Abstract
One fundamental role of the centriole in eukaryotic cells is to nucleate the growth of cilia. The unicellular alga Chlamydomonas reinhardtii provides a simple genetic system to study the role of the centriole in ciliogenesis. Wild-type cells are biflagellate, but “uni” mutations result in failure of some centrioles (basal bodies) to assemble cilia (flagella). Serial transverse sections through basal bodies in uni1 and uni2 single and double mutant cells revealed a previously undescribed defect in the transition of triplet microtubules to doublet microtubules, a defect correlated with failure to assemble flagella. Phosphorylation of the Uni2 protein is reduced in uni1 mutant cells. Immunogold electron microscopy showed that the Uni2 protein localizes at the distal end of the basal body where microtubule transition occurs. These results provide the first mechanistic insights into the function of UNI1 and UNI2 genes in the pathway mediating assembly of doublet microtubules in the axoneme from triplet microtubules in the basal body template.
INTRODUCTION
Cilia are utilized in cell motility, fluid flow, food capture, sexual reproduction, and sensation. In mammals, cilia are essential organelles that function in numerous sensory and developmental processes (for review see Christensen et al., 2007). Formation of microtubules in the ciliary axoneme requires a unique template provided by the centriole. An increased knowledge of the pathways mediating ciliogenesis will provide insights key to understanding several human genetic disorders, collectively known as ciliopathies, that can be attributed to defects in centrioles or cilia (for reviews see Afzelius, 2004; Marshall, 2008).
Centrioles and cilia both contain radially arranged sets of microtubules. A ring of nine triplet microtubules forms the wall of the centriole. Each triplet typically contains a complete A-tubule composed of 13 protofilaments adjoined by partial B- and C-tubules, each of which contains 10 protofilaments and an unidentified eleventh component (for review see Linck and Stephens, 2007). Axonemes of cilia contain a ring of nine doublet microtubules, each assembling from a template provided by the A- and B-tubules of the centriole. The conversion of triplet microtubules to doublet microtubules occurs uniformly at a distinct point at the distal end of the centriole (for example see Cavalier-Smith, 1974; Albrecht-Buehler and Bushnell, 1980; Paintrand et al., 1992).
Because the genes required for the formation and function of cilia and centrioles are highly conserved, the unicellular biflagellate green alga Chlamydomonas reinhardtii provides a relatively simple model system to study these organelles. Powerful genetic approaches take advantage of the fact that vegetative cells are haploid and that neither cilia nor centrioles are essential for viability (Matsuura et al., 2004). The availability of the genome sequence (Merchant et al., 2007) combined with a well-characterized molecular map (Kathir et al., 2003) and efficient transformation methods (Kindle, 1990) add to the utility of this model organism. Centrioles (basal bodies) and cilia (flagella) in wild-type (WT) cells have been characterized at the ultrastructural level (for examples see Ringo, 1967; Cavalier-Smith, 1974; O'Toole et al., 2003; Geimer and Melkonian, 2004).
In nature, the arrangement of nine triplet microtubules of the centriole is almost omnipresent in organisms that retain these organelles. Mutant analysis in C. reinhardtii has revealed genes essential for assembly of triplet microtubules and for the ninefold rotational symmetry of the basal body. For example, mutations in the gene BLD2, which encodes ε-tubulin, result in formation of basal bodies with only nine singlet A-tubules (Dutcher et al., 2002). Mutation of the gene UNI3, which encodes δ-tubulin, results in basal bodies with doublet rather than triplet microtubules (Dutcher and Trabuco, 1998). Mutations in the SAS6 gene, which encodes a protein of the cartwheel structure at the base of the basal body, result in variable numbers, from seven to eleven, of complete triplet microtubules (Nakazawa et al., 2007). The SAS6 gene was originally identified in Caenorhabditis elegans as an early component of centriolar assembly (Dammermann et al., 2004; Leidel et al., 2005). Analysis of these mutant phenotypes reveals the essential role of the ninefold arrangement of microtubules in the basal body for the normal assembly of axonemal doublet microtubules.
Like other green algae, Chlamydomonas is a bikont organism with two flagella assembled from basal bodies of different chronological ages (Beech et al., 1991; Cavalier-Smith, 2002). In each cell cycle, the younger basal body, positioned cis to the eyspot, undergoes transformation to become an older basal body, positioned trans to the eyespot (Holmes and Dutcher, 1989). The uni1, uni2, and uni3 mutations preferentially affect the growth of a flagellum from the younger of the two basal bodies (Huang et al., 1982; Dutcher and Trabuco, 1998; Piasecki et al., 2008). These mutations also affect the ability of basal bodies to properly assemble a transition zone (TZ), the region just distal to the point of triplet to doublet microtubule transition (Huang et al., 1982; O'Toole et al., 2003; Piasecki et al., 2008). Mutations in either the UNI1 or the UNI2 gene do not appear to affect triplet microtubule assembly in basal bodies as in the uni3 mutant, but rather result in similarly aberrant and elongated TZ structures (Huang et al., 1982; Piasecki et al., 2008). The UNI2 gene was shown to encode an alanine-rich phosphoprotein that localizes to both basal bodies and probasal bodies (Piasecki et al., 2008). The UNI1 gene product has not been identified. The similarity in the ultrastructural phenotypes of the uni1 and uni2 mutations suggests that these genes may function in the same pathway.
In this study, we explored the interaction between the UNI1 and UNI2 genes. We show that phosphorylation of the Uni2 protein is greatly reduced in uni1 mutant cells. A detailed ultrastructural analysis of uni1 and uni2 single and double mutant cells demonstrated a similar defect that likely explains the function of the UNI1 and UNI2 genes in flagellar formation. We found that failure to transition from triplet to doublet microtubules at the distal end of the basal body is strongly correlated with failure to assemble flagella. Further, the Uni2 protein was localized to the point where microtubule transition occurs. These results suggest that the UNI1 and UNI2 genes function in the pathway controlling the transition from triplet to doublet microtubules.
MATERIALS AND METHODS
Strains, Culture Conditions, and Fixation
Strains of uni1-1 (CC-1926), uni2-3 (CC-4162), and uni2-3::NIT1 UNI2-HA (CC-4163) were obtained from the Chlamydomonas Resource Center at The University of Minnesota. The uni3-1 mutant was provided by Dr. Susan K. Dutcher (Washington University) and is now deposited in the Chlamydomonas Resource Center (CC-4179). Cultures were typically grown axenically in minimal medium I (Sager and Granick, 1953). Cultures of strain CC-4179 and all cultures grown for immunoblot analyses were grown in modified minimal medium supplemented with 22 μM sodium acetate. All cultures were maintained at 24°C by bubbling continuously with filtered air and were illuminated by fluorescent white light at ∼60 μmol photons/m2/s1 on a 14:10-h light:dark cycle.
Tetrad analysis was performed at 24°C using standard techniques (Levine and Ebersold, 1960). The uni2-3 and uni3-1 mutations are gene deletions, generated through insertional mutagenesis (Tam and Lefebvre, 1993; Dutcher and Trabuco, 1998). Among progeny from complete tetrads, genotypes were confirmed using a PCR screen with template DNA from putative mutant strains. Within tetrads, the two strains with the uni1-1 mutation were deduced from the flagellar number phenotypes. Double mutant progeny have sharply reduced numbers of flagella compared with the parental strains (Dutcher and Trabuco, 1998).
Phenotypic rescue of the uni2 mutation was accomplished using glass bead cotransformation (Kindle, 1990) with the wild-type UNI2 gene encoding an HA epitope tag on plasmid pML9.7-3xHA (Piasecki et al., 2008) together with the pSI103 plasmid, which confers paromomycin resistance (Sisova et al., 2001). Transformants expressing the HA-tagged Uni2 protein and showing a rescue of the uni2 flagellar number defect were chosen for further experiments. Strains generated for this study were deposited in the Chlamydomonas Resource Center at The University of Minnesota.
For conducting flagellar counts, cells were suspended in 10 mM HEPES buffer (pH 7.4) and gently agitated on an orbital shaker for 1 h before fixation in 2% glutaraldehyde. A Zeiss (Thornwood, NY) compound microscope equipped with phase contrast optics was used to count the number of flagella per cell.
Immunoblotting and Densitometry
Immunoblotting was performed as described by Silflow et al. (2001) using protein extracts from ∼3 × 106 cells per lane on 7% SDS-PAGE minigels. The transfer buffer included 0.01% SDS. The hemagglutinin (HA)-tagged protein was detected with a rat anti-HA (3F10) high-affinity antibody (Roche Molecular Biochemical, Indianapolis, IN) at a 1:1200 dilution. The primary antibody was detected using a secondary goat anti-rat IgG-POD (Sigma Aldrich, St. Louis, MO) at a 1:10,000 dilution. For a loading control, a mouse anti-β-tubulin (2-10-B6) mAb (a gift from Dr. G. Piperno, Mount Sinai School of Medicine, New York, NY) was used at a 1:75 dilution. To detect the primary antibody, a goat anti-mouse IgG-POD (Sigma Aldrich) was used at 1:25,000 dilution. Densitometry of the scanned HA-tagged protein blot was performed as described in Piasecki et al., (2008).
Indirect Immunofluorescence Labeling and Ultrastructural Analysis
Indirect immunofluorescence microscopy and transmission electron microscopy in whole cells was conducted as described by Piasecki et al. (2008). For postembedment immunoelectron microscopy, cells were pelleted by centrifugation, suspended in 4% percent paraformaldehyde and microtubule fixation/stabilization buffer (pH 7.0), and fixed on ice for 30 min (Sanders and Salisbury, 1995). Fixed cells were rinsed three times in 10 mM HEPES (pH 7.0) for 15 min/step. An ETOH dehydration series was conducted by pelleting cells and suspending them in a gradation series of (25, 50, 75, 95, and 2 × 100%) ETOH for 15 min/step on ice. Cells were substituted with LR White Medium Grade resin (Ted Pella, Redding, CA) at 4°C following the manufacturer's specifications, including the addition of accelerator for polymerization. Ultrathin sections were cut, hydrated in PBS, and incubated in blocking buffer for 30 min at room temperature. Sections were labeled with rat anti-HA (3F10, Roche Molecular Biochemical) antibodies at a 1:50 dilution in blocking buffer at 4°C overnight. An unlabeled control was incubated overnight in PBS. After rinsing four times in PBS, sections were incubated with 12-nm gold–conjugated goat anti-RAT (112-205-143, Jackson ImmunoResearch, Balitmore, PA) antibodies at a 1:20 dilution for 30–60 min at room temperature. Sections were rinsed four times in PBS and fixed in 1% glutaraldehyde in PBS for 5 min. The grids were washed five times in double-distilled H2O and stained with 3% uranyl acetate for 20 min followed by Sato triple lead stain for 3 min (Sato, 1968).
RESULTS
Chlamydomonas cells in interphase have two flagella-bearing basal bodies of different chronological ages and two immature probasal bodies of the same age (Gould, 1975; Gaffal, 1988; Holmes and Dutcher, 1989). Mutations in the UNI1, UNI2, or UNI3 genes result in the preferential assembly of a single flagellum from the older basal body (Huang et al., 1982; Dutcher and Trabuco, 1998; Piasecki et al., 2008). The uni3 mutant shows defects in the assembly of the C-tubule of the basal body triplet microtubule as well as defects in structure and positioning of the TZ, an H-shaped electron-dense structure located just distal to the basal body proper (Figure 1A, Dutcher and Trabuco, 1998; O-Toole et al., 2003). In contrast, ultrastructural analysis of uni1 and uni2 mutant cells revealed properly assembled triplet microtubules in the basal bodies but similarly elongated and malformed TZs (Huang et al., 1982; Piasecki et al., 2008). The similarity in the phenotypes of the uni1 and uni2 mutations prompted us to examine the potential interaction of UNI1 and UNI2 genes.
Figure 1.
Median longitudinal sections of basal bodies from uni1-1 uni2-3 double mutant cells (CC-4201) reveal TZ and docking defects. (A) The TZ in WT cells (white bracket) is composed of distal (d) and proximal (p) cylinders separated by an electron dense transitional plate (tp). The basal body docks with the plasma membrane (pm) allowing the TZ and axoneme to extend through an opening in the cell wall (cw). Projections extend from triplet microtubules into the lumen of the basal body. The transition from triplet to doublet microtubules occurs near the distal end of the basal body (black arrow). (B and C) Mutant uni1 uni2 (CC-4201) cells have highly elongated or multiplied and stacked TZ regions. The basal body in B is docked at the plasma membrane; the basal body in C is undocked. The distal striated fiber (df, dark arrow head) typically links the two basal bodies together and appears to be properly connected to each of the mutant basal bodies.
Genetic Interactions among Uniflagellar Mutants
To characterize genetic interactions with the UNI2 gene, uni2 mutant cells were crossed with uni1 and uni3 cells to obtain double mutant strains (Table 1). Wild-type C. reinhardtii cells are almost entirely biflagellate (>97%), whereas populations of uni1, uni2, and uni3 mutant cells all contain a high percentage of uniflagellate cells together with some aflagellate and biflagellate cells (Table 1). Double-mutant combinations of uni1, uni2, and uni3 show enhancement of the defect in flagellar assembly (Table 1; Dutcher and Trabuco, 1998). Only 1% of uni1 uni2 double mutant cells and 6% of uni2 uni3 double mutant cells were able to assemble one or two flagella.
Table 1.
Flagellar number in wild-type and mutant cultures
| Strainsa | Genotype | Percent flagellar number |
n | |||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | ≥4 | >0 | |||
| CC-124 | Wild type | 0.9 | 1.3 | 97.8 | 0 | 0 | 99.1 | 225 |
| CC-1926 | uni1-1 | 51.5 | 45.2 | 3.3 | 0 | 0 | 48.5 | 305 |
| CC-4162 | uni2-3 | 52.9 | 36.7 | 9.5 | 0.9 | 0 | 47.1 | 452 |
| CC-4179 | uni3-1 | 47.9 | 19.5 | 29.4 | 2.6 | 0.6 | 52.1 | 313 |
| CC-4201 | uni1-1 uni2-3 | 98.9 | 0.8 | 0.4 | 0 | 0 | 1.2 | 524 |
| CC-4202 | uni2-3 uni3-1 | 93.7 | 4.4 | 1.9 | 0 | 0 | 6.3 | 319 |
| CC-4163 | uni2-3::NIT1 UNI2-HA | 3.9 | 2.2 | 93.3 | 0.6 | 0 | 96.1 | 359 |
| CC-4204 | uni1-1 uni2-3::pSI103 UNI2-HA | 51.6 | 42.2 | 5.9 | 0 | 0 | 48.1 | 353 |
| CC-4205 | uni2-3 uni3-1::pSI103 UNI2-HA | 50.8 | 25.4 | 21.1 | 2.3 | 0.3 | 49.1 | 303 |
a Strains are deposited in the Chlamydomonas Resource Center (http://www.chlamy.org/).
The uni1 uni2 Double Mutant Cells Display Enhanced Transition Zone Defects
In WT cells the proximal end of the basal body is freely exposed in the cytoplasm of the cell whereas the distal end is docked with the plasma membrane (Figure 1A). At the distal end of the basal body, the triplet microtubules transition uniformly into the doublet microtubules of the axoneme (black arrow). The TZ, positioned just distal to this transition point, consists of electron dense proximal (p) and distal cylinders (d) separated by a transitional plate (tp), and is highly uniform in size and shape (Figure 1A; O'Toole et al., 2003). Because similar elongated TZs were observed previously in both uni1 and uni2 cells (Huang et al., 1982; Piasecki et al., 2008), ultrastructural analysis of TZs from uni1-1 uni2-3 double mutant cells was performed. In fortuitous longitudinal sections through 36 TZs from double mutant cells, we consistently observed elongated or multiplied and stacked TZs (Figure 1, B and C). Additionally, basal body docking defects in the uni1-1 uni2-3 double mutant cells are more pronounced than have been reported for either single mutant strain. Although WT basal bodies are always docked with the plasma membrane (Figure 1A), 24.4% (x̄ = 10, n = 41) of basal bodies in double mutant cells fail to dock with the plasma membrane and lie within the cytoplasm, usually at the anterior end of the cell (Figure 1C). Both docked and undocked basal bodies are able to assemble defective TZs.
Phosphorylation of the Uni2 Protein Is Decreased in uni1 Strains
We explored the possibility that the expression or localization of the Uni2 protein might be altered in the uni1 or uni3 mutant cells. A tagged UNI2 gene encoding a triple-HA-epitope was used previously to rescue the phenotype of the uni2-3 mutation to the WT flagellar number (Piasecki et al., 2008). For double mutants containing the uni2-3 mutation, we rescued the uni2 phenotype by transforming cells with the HA-tagged UNI2 gene. Multiple independent transformants were shown to be rescued to the flagellar number phenotype of the corresponding single mutant strain (Table 1).
We showed previously that the HA-tagged UNI2 gene expresses proteins in at least two distinct molecular-weight variants, the larger one being a phosphoprotein (Piasecki et al., 2008). To determine whether the Uni2 protein isoform pattern is altered in the genetic background of the uni1-1 or uni3-1 mutations, we analyzed protein extracts from the uni1-1 and uni3-1 mutants expressing the Uni2 HA-tagged protein and compared them with extracts from the uni2-3 mutant rescued to WT with the HA-tagged UNI2 gene. Protein extracts were subjected to SDS-PAGE and immunoblotting using an anti-HA antibody (Figure 2). Although no HA-tagged protein is detected in the untransformed WT control, the HA-tagged Uni2 protein migrates as two distinct molecular-weight variants in a uni2-3 strain rescued to WT. In contrast, the Uni2 protein in the uni1-1 mutant background is expressed primarily as a single form, corresponding to the lower molecular-weight or nonphosphorylated isoform. This dramatic decrease of the phosphorylated isoform is not detected in the uni3-1 mutant cells. The reduction in phosphorylation of the Uni2 protein in the uni1-1 cells was quantified using densitometry (Figure 2B). In different experiments, we observed a 10–13-fold decrease in the phosphorylated isoform in the uni1-1 mutant cells compared with WT cells. The expression patterns shown in Figure 2 for the HA-tagged Uni2 protein in the uni1-1 or the uni3-1 mutant background were observed in multiple independent transformants, suggesting that the results are not dependent on integration of the UNI2 transgene in a particular context in the nuclear genome (Supplemental Figure S1).
Figure 2.
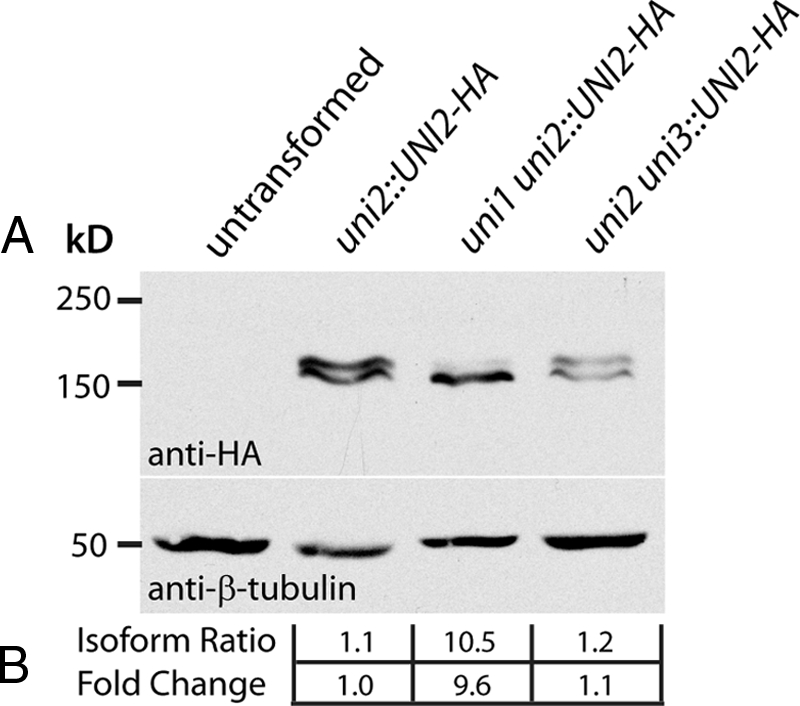
(A) Immunoblot analysis of uniflagellar mutants expressing the HA-tagged Uni2 protein. Protein extracts from an untransformed WT strain (CC-124) and single and double mutant strains expressing the HA-tagged Uni2 protein (CC-4163, CC-4204, CC-4205) were separated by SDS-PAGE and transferred to a PVDF membrane. Top, a high-affinity anti-HA antibody was used to identify the HA-tagged Uni2 protein; bottom, an antibody against β-tubulin was used as a loading control. (B) Expression of the two distinct Uni2 protein isoforms was compared using densitometry. The ratio of the lower to the higher molecular-weight isoform was calculated and the fold change of this ratio as compared with the uni2-3 strain rescued to WT (lane 2) was determined.
The Uni2 Protein Localizes to Basal Bodies and Probasal Bodies in uni1 and uni3 Mutant Cells
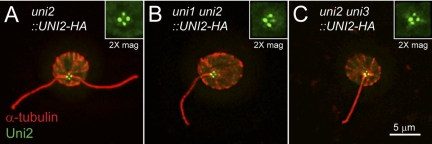
To determine whether the reduction in the phosphorylated Uni2 protein isoform in the uni1 mutant background can be attributed to a mislocalization of the protein, we examined its distribution in uni1-1 mutant cells using immunofluorescence microscopy. In a uni2-3 mutant rescued to WT with the HA-tagged UNI2 gene, the Uni2 protein localizes to four distinct spots corresponding to basal bodies and probasal bodies (Figure 3A; Piasecki et al., 2008). In uni1 uni2 double mutant cells rescued to the uni1 phenotype, the Uni2 protein localizes to four distinct spots at the base of a single flagellum in uniflagellate cells (Figure 3B) or at the base of the two flagella in biflagellate cells (data not shown). In multiple rescued cell lines, the overall intensity of staining was similar to that seen in uni2-3 single mutant cells rescued to WT. These results indicate that the diminished phosphorylation of the Uni2 protein does not result in its mislocalization.
Figure 3.
Indirect immunofluorescence microscopy of the HA-tagged Uni2 protein expressed in single and double mutant strains (A) uni2, strain CC-4163; (B) uni1 uni2, strain CC-4204; (C) uni2 uni3, strain CC-4205. Fixed whole cells were doubly stained with rat anti-HA and rabbit anti-α-tubulin antibodies. Secondary anti-rat Alexa Fluor 488 (green) and anti-rabbit Texas Red (red) antibodies were used to detect primary antibodies. Basal body regions were enlarged two times.
In uni2 uni3 double mutant cells rescued to the uni3 phenotype, the Uni2 protein also localizes to basal bodies and probasal bodies (Figure 3C). However, we observed many cells with an abnormal number and positioning of these organelles (Supplemental Figure S2). These results are consistent with those of O'Toole et al. (2003) showing that some basal bodies in uni3-1 cells fail to associate with fibers that are involved in segregation and positioning of basal bodies.
Some Basal Bodies in uni1 and uni2 Mutant Cells Show a Normal Transition of Triplet to Doublet Microtubules
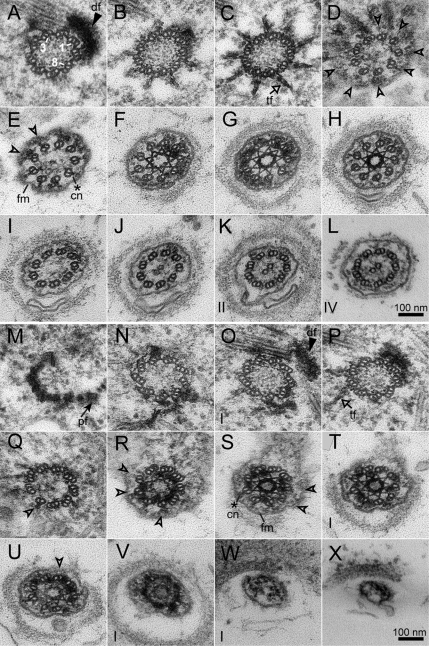
To further explore the basis for the TZ defects in the uniflagellar mutants, we examined serial transverse sections of basal bodies from uni1-1 and uni2-3 single and double mutant cells. These experiments were informed by results from several exhaustive studies of WT cells using either electron microscopy (Ringo, 1967; Cavalier-Smith, 1974; Geimer and Melkonian, 2004) or electron tomography (O'Toole et al., 2003). In the series shown in Figures 4 and 5, the basal-most section of each basal body is positioned on the upper left, and the distal-most flagellar region is positioned on the bottom right. All images are displayed as if the observer is looking down at the axoneme and basal body from outside the cell. The distal striated fiber (df, solid arrow head) adjoins the two mature basal bodies approximately midway along the length of a basal body and provides a useful marker for numbering triplet, and in subsequent sections doublet, microtubules (Hoops and Witman, 1983). In Figure 4A, the triplet microtubules numbered 1, 3, and 8 are labeled. All images are rotated in a similar orientation with respect to the numbering of microtubules.
Figure 4.
Transverse serial sections through two individual basal bodies and axonemal regions in uni2-3 mutant cells (CC-4162) reveal that for some basal bodies, a normal transition of triplet to doublet microtubules occurs before assembly of a TZ and flagellum (A–L), whereas other basal bodies fail to transition all triplet microtubules to doublet microtubules at the distal end of the transitional fibers (M–X). Triplet numbers (labeled 1, 3, and 8 in A) can be determined based on the location of the distal striated fiber (df, solid arrowhead) or the proximal fiber (pf, solid arrow). Open arrowheads demarcate the transition from triplet to doublet microtubules, which typically occurs at the distal end of transitional fibers (tf, open arrow) in WT cells. The ciliary necklace (cn, asterisk) links the doublet microtubules at the base of the axoneme to the flagellar membrane (fm). Roman numerals in the bottom left of some panels represent the number of omitted sections between that panel and the previous panel.
Figure 5.
Transverse serial sections through a single basal body and axonemal region in A–L the uni1-1 mutant (CC-1926) and in M–X the uni1-1 uni2-3 double mutant (CC-4201) reveal an incomplete transition of triplet to doublet microtubules and flagellar assembly failure. The basal body microtubule triplets were oriented based on the location of the proximal fiber (pf, solid arrow), which is in congruence with a normal localization of the distal striated fiber (df, solid arrowhead) in series A–L, but not in series M–X. Open arrowheads demarcate the transition from triplet to doublet microtubules in all series, which typically occurs at the distal end of transitional fibers (tf, open arrow) in WT cells. The ciliary necklace (cn, asterisk) links the microtubules of the axoneme to the flagellar membrane (fm). Roman numerals in the bottom left of some panels represent the number of omitted sections between that panel and the previous panel.
In both the uni1-1 and uni2-3 mutant cells, serial sections through several basal bodies and TZ regions showed ultrastructural features similar to those of WT cells. For example, the series in Figure 4, A–L, is representative of four series from uni2-3 mutant cells showing a normal basal body, TZ, and flagellum. The transitional fibers (tf, open arrow) extend as “propellers” from the wall of each triplet microtubule in the basal body to the base of the ciliary membrane (Figure 4, B and C; Weiss et al., 1977). The conversion of triplet microtubules to doublet microtubules occurs just distal to the point of attachment of the transitional fibers to the basal body (open arrowheads, Figure 4, D and E) and is completed before the formation of the stellate structure of the TZ (Figure 4, F–H). This basal body is docked with the plasma membrane and the flagellar membrane can be resolved (fm; Figure 4, E–L). Beginning at the base of the proximal stellate structure (Figure 4E), links between each doublet microtubule and the flagellar membrane constitute the “ciliary necklace” (cn, asterisk; Gilula and Satir, 1972). The ciliary necklace extends all the way through the TZ region in Chlamydomonas and is observed in the most distal TZ section of this series (Figure 4H). Note that the stellate structure of the TZ region in this cell is fully included over three sections. Because each section is ∼60 nm thick, this TZ is ∼180 nm in length (Figure 4, F–H), similar to the length of a WT TZ, which typically measures between 150 and 175 nm (Figure 1A). The central pair microtubules of the flagellum are observed in sections distal to the TZ (Figure 4, J–L). The axoneme extends from the TZ of this basal body for at least eleven sections, spanning ∼660 nm in length, indicating assembly of a flagellum. Because the two flagellated basal bodies of C. reinhardtii are positioned approximately at a 90° angle relative to each other, both basal bodies of a single cell are never in an orientation conducive to simultaneous transverse sectioning. Thus, we were unable to determine whether both basal bodies of a single cell retained this normal transition of triplet to doublet microtubules.
Some Basal Bodies in uni2 Cells Retain Triplet Microtubules in the Transition Zone
A striking new ultrastructural defect was uncovered in serial sections through some basal bodies in uni2-3 cells. In WT cells, the triplet microtubules never extend past the distal end of the transitional fibers (Geimer and Melkonian, 2004). However, five of nine basal bodies from uni2-3 cells showed the aberrant presence of one or more triplet microtubules in the wall of the axoneme surrounding the TZ (Figure 4, M–X). The proximal fiber (pf, dark arrowhead) at the base of the basal body (Figure 4M) connects to triplet microtubule number eight and provides a marker for the numbering of triplet microtubules (Hoops and Witman, 1983; Geimer and Melkonian, 2004). In this series, the transitional fibers (Figure 4, P and Q) appear less pronounced than those in the uni2-3 basal body that assembles a flagellum (Figure 4, B and C). Although this basal body appears docked with the plasma membrane, the flagellar membrane is less pronounced and is malformed on the side including microtubule triplet numbers 1–3 (Figure 4, R–X). Five triplet microtubules are present at the proximal end of the TZ (Figure 4S). Over the length of the TZ, three of these triplet microtubules transition into doublet microtubules but two triplet microtubules remain at the distal end (Figure 4, S–U). The TZ in this series extends over at least six sections (Figure 4, R–V), which would span at least 360 nm in length and corresponds to a length approximately double that of a WT TZ. Microtubule dissociation and flagellar termination occur just distal to the TZ and a membrane plug appears to seal off the flagellar stub (Figure 4X).
Some Basal Bodies in uni1 Cells Retain Triplet Microtubules in the Transition Zone
In serial sections through seven uni1-1 cells in which all nine sets of microtubules from a single basal body could be resolved, four cells contained the normal arrangement of nine doublet microtubules in the TZ, whereas three cells contained numerous triplet microtubules in the TZ. A series of transverse sections through an aberrant basal body and TZ from the uni1-1 mutant is displayed in Figure 5, A–L. In this series, the proximal fiber and distal striated fiber are clearly discernable. The transitional fibers appear less pronounced than those in WT cells (Figure 5, D–F). This basal body is docked with the plasma membrane and the flagellar membrane appears well formed (Figure 5, F–L). Three triplet microtubules are retained at the base of the TZ (Figure 5G). Surprisingly, some triplet microtubules in the TZ of this series appear to form ciliary necklace links extending between the junction of the A- and B-tubule walls to the flagellar membrane (Figure 5, G and H). No link was observed at the junction of the B- and C-tubule walls. The TZ region of this cell extends for at least five sections (Figure 5, G–J), which would span at least 300 nm in length, nearly double the length of a WT TZ. Although only one triplet microtubule remains present in the most distal section in which all nine microtubules exist (Figure 5J), microtubule dissociation and flagellar termination occurs at the distal end of the TZ (Figure 5, K and L).
Failure of Flagellar Assembly Correlates with a Failure in Microtubule Transition
The basal body serial images, including seven series from uni1-1 cells and nine series from uni2-3 cells, allowed us to correlate flagellar assembly with defects in microtubule transition. Of the four uni2-3 basal bodies that displayed only doublet microtubules in the TZ, subsequent sections in three of the four series demonstrated that flagellar assembly occurred; sections through the other cell did not extend far enough to determine whether axonemal assembly occurred. Similarly, three of the four uni1-1 basal bodies with a normal arrangement of microtubules in the TZ were able to assemble a flagellum, whereas one was not. In contrast, basal bodies that failed to transition normally from triplet to doublet microtubules were uniformly defective in flagellar assembly. In all five uni2-3 basal bodies showing an abnormal transition, the number of triplets varied from between two and nine triplets at the proximal end of the TZ and between zero and nine triplet microtubules at the distal end. Subsequent sections through three cells showed that microtubule dissociation and flagellar termination occurred at the distal end of the TZ or at the base of the axoneme. Sections through the remaining two cells did not extend far enough to assess axonemal assembly. In three of the five cells with aberrant microtubule transition, the central pair of microtubules was able to form, although these microtubules were significantly mislocalized in one case. In the three uni1-1 basal bodies showing an abnormal transition of triplet to doublet microtubules, the number of triplets varied from between two and nine triplet microtubules at the proximal end of the TZ to between zero and nine triplet microtubules at the distal end of the TZ or disintegrating axoneme. Subsequent sections in each series showed that flagellar assembly did not occur. Microtubule termination and axonemal disintegration were observed at or near the distal end of the TZ.
Basal Bodies in uni1 uni2 Cells Retain Triplet Microtubules in the Transition Zone
The anomalous presence of triplet microtubules in and above the TZ is more pronounced in uni1-1 uni2-3 double mutant cells. Serial cross sections through eight basal bodies in which all nine sets of microtubules could be resolved, such as the one in Figure 5, M–X, showed multiple triplet microtubules in each TZ. In this series, the proximal fiber (Figure 5M) and distal striated fiber (Figure 5O) attached to this basal body do not reside in an orientation consistent with those previously displayed in either single mutant strain (Figures 4 and 5) or with that observed in WT strains (Hoops and Witman, 1983; Geimer and Melkonian, 2004). Thus, either the proximal fiber or the distal striated fiber is mislocalized on this basal body. Transitional fibers are less pronounced than those in a WT cell (Figure 5, P and Q). This basal body is likely docked with the plasma membrane; however, the flagellar membrane appears significantly malformed around the entire length of the TZ (Figure 5, R–X). Eight triplet microtubules are present at the base of the TZ (Figure 5Q) and remain throughout the entire length of the TZ, which spans at least 10 sections (Figure 5, Q–T). The length of this TZ is ∼600 nm, which is over three times the length of a typical TZ region in WT cells. Microtubule dissociation and flagellar termination occur at the distal end of the TZ (Figure 5X). In the eight basal bodies from double mutant cells, the number of triplet microtubules varied between two and nine at the proximal end of the TZ and between one and six at the distal end of the TZ. In five of these basal bodies, subsequent sections in each series demonstrated that central pair formation and flagellar assembly never occurred. Sections through the remaining three cells did not extend far enough to determine whether axonemal assembly occurred.
Some common features were observed in uni1-1 and uni2-3 single and double mutant cells. In a total of 13 basal bodies, all showing an aberrant transition of triplet to doublet microtubules, serial sectioning allowed us to determine the rotational polarity of the affected microtubule(s). This analysis demonstrated that the specific triplet microtubules defective in the microtubule transitioning process were positioned randomly (Table 2). Further, the transition of a triplet microtubule to a doublet microtubule was never observed to reverse back to the triplet structure.
Table 2.
Rotational polarity of affected triplet microtubules at the proximal end of the TZ
| Mutant background | Affected triplet microtubule |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| uni1-1a | × | ||||||||
| uni1-1a,b | × | × | × | ||||||
| uni2-3c | × | × | |||||||
| uni2-3a | × | × | × | × | |||||
| uni2-3a,d | × | × | × | × | × | ||||
| uni2-3b,c | × | × | × | × | × | × | × | × | × |
| uni1-1 uni2-3a | × | × | |||||||
| uni1-1 uni2-3c | × | × | × | × | |||||
| uni1-1 uni2-3a | × | × | × | × | |||||
| uni1-1 uni2-3c | × | × | × | × | × | × | × | × | |
| uni1-1 uni2-3a | × | × | × | × | × | × | × | × | |
| uni1-1 uni2-3a,e | × | × | × | × | × | × | × | × | |
| uni1-1 uni2-3a | × | × | × | × | × | × | × | × | × |
| Total | 7 | 8 | 7 | 7 | 8 | 8 | 7 | 8 | 7 |
The Uni2 Protein Localizes to the Region of Microtubule Transition
We previously showed that the Uni2 protein is a component of both basal bodies and probasal bodies (Piasecki et al., 2008). Because the HA-tagged Uni2 protein colocalizes with basal bodies and probasal bodies during mitosis when the cells have resorbed flagella and TZs (Cavalier-Smith, 1974; Gould, 1975), we proposed that the Uni2 protein localizes to the basal body in a region proximal to the TZ. Although immunofluorescence microscopy of interphase cells consistently shows staining of four distinct spots in the expected location of basal bodies and probasal bodies, the preembedding immunogold labeling method confirmed the localization of Uni2 protein only to probasal bodies (Piasecki et al., 2008).
The unique ultrastructural phenotypes of the uni1-1 and uni2-3 single and double mutants emphasized the need to determine the specific localization of the Uni2 protein on basal bodies. In this study, we used a post embedding strategy with fixation modeled after conditions that consistently have proven successful for fluorescence microscopy (Sanders and Salisbury, 1995; Silflow et al., 2001; Piasecki et al., 2008). Although the overall ultrastructural preservation was reduced using this method, it allowed us to detect the localization of the Uni2 protein on basal bodies in intact cells. Thin sections from the uni2-3 mutant cells rescued to WT with the HA-tagged UNI2 gene were labeled with an antibody against the HA epitope. Both labeled and unlabeled control sections were then labeled with 12-nm gold–conjugated secondary antibodies.
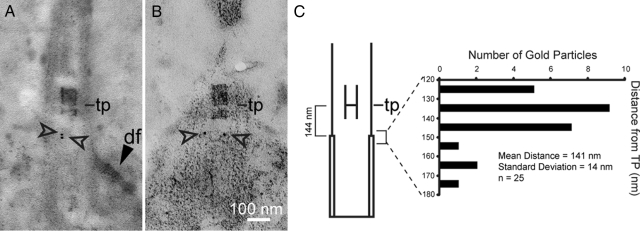
Gold staining at the distal end of the basal body was detected in 26 longitudinal sections through the basal body, TZ, and flagellum (Figure 6, A and B). Among these images, 17 showed no additional staining outside the basal body structure; background gold particles in the other images did not consistently demarcate any other discernable feature. Control sections labeled with secondary antibody alone did not contain gold particles on any specific region of basal bodies (data not shown). The localization of gold particles was determined with respect to the transitional plate (tp), a highly consistent morphological feature separating the distal and proximal cylinders of the TZ (Figures 1A and 6C; Ringo, 1967; Cavalier-Smith, 1974; Geimer and Melkonian, 2004). In 23 of 26 basal body images showing gold particles, we could resolve the transitional plate. In each of these images, a line was drawn parallel to the length of the basal body between the transitional plate and each gold particle. The mean distance between the transitional plate and a gold particle was 140.7 nm (SD = 13.7; Figure 6C).
Figure 6.
Postembedment immunogold electron microscopy of whole cells reveals the localization of the Uni2 protein at the distal end of basal bodies. (A and B) Thin sections of uni2-3 mutant cells rescued to WT with an HA-tagged UNI2 gene (CC-4163) were labeled with rat anti-HA antibodies and then labeled with secondary goat anti-rat antibodies conjugated with 12-nm gold particles. Gold particles are indicated by open arrowheads. The distal striated fiber (df, dark arrowhead) attaches to one side of the basal body. (C) The transitional plate (tp) provided a reference point for quantifying the localization of gold particles. The position of each gold particle with respect to the position of the transitional plate was determined (right bracket and bar graph). The position of the distal end of the triplet microtubules with respect to the transitional plate (left bracket) was determined from six basal body images similar to the one in Figure 1A.
In median longitudinal sections through basal bodies, triplet microtubules can be distinguished from doublet microtubules by the presence of projections extending from triplet microtubules into the basal body lumen (solid arrow, Figure 1A; Geimer and Melkonian, 2004). Although this structural feature was not resolved in the immunogold-labeled images, we used images of WT basal bodies from conventional fixation and embedment methods such as the one in Figure 1A to determine the distance between the transitional plate and the distal end of triplet microtubules (the site of triplet to doublet microtubule transition). Measurements of six such WT basal body images provided a distance between the transitional plate and the distal end of the triplet microtubule of 143.7 nm (SD = 10.6; Figure 6C). We conclude that the Uni2 protein localizes near the site of microtubule transition.
The Vfl1 protein was shown to localize at the distal end of the basal body but in a rotationally asymmetrical pattern, near the side of the basal body closest to the distal striated fiber (Silflow et al., 2001). We looked for rotational asymmetry in the position of the Uni2 protein. In five gold-labeled basal bodies with a distal striated fiber, the gold particles were cis to the distal striated fiber and three were trans to the distal striated fiber. Thus, a rotationally asymmetric localization of the Uni2 protein seems unlikely. Fixation conditions did not allow us to resolve the structures of transverse sections of basal bodies in this material, preventing further analysis of rotational positioning of the gold particles.
DISCUSSION
The transition from triplet microtubules to doublet microtubules between the basal body and flagellum is an essential process for flagellar formation. In 11 series of transverse sections that contained one or more triplet microtubules in the TZ, including those from uni1 and uni2 single and double mutant cells, we did not observe flagellar formation. However, in both the uni1 and uni2 single mutant strains, each of which contain a high percentage of uniflagellate cells, flagellar formation was almost always associated with basal bodies in which the transition of triplet to doublet microtubules was completed before assembly of the TZ. These data both implicate the uni1 and uni2 genes in the process of microtubule transitioning and provide a functional explanation for why the uni1 and uni2 mutants sometimes fail to form flagella.
The uni1 and uni2 mutations preferentially affect assembly of flagella from the younger basal body located cis to the eyespot (Huang et al., 1982; Dutcher and Trabuco, 1998; Piasecki et al., 2008). Although the alignment of the basal body apparatus makes it impossible to obtain transverse sections from both basal bodies from a single cell, our results demonstrate a correlation between the ability to transition triplet to doublet microtubules before forming the TZ and the ability to nucleate flagellar assembly. Thus, it is likely that in the uni1 and uni2 mutant cells, the younger basal body is less competent to transition from triplet to doublet microtubules, suggesting that the pathway of microtubule transition is interrelated with the basal body development pathway.
The UNI1 and UNI2 genes likely function in the same pathway. A synthetic phenotype with apparently additive defects in flagellar assembly is observed in uni1 uni2 double mutant cells. Our results confirm the report of Dutcher and Trabuco (1998) of the enhanced aflagellate phenotype of the uni1 uni2-double mutant. Although 50% or more of the cells in each single mutant population can assemble one or more flagella, only 1% of double mutant cells are able to assemble a flagellum. Longitudinal sections through basal bodies in the uni1-1 or uni2-3 mutant cells revealed elongated or multiplied and stacked TZs, a defect that is more severe in double mutant cells. A defect in docking of basal bodies at the plasma membrane is more severe in double mutant cells compared with either single mutant strain. Transverse serial sections demonstrated that a specific ultrastructural defect, the inability to transition triplet to doublet microtubules, was present in both the uni1-1 and uni2-3 single mutants and was significantly more pronounced in the uni1-1 uni2-3 double mutant. The Uni2 protein shows a greatly reduced level of phosphorylation in uni1-1 mutant cells, indicating that phosphorylation requires the function of the UNI1 gene. These results, together with localization of the Uni2 protein at the distal end of basal bodies where the triplet to doublet microtubule transition occurs, suggest that the UNI1 and UNI2 genes work in a process to block elongation of the C-tubule. Determining the biochemical basis of any gene interaction will require identification of the UNI1 gene.
Phosphorylation of the Uni2 protein appears not to be required for localization of the Uni2 protein to basal bodies and probasal bodies. In this study we demonstrated that the Uni2 protein localizes to both basal bodies and probasal bodies in uni1 mutant cells, which show over a 10-fold reduction in the phosphorylation level of the Uni2 protein. Consistent with this observation are results from our previous analysis of Uni2 protein expression during the cell division cycle (Piasecki et al., 2008). In preparation for cell division, cells resorb the entire length of both flagella, including TZ regions (Gaffal, 1988). Probasal bodies elongate, resulting in two basal body pairs which then segregate with the spindle poles as new probasal bodies are assembled during mitosis. At the completion of multiple rounds of mitotic division, the daughter cells reassemble TZs and flagella for swimming out of the mother cell wall. We demonstrated that the unphosphorylated isoform of the Uni2 protein is up-regulated first during mitosis as new Uni2 protein foci were detected in association with probasal body assembly (Piassecki et al., 2008). We found that phosphorylation of the Uni2 protein occurs at the end of the mitotic cycles as daughter cells assemble TZs and flagella.
The results from analysis of Uni2 protein accumulation also provide some insight into possible roles for the UNI1 and UNI2 genes in the basal body development pathway. Although the Uni2 phosphoisoform does not increase in abundance until the end of the cell division period, the same total level of phosphorylated Uni2 protein present in a late interphase cell remains throughout the division period (Piasecki et al., 2008). We suggested a model in which the accumulation of the Uni2 phosphoprotein on a basal body occurs over successive dark-phase periods. On accumulation of a threshold level of Uni2 phosphoprotein, a basal body becomes competent for flagellar assembly. This model predicts that the older basal body would contain a higher level of the phosphoprotein, making it more competent for transition of triplet to doublet microtubules. In this way, the sequential accumulation of Uni2 phosphoprotein becomes part of the basal body development cycle.
Although the Uni2 protein functions in the transition of triplet to doublet microtubules, it is unclear whether it requires triplet microtubules for binding to basal bodies and probasal bodies. Soon after their assembly during mitosis, probasal bodies in Chlamydomonas cells acquire triplet microtubules (Weiss, 1984; Gaffal, 1988) and the Uni2 protein (Piasecki et al., 2008), but we have not yet resolved the order of these two events. Mutation of the UNI3 gene results in basal bodies assembled primarily with doublet microtubules, although some truncated triplet microtubules were observed at the proximal and distal ends (O'Toole et al., 2003; Fromherz et al., 2004). The UNI3 gene encodes a variant tubulin known as δ-tubulin (Dutcher and Trabuco, 1998). We found that the uni3 mutation did not affect Uni2 phosphorylation or prevent its localization to basal bodies and probasal bodies. It is possible that truncated triplet microtubules at the distal end of basal bodies in the uni3-1 mutant may be sufficient for the localization of the Uni2 protein. Alternatively, we cannot exclude the possibility that the association of the Uni2 protein with basal bodies and probasal bodies may not require triplet microtubules.
Previously published ultrastructural analyses have demonstrated that the transition of triplet to doublet microtubules occurs in all organisms at or around the transitional fibers or their analogous metazoan structures, termed distal appendages (for example see Albrecht-Buehler and Bushnell, 1980; Vorobjev and Chentsov, 1980, 1982; Paintrand et al., 1992; Geimer and Melkonian, 2004). Our work emphasizes the importance of the microtubule transition process occurring in this region. The transitional fibers in Chlamydomonas facilitate docking of the basal bodies at the plasma membrane (Weiss et al., 1977), a process that is defective for ∼25% of basal bodies in uni1 uni2 double mutant cells. Some part of the failure to assemble flagella in these cells can be attributed to this failure in basal body positioning.
In addition to their role in basal body docking, transitional fibers have been implicated in flagellar assembly. Intraflagellar transport (IFT), a bidirectional transport process that mediates flagellar assembly and maintenance, is conserved from algae to humans (for reviews see Rosenbaum and Witman, 2002; Cole, 2003; Scholey, 2008; Pedersen et al., 2008). Anterograde IFT is driven by kinesin-2 motor proteins while retrograde IFT depends on cytoplasmic dynein 1b. The motor proteins move along the axonemal doublet microtubules, transporting protein complexes (IFT particles) containing at least 17 different polypeptides. The protein precursors required for IFT-mediated flagellar formation reside in a pool around the distal end of the basal body in stoichiometrically greater amounts than reside in the flagellum itself (for example see Cole et al., 1998; Pazour et al., 1999; Vashishtha et al., 1996; Deane et al., 2001). The Chlamydomonas transitional fibers were proposed to demarcate the docking site for IFT based on the localization of the IFT52 protein primarily to this region (Deane et al., 2001). Because the movement of IFT particles in both directions occurs on the B-tubule of axonemal doublet microtubules (Kozminski et al., 1993), the discontinuation of the C-tubule may be essential for the docking of the IFT machinery on the B-tubule. This requirement may provide a functional explanation for the evolutionary conservation of a precise point for conversion of triplet microtubules to doublet microtubules at the end of the centriole.
Supplementary Material
ACKNOWLEDGMENTS
Staff in the Imaging Center, University of Minnesota, provided serial sectioning (Gail J. Celio) and technical advice (Mark A. Sanders and Gilbert G. Ahlstrand). Batch deconvolution was conducted using The Supercomputing Institute, University of Minnesota. William L. Dentler, University of Kansas, helped us to assess the electron micrographs. Paul A. Lefebvre and Lai-Wa Tam, University of Minnesota, critically reviewed the manuscript. We thank Richard W. Link, University of Minnesota, for helpful comments. This work was supported by the National Science Foundation Grant MCB-0344661 to C.D.S. and a Fellowship from the Microbial and Plant Genomics Institute at The University of Minnesota to B.P.P. Susan K. Dutcher, Washington University, provided the uni3-1 strain. We thank Matthew LaVoie for assistance with SDS-PAGE and immunoblotting. The College of Biological Sciences Imaging Center, University of Minnesota, provided facilities and expertise for electron microscopy.
Abbreviations used:
- IFT
intraflagellar transport
- TZ
transition zone
- WT
wild-type.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E08-09-0900) on November 12, 2008.
REFERENCES
- Afzelius B. A. Cilia-related diseases. J. Pathol. 2004;204:470–477. doi: 10.1002/path.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht-Buehler G., Bushnell A. The ultrastructure of primary cilia in quiescent 3T3 cells. Exp. Cell Res. 1980;126:427–437. doi: 10.1016/0014-4827(80)90282-7. [DOI] [PubMed] [Google Scholar]
- Beech P. L., Heimann K., Melkonian M. Development of the flagellar apparatus during the cell cycle in unicellular algae. Protoplasma. 1991;164:23–37. [Google Scholar]
- Cavalier-Smith T. Basal body and flagellar development during the vegetative cell cycle and the sexual cycle of Chlamydomonas reinhardtii. J. Cell Sci. 1974;16:529–556. doi: 10.1242/jcs.16.3.529. [DOI] [PubMed] [Google Scholar]
- Cavalier-Smith T. The phagotrophic origin of eukaryotes and phylogenetic classification of Protozoa. Int. J. Syst. Evol. Microbiol. 2002;52:297–354. doi: 10.1099/00207713-52-2-297. [DOI] [PubMed] [Google Scholar]
- Christensen S. T., Pedersen L. B., Schneider L., Satir P. Sensory cilia and integration of signal transduction in human health and disease. Traffic. 2007;2:97–109. doi: 10.1111/j.1600-0854.2006.00516.x. [DOI] [PubMed] [Google Scholar]
- Cole D. G. The intraflagellar transport machinery of Chlamydomonas reinhardtii. Traffic. 2003;4:435–442. doi: 10.1034/j.1600-0854.2003.t01-1-00103.x. [DOI] [PubMed] [Google Scholar]
- Cole D. G., Diener D. R., Himelblau A. L., Beech P. L., Fuster J. C., Rosenbaum J. L. Chlamydomonas kinesin-II-dependent intraflagellar transport (IFT): IFT particles contain proteins required for ciliary assembly in Caenorhabditis elegans sensory neurons. J. Cell Biol. 1998;141:993–1008. doi: 10.1083/jcb.141.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dammermann A., Müller-Reichert T., Pelletier L., Habermann B., Desai A., Oegema K. Centriole assembly requires both centriolar and percentriolar material proteins. Dev. Cell. 2004;7:815–829. doi: 10.1016/j.devcel.2004.10.015. [DOI] [PubMed] [Google Scholar]
- Deane J. A., Cole D. G., Seeley E. S., Diener D. R., Rosenbaum J. L. Localization of intraflagellar transport protein IFT52 identifies basal body transitional fibers as the docking site for IFT particles. Curr. Biol. 2001;11:1586–1590. doi: 10.1016/s0960-9822(01)00484-5. [DOI] [PubMed] [Google Scholar]
- Dutcher S. K., Morrissette N. S., Preble A. M., Rackley C., Stanga J. ε-tubulin is an essential component of the centriole. Mol. Biol. Cell. 2002;13:3859–3869. doi: 10.1091/mbc.E02-04-0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutcher S. K., Trabuco E. C. The UNI3 gene is required for assembly of basal bodies of Chlamydomonas and encodes δ-tubulin, a new member of the tubulin superfamily. Mol. Biol. Cell. 1998;9:1293–1308. doi: 10.1091/mbc.9.6.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromherz S., Giddings T. H., Jr., Gomex-Ospina N., Dutcher S. K. Mutations in α-tubulin promote basal body maturation and flagellar assembly in the absence of δ-tubulin. J. Cell Sci. 2004;117:303–314. doi: 10.1242/jcs.00859. [DOI] [PubMed] [Google Scholar]
- Gaffal K. P. The basal body-root complex of Chlamydomonas reinhardtii during mitosis. Protoplasma. 1988;143:139–148. [Google Scholar]
- Geimer S., Melkonian M. The ultrastructure of the Chlamydomonas reinhardtii basal apparatus: identification of an early marker of radial asymmetry inherent in the basal body. J. Cell Sci. 2004;117:2663–2674. doi: 10.1242/jcs.01120. [DOI] [PubMed] [Google Scholar]
- Gilula N. B., Satir P. The ciliary necklace. A ciliary membrane specialization. J. Cell Biol. 1972;53:494–509. doi: 10.1083/jcb.53.2.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould R. R. The basal bodies of Chlamydomonas reinhardtii. Formation from probasal bodies, isolation, and partial characterization. J. Cell Biol. 1975;65:65–74. doi: 10.1083/jcb.65.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes J. A., Dutcher S. K. Cellular asymmetry in Chlamydomonas reinhardtii. J. Cell Sci. 1989;94:273–285. doi: 10.1242/jcs.94.2.273. [DOI] [PubMed] [Google Scholar]
- Hoops H. J., Witman G. B. Outer doublet heterogeneity reveals structural polarity related to beat direction in Chlamydomonas flagella. J. Cell Biol. 1983;97:902–908. doi: 10.1083/jcb.97.3.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B., Ramanis Z., Dutcher S. K., Luck D. J. Uniflagellar mutants of Chlamydomonas: evidence for the role of basal bodies in transmission of positional information. Cell. 1982;29:745–753. doi: 10.1016/0092-8674(82)90436-6. [DOI] [PubMed] [Google Scholar]
- Kathir P., LaVoie M., Brazelton W. J., Haas N. A., Lefebvre P. A., Silflow C. D. Molecular map of the Chlamydomonas reinhardtii nuclear genome. Eukaryot. Cell. 2003;2:362–379. doi: 10.1128/EC.2.2.362-379.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindle K. L. High-frequency nuclear transformation of Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. USA. 1990;87:1228–1232. doi: 10.1073/pnas.87.3.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozminski K. G., Johnson K. A., Forscher P., Rosenbaum J. L. A motility in the eukaryotic flagellum unrelated to flagellar beating. Proc. Natl. Acad. Sci. USA. 1993;90:5519–5523. doi: 10.1073/pnas.90.12.5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leidel S., Delattre M., Cerutti L., Baumer K., Gonczy P. SAS-6 defines a protein family required for centrosome duplication in C. elegans and in human cells. Nat. Cell Biol. 2005;7:115–125. doi: 10.1038/ncb1220. [DOI] [PubMed] [Google Scholar]
- Levine R. P., Ebersold W. T. The genetics and cytology of Chlamydomonas. Annu. Rev. Microbiol. 1960;14:197–216. doi: 10.1146/annurev.mi.14.100160.001213. [DOI] [PubMed] [Google Scholar]
- Linck R. W., Stephens R. E. Functional protofilament numbering of ciliary, flagellar, and centriolar microtubules. Cell Motil. Cytoskelet. 2007;64:489–495. doi: 10.1002/cm.20202. [DOI] [PubMed] [Google Scholar]
- Marshall W. F. The cell biological basis of ciliary disease. J. Cell Biol. 2008;180:17–21. doi: 10.1083/jcb.200710085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura K., Lefebvre P. A., Kamiya R., Hirono M. Bld10p, a novel protein essential for basal body assembly in Chlamydomonas: localization to the cartwheel, the first ninefold symmetrical structure appearing during assembly. J. Cell Biol. 2004;165:663–671. doi: 10.1083/jcb.200402022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant S. S., et al. The Chlamydomonas genome reveals the evolution of key animal and plant functions. Science. 2007;318:245–250. doi: 10.1126/science.1143609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa Y., Hiraki M., Kamiya R., Hirono M. SAS-6 is a cartwheel protein that establishes the 9-fold symmetry of the centriole. Curr. Biol. 2007;17:2169–2174. doi: 10.1016/j.cub.2007.11.046. [DOI] [PubMed] [Google Scholar]
- O'Toole E. T., Giddings T. H., McIntosh J. R., Dutcher S. K. Three-dimensional organization of basal bodies from wild-type and δ-tubulin deletion strains of Chlamydomonas reinhardtii. Mol. Biol. Cell. 2003;14:2999–3012. doi: 10.1091/mbc.E02-11-0755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paintrand M., Moudjou M., Delacroix H., Bornens M. Centrosome organization and centriole architecture: their sensitivity to divalent cations. J. Struct. Biol. 1992;108:107–128. doi: 10.1016/1047-8477(92)90011-x. [DOI] [PubMed] [Google Scholar]
- Pazour G. J., Dickert B. L., Witman G. B. The DHC1b (DHC2) isoform of cytoplasmic dynein is required for flagellar assembly. J. Cell Biol. 1999;144:473–481. doi: 10.1083/jcb.144.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen L. B., Veland I. R., Schroder J. M., Christensen S. T. Assembly of primary cilia. Dev. Dyn. 2008;237:1993–2006. doi: 10.1002/dvdy.21521. [DOI] [PubMed] [Google Scholar]
- Piasecki B. P., Lavoie M., Tam L. W., Lefebvre P. A., Silflow C. D. The Uni2 phosphoprotein is a cell cycle-regulated component of the basal body maturation pathway in Chlamydomonas reinhardtii. Mol. Biol. Cell. 2008;19:262–273. doi: 10.1091/mbc.E07-08-0798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringo D. L. Flagellar motion and fine structure of the flagellar apparatus in Chlamydomonas. J. Cell Biol. 1967;33:543–571. doi: 10.1083/jcb.33.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum J. L., Witman G. B. Intraflagellar transport. Nat. Rev. Mol. Cell Biol. 2002;3:813–825. doi: 10.1038/nrm952. [DOI] [PubMed] [Google Scholar]
- Sager R., Granick S. Nutritional studies with Chlamydomonas reinhardtii. Ann. NY Acad. Sci. 1953;56:831–838. doi: 10.1111/j.1749-6632.1953.tb30261.x. [DOI] [PubMed] [Google Scholar]
- Sanders M. A., Salisbury J. L. Immunofluorescence microscopy of cilia and flagella. Methods Cell Biol. 1995;47:163–169. doi: 10.1016/s0091-679x(08)60805-5. [DOI] [PubMed] [Google Scholar]
- Sato T. A modified method for lead staining of thin sections. J. Electron Microsc. 1968;17:158–159. [PubMed] [Google Scholar]
- Scholey J. M. Intraflagellar transport motors in cilia: moving along the cell's antenna. J. Cell Biol. 2008;180:23–29. doi: 10.1083/jcb.200709133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silflow C. D., LaVoie M., Tam L. W., Tousey S., Sanders M., Wu W., Borodovsky M., Lefebvre P. A. The Vfl1 Protein in Chlamydomonas localizes in a rotationally asymmetric pattern at the distal ends of the basal bodies. J. Cell Biol. 2001;153:63–74. doi: 10.1083/jcb.153.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sizova I., Fuhrmann M., Hegemann P. A streptomyces rimosus aph VIII gene coding for a new type of phosphotransferase provides stable antibiotic resistance to Chlamydomonas reinhardtii. Gene. 2001;277:221–229. doi: 10.1016/s0378-1119(01)00616-3. [DOI] [PubMed] [Google Scholar]
- Tam L. W., Lefebvre P. A. Cloning of flagellar genes in Chlamydomonas reinhardtii by DNA insertional mutagenesis. Genetics. 1993;135:375–384. doi: 10.1093/genetics/135.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vashishtha M., Walther Z., Hall J. L. The kinesin-homologous protein encoded by the Chlamydomonas FLA10 gene is associated with basal bodies and centrioles. J. Cell Sci. 1996;109:541–549. doi: 10.1242/jcs.109.3.541. [DOI] [PubMed] [Google Scholar]
- Vorobjev I. A., Chentsov Y. S. The ultrastructure of centriole in mammalian tissue culture cells. Cell Biol. Int. Rep. 1980;4:1037–1044. doi: 10.1016/0309-1651(80)90177-0. [DOI] [PubMed] [Google Scholar]
- Vorobjev I. A., Chentsov Y. S. Centrioles in the cell cycle. I. Epithelial cells. J. Cell Biol. 1982;93:938–949. doi: 10.1083/jcb.93.3.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R. L. Ultrastructure of the flagellar roots in Chlamydomonas gametes. J. Cell Sci. 1984;67:133–143. doi: 10.1242/jcs.67.1.133. [DOI] [PubMed] [Google Scholar]
- Weiss R. L., Goodenough D. A., Goodenough U. W. Membrane particle arrays associated with the basal body and with contractile vacuole secretion in Chlamydomonas. J. Cell Biol. 1977;72:133–143. doi: 10.1083/jcb.72.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.