Abstract
Background
Malaria is the infectious disease causing the highest morbidity and mortality in Angola and due to widespread chloroquine (CQ) resistance, the country has recently changed its first-line treatment recommendations for uncomplicated malaria, from CQ to artemisinin combination therapies (ACT) in adults, and sulphadoxine/pyrimethamine (S/P) in pregnant women. Loss of SP sensitivity is, however, progressing rapidly in Africa and, in this study, were investigated a number of molecular markers associated to CQ and S/P.
Methods
Blood samples were collected from 245 children with uncomplicated malaria, admitted at the Pediatric Hospital Dr. David Bernardino (HPDB), Angola, and the occurrence of mutations in Plasmodium falciparum was investigated in the pfmdr1 (N86Y) and pfcrt (K76T) genes, associated with CQ resistance, as well as in pfdhfr (C59R) and pfdhps (K540E), conferring SP resistance.
Results
The frequencies of pfmdr1 mutations in codon 86 were 28.6% N, 61.3% Y and 10.1% mixed infections (NY). The frequency of pfcrt mutations in codon 76 were 93.9% K, 5.7% T and 0.4% mixed infections (KT). For pfdhfr the results were in codon 59, 60.6% C, 20.6% R and 18.8% mixed infections (CR). Concerning pfdhps, 6.3% of the isolates were bearers of the mutation 540E and 5.4% mixed infections (K540E).
Conclusion
The results of this epidemiologic study showed high presence of CQ resistance markers while for SP a much lower prevalence was detected for the markers under study.
Background
Malaria is endemic throughout much of the Angolan territory, and is by far the highest cause of morbidity and mortality particularly among children under five years old and pregnant women. Malaria continues to be responsible for 50% of all outpatient attendance and around 25% of all hospital deaths http://www.rollbackmalaria.org/wmr2005/. Recently and according to the Angola Malaria Indicator Survey, 2007, http://www.procaare.org/archive/procaare/200804 these figures have been reduced due to control programmes, but about 35% of all cases and 70% of all deaths reported annually occur in children under five years of age.
The first cases of resistance to chloroquine (CQ) were described in the eighties [1-4]. As a consequence of increasing rates of clinical resistance to CQ, the protocol at the Pediatric Hospital David Bernardino (HPDB) was changed, in 2006, two combined therapies artesunate with lumefantrine (Coartem®), and amodiaquine (AQ) with sulphadoxine-pyrimethamine (SP – Fansidar®) as first-line treatments of uncomplicated malaria.
The pfmdr1 gene (multidrug resistance) has been described as associated with CQ resistance [5-7], with a polymorphism resulting from the substitution of an asparagine for a tyrosine in amino acid 86 (N86Y) in Plasmodium falciparum [8-14]. CQ resistance is also associated with a mutation in the transporter gene pfcrt where the amino acid substitution at pfcrt codon 76 (K to T) has been shown to have a determinant association with the resistance phenotype [15-19].
Plasmodium falciparum resistance to sulphadoxine and pyrimethamine (SP) is conferred by mutations of the dihydropteroate synthase (pfdhps) and dihydrofolate reductase (pfdhfr) genes, respectively [20-23]. pfdhfr 108N mutation seems to be enough to confer resistance to pyrimethamine [24,25]. The presence of a mutation at positions 51 (N51I) or 59 (C59R), together with S108N confer a considerable increase in the resistance level to pyrimethamine when compared with mutation S108N by itself [26-28].
Mutations in codons 437 (A437G) and 540 (K540E) of P. falciparum pfdhps are associated with resistance to sulphadoxine. The quintuple mutant (triple pfdhfr: 51I, 59R, 108N and double pfdhps: 437G, 540E) is considered as the molecular marker of SP treatment failure [29-34]. Studies of genetic transfection of P. falciparum, confirmed that the amino acid substitution at pfdhfr codon 108 (S→N), increases approximately ten times the resistance to pyrimethamine [35]. Epidemiology studies also demonstrated that the presence of mutations 59R in pfdhfr and 540E in pfdhps are significantly associated to resistance as well as to the presence of the other above mentioned mutations [30,36,37].
In this work were investigated the frequencies of mutations associated with chloroquine resistance (pfmdr1 N86Y and pfcrt K76T) and also screened for the two mutations associated to the resistance of P. falciparum to SP as used in populations from high malaria transmission areas.
Materials and methods
Sample collection
Blood samples included in this study were collected from children between 1 and 16 years, at the HPDB Hospital in Luanda, Angola, and individually spotted on Whatman n.°4 filter paper, after microscopic confirmation of P. falciparum infection. Parent's informed consent was obtained before inclusion of the blood samples in the study which was reviewed and approved by the Ethical Committees from the Ministry of Health of Angola and of the HPDB. Original study was associated to quinine treatment and published elsewhere [38].
Genetic characterization of the parasites
Parasite DNA was extracted from dried blood spots, using Chelex as described elsewhere [39]. P. falciparum mutations associated with resistance to CQ and SP were typed by PCR-RFLP as described elsewhere [39,40], primers sequences, amplification cycles and restrictions enzymes are described in table 1 and 2, respectively. In this study, the following codons and polymorphisms were analysed: pfmdr1 86, pfcrt 76; pfdhfr 59 and pfdhps 540. Amplicons and fragments were separated on 2% or 3% agarose gels stained with ethidium bromide and visualized under UV.
Table 1.
PCR Programme for the genes pfmdr1 86, pfcrt 76, pfdhps 540, pfdhfr 59, primer and product size
| Gene | Primers | PCR Programme | Product size (bp) | ||
| Pfmdr1 86 | 754N 754R | Initial step | 92°C | 3 min | 321 |
| Denaturation (35×) | 92°C | 1 min | |||
| Annealing (35×) | 51°C | 30 s | |||
| Extension (35×) | 72°C | 1 min | |||
| Final step | 72°C | 3 min | |||
| Pfcrt 76 1° nested | 76o1F N1R | Initial step | 94°C | 3 min | 528 |
| Denaturation (40×) | 94°C | 45 s | |||
| Annealing (40×) | 55°C | 45 s | |||
| Extension (40×) | 72°C | 45 s | |||
| Final step | 72°C | 3 min | |||
| Pfcrt 76 2° nested | 76 N2F 76 N2R | Initial step | 94°C | 3 min | 271 |
| Denaturation (35×) | 94°C | 45 s | |||
| Annealing (35×) | 53°C | 45 s | |||
| Extension (35×) | 72°C | 45 s | |||
| Final step | 72°C | 3 min | |||
| Pfdhps 540 | 540 F 540 R | Initial step | 94°C | 3 min | 439 |
| Denaturation (40×) | 94°C | 1 min | |||
| Annealing (40×) | 45°C | 1 min | |||
| Extension (40×) | 72°C | 1 min | |||
| Final step | 72°C | 3 min | |||
| Pfdhfr 59 | 59 F 59 R | Initial step | 94°C | 3 min | 326 |
| Denaturation (35×) | 94°C | 1 min | |||
| Annealing (35×) | 55°C | 1 min | |||
| Extension (35×) | 72°C | 1 min | |||
| Final step | 72°C | 3 min | |||
Table 2.
Fragments length, clones and enzymes used for digestion of the codons 86 Pfmdr 1, 76 Pfcrt, 540 Pfdhps and 59 Pfdhfr.
| Gene | Enzyme | clone | Codon | Fragments length |
| Pfmdr1 86 | ApoI | 3D7 | AAT (Asn) | 249 + 72 bp |
| Dd2 | TAT (Tyr) | 321 bp | ||
| Pfcrt 76 | ApoI | 3D7 | AAA (Lys) | 137 + 124 + 10 bp |
| K1 | ACA (Thr) | 261 + 10 bp | ||
| Pfdhps 540 | BseGI | N3 | GAA (Glu) | 354 + 85 bp |
| T9/94 | AAA (Lys) | 439 bp | ||
| Pfdhfr 59 | PdmI | K1 | CGT (Arg) | 354 + 85 bp |
| HB3 | TGT (Cys) | 439 bp |
Statistical analysis
Associations between the different mutations were tested by Fisher's exact test.
Results
Patients and parasites
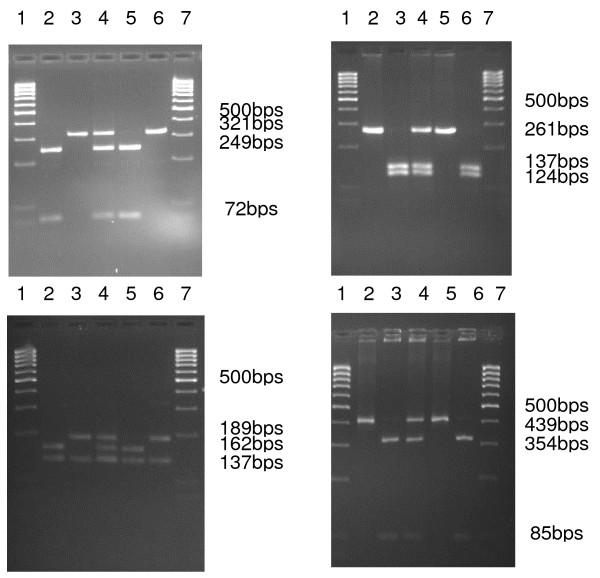
From the 245 isolates of P. falciparum parasites used in this study, 199 samples were successfully typed by PCR-RFLP for pfmdr1, for pfcrt 245, for pfdhps 221 and for pfdhfr 224. Mutant alleles were detected in four loci: pfcrt 76T, pfmdr1 86Y, pfdhps 540E and pfdhfr 59R (Figure 1).
Figure 1.
Agarose gels showing pfmdr1 86, pfcrt 76, pfdhfr 59 and pfdhps 540 PCR-RFLP products of control and field-collected samples of P. falciparum. pfmdr1: 2-N86; 3-Y86; 4-N/Y86; 5-3D7 (N86); 6-Dd2 (Y86). pfcrt: 2-k76; 3-T76; 4-K/T76; 5-K1 (K76); 6-3D7 (T76). pfdhfr: 2-C59; 3-R59; 4-R/C59; 5-N3 (C59); 6-T9/94 (R59). pfdhps: 2-E540; 3-K540; 4-K/E540; 5-HB3 (E540); 6-K1 (K540). In all gels lanes 1 and 7 shows the molecular weight marker.
pfmdr1 and pfcrt
As shown in Table 3, the frequency of the pure mutant allele pfcrt T76 was 93.9%. Only one isolate carried a mixed population KT for this allele (0.41%). The frequency of the mutant pfmdr1 Y86 allele was 61.3% and mixed pfmdr1 N and Y allele was detected in 10.1% of the isolates.
Table 3.
Prevalence of mutations conferring resistance to chloroquine and sulphadoxine/pyrimethamine in Plasmodium falciparum isolates from Angola
| Gene | Mutation | n | Mutation (%) | Mixed* (%) |
| Pfcrt | T76 | 245 | 93.9 (230/245) | 0.4 (1/245) |
| pfmdr1 | Y86 | 199 | 61.3 (122/199) | 10.1 (20/199) |
| pfdhfr | R59 | 224 | 20.6 (46/224) | 18.8 (42/224) |
| pfdhps | E540 | 221 | 6.3 (14/221) | 5.4 (12/221) |
*refers to mixed populations of P. falciparum parasites.
To study the associations between the loci pfcrt K76T and pfmdr1 N86Y, 176 single infection isolates were analysed. Among the 165 isolates with the pfcrt T76 mutation, 115 (70%) also carried the mutant pfmdr1 Y86 allele. Whether or not the mixed infections were excluded from the analysis, the association between the two mutant alleles was significant (P = 0.029, mixed infections excluded).
pfdhps and pfdhfr
One locus of pfdhps (K540E) and one of pfdhfr (C59R) were investigated (Table 3). For the pfdhps 88.3% of the isolates carried the mutant allele E540 and 5.4% carried mixed (K and E) alleles. For pfdhfr 60.6% were wild type (C59) and 18.8% were mixed populations. To study the associations between the pfdhps E540 and pfdhfr R59 loci, only 61 single infection isolates were analysed, and there was no association between pfdhps E540 and pfdhfr R59 (P = 0.159).
Discussion
In vivo resistance to CQ and SP have reached high levels in some regions of Angola, where resistance to these drugs is high (25%) [41]. This study reveals a high frequency of drug resistance molecular markers for, CQ (pfcrt T76; 93.9% and pfmdr1 Y86; 61.3%) and SP (9% of bearers with quintuple mutant).
In this study, associations within and between the mutations that confer resistance against CQ and those considered predictive of SP treatment failure were evaluated. Obtained data revealed an association (P = 0.029) between pfcrt T76 (chromosome 7) and pfmdr1 Y86 (chromosome 5) as reported by other authors [42-45].
Presence of mutations R59 and E540 in pfdhfr and pfdhps, respectively, is considered a marker of confirmed resistance to SP and our data indicates that 9% of the children attending the HPDB, were bearers of P. falciparum parasites with the SP quintuple mutant associated usually to treatment failure. These results are in line with others from western Africa, where low prevalence of the quintuple mutant has been observed [46,47]. Although the predictive value of these markers for SP treatment failure has not been established in this study, these results emphasize the need for close monitoring of mutation prevalence with treatment outcome, since these antimalarials are now used as first line treatment of uncomplicated malaria in children attending the HPDB as well as prophylactic treatment in pregnant women.
Inter- and intragenic association of pfdhfr and pfdhps mutant codons was indirectly proven in other studies where SP resistance was found to be associated with double up to quintuple mutations in both genes [48,49]. Here, only 9% of the samples, with concurrence of the pfdhfr and pfdhps variants, R59 and E450 simultaneously were observed, which are considered to be predictive of the quintuple mutant (pfdhfr I51, R59, N108, pfdhps G437, E540) associated to SP treatment failure [37,50-53]. All other samples had presence of one or the other mutation suggesting that fixation of the quintuple mutant was still an on going process.
Association between mutations on pfcrt, pfmdr1, pfdhfr and pfdhps, have been reported from West Africa [54,55] but reports on linkage between response to treatment with CQ and SP, in the same patients, is rare. Even though no statistic association was found, 43 double mutants for pfdhfr/pfcrt, 21 for pfdhfr/pfmdr1, 12 pfdhps/pfcrt and 6 pfdhps/pfmdr1 were detected. Regarding the association between mutations conferring resistance to SP and CQ; almost all of the isolates carrying the mutant genotype for pfdhfr (43 out of 46) or pfdhps (12 out of 14) carried at least one of the CQ associated mutations in the genes pfcrt or pfmdr1. This reflects the possible association and accumulation of at least three or four out of 7 mutations (pfcrt T76, pfmdr1 Y86, pfdhfr: N108, I51, R59 and pfdhps: G437, E540) scattered on four different chromosomes and involved in resistance to three different antimalarials, chloroquine, sulfadoxine and pyrimethamine.
As a result of this study it is not possible to comment whether the presence of pfcrt T76 favors the presence of pfdhfr and pfdhps mutations or vice versa because this requires longitudinal and long lasting studies and observations. Despite these difficulties in drawing firm conclusions, several previous findings support the hypothesis of linkage disequilibrium between mutations associated with SP resistance and CQ resistance. In a murine malaria model, CQ resistance could be induced in pyrimethamine-resistant parasites, but not in sensitive ones [56].
In field isolates, several observations indicate that it is more likely to find mutations in pfdhfr and pfdhps in isolates exhibiting already pfcrt T76 mutation than in isolates comprising pfcrt wild type parasites [57-59]. The reason for this apparent association between resistance to CQ and SP is obscure since both drugs have distinct modes of action and resistance to these is determined by mutations on different chromosomes [60-62]. The accelerated acquisition of resistance to multiple drugs (ARMD) possibly reflecting a rapid mutator phenotype [63] could be one explanation.
Multiple CQ and SP resistance mutations are thought to have higher fitness cost of the parasite asexual reproduction (reviewed in [64,65]). In line with the previous considerations, the reproductive capacity of mutant strains is believed to be jeopardized [64-66]. The results did not reflect these since gametocyte prevalence in infections with mutant-type parasites, was not higher than in infections with wild-type isolates.
Conclusion
This was the first molecular study carried out in this geographical area including a considerable number of samples (245) and focused in the mutations of pfmdr1, pfcrt, pfdhps and pfdhfr genes, strongly associated to CQ and SP resistance. The results of the epidemiological study on prevalence of genotypes associated with drug resistance, carried out at a HPDB in Luanda, showed high presence of CQ resistance markers, while for SP a much lower prevalence was detected. This work can be important to evaluate the implementation of new therapeutics strategies based on combinations that includes SP, like the protocol that are now implemented in HPDB (artesunate combined with SP) as first-line drug for uncomplicated malaria treatment.
Competing interests
The authors declare that they have no competing interests
Authors' contributions
PF carried out the molecular analyses and drafted this manuscript. FN participated in the design of the study performed the statistical analysis and helped to draft the manuscript. CB carried out the selection of children and sample collection. DL helped in molecular analysis and the draft of the manuscript. LB coordinated sample collection in PHDB as hospital director. VEdR helped in coordination and design of the study. LV coordinated the project and designing of the study. All authors read and approved the final manuscript.
Acknowledgments
Acknowledgements
This study was supported by projects (54965 and 65472) financed by Fundação Calouste Gulbenkian. The authors are grateful to the children involved in the study and the staff of Pediatric Hospital Dr. David Bernardino who participated in sample collection.
Contributor Information
Paula Figueiredo, Email: paulafigueiredo@ihmt.unl.pt.
Carla Benchimol, Email: Pediatria@netangola.com.
Dinora Lopes, Email: dferreira@ihmt.unl.pt.
Luís Bernardino, Email: Pediatria@netangola.com.
Virgílio E do Rosário, Email: CMDT@ihmt.unl.pt.
Luís Varandas, Email: varandas@ihmt.unl.pt.
Fátima Nogueira, Email: fnogueira@ihmt.unl.pt.
References
- Olsen VV, Jensen T, Jorgensen M. Chloroquine-resistant Plasmodium falciparum malaria from Angola. Lancet. 1984;1:1462–1463. doi: 10.1016/s0140-6736(84)91949-4. [DOI] [PubMed] [Google Scholar]
- Kyronseppa H, Lumio J, Ukkonen R, Pettersson T. Chloroquine-resistant malaria from Angola. Lancet. 1984;1:1244. doi: 10.1016/s0140-6736(84)91731-8. [DOI] [PubMed] [Google Scholar]
- Silva Z, Sampaio MM, Henne A, Bohm A, Gutzat R, Boos W, da Costa MS, Santos H. The high-affinity maltose/trehalose ABC transporter in the extremely thermophilic bacterium Thermus thermophilus HB27 also recognizes sucrose and palatinose. J Bacteriol. 2005;187:1210–1218. doi: 10.1128/JB.187.4.1210-1218.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg J, Sandberg T, Bjorkholm B, Bjorkman A. Chloroquine and Fansidar resistant malaria acquired in Angola. Lancet. 1985;1:765. doi: 10.1016/s0140-6736(85)91315-7. [DOI] [PubMed] [Google Scholar]
- Volkman S, Wirth D. Functional analysis of pfmdr1 gene of Plasmodium falciparum. Methods Enzymol. 1998;292:174–181. doi: 10.1016/s0076-6879(98)92014-6. [DOI] [PubMed] [Google Scholar]
- Povoa MM, Adagu IS, Oliveira SG, Machado RL, Miles MA, Warhurst DC. Pfmdr1 Asn1042Asp and Asp1246Tyr polymorphisms, thought to be associated with chloroquine resistance, are present in chloroquine-resistant and -sensitive Brazilian field isolates of Plasmodium falciparum. Exp Parasitol. 1998;88:64–68. doi: 10.1006/expr.1998.4195. [DOI] [PubMed] [Google Scholar]
- Char S, Kelly P, Naeem A, Farthing MJ. Codon usage in Cryptosporidium parvum differs from that in other Eimeriorina. Parasitology. 1996;112:357–362. doi: 10.1017/s0031182000066580. [DOI] [PubMed] [Google Scholar]
- Duraisingh MT, Jones P, Sambou I, von Seidlein L, Pinder M, Warhurst DC. The tyrosine-86 allele of the pfmdr1 gene of Plasmodium falciparum is associated with increased sensitivity to the anti-malarials mefloquine and artemisinin. Mol Biochem Parasitol. 2000;108:13–23. doi: 10.1016/s0166-6851(00)00201-2. [DOI] [PubMed] [Google Scholar]
- Holmgren G, Gil JP, Ferreira PM, Veiga MI, Obonyo CO, Bjorkman A. Amodiaquine resistant Plasmodium falciparum malaria in vivo is associated with selection of pfcrt 76T and pfmdr1 86Y. Infect Genet Evol. 2006;6:309–314. doi: 10.1016/j.meegid.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Khalil IF, Alifrangis M, Tarimo DS, Staalso T, Satti GM, Theander TG, Rønn AM, Bygbjerg IC. The roles of the pfcrt 76T and pfmdr1 86Y mutations, immunity and the initial level of parasitaemia, in predicting the outcome of chloroquine treatment in two areas with different transmission intensities. Ann Trop Med Parasitol. 2005;99:441–448. doi: 10.1179/136485905X46441. [DOI] [PubMed] [Google Scholar]
- Coppel RL, Favaloro JM, Crewther PE, Burkot TR, Bianco AE, Stahl HD, Kemp DJ, Anders RF, Brown GV. A blood stage antigen of Plasmodium falciparum shares determinants with the sporozoite coat protein. Proc Natl Acad Sci USA. 1985;82:5121–5125. doi: 10.1073/pnas.82.15.5121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edrissian GH, Ghorbani M, Afshar A. IFA serological surveys of malaria in north, north-west and south-west parts of Iran. Bull Soc Pathol Exot Filiales. 1985;78:349–359. [PubMed] [Google Scholar]
- Li JL, Li YJ. Plasmodium falciparum: correlation between immunofluorescent properties of monoclonal antibodies and their protective activities. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 1988;6:115–117. [PubMed] [Google Scholar]
- Keppler D, Cui Y, Konig J, Leier I, Nies A. Export pumps for anionic conjugates encoded by MRP genes. Adv Enzyme Regul. 1999;39:237–246. doi: 10.1016/s0065-2571(98)00015-6. [DOI] [PubMed] [Google Scholar]
- Babiker HA, Pringle SJ, Abdel-Muhsin A, Mackinnon M, Hunt P, Walliker D. High-level chloroquine resistance in Sudanese isolates of Plasmodium falciparum is associated with mutations in the chloroquine resistance transporter gene pfcrt and the multidrug resistance Gene pfmdr1. J Infect Dis. 2001;183:1535–1538. doi: 10.1086/320195. [DOI] [PubMed] [Google Scholar]
- Best Plummer W, Pinto Pereira LM, Carrington CV. Pfcrt and pfmdr1 alleles associated with chloroquine resistance in Plasmodium falciparum from Guyana, South America. Mem Inst Oswaldo Cruz. 2004;99:389–392. doi: 10.1590/s0074-02762004000400008. [DOI] [PubMed] [Google Scholar]
- Echeverry DF, Holmgren G, Murillo C, Higuita JC, Björkman A, Gil JP, Osorio L. Short report: polymorphisms in the pfcrt and pfmdr1 genes of Plasmodium falciparum and in vitro susceptibility to amodiaquine and desethylamodiaquine. Am J Trop Med Hyg. 2007;77:1034–1038. [PubMed] [Google Scholar]
- Lopes D, Nogueira F, Gil JP, Ferreira C, do Rosario VE, Cravo P. pfcrt and pfmdr1 mutations and chloroquine resistance in Plasmodium falciparum from Sao Tome and Principe, West Africa. Ann Trop Med Parasitol. 2002;96:831–834. doi: 10.1179/000349802125002284. [DOI] [PubMed] [Google Scholar]
- Basco LK, Le Bras J. In vitro activity of artemisinin derivatives against African isolates and clones of Plasmodium falciparum. Am J Trop Med Hyg. 1993;49:301–307. doi: 10.4269/ajtmh.1993.49.301. [DOI] [PubMed] [Google Scholar]
- Wongsrichanalai C, Pickard AL, Wernsdorfer WH, Meshnick SR. Epidemiology of drug-resistant malaria. Lancet Infect Dis. 2002;2:209–218. doi: 10.1016/s1473-3099(02)00239-6. [DOI] [PubMed] [Google Scholar]
- Le Bras J, Durand R. The mechanisms of resistance to antimalarial drugs in Plasmodium falciparum. Fundam Clin Pharmacol. 2003;17:147–153. doi: 10.1046/j.1472-8206.2003.00164.x. [DOI] [PubMed] [Google Scholar]
- Jelinek T, Rønn AM, Lemnge MM, Curtis J, Mhina J, Duraisingh MT, Bygbjerg IC, Warhurst DC. Polymorphisms in the dihydrofolate reductase (DHFR) and dihydropteroate synthetase (DHPS) genes of Plasmodium falciparum and in vivo resistance to sulphadoxine/pyrimethamine in isolates from Tanzania. Trop Med Int Health. 1998;3:605–609. doi: 10.1046/j.1365-3156.1998.00280.x. [DOI] [PubMed] [Google Scholar]
- Ngo T, Duraisingh M, Reed M, Hipgrave D, Biggs B, Cowman AF. Analysis of pfcrt, pfmdr1, dhfr, and dhps mutations and drug sensitivities in Plasmodium falciparum isolates from patients in Vietnam before and after treatment with artemisinin. Am J Trop Med Hyg. 2003;68:350–356. [PubMed] [Google Scholar]
- Mockenhaupt FP, Eggelte TA, Till H, Bienzle U. Plasmodium falciparum pfcrt and pfmdr1 polymorphisms are associated with the pfdhfr N108 pyrimethamine-resistance mutation in isolates from Ghana. Trop Med Int Health. 2001;6:749–755. doi: 10.1046/j.1365-3156.2001.00792.x. [DOI] [PubMed] [Google Scholar]
- Durand R, Jafari S, Bouchaud O, Ralaimazava P, Keundjian A, Le Bras J. Plasmodium falciparum: pfcrt and DHFR mutations are associated with failure of chloroquine plus proguanil prophylaxis in travelers. J Infect Dis. 2001;184:1633–1634. doi: 10.1086/324616. [DOI] [PubMed] [Google Scholar]
- Tarnchompoo B, Sirichaiwat C, Phupong W, Intaraudom C, Sirawaraporn W, Kamchonwongpaisan S, Vanichtanankul J, Thebtaranonth Y, Yuthavong Y. Development of 2,4-diaminopyrimidines as antimalarials based on inhibition of the S108N and C59R+S108N mutants of dihydrofolate reductase from pyrimethamine-resistant Plasmodium falciparum. J Med Chem. 2002;45:1244–1252. doi: 10.1021/jm010131q. [DOI] [PubMed] [Google Scholar]
- Sirawaraporn W, Sathitkul T, Sirawaraporn R, Yuthavong Y, Santi DV. Antifolate-resistant mutants of Plasmodium falciparum dihydrofolate reductase. Proc Natl Acad Sci USA. 1997;94:1124–1129. doi: 10.1073/pnas.94.4.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourier T, Pain A, Barrell B, Griffiths-Jones S. A selenocysteine tRNA and SECIS element in Plasmodium falciparum. RNA. 2005;11:119–122. doi: 10.1261/rna.7185605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nzila AM, Mberu EK, Sulo J, Dayo H, Winstanley PA, Sibley CH, Watkins WM. Towards an understanding of the mechanism of pyrimethamine-sulfadoxine resistance in Plasmodium falciparum: genotyping of dihydrofolate reductase and dihydropteroate synthase of Kenyan parasites. Antimicrob Agents Chemother. 2000;44:991–996. doi: 10.1128/aac.44.4.991-996.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kublin JG, Dzinjalamala FK, Kamwendo DD, Malkin EM, Cortese JF, Martino LM, Mukadam RA, Rogerson SJ, Lescano AG, Molyneux ME, Winstanley PA, Chimpeni P, Taylor TE, Plowe CV. Molecular markers for failure of sulfadoxine-pyrimethamine and chlorproguanil-dapsone treatment of Plasmodium falciparum malaria. J Infect Dis. 2002;185:380–188. doi: 10.1086/338566. [DOI] [PubMed] [Google Scholar]
- Bwijo B, Kaneko A, Takechi M, Zungu IL, Moriyama Y, Lum JK, Tsukahara T, Mita T, Takahashi N, Bergquist Y, Björkman A, Kobayakawa T. High prevalence of quintuple mutant dhps/dhfr genes in Plasmodium falciparum infections seven years after introduction of sulfadoxine and pyrimethamine as first line treatment in Malawi. Acta Trop. 2003;85:363–373. doi: 10.1016/s0001-706x(02)00264-4. [DOI] [PubMed] [Google Scholar]
- Appawu M, Owusu-Agyei S, Dadzie S, Asoala V, Anto F, Koram K, Rogers W, Nkrumah F, Hoffman SL, Fryauff DJ. Malaria transmission dynamics at a site in northern Ghana proposed for testing malaria vaccines. Trop Med Int Health. 2004;9:164–170. doi: 10.1046/j.1365-3156.2003.01162.x. [DOI] [PubMed] [Google Scholar]
- Marti HR. Hemoglobinopathies in developing countries. Wien Klin Wochenschr. 1984;96:535–539. [PubMed] [Google Scholar]
- Bhatt KM, Bhatt SM, Okello GB, Watkins WM. Chloroquine resistant Plasmodium falciparum malaria in a local Kenyan: a case report. East Afr Med J. 1984;61:745–747. [PubMed] [Google Scholar]
- Dormer P, Dietrich M, Kern P, Horstmann RD. Ineffective erythropoiesis in acute human P. falciparum malaria. Blut. 1983;46:279–288. doi: 10.1007/BF00319868. [DOI] [PubMed] [Google Scholar]
- Kyabayinze D, Cattamanchi A, Kamya MR, Rosenthal PJ, Dorsey G. Validation of a simplified method for using molecular markers to predict sulfadoxine-pyrimethamine treatment failure in African children with falciparum malaria. Am J Trop Med Hyg. 2003;69:247–252. [PubMed] [Google Scholar]
- Baruch DI, Ma XC, Pasloske B, Howard RJ, Miller LH. CD36 peptides that block cytoadherence define the CD36 binding region for Plasmodium falciparum-infected erythrocytes. Blood. 1999;94:2121–2127. [PubMed] [Google Scholar]
- Varandas L, Vandunem J, Benchimol C, Quinhentos V, Ferrinho P, Gonçalves L, Bernardino L. Tratamento efectivo na malária com moderada/alta parasitémia, em crianças, com uma toma diária de quinino em Luanda, Angola. Acta Med Angolana. 2007;16:15–20. [Google Scholar]
- Lopes D, Nogueira F, Gil JP, Ferreira C, do Rosario VE, Cravo P. pfcrt and pfmdr1 mutations and chloroquine resistance in Plasmodium falciparum from Sao Tome and Principe, West Africa. Ann Trop Med Parasitol. 2002;96:831–834. doi: 10.1179/000349802125002284. [DOI] [PubMed] [Google Scholar]
- Cravo P, Figueiredo S, Nogueira F, Lopes D, Ferreira ID, Ferreira C, Gil JP, do Rosario VE. High frequency of the genetic polymorphisms associated with sulfadoxine-pyrimethamine resistance, among Plasmodium falciparum isolates from Sao Tome and Principe, West Africa. Ann Trop Med Parasitol. 2004;98:293–296. doi: 10.1179/000349804225003262. [DOI] [PubMed] [Google Scholar]
- Guthmann JP, Ampuero J, Fortes F, van Overmeir C, Gaboulaud V, Tobback S, Dunand J, Saraiva N, Gillet P, Franco J, Denoncin A, van Herp M, Balkan S, Dujardin JC, D'Alessandro U, Legros D. Antimalarial efficacy of chloroquine, amodiaquine, sulfadoxine-pyrimethamine, and the combinations of amodiaquine + artesunate and sulfadoxine-pyrimethamine + artesunate in Huambo and Bie provinces, central Angola. Trans R Soc Trop Med Hyg. 2005;99:485–492. doi: 10.1016/j.trstmh.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Adagut IS, Warhurst DC. Plasmodium falciparum: linkage disequilibrium between loci in chromosomes 7 and 5 and chloroquine selective pressure in Northern Nigeria. Parasitology. 2001;123:219–224. doi: 10.1017/s0031182001008344. [DOI] [PubMed] [Google Scholar]
- Mockenhaupt FP, Eggelte TA, Till H, Bienzle U. Plasmodium falciparum pfcrt and pfmdr1 polymorphisms are associated with the pfdhfr N108 pyrimethamine-resistance mutation in isolates from Ghana. Trop Med Int Health. 2001;6:749–755. doi: 10.1046/j.1365-3156.2001.00792.x. [DOI] [PubMed] [Google Scholar]
- Sutherland CJ, Alloueche A, Curtis J, Drakeley CJ, Ord R, Duraisingh M, Greenwood BM, Pinder M, Warhurst D, Targett GA. Gambian children successfully treated with chloroquine can harbor and transmit Plasmodium falciparum gametocytes carrying resistance genes. Am J Trop Med Hyg. 2002;67:578–585. doi: 10.4269/ajtmh.2002.67.578. [DOI] [PubMed] [Google Scholar]
- Giha HA, Elbashir MI, A-Elbasit IE, A-Elgadir TM, ElGhazali GE, Mackinnon MJ, Babiker HA. Drug resistance-virulence relationship in Plasmodium falciparum causing severe malaria in an area of seasonal and unstable transmission. Acta Trop. 2006;97:181–187. doi: 10.1016/j.actatropica.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Aubouy A, Jafari S, Huart V, Migot-Nabias F, Mayombo J, Durand R, Bakary M, Le Bras J, Deloron P. DHFR and DHPS genotypes of Plasmodium falciparum isolates from Gabon correlate with in vitro activity of pyrimethamine and cycloguanil, but not with sulfadoxine-pyrimethamine treatment efficacy. J Antimicrob Chemother. 2003;52:43–49. doi: 10.1093/jac/dkg294. [DOI] [PubMed] [Google Scholar]
- Nsimba B, Jafari-Guemouri S, Malonga DA, Mouata AM, Kiori J, Louya F, Yocka D, Malanda M, Durand R, Le Brás J. Epidemiology of drug-resistant malaria in Republic of Congo: using molecular evidence for monitoring antimalarial drug resistance combined with assessment of antimalarial drug use. Trop Med Int Health. 2005;10:1030–1037. doi: 10.1111/j.1365-3156.2005.01490.x. [DOI] [PubMed] [Google Scholar]
- Osman ME, Mockenhaupt FP, Bienzle U, Elbashir MI, Giha HA. Field-based evidence for linkage of mutations associated with chloroquine (pfcrt/pfmdr1) and sulfadoxine-pyrimethamine (pfdhfr/pfdhps) resistance and for the fitness cost of multiple mutations in P. falciparum. Infect Genet Evol. 2007;7:52–59. doi: 10.1016/j.meegid.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Wang P, Lee CS, Bayoumi R, Djimde A, Doumbo O, Swedberg G, Dao LD, Mshinda H, Tanner M, Watkins WM, Sims PF, Hyde JE. Resistance to antifolates in Plasmodium falciparum monitored by sequence analysis of dihydropteroate synthetase and dihydrofolate reductase alleles in a large number of field samples of diverse origins. Mol Biochem Parasitol. 1997;89:161–177. doi: 10.1016/s0166-6851(97)00114-x. [DOI] [PubMed] [Google Scholar]
- Bwijo B, Kaneko A, Takechi M, Zungu IL, Moriyama Y, Lum JK, Tsukahara T, Mita T, Takahashi N, Bergqvist Y, Björkman A, Kobayakawa T. High prevalence of quintuple mutant dhps/dhfr genes in Plasmodium falciparum infections seven years after introduction of sulfadoxine and pyrimethamine as first line treatment in Malawi. Acta Trop. 2003;85:363–373. doi: 10.1016/s0001-706x(02)00264-4. [DOI] [PubMed] [Google Scholar]
- Mayor A, Serra-Casas E, Sanz S, Aponte JJ, Macete E, Mandomando I, Puyol L, Berzosa P, Dobaño C, Aide P, Sacarlal J, Benito A, Alonso P, Menéndez C. Molecular markers of resistance to sulfadoxine-pyrimethamine during intermittent preventive treatment for malaria in Mozambican infants. J Infect Dis. 2008;197:1737–1742. doi: 10.1086/588144. [DOI] [PubMed] [Google Scholar]
- Mbugi EV, Mutayoba BM, Malisa AL, Balthazary ST, Nyambo TB, Mshinda H. Drug resistance to sulphadoxine-pyrimethamine in Plasmodium falciparum malaria in Mlimba, Tanzania. Malar J. 2006;5:94. doi: 10.1186/1475-2875-5-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przyborski JM, Bartels K, Lanzer M, Andrews KT. The histone H4 gene of Plasmodium falciparum is developmentally transcribed in asexual parasites. Parasitol Res. 2003;90:387–389. doi: 10.1007/s00436-003-0874-x. [DOI] [PubMed] [Google Scholar]
- Mockenhaupt FP, Eggelte TA, Till H, Bienzle U. Plasmodium falciparum pfcrt and pfmdr1 polymorphisms are associated with the pfdhfr N108 pyrimethamine-resistance mutation in isolates from Ghana. Trop Med Int Health. 2001;6:749–755. doi: 10.1046/j.1365-3156.2001.00792.x. [DOI] [PubMed] [Google Scholar]
- Mockenhaupt FP, Ehrhardt S, Eggelte TA, Agana-Nsiire P, Stollberg K, Mathieu A, Markert M, Otchwemah RN, Bienzle U. Chloroquine-treatment failure in northern Ghana: roles of pfcrt T76 and pfmdr1 Y86. Ann Trop Med Parasitol. 2005;99:723–732. doi: 10.1179/136485905X75395. [DOI] [PubMed] [Google Scholar]
- Powers KG, Jacobs RL, Good WC, Koontz LC. Plasmodium vinckei: production of chloroquine-resistant strain. Exp Parasitol. 1969;26:193–202. doi: 10.1016/0014-4894(69)90112-x. [DOI] [PubMed] [Google Scholar]
- Basco LK, Ringwald P. Molecular epidemiology of malaria in Yaounde, Cameroon IV. Evolution of pyrimethamine resistance between 1994 and 1998. Am J Trop Med Hyg. 1999;61:802–806. doi: 10.4269/ajtmh.1999.61.802. [DOI] [PubMed] [Google Scholar]
- Ringwald P, Basco LK. Comparison of in vivo and in vitro tests of resistance in patients treated with chloroquine in Yaounde, Cameroon. Bull World Health Organ. 1999;77:34–43. [PMC free article] [PubMed] [Google Scholar]
- White NJ. Antimalarial drug resistance. J Clin Invest. 2004;113:1084–1092. doi: 10.1172/JCI21682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Read M, Sims PF, Hyde JE. Sulfadoxine resistance in the human malaria parasite Plasmodium falciparum is determined by mutations in dihydropteroate synthetase and an additional factor associated with folate utilization. Mol Microbiol. 1997;23:979–986. doi: 10.1046/j.1365-2958.1997.2821646.x. [DOI] [PubMed] [Google Scholar]
- Peterson DS, Walliker D, Wellems TE. Evidence that a point mutation in dihydrofolate reductase-thymidylate synthase confers resistance to pyrimethamine in falciparum malaria. Proc Natl Acad Sci USA. 1988;85:9114–118. doi: 10.1073/pnas.85.23.9114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidock DA, Nomura T, Talley AK, Cooper RA, Dzekunov SM, Ferdig MT, Ursos LM, Sidhu AB, naudé B, Deitsch KW, Su XZ, Wootton JC, Roppe PD, Wellems TE. Mutations in the P. falciparum digestive vacuole transmembrane protein PfCRT and evidence for their role in chloroquine resistance. Mol Cell. 2000;6:861–871. doi: 10.1016/s1097-2765(05)00077-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathod PK, McErlean T, Lee PC. Variations in frequencies of drug resistance in Plasmodium falciparum. Proc Natl Acad Sci USA. 1997;94:9389–9393. doi: 10.1073/pnas.94.17.9389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings IM, Donnelly MJ. The impact of antimalarial drug resistance mutations on parasite fitness, and its implications for the evolution of resistance. Drug Resist Updat. 2005;8:43–50. doi: 10.1016/j.drup.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Walliker D, Hunt P, Babiker H. Fitness of drug-resistant malaria parasites. Acta Trop. 2005;94:251–259. doi: 10.1016/j.actatropica.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Hallett RL, Sutherland CJ, Alexander N, Ord R, Jawara M, Drakeley CJ, inder M, Walraven G, Targett GA, Alloueche A. Combination therapy counteracts the enhanced transmission of drug-resistant malaria parasites to mosquitoes. Antimicrob Agents Chemother. 2004;48:3940–3943. doi: 10.1128/AAC.48.10.3940-3943.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]