Abstract
In this issue of Developmental Cell, Rane et al. report a cellular pathway to link PrPSc, via ER stress and the activation of a preemptive quality control process, to neurodegeneration in a PrP-dependent manner. This pathway puts together several pieces in the puzzle of the relationship between PrPSc and brain damage and may in part explain the mechanism of prion neurodegeneration.
Transmissible spongiform encephalopathies (TSEs), also known as prion disorders, are a group of fatal and infectious neurodegenerative diseases. The central event in TSEs is the misfolding, aggregation, and brain accumulation of the prion protein. The misfolded form of the prion protein (termed PrPSc) is not only the typical pathological feature of the disease and a possible triggering event in the pathogenesis, but it is also the major (and perhaps the sole) component of the infectious agent.
Despite the impressive knowledge about the characteristics of the TSE infectious agent and its mechanism of replication, the association of PrPSc and neurodegeneration and the molecular pathways leading to cerebral damage are for the most part unknown. The most accepted idea is that misfolding and aggregation result in a gain of toxic activity of PrPSc. The initial model, that PrPSc aggregates cause direct neurotoxicity, seemed reasonable considering that other, more prevalent neurodegenerative disorders such as Alzheimer’s and Parkinson’s diseases are also characterized by cerebral accumulation of misfolded protein aggregates (Soto, 2003). Although purified PrPSc was shown to be neurotoxic at very low concentrations, a number of observations argue that this model is an oversimplification. Indeed, a discrepancy between the amount of PrPSc aggregates and the extent of brain damage and disease has been reported in various experiments (Piccardo et al., 2007, and references therein). Furthermore, in studies with postnatal PrP knockout animals, it was observed that depletion of PrPC in mice with established prion infection reversed early spongiform degeneration and prevented neuronal loss and progression to clinical disease (Mallucci et al., 2003). Importantly, this occurred despite the accumulation of extraneuronal PrPSc to levels similar to terminally ill, wild-type infected animals (Mallucci et al., 2003). These data suggested that PrPSc might not be directly responsible for neurodegeneration and that expression of PrPC plays an essential role in this process.
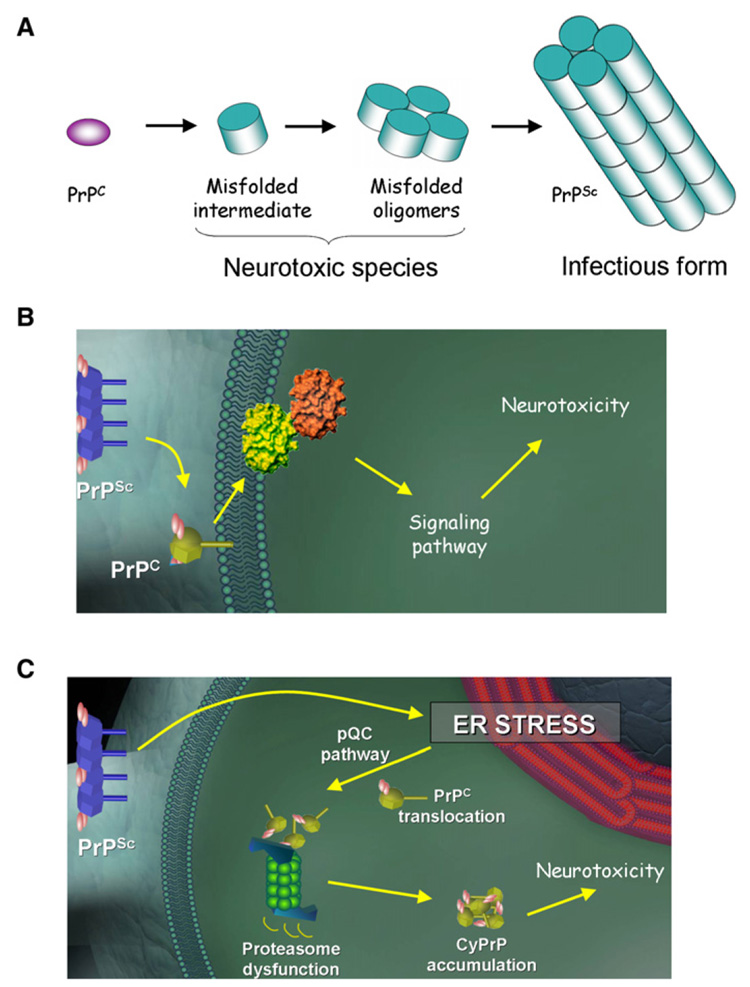
At least two models can explain these discrepancies (Figure 1): first, the neurotoxic species may not be mature aggregated PrPSc, but a cell-associated and transient oligomeric intermediate that requires PrPC expression for its continuous formation (Figure 1A). This model is appealing, as a similar paradigm change is occurring in other disorders associated with misfolding and aggregation of proteins. Indeed, studies from Alzheimer’s, Parkinson’s, and other misfolding disorders have provided compelling evidence that small (difficult to detect) oligomers appear to be the culprit in causing brain damage (Caughey and Lansbury, 2003). A second possibility is that PrPSc interacts with cells through its binding to PrPC, triggering a signal transduction pathway that leads to neuronal damage (Figure 1B). This hypothesis is supported by the fact that the normal and pathological forms of PrP bind tightly in the context of the cell membrane. Furthermore, PrPC-antibody cross-linking experiments have shown the induction of a cellular signaling pathway, resulting in neuronal dysfunction (Solforosi et al., 2004).
Figure 1. Role of PrPSc and Mechanism of Neurodegeneration.
Although PrPSc is certainly associated to the pathogenesis of TSEs, its role is neurodegeneration is controversial. Several observations suggest that PrPSc levels do not correlate with the extent of brain damage and that the natively folded PrPC is required for neurodegeneration. Three non-mutually exclusive models can be proposed to explain the discrepancies and the implication of PrPC.
(A) First, the infectious and neurotoxic PrP species might not be the same. Indeed, it is possible that an undetectable misfolded intermediate might be responsible for neurotoxicity in a way similar to that proposed for other neurodegenerative diseases. The transient nature of these intermediates determines that its presence depends on the permanent synthesis of new PrPC.
(B) PrPC located in the cell surface may act as a receptor for PrPSc, triggering a signal transduction pathway leading to neurodegeneration.
(C) As proposed by Rane and colleagues in this issue (Rane et al., 2008), induction of ER stress by PrPSc may lead to translocation of nascent PrPC molecules to the cytosol for proteasomal degradation as a way to alleviate the damaged ER (pQC pathway). However, this mechanism of defense turns negative under chronic ER stress conditions, overwhelming the proteasome and leading to the cytosolic accumulation of potentially toxic PrP molecules.
In the current issue of Developmental Cell, Rane and colleagues report an interesting third possibility (Figure 1C) (Rane et al., 2008). The authors’ hypothesis, supported by a set of elegant experiments, is that TSE neurodegeneration might be dependent on the chronic endoplasmic reticulum (ER) stress produced by PrPSc accumulation, which in turn lead to persistent activation of a “preemptive” quality control system (pQC) that aborts the ER translocation of PrP, allowing its proteasome-mediated degradation in the cytosol. This pathway constitutes a defense mechanism to prevent nascent protein entry into the ER lumen during conditions of compromised ER function. However, under chronic ER stress conditions, the proteasome may become overwhelmed, resulting in PrP accumulation in the cytosol. It has been shown in cell culture experiments that cytosolic accumulation of PrP (cyPrP) produced by pharmacological inhibition of the proteasome lead to PrPSc-like formation and neurotoxicity (Ma et al., 2002). Furthermore, artificial expression of PrP in the cytosol in transgenic animals expressing a construct lacking ER-targeting and GPI-anchoring signals cause severe neurodegeneration (Ma et al., 2002). To test their hypothesis, Rane and coworkers created transgenic mice with a greater or lower proportion of PrP routed to the pQC pathway, independent of PrPSc accumulation or ER stress. The results showed that even a modest increase in PrP routing to pQC degradation for prolonged periods of time caused neurodegenerative changes (clinical and histological) that partially resemble those observed in prion diseases (Rane et al., 2008). These results provide a plausible model for PrPSc cell-type selective neurotoxicity in a PrPC-dependent pathway, implicating ER stress and changes of protein trafficking. The findings also suggest that pharmacological agents able to reduce ER stress or the pQC pathway might have potential for the treatment of prion diseases. However, both mild ER stress and the pQC pathway can be beneficial. Indeed, it has been shown that the first response to ER stress is the launching of the unfolded protein response (UPR), resulting in the upregulation of various molecular chaperones and decreased general protein synthesis (Hetz and Soto, 2006). Also, as Rane and coworkers pointed out, the ability of PrP to be delivered to the pQC pathway appears to have evolved for avoiding its misfolding under ER stress conditions. Therefore, it seems that the pathogenic mechanisms in prion diseases may be the result of overwhelming a normally beneficial quality control defense mechanism.
Although the authors’ hypothesis is appealing and the role of ER stress in prion diseases is well documented (Hetz and Soto, 2006), the relevance or even the existence of cyPrP is highly controversial (Fioriti et al., 2005). Furthermore, the transgenic mice with increased PrP translocation to the pQC pathway reported in this study show a relatively mild neurodegenerative phenotype that recapitulates only a subset of TSE pathology (Rane et al., 2008). Thus, as the authors acknowledge, it is likely that other cellular pathways are also contributing to prion-induced neurodegeneration. Finally, an intriguing question not yet explored is whether altered PrP translocation leading to neurodegeneration might be implicated in other brain diseases in which ER stress occurs. Indeed, extensive reports have shown sustained ER stress in several neurodegenerative diseases associated to the accumulation of misfolded aggregated proteins (Lindholm et al., 2006). It could be interesting to assess whether under these PrPSc-unrelated ER stress conditions, nascent PrP is also routed to the pQC pathway, leading to proteasomal dysfunction and brain degeneration. It would also be interesting to study whether other proteins besides PrP are abnormally translocated and delivered to the pQC pathway during ER stress and their potential contribution to brain degeneration in TSEs and other neurodegenerative diseases.
ACKNOWLEDGMENTS
This work is supported in part by grant R01 NS050349 from NIH to C.S.
REFERENCES
- Caughey B, Lansbury PT. Annu. Rev. Neurosci. 2003;26:267–298. doi: 10.1146/annurev.neuro.26.010302.081142. [DOI] [PubMed] [Google Scholar]
- Fioriti L, Dossena S, Stewart LR, Stewart RS, Harris DA, Forloni G, Chiesa R. J. Biol. Chem. 2005;280:11320–11328. doi: 10.1074/jbc.M412441200. [DOI] [PubMed] [Google Scholar]
- Hetz CA, Soto C. Curr. Mol. Med. 2006;6:37–43. doi: 10.2174/156652406775574578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindholm D, Wootz H, Korhonen L. Cell Death Differ. 2006;13:385–392. doi: 10.1038/sj.cdd.4401778. [DOI] [PubMed] [Google Scholar]
- Ma J, Wollmann R, Lindquist S. Science. 2002;298:1781–1785. doi: 10.1126/science.1073725. [DOI] [PubMed] [Google Scholar]
- Mallucci G, Dickinson A, Linehan J, Klohn PC, Brandner S, Collinge J. Science. 2003;302:871–874. doi: 10.1126/science.1090187. [DOI] [PubMed] [Google Scholar]
- Piccardo P, Manson JC, King D, Ghetti B, Barron RM. Proc. Natl. Acad. Sci. USA. 2007;104:4712–4717. doi: 10.1073/pnas.0609241104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rane NS, Kang S-W, Chakrabarti O, Feigenbaum L, Hegde RS. Dev. Cell. 2008;15:359–370. doi: 10.1016/j.devcel.2008.06.015. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solforosi L, Criado JR, McGavern DB, Wirz S, Sanchez-Alavez M, Sugama S, DeGiorgio LA, Volpe BT, Wiseman E, Abalos G, et al. Science. 2004;303:1514–1516. doi: 10.1126/science.1094273. [DOI] [PubMed] [Google Scholar]
- Soto C. Nat. Rev. Neurosci. 2003;4:49–60. doi: 10.1038/nrn1007. [DOI] [PubMed] [Google Scholar]