Abstract
Anchorage dependence of cell growth and survival is a critical trait that distinguishes nontransformed cells from transformed cells. We demonstrate that anchorage dependence is determined by anchorage-dependent nuclear retention of cyclin D1, which is regulated by the focal adhesion protein, Hic-5, whose CRM1-dependent nuclear export counteracts that of cyclin D1. An adaptor protein, PINCH, interacts with cyclin D1 and Hic-5 and potentially serves as an interface for the competition between cyclin D1 and Hic-5 for CRM1. In nonadherent cells, the nuclear export of Hic-5, which is redox-sensitive, was interrupted due to elevated production of reactive oxygen species, and cyclin D1 was exported from the nucleus. When an Hic-5 mutant that was continuously exported in a reactive oxygen species-insensitive manner was introduced into the cells, cyclin D1 was retained in the nucleus under nonadherent conditions, and a significant population of cells escaped from growth arrest or apoptosis. Interestingly, activated ras achieved predominant cyclin D1 nuclear localization and thus, growth in nonadherent cells. We report a failsafe system for anchorage dependence of cell growth and survival.
INTRODUCTION
Anchorage dependence of cell growth is the phenomenon whereby nontransformed cells adhere to the substratum for cell cycle progression from G1 to S phase. Numerous studies have defined the roles of adhesion signals mediated by the integrin–extracellular matrix (ECM) interaction in cell cycle progression. Basically, integrin-ECM–mediated signaling potentiates and prolongs the growth factor receptor–mediated mitogenic signaling and is required from mid- to late-G1 phase in various events associated with cell cycle progression, such as up-regulation of G1-phase CDK activity, Cip/Kips down-regulation, association of cyclin E with CDK2, pRb phosphorylation, and cyclin A expression (Fang et al., 1996; Zhu et al., 1996; Assoian, 1997; Schwartz and Assoian, 2001). As a result, loss of adhesion generally causes complete G1-phase cell cycle arrest in nontransformed cells; moreover, in susceptible cells, it leads to anoikis, a specific type of apoptosis caused by the detachment of a cell from its supportive matrix, which was first described in epithelial and endothelial cells (Frisch and Screaton, 2001; Reddig and Juliano, 2005).
In contrast, transformed cells usually circumvent the anchorage requirement in cell cycle progression. Their anchorage-independent survival and growth is well known as a hallmark of cellular transformation and correlates with tumorigenicity in vivo (Freedman and Shin, 1974). Mechanistically, the anchorage-independent growth is considered to be based on an abnormal activation of the G1-phase cyclin—cyclin-dependent kinases (CDKs) uncoupled from anchorage. In general, an oncogenic pathway activates a robust and/or constitutive mitogenic signal, which is presumed to reduce the requirement for integrin–ECM-mediated signaling and its importance as a booster of growth factor receptor–mediated mitogenic signaling in the transformed cells. Among the downstream pathways of oncogenic signals, the activation of the phosphatidylinositol 3-kinase/Akt pathway is crucial for the induction of anchorage-independent growth and cell survival (Wang, 2004; Reddig and Juliano, 2005).
Cyclin D1 is a proto-oncogene whose amplification and overexpression are frequently associated with human cancers (Diehl, 2002). Until recently, cyclin D1 was believed to play a critical role as a CDK4-dependent regulator in G1-to-S cell cycle progression (Sherr and Roberts, 1999). These intensive studies revealed that cyclin D1 is distinct from other cyclins and acts as a dual sensor for mitogenic and adhesion signaling. Thus, the level of cyclin D1 is affected by both anchorage and mitogens at multiple levels, including induction, stability, and translation of mRNA as well as protein degradation; however, the details are still controversial (Fang et al., 1996; Schulze et al., 1996; Zhu et al., 1996). Similar to the expression level, subcellular localization of cyclin D1 is also cell cycle-dependent, and cyclin D1 is exported from the nucleus at the onset of S phase, which is dependent on phosphorylation at threonine (Thr)-286 by glycogen synthase kinase-3β (GSK-3β) and the nuclear exportin CRM1 (Diehl et al., 1998; Alt et al., 2000). Although several regulators of cyclin D1 subcellular localization have been identified (Lin et al., 2000; Alt et al., 2002), knowledge of the regulating mechanisms is relatively scarce.
Recent studies on the cyclin D-null mouse have provided substantial information on the biological functions of cyclin D in vivo (Kozar et al., 2004; Sherr and Roberts, 2004). These studies recapitulate the close relationship between cyclin D1 and neoplastic transformation; however, they do not support its essential role in the G1-to-S progression, which is the basis of the long-standing interpretation of the oncogenicity of cyclin D1. Accordingly, there is no satisfactory explanation of how cyclin D1 contributes to tumorigenesis. Overexpression alone is unlikely to be adequate for cell transformation (Quelle et al., 1993; Resnitzky et al., 1994). The importance of cyclin-D1 nuclear localization was emphasized in a recent review (Gladden and Diehl, 2005).
To our knowledge, we are the first to report that cyclin D1 nuclear localization is dependent on cell adhesion to the substratum and that its nuclear localization decreases in nonadherent cells. This anchorage dependence was regulated by a focal adhesion (FA) protein, Hic-5 and its binding partner, PINCH (particularly interesting new Cys-His protein), which translocated in and out of the nucleus and counteracted the nuclear export of cyclin D1 through CRM1. Under nonadherent conditions, the cellular level of reactive oxygen species (ROS) increased and inhibited the nuclear export of Hic-5 resulting in the nuclear export of cyclin D1. Importantly, the forced localization of cyclin D1 in the nuclei of nonadherent cells enabled them to progress through the cell cycle and to partially circumvent anoikis. Interestingly, the ras oncogene localized cyclin D1 predominantly in the nucleus of nonadherent cells, thereby inducing anchorage-independent cell growth. These results demonstrate the existence of a failsafe system for anchorage-dependent cell growth and survival that can prevent anchorage-independent growth and is based on the competitive nuclear export of cyclin D1 and Hic-5 as a result of competition for CRM1.
MATERIALS AND METHODS
Cell Culture and Reagents
Mouse C3H10T1/2 fibroblasts, NIH3T3 cells, primary embryo fibroblasts (mouse embryo fibroblasts [MEFs]), and HEK293 cells were grown in Dulbecco's modified MEM supplemented with 10% fetal calf serum (C3H10T1/2, primary embryo fibroblasts and HEK293 cells) or calf serum (NIH3T3) as described previously (Nishiya et al., 2001; Shibanuma et al., 2003). Mouse mammary epithelial NMuMG cells were maintained as reported previously (Kanome et al., 2007).
Cells were trypsinized with TrypLE Express (Invitrogen, Carlsbad, CA), incubated in suspension, and seeded in silane-coated and normal tissue culture dishes in conditioned medium. Cell viability was maintained at >90% for 5 h, and the observations were conducted within 4 h. For the examinations of cell growth and apoptosis, the cells were placed in suspension for 24–72 h. To obtain a mixed population of cells with different adhesive properties, the suspended cells were replated onto the same coverslip at defined intervals. After a given period, the populations were distinguished by the formation of focal adhesions, which was monitored by immunostaining with antibody to the Hic-5 focal adhesion protein. Cells were designated as FA+ if they showed a twofold higher cytoplasmic fluorescence intensity than nuclear intensity and if discernible signals were clustered at substratal attachment sites. The remaining cells that were round and poorly adherent and exhibited faint signals for Hic-5 at the periphery (a cytoplasmic/nuclear fluorescence ratio of <2) were designated as FA−.
Leptomycin B (LMB), MG132, and cytochalasin D were purchased from LC Laboratories (Woburn, MA), Sigma (St. Louis, MO), and Merck Chemicals (Nottingham, United Kingdom), respectively. Tiron (1,2-dihidroxybenzene-3,5-disulphonic acid) and PDTC (pyrrolidine dithiocarbamate) were obtained from Sigma.
Expression Vectors, Transfection, and Infection
Expression vectors for hemagglutinin (HA)- and Flag-tagged wild-type proteins (Hic-5, paxillin, and PINCH-1) were used as described previously (Mori et al., 2006).
The Flag-tagged LD (mLD3) and Cfl/ns Hic-5 constructs were enzymatically generated by excising full-length mhic-5 cDNA fragments from the corresponding constructs of the HA-tagged series (Shibanuma et al., 2003) and inserting the fragments into pcDNA3 (Invitrogen). For LD/N-NES Hic-5 (N), PCR-amplified CRM1-independent nuclear export signal (NES; LEKIRRERNY; Gotoh et al., 2007) was linked to the N-terminus of LD Hic-5. The nuclear-targeted Hic-5 (nuclear localization signal [NLS]), the LIM4 deletion mutant (Δ4; Shibanuma et al., 2003), a deletion mutant of PINCH whose LIM5 was removed (PINCH Δ LIM5; Mori et al., 2006), and PTP-PEST (protein-tyrosine phosphatase PEST; Shibanuma et al., 2005) were used as described previously.
The expression vectors for the other proteins were constructed with a PCR-based method using pCG-N-BL (HA-tagged) or pcDNA3.1(−)/myc-HisA (Myc-tagged, Invitrogen) as a vector (Shibanuma et al., 2003; Mori et al., 2006). To generate the HA-tagged cyclin D1 mutants, T286A and CRM(−) (V290A, V293A, I295A), point mutations were introduced into HA-tagged cyclin D1 using a site-directed mutagenesis kit (Stratagene, La Jolla, CA) with a mutated primer according to the manufacturer's instructions (Kanome et al., 2007). A coding region of v-Ki-ras/pBR322 was PCR-amplified and inserted into pcDNA3 for the Flag-tagged v-Ki-ras expression vector.
The expression vectors were introduced into the cells by the conventional calcium phosphate precipitation method, and the cells were processed for analysis 24 h after transfection.
The retroviral expression vectors and the procedure for infection have previously been described previously (Kanome et al., 2007). The efficiency of the infection, as monitored by enhanced green fluorescent protein (EGFP) expression, was above 80%.
RNA Interference
The small interfering RNAs (siRNAs) for mouse Hic-5 seqA and seqB were designed by a custom siRNA service (Qiagen K.K., Tokyo, Japan) and siDirect, a Web-based online software (http://genomics.jp/sidirect), respectively. The sequences were as follows: seqA, 5′-AGUUCAACAUUACAGAUGAdTdT-3′ and 5′-UCAUCUGUAAUGUUGAACUdGdG-3′; seqB, 5′-CCAACCCAUCCGACAGAAAdTdT-3′ and 5′-UUUGUGUCGGAUGGGUUGGdTdT-3′. The siRNA for mouse PINCH (PINCH-1) seqA and seqB were purchased from Sigma. Seq A was 5′-CUGUGAAACUCACUAUAAUdTdT-3′ and 5′-AUUAUAGUGAGUUUCACAGdTdT-3′, and seqB was 5′-CUAUCUGAGACCUUAGGAAdTdT-3′ and 5′-UUCCUAAGGUCUCAGAUAGdTdT-3′. The validated siRNA duplexes for mouse cyclin D1 and the negative control were purchased from GE Healthcare (Little Chalfont, Buckinghamshire, United Kingdom) and Applied Biosystems (Foster City, CA), respectively. Cells were transfected with the siRNA duplexes (30 nM) using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. The cells were processed for analysis 48 h after transfection.
Antibodies, Immunocytochemistry, Immunoprecipitation, and Western Blotting
Monoclonal and polyclonal anti-HA, monoclonal anti-Flag, and polyclonal anti-Hic-5 antibodies used were described previously (Mori et al., 2006). Other antibodies used were Hic-5, cyclin D1, paxillin, PINCH, integrin-linked kinase (ILK), and extracellular signal–regulated kinase 2 (ERK2; BD Biosciences, San Jose, CA); myc (9E10) (Upstate Biotechnology, Lake Placid, NY); focal adhesion kinase (FAK) and Rb (Santa Cruz Biotechnology, Santa Cruz, CA); and phospho-Rb (Ser807/811), (Ser780), and phosphocyclin D1 (Thr-286; Cell Signaling, Beverly, MA).
Immunocytochemistry, immunoprecipitation, and Western blotting were performed as described previously (Mori et al., 2006). When endogenous Hic-5 and cyclin D1 were immunoprecipitated, the primary antibodies were covalently coupled to protein G beads (Amersham Biosciences, Piscataway, NJ) with the cross-linking agent dimethyl pimelimidate (Sigma, St. Louis, MO). For immunocytochemistry, the suspended cells were centrifuged onto coverslips and fixed. Fluorescence microscopy was performed using a RTS-2000 MP confocal laser microscope (Bio-Rad Laboratories, Hercules, CA) and a BZ-8000 microscope (Keyence, Osaka, Japan), and the data were analyzed using BZ-Analyzer software (Keyence). The antibody-specific fluorescence signal was quantified by subtracting the background signal (no primary antibody).
After scanning and normalizing the Western blot bands to their corresponding control or nonspecific bands, the results were quantitatively assessed using the Image J freeware (http://rsb.info.nih.gov/ij/).
Subcellular Fractionation
The cells were suspended and allowed to swell on ice in a buffer (10 mM HEPES-KOH, pH 7.9, 1.5 mM MgCl2, 10 mM KCl, and 1 mM dithiothreitol) with a protease inhibitor cocktail (Sigma) for 20 min and were homogenized using a Dounce homogenizer until disruption of the plasma membrane was confirmed by microscopic observation. After centrifugation at 5500 rpm for 10 min at 4°C, the supernatant containing the cytoplasmic fraction was concentrated by acetone precipitation and dissolved in RIPA buffer (50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 2 mM MgCl2, 1% Triton X-100, 0.5% sodium deoxycholate, and 0.1% SDS). The nuclear pellet was resuspended in a buffer (0.25 M sucrose, 10 mM Tris-HCl, pH 7.9, 5 mM MgCl2, 1 mM dithiothreitol, and 0.1% Triton X-100) supplemented with the protease inhibitor cocktail, loaded onto 0.5 M sucrose buffer (0.5 M sucrose, 10 mM Tris-HCl, pH 7.9, 5 mM MgCl2, 1 mM dithiothreitol, and 0.1% Triton X-100) and centrifuged at 5500 rpm for 10 min at 4°C. The sucrose-purified nuclear fraction was then dissolved in RIPA buffer and analyzed by Western blotting.
In this study, MG132 (20 μM) was included in the medium and buffer during cell disruption to inhibit the protein degradation accompanying redistribution of the protein in the cytoplasm.
Mammalian Two-Hybrid Assay
The luciferase reporter pGL2 T + I 5 × GAL4 was kindly provided by Dr. Wang (Duke University; Li et al., 1998). The GAL4 expression vectors were engineered based on pCG-N-BL. The DNA-binding domain of GAL4 (1-147 amino acids; GalDB) was PCR-amplified and fused in-frame to the C-terminal of Hic-5 in pCG-LD1 mhic-5 (Nishiya et al., 2001) to produce pCG-LD1 mhic-5/GalDB (Gal-Hic, GH), or inserted into the vector to engineer a control, pCG-GalDB (Gal, G). Expression vectors for the transcriptional-activation domain of herpes simplex virus VP16 were constructed based on pcDNA3.1(−)/myc-HisA (Invitrogen), and the PCR-amplified fragment of VP16 (411–490 amino acids) was linked to the C-terminal of CRM1 to produce pcDNA3.1-CRM1/VP (CRM-VP, CrmV), or inserted into the vector to yield pcDNA3.1-VP (VP, V).
The DNA mixture containing the reporter (1 μg), the expression vectors for the GAL4 (0.02 μg) and VP16 (1 μg) fusion proteins, and those for the effectors (1 μg, unless otherwise noted) was transiently introduced into HEK293 cells together with an internal control plasmid, pRL/CMV. Luciferase activity was quantified 24 h after transfection using a Dual Luciferase Assay Kit (Promega). Each assay was performed in duplicate and repeated at least three times, and the value was corrected with Renilla luciferase activity expressed from the internal-control plasmid.
To obtain the valid results of the effectors on the Hic-5 and CRM1 interaction, we estimated the ratio of GH + CrmV to GH + V to cancel the irrelevant effects on the assay system.
Monitoring of Intracellular ROS Production
For monitoring of intracellular ROS production, 2′,7′-dichlorofluorescein diacetate (H2DCFDA, 10 μM) (Invitrogen) and 2 μM calcein (Invitrogen) were added to the medium and incubated for 5 min. Fluorescence was visualized with excitation at 460–500 (DCF) or 365 (calcein) nm and emission at 510–560 (DCF) or 400 (calcein) nm. The images were immediately captured on a microscope (Eclipse TE2000-U; Nikon, Tokyo, Japan) with identical parameters and analyzed by Aquacosmos software (Hamamatsu Photonics, Hamamatsu, Japan). The level of intracellular ROS was evaluated as the intensity of DCF normalized to that of calcein in individual cells.
Bromodeoxyuridine Incorporation
Bromodeoxyuridine (BrdU; 5-bromo-2′-deoxyuridine; 1 μg/ml) was added to culture medium containing 1 × 105 cells. After 12 h (C3H10T1/2) or 48 h (NMuMG), the cells were fixed with 70% ethanol for 30 min at room temperature and processed for immunocytochemistry with a Cell Proliferation Kit (Amersham Biosciences) according to the manufacturer's directions.
BrdU was incorporated into ∼60% of NMuMG and 70% of C3H10T1/2 cell monolayers.
Apoptosis Assay
Apoptosis was examined quantitatively using the APOPercentage apoptosis assay (Biocolor, Newtownabbey, Northern Ireland, United Kingdom). First, 5 × 105 cells were placed in suspension for 48 h, collected, and stained with APOPercentage dye according to the manufacturer's instructions and as previously described (Kanome et al., 2007). The values were normalized to the total cell number.
RESULTS
Anchorage-dependent Nuclear Localization of Cyclin D1
Cyclin D1 displayed clear nuclear localization only in fully adherent cells among a mixed population with different adhesive properties (Figure 1A). In this experiment, the cells were detached and replated, one population after another, on a coverslip at specific intervals. After a given period, the cells were distinguished based on the formation of focal adhesions described in Materials and Methods (focal adhesion; FA, +/−), which was monitored by incorporation of a focal adhesion protein, Hic-5 (Matsuya et al., 1998), in the structures. For the observation of short-term responses to deprivation of substratum, including changes in subcellular localization of the concerned proteins, the cells were in suspension for <4 h throughout the study, ensuring more than 90% viability. Immunostaining of cyclin D1 with an antibody revealed distinct nuclear signals in fully adherent cells with strong Hic-5 signals at the focal adhesions (Figure 1A; FA+, arrow), whereas in the case of nonadherent cells with small-dotted Hic-5 signals at the cell periphery, only a vague signal was detected in the nucleus (Figure 1A; FA−, arrowheads). A clear cyclin D1 nuclear signal was restored 120 min after replating (Figure 1B). It is to be noted that when the cells were pretreated with an inhibitor of CRM1-dependent nuclear export, LMB, cyclin D1 remained in the nucleus even in the FA− cells (Figure 1B; 30 min + LMB vs. 30 min). These optical observations are quantified in Figure 1C, based on the fluorescence intensity in the nucleus. Nuclear localization of the transcription factor Sp1 was evaluated simultaneously to validate the entire process.
Figure 1.
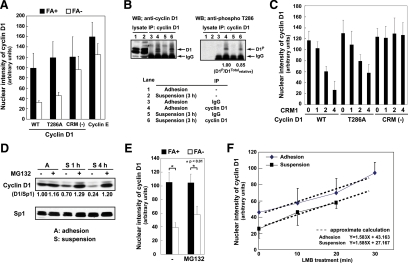
Anchorage dependence of cyclin D1 nuclear localization. (A–C) To obtain a mixed population of cells with different adhesive properties, the suspended cells were replated onto the same coverslip at defined intervals, allowed to adhere for 120 or 30 min, and then processed for immunocytochemistry with antibodies for Hic-5, cyclin D1, or Sp1. The nuclei were labeled with 4′,6-diamidino-2-phenylindole (DAPI). Photographs were taken under a confocal fluorescence microscope. (A) arrow, FA+; arrowheads, FA− cells (see Materials and Methods). Scale bars, 50 μm. (B and C) The cells were pretreated with or without LMB (10 ng/ml) for 1 h. The optical observations were quantified and the nuclear intensity (n ≥ 50) is shown. Data are expressed as mean ± SD (n ≥ 3). (D) The cells were treated with cytochalasin D (1 μM) in monolayers and then immunostained with the antibody for cyclin D1 and Hic-5. The photographs show the presence and absence of distinctive signals of Hic-5 at FAs representing the adherent status of the cells at the indicated time. Scale bars, 50 μm. Nuclear localization of cyclin D1 was assessed as above. (E) The cells pretreated with MG132 (20 μM) for 1 h were incubated in monolayer (A) or suspension (S) cultures for 3 h and subsequently fractionated and analyzed by Western blotting. Sp1 and ERK2 served as monitors of successful fractionation as well as loading controls. Quantification of the band densities was performed using Image J software, and relative intensities are shown after normalization to Sp1 or ERK2.
Cytochalasin D treatment, which interferes with the formation of actin stress fibers, disorganizes the focal adhesions, and causes eventual detachment of the cells from the substratum, decreases cyclin D1 nuclear localization in 60 min (Figure 1D), thus supporting the role of adhesion as a cause for the altered localization of cyclin D1 and excluding any unintended effect of trypsinization. Biochemical fractionation of cells demonstrated that a 3-h incubation of suspended cells resulted in a decrease in cyclin D1 in the nuclear fraction with a concomitant increase in the cytoplasmic fraction in support of the results of immunostaining (Figure 1E). Hic-5 and its binding partner PINCH, focal adhesion proteins that form a complex and translocate in and out of the nucleus (Shibanuma et al., 2003; Mori et al., 2006), were regulated in a reverse manner to cyclin D1, implying a competitive transport of cyclin D1 and the Hic-5–PINCH complex. Similar changes were observed in cyclin D1 localization in other immortalized and primary fibroblasts (data not shown) and with tagged cyclin D1 (Figure 2A; WT). Another G1 cyclin, cyclin E, showed marginal anchorage-dependent nuclear localization (Figure 2A). PML, HDAC3, p21Waf1/Cip1, and AP2 are nuclear proteins, and similar to cyclin D1, these proteins have the potential to be exported from the nucleus through CRM1 (Henderson and Eleftheriou, 2000; de Ruijter et al., 2003); nuclear localization of all except AP2 remained unchanged upon detachment of the cells (data not shown).
Figure 2.
Nuclear export of cyclin D1 independent of phosphorylation at Thr-286 and proteasomal proteolysis upon loss of adhesion. (A) The expression vectors for HA-tagged wild-type (WT), T286A, and CRM(−) mutants of cyclin D1 and cyclin E were introduced into C3H10T1/2 cells; and after 24 h, the cells were detached, replated, and processed as shown in Figure 1, A–C. The expressed proteins were detected with the antibody to the tag, and nuclear localization was evaluated as in Figure 1C. (B) Cell lysates from C3H10T1/2 cells incubated in monolayers or in suspension for 3 h were immunoprecipitated with the antibody to cyclin D1 followed by Western blotting with anti-cyclin D1 and anti-phosphocyclin D1 (Thr286). The band intensities of the phospho-cyclin D1 were quantified using ImageJ software, and the values relative to the adhesion are indicated after normalization to the total cyclin D1 immunoprecipitated. (C) Expression vectors for HA-tagged wild-type (WT), T286A, and CRM(−) mutant cyclin D1 were introduced into the cells along with the vector for myc-tagged CRM1 at the indicated CRM1:cyclin D1 ratios (0, 0:1; 1, 1:1; 2, 2:1; 4, and 4:1). At 24 h after introduction, cells in monolayers were immunostained with antibody to the HA tag, and the nuclear localization of HA-tagged cyclin D1 was examined as above. (D) Cells incubated in monolayers for 4 h (A) or in suspension for 1 (S 1 h) and 4 h (S 4 h) in the presence or absence of MG132 (20 μM) were lysed and analyzed by Western blotting with antibodies for cyclin D1 and Sp1 as a reference. The results were quantitatively assessed as above. (E) The cells were detached, replated one population after another, and attached onto coverslips as above in the presence or absence of MG132 (20 μM). After immunocytochemistry with the antibody to cyclin D, nuclear localization of cyclin D1 was examined as above. (F) Cells overexpressing CRM1 and HA-cyclin D1 were incubated in monolayers or in suspensions for 2 h and then treated with LMB (10 ng/ml) at time 0. After the indicated time, the cells were fixed and the accumulation of cyclin D1 in the nucleus was evaluated by immunostaining with the antibody to the tag. The nuclear signal was plotted against the time after the addition of LMB.
The nuclear export of cyclin D1 at the onset of S phase is dependent on CRM1 as well as GSK-3β phosphorylation at Thr-286 (Diehl et al., 1998; Alt et al., 2000). The decrease in cyclin D1 nuclear localization upon detachment in unsynchronized cell populations, 50–70% cells of which were at G1/G0 phase (Figure S2), was also dependent on CRM1 (Figure 1B, C). However, unlike S phase–dependent regulation, the export seemed to be independent of Thr-286 phosphorylation based on the following observations. First, there was no detectable increase in Thr-286 phosphorylation in nonadherent cells using a phosphospecific antibody to phosphorylated Thr-286 (Figure 2B). Second, the decrease in the nuclear localization of a nonphosphorylable T286A mutant in FA− cells was indistinguishable from that of the wild type (Figure 2A, T286A). The nuclear export induced by CRM1 overexpression in the mutant was also similar to those in the wild type, albeit with slightly lower efficiency (Figure 2C). In contrast, a mutant that was phosphorylable at Thr-286 but defective in CRM1 binding (CRM(−); Benzeno and Diehl, 2004) remained in the nucleus of FA− cells (Figure 2A). The behavior of this mutant was consistent with the result obtained using a CRM1-inhibitor of LMB, which inhibited the nuclear export of cyclin D1 in nonadherent conditions (Figure 1, B and C). These results suggested that cyclin D1 was exported from the nucleus upon loss of adhesion in a CRM1-dependent manner but independent of phosphorylation at Thr-286. The CRM(−) mutant could not be exported even with CRM1 overexpression, which verified that the cyclin D1 interaction with CRM1 was fundamentally required for cyclin D1 to be exported from the nucleus (Figure 2C, CRM(−)).
Western blotting showed that the total amount of cyclin D1 decreased after 4 h of incubation in suspension and that MG132, a proteasome inhibitor, prevented this decrease (Figure 2D). Given that cyclin D1 is subjected to proteolysis by the ubiquitin–proteasome pathway in the cytoplasm (Diehl et al., 1997), this observation supported the export of cyclin D1 to the cytoplasm in suspended cells. This result might also suggest that the decrease in cyclin D1 nuclear localization was a consequence of the net decrease in the amount of the protein or was somehow dependent on the proteolysis in the cytoplasm. However, a similar decrease in cyclin D1 nuclear localization was observed in a suspension culture supplemented with MG132 (Figure 2E) maintaining the total protein level as high as that in the monolayer culture (Figure 2D). This implies that the degradation process in the cytoplasm was not responsible for the decrease in nuclear localization.
We also examined the rate of nuclear entry of cyclin D1 in adherent and nonadherent cells. Once CRM1 was overexpressed and cyclin D1 was exported from the nucleus (Figure 2C), the cells were placed on substratum or in suspension. Using fluorescence intensity, we monitored the cyclin D1 accumulation in the nucleus, i.e., the nuclear entry of the protein, following the addition of LMB, which blocked further nuclear export. It was clear that cyclin D1 accumulation occurred at a similar rate in both nonadherent and adherent cells, indicating that nuclear entry did not deteriorate upon loss of adhesion (Figure 2F).
In summary, nuclear localization of cyclin D1 was anchorage dependent, and upon loss of adequate adhesion to the substratum, it was transported out of the nucleus by the CRM1 nuclear-export system, resulting in a decrease in its nuclear localization.
Hic-5–PINCH Complex Regulates Cyclin D1 Nuclear Localization
Hic-5 is a multidomain LIM protein providing a molecular scaffold for various cellular activities, including integrin signaling at focal adhesions (Nishiya et al., 2001) and transcriptional activities in the nucleus (Guerrero-Santoro et al., 2004; Shibanuma et al., 2004). Notably, Hic-5 translocates in and out of the nucleus dependent on CRM1 and potentially coordinates cytoplasmic and nuclear activities (Shibanuma et al., 2003; Mori et al., 2006). It should be emphasized that the nuclear-to-cytoplasm export of Hic-5 is distinctive because of its sensitivity to the cellular redox state.
In our previous study, we demonstrated that Hic-5, another LIM protein of PINCH, and integrin-linked kinase (ILK) interacted to form a complex (Mori et al., 2006). Furthermore, Hic-5 and PINCH not only create a complex with each other but also translocate in and out of the nucleus together. In the present study, we found that cyclin D1 was present in the complex with Hic-5 and PINCH. As shown in Figure 3A, cyclin D1 bound PINCH when they were coexpressed in HEK293. Although direct interaction between cyclin D1 and Hic-5 was not detected, the copresence of Hic-5 clearly reinforced the interaction between cyclin D1 and PINCH (Figure 3B), suggesting the formation of a trimeric-complex mediated by the binding of PINCH to cyclin D1 and Hic-5. Immunoprecipitation of the endogenous proteins using primary MEFs demonstrated that cyclin D1 and PINCH were coimmunoprecipitated with Hic-5 and that Hic-5 and PINCH were coimmunoprecipitated with cyclin D1 (Figure 3C). Interestingly, the interaction displayed sensitivity to the adhesion state. Although the interaction between Hic-5 and PINCH was increased, the interaction between cyclin D1 and PINCH was decreased in the nonadherent condition, suggesting that increased cyclin D1 was released from the Hic-5–PINCH complex (Figure 3C).
Figure 3.
Interaction of cyclin D1 with PINCH and Hic-5. (A and B) HEK293 cells overexpressing HA-cyclin D1 together with Flag-PINCH and Hic-5, as indicated, were lysed, and the lysate was immunoprecipitated with the anti-HA antibody (H) or control IgG (C) and immunoblotted with the antibodies for the tags. In B, the amount of PINCH coimmunoprecipitated with cyclin D1 was quantified as above, normalized with that of immunoprecipitated cyclin D1, and compared in the presence or absence of Hic-5. (C) Primary MEFs pretreated with MG132 for 1 h, as in Figure 2D, were incubated in monolayers (A) or in suspensions (S) for 4 h, lysed, and subjected to the immunoprecipitation using the antibodies for Hic-5 or cyclin D1. The coimmunoprecipitated proteins were detected by Western blotting with the indicated antibodies, quantified as above, and compared between the adhesion and suspension cultures.
These results prompted us to investigate the role of Hic-5 and PINCH in the regulation of cyclin D1 nuclear localization. Using siRNAs against Hic-5 and PINCH that showed a specific knockdown effect (Supplemental Figure S1, A and B), we found that two unrelated siRNA sequences for each protein appreciably reduced cyclin D1 nuclear localization in monolayers (Figure 4A, Hic-5, and B, PINCH). This suggested that both proteins were required for the nuclear localization of cyclin D1 in adherent cells. Nuclear localization of the exogenously expressed wild-type cyclin D1 was similarly affected by the siRNA (Figure 4C). Similar to the wild-type, nuclear localization of the T286A mutant was reduced, whereas the CRM1 binding–defective mutant remained in the nucleus (Figure 4C), implying that the loss of Hic-5 function lead to the exportation of cyclin D from the nucleus in a manner that was independent of Thr-286 phosphorylation but was dependent on CRM1, as was the case upon loss of adhesion. On the other hand, overexpression of Hic-5, but not the Hic-5 homolog paxillin, (Thomas et al., 1999) increased nuclear retention of cyclin D1 in FA− cells (Figure 4D). PINCH alone had no effect but slightly enhanced the Hic-5 effect. Taken together, these results suggest that Hic-5 is required for cyclin D1 nuclear localization in adherent cells and plays a substantial role in retaining cyclin D1 in the nucleus. Although PINCH was also involved in the regulation, its role appears to be auxiliary based on the marginal effect of its overexpression on cyclin D1 nuclear localization. This might be related to the role of PINCH as a molecular scaffold that mediates communication between Hic-5 and cyclin D1, because PINCH has no intrinsic activity and its translocation must be directed by Hic-5 (Mori et al., 2006).
Figure 4.
Role of Hic-5 and PINCH in nuclear localization of cyclin D1 in adherent cells. (A–C) siRNA against Hic-5 or PINCH (seqA or seqB; see Materials and Methods) or control siRNA (Ctr) was introduced into the cells. (C) The expression vectors for HA-tagged wild-type or T286A and CRM(−) mutant cyclin D1 were cotransfected with the siRNA. After 48 h, the cell monolayers were immunostained with the antibody to cyclin D1 (A and B) or the HA tag (C), and nuclear localization of endogenous (A and B) and exogenous (C) cyclin D1 was quantified as described above. (D) HA-tagged Hic-5, PINCH, and paxillin were expressed from the vectors in C3H10T1/2 cells as indicated, and 24 h after transfection, the cells were detached and replated as above, followed by coimmunostaining with antibodies for cyclin D1 and the HA tag. Nuclear localization of cyclin D1 in the cells expressing the HA-tagged proteins was assessed.
Functional Competition of Hic-5 with Cyclin D1 for CRM1-dependenet Nuclear Export
On the basis of the above findings and the fact that the nuclear export of cyclin D1 and Hic-5 is CRM1 dependent, we speculated that the nuclear export of Hic-5 competed with the export of cyclin D1 and lead to the nuclear retention of cyclin D1. Therefore, we studied the effects of Hic-5 and a NES mutant on cyclin D1 nuclear export driven by CRM1. CRM1 decreased cyclin D1 nuclear localization in adherent cells (Figure 5, lane 1 vs. 2), whereas wild-type Hic-5 coexpression counteracted the effect of CRM1 (Figure 5, lane 2 vs. 3). The effect of Hic-5 was slightly augmented by the presence of PINCH (Figure 5, lane 3 vs. 4) as observed on cyclin D1 nuclear localization in FA− cells (Figure 4D). Importantly, one of the Hic-5 mutants (LD) whose NES was disrupted (Shibanuma et al., 2003) almost completely lost its counteracting ability (Figure 5, lanes 2 and 3 vs. 5). Another type of mutant (Cfl/ns) that retained the NES but specifically lost its sensitivity to oxidants (Shibanuma et al., 2003) showed counteracting ability comparable to the wild type (Figure 5; lanes 2 and 3 vs. 6), suggesting the importance of the NES of Hic-5 in this effect.
Figure 5.
Functional competition between Hic-5 and cyclin D1 for CRM1-driven nuclear export. Expression vectors for myc-tagged CRM1, Flag-tagged wild-type or mutant Hic-5 as schematically presented (see details in Materials and Methods), and PINCH and PTP-PEST were introduced into the cells in the indicated combinations together with HA-tagged cyclin D1. Nuclear localization of cyclin D1 was visualized in cell monolayers by immunostaining with the antibody to the HA tag and quantified as described above. Expression levels of each protein in the combinations were examined by Western blotting with antibodies for each tag.
The counteracting activity might be associated with the localization of Hic-5 in the cytoplasm or at focal adhesions. However, this is unlikely because the mutant that was predominantly of nuclear-localized form (NLS) showed the same ability to counteract with the wild-type localized at focal adhesions (Figure 5, lanes 2 and 3 vs. 7). Alternatively, the addition of CRM1-independent NES (Gotoh et al., 2007) to the LD mutant whose CRM1-dependent NES was disrupted (this modified mutant was designated LD/N-NES [N]) highlights the importance of CRM1-dependent NES but not the localization at focal adhesions. The heterokaryon assay confirmed that the CRM1-independent NES was functional and that the N mutant shuttled from nucleus to nucleus (data not shown), although the mutant was still largely localized in the nucleus as LD in a steady state. The coexpression of PTP-PEST, however, which functions as a retention mechanism for Hic-5 at FAs (Shibanuma et al., 2005), localized the protein mostly at FAs. Thus, the mutant was manipulated to be localized predominantly in the nucleus (Figure 5, −PTP-PEST; lane 9) or at focal adhesions (+PTP-PEST; lane 10). In either case, the mutant (N) could not counteract the nuclear export of cyclin D1 by CRM1 (Figure 5, lanes 2 and 3 vs. 9 and 10). This result argues that CRM1-dependent NES per se and not specific subcellular localization or shuttling itself allowed Hic-5 to compete. Altogether, these observations supported the assumption that Hic-5, by its NES, competes with cyclin D1 and consequently promotes cyclin D1 nuclear localization in adherent cells. Another Hic-5 mutant with an LIM4 deletion (Figure 5, Δ4, lane 8; Mori et al., 2006) that could not bind to PINCH also lost counteracting ability, suggesting that the counteraction of Hic-5 over the CRM1-driven cyclin D1 nuclear export required PINCH interaction as well as CRM1-dependent NES.
Physical Competition of Hic-5 with Cyclin D1 for CRM1
To substantiate these characteristics of Hic-5, we next addressed the physical competition between Hic-5 and cyclin D1 for CRM1 and the assisting role of PINCH based on a mammalian cell-based two-hybrid quantitative assay. We first addressed the physical interaction of Hic-5 with CRM1; cyclin D1 was demonstrated to interact directly with CRM1 (Benzeno and Diehl, 2004). The two-hybrid assay generally monitors luciferase activity derived from a reporter that is transcriptionally directed by the GAL4-DNA–binding domain (Gal, G). The transcription is activated by the herpes simplex virus VP16 (VP, V) activation domain tethered in proximity to the GAL4-DNA domain by an interaction between moieties fused to each domain in the nucleus. In this study, we fused Hic-5 to the GAL4-DNA–binding domain and CRM1 to the VP16 activation domain, designated Gal-Hic (GH) and CRM-VP (CrmV), respectively. In this way, we evaded the localization of Hic-5 at focal adhesions and were able to monitor the interaction of Hic-5 with CRM1 in the nucleus. We confirmed the exclusive nuclear localization of GH based on the nuclear localization signal within the GAL4-DNA–binding domain (data not shown).
Supporting the interaction between Hic-5 and CRM1, prominent activity was observed when Gal-Hic and CRM-VP were coexpressed (Figure 6A, GH + CrmV). In contrast, when Gal, Hic-5, or CRM1 were absent (Figure 6A, H + CrmV, G + CrmV, and GH + V), luciferase activity was <10% of that in the GH + CrmV condition, indicating that GH + CrmV activity was largely dependent on the presence and interaction of the Hic-5 and CRM1 moieties. Limited activity was detected for Gal-Hic and VP alone (GH + V) possibly because of the weak transactivational activity occurring in the N-terminal half of Hic-5 (Yang et al., 2000).
Figure 6.
Physical competition between Hic-5 and cyclin D1 for CRM1. (A) The plasmid mixture containing the luciferase reporter (1 μg/assay), the expression vectors for Gal-Hic (GH) or control Gal (G) and Hic-5 only (H; 0.02 μg/assay), and CRM-VP (CrmV) or control VP (V; 1 μg/assay) proteins was transiently introduced into HEK293 cells together with an internal control plasmid. Luciferase activity was quantified 24 h after transfection, as described in Materials and Methods. Each assay was performed in duplicate and repeated at least three times. Data are expressed as mean ± SD (B–D) The plasmid mixture containing the luciferase reporter, expression vectors for Gal-Hic (GH) and CRM-VP (CrmV) or control VP (V) proteins, and those for effectors was transiently introduced into the HEK293 cells as described above, and luciferase activity was evaluated as in A. The effectors introduced were (B) the wild-type cyclin D1 (D1) at the indicated amount (μg/assay); (C) the wild-type (Dw), T286A (D286), and CRM(−) (Dcrm(−)) mutants of cyclin D1, and cyclin E (E; 0.5 μg/assay); and (D) the wild-type cyclin D1 (Dw) and PINCH or its mutant (ΔLIM5) in the indicated combinations at a concentration of 0.25 μg/assay. The expression levels of the effectors were examined by Western blotting using the antibodies for the tag (bottom).
Using this system, we studied the competitive effect of cyclin D1 on the Hic-5 and CRM1 interaction. The GH + CrmV luciferase activity decreased in a concentration-dependent manner in the presence of cyclin D1 (Figure 6B), supporting the expected effect; however, more than a 10-fold amount compared with the Gal-Hic fusion protein was required for cyclin D1 to compete, suggesting that the affinity of Hic-5 to CRM1 was higher than that of cyclin D1. Similar to the wild type, the T286A and CRM1-binding–defective mutants decreased the activity, implying that the competitive effect of cyclin D1 was dependent neither on Thr-286 phosphorylation nor on CRM1 binding (Figure 6C). Cyclin E minimally affected the activity (Figure 6C). Thus, cyclin D1 competed with Hic-5 for CRM1, but the mechanism did not appear to occur by simple binding to CRM1. Instead, it was likely that the mechanism involved PINCH, an intermediary for the interaction with cyclin D1 and Hic-5 as described above. In fact, exogenous PINCH potentiated the competitive effect of cyclin D1 (Figure 6D). These observations were all consistent with the results addressing the functional competition (Figure 5). Together, we concluded that Hic-5 and cyclin D1 compete physically and functionally with each other for CRM1 with the aid of PINCH. The role of PINCH as an interface for the communication between Hic-5 and cyclin D1 was further supported by the behavior of the PINCHΔLIM5 mutant that could not bind to either Hic-5 or cyclin D1 (Mori et al., 2006 and unpublished data). This mutant could not potentiate the competitive effect of cyclin D1 (Figure 6D, PINCHΔLIM5).
Interruption of Hic-5 Nuclear Export by an Elevated Level of ROS in Nonadherent Cells and Its Contribution to the Prevention of Anchorage-independent Cellular Growth and Survival
Based on the above findings, in nonadherent cells, where nuclear localization of cyclin D1 was decreased, the competitive effect of Hic-5 on cyclin D1 for CRM1 was assumed to be somehow deteriorated, leading to facilitation of the nuclear export of cyclin D1. One of the possible mechanisms was dissociation of cyclin D1 from the Hic-5–PINCH complex to be free from the regulation by Hic-5. Actually, as shown in Figure 3C, the amount of cyclin D1 interacting with the Hic-5–PINCH complex was reduced in nonadherent conditions. Another possible mechanism was that in nonadherent cells, the NES function of Hic-5 was disturbed. Theoretically, both possibilities could contribute to the nuclear export of cyclin D1 in nonadherent cells. Here we investigated a change in the oxidant-sensitive nuclear export of Hic-5 (Shibanuma et al., 2003) and its involvement in the decreased nuclear localization of cyclin D1 in nonadherent conditions. Actually, evaluation by LMB-sensitive nuclear accumulation showed that the export of Hic-5 was severely impeded in nonadherent cells (Figure 7A; Hic-5, Suspension, WT).
Figure 7.
Nuclear export of Hic-5 and PINCH is interrupted by elevated levels of ROS in nonadherent cells. (A) The vector for HA-tagged wild-type (WT) or mutant Hic-5, Cfl/ns, was introduced into C3H10T1/2 cells. After 24 h, the cells were detached, transferred to suspension culture (Suspension), or replated onto coverslips (Adhesion) and further incubated for 2 h with or without LMB (10 ng/ml). After coimmunostaining with the antibodies for the HA tag (Hic-5) and PINCH, the ratio of the intensity in the nucleus to that in the whole cell was obtained and graphed (n ≥ 50). Data are expressed as mean ± SD (n ≥ 3). (B) The cells were detached and transferred to a suspension culture, and the ROS level was measured after the indicated periods (n ≥ 20). Data are expressed as mean ± SD (n ≥ 5). (C) Cells expressing HA-tagged wild-type Hic-5 as in A were preincubated with ROS scavengers (1 mM tiron and 100 μM PDTC) for 30 min, detached, transferred to suspension culture, and then incubated for 2 h with or without LMB (10 ng/ml). Nuclear localization of Hic-5 was monitored as in A. (D) The cells expressing wild-type (WT) or Cfl/ns mutant Hic-5 and the HA-tagged cyclin D1 were transferred to suspension cultures and, after 2 h, were immunostained with the antibody to the HA tag. Nuclear localization of cyclin D1 in the cells was examined (n ≥ 50).
On the other hand, the Hic-5 mutant Cfl/ns was exported in nonadherent cells, as it was in adherent cells (Figure 7A; Hic-5, Suspension, Cfl/ns). Considering that Cfl/ns is a mutant that has lost oxidant sensitivity through mutation at specific cysteine residues (Shibanuma et al., 2003), it was predictable that the cellular redox state in nonadherent cells would be shifted to oxidative conditions, whereby the wild type of Hic-5 was modified to lose its nuclear export capability. To confirm this assumption, we used a fluorescent indicator to monitor the ROS level in cells detached from the substratum and observed a burst of ROS production followed by a sustained increase (Figure 7B). When the nonadherent cells were pretreated with chemical scavengers of ROS such as tiron and PDTC, even wild-type Hic-5 retained its nuclear export capability (Figure 7C). These observations provided substantial evidence that under nonadherent conditions, the increased ROS production halted the nuclear export of Hic-5. Consistent with a previous finding of the role of Hic-5 in the translocation of PINCH between the cellular compartments (Mori et al., 2006), the behaviors of PINCH were similar with those of Hic-5 in either monolayers or suspended cells (Figure 7A, PINCH).
On the basis of these findings, we hypothesized that the Cfl/ns mutant, which can translocate in and out of the nucleus independent of oxidative conditions with the ability to interact with cyclin D1 through PINCH (Figure 3B), localizes cyclin D1 to the nucleus even in nonadherent cells. This was found to be the case, and the introduction of the mutant into the cells in suspension increased the number of cells in which cyclin D1 was localized to the nucleus (Figure 7D). This result also supports the idea that the nuclear export of Hic-5 indeed acted as a driving force in localization cyclin D1 to the nucleus.
Next, we evaluated the significance of the anchorage dependence of cyclin D1 nuclear localization, which is regulated by the redox-sensitive nuclear export of Hic-5. We used the Cfl/ns mutant, achieved the aberrant nuclear localization of cyclin D1 in nonadherent cells and observed the consequences (escape from growth arrest and apoptosis). We used two cell lines, NMuMG and C3H10T1/2, as representatives of epithelial and fibroblastic cells, respectively. In general, epithelial cells were susceptible to apoptosis upon deprivation of the substratum, whereas when the fibroblasts were deprived of the substratum, they arrested the growth. We first retrovirally overexpressed the wild-type and oxidant-resistant Cfl/ns mutant of Hic-5, or NLS-cyclin D1, a derivative of cyclin D1 with an NLS, in NMuMG cells. Then, we placed the cells in suspension and performed a BrdU incorporation analysis. NLS-cyclin D1 overexpression resulted in a fourfold stimulation of BrdU incorporation (Figure 8A, D1/Hic-5, NLS/−). Notably, the C/fl/ns mutant alone also increased incorporation more than twofold compared with the control (Figure 8A, D1/Hic-5; −/Cfl/ns). Similarly, in the suspended C3H10T1/2 cell culture, the amount of BrdU incorporated was increased by the introduction of NLS-cyclin D1 (Figure 8B, D1/Hic-5; NLS/v). In accordance with previous findings emphasizing the importance of nuclear-localizing ability (Quelle et al., 1993; Resnitzky et al., 1994), the effect of wild-type cyclin D1 was much weaker than that of NLS-cyclin D1 (Figure 8B, D1/Hic-5; +/v); the Cfl/ns mutant of Hic-5 when coexpressed with wild-type cyclin D1 potentiated its effect (Figure 8B, D1/Hic-5; +/Cfl/ns). In parallel, moderate but reproducible increases in the S-phase fraction were observed in the culture overexpressing NLS-cyclin D1 and Cfl/ns (Supplemental Figure S2A).
Figure 8.
Anchorage-independent cell growth and survival is induced by oxidant-resistant nuclear-to-cytoplasmic export of Hic-5 in suspension culture. The cells were infected with retroviral vectors: empty vector (v), Flag-tagged wild type (WT), or Cfl/ns mutant Hic-5, HA-tagged nuclear-targeted cyclin D1 (NLS). At 72 h after infection, the cells were placed in suspension for 24 h, and BrdU incorporation was examined (A and B; n ≥ 50). Data are expressed as mean ± SD (n ≥ 3). Total lysates of the infected cells were subjected to Western blotting (bottom panel). In C, infected cells placed in suspension for 48 h were examined with a colorimetric APOPercentage apoptosis assay kit as described in Materials and Methods. Data are relative to the adherent condition and are expressed as mean ± SD (n ≥ 3). (D) Infected cells were transferred to suspension cultures and counted at the indicated time. Data are expressed as mean ± SD (n ≥ 3). (E) Cell lysates were prepared from infected C3H10T1/2 cells that were transferred into suspension or kept in monolayer cultures for 72 h and examined by Western blotting with antibodies for Rb (Total Rb) and Rb phosphorylated at Ser 780 (pRb S780) and Ser 807/811 (pRb S807/811). The intensities of the bands were quantified and normalized with that of total Rb and are shown relative to the control.
In the NMuMG-epithelial cells, we detected a population with sub-G1 DNA content (data not shown), which suggested that a fraction of the epithelial cells underwent apoptosis. In fact, the apoptotic cells were detected in the suspended culture, and the population was reduced by the Cfl/ns mutant and NLS-cyclin D1 expression (Figure 8C), suggesting that nuclear-localized cyclin D1 had an anti-apoptotic effect in the suspended cells. Importantly, in the presence of the Hic-5 Cfl/ns mutant and NLS-cyclin D1, there were a significant number of viable cells after the long incubation in suspension (Figure 8D). Overall, these analyses demonstrated a partial but appreciable escape from growth arrest and/or acquisition of resistance to apoptosis in nonadherent cells by ROS-insensitive, continuous Hic-5 nuclear export that increased nuclear localization of cyclin D1. Incompleteness of the escape may have been due to the dissociation of cyclin D1 from the Hic-5–PINCH complex in the nonadherent conditions (Figure 3C). This population was presumed to be out of the control by Hic-5 that was based on the association of cyclin D1 with the Hic-5–PINCH complex.
The mechanisms underlying the escape from growth arrest or apoptosis mediated by the nuclear-localized cyclin D1 merit further investigation. The phosphorylation of Rb at serine 780 by cyclin D1-CDK4/6 showed no robust changes under the above conditions (Figure 8E), making the involvement of hyperphosphorylation of Rb, a classic hallmark of the transition from G1 to S phase, obscure.
Interconnection of the Oncogenic Potential of Two Oncogenes, Cyclin D1 and Ras
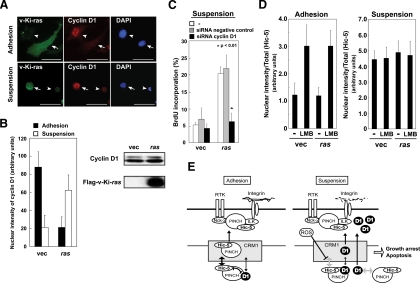
Finally, to investigate the possible implications of the above phenomenon in tumorigenesis, we introduced v-Ki-ras into cells and examined the subcellular localization of cyclin D1. In contrast to the control cells, there were strong nuclear signals in the suspended culture rather than in the monolayer in cells expressing v-Ki-ras (Figure 9, A and B), suggesting that the ras signal uncouples cyclin D1 nuclear localization from the anchorage. The nuclear signal was diminished by cyclin D1 knockdown with siRNA, which ruled out antibody cross-reactivity (Supplemental Figure S3B). Most importantly, when cyclin D1 expression was knocked down in NIH3T3 cells, the suspended cells that expressed ras lost the ability to incorporate BrdU (Figure 9C), suggesting that ras was dependent on nuclear localization of cyclin D1 to induce anchorage-independent growth in this permanent cell line. Because the nuclear-to-cytoplasmic export of Hic-5 was halted in the suspended cells expressing ras as well as the nonexpressing cells, the ras signal was likely to modify cyclin D1 itself to achieve anchorage-independent nuclear localization rather than to modify the NES function of Hic-5 (Figure 9D). Alternatively, the complex formation among cyclin D1, PINCH and Hic-5, or the interaction of cyclin D1 with other binding partners might be altered to allow anchorage-independent nuclear localization of cyclin D1.
Figure 9.
Oncogenic ras disrupted the anchorage dependence of cyclin D1 nuclear localization and induced anchorage-independent cell growth. (A and B) The expression vector for Flag-tagged v-Ki-ras or an empty vector was introduced into NIH3T3 cells and, after 24 h, the cells were detached and transferred to suspension culture (Suspension) or cultured in monolayers (Adhesion). After 3 h, the cells were coimmunostained with the antibodies for cyclin D1 and the Flag tag. Photographs were taken using a confocal fluorescence microscope (A; arrow, Flag-positive; arrowhead, Flag-negative). Scale bars, 50 μm. (B) Cyclin D1 nuclear localization was examined in the Flag-positive cells. Total cell lysates were analyzed by Western blotting. (C) The expression vector for ras or an empty vector was introduced into the cells with the siRNA for cyclin D1 or a control, and after 48 h, the cells were placed in suspension. BrdU incorporation was examined after coimmunostaining with the antibodies for the tag and BrdU. (D) Vectors for HA-tagged wild-type Hic-5 and Flag-tagged v-Ki-ras were introduced into C3H10T1/2 cells. After 24 h, the cells were detached, transferred to suspension cultures (Suspension), or replated onto coverslips (Adhesion) and further incubated for 2 h with or without LMB (10 ng/ml). The cells were immunostained with antibodies for the tags and Hic-5 nuclear localization was evaluated as in Figure 7A. (E) Model of a failsafe system for anchorage dependence of cell growth and survival. Cyclin D1 is retained in the nucleus in adherent cells by the CRM1-dependent nuclear export of Hic-5 with the aid of PINCH, which counteracts the nuclear export of cyclin D1. The higher affinity of Hic-5 for CRM1 favors the export of Hic-5. In suspended cells, the nuclear export of Hic-5 is inhibited by elevated levels of ROS, and cyclin D1 is actively transported out of the nucleus, which results in a decrease in its nuclear localization. Otherwise, cells acquire the capability to survive and/or grow in anchorage-independent conditions, as demonstrated in Figure 8D.
DISCUSSION
We summarize the findings of this study in a model proposing a failsafe system for the anchorage dependence of cell growth and survival (Figure 9E). CRM1-dependent NES of Hic-5 and the Hic-5–cyclin D1 interaction through PINCH enables Hic-5 to counteract cyclin D1 nuclear export and retain cyclin D1 in the nucleus of adherent cells. The high affinity of Hic-5 for CRM1 (Figure 6) favors Hic-5 export rather than cyclin D1 export. On the loss of adhesion, two mechanisms operate to liberate cyclin D1 from the counteracting regulation by Hic-5-PINCH. First, the interaction of cyclin D1 with the Hic-5–PINCH complex is weakened (Figure 3C). Second, the NES function of Hic-5 is turned off in response to ROS production (Figure 7). Both mechanisms potentially contribute to cyclin D1 nuclear export, prevent irrelevant cyclin D1 nuclear localization under nonadherent conditions, and couple cellular growth and survival to an appropriate attachment to matrix. Given that the effects of Cfl/ns, which drove cyclin D1 back into the nucleus by its ROS-insensitive continuous nuclear export, were intermediate between those of NLS-cyclin D1 and the control (Figure 8), the above two mechanisms seemingly contribute equally to the nuclear cyclin D1 export in suspended cells (Figure 9E; upward arrows in suspension).
According to recent studies, cyclin D is not required for proliferation in most types of cells in vivo. However, hematopoietic stem cells are exceptional (Kozar et al., 2004; Sherr and Roberts, 2004), which suggests a proproliferative potential of cyclin D1 in nonadherent, but not adherent cells. It follows that in the event of detachment or inappropriate matrix attachment of adherent cells, a failsafe system is required to prevent an irrelevant manifestation of the proproliferative potential that might lead to the malignant transformation of cells. A likely scenario implicating cyclin D1 in tumorigenesis is that once an oncogenic pathway ignores or disrupts the failsafe system, cyclin D1 acquires constitutive nuclear localization and confers resistance to anoikis and/or anchorage-independent growth in cells. Supporting this scenario is a line of evidence that addresses the contribution of cyclin D1 in the induction of anchorage-independent cell growth rather than anchorage-dependent G1 progression in neoplastic transformation (Resnitzky, 1997; Li et al., 2003; Holley et al., 2005).
It is unclear how nuclear-localized cyclin D1 enables cells to escape from growth arrest and anoikis under nonadherent conditions. The marginal change in Rb phosphorylation (Figure 8E) suggests the involvement of undefined functions of cyclin D1. An increasing amount of in vivo evidence, including a surprising lack of correlation between increased cyclin D1 expression and increased DNA synthesis in tumors, has cast doubt on the notion that cyclin D1 contributes to tumorigenesis by promoting proliferation in a CDK-dependent manner (Weinstat-Saslow et al., 1995; Oyama et al., 1998; Ewen and Lamb, 2004; Fu et al., 2004). More recent studies have proposed a new framework in which cyclin D1 is viewed as a transcriptional coregulator (Coqueret, 2002; Lamb et al., 2003). In one example, cyclin D1 induced Bcl-2, resulting in resistance to apoptosis (Rieber and Rieber, 2006). In the near future, a better understanding of cyclin D1 function may define a new mechanism of tumorigenesis that will clarify its role.
Apart from a failsafe system for the anchorage dependence of cell growth and survival, our findings also offer a new molecular mechanism of cellular transformation by ras. Although in vivo experimental models for mammary tumor propose cyclin D1 as a downstream effector of oncogenic ras signaling and a necessary target of the ras and ErbB-2 oncogenes (Yu et al., 2001), the details remain unknown. In this study, we uncovered the interdependency between ras and cyclin D1, whereby the ras signal uncouples cyclin D1 nuclear localization from the failsafe system and achieves its constitutive nuclear localization independent of anchorage as ras uses the nuclear-localized cyclin D1 to induce anchorage-independent cellular growth (Yang et al., 1998; Figure 9). Similarly, disturbing the molecular modalities that constitute the failsafe system could be a target for other oncogenic signals.
ROS have now been widely accepted as physiological signaling molecules mediating a diverse array of cellular activities (Lander, 1997; Nose, 2000; Thannickal and Fanburg, 2000; Finkel, 2003). The extracellular cues stimulating cells to produce ROS include soluble growth factors, cytokines, and integrin-mediated adhesion (Chiarugi and Fiaschi, 2007). Because the quality (i.e., sources and species) and quantity (i.e., amount and duration) of ROS differ according to the stimuli, it can be reasonably assumed that specific effectors are selectively activated by ROS in a given situation; however, little is known about the specificity of ROS signaling. Until now, limited categories of molecules have been identified as direct targets and effectors of ROS (see the above reviews). Among them are transcription factors, protein tyrosine kinases and phosphatases, which mediate the biological effects as modified by ROS. In general, ROS produced upon activation of a receptor tyrosine-kinase activate protein kinases and inactivate phosphatases through oxidative modification of specific cysteine residues, which leads to a prolonged activation of the protein kinases and a potentiation of the mitogenic signal. Similar potentiation of protein kinase signaling by ROS occurs upon integrin-mediated cell adhesion, which plays a role in the regulation of cell growth, actin cytoskeleton organization, and gene expression (Chiarugi et al., 2003).
In this study, we observed ROS generation upon loss of adhesion. Detachment of cells from the substratum induced a transient burst followed by a sustained production of hydrogen peroxide (Figure 7B). Although the details have not yet been examined, this biphasic pattern implies the involvement of multiple ROS generating systems. On integrin engagement, the released ROS inactivate low-molecular-weight phosphotyrosine phosphatase and contribute to cell adhesion by reinforcing FAK signaling (Chiarugi et al., 2003). On loss of adhesion, we found that ROS played a role in preventing anchorage-independent growth by modifying the nuclear export of Hic-5. This finding does not exclude the role of other ROS effectors, such as low-molecular-weight phosphotyrosine phosphatase, in ROS signaling upon loss of adhesion. Under adherent and nonadherent conditions, it is conceivable that both common and distinct molecules are modified by ROS and that overlapping and nonoverlapping signaling cascades are activated, which eventually lead to a distinctive outcome in cooperation with other signaling processes. To establish ROS as authentic signaling molecules, the specificity and its determinant, as well as the biological significance of these signaling processes must be addressed.
Anchorage-independent cell growth and survival play a critical role in tumor progression by potentially allowing increased survival of cancer cells in the absence of an appropriate matrix attachment, e.g., upon detachment from the basement membrane or during systemic circulation, thereby facilitating colonization at distant sites. The strategy of targeting cyclin D1 and preventing its access to sites of irrelevant action by manipulating subcellular localization might deprive the oncogene of its contribution to the metastatic process. Hopefully, this study will contribute to the development of new therapies for the prevention of metastasis.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. N. Huh (Okayama University) for critical reading and helpful comments. We also thank Dr. X.-F. Wang (Duke University) for the generous gift of the expression plasmids for the two-hybrid system and Dr. T. Miyazaki (Showa University) for his technical help. We also thank Y. Araki, R. Sampei, T. Ohata, and S. Okugawa for contributing to this work as the theme for their bachelor's degrees. This work was supported in part by Grants-in-Aid for Scientific Research, the High-Technology Research Center Project, and by the High-Technology Research Center Project for Private Universities: matching fund subsidy from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT) 2000–2003.
Abbreviations used:
- BrdU
5-bromo-2′-deoxyuridine
- CDK
cyclin-dependent kinase
- DAPI
4′6-diamidino-2-phenylindole
- ECM
extracellular matrix
- ERK
extracellular signal-regulated kinase
- FA
focal adhesions
- FAK
focal adhesion kinase
- GSK
glycogen synthase kinase
- ILK
integrin-linked kinase
- LMB
leptomycin B
- MEF
mouse embryo fibroblast
- NLS
nuclear localization signal
- PDTC
pyrrolidine dithiocarbamate
- PINCH
particularly interesting new Cys-His protein
- PTP-PEST
protein-tyrosine phosphatase PEST
- ROS
reactive oxygen species
- tiron
1,2-dihidroxybenzene-3,5-disulphonic acid.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E08-04-0428) on October 22, 2008.
REFERENCES
- Alt J. R., Cleveland J. L., Hannink M., Diehl J. A. Phosphorylation-dependent regulation of cyclin D1 nuclear export and cyclin D1-dependent cellular transformation. Genes Dev. 2000;14:3102–3114. doi: 10.1101/gad.854900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alt J. R., Gladden A. B., Diehl J. A. p21(Cip1) Promotes cyclin D1 nuclear accumulation via direct inhibition of nuclear export. J. Biol. Chem. 2002;277:8517–8523. doi: 10.1074/jbc.M108867200. [DOI] [PubMed] [Google Scholar]
- Assoian R. K. Anchorage-dependent cell cycle progression. J. Cell Biol. 1997;136:1–4. doi: 10.1083/jcb.136.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzeno S., Diehl J. A. C-terminal sequences direct cyclin D1-CRM1 binding. J. Biol. Chem. 2004;279:56061–56066. doi: 10.1074/jbc.M411910200. [DOI] [PubMed] [Google Scholar]
- Chiarugi P., Fiaschi T. Redox signalling in anchorage-dependent cell growth. Cell Signal. 2007;19:672–682. doi: 10.1016/j.cellsig.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Chiarugi P., Pani G., Giannoni E., Taddei L., Colavitti R., Raugei G., Symons M., Borrello S., Galeotti T., Ramponi G. Reactive oxygen species as essential mediators of cell adhesion: the oxidative inhibition of a FAK tyrosine phosphatase is required for cell adhesion. J. Cell Biol. 2003;161:933–944. doi: 10.1083/jcb.200211118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coqueret O. Linking cyclins to transcriptional control. Gene. 2002;299:35–55. doi: 10.1016/s0378-1119(02)01055-7. [DOI] [PubMed] [Google Scholar]
- de Ruijter A. J., van Gennip A. H., Caron H. N., Kemp S., van Kuilenburg A. B. Histone deacetylases (HDACs): characterization of the classical HDAC family. Biochem. J. 2003;370:737–749. doi: 10.1042/BJ20021321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl J. A. Cycling to cancer with cyclin D1. Cancer Biol. Ther. 2002;1:226–231. doi: 10.4161/cbt.72. [DOI] [PubMed] [Google Scholar]
- Diehl J. A., Cheng M., Roussel M. F., Sherr C. J. Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis and subcellular localization. Genes Dev. 1998;12:3499–3511. doi: 10.1101/gad.12.22.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl J. A., Zindy F., Sherr C. J. Inhibition of cyclin D1 phosphorylation on threonine-286 prevents its rapid degradation via the ubiquitin-proteasome pathway. Genes Dev. 1997;11:957–972. doi: 10.1101/gad.11.8.957. [DOI] [PubMed] [Google Scholar]
- Ewen M. E., Lamb J. The activities of cyclin D1 that drive tumorigenesis. Trends Mol. Med. 2004;10:158–162. doi: 10.1016/j.molmed.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Fang F., Orend G., Watanabe N., Hunter T., Ruoslahti E. Dependence of cyclin E-CDK2 kinase activity on cell anchorage. Science. 1996;271:499–502. doi: 10.1126/science.271.5248.499. [DOI] [PubMed] [Google Scholar]
- Finkel T. Oxidant signals and oxidative stress. Curr. Opin. Cell Biol. 2003;15:247–254. doi: 10.1016/s0955-0674(03)00002-4. [DOI] [PubMed] [Google Scholar]
- Freedman V. H., Shin S. I. Cellular tumorigenicity in nude mice: correlation with cell growth in semi-solid medium. Cell. 1974;3:355–359. doi: 10.1016/0092-8674(74)90050-6. [DOI] [PubMed] [Google Scholar]
- Frisch S. M., Screaton R. A. Anoikis mechanisms. Curr. Opin. Cell Biol. 2001;13:555–562. doi: 10.1016/s0955-0674(00)00251-9. [DOI] [PubMed] [Google Scholar]
- Fu M., Wang C., Li Z., Sakamaki T., Pestell R. G. Minireview: Cyclin D1, normal and abnormal functions. Endocrinology. 2004;145:5439–5447. doi: 10.1210/en.2004-0959. [DOI] [PubMed] [Google Scholar]
- Gladden A. B., Diehl J. A. Location, location, location: the role of cyclin D1 nuclear localization in cancer. J. Cell. Biochem. 2005;96:906–913. doi: 10.1002/jcb.20613. [DOI] [PubMed] [Google Scholar]
- Gotoh I., Uekita T., Seiki M. Regulated nucleo-cytoplasmic shuttling of human aci-reductone dioxygenase (hADI1) and its potential role in mRNA processing. Genes Cells. 2007;12:105–117. doi: 10.1111/j.1365-2443.2006.01035.x. [DOI] [PubMed] [Google Scholar]
- Guerrero-Santoro J., Yang L., Stallcup M. R., DeFranco D. B. Distinct LIM domains of Hic-5/ARA55 are required for nuclear matrix targeting and glucocorticoid receptor binding and coactivation. J. Cell. Biochem. 2004;92:810–819. doi: 10.1002/jcb.20109. [DOI] [PubMed] [Google Scholar]
- Henderson B. R., Eleftheriou A. A comparison of the activity, sequence specificity, and CRM1-dependence of different nuclear export signals. Exp. Cell Res. 2000;256:213–224. doi: 10.1006/excr.2000.4825. [DOI] [PubMed] [Google Scholar]
- Holley S. L., Heighway J., Hoban P. R. Induced expression of human CCND1 alternative transcripts in mouse Cyl-1 knockout fibroblasts highlights functional differences. Int. J. Cancer. 2005;114:364–370. doi: 10.1002/ijc.20750. [DOI] [PubMed] [Google Scholar]
- Kanome T., Itoh N., Ishikawa F., Mori K., Kim-Kaneyama J. R., Nose K., Shibanuma M. Characterization of Jumping translocation breakpoint (JTB) gene product isolated as a TGF-beta1-inducible clone involved in regulation of mitochondrial function, cell growth and cell death. Oncogene. 2007;26:5991–6001. doi: 10.1038/sj.onc.1210423. [DOI] [PubMed] [Google Scholar]
- Kozar K., et al. Mouse development and cell proliferation in the absence of D-cyclins. Cell. 2004;118:477–491. doi: 10.1016/j.cell.2004.07.025. [DOI] [PubMed] [Google Scholar]
- Lamb J., Ramaswamy S., Ford H. L., Contreras B., Martinez R. V., Kittrell F. S., Zahnow C. A., Patterson N., Golub T. R., Ewen M. E. A mechanism of cyclin D1 action encoded in the patterns of gene expression in human cancer. Cell. 2003;114:323–334. doi: 10.1016/s0092-8674(03)00570-1. [DOI] [PubMed] [Google Scholar]
- Lander H. M. An essential role for free radicals and derived species in signal transduction. FASEB J. 1997;11:118–124. [PubMed] [Google Scholar]
- Li J. M., Datto M. B., Shen X., Hu P. P., Yu Y., Wang X. F. Sp1, but not Sp3, functions to mediate promoter activation by TGF-beta through canonical Sp1 binding sites. Nucleic Acids Res. 1998;26:2449–2456. doi: 10.1093/nar/26.10.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y. J., Song R., Korkola J. E., Archer M. C., Ben-David Y. Cyclin D1 is necessary but not sufficient for anchorage-independent growth of rat mammary tumor cells and is associated with resistance of the Copenhagen rat to mammary carcinogenesis. Oncogene. 2003;22:3452–3462. doi: 10.1038/sj.onc.1206411. [DOI] [PubMed] [Google Scholar]
- Lin X., Nelson P., Gelman I. H. SSeCKS, a major protein kinase C substrate with tumor suppressor activity, regulates G(1)→S progression by controlling the expression and cellular compartmentalization of cyclin D. Mol. Cell. Biol. 2000;20:7259–7272. doi: 10.1128/mcb.20.19.7259-7272.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuya M., Sasaki H., Aoto H., Mitaka T., Nagura K., Ohba T., Ishino M., Takahashi S., Suzuki R., Sasaki T. Cell adhesion kinase beta forms a complex with a new member, Hic-5, of proteins localized at focal adhesions. J. Biol. Chem. 1998;273:1003–1014. doi: 10.1074/jbc.273.2.1003. [DOI] [PubMed] [Google Scholar]
- Mori K., Asakawa M., Hayashi M., Imura M., Ohki T., Hirao E., Kim-Kaneyama J. R., Nose K., Shibanuma M. Oligomerizing potential of a focal adhesion LIM protein Hic-5 organizing a nuclear-cytoplasmic shuttling complex. J. Biol. Chem. 2006;281:22048–22061. doi: 10.1074/jbc.M513111200. [DOI] [PubMed] [Google Scholar]
- Nishiya N., Tachibana K., Shibanuma M., Mashimo J. I., Nose K. Hic-5-reduced cell spreading on fibronectin: competitive effects between paxillin and Hic-5 through interaction with focal adhesion kinase. Mol. Cell. Biol. 2001;21:5332–5345. doi: 10.1128/MCB.21.16.5332-5345.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nose K. Role of reactive oxygen species in the regulation of physiological functions. Biol. Pharm. Bull. 2000;23:897–903. doi: 10.1248/bpb.23.897. [DOI] [PubMed] [Google Scholar]
- Oyama T., Kashiwabara K., Yoshimoto K., Arnold A., Koerner F. Frequent overexpression of the cyclin D1 oncogene in invasive lobular carcinoma of the breast. Cancer Res. 1998;58:2876–2880. [PubMed] [Google Scholar]
- Quelle D. E., Ashmun R. A., Shurtleff S. A., Kato J. Y., Bar-Sagi D., Roussel M. F., Sherr C. J. Overexpression of mouse D-type cyclins accelerates G1 phase in rodent fibroblasts. Genes Dev. 1993;7:1559–1571. doi: 10.1101/gad.7.8.1559. [DOI] [PubMed] [Google Scholar]
- Reddig P. J., Juliano R. L. Clinging to life: cell to matrix adhesion and cell survival. Cancer Metastasis Rev. 2005;24:425–439. doi: 10.1007/s10555-005-5134-3. [DOI] [PubMed] [Google Scholar]
- Resnitzky D. Ectopic expression of cyclin D1 but not cyclin E induces anchorage-independent cell cycle progression. Mol. Cell. Biol. 1997;17:5640–5647. doi: 10.1128/mcb.17.9.5640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnitzky D., Gossen M., Bujard H., Reed S. I. Acceleration of the G1/S phase transition by expression of cyclins D1 and E with an inducible system. Mol. Cell. Biol. 1994;14:1669–1679. doi: 10.1128/mcb.14.3.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieber M., Rieber M. S. Cyclin D1 overexpression induces epidermal growth factor-independent resistance to apoptosis linked to BCL-2 in human A431 carcinoma. Apoptosis. 2006;11:121–129. doi: 10.1007/s10495-005-3084-4. [DOI] [PubMed] [Google Scholar]
- Schulze A., Zerfass-Thome K., Berges J., Middendorp S., Jansen-Durr P., Henglein B. Anchorage-dependent transcription of the cyclin A gene. Mol. Cell. Biol. 1996;16:4632–4638. doi: 10.1128/mcb.16.9.4632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M. A., Assoian R. K. Integrins and cell proliferation: regulation of cyclin-dependent kinases via cytoplasmic signaling pathways. J. Cell Sci. 2001;114:2553–2560. doi: 10.1242/jcs.114.14.2553. [DOI] [PubMed] [Google Scholar]
- Sherr C. J., Roberts J. M. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- Sherr C. J., Roberts J. M. Living with or without cyclins and cyclin-dependent kinases. Genes Dev. 2004;18:2699–2711. doi: 10.1101/gad.1256504. [DOI] [PubMed] [Google Scholar]
- Shibanuma M., Kim-Kaneyama J. R., Ishino K., Sakamoto N., Hishiki T., Yamaguchi K., Mori K., Mashimo J., Nose K. Hic-5 communicates between focal adhesions and the nucleus through oxidant-sensitive nuclear export signal. Mol. Biol. Cell. 2003;14:1158–1171. doi: 10.1091/mbc.02-06-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibanuma M., Kim-Kaneyama J. R., Sato S., Nose K. A LIM protein, Hic-5, functions as a potential coactivator for Sp1. J. Cell. Biochem. 2004;91:633–645. doi: 10.1002/jcb.10754. [DOI] [PubMed] [Google Scholar]
- Shibanuma M., Mori K., Kim-Kaneyama J. R., Nose K. Involvement of FAK and PTP-PEST in the regulation of redox-sensitive nuclear-cytoplasmic shuttling of a LIM protein, Hic-5. Antioxid. Redox Signal. 2005;7:335–347. doi: 10.1089/ars.2005.7.335. [DOI] [PubMed] [Google Scholar]
- Thannickal V. J., Fanburg B. L. Reactive oxygen species in cell signaling. Am. J. Physiol. Lung Cell Mol. Physiol. 2000;279:L1005–L1028. doi: 10.1152/ajplung.2000.279.6.L1005. [DOI] [PubMed] [Google Scholar]
- Thomas S. M., Hagel M., Turner C. E. Characterization of a focal adhesion protein, Hic-5, that shares extensive homology with paxillin. J. Cell Sci. 1999;112(Pt 2):181–190. doi: 10.1242/jcs.112.2.181. [DOI] [PubMed] [Google Scholar]
- Wang L. H. Molecular signaling regulating anchorage-independent growth of cancer cells. Mt. Sinai J. Med. 2004;71:361–367. [PubMed] [Google Scholar]
- Weinstat-Saslow D., Merino M. J., Manrow R. E., Lawrence J. A., Bluth R. F., Wittenbel K. D., Simpson J. F., Page D. L., Steeg P. S. Overexpression of cyclin D mRNA distinguishes invasive and in situ breast carcinomas from non-malignant lesions. Nat. Med. 1995;1:1257–1260. doi: 10.1038/nm1295-1257. [DOI] [PubMed] [Google Scholar]
- Yang J. J., Kang J. S., Krauss R. S. Ras signals to the cell cycle machinery via multiple pathways to induce anchorage-independent growth. Mol. Cell. Biol. 1998;18:2586–2595. doi: 10.1128/mcb.18.5.2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Guerrero J., Hong H., DeFranco D. B., Stallcup M. R. Interaction of the tau2 transcriptional activation domain of glucocorticoid receptor with a novel steroid receptor coactivator, Hic-5, which localizes to both focal adhesions and the nuclear matrix. Mol. Biol. Cell. 2000;11:2007–2018. doi: 10.1091/mbc.11.6.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q., Geng Y., Sicinski P. Specific protection against breast cancers by cyclin D1 ablation. Nature. 2001;411:1017–1021. doi: 10.1038/35082500. [DOI] [PubMed] [Google Scholar]
- Zhu X., Ohtsubo M., Bohmer R. M., Roberts J. M., Assoian R. K. Adhesion-dependent cell cycle progression linked to the expression of cyclin D1, activation of cyclin E-cdk2, and phosphorylation of the retinoblastoma protein. J. Cell Biol. 1996;133:391–403. doi: 10.1083/jcb.133.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.