Abstract
Chronic pain is a major health concern with up to 50% of patients finding little if any relief following traditional pharmacotherapy. This review describes the treatment of chronic pain using herpes simplex virus type 1 (HSV)-based vectors. HSV can be effectively used to deliver pain-modulating transgenes to sensory neurons in vivo following intradermal inoculation. The vector genome persists in peripheral nerve bodies in an episomal state and serves as a platform for expression of natural pain-relieving molecules that access endogenous antinociceptive circuitry. The vectors are mutated to prevent reactivation from latency or spread to the central nervous system. Dermatome selection for administration of HSV vectors provides targeted delivery of pain gene therapy to primary afferent neurons. This novel approach alleviates pain without systemic side effects or the induction of tolerance and can be used in combination with standard pain treatments.
Acute pain is defined by the International Association for the Study of Pain as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage or described in terms of such damage.”1 The perception of acutely painful stimuli—along with the cognitive and affective correlates of that perception—plays a key role in the adaptation of the organism to the environment to allow its survival. In the setting of persistent injury, molecular, structural, and biochemical alterations in the properties of components of the pain pathway result in a syndrome of chronic pain. There is an emerging consensus in the field that chronic pain may not be a symptom of disease, but rather a disease entity that itself; in any case chronic pain represents a major cause of morbidity, significantly impairing individual quality of life2 and imposing a substantial burden on society.
The pathways subserving perception of pain are well established. Primary nociceptors are pseudounipolar neurons with cell bodies in the dorsal root ganglion (DRG) and axons that terminate peripherally in the skin or organs and project centrally to terminate in the dorsal horn of spinal cord in an anatomically defined (dermatomal or radicular) pattern. Second-order neurons project from the spinal cord principally to sensory nuclei in the thalamus. Third-order neurons in the thalamus project to sensory cortex that subserves the discriminative aspects of pain perception, and to limbic cortex to subserve the hedonic aspects of the pain experience. Many regions of brain are subsequently recruited. In addition to the ascending pathways, descending pathways that ultimately project from the brainstem back to the spinal dorsal horn act to modulate primary nociceptive neurotransmission at that first synapse in the pain pathway.
Two categories of chronic pain are commonly distinguished. Inflammatory (also termed nociceptive) pain is that pain which results from tissue injury. Many factors including a drop in pH and release of inflammatory mediators such as histamine, bradykinin, endothelin, or interleukins (ILs) result in recruitment of inflammatory cells and continuous activation of nociceptors. Neuropathic pain is that pain which results from damage to neural structures, which arises in the absence of accompanying injury to non-neural tissues. A common form of neuropathic pain occurs in diabetes, where nerve damage (diabetic neuropathy) is accompanied by spontaneously occurring pain. Recent work strongly suggests that chronic peripheral nerve damage is accompanied by activation of microglia in the spinal cord, resulting in a spinal “neuroimmune” process that plays a critical role in the establishment of chronic neuropathic pain.
Chronic nociceptive pain can be treated by removing the inciting injury, or if that is not possible, employing treatment with nonsteroidal anti-inflammatory drugs to reduce peripheral inflammation and coincidentally block neuroimmune changes in the dorsal horn of spinal cord. Neuropathic pain treatment regimens commonly employ anticonvulsant drugs that are used to reduce neuronal excitability and selective or nonselective serotonin reuptake inhibitors that, among other effects, modulate nociceptive neurotransmission in the dorsal horn. Treatment of both nociceptive and neuropathic pain often ends in the use of opioid drugs that act at endogenous receptors at multiple sites in the neuraxis to modulate nociceptive neurotransmission. Because the currently available drugs act through mechanisms that are not specific to pain-related neural pathways, off-target effects unrelated to the relief of pain often limit the maximum dose of analgesic medication that can be prescribed. In the special case of the potent opioid analgesic morphine, for example, activation of opioid receptors in regions of brain unrelated to pain perception results in somnolence, confusion, and ultimately impaired respiration, whereas activation of opioid receptors in the gut and the urinary bladder result in nausea, constipation, and urinary retention. In addition, continued treatment with opioids results in the development of tolerance leading to a requirement for escalating doses of medication to achieve the same result and treatment is further complicated by the possibility of drug abuse, unrelated to pain relief.
The wide distribution of a limited range of neurotransmitters, receptors, and voltage-gated ion channels in the nervous system makes it difficult to selectively target pain-related pathways using drugs that are administered systemically. Gene transfer methods have been used to achieve continuous expression of short-lived analgesic peptides at several sites in the ascending pain pathway. The first synapse between the primary nociceptor and the second-order projection neuron in the spinal cord is a particularly attractive target because of the ability to selectively block nociception at that level. This site has been exploited using conventional analgesic drugs (e.g., morphine, baclofen) by delivery through chronic intrathecal infusion. Intrathecal administration results in a tenfold reduction in dose requirement and a reduction in off-target adverse events. However, despite this directed approach, problems remain in effectiveness and tolerance.
Over the past several years, a number of investigators have used herpes simplex virus (HSV)-based vectors to achieve a similar targeted delivery of inhibitory neurotransmitters at the spinal level to block nociceptive neurotransmission at the first synapse between the primary peripheral nociceptor and the second-order neuron in the spinal cord. HSV-based vectors have also been used to express anti-inflammatory cytokines to block central neuroimmune activation that appears to play an important role in the transition from acute pain perception to the chronic pain state, and more recently, have been employed to alter the expression or activation of ion channels involved in pain-sensing changes in the local environment. These include the use of genes that encode antisense or microRNA sequences and genes that antagonize ion channel function whose activities are essential to the development of chronic pain. Intrathecal injection of plasmid, adenovirus, and adeno-associated virus–based vectors has been tested in animal models of pain, but this review is limited to the published preclinical studies of HSV vectors in the treatment of chronic pain. The use of HSV vectors exploits the advantage of local vector application to the skin where virus entry into sensory nerve endings leads to vector transport to the nerve cell body. Here the vector genome serves as a platform for the exclusive expression of transgene(s) that encode analgesic substances or alter the nerve cell physiology in a manner to prevent responses to pain-related stimuli (Figure 1).
Figure 1.
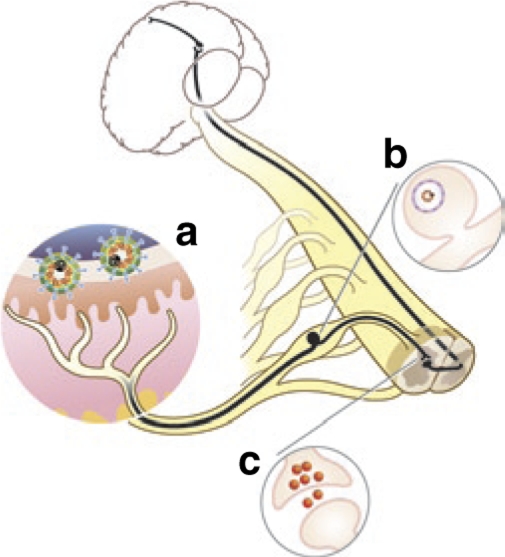
Herpes simplex virus (HSV) gene transfer to modify pain perception. The ascending pain transmission pathway is shown in black. Nonreplicating recombinant HSV vectors injected into the skin (a) are carried by retrograde axonal transport to the dorsal root ganglion (DRG) (b) where the vector establishes a pseudo-latent state. Transgene products produced in the DRG are released from afferent nerve terminals in the dorsal horn of spinal cord (c) to modulate nociceptive neurotransmission.
HSV is a large double-stranded DNA virus that in its natural life cycle is spread by contact, infecting and replicating in skin or mucous membrane. Viral particles released in the skin are taken up by sensory nerve terminals and then carried by retrograde axonal transport to the neuronal perikaryon in the DRG, where the wild-type virus may either re-enter the lytic cycle, or establish a latent state from which the (wild-type) virus can subsequently reactivate and spread to other individuals. Recombinant HSV vectors, by virtue of the deletion of essential lytic viral functions establish a persistent state similar to latency characterized by the inability to reactivate or re-establish active virus growth, but retain the DRG-targeting properties of the wild-type virus and remain active in their ability to express transgene products.
HSV is a complex virus with >85 genes coded in the 150-kb genome. These gene functions can be classified as either essential for virus replication or accessory playing an important role in the natural history of virus infection in the host. Two approaches have been used to construct recombinant HSV vectors for use in gene transfer. One class of vectors is deleted for accessory viral functions (e.g., thymidine kinase) that are important to virus replication in neurons and thus contribute to neurovirulence. Recombinant herpes viruses defective in tk can be propagated in culture and will replicate in skin, but are unable to replicate in the DRG and are thus forced into a pseudo-latent state. A second class of HSV vectors is rendered defective in essential virus genes and thus fails to replicate in all cell types. Attractive targets for deletion include the immediate-early (IE) class of genes that are required to launch the cascade of viral gene expression; recombinants deleted in these genes are blocked at the beginning of the replication cycle. These gene products include infected cell polypeptides (ICPs) ICP4 and ICP27 without which the virus genome is highly repressed.3 Such recombinants can be propagated in cells that provide the missing gene product(s) in trans, but are incapable of replication in vivo4 (Figure 2). More recently, nonreplicating HSV vectors have been constructed that are functionally deleted for ICP22 and ICP47 in addition to ICP4 while retaining ICP0, a product required for the vector genome to remain transcriptionally active.5 These vectors are completely incapable of replication in vivo, but because the recombinant particles retain the targeting properties of the wild-type virus, it can be used to effectively deliver genes from the skin to the sensory ganglion in vivo where the vector establishes a latency-like state in which all the viral lytic genes are repressed.
Figure 2.
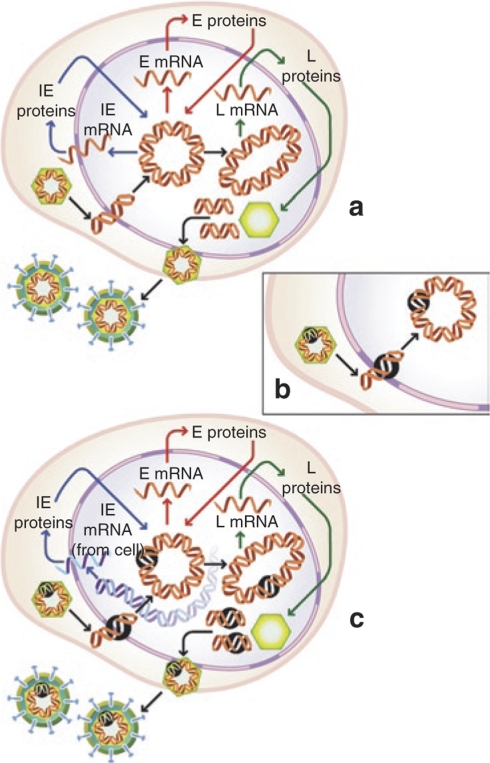
Propagation of nonreplicating herpes simplex virus (HSV) vectors. Replication of wild-type HSV requires the expression of essential immediate-early (IE) genes that are expressed on entry of the viral genome into the nucleus (a). Recombinants for gene transfer are rendered replication incompetent by disruption of expression of one or more essential IE genes (indicated by black circle, b). These replication-incompetent recombinants can be propagated to high titers in complementing cells engineered to express the required IE functions from the cellular genome (c).
Opioid receptors are found presynaptically on the terminals of primary nociceptive afferents in the spinal cord, and postsynaptically on second-order neurons in the dorsal horn. Activation of receptors naturally by endogenous ligands enkephalin or endomorphin (EM), or therapeutically by opiate drugs such as morphine, inhibits pain-related neurotransmission at the spinal level. The efficacy of HSV-mediated gene transfer of enkephalin has been tested in several different models of pain in rodents (Figure 3). Pohl et al. first showed that a tk-defective HSV recombinant injected subcutaneously in the paw will transduce DRG neurons that express enkephalin in DRG;6 Wilson et al. subsequently demonstrated that a similar tk− HSV-based vector containing the human proenkephalin gene and injected subcutaneously into the paw reduces hyperalgesic C-fiber responses ipsilateral to the injection.7 Pohl et al. further showed that subcutaneous inoculation of the vector reduces pain-related behaviors in a rodent model of chronic pain related to polyarthritis induced by injection of complete Freund's adjuvant.8 Expression of enkephalin from the vector did not only reduce pain-related behaviors, but also prevented cartilage and bone destruction in the inflamed joints, presumably because of release of enkephalin from the peripheral sensory terminals in the joint,8 and correlating with the demonstration that axonal transport of the transgene product from transduced cells carrying that product to the periphery (as well as toward the spinal cord) could be demonstrated by the application of a ligature to the nerve.9
Figure 3.
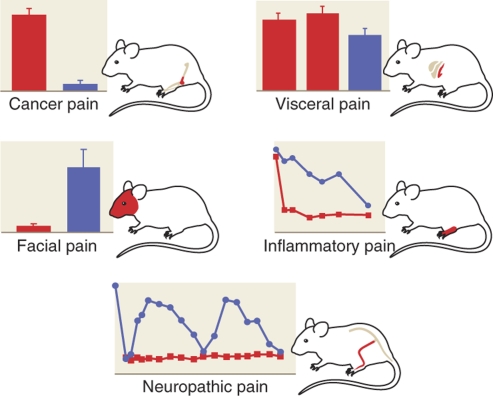
Herpes simplex virus vectors have been reported to show analgesic effects in several different rodent models of chronic pain including: inflammatory pain,22 somatic neuropathic pain,11 facial neuropathic pain,12 visceral pain,15 and pain from cancer in bone.18
Similar effects were subsequently demonstrated using a nonreplicating HSV vector deleted for both copies of the essential HSV IE gene ICP4. Subcutaneous inoculation of an enkephalin-producing nonreplicating vector produces an analgesic effect in the delayed phase of the formalin model of inflammatory pain.10 The time course of the analgesic effect of this vector, maximal at 1 week and declining over time, is consistent with the known time course of expression driven by the human cytomegalovirus IE promoter (HCMV IEp) inserted to drive transgene expression from this vector. The observation that reinoculation of the vector at 4 weeks restored the analgesic effect is consistent with the interpretation that the animals do not develop tolerance to the vector-mediated transgene product released from synaptic terminals in vivo. The observations regarding reinoculation, which have been repeated in several different models of pain and with different transgene products, indicates the absence of any significant immune response to vector inoculation in the rodent.
HSV-mediated enkephalin expression produces antinociceptive effects in neuropathic pain as well. In the selective spinal nerve ligation (SNL) model, injection of the HSV vector 1 week after SNL results in an analgesic effect that is additive with morphine (reducing the ED50 of coadministered morphine by an order of magnitude), and persists in the face of morphine tolerance.11 In this model as well, the effect of enkephalin expression driven by the HCMV IEp produced an analgesic effect persisted several weeks, and could be re-established by reinoculation.11 In the infraorbital nerve constriction model of craniofacial pain, Pohl et al. demonstrated that inoculation of a tk− enkephalin-expressing vector into the vibrissal pad produced a significant reduction in mechanical hypersensitivity on the affected side.12
In experiments designed to test the effect of the vector on visceral pain, investigators have injected the vector directly into the end organ, rather than the skin. Yoshimura et al. demonstrated that injection of the nonreplicating enkephalin-expressing HSV vector into the rat bladder wall results in enkephalin expression in relevant DRG, and that vector-mediated enkephalin effectively attenuated capsaicin-induced bladder irritation and resultant bladder hyperactivity.13,14 Similarly, Westlund et al. have shown in rodent models of acute and chronic pancreatitis that direct injection of an enkephalin-expressing HSV vector into the pancreas attenuates evoked nocisponsive behaviors.15,16 In the pancreas, enkephalin expression appeared to reduce the inflammatory response in the pancreas, analogous to the effect that reported in polyarthritis.8
Pain resulting from cancer has characteristics of both neuropathic and inflammatory pain.17 In a mouse model of bone cancer pain, subcutaneous inoculation of the HSV vector–expressing enkephalin resulted in an attenuation of spontaneous nocisponsive behaviors.18
Studies of the enkephalin-expressing HSV vector have been extended to primates. Yeomans et al. demonstrated that peripheral application of the HSV vector–expressing enkephalin to the dorsal surface of the foot of macaques reduced an A-delta and C-fiber-mediated pain-related responses.19 Taken together, these results from several different groups of investigators provide proof-of-principle evidence that HSV vector–mediated delivery of enkephalin can provide an analgesic effect and set the stage for a human trial to treat chronic pain using HSV vector–expressed enkephalin (see below).
Our groups have examined HSV vectors constructed to express other inhibitory neurotransmitters. EM-2 (Tyr-Pro-Phe-Phe-NH2) is an endogenous highly selective µ-receptor agonist,20 but the gene coding for EM-2 has not yet been identified. We therefore constructed a tripartite synthetic gene cassette with the N-terminal signal sequence of human preproenkephalin followed by a pair of EM-2 coding elements including the addition a C-terminal glycine residue flanked by dibasic cleavage sites. The gene product is processed by the cellular machinery that processes preproenkephalin to enkephalin,21 with the C-terminal glycine in the cleaved product directing amidation of the cleaved peptide by the widely distributed enzyme peptidylglycine α-amidating monooxygenase. Subcutaneous inoculation of the EM-expressing HSV vector into the footpad of rats with neuropathic pain from selective L5 SNL resulted in a significant reduction in both mechanical allodynia and thermal hyperalgesia that could be blocked by the highly selective µ-opioid receptor antagonist D-Phe-Cys-Tyr-D-Trp-Orn-Thr-Pen-Thr-amide,21 and a substantial reduction in nocisponsive behaviors in the delayed phase of the formalin test and in the complete Freund's adjuvant model of inflammatory pain.22
Nocisponsive behaviors can also be attenuated by using HSV-mediated gene transfer to modulate expression of µ-opioid receptors in primary sensory afferents. Cutaneous application of an HSV vector defective in expression of the viral thymidine kinase gene and with the human µ-opioid receptor cDNA in reverse orientation results in decreased expression of µ-opioid receptors on the central primary sensory afferent terminals in the dorsal horn of spinal cord resulting in a reduced potency of intrathecal [D-Ala2,N-MePhe4,Gly-ol5] enkephalin on C-fiber nociceptive responses23 consistent with a centrally mediated effect. Conversely, cutaneous application of an HSV vector expressing the µ-opioid receptor gene in the sense orientation increases µ-opioid receptor immunoreactivity in primary sensory afferents and a leftward shift in the dose–response to intraperitoneal lopiramide, indicating an effect at transgene-mediated µ-opioid receptors expressed on the peripheral terminals of the primary sensory neurons.24
γ-amino butyric acid (GABA) is the principal inhibitory neurotransmitter in the nervous system, but the use of GABA agonists (e.g., baclofen) for pain relief is severely limited by substantial off-target depressant central nervous system effects. Glutamic acid decarboxylase (GAD) decarboxylates glutamic acid to produce GABA; we constructed a replication-incompetent HSV vector encoding the 67-kd isoform of human.25 Subcutaneous inoculation of the GAD-expressing HSV vector transduces DRG neurons to produce GAD and release GABA. In the selective SNL model of neuropathic pain, inoculation of the GAD-expressing vector results in a substantial reduction in mechanical allodynia and thermal hyperalgesia as shown in ref. 26. In neuropathic pain, the analgesic effect of the GAD-expressing vector is greater in magnitude than the effect produced by either the enkephalin or EM-expressing vectors. This finding is consistent with the evidence that development of chronic pain after peripheral nerve injury is accompanied by the loss of GABAergic tone in the dorsal horn of spinal cord,27 and the clinical observation that opiate drugs are relatively ineffective in the treatment of neuropathic pain. The GAD-expressing HSV vector also reduces pain-related behaviors in a model of central neuropathic pain created by T13 spinal cord hemisection.25 In the latter study, the vector-mediated analgesic effect was reversed in part by intrathecal administration of the GABA receptor antagonists, bicuculline or phaclofen, indicating that the analgesic effect of the vector is mediated by spinal GABA(A) and GABA(B) receptors.
In addition to the expression of inhibitory neurotransmitters to block nociceptive neurotransmission at the spinal level, we have more recently begun to examine the feasibility of using HSV vectors to modulate spinal neuroimmune activation, a phenomenon that is increasingly recognized for its important role in the development of chronic pain.28,29,30,31 Among the proinflammatory substances released by activated microglia and astrocytes in the spinal cord, tumor necrosis factor α (TNFα) appears to play a central role. Intraperitoneal inoculation of neutralizing antibodies directed against the p55 TNF receptor (TNFR) reduces thermal hyperalgesia and mechanical allodynia, whereas the intrathecal administration of the recombinant p75 soluble TNFR (sTNFR) peptide (etanercept) reduces mechanical allodynia in a rat model of neuropathic pain.32,33,34 Because long-term systemic administration of these potent immune modulators results in substantial risks, we examined the effects of an HSV coding for the truncated soluble TNF receptor (p55 sTNFR) in chronic pain.35 Following subcutaneous inoculation in the foot, vector-mediated release of p55 sTNFR reduced the behavioral manifestations of neuropathic pain while simultaneously reducing the phosphorylation of p38α, the expression of IL-1β, and the expression of membrane-associated TNFα in the spinal cord.35 Similar results were found in a separate study using a model of below-level central neuropathic pain following thoracic spinal cord hemisection.36
IL-4 is a prototypical anti-inflammatory cytokine.37,38 HSV-expressed IL-4 prevents the development of autoimmune encephalitis in Biozzi AB/H mice and in rhesus monkeys.39 We examined the analgesic effects of locally delivered IL-4 in neuropathic pain by using a replication-defective HSV vector to express the coding sequence for IL-4 under the transcriptional control of the HSV ICP4 IEp.40 Vector-mediated expression of IL-4 did not alter paw withdrawal latency to thermal stimuli or tactile threshold in normal animals, but inoculation of the vector 1 week after SNL reduced mechanical allodynia and reversed thermal hyperalgesia. Inoculation of the vector 1 week before SNL delayed, but did not ultimately prevent, the development of neuropathic pain. We also found that injection of a nonreplicating HSV vector coding for the anti-inflammatory peptide IL-10 reduces pain-related behaviors in the delayed phase of the formalin test,41 an effect that corresponds with downregulation of the expression of TNFα in spinal microglia.
On the basis of the demonstration that direct intrathecal infusion of glial cell line–derived neurotrophic factor reduces ectopic nerve discharges and pain-related behaviors in the partial sciatic nerve injury model of neuropathic pain in rodents,42 we examined the effect of a nonreplicating glial cell line–derived neurotrophic factor–expressing HSV vector in the SNL model of neuropathic pain. Subcutaneous inoculation of the vector into the paw 1 week after SNL produced a significant antiallodynic effect.43 Like the effects of other HSV vectors in which transgene expression is driven by the HCMV IEp, the antiallodynic effect produced by inoculation of the glial cell line–derived neurotrophic factor–expressing vector persisted for several weeks, and was re-established by reinoculation of the vector.43
Recent advances in RNA technology have made it possible to reduce endogenous gene expression in vivo. There is substantial evidence from animal studies and human genetics that the voltage-gated sodium channel isoform Nav1.7 and the peptide neurotransmitter calcitonin gene–related peptide play important roles in pain perception and chronic pain states. Yeomans et al. have reported that an HSV vector coding a sequence antisense to Nav1.7 applied to the skin prevents the increase in Nav1.7 expression caused by complete Freund's adjuvant injection. The effect correlates with a reduction in the development of hyperalgesia in C and A-delta thermonociceptive tests.44 More recently, the same group has shown that an HSV vector coding a sequence antisense to calcitonin gene–related peptide reduces calcitonin gene–related peptide expression in transduced DRG neurons with concomitant reduction in nociceptive neurotransmission in vivo.45
HSV vectors can also be used as tools to explore the nature of changes in the physiology of nociceptive neurons that lead to chronic pain states, and the full range of mechanisms underlying the vector-mediated effects. Glorioso et al. have launched new studies using HSV vectors designed to investigate signaling pathways that result in altered ion channel functions that have become hyper-responsive to channel activation and pain signaling. Methods to identify novel Trpv1 channel modulators are underway using libraries of HSV gene vectors that represent expressed genes in sensory nerves,46 and expression of a dominant negative form of PKCε interferes with translocalization of the Trpv1 channel to the cell surface and can inhibit the thermosensitivity response in rats treated with capsaicin following HSV gene transfer.47 Fink and colleagues have found that continuous HSV-mediated expression of enkephalin in DRG reverses the increase in the voltage-gated sodium channel Nav1.7 characteristic of painful diabetic neuropathy.48 These experiments underscore the potential for exploiting HSV vectors to reverse changes in nociceptor function that result in chronic pain signaling.
In in vivo experiments in rodents, even strong promoters such as the HCMV IE gene promoter only drive transgene expression for a period of weeks. It appears that the same mechanisms involved in shutoff of HSV gene expression during latency repress transgene expression in vivo. In wild-type HSV, the latency-active promoter escapes promoter silencing to drive expression of the latency-associated transcript, a nonpolyadenylated RNA that can be detected years after primary infection. The latency-active promoter sequence has been identified as a transposable element49 that has been demonstrated to be capable of driving prolonged biologically active transgene expression in both the central50 and peripheral nervous system51,52 in rodents.
The preclinical studies provide proof-of-principle for the potential utility of HSV-based vectors in the treatment of chronic pain. The animal studies demonstrate that different genes can effectively be delivered to DRG by subcutaneous inoculation of HSV to provide analgesic effects in different models of pain. Although comparison of different models is difficult, it would appear that the most effective gene product may vary according to the type of pain being treated. Experience garnered from studies of replication-compromised (but not incompetent) HSV vectors directly into brain in patients with glioblastoma have not demonstrated significant toxicity,53,54 suggesting that the intradermal inoculation of replication-incompetent vectors should be safe. A commonly expressed concern is whether the nonreplicating vector might recombine with endogenous viruses to produce a wild-type lytic virus carrying the transgene. For this reason, an HSV vector engineered for human use has been constructed to incorporate the transgene within the essential IE gene ICP4 locus; thus, a recombination event incorporating the transgene would render the recombinant replication incompetent. Extensive preclinical biodistribution studies showed no evidence of vector spread from the site of inoculation, and, in parallel, toxicology studies showed no evidence of vector-induced toxicity. An IND for the first phase 1 human trial of HSV, to examine the well-characterized enkephalin-expressing nonreplicating HSV vector in patients with intractable pain caused by cancer, was approved by the Food and Drug Administration in February 2008. This single-site phase 1 trial will be carried out at the University of Michigan by one of us (D.J.F.) with sponsorship provided by Diamyd Inc.
Several important questions will need to be addressed in subsequent phase 2 efficacy trials: (i) Will delivery from intradermal inoculation in humans—where a dermatome covers a much larger area than the dermatome of a rodent and the density of innervation may be lower—allow for effective transduction of the DRG as has been demonstrated in the rodent models? (ii) Will patients experience a local immune response in the skin that will limit the amount of vector that can be inoculated? (iii) Will transduction of DRG by the vector result in reactivation of latent HSV or related herpes viruses from latently infected ganglia in humans? (iv) Will pre-existing anti-HSV antibodies limit the effectiveness of subcutaneously inoculated HSV in patients (representing >50% of the population in most American cities) who have such antibodies? (v) Can prolonged gene expression be achieved from HSV vectors in humans administered subcutaneously to patients, and if not, can repeated injections of the vector be delivered without complications? Ultimately, the results of human studies will allow us to determine whether HSV-mediated gene transfer can be moved from preclinical success in rodents to add to the armamentarium of available pain treatments for patients.
Acknowledgments
This work was supported by grants from the Department of Veterans Affairs, the Juvenile Diabetes Research Foundation, and the National Institutes of Health grants NS038850 and DK044935 (D.J.F.) and grants DK044935, CA119298, U54AR050733, NS40923, and NS059003 (J.C.G.). The figures were created by Chris Burke (cjburke@umich.edu). Joseph Glorioso is a consultant for Diamyd Medical and owns stock in the company. David Fink has research grants from Diamyd in support of the clinical trial of HSV-mediated gene transfer; he does not have any paid consulting relationships with or equity interest in Diamyd.
REFERENCES
- Merskey H, Albe-Fessard D, Bonica JJ, Carmon A, Dubner R, Kerr FWL, et al. Pain terms: a list with definitions and notes on usage. Recommended by the IASP Subcommittee on Taxonomy. Pain. 1979;6:249–252. [PubMed] [Google Scholar]
- Niv D., and , Devor M. Chronic pain as a disease in its own right. Pain Pract. 2004;4:179–181. doi: 10.1111/j.1533-2500.2004.04301.x. [DOI] [PubMed] [Google Scholar]
- Roizman B., and , Knipe DM.Knipe DM., and , Howley PM.Herpes simplex viruses and their replication Fields Virology 2001Lippincott Williams & Wilkins: Philadelphia, PA; 2399–2460.eds [Google Scholar]
- DeLuca NA, McCarthy AM., and , Schaffer PA. Isolation and characterization of deletion mutants of herpes simplex virus type 1 in the gene encoding immediate-early regulatory protein ICP4. J Virol. 1985;56:558–570. doi: 10.1128/jvi.56.2.558-570.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe D, Goins WF, Yamada M, Moriuchi S, Krisky DM, Oligino TJ, et al. Engineering herpes simplex virus vectors for CNS applications. Exp Neurol. 1999;159:34–46. doi: 10.1006/exnr.1999.7158. [DOI] [PubMed] [Google Scholar]
- Antunes Bras JM, Epstein AL, Bourgoin S, Hamon M, Cesselin F., and , Pohl M. Herpes simplex virus 1-mediated transfer of preproenkephalin A in rat dorsal root ganglia. J Neurochem. 1998;70:1299–1303. doi: 10.1046/j.1471-4159.1998.70031299.x. [DOI] [PubMed] [Google Scholar]
- Wilson SP, Yeomans DC, Bender MA, Lu Y, Goins WF., and , Glorioso JC. Antihyperalgesic effects of infection with a preproenkephalin-encoding herpes virus. Proc Natl Acad Sci USA. 1999;96:3211–3216. doi: 10.1073/pnas.96.6.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braz J, Beaufour C, Coutaux A, Epstein AL, Cesselin F, Hamon M, et al. Therapeutic efficacy in experimental polyarthritis of viral-driven enkephalin overproduction in sensory neurons. J Neurosci. 2001;21:7881–7888. doi: 10.1523/JNEUROSCI.21-20-07881.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antunes bras J, Becker C, Bourgoin S, Lombard M, Cesselin F, Hamon M, et al. Met-enkephalin is preferentially transported into the peripheral processes of primary afferent fibres in both control and HSV1-driven proenkephalin A overexpressing rats. Neuroscience. 2001;103:1073–1083. doi: 10.1016/s0306-4522(01)00034-3. [DOI] [PubMed] [Google Scholar]
- Goss JR, Mata M, Goins WF, Wu HH, Glorioso JC., and , Fink DJ. Antinociceptive effect of a genomic herpes simplex virus-based vector expressing human proenkephalin in rat dorsal root ganglion. Gene Ther. 2001;8:551–556. doi: 10.1038/sj.gt.3301430. [DOI] [PubMed] [Google Scholar]
- Hao S, Mata M, Goins W, Glorioso JC., and , Fink DJ. Transgene-mediated enkephalin release enhances the effect of morphine and evades tolerance to produce a sustained antiallodynic effect. Pain. 2003;102:135–142. doi: 10.1016/s0304-3959(02)00346-9. [DOI] [PubMed] [Google Scholar]
- Meunier A, Latremoliere A, Mauborgne A, Bourgoin S, Kayser V, Cesselin F, et al. Attenuation of pain-related behavior in a rat model of trigeminal neuropathic pain by viral-driven enkephalin overproduction in trigeminal ganglion neurons. Mol Ther. 2005;11:608–616. doi: 10.1016/j.ymthe.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Goins WF, Yoshimura N, Phelan MW, Yokoyama T, Fraser MO, Ozawa H, et al. Herpes simplex virus mediated nerve growth factor expression in bladder and afferent neurons: potential treatment for diabetic bladder dysfunction. J Urol. 2001;165:1748–1754. [PubMed] [Google Scholar]
- Yoshimura N, Franks ME, Sasaki K, Goins WF, Goss J, Yokoyama T, et al. Gene therapy of bladder pain with herpes simplex virus (HSV) vectors expressing preproenkephalin (PPE) Urology. 2001;57 6 suppl. 1:116. doi: 10.1016/s0090-4295(01)01060-3. [DOI] [PubMed] [Google Scholar]
- Lu Y, McNearney TA, Lin W, Wilson SP, Yeomans DC., and , Westlund KN. Treatment of inflamed pancreas with enkephalin encoding HSV-1 recombinant vector reduces inflammatory damage and behavioral sequelae. Mol Ther. 2007;15:1812–1819. doi: 10.1038/sj.mt.6300228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, McNearney TA, Chu R, Lu Y, Ren Y, Yeomans DC, et al. Enkephalin-encoding herpes simplex virus-1 decreases inflammation and hotplate sensitivity in a chronic pancreatitis model. Mol Pain. 2008;4:8. doi: 10.1186/1744-8069-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honore P, Rogers SD, Schwei MJ, Salak-Johnson JL, Luger NM, Sabino MC, et al. Murine models of inflammatory, neuropathic and cancer pain each generates a unique set of neurochemical changes in the spinal cord and sensory neurons. Neuroscience. 2000;98:585–598. doi: 10.1016/s0306-4522(00)00110-x. [DOI] [PubMed] [Google Scholar]
- Goss JR, Harley CF, Mata M, O'Malley ME, Goins WF, Hu X, et al. Herpes vector-mediated expression of proenkephalin reduces pain-related behavior in a model of bone cancer pain. Ann Neurol. 2002;52:662–665. doi: 10.1002/ana.10343. [DOI] [PubMed] [Google Scholar]
- Yeomans DC, Lu Y, Laurito CE, Peters MC, Vota-Vellis G, Wilson SP, et al. Recombinant herpes vector-mediated analgesia in a primate model of hyperalgesia. Mol Ther. 2006;13:589–597. doi: 10.1016/j.ymthe.2005.08.023. [DOI] [PubMed] [Google Scholar]
- Zadina JE, Hackler L, Ge LJ., and , Kastin AJ. A potent and selective endogenous agonist for the mu-opiate receptor. Nature. 1997;386:499–502. doi: 10.1038/386499a0. [DOI] [PubMed] [Google Scholar]
- Wolfe D, Hao S, Hu J, Srinivasan R, Goss J, Mata M, et al. Engineering an endomorphin-2 gene for use in neuropathic pain therapy. Pain. 2007;133:29–38. doi: 10.1016/j.pain.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Hao S, Wolfe D, Glorioso JC, Mata M., and , Fink DJ. Eur J Pain. epub ahead of print; 2008. Effects of transgene-mediated endomorphin-2 in inflammatory pain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones TL, Sweitzer SM, Wilson SP., and , Yeomans DC. Afferent fiber-selective shift in opiate potency following targeted opioid receptor knockdown. Pain. 2003;106:365–371. doi: 10.1016/j.pain.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Zhang G, Mohammad H, Peper BD, Raja S, Wilson SP., and , Sweitzer SM. Enhanced peripheral analgesia using virally mediated gene transfer of the mu-opioid receptor in mice. Anesthesiology. 2008;108:305–313. doi: 10.1097/01.anes.0000299836.61785.79. [DOI] [PubMed] [Google Scholar]
- Liu J, Wolfe D, Hao S, Huang S, Glorioso JC, Mata M, et al. Peripherally delivered glutamic acid decarboxylase gene therapy for spinal cord injury pain. Mol Ther. 2004;10:57–66. doi: 10.1016/j.ymthe.2004.04.017. [DOI] [PubMed] [Google Scholar]
- Hao S, Mata M, Wolfe D, Glorioso JC., and , Fink DJ. Gene transfer of glutamic acid decarboxylase reduces neuropathic pain. Ann Neurol. 2005;57:914–918. doi: 10.1002/ana.20483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore KA, Kohno T, Karchewski LA, Scholz J, Baba H., and , Woolf CJ. Partial peripheral nerve injury promotes a selective loss of GABAergic inhibition in the superficial dorsal horn of the spinal cord. J Neurosci. 2002;22:6724–6731. doi: 10.1523/JNEUROSCI.22-15-06724.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLeo JA., and , Yezierski RP. The role of neuroinflammation and neuroimmune activation in persistent pain. Pain. 2001;90:1–6. doi: 10.1016/s0304-3959(00)00490-5. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Milligan ED., and , Maier SF. Glial activation: a driving force for pathological pain. Trends Neurosci. 2001;24:450–455. doi: 10.1016/s0166-2236(00)01854-3. [DOI] [PubMed] [Google Scholar]
- Wieseler-Frank J, Maier SF., and , Watkins LR. Glial activation and pathological pain. Neurochem Int. 2004;45:389–395. doi: 10.1016/j.neuint.2003.09.009. [DOI] [PubMed] [Google Scholar]
- DeLeo JA, Tanga FY., and , Tawfik VL. Neuroimmune activation and neuroinflammation in chronic pain and opioid tolerance/hyperalgesia. Neuroscientist. 2004;10:40–52. doi: 10.1177/1073858403259950. [DOI] [PubMed] [Google Scholar]
- Sommer C, Schmidt C., and , George A. Hyperalgesia in experimental neuropathy is dependent on the TNF receptor 1. Exp Neurol. 1998;151:138–142. doi: 10.1006/exnr.1998.6797. [DOI] [PubMed] [Google Scholar]
- Schafers M, Svensson CI, Sommer C., and , Sorkin LS. Tumor necrosis factor-alpha induces mechanical allodynia after spinal nerve ligation by activation of p38 MAPK in primary sensory neurons. J Neurosci. 2003;23:2517–2521. doi: 10.1523/JNEUROSCI.23-07-02517.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson CI, Schafers M, Jones TL, Powell H., and , Sorkin LS. Spinal blockade of TNF blocks spinal nerve ligation-induced increases in spinal P-p38. Neurosci Lett. 2005;379:209–213. doi: 10.1016/j.neulet.2004.12.064. [DOI] [PubMed] [Google Scholar]
- Hao S, Mata M, Glorioso JC., and , Fink DJ. Gene transfer to interfere with TNFalpha signaling in neuropathic pain. Gene Ther. 2007;14:1010–1016. doi: 10.1038/sj.gt.3302950. [DOI] [PubMed] [Google Scholar]
- Peng X, Zhou Z, Glorioso JC, Fink DJ., and , Mata M. Tumor necrosis factor alpha contributes to below-level neuropathic pain after spinal cord injury. Ann Neurol. 2006;59:843–851. doi: 10.1002/ana.20855. [DOI] [PubMed] [Google Scholar]
- te Velde AA, Huijbens RJ, Heije K, de Vries JE., and , Figdor CG. Interleukin-4 (IL-4) inhibits secretion of IL-1 beta, tumor necrosis factor alpha, and IL-6 by human monocytes. Blood. 1990;76:1392–1397. [PubMed] [Google Scholar]
- Mijatovic T, Kruys V, Caput D, Defrance P., and , Huez G. Interleukin-4 and -13 inhibit tumor necrosis factor-alpha mRNA translational activation in lipopolysaccharide-induced mouse macrophages. J Biol Chem. 1997;272:14394–14398. doi: 10.1074/jbc.272.22.14394. [DOI] [PubMed] [Google Scholar]
- Poliani PL, Brok H, Furlan R, Ruffini F, Bergami A, Desina G, et al. Delivery to the central nervous system of a nonreplicative herpes simplex type 1 vector engineered with the interleukin 4 gene protects rhesus monkeys from hyperacute autoimmune encephalomyelitis. Hum Gene Ther. 2001;12:905–920. doi: 10.1089/104303401750195872. [DOI] [PubMed] [Google Scholar]
- Hao S, Mata M, Glorioso JC., and , Fink DJ. HSV-mediated expression of interleukin-4 in dorsal root ganglion neurons reduces neuropathic pain. Mol Pain. 2006;2:6. doi: 10.1186/1744-8069-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Peng X, Hao S, Fink DJ., and , Mata M. HSV-mediated transfer of interleukin-10 reduces inflammatory pain through modulation of membrane tumor necrosis factor alpha in spinal cord microglia. Gene Ther. 2008;15:183–190. doi: 10.1038/sj.gt.3303054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DL, Boucher TJ, Armanini MP, Poulsen KT, Michael GJ, Priestley JV, et al. The glial cell line-derived neurotrophic factor family receptor components are differentially regulated within sensory neurons after nerve injury. J Neurosci. 2000;20:427–437. doi: 10.1523/JNEUROSCI.20-01-00427.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao S, Mata M, Wolfe D, Huang S, Glorioso J., and , Fink DJ. HSV-mediated gene transfer of the glial cell derived neurotrophic factor (GDNF) provides an anti-allodynic effect in neuropathic pain. Mol Ther. 2003;8:367–375. doi: 10.1016/s1525-0016(03)00185-0. [DOI] [PubMed] [Google Scholar]
- Yeomans DC, Levinson SR, Peters MC, Koszowski AG, Tzabazis AZ, Gilly WF, et al. Decrease in inflammatory hyperalgesia by herpes vector-mediated knockdown of Nav1.7 sodium channels in primary afferents. Hum Gene Ther. 2005;16:271–277. doi: 10.1089/hum.2005.16.271. [DOI] [PubMed] [Google Scholar]
- Tzabazis AZ, Pirc G, Votta-Velis E, Wilson SP, Laurito CE, Yeomans DC. Antihyperalgesic effect of a recombinant herpes virus encoding antisense for calcitonin gene-related peptide. Anesthesiology. 2007;106:1196–1203. doi: 10.1097/01.anes.0000267603.32634.03. [DOI] [PubMed] [Google Scholar]
- Srinivasan R, Huang S, Chaudhry S, Sculptoreanu A, Krisky D, Cascio M, et al. An HSV vector system for selection of ligand-gated ion channel modulators. Nat Methods. 2007;4:733–739. doi: 10.1038/nmeth1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan R, Wolfe D, Goss J, Watkins S, de Groat W, Sculptoreanu A, et al. Eur J pain. in the press; 2008. PKCε contributes to basal and sensitizing responses of Trpv1 to capsaicin in dorsal root ganglion neurons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay M, Mata M., and , Fink DJ. Continuous delta opioid receptor activation reduces neuronal voltage gated sodium channel (NaV1.7) levels through activation of protein kinase C in painful diabetic neuropathy. J Neurosci. 2008;28:6652–6658. doi: 10.1523/JNEUROSCI.5530-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goins WF, Sternberg LR, Croen KD, Krause PR, Hendricks RL, Fink DJ, et al. A novel latency-active promoter is contained within the herpes simplex virus type 1 UL flanking repeats. J Virol. 1994;68:2239–2252. doi: 10.1128/jvi.68.4.2239-2252.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puskovic V, Wolfe D, Goss J, Huang S, Mata M, Glorioso JC, et al. Prolonged biologically active transgene expression driven by HSV LAP2 in brain in vivo. Mol Ther. 2004;10:67–75. doi: 10.1016/j.ymthe.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay M, Mata M, Goss J, Wolfe D, Huang S, Glorioso JC, et al. Prolonged preservation of nerve function in diabetic neuropathy in mice by herpes simplex virus-mediated gene transfer. Diabetologia. 2007;50:1550–1558. doi: 10.1007/s00125-007-0702-4. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay M, Wolfe D, Mata M, Huang S, Glorioso JC., and , Fink DJ. Long-term neuroprotection achieved with latency-associated promoter-driven herpes simplex virus gene transfer to the peripheral nervous system. Mol Ther. 2005;12:307–313. doi: 10.1016/j.ymthe.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Shand N, Weber F, Mariani L, Bernstein M, Gianella-Borradori A, Long Z, et al. A phase 1-2 clinical trial of gene therapy for recurrent glioblastoma multiforme by tumor transduction with the herpes simplex thymidine kinase gene followed by ganciclovir. GLI328 European-Canadian Study Group. Hum Gene Ther. 1999;10:2325–2335. doi: 10.1089/10430349950016979. [DOI] [PubMed] [Google Scholar]
- Rainov NG. A phase III clinical evaluation of herpes simplex virus type 1 thymidine kinase and ganciclovir gene therapy as an adjuvant to surgical resection and radiation in adults with previously untreated glioblastoma multiforme. Hum Gene Ther. 2000;11:2389–2401. doi: 10.1089/104303400750038499. [DOI] [PubMed] [Google Scholar]