Abstract
OBJECTIVES
To test whether patients with coronary atherosclerosis have increases in circulating endothelial progenitor cells (EPCs) expressing an osteogenic phenotype.
BACKGROUND
Increasing evidence indicates a link between bone and the vasculature, and bone marrow and circulating osteogenic cells have been identified by staining for the osteoblastic marker, osteocalcin (OCN). EPCs contribute to vascular repair, but repair of vascular injury may result in calcification. Using cell surface markers (CD34, CD133, KDR) to identify EPCs, we examined whether patients with coronary atherosclerosis had increases in the percentage of EPCs expressing OCN.
METHODS
We studied 72 patients undergoing invasive coronary assessment: Controls (normal coronary arteries and no endothelial dysfunction, n = 21) versus two groups with coronary atherosclerosis: early coronary atherosclerosis (ECA: normal coronary arteries but with endothelial dysfunction, n = 22) and late coronary atherosclerosis (LCA: severe, multi-vessel coronary artery disease [CAD], n = 29). Peripheral blood mononuclear cells were analyzed using flow cytometry.
RESULTS
Compared to controls, patients with ECA or LCA had significant increases (~2-fold) in the percentage of CD34+/KDR+ and CD34+/CD133+/KDR+ cells co-staining for OCN; even larger increases were noted in the ECA and LCA patients in the percentage of CD34+/CD133−/KDR+ cells co-staining for OCN (5- and 2-fold, P < 0.001 and 0.05, respectively).
CONCLUSIONS
A higher percentage of EPCs express OCN in patients with coronary atherosclerosis compared with subjects with normal endothelial function and no structural CAD. These findings have potential implications for the mechanisms of vascular calcification and for the development of novel markers for coronary atherosclerosis.
Keywords: calcification, bone, atherosclerosis
Introduction
There is increasing evidence for a link between bone metabolism and the vasculature. Thus, elderly women with osteoporosis (1) or with high bone turnover (2) have increased cardiovascular mortality. In addition, mice with deletion of osteoprotegerin (OPG), which binds and neutralizes the key osteoclastogenic factor, receptor activator of NFκB ligand (RANKL), not only have severe osteoporosis and a marked increase in bone turnover but also diffuse vascular calcification (3). Moreover, it is now recognized that vascular calcification is not simply a dystrophic calcifying process but rather involves active bone formation that recapitulates the normal sequence of osteoblast development and activity on bone surfaces (4,5). These findings collectively suggest that changes in bone turnover affect the vasculature, although the possible mechanism(s) of this are unclear.
A potential candidate for providing a link between bone metabolism and the vasculature are endothelial progenitor cells (EPCs) which reside, at least in part, in bone marrow, are mobilized in response to vascular injury, and contribute to vascular repair (6). While there is ongoing controversy regarding the true identity of EPCs (6), cell surface expression of CD34, CD133, and vascular endothelial growth factor receptor 2/kinase insert domain receptor (KDR) have been used to define populations of circulating cells that include cells participating in the response to vascular injury (6,7). In addition, recent work has suggested that expression of these markers may be used to classify CD34+ cells as less differentiated (CD34+/CD133+/KDR+) vs. relatively more differentiated (CD34+/CD133−/KDR+) EPCs (6–8).
In addition to EPCs, osteoprogenitor cells also reside in the bone marrow, and studies by Friedenstein and colleagues (9) established almost 40 years ago that the bone marrow stroma contains plastic adherent cells that can give rise to a broad spectrum of fully differentiated connective tissues, including osteoblasts. Concurrent with this work, however, Long and colleagues identified, over a decade ago, a non-adherent population of cells in bone marrow with osteogenic potential (10,11). These investigators used flow cytometry and magnetic activated cell sorting with an anti-osteocalcin (OCN) antibody to isolate osteoprogenitor cells that expressed bone-related proteins and were capable of mineralization in vitro (10–13). Reasoning that these non-adherent osteogenic cells likely accessed the peripheral circulation, our group used identical methods to characterize OCN+ cells in peripheral blood that expressed osteoblastic markers and were capable of forming mineralized nodules in vitro and bone in vivo (14,15).
While endothelial and osteoblastic cells have traditionally been believed to derive from distinct progenitor populations, there is increasing evidence for overlap between these lineages. In fact, the Stro-1 antibody, which is widely used to identify mesenchymal stem cells in bone marrow and other tissues, was originally derived by immunizing mice with purified human CD34+ cells (16). Moreover, human bone marrow CD34+ cells can differentiate into osteoblastic cells capable of forming mineralized nodules in vitro (17) and Tondreau et al. have found that CD133+ cells from human bone marrow, umbilical cord blood, or peripheral blood from G-CSF-treated donors are enriched for mesenchymal stem cells capable of differentiating into osteoblasts (18). Recent studies using single cell reverse-transcriptase polymerase chain reaction have also demonstrated that ~20% of human peripheral blood CD34+ cells express the mRNA for OCN (19). In addition, when infused into immunocompromised rats following femur fractures, these cells can localize to the fracture site and differentiate into endothelial cells as well as osteoblasts and enhance fracture healing (19).
Given this potential overlap between endothelial and osteoblastic lineages, in the current study we tested the hypothesis that there are an increased percentage of circulating EPCs expressing an osteogenic phenotype, as assessed by staining for OCN (10–15), in patients with early or established coronary atherosclerosis. Our findings suggest a novel link between bone metabolism and the vasculature, and may also help define new diagnostic approaches for coronary atherosclerosis.
Methods
Study subjects
The study was approved by the Institutional Review Board of Mayo Foundation and all study subjects provided written, informed consent. A total of 72 patients who were undergoing coronary angiography and coronary endothelial function testing for clinical purposes, and met the criteria outlined below for inclusion into the 3 study groups, were recruited. Patients with acute coronary syndromes (unstable angina or acute myocardial infarction), heart failure (ejection fraction < 50%), or severe renal or liver disease were excluded. The Framingham risk score was calculated as previously described (20). The 3 groups were defined as follows: control, patients without significant structural coronary lesions on angiography (< 30% stenosis) and normal endothelial function, as assessed by intra-coronary acetylcholine challenge (see below), n = 21; early coronary atherosclerosis (ECA), defined by the absence of significant structural coronary lesions on angiography but with abnormal endothelial function, n = 22; and late coronary atherosclerosis (LCA), defined as patients with severe, multi-vessel CAD (> 50% stenosis in 2 or more major epicardial arteries), n = 29.
Coronary angiography and invasive endothelial function testing
Patients underwent coronary angiography using standard clinical protocols. Arterial blood (20 mL) was drawn from the aortic catheter prior to cardiac catheterization for flow cytometry and biochemical analyses. After diagnostic angiography, subjects without significant structural coronary lesions underwent assessment of endothelium-dependent coronary vasoreactivity, as previously described (21–24). In brief, a Doppler guidewire (Flowire, Volcano Inc) within a coronary-infusion catheter (Ultrafuse, SciMed Life System) was positioned into the mid-portion of the left anterior descending coronary artery. Acetylcholine at increasing concentrations was infused into the left anterior descending coronary artery to assess endothelium-dependent vasoreactivity. Hemodynamic data, Doppler measurements, and a coronary angiogram were obtained after each infusion. Coronary artery diameter was measured by an independent investigator in the segment 5 mm distal to the tip of the Doppler wire using a computer-based image analysis system. Average peak velocity (APV) was derived from the Doppler flow velocity spectra and coronary blood flow (CBF) was determined as π(coronary artery diameter/2)2 X (APV/2). As previously described, microvascular endothelial dysfunction was defined as an increase in CBF of < 50% and epicardial endothelial dysfunction was defined as a decrease in epicardial coronary artery diameter of more than 20% in response to the maximal dose of acetylcholine (10−4 M) (21–24). Endothelium-independent microvascular function was determined by the coronary flow reserve (CFR), which is the ratio of the APV at maximal hyperemia (induced by intracoronary adenosine [18–60 ug]) to the APV at baseline.
Flow cytometry
Peripheral blood mononuclear cells (PBMNCs) were isolated using a Ficoll density gradient, and immunofluorescent cell staining was performed using the following fluorescent conjugated antibodies: CD34-PerCP Cy 5.5 (Beckton-Dickinson), CD133-phycoerythrin (PE) (Miltenyi Biotec GmbH) and kinase insert domain receptor KDRAPC (R & D Systems) and the appropriate isotype controls. In addition, OCN+ cells were identified using an anti-human OCN antibody (Santa Cruz Biotechnology) and a fluorescein isothiocyanate (FITC) secondary antibody (Jackson ImmunoResearch), as previously described (14,15). Cell fluorescence was measured immediately after staining (Becton Dickinson, FACS Calibur) and data were analyzed using CellQuest software (Becton Dickinson).
Confocal microscopy
For confocal microscopy, cells were stained for OCN-FITC and CD34-PerCP Cy 5.5 and isolated by magnetic activated cell sorting (MACS) (AutoMACS™, Miltenyi Biotec GmbH) with the appropriate magnetic beads. The cells were then co-stained with the CD133-PE and KDR-APC antibodies. Following fixation, the cells were mounted in ProLong® Gold antifade reagent with DAPI (Invitrogen) to stain the nucleus. Samples were then examined on an LSM510 confocal laser scanning microscope (Carl Zeiss, Inc).
Cell Culture
CD34+ cells isolated by MACS or human bone marrow stromal cells (hMSCs, Lonza) were cultured in uncoated plastic culture plates in regular culture media [αMEM, 10% heat-inactivated FBS, 1% penicillin/streptomycin/amphotericin B (all from GIBCO/Invitrogen Corporation)] containing an additional 5 mM Ca2+ for the first 5 days. Subsequently, the medium was changed to osteoblast differentiation medium [αMEM, 10% heat-inactivated FBS, 1% penicillin/streptomycin/amphotericin B plus 50 µg/ml ascorbic acid phosphate magnesium salt n-Hydrate (Wako), 10 mM β-glycerophosphate, 10−8 M 1,25-dihydroxyvitamin D3, 10−8 M dexamethasone (Sigma)] for an additional 7 days. The mineralization assay was done using MACS-sorted CD34+ cells and unsorted post-Ficoll PBMNCs, which were cultured in uncoated plastic culture plates in regular culture media for 2 days and than were either maintained in regular culture medium or were changed to regular culture medium containing an additional 2.1 mM Ca2+ for 5 days (days 3–7) of culture. Subsequently, the Ca2+ was removed and the cells were cultured in osteoblast differentiation medium (as described before) for an additional 21 days (days 8–28) and the presence of mineralization determined by Alizarin Red and Von Kossa staining.
Assessment of gene expression in CD34+ cells
CD34+ cells were isolated using MACS, as described above. Total RNA from CD34+ cells and undifferentiated human bone marrow stromal cells (hMSCs, Lonza) was isolated using spin columns (Micro columns, Qiagen) followed by a DNase digestion. Because the overall number of the CD34+ cells was limited and therefore the yield of total RNA was low, we used the WT-Ovation™ Pico RNA amplification system (NuGEN, Technologies, Inc) to synthesize micrograms quantities of amplified cDNA starting with total RNA input amounts of 50 ng for all the samples. In this linear amplification system, the relative representation of each transcript species in the original sample is maintained during and after amplification. The pre-amplified cDNA was then used in quantitative polymerase chain reaction (QPCR) analyses using primer pairs for a panel of bone- and stem cell-related genes (Table 1). Normalization of the samples was performed using the geNorm method (25,26), which determines the most stable housekeeping genes within the experiment. Further analysis was performed by calculating the difference in cycle threshold (ΔCT) values between the sample and the geometric mean CT of geNorm selected housekeeping genes. The expression level for each individual gene was determined by 2−ΔCT.
Table 1.
Gene identification and primer sequences
| Gene | ACC number | Forward Primer | Reverse Primer |
|---|---|---|---|
| Runx2 | NM_001024630F | CCAGATGGGACTGTGGTTACTG | TTCCGGAGCTCAGCAGAATAA |
| β-catenin | NM_001904F | GGGTCCGCATGGAAGAAATAG | TGTGAACATCCCGAGCTAGGA |
| BMP2 | NM_001200F | GCCCTTTTCCTCTGGCTGAT | TTGACCAACGTCTGAACAATGG |
| Msx-2 | NM_002449F | AGACTGCAGGAGGCAGAACTG | GGAGGGCAGCATAGGTTTTG |
| Notch1 | NM_017617F | CACCAGTTTGAATGGTCAATGC | CCGCAGAGGGTTGTATTGGTT |
| OCN | NM_199173F | TGTGAGCTCAATCCGGACTGT | CCGATAGGCCTCCTGAAAGC |
| Alkaline Phosphatase | NM_000478F | TCGTTGACACCTGGAAGAGCTT | CGTGCGGTTCCAGATGAAGT |
| Osteonectin | NM_003118F | CTACATCGGGCCTTGCAAAT | GGGAATTCGGTCAGCTCAGA |
| Osteopontin | NM_00104058F | TGAGCATTCCGATGTGATTGA | TGTGGAATTCACGGCTGACTT |
| MGP | NM_000900F | AGTCCAAGAGAGGATCCGAGAA | GCGTTCGCAAAGTCTGTAGTCA |
| Col1α1 | NM_000088F | TACCCCACTCAGCCCAGTGT | ACCAGACATGCCTCTTGTCCTT |
| Col1α2 | NM_000089F | GCTACCCAACTTGCCTTCATG | TGCAGTGGTAGGTGATGTTCTGA |
| CD44 | NM_000610F | GATCATCTTGGCATCCCTCTTG | CGACTGTTGACTGCAATGCAA |
| Nanog | NM_024865F | AAAGGCAAACAACCCACTTCTG | TTCTTGACCGGGACCTTGTC |
| HIF1-α | NM_017902F | ACGTTCCTTCGATCAGTTGTCA | TTTGAGGACTTGCGCTTTC |
| CXCR4 | NM_001008540F | TCATCCTCCTGGAAATCATCAA | TGGAAATCCACTTGTGCACAGT |
| CD45 | NM_002838F | TGTCATGGTCACTCGATGTGAA | CCATTGACGGCCAGTATTCTG |
| CD34 | NM_001025109F | TTCACTGAGCAAGATGTTGCAA | ACCAGTGCAATCAGGGTCTTTT |
Biochemical assays
Serum lipids were measured using enzymatic colorimetry and LDL cholesterol calculated from these parameters. High sensitivity C-reactive protein (hs-CRP) levels were measured using a latex particle-enhanced immunoturbidemetric assay on a Hitachi 912 automated analyzer. Serum creatinine was measured using an enzymatic colorimetic assay (Roche Diagnostics) and glomerular filtration rate (GFR) was estimated using the IDMS-traceable Modification of Diet in Renal Disease (MDRD) Study equation [eGFR (mL/min/1.73 m2 = 175 × serum creatinine (−1.154) × age (−0.203) × (0.742 if female) × (1.210 if African American)].
RANKL (receptor activator of nuclear factor κB ligand) was measured using the ampli-sRANKL ELISA (ALPCO Diagnostics). Ampli-sRANKL has increased sensitivity compared to standard ELISA-kits, since it uses a NADPH-based enhancement system. Osteoprotegerin (OPG) and matrix gla protein (MGP) were also measured using ELISAs (ALPCO Diagnostics). The inter-assay CVs for the RANKL, OPG, and MGPs ELISAs were 6%, 8%, and 9%, respectively.
Statistical analyses
Pre-specified comparisons between the ECA or LCA patients and control subjects were made using the Wilcoxon rank-sum test for continuous variables and the Fisher’s Exact test for categorical variables. Since some of these data were not normally distributed, they are presented as median (inter-quartile [25th-75th percentile] range, IQR). Spearman correlations were used to describe relationships between the cell populations with circulating RANKL, OPG, and MGP levels. Logistic regression models following log (base 2) transformation, where appropriate, of the CD34+/CD133−/KDR+/OCN+ and the percentage of CD34+/CD133−/KDR+ cells co-staining for OCN or of hs-CRP were used to assess the ability of these variables to predict early coronary atherosclerosis (ECA) following adjustment for age and sex. Model assumptions and conclusions were checked. Receiver-operator characteristic (ROC) curve analysis was used to describe the ability of the CD34+/CD133−/KDR+/OCN+ and the percentage of CD34+/CD133−/KDR+ cells co-staining for OCN or of hs-CRP to predict early coronary atherosclerosis (ECA), and the areas under the ROCs were compared using a non-parametric test (27). The gene expression data are presented as mean ± SEM. For comparison with hMSCs, the expression of each gene was normalized to the level of expression in the hMSCs and compared to 1.0 using a one-sample t-test. Differences between freshly isolated and cultured CD34+ cells were analyzed using a two-sample t-test. P-values less than 0.05 were considered statistically significant.
Results
Patient characteristics
The relevant clinical and biochemical data as well as the detailed cardiac catheterization data in the study subjects are shown in Table 2. Subjects with LCA were significantly older than the control subjects, and were predominantly male. However, subjects with ECA had an age and gender mix not significantly different from the control subjects. BMI was similar across groups, but a higher percentage of the LCA subjects were on statins as compared to control subjects. The prevalence of diabetes, hypertension, and smoking was virtually identical in the control and ECA subjects, but higher for all three parameters in the LCA compared with the control subjects. There was no significant difference in the Framingham score between the subjects with ECA and control subjects and significantly higher in the subjects with LCA as compared to the control subjects. Blood lipids were similar in the ECA subjects compared to the control subjects, with the LCA subjects having lower total and HDL cholesterol levels as compared to the control subjects. There was a non-significant trend for hs-CRP levels to be higher in the ECA as compared to control subjects; by contrast, patients with LCA had hs-CRP levels that were significantly lower than control subjects. Since statin therapy is known to reduce CRP levels (28), the lower hs-CRP levels in the LCA patients were likely related to the fact that most of these patients were on statin therapy. Overall, therefore, while there were significant differences in the clinical characteristics of the LCA patients compared to subjects with normal coronary endothelial function, the patients with coronary endothelial dysfunction had clinical and biochemical features that were virtually identical to the control subjects.
Table 2. Clinical, biochemical, and cardiac catheterization data of the study subjects.
ECA, early coronary atherosclerosis; LCA, late coronary atherosclerosis. Data are median (IQR).
| Control | ECA | LCA | |
|---|---|---|---|
| N | 21 | 22 | 29 |
| Clinical data | |||
| Age, years | 45 (42, 52) | 50 (42, 60) | 72 (63, 80)*** |
| Male, n (%) | 5 (24) | 9 (41) | 26 (90)*** |
| BMI, kg/m2 | 28 (24, 33) | 32 (25, 38) | 29 (27, 33) |
| Statin use, n (%) | 9 (43) | 13 (59) | 24 (83)** |
| Diabetes, n (%) | 0 (0) | 0 (0) | 7 (24) * |
| Hypertension, n (%) | 9 (43) | 9 (41) | 23 (79)* |
| Smoking, n (%) | 4 (19) | 6 (27) | 18 (62)** |
| Framingham score | 1.0 (−2.0, 5.0) | 2.5 (−1.2, 6.0) | 7.0 (5.0, 9.0)*** |
| Biochemical data | |||
| Total cholesterol, mg/dL | 187 (175, 206) | 188 (149, 227) | 154 (129, 184)** |
| HDL cholesterol, mg/dL | 55 (48, 67) | 56 (44, 72) | 45 (40, 53)** |
| Triglycerides, mg/dL | 109 (78, 168) | 94 (69, 132) | 105 (88, 169) |
| LDL cholesterol, mg/dL | 106 (98, 120) | 106 (77, 133) | 78 (58, 103)* |
| hs-CRP, mg/L | 1.2 (0.5, 4.4) | 1.6 (1.2, 3.8) | 0.6 (0.1, 1.4) * |
| Creatinine, mg/dL | 0.8 (0.8, 1.0) | 0.9 (0.8, 1.1) | 1.1 (0.9, 1.2) *** |
| Estimated GFR, mL/min | 83 (70, 95) | 76 (69, 85) | 69 (62, 86) * |
| Cardiac catheterization data | |||
| Mean coronary artery stenosis based on all angiographically identified stenoses, %) | 10 (0, 20) | 18 (0, 23) | 70 (60, 78)*** |
| Mean coronary artery stenosis based on coronary arteries with >50% stenosis, % | ND | ND | 90 (86, 94) |
| Microvascular endothelial function (Δcoronary blood flow to 10−4 M acetylcholine, %) | 121.4 (62.0, 191.1) | −19.1 (−47.7, 17.0)*** | ND |
| Epicardial endothelial function (Δcoronary diameter to 10−4 M acetylcholine, %) | −0.4 (−9.4, 2.5) | −36.1 (−48.6, −19.4)*** | ND |
| Endothelium−independent microvascular function (coronary flow reserve) | 2.7 (2.5, 3.4) | 2.9 (2.4, 3.4) | ND |
ND, not determined
P < 0.05
P < 0.01
P < 0.001 vs. Control
Table 2 also shows the detailed cardiac catheterization data in the study subjects. Based on the definition of the groups, the ECA subjects did not have significant structural coronary artery disease, whereas the LCA patients had a mean coronary artery stenosis of 90%, when calculated based on coronary arteries with at least a 50% stenosis (29,30). As expected, parameters of epicardial and microvascular endothelial function were significantly different in the ECA as compared to the control subjects.
Flow cytometry
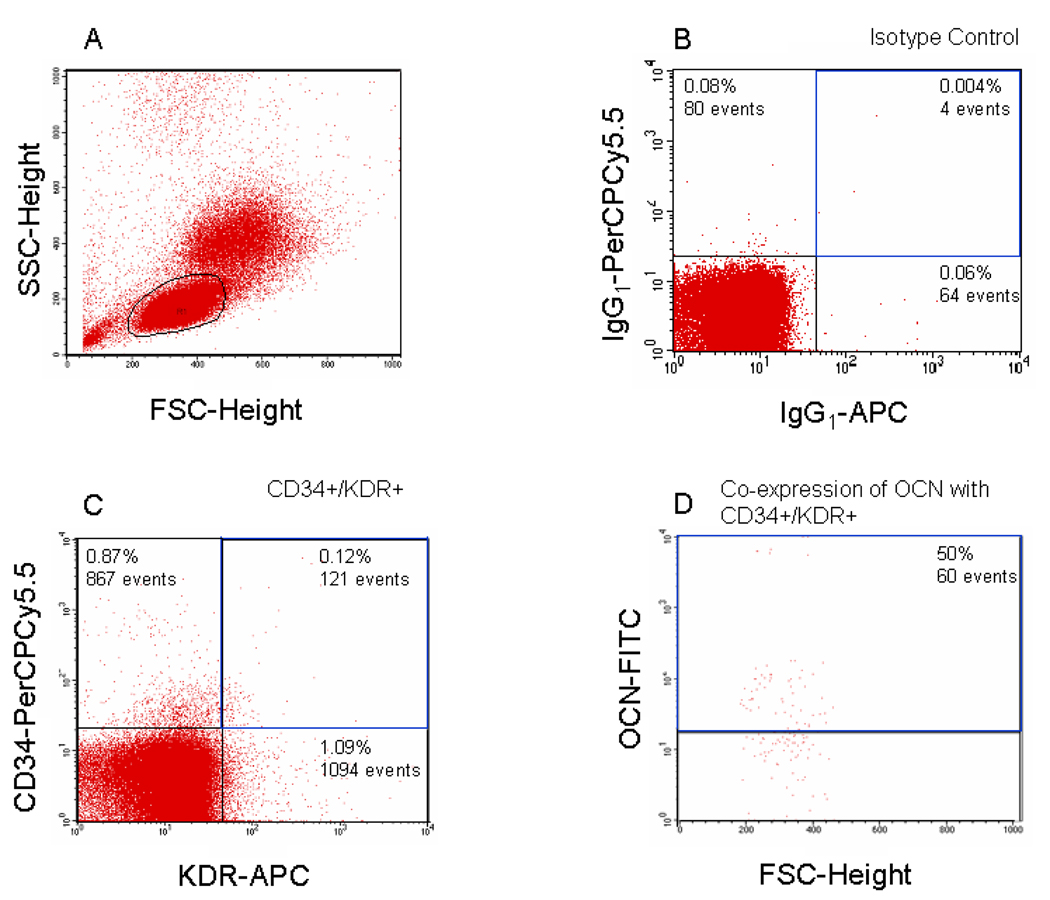
We performed flow cytometry using antibodies to the EPC markers and OCN, as described in detail in the Methods, and Figure 1 shows a representative example of the flow analysis for CD34, KDR, and OCN. In preliminary experiments, we determined that the vast majority of the CD34+/KDR+ cells were in the small lymphocyte gate (Figure 1A), as has also been noted by other investigators (29,31). Figure 1B and C show the flow cytometry analysis for CD34+/KDR+ cells and Figures 1D shows the further analysis of these cells with the OCN antibody.
Figure 1. Example of flow analysis.
Panel A shows the forward/side scatter and the gates used in the analysis. Panel B shows the staining with the isotype controls for the CD34- and KDR-specific antibodies and panel C shows the specific staining with these antibodies. Panel D shows the CD34/KDR+ cells stained with the OCN antibody. We required that < 0.5% of the cells were positive with the isotype controls, and for all data reported in the paper, we subtracted the signal of the isotype from the signal due to the specific antibodies.
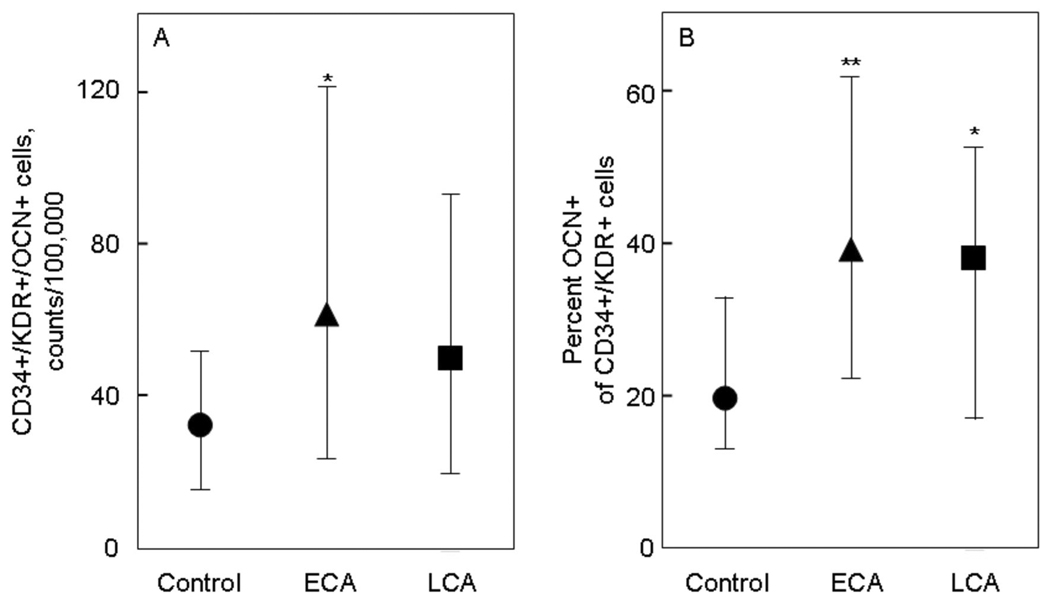
Figure 2 shows the analysis of peripheral blood mononuclear cells for the absolute counts of CD34+/KDR+/OCN+ cells (per 100,000 counts, Figure 2A) as well as the percent of CD34+/KDR+ cells that were OCN+ (Figure 2B). As is evident, the number of CD34+/KDR+/OCN+ cells were increased (by ~2-fold) in the ECA patients, with somewhat smaller increases in the LCA patients. Similarly, there were ~2-fold increases in the percent OCN+ of CD34+/KDR+ cells in the ECA and LCA patients as compared to the control subjects.
Figure 2. OCN co-staining of CD34+/KDR+ cells.
(A) CD34+/KDR+/OCN+ cells, expressed as absolute counts per 100,000 counts, and (B) percent OCN+ of CD34+/KDR+ cells in control subjects (circles), patients with early coronary atherosclerosis (ECA, triangles), and in patients late coronary atherosclerosis (LCA, squares). *P < 0.05 and **P < 0.01 compared with control subjects. Shown are the median values and IQRs.
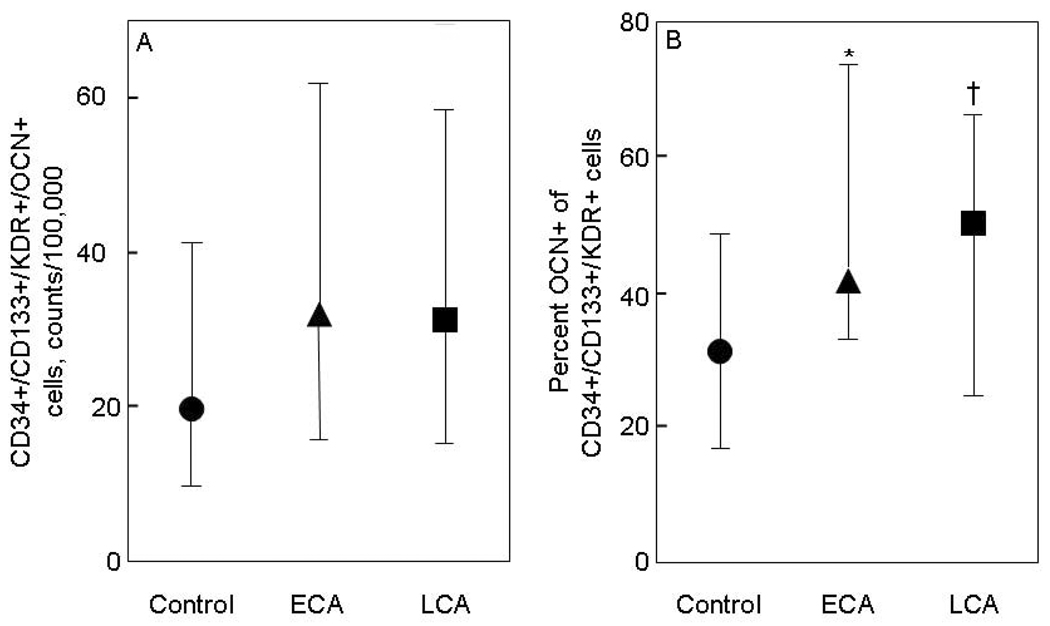
To gain further insight into these changes in CD34+/KDR+ cells in the vascular disease groups, we examined sub-populations of the CD34+/KDR+ cells based on CD133 expression. As shown in Figure 3A, the absolute number of CD34+/CD133+/KDR+/OCN+ cells were ~60% higher in the ECA and LCA as compared to the control subjects, but these differences did not achieve statistical significance. By contrast, the percent OCN+ of CD34+/CD133+/KDR+ cells were significantly higher in the ECA as compared to the control subjects. LCA patients also had higher levels of percent OCN+ of CD34+/CD133+/KDR+ cells as compared to the control subjects, but the P-value for this difference was 0.07.
Figure 3. OCN co-staining of CD34+/CD133+/KDR+ cells.
(A) CD34+/CD133+/KDR+/OCN+ cells, expressed as absolute counts per 100,000 counts, and (B) percent OCN+ of CD34+/CD133+/KDR+ cells in control subjects (circles), patients with early coronary atherosclerosis (ECA, triangles), and in patients late coronary atherosclerosis (LCA, squares). *P < 0.05, **P < 0.01, and †P = 0.071 compared with control subjects. Shown are the median values and IQRs.
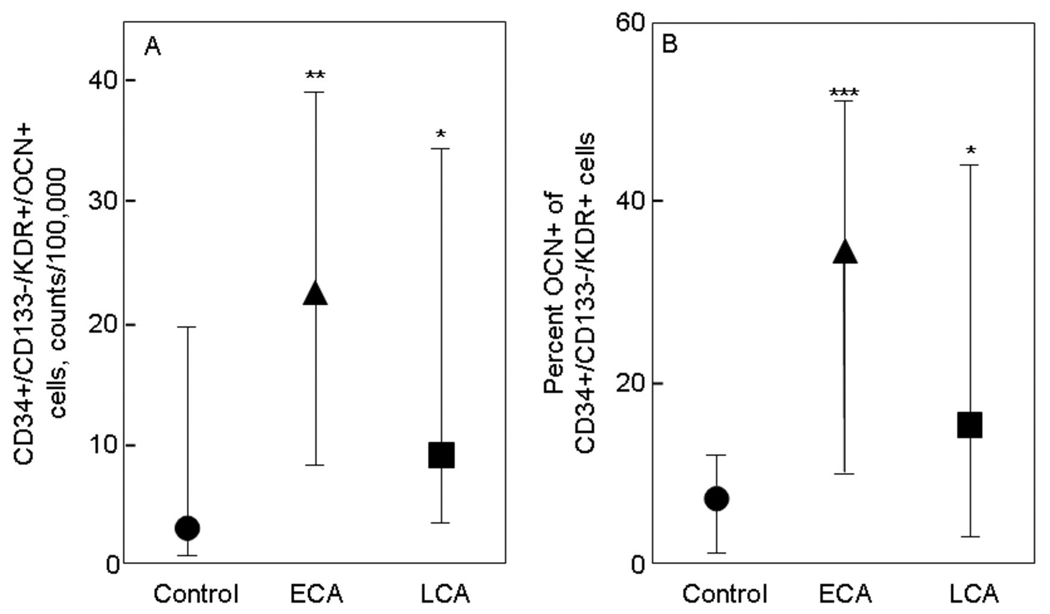
Figure 4 shows the corresponding results for CD34+/CD133−/KDR+/OCN+ cells (Figure 4A) and percent OCN+ of CD34+/CD133−/KDR+ cells (Figure 4B). As is evident, there were highly significant, 5- to 7-fold increases in these parameters in the ECA as compared to the control subjects, and 2- to 3-fold increases in the LCA as compared to the control subjects.
Figure 4. OCN co-staining of CD34+/CD133−/KDR+ cells.
(A) CD34+/CD133−/KDR+/OCN+ cells, expressed as absolute counts per 100,000 counts, and (B) percent OCN+ of CD34+/CD133−/KDR+ cells in control subjects (circles), patients with early coronary atherosclerosis (ECA, triangles), and in patients late coronary atherosclerosis (LCA, squares). *P < 0.05 and ***P < 0.001 compared with control subjects. Shown are the median values and IQRs.
Table 3 shows the absolute numbers of CD34+/KDR+, CD34+/CD133+/KDR+, and CD34+/CD133−/KDR+ cells in the three groups. In contrast to the significant differences in the cells co-staining for OCN noted above, the absolute numbers of these cells did not differ significantly in these study subjects. CD34+/CD133+/KDR+ cells were ~10% lower in the LCA as compared to the control subjects, but this difference was not statistically significant.
Table 3. Differences in CD34+ sub-populations.
Absolute number (in counts per 100,000) of CD34+/KDR+, CD34+/CD133+/KDR+, and CD34+/CD133−/KDR+ cells in the study subjects. ECA, early coronary atherosclerosis; LCA, late coronary atherosclerosis. Data are median (IQR).
| Control | ECA | LCA | |
|---|---|---|---|
| CD34+/KDR+ | 181 (56, 378) | 173 (70, 323) | 180 (100, 240) |
| CD34+/CD133+/KDR+ | 80 (37, 156) | 77 (36, 126) | 73 (48, 107) |
| CD34+/CD133−/KDR+ | 75 (23, 200) | 99 (29, 190) | 98 (49, 119) |
Serum RANKL, OPG, and MGP levels
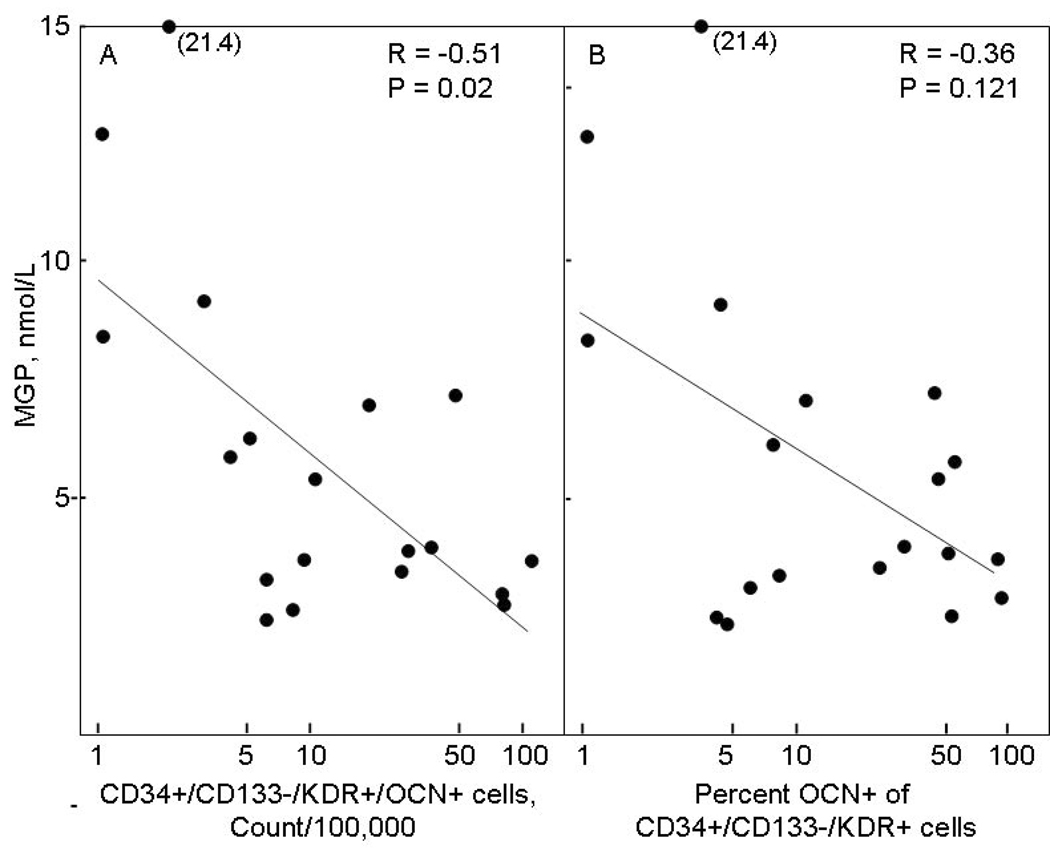
Since changes in OPG levels have been associated with vascular disease (32) and MGP is a known inhibitor of vascular calcification (33), we also measured serum RANKL, OPG, and MGP levels in the study subjects. As shown in Table 4, while serum RANKL levels did not differ in the study subjects, serum OPG levels were significantly higher and serum MGP levels significantly lower in the LCA as compared to the control subjects. In correlation analyses, serum RANKL or OPG levels did not correlate with any of the cell populations in the three study groups and serum MGP levels did not correlate with the cell populations in the control or ECA patients (data not shown). However, in the LCA patients, serum MGP were significantly inversely correlated with the absolute numbers of CD34+/CD133−/KDR+/OCN+ cells (Figure 5A), with a similar trend seen for the percent OCN+ of CD34+/CD133−/KDR+ cells (Figure 5B).
Table 4. Serum RANKL, OPG, and MGP levels in the study subjects.
ECA, early coronary atherosclerosis; LCA, late coronary atherosclerosis. Data are median (IQR).
| Control | ECA | LCA† | |
|---|---|---|---|
| RANKL, pmol/L | 0.02 (0.00, 0.12) | 0.05 (0.00, 0.10) | 0.06 (0.00, 0.21) |
| OPG, pmol/L | 4.90 (3.20, 6.28) | 4.80 (3.63, 6.69) | 7.01 (4.90, 8.97)* |
| MGP, nmol/L | 7.08 (6.07, 10.58) | 8.29 (6.52, 10.18) | 4.19 (3.35, 7.16)** |
P < 0.05
P < 0.01
P < 0.001 vs. Control
Serum was available in 20 of the 29 subjects in the LCA group for these measurements.
Figure 5. Correlation with serum MGP levels.
Correlation between serum MGP levels in the LCA patients and (A) CD34+/CD133−/KDR+/OCN+ cells, expressed as absolute counts per 100,000 counts, and (B) percent OCN+ of CD34+/CD133−/KDR+ cells.
Confocal microscopy
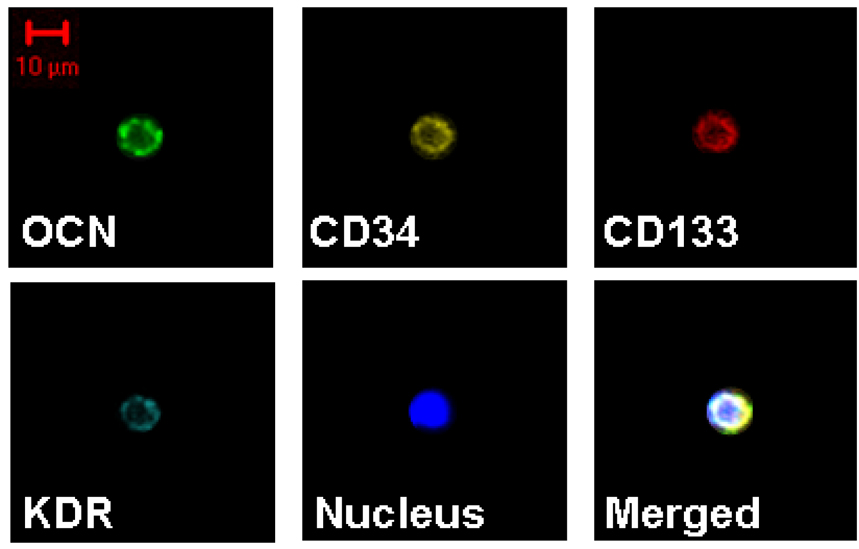
Figure 6 shows evidence, using confocal microscopy, that each of the antibodies used in the study was binding to the surface of the cell. Note also in this Figure the relatively small size (~10 µm) of these cells, as represented by a cell positive with all four antibodies (CD34, CD133, KDR, and OCN).
Figure 6. Confocal microscopy of cells.
Confocal microscopy showing a cell staining with all four antibodies used in the study (OCN, CD34, CD133, KDR). Shown also is a stain for nuclei (DAPI) and the merged images.
Cell culture
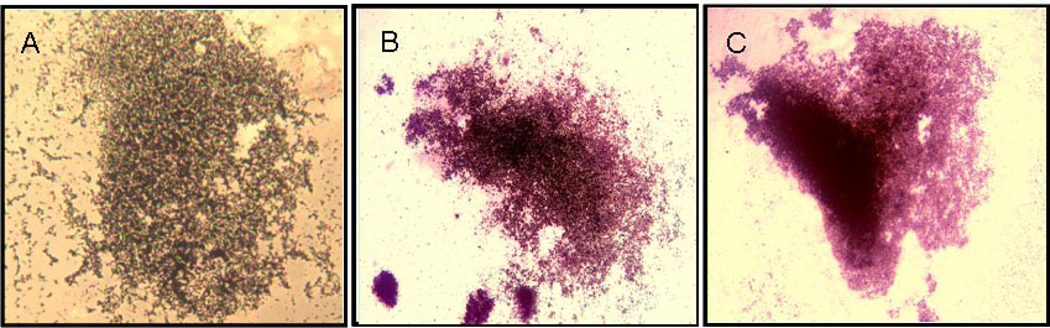
While previous work from our group (14,15) and others (10–13) has shown that staining for OCN identifies cells with osteogenic potential both in bone marrow and in peripheral blood, we next assessed whether peripheral blood CD34+ cells were capable of mineralization in vitro. For these experiments, we used CD34+ cells isolated from anonymous blood donors and found that when cultured on uncoated plastic plates, these cells failed to adhere and by 21 days in osteoblast differentiation medium, the culture plates contained no visible cells (data not shown). Reasoning that local calcium deposition in the vasculature due either to cell death (34) or vascular microcalcifications might induce these cells to adhere, we next exposed CD34+ cells to an additional 2.1 mM of extracellular Ca2+ only for 5 days at the beginning of culture (days 3–7). Under these conditions, we found that peripheral blood CD34+ cells routinely adhered to the plastic dishes and, when placed in osteogenic differentiation medium for an additional 21 days, formed mineralized nodules, as assessed either by Von Kossa (Figure 7A) or Alizarin Red (Figure 7B) staining. Note that in these assays, from days 8 to 28 (21 days of differentiation media) the cells were in osteoblast differentiation medium without additional calcium. Since we could not obtain sufficient numbers of cells from the patients undergoing cardiac catheterization in order to specifically isolate CD34+ cells for culture, we next tested whether PBMNCs either from normal subjects (n = 3) or patients with LCA (n = 3) could be induced to mineralize under similar culture conditions. PBMNCs from normal subjects, who had percent OCN+ of CD34+ cells of 4.2%, 9.9%, and 12.7%, respectively, did not form mineralized colonies, without or with calcium exposure (data not shown). Of the LCA patients, PBMNCs from the subject with the highest percent OCN+ of CD34+ cells (31.2%) were able to form mineralized deposits in the presence, but not in the absence of the brief 5 day exposure to calcium (Figure 7C), whereas PBMNCs from the other 2 LCA patients who had lower percent OCN+ of CD34+ cells (18.3% and 24.9%) failed to mineralize under these conditions (data not shown).
Figure 7. Mineralization assays.
In vitro mineralization assay of CD34+ cells from an anonymous blood donor (A) using von Kossa staining and (B) using Alizarin Red staining and (C) of PBMNCs from a patient with LCA (Alizarin Red stain). In these cultures, cells were exposed to Ca2+ for 5 days followed by 21 days of culture in osteoblast differentiation medium. All pictures are at x 4 magnification.
Expression of bone-related genes by CD34+ cells
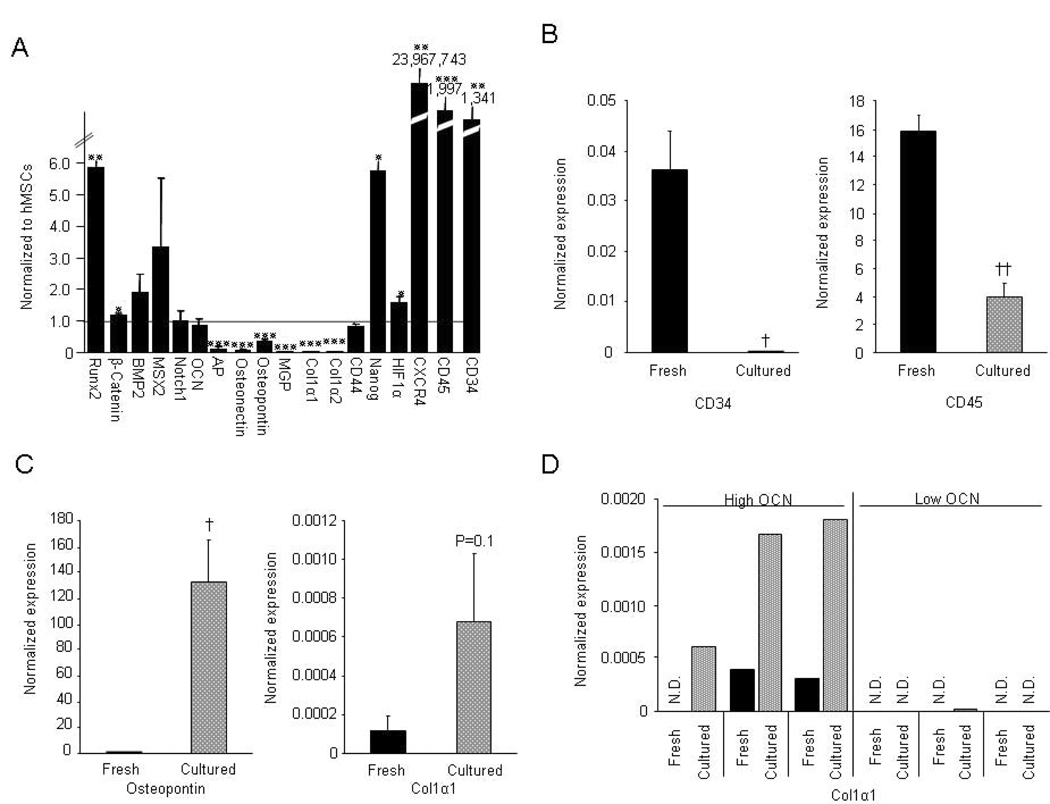
For the gene expression analyses, we isolated CD34+ cells using MACS from 6 anonymous donors from the Mayo Blood Bank and compared expression of selected bone-related genes in the CD34+ samples in these cells to human bone marrow stromal cells (hMSCs), which are authentic osteoblast precursors (9). As shown in Figure 8A, CD34+ cells expressed significantly higher levels of runx2 and β-catenin than hMSCs; additional bone-related genes (BMP-2, Msx-2, Notch, and OCN) were expressed at levels similar to hMSCs. AP, osteonectin, osteopontin (OPN), MGP, Col1α1 and Col1α2 were expressed by CD34+ cells at low levels. The stem cell marker, Nanog, was expressed at significantly higher levels in CD34+ compared to hMSCs, whereas CD44, another stem cell marker, was expressed at similar levels. HIF-1α and the chemokine receptor, CXCR4, both which are involved in homing of cells to ischemic tissues, were expressed at very high levels in the CD34+ cells. CD34+ cells also expressed mRNAs for CD34 and CD45. Following in vitro culture of the CD34+ cells (first 5 days in the presence of Ca2+ and than an additional 7 days in osteoblast differentiation medium), mRNA expression of CD34 virtually disappeared, and CD45 mRNA was significantly decreased (Figure 8B). Expression of OPN, a non-collagenous bone matrix protein which is known to have chemoattractant properties (35), increased dramatically (by 36,000-fold) following culture (Figure 8C). We also observed a 5-fold increase in the expression of Col1α1 under the same culture conditions (Figure 8C). However, of the 6 CD34+ cell samples, the 3 with the highest expression of OCN mRNA prior to culture significantly (by 600-fold) upregulated Col1α1 expression, whereas the 3 with low OCN mRNA levels prior to culture did not (Figure 8D).
Figure 8. Gene expression data.
Gene expression analysis of CD34+ cells from 6 anonymous blood donors. (A) Expression of bone-related and other genes normalized to undifferentiated hMSCs (expression of each gene in hMSCs set at 1.0); (B) Changes in expression of mRNAs for CD34 and CD45 following in vitro culture; (C) Changes in expression of mRNAs for osteopontin and Col1α1 following in vitro culture; and (D) Changes in mRNA expression for Col1α1 following in vitro culture in the 3 samples with the highest versus the lowest expression of OCN mRNA prior to culture. *P < 0.05, **P < 0.01 and ***P < 0.001 compared to 1.0 using a one-sample t-test; †P < 0.01 and ††P < 0.001 compared to freshly sorted CD34 cells using a two-sample t-test. N.D., not detected.
Ability of OCN+ cells to predict early coronary atherosclerosis
To evaluate the potential utility of OCN+ cells as a biomarker for early atherosclerosis (ECA), we performed logistic regression models using control and ECA subjects and tested the ability of the cell population that differed most between the normal and ECA patients (CD34+/CD133−/KDR+/OCN+ cells or percent OCN+ of CD34+/CD133−/KDR+ cells) to predict ECA in multivariable logistic regression models that included age and gender. In these age- and gender-adjusted models, both parameters were significant (P = 0.003 for both) predictors of ECA, with odds ratios (95% confidence interval) of 1.9 (1.3–3.0) and 3.0 (1.5–6.1) for the CD34+/CD133−/KDR+/OCN+ and percent OCN+ of CD34+/CD133−/KDR+ cells, respectively. Since in these models a log2 transformation of both the CD34+/CD133−/KDR+/OCN+ and the percent OCN+ of CD34+/CD133−/KDR+ cells was used, the odds ratio represents the increase in risk associated with a doubling in the percentage of these cells. Results were similar when models were adjusted for any of the following: statin use, diabetes, hypertension, smoking, hs-CRP, cholesterol, HDL, triglyceride, LDL, creatinine, and GFR.
Finally, we also performed ROC analyses to further assess the ability of these cell populations to predict early coronary atherosclerosis (ECA) and placed this in the context of a similar analysis using the currently available marker, hs-CRP. As shown in Table 5, both for unadjusted and for age-adjusted models, the percent OCN+ of CD34+/CD133−/KDR+ cells performed better than hs-CRP as predictors of ECA, with the CD34+/CD133−/KDR+/OCN+ having intermediate areas under the curve (AUCs).
Table 5. Receiver operator characteristic analysis.
Unadjusted and age-adjusted areas under the curve (AUC) for receiver operator characteristic (ROC) curves for prediction of early coronary atherosclerosis (ECA), using the OC+/CD34+/CD133−/KDR+ per 100,000, percent OCN+ of CD34+/CD133−/KDR+ cells, or hs-CRP levels as predictors. This analysis was done using the control and ECA patients.
| Predictor | Unadjusted AUC (95% confidence interval) | Age-adjusted AUC (95% confidence interval) |
|---|---|---|
| hs-CRPa | 0.59(0.41-0.77) | 0.58(0.40-0.76) |
| OC+/CD34+/CD133−/KDR+ per 100Ka | 0.78(0.64-0.92) | 0.78(0.63-0.92) |
| % OCN+ of CD34+/CD133−/KDR+ cellsa | 0.84(0.72-0.96)* | 0.84(0.72-0.96)* |
log2 used
P < 0.05 vs. corresponding AUC for hs-CRP
Discussion
We demonstrate in the present study that, as compared to control subjects with normal endothelial function and no structural CAD, patients with ECA, characterized by coronary endothelial dysfunction, or those with established LCA, characterized by severe, multi-vessel CAD, have a greater percentage of CD34+/KDR+ cells co-staining for OCN. Similar increases in the percent OCN+ of CD34+/CD133+/KDR+ cells were present in the vascular disease patients, with these subjects having even more marked increases in the percent OCN+ of CD34+/CD133−/KDR+ cells. In addition, the absolute numbers of CD34+/CD133−/KDR+/OCN+ cells were also significantly higher in the vascular disease as compared to the control subjects. These findings have potentially important implications for our understanding of the mechanisms of coronary vascular calcification and of the link between osteoporosis and vascular disease, as well as for the development of possible new markers for vascular disease.
While novel, our findings are nonetheless consistent with recent reports by Gabbasov et al. comparing circulating cells staining with an antibody for another bone-related protein, osteonectin, in patients with CAD compared with normal subjects (36,37). In those studies, osteonectin-positive cells were not characterized as EPCs; however, the authors did find that osteonectin-positive cells were increased in the circulation of CAD as compared to control patients. In the context of the increasing overlap between endothelial and osteoblastic lineages noted earlier (17–19), our findings and those of Gabbasov et al. (36,37) suggest that vascular injury or disease may be associated with activation of osteogenic genes by EPCs. Since circulating EPCs may constitute the initial response to vascular injury (6,7), the expression of an osteogenic “transcriptosome” by these cells may promote vascular calcification rather than normal repair processes. Consistent with this, our gene expression analysis demonstrated that human circulating CD34+ cells expressed a spectrum of bone-related genes, including runx2, β-catenin, BMP-2, Msx-2, Notch, and OCN at levels similar to (or even higher than) cultured hMSCs, which are authentic osteoblast precursors (9).
Our cell culture studies using MACS-sorted CD34+ cells suggest that increases in local calcium concentrations in the vessel wall, perhaps due to cell death (34) or microcalcifications, may play a role in the adherence of circulating EPC populations and perhaps their differentiation towards an osteogenic phenotype. Along these lines, it is also of interest that, even using exposure to increased extracellular Ca2+, we could not successfully culture mineralizing cells from unsorted PBMNCs of normal subjects, but were able to do so from PBMNCs of a patient with LCA who had the highest OCN+ of CD34+ cells of the 3 LCA patients tested, consistent with the hypothesis that there are increased concentrations of cells with mineralizing ability in the circulation of patients with advanced atherosclerosis. Our culture data are also consistent with a recent preliminary report by Bick et al. (38) showing that circulating EPCs from sheep and humans can be differentiated into osteoblastic cells, thus providing further support for a possible role for these cells in vascular calcification.
Of particular interest, OPN expression was markedly up-regulated (by 36,000-fold) following in vitro culture in the presence of Ca2+. OPN is a non-collagenous bone matrix protein produced by osteocytes, osteoblasts, and osteoclasts that can interact with the cell surface receptor, CD44 (39). However, it also appears that OPN is associated with the presence and the extent of cardiovascular disease (40), and it may serve as a chemoattractant for a number of cell types (35,41). Thus, production of OPN by EPCs at sites of tissue injury may modulate proliferation, migration, and accumulation of endothelial and vascular smooth muscle cells, thereby promoting vascular repair, but also perhaps initiating vascular calcification.
Our cell culture studies also revealed that CD34+ cells with higher expression of OCN mRNA prior to culture were the ones that upregulated expression of Colα1 mRNA following in vitro culture. Thus, the level of OCN expression by CD34+ cells may be a predictor of the ability of these cells to develop an osteogenic phenotype. Clearly, further studies are needed to address this possibility. In addition, further studies using animal models are needed to determine whether it is these circulating cells themselves that lead to vascular calcification or whether they initiate this process, resulting in the recruitment of local cells, such as vascular smooth muscle cells or pericytes (42,43), to develop an osteogenic phenotype, or both. These additional studies should help to define the true biological role in vivo of EPC populations in regulating the process of vascular calcification. Additionally, further studies using coronary histopathology are needed to identify whether the EPC populations differentially impact microcalcifications of fibrous versus lipidaceous origin (44) and the outward remodeling that characterizes acute coronary syndromes (45).
Interestingly, we also found that in the LCA patients, CD34+/CD133−/KDR+/OCN+ cells were significantly inversely correlated with serum MGP levels. Since MGP is a known inhibitor of vascular calcification (33), these findings suggest a biological connection between the cell populations we have identified and the process of vascular calcification that warrants further investigation. In addition, the use of newer assays for undercarboxylated MGP (46), which has impaired biological function, may identify further associations between this form of MGP and the cell populations described here and may also provide additional risk stratification to identify patients who progress from early to late coronary atherosclerosis.
The current study also demonstrates for the first time that the percent OCN+ of EPCs, particularly the CD34+/CD133−/KDR+/OCN+ and percent OCN+ of CD34+/CD133−/KDR+ cells, can serve as peripheral markers of early coronary atherosclerosis. In fact, from a diagnostic standpoint, these cells outperformed the commonly used systemic marker, hsCRP, in ROC analyses. We recognize, however, that additional studies using larger numbers of subjects are needed to formally compare the predictive ability of these cells with other biological markers for coronary atherosclerosis.
While previous studies (29,47) have found associations between the numbers of EPCs defined as CD34+/KDR+ and vascular outcomes, we did not find significant differences in these cells between our study groups. The reasons for this are somewhat unclear, but may have to do with the specific patient populations studied, including the fact that the majority of our patients with LCA were on statins, which are known to increase circulating EPCs (48). In this context, our findings suggest that the addition of OCN as a biomarker to CD34+/KDR+ or CD34+/CD133−/KDR+ cells may be of utility in predicting cardiovascular outcomes, and prospective studies addressing this possibility are needed.
In summary, our findings demonstrate that a higher number and percentage of circulating cell populations containing EPCs co-stain for OCN in patients with early or late coronary atherosclerosis compared with control subjects with normal endothelial function and no structural CAD. These data suggest that the activation of an osteogenic program by EPCs may play a role in the response to vascular injury and contribute to vascular calcification, as opposed to non-calcifying vascular repair. Additionally, the same pro-inflammatory factors involved in the pathogenesis of osteoporosis may lead to the expression of an osteogenic phenotype by endothelial lineage cells, providing a potential mechanism for the link between osteoporosis and vascular calcifications. Finally, our finding that the number of CD34+/CD133−/KDR+/OCN+ cells and percent OCN+ of CD34+/CD133−/KDR+ cells can predict coronary atherosclerosis suggests that these (or similar) cell populations may hold promise as novel markers for vascular disease.
Acknowledgements
We would like to thank James Peterson and Sara Achenbach for valuable assistance with the statistical analyses, Darrel Loeffler for helping with the flow cytometry analyses, and B. Lawrence Riggs for helpful comments and discussions.
This work was supported by NIH Grants AG-004875, HL63911, and HL39840.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicts of interest.
References
- 1.Tanko LB, Christiansen C, Cox CA, Geiger MJ, McNabb MA, Cummings SR. Relationship between osteoporosis and cardiovascular disease in postmenopausal women. J Bone Miner Res. 2005;20:1912–1920. doi: 10.1359/JBMR.050711. [DOI] [PubMed] [Google Scholar]
- 2.Sambrook PN, Chen CJS, March LM, et al. High bone turnover is an independent predictor of mortality in the frail elderly. J Bone Miner Res. 2006;21:549–555. doi: 10.1359/jbmr.060104. [DOI] [PubMed] [Google Scholar]
- 3.Bucay N, Sarosi I, Dunstan CR, et al. Osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev. 1998;12:1260–1268. doi: 10.1101/gad.12.9.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson RC, Leopold JA, Loscalzo J. Vascular calcification: pathobiological mechanisms and clinical implications. Circ Res. 2006;99:1044–1059. doi: 10.1161/01.RES.0000249379.55535.21. [DOI] [PubMed] [Google Scholar]
- 5.Rajamannan NM, Bonrow RO, Rahimtoola SH. Calcific aortic stenosis: an update. Nat Clin Pract Cardiovasc Med. 2007;4:254–262. doi: 10.1038/ncpcardio0827. [DOI] [PubMed] [Google Scholar]
- 6.Urbich C, Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res. 2004;95:343–353. doi: 10.1161/01.RES.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- 7.Friedrich EB, Walenta K, Scharlau J, Nickenig G, Werner N. CD34-/CD133+/VEGFR-2+ endothelial progenitor cell subpopulation with potent vasoregenerative capacities. Circ Res. 2006;98:e20–e25. doi: 10.1161/01.RES.0000205765.28940.93. [DOI] [PubMed] [Google Scholar]
- 8.Rafii S, Lyden D. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat Med. 2003;9:702–712. doi: 10.1038/nm0603-702. [DOI] [PubMed] [Google Scholar]
- 9.Friedenstein AJ, Petrakova KV, Kurolesova AI, Frolova GP. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968;6:230–247. [PubMed] [Google Scholar]
- 10.Long MW, Williams JL, Mann KG. Expression of human bone-related proteins in the hematopoietic microenvironment. J Clin Invest. 1990;86:1387–1395. doi: 10.1172/JCI114852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Long MW, Robinson JA, Ashcraft EA, Mann KG. Regulation of human bone marrow-derived osteoprogenitor cells by osteogenic growth factors. J Clin Invest. 1995;95:881–887. doi: 10.1172/JCI117738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Long MW, Ashcraft EK, Normalle D, Mann KG. Age-related phenotypic alterations in populations of purified human bone precursor cells. J Gerontol. 1999;54A:B54–B62. doi: 10.1093/gerona/54.2.b54. [DOI] [PubMed] [Google Scholar]
- 13.Eipers PG, Kale S, Taichman RS, et al. Bone marrow accessory cells regulate human bone precursor cell development. Exp Hematol. 2000;28:815–825. doi: 10.1016/s0301-472x(00)00183-1. [DOI] [PubMed] [Google Scholar]
- 14.Eghbali-Fatourechi GZ, Lamsam J, Fraser D, Nagel DA, Riggs BL, Khosla S. Circulating osteoblast-lineage cells in humans. N Engl J Med. 2005;352:1959–1966. doi: 10.1056/NEJMoa044264. [DOI] [PubMed] [Google Scholar]
- 15.Eghbali-Fatourechi GZ, Moedder UI, Charatcharoenwitthaya N, et al. Characterization of circulating osteoblast linage cells in humans. Bone. 2007;40:1370–1377. doi: 10.1016/j.bone.2006.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simmons PJ, Torok-Storb B. Identification of stromal cell precursors in human bone marrow by novel monoclonal antibody, STRO-1. Blood. 1991;78:55–62. [PubMed] [Google Scholar]
- 17.Chen JL, Hunt P, McElvain M, Black T, Kaufman S, Choi ESH. Osteoblast precursor cells are found in CD34+ cells from human bone marrow. Stem Cells. 1997;15:368–377. doi: 10.1002/stem.150368. [DOI] [PubMed] [Google Scholar]
- 18.Tondreau T, Meuleman N, Delforge A, et al. Mesenchymal stem cells derived from CD133-positive cells in mobilized peripheral blood and cord blood: proliferation, Oct4 expression, and plasticity. Stem Cells. 2005;23:1105–1112. doi: 10.1634/stemcells.2004-0330. [DOI] [PubMed] [Google Scholar]
- 19.Matsumoto T, Kawamoto A, Kuroda R, et al. Therapeutic potential of vasculogenesis and osteogenesis promoted by peripheral blood CD34-positive cells for functional bone healing. Am J Pathol. 2006;169:1440–1457. doi: 10.2353/ajpath.2006.060064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilson PWF, D'Agostino RB, Sr, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 21.Suwaidi JA, Higano ST, Hamasaki S, Holmes DR, Jr, Lerman A. Association between obesity and coronary atherosclerosis and vascular remodeling. Am J Cardiol. 2001;88:1300–1303. doi: 10.1016/s0002-9149(01)02093-8. [DOI] [PubMed] [Google Scholar]
- 22.Hasdai D, Gibbons RJ, Holmes DR, Jr, Higano ST, Lerman A. Coronary endothelial dysfunction in humans is associated with myocardial perfusion defects. Circulation. 1997;96:3390–3395. doi: 10.1161/01.cir.96.10.3390. [DOI] [PubMed] [Google Scholar]
- 23.Lerman A, Zeiher AM. Endothelial function: cardiac events. Circulation. 2005;111:363–368. doi: 10.1161/01.CIR.0000153339.27064.14. [DOI] [PubMed] [Google Scholar]
- 24.Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes DR, Jr, Lerman A. Long-term follow-up of patients with mild artery disease and endothelial dysfunction. Circulation. 2000;101:948–954. doi: 10.1161/01.cir.101.9.948. [DOI] [PubMed] [Google Scholar]
- 25.Vandesompele J, De Preter K, Pattyn F, et al. Accurate normalization of real-time quantitative RT-PCR data by geometeric averaging of multiple internal control genes. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-7-research0034. research0034.1-0-34.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Radonic A, Thulke S, Mackay IM, Landt O, Siegert W, Nitsche A. Guideline to reference gene selection for quantitative real-time PCR. Biochem Biophys Res Commun. 2004;313:856–862. doi: 10.1016/j.bbrc.2003.11.177. [DOI] [PubMed] [Google Scholar]
- 27.DeLong ER, Delong DM. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 28.Asher J, Houston M. Statins and C-reactive protein levels. J Clin Hypertens. 2007;9:622–628. doi: 10.1111/j.1524-6175.2007.06639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Werner N, Kosiol S, Schiegl T, et al. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med. 2005;353:999–1007. doi: 10.1056/NEJMoa043814. [DOI] [PubMed] [Google Scholar]
- 30.Holmes DR, Jr, Teirstein P, Satler L, et al. Sirolimus-eluting stents vs vascular brachytherapy for in-stent restenosis within bare-metal stents. JAMA. 2006;295:1264–1273. doi: 10.1001/jama.295.11.1264. [DOI] [PubMed] [Google Scholar]
- 31.Vasa M, Fichtlscherer S, Aicher A, et al. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89:e1–e7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- 32.Schoppet M, Sattler AM, Schaefer JR, Herzum M, Maisch B, Hofbauer LC. Increased osteoprotegerin serum levels in men with coronary artery disease. J Clin Endocrinol Metab. 2003;88:1024–1028. doi: 10.1210/jc.2002-020775. [DOI] [PubMed] [Google Scholar]
- 33.Doherty TM, Fitzpatrick LA, Inoue D, et al. Molecular, endocrine, and genetic mechanisms of arterial calcification. Endocr Rev. 2004;25:629–672. doi: 10.1210/er.2003-0015. [DOI] [PubMed] [Google Scholar]
- 34.Deniaud A, el dein OS, Maillier E, et al. Endoplasmic reticulum stress induces calcium-dependent permeability transition, mitochonchondrial outer membrane permeabilization and apoptosis. Oncogene. doi: 10.1038/sj.onc.1210638. in press. [DOI] [PubMed] [Google Scholar]
- 35.Liaw L, Almeida M, Hart CE, Schwartz SM, Giachelli CM. Osteopontin promotes vascular cell adhesion and spreading and is chemtactic for smooth muscle cells in vitro. Circ Res. 1994;74:214–224. doi: 10.1161/01.res.74.2.214. [DOI] [PubMed] [Google Scholar]
- 36.Gabbasov ZA, Agapov AA, Saburova OS, Obedzinskii EA, Soboleva EL. Detection of circulating stromal stem cells with osteogenic potential in the blood of coronary patients by laser flow cytometry. Bull Exp Biol Med. 2005;139:266–268. doi: 10.1007/s10517-005-0266-6. [DOI] [PubMed] [Google Scholar]
- 37.Gabbasov ZA, Agapov AA, Saburova OS, et al. Circulating stromal osteonectin-positive progenitor cells and stenotic coronary atherosclerosis. Can J Physiol Pharmacol. 2007;85:295–300. doi: 10.1139/y07-001. [DOI] [PubMed] [Google Scholar]
- 38.Bick T, Rozen N, Dreyfuss E, Soundry M, Lewinson D. Osteogenic differentiation of circulating endothelial progenitor cells. J Bone Miner Res. 2007;22:S143. [Google Scholar]
- 39.Weber GF, Ashkar S, Glimcher MJ, Cantor H. Receptor-ligand interaction between CD44 and osteopontin (Eta-1) Science. 1996;271:509–512. doi: 10.1126/science.271.5248.509. [DOI] [PubMed] [Google Scholar]
- 40.Ohmori R, Momiyama Y, Taniguchi H, et al. Plasma osteopontin levels are associatated with the presence and extent of coronary artery disease. Atherosclerosis. 2003;170:333–337. doi: 10.1016/s0021-9150(03)00298-3. [DOI] [PubMed] [Google Scholar]
- 41.Standal T, Borset M, Sundan A. Role of osteopontin in adhesion, migration, cell survival and bone remodeling. Exp Oncol. 2004;26:179–184. [PubMed] [Google Scholar]
- 42.Abedin M, Tintut Y, Demer LL. Mesenchymal stem cells and the artery wall. Circ Res. 2004;95:671–676. doi: 10.1161/01.RES.0000143421.27684.12. [DOI] [PubMed] [Google Scholar]
- 43.Shao JS, Cheng SL, Pingsterhaus JM, Charlton-Kachigian N, Loewy AP, Towler DA. Msx2 promotes cardiovascular calcification by activating paracrine Wnt signals. J Clin Invest. 2005;115:1210–1220. doi: 10.1172/JCI24140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burke AP, Kolodgie FD, Farb A, Weber D, Virmani R. Morphological predictors of arterial remodeling in coronary atherosclerosis. Circulation. 2002;105:297–303. doi: 10.1161/hc0302.102610. [DOI] [PubMed] [Google Scholar]
- 45.Ward MR, Pasterkamp G, Yeung AC, Borst C. Arterial remodeling. Mechanisms and clinical implications. Circulation. 2000;102:1186–1190. doi: 10.1161/01.cir.102.10.1186. [DOI] [PubMed] [Google Scholar]
- 46.Cranenburg EC, Vermeer C, Koos R, et al. The circulating inactive form of matrix Gla protein (ucMGP) as a biomarker for cardiovascular calcification. J Vasc Res. 2008;45:427–436. doi: 10.1159/000124863. [DOI] [PubMed] [Google Scholar]
- 47.Hill JM, Zalos G, Halcox JPJ, et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 48.Gomez-Cerezo JF, Pagan-Munoz B, Lopez-Rodriguez M, Estebanez-Munoz M, Barbaro-Hernandez FJ. The role of endothelial progenitor cells and statins in endothelial function: a review. Cardiovasc Hematol Agents Med Chem. 2007;5:265–272. doi: 10.2174/187152507782109836. [DOI] [PubMed] [Google Scholar]