Abstract
The molecular mechanism via which keratinocyte differentiation assembles multiple layers of cells (stratification) is poorly understood. We describe here a novel function of the Rho family member RhoE as a regulator of epidermal morphogenesis. RhoE protein levels are specifically and transiently up-regulated upon keratinocyte differentiation. RhoE up-regulation requires the activity of Rho kinase (ROCK) I, suggesting that both RhoE and ROCKI are important during keratinocyte differentiation. RhoE overexpression results in a striking enlargement of cell size and the number of stratified cells. In contrast, RhoE depletion induces hyperproliferation and delays initiation of keratinocyte differentiation. Interestingly, up-regulation of RhoE protein is seen primarily in basal, undifferentiated cells, in which commitment to differentiation and stratification takes place. RhoE activation in basal cells negatively modulates integrin adhesion, thereby facilitating detachment from the substratum and migration to form suprabasal layers. Thus, RhoE integrates two processes essential for keratinocyte differentiation and stratification: regulation of proliferative status and integrin adhesion.
INTRODUCTION
During epidermal development, a single layer of keratinocytes is converted into multiple layers of terminally differentiating cells that continuously migrate outward and are shed from the body surface. This tissue architecture is precisely maintained and renewed throughout life: the basal cell population attached to the basement membrane retains proliferative capacity and, upon withdrawal from the cell cycle, initiates differentiation to assemble suprabasal, stratified layers (reviewed by Watt, 2002). Thus, the keratinocyte differentiation program involves a tight balance between the regulation of proliferation and differentiation, coupled with a specialized migratory phenotype.
Previous work has defined the importance of cell adhesion receptors in keratinocyte differentiation (Watt, 2002; Fuchs, 2007). Although the contribution of different adhesive structures cannot be underestimated (Garrod et al., 2002), the initiation of stratification in human keratinocytes requires the function of two families of adhesion receptors that provide attachment to the basement membrane (integrins) or to neighboring cells (cadherins). Integrins are normally expressed only in the basal, proliferative layer of the epidermis (Watt, 2002). The reduction of integrin levels observed when keratinocytes differentiate is thought to allow detachment from the basement membrane to migrate upward (Hodivala and Watt, 1994).
In contrast, cadherins are expressed in all living layers of the epidermis and are essential to hold cells together as a multilayered tissue. For example, preventing cadherin adhesion by depletion of calcium ions or inhibitory antibodies abolishes stratification (Wheelock and Jensen, 1992; Hodivala and Watt, 1994). In addition, the function of E- and P-cadherin, the main classical cadherins expressed in the epidermis, is required to induce expression of the differentiation marker transglutaminase but not involucrin (Hines et al., 1999). Together, these studies indicate that adhesive events regulate differentiation-specific and other signaling pathways in keratinocytes (Braga and Yap, 2005; Fuchs, 2007). Interestingly, even in the absence of cadherin adhesion, a pool of keratinocytes can still initiate differentiation (Hodivala and Watt, 1994), suggesting that stratification and initiation of differentiation are tightly coordinated processes but can be separated in vitro. Indeed, keratinocyte differentiation can be induced in a suspension model, in which no attachment to extracellular matrix or neighbors is allowed (Watt, 2002). Thus, keratinocyte differentiation program is a complex, multistep process, in which stratification occurs after cells have committed to differentiate.
During epidermal differentiation, the expression profile of transcription factors, differentiation markers and a number of signaling processes have been mapped (Watt, 2002; Watt et al., 2006; Fuchs, 2007). However, the specific signaling pathways required for keratinocyte stratification per se are not fully understood. At the molecular level, the transcription factor p63 is essential for keratinocyte differentiation and stratification in vivo (reviewed by Morasso and Tomic-Canic, 2005). Expression of p63 can induce transcription of integrins and extracellular matrix components among others (Carroll et al., 2006; Koster et al., 2007), but further links with adhesive receptor-dependent signaling are not known.
The small GTPase Rho (refers collectively to the closely related members RhoA, RhoB, and RhoC) also has been implicated in epithelial morphogenesis and wound closure (Van Aelst and Symons, 2002). During keratinocyte adhesion and differentiation, Rho is activated upon assembly of cell–cell contacts and has been shown to regulate the stability of cadherin receptors in keratinocytes (Braga et al., 1997; Calautti et al., 2002). Inhibition of Rho contributes to skin blistering in pemphigus (Waschke et al., 2006), whereas blocking Rho signaling with C3 transferase prevents keratinocyte terminal differentiation, stimulates growth and induces skin hyperplasia (Sugai et al., 1992; McMullan et al., 2003). Downstream of Rho signaling, the Rho serine-threonine kinase effectors PRK2 and Rho kinase (ROCK) II may participate in keratinocyte differentiation (Calautti et al., 2002; McMullan et al., 2003). PRK2 is specifically activated after calcium-induced differentiation, but the functional outcome is not known (Calautti et al., 2002). In contrast, overexpression/activation of ROCKII during suspension-induced differentiation leads to cell cycle arrest and an increase in the expression levels of differentiation markers (McMullan et al., 2003). Consistent with these findings, remodelling of the actin cytoskeleton in vivo in mouse keratinocytes seems to depend on Rho and ROCK signaling (Vaezi et al., 2002). Thus, it seems that activation of Rho and ROCK participates in the acquisition of actin reorganization and regulation of keratinocyte proliferation and differentiation.
Here, we aim to identify additional members of the Rho family that are important for keratinocyte differentiation. We used calcium-induced differentiation of normal human keratinocytes, a well established model of keratinocyte differentiation that mimics the adhesive events that occur in vivo during stratification (Wheelock and Jensen, 1992; Hodivala and Watt, 1994). We found that expression of the GTP-binding protein RhoE (also known as Rnd3) is regulated in a temporal and spatial manner during keratinocyte differentiation. RhoE expression contributes to the withdrawal from cell cycle and migration upward to assemble multiple layers. Therefore, we identify a novel and specific function of RhoE in the regulation of epidermal morphogenesis.
MATERIALS AND METHODS
Cells
Normal human keratinocytes (strains Kb and Sf, passages 3–7) were cultured in standard medium containing 1.8 mM CaCl2 as described previously (Braga et al., 1997, and references therein). Cultures grown in the absence of calcium-dependent cell–cell contacts used 0.1 mM calcium ions and fetal calf serum-depleted from divalent cations (low calcium medium; Hodivala and Watt, 1994). For induction of differentiation, confluent monolayers of keratinocytes grown in low calcium medium were induced to differentiate and stratify by adding calcium ions to 1.8 mM. Differentiation was also induced in low calcium cultures by addition of 12-0-tetradecanoylphorbol-13-acetate (50 nM) to low calcium medium (Todd and Reynolds, 1998).
Small Interfering RNA (siRNA)
Keratinocytes grown in low calcium medium were transfected with siRNA oligonucleotides (oligos) by using RNAiFect (QIAGEN, Dorking, Surrey, United Kingdom) according to the manufacturer's instructions. Two different predesigned oligos were used against RhoE (Dharmacon RNA Technologies, Lafayette, CO; D-007794-04 UAAGUAGAGCUCUCCAAUCAUU and D-007794-03, GAAAUUAUCCAGCAAAUCUUU), ROCK1 (Ambion, Austin, TX; oligo1, #681, ACCUUUUAAAUUGUCUGCCtc, and oligo 2, Dharmacon RNA Technologies, D-003536-02; GAAGAAACAUUCCCUAUUC) and control scrambled oligo (Dharmacon RNA Technologies; D-001206-13). Different concentrations of each oligo were tested, and the optimum time for knockdown of each protein was determined by Western blots at 72 h before induction of differentiation. To prevent RhoE up-regulation during induction of keratinocyte differentiation, an additional transfection with RhoE siRNA oligos was required 4 h before (24-h time point) and just before adding calcium ions (1.8 mM) for other time points (3 and 6 h).
Treatment with Inhibitors
To inhibit protein synthesis, confluent keratinocytes grown in low calcium medium were pretreated with 50 μM cycloheximide (Sigma Chemical, Poole, Dorset, United Kingdom) for 30 min or 100 μM emetine (Sigma Chemical) for 15 min. Cell–cell contacts were induced by addition of calcium ions for 3 h in the presence of inhibitors. The ROCK inhibitor Y27632 (gift from Dr. A. Yoshimura, Yoshitomi Pharmaceuticals) was added to keratinocytes at a final concentration of 5 μM for 30 min before the calcium switch. During longer incubation, the inhibitor was added every 3 h after the calcium switch.
Determination of Protein Levels
Lysates were obtained at 3, 6, and 24 h to ensure that the peak of RhoE up-regulation was detected (between 2 and 4 h). All protein lysates were prepared in lysis buffer (50 mM Tris-HCl, pH 7.5, 1% Triton X-100, 150 mM NaCl, 0.1% SDS, 50 mM sodium fluoride, 1 mM phenylmethylsulfonyl fluoride, 5 μg/ml each leupeptin, pepstatin, and Pefabloc [Roche Biochemicals, Indianapolis, IN], 1 mM sodium molybdate, and 1 mM sodium ortho-vanadate). Western blots were carried out with equal amount of protein per sample (bicinchoninic acid; Pierce Chemical, Rockford, IL).
Antibodies
Primary mouse monoclonal antibodies used were against: myc epitope (9E10; Cancer Research UK [CRUK], London, United Kingdom), hemagglutinin (HA) (262K; Cell Signaling Technology, Danvers, MA), and FLAG epitope (M2, Sigma Chemical), E-cadherin (HECD1), α3β1 integrin (VM-2), Rac (23A8; Millipore, Billerica, MA), RhoA (26C4; Santa Cruz Biotechnology, Santa Cruz, CA), RhoE (4; Millipore), involucrin (SY5; Sigma Chemical), vinculin (hVIN; Sigma Chemical), epidermal growth factor receptor (EGFR) (CRUK), actin (C4; MP Biomedicals, Irvine, CA), phospho-p44/42 mitogen-activated protein kinase (MAPK) 1/2 (Cell Signaling Technology), and 5-bromo-2′-deoxyuridine (BrdU) (BD Biosciences, San Jose, CA). Primary rat monoclonal antibodies used were anti-E-cadherin (ECCD-2; Invitrogen, Paisley, United Kingdom), and anti-myc epitope (JAC6; Serotec, Oxford, United Kingdom). Rabbit polyclonal sera used were raised against β1-integrin cytoplasmic domain (rabbit polyclonal; gift from F. Giancotti, Sloane-Kettering Cancer Center), α-catenin and β-catenin (Braga et al., 1995), transglutaminase (Abcam), keratin 1 (Covance Research Products, Princeton, NJ). Anti-ROCKI (goat polyclonal; K-18) and anti-ROCKII (rabbit polyclonal; H-85) were purchased from Santa Cruz Biotechnology. Directly conjugated reagents used in flow cytometry were as follows: mouse anti-β1 integrins (fluorescein isothiocyanate [FITC]-CD29) and lectin-binding protein peanut lectin agglutin ([PNA]-FITC; Vector laboratories, Burlingame, CA). Negative controls were F(ab′)2 goat anti-human immunoglobulin G (IgG)-FITC (Serotec) and FITC-rabbit anti-mouse F(ab′)2 fragments (Dako UK, Ely, Cambridgeshire, United Kingdom). Other secondary antibodies were purchased from Jackson ImmunoResearch Laboratories (West Chester, PA). Staining was performed and collected as described previously (Braga et al., 1997). Pictures were processed using Adobe Photoshop and Adobe Illustrator (Adobe Systems, Mountain View, CA).
Stratification Assays
Keratinocytes grown to confluence in low calcium medium were transfected using Trans-IT-keratinocyte transfection reagent (MirusBio, Cambridge Biosciences, Cambridge, United Kingdom) and the following constructs: pCMV5-FLAG-RhoEWT (wild-type), pCMV5-FLAG-RhoEN37T (a RhoE mutant that does not detectably bind GTP or affect stress fibers; Chardin, 2006), pCS2-myc-Rac1bQ61L (gift from P. Jordan, Center for Human Genetics, Portugal) and empty vector EGFP-C1 (Clontech, Mountain View, CA). After overnight expression, calcium ions were added for 8 or 12 h.
Quantification
Results were obtained from at least three independent experiments. The number of experiments is shown in figure legends, where N is the number of repetitions (e.g., N = 3). Statistical analysis was performed by Student's t test. In stratification assays, confocal pictures were collected at different levels to image the basal and suprabasal layers. The percentage of expressing cells present in suprabasal layers was quantified for all transfected constructs. To measure changes in cell size after expression of different constructs, expressing cells (basal and suprabasal layers) were marked around the cell border and the cell size was calculated by ImageJ. At least 100 cells per coverslip were counted for each replicate experiment and the fold change in cell size was calculated.
To determine the number of cells expressing involucrin, the involucrin signal intensity of every cell within the field of view was determined using ImageJ (National Institutes of Health, Bethesda, MD), and a threshold value picked from cells that were negative for involucrin expression. At least 500 cells were counted per coverslip and the fold change in the number of involucrin-positive cells was calculated.
To quantify protein levels in Western blots, films were scanned in the linear range using LAS-3000 image analyser (Fujifilm, Edenbridge, Kent, United Kingdom), and pixel intensity was quantified using Aida image analyzer software 4.15 (Raytest, Pittsburgh, Germany). Values were expressed as -fold induction relative to the amount found at time zero (without cell–cell contacts). In clustering experiments, values were normalized to control bovine serum albumin (BSA)-coated beads (arbitrarily set at 1).
Assessing Integrin and Cadherin Adhesive Functions
For receptor clustering, latex beads (15-μm polystyrene microsphere Polybead; Polysciences, Warrington, PA) were coated with different antibodies essentially as described previously (Braga et al., 1997). After incubation of the beads on keratinocytes, cell lysates were prepared in lysis buffer, and an equal amount of protein was used in Western blots to determine RhoE levels. Alternatively, coverslips were fixed after 30 min, and the amount of attached beads was calculated from phase-contrast images. Adhesion of cells to collagen 1 (Collaborative Research, Bedford, MA) was performed using Calcein-AM (Invitrogen) as described previously (Mahalingam et al., 2007). The amount of attached cells was assessed by fluorometry (BMG Labtech, Offenburg, Germany), and the percentage of attached cells was expressed relative to attachment to BSA-coated wells.
Cell Sorting and Flow Cytometry
Keratinocytes maintained in low calcium medium or induced to differentiate for 3 h were sorted (FACSDiva; BD Biosciences) into proliferating and differentiating pools by using two methods: forward- and side-scattering properties or binding to PNA-FITC (labels differentiating cells) as described previously (Jones and Watt, 1993). Cells with a very low forward- (FSC) and side-scatter (SSC) were gated using FlowJo software version 8.1.1 (Tree Star, San Carlos, CA).
To determine β1-integrin surface levels, keratinocytes were labeled with CD29-FITC or IgG isotype control antibodies. For proliferation assays, siRNA-treated keratinocytes were labeled with 10 μM BrdU (Sigma Chemical) for 24 h after induction of differentiation. Samples were analyzed with a FACSCalibur (BD Biosciences), and data were analyzed using Cell Quest Pro version 4.0.2 (BD Biosciences) or FlowJo version 8.1.1 software.
RESULTS
RhoE Is Up-Regulated during Keratinocyte Differentiation
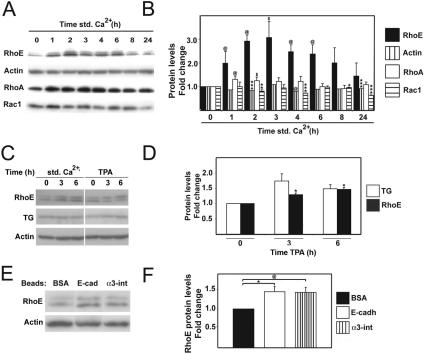
We analyzed the expression levels of different Rho small GTPases during keratinocyte differentiation. Protein extracts were prepared from cultures grown in the absence of cell–cell contacts (low calcium), and cultures were induced to differentiate up to 24 h by addition of calcium ions (Std Ca2+). Under these conditions, cell–cell contacts assemble and keratinocytes polarize within 1 h, whereas migration upward to form suprabasal layers initiates by 4–6 h (stratification) (Hodivala and Watt, 1994). We found a threefold increase in RhoE protein levels, peaking around 3 h after induction of differentiation (Figure 1, A and B). A smaller, transient increase in RhoA protein was observed at 1–2 h, whereas Rac1 levels varied but overall were slightly reduced throughout the time course (Figure 1, A and B). In contrast to RhoA, Rac1 and Cdc42, RhoE is unable to hydrolyze GTP and is thus not regulated by GTP loading/hydrolysis (Chardin, 2006). Instead, RhoE function is believed to be regulated primarily by expression levels.
Figure 1.
RhoE protein levels are up-regulated during calcium-induced keratinocyte differentiation. Keratinocytes were induced to stratify for up to 24 h, and total protein lysates were extracted. Equal amounts of protein were separated in SDS-polyacrylamide gel electrophoresis and blotted with the indicated antibodies. (A) RhoE protein levels were increased during keratinocyte differentiation. As controls, actin, RhoA and Rac1 protein levels are shown. (B) Quantification of the results shown in B. Values are expressed as -fold change relative to controls (keratinocytes grown in low calcium medium; time zero). (C) Induction of keratinocyte differentiation in the absence of calcium ions by addition of TPA for 3 and 6 h. Protein levels for RhoE, transglutaminase (TG) and actin were assessed by Western blots. (D) Quantification of results shown in C normalized to low calcium controls (t = 0). (E) Up-regulation of RhoE protein is mediated by clustering of adhesive receptors. Keratinocytes grown in low calcium medium were exposed for 3 h to beads coated with BSA (control) or coated with antibodies against E-cadherin or α3-integrins. (F) Quantification of the results from E normalized to BSA-coated beads. Quantifications were performed with three independent experiments (N = 3). Error bars represent SE. ***p < 0.0008, **p < 0.0007, *p < 0.004, @p < 0.03, and $p < 0.05.
To determine whether RhoE up-regulation participates in the keratinocyte differentiation program, we induced differentiation by adding TPA (50 μg/ml) to cells maintained in low calcium medium (Todd and Reynolds, 1998). It is thought that TPA treatment by-passes some of the differentiation signaling that is provided by addition of calcium ions. This is a well-established method to induce expression of differentiation markers such as transglutaminase (Figure 1C). Induction of differentiation by treatment with TPA also increased RhoE protein levels (Figure 1D), similar to the calcium-induced differentiation model (Figure 1B). To our knowledge, our data provide the first example of modulation of endogenous RhoE levels during differentiation.
Calcium-induced differentiation triggers an increase in intracellular calcium ions as well as signaling from cell–cell adhesive receptors after relocalization and clustering of receptors at junctions. We addressed whether RhoE up-regulation could be driven by signaling from intracellular calcium or cell–cell adhesion receptors. Using beads coated with antibodies, we clustered E-cadherin or α3-integrin receptors in cells grown in low calcium medium, an established technique to induce early signaling events downstream of specific receptors (Figure 1E) (Miyamoto et al., 1995; Braga et al., 1997). Interestingly, clustering of E-cadherin and α3-integrin receptors induced a reproducible increase in RhoE protein levels in the absence of calcium ions (Figure 1, E and F). Together, our results suggest that the up-regulation of RhoE protein does not result from calcium signaling per se (TPA-induced differentiation and clustering receptors with beads [Figure 1, C–F]).
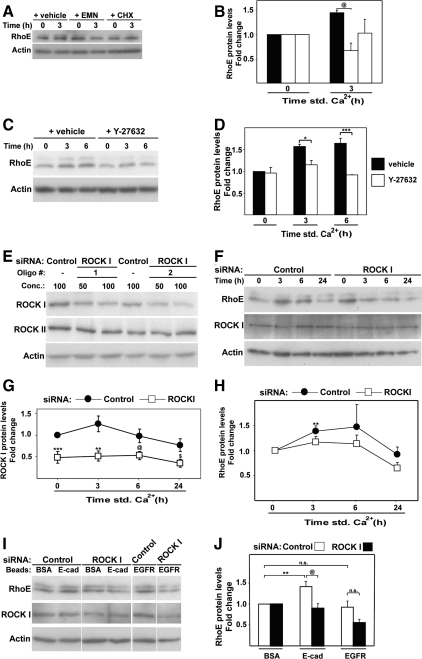
We found that the increase in RhoE protein levels during differentiation requires de novo protein synthesis. Inhibition of protein synthesis by treatment with cycloheximide or emetine prevented RhoE up-regulation (Figure 2, A and B), indicating that regulation of RhoE translation plays a role during keratinocyte differentiation. However, RhoE protein is known to be unstable (Chardin, 2006). Thus, one would predict that protein stabilization should also be in place to sustain elevated RhoE levels for up to 8 h (Figure 1B). Stabilization and localization of RhoE protein has been shown to be regulated by ROCKI-mediated phosphorylation (Riento et al., 2005). We directly tested whether the transient, differentiation-specific increase of endogenous RhoE protein required ROCKI. Inhibition of ROCK kinase activity with Y27632 during induction of keratinocyte differentiation significantly blocked RhoE up-regulation (Figure 2, C and D).
Figure 2.
Increase in RhoE protein levels requires ROCKI function. Keratinocytes grown in low calcium medium were treated as described below and induced to differentiate by adding calcium ions to the medium (A–H) or left in low calcium medium (I and J). Equal amount of protein was probed with antibodies against the indicated proteins. (A) Inhibition of protein synthesis by cycloheximide (CHX) or emetine (EMN) prevents differentiation-specific RhoE up-regulation. Keratinocytes were pre-incubated with inhibitors and induced to differentiate for 3 h in the presence of each inhibitor. (B) Quantification of the results shown in A. (C) Keratinocytes were incubated in the presence or absence of Y27632 to inhibit ROCK kinase activity. (D) Quantification of results from C. (E) ROCKI siRNA optimization using two different siRNA oligos against ROCKI or nontargeting control. (F) Time course of keratinocyte differentiation after treatment with control or ROCKI siRNA oligos. (G) Protein levels for ROCKI were quantified and expressed relative to control time zero. (H) RhoE protein levels were quantified and normalized for time zero of each group (control or ROCKI RNAi). (I) siRNA-treated keratinocytes (nontargeting control or ROCKI) were exposed for 1 h in low calcium medium to beads coated with BSA (control) or antibodies against E-cadherin or EGFR. (J) Quantification of results from I. RhoE levels found in cells treated with control BSA-beads were arbitrarily set as 1. Error bars represent SE. N = 3 experiments, apart from panels H, J (N = 4) and G (N = 5); ***p < 0.006, **p < 0.009, *p < 0.02, @p < 0.04, and $p < 0.05.
In support of this finding, we performed ROCKI siRNA by using two different oligos, both of which induced a reduction of around 50% in ROCKI protein levels but did not affect ROCKII (Figure 2E). Depletion of ROCKI was partial but significant (Figure 2, F and G) and efficiently prevented the increase in RhoE protein levels in control cells between 3 and 6 h (∼70–80%; Figure 2, F and H). Similar results were obtained with a second oligo against ROCKI (data not shown). These results were substantiated by experiments in which RhoE was up-regulated by clustering of cadherin receptors: ROCKI siRNA significantly reduced RhoE levels after cadherin clustering but not EGFR clustering (Figure 2, I and J). Thus, ROCKI is required for up-regulation of RhoE expression during keratinocyte differentiation, possibly by stabilizing RhoE protein levels as reported in other cell types (Riento et al., 2005).
RhoE Overexpression Promotes Keratinocyte Stratification
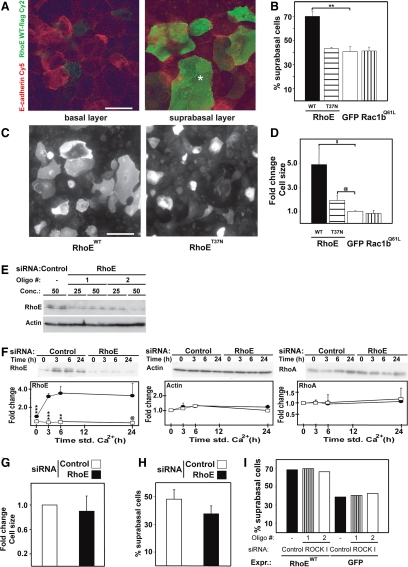
The precise temporal regulation of RhoE expression at early time points of keratinocyte differentiation leads to the question of what function RhoE has during this process. RhoE was identified for its ability to promote cell rounding and detachment from the substratum when overexpressed in fibroblasts (RhoEWT) (Guasch et al., 1998; Nobes et al., 1998). However, the function of endogenous RhoE remains elusive. Because the timing of RhoE activation correlates with the assembly of stratified layers (Figure 1; Hodivala and Watt, 1994), we addressed the effects of RhoE on the ability of keratinocytes to stratify.
Wild-type RhoE was transiently transfected in keratinocytes for 12 h and calcium ions added for 12–14 h to allow stratification (Figure 3, A and B). Confocal images were collected at two different levels to visualize the basal and suprabasal layers and to identify the position of expressing cells (Figure 3A). The baseline for stratification was set with green fluorescent protein (GFP) expression: 40% of GFP-expressing cells localized in suprabasal layers. In contrast, 72% of keratinocytes expressing RhoE were found in top layers (Figure 3B). No changes in the proportion of stratified, expressing cells was observed after transfection of the inactive mutant RhoET37N or Rac1b, another GTPase-deficient family member (Figure 3B). Our results suggest that RhoE expression increases the potential of keratinocytes to stratify.
Figure 3.
RhoE expression promotes keratinocyte stratification and an increase in cell size. Keratinocytes were transfected with wild-type RhoE (RhoEWT), mutant RhoE (RhoET37N), GFP or activated Rac1b (Rac1bQ61L) and induced to differentiate and stratify for 12 h, fixed, and stained for E-cadherin and the FLAG-tag (A–D). (A) Confocal images were collected at two levels to show the basal (left) and suprabasal layers (right). Asterisk show a suprabasal cell. (B) Quantification of the number of suprabasal cells expressing the different constructs. Values are expressed as percentage of expressing cells in suprabasal layers. (C) Enlargement of keratinocyte size upon RhoE overexpression and differentiation. (D) Quantification of keratinocyte cell size using ImageJ. The size of GFP-expressing cells was arbitrarily set as 1. (E–H) Keratinocytes were transfected with nontargeting siRNA control (control) or with two different siRNA oligos against RhoE and induced to differentiate for up to 24 h. (E) Optimization of RhoE RNAi conditions. Different concentrations of oligos were tested and RhoE depletion was confirmed by Western blots, actin was used as loading control. (F) Time course of keratinocyte differentiation after treatment with control oligos (closed circles) or RhoE-specific oligos (open squares). Equal amount of proteins was probed as described. (G) Quantification of cell size in keratinocytes incubated with RhoE or control siRNA oligos and differentiated for 8 h (see Materials and Methods for details). (H) Keratinocytes incubated with RhoE or control siRNA oligos were transfected with GFP and induced to stratify for 8 h. The number of stratified cells expressing GFP was calculated. (I) Cells treated with ROCKI siRNA oligos or controls were transfected with wild-type RhoE, induced to stratify for 12 h and the proportion of stratified cells was quantified as described above. Error bars represent SE. N = 3; ***p < 0.001, **p < 0.002, *p < 0.004, @p < 0.03, and $p < 0.045. Bar, 20 mm.
Keratinocyte differentiation and stratification is usually accompanied by an increase in cell size (Watt, 2002). Strikingly, RhoE-expressing, stratifying keratinocytes showed a significant enlargement of cell area (4.9-fold; RhoEWT) over the increase observed by control-expressing cells (GFP and Rac1bQ61L; Figure 3, C and D). Expression of the RhoE mutant (RhoET37N) also increased cell area (1.9-fold), albeit to a smaller extent than RhoEWT. These results were intriguing, considering the rounding response to RhoE expression in fibroblasts (Guasch et al., 1998). Overall, our data argue that RhoE has specific functions in epithelia and in particular during keratinocyte differentiation.
We reasoned that depletion of RhoE protein might have the opposite effect: a decrease in cell size and a reduction in stratification levels. Efficient knock down of RhoE protein was observed with two distinct siRNA oligos in keratinocytes grown in low calcium medium (Figure 3E). Optimization of this protocol was performed to ensure that the calcium-induced up-regulation of RhoE levels was eliminated (see Materials and Methods; Figure 3F). Controls showed that actin and RhoA protein levels were not changed after RhoE siRNA during the same time course, indicating the specificity of RhoE depletion (Figure 3F). After RhoE RNA interference (RNAi), keratinocytes were transfected with GFP and allowed to stratify for 8 h (Figure 3, G and H). Interestingly, RhoE depletion did not reduce significantly keratinocyte cell area compared with nontargeting siRNA controls (Figure 3G; data not shown with additional oligo). This result implies that the keratinocyte population that had initiated differentiation before RhoE depletion is able to enlarge cell size similar to controls.
Consistent with the above-mentioned data, RhoE RNAi did not prevent stratification. When calcium ions were added to the medium, keratinocytes that had already entered the differentiation program before RhoE depletion were able to proceed and stratify (Figure 3H; data not shown with additional oligo). We interpret these results as RhoE is required for the de novo commitment to stratification after keratinocyte cell–cell adhesion. Together, our results indicate that the basal level of stratification potential found in low calcium cultures (Hodivala and Watt, 1994) is not affected by RhoE depletion.
Because ROCKI is required for RhoE stabilization, an increase in RhoE protein levels by overexpression of exogenous RhoEWT should bypass the requirement of ROCKI function. We predict that ROCKI inhibition should not affect the ability of keratinocytes overexpressing RhoE to stratify. To test this possibility, keratinocytes treated with two distinct siRNA oligos against ROCKI or control oligos were transfected with RhoEWT or GFP and allowed to differentiate and stratify for 12 h (Figure 3I). Controls (GFP-expressing cells) showed that depletion of ROCKI did not interfere with the basal level of stratification found in keratinocyte cultures (Figure 3I). Our result is consistent with RhoE RNAi data: stratification levels in cells that had already committed to stratify are slightly reduced but not abolished (Figure 3H). After treatment with control oligos, 70% of RhoE-expressing cells were found in suprabasal layers (Figure 3I), similar to what was observed in Figure 3, A and B. Depletion of ROCKI protein using two different siRNA oligos did not interfere with the increase in stratification induced by RhoE overexpression (Figure 3I). These data indicate that ROCKI function per se is not sufficient to drive keratinocyte stratification but rather RhoE up-regulation is essential.
RhoE Knockdown Delays Expression of Differentiation Markers and Reduces the Number of Differentiating Keratinocytes
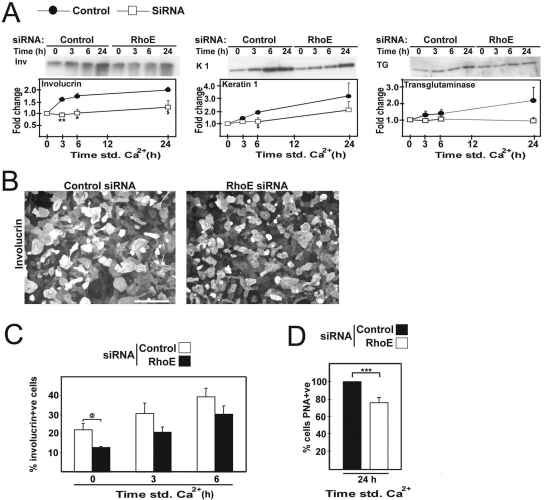
Because RhoE overexpression induced an enlargement of cell size and acceleration of keratinocyte stratification, we addressed whether RhoE siRNA affects the expression of differentiation markers. Lysates from siRNA-treated cells were probed with antibodies against the differentiation markers involucrin, keratin 1, or transglutaminase (Figure 4A), which are up-regulated during calcium-induced differentiation (Watt, 2002). Compared with controls, RhoE depletion led to a significant delay in the up-regulation of the early marker involucrin, and levels of keratin 1 and transglutaminase also were reduced (Figure 4A and additional oligo; data not shown). The same results were obtained with a different oligo against RhoE (data not shown). The stronger effect of RhoE RNAi on involucrin levels compared with transglutaminase is similar to observations after different stimuli such as protein kinase C stimulation or receptor clustering (Todd and Reynolds, 1998; Hines et al., 1999).
Figure 4.
RhoE siRNA reduces the differentiation potential of keratinocytes. Keratinocytes treated with control oligos (closed circles) or RhoE-specific oligos (open squares) were induced to differentiate for up to 24 h. (A) Western blots show the expression of the early differentiation marker involucrin, keratin 1, and transglutaminase. (B) Keratinocytes treated with RhoE or control siRNA oligos were induced to differentiate and stained for involucrin to label differentiating cells. Bar, 80 μm. (C) Quantification of the number of involucrin-expressing cells after a time course of differentiation. (D) Keratinocytes differentiated for 24 h were incubated with the lectin PNA to label differentiated cells and FACS sorted. Error bars represent SE. N = 3; ***p < 0.0006, **p < 0.001, *p < 0.008, $p < 0.04, and @p < 0.05.
We predict that the decrease in involucrin protein levels could be due in part to a decline in the pool of differentiating keratinocytes. This prediction would be consistent with our data that depletion of RhoE prevents the de novo commitment of keratinocytes to stratify (Figure 3H). Indeed, after RhoE siRNA, a significant reduction in the number of differentiating, involucrin-positive cells in low calcium medium was observed (44%) and persisted for 3 and 6 h after cell–cell contact induction (Figure 4, B and C).
Sorting differentiating keratinocytes by labeling with another differentiation marker, PNA+ve, obtained similar results. A decrease in the percentage of cells labeled with PNA was observed in low calcium (20%; data not shown) or after differentiation for 24 h (24%; Figure 4D). Together, our data indicate that, in the absence of RhoE, fewer keratinocytes were able to express differentiation markers upon addition of calcium ions, and fewer cells initiate differentiation at steady state (basal level in low calcium medium).
RhoE Knockdown Enhances the Pool of Undifferentiated Keratinocytes and Proliferation Potential
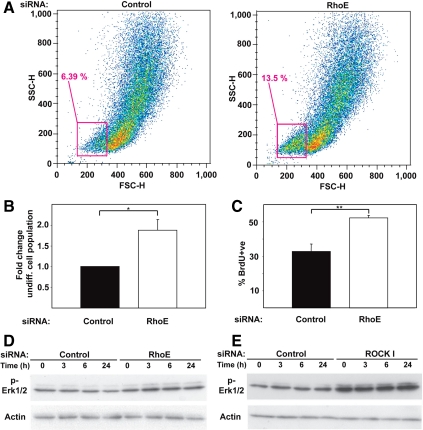
Keratinocyte differentiation and proliferation are tightly linked, because initiation of the differentiation program requires prior withdrawal from the cell cycle (Watt, 2002). In the absence of RhoE, the reduced number of differentiating keratinocytes observed suggests that RhoE could affect proliferation, as shown previously in several cell types (Chardin, 2006). If this hypothesis is correct, RhoE depletion should enhance the number of undifferentiated, proliferative keratinocytes, which would in turn affect differentiation.
We quantified the percentage of undifferentiated cells in the presence (control) or absence of RhoE (RhoE siRNA) by using light side- and forward-scattering to separate basal from differentiating keratinocytes (Figure 5A) (Jones and Watt, 1993). Light scattering varies with cell size and cytoplasmic composition: basal, undifferentiated cells are smaller and show low side- and forward-scattering properties (Jones and Watt, 1993). Because RhoE siRNA did not alter cell size (Figure 3G), the use of light scattering technique is suitable to separate/analyze our model system. RhoE siRNA induced a significant increase in the pool of undifferentiated keratinocytes compared with controls treated with scrambled oligos (Figure 5, A and B).
Figure 5.
RhoE siRNA increases the pool of undifferentiated keratinocytes and promoted proliferation. (A) Cells treated with control or RhoE siRNA were induced to differentiate for 3 h and assessed for their FSC and SSC properties by fluorescence-activated cell sorting sorting. The gate used for separation of undifferentiated cells is shown. (B) Quantification of the data shown in A. (C) Keratinocytes treated with siRNA control oligos or RhoE-specific oligos were induced to differentiate, labeled with BrdU and analyzed by FACS (BrdU +ve). (D and E) Keratinocytes treated with nontargeting control, RhoE (D) or ROCK1 (E) siRNA oligos were induced to differentiate for up to 24 h, and lysates probed for phosphorylated Erk1/2, and actin. Error bars represent SE. N = 3; **p < 0.02 and *p < 0.03.
To confirm the above-mentioned data, we assessed the levels of proliferation in RhoE-depleted cells by measuring DNA synthesis (BrdU incorporation; Figure 5C). We predict that an increase in proliferation should be seen after RhoE RNAi, because the undifferentiated pool is enlarged (Figure 5, A and B). Indeed, RhoE-depleted cells showed higher BrdU incorporation than control siRNA-treated keratinocytes (Figure 5C). Significantly higher levels of proliferation were observed 24 h after initiation of calcium-induced differentiation. Furthermore, RhoE or ROCKI depletion enhanced the levels of extracellular signal-regulated kinase (Erk) 1/2 phosphorylation (Figure 5, D and E), which could contribute to the increase in proliferating cells (Zhu et al., 1999; Schmidt et al., 2000). This effect seemed to be a direct consequence of RhoE (and ROCKI) depletion per se (0 h; Figure 5, D and E), but it was further enhanced during calcium-induced differentiation. We conclude that RhoE depletion increases the undifferentiated pool of keratinocytes, and this correlated with hyperproliferation. In turn, these events reduce the proportion of keratinocytes undergoing differentiation (Figure 4, B–D).
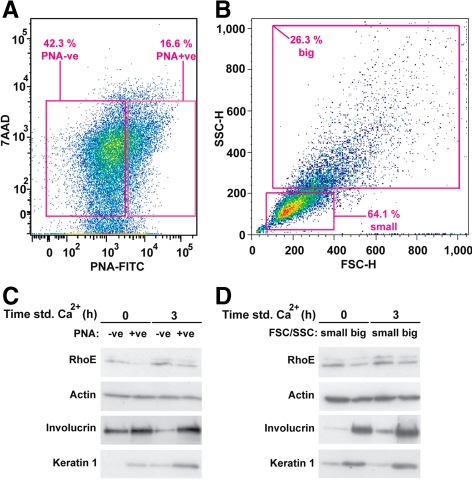
Because RhoE seemed to regulate keratinocyte proliferation, and proliferation is restricted to basal, undifferentiated keratinocytes, we reasoned that the differentiation-induced activation of RhoE (Figure 1) may occur in those cells destined to withdraw from the cell cycle to initiate differentiation. To test this hypothesis, keratinocytes were FACS-sorted according to their differentiation status by two distinct methods: using PNA to label differentiating keratinocytes (Figure 6A) or their light scattering properties (Figure 6B) (Jones and Watt, 1993). Controls showed that the sorted samples were representative: differentiated cells (PNA-positive or big) had higher expression levels of keratin 1 and involucrin compared with controls (Figure 6, C and D). Stimulation of differentiation by adding calcium ions resulted in a further increase in the levels of keratin 1 and involucrin (Figure 6, C and D). Interestingly, the increase in RhoE protein triggered by keratinocyte differentiation (Figure 1) was detected primarily in the undifferentiated keratinocyte pool (Figure 6, C and D). These results are consistent with RhoE participation in down-regulation of proliferation and initiation of differentiation.
Figure 6.
RhoE is up-regulated in undifferentiated keratinocytes. (A and B) Keratinocytes were treated with RhoE-specific or control siRNA oligos and induced to differentiate for 3 h. Undifferentiated keratinocytes were obtained by FACS sorting using PNA +ve) (A; N = 1). Alternatively, cells were separated by their forward- and side-scatter properties to sort small, undifferentiated cells from larger, differentiated keratinocytes (B; N = 2). Gates used to sort the different keratinocytes populations are shown. (C and D) Lysates were extracted from the different FACS-sorted keratinocytes and probed with RhoE, actin, and the differentiation markers involucrin and keratin 1.
Changes in Keratinocyte Adhesive Properties Induced by RhoE Depletion
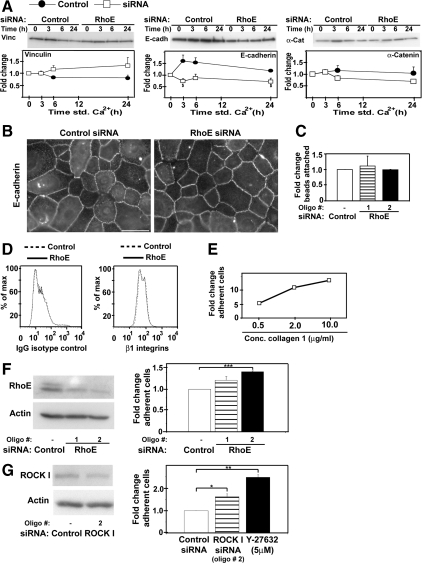
Because keratinocyte stratification requires both cadherin adhesion and a decrease in attachment to the substratum, we investigated whether the protein levels and function of cell–cell adhesion and cell–matrix adhesive receptor were affected after RhoE siRNA. Upon RhoE depletion and calcium-induced differentiation, no significant changes were observed in vinculin or α-catenin expression levels, whereas E-cadherin levels were slightly reduced (Figure 7A). However, this reduction in cadherin levels did not affect cell–cell adhesion, because cadherins were able to relocalize to junctions (Figure 7B) and keratinocytes that had initiated differentiation (26%; Figure 6B) were able to stratify (Figure 3H; data not shown). Moreover, attachment of beads coated with anti-cadherin antibodies was not affected by RhoE-depletion (Figure 7C), suggesting that surface cadherin levels were similar to controls. We conclude that cadherin function was not significantly perturbed by RhoE depletion.
Figure 7.
RhoE depletion enhances keratinocyte cell-matrix adhesion, but not cell-cell adhesion. (A) Keratinocytes were treated with control siRNA (closed circles) or RhoE-specific oligos (open squares) and induced to differentiate for different time points (std Ca2+). Lysates were probed with antibodies against vinculin, E-cadherin, and α-catenin. (B) Control or RhoE siRNA-treated cells were induced to differentiate for 3 h, fixed, and stained for E-cadherin. (C) Keratinocytes were incubated with beads coated with anti-E-cadherin antibodies. The number of attached beads was counted and expressed as -fold induction relative to attachment of beads to control siRNA-treated cells. (D) Keratinocytes grown in low calcium medium were labeled with anti-β1-integrin antibody or isotype matched controls and analyzed by FACS. (E) Optimization of adhesion assays. Keratinocytes grown in low calcium medium were allowed to adhere to different concentrations of collagen 1. (F) Keratinocytes grown in low calcium medium were transfected with nontargeting control or two different RhoE siRNA oligos (left) and used in adhesion assays as optimized above (0.5 μg/ml collagen 1). (G) Keratinocytes depleted of ROCKI protein (left) or treated with the ROCK inhibitor Y27632 were allowed to adhere to collagen 1. Quantification of the data expressed as -fold change relative to control siRNA (arbitrarily set as 1; right). Bar, 40 μm. Error bars represent SE. N = 3; ***p < 0.001, **p < 0.002, *p < 0.01, and $p < 0.04.
After RhoE siRNA, no difference in β1-integrin levels was observed by FACS analysis in keratinocytes (Figure 7D). Integrin function was then assessed by adhesion assays on collagen 1 using normal keratinocytes. Adhesion of keratinocytes to collagen 1 increased between 0.5 and 10 μg/ml (Figure 7E). Attachment of RhoE-depleted cells grown in low calcium medium to collagen 1 (0.5 μg/ml) was expressed as -fold induction over control cells (scrambled oligo; Figure 7F). Reduction of RhoE protein levels induced up to 41% increase in attached keratinocytes (Figure 7F). This implies that RhoE depletion increases integrin function but not protein levels. Inhibition of ROCK kinase activity by treatment with Y27632 induced even higher levels of attachment (2.5-fold; Figure 7G). Consistent with our observation that ROCKI regulates RhoE protein levels in keratinocytes (Figure 2), ROCKI RNAi increased attachment of keratinocytes cells to collagen 1 (1.6-fold; Figure 7G). Thus, inhibition of ROCKI mimicked the phenotype on integrin adhesion seen after reduction of RhoE protein levels by siRNA.
DISCUSSION
Our work establishes a novel function of RhoE in primary keratinocyte differentiation. RhoE/Rnd3 is an atypical GTP-binding protein of the Rnd subfamily that is regulated by protein expression levels, localization and phosphorylation rather than GTP hydrolysis (Chardin, 2006). Rnd proteins emerged late in evolution (chordates), suggesting more specialized functions than the ancient members Rho, Rac, and Cdc42 (Boureux et al., 2007). Overexpression of exogenous RhoE regulates cell proliferation, actin cytoskeleton, and migration and transiently promotes cell rounding (Guasch et al., 1998; Nobes et al., 1998; Villalonga et al., 2004). However, whether these distinct functions reflect a response to different stimuli or cell type specificity is unclear.
Calcium-induced keratinocyte differentiation and stratification (assembly of multiple layers) is a tightly regulated, multistep process. Commitment to differentiation and withdrawal from the cell cycle precedes the expression of differentiation markers and detachment from the substratum to assemble suprabasal layers (Watt, 2002; Fuchs, 2007). In the absence of calcium ions, initiation of differentiation occurs, but keratinocytes are unable to stratify because of the absence of cell–cell contacts. Thus, the keratinocyte differentiation program has highly coordinated but separable steps, most likely regulated by distinct events.
We demonstrate a transient increase in RhoE protein expression that temporally correlates with keratinocyte stratification. RhoE up-regulation occurs primarily in small, undifferentiated cells present in the basal layer, where the decision to stratify takes place. Interestingly, the functional consequence of RhoE overexpression in keratinocytes is an increase in stratification potential, as assessed by higher numbers of stratifying cells and enlargement of cell size, which is indicative of terminal differentiation. Because ∼20% of keratinocytes in low calcium cultures are already differentiating (Hodivala and Watt, 1994), it is possible that RhoE siRNA would not interfere with stratification in this pool. Indeed, after RhoE depletion no significant changes are observed in cell size or stratification within the time frame investigated. Our data imply that RhoE is required for de novo commitment to stratification and has no effect on keratinocytes that have already initiated the differentiation program.
The striking effects of RhoE expression on stratification provide insight into its potential mechanism of action. Stratification occurs after detachment of cells committed to differentiate from the basement membrane. This process requires modulation of integrin binding affinity and a decrease in integrin mRNA and protein levels (Watt, 2002). RhoE depletion per se does not affect β1-integrin levels, but rather increases attachment of keratinocytes to collagen. These results suggest that RhoE may regulate inside-out signals that modulate integrin function, a previously unreported role.
Our results are consistent with the reduction in integrin adhesion that occurs after 2–4 h of induction of cell–cell contacts (Hodivala and Watt, 1994) and the rounding up caused by RhoE overexpression in fibroblasts (Guasch et al., 1998). However, in keratinocytes the net effect of substratum detachment is increased stratification, rather than rounding up. We speculate that because cadherin-mediated contacts are not perturbed by RhoE RNAi, detached keratinocytes can instead assemble suprabasal layers by adhering to and climbing on top of neighboring cells.
RhoE depletion decreases the number of cells committed to differentiate and leads to a striking increase in keratinocyte proliferation, most likely via activation of Erk1/2 (Zhu et al., 1999; Schmidt et al., 2000). Both phenotypes are consistent with the up-regulation of RhoE in basal cells, the pool in which proliferation and initiation of differentiation occur (Watt et al., 2006; Fuchs, 2007). Our interpretation is that the RhoE depletion-induced hyperproliferation and associated increase in the undifferentiated pool of keratinocytes have a knockon effect on differentiation as a secondary event (less keratinocytes starting the differentiation program).
These results are supported by two lines of evidence: 1) RhoE overexpression induces cell cycle arrest in other cell types (Villalonga et al., 2004; Chardin, 2006); and 2) inhibition of keratinocyte differentiation and skin hyperplasia is also seen upon treatment with EDIN toxin, a toxin that modifies RhoE (Sugai et al., 1990, 1992; Wilde et al., 2001). Together, our data suggest that the transient RhoE up-regulation in keratinocytes inhibits cell cycle progression and facilitates entry into the keratinocyte differentiation program, including stratification and enlargement of cell size.
Although we have identified the cellular processes that RhoE regulates during keratinocyte differentiation, the molecular mechanisms whereby RhoE is up-regulated remain to be identified. We show that elevated RhoE protein levels is not due to calcium signaling, but rather an adhesive event induced by clustering of cadherin and integrin receptors. Stabilization of RhoE protein by ROCKI (Riento et al., 2005) may also contribute to the precise temporal activation of RhoE. The novelty of our results is to demonstrate a differentiation-specific stabilization of endogenous RhoE protein by endogenous ROCKI.
Our data can be interpreted in two ways. First, RhoE has been previously shown to inhibit RhoA-dependent cellular processes by activating p190-RhoGAP or inhibiting ROCKI function in a feedback loop (Chardin, 2006). Thus, in keratinocytes, it is possible that a RhoE-dependent inhibition of ROCKI may play a role in the cellular processes investigated; hence, similar phenotypes are seen after RhoE or ROCKI siRNA. However, depletion of ROCKI is not sufficient to increase stratification. Moreover, RhoE siRNA does not noticeably increase stress fiber formation in keratinocytes as expected if ROCKI is activated (data not shown). Alternatively, ROCKI acts upstream of RhoE during keratinocyte differentiation. Indeed, after ROCKI RNAi, endogenous RhoE is not transiently up-regulated. Consistent with this, inhibition of ROCKI function in keratinocytes phenocopies RhoE siRNA: increased attachment to collagen and phosphorylation of Erk1/2. Thus, our data strongly support the latter interpretation.
Yet, although the links between RhoE/ROCKI signaling are apparent in the above-mentioned processes, their joint involvement on the regulation of stratification is less clear. RhoE expression is sufficient to increase stratification potential. However, we have been unable to formally demonstrate that ROCKI function is required for stratification due to technical issues and difficulties in interpreting multiple ROCK1 effects (e.g., contractility, focal adhesions, stress fibers). RhoE also may have distinct functions from ROCKI in keratinocytes, as shown by the identification of RhoE-specific targets that do not interact with RhoA (Chardin, 2006) and experiments using bacterial toxins (Sugai et al., 1992; McMullan et al., 2003). Together, we argue that RhoE and RhoA/ROCKI may have overlapping but also distinct functions in keratinocytes.
The involvement of ROCKI and RhoE in the keratinocyte differentiation program is novel and further supports the importance of Rho small GTPase-dependent signaling pathways for determination of the epidermal phenotype (Sugai et al., 1992; Calautti et al., 2002; McMullan et al., 2003; Grossi et al., 2005). Interestingly, ROCKII activation has been implicated in inhibition of keratinocyte proliferation and expression of differentiation markers using a suspension-induced differentiation model (McMullan et al., 2003). The latter model excludes adhesive and stratification events and thus is not directly comparable with the calcium-induced differentiation model used in our study. Importantly, ROCKII is not known to regulate RhoE stability (Riento et al., 2005). Thus, it is feasible that both ROCKI and ROCKII may be activated during keratinocyte differentiation and that distinct cellular processes are regulated by each isoform (Riento et al., 2005).
The effects of RhoE siRNA on keratinocyte proliferation and stratification are clearly different from the effects caused by depletion of other small GTPases. For example, Rac1 siRNA in keratinocytes in vitro increases expression of differentiation markers without interfering with proliferation levels (Nikolova et al., 2008). Epidermis-specific Rac1 (Benitah et al., 2005) or Cdc42 (Wu et al., 2006) conditional knockout results in perturbation of hair follicle formation by depletion of follicular stem cells. Yet, Rac knockdown does not perturb the maintenance of interfollicular epidermis in some cases (Chrostek et al., 2006; Castilho et al., 2007). Together, these results indicate that the phenotype reported here is unique for RhoE depletion.
In conclusion, our data strongly support a role for RhoE in epidermal morphogenesis: RhoE up-regulation decreases keratinocyte proliferation, reduces integrin attachment, promotes cell enlargement, and increases stratification. Thus, RhoE initiates a broad signaling program to regulate keratinocyte differentiation and a previously unreported coordination of these cellular processes. Because RhoE mRNA is found in different epithelia-containing organs (Nobes et al., 1998), our results may have implications for other types of epithelia. For example, overexpression of RhoE in simple epithelial cells such as Madin-Darby canine kidney induces multilayering (Hansen et al., 2000), similar to the increase in stratification seen in keratinocytes. We propose that RhoE is a key switch of the morphogenetic program leading from simple to stratified epithelia. Thus, it is tempting to speculate that activation of RhoE may be essential for the regulatory events that lead to multilayering during disease and differentiation of stratified epithelia.
ACKNOWLEDGMENTS
We thank Prof Fiona Watt for helpful discussions and all researchers for the gifts of reagents mentioned in Materials and Methods. T. L. and R. K. are supported by studentships from the National Heart and Lung Institute and the Cell Biology Initiative, respectively. We thank the generous support of the Medical Research Council, Cancer Research UK (V. B.) and BBSRC (A. R.).
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E07-11-1197) on October 15, 2008.
REFERENCES
- Benitah S. A., Frye M., Glogauer M., Watt F. M. Stem cell depletion through epidermal deletion of Rac1. Science. 2005;309:933–935. doi: 10.1126/science.1113579. [DOI] [PubMed] [Google Scholar]
- Boureux A., Vignal E., Faure S., Fort P. Evolution of the Rho family of ras-like GTPases in eukaryotes. Mol. Biol. Evol. 2007;24:203–216. doi: 10.1093/molbev/msl145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga V. M., Yap A. S. The challenges of abundance: epithelial junctions and small GTPase signalling. Curr. Opin. Cell Biol. 2005;17:466–474. doi: 10.1016/j.ceb.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Braga V.M.M., Hodivala K. J., Watt F. M. Calcium-induced changes in distribution and solubility of cadherins and their associated cytoplasmic proteins in human keratinocytes. Cell Adhes. Comm. 1995;3:201–215. doi: 10.3109/15419069509081287. [DOI] [PubMed] [Google Scholar]
- Braga V.M.M., Machesky L. M., Hall A., Hotchin N. A. The small GTPases Rho and Rac are required for the establishment of cadherin-dependent cell-cell contacts. J. Cell Biol. 1997;137:1421–1431. doi: 10.1083/jcb.137.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calautti E., Grossi M., Mammucari C., Aoyama Y., Pirro M., Ono Y., Li J., Dotto G. P. Fyn tyrosine kinase is a downstream mediator of Rho/PRK2 function in keratinocyte cell-cell adhesion. J. Cell Biol. 2002;156:137–148. doi: 10.1083/jcb.200105140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll D. K., Carroll J. S., Leong C.-O., Cheng F., Brown M., Mills A. A., Brugge J. S., Ellisen L. W. p63 regulates an adhesion programme and cell survival in epithelial cells. Nat. Cell Biol. 2006;8:551–561. doi: 10.1038/ncb1420. [DOI] [PubMed] [Google Scholar]
- Castilho R. M., Squarize C. H., Patel V., Millar S. E., Zheng Y., Molinolo A., Gutkind J. S. Requirement of Rac1 distinguishes follicular from interfollicular epithelial stem cells. Oncogene. 2007;26:5078–5085. doi: 10.1038/sj.onc.1210322. [DOI] [PubMed] [Google Scholar]
- Chardin P. Function and regulation of Rnd proteins. Nature Rev. Mol. Cell Biol. 2006;7:54–62. doi: 10.1038/nrm1788. [DOI] [PubMed] [Google Scholar]
- Chrostek A., Wu X., Quondamatteo F., Hu R., Sanecka A., Niemann C., Langbein L., Haase I., Brakebusch C. Rac1 is crucial for hair follicle integrity but is not essential for maintenance of the epidermis. Mol. Cell Biol. 2006;26:6957–6970. doi: 10.1128/MCB.00075-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E. Scratching the surface of skin development. Nature. 2007;445:834–842. doi: 10.1038/nature05659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrod D. R., Merritt A. J., Nie Z. Desmosomal cadherins. Curr. Opin. Cell Biol. 2002;14:537–545. doi: 10.1016/s0955-0674(02)00366-6. [DOI] [PubMed] [Google Scholar]
- Grossi M., Hiou-Feige A., Di Vignano A. T., Calautti E., Ostano P., Lee S., Chiorino G., Dotto G. P. Negative control of keratinocyte differentiation by Rho/CRIK signaling coupled with up-regulation of KyoT1/2 (FHL1) expression. Proc. Natl. Acad. Sci. USA. 2005;102:11313–11318. doi: 10.1073/pnas.0505011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guasch R. M., Scambler P., Jones G. E., Ridley A. J. RhoE regulates actin cytoskeleton organization and cell migration. Mol. Cell Biol. 1998;18:4761–4771. doi: 10.1128/mcb.18.8.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen S. H., Zegers M.M.P., Woodrow M., Rodriguez-Viciana P., Chardin P., Mostov K. E., McMahon M. Induced expression of Rnd3 is associated with transformation of polarized epithelial cells by the Raf-MEK-extracellular-signal-regulated kinase pathway. Mol. Cell Biol. 2000;20:9364–9375. doi: 10.1128/mcb.20.24.9364-9375.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines M. D., Jin H. C., Wheelock M. J., Jensen P. J. Inhibition of cadherin function differentially affects markers of terminal differentiation in cultured human keratinocytes. J. Cell Sci. 1999;112:4569–4579. doi: 10.1242/jcs.112.24.4569. [DOI] [PubMed] [Google Scholar]
- Hodivala K. J., Watt F. M. Evidence that cadherins play a role in the downregulation of integrin expression that occurs during keratinocyte terminal differentiation. J. Cell Biol. 1994;124:589–600. doi: 10.1083/jcb.124.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P. H., Watt F. M. Separation of human epidermal stem cells from transit amplifying cells on the basis of differences in integrin function and expression. Cell. 1993;73:713–724. doi: 10.1016/0092-8674(93)90251-k. [DOI] [PubMed] [Google Scholar]
- Koster M. I., Dai D., Marinari B., Sano Y., Costanzo A., Karin M., Roop D. R. p63 induces key target genes required for epidermal morphogenesis. Proc. Natl. Acad. Sci. USA. 2007;104:3255–3260. doi: 10.1073/pnas.0611376104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahalingam Y., Gallagher J. T., Couchman J. R. Cellular adhesion responses to the heparin-binding (HepII) domain of fibronectin require heparan sulfate with specific properties. J. Biol. Chem. 2007;282:3221–3230. doi: 10.1074/jbc.M604938200. [DOI] [PubMed] [Google Scholar]
- McMullan R., Lax S., Robertson V. H., Radford D. J., Broad S., Watt F. M., Rowles A., Croft D. R., Olson M. F., Hotchin N. A. Keratinocyte differentiation is regulated by the Rho and ROCK signaling pathway. Curr. Biol. 2003;13:2185–2189. doi: 10.1016/j.cub.2003.11.050. [DOI] [PubMed] [Google Scholar]
- Miyamoto S., Akiyama S., Yamada K. M. Synergistic roles for receptor occupancy and aggregation in integrin transmembrane function. Science. 1995;267:883–885. doi: 10.1126/science.7846531. [DOI] [PubMed] [Google Scholar]
- Morasso M. I., Tomic-Canic M. Epidermal stem cells: the cradle of epidermal determination, differentiation and wound healing. Biol. Cell. 2005;97:173–183. doi: 10.1042/BC20040098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolova E., Mitev V., Minner F., Deroanne C. F., Poumay Y. The inhibition of the expression of the small Rho GTPase Rac1 induces differentiation with no effect on cell proliferation in growing human adult keratinocytes. J. Cell. Biochem. 2008;103:857–864. doi: 10.1002/jcb.21455. [DOI] [PubMed] [Google Scholar]
- Nobes C. D., Lauritzen I., Mattei M., Paris S., Hall A., Chardin P. A new member of the Rho family, Rnd1, promotes disassembly of actin filament structures and loss of cell adhesion. J. Cell Biol. 1998;141:187–197. doi: 10.1083/jcb.141.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riento K., Totty N., Villalonga P., Garg R., Guasch R., Ridley A. J. RhoE function is regulated by ROCK I-mediated phosphorylation. EMBO J. 2005;24:1170–1180. doi: 10.1038/sj.emboj.7600612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M., Goebeler M., Posern G., Feller M. S., Seitz C. S., Brocker E.-B., Rapp U. R., Ludwig S. Ras-independent activation of Raf/MEK/ERK pathway upon calcium-induced differentiation of keratinocytes. J. Biol. Chem. 2000;275:41011–41017. doi: 10.1074/jbc.M003716200. [DOI] [PubMed] [Google Scholar]
- Sugai M., Enomoto T., Hashimoto K., Matsumoto K., Matsuo Y., Ohgai H., Hong Y.-M., Inoue S., Yoshikawa K., Suginaka H. A novel epidermal cell differentiation inhibitor (EDIN): purification and characterization from Staphylococcus aureus. Biochem. Biophys. Res. Commun. 1990;173:92–98. doi: 10.1016/s0006-291x(05)81026-5. [DOI] [PubMed] [Google Scholar]
- Sugai M., Hashimoto K., Kikuchi A., Inoue S., Okumura H., Matsumoto K., Goto Y., Ohgai H., Moriishi K., Syuto B. Epidermal cell differentiation inhibitor ADP-ribosylates small GTP-binding proteins and induces hyperplasia of epidermis. J. Biol. Chem. 1992;267:2600–2604. [PubMed] [Google Scholar]
- Todd C., Reynolds N. J. Up-regulation of p21WAF1 by phorbol ester and calcium in human keratinocytes through a protein kinase C-dependent pathway. Am. J. Pathol. 1998;153:39–45. doi: 10.1016/S0002-9440(10)65543-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaezi A., Bauer C., Vasioukhin V., Fuchs E. Actin cable dynamics and Rho/Rock orchestrate a polarized cytoskeletal architecture in the early steps of assembling a stratified epithelium. Dev. Cell. 2002;3:367–381. doi: 10.1016/s1534-5807(02)00259-9. [DOI] [PubMed] [Google Scholar]
- Van Aelst L., Symons M. Role of Rho family GTPases in epithelial morphogenesis. Genes Dev. 2002;16:1032–1054. doi: 10.1101/gad.978802. [DOI] [PubMed] [Google Scholar]
- Villalonga P., Guasch R. M., Riento K., Ridley A. J. RhoE inhibits cell cycle progression and Ras-induced transformation. Mol. Cell Biol. 2004;24:7829–7840. doi: 10.1128/MCB.24.18.7829-7840.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waschke J., Spindler V., Bruggeman P., Zillikens D., Schmidt G., Drenckhahn D. Inhibition of Rho A activity causes pemphigus skin blistering. J. Cell Biol. 2006;175:721–727. doi: 10.1083/jcb.200605125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt F. M. Role of integrins in regulating epidermal adhesion, growth and differentiation. EMBO J. 2002;21:3919–3926. doi: 10.1093/emboj/cdf399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt F. M., Celso C. L., Silva-Vargas V. Epidermal stem cells: an update. Curr. Opin. Genet. Dev. 2006;16:518–524. doi: 10.1016/j.gde.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Wheelock M. J., Jensen P. J. Regulation of keratinocyte intercellular junction organization and epidermal morphogenesis by E-cadherin. J. Cell Biol. 1992;117:415–425. doi: 10.1083/jcb.117.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde C., Chhatwal G. S., Schmalzing G., Aktories K., Just I. A novel C3-like ADP-ribosyltransferase from Staphylococcus aureus modifying RhoE and Rnd3. J. Biol. Chem. 2001;276:9537–9542. doi: 10.1074/jbc.M011035200. [DOI] [PubMed] [Google Scholar]
- Wu X., Quondamatteo F., Lefever T., Czuchra A., Meyer H., Chrostek A., Paus R., Langbein L., Brakebusch C. Cdc42 controls progenitor cell differentiation and β-catenin turnover in skin. Genes Dev. 2006;20:571–585. doi: 10.1101/gad.361406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu A. J., Haase I., Watt F. I. Signaling via β1 integrins and mitogen-activated protein kinase determines human epidermal stem cell fate in vitro. Proc. Natl. Acad. Sci. USA. 1999;96:6728–6733. doi: 10.1073/pnas.96.12.6728. [DOI] [PMC free article] [PubMed] [Google Scholar]