Abstract
Background
Fluid refractory septic shock can develop into a hypodynamic cardiovascular state in both children and adults. Despite management of these patients with empiric inotropic therapy (with or without a vasodilator), mortality remains high.
Objectives
Here, the effect of cardiovascular support using intraaortic balloon counterpulsation (IABC) was investigated in a hypodynamic, mechanically ventilated canine sepsis model in which cardiovascular and pulmonary support were titrated based on treatment protocols.
Methods
Each week, three animals (n = 33, 10-12 kg) were administered intrabronchial S. aureus challenge and then randomized to receive IABC for 68 h or no IABC (control). Bacteria doses were increased over the study (4 to 8 × 109 cfu/kg) to assess the effects of IABC during sepsis with increasing risk of death.
Main Results
Compared to lower bacteria doses (4 to 7 × 109 cfu/kg), control animals challenged with the highest dose (8 × 109 cfu/kg) had a greater risk of death (mortality rate 86% vs. 17%), with worse lung injury (A-aO2), and renal dysfunction (creatinine). These sicker animals required higher norepinephrine infusion rates to maintain blood pressure and higher FiO2 and PEEP levels to maintain oxygenation (p ≤ 0.04 for all). In animals receiving the highest bacterial dose, IABC improved survival time (23.4 ± 10 h longer; p = 0.003) and lowered norepinephrine requirements (0.43 ± 0.17 mcg/kg/min; p = 0.002) and systemic vascular resistance index (1.44 ± 0.57 dynes*s-1cm-5kg-1; p < 0.0001) compared to controls. Despite these beneficial effects, IABC was associated with an increase in BUN (p = 0.002) and creatinine (p = 0.12). In animals receiving lower doses of bacteria, IABC had no significant effects on survival or renal function.
Conclusions
In a canine model of severe septic shock with a low cardiac index, IABC prolongs survival time and lowers vasopressor requirements.
Keywords: sepsis, balloon pumping, S. Aureus, canine, septic shock, intra-aortic
Introduction
Septic shock is the leading cause of death in medical intensive care units [1] and is typically characterized as a hyperdynamic, high cardiac output, low systemic vascular resistance state. However, data suggests that as many as one fourth of adult patients had relatively low cardiac outputs after fluid resuscitation [2, 3]. In addition, children with fluid refractory septic shock are even more likely than adults to have a hypodynamic cardiovascular profile. After fluid resuscitation, up to 58% of pediatric patients with septic shock have cardiac indices < 3.3 L/min/m2 [4]. Notably, children with this hypodynamic septic shock phenotype had a mortality rate that was almost three times higher than their hyperdynamic counterparts. Likewise, mortality in adults with this hemodynamic pattern is high, exceeding 50% [2, 3]. Inotropes (dopamine, epinephrine), inodilators (dobutamine, milrinone) and vasodilators (nitroprusside, nitroglycerin) are commonly used in cardiogenic shock and occasionally used in the setting of hypodynamic septic shock [4]. However, these therapeutic interventions have potential risks and have not been validated in clinical trials.
The intra-aortic balloon counterpulsation (IABC) cardiac assist device is presently approved by the United States Food and Drug Administration (FDA) for use in various forms of shock including septic shock [5]. The first IABC device was tested in animal experiments in the 1950’s [6, 7]. In 1967, Kantrowitz et al. used IABC in 3 patients in cardiogenic shock [8]. This study [8] and two other clinical series [9, 10] had limited success on outcome but interest grew due to the demonstrated beneficial hemodynamic effects. A surgically placed counter-pulsation device with the ability to inflate and deflate in synchronization with cardiac function was available for clinical studies by the 1970’s [11]. The septic shock indication was largely based on preclinical and clinical studies conducted at that time. In large animals challenged with gram negative bacteria, IABC improved survival times and hemodynamics, and had variable effects on organ injury [12-14] in contrast to no effect observed in a model using endotoxin infusion over a short time period [15]. Clinically, several open label case series supported the use of IABC during sepsis in the setting of severe coronary disease or evidence of a low output state [16-19].
Despite the approval of IABC for septic shock, the well-established associated risks (limb ischemia, bleeding, vascular injury, renal injury, and infection) and difficulty of placement largely precluded the clinical adoption of this approach [20-22]. However, the development of a bedside percutaneously inserted device has made the procedure easier and has led to the widespread use of IABC for several cardiac indications [23, 24]. The last two decades have seen further technical advances including smaller sheaths and advanced computer software programs that have improved synchronization and augmentation [25]. The technologically advanced IABC device with a better risk/benefit ratio and simplified operation has not been studied in clinical septic shock.
The purpose of the present investigation was to test the efficacy of the current generation of the IABC device in a large animal model of septic shock and determine whether clinical studies including randomized, controlled trials are warranted. Mechanically ventilated dogs were challenged intrabronchially with Staphylococcus aureus to induce pneumonia, bacteremia, and septic shock. Following bacterial challenge, animals were randomized to receive IABC or no pump (controls) for a 68 h period. Since the risk/benefit ratio of IABC may vary depending on the risk of death, bacteria doses were escalated over the course of the study to increase mortality. All animals received conventional hemodynamic and pulmonary support based on treatment algorithms similar to those employed in the routine clinical care of critically ill septic patients [26]. The effect of IABC on survival, cardiac performance, organ injury and vasopressor dependence was measured.
Materials and Methods
Study Design
The experiments described below were performed as part of an approved protocol by the Animal Care and Use Committee of the Clinical Center at the National Institutes of Health. For 11 consecutive weeks, three purpose-bred beagles (12-28 months, 10-12 kg) were challenged intrabronchially with S. aureus (n = 33) and followed for 96 h. Each week, two of the animals were assigned to IABC from 4 to 72 h or no pump (control). The third animal studied each week was assigned to receive whichever therapy the other two animals were not randomized to that week. We gave progressively larger doses of bacteria (4 to 8 × 109 cfu/kg) to increase mortality rates (i.e. risk of death) as weeks progressed. This allowed us to test initially whether the balloon pump was safe using relatively non-lethal challenges (low risk of death); the low mortality bacterial dose would favor the detection of IABC-associated harm. Later in the study, high lethality (high risk of death) challenges were used to enhance our ability to detect benefit.
Each week, general anesthesia (propofol, isoflurane) with mechanical ventilation via an endotracheal tube was used both to perform a tracheostomy and to place percutaneous femoral arterial, external jugular venous and urinary bladder catheters. Following tracheostomy, the dogs received continuous sedation (fentanyl, midazolam, and medetomidine) and were maintained with mechanical ventilation and fluids and vasopressors. Baseline blood cultures and routine blood samples were taken for analysis. A bronchoscope was then used at time 0 to place S. aureus in the lower lobe of the right lung. During the first 4 h after S. aureus challenge while sepsis was developing, maintenance fluids (2 ml/kg/h) and phenylephrine, titrated to mean arterial pressure (MAP) > 80 mmHg were administered. Phenylephrine was used to counteract the hypotensive effects related to sedation while sepsis was developing. After 4 h, when symptoms of sepsis were fully developed (based on prior experience with the model), vasopressor support was terminated, intravascular hemodynamics were measured, an echocardiogram was obtained, and blood samples were taken. Treatment for sepsis was then initiated and similar to human care was individualized to the hemodynamic, oxygenation and ventilation needs of each dog. Specifically, the level of fluid, vasopressor and ventilatory support was dictated by algorithms and adjusted based on transcutaneous oxygen saturation, MAP, pulmonary arterial occlusion pressure (PAOP) determinations and arterial blood gas monitoring. In addition to the start of standard sepsis treatment, at 4 h the dogs were also randomized for treatment with IABC or no pump (control). In those randomized to IABC, balloon counterpulsation was initiated and continued for 68 h or until death. The above hemodynamic and blood measurements were repeated at 8 h, and on days 1, 2, 3, and 4. Antibiotics (ceftriaxone, 50 mg/kg IV q24 h) were started 4 h after bacterial inoculation and administered daily through day 4. Animals alive at 96 h were considered survivors. Nonsurvivors progressed to terminal shock and death despite the application of fluids and vasopressors per protocol. Veterinarians unaware of the hypothesis being tested could euthanize an animal at any time if they felt it necessary. This did not occur, as the titration of sedatives and narcotics based on a set protocol kept animals pain-free.
Surgical Procedures
Animals were fasted for 18 h prior to surgery. After intravenous induction with propofol (4-6 mg/kg), tracheal intubation (Rusch, 6 F, Duluth, GA) and mechanical ventilation (Fabius Trio, Drager Medical, Telford, PA), anesthesia was maintained with isoflurane (0.5 – 1.5 %). During surgery animals received 500 ml of normal saline over 30 min in addition to maintenance fluids (2 ml/kg/h) until the end of the procedure. A tracheostomy was performed and a tracheostomy tube was placed and secured.
Catheter Placement
Femoral arterial and external jugular venous catheters (Maxxim Medical, Athens, TX) were placed percutaneously using aseptic technique. A 7 F pulmonary artery thermodilution catheter (Abbott Critical Care, Chicago, IL) was introduced through an 8 F introducer via the external jugular vein into the pulmonary artery. A 20-gauge arterial catheter was placed into right femoral artery. An 8 F sheath was placed in the left femoral artery for placement of IABC device. A urinary catheter (Cook, Foley 8 F, 55 cm) was also placed in the bladder aseptically and connected to a bag.
Intra-aortic balloon and pump
At T4 h, fluoroscopy was used to place the tip of the intra-aortic balloon (8 F, 9 cc; Datascope Corp. Fairfield, NJ) in the descending thoracic aorta, just distal to the left subclavian artery and then secured in place. The catheter was connected to the console (98XT, Datascope Corp. Fairfield, NJ) for the IABC device and balloon counterpulsation (1:1 augmentation) was initiated. Balloon counterpulsation was optimized by appropriately timing the inflation-deflation cycle of the balloon with the electrocardiogram or arterial blood pressure tracings to maximize diastolic augmentation (the maximum pressure in the proximal aorta during balloon inflation in diastole) while appropriately decreasing assisted systolic and end diastolic pressures [27]. Controls received an intraarterial 8 F sheath without an IABC device. At 72 h, IABC was terminated over the course of 1 h and the balloon catheter was removed.
Bacterial Inoculation
Under general anesthesia (see above), the dogs received preoxygenation with 100% oxygen for 5 min, the tracheal tube was removed and a sterile bronchoscope (Olympus BF 1T20, Center Valley, PA) was advanced via the tracheal stoma under direct vision into a right lower lobe segmental bronchus [28]. A Swan-Ganz catheter was advanced via the suction port of the bronchoscope and wedged with the balloon inflated into a subsegmental bronchus. Ten ml of a solution with a known amount of S. aureus bacteria (4 to 8 × 109 cfu/kg) was administered via the catheter into the right lower lobe. The balloon was deflated and the bronchoscope and swan-ganz catheter were removed. Preparation of the bacteria has been previously described [29, 30].
Mechanical Ventilation
The ventilator (Servovent 300, Siemans Medical, Sweden) was initially set with a FiO2 = 25%, PEEP = 5 cm H2O, tidal volume = 15 ml/kg, and respiratory rate = 15 breaths/min. These tidal volumes are standard in intensive care units treating critically ill septic canines [31-33]. If the O2 saturation fell below 92%, the FiO2 was increased in order to 50, 75 and 100% alternating with increases in PEEP, initially increased by 5 followed by 2 cm H20 to reach an O2 saturation ≥ 92%. The maximum settings were FiO2 = 100% and PEEP = 12 cm H2O. Support was reduced if the O2 saturation was above 93% for 6 h by similar decrements. Blood gas determinations (q2 h until T8 h and q8 h thereafter) were used to set breath rates on the mechanical ventilator. Breath rate was increased by increments of 5 to maintain pCO2 under 35 mmHg or decreased by 5 if pH > 7.35 and pCO2 ≤ 30 mmHg. The maximum setting was 35 and the minimum was 15 breaths per min.
Fluids and Vasopressors
During the first 4 h after bacterial inoculation before the development of signs and symptoms of sepsis in this model, a phenylephrine infusion (10 mg/250 ml, titrated to effect) was used to maintain the animal’s blood pressure at a mean of 80 mmHg (low normal for dogs). This was to insure that sedation did not cause hypotension in any animal while sepsis was developing. During this time, maintenance intravenous fluid infusion of normosol-M with 27 mEq KCl added (2 ml/kg/h) was administered. This is standard maintenance fluid in critically ill septic shock canines in intensive care units [31-33]. Four hours after bacterial inoculation (T4 h), the phenylephrine was turned off for a washout period of 10 min.
At T4 h, to simulate hemodynamic support practiced clinically during sepsis, if PAOP was < 10 mmHg, a fluid challenge (20 ml/kg) was given. If after 3 fluid challenges, the MAP was < 80 mmHg, an infusion of norepinephrine was initiated. Norepinephrine was adjusted incrementally (0, 0.2, 0.6, 1.0, and 2.0 μg/kg/min) to maintain MAP between 80 mmHg and 100 mmHg. At subsequent time points (q2 h until T12 h and q4 h thereafter) until the end of the study, if PAOP was < 10 mmHg, an additional intravenous fluid challenge (20 ml/kg) was administered. If one averages all the fluids given, including for cardiac output measurements, carriers needed to infuse intravenous medications, i.e. sedation, antibiotics, etc., and all fluid challenges and maintenance fluid given over the 96 h experiments, on average each septic animal received 2.5 to 3.5 liters of crystalloid or the equivalent of approximately 18 to 25 liters of fluid in a 70 kg (4.5 – 6.3 L/d) septic human.
Other ICU Therapies
Other care was instituted based on the standard veterinary practices for critically ill dogs requiring sustained mechanical ventilation in the clinical setting [31, 33]. Every 4 h, the animal’s mouth was flushed with chlorhexidine solution and the eyes were lubricated with a sterile petroleum gel [34]. The forelimbs were placed square with the slightly elevated head and the hind limbs were serially rotated between left and right positions. Passive limb movement (fore and hind limbs) were performed every 4 h. Every 12 h, sterile saline (3 ml) was instilled in the trachea followed by tracheal suctioning. The inner cannula of the tracheostomy was cleaned with chlorhexidine and then rinsed with sterile saline two times each day or more frequently if secretions accumulated. All dressings of catheter sites were changed daily. Throughout the study, a heated water blanket and regular blankets were used to maintain core temperature between 36.5° C and 37.5° C. Humidity in the tubing was maintained using a humidifier (Conchatherm III, Hudson RCI-AB) attached to the airway system. To protect the dogs from stress induced stomach ulcers, famotidine, an H2 blocker (1 mg/kg IV q12 h), was administered and to protect from venous thrombosis during mechanical ventilation and sedation, heparin (3000 IU IM, q8 h) was administered until the end of the study.
Sedation Management
The adequacy of sedation was evaluated and adjusted by a clinician or trained technician continuously at the bedside for 96 h after initiation of midazolam (0.2 mg/kg loading dose, 50 μg/kg/min infusion) and fentanyl (5 μg/kg loading dose, 0.7 μg/kg/min infusion). Both the fentanyl and midazolam infusions were increased in increments of one fourth of the dose, every 5 min until adequate sedation had been obtained. Medetomidine infusion (2-5 μg/kg/min) was used to supplement sedation as needed according to set criteria. Criteria for adequacy of sedation were continuously monitored as follows: 1) the animal should be breathing comfortably in synchrony with the ventilator with jaw tone present but without voluntary limb movement; 2) the eyes should remain central in the orbit; 3) the animal should be unresponsive to light tactile stimuli. Criteria for reducing sedation were also monitored and included: 1) palpebral reflexes not present; 2) the animal not responsive to painful stimuli (toe squeeze).
Physiologic Measurements
Cardiac output (CO), mean pulmonary artery pressure (MPAP), PAOP, and central venous pressure (CVP) were determined via a pulmonary artery thermodilution catheter placed in the external jugular vein. MAP was measured and heart rate (HR) calculated via the femoral arterial pressure recording. Left ventricular ejection fraction (LVEF) was determined by ultrasound (Sonos 5500, Philips Medical). The CO was standardized to the animal’s weight in kilograms (CI). These measurements were performed at baseline, 4, 24, 48, 72 and 96 h after intra-bronchial bacterial inoculation.
Laboratory Data
Arterial and mixed venous blood gases were measured every 2 h until T8 h and every 8 h thereafter with a blood gas system (ABL 500; Radiometer, Copenhagen, Denmark). Complete blood counts (STK-S; Coulter Electronics, Hialeah, FL) and chemistries were performed with an automatic analyzer at 24 h and every 24 h thereafter. Blood (isolator tubes) was also obtained for quantitative blood cultures at 24 h and every 24 h thereafter.
Statistical Methods
Survival times were analyzed using a Cox Proportional Hazards model [35] with dose of bacteria identified as low (first six weeks of study) and high (last 5 weeks of study) based on control (no pump) mortality. As each week of the study had both IABC and no pump animals, the effect of IABC during low and high lethality challenges were estimated controlling for study week. Survival time differences between IABC and no pump animals were presented as an odds ratio reflecting the beneficial (or harmful) mortality effects of treatment compared to control. Hemodynamic, laboratory, and measures of pulmonary and cardiac support data were analyzed using a four-way analysis of variance (ANOVA) procedure [36]. The four factors in the ANOVA model included lethality (low and high), group (IABC vs. control), dog nested within group, and time. Interactions among lethality, group, and time were included in the model. Interactions between dog and all other factors formed the error term. Time was treated as a continuous variable, so that the group-time interaction tested for differences among slopes, and as a class variable to compute an area under the curve to summarize the complete time course of the study.
Results
Survival, organ injury, and treatment differences with low vs. high bacterial challenges
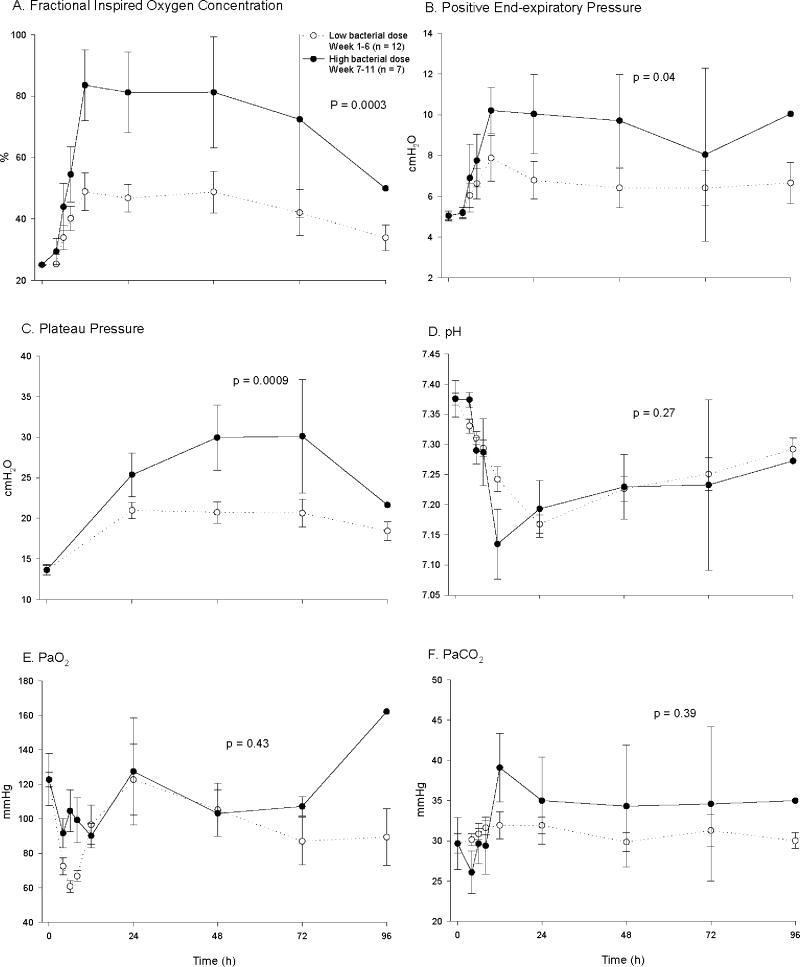
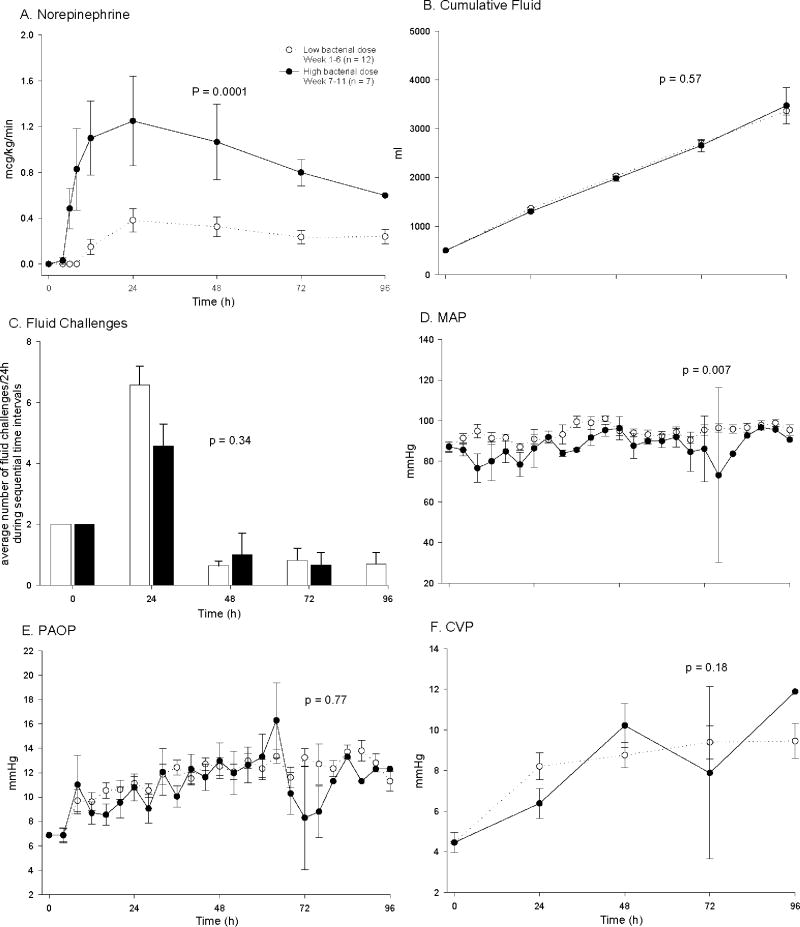
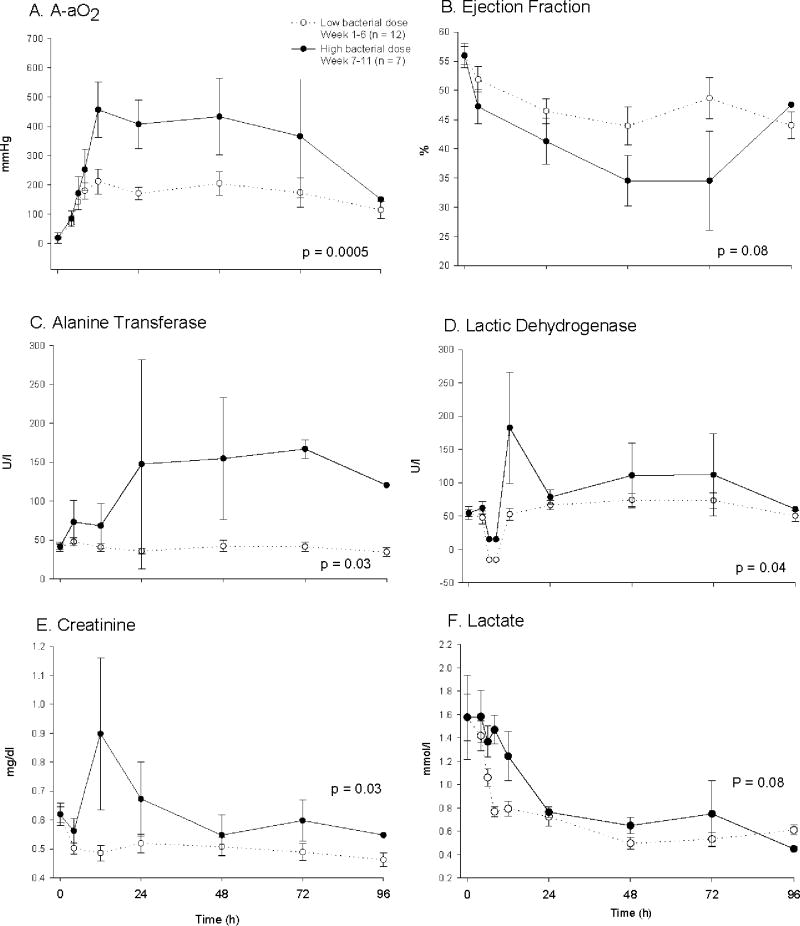
There were no significant differences in baseline hemodynamic measurements and blood chemistries except for a lower serum creatinine, arterial pO2 and pH, and plateau pressure in control animals challenged with high compared to low doses of bacteria (all p < 0.05) (table 1). Although statistically significant, these differences were small and of uncertain physiologic relevance. In control animals (no pump), mortality rates increased from 17% to 86% during the first six weeks of the study compared to the last five weeks [bacterial doses of 4 - 7 vs. 8.0 × 109 cfu/kg] with a significant decrease in survival time in animals receiving the highest dose of bacteria. Compared to control animals receiving the lower bacterial doses, controls receiving the highest dose required significantly greater mechanical ventilatory support including higher FiO2 concentrations (p = 0.0003) and higher PEEP levels (p = 0.04) to maintain similar O2 saturation goals (≥ 92%) and PaO2 levels (fig. 1A, B, E). Set breath rates on mechanical ventilation rates were not significantly different (data not shown; p = ns) comparing control animals receiving high and low bacterial dose. These breath rates maintained comparable arterial pH and PaCO2 levels in high and low bacterial dose control animals (all p = ns; fig. 1D, F). In controls, plateau pressures were significantly greater in animals receiving high versus low doses of bacteria (p < 0.0009), but on average, always < 30 mmHg (fig. 1C), and therefore not indicative of a need to institute lower tidal volumes [37]. Futhermore, control animals receiving the highest bacterial dose required higher norepinephrine doses (p = 0.0001), but a similar volume of cumulative fluid intake (p = 0.6) and number of fluid challenges (p = 0.34) (fig. 2A-C) to maintain comparable MAP (~80 mmHg) and PAOP goals (8 to 10 mmHg) and CVP levels (fig. 2D-F). Indicators of organ injury such as A-aO2 gradient (p = 0.0005), left ventricular ejection fraction (p = 0.08), liver function test [alanine transferase(p = 0.03), lactic dehydrogenase (p = 0.04)] and serum creatinine levels (p = 0.03) were significantly worse with the highest vs. lower doses of bacteria (fig. 3A-E). Lactate levels fell similarly throughout the 96 h experiment with low versus high dose of bacteria (p = ns; fig. 3F).
Table 1.
Baseline Values (Mean ± SE)
| Low bacterial dose
|
High bacterial dose
|
|||||
|---|---|---|---|---|---|---|
| IABC
|
No Pump
|
IABC
|
No Pump
|
p-value* | p-value+ | |
| Mean Arterial Pressure (mmHg) | 88(5) | 86(3) | 89(2) | 89(2) | 0.44 | 0.66 |
| Pulmonary Artery Occlusion Pressure (mmHg) | 7.2(0.4) | 7.0(0.5) | 7.0(0.6) | 8.3(0.6) | 0.07 | 0.65 |
| PaO2 (mmHg, FiO2 = 25%) | 146(7) | 141(4) | 105(15) | 124(15) | 0.0006 | 0.75 |
| Cardiac Index (l/min/kg) | 0.17(0.04) | 0.15(0.02) | 0.13(0.01) | 0.17(0.02) | 0.37 | 0.52 |
| Ejection Fraction (%) | 56(4) | 57(2) | 56(2) | 55(2) | 0.83 | 0.94 |
| Alanine Transferase (U/l) | 36.5(3.3) | 40.6(3.5) | 43.8(7.4) | 42.1(6.1) | 0.39 | 0.99 |
| Lactic Dehydrogenase (U/l) | 53(8) | 70(10) | 65(13) | 39(5) | 0.19 | 0.77 |
| Creatinine (mg/dl) | 0.63(0.05) | 0.67(0.03) | 0.5(0.03) | 0.57(0.04) | 0.002 | 0.50 |
| Blood Urine Nitrogen (mg/dl) | 10.8(1.0) | 9.6(0.6) | 9.4(0.7) | 10.6(1.6) | 0.79 | 0.99 |
| pH | 7.43(0.04) | 7.40(1.71) | 7.36(0.02) | 7.35(0.03) | 0.02 | 0.54 |
| Lactate (mmol/l) | 2.4(1.0) | 1.6(0.2) | 0.74(0.1) | 1.53(0.4) | 0.09 | 0.08 |
| SVRI (dyne* s-1cm-5kg-1) | 3.6(0.8) | 3.8(0.4) | 5.3(0.6) | 3.6(0.4) | 0.35 | 0.49 |
| Plateau Pressure (cmH20) | 12.7(0.4) | 12.8(0.7) | 14.2(0.7) | 14.4(0.6) | 0.02 | 0.99 |
| SvO2 (%) | 46.4(3.1) | 46.0(1.7) | 35.6(3.7) | 46.7(6.2) | 0.13 | 0.11 |
| Central Venous Pressure (mmHg) | 4.3(0.7) | 4.3(0.5) | 6.5(1.5) | 4.6(0.0) | 0.18 | 0.31 |
| n = | 6 | 12 | 8 | 7 | ||
, low versus high bacterial dose;
, Interaction IABC versus no pump
Figure 1. Effects of S. aureus dose on levels of pulmonary supportive therapies required to normalize measures over 96 h in controls (no pump).
Shown are A) fractional inspired oxygen concentration, B) positive end-expiratory pressure, C) plateau pressure, and D) arterial pH, E) PaO2, and F) PaCO2. The dashed lines with open circles represent (mean ± SE) values weeks 1-6 when animals received low bacterial dose, low lethality challenges and the solid lines with closed circles represent weeks 7-11, when high bacterial dose, high lethality challenges were used. All data are plotted from a common origin based on values for all animals at baseline. For actual baseline values for the individual treatment groups, see Table 1. P-value compares groups over time.
Figure 2. Effects of S. aureus dose on cardiac support measures over 96 h in controls (no pump).
Shown are A) norephinephrine dose, B) cumulative fluid, C) fluid challenges, D) MAP, E) PAOP and F) CVP. The format is the same as Fig. 1.
Figure 3. Effects of S. aureus dose on measures of organ injury over 96 h in controls (no pump).
Shown are A) arterial-alveolar oxygen gradient, B) cardiac ejection fraction, C) alanine transferase, D) lactic dehydrogenase, E) creatinine and F) lactate. The format is the same as Fig. 1.
Effects of IABC on Mortality
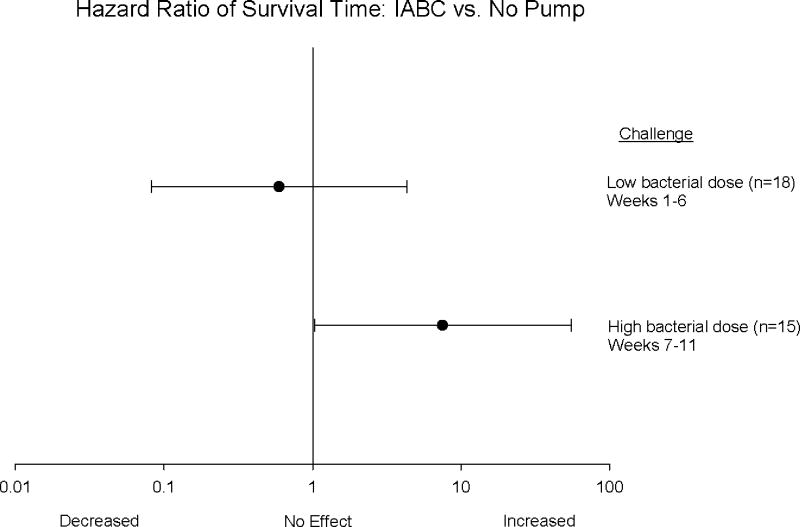
IABC did not improve overall mortality rates (controls 17% vs. IABC 33%) or survival time amongst animals challenged with the lower doses of bacteria (weeks 1 to 6) (fig. 4A). In animals receiving the highest dose of bacteria (weeks 7 to 11), IABC did not improve overall mortality rates (controls 86% vs. IABC 75%), but did improve survival time (+23.4 ± 10 h longer). In each of the last five weeks of the study with the highest dose of bacteria, of the 3 animals studied, a control always died first (fig. 4B). In three of these five weeks, 2 IABC animals, despite receiving the same dose of bacteria as the control, survived longer and in one week the IABC animal survived longer then the two controls. Only in one of these five weeks did one control animal survive longer than an IABC animal (fig. 4B). During these weeks using the highest bacterial challenge, IABC significantly increased the hazard ratio of survival time compared to controls (p = 0.003; fig. 5). In addition, IABC with high lethality bacterial challenges had a greater beneficial effect on survival time than during the low lethality challenges (p < 0.05 for interaction; fig. 5).
Figure 4. Survival time in control and IABC treated dogs by study weeks.
In panel A, study weeks 1 – 6 when low dose bacterial challenges were used (4 – 7 × 109 cfu/kg) and in panel B, weeks 7 – 11 when high dose bacterial challenges were used (8 × 109 cfu/kg)
Figure 5. The hazard ratio of survival time and 95% confidence interval (horizontal line) comparing IABC to no pump.
There was an increase in survival time with IABC during weeks 7-11 at high bacterial dose (solid circle), high lethality producing S. aureus challenges (p = 0.003). This beneficial effect on survival time was significantly greater weeks 7-11 than weeks 1-6 when low bacterial dose (open circle), low lethality challenges were employed. In those animals receiving treatment, IABC was active from 4 to 72h.
Differences in outcome other than survival with IABC: High vs. low lethality challenges
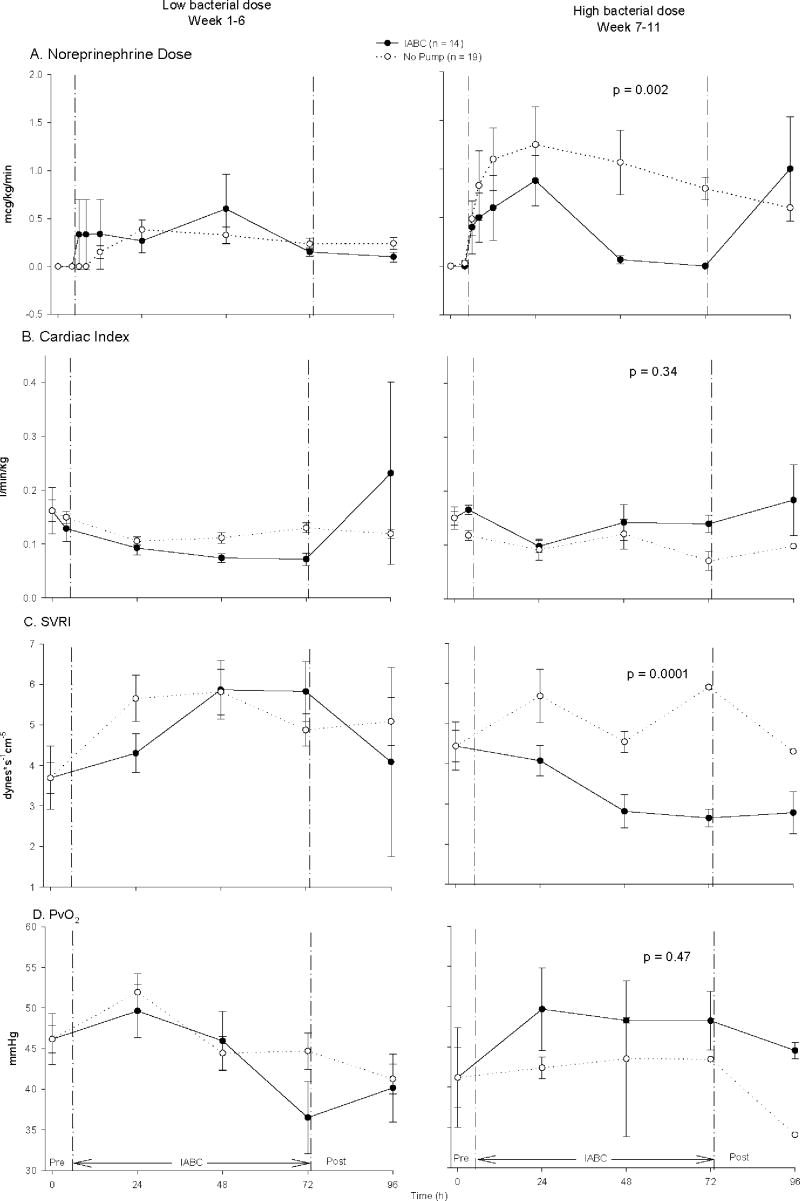
Compared to controls, animals receiving IABC had significantly lower norepinephrine requirements and significantly lower systemic vascular resistance indices with high, but not low bacterial dose challenges (fig. 6A, p = 0.002 for interaction; fig. 6C, p < 0.0001 for interaction). Despite these lower norepinephrine requirements and systemic vascular resistance indices there were no significant differences compared to controls in CI (p = 0.34, interaction)(fig. 6B), mixed venous oxygen saturation, PvO2 (p = 0.47, interaction)(fig. 6D), MAP (p = 0.72, interaction)(data not shown), or PAOP (p = 0.38, interaction)(data not shown) in high versus low dose bacterial challenges. Compared to controls, IABC was associated with a trend toward a greater increase in serum creatinine (p = 0.12, interaction) and a significantly greater increase in BUN (p = 0.002, interaction) with high compared to low dose bacterial challenges (Fig. 7). There was no effect of IABC on liver function tests at high or low dose bacterial challenges (data not shown).
Figure 6. Norepinephrine doses and systemic vascular resistance index had significantly different outcomes in animals treated with IABC vs. no pump at low bacterial dose, low lethality challenges (left panel) compared to high bacterial dose, high lethality challenges (right panel) of S. aureus (p = 0.002 interaction, Panel A; p < 0.0001 interaction, Panel C).
During high dose bacterial challenges, the IABC (solid circle, solid line) compared to no pump (open circle, dashed line) significantly lowered the dose of norepinephrine required and systemic vascular resistance index obtained, but this was not true at low dose bacterial challenges. There was no significant difference for CI and PvO2 between the effect of the IABC versus no pump comparing low to high dose bacterial challenges (all, p = ns for an interaction, Panel B and D).
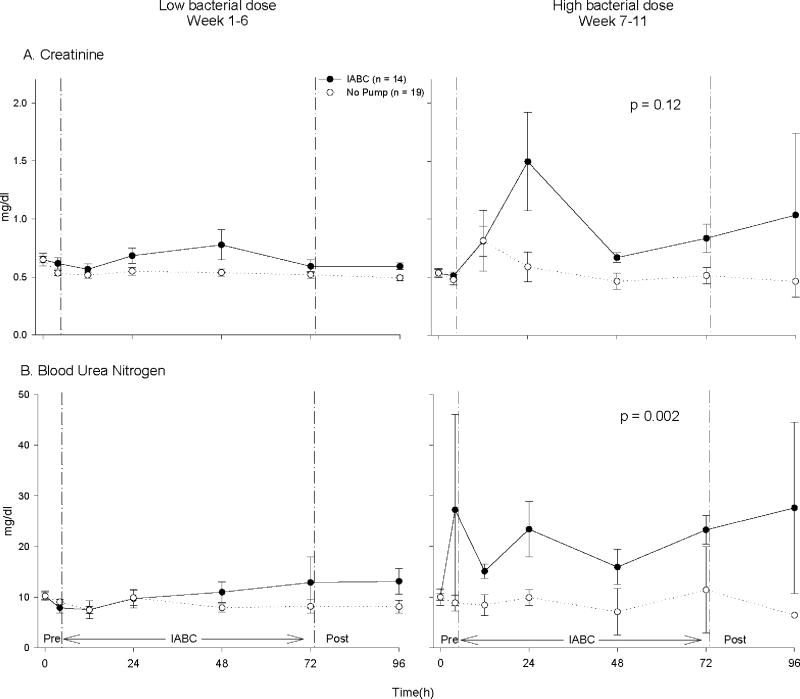
Figure 7. Effects of IABC on renal function.
Shown are A) creatinine and B) blood urea nitrogen (BUN). The format is the same as Fig. 6. At high dose bacterial challenges, the IABC compared to no pump caused a trend toward a greater rise in creatinine (p = 0.12, interaction) and a significantly greater rise in BUN (p = 0.002, interaction) over the 68 h study compared to low dose bacterial challenges.
Other laboratory measurements
There were no significant differences in complete blood counts or electrolytes that could explain the effects of IABC on survival times, norepinephrine doses, or organ injury (table 2).
Table 2.
Laboratory measures
| Parameter | Bacterial Dose | No Pump | IABC | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time after bacterial inoculation (mean (+/- SE)) | p-value* | Time after bacterial inoculation (mean (+/- SE)) | p-value+ | ||||||||||
| Baseline
|
24 h
|
48 h
|
72 h
|
96 h
|
Baseline
|
24 h
|
48 h
|
72 h
|
96 h
|
||||
| Animals alive | 19 | 16 | 14 | 13 | 11 | 14 | 11 | 8 | 7 | 6 | |||
| White blood cells (K/uL) | Low | 6.6(0.5) | 8.6(1.7) | 14.3(1.4) | 14.7(1.7) | 8.7(2.0) | 7.5(0.8) | 8.4(2.6) | 15.5(2.9) | 15.7(2.3) | 7.4(2.3) | ||
| High | 6.4(0.4) | 2.5(0.8) | 8.7(0.8) | 11.9(4.6) | 16.3(0.0) | 0.29 | 7.5(0.9) | 2.3(0.6) | 8.5(1.0) | 10.9(3.7) | 7.7(1.5) | 0.99 | |
| Hemoglobin (g/dL) | Low | 12.5(0.4) | 16.5(0.7) | 13.4(0.8) | 11.9(1.0) | 11.3(0.8) | 12.0(0.2) | 16.6(1.0) | 14.0(1.5) | 10.7(1.8) | 11.7(1.1) | ||
| High | 11.4(0.4) | 18.2(0.6) | 14.0(0.5) | 12.8(1.1) | 11.2(0) | 0.18 | 13.7(0.8) | 19.6(1.5) | 15.1(1.4) | 14.6(0.8) | 12.0(1.2) | 0.18 | |
| Platelets (K/uL) | Low | 221(14) | 189(14) | 105(9) | 86(13) | 80(14) | 190(15) | 149(13) | 52.4(5.0) | 21.3(4.5) | 36.3(4.3) | ||
| High | 187(17) | 170(17) | 102(27) | 63(71) | 137(0) | 0.55 | 247(19) | 129(20) | 47(4) | 28.3(17.7) | 45(13) | 0.04 | |
| Bicarbonate (mmol/L) | Low | 17.1(0.4) | 11.1(0.5) | 11.8(0.3) | 13.2(0.7) | 14.3(1.0) | 15.5(0.3) | 11.1(0.8) | 11.9(0.7) | 12.3(0.6) | 12.8(0.5) | ||
| High | 16.8(1.5) | 12.6(1.0) | 13.0(1.1) | 13.3(1.1) | 15.1(0) | 0.48 | 13.2(1.2) | 9.6(1.2) | 11.2(1.2) | 11.3(1.2) | 13.1(1.1) | 0.81 | |
| Sodium (mmol/L) | Low | 145 (1) | 144(1) | 142(1) | 142(1) | 144(1) | 143 (1) | 143(2) | 141(2) | 142(3) | 144(4) | ||
| High | 144(1) | 139(2) | 139(4) | 141(8) | 145(0) | 0.006 | 145(1) | 142(2) | 138(2) | 136(2) | 136(6) | 0.62 | |
| Chloride (mmol/L) | Low | 117(1) | 126(1) | 124(1) | 123(1) | 124(1) | 117(1) | 125(1) | 124(2) | 123(3) | 125(4) | ||
| High | 115(1) | 119(2) | 122(4) | 122(4) | 121(0) | 0.01 | 118(1) | 122(2) | 122(3) | 121(3) | 118(1) | 0.91 | |
| Potassium (mmol/L) | Low | 3.7(0.1) | 3.6(0.1) | 3.4(0.1) | 3.2(0.2) | 3.2(0.2) | 3.5(0.1) | 3.3(0.2) | 3.5(0.1) | 3.4(0.1) | 3.4(0.3) | ||
| High | 3.8(0.1) | 4.3(0.3) | 4.0(0.4) | 4.5(1.3) | 4.2(0) | 0.01 | 3.8(0.1) | 4.7(0.4) | 3.9(0.2) | 4.2(1.0) | 4.3(0) | 0.41 | |
, low vs. high bacterial dose;
, Interaction IABC vs. no pump comparing low vs. high bacterial dose
Discussion
We tested the effect of intra-aortic balloon counterpulsation therapy on outcome after S. aureus intrabronchial challenge. As expected, animals challenged with high doses of S. aureus had higher mortality rates, and worse cardiopulmonary, renal and hepatic function compared to lower less lethal bacterial doses. Likewise, animals challenged with high doses of bacteria required increased amounts of vasopressors, FiO2 and PEEP to maintain normal blood pressures and oxygenation levels. IABC therapy increased survival times and decreased vasopressor dose requirements and lowered systemic vascular resistance index in animals that had been challenged with high doses of bacteria. Despite lower vasopressor doses and systemic vascular resistance index in these animals, CI and PvO2 were similar in the IABC and control groups. During low lethality challenges (17% mortality), IABC had no significant beneficial effects on vasopressor dose or outcome.
In a severe pneumonia model with depressed cardiac function, optimal fluid management is complex. Excessive fluid may raise cardiac filling pressures to the point of markedly worsening oxygenation while inadequate fluid may fail to optimize cardiac preload and impair organ perfusion. These 10 kg animals on average received 2.5 to 3.5 liters of crystalloids over the 96 h experiment. Giving an equivalent volume of fluid to a 70 kg patient with sepsis would require 18 to 25 liters of crystalloid resuscitation over 4 days (4.5 – 6.3 L/d). Indications that fluid resuscitation in our study was sufficient include the facts that lactate levels fell throughout the experiment (fig. 3F), and at least with the highest doses of bacteria (associated with a survival time benefit for IABC) systemic vascular resistance index never rose (fig.6C) and PvO2 did not fall (fig. 6D). Conversely, cardiac filling pressures were within an acceptable range for fluid resuscitation throughout the experiment (CVP 4-10 and PAOP 10-12 mmHg; fig. 2E, F) suggesting that this volume of fluid was not excessive and therefore did not inappropriately worsen the degree of respiratory failure. Finally, plateau pressures (fig. 1C) were on average always less than 30 mmHg throughout the experiment, within a clinically acceptable range that does not mandate reductions in tidal volume [37]. Thus, there is no clinical evidence to suggest that preload was inadequate, or that cardiac filling pressures or airway pressure were too high in our study.
After high dose bacterial challenges, renal function as measured by increases in BUN and creatinine worsened during IABC. We initially positioned the IABC catheter tip under fluoroscopy and then sutured the insertion site to hold the tip in place. It is possible that with changing the animal’s position every 4 hours, the catheter migrated and the balloon potentially intermittently obstructed renal artery flow. However, this effect of IABC on renal function was greater in high compared to low dose bacterial challenge. This suggests that catheter migration is not the sole explanation for IABC-induced renal dysfunction, which would have been expected to be similar between low and high dose bacterial challenges. We speculate that the IABC may worsen renal blood flow during sepsis through a deflation-induced steal mechanism that may not be fully balanced by the salutary effects of IABC on cardiac performance. This phenomenon could also be a problem in septic humans.
It is also of concern that the IABC was only beneficial on survival times but not rates, and only with high but not low dose bacterial challenges. Of note in this study, the balloon pump was neither applied nor discontinued based on therapeutic indications. All animals were randomized for treatment at 4 h after bacterial challenge and received IABC independent of severity of cardiac abnormalities or vasopressor needs for 72 h or until death, whether or not a clinical indication for continuing was still present. It is unknown whether or not IABC would have a more marked beneficial effect if only animals with the most severe cardiovascular abnormalities were randomized and IABC was continued until sufficient recovery of cardiovascular function. Lastly, in animals exposed to low dose bacterial challenges with minimal cardiovascular abnormalities, the ability of the IABC to improve cardiac function and thereby outcome would be expected to be reduced and consistent with this, we found no benefit to IABC therapy in this group.
In our study, cardiac index was always below baseline despite adequate preload, suggesting therapies that enhance cardiac performance without increasing myocardial demand might be beneficial. In patients with low cardiac output septic shock, dobutamine and sodium nitroprusside are sometimes used to improve cardiac performance. However, neither of these agents has been tested in randomized controlled trials for this indication and their efficacy is uncertain. Dobutamine itself can cause hypotension and arrhythmias, and in high doses has been associated with worsening outcome during septic shock [38]. Sodium nitroprusside can cause life threatening hypotension and in renal failure, a common complication of septic shock, toxic metabolites accumulate [39]. Another approach for low cardiac output septic shock is to switch from norepinephrine to epinephrine. However, in canine sepsis, epinephrine compared to other vasopressors has been associated with delayed cardiac recovery [28] and worsened survival [40]. The risks associated with the use of these vasoactive agents during septic shock may, in part, be why the mortality for low cardiac output septic shock remains so high [3]. Thus, new therapeutic options for low cardiac output septic shock such as IABC therapy need to be considered.
The mechanism for improved survival times and reduced vasopressor requirements with IABC therapy in severe septic shock is unknown. High dose vasopressors have been shown to maldistribute blood flow [41] and in similar sepsis models worsen outcome [40]. IABC reduces cardiac workload by decreasing afterload improving left ventricular ejection and outflow. This increase in ventricular outflow on a beat-to-beat basis over time increases mean arterial pressure, thus, lowering vasopressor requirements. In this study with high dose bacterial challenge, as norepinephrine doses decreased in IABC animals, systemic vascular resistance index was lowered and cardiac index was maintained and by 72 h mixed venous oxygen saturation, and cardiac index was improved compared to control animals (Fig. 6A-D). In addition, by inflating at the dicrotic notch, IABC augments diastolic pressure resulting in an increase in coronary perfusion. The decrease in afterload (balloon deflation at end diastole) and increase in coronary perfusion (diastolic augmentation) by IABC is in contrast to vasopressors that increase coronary flow at the expense of reducing cardiac performance. The ability of the IABC to improve cardiac function and reduce vasopressor dependence, thereby decreasing its deleterious effects on cardiac performance and the microcirculation may have improved survival time.
The question remains, have we provided adequate evidence to support IABC studies in septic humans. At low bacterial doses and less severe sepsis, we found no significant harmful effects of IABC. However, the number of animals studied was small. As risk of death from infection increased, IABC therapy improved two outcomes that may be related, i.e. longer survival times and lower vasopressor requirements. Animal models are not proof that a therapy will or will not be beneficial in humans. Accordingly, this animal study should not be used to justify use of IABC clinically in septic patients. Moreover, we found no beneficial effect of IABC on survival rates. Nonetheless, clinical septic shock still has an unacceptably high mortality rate (30 to 50%) and new therapies are needed [1]. In the setting of highly lethal septic shock resulting in a low or normal cardiac output and dependence on high dose vasopressors, our data suggest IABC therapy should be further investigated.
This study used a persistent sepsis model never used before to study IABC therapy in the treatment of sepsis. The IABC device was in place over 4 days and easily managed by clinicians and technicians without obvious technical problems. The animals were individually treated over days based on standardized algorithms and therapies commonly used in human septic shock. Although there were only 30 IABC-days in these critically ill septic animals, there was no evidence of complications related to catheter placement, limb ischemia, bleeding, infection, or vascular injury. The data in this model with a modern IABC device were consistent with previous studies in animals using older IABC devices showing survival benefits [12, 13, 15] and human sepsis studies showing improvement in low cardiac output states [16, 17].
Conclusions
In summary, with high risk, high dose S. aureus pneumonia-induced septic shock, we found that IABC therapy improved survival time and decreased vasopressor requirements. The modern IABC device has never been formally tested in a sepsis trial and the decrease in renal function demonstrated in our study is concerning. However, in carefully selected patients with low cardiac output septic shock and a high risk of death, a randomized controlled trial of IABC therapy may be indicated.
Acknowledgments
This study was funded by the National Institutes of Health intramural program.
Datascope Corporation provided funding ($19,378) designated to support a six-month lease of the ultrasound machine used for this animal study. In addition to the funds for the ultrasound, Datascope loaned the Critical Care Medicine Department 3 intra-aortic balloon counterpulsation devices (system 98XT) and supplied 26 sterile intra-aortic balloons. This funding represents less then 5% of the cost of the study. Datascope Corporation, as part of the agreement with the National Institutes of Health, did not participate in the collection or analysis of the data or the manuscript.
Footnotes
The authors have not disclosed any potential conflicts of interest.
References
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.D’Orio V, Mendes P, Saad G, Marcelle R. Accuracy in early prediction of prognosis of patients with septic shock by analysis of simple indices: prospective study. Crit Care Med. 1990;18(12):1339–1345. doi: 10.1097/00003246-199012000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Parker MM, Shelhamer JH, Natanson C, Alling DW, Parrillo JE. Serial cardiovascular variables in survivors and nonsurvivors of human septic shock: heart rate as an early predictor of prognosis. Crit Care Med. 1987;15(10):923–929. doi: 10.1097/00003246-198710000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Ceneviva G, Paschall JA, Maffei F, Carcillo JA. Hemodynamic support in fluid-refractory pediatric septic shock. Pediatrics. 1998;102(2):e19. doi: 10.1542/peds.102.2.e19. [DOI] [PubMed] [Google Scholar]
- 5.www.fda.gov.
- 6.Kantrowitz A. Experimental augmentation of coronary flow by retardation of the arterial pressure pulse. Surgery. 1953;34(4):678–687. [PubMed] [Google Scholar]
- 7.Kantrowitz A, Mc KW. The experimental use of the diaphragm as an auxiliary myocardium. Surg Forum. 1958;9:266–268. [PubMed] [Google Scholar]
- 8.Kantrowitz A, Tjonneland S, Freed PS, Phillips SJ, Butner AN, Sherman JL., Jr Initial clinical experience with intraaortic balloon pumping in cardiogenic shock. Jama. 1968;203(2):113–118. [PubMed] [Google Scholar]
- 9.Bregman D, Kripke DC, Goetz RH. The effect of synchronous unidirectional intraaortic balloon pumping on hemodynamics and coronary blood flow in cardiogenic shock. Trans Am Soc Artif Intern Organs. 1970;16:439–446. [PubMed] [Google Scholar]
- 10.Buckley MJ, Leinbach RC, Kastor JA, Laird JD, Kantrowitz AR, Madras PN, Sanders CA, Austen WG. Hemodynamic evaluation of intraaortic balloon pumping in man. Circulation. 1970;41(5 Suppl):II130–136. doi: 10.1161/01.cir.41.5s2.ii-130. [DOI] [PubMed] [Google Scholar]
- 11.Weber KT, Janicki JS. Intraaortic balloon counterpulsation. A review of physiological principles, clinical results, and device safety. Ann Thorac Surg. 1974;17(6):602–636. doi: 10.1016/s0003-4975(10)65706-2. [DOI] [PubMed] [Google Scholar]
- 12.Pribble CG, Shaddy RE. Intra-aortic balloon counterpulsation in newborn lambs infected with group B streptococcus. ASAIO Trans. 1991;37(1):33–37. doi: 10.1097/00002480-199101000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Roberts AJ, Hoover EL, Alonso DR, Combes JR, Dineen P, Gay WA, Jr, Subramanian VA. Prolonged intraaortic balloon pumping in klebsiella-induced hypodynamic shock: cardiopulmonary, hematological, metabolic, and pathological observations. Ann Thorac Surg. 1979;28(1):73–86. doi: 10.1016/s0003-4975(10)63397-8. [DOI] [PubMed] [Google Scholar]
- 14.Dunn JM, Kirsh MM, Harness J, Lee R, Straker J, Sloan H. The role of assisted circulation in the management of endotoxic shock. Ann Thorac Surg. 1974;17(6):575–583. doi: 10.1016/s0003-4975(10)65700-1. [DOI] [PubMed] [Google Scholar]
- 15.Engoren M, Habib RH. Effects of intraaortic balloon augmentation in a porcine model of endotoxemic shock. Resuscitation. 2004;60(3):319–326. doi: 10.1016/j.resuscitation.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 16.Berger RL, Saini VK, Long W, Hechtman H, Hood W., Jr The use of diastolic augmentation with the intra-aortic balloon in human septic shock with associated coronary artery disease. Surgery. 1973;74(4):601–606. [PubMed] [Google Scholar]
- 17.Foster ED, Subramanian VA, Hechtman HB, Berger RL, Vito L. Response to intra-aortic balloon pumping. Am J Surg. 1975;129(4):464–471. doi: 10.1016/0002-9610(75)90194-4. [DOI] [PubMed] [Google Scholar]
- 18.Mercer D, Doris P, Salerno TA. Intra-aortic balloon counterpulsation in septic shock. Can J Surg. 1981;24(6):643–645. [PubMed] [Google Scholar]
- 19.Chiu YH, How CK, Chern CH, Wang LM, Huang CI. Cardiac rescue with intra-aortic balloon counterpulsation in refractory shock due to acute meningococcemia. Am J Emerg Med. 2007;25(2):253–254. doi: 10.1016/j.ajem.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 20.Alvarez JM, Gates R, Rowe D, Brady PW. Complications from intra-aortic balloon counterpulsation: a review of 303 cardiac surgical patients. Eur J Cardiothorac Surg. 1992;6(10):530–535. doi: 10.1016/1010-7940(92)90003-g. [DOI] [PubMed] [Google Scholar]
- 21.Curtis JJ, Boland M, Bliss D, Walls J, Boley T, Schmaltz R, Flaker G, Anderson SK. Intra-aortic balloon cardiac assist: complication rates for the surgical and percutaneous insertion techniques. Am Surg. 1988;54(3):142–147. [PubMed] [Google Scholar]
- 22.Makhoul RG, Cole CW, McCann RL. Vascular complications of the intra-aortic balloon pump: an analysis of 436 patients. Am Surg. 1993;59(9):564–568. [PubMed] [Google Scholar]
- 23.Subramanian VA, Goldstein JE, Sos TA, McCabe JC, Hoover EA, Gay WA., Jr Preliminary clinical experience with percutaneous intraaortic balloon pumping. Circulation. 1980;62(2 Pt 2):I123–129. [PubMed] [Google Scholar]
- 24.Subramanian VA, McCabe JC, Hoover EI, Gay WA, Jr, Sos TA, Goldstein J. Percutaneous intra-aortic balloon counterpulsation. A new technique. N Y State J Med. 1981;81(6):923–924. [PubMed] [Google Scholar]
- 25.Nanas JN, Moulopoulos SD. Counterpulsation: historical background, technical improvements, hemodynamic and metabolic effects. Cardiology. 1994;84(3):156–167. doi: 10.1159/000176394. [DOI] [PubMed] [Google Scholar]
- 26.Hollenberg SM, Ahrens TS, Annane D, Astiz ME, Chalfin DB, Dasta JF, Heard SO, Martin C, Napolitano LM, Susla GM, et al. Practice parameters for hemodynamic support of sepsis in adult patients: 2004 update. Crit Care Med. 2004;32(9):1928–1948. doi: 10.1097/01.ccm.0000139761.05492.d6. [DOI] [PubMed] [Google Scholar]
- 27.Maccioli GA, Lucas WJ, Norfleet EA. The intra-aortic balloon pump: a review. J Cardiothorac Anesth. 1988;2(3):365–373. doi: 10.1016/0888-6296(88)90320-1. [DOI] [PubMed] [Google Scholar]
- 28.Freeman BD, Quezado Z, Zeni F, Natanson C, Danner RL, Banks S, Quezado M, Fitz Y, Bacher J, Eichacker PQ. rG-CSF reduces endotoxemia and improves survival during E. coli pneumonia. J Appl Physiol. 1997;83(5):1467–1475. doi: 10.1152/jappl.1997.83.5.1467. [DOI] [PubMed] [Google Scholar]
- 29.Natanson C, Danner RL, Elin RJ, Hosseini JM, Peart KW, Banks SM, MacVittie TJ, Walker RI, Parrillo JE. Role of endotoxemia in cardiovascular dysfunction and mortality. Escherichia coli and Staphylococcus aureus challenges in a canine model of human septic shock. J Clin Invest. 1989;83(1):243–251. doi: 10.1172/JCI113866. published erratum appears in J Clin Invest 1989 Mar;83(3):1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Natanson C, Fink MP, Ballantyne HK, MacVittie TJ, Conklin JJ, Parrillo JE. Gram-negative bacteremia produces both severe systolic and diastolic cardiac dysfunction in a canine model that simulates human septic shock. J Clin Invest. 1986;78(1):259–270. doi: 10.1172/JCI112559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gronert GA, H S, Steffey EP, Fung D. Plasma electrolyte and metabolite concentrations associated with pentobarbital or pentobarbitol-propofol anesthesia during three weeks of mechanical ventilation and intensive care in dogs. Lab Animal Science. 1998;48:513–519. [PubMed] [Google Scholar]
- 32.Hansen B. Mechanical ventilation. 2005 [Google Scholar]
- 33.Moon PF, C K. Mechanical Ventilation. 9. Philadelphia: WB Saunders Co.; 1992. [Google Scholar]
- 34.Slatter d. Diseases of the trachea and bronchi. Philadelphia: WB Saunders; 2002. [Google Scholar]
- 35.Cox D. Regression models and life tables. Journal of Royal Statistical Society. 1972;B(34):187–200. [Google Scholar]
- 36.Scheffe Models in the analysis of variance. Annals of Mathematical Statistics. 1956;27:251–71. [Google Scholar]
- 37.Dellinger RP, Carlet JM, Masur H, Gerlach H, Calandra T, Cohen J, Gea-Banacloche J, Keh D, Marshall JC, Parker MM, et al. Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Crit Care Med. 2004;32(3):858–873. doi: 10.1097/01.ccm.0000117317.18092.e4. [DOI] [PubMed] [Google Scholar]
- 38.Hayes MA, Timmins AC, Yau EH, Palazzo M, Hinds CJ, Watson D. Elevation of systemic oxygen delivery in the treatment of critically ill patients. N Engl J Med. 1994;330(24):1717–1722. doi: 10.1056/NEJM199406163302404. [DOI] [PubMed] [Google Scholar]
- 39.Gauss A, Anhaupl T, Schutz W. The basics of catecholamine therapy. 2. A guide to clinical use. Anasthesiol Intensivmed Notfallmed Schmerzther. 2000;35(3):131–136. doi: 10.1055/s-2000-10851. [DOI] [PubMed] [Google Scholar]
- 40.Minneci PC, Deans KJ, Banks SM, Costello R, Csako G, Eichacker PQ, Danner RL, Natanson C, Solomon SB. Differing effects of epinephrine, norepinephrine, and vasopressin on survival in a canine model of septic shock. Am J Physiol Heart Circ Physiol. 2004;287(6):H2545–2554. doi: 10.1152/ajpheart.00450.2004. [DOI] [PubMed] [Google Scholar]
- 41.Carlyle PF, Cohn JN. Systemic and regional hemodynamic effects of alpha-adrenoceptor blockade in chronic left ventricular dysfunction in the conscious dog. Am Heart J. 1990;120(3):619–624. doi: 10.1016/0002-8703(90)90020-x. [DOI] [PubMed] [Google Scholar]