Abstract
Objective
Therapeutic hypothermia is being clinically employed to reduce neurological deficits after cardiac arrest (CA). Patients receiving hypothermia after CA receive a wide-array of medications. During hypothermia, drug metabolism is markedly reduced. Little, however, is known about the impact of hypothermia on drug metabolism after re-warming. The objective of this study was to examine the effect of CA and hypothermia on the functional regulation of two major drug metabolizing cytochrome P450 (CYP) isoforms.
Design
Laboratory investigation.
Setting
University pharmacy school and animal research facility.
Subjects
Thirty-six male Sprague-Dawley rats.
Interventions
Hypothermia was induced via surface cooling in a rat CA model and maintained for 3h. Animals were sacrificed at 5 or 24h and liver was analyzed for hepatic activity and mRNA expression of CYP3A2 and CYP2E1. Plasma interleukin-6 (IL-6) concentrations were determined. The effect of IL-6 on PXR mediated transcription of the rat CYP3A2 promoter was evaluated via luciferace reporter in HepG2 cells.
Measurements and Main Results
At 24h after CA a decrease in CYP3A2 and CYP2E1 activity was observed, 55.7±12.8% and 46.8±29.7% of control, respectively (p<0.01). CA decreased CYP3A2 mRNA(p<0.05), but not CYP2E1 mRNA. Expression of other PXR target enzymes and transporter genes were similarly down-regulated. CA also produced a ∼10-fold increase in plasma IL-6. CA mediated inhibition of CYP3A2 and CYP2E1 was attenuated by hypothermia, as was the increase in IL-6. Furthermore, IL-6 attenuated PXR-mediated transcription of the CYP3A2 promoter.
Conclusions
CA produces CYP3A2 down-regulation at 24h, potentially via IL-6 effects on PXR-mediated transcription. Also, hypothermia attenuates the CA mediated down-regulation, thereby normalizing drug metabolism after re-warming.
Keywords: Heart Arrest, Pharmacology, Metabolism, Interleukin-6, cytochrome P450, Pregnane-X-Receptor
Introduction
Mild hypothermia improves long-term neurological outcome and reduces mortality when applied after out-of-hospital cardiac arrest (CA).(1, 2) Benefits have also been observed in stroke,(3) traumatic brain injury (TBI),(4) and perinatal asphyxia with up to a 72 h duration of hypothermia.(5) Results from these trials have led to increasing clinical implementation with increased interest in the factors that may influence the outcome of safety and efficacy trials.(6)
CA patients receive an extensive pharmacotherapeutic regimen. Many of the medications are eliminated by hepatic cytochrome P450 (CYP) metabolism. Some of the drugs that rely on CYP elimination include amiodarone, calcium channel blockers, fentanyl, and midazolam. Several of these medications produce adverse drug reactions, which are difficult to diagnose in critically ill patients. During cooling (immediately applied after injury), CYP-mediated metabolism of drugs is decreased.(7-9) However, alterations in CYP metabolism in experimental models after re-warming have not been reported.
The CYP3A family metabolizes greater than 50% of clinically relevant drugs in humans.(10) CYP3A2 is regulated through transcriptional mechanisms, including via the pregnane X receptor (PXR).(11) CYP2E1 is also constitutively expressed in the rat liver and catalyzes the oxidation of drugs and environmental contaminants.(12, 13) Disease states with a potent acute phase response decrease the expression and activity of CYP3A2 and CYP2E1.(14, 15) The down-regulation of these enzyme systems is believed to be mediated by pro-inflammatory cytokines which decrease the basal transcriptional rates.
Based on these previous studies, we set out to examine the effects of CA and therapeutic hypothermia on hepatic CYP3A2 and CYP2E1 isoforms after re-warming. The purpose of this study was to examine the effects of CA and moderate, transient hypothermia on the functional activity and mRNA expression of CYP3A2 and CYP2E1 in the rat liver at 5 and 24 h after injury and to determine the effects of CA with and without hypothermia on the regulation of PXR target genes in the rat liver. We also evaluated the potential role for increased cytokines after CA on the transcriptional regulators, expression, and functional activity of CYP3A2 and CYP2E1. Moderate hypothermia for 3 h was selected as a proof-of-concept study to determine if CA in the presence or absence of hypothermia alters drug metabolism.
Materials and Methods
Materials and Chemicals
6β-hydroxytestosterone (6β-OH TST) and 11β-hydroxytestosterone (11β-OH TST) were purchased from Steraloids (Newport, RI). Solutions for nitric oxide analysis were purchased from EiCOM Corporation (San Diego, CA). Organic solvents were purchased from Fisher Scientific (Pittsburgh, PA). All other chemicals were purchased from Sigma Aldrich.
Animal CA and Hypothermia Protocol
The University of Pittsburgh Animal Care and Use Committee approved all studies. Adult male Sprague-Dawley rats (body weight, 300-400g) were purchased from Harlan Laboratories (Indianapolis, IN). CA was produced as previously described by Katz et al.(16) as modified by Fink et al.(17) Rats were anesthetized with 3%halothane, endotracheally intubated, and mechanically ventilated. Halothane concentration was then reduced to 0.5 to 1%. Mean arterial pressure was measured via the right femoral artery and the femoral veins were cannulated bilaterally for drug administration and sampling. Neuromuscular blockade was induced with vecuronium 2mg/kg. Body temperature was measured by rectal probe. Halothane was washed out with 100%O2 for 3 min and room air for 2 min and rats were randomized to receive anesthesia control normothermia (control), CA with normothermia (CA normothermia), or CA with hypothermia (CA hypothermia). Animals received a second dose of vecuronium 1mg/kg IV and CA was initiated by disconnection of mechanical ventilation. Asphyxial arrest was conducted for 8 min, with ∼5 min of asystole, confirmed by ECG.(17) Resuscitation was started by ventilator reconnection, IV epinephrine (0.005mg/kg), IV bicarbonate (1mEq/kg), and performing manual chest compressions for 1 min until restoration of spontaneous circulation. Halothane was not resumed after asphyxiation based on the known burst-suppression pattern on EEG.(17) Arterial blood gases and glucose were determined before asphyxia and at the end of re-warming. Acidosis was corrected via bicarbonate to maintain pH at 7.4.
After resuscitation, rats were maintained at 37°C or temperature was decreased to 30°C via surface cooling. Rats remained hypothermic for 3 h followed by 1 h of re-warming. Control rats were maintained at 37°C and received the same surgery protocol without asphyxiation.
Rats were randomized to be sacrificed at 5 h (1 h after re-warming) or 24 h after injury (20 h after re-warming) and liver tissue was excised. Blood samples (0.3ml) were obtained at 60, 70, 90, 120, and 180 min after injury. Rats randomized to the 24 h sacrifice group were weaned from ventilation at the end of re-warming. Thirty-six rats were used for this study, thereby, providing an n=6 per group (control, CA normothermia, and CA hypothermia) at the 5 h and 24 h times, respectively.
Microsomal Preparation
Liver tissue was homogenized and microsomes were prepared by standard methods.(18) Total protein was determined by the method of Lowry et al.(19)
CYP3A2 Activity Analysis
Microsomal incubations contained 400μg protein, 1mM NADPH and 250μM TST in a 1ml volume. Samples were incubated for 10 min at 37°C in a water bath. Reactions were stopped on ice and addition of internal standard, 11β-OH TST (164μmol/L). 6β-OH TST was determined as described previously.(20) 6β-OH TST, 11β-OH TST, and TST were separated using a NovaPak C-18 (5μm, 3.9×150mm) column with UV detection at 254nm. The ratio of 6β-OH TST to 11β-OH TST was used for quantitation.
CYP2E1 Activity Analysis
Microsomal incubations contained 400μg protein, 1mM NADPH and 400μmol/L of CZN. Samples were incubated for 20 min at 37°C in a water bath. Reactions were stopped by adding 50μl of 42.5% o-phosphoric acid and internal standard, umbelliferone (78μmol/L). 6-OH CZN was measured as described by Rockich et al.(18) CZN, umbelliferone, and 6-OH CZN were separated using a NovaPak C-18 (5μm, 3.9×150mm) column. UV-detection was set at 287nm for CZN and 296nm for 6-OH CZN. The ratio of CZN or 6-OH CZN to umbelliferone was used for quantitation.
Northern Blot Analysis and Real-time PCR
Total RNA was prepared from liver tissue using TRIZOL reagent (Invitrogen). Northern hybridization was carried out as previously described.(21) The probes of CYP3A2, CYP2E1 were cloned from wild-type mouse liver mRNA as described (22). Northern blot analysis was performed as previously described.(21, 23, 24) The membranes were stripped and re-probed with GAPDH cDNA as loading control.(25) Real-time PCR using predesigned Assay-On-Demand TaqMan reagents or SYBR Green was performed with the ABI 7300 Real-Time PCR System.(26) Sequences for the real-time PCR oligonucleotides are available upon request.
Analysis of IL-6 plasma concentrations
IL-6 was measured in the plasma using an enzyme linked immunosorbent assay kit specific for Rat IL-6 (R&D Systems, Minneapolis, MN). The optical density was determined using a Victor-2 Plate Reader (PerkinElmer; Shelton, CT) set at 450nm plus a correction at 540 to 570nm.
Plasmid and Transient Transfection
The reporter plasmid (PGL3-CYP3A2-Luc) and the expression vector for the human pregnane X receptor (CMX-hPXR) have been described.(23) Transient transfection was performed on HepG2 cells as described.(21, 24, 26) For each well, the plasmid- Lipofectamine 2000 complex was formed by incubating 2.4μg of expression vector for the nuclear receptor and 4.8μg of reporter gene and 1.5μ g ofβ-gal plasmid with 60μl of Lipofectamine 2000 at room temperature for 20min in serum-free Eagle’s Minimum Essential Medium (EMEM). After 12h of incubation, the transfection medium was replaced with EMEM that contains 10% fetal bovine serum and appropriate solvent or drugs. Cells were lysed 24h later and assayed for luciferase and β-gal activities. Luciferase activity was normalized against the co-transfected and β-galactosidase activities.
Statistical Analysis
Physiological variables and IL-6 concentrations were compared via repeated measures one-way analysis of variance (ANOVA) with a Bonferroni correction. CYP2E1, CYP3A2, and PXR measures within each time period were compared via one-way ANOVA with a Bonferroni correction. Comparisons were made between control, CA normothermia, and CA hypothermia groups within each sacrifice time with 6 rats per treatment group. All analyses were conducted using the software program Prism (GraphPad Software, San Diego, CA).
Results
Physiologic Variables
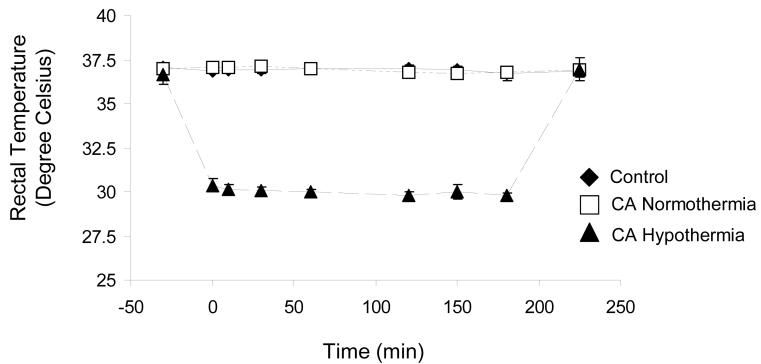
CA hypothermia rats were cooled to 30°C over 30 min and maintained for 3h followed by 1h of re-warming (Figure 1). Physiological parameters (MABP, blood pH, PaO2, PaCO2, and glucose) were measured (Table 1). No differences in physiologic parameters were observed before CA. Post-CA, the CA normothermia and CA hypothermia groups had an anticipated increase in PaO2 and decrease in PaCO2 (***p<0.01).(17) Furthermore, post-CA/hypothermia values of PaO2 and PaCO2 in CA normothermia and CA hypothermia groups were significantly different from control (***p<0.001).
Figure 1.
Time-course of rectal temperature beginning 15 min after asphyxial arrest, and ending after 3 hrs of moderate hypothermia plus 1 hr of re-warming. Control and Cardiac Arrest Normothermia rats were maintained at 37°C, and CA Hypothermia rats were maintained at 30°C for 3 hrs followed by 1 hr of re-warming.
Table 1.
Physiological variables in control, cardiac arrest normothermia and hypothermia rats
| Experimental Group | Pre-CA/hypothermia | Post-CA/hypothermia | |
|---|---|---|---|
| MABP (mmHg) | Control | 98.58 ± 8.55 | 102.8 ± 13.7 |
| CA Normothermia | 105.0 ± 13.7 | 122.5 ± 20.1 | |
| CA Hypothermia | 105.8 ± 8.49 | 111.7 ± 34.5 | |
| pH | Control | 7.37 ± 0.022 | 7.36 ± 0.077 |
| CA Normothermia | 7.38 ± 0.032 | 7.39 ± 0.047 | |
| CA Hypothermia | 7.38 ± 0.19 | 7.37 ± 0.085 | |
| PaCO2 (mmHg) | Control | 39.93 ± 3.84 | 39.85 ± 7.83 |
| CA Normothermia | 38.16 ± 2.55 | 25.22 ± 6.89***,‡‡‡ | |
| CA Hypothermia | 38.50 ± 3.50 | 21.81 ± 5.71 ***,‡‡‡ | |
| PaO2 (mmHg) | Control | 244.4 ± 15.7 | 227.38 ± 58.7 |
| CA Normothermia | 236.3 ± 25.8 | 417.54 ± 76.2 ***,‡‡‡ | |
| CA Hypothermia | 234.4 ± 38.1 | 463.54 ± 67.4 ***,‡‡‡ | |
| Glucose (mg/dL) | Control | 114.5 ± 20.2 | 121.9 ± 27.1 |
| CA Normothermia | 120.6 ± 32.5 | 108.5 ± 24.4 | |
| CA Hypothermia | 116.3 ± 16.9 | 136.5 ± 28.2 |
Statistically significant differences: p<0.001 represent differences of Pre-CA/hypothermia to Post-CA/hypothermia.
p<0.001 represents differences between groups Post-CA/hypothermia.
Effect of Cardiac Arrest and Hypothermia on 6β-hydroxytestosterone Formation Rate
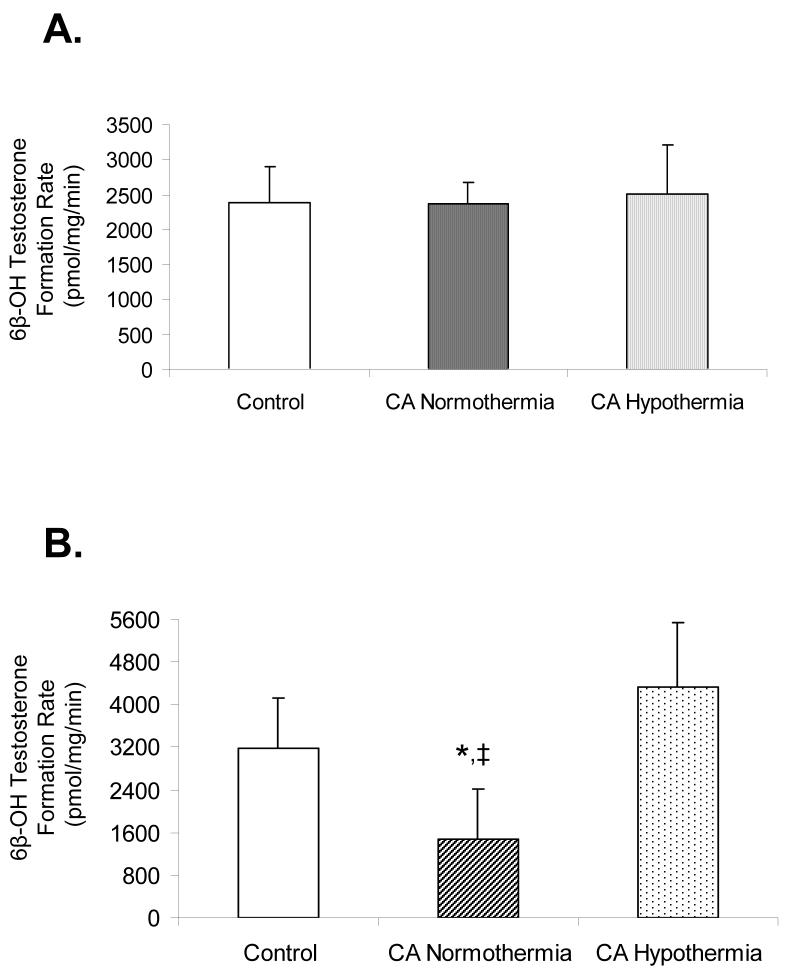
Figure 2, Panel A depicts the microsomal 6β-OH TST formation rate in control, CA normothermia, and CA hypothermia (n=6 per group) rats at 5h after injury. 6β-OH TST formation rates were not different between control (2388±513pmol/mg/min), CA normothermia (2374 ± 299pmol/mg/min), and CA hypothermia (2521±689pmol/mg/min) groups. Figure 2, Panel B depicts the microsomal formation rate of 6β-OH TST (n=6 per group) rats at 24h after injury. 6β-OH TST formation rate was decreased in the CA normothermia compared to control rats (1484±942.9pmol/mg/min vs. 3174±965.4pmol/mg/min;*p<0.05, respectively). 6β-OH TST formation rate (4345±1198pmol/mg/min) was not different between CA hypothermia and control rats, however a difference was observed between CA hypothermia and CA normothermia rats; ‡p<0.05.
Figure 2.
6β-hydroxytestosterone formation rate as a measure of CYP3A2 functional activity in liver microsomes from control, CA normothermia, and CA hypothermia treated rats. Panel A shows 6β-hydroxytestosterone formation rate in rats sacrificed at 5 h after injury (1 hr after re-warming). No differences were observed between groups. Panel B shows 6β-hydroxytestosterone formation rate in rats sacrificed at 24 h after injury (20 h after re-warming). A significant decrease in 6β-hydroxytestosterone formation rate in CA normothermia rats was observed compared to both control and CA hypothermia;‡p<0.05.
Effect of Cardiac Arrest and Hypothermia on CYP3A2 Expression
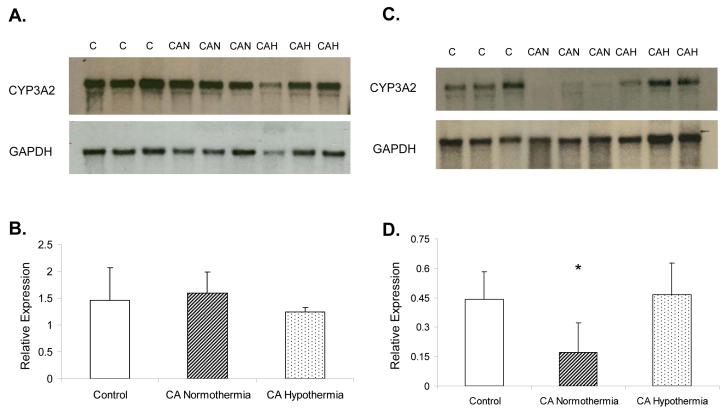
Rats sacrificed at 5 h after injury in all three groups showed no difference in CYP3A2 mRNA expression (Figure 3, Panel A and B). However, a decrease in CA normothermia group CYP3A2 mRNA expression compared to both control and CA hypothermia was observed at 24h (Figure 3, Panel C and D; n=6 per group; *p<0.05). No difference was observed at 24h in the CA hypothermia rats vs control.
Figure 3.
CYP3A2 mRNA hepatic expression in control, CA normothermia, and CA hypothermia rat liver. Panel A shows CYP3A2 mRNA expression at 5 h after injury. Panel B shows no difference in band density between all 3 groups(n=6). Panel C shows CYP3A2 mRNA expression at 24h after injury. Panel D shows a significant decrease in CYP3A2 relative hepatic expression in the CA normothermia compared to control and CA hypothermia;*p<0.05.
Effect of Cardiac Arrest and Hypothermia on 6-hydroxychlorzoxazone Formation Rate
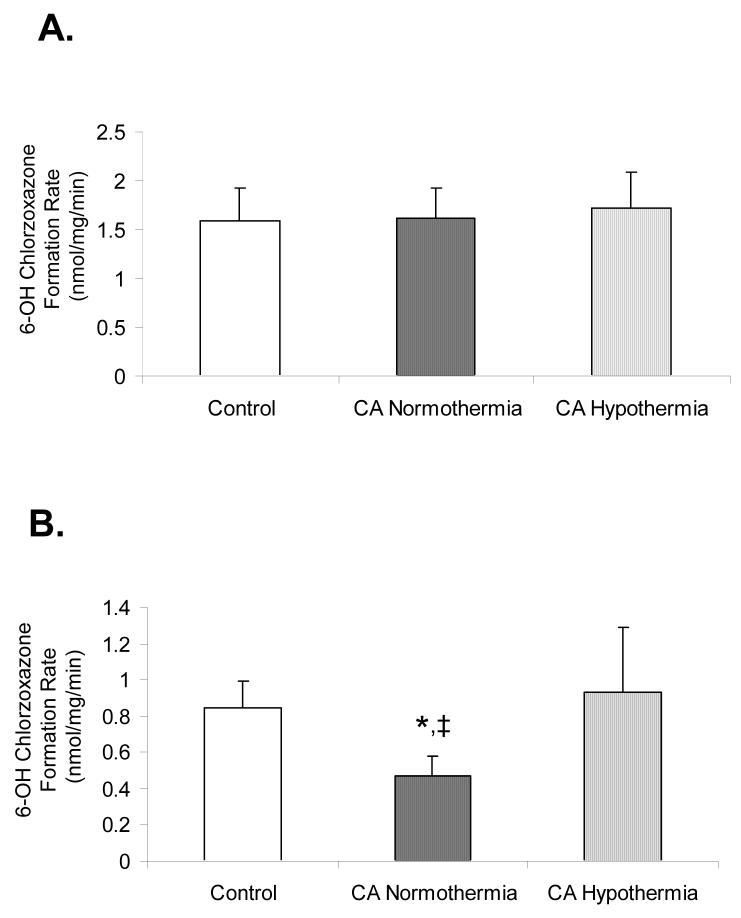
No significant difference in 6-OH CZN formation rate was observed between control (1.59±0.34nmol/mg/min), CA normothermia (1.62±0.31nmol/mg/min), and CA hypothermia (1.73±0.36nmol/mg/min) groups (Figure 4 Panel A). At 24h, CA normothermia showed a significant decrease in 6-OH CZN formation rate (0.47±0.10nmol/mg/min) vs control (0.85±0.14nmol/mg/min) or CA hypothermia rats (0.93±0.36nmol/mg/min), whereas, 6-OH CZN formation rate was not different between CA hypothermia and control (Figure 4 Panel B).
Figure 4.
6-hydroxychlorzoxazone formation rate as a measure of CYP2E1 functional activity in liver microsomes from control, CA normothermia, and CA hypothermia treated rats. Panel A shows 6-hydroxychlorzoxazone formation rate at 5 h after injury. No differences were observed between groups. Panel B shows 6-hydroxychlorzoxazone formation rate at 24 h after injury. Activity in CA normothermia was significantly decreased compared to both control and CA hypothermia; ‡p<0.05.
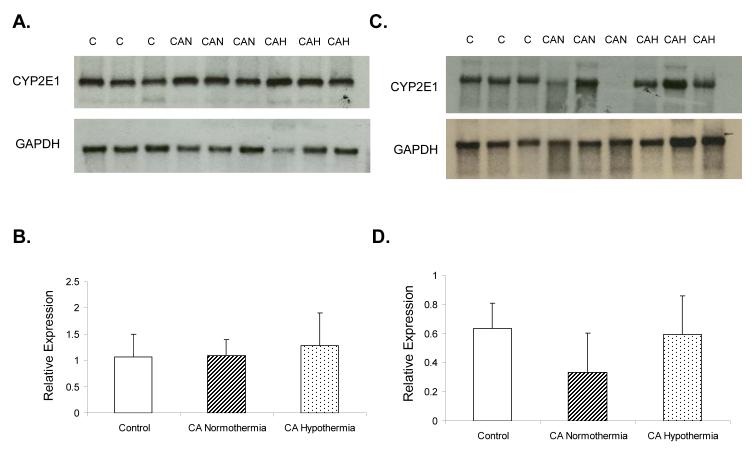
Effect of Cardiac Arrest and Hypothermia on CYP2E1 mRNA Expression
Rats sacrificed at 5h after injury showed no difference in CYP2E1 mRNA expression (Figure 5, Panel A and B; n=6 per group). A non-significant trend towards a decrease in CYP2E1 mRNA expression was observed at 24h between CA normothermia vs control and CA hypothermia (Figure 5, Panel C and D).
Figure 5.
CYP2E1 mRNA hepatic expression in control, CA normothermia, and CA hypothermia rat liver. Panel A shows CYP2E1 mRNA expression at 5 h after injury. Panel B shows no difference between groups(n=6). Panel C shows CYP2E1 mRNA expression at 24 h after injury. Panel D shows CYP2E1 relative hepatic expression in the CA normothermia rats compared to control and CA hypothermia. No significant difference was observed between treatment groups.
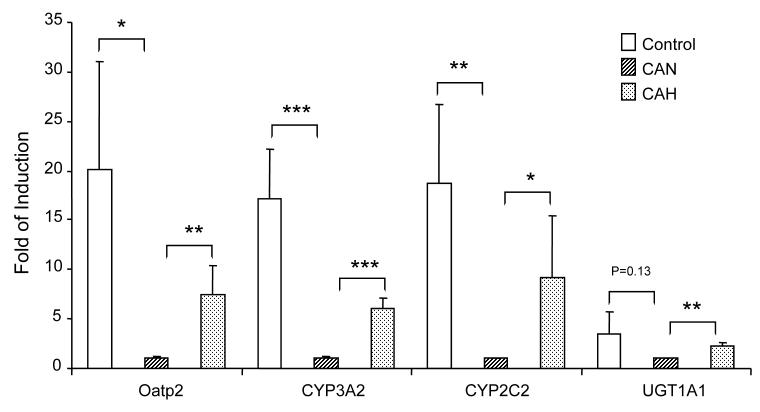
Effect of Cardiac Arrest and Hypothermia on the Expression of Pregnane X Receptor (PXR) Target Genes
The mRNA expression of an organic anion transporter (Oatp2), UDP- glucuronosyltransferase (UGT1A1), and two CYP genes that are known to have common regulatory control by the pregnane X receptor were evaluated in the rat liver. Figure 6 depicts the mRNA expression of Oatp2, UGT1A1, CYP3A2, and CYP2C2 in the liver from control, CA normothermia, and CA hypothermia rats. A significant reduction in mRNA expression of Oatp2, CYP3A2, and CYP2C2 was observed in CA normothermia as compared to control animals. The expression of Oatp2, UGT1A1, CYP3A2, and CYP2C2 was increased in rats that received hypothermia after CA vs normothermia.
Figure 6.
Expression levels of PXR target genes were evaluated to determine the effect of CA and hypothermia on drug metabolism and detoxification pathways. Real-time PCR analyses of liver RNA levels were determined from control, CA normothermia, and CA hypothermia groups. The hepatic expression of Oatp2, CYP3A2, CYP 2C2, and UGT1A1 are presented as average with standard division from 4-6 rats per treatment. (*p<0.05,**p<0.01,***p<0.001).
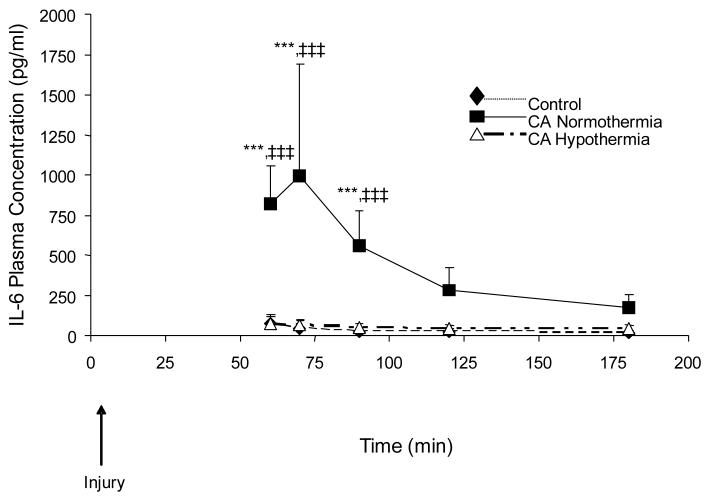
Effect of Cardiac Arrest and Hypothermia on IL-6 Concentrations in Rat Plasma
Figure 7 depicts the time-course of plasma concentrations of IL-6 in 3 treatment groups, control, CA normothermia, and CA hypothermia; n=6 per group. CA normothermia produced increases in IL-6 concentrations compared to control at 60, 70, and 90min after injury;***p<0.001. When hypothermia was applied in the CA model, the rise in IL-6 plasma concentrations was attenuated and did not differ from control.
Figure 7.
Plasma IL-6 levels in control, CA normothermia, and CA hypothermia after injury. CA normothermia IL-6 plasma concentrations at 60, 70, and 90 min after injury were significantly increased as compared to control or CA hypothermia (***p<0.001). The CA mediated increase in plasma IL-6 was attenuated in the CA hypothermic animals.
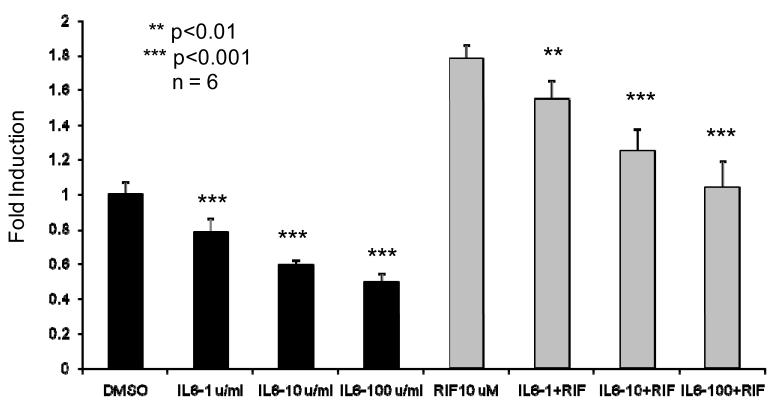
Effect of IL-6 on hPXR Expression of CYP3A2 in HepG2 Cells
Figure 8 depicts the dose dependent effect of IL-6 on luciferase reporter plasmid expression in the presence and absence of rifampin. Data demonstrates that IL-6 (1 unit — 100 units) significantly inhibits both the basal and inducible expression of the PGL3-rCYP3A2-Luc natural promoter. An IL-6 dose dependent reduction in luciferase activity was observed in both vehicle control and rifampin induced expression of the natural promoter.
Figure 8.
Co-transfection of hPXR and pGL3-rCYP3A2 natural promoter treated by human IL6 in HepG2 cells showed suppression of PXR activation. HepG2 cells were cotransfected with 1.4kb natural promoter pGL3-rCYP3A2-Luc reporter and human PXR. Transfected cells were treated with indicated IL6 concentration and/or rifampicin 10mM for 24 h before luciferase assay. Results are shown as —fold induction over vehicle control, which are represented as mean ± standard deviation from hexaplicate assay.
Discussion
We report four significant findings. First, CA significantly decreases CYP3A2 and CYP2E1 activity 24h after injury. Second, in IL-6 plasma concentrations increase after CA. Third, moderate hypothermia prevented the down-regulation of CYP3A2 and CYP2E1-metabolism and blocked the early increase in IL-6 after CA. Fourth, multiple PXR target genes follow a similar pattern of altered expression, which may be due to the effects of IL-6 on PXR activity in the gene regulatory region genes. These findings suggest that by decreasing IL-6 concentrations after CA, hypothermia may favorably alter the course of disease and normalize CYP-drug metabolism.
CA produces a decrease in hepatic activity and expression of CYP3A2 and CYP2E1
CA decreased the activity of both CYP3A2 and CYP2E1 at 24h. This change was paralleled by a decrease in CYP3A2 mRNA. This is the first reported results on CA effects on CYP-mediated metabolism; however studies in other injury models show decreased functional expression of CYP isoforms.(15, 27, 28) Toler et al. showed that cortical concussion decreases hepatic CYP2C11 and CYP3A mRNA expression to ∼50% at 24h.(29) Similarly, we reported a significant reduction in hepatic CYP2E1 activity at 48h after TBI.(30) These studies suggest that ischemic and traumatic injuries down-regulate CYP activity via changes in gene expression. These results are important given that amiodarone, fentanyl, and midazolam are highly dependent on CYP3A for the majority of their elimination.
CA produces increases in IL-6 plasma concentrations that are attenuated by hypothermia after injury
We found that CA produced a ∼10-fold increase in IL-6 plasma concentrations after injury. These results are consistent with clinical studies that have described CA as a sepsis-like syndrome.(31, 32) IL-6 a mediator of the hepatic acute phase response and a potent inhibitor of CYPs.(33) Other ischemia models have shown that increased plasma IL-6 after injury is associated with decreased CYP activity.(15) The addition of IL-6 to hepatocytes decreases the CYP2E1 and CYP3A2 mRNA.(34) Our data also show that hypothermia attenuated the CA mediated increase in IL-6. Prior studies show that hypothermia reduced IL-6 levels in animal models of TBI and hemorrhagic shock.(35) These data are consistent with the putative role of cytokines in the metabolic phase after CA, as proposed by Weisfeldt and Becker.(36) Thus, cytokine alterations after a global ischemic insult are likely to contribute to the reduction in the functional regulation of CYP3A2 and hypothermia attenuates this pathological response.
Moderate hypothermia provides a protective effect on CYP3A2 and CYP2E1 activity and expression
Moderate hypothermia attenuated the CA-mediated reduction in CYP3A2 and CYP2E1. These results are consistent with a study in TBI patients where the systemic clearance of phenytoin was reduced during cooling; however, after re-warming, the systemic clearance returned to normal. (37) Similarly, Nishida et al. recently reported that hepatic metabolism and biliary excretion was reduced during hypothermia, whereas, renal filtration was unchanged at 32°C.(38) These studies suggest that the results in our model parallel observations in the limited number of clinical reports.
Our data suggest that several PXR target genes are similarly regulated and that IL-6 reduces PXR functional activity. IL-6 produces a greater reduction in Oatp2 and CYP3A11 expression in the PXR+/+ mice vs the PXR-/-; thereby suggesting that IL-6 mediated downregulation is via the PXR pathway.(39) Previous studies in hPXR transfected HepG2 cells containing the pGL3–3A4-Luc reporter demonstrated that lipopolysaccharide and TNFα downregulation of CYP3A4 transcription is dependent on cytokine activation of NFκB.(40, 41) These studies suggest that NFκB directly inhibits the binding of PXR to its target regulatory sequence, thereby, inhibiting PXR responsive gene transcription. Future studies to delineate the potential relationship between individual cytokines, NFκB and PXR-regulated genes will yield additional insight into these mechanisms.
Limitations
One consideration in the interpretation of this work is the fact that these studies were conducted under moderate hypothermic temperatures (30°C) for 3h. Temperatures of 30°C were targeted due to the 3h duration in this rat model. Clinical hypothermia for CA is conducted at mild hypothermic temperatures (32 - 35°C) for 12 to 72h. Whereas, deep hypothermia during cardiac surgery targets core temperatures well below 30°C for short intervals. Therefore, 30°C temperatures were selected to maximize the effect of hypothermia given the short duration of hypothermia used. Our results provide proof-of-concept by demonstrating that therapeutic hypothermia attenuates the cytokine-mediated effects of CA on drug metabolizing enzymes. Future studies to delineate the effects of mild hypothermia for longer durations are needed.
Conclusions
We report that in addition to the known reduction in drug metabolism during therapeutic hypothermia, this therapy mitigates the pathological reduction in metabolism after re-warming in the experimental CA rat model. Hypothermia also attenuates the large increases in plasma IL-6 concentration after CA. Although clinical data are needed to fully understand the effects of hypothermic therapy on the disposition of medications in CA patients, our data suggest that hypothermia-drug metabolic alterations are time dependent with reductions in metabolism during cooling and protection from CA mediated down-regulation after re-warming. Thus, hypothermia-drug metabolic interactions are time dependent and are an important consideration in the pharmacotherapy of CA patients both during temperature reduction and after re-warming.
Acknowledgments
The authors would like to acknowledge John Melick for his technical expertise in conducting this work. The authors would also like to thank Dr. Dianxu Ren for his expertise in providing the biostatistical review for this manuscript. Finally, the authors would also like to thank David Toma for his technical contributions.
Financial Support: This work was supported in part by grants from the National Institute of General Medical Sciences (Primary) and the National Heart, Lung, and Blood Institute (Secondary) GM073031(SMP) and the National Center for Research Resources S10 RR023461(SMP). National Institute of Neurological Disorders and Stroke NS30318(PMK) and NS 38087(PMK).
Footnotes
Disclosures: Dr. Kochanek is a co-patent holder for emergency Preservation and Resuscitation (EPR), a method that involves the use of therapeutic hypothermia.
References
- 1.Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346(8):557–563. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 2.The Hypothermia After Cardiac Arrest Study (THACAS) Group Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346(8):549–556. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 3.Guluma KZ, Oh H, Yu SW, et al. Effect of Endovascular Hypothermia on Acute Ischemic Edema: Morphometric Analysis of the ICTuS Trial. Neurocritical care. 2008;8(1):42–47. doi: 10.1007/s12028-007-9009-z. [DOI] [PubMed] [Google Scholar]
- 4.Marion DW, Penrod LE, Kelsey SF, et al. Treatment of traumatic brain injury with moderate hypothermia. N Engl J Med. 1997;336(8):540–546. doi: 10.1056/NEJM199702203360803. [DOI] [PubMed] [Google Scholar]
- 5.Shankaran S, Laptook AR, Ehrenkranz RA, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353(15):1574–1584. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- 6.Zhao H, Steinberg GK, Sapolsky RM. General versus specific actions of mild-moderate hypothermia in attenuating cerebral ischemic damage. J Cereb Blood Flow Metab. 2007;27(12):1879–1894. doi: 10.1038/sj.jcbfm.9600540. [DOI] [PubMed] [Google Scholar]
- 7.Tortorici MA, Kochanek PM, Bies RR, et al. Therapeutic hypothermia-induced pharmacokinetic alterations on CYP2E1 chlorzoxazone-mediated metabolism in a cardiac arrest rat model. Crit Care Med. 2006;34(3):785–791. doi: 10.1097/01.ccm.0000201899.52739.4f. [DOI] [PubMed] [Google Scholar]
- 8.Fukuoka N, Aibiki M, Tsukamoto T, et al. Biphasic concentration change during continuous midazolam administration in brain-injured patients undergoing therapeutic moderate hypothermia. Resuscitation. 2004;60(2):225–230. doi: 10.1016/j.resuscitation.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 9.McAllister RG, Jr., Tan TG. Effect of hypothermia on drug metabolism. In vitro studies with propranolol and verapamil. Pharmacology. 1980;20(2):95–100. doi: 10.1159/000137349. [DOI] [PubMed] [Google Scholar]
- 10.Rendic S. Summary of information on human CYP enzymes: human P450 metabolism data. Drug Metab Rev. 2002;34(12):83–448. doi: 10.1081/dmr-120001392. [DOI] [PubMed] [Google Scholar]
- 11.Miyata M, Nagata K, Yamazoe Y, et al. Transcriptional elements directing a liver-specific expression of P450/6 beta A (CYP3A2) gene-encoding testosterone 6 beta-hydroxylase. Arch Biochem Biophys. 1995;318(1):71–79. doi: 10.1006/abbi.1995.1206. [DOI] [PubMed] [Google Scholar]
- 12.Tanaka E, Terada M, Misawa S. Cytochrome P450 2E1: its clinical and toxicological role. J Clin Pharm Ther. 2000;25(3):165–175. doi: 10.1046/j.1365-2710.2000.00282.x. [DOI] [PubMed] [Google Scholar]
- 13.Poloyac SM, Tortorici MA, Przychodzin DI, et al. The effect of isoniazid on CYP2E1-and CYP4A-mediated hydroxylation of arachidonic acid in the rat liver and kidney. Drug Metab Dispos. 2004;32(7):727–733. doi: 10.1124/dmd.32.7.727. [DOI] [PubMed] [Google Scholar]
- 14.Roe AL, Poloyac SM, Howard G, et al. The effect of endotoxin on hepatocyte nuclear factor 1 nuclear protein binding: potential implications on CYP2E1 expression in the rat. J Pharm Pharmacol. 2001;53(10):1365–1371. doi: 10.1211/0022357011777864. [DOI] [PubMed] [Google Scholar]
- 15.Toler SM, Young AB, McClain CJ, et al. Head injury and cytochrome P-450 enzymes. Differential effect on mRNA and protein expression in the Fischer-344 rat. Drug Metab Dispos. 1993;21(6):1064–1069. [PubMed] [Google Scholar]
- 16.Katz L, Ebmeyer U, Safar P, et al. Outcome model of asphyxial cardiac arrest in rats. J Cereb Blood Flow Metab. 1995;15(6):1032–1039. doi: 10.1038/jcbfm.1995.129. [DOI] [PubMed] [Google Scholar]
- 17.Fink EL, Alexander H, Marco CD, et al. Experimental model of pediatric asphyxial cardiopulmonary arrest in rats. Pediatr Crit Care Med. 2004;5(2):139–144. doi: 10.1097/01.pcc.0000112376.29903.8f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rockich K, Blouin R. Effect of the acute-phase response on the pharmacokinetics of chlorzoxazone and cytochrome P-450 2E1 in vitro activity in rats. Drug Metab Dispos. 1999;27(9):1074–1077. [PubMed] [Google Scholar]
- 19.Lowry OH, Rosebrough NJ, Farr AL, et al. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193(1):265–275. [PubMed] [Google Scholar]
- 20.Sonderfan AJ, Arlotto MP, Dutton DR, et al. Regulation of testosterone hydroxylation by rat liver microsomal cytochrome P-450. Arch Biochem Biophys. 1987;255(1):27–41. doi: 10.1016/0003-9861(87)90291-8. [DOI] [PubMed] [Google Scholar]
- 21.Mu Y, Stephenson CR, Kendall C, et al. A pregnane X receptor agonist with unique species-dependent stereoselectivity and its implications in drug development. Mol Pharmacol. 2005;68(2):403–413. doi: 10.1124/mol.105.013292. [DOI] [PubMed] [Google Scholar]
- 22.Caron E, Rioux N, Nicolas O, et al. Quantification of the expression and inducibility of 12 rat cytochrome P450 isoforms by quantitative RT-PCR. J Biochem Mol Toxicol. 2005;19(6):368–378. doi: 10.1002/jbt.20103. [DOI] [PubMed] [Google Scholar]
- 23.Xie W, Barwick JL, Downes M, et al. Humanized xenobiotic response in mice expressing nuclear receptor SXR. Nature. 2000;406(6794):435–439. doi: 10.1038/35019116. [DOI] [PubMed] [Google Scholar]
- 24.Saini SP, Mu Y, Gong H, et al. Dual role of orphan nuclear receptor pregnane X receptor in bilirubin detoxification in mice. Hepatology. 2005;41(3):497–505. doi: 10.1002/hep.20570. [DOI] [PubMed] [Google Scholar]
- 25.Pohjanvirta R, Niittynen M, Linden J, et al. Evaluation of various housekeeping genes for their applicability for normalization of mRNA expression in dioxin-treated rats. Chemico-biological interactions. 2006;160(2):134–149. doi: 10.1016/j.cbi.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 26.Zhou J, Zhai Y, Mu Y, et al. A novel pregnane X receptor-mediated and sterol regulatory element-binding protein-independent lipogenic pathway. J Biol Chem. 2006;281(21):15013–15020. doi: 10.1074/jbc.M511116200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boucher BA, Hanes SD. Pharmacokinetic alterations after severe head injury. Clinical relevance. Clin Pharmacokinet. 1998;35(3):209–221. doi: 10.2165/00003088-199835030-00004. [DOI] [PubMed] [Google Scholar]
- 28.Kalsotra A, Turman CM, Dash PK, et al. Differential effects of traumatic brain injury on the cytochrome p450 system: a perspective into hepatic and renal drug metabolism. J Neurotrauma. 2003;20(12):1339–1350. doi: 10.1089/089771503322686139. [DOI] [PubMed] [Google Scholar]
- 29.Toler SM, Young AB, McClain CJ, et al. Head Injury and Cytochrome P450 Enzymes. Drug Metabolism and Disposition. 1993;21(6):1064–1068. [PubMed] [Google Scholar]
- 30.Poloyac SM, Perez A, Scheff S, et al. Tissue-specific alterations in the 6-hydroxylation of chlorzoxazone following traumatic brain injury in the rat. Drug Metab Dispos. 2001;29(3):296–298. [PubMed] [Google Scholar]
- 31.Adrie C, Adib-Conquy M, Laurent I, et al. Successful cardiopulmonary resuscitation after cardiac arrest as a “sepsis-like” syndrome. Circulation. 2002;106(5):562–568. doi: 10.1161/01.cir.0000023891.80661.ad. [DOI] [PubMed] [Google Scholar]
- 32.Oppert M, Gleiter CH, Muller C, et al. Kinetics and characteristics of an acute phase response following cardiac arrest. Intensive Care Med. 1999;25(12):1386–1394. doi: 10.1007/s001340051086. [DOI] [PubMed] [Google Scholar]
- 33.Hakkola J, Hu Y, Ingelman-Sundberg M. Mechanisms of down-regulation of CYP2E1 expression by inflammatory cytokines in rat hepatoma cells. J Pharmacol Exp Ther. 2003;304(3):1048–1054. doi: 10.1124/jpet.102.041582. [DOI] [PubMed] [Google Scholar]
- 34.Abdel-Razzak Z, Loyer P, Fautrel A, et al. Cytokines down-regulate expression of major cytochrome P-450 enzymes in adult human hepatocytes in primary culture. Mol Pharmacol. 1993;44(4):707–715. [PubMed] [Google Scholar]
- 35.Gundersen Y, Vaagenes P, Pharo A, et al. Moderate hypothermia blunts the inflammatory response and reduces organ injury after acute haemorrhage. Acta Anaesthesiol Scand. 2001;45(8):994–1001. doi: 10.1034/j.1399-6576.2001.450812.x. [DOI] [PubMed] [Google Scholar]
- 36.Weisfeldt ML, Becker LB. Resuscitation after cardiac arrest: a 3-phase time-sensitive model. JAMA. 2002;288(23):3035–3038. doi: 10.1001/jama.288.23.3035. [DOI] [PubMed] [Google Scholar]
- 37.Iida Y, Nishi S, Asada A. Effect of mild therapeutic hypothermia on phenytoin pharmacokinetics. Ther Drug Monit. 2001;23(3):192–197. doi: 10.1097/00007691-200106000-00002. [DOI] [PubMed] [Google Scholar]
- 38.Nishida K, Okazaki M, Sakamoto R, et al. Change in pharmacokinetics of model compounds with different elimination processes in rats during hypothermia. Biol Pharm Bull. 2007;30(9):1763–1767. doi: 10.1248/bpb.30.1763. [DOI] [PubMed] [Google Scholar]
- 39.Teng S, Piquette-Miller M. The involvement of the pregnane X receptor in hepatic gene regulation during inflammation in mice. J Pharmacol Exp Ther. 2005;312(2):841–848. doi: 10.1124/jpet.104.076141. [DOI] [PubMed] [Google Scholar]
- 40.Zhou C, Tabb MM, Nelson EL, et al. Mutual repression between steroid and xenobiotic receptor and NF-kappaB signaling pathways links xenobiotic metabolism and inflammation. J Clin Invest. 2006;116(8):2280–2289. doi: 10.1172/JCI26283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gu X, Ke S, Liu D, et al. Role of NF-kappaB in regulation of PXR-mediated gene expression: a mechanism for the suppression of cytochrome P-450 3A4 by proinflammatory agents. J Biol Chem. 2006;281(26):17882–17889. doi: 10.1074/jbc.M601302200. [DOI] [PubMed] [Google Scholar]