Abstract
Low-density lipoprotein receptor–related protein 1 (LRP1) is an endocytic recycling receptor with two cytoplasmic tyrosine-based basolateral sorting signals. Here we show that during biosynthetic trafficking LRP1 uses AP1B adaptor complex to move from a post-TGN recycling endosome (RE) to the basolateral membrane. Then it recycles basolaterally from the basolateral sorting endosome (BSE) involving recognition by sorting nexin 17 (SNX17). In the biosynthetic pathway, Y29 but not N26 from a proximal NPXY directs LRP1 basolateral sorting from the TGN. A N26A mutant revealed that this NPXY motif recognized by SNX17 is required for the receptor's exit from BSE. An endocytic Y63ATL66 motif also functions in basolateral recycling, in concert with an additional endocytic motif (LL86,87), by preventing LRP1 entry into the transcytotic apical pathway. All this sorting information operates similarly in hippocampal neurons to mediate LRP1 somatodendritic distribution regardless of the absence of AP1B in neurons. LRP1 basolateral distribution results then from spatially and temporally segregation steps mediated by recognition of distinct tyrosine-based motifs. We also demonstrate a novel function of SNX17 in basolateral/somatodendritic recycling from a different compartment than AP1B endosomes.
INTRODUCTION
Epithelial cells posses functional, morphological, and biochemically distinct apical and basolateral cell surface domains and maintain this polarized phenotype addressing specific plasma membrane proteins into each domain (Yeaman et al., 1999; Mostov, 2003; Rodriguez-Boulan et al., 2005). Apical and basolateral proteins are sorted in the biosynthetic route at the level of the trans-Golgi network (TGN; Rindler et al., 1984; Fuller et al., 1985; Griffiths and Simons, 1986), and those proteins that undergo endocytosis can be additionally sorted in recycling endosomes (RE; Matter and Mellman, 1994; Mostov and Cardone, 1995; Odorizzi and Trowbridge, 1997). Evidence accumulated over a decade and consolidated in the most recent studies (Ang et al., 2004; Lock and Stow, 2005; Cancino et al., 2007; Cresawn et al., 2007; Gravotta et al., 2007) have shown that the biosynthetic route of at least some proteins includes a post-TGN transit through RE. Under this scenery, it is now important to define the relative contribution of the TGN and RE in the polarized sorting mechanisms of different cargo and in different kind of polarized cells. Neurons, for instance, have to direct distinct proteins to somato-dendritic or axonal plasma membrane domains (Rodriguez-Boulan and Powell, 1992; Winckler and Mellman, 1999), yet their protein-sorting mechanisms remain less known than in epithelial cells. A comparative analysis in epithelial cells and neurons could indeed help to understand the underlying mechanisms of the polarized phenotype.
Studies in MDCK cells, the most currently used model of cell polarity, settled the basics of apical and basolateral protein sorting (Rodriguez-Boulan et al., 2005). Apical membrane proteins possess sorting information located in their extracellular, transmembrane, or cytosolic regions (Rodriguez-Boulan and Gonzalez, 1999; Marzolo et al., 2003), and their polarized sorting has been mainly linked to lipid raft association (Fullekrug and Simons, 2004) and glycosylation (Fiedler and Simons, 1995). Glycosylation-independent apical pathways have been also reported (Marzolo et al., 1997, 2003; Rodriguez-Boulan and Gonzalez, 1999; Bravo-Zehnder et al., 2000; Marmorstein et al., 2000). In contrast, basolateral transmembrane proteins hold discrete sorting signals exclusively in their cytoplasmic domains and frequently based on tyrosine (NPxY, Yxxφ) or dihydrophobic (LL; IL) residues. Noncanonic basolateral motifs lacking any consensus sequence have been also described (Casanova et al., 1991; Aroeti and Mostov, 1994; Le Gall et al., 1997; Odorizzi and Trowbridge, 1997; Deora et al., 2004). In addition, many basolateral proteins possess recessive apical-sorting information that becomes apparent after abrogation of their basolateral motifs (Rodriguez-Boulan et al., 2005). The frequent finding that Y-dependent basolateral motifs are collinear with endocytic determinants has for a long time suggested that the basolateral and the endocytic-sorting machineries share some common elements (Hunziker and Fumey, 1994; Matter and Mellman, 1994; Matter et al., 1994; Rodriguez-Boulan et al., 2005). Studies involving clathrin adaptors (Folsch et al., 1999; Ohno et al., 1999; Simmen et al., 2002) and, most recently clathrin itself, (Deborde et al., 2008) in basolateral sorting support this notion.
Because the TGN and endosomal compartments cooperate in the process of polarized protein sorting (Rodriguez-Boulan et al., 2005), it is important to define where and how the variety of sorting signals become decoded. In MDCK cells, newly synthesized apical and basolateral membrane proteins segregate first at the TGN (Rodriguez-Boulan et al., 2005). Then, membrane proteins leaving the Golgi apparatus may traverse RE compartments before arrival to the cell surface. This pathway has been better documented for basolateral proteins (Ang et al., 2004; Lock and Stow, 2005; Cancino et al., 2007; Gravotta et al., 2007). At least for some basolateral proteins, such as the transferrin receptor (TfR) and vesicular stomatitis virus glycoprotein (VSVG) protein, but not the low-density lipoprotein receptor (LDLR), biosynthetic trafficking through RE seems to be an obligate station (Cancino et al., 2007). Some apical proteins may also pass through endosomal intermediates (Cresawn et al., 2007). Once at the plasma membrane, proteins internalized from each cell surface domain can be recycled back to the same domain or transported by transcytosis to the opposite pole (Matter et al., 1993; Aroeti and Mostov, 1994; Matter and Mellman, 1994; Mostov and Cardone, 1995; Odorizzi and Trowbridge, 1997). Again, this view mainly derives from observations in basolateral proteins, as many of them are also endocytic proteins that recycle several times without losing polarity (Rodriguez-Boulan et al., 2005), indicating that they are sorted first during their biosynthetic trafficking and then several times during recycling (Matter et al., 1993; Gan et al., 2002; Marzolo et al., 2003; Cancino et al., 2007; Gravotta et al., 2007).
Studies on the LDLR (Matter et al., 1993) and polymeric immunoglobulin receptor (pIgR; Aroeti and Mostov, 1994) led to the concept that the same sorting motifs are used both at the TGN and recycling endosomes. However, the TfR seems to use distinct motifs at these locations (Odorizzi and Trowbridge, 1997). The sorting machinery of the TGN can discriminate between different basolateral sorting signals in vitro (Müsch et al., 1996) and in vivo (Soza et al., 2004) and could in principle also discriminate between otherwise similar basolateral and recycling motifs. So far, the proteins involved in decoding basolateral sorting signals have been the widely expressed AP4 complex (Simmen et al., 2002) and the clathrin adaptor complexes AP1B complex specifically expressed by certain epithelial cells (Folsch et al., 1999; Ohno et al., 1999; Gan et al., 2002), both acting predominantly upon Y-dependent motifs. AP1B has been localized in TfR-containing recycling endosomes as part of the sorting machinery operating in post-TGN biosynthetic and recycling pathways (Cancino et al., 2007; Gravotta et al., 2007). It is necessary to extend the analysis of the sorting signals that could operate at the TGN and/or endosomes and to search for additional decoding elements that might be involved in distinct endocytic compartments.
All these aspects are less known in neurons, even though they are considered to share elements of the sorting machinery with epithelial cells (Horton and Ehlers, 2003; Silverman et al., 2005). There are several examples of apical and basolateral proteins handled, respectively, as axonal and somatodendritic proteins in neurons, and vice versa in epithelial cells (Dotti and Simons, 1990; Dotti et al., 1991; Pietrini et al., 1994; Bradke and Dotti, 1998). However, there are also examples that do not fit into this pattern. For example, there is evidence suggesting that neurons cannot interpret dihydrophobic sorting signals as epithelial cells do (Silverman et al., 2005). Interestingly, even though neurons do not express AP1B (Ohno et al., 1999), they still direct proteins to dendrites, such as the TfR (Bradke and Dotti, 1998), LDLR (Jareb and Banker, 1998), and LDLR-related protein 1 (LRP1; Brown et al., 1997), whose basolateral distribution in epithelial cells is AP1B-dependent (Folsch et al., 1999; Gan et al., 2002; Marzolo et al., 2003).
The variety of functions played by LRP1 and what we known about its trafficking behavior makes it an interesting model protein to explore how epithelial cells and neurons organize their protein-sorting machineries. This receptor is essential for early embryonic development (Herz et al., 1992, 1993) and also plays roles in blood coagulation, cell adhesion and migration, neuronal process outgrowth, and the pathogenesis of Alzheimer's disease (Hussain, 2001; Herz and Bock, 2002). LRP1 ligands include proteinases, proteinase-inhibitor complexes, lipoprotein particles, amyloid precursor protein, and extracellular matrix proteins (Bu et al., 1992; Godyna et al., 1995; Hussain, 2001; Salicioni et al., 2002; Brandan et al., 2006). On binding, LRP1 mediates catabolism of these ligands and/or their signal transduction effects (Goretzki and Mueller, 1998; Bacskai et al., 2000; Zhuo et al., 2000). LRP1 is expressed broadly, being basolateral in epithelial cells and somato-dendritic in neurons (Brown et al., 1997; Marzolo et al., 2003).
We previously described that basolateral sorting of LRP1 depends on two critical tyrosine residues (Y29 and Y63, after the first amino acid residue of the cytoplasmic domain after the transmembrane domain) and the adaptor complex AP1B (Marzolo et al., 2003). Studies in nonpolarized cells have shown that Y63, within the YATL motif, contributes to the very fast internalization of LRP1 (Li et al., 2000). It is important to define whether Y63 operates as a basolateral signal within the context of NPxY63 or Y63xxφ motifs that share this residue (Marzolo et al., 2003). In addition, our studies in nonpolarized cells demonstrated that Y29 belongs to a recycling motif N26PxY29 that provides a binding site for sorting nexin 17 (SNX17). These studies also showed that SNX17 is required for LRP1 recycling (van Kerkhof et al., 2005 3518). SNX17 belongs to a family of proteins involved in sorting processes (Worby and Dixon, 2002) and binds to the cytosolic domain of several LDLR family members (Stockinger et al., 2002). However, the role of SNX17 and its binding motif N26PxY29 has not been explored in polarized cells, neither in epithelial cells nor in neurons. Actually, no sorting signal has been identified as somatodendritic determinant in LRP1 (Brown et al., 1997). Thus, LRP1 provides unique opportunities to study the relative contribution of different motifs in basolateral sorting processes taking place at the TGN and at post-TGN pathways, including AP1B- and SNX17-containing endocytic compartments. It is also an interesting model protein to assess the role of SNX17 in the sorting machinery of epithelial cells and neurons.
MATERIALS AND METHODS
Materials
All tissue culture media, serum, and plastic ware were from Invitrogen (Carlsbad, CA). Tissue culture-treated Transwell polycarbonate filters were from Costar (Costar, Cambridge, MA). Complete protease inhibitor tablets were from Roche Applied Science (Indianapolis, IN). Rabbit polyclonal anti-human LRP1 (RRR) and the monoclonal anti-HA antibody have been described before (Obermoeller et al., 1998). Monoclonal anti-HA (12CA5) was from BabCO (Richmond, CA). Polyclonal anti-HA antibody was from Upstate Biotechnology (Lake Placid, NY) and a rabbit polyclonal anti-HA (HA, Y-11) was from Santa Cruz Biotechnology (Santa Cruz, CA). Monoclonal anti-myc (9E10) was purchased from Roche Diagnostics (Alameda, CA). Mouse anti-E-cadherin mAb was from BD Biosciences (San Jose, CA). Rat anti-uvomorulin/E-cadherin and mouse anti-acetylated tubulin (clone 6-11B-1) monoclonal antibodies were from Sigma Chemical (St. Louis, MO). Polyclonal anti-human megalin was generated against a recombinant megalin tail obtained as described previously (Marzolo et al., 2003). Human serum with reactivity against EEA1 was obtained from a patient with systemic lupus erythematosus controlled in our rheumatology laboratory. Rabbit anti-human SNX17 was made against the peptide “HGNFAFEGIGDEDL” present in the carboxyl terminal region. This sequence is completely conserved between human and mouse SNX17 and does not have homology to other proteins. μ1B-specific blocking-function antibody was previously described (Cancino et al., 2007) and recognized the human as well as the rat adaptor subunit. The secondary antibodies sheep anti-mouse– and goat anti-rabbit–conjugated to horseradish peroxidase (HRP) were from Chemicon (Temecula, CA). The mAb against MAP2 (clone AP14, mouse IgG) was described previously (Caceres et al., 1992). Cy-3–conjugated goat anti-mouse IgG was from Chemicon. Alexa488- and Alexa594-conjugated goat anti-rabbit antibodies, goat anti-chicken Alexa594, and goat anti-mouseAlexa350 were obtained from Molecular Probes (Leiden, The Netherlands). TRITC-conjugated goat-anti-human IgG was from Sigma Chemical. EZ-link Sulfo-NHS-LC-biotin, EZ-link Sulfo-NHS-SS-biotin, and Immunopure streptavidin-agarose were from Pierce Biochemical (Rockford, IL). Protein A-agarose was from Repligen (Waltham, MA). Immobilon-P transfer membrane was from Millipore (Billerica, MA). Kaleidoscope molecular weight markers were from Bio-Rad (Hercules, CA). The ECL system was from Amersham Pharmacia Biotech (Piscataway, NJ).
Construction of LRP1 Minireceptors
All the constructs have the hemagglutinin (HA) epitope in their amino termini and are composed of the fourth ligand binding, transmembrane and cytoplasmic domains of LRP1 (mLRP4). The construction of wild-type and mutant forms of the minireceptor (N26A, Y29A, LL43,44AA, N60A, Y63A, L66A, S76A, LL86,87AA, and Y63A/LL86,87AA), have already been described (Li et al., 2000, 2001a,b). The new mutant mLRP4 DD38,39AA was made using the QuickChange site-directed mutagenesis kit from Stratagene, according to the manufacturer's instructions. The oligonucleotides synthesized at the Washington University School of Medicine Protein Chemistry Laboratory. All the constructs were verified by DNA sequencing.
Plasmids for the Expression of μ1B-HA and myc-SNX17 and of a Dominant Negative Form of eps15
The plasmid encoding μ1B with an internal HA tag was kindly provided by Dr. Ira Mellman (Folsch et al., 2001). The plasmid encoding the myc-tagged SNX17 was obtained as described (van Kerkhof et al., 2005). The expression plasmid for GFP-EPS15 DD (EΔ95/295), a dominant form of eps15 (D/N-eps15; Benmerah et al., 1999) was kindly provided by Dr. Alexander Benmerah (Institut Cochin-U567 INSERM/UMR8104 CNRS-Paris, France).
Cell Culture Conditions and Transfection
Madin-Darby canine kidney (MDCK) cells (strain II) were maintained in DMEM (Invitrogen) supplemented with 7.5% fetal bovine serum (FBS; Invitrogen) containing 100 U/ml penicillin and 100 mg/ml streptomycin sulfate. Fisher rat thyroid (FRT) cells were grown in Coon's modified F12 medium with 10% FBS and antibiotics as described (Marzolo et al., 1997). Clonal cell lines were derived from MDCK and FRT cells stably transfected with 2 μg DNA by using Lipofectamine Plus transfection reagent (Invitrogen) according to the supplier's protocol, followed by 10–14 d of selection with 0.8 mg/ml G418 (Invitrogen). Cells were screened and analyzed by Western blot and indirect immunofluorescence as described below. Selected clones were maintained in the same medium plus 0.4 mg/ml G418.
Hippocampal neurons were cultured as described (Banker and Cowan, 1977). Dissociated cells were plated on glass coverslips coated with 1 mg/ml poly-l-lysine in medium containing 10% horse serum (Invitrogen-BRL). After 3 h, the medium was supplemented with N2 (Invitrogen-BRL; Bottenstein and Sato, 1979). Cells were transiently transfected at 7 d using LipofectAMINE 2000 (Invitrogen) (Paglini et al., 1998). Cells were then analyzed at different posttransfection intervals ranging from 12 to 18 h.
For most of the experiments related to epithelial polarity analysis, cells were plated at high density onto 12- or 24-mm Transwell polycarbonate filter units (0.4-μm pore size). Cells were grown until the transepithelial resistance reached 200–350 Ù/cm2 for MDCK cells, 5000–8000 Ù/cm2 for FRT cells, measured with an EVOM electrometer (World Precision Instruments, Sarasota, FL). The formation of a properly polarized monolayer of MDCK cells was determined by the analysis of the lateral expression of E-cadherin either by indirect immunofluorescence or by biotinylation, as indicated.
Immunofluorescence Microscopy
MDCK and FRT cells were plated at 1 × 105 on glass coverslips in 24-well dishes and grown for 3 d. Cells were fixed in 2% paraformaldehyde (PFA) in PBSc (phosphate-buffered saline containing 0.1 mM calcium and 1 mM magnesium) and then permeabilized or not with 0.2% Triton X-100 in PBSc. Before being incubated with the primary antibody, cells were blocked with 0.2% gelatin in PBS. Successive incubations with the first antibody and Cy3 or Alexa 488 secondary antibody were carried out. After washing with PBS and with 0.2% gelatin in PBS, the coverslips were mounted with Mowiol (Calbiochem, San Diego, CA).
Hippocampal neurons were fixed with 4% PFA and 4% sucrose for 20 min at 37°C, and processed for immunofluorescence as described previously (Rosso et al., 2004). For cell surface staining, incubation with primary or secondary antibodies was performed before permeabilization with detergents. Stained cells were observed and analyzed with an inverted microscope (Carl Zeiss, Thornwood, NY; Axiovert 35M) equipped with epifluorescence and photographed using a 63× objective (Carl Zeiss) using Axiovision (version 3.0.6), or a Zeiss laser scanning confocal microscope. X-Y sections, in 0.45-μm steps, were collected sequentially at 1024 × 1024 resolution.
Cell Surface Biotinylation and Flow Cytometry in Epithelial Cell Lines
The cell surface distribution of the receptors was assessed by cell surface biotinylation, immunofluorescence, and flow cytometry. Cell surface biotinylation was performed at 4°C, as described (Le Bivic et al., 1989; Marzolo et al., 2003). Briefly, the cell monolayers grown on filters were washed in PBSc and then biotinylated with sulfo-NHS-LC-biotin from either the apical (0.7 ml) or basolateral (1.5 ml) chamber compartment. The chamber not receiving biotin was incubated with PBSc. Filters were incubated twice for 30 min. After biotinylation steps, biotin was quenched by incubation with 50 mM NH4Cl in PBSc for 10 min. Cells were lysed in ice-cold lysis buffer (150 mM NaCl, 20 mM Tris, pH 8.0, 5 mM EDTA, 1% Triton X-100, 0.2% BSA, and protease inhibitors). Biotinylated cell surface proteins were then adsorbed to streptavidin-agarose beads for 2 h. Beads were washed, and the biotinylated proteins analyzed by SDS-PAGE followed by immunoblotting (see below). For analysis of the cell surface receptor by flow cytometry, MDCK cells expressing the different minireceptors were grown on 100-mm dishes until 80% confluent. The cells were detached by incubation with PBS/5 mM EDTA and trypsin. Detection of cell surface minireceptor was performed by using monoclonal anti-HA antibody, followed by incubation with goat anti-mouse R-phycoerythrin (RPE) antibody (Dako, Glostrup, Denmark). The total amount of receptor expressed en each cell was assessed in cells previously permeabilized with PBS 0.1% saponin. Background fluorescence intensity was assessed in the absence of primary antibody and subtracted. Mean fluorescence values were obtained in triplicate with a FACScalibur (BD Biosciences-PharMingen, Sweden), and data were analyzed with Cell Quest software (BD Biosciences-PharMingen).
Immunoblotting and Coimmunoprecipitation Assays
For Western blotting, cells were lysed with lysis buffer (PBS containing 1% Triton X-100, 1 mM PMSF, 1 μg/ml each of pepstatin, antipain, leupeptin, and aprotinin) for 1 h at 4°C. Lysates were resolved by SDS-PAGE under reducing conditions, transferred to polyvinylidene difluoride (PVDF) membrane, and incubated with the corresponding primary antibody (mouse anti-HA 1:200 for the 12CA5 mAb and 1:500 for the BabCO antibody, anti-human LRP1 1:500, anti-E-cadherin 1:1000, mouse anti-myc 1: 500) overnight at 4°C. Membranes were washed, incubated with species-specific secondary antibodies conjugated to HRP (anti-mouse HRP 1:5000 and anti-rabbit HRP 1:10000) for 1 h at room temperature, and immunoreactive proteins were detected using the ECL system.
For the coimmunoprecipitation, cells were grown to subconfluence on 10-cm tissue culture dishes. Cells were rinsed twice with cold PBS and lysed in 800 μl of buffer lysis (150 mM NaCl, 50 mM HEPES, pH 7.5, 10% glycerol, 1 mM EGTA, 1% Triton X-100, 1.5 mM MgCl2, 100 mM NaF, 10 mM sodium pyrophosphate, 500 μM orthovanadate, 1 mM PMSF, 1 mM aprotinin, 1 mM leupeptin, and 1 mM pepstatin). Lysates were cleared by centrifugation at 10,000 rpm at 4°C, incubated with 10 μg of anti-LRP1 or anti-MegT antibody (Marzolo et al., 2003) for 2 h at 4°C, and subsequently mixed with 40 μl of protein A-Sepharose for 1 h at 4°C. Sepharose beads were collected by centrifugation and washed three times with lysis buffer. Immunoprecipitates were boiled 3 min and resolved by SDS-PAGE and immunoblotting.
Cell Surface Fluorescence Quenching Recycling Assay
This assay was basically performed as described (van Kerkhof et al., 2005). In our assay we also included nontransfected cells to subtract the nonspecific fluorescence. MDCK cells, wild-type and stably expressing minireceptors were incubated with Alexa488-labeled anti-HA antibodies for 20 min at 37°C. After removal of fluorescent antibody in the medium, the cells were incubated for the indicated time periods (0–10 min) in the absence or presence of 24 μg/ml anti-Alexa488 IgG (Molecular Probes) to quench the fluorescence and then rapidly chilled, detached, and analyzed by flow cytometry for fluorescence intensity. Background fluorescence intensity assessed in the absence of primary antibody was subtracted. Mean fluorescence values were obtained in triplicate with a FACScalibur (BD Biosciences-PharMingen), and data were analyzed with Cell Quest software (BD Biosciences-PharMingen). The percentage of initial fluorescence (pulse) remaining at each time point was calculated as the difference between nonchased (time 0) and chased cell fluorescence and then normalized to the nonchased value in order to calculate the % of recycling efficiency.
Microinjection Experiments
Minireceptor′s Trafficking Assays.
The microinjection approach to express and accumulate exogenous cargo at the TGN and then to assess its subsequent trafficking to the plasma membrane, was performed essentially as described (Kreitzer et al., 2000; Cancino et al., 2007). MDCK and FRT cells were plated at subconfluent levels on glass coverslips and microinjected after 3 d of reaching confluence. The expression plasmids (25 μg/ml) and antibodies (40 μg/ml) were dissolved in HKCl microinjection buffer (10 mM HEPES, 140 mM potassium chloride, pH 7.4). Microinjections were performed in the cell nucleus (plasmids) and cytosol (antibodies) using back-loaded glass capillaries and an Eppendorf Transjector 5246 system mounted on a Zeiss Axiovert S100 inverted microscope, keeping the cells in bicarbonate-free DMEM supplemented with 5% FBS, 20 mM HEPES, pH 7.4. The microinjected cells were incubated for 1 h at 37°C, and newly synthesized protein was accumulated in the TGN at 20°C for 2 h in the presence of 100 μg/ml of cycloheximide. After the 20°C block the cells were incubated for different times at 37°C to resume TGN-to-cell-surface protein traffic and then fixed with 4% PFA in PBS-CM buffer for 30 min. The cells were then incubated with rabbit polyclonal anti-HA antibody before permeabilization for 30 min. Cells were washed twice 5 min with PBS and refixed in 4% PFA for 15 min and then permeabilized with 0.2% Triton X-100 for 10 min. The cells were then incubated with chicken anti HA-antibody for 30 min. The apical localization of mini-receptors was detected with secondary goat anti-rabbit-Alexa488 or goat anti-rabbit-Alexa555 antibodies. Intracellular/basolateral localization of mini-receptors was detected with goat anti-chickenAlexa-594 antibody. Confocal microscopy was performed using a laser scanning LSM 510 Zeiss microscope, 63× oil immersion lens, at 22°C. Images were processed using MetaMorph software version 6.0r1.
B) Colocalization Analysis.
MDCK cells were microinjected as above with plasmids for the expression of RAP, SNX17-myc and HA-tagged minireceptors. The microinjected cells were incubated for 2 h at 37°C, newly synthesized proteins were left 2 h in the presence of 100 μg/ml of cycloheximide, and then cells were fixed, permeabilized and stained with rabbit anti-HA, mouse anti-myc and human anti-EEA1 antibodies. The secondary antibodies used were goat-anti-mouse-Alexa350, goat-anti-rabbit-Alexa488 and goat-anti-human TRITC. Images were captured in a Zeiss Axiophot microscope using 100x lens and Axiocam camera. Images were processed using MetaMorph software version 6.0r1. For quantification, all images from a single experiment, five for each coverslip, were acquired under identical settings (14 bits; 1300 × 1030 pixels, and the same exposure times, avoiding signal saturation), and their integrated fluorescence intensities (equivalent to the sum of all grayscale values for every pixel in the region) ere analyzed after 2D deconvolution and threshold adjustment to select positive staining for each fluorescent probe. The percentage of colocalization, measured as integrated pixel intensity in the regions of overlap, of mLRP4 and mLRP4N26A with EEA-1 and SNX17 was calculated for each individual cell (n = 30). Objects with saturated pixels were omitted from quantifications.
Transcytosis Assay
To assess basolateral-to-apical transcytosis, we applied a described strategy that uses sulfo-NHS-SS-biotin and glutathione stripping (Burgos et al., 2004) with modifications. Cells were grown in transwell filters until confluent. On the day of the experiment, cells were washed two times with ice-cold PBSc for 5 min, and the basolateral domain was biotinylated with 1 mM of the cleavable sulfo-NHS-SS-biotin for 20 min at 4°C. The reaction was then quenched twice with 50 mM NH4Cl for 10 min at 4°C. After three washes with PBSc for 5 min each, the cells were incubated with DMEM/HEPES for 45 min at 37°C and then chilled and washed again three times with ice-cold PBS. The biotin of proteins appearing at the apical cell surface was stripped with 50 mM GSH, added twice to the apical compartment for 30 min at 4°C. Reduction was also performed at the basolateral domain in different filters as control. The reaction was stopped by washing with PBS and incubating with 5 mg/ml iodoacetamide in PBS, containing 10% BSA, for 30 min at 4°C. Cells were washed with PBS three times and then were lysed in ice-cold lysis buffer containing 2.5 mM iodoacetamide for 1 h at 4°C. Lysates were centrifuged at 14,000 rpm for 10 min at 4°C and incubated with streptoavidin beads as in the biotinylation protocol. Precipitated proteins were identified by Western blot with anti-HA to detect the minireceptors and anti-E-cadherin to control loading and domain specific reduction. All the experiments were repeated three times, with 1–2 filters per point, depending on the expression level of the minireceptor analyzed. The band corresponding to the minireceptor in each condition was quantified by densitometry. The transcytosis efficiency was determined after 45 min of basolateral-to-apical trafficking at 37°C after the basolateral biotinylation, as follows: Basolateral-to-apical transcytosed minireceptor = Total basolaterally biotinylated minireceptor − Biotinylated minireceptor remaining after reduction at the apical surface. Basolaterally Recycled receptor = Total Basolaterally Biotinylated receptor − Biotinylated minireceptor remaining after reduction at the basolateral surface.
RESULTS
LRP1 Is Transported to the Basolateral Surface in MDCK and FRT Cells
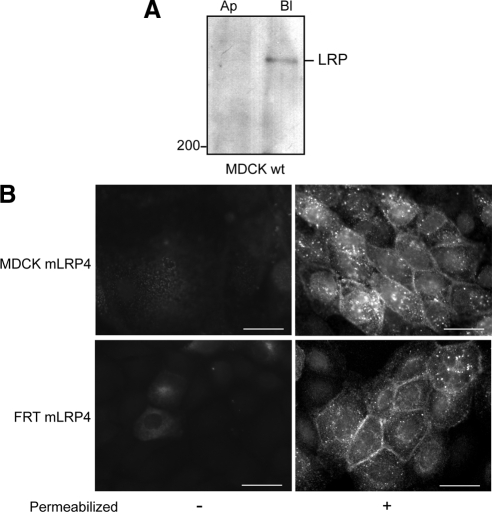
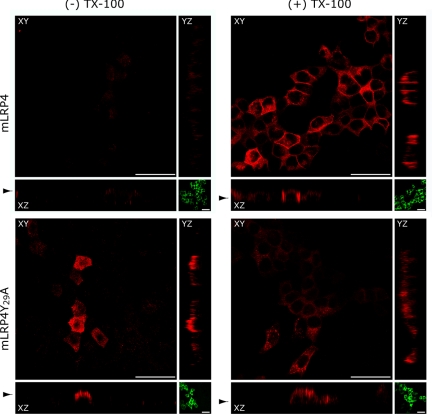
To assess the sorting behavior of LRP1, we used HA-tagged minireceptors containing the fourth ligand binding domain, the transmembrane domain, and the cytoplasmic tail of the receptor (mLRP4; Marzolo et al., 2003). We first examined whether two different epithelial cell lines, MDCK and FRT, of kidney and thyroid origin, respectively, express and sort endogenous LRP1 similarly. The LRP1 antibody against the human protein readily localized the endogenous LRP1 in the basolateral domain of MDCK cells, as we previously described (Marzolo et al., 2003; Figure 1A). In contrast, FRT cells showed much lower expression levels (not shown). However, both cell lines distributed the transfected mLRP4 predominantly in the basolateral domain (Figure 1B), indicating similar decoding of the LRP1 basolateral sorting information.
Figure 1.
Basolateral expression of LRP1 and its minireceptors in polarized epithelial cells. (A) MDCK cells biotinylated either at the apical (Ap) or basolateral (Bl) domain at 4°C were lysed, and the surface biotinylated proteins were precipitated with streptavidin-agarose and resolved in 5% SDS-PAGE followed by immunoblot with anti-human LRP1. Endogenous LRP1 distributes at the basolateral domain. (B) MDCK and FRT cells stably expressing the mLRP4 minireceptor were grown until confluent on coverslips, and the distribution of the minireceptor was visualized by indirect immunofluorescence with monoclonal anti-HA. The minireceptor was detected only after cell permeabilization at the basolateral cell borders and in intracellular vesicles. Scale bars, 10 μm.
AP1B Regulates LRP1 Trafficking at a Post-Golgi Recycling Endosome
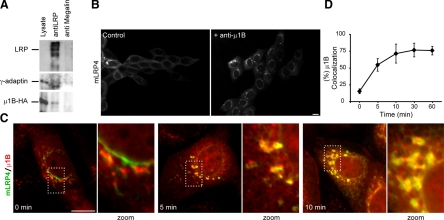
We have previously shown that LLC-PK1 cells, which lack the adaptor subunit μ1B of the AP1B complex that recognizes tyrosine-based basolateral sorting motifs (Ohno et al., 1999), distributes LRP1 in a nonpolarized manner unless exogenous μ1B is expressed by transfection (Marzolo et al., 2003). To determine whether LRP1 interacts with AP1B, we transiently transfected MDCK cells with HA-μ1B and then LRP1 was immunoprecipitated from the cell lysates. The adaptor complex subunits, HA-μ1B and γ-adaptin, were found in the immunoprecipitates (Figure 2A), suggesting that LRP1 does interact with AP1B.
Figure 2.
Physical and functional interaction between LRP1 and AP-1B. (A) MDCK cells were transiently transfected with a plasmid encoding μ1B-HA. Cells were lysed and the lysates were immunoprecipitated with anti-LRP1 or anti-megalin antibodies. The immunoprecipitates were resolved on 4–15% SDS-PAGE. The presence of LRP1 was detected by immunoblotting with anti-LRP antibody, and the complex AP-1B was evidenced by the presence of γ-adaptin and μ1-B. (B) FRT cells were microinjected with mLRP4 minireceptor plus RAP cDNAs with or without blocking-function anti-μ1B antibody. Cells were maintained for 1 h at 37°C, followed by 2 h at 20°C to accumulate the receptor at the TGN. After 1 h of exit at 37°C cells were fixed and processed for immunofluorescence with anti-HA to detect the minireceptor. After 60 min, release from TGN block mLRP4 was located on surface in control cells. In contrast, in μ1B-microinjected cells, mLRP4 was located mainly in a perinuclear localization, most likely RE, indicating that AP1B adaptor complex is involved in the intracellular trafficking of the receptor. (C and D) Kinetic analyses of mLRP4/μ1B colocalization of newly synthesized mLRP4 demonstrates that perinuclear accumulation was achieved during biosynthetic trafficking. Scale bar, 10 μm.
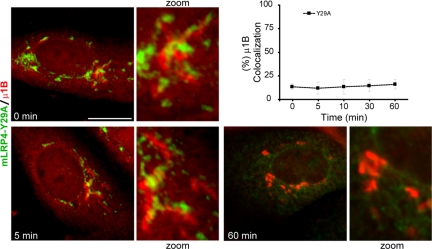
We recently reported that an anti-μ1B antibody is able to block the biosynthetic trafficking of VSVG and TfR at a μ1B-containing RE that most likely represents the common recycling endosome (CRE) in FRT cells, 5–10 min after exiting the TGN (Cancino et al., 2007). The antibody also blocked the postendocytic trafficking of LDLR at μ1B-RE. Here we performed similar experiments to define which μ1B-dependent route is used by LRP1. FRT cells were microinjected with mLRP4 and RAP plasmids with or without anti μ1B antibody. After 1 h of synthesis at 37°C, 2 h of TGN accumulation at 20°C, and 1 h of releasing the TGN block at 37°C, the mLRP4 achieved a basolateral distribution that contrasted with the perinuclear retention caused by the μ1B antibody (Figure 2B). This experiment revealed a μ1B-dependence of LRP1 trafficking at μ1B-RE but did not allow to define whether this occurs immediately after exiting the TGN or after endocytosis. A time course experiment resolved this question (Figure 2, C and D). Before releasing from the 20°C TGN block, the receptor does not colocalize with μ1B. After 5 min of TGN block release, more than 50% of mLRP4 colocalizes with μ1B in a perinuclear compartment and reaches up to a 75% of colocalization at 10 min, maintaining this distribution even after 1 h at 37°C. These results indicate that after exiting the TGN the LRP1 is first transported to the μ1B-RE while en route to the basolateral cell surface, thus sharing with VSVG and TfR this indirect basolateral pathway (Cancino et al., 2007).
Identification of Sorting Motifs in the LRP Cytoplasmic Domain
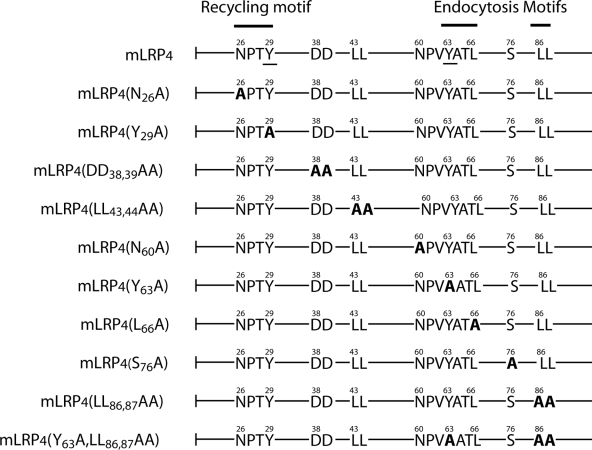
To define sorting motifs within the LRP1 cytoplasmic domain, as well as the context sequence of the basolateral information conveyed by the Y29 and Y63 residues (Marzolo et al., 2003), we analyzed new site-directed mutants of LRP1 minireceptors, as depicted in Figure 3, The wild-type minireceptor mLRP4 has a cytoplasmic domain composed of 100 amino acids containing several putative sorting motifs (Figure 3). These include two NPxY motifs (N26PTY29 and N60PVY63), one YxxΦ (Y63ATL66) motif that shares the tyrosine with the N60PVY63 motif, two dileucine motifs (LL43,44 and LL86,87), and a pair of negatively charged amino acids (DD38,39), similar to those involved in LDLR basolateral sorting (Matter et al., 1992). There is also a protein kinase A (PKA) phosphorylation site involving the serine in position 76 (S76) that has a role in LRP endocytosis (Li et al., 2001b).
Figure 3.
Schematic representation of mLRPs with the potential cytoplasmic basolateral sorting signals and their mutations. The sequence of the wild-type tail (mLRP4) is depicted with the already known recycling and endocytic-sorting motifs described in nonpolarized cells (Li et al., 2000; van Kerkhof et al., 2005) and the two tyrosines (Y29 and Y63, underlined) involved in basolateral distribution (Marzolo et al., 2003). All the mutants made are highlighted in bold where the critical residues were replaced by alanine.
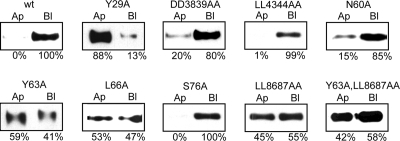
We generated stable MDCK cells lines and assessed the steady-state distribution and trafficking properties of the different minireceptor constructs by analyzing two or three MDCK clones for each construct. The results of domain specific biotinylation assays are presented from the proximal to distal location of the analyzed motifs (Figure 4). As described (Marzolo et al., 2003), the mutation in the first NPxY motif (NPTY29A) completely redistributes LRP1 to the apical domain. Neither the DD38,39AA mutant nor the LL43,44AA mutant affected the LRP1 basolateral distribution. However, a truncated LRP1 construct lacking the last 41 amino acids revealed a potential basolateral sorting function of DD38,39 residues (data not shown). The already described mutation of the Y63 residue leads to a nonpolarized distribution of the receptor (Marzolo et al., 2003). This tyrosine is shared by the distal N60PVY63 motif and the endocytic motif Y63ATL66 (Li et al., 2000). Here, we determined that its basolateral sorting function is exerted independently of N60, since the N60A mutant distributed predominantly basolateral. Instead, the L66A mutation mistargeted the receptor to the apical domain by more than 50%. Thus, Y63 constitutes a YxxΦ and not a NPxY basolateral sorting motif.
Figure 4.
Changes in the steady-state distribution of mLRP4 mutants uncover the critical basolateral sorting motifs within the LRP1 tail. MDCK cells stably expressing the different mLRP4 constructs were grown on filters to determine the membrane distribution of the receptors by domain-specific biotinylation. Biotinylated minireceptors were precipitated with streptavidin-agarose and resolved in 6% SDS-PAGE followed by immunoblot with monoclonal anti-HA. The receptor's relative expression level in each membrane domain was estimated by densitometry of the resulting bands. The critical basolateral determinants include the Y29 of the proximal NPxY, the Y63ATL66, and the distal LL86,87 motifs. Similar results were obtained from two to three clones of each construct.
In nonpolarized cells, the Y63ATL66 and LL86,87 motifs contribute independently to the rate of LRP1 endocytosis (Li et al., 2000). We found here that LL86,87 is also required for LRP1 basolateral distribution. The mutant LL86,87AA, similarly to the Y63A mutant, showed a nonpolarized distribution. Furthermore, the double mutant Y63A/LL86,87AA also distributed in a nonpolarized manner. Neither of these motifs seem to compensate for the loss of function of the other. These signals seem to act in concert, as if forming part of a single complex sorting signal. Finally, the unique PKA phosphorylation site S76 was not required for LRP1 basolateral sorting.
These results define the context of the previously described Y63-dependent basolateral sorting signal and revealed novel sorting requirements involving L66 and LL86,87 residues. The contrasting apical segregation of Y29A and nonpolarized distribution of Y63A and LL86,87AA mutants prompts to further analyzing the proximal NPxY motif and the sorting pathways where these motifs exert their function.
The Basolateral Information Conveyed by Y29 Operates at the TGN
The different phenotypes produced by alanine replacement in either the Y29 or the Y63 and LL86,87 residues suggest that sorting signals associate with temporally–spatially compartmentalized functions. An interesting possibility is that the Y29-dependent motif operates during biosynthetic trafficking at the TGN level, whereas Y63 and LL86,87 operates biosynthetically in a post-TGN station and/or at a basolateral recycling route after internalization. In this hypothetic model, mutant receptors lacking the function of Y63 or LL86,87 residues would be targeted basolaterally from TGN due to the Y29-dependent sorting, but would enter into basolateral-to-apical transcytotic pathways and lose polarity during postendocytic recycling.
To test the hypothesis that the Y29-dependent motif acts at the biosynthetic route, we performed cDNA microinjection experiments in MDCK cells grown on glass coverslips and microinjected after 3 d of reaching confluence. Under these conditions the cells are properly polarized, as is shown by the basolateral distribution of E-cadherin and the presence of detectable primary cilium, which is labeled with a mAb recognizing acetylated tubulin (Supplemental Figure S1). We used an established approach that assesses transport from the TGN to the cell surface (Kreitzer et al., 2000). The wild-type or mutant receptor was expressed together with the chaperone RAP, which is required for correct folding and subsequent transport of LRP1 out of the endoplasmic reticulum (Bu and Marzolo, 2000). In addition, we coexpressed a dominant negative form of the accessory protein eps15 (D/N-eps15), known to interfere with the clathrin-mediated endocytosis of the TfR, as a fusion protein with green fluorescent protein (GFP; Benmerah et al., 1999) and also previously used to block the endocytosis of ApoER2, another member of the LDLR family (Cuitino et al., 2005). We checked that this D/N-eps15 effectively interrupts the internalization of mLRP4wt in transient transfection conditions (Supplemental Figure S2, A and B) and also under the microinjection conditions used, in this case for the mLRP4 Y29A (Supplemental Figure S2C). In this way, we attempted to inhibit the rapid endocytosis of mLRP4 from the basolateral membrane and thus increase the probability of its detection at the cell surface. In addition, this strategy allow us to determine whether the apical distribution of the Y29A receptor mutant derives from a sorting defect at the TGN, resulting in its direct segregation to the apical domain, or at an endosomal compartment, which would determine basolateral to apical transcytosis instead of basolateral recycling.
The cells microinjected with the corresponding plasmids were first incubated for 1 h at 37°C to allow expression and then for 2 h at 20°C to accumulate the newly synthesized proteins at the TGN. After releasing the TGN block for 1 h at 37°C, both the wild-type mLRP4 and the Y29A minireceptor showed a predominant intracellular vesicular pattern, with some apical distribution of Y29A (Supplemental Figure S3). Then, in the presence of D/N-eps15, mLRP4 distributed basolaterally, indicating that its endocytosis was effectively reduced (Figure 5). Instead, in these conditions the Y29A mutant showed an apical cell surface distribution, albeit with some intracellular staining. Supplemental Figure S4 shows higher magnification images corresponding to the same confocal plane, for mLRP4Y29A, mLRP4wt and E-cadherin, which clearly illustrate the basolateral distribution of wt minireceptor (coincident with E-cadherin distribution), whereas the mutant was mostly intracellular. These results are representative of the analysis of several fields, quantifying the distribution of minireceptors in 200 microinjected cells (D/N-eps15-GFP–positive cells) per condition (Supplemental Figure S5A). The wild-type receptor distributed basolaterally in about 70% of microinjected cells. In 25% of the cells we could detect apical staining, representing just 7.4% of the total fluorescence (Supplemental Figure S5B), suggesting missorting by overexpression. In contrast, the Y29A mutant distributed apically in 70% of cells, accounting for 50% of the total fluorescence (Supplemental Figure S5B), with the remaining fluorescence being only intracellular.
Figure 5.
The mLRP4Y29A mutant is directly addressed to the apical domain in MDCK cells. Three days after reaching confluency, MDCK cells plated on glass coverslips were microinjected in the nucleus with the expression plasmids mLRP4 or Y29A together with pcDNA-RAP and a plasmid encoding the dominant negative form of eps15 (D/N-eps15), GFP- Eps15 DD (EΔ95/295). After incubating the cells for 1 h at 37°C, the newly synthesized receptors were accumulated in the TGN at 20°C for 2 h in the presence of cycloheximide. TGN-to-cell-surface protein traffic was then allowed to proceed at 37°C for 60 min, and the cells were treated for indirect immunofluorescence. The apical or intracellular/basolateral surface distributions of the receptors were assessed in nonpermeabilized and permeabilized cells, respectively. Expression of the D/N-eps15 increased mLRP4wt detection at the cell surface. The cells were analyzed by confocal microscopy and representative x-y plane and x-z and y-z sections are shown (red). D/N-eps15 expression is shown in the x-y plane, medial to x-z sectioning (green). The mLRP4wt appears in intracellular vesicles and in the basolateral membrane, whereas the Y29A mutant was present only in intracellular vesicles and at the apical surface, indicating direct TGN-to-apical addressing. Scale bar: 20 μm. Arrowhead indicates selected x-y plane of x-z sectioning.
These results discard two possibilities: First, that the Y29A mutant is sorted without polarity to both cell surfaces and upon endocytosis from the basolateral domain becomes intracellularly arrested at endosomal compartments and subsequently disappears from this domain, and second, that the Y29A mutant reaches the apical pole by transcytosis after rapid endocytosis from the basolateral domain. In both cases, the endocytic inhibition caused by coexpressing D/N-eps15 would have increased the basolateral location of the mutant. The Y29A mutation most likely released otherwise recessive apical sorting information present in the ectodomain (Marzolo et al., 2003).
Experiments in FRT cells showed that after releasing the TGN block in the presence of the function blocking anti-μ1B antibody the Y29A mutant did not accumulate in the post-Golgi RE (Figure 6), contrasting with the wild-type receptor (see Figure 2). Under these conditions, the Y29A mutant distributed in peripheral vesicles and was not detected at any moment in μ1B-RE, indicating that it was very likely missorted directly from the TGN. Therefore, the Y29 seems to be part of a dominant basolateral sorting signal that operates in the TGN during biosynthetic trafficking. The results cannot exclude the possibility that the same sorting signal also operates at RE during post-TGN trafficking or that additional biosynthetic basolateral sorting signals decoded by AP1B direct LRP1 to the basolateral plasma membrane.
Figure 6.
The mutant Y29A does not traffic through post-Golgi compartment to get the apical plasma membrane. FRT cells were microinjected with mLRP4-Y29A minireceptor plus the chaperone RAP cDNAs and the blocking-function anti-μ1B antibody. Cells were maintained for 1 h at 37°C, followed by 2 h at 20°C to accumulate the receptor at the TGN, and then the cells were fixed at the indicated time and processed for immunofluorescence with anti-HA to detect the minireceptor. Kinetic analyses of mLRP4-Y29A/μ1B colocalization of newly synthesized mLRP4-Y29A show that the μ1B compartment is not involved in biosynthetic trafficking of this mutant. Scale bar, 10 μm.
The N26PTY29 Motif Is Involved in Basolateral Recycling and in Recruiting SNX17 to BSEs
It is currently believed that the functional integrity of NPxY motifs depend equally on both the N and the Y residues (Bansal and Gierasch, 1981). Mutational analysis has revealed that the Y residue can be engaged in functions different from those of the NPxY motif. This was first demonstrated in the LDLR, in which the NPxY motif also acts as an endocytic signal, but the Y residue participates independently of the N residue in basolateral sorting (Matter et al., 1992).
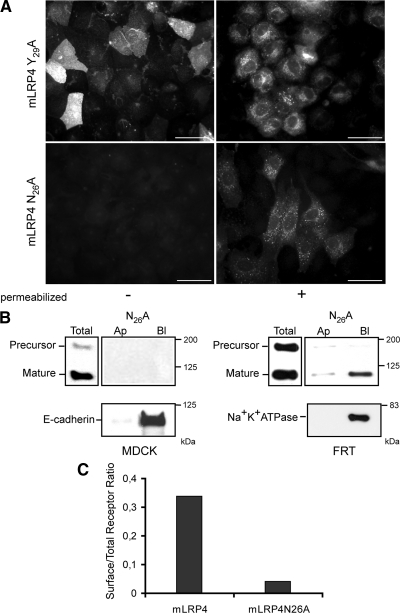
In LRP1, the proximal NPxY motif has no role in endocytosis (Li et al., 2000) but regulates receptor's recycling (van Kerkhof et al., 2005). Because the Y29A mutation caused missorting to the apical domain, we decided to study the effect an N26 A mutation. Surprisingly, in FRT cells, most of the mLRP4N26A mutant displayed an intracellular location instead of an apical distribution (Figure 7A). MDCK cells showed the same effect (not shown). Biotinylation assays could not detect mLRP4N26A mutant at the cell surface of MDCK cells, whereas a rather small amount appeared basolaterally in FRT cells, relative to its total level of expression (Figure 7B). By detecting E-cadherin and Na+K+ ATPase at the basolateral domain of MDCK and FRT cells, respectively, we ensured that the cells were correctly polarized and that the biotin had access to the basolateral cell surface.
Figure 7.
Mutations within the proximal NPxY motif of the LRP1 tail result in different receptor distributions. (A) FRT cells stably transfected with either mLRP4Y29A or mLRP4N26A were grown to confluence on coverslips and treated for indirect immunofluorescence with anti-HA under nonpermeabilized and permeabilized conditions, as indicated. Y29A distributed both at the apical cell surface (right panel) and intracellular vesicles (left panel), whereas N26A show only an intracellular localization, not being detectable at the cell surface. MDCK cells gave similar results (not shown). Scale bars, 10 μm. (B) Domain-specific cell surface biotinylation of MDCK and FRT cells expressing mLRP4N26A. In MDCK cells the protein was not detected at the cell surface. Controls confirmed the receptor expression (immunoblot of total lysate) and proper basolateral expression of E-cadherin. In FRT cells, however, a low amount of minireceptor was detected at the basolateral cell surface. Na+K+ ATPase was used as an endogenous basolateral marker. (C) MDCK cells expressing either mLRP4wt or the N26A mutant were consecutively incubated, either intact or after permeabilization with 0.1% saponin with an anti-HA and anti-mouse RPE-conjugated antibody, and analyzed by flow cytometry. The ratio of expression levels observed in intact versus permeabilized cells show 30% of the wild-type receptor versus no more than 4% of the N26A mutant at the cell surface.
In a complementary approach, we used flow cytometry to compare the expression level of the wild-type and mutant receptors at the cell surface of MDCK cells. Only 4% of the total mLRP4N26A was at the cell surface, compared with ∼33% of the wild-type minireceptor, indicating the mutant minireceptor is able to get the cell surface but with an almost 10-fold less efficiency than mLRP4 wild type (Figure 7C). Thus, our results suggest that after rapid internalization the N26A mutant stays longer in rate-limiting recycling compartments. If this is so, one might expect that inhibiting the LRP1 endocytosis would increase the detection of the mutant at the basolateral cell surface. The microinjection protocol, detailed in Figure 5, to coexpress D/N-eps15 allowed detecting the N26A mutant at the basolateral membrane, albeit in a few cells (Figure 8A). As shown for the wild-type and Y29A mutant minireceptors, we quantified the % of microinjected cells expressing the N26A mutant at the cell surface, finding that no more than 5% of the cells had surface staining, being almost all basolateral (Supplemental Figure S5).
Figure 8.
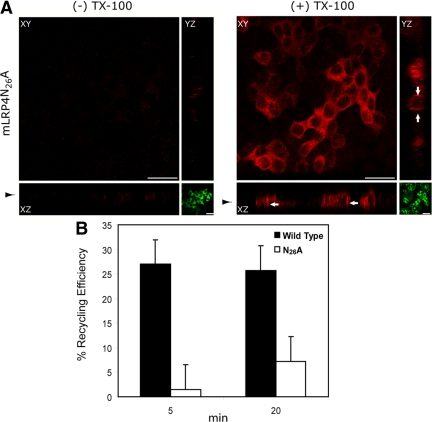
The mutant N26A reaches the basolateral membrane, but its recycling efficiency is severely impaired. (A) MDCK cells were microinjected 3 d after reaching confluence with the plasmid encoding the D/N-eps15, GFP- EPS15 DD (EΔ95/295) as described and analyzed in Figure 5. Confocal microscopy and representative x-y plane and x-z and y-z sections are shown (red). D/N-eps15 expression is shown as x-y plane medial of x-z sectioning (green). The distribution of the N26A mutant was mainly intracellular, but was also detectable at the basolateral membrane (arrows). Scale bar, 20 μm. Arrowhead indicates selected x-y plane of x-z sectioning. (B) The recycling efficiency of the minireceptors was evaluated in MDCK cell lines by a fluorescence quenching assay. The mLRP4wt or N26A mutant were labeled with Alexa488-conjugated anti-HA antibody at 37°C for 20 min and then chased for the indicated time periods in the presence of quenching anti-Alexa488 IgG. Cells were processed for flow cytometry, and the recycling efficiency was calculated. The data were plotted and are shown in the graph, corresponding to two experiments measured in duplicate. Average values are shown; error bars, SEM.
To test directly whether recycling of the N26A mutant is defective in polarized epithelial cells, as was previously shown in nonpolarized cells (van Kerkhof et al., 2005), we used a described cell surface fluorescence quenching recycling assay (van Kerkhof et al., 2005). In this procedure, the receptor that becomes exposed to the cell surface binds Alexa488-labeled anti-HA and after 20 min of internalization, the fluorescent antibody is quenched by an anti-Alexa488 antibody; the magnitude of the quenching being proportional to the receptor's recycling rate. Accordingly to the difference in the cell surface expression of the minireceptors (Figure 7C and Supplemental Figure S5), cells expressing the wild-type protein show a fivefold increase in the total fluorescence intensity compared with the N26A mutant after 20 min of anti-HA internalization (not shown). At 5 min of antibody quenching, the measured recycling efficiency of cells expressing the wild-type mLRP4 was around 27%, compared with <2% for cells expressing the N26A mutant. After 20 min of quenching, the efficiency of recycling for the mutant was still very low (7% compared with 26% of the control).
Taken together, all these results demonstrate that recycling of the mutant N26A receptor is severely impaired (Figure 8B) and more dramatically than in nonpolarized cells (van Kerkhof et al., 2005). Thus the lack of surface localization of this mutant at the steady state is most likely due to abrogation of a motif that promotes recycling.
To determine the sorting compartment where the N26A mutant minireceptor is intracellularly arrested and its relationship with SNX17, a cytosolic protein involved in LRP1 recycling (van Kerkhof et al., 2005), we performed quantitative analysis of immunofluorescent colocalization with the EEA1, an early endosomal marker. We found a significantly increased colocalization of the N26A mutant minireceptor with EEA1 (62 ± 2.7% of the N26A mutant vs. 54 ± 2.8% of the mLRP4 wild-type; p < 0.05, unpaired t test; Figure 9). Because the proximal NPxY is the motif that binds SNX17 and mediates LRP1's recycling (van Kerkhof et al., 2005), we assessed the distribution of SNX17 in cells expressing either the wild-type or the N26A mutant receptor. The two available antibodies recognizing the human protein (van Kerkhof et al., 2005) detect the endogenous SNX17 in MDCK cells in immunoblot (not shown), but not by immunofluorescence. Therefore we performed microinjection experiments to express myc-tagged human SNX17 together with the minireceptors and RAP.
Figure 9.
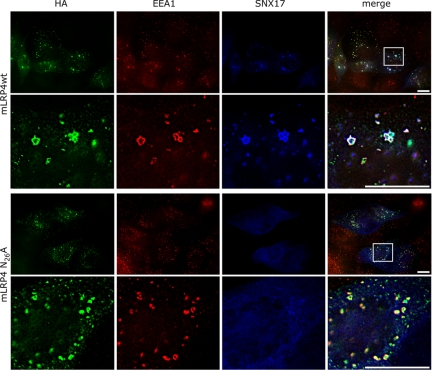
Colocalization of minireceptors with endosomal markers EEA1 and SNX17. MDCK cells were microinjected with the plasmids encoding for RAP, SNX17-myc, and either the mLRP4wt or N26A minireceptors. Cells were processed for immunofluorescence to detect the HA-tagged minireceptor (green), the early endosome marker EEA1 (red), and the myc-tagged SNX17 (blue). In the amplified merged images, colocalization of the wild-type minireceptor with EEA1 in endosome-like structures was clearly visible; some of these structures also contain SNX17 (white dots). The N26A minireceptors practically did not colocalize with SNX17 and exhibited an increased colocalization with EEA1 (amplified merge image, yellow dots). Scale bars, 10 μm.
Interestingly, SNX17 displayed a better endosomal distribution when coexpressed with the wild-type minireceptor than with the N26A mutant receptor. In cells expressing the mLRP4wt 37 ± 3.3% of EEA1 endosomes contained SNX17, versus 5.8 ± 1.8% in cells expressing the N26A mutant. The wild-type minireceptor colocalized 33 ± 3% with vesicular SNX17, versus 7 ± 2.1% by the N26A mutant receptor. These results show that mLRP4 promotes SNX17 recruitment to EEA1 early endosomes and provide further evidence that N26 is part of a sorting signal that mediates LRP1 basolateral recycling. As judged by the immunofluorescence patterns, the rate-limiting step of LRP1 recycling could be EEA1/SNX17 containing compartments.
All these results indicate that N26 and Y29 residues conform distinct sorting determinants that act at different steps of LRP1 trafficking. The Y29 constitutes a biosynthetically basolateral sorting signal that functions at the TGN, and acts independently of the NPxY motif, whereas the N26 and the Y29 residues within the proximal NPxY motif regulate an additional sorting event occurring at the recycling route of the receptor. This recycling step most likely involves SNX17 recruitment to the EEA1 endosomes that would represent an earlier compartment than μ1B-containing endosomes.
The Endocytic Motifs, Y63ATL and LL86,87, Also Contribute to Receptor Basolateral Recycling
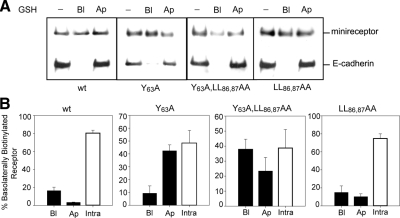
The nonpolarized cell surface distribution of LRP1 mutants Y63A and LL86,87A resemble LRP1 in the absence of AP1B (Marzolo et al., 2003), thus suggesting that these motifs are involved in basolateral sorting from a μ1B recycling compartment. Indeed, μ1B could recognize tyrosine-based motifs of the kind of Y63ATL66. In addition, both Y63A and LL86,87A motifs have been reported to decrease, but not completely abrogate, the endocytic rate of LRP1 (Li et al., 2000). Therefore, these motifs could also contribute to the polarized recycling, maybe at a different step than that mediated by N26PxY29 motif. To test this hypothesis, we performed transcytosis assays in MDCK cells grown on filters, using glutathione stripping of cleavable sulfo-NHS-SS-biotin (Figure 10). After basolateral biotinylation the cells were incubated for 45 min at 37°C. The majority (80 ± 5%) of the mLRP4 wild-type becomes insensitive to glutathione stripping from the apical or basolateral cell surfaces, indicating internalization and steady-state distribution at intracellular compartments, with almost undetectable transcytosis (3.3 ± 0.3%). On the contrary, a percentage of the basolaterally biotinylated mutants, 42.4 ± 4.6% of the Y63A, 24 ± 6% of double mutant Y63A/LL86,87AA, and 11 ± 3% of LL86,87AA mutants, became sensitive to glutathione stripping from the apical cell surface, reflecting an increased basolateral-to-apical transcytosis. These results indicate that Y63 and the LL86,87 promote basolateral recycling, in addition to internalization of LRP1. Disruption of these residues causes nonpolarized recycling, a fraction to the basolateral surface and a fraction to the opposite domain by transcytosis.
Figure 10.
Basolateral to apical transcytosis of mLRPs in MDCK cells. MDCK cells stably expressing one of the following constructs: mLRP4wt, mLRP4LL86,87AA, mLRPY63A, or the double mutant minireceptor, were grown on filters to confluence. Cells were biotinylated with reducible biotin at 4°C, shifted to 37°C for 45 min, and reduced with glutathione either at the apical or basolateral surface. Cells were lysed, biotinylated proteins were precipitated with streptavidin beads, and the complexes resolved by reducing 6% SDS-PAGE and Western blot. (A) Minireceptor detection with anti-HA and E-cadherin blot. The absence of apical reduction of biotinylated E-cadherin indicates that the observed reduction of the minireceptor was due to its transcytosis from the basolateral to the apical surface. (B) Bands were quantified, and the total biotinylated minireceptor was taken as 100% (see Material and Methods). Minireceptor present in the basolateral surface, apical surface and within the cells was determined as a percentage of total. The determinations were performed in triplicate for each construct.
The lack of colocalization of SNX17 and μ1B in polarized MDCK cells (Supplemental Figure S6) strongly suggests that LRP1 experiences two postendocytic recycling steps spatially and temporally separated. The dominant recycling step would depend on the proximal NPxY motif and SNX17 operating at the basolateral sorting endosome (BSE), whereas the few receptors that escape this sorting event would enter into the μ1B-RE and rescued to a basolateral sorting.
Neurons Decode LRP1-sorting Motifs with Mechanisms Similar to Those in Epithelial Cells
LRP1 has been localized in the somatodendritic domain (Brown et al., 1997), but the sorting elements that determine this distribution remain unknown. Having defined the effect of our LRP1 mutants in polarized epithelial cells and the role of μ1B and SNX17 in LRP1 basolateral sorting, we went on to assess their sorting behavior in neurons that are known to lack AP1B (Ohno et al., 1999).
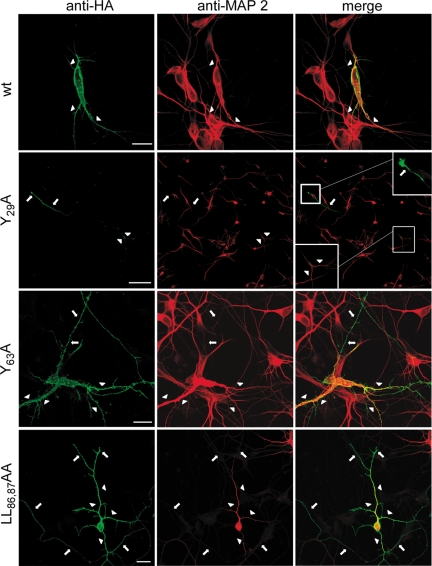
We transfected primary cultured hippocampal neurons with each of the mLRP4 constructs and, by indirect immunofluorescence with anti-HA monoclonal antibodies, assessed the cell surface distribution of the minireceptor in nonpermeabilized cells. Then, we permeabilized the cells to define the somatodendritic domain by MAP2 staining (Caceres et al., 1984). As expected for a somatodendritic protein, the wild-type minireceptor mLRP4 completely colocalized with MAP2 (Figure 11). Minireceptors with Y63A and LL86,87AA mutations mimicked epithelial-sorting behavior when expressed in neurons. Both distributed without polarity, evenly in somatodendritic and axonal domains. Strikingly, the Y29A mutant distributed exclusively at the distal part of the axon.
Figure 11.
The somatodendritic distribution of mLRP in hippocampal neurons depends on the same motifs required for its basolateral distribution in epithelial cells. Primary cultured hippocampal neurons were transfected as described in Materials and Methods. The distribution of the transfected mLRP was determined by confocal microscopy, using anti-HA to detect the receptor at the cell surface (green) and, after cell permeabilization, using anti-MAP2 (red) to determine the somatodendritic domain. The restricted cell surface somatodendritic distribution of mLRP (arrowheads) was lost when the critical basolateral determinants, based on tyrosines and dileucines, were replaced by alanines. This was visualized by the axonal staining of the receptor in MAP2-negative structures (arrows). The most striking distribution was the mLRPY29A mutant, for which only the distal region of the axon was positively stained with anti-HA. Scale bars, 20 μm (wt, Y63A and LL86,87AA) and 100 μm (Y29A).
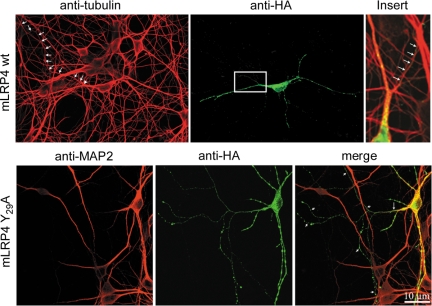
In permeabilized neurons, the wild-type minireceptor exhibits an exclusively somato-dendritic vesicular distribution (Figure 12), resembling the TfR that is also excluded from the axon (Cameron et al., 1991). The intracellular vesicles carrying the Y29A mutant, however, distributed both in axons and dendrites. The mechanistic relationship between such intracellular nonpolarized distribution and the distal axonal sorting that this mutant displays at the cell surface remains unknown.
Figure 12.
Intraneuronal distribution and unrestricted transport of the mLRP4Y29A mutant to the axons. Top, confocal micrographs showing the distribution of tubulin (red) and HA-tagged mLRP4wt (green) in 7 DIV (days in vitro) permeabilized cultured hippocampal neurons. HA-tagged mLRP4 localized to short, dendritic-like processes. Note that a thin long axon-like neurite (arrows) that emerges from a dendritic shaft (arrowhead) and that is positive for tubulin does not contain HA-tagged mLRP4. The inset on the right panel (merge color) shows a high-magnification view of the region where the axon-like neurite originates. Bottom, confocal micrographs showing the distribution of the somatodendritic marker MAP2 (red) and the mutant HA-tagged mLRP4Y29A (green) in 7 DIV cultured hippocampal neurons. Note that the ectopic mutant variant of mLRP4 not only localized to dendritic-like processes, but also to MAP2 (−), axon-like neurites (short arrows in the merge panel). The long arrow in the merge panel shows the dendritic site from which the axon merges. For these experiments cells were fixed and permeabilized 18 h after transfection. Scale bar, 10 μm.
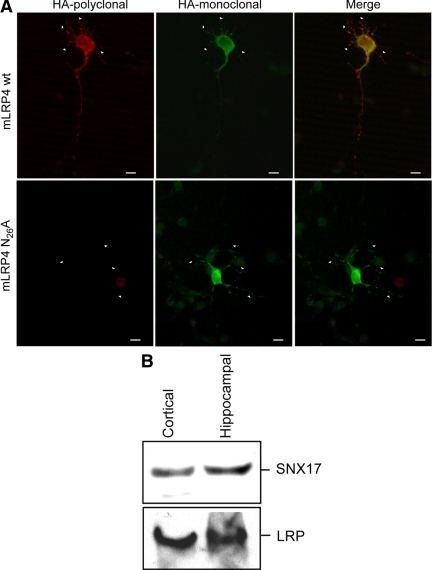
The sorting behavior of the N26A mutant was similar to that as seen in MDCK cells. We could not detect the minireceptor mLRP4N26A at the cell surface of nonpermeabilized neurons, whereas permeabilized cells show intense immunofluorescence staining, indicating intracellular accumulation (Figure 13A). These results suggest that in hippocampal neurons, LRP1 also recycles through a mechanism that depends on the N26PxY29 motif. Supporting this possibility, SNX17 is shown to be endogenously expressed in cultured hippocampal and cortical neurons (Figure 13B).
Figure 13.
Intraneuronal distribution and absence of cell surface expression of the mLRP4N26A mutant in hippocampal neurons. (A) Differential cell surface expression of mLRP4wt (top panel) and N26A (bottom panel) in hippocampal neurons in primary culture was evidenced by immunofluorescence detection of the surface minireceptor using a polyclonal anti-HA (red), followed by permeabilization and detection of the intracellular minireceptor using a monoclonal anti-HA (green). The wild-type mLRP4 was expressed in the somato-dendritic surface, but the N26A mutant was only intracellular and somatodendritic. Scale bars, 10 μm. (B) Hippocampal neurons cultured 7 d in vitro and cortical neurons were lysed, 100 μg of proteins were resolved in reducing SDS-PAGE, and the presence of endogenous SNX17 and LRP1 was determined by Western blot.
All these results suggest that the same sorting motifs that address LRP1 basolaterally in epithelial cells operate to somatodendritically target the receptor in neurons, despite the differences in the expression of AP1B. In this system the recycling step would be only mediated by SNX17.
DISCUSSION
This work shows novel features of the sorting mechanisms involved in the polarized distribution of LRP1, by defining the trafficking behavior of sorting mutants and the participation of AP1B and SNX17. The biosynthetic basolateral pathway of LRP1 includes a TGN sorting event, mediated by Y29, and a post-TGN trafficking through μ1B-containing RE, which is blocked by an μ1B antibody (Cancino et al., 2007). Y29A mutants were apically missorted without entering this μ1B-RE, indicating that Y29 directs LRP1 from TGN to RE. We also detected an interaction of LRP1 with AP1B by coimmunoprecipitation, in agreement with our previous observation of LRP1 missorting in cells lacking μ1B (Marzolo et al., 2003). We also found that a different Y-dependent sorting signal mediates LRP1 basolateral recycling, implicating distinct sorting elements operating during biosynthetic and recycling trafficking. These sorting elements include, for the first time, SNX17 recruitment to BSEs, which are distinct from the AP1B endosomes. Finally, sorting signals of LRP1 seem to be similarly decoded in neurons. We found that neurons, which lack AP1B, express SNX17 and the LRP1 mutant with a defective SNX17-binding site distributes intracellularly as expected for a recycling defect. This suggests that SNX17 constitutes a conserved element of the polarized protein-sorting machinery between neurons and epithelial cells.
Mutational analysis revealed multiple motifs contributing to basolateral distribution of LRP1, including Y29, N26, Y63, and LL85,86 residues. The individual silencing of these residues produces distinct LRP1 distribution patterns, indicating that they are not functionally redundant and cannot compensate the lack of each other. The different phenotypes could be explained by temporal-spatial differences in the sorting events that these motifs mediate.
In nonpolarized cells the proximal NPxY motif of LRP1 constitutes both a binding site for SNX17 and a sorting motif for SNX17-mediated receptor recycling (van Kerkhof et al., 2005). The mutant NPxA29 showed lower recycling efficiency, and SNX17 knockdown by siRNA inhibited LRP1 recycling (van Kerkhof et al., 2005). Although these studies only analyzed the recycling effect of the Y29A mutant, they show that both Y29 and N29 residues are required for SNX17 binding in vitro. Therefore, one might expect that both N26A and Y29A mutant receptors display similar sorting defects unless they follow different pathways. Our current results clearly demonstrate that N26 and Y29 residues exert separable functions at different sorting places in polarized cells.
In polarized cells, the N26A mutation dramatically decreased the cell surface expression of LRP1. The receptor became intracellularly accumulated in endosomes that contain EEA1, thus corresponding to described BSE (Wilson et al., 2000). LRP1 has a very high internalization rate, ∼10 times higher than LDLR's (Li et al., 2001a), thus at steady state, it distributes mainly at intracellular endocytic pools, but also basolaterally in small yet detectable levels (Marzolo et al., 2003). In MDCK cells, we performed microinjection experiments to follow the sorting of LRP1 minireceptors from the TGN to the cell surface. Coexpression of D/N-eps15 to inhibit LRP1 endocytosis increased the basolateral distribution of the wild-type minireceptors. However, the distribution of the N26A mutants was much less affected, as expected for a defect in recycling, which was demonstrated using a sensitive recycling assay and cytometry. The results showed that recycling efficiency of N26A mutant decreased by ∼90%.
The only proteins so far described to mediate Y-dependent basolateral sorting have been the clathrin adaptors AP4 (Simmen et al., 2002) and AP1B (Folsch et al., 1999; Sugimoto et al., 2002). In nonpolarized cells, LRP1 recycling involves SNX17 (van Kerkhof et al., 2005). Strikingly, we found here that the wild-type minireceptor, but not the N26A mutant, recruits SNX17 to EEA1-containing BSE, the same compartments in which the mutant accumulates. This previously unnoticed correspondence between NPxY-mediated SNX17 endosomal recruitment and LRP1 recycling indicates that SNX17 constitutes a novel element of the basolateral recycling machinery.
When the Y29A mutant was coexpressed with D/N-eps15 and after having exited the TGN, it appeared at the apical cell surface, without displaying any basolateral location. Therefore, in epithelial cells the Y29A mutant does not follow the same pathway as the N26A mutant; otherwise, a recycling arrest would have led to its intracellular accumulation, with very little or undetectable cell surface expression. Experiments performed in FRT cells, which first accumulated the Y29A in the TGN by the 20°C block and then followed its exit from this compartment, did not detect a transit through the μ1B-RE, in contrast to the wild-type receptor. Thus, the data fit better with a scenario in which the Y29A mutant, due to recessive apical information possibly contained in its ectodomain (Marzolo et al., 2003), reaches the apical surface directly from the TGN rather than from the post-Golgi endosomal compartment or from the basolateral cell surface by transcytosis (Figure 14). Thus, LRP1 contains a Y29-dependent, but N26-independent, sorting signal for biosynthetic basolateral sorting at the TGN, whereas the N26PxY29 motif directs postendocytic exit from BSE, through a mechanism involving membrane recruitment of SNX17 (Figure 14).
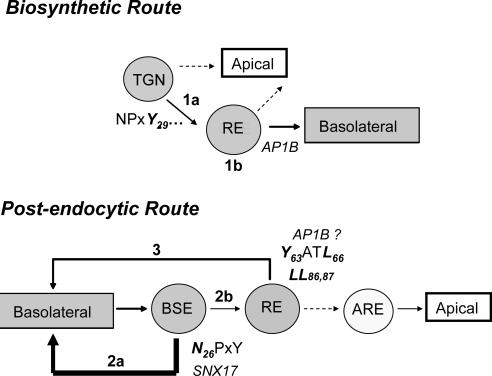
Figure 14.
Working model for LRP1 intracellular trafficking in epithelial cells. The data obtained in this work and others previously published (Marzolo et al., 2003; Rodriguez-Boulan et al., 2005; van Kerkhof et al., 2005; Cancino et al., 2007) allow us to derive a working model for LRP1 trafficking. During the biosynthetic pathway, LRP1 is primarily segregated into basolateral-destined vesicles through the recognition of Y29, likely without an NPxY motif. This recognition step occurs at the TGN and likely requires a not yet known adaptor protein(s) that recognizes the critical tyrosine residue (1a). Most of LRP1 should be then segregated by AP1B in the RE (1b), probably recognized through the distal tyrosine-based signal YATL. When the sorting signal is mutated or the corresponding sorting machinery is functionally absent, the receptor fails to be correctly segregated and is directed to the apical membrane or to both domains (punctuate arrow). Once LRP1 arrives at the basolateral membrane, it is actively endocytosed and has to be postendocytically segregated from the basolateral sorting endosome (BSE) in a manner that depends on the proximal NPxY motif and SNX17 (2a; van Kerkhof et al., 2005). A failure in this step causes LRP1 to be trapped in this compartment. After BSE (2b), a secondary recycling step may occur at the common RE and depend on the Y63ATL66/LL86,87 motifs and probably on the AP1B adaptor complex as well (Marzolo et al., 2003) (3).
Exhaustive studies on LDLR have led to the prevalent notion that the same Y-dependent basolateral information is decoded during biosynthetic and recycling trafficking (Matter et al., 1993). In this sense, our results constitute the first indication that the biosynthetic and recycling sorting machineries can distinguish between Y-based sorting motifs. Furthermore, we provide evidence that such distinction could be due to temporally and spatially separated sorting events accomplished by yet unknown adaptors at the TGN, AP1B in RE, and SNX17 in BSEs. In FRT cells, where our anti-μ1B antibody is suitable for immunofluorescent staining of endogenous μ1B, we found that the SNX17 endosomes contain EEA1, which is considered a marker of BSEs (Wilson et al., 2000), but do not contain μ1B. AP1B distributes poorly in early endosomes labeled with rab 5 (Gan et al., 2002) and distributes in a compartment with typical characteristics of CRE (Cancino et al., 2007). Because CRE is downstream BSE, all these observations mean that most recycling of LRP1 is SNX17-mediated at BSEs before reaching CRE.
Our results reinforce the notion that NPxY motifs can have distinct sorting functions and even dual functions in a same protein depending on the kind of cell. The trafficking role of NPxY motifs of other proteins seems to be mainly endocytic (Chen et al., 1990; Hsu et al., 1994; Cuitino et al., 2005). However, neither of the two cytosolic NPxY motifs of LRP1 play roles in internalization (Li et al., 2000). In the LDLR, the proximal NPxY motif is an endocytic motif in which the tyrosine residue, independent of the N and P residues and together with downstream acidic residues, constitutes a mild basolateral sorting signal, as it is easily overcome by receptor overexpression and becomes apparent only in truncated receptors (Matter et al., 1992, 1994). The strongest basolateral sorting signal of the LDLR depends on a more distal tyrosine and also includes distal acidic residues (Matter et al., 1994). In contrast, the proximal Y29-dependent basolateral sorting signal of LRP1 is rather independent of downstream acidic residues and exerts basolateral destination even upon receptor overexpression and in the context of the entire cytosolic tail, thus acting as a strong basolateral determinant. This Y29 residue also belongs to an NPxY motif, but it is only crucial for basolateral recycling. These results show for the first time that trafficking information embedded within a single NPxY motif can assign distinct temporal-spatial sorting functions to the Y residue.
We previously showed that Y63 also exerts a basolateral sorting function (Marzolo et al., 2003). Y63 is shared by a distal N60PxY63 motif and by the previously identified endocytic motif Y63ATL (Li et al., 2000). Here, we demonstrate that the Y63ATL is a basolateral sorting motif that seems to function in concert with the other LRP1 endocytic signal LL86,87 (Li et al., 2000). Disruption of any of these motifs separately or together in a double mutant Y63A/LL86,87AA results in nonpolarized receptor distribution, suggesting that both act as a single complex sorting signal. Furthermore, the sorting information conveyed by Y63ATL and LL86,87 does not compensate for the Y29A mutation, thus ruling out their participation at the TGN.
Because Y63A mutant has been shown to decrease but not completely abrogate the endocytic rate of LRP1 (Li et al., 2000), the loss of polarity of this mutant could be achieved during its recycling traffic through a compartment different from BSE, in which the N26PxY29/SNX17 system mediates basolateral recycling. Our biotinylation assays demonstrated that these mutants undergo basolateral-to-apical transcytosis. Taken together, the overall results suggest that Y63A mutants are first addressed basolaterally by Y29-dependent sorting at the TGN and then become missorted to both plasma membrane domains when they reach the CRE compartment, presumably after several rounds of recycling from the SNX17-containing BSE. Because the nonpolarized distribution of the endocytosis mutants is similar to the distribution of LRP1 in cells lacking μ1B (Marzolo et al., 2003), the most plausible model is that Y63ATL and LL86,87 motifs contribute to AP1B-dependent basolateral sorting of LRP1 in CRE compartment, both during post-TGN biosynthetic trafficking and post-SNX17 recycling trafficking. This model implies that AP1B and SNX17 sort LRP1 at different stations of its endocytic recycling itinerary recognizing distinct sorting motifs (see proposed model in Figure 14).
A matter still under scrutiny and debate is the proposed correspondence between basolateral/apical sorting in epithelial cells and somatodendritic/axonal sorting in neurons (Dotti and Simons, 1990; Silverman et al., 2005). It could be dependent on the specific type of sorting signals possessed by individual proteins. For instance, mutants of the LDLR and TfR lacking basolateral sorting signals are directed apically in MDCK cells but without polarity in neurons (Jareb and Banker, 1998). This suggests that neurons cannot handle recessive apical information contained in these proteins. There is also evidence suggesting that neurons do not recognize dihydrophobic-based basolateral sorting signals (Silverman et al., 2005). However, the sorting behavior of wild type and all our mutant receptors expressed in hippocampal neurons show striking congruency with the sorting correspondence hypothesis. Even the apical Y29A mutant segregate exclusively into the distal part of the axon, whereas the Y63A and LL86,87AA mutants segregate without polarity. This indicates that neurons can effectively decode recessive apical information as well as dihydrophobic somatodendritic sorting signals in recycling proteins. Interestingly, both the axon and dendrites contained intracellular vesicles carrying the Y29A mutant, suggesting a complex mechanism of distal axonal regionalization that, as described for other axonal proteins such as NgCAM (Horton and Ehlers, 2003), might involve selective fusion/retention processes. Neurons do not express AP1B (Ohno et al., 1999), but we found that they do express SNX17. Furthermore, neurons also intracellularly distributed the N26A mutant lacking the SNX17 binding motif. In principle, AP4, which has been involved in somatodendritic addressing of the glutamate-receptor δ2 (Yap et al., 2003), could sort LRP1 at the TGN, whereas SNX17 could play a predominant role in LRP1 somatodendritic recycling. The missorting similarities of the LRP1 mutants suggest conservation of the compartmentalized use of sorting signals at the TGN and recycling compartments in epithelial cells and neurons.
LRP1 is required for development and exerts crucial physiological functions in tissues such as brain, liver and different epithelia, which rely on polarized cells (Herz et al., 1992; Herz et al., 1993). We could anticipate, in accordance with recent data (Roebroek et al., 2006), that natural mutants affecting those critical sorting motifs that we have defined, especially the proximal NPxY, would be relatively unviable.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Alexander Benmerah (Institut Cochin, U567 INSERM/UMR8104 CNRS, Paris, France) for giving us the plasmid encoding the dominant negative forms of eps15 with GFP and Dr. Ira Mellman (Genentech, South San Francisco, CA) for providing us the cDNA of μ1B-HA-tagged. From the Facultad de Ciencias Biológicas, PUC, we thank Dr. Alejandra Alvarez for providing us some of the hippocampal neurons used in this work, and Dr. Alexis Kalergis and Leandro Carreño for providing access to the FACS equipment. We also thank Dr. María Isabel Yuseff for the characterization of the human anti-EEA1 antibodies in our laboratory and Olivia Ying for reviewing this manuscript. This work was supported by FIRCA grant TW006456 to G.B. and M.P.M., National Institutes of Health Grant R01 AG027924 to G.B., an Alzheimer's Disease Research grant from the American Health Assistance Foundation (AHAF) to G.B., Grant 1020746 from the Fondo Nacional de Investigación Científica y Tecnológica (FONDECYT) to M.P.M., Fondo de Investigación Avanzada en Areas Prioritarias (FONDAP) Grant 13980001 and MIFAB to M.P.M. and A.G. It was also supported by grants from Comisión Nasional de Investigación Cientifico y Technológico (CONICET) and Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT; Argentina) to A.C. G.B. is an Established Investigator of the American Heart Association.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E08-08-0805) on November 19, 2008.
REFERENCES
- Ang A. L., Taguchi T., Francis S., Folsch H., Murrells L. J., Pypaert M., Warren G., Mellman I. Recycling endosomes can serve as intermediates during transport from the Golgi to the plasma membrane of MDCK cells. J. Cell Biol. 2004;167:531–543. doi: 10.1083/jcb.200408165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroeti B., Mostov K. E. Polarized sorting of the polymeric immunoglobulin receptor in the exocytotic and endocytotic pathways is controlled by the same amino acids. EMBO J. 1994;13:2297–2304. doi: 10.1002/j.1460-2075.1994.tb06513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacskai B. J., Xia M. Q., Strickland D. K., Rebeck G. W., Hyman B. T. The endocytic receptor protein LRP also mediates neuronal calcium signaling via N-methyl-D-aspartate receptors. Proc. Natl. Acad. Sci. USA. 2000;97:11551–11556. doi: 10.1073/pnas.200238297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banker G. A., Cowan W. M. Rat hippocampal neurons in dispersed cell culture. Brain Res. 1977;126:397–425. doi: 10.1016/0006-8993(77)90594-7. [DOI] [PubMed] [Google Scholar]
- Bansal A., Gierasch L. M. The NPXY internalization signal of the LDL receptor adopts a reverse-turn conformation. Cell. 1981;67:1195–1201. doi: 10.1016/0092-8674(91)90295-a. [DOI] [PubMed] [Google Scholar]
- Benmerah A., Bayrou M., Cerf-Bensussan N., Dautry-Varsat A. Inhibition of clathrin-coated pit assembly by an Eps15 mutant. J. Cell Sci. 1999;112:1303–1311. doi: 10.1242/jcs.112.9.1303. [DOI] [PubMed] [Google Scholar]
- Bottenstein J. E., Sato G. H. Growth of a rat neuroblastoma cell line in serum-free supplemented medium. Proc. Natl. Acad. Sci. USA. 1979;76:514–517. doi: 10.1073/pnas.76.1.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradke F., Dotti C. G. Membrane traffic in polarized neurons. Biochim. Biophys. Acta. 1998;1404:245–258. doi: 10.1016/s0167-4889(98)00060-3. [DOI] [PubMed] [Google Scholar]
- Brandan E., Retamal C., Cabello-Verrugio C., Marzolo M. P. The low density lipoprotein receptor-related protein functions as an endocytic receptor for decorin. J. Biol. Chem. 2006;281:31562–31571. doi: 10.1074/jbc.M602919200. [DOI] [PubMed] [Google Scholar]
- Bravo-Zehnder M., Orio P., Norambuena A., Wallner M., Meera P., Toro L., Latorre R., Gonzalez A. Apical sorting of a voltage- and Ca2+-activated K+ channel alpha-subunit in Madin-Darby canine kidney cells is independent of N-glycosylation. Proc. Natl. Acad. Sci. USA. 2000;97:13114–13119. doi: 10.1073/pnas.240455697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. D., Banker G. A., Hussaini I. M., Gonias S. L., Vandenberg S. R. Low density lipoprotein receptor-related protein is expressed early and becomes restricted to a somatodendritic domain during neuronal differentiation in culture. Brain Res. 1997;747:313–317. doi: 10.1016/s0006-8993(96)01321-2. [DOI] [PubMed] [Google Scholar]
- Bu G., Marzolo M. P. Role of rap in the biogenesis of lipoprotein receptors. Trends Cardiovasc. Med. 2000;10:148–155. doi: 10.1016/s1050-1738(00)00045-1. [DOI] [PubMed] [Google Scholar]
- Bu G., Williams S., Strickland D. K., Schwartz A. L. Low density lipoprotein receptor-related protein/alpha 2-macroglobulin receptor is an hepatic receptor for tissue-type plasminogen activator. Proc. Natl. Acad. Sci. USA. 1992;89:7427–7431. doi: 10.1073/pnas.89.16.7427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgos P. V., Klattenhoff C., de la Fuente E., Rigotti A., Gonzalez A. Cholesterol depletion induces PKA-mediated basolateral-to-apical transcytosis of the scavenger receptor class B type I in MDCK cells. Proc. Natl. Acad. Sci. USA. 2004;101:3845–3850. doi: 10.1073/pnas.0400295101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caceres A., Banker G., Steward O., Binder L., Payne M. MAP2 is localized to the dendrites of hippocampal neurons which develop in culture. Brain Res. 1984;315:314–318. doi: 10.1016/0165-3806(84)90167-6. [DOI] [PubMed] [Google Scholar]
- Caceres A., Mautino J., Kosik K. S. Suppression of MAP2 in cultured cerebellar macroneurons inhibits minor neurite formation. Neuron. 1992;9:607–618. doi: 10.1016/0896-6273(92)90025-9. [DOI] [PubMed] [Google Scholar]
- Cameron P. L., Südhof T. C., Jahn R., Camilli P. d. Colocalization of synaptophysin with transferrin receptors: implications of synaptic vesicle biogenesis. J. Cell Biol. 1991;1991:151–164. doi: 10.1083/jcb.115.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancino J., Torrealba C., Soza A., Yuseff M. I., Gravotta D., Henklein P., Rodriguez-Boulan E., Gonzalez A. Antibody to AP1B adaptor blocks biosynthetic and recycling routes of basolateral proteins at recycling endosomes. Mol. Biol. Cell. 2007;18:4872–4884. doi: 10.1091/mbc.E07-06-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova J. E., Apodaca G., Mostov K. E. An autonomous signal for basolateral sorting in the cytoplasmic domain of the polymeric immunoglobulin receptor. Cell. 1991;66:65–75. doi: 10.1016/0092-8674(91)90139-p. [DOI] [PubMed] [Google Scholar]
- Chen W. J., Goldstein J. L., Brown M. S. NPXY, a sequence often found in cytoplasmic tails, is required for coated pit-mediated internalization of the low density lipoprotein receptor. J. Biol. Chem. 1990;265:3116–3123. [PubMed] [Google Scholar]
- Cresawn K. O., Potter B. A., Oztan A., Guerriero C. J., Ihrke G., Goldenring J. R., Apodaca G., Weisz O. A. Differential involvement of endocytic compartments in the biosynthetic traffic of apical proteins. EMBO J. 2007;26:3737–3748. doi: 10.1038/sj.emboj.7601813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuitino L., Matute R., Retamal C., Bu G., Inestrosa N. C., Marzolo M.-P. ApoER2 is endocytosed by a clathrin-mediated process involving the adaptor protein Dab2 independent of its rafts association. Traffic. 2005;6:820–838. doi: 10.1111/j.1600-0854.2005.00320.x. [DOI] [PubMed] [Google Scholar]
- Deborde S., Perret E., Gravotta D., Deora A., Salvarezza S., Schreiner R., Rodriguez-Boulan E. Clathrin is a key regulator of basolateral polarity. Nature. 2008;452:719–723. doi: 10.1038/nature06828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deora A. A., Gravotta D., Kreitzer G., Hu J., Bok D., Rodriguez-Boulan E. The basolateral targeting signal of CD147 (EMMPRIN) consists of a single leucine and is not recognized by retinal pigment epithelium. Mol. Biol. Cell. 2004;15:4148–4165. doi: 10.1091/mbc.E04-01-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotti C. G., Parton R. G., Simons K. Polarized sorting of glypiated proteins in hippocampal neurons. Nature. 1991;349:158–161. doi: 10.1038/349158a0. [DOI] [PubMed] [Google Scholar]
- Dotti C. G., Simons K. Polarized sorting of viral glycoproteins to the axon and dendrites of hippocampal neurons in culture. Cell. 1990;62:63–72. doi: 10.1016/0092-8674(90)90240-f. [DOI] [PubMed] [Google Scholar]
- Fiedler K., Simons K. The role of N-glycans in the secretory pathway. Cell. 1995;81:309–312. doi: 10.1016/0092-8674(95)90380-1. [DOI] [PubMed] [Google Scholar]
- Folsch H., Ohno H., Bonifacino J. S., Mellman I. A novel clathrin adaptor complex mediates basolateral targeting in polarized epithelial cells. Cell. 1999;99:189–198. doi: 10.1016/s0092-8674(00)81650-5. [DOI] [PubMed] [Google Scholar]
- Folsch H., Pypaert M., Schu P., Mellman I. Distribution and function of AP-1 clathrin adaptor complexes in polarized epithelial cells. J. cell Biol. 2001;152:595–606. doi: 10.1083/jcb.152.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullekrug J., Simons K. Lipid rafts and apical membrane traffic. Ann. NY Acad. Sci. 2004;1014:164–169. doi: 10.1196/annals.1294.017. [DOI] [PubMed] [Google Scholar]
- Fuller S. D., Bravo R., Simons K. An enzymatic assay reveals that proteins destined for the apical and basolateral domains of an epithelial cell line share the same late Golgi compartments. EMBO J. 1985;4:297–307. doi: 10.1002/j.1460-2075.1985.tb03629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan Y., McGraw T. E., Rodriguez-Boulan E. The epithelial-specific adaptor AP1B mediates post-endocytic recycling to the basolateral membrane. Nat. Cell Biol. 2002;4:605–609. doi: 10.1038/ncb827. [DOI] [PubMed] [Google Scholar]
- Godyna S., Liau G., Popa I., Stefansson S., Argraves W. S. Identification of the low density lipoprotein receptor-related protein (LRP) as an endocytic receptor for thrombospondin-1. J. Cell Biol. 1995;129:1403–1410. doi: 10.1083/jcb.129.5.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goretzki L., Mueller B. M. Low-density-lipoprotein-receptor-related protein (LRP) interacts with a GTP-binding protein. Biochem. J. 1998;336:381–386. doi: 10.1042/bj3360381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravotta D., Deora A., Perret E., Oyanadel C., Soza A., Schreiner R., Gonzalez A., Rodriguez-Boulan E. AP1B sorts basolateral proteins in recycling and biosynthetic routes of MDCK cells. Proc. Natl. Acad. Sci. USA. 2007;104:1564–1569. doi: 10.1073/pnas.0610700104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths G., Simons K. The trans-Golgi network: sorting at the exit site of the Golgi complex. Science. 1986;234:4381–4443. doi: 10.1126/science.2945253. [DOI] [PubMed] [Google Scholar]
- Herz J., Bock H. H. Lipoprotein receptors in the nervous system. Annu. Rev. Biochem. 2002;71:405–434. doi: 10.1146/annurev.biochem.71.110601.135342. [DOI] [PubMed] [Google Scholar]
- Herz J., Clouthier D. E., Hammer R. E. LDL receptor-related protein internalizes and degrades uPA-PAI-1 complexes and is essential for embryo implantation [published erratum appears in Cell 1993 May 7;73(3):428] Cell. 1992;71:411–421. doi: 10.1016/0092-8674(92)90511-a. [DOI] [PubMed] [Google Scholar]
- Herz J., Couthier D. E., Hammer R. E. Correction: LDL receptor-related protein internalizes and degrades uPA-PAI-1 complexes and is essential for embryo implantation [letter] Cell. 1993;73:428. doi: 10.1016/0092-8674(93)90130-i. [DOI] [PubMed] [Google Scholar]
- Horton A. C., Ehlers M. D. Neuronal polarity and trafficking. Neuron. 2003;40:277–295. doi: 10.1016/s0896-6273(03)00629-9. [DOI] [PubMed] [Google Scholar]
- Hsu D., Knudson P. E., Zapf A., Rolband G. C., Olefsky J. M. NPXY motif in the insulin-like growth factor-I receptor is required for efficient ligand-mediated receptor internalization and biological signaling. Endocrinology. 1994;134:744–750. doi: 10.1210/endo.134.2.8299569. [DOI] [PubMed] [Google Scholar]
- Hunziker W., Fumey C. A di-leucine motif mediates endocytosis and basolateral sorting of macrophage IgG Fc receptors in MDCK cells. EMBO J. 1994;13:2963–2967. doi: 10.1002/j.1460-2075.1994.tb06594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain M. M. Structural, biochemical and signaling properties of the low-density lipoprotein receptor gene family. Front. Biosci. 2001;6:D417–D428. doi: 10.2741/hussain1. [DOI] [PubMed] [Google Scholar]
- Jareb M., Banker G. The polarized sorting of membrane proteins expressed in cultured hippocampal neurons using viral vectors. Neuron. 1998;20:855–867. doi: 10.1016/s0896-6273(00)80468-7. [DOI] [PubMed] [Google Scholar]
- Kreitzer G., Marmorstein A., Okamoto P., Vallee R., Rodriguez-Boulan E. Kinesin and dynamin are required for post-Golgi transport of a plasma-membrane protein. Nat. Cell Biol. 2000;2:125–127. doi: 10.1038/35000081. [DOI] [PubMed] [Google Scholar]
- Le Bivic A., Real F. X., Rodriguez-Boulan E. Vectorial targeting of apical and basolateral plasma membrane proteins in a human adenocarcinoma epithelial cell line. Proc. Natl. Acad. Sci. USA. 1989;86:9313–9317. doi: 10.1073/pnas.86.23.9313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Gall A. H., Powell S. K., Yeaman C. A., Rodriguez-Boulan E. The neural cell adhesion molecule expresses a tyrosine-independent basolateral sorting signal. J. Biol. Chem. 1997;272:4559–4567. doi: 10.1074/jbc.272.7.4559. [DOI] [PubMed] [Google Scholar]
- Li Y., Lu W., Marzolo M. P., Bu G. Differential functions of members of the low density lipoprotein receptor family suggested by their distinct endocytosis rates. J. Biol. Chem. 2001a;276:18000–18006. doi: 10.1074/jbc.M101589200. [DOI] [PubMed] [Google Scholar]
- Li Y., Marzolo M. P., Kerkhof P., Strous G. J., Bu G. The YXXL motif, but not the two NPXY motifs, serves as the dominant endocytosis signal For LDL receptor-related protein (LRP) J. Biol. Chem. 2000;275:17187–17194. doi: 10.1074/jbc.M000490200. [DOI] [PubMed] [Google Scholar]
- Li Y., van Kerkhof P., Marzolo M. P., Strous G. J., Bu G. Identification of a major cyclic AMP-dependent protein kinase A phosphorylation site within the cytoplasmic tail of the low-density lipoprotein receptor-related protein: implication for receptor-mediated endocytosis. Mol. Cell. Biol. 2001b;21:1185–1195. doi: 10.1128/MCB.21.4.1185-1195.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock J. G., Stow J. L. Rab11 in recycling endosomes regulates the sorting and basolateral transport of E-cadherin. Mol. Biol. Cell. 2005;16:1744–1755. doi: 10.1091/mbc.E04-10-0867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmorstein A. D., Csaky K. G., Baffi J., Lam L., Rahaal F., Rodriguez-Boulan E. Saturation of, and competition for entry into, the apical secretory pathway. Proc. Natl. Acad. Sci. USA. 2000;97:3248–3253. doi: 10.1073/pnas.070049497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzolo M. P., Bull P., Gonzalez A. Apical sorting of hepatitis B surface antigen (HBsAg) is independent of N-glycosylation and glycosylphosphatidylinositol-anchored protein segregation. Proc. Natl. Acad. Sci. USA. 1997;94:1834–1839. doi: 10.1073/pnas.94.5.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzolo M. P., Yuseff M. I., Retamal C., Donoso M., Ezquer F., Farfan P., Li Y., Bu G. Differential distribution of low-density lipoprotein-receptor-related protein (LRP) and megalin in polarized epithelial cells is determined by their cytoplasmic domains. Traffic. 2003;4:273–288. doi: 10.1034/j.1600-0854.2003.00081.x. [DOI] [PubMed] [Google Scholar]
- Matter K., Hunziker W., Mellman I. Basolateral sorting of LDL receptor in MDCK cells: the cytoplasmic domain contains two tyrosine-dependent targeting determinants. Cell. 1992;71:741–753. doi: 10.1016/0092-8674(92)90551-m. [DOI] [PubMed] [Google Scholar]
- Matter K., Mellman I. Mechanisms of cell polarity: sorting and transport in epithelial cells. Curr. Opin. Cell Biol. 1994;6:545–554. doi: 10.1016/0955-0674(94)90075-2. [DOI] [PubMed] [Google Scholar]
- Matter K., Whitney J. A., Yamamoto E. M., Mellman I. Common signals control low density lipoprotein receptor sorting in endosomes and the Golgi complex of MDCK cells. Cell. 1993;74:1053–1064. doi: 10.1016/0092-8674(93)90727-8. [DOI] [PubMed] [Google Scholar]
- Matter K., Yamamoto E. M., Mellman I. Structural requirements and sequence motifs for polarized sorting and endocytosis of LDL and Fc receptors in MDCK cells. J. Cell Biol. 1994;126:991–1004. doi: 10.1083/jcb.126.4.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostov K. E. Epithelial polarity and morphogenesis. Methods. 2003;30:189–190. doi: 10.1016/s1046-2023(03)00024-0. [DOI] [PubMed] [Google Scholar]
- Mostov K. E., Cardone M. H. Regulation of protein traffic in polarized epithelial cells. Bioessays. 1995;17:129–138. doi: 10.1002/bies.950170208. [DOI] [PubMed] [Google Scholar]
- Müsch A., Xu H., Schields D., Rodriguez-Boulan E. Transport of vesicular stomatitis virus G protein to the cells surface is signal mediated in polarized and nonpolarized cells. J. Cell Biol. 1996;133:543–558. doi: 10.1083/jcb.133.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obermoeller L. M., Chen Z., Schwartz A. L., Bu G. Ca2+ and receptor-associated protein are independently required for proper folding and disulfide bond formation of the low density lipoprotein receptor-related protein. J. Biol. Chem. 1998;273:22374–22381. doi: 10.1074/jbc.273.35.22374. [DOI] [PubMed] [Google Scholar]
- Odorizzi G., Trowbridge I. S. Structural requirements for basolateral sorting of the human transferrin receptor in the biosynthetic and endocytic pathways of Madin-Darby canine kidney cells. J. Cell Biol. 1997;137:1255–1264. doi: 10.1083/jcb.137.6.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno H., Tomemori T., Nakatsu F., Okazaki Y., Aguilar R. C., Foelsch H., Mellman I., Saito T., Shirasawa T., Bonifacino J. S. Mu1B, a novel adaptor medium chain expressed in polarized epithelial cells. FEBS Lett. 1999;449:215–220. doi: 10.1016/s0014-5793(99)00432-9. [DOI] [PubMed] [Google Scholar]
- Paglini G., Pigino G., Kunda P., Morfini G., Maccioni R., Quiroga S., Ferreira A., Caceres A. Evidence for the participation of the neuron-specific CDK5 activator P35 during laminin-enhanced axonal growth. J. Neurosci. 1998;18:9858–9869. doi: 10.1523/JNEUROSCI.18-23-09858.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrini G., Suh Y. J., Edelmann L., Rudnick G., Caplan M. J. The axonal gamma-aminobutyric acid transporter GAT-1 is sorted to the apical membranes of polarized epithelial cells. J. Biol. Chem. 1994;269:4668–4674. [PubMed] [Google Scholar]
- Rindler M. J., Ivanov I. E., Plesken H., Rodriguez-Boulan E., Sabatini D. D. Viral glycoproteins destined for apical or basolateral plasma membrane domains traverse the same Golgi apparatus during their intracellular transport in doubly infected Madin-Darby canine kidney cells. J. Cell Biol. 1984;98:1304–1319. doi: 10.1083/jcb.98.4.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Boulan E., Gonzalez A. Glycans in post-Golgi apical targeting: sorting signals or structural props? Trends Cell Biol. 1999;9:291–294. doi: 10.1016/s0962-8924(99)01595-0. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Boulan E., Kreitze G., Musch A. Organization of vesicular trafficking in epithelia. Nat. Rev. Mol. Cell Biol. 2005;6:233–247. doi: 10.1038/nrm1593. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Boulan E., Powell S. K. Polarity of epithelial and neuronal cells. Annu. Rev. Cell Biol. 1992;8:395–427. doi: 10.1146/annurev.cb.08.110192.002143. [DOI] [PubMed] [Google Scholar]
- Roebroek A. J., Reekmans S., Lauwers A., Feyaerts N., Smeijers L., Hartmann D. Mutant Lrp1 knock-in mice generated by recombinase-mediated cassette exchange reveal differential importance of the NPXY motifs in the intracellular domain of LRP1 for normal fetal development. Mol. Cell. Biol. 2006;26:605–616. doi: 10.1128/MCB.26.2.605-616.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosso S., Bollati F., Bisbal M., Peretti D., Sumi T., Nakamura T., Quiroga S., Ferreira A., Caceres A. LIMK1 regulates Golgi dynamics, traffic of Golgi-derived vesicles, and process extension in primary cultured neurons. Mol. Biol. Cell. 2004;15:3433–3449. doi: 10.1091/mbc.E03-05-0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salicioni A. M., Mizelle K. S., Loukinova E., Mikhailenko I., Strickland D. K., Gonias S. L. The low density lipoprotein receptor-related protein mediates fibronectin catabolism and inhibits fibronectin accumulation on cell surfaces. J. Biol. Chem. 2002;277:16160–16166. doi: 10.1074/jbc.M201401200. [DOI] [PubMed] [Google Scholar]
- Silverman M. A., Peck R., Glover G., He C., Carlin C., Banker G. Motifs that mediate dendritic targeting in hippocampal neurons: a comparison with basolateral targeting signals. Mol. Cell Neurosci. 2005;29:173–180. doi: 10.1016/j.mcn.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Simmen T., Honing S., Icking A., Tikkanen R., Hunziker W. AP-4 binds basolateral signals and participates in basolateral sorting in epithelial MDCK cells. Nat. Cell Biol. 2002;4:154–159. doi: 10.1038/ncb745. [DOI] [PubMed] [Google Scholar]
- Soza A., Norambuena A., Cancino J., De La Fuente E., Henklein P., Gonzalez A. Sorting competition with membrane-permeable peptides in intact epithelial cells revealed discrimination of transmembrane proteins not only at the trans-golgi network but also at pre-golgi stages. J. Biol. Chem. 2004;279:17376–17383. doi: 10.1074/jbc.M313197200. [DOI] [PubMed] [Google Scholar]
- Stockinger W., Sailler B., Strasser V., Recheis B., Fasching D., Kahr L., Schneider W. J., Nimpf J. The PX-domain protein SNX17 interacts with members of the LDL receptor family and modulates endocytosis of the LDL receptor. EMBO J. 2002;21:4259–4267. doi: 10.1093/emboj/cdf435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto H., et al. Differential recognition of tyrosine-based basolateral signals by AP-1B subunit mu1B in polarized epithelial cells. Mol. Biol. Cell. 2002;13:2374–2382. doi: 10.1091/mbc.E01-10-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kerkhof P., Lee J., McCormick L., Tetrault E., Lu W., Schoenfish M., Oorschot V., Strous G. J., Klumperman J., Bu G. Sorting nexin 17 facilitates LRP recycling in the early endosome. EMBO J. 2005;24:2851–2861. doi: 10.1038/sj.emboj.7600756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. M., de Hoop M., Zorzi N., Toh B. H., Dotti C. G., Parton R. G. EEA1, a tethering protein of the early sorting endosome, shows a polarized distribution in hippocampal neurons, epithelial cells, and fibroblasts [In Process Citation] Mol. Biol. Cell. 2000;11:2657–2671. doi: 10.1091/mbc.11.8.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winckler B., Mellman I. Neuronal polarity: controlling the sorting and diffusion of membrane components. Neuron. 1999;23:637–640. doi: 10.1016/s0896-6273(01)80021-0. [DOI] [PubMed] [Google Scholar]
- Worby C. A., Dixon J. E. Sorting out the cellular functions of sorting nexins. Nat. Rev. Mol. Cell Biol. 2002;3:919–931. doi: 10.1038/nrm974. [DOI] [PubMed] [Google Scholar]
- Yap C. C., Murate M., Kishigami S., Muto Y., Kishida H., Hashikawa T., Yano R. Adaptor protein complex-4 (AP-4) is expressed in the central nervous system neurons and interacts with glutamate receptor delta2. Mol. Cell Neurosci. 2003;24:283–295. doi: 10.1016/s1044-7431(03)00164-7. [DOI] [PubMed] [Google Scholar]
- Yeaman C., Grindstaff K. K., Nelson W. J. New perspectives on mechanisms involved in generating epithelial cell polarity. Physiol. Rev. 1999;79:73–98. doi: 10.1152/physrev.1999.79.1.73. [DOI] [PubMed] [Google Scholar]
- Zhuo M., Holtzman D. M., Li Y., Osaka H., DeMaro J., Jacquin M., Bu G. Role of tissue plasminogen activator receptor LRP in hippocampal long-term potentiation. J. Neurosci. 2000;20:542–549. doi: 10.1523/JNEUROSCI.20-02-00542.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.