Abstract
Endogenously produced nitric oxide synthase inhibitor, asymmetric methylarginine (ADMA) is associated with vascular dysfunction and endothelial leakage. We studied the role of ADMA, and the enzymes metabolizing it, dimethylarginine dimethylaminohydrolases (DDAH) in the regulation of endothelial barrier function in pulmonary macrovascular and microvascular cells in vitro and in lungs of genetically modified heterozygous DDAHI knockout mice in vivo. We show that ADMA increases pulmonary endothelial permeability in vitro and in in vivo and that this effect is mediated by nitric oxide (NO) acting via protein kinase G (PKG) and independent of reactive oxygen species formation. ADMA-induced remodeling of actin cytoskeleton and intercellular adherens junctions results from a decrease in PKG-mediated phosphorylation of vasodilator-stimulated phosphoprotein (VASP) and a subsequent down-regulation of Rac1 activity. The effects of ADMA on endothelial permeability, Rac1 activation and VASP phosphorylation are prevented by overexpression of active DDAHI and DDAHII, whereas inactive DDAH mutants have no effect. These findings demonstrate for the first time that ADMA metabolism critically determines pulmonary endothelial barrier function by modulating Rac1-mediated remodeling of the actin cytoskeleton and intercellular junctions.
INTRODUCTION
The endothelium constitutes the inner lining of blood vessels and regulates exchange of fluids, macromolecules, and leukocytes between blood and interstitial tissues. Precise control of endothelial barrier function strongly depends on endothelial nitric oxide (NO) production, as both inhibition and excessive amounts of NO can induce vascular leakage (Predescu et al., 2005; Hatakeyama et al., 2006). Methylarginines such as the guanidino-methylated arginine analogue N(G)-mono-methyl-l-arginine (L-NMMA) or asymmetric dimethylarginine (ADMA) are naturally occurring molecules that inhibit the activity of the enzymes responsible for NO production, nitric oxide synthases (NOS; Vallance and Leiper, 2004). ADMA is a cardiovascular risk factor and its plasma levels are increased in conditions associated with endothelial dysfunction and vascular leakage such as diabetes, atherosclerosis, pulmonary and systemic hypertension, hypercholesterolemia, hyperglycemia and heart failure (Vallance and Leiper, 2004; Cooke, 2005). Symmetric dimethylarginine (SDMA) does not act as an inhibitor of NOS (Vallance and Leiper, 2004). The level of methylarginines is controlled in part by the enzymes dimethylarginine dimethylaminohydrolases I and II (DDAH I and II; Vallance and Leiper, 2004). Analysis of methylarginine metabolism in the cardiovascular system has identified the lung as a major source of ADMA (Bulau et al., 2007). However, the role of this prevailing methylarginine in vivo, in the regulation of pulmonary endothelial barrier function is not known.
Methylarginines reduce synthesis of NO. Reduction of NO generation in vivo induces microvessel leakage in pulmonary circulation of eNOS−/− mice treated with the NOS inhibitor L-NAME [N(G)-nitro-l-arginine methyl ester; Predescu et al., 2005] and in the isolated perfused rabbit lung after NOS inhibition (Mundy and Dorrington, 2000), but the mechanisms are unclear. NOS inhibitors appear to have a direct effect on endothelial barrier function in vivo (Predescu et al., 2005), but their actions may also involve indirect, secondary mechanisms such as increased neutrophil adhesion (Rumbaut et al., 2000). In addition to inhibition of NO production, it has been proposed that ADMA might uncouple NOS leading to superoxide production (Cardounel et al., 2005). Oxidative stress increases in many models of pulmonary hypertension and both increases (van Wetering et al., 2002) and decreases in ROS (Wojciak-Stothard et al., 2005) can disrupt endothelial barrier function.
We have recently shown that ADMA differentially regulates the activities of the known regulators of actin dynamics, Rho GTPases RhoA, Rac1, and Cdc42 in PAECs (Wojciak-Stothard et al., 2007). Rho GTPases are important in the regulation of endothelial barrier function. Activation of RhoA increases endothelial permeability by increasing endothelial actomyosin contractility and intercellular gap formation (van Nieuw Amerongen and van Hinsbergh, 2001). In contrast, Rac1 activity is required for the assembly, maintenance, and recovery of endothelial intercellular junctions (Waschke et al., 2004; Wojciak-Stothard et al., 2005). Cdc42 has been shown to promote junction recovery after thrombin treatment in human pulmonary arterial endothelial cells (Kouklis et al., 2004).
Downstream effectors of NO, cAMP-activated protein kinase A (PKA) and cGMP-activated protein kinase G (PKG) may also mediate the effects of methylarginines. PKA and PKG have been implicated in the control of endothelial permeability but, depending on the vascular bed, their effects have been either protective or detrimental (van Nieuw Amerongen and van Hinsbergh, 2002). Both kinases phosphorylate vasodilator-stimulated phosphoprotein (VASP), which links intercellular junction proteins and integrins with the actin cytoskeleton (Krause et al., 2003). Genetically altered mice have indicated a crucial role of VASP for vascular integrity (Furman et al., 2007) but the role of VASP phosphorylation in control of junctional integrity remains unclear. VASP phosphorylation by PKA was postulated to strengthen intercellular adhesions (Comerford et al., 2002), but others have shown that it may interfere with the assembly of cortical actin cytoskeleton and decrease intercellular adhesion (Benz et al., 2008). VASP phosphorylation by PKG has been shown to induce reorganization of endothelial cytoskeleton and enhance angiogenesis (Chen et al., 2008), but its effects on endothelial barrier function are not known. Interestingly, VASP localization and activity in endothelial cells is closely associated with Rac1 activation and junctional integrity (Schlegel et al., 2008).
We studied the role of ADMA metabolism in the regulation of endothelial permeability in cultured primary macrovascular and microvascular pulmonary endothelial cells in vitro and in mouse lungs in vivo. We also sought to elucidate the mechanism by which methylarginines affect the organization of junction-associated cytoskeleton and intercellular junctions in endothelial cells.
MATERIALS AND METHODS
Cell Culture
Porcine pulmonary artery endothelial cells (PAECs) were purchased from Cell Applications (San Diego, CA) and cultured as in Wojciak-Stothard et al. (2005, 2007). Mouse pulmonary microvascular cells (PMVECs) were isolated from peripheral parts of the lung of DDAHI heterozygous (HT) knockout mice, purified, and cultured as described in Wojciak-Stothard et al. (2007). PMVECs from normal wild-type (WT) littermates were used as controls. Heterozygous DDAHI knockout mice are described in Leiper et al. (2007).
The Use of Drugs
ADMA (100 μmol/l), SDMA (100 μmol/l), or L-NAME (1 mmol/l, Calbiochem, La Jolla, CA) were added to cell cultures for 24 h. S-nitroso-N-acetyl-d, l-penicillamine (SNAP; 10–100 μmol/l, Calbiochem); guanosine 3′,5′-cyclic monophosphate, 8-bromo-, sodium salt (Br-cGMP, Na; 500 μmol/l, Calbiochem); guanosine, 3′,5′-cyclic monophosphorothioate,8-(4-chlorophenylthio)-, Rp-Isomer, triethylammonium (Rp-8-pCPT-cGMPS, TEA; 100 nmol/l, Calbiochem); or the flavoprotein inhibitor diphenylene iodonium (DPI, 10 μmol/l; Calbiochem) were added to untreated cells or ADMA-treated cells 1 h before the end of experiment. The Rho kinase inhibitor, Y-27632 (Calbiochem, 5 μmol/l) was added to the cells 2 h before the end of experiment, and superoxide dysmutase mimic, Mn(III)tetrakis(1-methyl-4-pirydyl)porphyrin pentachloride (MnTMPyP; 50 μmol/l, Calbiochem,) or gap junction inhibitor, 18β-glycyrrhetinic acid (18β-GA, 10 μmol/l, Sigma, St. Louis, MO) were added to the cells together with ADMA and incubated for 24 h.
Plasmids and Transfection
WT GFP-VASP, S239A GFP-VASP, and S239D GFP-VASP were prepared as in Lindsay et al. (2007). Transfection was carried out using Amaxa Nucleofector system (Köln, Germany, program U-01). The cells were seeded at the density 3 × 104 cells/ml and studied 48 h after transfection.
Rho, Rac, and Cdc42 GTP-binding assays and nitrite determination were performed as described earlier (Wojciak-Stothard et al., 2007).
Construction of Recombinant Adenoviruses
Adenoviral vectors for mutant Rho GTPases, DDAHI, DDAHII, and inactive mutants of DDAHI and DDAHII were generated as described previously (Wojciak-Stothard et al., 2005, 2007). Inactive DDAH mutants have a Cys249 to Ser mutation in their active site, which completely inactivates the enzyme, but does not affect protein folding.
Transendothelial Permeability Assay In Vitro
PAECs were plated in Transwell-Clear chambers (3-μm pore size, 12-mm diameter; Costar Corning, Costar, High Wycombe, United Kingdom) at cell density of 1 × 104 cells/well, and grown till confluence. The cells were left untreated or were infected with recombinant adenoviruses to induce overexpression of DDAH or mutant Rho GTPases. Two hours after infection, ADMA was added to the upper and lower chambers of Transwell dishes and incubated with the cells for a further 24 h. Alternatively, PAECs were transfected with VASP mutants, plated at cell density of 3 × 104 cells/well, and left for 24 h to form a monolayer. After this time, ADMA was added to transfected cells for a further 24 h. For dextran flux measurements, FITC-dextran (molecular wt 42,000, 1 mg/ml, Sigma) was added to the upper chamber of Transwell dishes, and samples were taken from the lower compartment after a 1-h incubation, as previously described (Wojciak-Stothard et al., 2001, 2005). The amount of FITC-dextran was determined with a TECAN GeNios microplate reader (TECAN, Reading, United Kingdom) using an excitation wavelength of 485 nm and emission at 510 nm.
Lung Permeability In Vivo
All experiments were carried out under a Home Office License and conducted according to the Animal Scientific Procedures Act 1986. DDAH HT knockout mice have been characterized elsewhere (Leiper et al., 2007).
The lungs of anesthetized (Hypnorm [fentanyl and fluanisone 0.25 ml/kg] and midazolam 25 mg/kg ip) DDAHI HT knockout mice and WT littermate controls (five animals per group) were flushed via a catheter inserted through right ventricle in situ in the open chest with 5 ml PBS at a flow rate of 1.8 ml/min with a nonpulsatile pump (Masterflex model 7518-00) until it was blood free (Zhao et al., 1999). The lungs were then perfused with 10 ml of 0.16 mg/ml Evans Blue (Sigma) in PBS and with 10 ml PBS afterward. The left lung lobe were dissected, weighed, homogenized, and suspended in 2.5 ml formamide for extraction overnight at room temperature. The homogenates were then centrifuged at 12,000 × g, and the concentration of Evans blue in supernatants was quantified by dual wavelength spectrophotometric analysis at 620 and 740 nm (Moitra et al.2007). This method corrects the specimen's absorbance at 620 nm for the contaminating heme pigments. The Evans blue concentration was calculated from the standard curve with the use of the following formula: Corrected absorbance = Real absorbance at 620 − [1.1649 × absorbance at 740 + 0.004] (Moitra et al., 2007). The Evans blue concentration read in μg/ml was converted to μg/g wet weight of lungs using the dilution factor of the original homogenate.
Measurement of ROS
Confluent PAECs grown on 96-well plates were treated with various pharmacological agents as described before. Culture medium was replaced with prewarmed Krebs saline, pH. 7.4, with or without drugs and containing 2′,7′-dichlorofluorescein diacetate (DCFH-DA; 5 μmol/l, Calbiochem; Wojciak-Stothard et al., 2005). After a 1-h incubation, DCFH fluorescence was read at excitation wavelength of 485 and emission 530 nm on TECAN GeNios microplate reader. Alternatively, N-acetyl-3,7-dihydroxyphenoxasine (Amplex Red; 50 μmol/l, Molecular Probes, Eugene, OR) and horseradish peroxidase (1 U/ml, Sigma) were dissolved in Krebs saline and added to the cells for 1 h, and then fluorescence was read on the microplate reader at 530-nm excitation and 590-nm emission (Takano et al., 2002).
Immunofluorescence and localization of F-actin were analyzed as described elsewhere (Wojciak-Stothard et al., 2007). Mouse monoclonal anti-phospho-VASP (Ser239) antibody (Upstate Biotechnology, Lake Placid, NY) and rabbit polyclonal anti-VASP antibody (Chemicon International, Temecula, CA) were used at 1:100.
Determination of p-Ser239 VASP was carried out with immunoprecipitation of the protein with mouse monoclonal anti-phospho-VASP (Ser239) antibody preadsorbed on A-Sepharose beads followed by Western blotting as in Wojciak-Stothard et al. (2007).
Statistical Analysis
All the experiments were performed in triplicate. Data are presented as means ± SD. Comparisons between two groups were carried out with two-tailed Student's t test. When more than conditions were being compared, a one-way ANOVA test followed by Dunnett's post-test was used. Statistical significance was accepted for p < 0.05.
RESULTS
ADMA But Not SDMA Induces Cytoskeletal Remodelling, Dispersion of Intercellular Adherens Junctions, and Increased Endothelial Permeability in PAECs
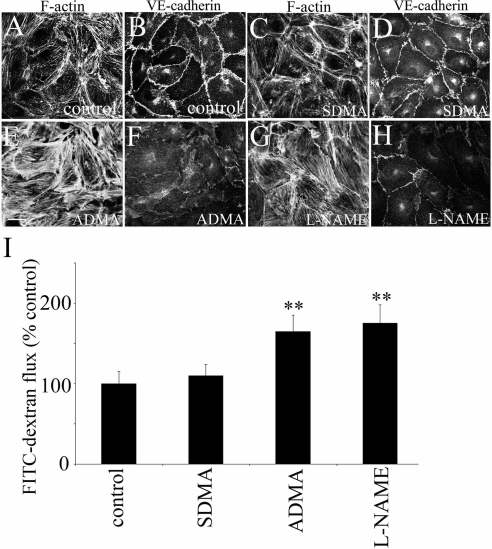
In confluent, control PAECs F-actin and VE-cadherin were predominantly localized in the cell periphery (Figure 1, A and B). NOS inhibitors, ADMA (100 μmol/l) and L-NAME (1 mmol/l) increased formation of stress fibers and induced dispersion of adherens junctions, whereas SDMA (100 μmol/l) had no effect (Figure 1, C–H). Consistent with their effects on intercellular junctions, ADMA and L-NAME, but not SDMA significantly increased endothelial permeability (160 ± 20 and 180 ± 15%, respectively, p < 0.01, comparison with controls; Figure 1I). The earliest effects of the inhibitors were noticed after 4 h, but the maximal effects were observed after 24-h incubation. The time- and dose-response permeability changes to ADMA and L-NAME are shown in Supplementary Figure S1. The basal rate of FITC-dextran passage through a confluent PAEC monolayer was 70 ng · h−1 · mm−2.
Figure 1.
ADMA and L-NAME but not SDMA increase endothelial permeability in vitro. (A, C, E, and G) Distribution of F-actin and VE-cadherin (B, D, F, and H) in PAECs treated with SDMA (100 μmol/l), ADMA (100 μmol/l), or L-NAME (1 mmol/l) for 24 h. Bar, 10 μm. (I) shows changes in endothelial permeability in PAECs treated with the inhibitors. ** p < 0.01, comparison with untreated controls, n = 5.
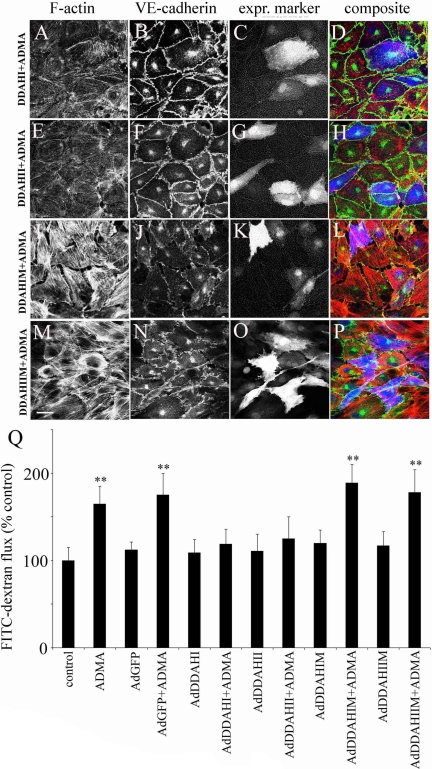
Overexpression of DDAH I and DDAHII did not affect endothelial phenotype or permeability in basal conditions but completely prevented the effects of ADMA (Figure 2, A–H and Q), whereas the inactive DDAH mutants had no significant effect (Figure 2, I–Q). As shown in Figure 2, A–H, changes induced by DDAH were also seen in cells in which expression of the recombinant protein was not detectable by fluorescence. To examine whether the effect of DDAH was passed onto the neighboring cells via diffusible factors present in medium, we studied permeability of PAECs grown on Transwell filters, whereas DDAH-overexpressing cells were grown on the bottom of the same Transwell chamber. In this system, overexpression of DDAHI and DDAHII did not significantly affect endothelial permeability with or without ADMA (Supplementary Figure S2A). To check if the intercellular communication plays a role, we cultured DDAHI-overexpressing cells in the presence of ADMA and a gap-junction inhibitor, 18β-GA. Inhibition of gap junctions significantly attenuated the effect of DDAHI on the ADMA-induced changes in endothelial permeability (Supplementary Figure S2B).
Figure 2.
DDAHI and DDAHII but not inactive DDAH mutants prevent ADMA-induced changes in the distribution of F-actin (A, E, I, and M), VE-cadherin (B, F, J, and N), and endothelial permeability (Q) in PAECs. (C, G, K, and O) Cells labeled with expression marker GFP; (D, H, L, and P) the corresponding composite images where F-actin is red, VE-cadherin is green, and GFP is blue (pseudocolors). Bar, 10 μm. DDAH and their inactive mutants were expressed via adenoviral gene transfer, and the cells were left untreated or were treated with 100 μM ADMA. * p < 0.05; ** p < 0.01, comparison with untreated controls, n = 5.
The Effects of ADMA on Endothelial Cytoskeletal Remodelling and Permeability Are Mediated by NO/PKG Pathway and Not Changes in ROS Levels
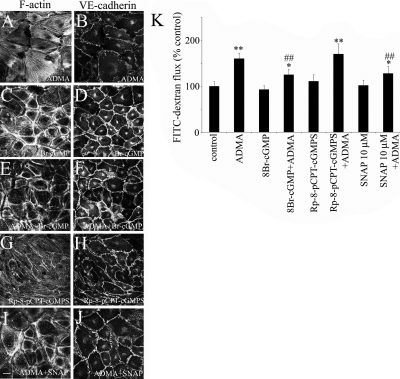
The NO donor SNAP (10 μmol/l) did not affect endothelial phenotype in control conditions, but prevented ADMA-induced effects (Figure 3, I, J, and L). However, higher concentrations of SNAP (>100 μmol/l) decreased endothelial barrier function, suggesting that an optimal level of NO is required (data not shown). Manipulation of PKG activity did not have a significant effect on cell morphology or permeability in basal conditions, though the PKG inhibitor, Rp-8-pCPT-cGMPS, induced a modest increase in stress fiber formation (Figure 3, G and K). The PKG activator, 8-Br-cGMP, significantly reduced the effects of ADMA on cell morphology and permeability (Figure 3, E, F, and K). Consistent with its function as NOS inhibitor, ADMA reduced nitrite levels in culture medium from 7 ± 1.3 to 3.5 ± 1.5 μmol/l (p < 0.01), the effect having been prevented by DDAHI and DDAHII. Changes in nitrite levels induced by ADMA and adenoviral overexpression of DDAH are shown in Supplementary Figure S3A, whereas changes induced by SNAP and L-NAME are shown in Supplementary Figure S3B.
Figure 3.
Activation of NO/PKG pathway prevents the effects of ADMA, whereas its inhibition mimics the effects of ADMA on the distribution of F-actin (A, C, E, G, and I), VE-cadherin (B, D, F, H, and J), and endothelial permeability (K) in PAECs. The cells were treated with ADMA for 24 h, and the drugs, SNAP (10–100 μmol/l), 8-Br-cGMP (500 μmol/l), and Rp-8-pCPT (100 nmol/l), were added 1 h before the end of the experiment, as described in Materials and Methods. * p < 0.05; ** p < 0.01, comparison with untreated controls. (K) ## p < 0.01, comparison with ADMA-treated cells. n = 5. Bar, 10 μm.
We studied ROS formation using two different, commonly used fluorescent probes, Amplex Red and DCFH-DA. Neither probe showed an increase in ROS formation after incubation of the cells with ADMA or L-NAME (Supplementary Figure S4). Amplex Red fluorescence was increased by H2O2 and menadione, a commonly used oxidant in a dose-response manner, showing that the probe was sensitive to ROS generation (Supplementary Figure S4). DCFH fluorescence was decreased by ADMA and substantially increased by SNAP, which suggests that this probe is not entirely specific for superoxide (Myhre et al., 2003) and may reflect the levels of intracellular NO (Imrich and Kobzik, 1997). Incubation of cells with a cell-permeable mimic of superoxide dismutase, MnTMPyP, did not prevent the effects of ADMA (Supplementary Figure S4), which suggests that the effects of ADMA in cultured PAECs were independent of ROS formation.
ADMA/NO/PKG-mediated Changes in the Activity of Rac1 Are Responsible for Remodelling of Endothelial Actin Cytoskeleton and Adherens Junctions and Changes in Endothelial Permeability
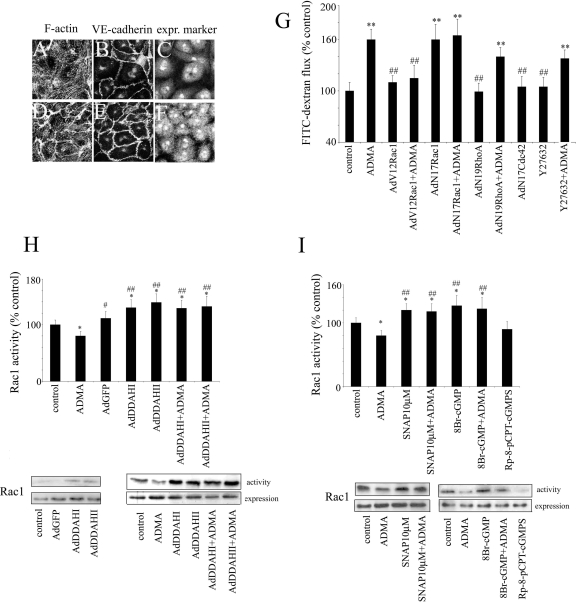
To verify the individual contributions of Rho GTPases to ADMA-induced effects, we overexpressed dominant negative and constitutively activated mutants of RhoA, Rac1, and Cdc42 in PAECs via adenoviral gene transfer.
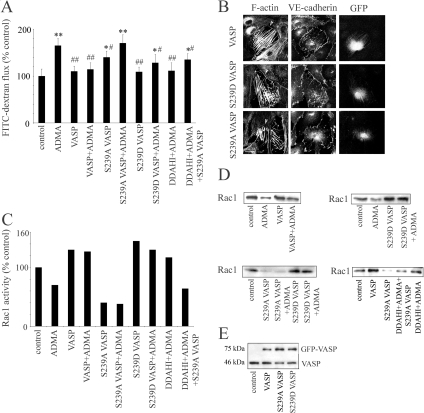
Dominant negative RhoA, N19RhoA reduced stress fiber formation (Figure 4, A–C) but did not significantly affect endothelial permeability in ADMA-treated cells (144 ± 15%; Figure 4G). Similarly, the inhibitor of Rho kinase, Y-27632, reduced stress fiber formation in cells (not shown) but did not prevent ADMA-induced endothelial leakage (140 ± 12%; Figure 4G). In contrast, the constitutively activated Rac1, V12Rac1, increased the levels of cortical F-actin, inhibited stress fiber formation and dispersion of intercellular junctions (Figure 4, D–F), and reduced endothelial permeability to control levels in ADMA-treated cells (Figure 4G). The dominant negative Rac1, N17Rac1, significantly increased endothelial permeability (160 ± 17%), consistent with previous reports (Wojciak-Stothard et al., 2005). This effect was not affected by ADMA, suggesting that inhibition of dextran flux by N17Rac1 was maximal. Rac1 activity was up-regulated by overexpression of DDAHI or DDAHII or by pretreatment with SNAP and 8-Br-cGMP (Figure 4, H and I), suggesting that NO/PKG are mediators in this regulation. DDAH, SNAP, and 8-Br-cGMP also restored Rac1 activity to control levels in cells treated with ADMA (Figure 4, H and I). The dominant negative Cdc42, N17Cdc42, did not affect endothelial permeability (Figure 4G).
Figure 4.
Rac1 but not RhoA or Cdc42 mediates the effects of ADMA on endothelial actin cytoskeleton (A and D), VE-cadherin (B and E), and endothelial permeability (G). (C and F) are corresponding images showing expression marker, c-myc. Cells in A–C were overexpressing N19RhoA in the presence of ADMA, whereas cells in D–F were overexpressing V12Rac1 in the presence of ADMA. Bar, 10 μm. (H) The graph shows the effects of recombinant DDAH on Rac1 activity; (I) the graph shows the effects of SNAP, 8Br-cGMP, and Rp-8-pCPT-cGMPS on Rac1 activity in untreated and ADMA-treated PAECs. Representative examples of Western blots are shown below the graphs. * p < 0.05; ** p < 0.01, comparison with untreated controls. # p < 0.05; ## p < 0.01, comparison with ADMA-treated cells. (G) n = 6; (H and I) n = 4. Adenoviral gene transfer was used to express recombinant Rho GTPases AdV12Rac1, AdN19RhoA, and AdN17Cdc42 as well as AdDDAHI, AdDDAHII, and inactive mutants AdDDAHIM, AdDDAHIIM.
p-Ser239 VASP Mediates the Effects of ADMA/DDAH on Endothelial Permeability and Rac1 Activation
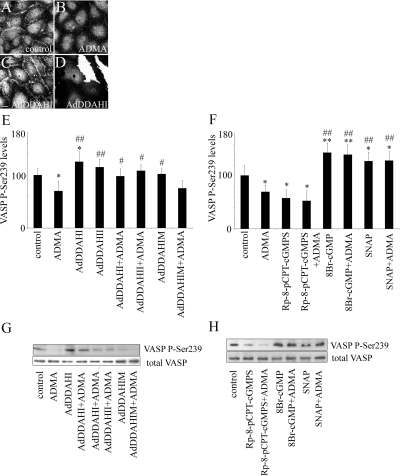
Ser239 phosphorylation of VASP correlates with barrier-enhancing effects of PKG (Draijer et al., 1995). We found that p-Ser239 VASP was concentrated in the junctional area in untreated PAECs (Figure 5A), whereas in ADMA-treated cells the staining was diffuse (Figure 5B). The levels of p-Ser239 VASP were increased in cells overexpressing DDAHI or DDAHII but not inactive DDAH mutants (Figure 5, C and E). We observed a substantial decrease in the levels of p-Ser239 VASP in PAECs treated with ADMA, which was prevented by active DDAHI and DDAHII (Figure 5, E and G). A decrease in VASP phosphorylation on Ser239 was also prevented by a 1-h incubation with an NO donor, SNAP, and a PKG activator, 8-Br-cGMP, whereas the PKG inhibitor, Rp-8-pCPT-cGMPS, mimicked the effects of ADMA (Figure 5, F and H).
Figure 5.
Distribution of p-Ser239 VASP in untreated cells (A), cells treated with ADMA (B), and cells overexpressing DDAHI (C). (D) A corresponding image showing expression marker, GFP. Bar, 10 μm. (E) Changes in the levels of p-Ser239 VASP in control and ADMA-treated cells, as well as cells overexpressing DDAH and their inactive mutants; n = 4. * p < 0.05, ** p < 0.01, comparison with untreated controls; #p < 0.05, ## p < 0.01, comparison with ADMA-treated cells. Western blots in G and H show levels of p-Ser239 VASP as well as total VASP in cells.
To address the contribution of VASP phosphorylation on Ser 239 in ADMA/DDAH-induced cytoskeleton-driven effects, we overexpressed WT GFP-VASP, the phosphomimetic mutant S239D GFP-VASP, and the nonphosphorylatable mutant S239A GFP-VASP in PAECs. WT VASP or S239D VASP did not have a significant effect on endothelial permeability in basal conditions, but significantly reduced endothelial leakage caused by ADMA (Figure 6A). In contrast, nonphosphorylatable mutant, S239A VASP increased basal endothelial permeability (124 ± 13%, p < 0.05, comparison with untreated controls) and attenuated the effects of DDAHI on ADMA-treated cells (Figure 6A). Consistent with VASP Thr278-phosphomimetic mutants (Blume et al., 2007), overexpression of all our VASP mutants induced formation of stress fibers in cells (Figure 6B), but only S239D VASP, and, to a lesser extent WT VASP increased cortical F-actin localization in the junctional area (Figure 6B, arrows). Overexpression of WT VASP and S239D VASP increased Rac1 activity in control PAECs (145 and 130% of controls, respectively), whereas S239A VASP reduced Rac1 activity to 40% of control levels (Figure 6, C and E). WT VASP and S239VASP prevented ADMA-induced reduction in Rac1 activity, whereas S239A VASP had no effect (Figure 6, C and E).
Figure 6.
Ser239 phosphorylation of VASP is required for the effects of DDAH on ADMA-treated cells. PAECs expressing WT GFP-VASP, the nonphosphorylatable mutant of VASP (S239A GFP-VASP), or the phosphomimetic mutant of VASP (S239D GFP-VASP) were left untreated, were treated with ADMA, or were overexpressed DDAHI. (A) Effects of VASP mutants on FITC-dextran flux in PAECs; n = 5. * p < 0.05, ** p < 0.01, comparison with untreated controls; # p < 0.05, ## p < 0.01, comparison with ADMA-treated cells. (B) The effects of VASP mutants on F-actin and VE-cadherin in recently confluent cells, as indicated. Bar, 10 μm. (C) Changes in Rac1 activity in PAECs overexpressing VASP mutants, whereas corresponding Western blots are shown in D. (E) Equal expression of VASP-GFP mutants in PAECs is shown.
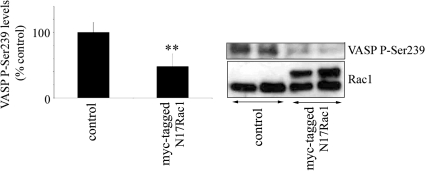
To test wheteher Rac can affect VASP in a reciprocal manner, we overexpressed dominant negative Rac1 (N17Rac1) in PAECs. N17Rac1 decreased the levels of p-Ser293 VASP in cells (Figure 7), suggesting the existence of a feedback regulatory mechanism.
Figure 7.
Rac1 inhibition decreases the levels of p-Ser239 VASP. VASP phosphorylation was studied in untreated PAECs and PAECs overexpressing N17Rac1. n = 4. ** p < 0.01.
DDAHI Gene Knockout Increases Pulmonary Endothelial Permeability In Vivo and In Vitro and Decreases Rac1 Activity and Ser-239 Phosphorylation of VASP.
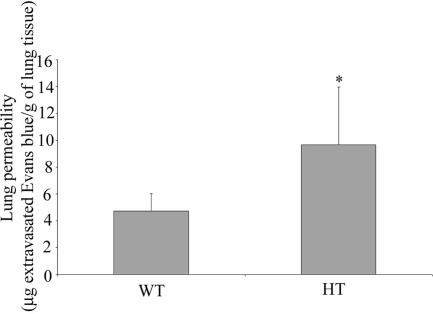
DDAHI HT knockout mice show increased plasma and tissue levels of ADMA (Leiper et al., 2007). We observed that vascular permeability in lungs of DDAH knockout mice was significantly higher than in WT mice (Figure 8). During perfusion, the lungs of DDAHI HT knockout mice accumulated 9.6 ± 4.3 μg Evans blue/g of wet lung weight, whereas lungs of WT mice accumulated 4.72 ± 1.2 μg Evans blue/g of wet lung weight (p < 0.05).
Figure 8.
DDAHI deficiency induces endothelial leakage in mouse lungs in vivo. The lungs of DDAHI heterozygous (HT) knockout mice and their wild-type littermates (WT) were perfused free of blood in situ with Evans Blue in PBS. The accumulation of the dye in the lung was quantified by dual wavelength spectrophotometric analysis at 620 and 740 nm (Moitra et al., 2007). * p < 0.05.
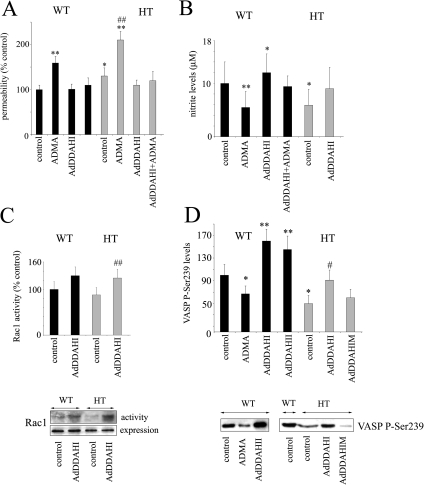
Consistently, cultured pulmonary microvascular cells from DDAH knockout mice showed an increase in basal permeability (130 ± 18% of control value, p < 0.05) and a significantly greater response to exogenous ADMA (Figure 9A; 210 ± 19% in DDAH HT cells and 159 ± 18% in WT controls, p < 0.01). Overexpression of DDAHI restored endothelial barrier function in DDAHI HT cells to control levels (Figure 9A).
Figure 9.
Inhibition of ADMA metabolism in cells from DDAHI heterozygous (HT) knockout mice affects endothelial permeability (A), nitrite levels (B), Rac1 activity (C), and p-Ser239 VASP levels (D). Pulmonary microvascular endothelial cells we obtained from HT mice and their wild-type (WT) littermates and used at two to three passages. The cells were left untreated or were treated with ADMA, or overexpressing DDAHI/DDAHIM with or without ADMA. * p < 0.05; ** p < 0.05, comparison with untreated controls from WT mice; # p < 0.05, comparison with HT controls. (A and B) n > 5; (C) n = 4; (D) n = 3.
The cultured DDAHI HT cells showed a decrease in nitrite levels from 10 ± 4 to 5.9 ± 3 μmol/l (p < 0.01), which was prevented by overexpression of DDAHI (Figure 9B). We reported previously that cultured pulmonary microvascular cells from DDAHI HT knockout mice show up-regulation of RhoA activity and down-regulation of Rac1 activity (Wojciak-Stothard et al., 2007). The cells from knockout animals showed small but noticeable reduction in the activity of Rac1–88 ± 16% (Figure 9C) and a twofold reduction in the levels of p-Ser239 VASP (Figure 9D). Overexpression of DDAHI in DDAHI HT cells restored activated Rac1 and p-Ser239 VASP to control levels (Figure 9, C and D).
DISCUSSION
These experiments elucidate for the first time that ADMA and the enzymes metabolizing it, DDAH, regulate pulmonary endothelial permeability in vitro and in vivo. The effects of ADMA involve reduction in NO/cGMP levels leading to decrease in Ser239 phosphorylation and Rac1 inhibition.
Precise control of NO levels by ADMA/DDAH system is important in the maintenance of basal endothelial junctional integrity. Exogenous and endogenous ADMA induced endothelial leakage in vitro, and its effects were reversed by overexpression of DDAH. Conversely, DDAH deficiency in mouse lungs compromised pulmonary endothelial barrier function in vivo. A moderate (twofold) increase in NO prevented the effects of ADMA, whereas a fourfold increase in NO induced endothelial leakage. In agreement with our data, others have reported that small increases in NO promote intercellular junction formation in HUVECs (Kameritsch et al., 2005), whereas a substantial (three- to fourfold) increase causes endothelial leakage (Miyawaki-Shimizu et al., 2006), likely to result from a significant increase in nitration and nitrosylation of proteins (Predescu et al., 2005).
ROS were postulated to mediate the effects induced by methylarginines in HEK293 cells (Cardounel et al., 2005). We did not observe any effects of superoxide dismutase mimetic on ADMA-induced permeability or detect changes in ROS levels induced by ADMA in pulmonary endothelial cells, indicating that ROS do not play a significant role in the ADMA/DDAH pathway in those cells.
ADMA can affect different physiological processes in endothelial cells by differentially regulating the Rho GTPases, RhoA and Rac1. In sparse endothelial cells, ADMA decreases the levels of cGMP, which leads to activation of RhoA and inhibition of spontaneous cell motility (Wojciak-Stothard et al., 2007). In confluent cells in basal conditions, inhibition of Rac1 by ADMA leads to leaky junctions, whereas changes in RhoA activity play a lesser role. However, when endothelial cells are challenged by thrombin, cGMP-dependent inhibition of RhoA is barrier-protective (Klinger et al., 2006). Although Rac1 appears more important in control of pulmonary endothelial barrier function in basal conditions, a role of RhoA cannot be completely excluded as Rac1 can regulate the activity of RhoA (Leeuwen et al., 1997; Alberts et al., 2005).
We identified NO/PKG as downstream mediators of ADMA/DDAH. Changes in PKG activity induced corresponding changes in the activity of Rac1. A role of PKG in the regulation of Rac1 was suggested in HEK-293, where 8-Br-cGMP induced a transient activation of Rac1 with kinetics similar to p38 MAPK phosphorylation (Hou et al., 2004). As Rac1 is not a substrate for PKG, an indirect activation mechanism must operate in cells that may involve modification of exchange factors for Rac1 or regulation of their association with scaffolding proteins (Hou et al., 2004).
Here we verified the role of VASP as a potential regulator of Rac1 activity in ADMA-mediated effects. VASP associates with adherens (Vasioukhin and Fuchs, 2001) and tight junctions (Comerford et al., 2002). Consistently, mice lacking proteins of the VASP family die from edema formation due to defective vascular barrier function (Furman et al., 2007). VASP-deficient endothelial cells show increased permeability and reduced Rac1 activity in basal conditions (Schlegel et al., 2008) and are more susceptible to conditions of impaired barrier function in vitro and in vivo (Benz et al., 2008). Changes in phosphorylation of VASP by PKA or PKG accompany changes in endothelial junctional integrity (Draijer et al., 1995; Comerford et al., 2002; Benz et al., 2008). The underlying mechanism is less understood and could involve altered interactions with the tight junction protein ZO-1 (Comerford et al., 2002) and αII spectrin (Benz et al., 2008) that are dependent on PKA-mediated Ser157 phosphorylation or impaired binding to F-actin after PKG-driven Ser239 phosphorylation. We observed that ADMA decreased phosphorylation of VASP on Ser239 and initiated translocation of the protein to the cytoplasm away from endothelial junctions, a change concomitant with inhibition of Rac1 and increase in endothelial permeability that was prevented by overexpression of DDAH. Overexpression of DDAH did not alter electrophoretic mobility of VASP in PAECs, indicating that Ser157 phosphorylation (that induces an electrophoretic shift from 46 to 50 kDa) was not affected by ADMA. In agreement with our data, PKG was shown to induce VASP phosphorylation on Ser239 but not on Ser157 in bovine aortic endothelial cells treated with atrial natriuretic peptide (ANP; Chen et al., 2008).
We used mutants of Ser239 VASP to directly verify the involvement of this phosphorylation site in control of endothelial permeability. Phosphomimetic mutant of VASP increased Rac1 activity and enhanced endothelial barrier function in untreated and ADMA-treated PAECs, whereas the nonphosphorylatable mutant had the opposite effect. VASP causes polymerization and bundling of actin filaments, leading to stress fiber formation and cell membrane ruffling (Krause et al., 2003). Ser239 phosphorylation of VASP did not affect stress fiber formation in PAECs, but increased the levels of junction-associated F-actin, thought to strengthen intercellular adhesion, in a manner similar to activated Rac1 (Schlegel et al., 2008). To a lesser extent, WT VASP also induced Rac1 activation and accumulation of peripheral F-actin, possibly resulting from Ser239 phosphorylation of the overexpressed protein.
The precise regulatory mechanisms between VASP and Rac1 and the role of VASP phosphorylation by PKA and PKG in control of endothelial barrier function under different conditions will require further studies. Though VASP was shown to act upstream of Rac1, the feedback regulatory mechanism may also exist. We observed that inhibition of Rac1 reduces the levels of Ser239-phosphorylated VASP in PAECs. Rac1 was also shown to increase junctional localization of VASP in immortalized mouse myocardial endothelial microvascular cells (MyEnd; Waschke et al., 2006).
Both exogenous and endogenous ADMA induced endothelial dysfunction. Although the dose of exogenous ADMA (100 μmol/l) was relatively high, the change in ADMA levels caused by heterozygous DDAHI gene deletion led to an increase in the plasma concentration of ADMA 0.5–0.7 μmol/l, similar to that reported in patients with multiple cardiovascular risk factors (Leiper et al., 2007). It is possible that lower doses of ADMA are required in chronic exposure to the inhibitor as mechanisms for the acute and chronic exposure to ADMA are different (Suda et al., 2004). Another possibility is that local changes in ADMA concentrations in cells and tissues are higher than estimated. Plasma levels of ADMA vary from 0.2–1 μmol/l, but tissue levels may be much higher reaching 15 μmol/l (Cardounel et al., 2005). We have recently reported a localized accumulation of DDAH at the leading edge that correlated with the motile activity of endothelial cells (Wojciak-Stothard et al., 2007). The extent and significance of changes in ADMA concentration determined by local expression of DDAH remain to be established. ADMA metabolism is important in the regulation of basal endothelial permeability in vitro and in perfused lungs in vivo, but DDAHI knockout HT mice do not appear edematous (Leiper et al., 2007). Interestingly, eNOS−/− mice also remain asymptomatic despite their leaky interendothelial junctions, suggesting the existence of a compensatory mechanism that prevents edema formation (Predescu et al., 2005).
The barrier-protective effects of DDAH required intact gap junctions. Formation of gap junctions is NO/cGMP-dependent (Bazzoni and Dejana, 2004). Gap junction proteins facilitate the assembly of adherens and tight junctions (Nagasawa et al., 2006) and mediate exchange of secondary signaling mediators between cells in the lung capillary bed (Parthasarathi et al., 2006). The role of gap junctions in the effects of DDAH will require further studies.
In conclusion, we show that ADMA and DDAH regulate pulmonary endothelial permeability in an NO/PKG-dependent manner. ADMA decreases the levels of Ser239-phosphorylated VASP, leading to inhibition of Rac1 and subsequent remodeling of the endothelial actin cytoskeleton and intercellular adherens junctions. These observations suggest that genetic or environmental factors that lead to decreased methylarginine metabolism have the potential to decrease pulmonary endothelial barrier function. Conversely, interventions that activate methylarginine metabolism might have therapeutic utility in a range of cardiovascular disease states including diabetes, atherosclerosis, pulmonary and systemic hypertension, and heart failure.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Professor Anne Ridley (King's College, London) for the gift of adenoviral constructs of mutant Rho GTPases. The work was supported by the British Heart Foundation Grants PG/06/01920328 and PG/02/165 and the Deutsche Forschungsgemeinschaft.
Abbreviations used:
- ADMA
asymmetric methylarginine
- DDAH
dimethylarginine dimethylaminohydrolases
- VASP
vasodilator-stimulated phosphoprotein.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E08-04-0395) on October 15, 2008.
REFERENCES
- Alberts A. S., Qin H., Carr H. S., Frost J. A. PAK1 negatively regulates the activity of the Rho exchange factor NET1. J. Biol. Chem. 2005;280:12152–12161. doi: 10.1074/jbc.M405073200. [DOI] [PubMed] [Google Scholar]
- Bazzoni G., Dejana E. Endothelial cell-to-cell junctions: molecular organization and role in vascular homeostasis. Physiol. Rev. 2004;84:869–901. doi: 10.1152/physrev.00035.2003. [DOI] [PubMed] [Google Scholar]
- Benz P. M., Blume C., Moebius J., Oschatz C., Schuh K., Sickmann A., Walter U., Feller S. M., Renne T. Cytoskeleton assembly at endothelial cell-cell contacts is regulated by alphaII-spectrin-VASP complexes. J. Cell Biol. 2008;180:205–219. doi: 10.1083/jcb.200709181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blume C., Benz P. M., Walter U., Ha J., Kemp B. E., Renne T. AMP-activated protein kinase impairs endothelial actin cytoskeleton assembly by phosphorylating vasodilator-stimulated phosphoprotein. J. Biol. Chem. 2007;282:4601–4612. doi: 10.1074/jbc.M608866200. [DOI] [PubMed] [Google Scholar]
- Bulau P., Zakrzewicz D., Kitowska K., Leiper J., Gunther A., Grimminger F., Eickelberg O. Analysis of methylarginine metabolism in the cardiovascular system identifies the lung as a major source of ADMA. Am. J. Physiol. Lung Cell Mol. Physiol. 2007;292:L18–L24. doi: 10.1152/ajplung.00076.2006. [DOI] [PubMed] [Google Scholar]
- Cardounel A. J., Xia Y., Zweier J. L. Endogenous methylarginines modulate superoxide as well as nitric oxide generation from neuronal nitric-oxide synthase: differences in the effects of monomethyl- and dimethylarginines in the presence and absence of tetrahydrobiopterin. J. Biol. Chem. 2005;280:7540–7549. doi: 10.1074/jbc.M410241200. [DOI] [PubMed] [Google Scholar]
- Chen H., Levine Y. C., Golan D. E., Michel T., Lin A. J. Atrial natriuretic peptide-initiated cGMP pathways regulate vasodilator-stimulated phosphoprotein phosphorylation and angiogenesis in vascular endothelium. J. Biol. Chem. 2008;283:4439–4447. doi: 10.1074/jbc.M709439200. [DOI] [PubMed] [Google Scholar]
- Comerford K. M., Lawrence D. W., Synnestvedt K., Levi B. P., Colgan S. P. Role of vasodilator-stimulated phosphoprotein in PKA-induced changes in endothelial junctional permeability. FASEB J. 2002;16:583–585. doi: 10.1096/fj.01-0739fje. [DOI] [PubMed] [Google Scholar]
- Cooke J. P. ADMA: its role in vascular disease. Vasc. Med. 2005;10(Suppl 1):S11–S17. doi: 10.1177/1358836X0501000103. [DOI] [PubMed] [Google Scholar]
- Draijer R., Vaandrager A. B., Nolte C., de Jonge H. R., Walter U., van Hinsbergh V. W. Expression of cGMP-dependent protein kinase I and phosphorylation of its substrate, vasodilator-stimulated phosphoprotein, in human endothelial cells of different origin. Circ. Res. 1995;77:897–905. doi: 10.1161/01.res.77.5.897. [DOI] [PubMed] [Google Scholar]
- Furman C., Sieminski A. L., Kwiatkowski A. V., Rubinson D. A., Vasile E., Bronson R. T., Fassler R., Gertler F. B. Ena/VASP is required for endothelial barrier function in vivo. J. Cell Biol. 2007;179:761–775. doi: 10.1083/jcb.200705002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatakeyama T., Pappas P. J., Hobson R. W., 2nd, Boric M. P., Sessa W. C., Duran W. N. Endothelial nitric oxide synthase regulates microvascular hyperpermeability in vivo. J. Physiol. 2006;574:275–281. doi: 10.1113/jphysiol.2006.108175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y., Ye R. D., Browning D. D. Activation of the small GTPase Rac1 by cGMP-dependent protein kinase. Cell Signal. 2004;16:1061–1069. doi: 10.1016/j.cellsig.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Imrich A., Kobzik L. Fluorescence-based measurement of nitric oxide synthase activity in activated rat macrophages using dichlorofluorescin. Nitric Oxide. 1997;4:359–369. doi: 10.1006/niox.1997.0135. [DOI] [PubMed] [Google Scholar]
- Kameritsch P., Khandoga N., Nagel W., Hundhausen C., Lidington D., Pohl U. Nitric oxide specifically reduces the permeability of Cx37-containing gap junctions to small molecules. J. Cell. Physiol. 2005;203:233–242. doi: 10.1002/jcp.20218. [DOI] [PubMed] [Google Scholar]
- Klinger J. R., Warburton R., Carino G. P., Murray J., Murphy C., Napier M., Harrington E. O. Natriuretic peptides differentially attenuate thrombin-induced barrier dysfunction in pulmonary microvascular endothelial cells. Exp. Cell Res. 2006;15:401–410. doi: 10.1016/j.yexcr.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Kouklis P., Konstantoulaki M., Vogel S., Broman M., Malik A. B. Cdc42 regulates the restoration of endothelial barrier function. Circ. Res. 2004;94:159–166. doi: 10.1161/01.RES.0000110418.38500.31. [DOI] [PubMed] [Google Scholar]
- Krause M., Dent E. W., Bear J. E., Loureiro J. J., Gertler F. B. Ena/VASP proteins: regulators of the actin cytoskeleton and cell migration. Annu. Rev. Cell Dev. Biol. 2003;19:541–564. doi: 10.1146/annurev.cellbio.19.050103.103356. [DOI] [PubMed] [Google Scholar]
- Leeuwen F. N., Kain H. E., Kammen R. A., Michiels F., Kranenburg O. W., Collard J. G. The guanine nucleotide exchange factor Tiam1 affects neuronal morphology; opposing roles for the small GTPases Rac and Rho. J. Cell Biol. 1997;139:797–807. doi: 10.1083/jcb.139.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiper J., et al. Disruption of methylarginine metabolism impairs vascular homeostasis. Nat. Med. 2007;13:198–203. doi: 10.1038/nm1543. [DOI] [PubMed] [Google Scholar]
- Lindsay S. L., Ramsey S., Aitchison M., Renne T., Evans T. J. Modulation of lamellipodial structure and dynamics by NO-dependent phosphorylation of VASP Ser239. J. Cell Sci. 2007;120:3011–3021. doi: 10.1242/jcs.003061. [DOI] [PubMed] [Google Scholar]
- Miyawaki-Shimizu K., Predescu D., Shimizu J., Broman M., Predescu S., Malik A. B. siRNA-induced caveolin-1 knockdown in mice increases lung vascular permeability via the junctional pathway. Am. J. Physiol. Lung Cell Mol. Physiol. 2006;290:L405–L413. doi: 10.1152/ajplung.00292.2005. [DOI] [PubMed] [Google Scholar]
- Moitra J., Sammani S., Garcia J. G. Re-evaluation of Evans Blue dye as a marker of albumin clearance in murine models of acute lung injury. Transl. Res. 2007;150:253–265. doi: 10.1016/j.trsl.2007.03.013. [DOI] [PubMed] [Google Scholar]
- Mundy A. L., Dorrington K. L. Inhibition of nitric oxide synthesis augments pulmonary oedema in isolated perfused rabbit lung. Br. J. Anaesth. 2000;85:570–576. doi: 10.1093/bja/85.4.570. [DOI] [PubMed] [Google Scholar]
- Myhre O., Andersen J. M., Aarnes H., Fonnum F. Evaluation of the probes 2′,7′-dichlorofluorescin diacetate, luminol, and lucigenin as indicators of reactive species formation. Biochem. Pharmacol. 2003;65:1575–1582. doi: 10.1016/s0006-2952(03)00083-2. [DOI] [PubMed] [Google Scholar]
- Nagasawa K., Chiba H., Fujita H., Kojima T., Saito T., Endo T., Sawada N. Possible involvement of gap junctions in the barrier function of tight junctions of brain and lung endothelial cells. 2006;208:123–132. doi: 10.1002/jcp.20647. [DOI] [PubMed] [Google Scholar]
- Parthasarathi K., Ichimura H., Monma E., Lindert J., Quadri S., Issekutz A., Bhattacharya J. Connexin 43 mediates spread of Ca2+-dependent proinflammatory responses in lung capillaries. J. Clin. Invest. 2006;116:2193–2200. doi: 10.1172/JCI26605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Predescu D., Predescu S., Shimizu J., Miyawaki-Shimizu K., Malik A. B. Constitutive eNOS-derived nitric oxide is a determinant of endothelial junctional integrity. Am. J. Physiol. Lung Cell Mol. Physiol. 2005;289:L371–L381. doi: 10.1152/ajplung.00175.2004. [DOI] [PubMed] [Google Scholar]
- Rumbaut R. E., Wang J., Huxley V. H. Differential effects of L-NAME on rat venular hydraulic conductivity. Am. J. Physiol. Heart Circ. Physiol. 2000;279:H2017–2023. doi: 10.1152/ajpheart.2000.279.4.H2017. [DOI] [PubMed] [Google Scholar]
- Schlegel N., Burger S., Golenhofen N., Walter U., Drenckhahn D., Waschke J. The role of VASP in regulation of cAMP- and Rac 1-mediated endothelial barrier stabilization. Am. J. Physiol. Cell Physiol. 2008;294:C178–C188. doi: 10.1152/ajpcell.00273.2007. [DOI] [PubMed] [Google Scholar]
- Suda O., et al. Asymmetric dimethylarginine produces vascular lesions in endothelial nitric oxide synthase-deficient mice: involvement of renin-angiotensin system and oxidative stress. Arterioscler Thromb. Vasc. Biol. 2004;24:1682–1688. doi: 10.1161/01.ATV.0000136656.26019.6e. [DOI] [PubMed] [Google Scholar]
- Takano M., Meneshian A., Sheikh E., Yamakawa Y., Wilkins K. B., Hopkins E. A., Bulkley G. B. Rapid upregulation of endothelial P-selectin expression via reactive oxygen species generation. Am. J. Physiol. Heart Circ. Physiol. 2002;283:H2054–H2061. doi: 10.1152/ajpheart.01001.2001. [DOI] [PubMed] [Google Scholar]
- Vallance P., Leiper J. Cardiovascular biology of the asymmetric dimethylarginine:dimethylarginine dimethylaminohydrolase pathway. Arterioscler Thromb. Vasc. Biol. 2004;24:1023–1030. doi: 10.1161/01.ATV.0000128897.54893.26. [DOI] [PubMed] [Google Scholar]
- van Nieuw Amerongen G. P., van Hinsbergh V. W. Cytoskeletal effects of rho-like small guanine nucleotide-binding proteins in the vascular system. Arterioscler Thromb. Vasc. Biol. 2001;21:300–311. doi: 10.1161/01.atv.21.3.300. [DOI] [PubMed] [Google Scholar]
- van Nieuw Amerongen G. P., van Hinsbergh V. W. Targets for pharmacological intervention of endothelial hyperpermeability and barrier function. Vascul. Pharmacol. 2002;39:257–272. doi: 10.1016/s1537-1891(03)00014-4. [DOI] [PubMed] [Google Scholar]
- van Wetering S., van Buul J. D., Quik S., Mul F. P., Anthony E. C., ten Klooster J. P., Collard J. G., Hordijk P. L. Reactive oxygen species mediate Rac-induced loss of cell-cell adhesion in primary human endothelial cells. J. Cell Sci. 2002;115:1837–1846. doi: 10.1242/jcs.115.9.1837. [DOI] [PubMed] [Google Scholar]
- Vasioukhin V., Fuchs E. Actin dynamics and cell-cell adhesion in epithelia. Curr. Opin. Cell Biol. 2001;13:76–84. doi: 10.1016/s0955-0674(00)00177-0. [DOI] [PubMed] [Google Scholar]
- Waschke J., Baumgartner W., Adamson R. H., Zeng M., Aktories K., Barth H., Wilde C., Curry F. E., Drenckhahn D. Requirement of Rac activity for maintenance of capillary endothelial barrier properties. Am. J. Physiol. Heart Circ. Physiol. 2004;286:H394–H401. doi: 10.1152/ajpheart.00221.2003. [DOI] [PubMed] [Google Scholar]
- Waschke J., Burger S., Curry F. R., Drenckhahn D., Adamson R. H. Activation of Rac-1 and Cdc42 stabilizes the microvascular endothelial barrier. Histochem. Cell Biol. 2006;125:397–406. doi: 10.1007/s00418-005-0080-2. [DOI] [PubMed] [Google Scholar]
- Wojciak-Stothard B., Potempa S., Eichholtz T., Ridley A. J. Rho and Rac but not Cdc42 regulate endothelial cell permeability. J. Cell Sci. 2001;114:1343–1355. doi: 10.1242/jcs.114.7.1343. [DOI] [PubMed] [Google Scholar]
- Wojciak-Stothard B., Tsang L. Y., Haworth S. G. Rac and Rho play opposing roles in the regulation of hypoxia/reoxygenation-induced permeability changes in pulmonary artery endothelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2005;288:L749–L760. doi: 10.1152/ajplung.00361.2004. [DOI] [PubMed] [Google Scholar]
- Wojciak-Stothard B., Torondel B., Tsang L. Y., Fleming I., Fisslthaler B., Leiper J. M., Vallance P. The ADMA/DDAH pathway is a critical regulator of endothelial cell motility. J. Cell Sci. 2007;120:929–942. doi: 10.1242/jcs.002212. [DOI] [PubMed] [Google Scholar]
- Zhao L., Long L., Morrell N. W., Wilkins M. R. NPR-A-Deficient mice show increased susceptibility to hypoxia-induced pulmonary hypertension. Circulation. 1999;99:605–607. doi: 10.1161/01.cir.99.5.605. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.