of β-Catenin
The above-mentioned results showed PCAF could improve the stability of β-catenin by direct acetylation. However, whether PCAF is essential for maintaining β-catenin protein level and transcriptional activity is still unclear. To address this question, PCAF-RNAi construct was made to knockdown PCAF. As shown in Figure 6A, the protein level of PCAF was significantly reduced by PCAF-RNAi, and the protein level of exogenous β-catenin or T41A-β-catenin was also markedly down-regulated by PCAF-RNAi. Similarly, endogenous β-catenin was down-regulated by PCAF-RNAi (Figure 6B). These data demonstrated PCAF is essential for maintenance of the protein level of β-catenin. The effect of PCAF-RNAi on β-catenin transcriptional activity was measured by luciferase assay. As shown in Figure 6C, knockdown of PCAF significantly inhibited the basal level β-catenin transcriptional activity, and the β-catenin transcriptional activity induced by overexpression of β-catenin, T41A-β-catenin or Wnt1. In addition, knockdown of PCAF dramatically decreased the acetylation level of β-catenin (Figure 6D). We found that K19 and K49 of β-catenin are important for the acetylation by PCAF (Figure 5C), then we tested whether PCAF-RNAi affected the protein level of K19,49R-β-catenin. As expected, knockdown of PCAF significantly down-regulated the protein level of β-catenin but not that of K19,49R-β-catenin (Figure 6E). These results showed that PCAF is essential for maintenance of the protein level, transcriptional activity and acetylation level of β-catenin.
Figure 6.
Knockdown of PCAF reduces the protein level, transcriptional activity, and acetylation level of β-catenin. (A) Knockdown of PCAF reduced the protein level of exogenous β-catenin. After transfection with β-catenin-FLAG (β-cat) or T41A-β-catenin-FLAG (T41A), and luc-RNAi or PCAF-RNAi in for 72 h, HEK293 cells were harvested, and β-catenin, PCAF, and tubulin in the cell lysates were detected by Western blot with anti-FLAG, anti-PCAF, or anti-tubulin antibody. (B) Knockdown of PCAF down-regulated endogenous β-catenin protein level. After transfected with luc-RNAi or PCAF-RNAi for 72 h, HEK293 cells were harvested, and endogenous β-catenin, PCAF, and tubulin in cell lysates were detected by Western blot. (C) Knockdown of PCAF inhibited β-catenin transcriptional activity measured by luciferase assay with Super8×TOPFlash. *p < 0.05, **p < 0.01, versus luc-RNAi. (D) Knockdown of PCAF decreased the acetylation level of β-catenin. HEK293 cells were transfected with luc-RNAi or PCAF-RNAi for 72 h. Immunoprecipitated proteins were detected by Western blot with anti-acetyl-Lys antibody. The same membrane was stripped and reblotted with anti-β-catenin antibody. The immunoprecipitated protein level was balanced to detect its acetylation level. (E) Knockdown of PCAF could not decrease the protein level of K19,49R-β-catenin. After transfecting HEK293 cells with WT-β-catenin or K19,49R-β-catenin, and luc-RNAi or PCAF-RNAi for 72 h, total β-catenin, PCAF, and tubulin in cell lysates were detected by Western blot.
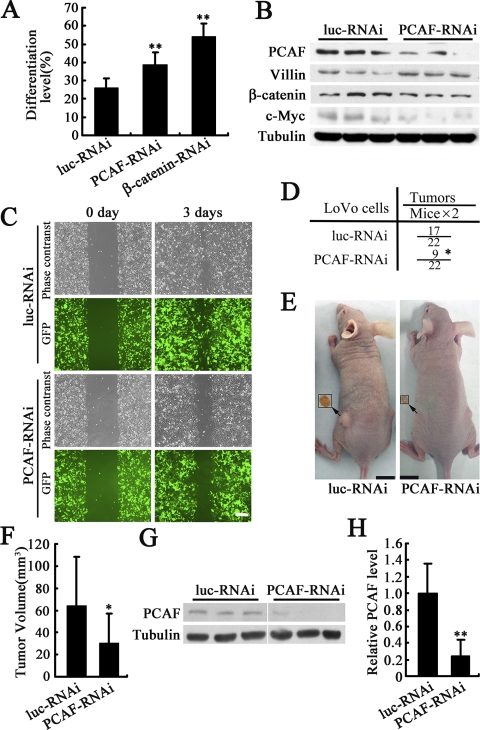
Knockdown of PCAF in Colon Cancer Cells Promotes Cell Differentiation, Inhibits Cell Migration and Tumor Growth
Disruption of β-catenin transcriptional activity could induce differentiation of colon cancer cells (Giles et al., 2003; Doucas et al., 2005); thus, it is possible that inhibition of β-catenin transcriptional activity by PCAF-RNAi might induce differentiation of colon cancer cells. To test this possibility, alkaline phosphatase activity, a marker of colon cancer cell differentiation (Aleman et al., 2005), was examined in LoVo cells. As shown in Figure 7A, knockdown of PCAF significantly induced the LoVo cell differentiation. Knockdown of β-catenin was used as a positive control. The differentiation of LoVo cells was further confirmed by examining the protein level of villin, another marker of colon cancer cell differentiation (van den Brink et al., 2004). As shown in Figure 7B, villin was significantly up-regulated by PCAF-RNAi. In addition, the protein level of β-catenin was down-regulated by PCAF-RNAi, and c-Myc, a β-catenin–regulated gene, its protein level was also decreased by PCAF-RNAi (Figure 7B). To test whether PCAF RNAi could inhibit colon cancer cell migration, wound healing assays were performed. The cells expressing luc-RNAi at the leading edges of the wound migrated and spread to cover the wound significantly faster than the cells expressing PCAF-RNAi (Figure 7C). The effect of PCAF RNAi on colon cancer cell differentiation and migration imply that PCAF RNAi might have an antitumor effect in vivo. To address this speculation, LoVo cells expressing PCAF RNAi or luciferase RNAi were injected subcutaneously into nude mice. Two months after inoculation, we found knockdown of PCAF markedly reduced the tumorigenesis and tumor growth in nude mice (Figure 7, D–F). The effect of PCAF RNAi to down-regulate PCAF in the tumors was confirmed by western blot (Figures 7, G and H). Together, these results showed that knockdown of PCAF in colon cancer cells induces cell differentiation, slows down cell migration, and potently suppressed tumorigenesis and tumor growth in vivo.
Figure 7.
Knockdown of PCAF in colon cancer cells promotes cell differentiation, blocks cell migration and tumor growth. (A) PCAF RNAi induced the differentiation of LoVo cells. After transfection with plasmids expressing luc-RNAi, PCAF-RNAi or β-catenin-RNAi for 3 d, differentiated LoVo cells were measured by alkaline phosphatase activity assay. **p < 0.01 versus luc-RNAi. (B) PCAF RNAi induced the expression of villin, a marker of colonic cell differentiation and down-regulated the protein level of β-catenin and c-Myc. After infection with lentivirus expressing luc-RNAi or PCAF-RNAi for 72 h, PCAF, villin, β-catenin, and tubulin in LoVo cells were detected by Western blot. (C) PCAF-RNAi slowed down the migration of LoVo cells. After infection with lentivirus expressing luc-RNAi or PCAF-RNAi for 7 d, LoVo cells were used for wound assay. GFP represented the cells expressing luc-RNAi or PCAF-RNAi. Bar, 200 μm. (D) PCAF RNAi reduced the tumor number. LoVo cells expressing luc-RNAi or PCAF-RNAi were injected subcutaneously into nude mice. The mice were killed 2 mo later to analyze the tumor number and tumor size. The chi-square test was used for the analysis of detection frequency of tumors in nude mice. *p < 0.05 versus luc-RNAi. (E) Gross appearance of xenografts and excised tumors. Bar, 1 cm. (F) PCAF RNAi reduced the tumor size. *p < 0.05 versus luc-RNAi. (G) Effect of PCAF-RNAi to down-regulate PCAF in the tumors was confirmed by Western blot. (H) Quantification of western blot results from G. **p < 0.01.
DISCUSSION
In this study, we demonstrated that PCAF could acetylate β-catenin and improve its stability, and knockdown of PCAF could reduce the protein level, transcriptional activity, and acetylation level of β-catenin, promote cell differentiation, and repress the migration and tumorigenesis of colon cancer cells. Thus, our observations provided a potential pharmaceutical target for treating colon cancer or other β-catenin signaling disorders.
The regulation of β-catenin stability and nuclear localization is a key event in Wnt signaling (Nelson and Nusse, 2004). Recent studies showed CBP and p300 could acetylate β-catenin and regulate the affinity of β-catenin for Tcf4 and the expression of Wnt-responsive genes (Hecht et al., 2000; Sun et al., 2000; Wolf et al., 2002; Levy et al., 2004). PCAF is an acetyltransferase and usually acts as a coactivator, which enhances the activity of numerous other activators and proteins involved in transcription, including class II transactivator (Spilianakis et al., 2000), MyoD (Sartorelli et al., 1999), E2F1 (Martinez-Balbas et al., 2000), and E1A (Zhang et al., 2000). Similarly, with CBP and p300, we found PCAF could also act as a coactivator to acetylate β-catenin and enhance its stability and transcriptional activity (Figures 1–4). It has been shown that CBP and p300 acetylate β-catenin at K49 and K345, respectively (Wolf et al., 2002; Levy et al., 2004). However, mutation of K49 or K345 failed to block the effect of PCAF on β-catenin (data not shown). So, the acetylation sites of β-catenin by PCAF should be different from CBP and p300. β-Catenin is usually degraded by ubiquitin proteasome system, and Lys-19 and Lys-49 are important sites for β-catenin ubiquitination (Aberle et al., 1997; Winer et al., 2006). Single amino acid substitutions of either Lys-19 or Lys-49 of β-catenin slightly affect its ubiquitination, but the K19/K49 double mutant β-catenin is stabilized as a result of significant reduced βTrCP-dependent ubiquitination (Winer et al., 2006). These findings implied that other posttranslational modifications at Lys-19 and Lys-49, such as acetylation, might affect the ubiquitination and stability of β-catenin. As expected, double mutation of the two lysines markedly decreased its acetylation by PCAF and subsequent ubiquitination (Figure 5). These data suggested that PCAF acetylates Lys-19 and Lys-49 and blocks the ubiquitination at the same sites.
β-Catenin plays an important role in development. However, mice lacking PCAF are developmentally normal without a distinct phenotype (Xu et al., 2000; Yamauchi et al., 2000), which raises the question that whether PCAF regulates β-catenin in vivo during development. PCAF mRNA is first detected on day 12.5, and the closely related PCAF-B/GCN5L2 mRNA is expressed at high levels already on day 8 (Yamauchi et al., 2000). Animals lacking PCAF-B/GCN5L2 die between days 9.5 and 11.5. In PCAF null-zygous mice, PCAF-B/GCN5L2 protein level is dramatically elevated in some tissues, where PCAF is abundantly expressed in wild-type mice, suggesting that PCAF-B/GCN5L2 functionally compensates for PCAF, which may explain the distinct knockout phenotypes between PCAF and PCAF-B (Yamauchi et al., 2000). It has been reported that the combined loss of PCAF and GCN5L2 leads to more severe defects than those observed for loss of GCN5L2 alone, indicating that these acetyltransferases have overlapping functions during embryogenesis (Xu et al., 2000). So, it is reasonable that PCAF can acetylate β-catenin and regulate its stability and biological functions in some specific tissues, although mice lacking PCAF are developmentally normal.
β-Catenin is usually localized in cytoplasm, and PCAF is mainly localized in nucleus. How PCAF could bind to and acetylate β-catenin needs to be elucidated in the future. As shown in Figure 1E, increased expression of PCAF recruited β-catenin into the nucleus, and this effect is dependent on its acetyltransferase activity. Several cytoplasmic Wnt regulators, including APC and Axin, have been found to shuttle in and out of nucleus with β-catenin (Willert and Jones, 2006). The shuttling of β-catenin between nucleus and cytoplasm provide the possibility for its interaction with PCAF. It seems like that this interaction or acetylation of β-catenin by PCAF inhibits the exportation of β-catenin, and facilitates the nuclear localization of β-catenin.
The correlation of colorectal cancer and aberrant Wnt signaling has been extensively reported (Clevers, 2004; Logan and Nusse, 2004; Reya and Clevers, 2005). Disruption of Wnt signaling is becoming a promising strategy for prevention or treatment of colon cancers (Clapper et al., 2004; Dihlmann and von Knebel Doeber, 2005; Dvory-Sobol et al., 2006). In this study, we found that knockdown of PCAF by RNAi could significantly decrease the protein level of β-catenin and inhibit its transcriptional activity (Figure 6). Furthermore, knockdown of PCAF promotes the differentiation and attenuates the migration and tumorigenesis of colon cancer cells (Figure 7). These results provide the possibility for the potential application of PCAF inhibition in colon cancer therapy. Consistent with what we observed that c-Myc mRNA level was increased by PCAF (Figure 2, A and B) and c-Myc protein level was down-regulated by PCAF-RNAi (Figure 7B), recent studies showed that the acetylation of oncoprotein c-Myc by PCAF results increased protein stability (Patel et al., 2004). Furthermore, acetylation of tumor suppresser PTEN by PCAF leads to reduced function of PTEN (Okumura et al., 2006), and acetylation of mouse p53 at lysine 317 by PCAF negatively regulates p53 apoptotic activities after DNA damage (Chao et al., 2006). Moreover, the deacetylase SIRT1 and acetyltransferase PCAF have common target proteins such as MyoD (Fulco et al., 2003), nuclear factor-κB (Yeung et al., 2004), and Foxo3 (Brunet et al., 2004), and even the common modification sites in Ku70 and NBS1 (Cohen et al., 2004b; Yuan et al., 2007). Consistent with our results, the deacetylase SIRT1 was shown to deacetylase β-catenin and negatively regulate β-catenin, and suppressed intestinal tumorigenesis and colon cancer growth (Firestein et al., 2008). These results implied that the acetyltransferase PCAF may have the opposite functions to SIRT1 and act as a tumor activator in some specific tumors.
In conclusion, PCAF acetylates β-catenin and improves its stability and transcriptional activity, and knockdown of PCAF regulates the differentiation, migration, and tumorigenesis of colon cancer cells. All these data suggested that inhibition of PCAF might be applied in β-catenin-driven diseases, such as colon cancer.
ACKNOWLEDGMENTS
We thank Drs. Randall Moon, Christine Neuveut, Eric Fearon, Chunming Liu, Xiang-Jiao Yang, and Lin Li for kindly providing the materials used in this study. This study was supported by grants from National Natural Science Foundation of China (30400083, 30570558, and 30825009), National Basic Research Program of China (973 Program, 2007CB914501 and 2009CB918403), the Chinese Academy of Sciences (KSCX2-2-25 and KSCX2-YW-N-034), and the Knowledge Innovation Program of Shanghai Institutes for Biological Sciences (2007KIP103). Q. Z. is a scholar of the Hundred Talents Program from Chinese Academy of Sciences, and a scholar of the Shanghai Rising-Star Program from Science and Technology Commission of Shanghai Municipality (08QH1402600).
Abbreviations used:
- CBP
CREB-binding protein
- HAT
histone acetyltransferase
- PCAF
p300/CBP-associated factor
- RNAi
RNA interference
- TCF
T cell factor
- WT
wild type.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E08-08-0792) on November 5, 2008.
REFERENCES
- Abbas-Terki T., Blanco-Bose W., Deglon N., Pralong W., Aebischer P. Lentiviral-mediated RNA interference. Hum. Gene Ther. 2002;13:2197–2201. doi: 10.1089/104303402320987888. [DOI] [PubMed] [Google Scholar]
- Aberle H., Bauer A., Stappert J., Kispert A., Kemler R. beta-Catenin is a target for the ubiquitin-proteasome pathway. EMBO J. 1997;16:3797–3804. doi: 10.1093/emboj/16.13.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleman M. J., DeYoung M. P., Tress M., Keating P., Perry G. W., Narayanan R. Inhibition of Single Minded 2 gene expression mediates tumor-selective apoptosis and differentiation in human colon cancer cells. Proc. Natl. Acad. Sci. USA. 2005;102:12765–12770. doi: 10.1073/pnas.0505484102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco J. C., Minucci S., Lu J., Yang X. J., Walker K. K., Chen H., Evans R. M., Nakatani Y., Ozato K. The histone acetylase PCAF is a nuclear receptor coactivator. Genes Dev. 1998;12:1638–1651. doi: 10.1101/gad.12.11.1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet A., et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- Chao C., Wu Z., Mazur S. J., Borges H., Rossi M., Lin T., Wang J. Y., Anderson C. W., Appella E., Xu Y. Acetylation of mouse p53 at lysine 317 negatively regulates p53 apoptotic activities after DNA damage. Mol. Cell. Biol. 2006;26:6859–6869. doi: 10.1128/MCB.00062-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapper M. L., Coudry J., Chang W. C. beta-Catenin-mediated signaling: a molecular target for early chemopreventive intervention. Mutat. Res. 2004;555:97–105. doi: 10.1016/j.mrfmmm.2004.05.021. [DOI] [PubMed] [Google Scholar]
- Clevers H. Wnt breakers in colon cancer. Cancer Cell. 2004;5:5–6. doi: 10.1016/s1535-6108(03)00339-8. [DOI] [PubMed] [Google Scholar]
- Clevers H. Wnt/β-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Cohen H. Y., Lavu S., Bitterman K. J., Hekking B., Imahiyerobo T. A., Miller C., Frye R., Ploegh H., Kessler B. M., Sinclair D. A. Acetylation of the C terminus of Ku70 by CBP and PCAF controls Bax-mediated apoptosis. Mol. Cell. 2004a;13:627–638. doi: 10.1016/s1097-2765(04)00094-2. [DOI] [PubMed] [Google Scholar]
- Cohen H. Y., Miller C., Bitterman K. J., Wall N. R., Hekking B., Kessler B., Howitz K. T., Gorospe M., de Cabo R., Sinclair D. A. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science. 2004b;305:390–392. doi: 10.1126/science.1099196. [DOI] [PubMed] [Google Scholar]
- Dihlmann S., von Knebel Doeber M. Wnt/beta-catenin-pathway as a molecular target for future anti-cancer therapeutics. Int. J. Cancer. 2005;113:515–524. doi: 10.1002/ijc.20609. [DOI] [PubMed] [Google Scholar]
- Doucas H., Garcea G., Neal C. P., Manson M. M., Berry D. P. Changes in the Wnt signalling pathway in gastrointestinal cancers and their prognostic significance. Eur. J. Cancer. 2005;41:365–379. doi: 10.1016/j.ejca.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Dvory-Sobol H., Sagiv E., Kazanov D., Ben-Ze'ev A., Arber N. Targeting the active beta-catenin pathway to treat cancer cells. Mol. Cancer Ther. 2006;5:2861–2871. doi: 10.1158/1535-7163.MCT-06-0122. [DOI] [PubMed] [Google Scholar]
- Feyt C., Kienlen-Campard P., Leroy K., N′Kuli F., Courtoy P. J., Brion J.-P., Octave J.-N. Lithium chloride increases the production of amyloid-{beta} peptide independently from its inhibition of glycogen synthase kinase 3. J. Biol. Chem. 2005;280:33220–33227. doi: 10.1074/jbc.M501610200. [DOI] [PubMed] [Google Scholar]
- Firestein R., et al. The SIRT1 deacetylase suppresses intestinal tumorigenesis and colon cancer growth. PLoS ONE. 2008;3:e2020. doi: 10.1371/journal.pone.0002020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulco M., Schiltz R. L., Iezzi S., King M. T., Zhao P., Kashiwaya Y., Hoffman E., Veech R. L., Sartorelli V. Sir2 regulates skeletal muscle differentiation as a potential sensor of the redox state. Mol. Cell. 2003;12:51–62. doi: 10.1016/s1097-2765(03)00226-0. [DOI] [PubMed] [Google Scholar]
- Gay F., Calvo D., Lo M.-C., Ceron J., Maduro M., Lin R., Shi Y. Acetylation regulates subcellular localization of the Wnt signaling nuclear effector POP-1. Genes Dev. 2003;17:717–722. doi: 10.1101/gad.1042403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles R. H., van Es J. H., Clevers H. Caught up in a Wnt storm: Wnt signaling in cancer. Biochim. Biophys. Acta. 2003;1653:1–24. doi: 10.1016/s0304-419x(03)00005-2. [DOI] [PubMed] [Google Scholar]
- Hecht A., Vleminckx K., Stemmler M. P., van Roy F., Kemler R. The p300/CBP acetyltransferases function as transcriptional coactivators of beta-catenin in vertebrates. EMBO J. 2000;19:1839–1850. doi: 10.1093/emboj/19.8.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ionov Y., Matsui S., Cowell J. K. A role for p300/CREB binding protein genes in promoting cancer progression in colon cancer cell lines with microsatellite instability. Proc. Natl. Acad. Sci. USA. 2004;101:1273–1278. doi: 10.1073/pnas.0307276101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia H., Yan T., Feng Y., Zeng C., Shi X., Zhai Q. Identification of a critical site in Wld(s): essential for Nmnat enzyme activity and axon-protective function. Neurosci. Lett. 2007;413:46–51. doi: 10.1016/j.neulet.2006.11.067. [DOI] [PubMed] [Google Scholar]
- Jiang H., Lu H., Schiltz R. L., Pise-Masison C. A., Ogryzko V. V., Nakatani Y., Brady J. N. PCAF interacts with tax and stimulates tax transactivation in a histone acetyltransferase-independent manner. Mol. Cell. Biol. 1999;19:8136–8145. doi: 10.1128/mcb.19.12.8136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy L., Wei Y., Labalette C., Wu Y., Renard C. A., Buendia M. A., Neuveut C. Acetylation of beta-catenin by p300 regulates beta-catenin-Tcf4 interaction. Mol. Cell. Biol. 2004;24:3404–3414. doi: 10.1128/MCB.24.8.3404-3414.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Li Y., Semenov M., Han C., Baeg G. H., Tan Y., Zhang Z., Lin X., He X. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell. 2002;108:837–847. doi: 10.1016/s0092-8674(02)00685-2. [DOI] [PubMed] [Google Scholar]
- Liu J., Stevens J., Rote C. A., Yost H. J., Hu Y., Neufeld K. L., White R. L., Matsunami N. Siah-1 mediates a novel β-catenin degradation pathway linking p53 to the adenomatous polyposis coli protein. Mol. Cell. 2001;7:927–936. doi: 10.1016/s1097-2765(01)00241-6. [DOI] [PubMed] [Google Scholar]
- Liu L., Scolnick D. M., Trievel R. C., Zhang H. B., Marmorstein R., Halazonetis T. D., Berger S. L. p53 sites acetylated in vitro by PCAF and p300 are acetylated in vivo in response to DNA damage. Mol. Cell. Biol. 1999;19:1202–1209. doi: 10.1128/mcb.19.2.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., et al. Mutations in AXIN2 cause colorectal cancer with defective mismatch repair by activating β-catenin/TCF signalling. Nat. Genet. 2000;26:146–147. doi: 10.1038/79859. [DOI] [PubMed] [Google Scholar]
- Logan C. Y., Nusse R. The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- Martinez-Balbas M. A., Bauer U. M., Nielsen S. J., Brehm A., Kouzarides T. Regulation of E2F1 activity by acetylation. EMBO J. 2000;19:662–671. doi: 10.1093/emboj/19.4.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzawa S.-I., Reed J. C. Siah-1, SIP, and Ebi collaborate in a novel pathway for β-catenin degradation linked to p53 responses. Mol. Cell. 2001;7:915–926. doi: 10.1016/s1097-2765(01)00242-8. [DOI] [PubMed] [Google Scholar]
- Morin P. J., Sparks A. B., Korinek V., Barker N., Clevers H., Vogelstein B., Kinzler K. W. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275:1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- Muraoka M., Konishi M., Kikuchi-Yanoshita R., Tanaka K., Shitara N., Chong J. M., Iwama T., Miyaki M. p300 gene alterations in colorectal and gastric carcinomas. Oncogene. 1996;12:1565–1569. [PubMed] [Google Scholar]
- Nelson W. J., Nusse R. Convergence of Wnt, {beta}-Catenin, and Cadherin Pathways. Science. 2004;303:1483–1487. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumura K., Mendoza M., Bachoo R. M., DePinho R. A., Cavenee W. K., Furnari F. B. PCAF Modulates PTEN Activity. J. Biol. Chem. 2006;281:26562–26568. doi: 10.1074/jbc.M605391200. [DOI] [PubMed] [Google Scholar]
- Pan W., Jia Y., Wang J., Tao D., Gan X., Tsiokas L., Jing N., Wu D., Li L. Beta-catenin regulates myogenesis by relieving I-mfa-mediated suppression of myogenic regulatory factors in P19 cells. Proc. Natl. Acad. Sci. USA. 2005;102:17378–17383. doi: 10.1073/pnas.0505922102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel J. H., et al. The c-MYC Oncoprotein Is a Substrate of the Acetyltransferases hGCN5/PCAF and TIP60. Mol. Cell. Biol. 2004;24:10826–10834. doi: 10.1128/MCB.24.24.10826-10834.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Y., Luo J., Leonard S. S., Harris G. K., Millecchia L., Flynn D. C., Shi X. Hydrogen peroxide formation and actin filament reorganization by Cdc42 are essential for ethanol-induced in vitro angiogenesis. J. Biol. Chem. 2003;278:16189–16197. doi: 10.1074/jbc.M207517200. [DOI] [PubMed] [Google Scholar]
- Reya T., Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- Sartorelli V., Puri P. L., Hamamori Y., Ogryzko V., Chung G., Nakatani Y., Wang J. Y., Kedes L. Acetylation of MyoD directed by PCAF is necessary for the execution of the muscle program. Mol. Cell. 1999;4:725–734. doi: 10.1016/s1097-2765(00)80383-4. [DOI] [PubMed] [Google Scholar]
- Schiltz R. L., Nakatani Y. The PCAF acetylase complex as a potential tumor suppressor. Biochim. Biophys. Acta. 2000;1470:M37–M53. doi: 10.1016/s0304-419x(99)00037-2. [DOI] [PubMed] [Google Scholar]
- Shibamoto S., Higano K., Takada R., Ito F., Takeichi M., Takada S. Cytoskeletal reorganization by soluble Wnt-3a protein signalling. Genes Cells. 1998;3:659–670. doi: 10.1046/j.1365-2443.1998.00221.x. [DOI] [PubMed] [Google Scholar]
- Spilianakis C., Papamatheakis J., Kretsovali A. Acetylation by PCAF enhances CIITA nuclear accumulation and transactivation of major histocompatibility complex class II genes. Mol. Cell. Biol. 2000;20:8489–8498. doi: 10.1128/mcb.20.22.8489-8498.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stambolic V., Ruel L., Woodgett J. R. Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signalling in intact cells. Curr. Biol. 1996;6:1664–1668. doi: 10.1016/s0960-9822(02)70790-2. [DOI] [PubMed] [Google Scholar]
- Sterner D. E., Berger S. L. Acetylation of Histones and Transcription-Related Factors. Microbiol. Mol. Biol. Rev. 2000;64:435–459. doi: 10.1128/mmbr.64.2.435-459.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stimson L., et al. Isothiazolones as inhibitors of PCAF and p300 histone acetyltransferase activity. Mol. Cancer Ther. 2005;4:1521–1532. doi: 10.1158/1535-7163.MCT-05-0135. [DOI] [PubMed] [Google Scholar]
- Sun Y., Kolligs F. T., Hottiger M. O., Mosavin R., Fearon E. R., Nabel G. J. Regulation of beta-catenin transformation by the p300 transcriptional coactivator. Proc. Natl. Acad. Sci. USA. 2000;97:12613–12618. doi: 10.1073/pnas.220158597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemaru K. I., Moon R. T. The transcriptional coactivator CBP interacts with beta-catenin to activate gene expression. J. Cell Biol. 2000;149:249–254. doi: 10.1083/jcb.149.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Brink G. R., et al. Indian Hedgehog is an antagonist of Wnt signaling in colonic epithelial cell differentiation. Nat. Genet. 2004;36:277–282. doi: 10.1038/ng1304. [DOI] [PubMed] [Google Scholar]
- Willert K., Jones K. A. Wnt signaling: is the party in the nucleus? Genes Dev. 2006;20:1394–1404. doi: 10.1101/gad.1424006. [DOI] [PubMed] [Google Scholar]
- Winer I. S., Bommer G. T., Gonik N., Fearon E. R. Lysine residues Lys-19 and Lys-49 of beta-catenin regulate its levels and function in T cell factor transcriptional activation and neoplastic transformation. J. Biol. Chem. 2006;281:26181–26187. doi: 10.1074/jbc.M604217200. [DOI] [PubMed] [Google Scholar]
- Wolf D., Rodova M., Miska E. A., Calvet J. P., Kouzarides T. Acetylation of beta-catenin by CREB-binding protein (CBP) J. Biol. Chem. 2002;277:25562–25567. doi: 10.1074/jbc.M201196200. [DOI] [PubMed] [Google Scholar]
- Xu W., Edmondson D. G., Evrard Y. A., Wakamiya M., Behringer R. R., Roth S. Y. Loss of Gcn5l2 leads to increased apoptosis and mesodermal defects during mouse development. Nat. Genet. 2000;26:229–232. doi: 10.1038/79973. [DOI] [PubMed] [Google Scholar]
- Yamauchi T., Yamauchi J., Kuwata T., Tamura T., Yamashita T., Bae N., Westphal H., Ozato K., Nakatani Y. Distinct but overlapping roles of histone acetylase PCAF and of the closely related PCAF-B/GCN5 in mouse embryogenesis. Proc. Natl. Acad. Sci. USA. 2000;97:11303–11306. doi: 10.1073/pnas.97.21.11303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X.-J., Ogryzko V. V., Nishikawa J.-i., Howard B. H., Nakatani Y. A p300/CBP-associated factor that competes with the adenoviral oncoprotein E1A. Nature. 1996;382:319–324. doi: 10.1038/382319a0. [DOI] [PubMed] [Google Scholar]
- Yeung F., Hoberg J. E., Ramsey C. S., Keller M. D., Jones D. R., Frye R. A., Mayo M. W. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004;23:2369–2380. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Z., Zhang X., Sengupta N., Lane W. S., Seto E. SIRT1 regulates the function of the Nijmegen breakage syndrome protein. Mol. Cell. 2007;27:149–162. doi: 10.1016/j.molcel.2007.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Yao H., Vo N., Goodman R. H. Acetylation of adenovirus E1A regulates binding of the transcriptional corepressor CtBP. Proc. Natl. Acad. Sci. USA. 2000;97:14323–14328. doi: 10.1073/pnas.011283598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L. Y., Liu Y., Bertos N. R., Yang X. J., Liao D. PCAF is a coactivator for p73-mediated transactivation. Oncogene. 2003;22:8316–8329. doi: 10.1038/sj.onc.1206916. [DOI] [PubMed] [Google Scholar]