Abstract
The assembly of metazoan Sm-class small nuclear ribonucleoproteins (snRNPs) is an elaborate, step-wise process that takes place in multiple subcellular compartments. The initial steps, including formation of the core RNP, are mediated by the survival motor neuron (SMN) protein complex. Loss-of-function mutations in human SMN1 result in a neuromuscular disease called spinal muscular atrophy. The SMN complex is comprised of SMN and a number of tightly associated proteins, collectively called Gemins. In this report, we identify and characterize the fruitfly ortholog of the DEAD box protein, Gemin3. Drosophila Gemin3 (dGem3) colocalizes and interacts with dSMN in vitro and in vivo. RNA interference for dGem3 codepletes dSMN and inhibits efficient Sm core assembly in vitro. Transposon insertion mutations in Gemin3 are larval lethals and also codeplete dSMN. Transgenic overexpression of dGem3 rescues lethality, but overexpression of dSMN does not, indicating that loss of dSMN is not the primary cause of death. Gemin3 mutant larvae exhibit motor defects similar to previously characterized Smn alleles. Remarkably, appreciable numbers of Gemin3 mutants (along with one previously undescribed Smn allele) survive as larvae for several weeks without pupating. Our results demonstrate the conservation of Gemin3 protein function in metazoan snRNP assembly and reveal that loss of either Smn or Gemin3 can contribute to neuromuscular dysfunction.
INTRODUCTION
Spinal muscular atrophy (SMA) is an autosomal recessive genetic disease with a carrier frequency of 1 in 50 unrelated individuals and is distinguished by degeneration of spinal motor neurons and severe atrophy of skeletal muscle (Pearn et al., 1978; Ogino and Wilson, 2004). The Survival Motor Neuron 1 gene (SMN1) was identified by positional cloning as the gene responsible for ∼95% of SMA cases (Lefebvre et al., 1995). Because of the observed variability in phenotypic severity, at least three classes of SMA have been established (Pearn, 1980; Ogino and Wilson, 2004). SMA type I, also known as Werdnig-Hoffman disease, is the most common and the most severe form of the disease, with an age of onset at <6 mo. SMA type I patients do not survive and typically die within the first 24 mo. SMA type II is an intermediate form, with an age of onset in the first 18 mo, and these patients often survive well into their teens. SMA type III, or Kugelberg-Welander syndrome, is characterized by late onset (after 18 mo) and chronic muscle weakness without a significant decrease in lifespan. All three classes of SMA are allelic, caused by mutations in SMN1 (Lefebvre et al., 1995).
Interestingly, the human genome contains a second locus, SMN2, which produces reduced amounts of full-length SMN protein and cannot fully compensate for the loss of SMN1 (Lorson et al., 1999; Monani et al., 1999). Complete loss of Smn function results in early embryonic lethality in mice (Schrank et al., 1997); animals that carry low-copy SMN2 transgenes survive embryogenesis but die postnatally, yet those with high-copy transgenes are completely viable (Hsieh-Li et al., 2000; Monani et al., 2000). Thus, SMA can be viewed as a “protein-dosage” disease, an interpretation that correlates well with the fact that SMA severity is inversely proportional to SMN protein levels (Coovert et al., 1997; Lefebvre et al., 1997).
SMN is part of a large, oligomeric protein complex that is essential for a number of distinct steps in the biogenesis of metazoan Sm-class small nuclear ribonucleoproteins (snRNPs; reviewed in Matera et al., 2007). SMN localizes diffusely throughout the cytoplasm, with intense nuclear signals corresponding to Cajal bodies (Liu and Dreyfuss, 1996; Matera and Frey, 1998). Based on the known protein–protein interactions, organization of the complex centers around SMN, which directly interacts with itself, Gemin2, Gemin3, Gemin5, and Gemin8 (Liu et al., 1997; Lorson et al., 1998; Charroux et al., 1999; Meister et al., 2000; Baccon et al., 2002; Gubitz et al., 2002; Pellizzoni et al., 2002a; Carissimi et al., 2006a; Battle et al., 2007; Otter et al., 2007). Gemin8 is thought to recruit Gemin6, Gemin7, and unr-interacting protein (UNRIP/STRAP), whereas Gemin3 brings Gemin4 into the complex (Charroux et al., 2000; Baccon et al., 2002; Carissimi et al., 2005, 2006b). The SMN complex binds directly to the snRNA and to Sm proteins in order to coordinate snRNP assembly (Fischer et al., 1997; Liu et al., 1997; Pellizzoni et al., 2002b; Yong et al., 2002; Battle et al., 2006). We previously demonstrated by RNA interference (RNAi) knockdown that SMN, Gemin2, Gemin3, and Gemin4 are each required for efficient snRNP assembly in HeLa cells (Shpargel and Matera, 2005). Current theories suggest that Gemins and associated proteins function together to mediate the various steps of snRNP biogenesis (Shpargel and Matera, 2005; Feng et al., 2005; Girard et al., 2006; Lemm et al., 2006). However, despite the excellent correlation between SMN protein levels and disease phenotype, mutations in other members of the SMN complex have not been associated with human disease.
Genetic analysis in model organisms provides a unique opportunity to study factors contributing to disease pathogenesis. Drosophila SMN (dSMN) has been identified on the basis of sequence and functional conservation, and null mutations within the gene are larval lethal in the second and third instar stages (Chan et al., 2003; Rajendra et al., 2007). These larvae exhibit motor and neuromuscular defects. We have also generated an adult model for Drosophila SMA. A hypomorphic mutation, called SmnE33, was created by imprecise excision of a P-element residing in the upstream control region (Rajendra et al., 2007). SmnE33 homozygotes exhibit reduced dSMN protein levels in the thorax of the adult fly. This deficiency leads to severe neuromuscular defects, including flightlessness, all of which can be rescued by expression of a YFP-Smn transgene (Rajendra et al., 2007). Notably, SMN is a sarcomeric protein in both flies and mice, and because snRNPs are absent from myofibrils, SMN likely performs a tissue-specific function in muscle (Rajendra et al., 2007). Other members of the Drosophila SMN complex have not been described.
Here, we identify and characterize dGemin3 (dGem3) as a member of the Drosophila SMN complex. Like its human counterpart, dGem3 interacts directly with dSMN in vitro and in vivo. Furthermore, these two proteins colocalize in the Drosophila Cajal body and are required for efficient assembly of Sm snRNPs. Previously uncharacterized transposon insertions in Gemin3 and Smn exhibit larval motor defects, developmental delay, and a failure to pupate. Our results demonstrate the conservation of Gemin3 function in the fruitfly SMN complex and establish its essential role in various aspects of Drosophila development.
MATERIALS AND METHODS
DNA Constructs
Smn and Gemin3 full-length cDNAs were PCR amplified with appropriate primers flanked by Gateway recombination sequences (Invitrogen, Carlsbad, CA). These products were recombined initially into pDONR221 (Invitrogen) before entry into glutathione S-transferase (GST)-tagged pDEST15, His-tagged pDEST17, green fluorescent protein (GFP)-tagged pAGW, or myc-tagged pAMW vectors (Invitrogen and the T. Murphy collection, housed at the Drosophila Genome Resource Center, Bloomington, IN).
Recombinant Protein Expression and S2 Cell Transfections
GST-dSMN and His-dGemin3 were expressed in BL21-star bacteria (Invitrogen) by 1 mM IPTG induction for 3 h. Lysate was extracted by sonication and passed over glutathione or Ni-agarose beads. S2 cells were transfected using Cellfectin as directed (Invitrogen).
Antibodies
GST (Santa Cruz Biotechnology, Santa Cruz, CA; 1:1000), His (Lab Vision, Fremont, CA; 1:1000), GFP (Roche, Indianapolis, IN; 1:1000), myc (Santa Cruz, 1:1000), SMN (Transduction Laboratories, Lexington, KY; 1:5000), SNF (U2B″, 1:1000), and tubulin (anti-rabbit; Sigma-Aldrich, St. Louis, MO) antibodies were used for Western blotting. Anti-myc (Santa Cruz, 1:40) was used for immunofluorescence. Anti-myc antibodies or Flag-conjugated agarose beads (Sigma) were used for immunoprecipitation in modified RIPA buffer.
Sm Assembly Assay
Smn, Gemin3, and LacZ dsRNAs were transcribed in vitro from PCR products flanked with T7 promoters. Drosophila S2 cells were placed in SF-900 media containing 14 μg/ml double-strand RNA (dsRNA). Extracts were generated 3 d after transfection using the Ne-Per nuclear/cytoplasmic extraction kit as directed (Pierce, Rockford, IL) and dialyzed in reconstitution buffer (20 mM HEPES-KOH, pH 7.9, 50 mM KCl, 5 mM MgCl2, and 0.2 mM EDTA (Pellizzoni et al., 2002a). Forty micrograms of cytoplasmic extract was loaded on a gel for Western blotting analysis to confirm knockdown. For the assembly assay, wild-type U1 snRNA and U1 snRNA containing a deletion of the Sm assembly site were in vitro transcribed from PCR products in the presence of P32-rUTP and m7G cap analogue (Promega). Equivalent amounts of radiolabeled U1 snRNA (n = 100,000 cpm) were incubated in 100 μg of cytoplasmic extract at 22°C for 40 min in reconstitution buffer. Assembled snRNPs were precleared with protein G beads before immunoprecipitation with 15 μl (1.5 μg) Y12 antibody in RSB-100 buffer (600 mM NaCl, 20 mM Tris-HCl, pH 7.4, 2.5 mM MgCl2, and 0.01% NP40). Immunoprecipitation products were denatured in formamide loading buffer, run on a 6% acrylamide TBE-urea gel, and exposed to a phosphorimager.
Fly Stocks
SmnA (Smn73Ao, G202S; Chan et al., 2003), SmnB (S201F; Chan et al., 2003), SmnC (PBac{WH}Smnf05960; Thibault et al., 2004), SmnD (PBac{WH}Smnf01109; Thibault et al., 2004), and SmnF (PBac{PL}Smn00733; Häcker et al., 2003) were maintained over TM3, P{ActGFP}JMR2, Ser[1] or TM6B, P{Ubi-GFP.S65T}PAD2, Tb[1] balancer chromosomes. Gem3A (Pbac{RB}Gem3e03688; Thibault et al., 2004) and Gem3B (P{PZ}Gem3rL562; Spradling et al., 1999) were maintained on TM6B, Tb balancer chromosomes. A deletion removing the Gemin3 region, Df(3L)ED4457, was obtained from the Bloomington Stock Center (Bloomington, IN). Alleles were recombined to create multiple insertions on a single chromosome. Gem3B-rev was created by precise excision of Gem3B. Timed matings were allowed to proceed for 6 h, and larvae were collected for phenotypic analyses on subsequent days. For the transgenic construct, the Flag tag was added to the Drosophila Gemin3 cDNA by PCR amplification. The Flag-Gemin3 product was cloned into pUAST and sent to BestGene, (Chino Hills, CA) for embryo injection and transgene screening. The YFP-Smn transgene (a gift from J. Gall, Carnegie Institution of Washington, Baltimore, MD) has been previously characterized (Liu et al., 2006; Rajendra et al., 2007).
RESULTS
Drosophila “Dhh1” Encodes a Putative Gemin3 Ortholog
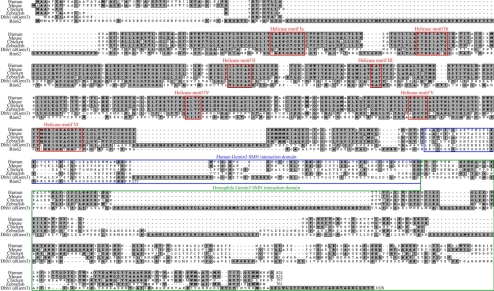
BLAST searches using human Gemin3 to probe the Drosophila proteome identified a number of potential orthologues, including the DEAD box proteins Dhh1 (CG6539) and Rm62 (CG10279). Alignment of Dhh1 and Rm62 with vertebrate orthologues of Gemin3 demonstrated strong conservation of the N-terminal helicase motifs among each of these proteins, with Dhh1 scoring slightly higher (Figure 1, red boxes). Although the C-terminal domain of Dhh1 is extensive, it bears little resemblance to vertebrate Gemin3 proteins; the C-terminus of Rm62 is truncated, and thus is also not conserved (Figure 1). A genome-wide Drosophila yeast two-hybrid analysis has been published, along with an accompanying searchable online database (Giot et al., 2003). Examination of dSMN (CG16725) in this database identified a high confidence interaction with Dhh1, but not with Rm62. Although dSMN did not interact with Rm62 in the yeast interaction screen, it is difficult to assign orthology on the basis of two-hybrid analysis and amino acid similarity alone. Human Gemin3 is characterized by its direct interaction and colocalization with SMN throughout the cytoplasm and in nuclear Cajal bodies, and by its function in efficient assembly of the Sm core snRNP. To identify the fruitfly Gemin3 ortholog, full-length Dhh1 and Rm62 cDNAs were cloned into myc-tagged expression vectors and cotransfected into S2 cells with GFP-dSMN.
Figure 1.
Drosophila Gemin3 is conserved throughout its helicase motifs. Gemin3 sequences from human, mouse, chicken, and zebrafish are aligned, along with two Drosophila proteins Dhh1 (dGem3) and Rm62. The seven helicase motifs are boxed in red, the Human SMN interaction domain is boxed in blue, and the Drosophila dSMN interaction domain is boxed in green.
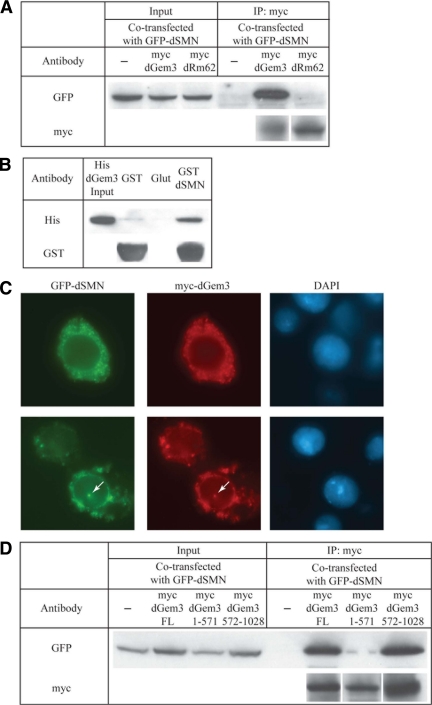
Coimmunoprecipitation analyses revealed that Dhh1 (myc-dGem3), but not Rm62 (myc-dRm62), forms complexes with GFP-dSMN in vivo (Figure 2A). The physical interaction between dSMN and Dhh1 (dGem3) is direct, as evidenced by in vitro GST-pulldown assays using purified recombinant proteins (Figure 2B). Furthermore, immunofluorescence of S2 cells cotransfected with dSMN (GFP-dSMN) and Dhh1 (myc-dGem3) constructs revealed complete colocalization throughout the cytoplasm and in nuclear Cajal bodies (Figure 2C). Interestingly, dSMN overexpression formed cytoplasmic aggregates, similar to its human ortholog (Shpargel et al., 2003). On the basis of these and other experiments (see below), we conclude that Dhh1 is the Drosophila ortholog of Gemin3. Because the well-studied yeast Dhh1 protein, a component of P-bodies, is actually orthologous to another Drosophila protein, called Me31B (CG4916), we have adopted the nomenclature dGemin3 (dGem3) for fruitfly Dhh1, to avoid confusion.
Figure 2.
dGemin3 interacts with dSMN in vitro and in vivo. (A) Drosophila S2 cells were transfected with GFP-dSMN alone or cotransfected with myc-dGemin3 (dGem3, Dhh1) or myc-dRm62. Immunoprecipitation with anti-myc antibodies coprecipitated GFP-dSMN only when myc-Gem3 was present. Inputs represent 10% of immunoprecipitation reactions. (B) Bacterially expressed His-dGem3 was passed over GST beads, glutathione beads (Glut), or GST-dSMN beads in a pulldown reaction. Western blotting demonstrated a specific, direct interaction with GST-dSMN. (C) GFP-dSMN and myc-dGem3 colocalize throughout the cytoplasm and in nuclear Cajal bodies (arrow). (D) dSMN interacts with the dGemin3 C-terminus. Myc-dGem3 deletion constructs were generated and transfected into S2 cells, repeating the experiment in A. GFP-dSMN is coimmunoprecipitated by myc-dGem3 amino acids 572-1028.
We generated myc-tagged dGem3 truncation constructs to assess the functional conservation of the C-terminal domain for its ability to interact with dSMN. Cotransfection with GFP-dSMN revealed that myc-dGem3 interacts with dSMN via amino acids 572-1028, a C-terminal region that is more distal to the equivalent SMN interaction domain in the human protein (Figure 2D). Thus, human and Drosophila Gemin3 proteins interact with SMN through divergent domains (Figure 1, blue and green boxes).
Drosophila Gemin3 Is Required for Efficient snRNP Assembly
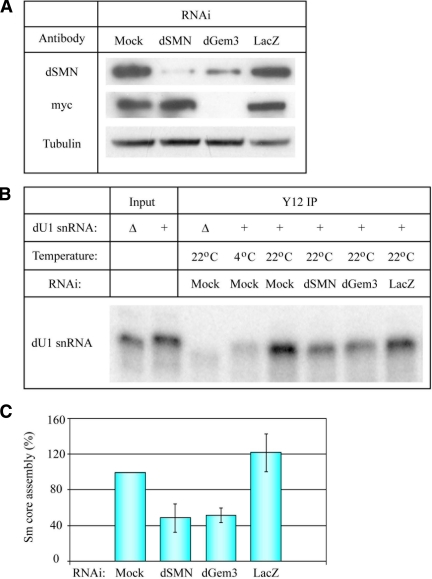
SMN and Gemin3 are essential for formation of the Sm protein core RNP in human cells (Shpargel and Matera, 2005). We utilized an Sm core assembly assay to investigate whether dGem3 plays a similar conserved role in flies. As described in Rajendra et al. (2007), this assay uses cytoplasmic extracts prepared from S2 cell lysates depleted for individual components by RNAi. Radiolabeled U1 snRNA was incubated in these lysates, and its assembly with Sm proteins was assayed by coimmunoprecipitation with anti-Sm antibodies (mAb Y12). We performed dsRNA-mediated RNAi on S2 cells to deplete dSMN and dGem3 proteins (Figure 3A). Western blotting of lysates derived from untransfected S2 cells (mock) or S2 cells treated with LacZ (control), Smn, or Gemin3 dsRNA demonstrated efficient and specific knockdown of dSMN and dGem3 compared with the Tubulin loading control. In each case, the cells were transfected with myc-dGem3 to monitor levels of dGem3 knockdown, because of the unavailability of an antibody targeting the endogenous protein. Interestingly, RNAi of dGem3 resulted in a moderate codepletion of dSMN (Figure 3A). As shown in Figure 3B, cytoplasmic extracts were incubated with either radiolabeled wild-type U1 snRNA (+) or mutant U1 snRNA (Δ), which lacks the Sm-binding site. Extracts were incubated at nonpermissive (4°C) or permissive (22°C) temperatures for the assembly assay (Figure 3B). Depletion of dSMN and dGem3 significantly reduced Sm core assembly (p < 0.005) relative to the mock or LacZ controls. Quantification of three separate experiments verified a 50% reduction in Sm core assembly activity when dSMN and dGem3 were depleted (Figure 3C). We conclude that the function of Gemin3 in snRNP assembly is conserved in invertebrates.
Figure 3.
Drosophila SMN and Gemin3 are required for efficient snRNA Sm core assembly. (A) Drosophila S2 cells were left untreated (Mock) or incubated with either Smn, Gem3, or LacZ dsRNAs for 24 h. Cells were then transfected with a myc-dGemin3 reporter construct and cytoplasmic extracts were collected after 3 additional days of incubation with the dsRNAs. Western blotting confirmed efficient knockdown of dSMN and dGem3 relative to the Tubulin loading control. (B) A radiolabeled U1 snRNA transcript was incubated in cytoplasmic extract and immunoprecipitated with Y12 (anti-Sm) antibody to assay for Sm core assembly. The U1 snRNA containing a deletion of the Sm protein assembly site (Δ) or incubating a wild-type U1 snRNA (+) at a nonpermissive temperature (4°C) serve as negative controls in the experiment. RNAi of dSMN and dGem3 significantly disrupted Sm core assembly compared with Mock and LacZ dsRNA treatments. (C) Quantification of Sm core assembly assays from three separate experiments. The results, normalized relative to the Mock control, were significant (p < 0.005). Approximately 50% reduction in Sm core assembly was observed for dSMN and dGem3 knockdown. LacZ RNAi had no significant effect (p > 0.2).
Gemin3 Is an Essential Gene in the Fly
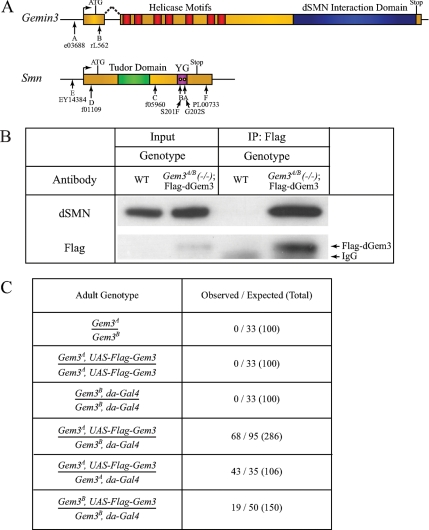
To investigate the function of Gemin3 at the organismal level, we obtained two transposon insertion lines, designated e03688 and rL562 (Figure 4A). The e03688 allele is a piggyBac insertion (PBac{RB}Gem3e03688) and will be referred to as Gem3A in the text; the rL562 allele contains a P-element insertion (P{PZ}Gem3rL562) and will be termed Gem3B. The Gem3A insertion is located in the Gemin3 promoter region and the Gem3B insertion is located immediately downstream of the translation start site (genomic insertion sites were verified by sequencing; see Flybase for details).
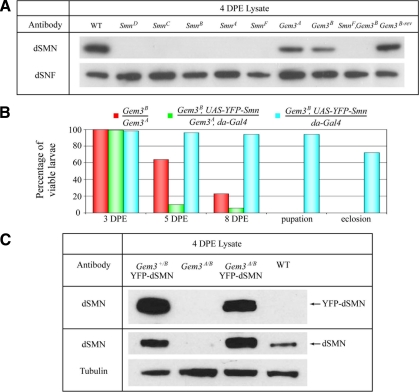
Figure 4.
Flag-dGem3 interacts with dSMN and rescues Gemin3 mutant larval lethality. (A) Schematic illustrating names and locations of the various Gemin3 and Smn alleles used in this study. (B) dGem3 interacts with dSMN in vivo. Adult fly lysates were prepared from either wild-type (WT) or Gem3A/B transheterozygotes expressing a Flag-dGem3 transgene driven by daughterless-Gal4 expression. Immunoprecipitation of Flag-dGem3 copurified dSMN. (C) Both Drosophila Gemin3 alleles are lethal, fail to complement each other, and can be rescued by exogenous transgene expression. The table illustrates the crosses performed (left) along with the adult progeny scored for the given genotype (right). The total numbers of flies analyzed are in parentheses. One-third of the total progeny resulting from the various heterozygous intercrosses (over balancer chromosomes) are expected to have the desired adult genotype.
Similar to all previously described Smn null alleles, homozygous mutants of both Gem3A and Gem3B die before pupation. Notably, these two Gemin3 alleles fail to complement each other and the recessive lethality can be rescued by ubiquitous expression of a Flag-Gemin3 transgene (Figure 4B,C). Additionally, when crossed over a deletion encompassing the Gemin3 region, Df(3L)ED4457, the Gem3A and Gem3B mutations failed in complementation tests and exhibited phenotypes indistinguishable from the individual homozygous mutations (data not shown). The pUAST-Flag-Gemin3 transgene was expressed ubiquitously by the Daughterless-GAL4 (da-GAL4) driver. Transgenic expression of Flag-dGem3 rescued the larval lethality and produced viable and fertile adults; the degree of rescue depended on the underlying combination of Gemin3 mutations, but was significant in each case (Figure 4C). As expected, no rescue was observed with the driver-only (da-Gal4) or responder-only (UAS-Flag-Gemin3) crosses (Figure 4C). Immunoprecipitation of Flag-dGem3 from total adult Drosophila lysates copurified dSMN (Figure 4B). Therefore, the dGem3 and dSMN interaction observed in S2 cells (Figure 2) is also maintained within the organism. The Gemin3 lethal phenotype was reverted by transposon excision repair of the disruption in Gem3B (data not shown). Taken together with the transgenic rescue experiments, these data lead us to conclude that the transposon insertions in the Gemin3 gene cause the observed lethal phenotype.
Long-lived Smn and Gemin3 Mutants Fail to Pupate
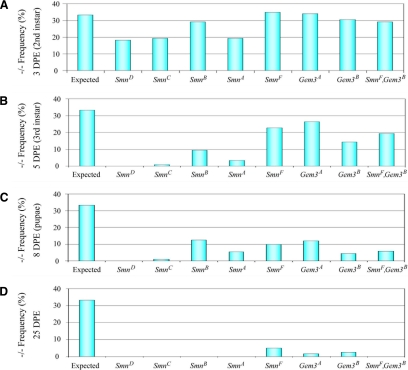
In an effort to draw correlations with SMN complex function, we compared Gem3A and Gem3B mutants to four previously characterized Smn alleles (SmnA to SmnD; Chan et al., 2003; Rajendra et al., 2007) and one previously uncharacterized allele, PBac{PL}Smn00733, which we designate SmnF (Figure 4A; Häcker et al., 2003). Similarly, each of the Smn and Gemin3 mutants are larval lethals and no homozygous pupae or adult flies were observed (data not shown). To better establish the critical lethal phases of the Smn and Gemin3 mutants, we performed a temporal analysis of heterozygous intercrosses. These experiments revealed that a certain fraction of the SmnD, SmnC, and SmnA homozygous larvae die by 3 d post egg laying (DPE; corresponding to the second instar larval stage in control animals, γ2 p < 0.001; Figure 5A). SmnD and SmnC appeared to be the most severely affected, with very few larvae surviving past day 5 (third instar; Figure 5B). Notably, whereas SmnB and SmnF exhibited moderate viability defects at the third instar time point (γ2 p < 0.0002), approximately one-third of the homozygous larvae (i.e., 10–12% of the total) survived beyond day 8, a period wherein the control wild-type and heterozygous larvae have already pupated (Figure 5C). Incredibly, a fraction of the SmnF larvae survived for more than 3 wk without pupating (Figure 5D). Although none of the SmnB homozygotes survived to day 25 (Figure 5D), a large fraction of them survived to day 8 (Figure 5C); some of these animals formed pseudopupae before dying (data not shown). On the basis of these and other phenotypic analyses, we conclude that the Smn alleles described to date (Chan et al., 2003; Rajendra et al., 2007; this study) can be ranked in order of decreasing severity as follows: D > C > A > B > F > E33.
Figure 5.
Smn and Gemin3 mutant larvae exhibit viability and pupation defects. (A–D) Smn and Gemin3 mutant larvae exhibit viability and pupation defects. Graphs illustrate the percentage of collected homozygous larvae at 3 DPE (A, second instar stage), 5 DPE (B, third instar stage), 8 DPE (C, pupation stage), or 25 DPE (D). γ2 analysis was performed for each genotype at each developmental time point. The expectation is that heterozygous intercrosses over balancer chromosomes will produce 33% homozygous mutant progeny (n ≥ 100 larvae scored for each genotype). Three days after egg laying, the numbers of SmnD, SmnC, and SmnA larvae were significantly reduced (p < 0.001); p for remaining genotypes was >0.3. At day 5, p for all genotypes was <0.004. At day 8, p for all genotypes was <3 × 10−5. Note that at 8 DPE, several Smn and Gemin3 mutants survived as larvae for extended periods, but failed to pupate (see text).
A temporal analysis of Gemin3 mutant intercrosses indicated that significant larval lethality also occurs during the second-third instar time points (Figure 5B; third instar γ2 p < 0.004). Similar to the results of the SmnF intercross, 30% of Gem3A and 15% of Gem3B homozygous larvae survived to day 8 after egg laying, but failed to pupate (Figure 5C). Comparable to SmnB, Gem3A mutants occasionally formed pseudopupal cases (data not shown). Unlike the stronger Smn alleles (A–D), but similar to SmnF, a small fraction of Gem3A and Gem3B homozygotes survived to day 25 (Figure 5D).
Double homozygotes for the Gem3B and SmnF insertions (illustrated as Gem3B, SmnF in Figure 5) displayed a phenotype similar to that of the individual mutations. In other words, homozygous loss of both genes did not significantly enhance the phenotype, except at the longest time point (Figure 5D). Thus, although dSMN and dGem3 work together in snRNP assembly, complete loss of function of both genes is equivalent to the loss of either one of them. Notably, a Gemin3 revertant allele, Gem3B-rev, recovered the ability to pupate and is fully viable (data not shown). Crossing Gem3A and Gem3B to the Df(3L)ED4457 deletion did not enhance the larval lethality phenotype (data not shown). We conclude that Smn and Gemin3 are essential for larval viability and pupation.
YFP-dSMN Overexpression Fails to Rescue Gemin3 Phenotypes
Because depletion of dGem3 by RNAi in S2 cells resulted in codepletion of dSMN (Figure 3A), we compared dSMN levels in the Gemin3 mutants to those of the five characterized Smn alleles. Larval lysates (4 DPE) were prepared and analyzed by Western blotting with anti-dSMN antibodies. As reported previously (Rajendra et al., 2007), the mutant Smn larvae expressed little or no dSMN during the phenocritical stage (Figure 6A). A certain degree of variability in the levels of dSMN was observed for the SmnA and SmnB alleles from preparation to preparation (Figure 6A and Rajendra et al., 2007). Interestingly, Gem3A and Gem3B homozygotes also expressed reduced levels of dSMN protein (Figure 6A), reminiscent of the results obtained in cell culture (Figure 3A). Importantly, transgenic expression of Flag-dGem3 in the Gemin3 mutant background (Figure 4B, compare dSMN input lanes) or reversion of the lethal phenotype by excision repair (Figure 6A) were each able to rescue dSMN levels. Thus, mutations in Gemin3 result in a corresponding depletion of dSMN, which may contribute to the phenotype.
Figure 6.
YFP-dSMN overexpression fails to rescue Gemin3 mutant phenotypes. (A) Western blotting of larval lysates at 4 DPE. At this time point, all of the Smn alleles were essentially protein nulls, whereas the two Gemin3 alleles displayed reduced dSMN levels compared with the controls (WT and Gem3B-rev). Anti-SNF (anti-U2B″) antibodies recognize Drosophila Sans fille, a homolog of this snRNP-specific protein, and were used as the loading control. (B) Gem3B/A transheterozygous larvae, Gem3B/A larvae with a YFP-Smn transgene, or Gem3B/+ larvae also bearing the YFP-Smn transgene were isolated at 2 DPE and scored for viability respective to the starting population at 3, 5, and 8 DPE. Gem3B/A larvae exhibited significant lethality at 5 and 8 DPE even in the presence of ectopic YFP-dSMN. Although a small percentage of these larvae survive to extended time points (i.e., well beyond 8 DPE), they failed to pupate and reach adulthood. n = 100–150 larvae scored for Gem3B/A, and Gem3B/A, and YFP-Smn larvae per time point. n = ∼50 larvae for Gem3+/B and YFP-Smn at each time point. (C) Western blot of lysate from Gem3B/A, Gem3B/A with YFP-Smn, Gem3B/+ larvae with YFP-Smn, or WT larvae. Expression of the YFP-Smn transgene rescued endogenous dSMN protein in Gem3B/A larvae. Note that for dSMN antibody detection, the membrane was cut and exposed separately to detect YFP-dSMN and endogenous dSMN because YFP-dSMN expression was much greater in intensity.
To test the hypothesis that dSMN function is compromised by loss of Gemin3 activity and that the observed lethality of Gemin3 mutants might be due to codepletion of dSMN, we overexpressed YFP-dSMN in the Gemin3 mutant background. The YFP-Smn transgene is fully functional and capable of rescuing Smn mutant phenotypes (Rajendra et al., 2007). To identify Gem3B/Gem3A transheterozygotes in the control cross, individual mutations were maintained over TM3, P{ActGFP} balancers, thus nonfluorescent larvae were selected as compound heterozygotes. In the YFP-dSMN rescue crosses, mutations were maintained over nonfluorescent TM3 balancers and the desired larvae were identified by fluorescent YFP-dSMN expression. Larvae of the desired genotypes were isolated at 2 DPE and scored for survival at 3, 5, and 8 DPE. Interestingly, although Gem3B/Gem3A transheterozygotes displayed a similar lethality profile to individual homozygotes (Figure 6B vs. Figure 5, A–C), expression of YFP-dSMN in this background did not rescue the larval lethality (Figure 6B). Furthermore, YFP-dSMN expression in the Gem3B/Gem3A larvae failed to rescue pupation, whereas Gem3+/B, YFP-dSMN control animals reached adulthood normally (Figure 6B). Notably, overexpression of YFP-dSMN appeared to stabilize endogenous dSMN protein levels (Figure 6C). Because Gemin3 mutant phenotypes persist when dSMN levels are restored, we conclude that dGem3's essential function in larval viability and pupation is not limited to regulating dSMN protein levels.
Smn and Gemin3 Mutant Larvae Exhibit Growth Defects
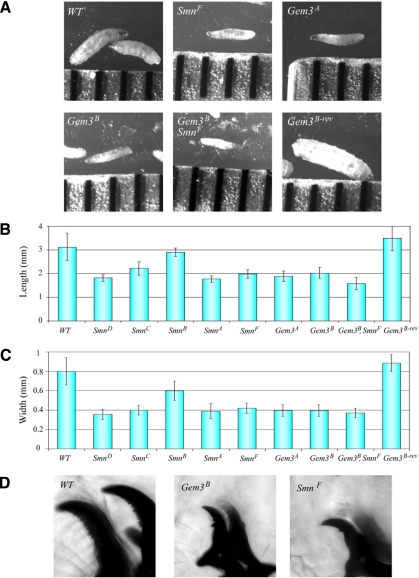
Although a fraction of the Gem3A, Gem3B, and SmnF homozygotes survived as larvae beyond 25 DPE, the mutants were by no means normal. In fact, by day 4, SmnF and Gemin3 mutant larvae appeared runted in size (Figure 7A). Wild-type or Gem3B-rev control larvae measured >3.0 mm in length and averaged ∼0.8 mm in width. Conversely, Smn and Gemin3 mutant alleles generally averaged only 1.5–2.0 mm in length and 0.4 mm in width (Figure 7, B and C; except for SmnB, all p < 0.0001). SmnB homozygotes were intermediate in size, averaging only 0.6 mm in width (a significant reduction, p < 0.002), but were of normal length (2.9 mm, p > 0.3). Analysis of the larval mouth hooks at 4 DPE revealed that the SmnF and Gemin3B homozygotes are more similar to second instar larvae, because similarly staged wild-type larvae have entered the third instar and have more highly serrated mouth hooks (Figure 7D). Therefore Smn and Gemin3 mutant larvae are significantly smaller than controls and appear to be developmentally arrested.
Figure 7.
Smn and Gemin3 mutants exhibit growth defects. (A) Images of Smn and Gemin3 second-third instar larvae (4 DPE) compared with WT and revertant (Gem3B-rev) controls. Tick marks on the ruler are 1 mm apart. (B) Graph of average length of 4-d-old larvae (n = 20 larvae scored for each genotype). Mutant length p < 0.0001 except for SmnB with a p > 0.3. (C) Graph of average width of 4-d-old larvae (mutant width p < 0.002). (D) Smn and Gemin3 mutants exhibit developmental delay. Four days after egg laying, the mouth hooks of Smn and Gemin3 larvae correspond to second instar larvae, whereas WT larvae have entered the third instar larval stage.
Smn and Gemin3 Are Required for Proper Motor Function
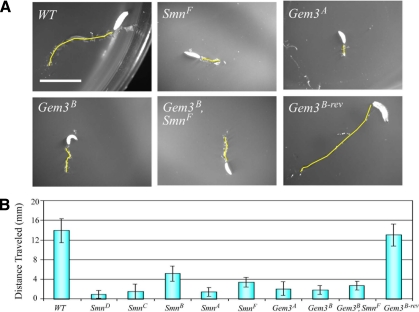
SmnA and SmnB were previously characterized as having defects in larval motility (Chan et al., 2003). To analyze the motor dysfunction of Smn and Gemin3 mutants, we placed second-third instar larvae on plates and stimulated movement with a needle. The distance they traveled over the next 20 s was traced and measured (Figure 8, A and B). Wild-type or Gem3B-rev larvae traveled an average of ∼14 mm during the 20 s; Smn and Gemin3 mutant larvae traveled at most 5 mm over the same interval (mutant p < 5 × 10−6). As shown in Figure 8B, SmnB again displayed the least severe phenotype. Thus, the motor defects originally observed in SmnA and SmnB larvae (Chan et al., 2003) are also recapitulated in the other Smn and Gemin3 mutants.
Figure 8.
Smn and Gemin3 mutant larvae exhibit defects in motor function. (A) Control (WT or Gem3B-rev), Smn, or Gemin3 homozygous larvae were prodded with a needle to stimulate movement and traced over 20 s (yellow line). Scale bar, 5 mm. (B) Graph of average distance traveled over 20 s (n = 10 larvae scored for each genotype). Larval movement was impaired for Smn and Gemin3 mutants relative to WT controls (p < 5 × 10−6).
DISCUSSION
To definitively demonstrate homologous Gemin3 activity in the fruitfly, we have shown that dGem3 interacts with dSMN in vitro and in vivo. Additionally, dGem3 and dSMN colocalize to Drosophila Cajal bodies and are required for efficient Sm core snRNP assembly in S2 cells. In human cells, Gemin3 interacts strongly with Gemin4 and forms a subcomplex with this protein (Charroux et al., 2000). Database searches have failed to identify putative Gemin4 orthologues in nonvertebrate species (Kroiss et al., 2008). Similarly, Gemins6–8, form distinct subcomplexes in human cells (Carissimi et al., 2006b; Battle et al., 2007; Otter et al., 2007), but orthologues of these proteins have not been identified in the Drosophila genome (Kroiss et al., 2008). With the possible exception of Gemin2 (Liu et al., 1997), budding yeast genomes do not appear to contain any of the known SMN complex proteins. Fission yeast, however, encode clear Smn and Gemin2 orthologues (Hannus et al., 2000; Owen et al., 2000). Despite these facts, there is little evidence for a role for Smn in snRNP assembly in Schizosaccharomyces pombe (Hannus et al., 2000; Paushkin et al., 2000) or even for a cytoplasmic phase for Sm core assembly in Saccharomyces cerevisiae (Murphy et al., 2004; Boon et al., 2007). These and other findings (see below) suggest that Drosophila contains a primitive version of the mammalian SMN complex.
The Drosophila SMN Complex and Ecdysone Signaling
Although database searches suggest that many of the SMN complex proteins are not conserved in the fly, putative orthologues of Gemin2 (dGemin2, CG10419), Gemin5 (rigor mortis/dGemin5; CG30149), and UNRIP/STRAP (wmd; CG3957) can be identified. Several lines of evidence suggest that these proteins function together. We have shown that endogenous dSMN copurifies with Flag-dGemin3 (Figures 2 and 4) and Flag-dGemin2 (K. Praveen and A.G. Matera, unpublished results). While this manuscript was under revision, Kroiss et al. (2008) also reported that dSMN forms complexes with dGem3 in S2 cells. However, dGem3 appears to be weakly or transiently associated with dSMN, as this protein was not recovered when Flag-dSMN or Flag-dGemin2 were used for the purification pulldowns. Thus it is possible that dGem3 is present in substoichiometric amounts relative to dSMN and dGem2. Despite the relative dearth of biochemical purification data linking these three factors into a single complex, we found that dGem3 is required for Sm core assembly in vitro (Figure 3). Moreover, RNAi knockdown of dGem3 in S2 cells and transposon insertions in the Gemin3 locus in vivo resulted in codepletion of dSMN (Figures 3 and 5). Importantly, overexpression of YFP-Smn in the Gemin3 null mutant background failed to rescue the lethality (Figure 6). Thus, although dGem3 may function to stabilize dSMN, it may have a separate function inside or outside of the SMN complex. Additional experiments will be required to distinguish among these possibilities.
Evidence supporting a role for the WD-repeat protein rigor mortis (rig/dGem5) in SMN complex function comes from phenotypic analyses. rigor mortis is an essential gene, and mutants therein display significant larval lethality; animals that escape the initial wave of larval lethality are developmentally delayed and fail to pupate (Gates et al., 2004). These phenotypes are strikingly similar to those of the SmnF and Gemin3 alleles described in this work. Thummel and colleagues have shown that rig/dGem5 interacts with several members of the ecdysone signaling pathway required for initiation of puparium formation (Gates et al., 2004). Mammalian Gemin5 is also involved in signal transduction (Kim et al., 2007). Similarly, UNRIP/STRAP, another WD repeat protein is an exclusively cytoplasmic member of the SMN complex (Carissimi et al., 2005; Grimmler et al., 2005) and is involved in intracellular signaling (Datta et al., 1998; Datta and Moses, 2000; Anumanthan et al., 2006). In the future, it will be interesting to determine whether rigor mortis interacts genetically and physically with other members of the Drosophila SMN complex.
Gemin3, Smn, and Neuromuscular Function
Irrespective of potential roles for the SMN complex in signal transduction, our results demonstrate the essential role that Gemin3 plays in organismal development. During manuscript revision of this article, Mouillet et al. (2008) showed that the murine ortholog of Gemin3 (Dp103/Ddx20) is essential for early embryonic development in mammals. Loss-of-function mutations in Gemin3 have not been described in other organisms. To date, several Smn and Gemin2 alleles have been characterized. Null mutations in mouse Smn and Gemin2 are also early embryonic lethals (Schrank et al., 1997; Jablonka et al., 2002). Expression of a low-copy human SMN2 transgene rescues the embryonic lethality, but the mice die shortly after birth and display severe motor neuron degeneration and muscular atrophy phenotypes (Monani et al., 2000). Depletion of Smn in zebrafish embryos by morpholino injection elicits defects in motor axon outgrowth, although the primary versus secondary nature of the reported Smn phenotypes is unclear and the results seem to depend on the extent of depletion (McWhorter et al., 2003; Winkler et al., 2005; Carrel et al., 2006; McWhorter et al., 2007). Interestingly, depletion of Gemin2 is reported to have conflicting effects on motor axon development, possibly because of differences in the levels of gene inhibition or in the methods of phenotypic analysis (Winkler et al., 2005; McWhorter et al., 2007).
The connection between snRNP biogenesis and SMA is certainly complicated and is not well understood. We have shown that mutation of two members of the Drosophila SMN complex, Smn and Gemin3, causes defects in larval motor function. In addition to larval Smn mutants, our laboratory has previously reported SMA-like phenotypes in adult flies containing a hypomorphic SmnE33 mutation (Rajendra et al., 2007). Thus, although it is clear that perturbations in the SMN complex can indeed result in neuromuscular dysfunction, the contribution that snRNP biogenesis plays in the etiology of these phenotypes remains a subject of ongoing investigation (Shpargel and Matera, 2005; Wan et al., 2005; Winkler et al., 2005; Gabanella et al., 2007). Further complicating interpretation of the various SMA models is the fact that the SMN complex appears to function in tissue-specific pathways involved in both neuronal (McWhorter et al., 2003; Zhang et al., 2006; Bowerman et al., 2007) and muscular development (Shafey et al., 2005; Rajendra et al., 2007). Clearly, animal models will play an important role in future research aimed at distinguishing among the various functions of the SMN complex.
ACKNOWLEDGMENTS
We thank A. Spradling (Carnegie Institution of Washington, Baltimore, MD), H. Bellen (Baylor College of Medicine, Houston, TX), and U. Häcker (Lund University, Lund, Sweden), as well as the Bloomington and Harvard/Exelixis stock centers for fly lines. We also thank J. Zhou (University of Massachusetts Medical School, Worcester, MA) for anti-dSMN antibodies and members of the Matera lab for helpful discussions and critical reading of the manuscript. This work was supported by National Institutes of Health (NIH) grants R01-GM53034 and R01-NS41617 (A.G.M.). K.B.S. was supported in part by an NIH predoctoral traineeship (T32-GM08613). K.P. was supported in part by an American Heart Association predoctoral fellowship.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E08-01-0024) on October 15, 2008.
REFERENCES
- Anumanthan G., Halder S. K., Friedman D. B., Datta P. K. Oncogenic serine-threonine kinase receptor-associated protein modulates the function of Ewing sarcoma protein through a novel mechanism. Cancer Res. 2006;66:10824–10832. doi: 10.1158/0008-5472.CAN-06-1599. [DOI] [PubMed] [Google Scholar]
- Baccon J., Pellizzoni L., Rappsilber J., Mann M., Dreyfuss G. Identification and characterization of Gemin7, a novel component of the survival of motor neuron complex. J. Biol. Chem. 2002;277:31957–31962. doi: 10.1074/jbc.M203478200. [DOI] [PubMed] [Google Scholar]
- Battle D. J., Kasim M., Wang J., Dreyfuss G. SMN-independent subunits of the SMN complex. Identification of a small nuclear ribonucleoprotein assembly intermediate. J. Biol. Chem. 2007;282:27953–27959. doi: 10.1074/jbc.M702317200. [DOI] [PubMed] [Google Scholar]
- Battle D. J., Lau C. K., Wan L., Deng H., Lotti F., Dreyfuss G. The Gemin5 protein of the SMN complex identifies snRNAs. Mol. Cell. 2006;23:273–279. doi: 10.1016/j.molcel.2006.05.036. [DOI] [PubMed] [Google Scholar]
- Boon K. L., Grainger R. J., Ehsani P., Barrass J. D., Auchynnikava T., Inglehearn C. F., Beggs J. D. prp8 mutations that cause human retinitis pigmentosa lead to a U5 snRNP maturation defect in yeast. Nat. Struct. Mol. Biol. 2007;14:1077–1083. doi: 10.1038/nsmb1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowerman M., Shafey D., Kothary R. Smn depletion alters profilin II expression and leads to upregulation of the RhoA/ROCK pathway and defects in neuronal integrity. J. Mol. Neurosci. 2007;32:120–131. doi: 10.1007/s12031-007-0024-5. [DOI] [PubMed] [Google Scholar]
- Carissimi C., Baccon J., Straccia M., Chiarella P., Maiolica A., Sawyer A., Rappsilber J., Pellizzoni L. Unrip is a component of SMN complexes active in snRNP assembly. FEBS Lett. 2005;579:2348–2354. doi: 10.1016/j.febslet.2005.03.034. [DOI] [PubMed] [Google Scholar]
- Carissimi C., Saieva L., Baccon J., Chiarella P., Maiolica A., Sawyer A., Rappsilber J., Pellizzoni L. Gemin8 is a novel component of the survival motor neuron complex and functions in small nuclear ribonucleoprotein assembly. J. Biol. Chem. 2006a;281:8126–8134. doi: 10.1074/jbc.M512243200. [DOI] [PubMed] [Google Scholar]
- Carissimi C., Saieva L., Gabanella F., Pellizzoni L. Gemin8 is required for the architecture and function of the survival motor neuron complex. J. Biol. Chem. 2006b;281:37009–37016. doi: 10.1074/jbc.M607505200. [DOI] [PubMed] [Google Scholar]
- Carrel T. L., McWhorter M. L., Workman E., Zhang H., Wolstencroft E. C., Lorson C., Bassell G. J., Burghes A. H., Beattie C. E. Survival motor neuron function in motor axons is independent of functions required for small nuclear ribonucleoprotein biogenesis. J. Neurosci. 2006;26:11014–11022. doi: 10.1523/JNEUROSCI.1637-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan Y. B., Miguel-Aliaga I., Franks C., Thomas N., Trulzsch B., Sattelle D. B., Davies K. E., van den Heuvel M. Neuromuscular defects in a Drosophila survival motor neuron gene mutant. Hum. Mol. Genet. 2003;12:1367–1376. doi: 10.1093/hmg/ddg157. [DOI] [PubMed] [Google Scholar]
- Charroux B., Pellizzoni L., Perkinson R. A., Shevchenko A., Mann M., Dreyfuss G. Gemin3, a novel DEAD box protein that interacts with SMN, the spinal muscular atrophy gene product, and is a component of gems. J. Cell Biol. 1999;147:1181–1194. doi: 10.1083/jcb.147.6.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charroux B., Pellizzoni L., Perkinson R. A., Yong J., Shevchenko A., Mann M., Dreyfuss G. Gemin4, A novel component of the SMN complex that is found in both gems and nucleoli. J. Cell Biol. 2000;148:1177–1186. doi: 10.1083/jcb.148.6.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coovert D. D., et al. The survival motor neuron protein in spinal muscular atrophy. Hum. Mol. Genet. 1997;6:1205–1214. doi: 10.1093/hmg/6.8.1205. [DOI] [PubMed] [Google Scholar]
- Datta P. K., Chytil A., Gorska A. E., Moses H. L. Identification of STRAP, a novel WD domain protein in transforming growth factor-beta signaling. J. Biol. Chem. 1998;273:34671–34674. doi: 10.1074/jbc.273.52.34671. [DOI] [PubMed] [Google Scholar]
- Datta P. K., Moses H. L. STRAP and Smad7 synergize in the inhibition of transforming growth factor beta signaling. Mol. Cell. Biol. 2000;20:3157–3167. doi: 10.1128/mcb.20.9.3157-3167.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng W., Gubitz A. K., Wan L., Battle D. J., Dostie J., Golembe T. J., Dreyfuss G. Gemins modulate the expression and activity of the SMN complex. Hum. Mol. Genet. 2005;14:1605–1611. doi: 10.1093/hmg/ddi168. [DOI] [PubMed] [Google Scholar]
- Fischer U., Liu Q., Dreyfuss G. The SMN-SIP1 complex has an essential role in spliceosomal snRNP biogenesis. Cell. 1997;90:1023–1029. doi: 10.1016/s0092-8674(00)80368-2. [DOI] [PubMed] [Google Scholar]
- Gabanella F., Butchbach M. E., Saieva L., Carissimi C., Burghes A. H., Pellizzoni L. Ribonucleoprotein assembly defects correlate with spinal muscular atrophy severity and preferentially affect a subset of spliceosomal snRNPs. PLoS ONE. 2007;2:e921. doi: 10.1371/journal.pone.0000921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates J., Lam G., Ortiz J. A., Losson R., Thummel C. S. rigor mortis encodes a novel nuclear receptor interacting protein required for ecdysone signaling during Drosophila larval development. Development. 2004;131:25–36. doi: 10.1242/dev.00920. [DOI] [PubMed] [Google Scholar]
- Giot L., et al. A protein interaction map of Drosophila melanogaster. Science. 2003;302:1727–1736. doi: 10.1126/science.1090289. [DOI] [PubMed] [Google Scholar]
- Girard C., Neel H., Bertrand E., Bordonne R. Depletion of SMN by RNA interference in HeLa cells induces defects in Cajal body formation. Nucleic Acids Res. 2006;34:2925–2932. doi: 10.1093/nar/gkl374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimmler M., Otter S., Müller F., Peter C., Chari A., Fischer U. Unrip, a factor implicated in cap-independent translation, associates with the cytosolic SMN-complex and influences its intracellular localization. Hum. Mol. Genet. 2005;14:3099–3111. doi: 10.1093/hmg/ddi343. [DOI] [PubMed] [Google Scholar]
- Gubitz A. K., Mourelatos Z., Abel L., Rappsilber J., Mann M., Dreyfuss G. Gemin5, a novel WD repeat protein component of the SMN complex that binds Sm proteins. J. Biol. Chem. 2002;277:5631–5636. doi: 10.1074/jbc.M109448200. [DOI] [PubMed] [Google Scholar]
- Häcker U., Nystedt S., Barmchi M. P., Horn C., Wimmer E. A. piggyBac-based insertional mutagenesis in the presence of stably integrated P elements in Drosophila. Proc Natl. Acad Sci. USA. 2003;100:7720–7725. doi: 10.1073/pnas.1230526100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannus S., Buhler D., Romano M., Seraphin B., Fischer U. The Schizosaccharomyces pombe protein Yab8p and a novel factor, Yip1p, share structural and functional similarity with the spinal muscular atrophy-associated proteins SMN and SIP1. Hum. Mol. Genet. 2000;9:663–674. doi: 10.1093/hmg/9.5.663. [DOI] [PubMed] [Google Scholar]
- Hsieh-Li H. M., Chang J. G., Jong Y. J., Wu M. H., Wang N. M., Tsai C. H., Li H. A mouse model for spinal muscular atrophy. Nat. Genet. 2000;24:66–70. doi: 10.1038/71709. [DOI] [PubMed] [Google Scholar]
- Jablonka S., Holtmann B., Meister G., Bandilla M., Rossoll W., Fischer U., Sendtner M. Gene targeting of Gemin2 in mice reveals a correlation between defects in the biogenesis of U snRNPs and motoneuron cell death. Proc. Natl. Acad Sci. USA. 2002;99:10126–10131. doi: 10.1073/pnas.152318699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E. K., Noh K. T., Yoon J. H., Cho J. H., Yoon K. W., Dreyfuss G., Choi E. J. Positive regulation of ASK1-mediated c-Jun NH(2)-terminal kinase signaling pathway by the WD-repeat protein Gemin5. Cell Death Differ. 2007;14:1518–1528. doi: 10.1038/sj.cdd.4402157. [DOI] [PubMed] [Google Scholar]
- Kroiss M., Schultz J., Wiesner J., Chari A., Sickmann A., Fischer U. Evolution of an RNP assembly system: a minimal SMN complex facilitates formation of UsnRNPs in Drosophila melanogaster. Proc. Natl. Acad Sci. USA. 2008;105:10045–10050. doi: 10.1073/pnas.0802287105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre S., et al. Identification and characterization of a spinal muscular atrophy-determining gene. Cell. 1995;80:155–165. doi: 10.1016/0092-8674(95)90460-3. [DOI] [PubMed] [Google Scholar]
- Lefebvre S., Burlet P., Liu Q., Bertrandy S., Clermont O., Munnich A., Dreyfuss G., Melki J. Correlation between severity and SMN protein level in spinal muscular atrophy. Nat. Genet. 1997;16:265–269. doi: 10.1038/ng0797-265. [DOI] [PubMed] [Google Scholar]
- Lemm I., Girard C., Kuhn A. N., Watkins N. J., Schneider M., Bordonne R., Luhrmann R. Ongoing U snRNP biogenesis is required for the integrity of Cajal bodies. Mol. Biol. Cell. 2006;17:3221–3231. doi: 10.1091/mbc.E06-03-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J. L., Buszczak M., Gall J. G. Nuclear bodies in the Drosophila germinal vesicle. Chromosome Res. 2006;14:465–475. doi: 10.1007/s10577-006-1062-5. [DOI] [PubMed] [Google Scholar]
- Liu Q., Dreyfuss G. A novel nuclear structure containing the survival of motor neurons protein. EMBO J. 1996;15:3555–3565. [PMC free article] [PubMed] [Google Scholar]
- Liu Q., Fischer U., Wang F., Dreyfuss G. The spinal muscular atrophy disease gene product, SMN, and its associated protein SIP1 are in a complex with spliceosomal snRNP proteins. Cell. 1997;90:1013–1021. doi: 10.1016/s0092-8674(00)80367-0. [DOI] [PubMed] [Google Scholar]
- Lorson C. L., Hahnen E., Androphy E. J., Wirth B. A single nucleotide in the SMN gene regulates splicing and is responsible for spinal muscular atrophy. Proc. Natl. Acad Sci. USA. 1999;96:6307–6311. doi: 10.1073/pnas.96.11.6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorson C. L., Strasswimmer J., Yao J. M., Baleja J. D., Hahnen E., Wirth B., Le T., Burghes A. H., Androphy E. J. SMN oligomerization defect correlates with spinal muscular atrophy severity. Nat. Genet. 1998;19:63–66. doi: 10.1038/ng0598-63. [DOI] [PubMed] [Google Scholar]
- Matera A. G., Frey M. R. Coiled bodies and gems: Janus or gemini? Am. J. Hum. Genet. 1998;63:317–321. doi: 10.1086/301992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matera A. G., Terns R. M., Terns M. P. Non-coding RNAs: lessons from the small nuclear and small nucleolar RNAs. Nat. Rev. Mol. Cell Biol. 2007;8:209–220. doi: 10.1038/nrm2124. [DOI] [PubMed] [Google Scholar]
- McWhorter M. L., Boon K. L., Horan E. S., Burghes A. H., Beattie C. E. The SMN binding protein gemin2 is not involved in motor axon outgrowth. Dev. Neurobiol. 2008;68:182–194. doi: 10.1002/dneu.20582. [DOI] [PubMed] [Google Scholar]
- McWhorter M. L., Monani U. R., Burghes A. H., Beattie C. E. Knockdown of the survival motor neuron (Smn) protein in zebrafish causes defects in motor axon outgrowth and pathfinding. J. Cell Biol. 2003;162:919–931. doi: 10.1083/jcb.200303168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister G., Buhler D., Laggerbauer B., Zobawa M., Lottspeich F., Fischer U. Characterization of a nuclear 20S complex containing the survival of motor neurons (SMN) protein and a specific subset of spliceosomal Sm proteins. Hum. Mol. Genet. 2000;9:1977–1986. doi: 10.1093/hmg/9.13.1977. [DOI] [PubMed] [Google Scholar]
- Monani U. R., Lorson C. L., Parsons D. W., Prior T. W., Androphy E. J., Burghes A. H., McPherson J. D. A single nucleotide difference that alters splicing patterns distinguishes the SMA gene SMN1 from the copy gene SMN2. Hum. Mol. Genet. 1999;8:1177–1183. doi: 10.1093/hmg/8.7.1177. [DOI] [PubMed] [Google Scholar]
- Monani U. R., et al. The human centromeric survival motor neuron gene (SMN2) rescues embryonic lethality in Smn(-/-) mice and results in a mouse with spinal muscular atrophy. Hum. Mol. Genet. 2000;9:333–339. doi: 10.1093/hmg/9.3.333. [DOI] [PubMed] [Google Scholar]
- Mouillet J. F., Yan X., Ou Q., Jin L., Muglia L. J., Crawford P. A., Sadovsky Y. DEAD-box protein-103 (DP103, Ddx20) is essential for early embryonic development and modulates ovarian morphology and function. Endocrinology. 2008;149:2168–2175. doi: 10.1210/en.2007-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy M. W., Olson B. L., Siliciano P. G. The yeast splicing factor Prp40p contains functional leucine-rich nuclear export signals that are essential for splicing. Genetics. 2004;166:53–65. doi: 10.1534/genetics.166.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogino S., Wilson R. B. Spinal muscular atrophy: molecular genetics and diagnostics. Expert. Rev. Mol. Diagn. 2004;4:15–29. doi: 10.1586/14737159.4.1.15. [DOI] [PubMed] [Google Scholar]
- Otter S., Grimmler M., Neuenkirchen N., Chari A., Sickmann A., Fischer U. A comprehensive interaction map of the human survival of motor neuron (SMN) complex. J. Biol. Chem. 2007;282:5825–5833. doi: 10.1074/jbc.M608528200. [DOI] [PubMed] [Google Scholar]
- Owen N., Doe C. L., Mellor J., Davies K. E. Characterization of the Schizosaccharomyces pombe orthologue of the human survival motor neuron (SMN) protein. Hum. Mol. Genet. 2000;9:675–684. doi: 10.1093/hmg/9.5.675. [DOI] [PubMed] [Google Scholar]
- Paushkin S., Charroux B., Abel L., Perkinson R. A., Pellizzoni L., Dreyfuss G. The survival motor neuron protein of Schizosaccharomyces pombe. Conservation of survival motor neuron interaction domains in divergent organisms. J. Biol. Chem. 2000;275:23841–23846. doi: 10.1074/jbc.M001441200. [DOI] [PubMed] [Google Scholar]
- Pearn J. Classification of spinal muscular atrophies. Lancet. 1980;1:919–922. doi: 10.1016/s0140-6736(80)90847-8. [DOI] [PubMed] [Google Scholar]
- Pearn J. H., Gardner-Medwin D., Wilson J. A clinical study of chronic childhood spinal muscular atrophy. A review of 141 cases. J. Neurol. Sci. 1978;38:23–37. doi: 10.1016/0022-510x(78)90242-3. [DOI] [PubMed] [Google Scholar]
- Pellizzoni L., Baccon J., Rappsilber J., Mann M., Dreyfuss G. Purification of native survival of motor neurons complexes and identification of Gemin6 as a novel component. J. Biol. Chem. 2002a;277:7540–7545. doi: 10.1074/jbc.M110141200. [DOI] [PubMed] [Google Scholar]
- Pellizzoni L., Yong J., Dreyfuss G. Essential role for the SMN complex in the specificity of snRNP assembly. Science. 2002b;298:1775–1779. doi: 10.1126/science.1074962. [DOI] [PubMed] [Google Scholar]
- Rajendra T. K., Gonsalvez G. B., Walker M. P., Shpargel K. B., Salz H. K., Matera A. G. A Drosophila melanogaster model of spinal muscular atrophy reveals a function for SMN in striated muscle. J. Cell Biol. 2007;176:831–841. doi: 10.1083/jcb.200610053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrank B., Gotz R., Gunnersen J. M., Ure J. M., Toyka K. V., Smith A. G., Sendtner M. Inactivation of the survival motor neuron gene, a candidate gene for human spinal muscular atrophy, leads to massive cell death in early mouse embryos. Proc. Natl. Acad. Sci. USA. 1997;94:9920–9925. doi: 10.1073/pnas.94.18.9920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafey D., Cote P. D., Kothary R. Hypomorphic Smn knockdown C2C12 myoblasts reveal intrinsic defects in myoblast fusion and myotube morphology. Exp. Cell Res. 2005;311:49–61. doi: 10.1016/j.yexcr.2005.08.019. [DOI] [PubMed] [Google Scholar]
- Shpargel K. B., Matera A. G. Gemin proteins are required for efficient assembly of Sm-class ribonucleoproteins. Proc. Natl. Acad Sci. USA. 2005;102:17372–17377. doi: 10.1073/pnas.0508947102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shpargel K. B., Ospina J. K., Tucker K. E., Matera A. G., Hebert M. D. Control of Cajal body number is mediated by the coilin C-terminus. J. Cell Sci. 2003;116:303–312. doi: 10.1242/jcs.00211. [DOI] [PubMed] [Google Scholar]
- Spradling A. C., Stern D., Beaton A., Rhem E. J., Laverty T., Mozden N., Misra S., Rubin G. M. The Berkeley Drosophila Genome Project gene disruption project: single P-element insertions mutating 25% of vital Drosophila genes. Genetics. 1999;153:135–177. doi: 10.1093/genetics/153.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibault S. T., et al. A complementary transposon tool kit for Drosophila melanogaster using P and piggyBac. Nat. Genet. 2004;36:283–287. doi: 10.1038/ng1314. [DOI] [PubMed] [Google Scholar]
- Wan L., Battle D. J., Yong J., Gubitz A. K., Kolb S. J., Wang J., Dreyfuss G. The survival of motor neurons protein determines the capacity for snRNP assembly: biochemical deficiency in spinal muscular atrophy. Mol. Cell. Biol. 2005;25:5543–5551. doi: 10.1128/MCB.25.13.5543-5551.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler C., Eggert C., Gradl D., Meister G., Giegerich M., Wedlich D., Laggerbauer B., Fischer U. Reduced U snRNP assembly causes motor axon degeneration in an animal model for spinal muscular atrophy. Genes Dev. 2005;19:2320–2330. doi: 10.1101/gad.342005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong J., Pellizzoni L., Dreyfuss G. Sequence-specific interaction of U1 snRNA with the SMN complex. EMBO J. 2002;21:1188–1196. doi: 10.1093/emboj/21.5.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Xing L., Rossoll W., Wichterle H., Singer R. H., Bassell G. J. Multiprotein complexes of the survival of motor neuron protein SMN with Gemins traffic to neuronal processes and growth cones of motor neurons. J. Neurosci. 2006;26:8622–8632. doi: 10.1523/JNEUROSCI.3967-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]