Abstract
The possible role of oxygen metabolism in supporting brain activation remains elusive. We have used a newly developed neuroimaging approach based on high-field in vivo 17O magnetic resonance spectroscopic (MRS) imaging to noninvasively image cerebral metabolic rate of oxygen (CMRO2) consumption in cats at rest and during visual stimulation. It was found that CMRO2 increases significantly (32.3%±10.8%, n = 6) in the activated visual cortical region as depicted in blood oxygenation level dependence functional maps; this increase is also accompanied by a CMRO2 decrease in surrounding cortical regions, resulting a smaller increase (9.7%±1.9%) of total CMRO2 change over a larger cortical region displaying either a positive or negative CMRO2 alteration. Moreover, a negative correlation between stimulus-evoked percent CMRO2 increase and resting CMRO2 was observed, indicating an essential impact of resting brain metabolic activity level on stimulus-evoked percent CMRO2 change and neuroimaging signals. These findings provide new insights into the critical roles of oxidative metabolism in supporting brain activation and function. They also suggest that in vivo 17O MRS imaging should provide a sensitive neuroimaging modality for mapping CMRO2 and its change induced by brain physiology and/or pathologic alteration.
Keywords: brain activation, cerebral metabolic rate of oxygen, functional MRI, in vivo 17O MRS, imaging CMRO2
Introduction
Normal brain function requires large amounts of energy. This high energy demand is met mainly through the chemical process of oxidative phosphorylation in the brain mitochondria to produce adequate quantities of adenosine triphosphate (ATP; Attwell and Laughlin, 2001; Du et al, 2008; Hyder et al, 2006; Lei et al, 2003; Raichle, 1987; Sokoloff, 1991). Under the resting condition, this process is responsible for the consumption of most glucose used by the brain, generating ~36 ATPs from each glucose molecule. It is also tightly coupled to cerebral oxygen utilization and is responsible for ~20% of the total oxygen consumption in the human body even though the brain constitutes only ~2% of the body weight. Therefore, the cerebral oxidative metabolism is essential for supplying brain energy and supporting brain function. Consequently, cerebral metabolic rate of oxygen (CMRO2) consumption provides an important physiologic parameter that can be used to evaluate cerebral bioenergetics in healthy and diseased brains at rest; as well as to investigate the functional neurometabolic relation during brain stimulation and/or task performance. However, the ability to directly and reliably image resting CMRO2 and CMRO2 alteration during brain activation has been a major challenge for several decades. Positron emission tomography (PET) combined with 15O radiotracers has been perhaps the only established in vivo imaging method able to directly image CMRO2 (Ibaraki et al, 2008; Mintun et al, 1984; Mintun et al, 2002). Despite its relatively coarse spatial resolution, its requirement for complex mathematical modeling, and the involvement of multiple measurement procedures (Mintun et al, 1984), the 15O-PET technique has been applied to investigate oxygen metabolism in the human brain under various conditions. In one prominent PET application, regional stimulus-evoked CMRO2 change in the human visual cortex was determined to be ~5% whereas alterations in cerebral blood flow (CBF) and cerebral metabolic rate of glucose (CMRglc) consumption were significantly higher (~50%; Fox et al, 1988). These metabolic rate changes only corresponded to ~7% increase in the stimulus-evoked ATP production rate, a surprising finding implying that energy cost of stimulus-evoked neuronal activity is relatively small and it might be met partially through the glycolysis process in the awake human brain (Barinaga, 1997; Fox et al, 1988; Raichle and Mintun, 2006). In contrast, the cerebral oxidative glucose consumption rate, determined through the in vivo 13C magnetic resonance spectroscopic (MRS) measurement, was reported to increase substantially up to 80%–100% in the anesthetized rat somatosensory cortex during forepaw electric stimulation (Hyder et al, 2001). One possible source of this sizable discrepancy has been speculated to be the difference in baseline CMRO2 levels between the awake human and the anesthetized rat brains, highlighting the importance of quantitative CMRO2 measurements under both baseline and stimulated brain states.
Qualitatively, the finding that the ratio between the stimulus-evoked percent CMRO2 change versus that of CBF or CMRglc is much smaller than 1 (Fox et al, 1988) is consistent with and underlies the interpretation of the blood oxygenation level dependence (BOLD) contrast (Ogawa et al, 1990; Raichle and Mintun, 2006) detected by functional magnetic resonance imaging (fMRI), which has become the most prominent neuroimaging modality for mapping brain activation (e.g., Bandettini et al, 1992; Kwong et al, 1992; Ogawa et al, 1992). However, fMRI is unable to directly detect neuronal activity; instead, it relies on a complex interplay among CBF, cerebral blood volume (CBV), and CMRO2 changes induced by altered brain activity (Ogawa et al, 1993). Precise interpretation of fMRI results requires a better understanding of the quantitative relationship between the fMRI BOLD contrast and the underlying neurophysiology—in particular the stimulus-evoked CMRO2 change.
Therefore, the ability to image absolute CMRO2 with relatively high spatial resolution and temporal resolution is crucial towards understanding cerebral metabolic events that occur during brain activation, and their implication on quantifying neuroimaging signals. Towards this goal, we recently established the three-dimensional (3D) 17O magnetic resonance spectroscopy imaging (MRSI) method at high/ultra-high magnetic fields (Zhu et al, 2002; Zhu et al, 2007) as a noninvasive neuroimaging modality for imaging CMRO2 in vivo; and have rigorously evaluated this method using an animal model (Zhang et al, 2004; Zhu et al, 2007). This CMRO2 imaging method relies on detecting the dynamic change of 17O-labeled metabolic water (H2 17O) produced by oxygen metabolism in the brain mitochondria during a short (2 to 3 mins) inhalation of 17O-isotope-labeled oxygen gas (Arai et al, 1990; Mateescu et al, 1989; Pekar et al, 1991; Zhu et al, 2005; Zhu et al, 2002; Zhu et al, 2007). In the present study, we exploit this newly developed high-field in vivo 17O MRSI approach to study the possible roles of cerebral oxygen metabolism in brain function and stimulus-evoked activation by imaging CMRO2 in the cat primary visual cortex (V1; cortical areas 17 and 18) at rest (defined as baseline condition) and during visual stimulation, ultimately generating functional metabolic activation maps of relative CMRO2 change (i.e., ΔCMRO2/CMRO2 maps) and comparing them with the BOLD-based fMRI mapping results. The overall results provide new insights into quantitative relationship of CMRO2 in a resting visual cortex and its change evoked by visual stimulation; they also provide vital evidence to potentially coincide several long-standing and unsolved issues in the literature regarding the quantitative relationships among CMRO2, brain energy expenditure, cerebral bioenergetics, and brain activation.
Materials and methods
Animal Preparation
Five female adolescent cats were used to conduct this study. Cats were initially anesthetized with a mixture of ketamine (15.0 mg/kg) and xylazine (2.5 mg/kg). After oral intubation, mechanical ventilation (30 to 33 stokes/mins) was applied and anesthesia was switched to 0.9% to 1.2% isoflurane (~1.5MAC) in a N2O/O2 mixture of 70:30 volume ratio throughout the experiment. The pupils of the cat were dilated with atropine sulfate solution; corrective contact lenses were placed to focus the eyes on the visual stimulus by refracting and locating the fovea of the cat retina with the aid of a fundus camera (Zeiss, Jena, Germany). The visual stimulus was a binocular high-contrast square-wave moving and rotating gratings (0.3 cycle/deg, 2 cycles/sec, and 16° rotation for every 4 secs) to achieve optimal visual stimulation of the neurons with different orientation preferences in the cat primary visual cortex. The cats were placed in a cradle with head position restrained by the mouth and ear bars. The animal physiologic condition was continuously monitored and maintained during the entire experiment. All animal surgical procedures and experimental protocol were approved by the Institutional Animal Care and Use Committee of the University of Minnesota.
Magnetic Resonance Spectroscopy Imaging and Magnetic Resonance Imaging Measurements
The 17O MRSI and 1H MRI measurements were conducted on a 9.4T horizontal animal magnet (Magnex Scientific, Oxford, UK) interfaced with a Varian INOVA console (Varian Inc., Palo Alto, CA, USA). A radiofrequency (RF) probe consisted of a 17O surface coil covering the cat V1 region for acquiring 17O MRSI and a larger 1H coil for brain anatomic images and fMRI was used in this study.
17O Magnetic Resonance Spectroscopy Imaging for Imaging Cerebral Metabolic Rate of Oxygen
The spatial localization of 17O MRSI was achieved by using the 3D Fourier series window chemical shift imaging approach with a total acquisition time of 12.5 secs per MRSI volume (Hendrich et al, 1994; Zhu et al, 2002). Other 17O acquisition parameters were: 3 × 3 × 2.5 cm3 field-of-view (FOV); 9 × 9 × 5 phase encodes; 12 µL voxel size, which is corresponding to a nominal voxel size of 43 µL. A 17 × 17 × 9 matrix of 17O free induced decay signals were generated from the original 9 × 9 × 5 phase encoded raw image data for generating each 3D 17O MRSI dataset.
Two paired measurements for collecting series of 3D 17O MRSI before and during 2 to 3 mins inhalation of 17O2 gas (up to 89% 17O enrichment purchased from ISOTEC Inc, Miamisburg, OH, USA) were performed for each cat in the absence and presence of visual stimulation, respectively; and only Cat 5 had repeated twice the paired 17O MRSI measurements in the same experimental session to test the reproducibility and reliability of the CMRO2 imaging approach.
The natural abundant 17O signal of brain water obtained before the 17O2 gas inhalation was used as internal reference for quantifying the absolute metabolic H2 17O concentration in the brain; and the dynamic change of the metabolic H2 17O concentration obtained during 17O2 gas inhalation was applied to determine CMRO2 using the linear regression model and to generate 3D CMRO2 images (Zhang et al, 2004; Zhu et al, 2002; Zhu et al, 2007). The functional CMRO2 activation maps were created based on the value of ΔCMRO2/CMRO2, in which ΔCMRO2 represents the difference between the stimulated and control CMRO2 values.
Two criterions were applied to quantify the CMRO2 values measured under resting and stimulated conditions, as well as ΔCMRO2/CMRO2. The 17O RF surface coil used in this study provides optimal sensitivity for detecting the 17O MRSI signal in the cat V1 and surrounding brain regions. In contrast, the detected 17O signal intensity reduces substantially in the brain regions, which are distant from the RF coil, resulting in a low signal-to-noise ratio (SNR). Thus, the first criterion was to exclude the 17O MRSI voxels with low SNR of natural abundance brain water signal from further CMRO2 quantification. The averaged SNR from the 17O MRSI voxels, which were included in CMRO2 quantification was approximately 10:1. The Monte Carlo simulation (unpublished results) has suggested that the CMRO2 fitting error measured by the high-field 17O MRSI approach can reach a few percents if the SNR of natural abundance brain water signal is below 10:1. Thus, the second criterion was to set a threshold of −5% > ΔCMRO2/CMRO2 > 5% for calculating the stimulus-evoked percent CMRO2 change (i.e., ΔCMRO2/CMRO2); and generating the functional metabolic activation maps of ΔCMRO2/CMRO2.
The functional ΔCMRO2/CMRO2 maps were further analyzed in two ways. The first way was to average the baseline and stimulated CMRO2 value, respectively, only from the same brain regions showing a positive stimulus-evoked CMRO2 change; and these averaged CMRO2 values were further used to calculate the averaged value of positive ΔCMRO2/CMRO2. The second way was to calculate the baseline and stimulated CMRO2 value, respectively, averaged from the brain regions showing either a positive or a negative stimulus-evoked CMRO2 change; and these CMRO2 values were used to present the averaged value of net change of ΔCMRO2/CMRO2 in these brain regions.
Functional Magnetic Resonance Imaging Mapping
At first, multislice anatomic images were acquired using a conventional T1-weighted imaging method. On the basis of these anatomic images, slices covering the cat V1 areas were appropriately selected for acquiring fMRI data using multislice gradient echo planar images (EPI) with the following acquisition parameters: repetition time (TR) = 1.65 sec; echo time (TE) = 19 ms; five adjacent axial EPI slices; FOV= 5 × 5 cm2; 780 µm × 780 µm in-plane spatial resolution; 1 mm slice thickness and 2 to 2.5 mm interval between adjacent slices.
The fMRI measurements were based on the block paradigm design: three control and two task periods in an interleaved way. Activation maps were generated using a time-shifted cross-correlation method (Bandettini et al, 1992; Xiong et al, 1995). The activated pixels were identified by correlating the fMRI time course with a trapezoidal function (the modified box-car function with the capability of accounting for hemodynamic delays). This procedure generated typical activation maps in V1, which were consistent across different cats. We chose a statistical threshold of P < 0.01 to generate fMRI activation maps.
The paired t-test was performed for statistical analysis and the results are presented as mean ± s.d.
Results, Discussion, and Conclusions
Stimulus-Evoked Cerebral Metabolic Rate of Oxygen Increase During Brain Activation
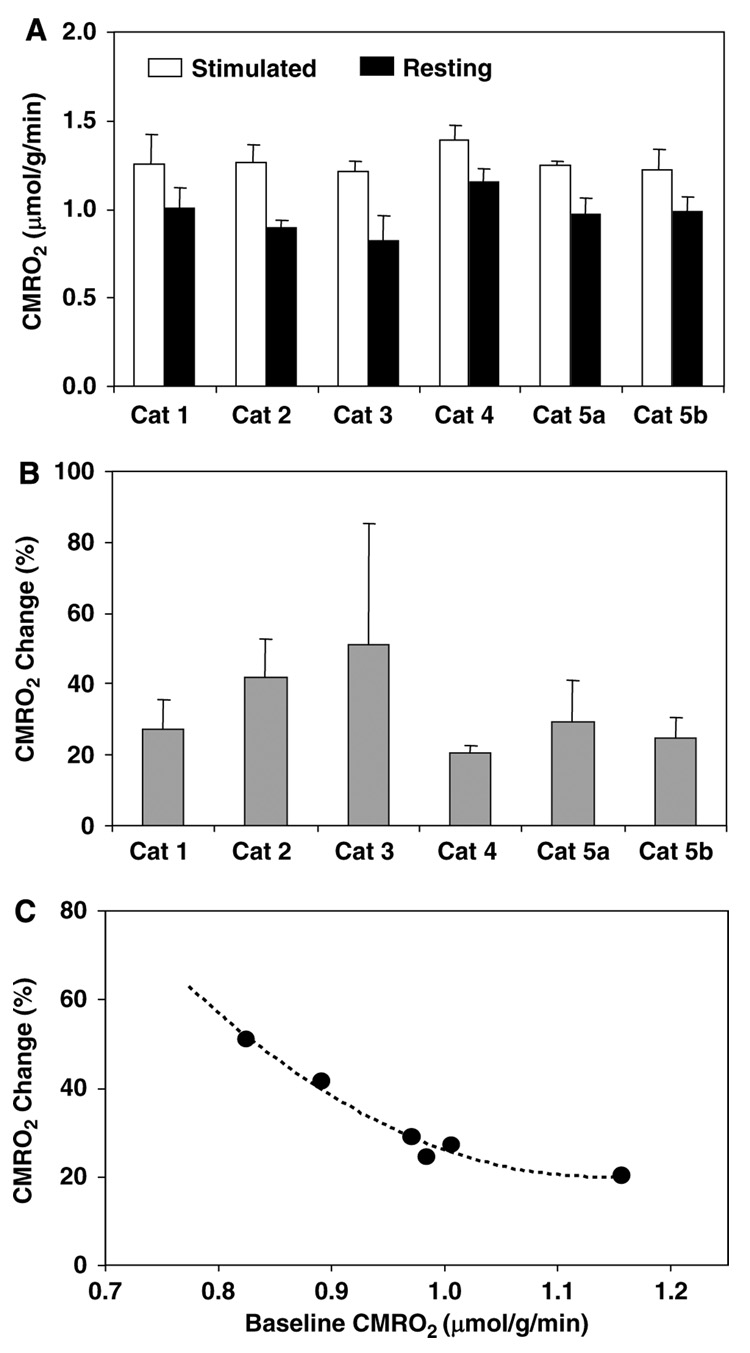
Figure 1 shows the 3D images of absolute CMRO2 values obtained at rest and during visual stimulation, functional metabolic activation maps of ΔCMRO2/CMRO2, BOLD-based fMRI maps, and their corresponding brain anatomic images in four adjacent image slices chosen from 3D image data in a representative cat brain. This figure clearly illustrates a significant CMRO2 increase in the activated brain region in the cat visual cortex (defined as activated V1 region herein) in response to a full-field, binocular grating visual stimulation. The size and location of the activated brain regions depicted in the functional ΔCMRO2/CMRO2 maps largely coincide with those regions determined by fMRI maps showing positive BOLD changes in the same cat brain despite of different spatial resolutions between CMRO2 and BOLD functional images. Figure 2 shows the absolute CMRO2 values averaged over these activated brain regions in different image slices from one representative cat, and the summarized results of six measurements from five cats (one cat having two repeated measurements under control and stimulated conditions). The CMRO2 value averaged spatially from the activated cat V1 region showing a positive change of ΔCMRO2/CMRO2 was 1.26 ± 0.09 µmol/g per min (n = 6) under the visual stimulation condition; and this value is significantly higher than that of 0.97 ± 0.04 µmol/g per min (n = 6) measured at resting condition from the same brain region, indicating a 32.3% ± 10.8% increase of CMRO2 in the activated V1 region (P < 0.005, paired t-test). Figures 3A and 3B summarize the intersubject averaged CMRO2 results measured under resting and stimulated brain states, indicating a substantial CMRO2 increase ranging approximately from 20% to 50% in the activated V1 region in different cats studied (see Figure 3B). These results suggest that the oxidative metabolic activity is significantly elevated in the anesthetized cat brain to support the stimulus-evoked enhancement in ATP production/consumption associated with the increased neuronal activity in the activated V1 region. Unlike previous studies, which relied on BOLD modeling to assess the relative (or percent) CMRO2 changes induced by brain stimulation (Davis et al, 1998; Hoge et al, 1999; Kim et al, 1999), the present study directly imaged the absolute CMRO2 values under both resting and activated brain states, which provide not only a quantitative measure of percent CMRO2 change elevated by visual stimulation but also crucial information regarding the relationship between the absolute CMRO2 change and increased brain activity during activation. The finding of significant CMRO2 increase in the activated brain region observed in the present study reveals a tight neurometabolic coupling during brain activation; suggesting the vital role of oxygen metabolism in supporting the intensified neuronal activity in a working brain.
Figure 1.
Significant CMRO2 increase in activated visual cortex. Three-dimensional CMRO2 maps obtained at resting (second column from the left) and stimulated (third column) conditions; 3D functional metabolic activation maps showing percent changes of CMRO2 elevated by visual stimulation (fourth column); BOLD-based fMRI maps (fifth column); and anatomic brain images (first column) in four adjacent image slices from a representative cat brain.
Figure 2.
Summary of resting and activated CMRO2 and percent changes. Summarized absolute CMRO2 values measured at resting and during visual stimulation (top inserts) and their percent changes (bottom inserts) from four CMRO2 image slices covering the visual cortex of cat anesthetized with isoflurane. (A) Results from a representative cat. (B) Averaged results from six experiments, for the 17O MRSI voxels with only positive CMRO2 change. (C) Averaged results from six experiments for the 17O MRSI voxels with both positive and negative CMRO2 changes. Paired t-test indicates a statistically significant difference between the control and activated CMRO2 values (P < 0.005 using paired t-test for both panels B and C).
Figure 3.
Stimulus-evoked CMRO2 increase and its relation to resting CMRO2. (A) Averaged absolute CMRO2 values for each cat brain at resting and during visual stimulation. (B) The averaged percent CMRO2 increases elevated by visual stimulation. (C) The negative correlation between the percent CMRO2 changes and baseline CMRO2 values. This figure shows that visual stimulation elevates a significant CMRO2 increase in the activated visual cortex of five cats studied (P < 0.005 using paired t-test for both panels A and B), and the percent CMRO2 changes are negatively correlated to the corresponding baseline CMRO2 levels.
Total Brain Energy Expenditure During Brain Activation
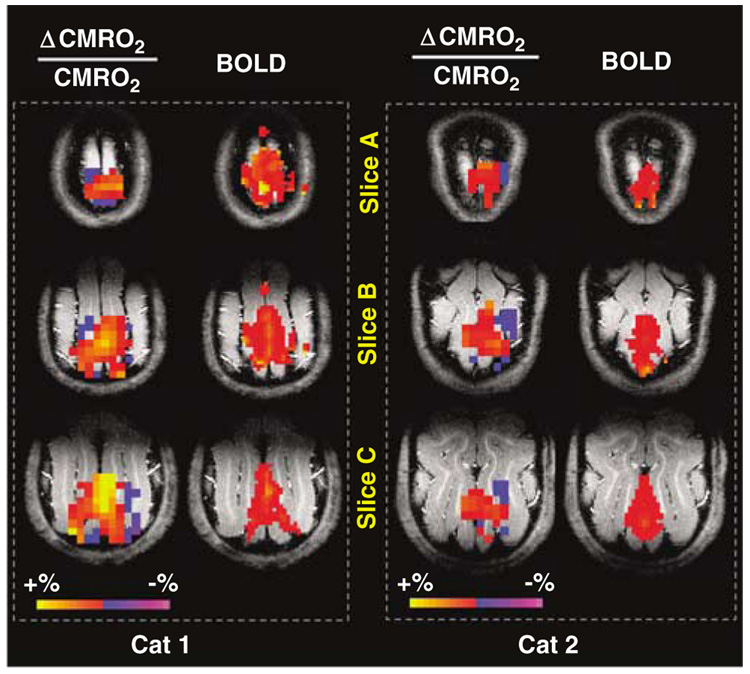
Interestingly, we also consistently detected a CMRO2 decrease in the brain region surrounding the territory showing positive CMRO2 or BOLD change in the cat V1. This phenomenon was observed in all cats, and the functional metabolic CMRO2 image results from two representative cats are shown in Figure 4. When both positive and negative CMRO2 alterations were counted and spatially averaged, the net CMRO2 increase induced by visual stimulation reduced to 9.7% ± 1.9% (n = 6, see Figure 2C) compared with the resting CMRO2 value measured from the same brain regions showing either a significant positive or negative CMRO2 alteration during the visual stimulation; the stimulus-evoked CMRO2 increase was still statistically significant (P < 0.005, paired t-test). This finding reveals that the stimulus-evoked CMRO2 change varied significantly across the examined brain regions and consistently displayed two distinct response patterns with positive and negative CMRO2 alterations in the cat brain during visual stimulation.
Figure 4.
Spatially compensated CMRO2 changes elevated by brain stimulation. Functional CMRO2 maps (show both positive and negative changes) and fMRI BOLD maps during visual stimulation from two representative cats. This figure shows that the CMRO2 increases in the central activated visual cortex regions are commonly accompanied by CMRO2 decreases in surrounding brain regions.
Similar spatial characteristics have been shown by other physiologic measurements based on the hemodynamic changes of CBV and CBF or BOLD contrast. For instance, negative CBV changes surrounding the central visual cortical regions showing stimulus-evoked increases in CBV were previously reported in the cat brain (Harel et al, 2002); and negative changes bordering on the territory of positive changes in BOLD and CBF maps were also observed in the human brain during retinotopic visual stimulation (Shmuel et al, 2002). In the latter, BOLD modeling was used to argue that the negative CBF/BOLD changes could correspond to a decrease in CMRO2 in these brain regions; however, ambiguities in the BOLD modeling was recognized and only a qualitative conclusion was reached (Shmuel et al, 2002).
Our results suggest that CMRO2 indeed decreases in the brain regions surrounding the primary foci of increased activity, suggesting that the neuronal activity in the regions with negative CMRO2 change might be suppressed owing to a tight neurometabolic coupling. Consistent with this notion, reduction in electrophysiologic signals of neuronal activity was recently reported in the negative BOLD regions surrounding the activated visual cortex in the primate (Shmuel et al, 2006). Therefore, these collective lines of evidence suggest that there are similar trends among CMRO2, CBF, CBV, and neuronal activity changes in response to visual stimulation in both activated cortical region and surrounding deactivated cortical region, indicating that a tight coupling among the metabolic and hemodynamic responses to neuronal activity change might qualitatively hold under both activated and deactivated conditions (Raichle and Mintun, 2006).
The negative CMRO2 changes observed during visual stimulation in this study could be driven by oxidative metabolic, other physiologic or neuronal process. Irrespective of the operative mechanism, however, this observed CMRO2 phenomenon may arise to limit total brain energy expenditure superimposed on an expensive energy budget used at the resting state, for instance, to maintain the resting brain network and neuronal connectivity sustained by spontaneous neuronal activity rhythm (Buzsaki and Draguhn, 2004; Fox et al, 2006) and to provide a significant amount of ‘house-keeping’ ATP energy (Du et al, 2008). This means that a resting brain is not truly at ‘rest’ and it costs sizeable brain energy. Thus, the increased energy demand in the activated brain regions could be partially compensated by the reduction of energy usage in other brain regions, leading to a smaller extent of total (or global) brain CMRO2 increase during activation. This energy compensation mechanism could provide a satisfactory explanation for the observation in the present study as well as to the surprising finding from a well-documented work published in 1955, showing no global CMRO2 change in the human brain during an intense mental arithmetic task (Sokoloff et al, 1955). Two speculations were discussed in this early publication to interpret the finding: one was that the total energy requirements of the brain, as reflected by the cerebral oxygen consumption, may be independent of the brain activity change elevated by task performance; the other speculation was that the task performance may lead to a spatial redistribution of neuronal and metabolic activities, that is, brain regions with increased functional and metabolic activities could then be counterbalanced by regions of reduced activities so that the global CMRO2 is unchanged (Sokoloff et al, 1955). Nevertheless, it was difficult to justify these two possibilities by the global CMRO2 measurements (Sokoloff et al, 1955) because lack of information regarding the spatial distribution of positive and negative CMRO2 changes inside the brain. The results from the present study provide vital evidence suggesting that the increased neuronal activity evoked by brain stimulation requires a significantly higher energy demand of oxygen consumption in the activated brain regions than that at rest; however, this increased regional energy demand could be partially compensated by the reduction of energy demand in other brain regions resulting in a smaller increase in CMRO2 over a larger brain volume. This mechanism could be critical for maintaining normal, sustained brain activation in a working brain.
Although, the present study is unable to examine whether the metabolic activity itself or neuronal activity is the primary origin driving the CMRO2 suppression in the surrounding brain regions during visual activation, it has been suggested that the decrease of CMRO2 likely indicates regional depression of synaptic activity (Pasley et al, 2007; Seitz and Roland, 1992; Shmuel et al, 2006).
One important implication of both negative and positive CMRO2 alterations in the nearby brain regions during stimulation as observed in this study is that the averaged stimulus-evoked CMRO2 change (in either relative or absolute scale) becomes substantially smaller when the spatial resolution of CMRO2 imaging is inadequate to differentiate the regions showing negative and positive CMRO2 alterations; with increased imaging voxel size (i.e., reducing spatial resolution) and increased partial volume effect, the change in CMRO2 may even become undetectable. This observation could provide key for potentially reconciling the long-standing controversy in the literature regarding the essential questions: if, and how much CMRO2 changes in response to brain stimulation; and whether the oxygen metabolism is vital in supporting brain work (Barinaga, 1997).
It is interesting to note that although the negative CMRO2 (this study) and CBV (Harel et al, 2002) changes were observed in the surrounding regions of cat visual cortex, the negative BOLD was not robustly seen in all cats in our study. This observation suggests the complexity of the BOLD signal nature owing to its dependence on the interplay among three stimulus-evoked physiologic parameter (i.e., CMRO2, CBF, and CBV) changes as well as on the baseline levels of these parameters as discussed in the following section. Thus, a single CMRO2 parameter may provide a better and more quantitative measure reflecting the brain energy state or the corresponding neuronal activity level if the limited 17O detection sensitivity is not a major concern; and it should be useful for understanding many neuroscience questions, for example, the relationship between brain energy usage and neuronal excitation versus neuronal inhibition (Buzsaki et al, 2007).
Importance of Baseline Cerebral Metabolic Rate of Oxygen
Another striking observation in the present study is that the positive percent CMRO2 changes evoked by visual stimulation were negatively correlated with their corresponding baseline CMRO2 values, as shown in Figure 3C. This finding indicates a strong influence of baseline metabolic activity level on the percent CMRO2 change in response to brain stimulation (Hyder et al, 2002; Pasley et al, 2007; Shulman et al, 2007); thus, the stimulus-evoked percent CMRO2 change alone can become less significant for quantitatively interpreting and fully understanding the neurometabolic relationship if the baseline CMRO2 is not determined.
According to the trend shown in Figure 3C, we could further speculate that the stimulus-evoked CMRO2 percentage change in the awake brains would be less pronounced as compared with the anesthetized brains because a higher baseline level of CMRO2 is expected under the awake state. The visual cortex of the awake human, for instance, has a relatively higher baseline CMRO2 value of ~1.7 µmol/g per min (Fox et al, 1988) compared with the value of 0.97 µmol/g per min in the anesthetized cat visual cortex as measured in the present study. On the basis of Figure 3C and the assumption of similar CMRO2 change behaviors between the human and cat visual cortices in response to visual stimulation, one could expect that the percent CMRO2 increase in the human visual cortex during visual stimulation should be significantly less than 32% observed in the anesthetized cat brain in this study, and certainly substantially smaller than the CBF change of 40% to 50% in the human visual cortex (Fox et al, 1988; Zhu et al, 1998). This notion is consistent with the fact that a hyperoxygenation level in the blood is elevated in the activated brain region because the mismatched CMRO2 and CBF changes, leading to a positive BOLD contrast in fMRI (Ogawa et al, 1990). This estimated range of percent CMRO2 increase in response to visual stimulation is also qualitatively consistent with the literature results reporting a wide range from 5% to 30% in the human visual cortex (Chen et al, 2001; Davis et al, 1998; Fox et al, 1988; Hoge et al, 1999; Kim et al, 1999). However, ultimately studies under the awake state either with the 17O CMRO2 imaging methodology or using this methodology as a calibration for BOLD modeling (Davis et al, 1998; Hoge et al, 1999; Kim et al, 1999; Ogawa et al, 1993) will be necessary to draw definitive conclusions in the human brain.
The possible mechanism to link the negative correlation between the stimulus-evoked percent CMRO2 change and its baseline CMRO2 level shown in Figure 3C can be explained by two possible models with distinct assumptions. The first one assumes that CMRO2 can rise from its baseline level to a higher and constant level in the activated brain region during stimulation (Hyder et al, 2002; Pasley et al, 2007) so that the absolute stimulus-evoked CMRO2 becomes independent of the baseline CMRO2 level. In this case, a relatively low baseline CMRO2 level should result in a larger stimulus-evoked percent increase in CMRO2; this is consistent with the negative correlation in Figure 3C. The second model is to assume that CMRO2 can rise from its baseline level to a higher level but with a constant CMRO2 increment in the activated brain region. On the basis of this model, the stimulus-evoked percent CMRO2 increase will be larger again if the baseline CMRO2 level is relatively low; this scenario will also lead to a similar negative correlation as shown in Figure 3C. Owing to the inevitable variation among animals in the CMRO2 data measured in the present study, these two models are difficult to be justified with statistical confidence; although the first model was slightly favored by our data showing a relatively smaller intersubject variation (quantified by s.d.) in the absolute values of stimulus-evoked CMRO2 compared with that of stimulus-evoked CMRO2 increments.
The tight correlation between the percent CMRO2 change and baseline CMRO2 level as shown in Figure 3C has several profound impacts on both fMRI methodology and functional brain electrophysiology. First, the percent change of stimulus-evoked CMRO2 can vary as a function of baseline CMRO2 level, and the influence of relative (percent) CMRO2 change on the percent change of BOLD signal measured by fMRI could rely on the baseline CMRO2 level, which can vary substantially among subjects or between awaked and anesthetized brains. Therefore, it is critical to consider the implication of baseline CMRO2 level on quantifying the relative changes of stimulus-evoked CMRO2 and/or BOLD (Pasley et al, 2007; Shulman et al, 2007). Second, the correlation shown in Figure 3C implies that a large portion of brain energy consumption remains in a resting brain. This resting brain energy expenditure should be essential for supporting spontaneous brain activity, coherent neuronal activity rhythm, neurotransmission cycling, and other neuronal signaling processes; and it could be superimposed on the extra stimulus-evoked energy change to perform brain work appropriately. This energy cost could also be crucial for supporting the resting brain network and neuronal connectivity sustained by spontaneous neuronal activity rhythm (Buzsaki and Draguhn, 2004; Fox et al, 2006), which has been linked to the slow frequency BOLD signal fluctuation for obtaining functional connectivity maps in a resting brain (Biswal et al, 1995; Fox and Raichle, 2007).
The overall findings lead to an important conclusion that both baseline and stimulus-evoked brain energies should be vital for normal brain function in performing specific tasks, and the fraction of these two brain energy expenditures relies on the baseline metabolic activity level, which could be susceptible among subjects, species, and physiologic/pathologic conditions; and the baseline level can also be manipulated readily by varying experimental conditions, for instance, by changing anesthetic depth. The isoflurane anesthetic applied in the present study could significantly low the baseline CMRO2 activity level in the cat visual cortex, ultimately, lead to a relatively larger increase in the percent CMRO2 change and a large absolute CMRO2 increment during brain stimulation as compared with the waked brain state. If the basal CMRO2 level can be further suppressed by using different anesthetic, for example, α-chloralose (Ueki et al, 1992), the percent CMRO2 increase could be even larger. Thus, the stimulus-evoked percent CMRO2 change is sensitive to the baseline CMRO2 level and can cover a wide range from deeply anesthetized to awaked brains. This notion provides an explanation to the large discrepancy of the CMRO2 percentage changes reported in the literature (e.g., Fox et al, 1988; Hyder et al, 2001).
In addition, our results suggest that both baseline CMRO2 level and the stimulus-evoked percent CMRO2 change are equally important for brain to work properly. Although the baseline CMRO2 level can be easily modulated, the total brain energy may have to be resumed and integrated for supporting brain activation during stimulation if the involved neurons can fully response to the stimulus.
Advantages and Promise of 17O-Based Cerebral Metabolic Rate of Oxygen Imaging Method
Overall findings in this study offer new insights into the underlying physiology of cerebral oxidative metabolism and its essential role in bioenergetics associated with brain activation; they also provide possible clarifications to a number of long-standing neuroscience questions. Moreover, they also highlight the importance of the neuroimaging capability to noninvasively imaging absolute CMRO2 values under both resting and activated brain states with adequate spatial resolution, for instance, to differentiate different brain regions with positive and negative CMRO2 changes in response to visual stimulation as illustrated in this study (Figure 4). This ability is crucial to quantitatively investigate the coupling relationship between the stimulus-evoked neuronal and oxidative metabolic activities. The high-field in vivo 17O MRSI approach for 3D mapping of absolute CMRO2 and its change provides a useful neuroimaging modality with satisfactory capability. In this study, we present the first exploration of this promising neuroimaging approach for noninvasively obtaining 3D functional CMRO2 maps in the cat brain during visual stimulation. The results clearly show the advantages of this approach in several aspects. First, the spatially averaged resting CMRO2 value (0.97 ± 0.04 µmol/g per min) measured in the isoflurane anesthetized cat V1 region in this study is in agreement with the value of 1.02 µmol/g per min reported in the literature (Todd and Drummond, 1984), which was measured in the cats under a similar anesthesia condition using the ‘golden-standard’ Kety–Schmidt method. Second, paired CMRO2 imaging measurements performed twice under resting and stimulated conditions in one cat (Cat 5) during the same experimental session show an excellent level of reproducibility for imaging CMRO2 under both conditions (see Figure 3A). This result suggests that the 17O MRSI detection sensitivity offered by high field is adequate for obtaining reliable 3D functional metabolic CMRO2 maps based on only two paired measurements: one under resting and another under stimulation, without averaging from repeated experiments. Third, based only on paired CMRO2 imaging measurements, functional metabolic activation maps of CMRO2 change were obtained reliably in all animals studied. Fourth, the spatial resolution of 3D CMRO2 image achieved in this study was able to provide distinct characteristics of CMRO2 changes in different brain regions in response to brain stimulation in a relatively small animal model. Another and perhaps the most important one is the merits of noninvasiveness, simplicity, robustness, and fair temporal resolution (a few minutes) offered by the high-field in vivo 17O MRSI approach, which should hold great promise for numerous biomedical applications beyond healthy subjects and normal brain function research.
Owing to the large differences in spatial resolution and voxel shape between the functional CMRO2 and BOLD maps in the present study, the investigation for correlating the stimulus-evoked CMRO2 change and BOLD signal was not pursued in this study. Nevertheless, the superior quality of high-field in vivo 17O MRSI approach for imaging CMRO2 change as shown in this study should be readily combined with MR-based functional imaging approaches for mapping BOLD, CBF, and CBV changes in the same animal model. This ability will provide a unique opportunity for further studying the implications of stimulus-evoked CMRO2 change on neuroimaging signals, in particular, on the BOLD signal; and for improving the reliability of BOLD calibration methods to estimate the percent CMRO2 change (Buxton et al, 2004; Davis et al, 1998; Hoge et al, 1999; Kim et al, 1999; Ogawa et al, 1993).
Acknowledgments
This work was supported in part by NIH Grants NS41262, EB00329, EB00513, P41 RR08079, and P30NS057091; the Keck foundation.
Footnotes
Conflict of interest
The authors declare no competing financial interests.
References
- Arai T, Nakao S, Mori K, Ishimori K, Morishima I, Miyazawa T, Fritz-Zieroth B. Cerebral oxygen utilization analyzed by the use of oxygen-17 and its nuclear magnetic resonance. Biochem Biophys Res Commun. 1990;169:153–158. doi: 10.1016/0006-291x(90)91447-z. [DOI] [PubMed] [Google Scholar]
- Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab. 2001;21:1133–1145. doi: 10.1097/00004647-200110000-00001. [DOI] [PubMed] [Google Scholar]
- Bandettini PA, Wong EC, Hinks RS, Tikofsky RS, Hyde JS. Time course EPI of human brain function during task activation. Magn Reson Med. 1992;25:390–397. doi: 10.1002/mrm.1910250220. [DOI] [PubMed] [Google Scholar]
- Barinaga M. What makes brain neurons run? Science. 1997;276:196–198. doi: 10.1126/science.276.5310.196. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Buxton RB, Uludag K, Dubowitz DJ, Liu TT. Modeling the hemodynamic response to brain activation. Neuroimage. 2004;23 Suppl 1:S220–S233. doi: 10.1016/j.neuroimage.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304:1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Kaila K, Raichle M. Inhibition and brain work. Neuron. 2007;56:771–783. doi: 10.1016/j.neuron.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Zhu XH, Gruetter R, Seaquist ER, Ugurbil K. Study of oxygen utilization changes of human visual cortex during hemifield stimulation using 1H-{13C} MRS and fMRI. Magn Reson Med. 2001;45:349–355. doi: 10.1002/1522-2594(200103)45:3<349::aid-mrm1045>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Davis TL, Kwong KK, Weisskoff RM, Rosen BR. Calibrated functional MRI: mapping the dynamic of oxidative metabolism. Proc Natl Acad Sci USA. 1998;95:1834–1839. doi: 10.1073/pnas.95.4.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du F, Zhu XH, Zhang Y, Friedman M, Zhang N, Ugurbil K, Chen W. Tightly coupled brain activity and cerebral ATP metabolic rate. Proc Natl Acad Sci USA. 2008;105:6409–6414. doi: 10.1073/pnas.0710766105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Zacks JM, Raichle ME. Coherent spontaneous activity accounts for trial-to-trial variability in human evoked brain responses. Nat Neurosci. 2006;9:23–25. doi: 10.1038/nn1616. [DOI] [PubMed] [Google Scholar]
- Fox PT, Raichle ME, Mintun MA, Dence C. Nonoxidative glucose consumption during focal physiologic neural activity. Science. 1988;241:462–464. doi: 10.1126/science.3260686. [DOI] [PubMed] [Google Scholar]
- Harel N, Lee SP, Nagaoka T, Kim DS, Kim SG. Origin of negative blood oxygenation level-dependent fMRI signals. J Cereb Blood Flow Metab. 2002;22:908–917. doi: 10.1097/00004647-200208000-00002. [DOI] [PubMed] [Google Scholar]
- Hendrich K, Hu X, Menon R, Merkle H, Camarata P, Heros R, Ugurbil K. Spectroscopic imaging of circular voxels with a two-dimensional Fourier-Series Window technique. J Magn Reson. 1994;105:225–232. doi: 10.1006/jmrb.1994.1128. [DOI] [PubMed] [Google Scholar]
- Hoge RD, Atkinson J, Gill B, Crelier GR, Marrett S, Pike GB. Linear coupling between cerebral blood flow and oxygen consumption in activated human cortex. Proc Natl Acad Sci USA. 1999;96:9403–9408. doi: 10.1073/pnas.96.16.9403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyder F, Kida I, Behar KL, Kennan RP, Maciejewski PK, Rothman DL. Quantitative functional imaging of the brain: towards mapping neuronal activity by BOLD fMRI. NMR Biomed. 2001;14:413–431. doi: 10.1002/nbm.733. [DOI] [PubMed] [Google Scholar]
- Hyder F, Patel AB, Gjedde A, Rothman DL, Behar KL, Shulman RG. Neuronal-glial glucose oxidation and glutamatergic-GABAergic function. J Cereb Blood Flow Metab. 2006;26:865–877. doi: 10.1038/sj.jcbfm.9600263. [DOI] [PubMed] [Google Scholar]
- Hyder F, Rothman DL, Shulman RG. Total neuroenergetics support localized brain activity: implications for the interpretation of fMRI. Proc Natl Acad Sci USA. 2002;99:10771–10776. doi: 10.1073/pnas.132272299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibaraki M, Miura S, Shimosegawa E, Sugawara S, Mizuta T, Ishikawa A, Amano M. Quantification of cerebral blood flow and oxygen metabolism with 3-dimensional PET and 15O: validation by comparison with 2-dimensional PET. J Nucl Med. 2008;49:50–59. doi: 10.2967/jnumed.107.044008. [DOI] [PubMed] [Google Scholar]
- Kim SG, Rostrup E, Larsson HB, Ogawa S, Paulson OB. Determination of relative CMRO2 from CBF and BOLD changes: significant increase of oxygen consumption rate during visual stimulation. Magn Reson Med. 1999;41:1152–1161. doi: 10.1002/(sici)1522-2594(199906)41:6<1152::aid-mrm11>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Kwong KK, Belliveau JW, Chesler DA, Goldberg IE, Weisskoff RM, Poncelet BP, Kennedy DN, Hoppel BE, Cohen MS, Turner R, Cheng HM, Brady TJ, Rosen BR. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc Natl Acad Sci USA. 1992;89:5675–5679. doi: 10.1073/pnas.89.12.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei H, Ugurbil K, Chen W. Measurement of unidirectional Pi to ATP flux in human visual cortex at 7 T by using in vivo31P magnetic resonance spectroscopy. Proc Natl Acad Sci USA. 2003;100:14409–14414. doi: 10.1073/pnas.2332656100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateescu GD, Yvars GM, Maylish-Kogovsek L, LaManna JC, Lust WD, Sudilovsky D. Oxygen-17 MRI and MRS of the brain, the heart and coronary arteries; Proceeding of Annual Meeting of Society of Magnetic Resonance in Medicine, Amsterdam, the Netherlands; 1989. p. 650. [Google Scholar]
- Mintun MA, Raichle ME, Martin WR, Herscovitch P. Brain oxygen utilization measured with O-15 radiotracers and positron emission tomography. J Nucl Med. 1984;25:177–187. [PubMed] [Google Scholar]
- Mintun MA, Vlassenko AG, Shulman GL, Snyder AZ. Time-related increase of oxygen utilization in continuously activated human visual cortex. Neuroimage. 2002;16:531–537. doi: 10.1006/nimg.2002.1114. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Lee T-M, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci USA. 1990;87:9868–9872. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Menon RS, Tank DW, Kim S-G, Merkle H, Ellermann JM, Ugurbil K. Functional brain mapping by blood oxygenation level-dependent contrast magnetic resonance imaging. Biophys J. 1993;64:800–812. doi: 10.1016/S0006-3495(93)81441-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Tank DW, Menon R, Ellermann JM, Kim S-G, Merkle H, Ugurbil K. Intrinsic signal changes accompanying sensory stimulation: functional brain mapping with magnetic resonance imaging. Proc Natl Acad Sci USA. 1992;89:5951–5955. doi: 10.1073/pnas.89.13.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasley BN, Inglis BA, Freeman RD. Analysis of oxygen metabolism implies a neural origin for the negative BOLD response in human visual cortex. Neuroimage. 2007;36:269–276. doi: 10.1016/j.neuroimage.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekar J, Ligeti L, Ruttner Z, Lyon RC, Sinnwell TM, van Gelderen P, Fiat D, Moonen CT, McLaughlin AC. In vivo measurement of cerebral oxygen consumption and blood flow using 17O magnetic resonance imaging. Magn Reson Med. 1991;21:313–319. doi: 10.1002/mrm.1910210217. [DOI] [PubMed] [Google Scholar]
- Raichle ME. Circulatory and metabolic correlates of brain function in normal humans. In: Mountcastle VB, Plum F, Geiger SR, editors. Handbook of physiology-the nervous system. Bethesda: American Physiological Society; 1987. pp. 643–674. [Google Scholar]
- Raichle ME, Mintun MA. Brain work and brain imaging. Annu Rev Neurosci. 2006;29:449–476. doi: 10.1146/annurev.neuro.29.051605.112819. [DOI] [PubMed] [Google Scholar]
- Seitz RJ, Roland PE. Vibratory stimulation increases and decreases the regional cerebral blood flow and oxidative metabolism: a positron emission tomography (PET) study. Acta Neurol Scand. 1992;86:60–67. doi: 10.1111/j.1600-0404.1992.tb08055.x. [DOI] [PubMed] [Google Scholar]
- Shmuel A, Augath M, Oeltermann A, Logothetis NK. Negative functional MRI response correlates with decreases in neuronal activity in monkey visual area V1. Nat Neurosci. 2006;9:569–577. doi: 10.1038/nn1675. [DOI] [PubMed] [Google Scholar]
- Shmuel A, Yacoub E, Pfeuffer J, Van de Moortele PF, Adriany G, Hu X, Ugurbil K. Sustained negative BOLD, blood flow and oxygen consumption response and its coupling to the positive response in the human brain. Neuron. 2002;36:1195–1210. doi: 10.1016/s0896-6273(02)01061-9. [DOI] [PubMed] [Google Scholar]
- Shulman RG, Rothman DL, Hyder F. A BOLD search for baseline. Neuroimage. 2007;36:277–281. doi: 10.1016/j.neuroimage.2006.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokoloff L. Relationship between functional activity and energy metabolism in the nervous system: whether, where and why? In: Lassen NA, Ingvar DH, Raichle ME, et al., editors. Brain work and mental activity. Copenhagen: Munksgaard; 1991. pp. 52–64. [Google Scholar]
- Sokoloff L, Mangold R, Wechsler RL, Kenney C, Kety SS. The effect of mental arithmetic on cerebral circulation and metabolism. J Clin Invest. 1955;34:1101–1108. doi: 10.1172/JCI103159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd MM, Drummond JC. A comparison of the cerebrovascular and metabolic effects of halothane and isoflurane in the cat. Anesthesiology. 1984;60:276–282. doi: 10.1097/00000542-198404000-00002. [DOI] [PubMed] [Google Scholar]
- Ueki M, Mies G, Hossmann KA. Effect of alpha-chloralose, halothane, pentobarbital and nitrous oxide anesthesia on metabolic coupling in somatosensory cortex of rat. Acta Anaesthesiol Scand. 1992;36:318–322. doi: 10.1111/j.1399-6576.1992.tb03474.x. [DOI] [PubMed] [Google Scholar]
- Xiong J, Gao JH, Lancaster JL, Fox PH. Clustered pixels analysis for functional MRI activation studies of the human brain. Hum Brain Mapp. 1995;3:287–301. [Google Scholar]
- Zhang N, Zhu XH, Lei H, Ugurbil K, Chen W. Simplified methods for calculating cerebral metabolic rate of oxygen based on 17O magnetic resonance spectroscopic imaging measurement during a short 17O2 inhalation. J Cereb Blood Flow Metab. 2004;24:840–848. doi: 10.1097/01.WCB.0000125885.54676.82. [DOI] [PubMed] [Google Scholar]
- Zhu XH, Kim SG, Andersen P, Ogawa S, Ugurbil K, Chen W. Simultaneous oxygenation and perfusion imaging study of functional activity in primary visual cortex at different visual stimulation frequency: quantitative correlation between BOLD and CBF changes. Magn Reson Med. 1998;40:703–711. doi: 10.1002/mrm.1910400510. [DOI] [PubMed] [Google Scholar]
- Zhu XH, Zhang N, Zhang Y, Zhang X, Ugurbil K, Chen W. In vivo17O NMR approaches for brain study at high field. NMR Biomed. 2005;18:83–103. doi: 10.1002/nbm.930. [DOI] [PubMed] [Google Scholar]
- Zhu XH, Zhang Y, Tian RX, Lei H, Zhang N, Zhang X, Merkle H, Ugurbil K, Chen W. Development of 17O NMR approach for fast imaging of cerebral metabolic rate of oxygen in rat brain at high field. Proc Natl Acad Sci USA. 2002;99:13194–13199. doi: 10.1073/pnas.202471399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu XH, Zhang Y, Zhang N, Ugurbil K, Chen W. Noninvasive and three-dimensional imaging of CMRO2 in rats at 9.4 T: reproducibility test and normothermia/ hypothermia comparison study. J Cereb Blood Flow Metab. 2007;27:1225–1234. doi: 10.1038/sj.jcbfm.9600421. [DOI] [PubMed] [Google Scholar]