Abstract
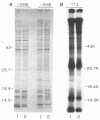
We isolated lipopolysaccharides (LPSs) from phase variants of Coxiella burnetii Nine Mile and compared the isolated LPS and C. burnetii cells by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotting. The LPSs were found to be the predominant component which varied structurally and antigenically between virulent phase I and avirulent phase II. A comparison of techniques historically used to extract the phase I antigenic component revealed that the aqueous phase of phenol-water, trichloroacetic acid, and dimethyl sulfoxide extractions of phase I C. burnettii cells all contained phase I LPS, although the efficiency and specificity of extraction varied. Our studies provide additional evidence that phase variation in C. burnetii is analogous to the smooth-to-rough LPS variation of gram-negative enteric bacteria, with phase I LPS being equivalent to smooth LPS and phase II being equivalent to rough LPS. In addition, we identified a variant with a third LPS chemotype with appears to have a structural complexity intermediate to phase I and II LPSs. All three C. burnetii LPS contain a 2-keto-3-deoxyoctulosonic acid-like substance, heptose, and gel Limulus amoebocyte lysates in subnanogram amounts. The C. burnetii LPSs were nontoxic to chicken embryos at doses of over 80 micrograms per embryo, in contrast to Salmonella typhimurium smooth- and rough-type LPSs, which were toxic in nanogram amounts.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amano K., Williams J. C. Chemical and immunological characterization of lipopolysaccharides from phase I and phase II Coxiella burnetii. J Bacteriol. 1984 Dec;160(3):994–1002. doi: 10.1128/jb.160.3.994-1002.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baca O. G., Paretsky D. Q fever and Coxiella burnetii: a model for host-parasite interactions. Microbiol Rev. 1983 Jun;47(2):127–149. doi: 10.1128/mr.47.2.127-149.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brezina R., Pospísil V. Study of the antigenic structure of Coxiella burneti. 8. Immunogenicity of phenol-extracted phase I antigenic component. Acta Virol. 1970 Jul;14(4):302–306. [PubMed] [Google Scholar]
- Burton P. R., Stueckemann J., Welsh R. M., Paretsky D. Some ultrastructural effects of persistent infections by the rickettsia Coxiella burnetii in mouse L cells and green monkey kidney (Vero) cells. Infect Immun. 1978 Aug;21(2):556–566. doi: 10.1128/iai.21.2.556-566.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DISCHE Z. Qualitative and quantitative colorimetric determination of heptoses. J Biol Chem. 1953 Oct;204(2):983–997. [PubMed] [Google Scholar]
- Delaney J. C., Roberts H. L. Q fever endocarditis and chronic liver involvement. Practitioner. 1975 Feb;214(1280):243–245. [PubMed] [Google Scholar]
- FISET P. Phase variation of Rickettsia (Coxiella) burneti; study of the antibody response in guinea pigs and rabbits. Can J Microbiol. 1957 Apr;3(3):435–445. doi: 10.1139/m57-046. [DOI] [PubMed] [Google Scholar]
- Fiset P., Ormsbee R. A., Silberman R., Peacock M., Spielman S. H. A microagglutination technique for detection and measurement of rickettsial antibodies. Acta Virol. 1969 Jan;13(1):60–66. [PubMed] [Google Scholar]
- Fiset P., Ormsbee R. A. The antibody response to antigens of Coxiella burneti. Zentralbl Bakteriol Orig. 1968 Apr;206(3):321–329. [PubMed] [Google Scholar]
- Fiset P., Wisseman C. L., Batawi Y. E. Immunologic evidence of human fetal infection with Coxiella burneti. Am J Epidemiol. 1975 Jan;101(1):65–69. doi: 10.1093/oxfordjournals.aje.a112072. [DOI] [PubMed] [Google Scholar]
- Fumarola D., Munno I., Monno R., Miragliotta G. Lipopolysaccharides from Rickettsiaceae: limulus endotoxin assay and pathogenetic mediators in rickettsiosis. Acta Virol. 1980 Mar;24(2):155–155. [PubMed] [Google Scholar]
- Hackstadt T., Todd W. J., Caldwell H. D. Disulfide-mediated interactions of the chlamydial major outer membrane protein: role in the differentiation of chlamydiae? J Bacteriol. 1985 Jan;161(1):25–31. doi: 10.1128/jb.161.1.25-31.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackstadt T., Williams J. C. Biochemical stratagem for obligate parasitism of eukaryotic cells by Coxiella burnetii. Proc Natl Acad Sci U S A. 1981 May;78(5):3240–3244. doi: 10.1073/pnas.78.5.3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock P. J., Brown T. M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983 Apr;154(1):269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimbrough R. C., 3rd, Ormsbee R. A., Peacock M., Rogers W. R., Bennetts R. W., Raaf J., Krause A., Gardner C. Q fever endocarditis in the United States. Ann Intern Med. 1979 Sep;91(3):400–402. doi: 10.7326/0003-4819-91-3-400. [DOI] [PubMed] [Google Scholar]
- Krauss H., Schiefer H. G., Schmatz H. D. Ultrastructural investigations on surface structures involved in Coxiella burnetii phase variation. Infect Immun. 1977 Mar;15(3):890–896. doi: 10.1128/iai.15.3.890-896.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUOTO L., CASEY M. L., PICKENS E. G. Q FEVER STUDIES IN MONTANA. DETECTION OF ASYMPTOMATIC INFECTION AMONG RESIDENTS OF INFECTED DAIRY PREMISES. Am J Epidemiol. 1965 May;81:356–369. doi: 10.1093/oxfordjournals.aje.a120522. [DOI] [PubMed] [Google Scholar]
- LUOTO L., HUEBNER R. J. Q fever studies in southern California; IX. Isolation of Q fever organisms from parturient placenta; of naturally infected dairy cows. Public Health Rep. 1950 Apr 21;65(16):541–544. [PubMed] [Google Scholar]
- Meiklejohn G., Reimer L. G., Graves P. S., Helmick C. Cryptic epidemic of Q fever in a medical school. J Infect Dis. 1981 Aug;144(2):107–113. doi: 10.1093/infdis/144.2.107. [DOI] [PubMed] [Google Scholar]
- Milner K. C., Finkelstein R. A. Bioassay of endotoxin: correlation between pyrogenicity for rabbits and lethality for chick embryos. J Infect Dis. 1966 Dec;116(5):529–536. doi: 10.1093/infdis/116.5.529. [DOI] [PubMed] [Google Scholar]
- ORMSBEE R. A., BELL E. J., LACKMAN D. B. Antigens of Coxiella burnetii. I. Extraction of antigens with non-aqueous organic solvents. J Immunol. 1962 Jun;88:741–749. [PubMed] [Google Scholar]
- ORMSBEE R. A., BELL E. J., LACKMAN D. B., TALLENT G. THE INFLUENCE OF PHASE ON THE PROTECTIVE POTENCY OF Q FEVER VACCINE. J Immunol. 1964 Mar;92:404–412. [PubMed] [Google Scholar]
- Olins A. L., Warner R. C. Physicochemical studies on a lipopolysaccharide from the cell wall of Azotobacter vinelandii. J Biol Chem. 1967 Nov 10;242(21):4994–5001. [PubMed] [Google Scholar]
- STOKER M. G., FISET P. Phase variation of the Nine Mile and other strains of Rickettsia burneti. Can J Microbiol. 1956 May;2(3):310–321. doi: 10.1139/m56-036. [DOI] [PubMed] [Google Scholar]
- SYRUCEK L., SOBESLAVSKY O., GUTVIRTH I. Isolation of Coxiella burneti from human placentas. J Hyg Epidemiol Microbiol Immunol. 1958;2(1):29–35. [PubMed] [Google Scholar]
- Schramek S., Brezina R. Characterization of an endotoxic lipopolysaccharide from Coxiella burnetii. Acta Virol. 1976 Apr;20(2):152–158. [PubMed] [Google Scholar]
- Schramek S., Brezina R., Kazár J. Influence of mild acid hydrolysis on the antigenic properties of phase I Coxiella burnetii. Acta Virol. 1978 Jul;22(4):302–308. [PubMed] [Google Scholar]
- Schramek S., Brezina R., Visacká E. Different antigenic properties of lipopolysaccharides isolated from Coxiella burnetii in phase I and pure phase II. Zentralbl Bakteriol Mikrobiol Hyg A. 1983 Sep;255(2-3):356–360. [PubMed] [Google Scholar]
- Schramek S., Mayer H. Different sugar compositions of lipopolysaccharides isolated from phase I and pure phase II cells of Coxiella burnetii. Infect Immun. 1982 Oct;38(1):53–57. doi: 10.1128/iai.38.1.53-57.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Turck W. P., Howitt G., Turnberg L. A., Fox H., Longson M., Matthews M. B., Das Gupta R. Chronic Q fever. Q J Med. 1976 Apr;45(178):193–217. [PubMed] [Google Scholar]
- WEISSBACH A., HURWITZ J. The formation of 2-keto-3-deoxyheptonic acid in extracts of Escherichia coli B. I. Identification. J Biol Chem. 1959 Apr;234(4):705–709. [PubMed] [Google Scholar]
- WELSH H. H., LENNETTE E. H., ABINANTI F. R., WINN J. F. Air-borne transmission of Q fever: the role of parturition in the generation of infective aerosols. Ann N Y Acad Sci. 1958 Jun 3;70(3):528–540. doi: 10.1111/j.1749-6632.1958.tb35409.x. [DOI] [PubMed] [Google Scholar]
- WELSH H. H., LENNETTE E. H., ABINANTI F. R., WINN J. F. Q fever in California. IV. Occurrence of Coxiella burnetii in the placenta of naturally infected sheep. Public Health Rep. 1951 Nov 9;66(45):1473–1477. [PubMed] [Google Scholar]
- Wagstaff D. J., Janney J. H., Crawford K. L., Dimijian G. G., Joseph J. M. Q fever studies in Maryland. Public Health Rep. 1965 Dec;80(12):1095–1099. [PMC free article] [PubMed] [Google Scholar]
- Williams J. C., Peacock M. G., McCaul T. F. Immunological and biological characterization of Coxiella burnetii, phases I and II, separated from host components. Infect Immun. 1981 May;32(2):840–851. doi: 10.1128/iai.32.2.840-851.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewski H. J., Kleiman M. M., Lackman D. B., Krumbiegel E. R. Demonstration of inapparent infection with disease agents common to animals and man. Health Lab Sci. 1969 Jul;6(3):173–177. [PubMed] [Google Scholar]