Abstract
Katanin is a microtubule-severing protein that participates in the regulation of cell cycle progression and in ciliary disassembly, but its precise role is not known for either activity. Our data suggest that in Chlamydomonas, katanin severs doublet microtubules at the proximal end of the flagellar transition zone, allowing disengagement of the basal body from the flagellum before mitosis. Using an RNA interference approach we have discovered that severe knockdown of the p60 subunit of katanin, KAT1, is achieved only in cells that also carry secondary mutations that disrupt ciliogenesis. Importantly, we observed that cells in the process of cell cycle-induced flagellar resorption sever the flagella from the basal bodies before resorption is complete, and we find that this process is defective in KAT1 knockdown cells.
INTRODUCTION
Almost all eukaryotic cells are ciliated (note that in some cells, such as Chlamydomonas, cilia have historically been called flagella, but eukaryotic flagella are in fact cilia). Although cells with highly specialized cilia, such as respiratory or olfactory epithelial cells or sperm, do not reenter the mitotic cell cycle, most ciliated cells do divide. With rare exception, there is a tightly orchestrated sequence involving loss of cilia before mitosis, and then, shortly after exit from mitosis, ciliogenesis occurs (Rieder et al., 1979). This coordination is thought to reflect the alternating roles of centrioles, as the basal bodies that nucleate cilia and as the organizing foci of mitotic spindle poles (Quarmby and Parker, 2005). The regulatory relationship between cilia and cell cycle progression has not been elucidated, but it has been proposed that one function of primary cilia is to sense environmental signals pertinent to cellular decisions about division, differentiation, and apoptosis (Marshall and Nonaka, 2006; Pan and Snell, 2007).
There are two distinct ways that cells can lose their cilia: by deciliation or by resorption. Deciliation, also known as deflagellation, refers to the shedding of cilia into the environment (reviewed by Quarmby, 2004). Most (if not all) ciliated cells deciliate in response to chemical or physical stress. When cilia are resorbed in advance of mitosis, both axonemal and membrane components are retained by the cell. Although these two processes seem to be distinct in both function and mechanism, we have previously shown that they share common aspects of regulation and that resorption likely involves activity at the base, in addition to the well-established disassembly that occurs at the ciliary tip (Marshall and Rosenbaum, 2001; Parker and Quarmby, 2003). We have previously proposed that elements of the deciliation machinery might play roles in resorption.
Deflagellation involves the precise severing of the axoneme at a specific site between the axoneme proper and the flagellar transition zone, known as the site of flagellar autotomy (SOFA) (Mahjoub et al., 2004). The microtubule-severing ATPase katanin has been implicated as the protein responsible for the breakage of the outer doublet microtubules during deflagellation, but the evidence has been indirect (Lohret et al., 1998, 1999). Purified sea urchin katanin is capable of breaking axonemes, antibodies against katanin inhibit calcium-activated axonemal severing in vitro, and immunogold labeling with an anti-katanin antibody places katanin at the site of action. However, a screen for deflagellation mutants in Chlamydomonas yielded multiple alleles of each of three genes, FA1, FA2, and ADF1, but not katanin (e.g., Finst et al., 1998). The failure to isolate katanin mutants potentially indicated that either katanin plays no role in deflagellation or it plays an additional role that is essential for survival of the cell. Consistent with the latter possibility, it is well-established in other systems that katanin plays a role in cell cycle progression (Buster et al., 2002; Toyo-Oka et al., 2005; McNally et al., 2006; Zhang et al., 2007).
Based on the idea that cells with partial loss of katanin, but still retaining enough katanin to survive, might reveal hypomorphic phenotypes, we have taken an RNA interference (RNAi) approach to reduce levels of expression the catalytic p60 subunit of katanin in the haploid unicellular alga Chlamydomonas reinhardtii. Katanin knockdown strains were recovered infrequently, and only in the context of additional mutations that affected flagellar assembly. We went on to show that there is a second site of axonemal severing proximal to the transition zone (the SOFA is distal) and that severing occurs at this site before complete premitotic resorption of flagella. Experiments with a cell wall-less strain revealed that cells deficient in katanin fail to release resorbing flagella from the basal bodies. Together, our data suggest that one important role of katanin is separation of the basal body from ciliary remnants before functional reassignment of the basal bodies to the spindle poles.
MATERIALS AND METHODS
Strains, Culture Conditions, and Nuclear Transformations
Chlamydomonas strains 137c, cw2, bld1, and ift88-1 were obtained from the Chlamydomonas Genetics Center (Durham, NC). fa2-3 mutants were generated previously in our laboratory (Finst et al., 1998). All cells were maintained on Tris-acetate-phosphate (TAP) medium plates (Harris, 1989) supplemented with 1.5% agar, under constant illumination at 22°C. Nuclear transformation was by the glass bead method (Kindle et al., 1989); recipient strains other than cw2 were treated with gametic lytic enzyme (Harris, 1989) before transformation. All par and ble transformants were grown on 1.5% TAP plates supplemented with 15 μg/ml paromomycin (Sigma-Aldrich, Oakville, ON, Canada) or 30 μg/ml Zeocin (Invitrogen, Burlington, ON, Canada).
For experiments involving mitotic cells, partial cell cycle synchrony was achieved by growing cells ∼2 d in liquid TAP, followed by starvation in minimal medium (MI) in foil-wrapped flasks for 24–30 h (Umen and Goodenough, 2001). Cells were then resuspended in fresh TAP in unwrapped flasks, and aliquots fixed at several time points starting 12 h after the shift to light. The exact timing and degree of synchrony are dependent upon culture conditions, but typically at 12 h ∼50% of cells were in division (as judged by the presence of furrows, unhatched cells, or phospho-histone H3 immunofluorescence).
Molecular Constructs
Nuclear transformation of Chlamydomonas is accomplished by nonhomologous insertion of the exogenous DNA into the genome, a process that can frequently involve deletions of the transforming and genomic DNA. To maximize the frequency with which selected colonies are competent to express the transgene, we constructed transformation vectors with different selectable markers at each end (pEZ-KAT1-RNAi; see Figure 1A). Briefly, pEZ-KAT1-RNAi is derived from pGenD (Fischer and Rochaix, 2001), pSI103 (Sizova et al., 2001), and pSP124S (Lumbreras et al., 1998). The aphVIII gene, along with its promoter and 3′ untranslated region (UTR) (the “par cassette”), was amplified from pSI103 by using primers Spe-par(+) and Spe-par(−) and cloned into the unique SpeI site in pGenD, whereas the ble cassette was removed from pSP124S by restriction digestion and ligated into a HindIII site in pGenD. The PsaD-derived promoter elements from pGenD drive expression of a cDNA hairpin such that transcription will result in double-stranded RNA. The hairpin was derived from the 5′ end of the katanin cDNA (KAT1, Chlamydomonas gene c_80022; base pairs 114-800, then 114-552 in reverse orientation). This same hairpin targeting KAT1 was also inserted into the pNI-537 vector (Rohr et al., 2004; generously provided by H. Cerutti (Cornell University). This region of the cDNA is outside of the coding regions for the AAA domain and has no significant similarity to the most similar genes in the Chlamydomonas genome (KAT2, C_620017, 46% identity to KAT1 and a predicted spastin-like protein, Chlamydomonas gene model e_gwW.1.632.1, 39% identity to KAT1). Primers used are in Table 1.
Figure 1.
Isolation of two KAT1 knockdown strains. (A) pEZ-KAT1-RNAi construct used to generate stable RNAi lines. Cells transformed with this construct were sequentially selected on plates containing paromomycin, and then on plates containing Zeocin, to confirm the presence of the PsaD-driven hairpin targeting KAT1 (large inward-pointing arrows). (B) Box plots of cell volume distributions of two KAT1-RNAi isolates and the parental cw2 strain. Boxes encompass 50% of all data points (the interquartile range) and error bars encompass 90% of all data points. The boundary between the black and white boxes is the median cell volume. (C) Box plots of cell volumes of both KAT1-RNAi isolates after backcross to a wild-type (cell-walled) strain, as well as the wild-type cell volume. Boxes encompass the interquartile range and error bars encompass 90% of all data points. (D) Anti-acetylated tubulin immunofluorescence visualization of typical cells from each isolate (right) shows lack of flagella in both KAT1-RNAi isolates. Bars, 7.5 μm (cw2) and2.5 μm (KAT1-RNAi#1 and KAT1-RNAi#4). (E) Quantification of mRNA from cw2 and the two KAT1-RNAi strains. Main graph shows relative KAT1 level (the amount of KAT1 mRNA in cw2 cells is set at 1). The inset graph shows relative control transcript (RPS14) level. (F) Agarose gel electrophoresis of reverse transcriptase-PCR products. Amplicons targeted putative genes disrupted by insertion of KAT1-RNAi constructs into strain KAT1-RNAi#4 (left) and KAT1-RNAi#1 (right). All cDNA pools showed accumulation of PCR products targeting a control gene (data not shown).
Table 1.
Sequences of the oligonucleotides used in this work
| Primer name | Sequence |
|---|---|
| Spe-par(+) | 5′-ATAACTAGTGAGCTCGCTGAGGCTTGA-3′ |
| Spe-par(−) | 5′-ATAACTAGTGGTACCCGCTTCAAATAC-3′ |
| Kat-Ecor1(+) | 5′-TAAGAATTCATCACTCGG TACGTCCGC-3′ |
| Kat-Spacer(−) | 5′-TAAGGATCCCGCCGAGCCGGCGCGCCC-3′ |
| Kat-IR(+) | 5′-ATATCTAGAGAATTCATCACTCGGTACGTCCGC-3′ |
| Kat-IR(−) | 5′-ATTGGATCCCAGACCCGCAATGTCGTC-3′ |
| K311A-1(+) | 5′-CGGGACCGGCGCGACCATGCTCGCC-3′ |
| KAT 1440-64 (−) | 5′-GGACCCGGCTCCTTGTCCTTTTCGC-3′ |
| PKG-RT(+) | 5′-AACCAAGCTTTTTGTTGTGAGTGG-3′ |
| PKG-RT(−) | 5′-CGGAAGACGTCGCGCTCCAGCA-3′ |
| IFT88-RT(+) | 5′-ATAGTGATATCATTCAAGCCCTGTTCCTAC-3′ |
| IFT88-RT(−) | 5′-GTGTGTGAATGTATGTGTGTGCTAGGTAAG-3′ |
| Qcp60(+) | 5′-AACAGCTACCCCGAGATGAA-3′ |
| Qcp60(−) | 5′-TTGTTGTTGTTAGCCCAGGA-3′ |
| Bubble1 | 5′-GAGAGGGAAGAGAGCAGGCAAGGAATGGAAGCTGTC-TGTCGCAGGAGGAAG-3′ |
| Bubble2 | 5′-GACTCTCCCTTCTCGAATCGTAACCGTTCGTACGAGA-ATCGCTGTCCTCTCCTTC-3′ |
| B-specific | 5′-CGAATCGTAACCGTTCGTACGAGAATCGCT-3′ |
| Par-specific | 5′-TGGCGTTTTACCGGCTGTTGGACGAGTTC-3′ |
All sequences are listed in the 5′-3′ direction. Plus and minus signs denote forward and reverse primer orientation, respectively.
Assessment of mRNA Levels
To assess relative transcript levels for our initial KAT1-RNAi experiments, we adopted a competitive polymerase chain reaction (PCR)-based approach (Wang et al., 1989). Experimental cDNA pools were normalized to equal concentrations and then independently mixed with increasing concentrations of a competitor that differs in sequence and length from the target sequence but that shares with the target the primer binding sites used for amplifying the target (or competitor) sequences. Primers K311A(+) and K1440-64R(−) give a product of 300 base pairs from the KAT1 cDNA. To make the competitor (pGEM-T-mimic), we performed low-stringency PCR by using those same primers on Chlamydomonas genomic DNA to obtain a product of 400 base pairs, followed by a high-stringency second round of PCR by using the same primers. The resulting product was cloned into pGEM-T Easy (Promega, Madison, WI). As a control, experimental cDNA pools were also assayed using the gene for ribosomal subunit RPS14 at the CPH1 locus using the “CRY1” primers described by Kathir et al. (2003).
Total Chlamydomonas RNA, mRNA and cDNA were obtained as described previously (Mahjoub et al., 2002). Varying concentrations of pGEM-T-mimic were mixed with the cDNA pool from pEZ-KAT1-RNAi cells as the substrate for PCR reactions. Quantification of PCR bands was performed with ImageQuant (GE Healthcare, Chalfont St. Giles, United Kingdom). For reverse transcriptase (RT)-PCR of genes putatively disrupted by insertion of pEZ-KAT1-RNAi, cDNA pools were tested with control primers against centrin; primers specific to IFT88 and PKG2 are shown in Table 1.
Real-Time Quantitative PCR
Chlamydomonas cDNA pools from wild-type (137c), KAT1-RNAi;ift88-1 and KAT1-RNAi;bld1-1 cells were prepared as described above. Primers specific to KAT1 (Qcp60+/Qcp60−; Table 1) were initially used to PCR-amplify a 227-base pair fragment from the 5′ end of the KAT1 cDNA cloned earlier into pEZ-KAT1-RNAi. Conditions that would yield a specific band corresponding to KAT1 were empirically determined. Next, Qcp60+/Qcp60− were used to quantitatively amplify target sequence in a 50-μl reaction mix containing 500 ng of cDNA pool (IQ SyberGreen Supermix; Bio-Rad, Mississauga, ON, Canada). The annealing and elongation steps of the amplification cycle were performed at 58 and 72°C, respectively. PCR was run for 44 cycles. Gene expression was standardized to the expression levels of ribosomal subunit RSP14. The MiniOpticon RT-PCR system (Bio-Rad) was used to monitor target sequence amplification and Opticon Monitor3 software (Bio-Rad) was used for data analysis. Quantitative PCR was carried out in triplicate, and the average reading is represented as the final KAT1 transcript level.
Identification of the Sites of Insertion of the RNAi Constructs
To determine the site of insertion of the RNAi constructs, we used the Vectorette PCR method (Riley et al., 1990). Briefly, this method is based on ligating DNA adapters that have large regions of mismatched nucleotides between the two DNA strands (the “bubble anchors”) to the ends of genomic DNA digested with blunt-cutting restriction endonucleases. Subsequent PCR, using one primer specific to one bubble anchor and the other primer specific to the integrated construct, amplifies genomic DNA between the restriction enzyme cut site and the insertion. Primer sequences used are in Table 1.
Measurements of Cell Size
The length and width of at least 75 cells of each strain were measured by differential interference contrast (DIC) microscopy and SoftWorx version 3.22 software package (Applied Precision, Issaquah, WA). Cell volumes were calculated using the formula 4/3 π[L/2][W/2]2 (Umen and Goodenough, 2001), where L and W describe length and width of the cells, respectively.
Generation of Antibodies and Immunoblotting
KAT1 antibodies were raised against two synthetic peptides (Sigma-Aldrich), N-terminal (KGSAGEKAKKQY) and C-terminal (QVDGVHGSEKDK), conjugated to KLH and injected into four rabbits (Sigma-Aldrich). KAT1 cDNA (KAT1 full length) and a derived construct lacking the sequence corresponding to the peptide epitopes (KAT1-Δ-epitope) were expressed using pET-DEST42 (Invitrogen) in BL21 Escherichia coli (Novagen, Madison, WI) and induced with 1 mM isopropyl β-d-thiogalactoside (IPTG; Invitrogen). Antisera from rabbit #8907 exhibited a reactive band of ∼60 kDa, which is the predicted size of KAT1, from IPTG-induced cells expressing KAT1 full length but not cells expressing KAT1-Δ-epitope or uninduced cells (data not shown). Characterization of antisera recognizing PKG2 will be described in a future report. Protein levels in cell lysates were determined using QuickStart Bradford reagent (Bio-Rad). Horseradish peroxidase-linked goat anti-rabbit IgG (1:10,000; Sigma-Aldrich) and ECL (GE Healthcare) were used for visualization of western blots. Densitometry was performed using Adobe Photoshop.
Immunofluorescence Microscopy
Indirect immunofluorescence was conducted as described (Mahjoub et al., 2004) using a DeltaVision imaging station (Applied Precision, Issaquah, WA). Primary antibodies used in this study were: mouse monoclonal anti-acetylated-tubulin Igγ2b (1:300; Sigma-Aldrich clone 6-11B-1), mouse monoclonal anti-α-tubulin (1:1000; Sigma−Aldrich clone DM1A) and anti-phosphohistone H3 (ser10, Cell Signaling). Secondary antibodies against mouse IgG2b, mouse IgG1, or rabbit were purchased conjugated to Alexa Fluor 488 or 594 (Invitrogen) and all were used at 1/500. Cells were also stained with DAPI.
For fixation of mitotic cells, a protocol graciously provided by Brian Piasecki and Carolyn Silflow (University of Minnesota, St. Paul, MN), and originally developed by M.A. Sanders, was modified. The fixative consists of 4% formaldehyde (added as Formalin, Anachemia), 0.01% glutaraldehyde, 10 mM HEPES buffer pH 7.0, 1 mM EGTA, 1 mM MgSO4 and 0.1% Igepal CA-630 (Sigma-Aldrich) and thus permeabilizes cells but also cross-links cyotological features. After incubation on ice, fixed cells were affixed to coverslips and methanol-extracted essentially as described previously (Mahjoub et al., 2004), except coverslips were washed with ammonium chloride in addition to PBS.
For flagellar remnant determination, cells were counted as “mitotic” if either positive for phospho-histone H3, unhatched and in mother cell walls, or showing either clear reorganization of rootlet microtubules or separated centrosomes.
For the experiment shown in Figure 4E, cells were pre-stained with Lugol's iodine solution (Harris, 1989) and then fixed and permeabilized in MeOH (Cole et al., 1998). Primary antibodies used were mouse anti-acetylated tubulin (Sigma), mouse anti-α tubulin (Sigma, clone DM 1A used at 1/300). Secondary antibodies (Jackson ImmunoResearch, West Grove, PA) used were CY5-conjugated goat anti-mouse immunoglobulin G (IgG), subclass I, fluorescein isothiocyanate-conjugated goat anti-mouse IgG, subclass 2b (used at 1/100 dilution), and tetramethylrhodamine B isothiocyanate-conjugated goat anti-rabbit (1/300).
Figure 4.
Basal bodies are freed from flagella by severing before mitosis. (A–D) Representative images of flagellar remnants associated with representative wild-type mitotic cells. Remnants are visible as dots staining for acetylated-α-tubulin (green) and are found at the anterior end of the cell (A, B, and D) or in a position consistent with the prior anterior of the mother cell, in cells that have completed at least one round of division (C). Blue is 4,6-diamidino-2-phenylindole (DAPI), which stains both nuclear and plastid DNA. (E) Longer flagella (stained with anti-acetylated tubulin; green) are observed more rarely in wild-type mitotic cells. The spindle of this cell visualized with anti-α-tubulin (red). (F–J) Remnants are found in mitotic fa2 mutant cells. (F) a fa2 cell early in cell division has relatively long flagella as stained by acetylated tubulin (green), counterstained with DAPI (blue). (G and I) fa2 cells later on in cell division, as indicated by the presence of cleavage furrows, have flagellar remnants as visualized by acetylated-tubulin staining (red; the primary antibody for these cells was a mixture of anti-acetylated- and anti-α-tubulin). (H and J) Overlay with corresponding DIC images. Bars, 5 μm.
RESULTS
Initial Isolation of Two KAT1 Knockdown Strains
Currently, the most effective approach to RNA interference in Chlamydomonas involves the nonhomologous insertion of a transgene that encodes the inhibitory RNA, into the nuclear genome (reviewed by Schroda, 2006). The insertion event is often associated with deletions of genomic sequence and part of the exogenous DNA (Gumpel et al., 1994). Because our initial attempts using conventional methods did not yield katanin knockdown strains, we sought to increase the frequency of recovery of intact insertions. To do this, we developed a vector wherein the inhibitory sequence (a hairpin loop corresponding to KAT1 sense and antisense sequence) is flanked by two different selectable markers (Figure 1A).
From 15 independent transformation experiments, ∼1500 colonies grew on plates containing both Zeocin and paromomycin. For initial characterization, we grew six of these isolates and assessed levels of KAT1 mRNA. Although all six had integrated the complete transgene, none of the colonies had reduced levels of KAT1 mRNA (data not shown). In Chlamydomonas, RNAi transgenes can be rapidly silenced or suppressed (especially those targeting genes involved in cell cycle progression; Schroda, 2006; Qin et al., 2007), and we were concerned that in growing up sufficient quantities of cells for RNA analysis, suppressed strains would rapidly overtake the culture preventing us from observing authentic knockdowns. To narrow our search for KAT1 knockdown isolates, we conducted a preliminary screen by phase-contrast microscopy. We examined live samples of the ∼1500 isolates for gross defects in motility or for defects in deflagellation in response to weak acid (as described in Finst et al., 1998). Fixed samples of each isolate were also examined by phase contrast microscopy for gross changes in ciliary length, number, or position or for aberrant cell size as a proxy for effects on cell cycle progression (Bradley and Quarmby, 2005).
Only two of the 1500 strains exhibited either flagellar or cell size defects, and both of these had the same phenotype: the cells were small relative to the cell wall-less parental strain (Figure 1B) and remained small after backcrossing into a wild-type (cell-walled) background (Figure 1C). In addition, both isolates also exhibited flagellar defects (Figure 1D). Katanin mRNA levels were dramatically reduced in both of these strains (Figure 1E). Although KAT1-RNAi#1 and #4 were clearly katanin knockdown strains, it was important to establish whether the phenotypes were due to the reduced levels of katanin. As described above, generation of transgenic strains in Chlamydomonas involves disruption of genomic DNA. We therefore set out to determine the sites of insertion of the RNAi transgenes to assess the possible role of these disruptions in affecting the observed phenotypes.
The Two Original KAT1 Knockdown Strains Are Also Insertional Mutants of Flagellar Assembly Genes
We used a PCR-based approach to clone the DNA flanking the insertions (see Materials and Methods). This approach identified unique sites of insertion in each of the two back-crossed strains. We found that the KAT1-RNAi#4 transgene had inserted into the genomic sequence for the 3′ UTR of the IFT88 gene (C_500002 in version 3 of the Chlamydomonas genome), which encodes an intraflagellar transport protein that is essential for ciliogenesis: ift88 mutants are flagella-less (Pazour et al., 2000). We confirmed that IFT88 expression is lost in the KAT1-RNAi#4 strain by RT-PCR (Figure 1F). This result raised the important possibility that the flagella-less phenotype was not a bona fide KAT1-RNAi phenotype but rather was a consequence of the inadvertent knockout of IFT88. It was thus imperative to determine the site of insertion of the transgene in the KAT1-RNAi#1 strain.
We determined that in the KAT1-RNAi#1 strain, the construct had inserted into the coding sequence of a predicted protein kinase G gene, which we refer to as PKG2 (C_740056). Primers specific to PKG2 amplified cDNA from the cw2 strain, but not from the KAT1-RNAi#1 strain (Figure 1F). We have subsequently determined that PKG2 plays an essential role in ciliogenesis but does not affect cell size (Rasi et al., unpublished observations.).
Is a Defect in Ciliogenesis Permissive for Knockdown of KAT1?
As described above, KAT1 knockdown strains were rare, and the only two that we identified were coincident with mutations that disrupted ciliogenesis. These observations lead us to hypothesize that KAT1 might indeed be performing an essential function, but whatever this function might be, it is only essential in cells with normal flagella. Thus, we hypothesized that defects in flagellar assembly are permissive for knockdown of katanin. This hypothesis led us to predict that we would recover KAT1 knockdown strains at a much higher frequency if the recipient strain for the transformations was already defective in ciliogenesis.
To test this idea, we independently transformed two flagella-less strains, ift88-1 and bld1-1 (which is mutant for IFT52; Brazelton et al., 2001; Deane et al., 2001), as well as wild-type (WT) cells, with the same KAT1-RNAi construct described in Figure 1A. In this experiment, we recovered only one isolate that was resistant to both selectable markers when the recipient strain was wild type. In contrast, 10 and 11 isolates were resistant to both Zeocin and paromomycin when the recipient strains were ift88-1 and bld1, respectively. The single KAT1-RNAi isolate from a wild-type background that grew on double selection was not knocked down for KAT1 mRNA (data not shown). In contrast, 9/10 and 8/11 double-selected isolates, from the transformations of ift88-1 and bld1, respectively, had significantly reduced levels of KAT1 mRNA (Figure 2, A and B; representative subset shown). Thus, 17 new KAT1 knockdown strains were isolated. All of these strains lack flagella (as do the parental strains), and, distinct from the parental strains, all had substantially reduced cell size (Figure 2, C and D; representative subset shown).
Figure 2.
KAT1 can be readily knocked down in flagella-less cells. (A) Relative KAT1 mRNA level, as determined by real-time PCR, from a representative subset of ift88 strains transformed with, and selected for, pEZ-KAT1-RNAi. (B) Relative KAT1 mRNA level, as determined by real-time PCR, from a representative subset of bld1 strains transformed with, and selected for, pEZ-KAT1-RNAi. (C) Box plots of cell volume distribution of the same ift88-KAT1-RNAi isolates examined in A. Error bars capture 90% of cells. (D) Box plots of cell volume distribution of the same bld1-KAT1-RNAi isolates examined in A. Error bars capture 90% of cells.
To more directly assess levels of KAT1, we raised an antibody against two peptides of KAT1 (see Materials and Methods). Western blotting reveals an ∼60-kDa band from wild-type Chlamydomonas cell lysates, not observed using preimmune serum from the same rabbit (Figure 3A). With this antibody in hand, we repeated the transformation of WT and bld1 cells with the KAT1-RNAi construct.
Figure 3.
KAT1 protein can be readily knocked down in bld1 mutants. (A) Characterization of anti-KAT1 antiserum. Wild-type cell lysate was blotted in duplicate and probed with either immune antiserum (left) or preimmune serum from the same rabbit (right). The expected molecular weight of KAT1 is 60 kDa. (B) Quantification of relative knockdown of KAT1 protein levels by anti-KAT1 Western blot (middle) in seven random isolates recovered from transformation of wild-type cells with pEZ-KAT1-RNAi. Anti-PKG2 was used as a loading control by reprobing the same blot (top). Gel films were scanned and densitometric analysis (bottom) performed as described in Materials and Methods. (C) Quantification of relative knockdown of KAT1 protein levels by anti-KAT1 Western blot (middle) in seven random isolates recovered from transformation of bld1 cells with pEZ-KAT1-RNAi. Anti-PKG2 was used as a loading control by reprobing the same blot (top). Gel films were scanned and densitometric analysis (bottom) performed as described in Materials and Methods. (D) Quantification of relative knockdown of KAT1 protein levels in a subset of the same isolates examined in C after an additional 2 wk of growth. Analysis as described for C. (E) Box plots showing cell volumes of the same bld1:KAT1-RNAi isolates examined in B and C. White boxes show cell volume distribution data from the time of the experiment shown in B and gray boxes are data from the time of the experiment shown in C. Boxes encompass the interquartile range and error bars encompass 90% of the total data points.
In this experiment, 52 isolates grew on double selection when the recipient strain was flagellated (137c). None of these 52 isolates had severely reduced levels of KAT1, but in a few lines KAT1 protein levels were moderately reduced (Figure 3B shows the data for eight of these 52 isolates, including two with moderately reduced levels of KAT1 protein). In this same experiment, 14 isolates grew on double selection when the recipient strain was bld1. In contrast to the flagellated cells transformed with the same construct, most of these flagella-less isolates showed reduced levels of KAT1 protein and in some the reduction in KAT1 levels was severe. Of the seven isolates shown in Figure 3C, three show substantial reduction in KAT1 levels, three are moderately reduced, and in the seventh KAT1 levels are unaffected.
In this experiment, the severely knocked down strains showed distorted cell size profiles (Figure 3E), but unlike the experiment in Figure 2, reduction in cell volume did not correlate with the extent of knockdown of KAT1. It is interesting to note that the knockdown of KAT1 is transient, even in the bld1 cells. Figure 3D shows that after an additional 2 wk, levels of KAT1 are no longer severely reduced. Concomitantly, the cell size profiles become closer to WT (Figure 3E).
What Are the Cellular Consequences of KAT1 Knockdown?
In addition to their ciliogenesis defects, the two original KAT1 knockdown isolates had small cells (Figure 1, B and C). The other genes disrupted in these strains, IFT88 and PKG2, are essential for ciliogenesis (Pazour et al., 2000; Rasi et al., unpublished observations), but do not affect cell size (data not shown). Furthermore, the 17 additional KAT1 knockdown strains generated in ift88-1 and bld1 backgrounds also had small cells (Figure 2, C and D). However, this pattern was not as clear in a subsequent experiment (Figure 3E). Figure 3, C and D, reveal that isolate #29 has the most severe knockdown of KAT1; yet, this isolate has unusually large cells (Figure 3E). Nevertheless, the data in Figure 3E also show that the cell size profiles approach wild-type as the levels of KAT1 return to normal. Taking all of these data together, we conclude that reduced levels of KAT1 can influence cell size, but the cell size phenotype is not consistent and seems to depend on some yet-to-be-defined differences in culture conditions.
Aberrant cell size is often an indication of defects in cell cycle progression. Unfortunately, none of the katanin knockdown strains remained phenotypically stable long enough for us to assess their cell cycle characteristics as we have previously done for other Chlamydomonas flagellar mutants with cell size defects (Mahjoub et al., 2002; Bradley and Quarmby, 2005). We have attempted several approaches to obtaining conditional KAT1 knockdown strains, but so far none of these strategies has succeeded. Nevertheless, an independent line of study has led us to a possible ciliary-cell cycle role for KAT1. The experiments in the next section may provide the explanation for the difference in susceptibility to KAT1 knockdown of WT compared with bld cells.
Basal Bodies Are Released by Severing at the Proximal Transition Zone before Complete Resorption of Flagella
Together, our data thus far indicate that KAT1 plays an important role in Chlamydomonas and that this role is obviated in cells that do not have flagella. This suggests that katanin may play a role in coupling the cycle of flagellar resorption and regeneration with cell cycle progression. Our earlier work predicted that the machinery of deflagellation plays a part in premitotic resorption of flagella. Indeed, we predicted that disassembly at the base of flagella might contribute to resorption (Parker and Quarmby, 2003).
The generally accepted model for premitotic resorption of flagella is that disassembly continues down from the tip, through the transition zone of the flagella, until all that is left is the basal body (Cavalier-Smith, 1974). Contrary to this view, the images in Figure 4 reveal that flagella or their derivatives (visualized with indirect immunofluorescence using an antibody against acetylated tubulin) remain with the mother cell wall when cells enter mitosis. In Figure 4, A–D, reveal pairs of acetylated tubulin spots associated with the mother cell walls of dividing wild-type cells. The spots, or flagellar remnants, were observed in the mother cell walls of 226 of 291 (78%) dividing cells. Flagellar remnants are not observed if glutaraldehyde, a cross-linking reagent, is omitted from the fixative, or in a cell wall-less mutant background, suggesting that the remnants are loosely associated with the cell walls (data not shown). Mitotic cells with more substantial incompletely resorbed flagellar remnants, in some cases almost full-length flagella, were uncommon, but they could be found (Figure 4E; also see Piasecki et al., 2007). Importantly, in all of these cells it is clear that the basal bodies have separated from the residual flagella (Figure 4).
These data indicate that a severing event occurs between the basal bodies and the axoneme, releasing the basal bodies for service at the spindle poles. Severing of the axoneme is a well-established event in deflagellation (reviewed in Quarmby, 2004), and we wondered whether the release of basal bodies might be using the same pathway. To test this idea, we examined the fa2 deflagellation-defective mutant. The fa2-3 strain is null for FA2; the cells are completely defective in the axonemal severing associated with deflagellation and exhibit a G2/M delay in cell cycle progression (Mahjoub et al., 2004). Figure 4, F–H, shows examples of fa2 cells clearly in mitosis, while retaining flagellar remnants. We examined 157 dividing fa2-3 cells and observed that 144 (92%) retained flagella or flagellar remnants associated with the mother cell wall. Thus, the production of flagellar remnants is at least as common in fa2 cells as it is in wild type. Severing in deflagellation occurs at the SOFA, which is distal to the transition zone and it is likely that the separation of basal bodies observed here is a consequence of severing at the proximal end of the transition zone (Figure 6). The proximal severing seems to be intact in fa2 mutants, consistent with the observation that the null mutation is not lethal. Together with the data of Mahjoub et al. (2004), our data indicate that FA2 plays a role in distal but not proximal severing. Katanin may play a role at both sites. Indeed, immunogold studies with an anti-katanin antibody revealed katanin at both sites (Lohret et al., 1999).
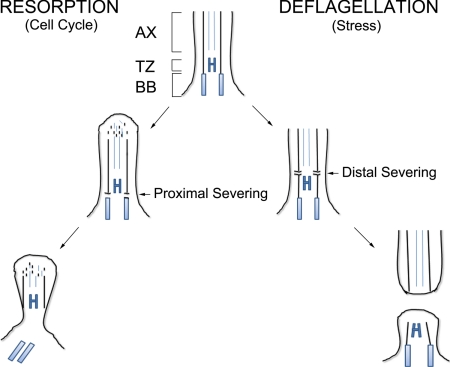
Figure 6.
Severing may occur at either end of the flagellar transition zone. Deflagellation in response to stress involves severing at the distal end of the transition zone (the SOFA), after which the flagellum is cast away from the cell, whereas the transition zone remains associated with the basal body and can nucleate the assembly of a new flagellum. Resorption before mitosis involves both shortening of the flagellum from its distal end and severing at the proximal transition zone. This proximal severing frees the basal body, leaving the transition zone. Any residual flagella, or flagellar remnants, remain associated with the cell wall. AX, axoneme; TZ, transition zone; BB, basal body.
Knockdown of KAT1 Prevents Premitotic Release of Flagella from Basal Bodies
We next asked whether the lethality of KAT1 knockdown in flagellated cells might be a consequence of failure to separate basal bodies from flagellar remnants. Our previous experiments indicated that flagellated recipient strains do not survive severe KAT1 knockdown, therefore we examined these colonies 5 d after transformation. We used a cw2 recipient strain in order to observe only flagella and flagellar remnants remaining attached to the basal bodies, without the complication of discarded flagellar remnants retained by association with the mother cell wall.
We examined 60 isolates that grew on paromomycin after transformation of cell wall-less (cw2) cells with the empty pNI-537 vector. We observed no flagella on cells with cytokinetic furrows (>20 presumptive dividing cells were examined in each colony; data not shown). These data support the idea that in cells with a normal allotment of katanin, release of the basal bodies from the flagella or flagellar remnants results in the shedding of the flagellar remnant from the cell. In the absence of cell walls, the flagellar remnants are washed away, whereas in walled cells remnants are observed because they are cross-linked to the mother cell wall (Figure 4). We next examined sixty isolates derived from pNI-537-KAT1-RNAi transformation of the same cell wall-less recipient strain. Dividing (furrowed) cells in 27/60 presumptive KAT1-RNAi colonies had flagella attached to almost all of the furrowed cells observed. Because these cells are wall-less, flagella will only be retained if still anchored at the basal body. Figure 5 shows examples of cw2 cells presumptively depleted for katanin. In addition, small malformed cells were common in these colonies (data not shown). These data suggest that depletion of KAT1 affects premitotic loss of flagella and leads to gross cellular abnormalities. Most importantly, the presence of flagella on the KAT1-RNAi transformed wall-less cells with apparent cytokinetic furrows is consistent with a failure to separate basal bodies from flagella before mitosis.
Figure 5.
DIC images of presumptive cw2:KAT1-RNAi cells. (A–C) Examples of presumptive KAT1 knockdown cells in a cw2, cell wall-less background. Note the presence of flagella on dividing cells. Similar cells were not seen in any control colonies. Bar, 5 μm.
DISCUSSION
A phenomenological correlation between ciliary disassembly and entry into mitosis has been known for some time (Tucker et al., 1979). More recently, several proteins have been implicated in coordinating ciliary resorption with cell cycle progression, including AurA, Hef1, and HDAC6 (Pugacheva et al., 2007), and IFT27 (Qin et al., 2007). The presumption has been that ciliary resorption is a consequence of down-regulation of anterograde intraflagellar transport (IFT) and possible up-regulation of retrograde IFT, resulting in a net transport of disassembled ciliary components from the tip back to the cell body. Although this is almost certainly the case based on the work of Qin et al. (2004), our previous work with Chlamydomonas mutants revealed that events at the base of the cilium are also important (Parker and Quarmby, 2003). The most dramatic instance of ciliary deconstruction at the base is deflagellation (also known as deciliation; reviewed in Quarmby, 2004). Deflagellation, which usually occurs as a stress response, involves the activation of a signaling pathway that culminates in an increase in intracellular calcium concentration leading to activation of the severing machinery that breaks all nine outer doublet microtubules at the SOFA. Although some elements of the signaling pathway have been established in Chlamydomonas, the actual machinery of severing has not been determined. Katanin has been implicated through biochemical and cell biological studies, but genetic evidence has been elusive. Because we were unable to sustain knockdown of katanin in flagellated cells, the current data do not allow us to test whether KAT1 or one of the other microtubule-severing proteins serves the stress-inducible deflagellation pathway.
Katanin is a conserved eukaryotic protein that has been implicated in the cell cycle since its original purification from M phase oocytes (McNally and Vale, 1993), but its specific role in cell cycle progression has been difficult to define. Evidence is accumulating that the microtubule-severing activity of katanin is required for rearrangement of the interphase microtubule cytoskeleton and concomitant spindle formation (Toyo-Oka et al., 2005), as well as spindle function and disassembly (Buster et al., 2002; McNally et al., 2006; Zhang et al., 2007). Mitotic spindle formation involves multiple pathways, even in a single organism or cell type (Wadsworth and Khodjakov, 2004; O'Connell and Khodjakov, 2007). In flies, but not worms, multiple microtubule-severing enzymes, katanin, spastin, and fidgetin, all have different roles in contributing to mitotic spindle function (Srayko et al., 2000; Zhang et al., 2007). In mice, katanin p60 is recruited to splitting centrosomes by Ndel1 in an Aurora A-dependent manner (Toyo-Oka et al., 2005; Mori et al., 2007), but no obvious Ndel-like proteins are identifiable by tBLASTn in the Chlamydomonas genome (version 3.0, accessed July 2008). It is possible that the development of important roles for microtubule severing in eukaryotic cell division preceded divergence of the microtubule severing proteins. This requires that, in divergent lineages, different microtubule-severing proteins cooperate with diverse partner proteins to undertake the various specialized severing tasks.
Early evolutionary divergence could also serve to explain differences in subunit interactions. In animal cells katanin is usually isolated as a dimer, composed of the p60 catalytic subunit and the p80 regulatory subunit (Hartman et al., 1998). A human tumor suppressor, LAPSER, associates with the human p80 subunit and localizes preferentially to the mother centriole, which nucleates the primary cilium (Sudo and Maru, 2008). The pf15 strain of Chlamydomonas, which carries a mutation in the sole katanin p80 subunit found in the genome, has defects in the formation and stability of the central pair of microtubules (Dymek et al., 2004). Intriguingly, the central pair microtubules are nucleated close to the SOFA, where the p60 subunit is implicated in axonemal severing.
We observed that a substantial fraction of mitotic cells retain flagella or flagellar remnants, even though their basal bodies are no longer associated with the retained structures, indicating that the axonemes of these flagella have been successfully severed from their basal bodies. This is consistent with previous observations by electron microscopy of Chlamydomonas (Johnson and Porter, 1968) and Chlorogonium (Hoops and Witman, 1985) and immunofluorescence microscopy (Piasecki et al., 2007). Based on these data, we propose that katanin-based severing of the axoneme proximal to the flagellar transition zone facilitates entry into mitosis (Figure 6). We propose that severing proximal to the transition zone results in release of the basal bodies and abandonment of the transition zone at the mother cell wall. Consequently, Chlamydomonas cells without flagella do not require katanin.
To be more precise, our model predicts that transition zone-less cells will not depend upon katanin for the release of their basal bodies. In spite of this, we were able to knockdown KAT1 in bld1 cells, which are capable of building transition zones (Brazelton et al., 2001; Deane et al., 2001). Our model suggests that the key event for mitosis is the release of basal bodies and that this it occurs by severing at the proximal end of the transition zone. In this case, why are flagella-less cells more tolerant of katanin knockdown than wild-type cells, even though both build transition zones? The higher frequency of recovery of knockdown strains might be indicative of a subpopulation of bld1 cells that do not assemble transition zones, or transition zones that are less stable than wild type. We think it more likely that there is an alternate mechanism to free basal bodies from transition zones, and in the absence of the need to disassemble flagella before mitosis, this alternate, albeit less efficient, pathway is taken.
It is interesting to note that in both bld1 and wild-type cells, katanin may itself contribute to the process of RNAi-mediated knockdown. Recently, in the plant Arabidopsis, Argonaute-mediated translational suppression via the siRNA pathway was shown to be dependent on katanin (Brodersen et al., 2007) acting through an as-yet-unknown mechanism. If this is true in Chlamydomonas, then it is likely that katanin knockdown may be unsustainable, even in the absence of direct effects on cell cycle or growth.
We propose that severing at the proximal transition zone is an important activity of katanin in Chlamydomonas and possibly mammalian cells, but not necessarily all ciliated cells. For example, in Tetrahymena, another genetic model system for the study of cilia, misexpression of katanin affects ciliary length but does not result in a cell cycle phenotype (Sharma et al., 2007). However, in Chlamydomonas but not in Tetrahymena, ciliary basal bodies must be detached from their axonemes before mitosis, thus any proteins that affect this process in Chlamydomonas may cause a cell cycle phenotype, whereas such an effect would not be predicted in Tetrahymena.
Flagella-less cells can survive and divide without katanin, but in some of the isolates the cells are small (or large), and the KAT1 knockdown is quickly lost. To date, these cultures have not survived as KAT1 knockdown strains long enough for us to determine the reason for the cell size defects and our attempts to develop conditional strains have failed. It is possible that the cell size phenotype might not be related to severing of the proximal transition zone because katanin has been implicated in several other mitotic events (Buster et al., 2002; Toyo-Oka et al., 2005; McNally et al., 2006). In addition, microtubule severing by katanin regulates noncentrosomal microtubule arrays (Roll-Mecak and Vale, 2006), and in Chlamydomonas, a cortical array of noncentrosomal microtubules play important roles in cytokinesis (Ehler et al., 1995). We speculate that katanin plays several fundamental roles essential to cell cycle progression in both Chlamydomonas and mammalian cells and that one of these roles is related to severing of the ciliary axoneme at the proximal transition zone before mitosis.
We have shown that Chlamydomonas cells free their basal bodies from flagella before mitosis. Katanin-mediated severing at the cell-proximal end of the transition zone is the likely mechanism for this disengagement, because of the relative ease with which katanin can be knocked down in flagella-less cells versus wild-type cells. This conclusion gains further support from our observation that presumptive KAT1 knockdown cells retain flagella during division. In retrospect, our initially perplexing observation that the two original KAT1 knockdown isolates both coincidentally disrupted genes required for ciliogenesis is predictable.
ACKNOWLEDGMENTS
We are grateful to Shazina Khan for excellent technical support and persistent good cheer. We thank Brian Piasecki and John Glover for stimulating discussions, and we further appreciate John Glover for valuable comments on the manuscript. We are deeply indebted to two persistent and thorough anonymous reviewers who guided us to substantial improvements in the quality of this work. We also thank Laura Hilton and Marc Champigny for comments that improved the quality of the final draft. We thank Heriberto Cerutti for providing pNI-537. We are deeply indebted to J. Salisbury (Mayo Clinic) for continuing to generously provide us with anti-centrin antibody. This work was supported primarily by an operating grant MOP-37861 from the Canadian Institutes of Health Research (to L.M.Q.) and in part by National Institutes of Health grant R01 GM-077004 (to W.F.M.). J.L.F. is supported by a National Science Foundation predoctoral fellowship and a UC Fletcher Jones fellowship.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E07-10-1007) on November 12, 2008.
REFERENCES
- Bradley B. A., Quarmby L. M. A NIMA-related kinase, Cnk2p, regulates both flagellar length and cell size in Chlamydomonas. J. Cell Sci. 2005;118:3317–3326. doi: 10.1242/jcs.02455. [DOI] [PubMed] [Google Scholar]
- Brazelton W. J., Amundsen C. D., Silflow C. D., Lefebvre P. A. The bld1 mutation identifies the Chlamydomonas osm-6 homolog as a gene required for flagellar assembly. Curr. Biol. 2001;11:1591–1594. doi: 10.1016/s0960-9822(01)00485-7. [DOI] [PubMed] [Google Scholar]
- Brodersen P., Sakvarelidze-Achard L., Bruun-Rasmussen M., Dunoyer P., Yamamoto Y. Y., Sieburth L., Voinnet O. Widespread translational inhibition by plant miRNAs and siRNAs. Science. 2008;320:1185–1190. doi: 10.1126/science.1159151. [DOI] [PubMed] [Google Scholar]
- Buster D., McNally K., McNally F. J. Katanin inhibition prevents the redistribution of gamma-tubulin at mitosis. J. Cell Sci. 2002;115:1083–1092. doi: 10.1242/jcs.115.5.1083. [DOI] [PubMed] [Google Scholar]
- Cavalier-Smith T. Basal body and flagellar development during the vegetative cell cycle and the sexual cycle of Chlamydomonas reinhardii. J. Cell Sci. 1974;16:529–556. doi: 10.1242/jcs.16.3.529. [DOI] [PubMed] [Google Scholar]
- Cole D. G., Diener D. R., Himelblau A. L., Beech P. L., Fuster J. C., Rosenbaum J. L. Chlamydomonas kinesin-II-dependent intraflagellar transport (IFT): IFT particles contain proteins required for ciliary assembly in Caenorhabditis elegans sensory neurons. J. Cell Biol. 1998;141:993–1008. doi: 10.1083/jcb.141.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deane J. A., Cole D. G., Seeley E. S., Diener D. R., Rosenbaum J. L. Localization of intraflagellar transport protein IFT52 identifies basal body transitional fibers as the docking site for IFT particles. Curr. Biol. 2001;11:1586–1590. doi: 10.1016/s0960-9822(01)00484-5. [DOI] [PubMed] [Google Scholar]
- Dymek E. E., Lefebvre P. A., Smith E. F. PF15p is the Chlamydomonas homologue of the Katanin p80 subunit and is required for assembly of flagellar central microtubules. Eukaryot. Cell. 2004;3:870–879. doi: 10.1128/EC.3.4.870-879.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehler L. L., Holmes J. A., Dutcher S. K. Loss of spatial control of the mitotic spindle apparatus in a Chlamydomonas reinhardtii mutant strain lacking basal bodies. Genetics. 1995;141:945–960. doi: 10.1093/genetics/141.3.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finst R. J., Kim P. J., Quarmby L. M. Genetics of the deflagellation pathway in Chlamydomonas. Genetics. 1998;149:927–936. doi: 10.1093/genetics/149.2.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer N., Rochaix J. D. The flanking regions of PsaD drive efficient gene expression in the nucleus of the green alga Chlamydomonas reinhardtii. Mol. Genet. Genomics. 2001;265:888–894. doi: 10.1007/s004380100485. [DOI] [PubMed] [Google Scholar]
- Gumpel N. J., Rochaix J. D., Purton S. Studies on homologous recombination in the green alga Chlamydomonas reinhardtii. Curr. Genet. 1994;26:438–442. doi: 10.1007/BF00309931. [DOI] [PubMed] [Google Scholar]
- Harris E. The Chlamydomonas Sourcebook: A Comprehensive Guide to Biology and Laboratory Use. San Diego, CA: Academic Press; 1989. [DOI] [PubMed] [Google Scholar]
- Hartman J. J., Mahr J., McNally K., Okawa K., Iwamatsu A., Thomas S., Cheesman S., Heuser J., Vale R. D., McNally F. J. Katanin, a microtubule-severing protein, is a novel AAA ATPase that targets to the centrosome using a WD40-containing subunit. Cell. 1998;93:277–287. doi: 10.1016/s0092-8674(00)81578-0. [DOI] [PubMed] [Google Scholar]
- Hoops H. J., Witman G. B. Basal bodies and associated structures are not required for normal flagellar motion or phototaxis in the green alga Chlorogonium elongatum. J. Cell Biol. 1985;100:297–309. doi: 10.1083/jcb.100.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson U. G., Porter K. R. Fine structure of cell division in Chlamydomonas reinhardi. Basal bodies and microtubules. J. Cell Biol. 1968;38:403–425. doi: 10.1083/jcb.38.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathir P., LaVoie M., Brazelton W. J., Haas N. A., Lefebvre P. A., Silflow C. D. Molecular map of the Chlamydomonas reinhardtii nuclear genome. Eukaryot. Cell. 2003;2:362–379. doi: 10.1128/EC.2.2.362-379.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindle K. L., Schnell R. A., Fernandez E., Lefebvre P. A. Stable nuclear transformation of Chlamydomonas using the Chlamydomonas gene for nitrate reductase. J. Cell Biol. 1989;109:2589–2601. doi: 10.1083/jcb.109.6.2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohret T. A., McNally F. J., Quarmby L. M. A role for katanin-mediated axonemal severing during Chlamydomonas deflagellation. Mol. Biol. Cell. 1998;9:1195–1207. doi: 10.1091/mbc.9.5.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohret T. A., Zhao L., Quarmby L. M. Cloning of Chlamydomonas p60 katanin and localization to the site of outer doublet severing during deflagellation. Cell Motil. Cytoskeleton. 1999;43:221–231. doi: 10.1002/(SICI)1097-0169(1999)43:3<221::AID-CM5>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Lumbreras V. S., David R., Purton Saul. Efficient foreign gene expression in Chlamydomonas reinhardtii mediated by an endogenous intron. Plant J. 1998;14:441–447. [Google Scholar]
- Mahjoub M. R., Montpetit B., Zhao L., Finst R. J., Goh B., Kim A. C., Quarmby L. M. The FA2 gene of Chlamydomonas encodes a NIMA family kinase with roles in cell cycle progression and microtubule severing during deflagellation. J. Cell Sci. 2002;115:1759–1768. doi: 10.1242/jcs.115.8.1759. [DOI] [PubMed] [Google Scholar]
- Mahjoub M. R., Qasim Rasi M., Quarmby L. M. A NIMA-related kinase, Fa2p, localizes to a novel site in the proximal cilia of Chlamydomonas and mouse kidney cells. Mol. Biol. Cell. 2004;15:5172–5186. doi: 10.1091/mbc.E04-07-0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall W. F., Nonaka S. Cilia: tuning in to the cell's antenna. Curr. Biol. 2006;16:R604–R614. doi: 10.1016/j.cub.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Marshall W. F., Rosenbaum J. L. Intraflagellar transport balances continuous turnover of outer doublet microtubules: implications for flagellar length control. J. Cell Biol. 2001;155:405–414. doi: 10.1083/jcb.200106141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally F. J., Vale R. D. Identification of katanin, an ATPase that severs and disassembles stable microtubules. Cell. 1993;75:419–429. doi: 10.1016/0092-8674(93)90377-3. [DOI] [PubMed] [Google Scholar]
- McNally K., Audhya A., Oegema K., McNally F. J. Katanin controls mitotic and meiotic spindle length. J. Cell Biol. 2006;175:881–891. doi: 10.1083/jcb.200608117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori D., et al. NDEL1 phosphorylation by Aurora-A kinase is essential for centrosomal maturation, separation, and TACC3 recruitment. Mol. Cell. Biol. 2007;27:352–367. doi: 10.1128/MCB.00878-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell C. B., Khodjakov A. L. Cooperative mechanisms of mitotic spindle formation. J. Cell Sci. 2007;120:1717–1722. doi: 10.1242/jcs.03442. [DOI] [PubMed] [Google Scholar]
- Pan J., Snell W. The primary cilium: keeper of the key to cell division. Cell. 2007;129:1255–1257. doi: 10.1016/j.cell.2007.06.018. [DOI] [PubMed] [Google Scholar]
- Parker J. D., Quarmby L. M. Chlamydomonas fla mutants reveal a link between deflagellation and intraflagellar transport. BMC Cell Biol. 2003;4:11. doi: 10.1186/1471-2121-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour G. J., Dickert B. L., Vucica Y., Seeley E. S., Rosenbaum J. L., Witman G. B., Cole D. G. Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene tg737, are required for assembly of cilia and flagella. J. Cell Biol. 2000;151:709–718. doi: 10.1083/jcb.151.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piasecki B. P., LaVoie M., Tam L-W., Lefebvre P. A., Silflow C. D. The Uni2 phosphoprotein is a cell cycle-regulated component of the basal body maturation pathway in Chlamydomonas reinhardtii. Mol. Biol. Cell. 2007;19:262–273. doi: 10.1091/mbc.E07-08-0798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugacheva E. N., Jablonski S. A., Hartman T. R., Henske E. P., Golemis E. A. HEF1-dependent Aurora A activation induces disassembly of the primary cilium. Cell. 2007;129:1351–1363. doi: 10.1016/j.cell.2007.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin H., Diener D. R., Geimer S., Cole D. G., Rosenbaum J. L. Intraflagellar transport (IFT) cargo: IFT transports flagellar precursors to the tip and turnover products to the cell body. J. Cell Biol. 2004;164:255–266. doi: 10.1083/jcb.200308132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin H., Wang Z., Diener D., Rosenbaum J. Intraflagellar transport protein 27 is a small G protein involved in cell-cycle control. Curr. Biol. 2007;17:193–202. doi: 10.1016/j.cub.2006.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quarmby L. M. Cellular deflagellation. Int. Rev. Cytol. 2004;233:47–91. doi: 10.1016/S0074-7696(04)33002-0. [DOI] [PubMed] [Google Scholar]
- Quarmby L. M., Parker J. D. Cilia and the cell cycle? J. Cell Biol. 2005;169:707–710. doi: 10.1083/jcb.200503053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder C. L., Jensen C. G., Jensen L. C. The resorption of primary cilia during mitosis in a vertebrate (PtK1) cell line. J. Ultrastruct. Res. 1979;68:173–185. doi: 10.1016/s0022-5320(79)90152-7. [DOI] [PubMed] [Google Scholar]
- Riley J., Butler R., Ogilvie D., Finniear R., Jenner D., Powell S., Anand R., Smith J. C., Markham A. F. A novel, rapid method for the isolation of terminal sequences from yeast artificial chromosome (YAC) clones. Nucleic Acids Res. 1990;18:2887–2890. doi: 10.1093/nar/18.10.2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohr J., Sarkar N., Balenger S., Jeong B., Cerutti H. Tandem inverted repeat system for selection of effective transgenic RNAi strains in Chlamydomonas. Plant J. 2004;40:611–621. doi: 10.1111/j.1365-313X.2004.02227.x. [DOI] [PubMed] [Google Scholar]
- Roll-Mecak A., Vale R. D. Making more microtubules by severing: a common theme of noncentrosomal microtubule arrays? J. Cell Biol. 2006;175:849–851. doi: 10.1083/jcb.200611149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroda M. RNA silencing in Chlamydomonas: mechanisms and tools. Curr. Genet. 2006;49:69–84. doi: 10.1007/s00294-005-0042-1. [DOI] [PubMed] [Google Scholar]
- Sharma N., Bryant J., Wloga D., Donaldson R., Davis R. C., Jerka-Dziadosz M., Gaertig J. Katanin regulates dynamics of microtubules and biogenesis of motile cilia. J. Cell Biol. 2007;178:1065–1079. doi: 10.1083/jcb.200704021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sizova I., Fuhrmann M., Hegemann P. A Streptomyces rimosus aphVIII gene coding for a new type phosphotransferase provides stable antibiotic resistance to Chlamydomonas reinhardtii. Gene. 2001;277:221–229. doi: 10.1016/s0378-1119(01)00616-3. [DOI] [PubMed] [Google Scholar]
- Srayko M., Buster D. W., Bazirgan O. A., McNally F. J., Mains P. E. MEI-1/MEI-2 katanin-like microtubule severing activity is required for Caenorhabditis elegans meiosis. Genes Dev. 2000;14:1072–1084. [PMC free article] [PubMed] [Google Scholar]
- Sudo H., Maru Y. LAPSER1/LZTS 2, a pluripotent tumor suppressor linked to the inhibition of katanin-mediated microtubule severing. Hum. Mol. Genet. 2008;17:2524–2540. doi: 10.1093/hmg/ddn153. [DOI] [PubMed] [Google Scholar]
- Toyo-Oka K., et al. Recruitment of katanin p60 by phosphorylated NDEL1, an LIS1 interacting protein, is essential for mitotic cell division and neuronal migration. Hum Mol Genet. 2005;14:3113–3128. doi: 10.1093/hmg/ddi339. [DOI] [PubMed] [Google Scholar]
- Tucker R. W., Pardee A. B., Fujiwara K. Centriole ciliation is related to quiescence and DNA synthesis in 3T3 cells. Cell. 1979;17:527–535. doi: 10.1016/0092-8674(79)90261-7. [DOI] [PubMed] [Google Scholar]
- Umen J. G., Goodenough U. W. Control of cell division by a retinoblastoma protein homolog in Chlamydomonas. Genes Dev. 2001;15:1652–1661. doi: 10.1101/gad.892101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadsworth P., Khodjakov A. E pluribus unum: towards a universal mechanism for spindle assembly. Trends Cell Biol. 2004;14:413–419. doi: 10.1016/j.tcb.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Wang A. M., Doyle M. V., Mark D. F. Quantitation of mRNA by the polymerase chain reaction. Proc. Natl. Acad. Sci. USA. 1989;86:9717–9721. doi: 10.1073/pnas.86.24.9717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D., Rogers G. C., Buster D. W., Sharp D. J. Three microtubule severing enzymes contribute to the “Pacman-flux” machinery that moves chromosomes. J. Cell Biol. 2007;177:231–242. doi: 10.1083/jcb.200612011. [DOI] [PMC free article] [PubMed] [Google Scholar]