Abstract
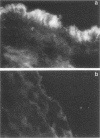
This study was designed to evaluate the role of Escherichia coli type 1 pili in adherence of the organism to porcine small intestines and the efficacy of pili as a vaccine antigen in controlling neonatal colibacillosis. Our results demonstrated that an E. coli phase cloned to express type 1 pili readily attached to the small intestines of colostrum-deprived newborn pigs. Immunofluorescent staining of intestine sections revealed the presence of E. coli expressing type 1 pili only on the brush border, suggesting involvement of type 1 pili in the colonization process. Administration of anti-type 1 serum to newborn pigs prior to challenge reduced the level of gut-associated E. coli sixfold compared with controls. Purified type 1 pilus vaccine induced significant protection against colibacillosis in newborn pigs following challenge with E. coli expressing type 1 pili. Pigs born to vaccinated gilts scoured less and gained more weight than pigs born to control gilts. Our results demonstrate that type 1 pili are a virulence factor, as well as an effective vaccine antigen.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham S. N., Hasty D. L., Simpson W. A., Beachey E. H. Antiadhesive properties of a quaternary structure-specific hybridoma antibody against type 1 fimbriae of Escherichia coli. J Exp Med. 1983 Oct 1;158(4):1114–1128. doi: 10.1084/jem.158.4.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertschinger H. U., Moon H. W., Whipp S. C. Association of Escherichia coli with the small intestinal epithelium. I. Comparison of enteropathogenic and nonenteropathogenic porcine strains in pigs. Infect Immun. 1972 Apr;5(4):595–605. doi: 10.1128/iai.5.4.595-605.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerina N. G., Kessler T. W., Guerina V. J., Neutra M. R., Clegg H. W., Langermann S., Scannapieco F. A., Goldmann D. A. The role of pili and capsule in the pathogenesis of neonatal infection with Escherichia coli K1. J Infect Dis. 1983 Sep;148(3):395–405. doi: 10.1093/infdis/148.3.395. [DOI] [PubMed] [Google Scholar]
- Harper M., Turvey A., Bramley A. J. Adhesion of fimbriate Escherichia coli to bovine mammary-gland epithelial cells in vitro. J Med Microbiol. 1978 May;11(2):117–123. doi: 10.1099/00222615-11-2-117. [DOI] [PubMed] [Google Scholar]
- Isaacson R. E., Dean E. A., Morgan R. L., Moon H. W. Immunization of suckling pigs against enterotoxigenic Escherichia coli-induced diarrheal disease by vaccinating dams with purified K99 or 987P pili: antibody production in response to vaccination. Infect Immun. 1980 Aug;29(2):824–826. doi: 10.1128/iai.29.2.824-826.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson R. E., Fusco P. C., Brinton C. C., Moon H. W. In vitro adhesion of Escherichia coli to porcine small intestinal epithelial cells: pili as adhesive factors. Infect Immun. 1978 Aug;21(2):392–397. doi: 10.1128/iai.21.2.392-397.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwahi T., Abe Y., Nakao M., Imada A., Tsuchiya K. Role of type 1 fimbriae in the pathogenesis of ascending urinary tract infection induced by escherichia coli in mice. Infect Immun. 1983 Mar;39(3):1307–1315. doi: 10.1128/iai.39.3.1307-1315.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemm P., Orskov I., Orskov F. F7 and type 1-like fimbriae from three Escherichia coli strains isolated from urinary tract infections: protein chemical and immunological aspects. Infect Immun. 1982 May;36(2):462–468. doi: 10.1128/iai.36.2.462-468.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levine M. M., Rennels M. B., Daya V., Hughes T. P. Hemagglutination and colonization factors in enterotoxigenic and enteropathogenic Escherichia coli that cause diarrhea. J Infect Dis. 1980 Jun;141(6):733–737. doi: 10.1093/infdis/141.6.733. [DOI] [PubMed] [Google Scholar]
- Levine M. M., Ristaino P., Sack R. B., Kaper J. B., Orskov F., Orskov I. Colonization factor antigens I and II and type 1 somatic pili in enterotoxigenic Escherichia coli: relation to enterotoxin type. Infect Immun. 1983 Feb;39(2):889–897. doi: 10.1128/iai.39.2.889-897.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungh A., Faris A., Wadström T. Hemagglutination by Escherichia coli in septicemia and urinary tract infections. J Clin Microbiol. 1979 Oct;10(4):477–481. doi: 10.1128/jcm.10.4.477-481.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan R. L., Isaacson R. E., Moon H. W., Brinton C. C., To C. C. Immunization of suckling pigs against enterotoxigenic Escherichia coli-induced diarrheal disease by vaccinating dams with purified 987 or K99 pili: protection correlates with pilus homology of vaccine and challenge. Infect Immun. 1978 Dec;22(3):771–777. doi: 10.1128/iai.22.3.771-777.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy B., Moon H. W., Isaacson R. E., To C. C., Brinton C. C. Immunization of suckling pigs against enteric enterotoxigenic Escherichia coli infection by vaccinating dams with purified pili. Infect Immun. 1978 Jul;21(1):269–274. doi: 10.1128/iai.21.1.269-274.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofek I., Beachey E. H. Mannose binding and epithelial cell adherence of Escherichia coli. Infect Immun. 1978 Oct;22(1):247–254. doi: 10.1128/iai.22.1.247-254.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orskov I., Orskov F., Smith H. W., Sojka W. J. The establishment of K99, a thermolabile, transmissible escherichia coli K antigen, previously called "Kco", possessed by calf and lamb enteropathogenic strains. Acta Pathol Microbiol Scand B. 1975 Feb;83(1):31–36. doi: 10.1111/j.1699-0463.1975.tb00066.x. [DOI] [PubMed] [Google Scholar]
- Silverblatt F. J., Cohen L. S. Antipili antibody affords protection against experimental ascending pyelonephritis. J Clin Invest. 1979 Jul;64(1):333–336. doi: 10.1172/JCI109458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirm S., Orskov I., Orskov F. K88, an episome-determined protein antigen of Escherichia coli. Nature. 1966 Jan 29;209(5022):507–508. doi: 10.1038/209507a0. [DOI] [PubMed] [Google Scholar]
- To S. C., Moon H. W., Runnels P. L. Type 1 pili (F1) of porcine enterotoxigenic Escherichia coli: vaccine trial and tests for production in the small intestine during disease. Infect Immun. 1984 Jan;43(1):1–5. doi: 10.1128/iai.43.1.1-5.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein R., Silverblatt F. J. Antibacterial mechanisms of antibody to mannose-sensitive pili of Escherichia coli. J Infect Dis. 1983 May;147(5):882–889. doi: 10.1093/infdis/147.5.882. [DOI] [PubMed] [Google Scholar]
- Welch H., Lee Mickle F. A Rapid Slide Test for the Serological Diagnosis of Typhoid and Paratyphoid Fevers. Am J Public Health Nations Health. 1936 Mar;26(3):248–255. doi: 10.2105/ajph.26.3.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Graaf F. K., Roorda I. Production, purification, and characterization of the fimbrial adhesive antigen F41 isolated from calf enteropathogenic Escherichia coli strain B41M. Infect Immun. 1982 May;36(2):751–758. doi: 10.1128/iai.36.2.751-758.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]