Abstract
We propose that working memory (WM) and reasoning share related capacity limits. These limits are quantified in terms of the number of items that can be kept active in WM, and the number of interrelationships between elements that can be kept active in reasoning. The latter defines the complexity of reasoning problems and the processing loads they impose. Principled procedures for measuring, controlling or limiting recoding and other strategies for reducing memory and processing loads have opened up new research opportunities, and yielded orderly quantification of capacity limits in both memory and reasoning. We argue that both types of limit may be based on the limited ability to form and preserve bindings between elements in memory.
The techniques that humans have for making the best use of available information processing capacity are of immense value, but they have to be controlled in order to study capacity limitations and effects of complexity. New, principled procedures for measuring, controlling or limiting recoding and other strategies whereby subjects reduce memory and processing loads have enabled complexity and capacity effects to be investigated independently of, and in interaction with, knowledge. In this paper we present an hypothesis that this development enables a new, integrated treatment of reasoning and working memory (WM), including an orderly quantification of capacity limits, and that this has opened up new research opportunities.
Working memory and reasoning
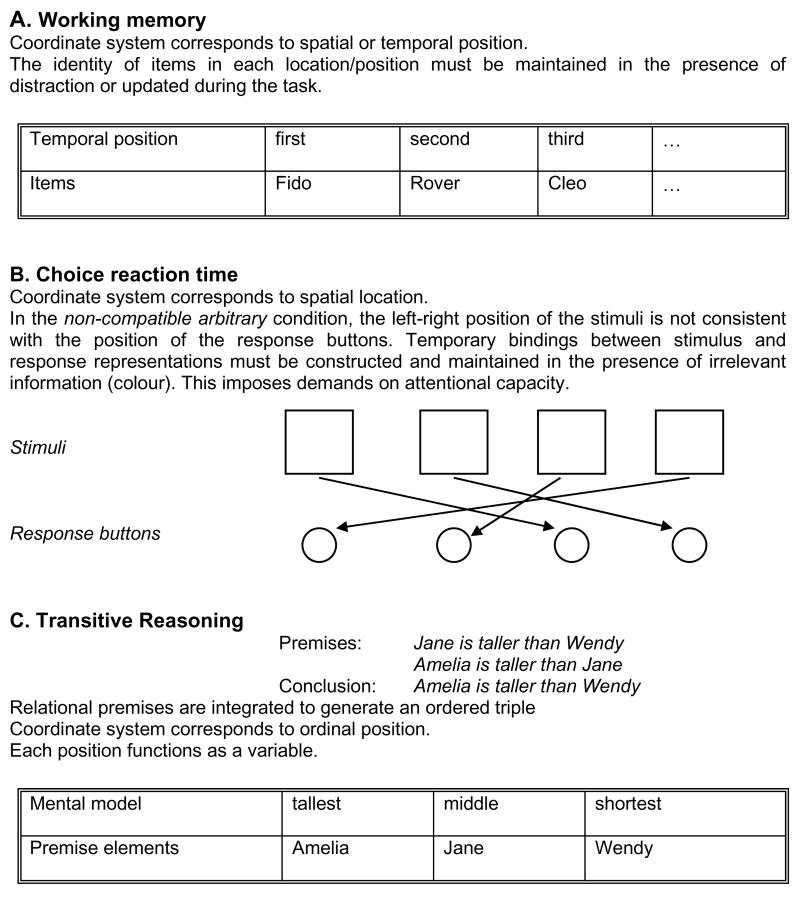
Developments in both theory and methodology have strengthened the links between WM and reasoning and some salient points are summarised in Box 1. We propose that the essential link between WM and reasoning is in the common requirement to bind elements to a coordinate system. Consider first short-term serial recall of the words, “Fido, Rover, Cleo”. The words are assigned to ordinal positions when presented (Figure 1A), but this assignment must be maintained for later recall, and this requires attention. Even in free recall (not shown), items on a trial must be bound to the present-trial concept or node in memory; binding may be even more extensive inasmuch as an associative network between items would greatly aid in recall. Now consider a choice reaction time task where participants press a different button in response to one of several lights, and the buttons are assigned to lights randomly (non-compatible mappings, Figure 1B). Maintaining bindings of button positions to light positions in WM requires attention [1]. Finally, consider a transitive inference problem such as “Jane is taller than Wendy, Amelia is taller than Jane”.
Box 1. Capacity effects in working memory (WM) and reasoning.
The core of WM is the temporary binding of elements to a coordinate system [5, 6] which is closely related to relational representations used in reasoning [4, 1]. Temporary binding to structural representations possibly accounts for the strong relationship between WM capacity and reasoning and fluid intelligence (Gf) [1].
Capacity limits in both WM and reasoning can be attributed to the number of bindings to slots in a coordinate system or relation. WM is limited to approximately four items that can be kept active [7], while representations in reasoning are limited to four interrelated variables [8].
Latent variable constructs of WM capacity account for approximately .60 of the variance in reasoning and Gf [2].
WM has a domain-general component that is critical to its prediction of reasoning and Gf [2].
New assessments of WM capacity measure how many elements fit in the focus of attention [3] or capacity-limited region [9] more explicitly than traditional sentence and operation spans. These include: Computer-paced reading of numbers, or performing simple operations of +1 or − 1, while retaining words or letters for later recall [10]; or presentations too rapid and unpredictable to allow rehearsal [3].
Figure 1.
Binding of elements into coordinate systems in (A) working memory (B) choice reaction time, and (C) reasoning.
This can be solved by mapping premise elements into an ordering schema as shown in Figure 1C.
Maintaining bindings between elements and slots using attention is common to WM and to reasoning. This is not the only common factor, but there is substantial evidence that working memory capacity (WMC) accounts for a sizeable proportion of the variance in reasoning [1, 2] and intelligence [3]. WM and reasoning differ in whether the binding is supplied with the input (as in short-term serial recall) or has to be constructed by the reasoner, as in syllogistic (including transitive) inference, where premise elements have to be mapped to slots in a mental model in a way that is consistent with the premises. There are intermediate cases, such as where the mapping of responses to inputs, although predetermined, must be coded by the performer, or where participants construct their own mnemonics. Coordinate systems in WM are less likely to include explicit relational representations than those in reasoning. Use of an explicit symbol that differentiates different kinds of links is a feature that distinguishes relations from associations [4]. In Figure 1C the relation “taller-than” is explicit whereas the ordering schemes used in WM might not be.
We further propose that the common demand for attention when binding elements into slots is a possible explanation for common capacity limitations in WM and reasoning. We will not present a detailed model of these processes [see 4], but will focus on methods for controlling chunking, recoding, rehearsal and other strategies, so that capacity limits can be measured.
Working memory capacity
Immediate memory capacity is roughly constant if measured in meaningful units, termed chunks [11]. For example, FBICIAIRS comprises up to 9 chunks, but only 3 chunks if the observer recognizes three familiar acronyms (FBI, CIA, IRS). WMC can only be estimated if the number of chunks can be determined, and techniques for this are summarised in Box 2.
Box 2: Some techniques to examine working memory capacity limits in chunks.
The following methods have been used to examine working memory capacity limits and show, we believe, 3 to 5 chunks on average. Various manipulations help to identify the number of items per retrieved chunk and, therefore, the number of chunks retrieved.
Spatial arrays: A visual array (spatial arrangement) is briefly presented for comparison with a probe array identical to the first array or differing in one item’s identity [12]. It is too fast for grouping, so presumably each item is a chunk.
Perfect serial recall: The length of list allowing errorless recall reflects the chunk capacity, assuming grouping strategies cannot be used consistently [13].
Running span: Items are presented rapidly, in a list with an unpredictable endpoint. The participant then recalls items from the end of the list. Given this presentation, it is impossible to group items as in ordinary serial recall, so each item is presumably one chunk [14].
Span with distraction or suppression: A visual or auditory list is presented. A secondary task prevents grouping and rehearsal, so each item is assumed to be one chunk [15].
Categorical retrieval: Items are to be recalled from a meaningful category (e.g., states). Each burst of items recalled is assumed to reflect working memory filled to capacity from long-term memory once [16].
Memory updating: Items are to be remembered, some of which are eligible to be changed (updated). Responses slow markedly as eligible items increase from 1 to 3 [9].
Probed recall: Participants indicate if a probe occurred in a just-presented list. At least 3 semantically-related items near the end of the list can show especially fast retrieval [17].There is interference from other, similar lists if the number of items exceeds basic capacity [18].
Multi-object tracking (examines attention limits related to WM): An array of items includes several cued as targets. The cues disappear; then all items move in different random trajectories. When they stop, the participant must identify the targets. Targets up to the capacity limit may be tracked together as one changing object [19].
Reconstruction by chunks: A structured configuration (chess setup) is examined and reconstructed from memory. The pieces reconstructed without pause presumably form a single chunk; capacity is estimated as chunks recalled per look [20].
Recollection by chunks: Word series with varying inter-item association strengths are presented; words recalled in the presented order are taken to reflect a single chunk [21, 22].
A review [7] of disparate procedures in which items cannot be grouped or rehearsed (see Box 2) converges on a range of 2–6 items in adults (usually 3–5), and fewer in children or the elderly [23]. In cases of exceptional or expert memory, it is apparently the size of recalled chunks, rather than their number, that is extraordinary [24].
Measuring complex chunks
A second approach to assessing capacity limits in chunks (see Box 2) is to manipulate and estimate chunking processes [21]. In a recent study using serial recall [25], pairs of words were taught with 0 to 4 paired exposures (and a complementary number of unpaired exposures, resulting in 4 exposures in each case) and cued recall was used to assess pair learning. A mathematical model that used cued recall and the order of items in serial recall to estimate items per chunk suggested that pair learning increased chunk size, but left the number of chunks recalled constant at about 3.5 on average.
Follow-up work [22] extended this method. With long lists and free recall or free scoring of serial recall, chunk capacity limits determined recall (e.g., 6 well-learned pairs were recalled with similar accuracy to 6 singletons; about 3.5 units in each case). With shorter lists and strict serial scoring, though, length limits predominated (e.g., 4 well-learned pairs and 8 singletons were recalled equally well, but more poorly than 4 singletons). Thus, participants may rehearse about 2 s of information without taxing the WM chunk limit.
Central capacity
Theoretically, capacity limits could occur separately in different parts of the processing system. Recent evidence begins to address questions such as (1) whether there is a central capacity that can be allocated across different modalities and codes, and (2) where the capacity may reside in the brain.
One can combine a spatial memory task with a verbal task to determine whether the amount retained from one task affects the amount retained from the other. Sometimes, very little interference between verbal and spatial tasks is observed [26, 27], consistent with the view that storage is modality-specific [28]. However, recent studies show that verbal and spatial tasks interfere with one another under some circumstances. It is not because both tasks share verbal rehearsal. Morey and Cowan obtained interference between a spatial memory task and a random 7-digit list, but not between spatial memory and a memorized 7-digit number or a random 2-digit number. Most of the interference occurred only when the verbal items were spoken aloud [29]. This suggests that some mnemonic processes are not part of the store used for visual memory (e.g., rehearsal or auditory memory) but that other processes are (e.g., overt verbal retrieval). In unpublished research, J. Scott Saults and N. Cowan presented concurrent arrays of spoken digits and colored squares and showed that, if sensory memory cues were eliminated, a 1-to-1 trade-off between modalities emerged. Subjects could retain either about 4 squares, in an attend-visual condition, or about 4 items including squares and digits together, in an attend-bimodal condition. Thus, the central capacity could be allocated across modalities.
Another study [5] examined memory for verbal-spatial associations (names shown in schematic houses at different screen locations). Adults could perform this task by combining modality-specific memories for the name sequence in the trial and for the spatial path between the houses in which the names appeared, provided that there was a 1-to-1 correspondence between names and houses. When that strategy was precluded cross-modal associations presumably had to be retained. That occurred when some houses contained two names and others contained none, when verbal rehearsal of the names was suppressed, or when the subjects were children too young to rehearse.
There is some evidence regarding where central capacity may reside. The parietal lobes take part in an attention system that integrates information across modalities and may correspond to the seat of attention, separate from frontal areas involved in controlling attention [30]. Although both areas are often active together in WM tasks that involve the storage and manipulation of information, transcranial magnetic stimulation (TMS) to the frontal lobes disrupts only the manipulation, whereas parietal stimulation disrupts storage as well [31]. Some parietal areas show fMRI responses to simple visual arrays that change across memory loads in a way quite similar to behavioural responses [32]. When feature complexity is added to the stimuli by requiring memory for several features (e.g., color as well as shape), some areas respond according to the number of objects and others respond according to complexity [33]. The brain data seem compatible with a central store that reflects the focus of attention used as a store [15] although it has not yet been proven that parietal storage does reflect the focus of attention.
Chunk capacity and chunk size limits
People recall lists best if they are separated into groups of 2 to 4 items, the optimal typically being 3 [34, 35]. It could be viewed as coincidental that the optimal group size is about the same as the maximal number of chunks that can be recalled. However, it may be more than coincidental, if the capacity limit is attention-related. Formation of a new chunk might be possible only if the elements are retained in the capacity-limited store (focus of attention?) concurrently, allowing rich associations to form between the items [15]. The optimal group size is therefore the largest number of items that can be reliably stored to be inter-associated at once. Chunks of any size can be built up, of course, using the capacity-limited region reiteratively. The fact that larger chunks can be built up with practice and attention and that WM no longer seems to limit performance [24] does not mean that chunk capacity limits cease to play a role; expertise leads to larger chunks [36] (like the configurations in a chess game) but, when independent estimates of capacity are obtained, they still equal about 3–4 chunks in adults, and fewer in children [3, 37].
Links between working memory and reasoning
Accumulated evidence indicates that estimates of WMC have some degree of domain-generality and have a central tendency of approximately 3.5 items. We hypothesise that the WM limit reflects capacity for attention, which determines the number of elements, whether items or chunks, that can be bound into a coordinate system. This hypothesis links WM capacity directly to a theory of capacity limitations in reasoning, Relational Complexity theory [4] considered next, which proposes that reasoning is limited by the number of variables or slots that can be related in a single representation.
Complexity in reasoning
Measures of the number of symbols required to define a concept [38, 39] have achieved significant successes, as have metrics based on rule hierarchies [40]. Complexity metrics have also been important in cognitive development [41]. However we focus on a metric that discriminates between complexity and knowledge effects, and which incorporates binding of problem elements to mental models.
The Relational complexity (RC) metric [4, 42, 43, 44] defines complexity by the number of slots or arity of a relation that must be represented to perform a specific cognitive process (e.g., a binary relation such as “larger-than” has two slots, one each for the larger and smaller entities). Each slot of a relation can be filled in a variety of ways, and corresponds to a variable or dimension, and an n-ary relation is a set of points in n-dimensional space. RC norms are summarised in Table 1. The relation is a co-ordinate system as noted earlier, so RC corresponds to the number of bindings to a co-ordinate system, consistent with the way we suggest capacity is defined in WM.
Table 1. Arity of relations, number of slots or variables, an example of each level and approximate median age of attainment.
| Arity of relation | Slots/variables | Example | Median age |
|---|---|---|---|
| Unary | one | class membership, e.g. cat(Marcus) | One year |
| Binary | two | larger(elephant, mouse) | 1½ years |
| Ternary | three | addition(2,3,5) | 5 years |
| Quaternary | four | proportion( 2,3,6,9) | 11 years |
In RC theory processing load depends on the complexity of relations processed in any step of a task. There are two mechanisms for reducing processing loads:
Conceptual chunking involves recoding concepts into less complex relations. For example, speed = distance/time, is a ternary relation, but speed can be recoded into a unary relation, speed(60kph) as when speed is indicated by a pointer on a dial. Chunking reduces processing load, but chunked variables cannot be accessed. Thus the chunked representation of speed does not permit us to answer questions such as “How does speed change if we cover the same distance in half the time?” and we must revert to the ternary relation to calculate the answer. (Similarly, if one needs to access part of a chunk in WM or its internal structure, the chunk must be unpacked.) Conceptual chunking is analogous to mnemonic chunking in that it compresses a representation into fewer variables, but explicit relations are defined between the variables, and they are not simply associated (e.g., velocity is not an association between speed, distance, time but a specific relation defined between them).
Segmentation entails breaking tasks into less complex steps, which can be processed serially. Strategies and algorithms are common ways of doing this (e.g., adding one column at a time in multi-digit addition). Representing complex structures hierarchically, and processing one level of the hierarchy at a time, is also an effective way to segment tasks [40]. Chunking and segmentation skills are important components of expertise.
There is common ground between WM and reasoning. First, both are limited by ability to map elements into coordinate systems, which in turn depends on attention [cf. 45]. Second, both involve compressing material into chunks, mnemonic or (for reasoning) conceptual in nature. Conceptual chunks contain more relational information than mnemonic chunks, because the coordinate systems in reasoning are explicit relations. Third, the limitation is in the number of independent components, irrespective of their size. Chunk-size in WM is analogous to the number of possible instantiations of a relational slot in reasoning (i.e. whether a slot can be instantiated in 2, 3, . . . , or arbitrarily many ways). In both cases, this factor has less influence on capacity than the number of chunks in WM, or the number of slots in reasoning.
Method for Analysis of Relational Complexity (MARC)
MARC principles that have been found consistently valid across many domains are shown in Box 3. Application of these principles shows some tasks are difficult because they resist decomposition. Two examples (see Figures 1C, 2) are discussed below.
Figure 2.
An application of the Method for Analysis of Relational Complexity to Knight and Knaves reasoning task.
Box 3 Principles of the Method of Analysis of Relational Complexity (MARC).
Complexity analyses begin with established models of reasoning processes, taking account of knowledge and strategies likely to be available to the reasoner. Assessments must be appropriate for the participant sample, and control tests are essential (e.g., closely matched binary-relational tasks are important controls for assessing ternary relations) [42].
Effective relational complexity for a cognitive process is the least complex relation required to represent it. The required mental models are determined from established process models, supported by empirical research.
Where tasks entail more than one step, the processing complexity of the task is the relation that must be represented to perform the most complex step, using the least demanding strategy available to humans for that task [4].
-
Variables cannot be chunked or segmented if relations between them must be processed, because relations between chunked or segmented variables become inaccessible. This leads to a number of corollaries:
Chunking sets. The relation between A and both B and C, is binary if the relation between B and C can be chunked, which is possible if the relation(s) between them need not be processed. In oddity tasks a strategy of noting that red is different from three blues has RC = 2 [46].
Interaction between variables. Variables cannot be chunked or segmented if they interact because the influence of each variable is modified by the others. Therefore interacting variables must be processed jointly.
Where two arguments of a relation function as a unit for the cognitive process being performed, they can be chunked. In Figure 2, “A is a knave and B is a knave” is one conceptual chunk for the first step. Similarly, if A is a knight and A says . . . (some proposition) then the predicates “is a knight” and “says” can be chunked because they are a unit, equivalent to “The knight, A says” [47].
Where a cognitive overload cannot be reduced by conceptual chunking or segmentation it is likely to be handled by default to a simpler representation, usually by ignoring one or more variables, leading to fragmentation of knowledge.
Transitive inference imposes a processing load [42] because both premises need to be considered to uniquely assign premise elements into slots in the representation in WM (Figure 1C). There are three slots, so RC = 3. Premise integration in transitive inference involves neural circuits in the prefrontal and parietal cortices [48, 49]. Prefrontal regions are also activated in other reasoning and Gf tasks that require relational integration including modified Ravens Matrices problems [49, 50] and analogy problems [51]. Moreover, as task complexity increases more anterior regions appear to be recruited [52, 53].
Knight and knave problems are segmented into separate inference steps (see Figure 2), but each inference entails compound propositions. In step 1, the proposition knight(A) contradicts the proposition knave(A) which implies knight(A) is false. Applications of MARC [47] result in three conceptual chunks: “Knight A says”, “A and B are knaves” (as a chunked argument of “says”), and “the hypothesis is false”. Therefore, for this step RC = 3.
The need to interpret inputs jointly constrains decomposition into simpler subtasks. Some tasks that are difficult or complex (e.g., according to Cognitive Complexity and Control theory [40]), incorporate this factor [54]. Interpretation of interactions was used to assess number of variables that humans can process in parallel, while controlling techniques for reducing processing load. Performance declined as complexity increased and was at chance level on 5-way interactions [8]. Given that an n-way interaction is defined on n variables, so content is assigned to n slots, the data indicate that humans are limited to processing four variables.
Summary
We have proposed similar limitations in WM and reasoning, which appear to reflect a central capacity limit. WM is limited to about 3–4 chunks, and reasoning is limited to relations between four variables. One priority is to identify the common underlying mechanisms. A promising hypothesis is that both WM and reasoning require items/concepts to be in the focus of attention concurrently. This would allow them to be inter-associated to form chunks or bound into coordinate systems, including relational representations that permit inferences. Another priority is to determine the underlying brain systems. Fronto-parietal networks have been implicated. Parietal regions appear most important for focus of attention and storage functions of WM, whereas prefrontal regions appear more important for manipulation functions in WM and reasoning. If manipulation demands increase with RC, then TMS applied to prefrontal regions should be more disruptive as task RC increases. Procedures for controlling simplifying strategies have opened the way for these and other lines of investigation into WM, reasoning, the links between them, and the underlying brain systems.
Box: Outstanding questions.
Techniques for quantification of reasoning complexity and the assessment of WM capacity imply potential advancements in several areas. Some of these have begun to be realised, but there is scope for further research. The following questions outline some possibilities.
WM Capacity. Does the WM limit of 3–5 items reflect the focus of attention? Is there a pool of capacity that is general across domains? Is there also a limit in how long ideas remain active when no longer attended, or not [55]? If not, then what is special about the attended information? Is it that only information in the focus of attention can be bound together [7, 45]?
WM and Reasoning. Are all possible inter-relations formed between items concurrently in the focus of attention? Is that why WM capacity is limited [7, 45]? Does such mutual binding allow new chunk formation and, simultaneously, comprehension?
Locus of capacity. Where in the brain might central capacity reside? In parietal cortex [31]?
Theoretical integration. Complexity analyses are bringing a more orderly interpretation to cognitive developmental findings [56]. Does this resolve some apparent conflicts, such as those between precocity and developmental change?
Classical findings. Hirst et al., [57] demonstrated improvement in concurrent tasks with practice, apparently indicating there was no capacity limit, but this might not hold if expertise and WM capacity were assessed independently. Chi’s finding of superior retention by child chess experts as compared with adult chess novices demonstrates a powerful effect of knowledge on short term memory [58], but when capacity is measured it has been found to increase with age. Should these findings be reassessed in the light of contemporary knowledge of WM?
Assessment criteria in reasoning. Given that the norms of logic are no longer seen as appropriate criteria for human reasoning, does cognitive complexity provide an alternative basis for assessment? [59]
Transition processes. Increasingly complex concepts can be acquired by adding variables to representations (e.g., density helps to account for variations in size and weight). Acquisition depends both on knowledge and on capacity to process the extra dimension, but chunking and segmentation can reduce processing loads. Do these factors have implications for transition processes in cognitive development?
Improving experimental design. Complexity effects are not always recognised. For example, the difference between categorisation by rules and by information integration could be accounted for by complexity differences [60]. Should the effect of complexity be considered in experimental design to avoid confounding with other variables?
Behavioural and brain imaging research. Recent research suggests that brain regions such as rostrolateral prefrontal cortex are more selective to cognitive complexity than to cognitive domain [52]. Can precise manipulation of cognitive complexity, with other factors controlled, increase precision of brain imaging research?
Contributor Information
Graeme S Halford, Griffith University, Mt Gravatt campus Griffith University, 170 Kessels Road, NATHAN QLD 4111, Australia.
Nelson Cowan, Department of Psychological Sciences, University of Missouri, 18 McAlester Hall, Columbia, MO65211, USA.
Glenda Andrews, Gold Coast campus Griffith University, PMB 50, GOLD COAST MAILCENTRE QLD 9726, Australia.
References
- 1.Wilhelm O, Oberauer K. Why are reasoning ability and working memory capacity related to mental speed? An investigation of stimulus-response compatibility in choice reaction time tasks. European Journal of Cognitive Psychology. 2006;18:18–50. [Google Scholar]
- 2.Kane, et al. The generality of working memory capacity: A latent variable approach to verbal and visuospatial memory span and reasoning. Journal of Experimental Psychology: General. 2004;133:189–217. doi: 10.1037/0096-3445.133.2.189. [DOI] [PubMed] [Google Scholar]
- 3.Cowan N, et al. On the capacity of attention: Its estimation and its role in working memory and cognitive aptitudes. Cognitive Psychology. 2005;51:42–100. doi: 10.1016/j.cogpsych.2004.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Halford GS, et al. Processing capacity defined by relational complexity: Implications for comparative, developmental, and cognitive psychology. Behavioral and Brain Sciences. 1998;21:803–831. doi: 10.1017/s0140525x98001769. [DOI] [PubMed] [Google Scholar]
- 5.Cowan N, et al. Development of working memory for verbal-spatial associations. Journal of Memory and Language. 2006;55:274–289. doi: 10.1016/j.jml.2006.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baddeley A. The episodic buffer: a new component of working memory? Trends in cognitive sciences. 2000;4:417–423. doi: 10.1016/s1364-6613(00)01538-2. [DOI] [PubMed] [Google Scholar]
- 7.Cowan N. The magical number 4 in short-term memory: A reconsideration of mental storage capacity. Behavioral and Brain Sciences. 2001;24:87–185. doi: 10.1017/s0140525x01003922. [DOI] [PubMed] [Google Scholar]
- 8.Halford GS, et al. How many variables can humans process? Psychological Science. 2005;16:70–76. doi: 10.1111/j.0956-7976.2005.00782.x. [DOI] [PubMed] [Google Scholar]
- 9.Oberauer K. Access to information in working memory: exploring the focus of attention. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2002;28:411–421. [PubMed] [Google Scholar]
- 10.Lepine R, et al. What makes working memory spans so predictive of high-level cognition? Psychonomic Bulletin & Review. 2005;12:165–170. doi: 10.3758/bf03196363. [DOI] [PubMed] [Google Scholar]
- 11.Miller GA. The magical number seven, plus or minus two: Some limits on our capacity for processing information. Psychological Review. 1956;63:81–97. [PubMed] [Google Scholar]
- 12.Luck SJ, Vogel EK. The capacity of visual working memory for features and conjunctions. Nature. 1997;390:279–281. doi: 10.1038/36846. [DOI] [PubMed] [Google Scholar]
- 13.Broadbent DE, editor. The magic number seven after fifteen years. John Wiley & Sons; 1975. [Google Scholar]
- 14.Bunting MF, et al. How does running memory span work? Quarterly Journal of Experimental Psychology. 2006;59:1691–1700. doi: 10.1080/17470210600848402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cowan N. Working memory capacity. Psychology Press; 2005. [Google Scholar]
- 16.Graesser A, II, Mandler G. Limited processing capacity constrains the storage of unrelated sets of words and retrieval from natural categories. Journal of Experimental Psychology: Human Learning and Memory. 1978;4:86–100. [Google Scholar]
- 17.McElree B. Working memory and focal attention. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2001;27:817–835. [PMC free article] [PubMed] [Google Scholar]
- 18.Cowan N, et al. Capacity limits in list item recognition: Evidence from proactive interference. Memory & Cognition. 2005;13:293–299. doi: 10.1080/09658210344000206. [DOI] [PubMed] [Google Scholar]
- 19.Yantis S. Multielement visual tracking: Attention and perceptual organization. Cognitive Psychology. 1992;24:295–340. doi: 10.1016/0010-0285(92)90010-y. [DOI] [PubMed] [Google Scholar]
- 20.Gobet F, et al. Chunking mechanisms in human learning. Trends in Cognitive Sciences. 2001;5:236–243. doi: 10.1016/s1364-6613(00)01662-4. [DOI] [PubMed] [Google Scholar]
- 21.Tulving E, Patkau JE. Concurrent effects of contextual constraint and word frequency on immediate recall and learning of verbal material. Canadian Journal of Psychology. 1962;16:83–95. doi: 10.1037/h0083231. [DOI] [PubMed] [Google Scholar]
- 22.Chen Z, Cowan N. Chunk limits and length limits in immediate recall: A reconciliation. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2005;31:1235–1249. doi: 10.1037/0278-7393.31.6.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Naveh-Benjamin M, Cowan N, Kilb A, Chen Z. Age-related differences in immediate serial recall: Dissociating chunk formation and capacity. Memory & Cognition. doi: 10.3758/bf03193310. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ericsson KA, et al. Uncovering the structure of a memorist ‘s superior “basic “memory capacity. Cognitive Psychology. 2004;49:191–237. doi: 10.1016/j.cogpsych.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 25.Cowan N, et al. Constant capacity in an immediate serial-recall task: A logical sequel to Miller (1956) Psychological Science. 2004;15:634–640. doi: 10.1111/j.0956-7976.2004.00732.x. [DOI] [PubMed] [Google Scholar]
- 26.Cocchini G, et al. Concurrent performance of two memory tasks: Evidence for domain-specific working memory systems. Memory & Cognition. 2002;30:1086–1095. doi: 10.3758/bf03194326. [DOI] [PubMed] [Google Scholar]
- 27.Fougnie D, Marois R. Distinct capacity limits for attention and working memory: Evidence from attentive tracking and visual working memory paradigms. Psychological Science. 2006;17:526–534. doi: 10.1111/j.1467-9280.2006.01739.x. [DOI] [PubMed] [Google Scholar]
- 28.Baddeley AD. Oxford Psychology Series #11. Clarendon Press; 1986. Working memory. [Google Scholar]
- 29.Morey CC, Cowan N. When do visual and verbal memories conflict? The importance of working-memory load and retrieval. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2005;31:703–713. doi: 10.1037/0278-7393.31.4.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Posner MI, Peterson SE. The attention system of the human brain. Annual Review of Neuroscience. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- 31.Postle BR, et al. Repetitive transcranial magnetic stimulation dissociates working memory manipulation from retention functions in the prefrontal, but not posterior parietal, cortex. Journal of Cognitive Neuroscience. 2006;18:1712–1722. doi: 10.1162/jocn.2006.18.10.1712. [DOI] [PubMed] [Google Scholar]
- 32.Todd JJ, Marois R. Capacity limit of visual short-term memory in human posterior parietal cortex. Nature. 2004;428:751–754. doi: 10.1038/nature02466. [DOI] [PubMed] [Google Scholar]
- 33.Xu Y, Chun MM. Dissociable neural mechanisms supporting visual short-term memory for objects. Nature. 2006;440:91–95. doi: 10.1038/nature04262. [DOI] [PubMed] [Google Scholar]
- 34.Ryan J. Grouping and short-term memory: Different means and patterns of groups. Quarterly Journal of Experimental Psychology. 1969;21:137–147. doi: 10.1080/14640746908400206. [DOI] [PubMed] [Google Scholar]
- 35.Wickelgren WA. Size of rehearsal group and short-term memory. Journal of Experimental Psychology. 1964;68:413–419. doi: 10.1037/h0043584. [DOI] [PubMed] [Google Scholar]
- 36.Chi MTH. Short-term memory limitations in children: Capacity or processing deficits? Memory and Cognition. 1976;4:559–572. doi: 10.3758/BF03213219. [DOI] [PubMed] [Google Scholar]
- 37.Burtis PJ. Capacity increase and chunking in the development of short-term memory. Journal of Experimental Child Psychology. 1982;34:387–413. doi: 10.1016/0022-0965(82)90068-6. [DOI] [PubMed] [Google Scholar]
- 38.Feldman J. Minimization of Boolean complexity in human concept learning. Nature. 2000;407:630–632. doi: 10.1038/35036586. [DOI] [PubMed] [Google Scholar]
- 39.Leeuwenberg ELL. Quantitative specification of information in sequential patterns. Psychological Review. 1969;76:216–220. doi: 10.1037/h0027285. [DOI] [PubMed] [Google Scholar]
- 40.Zelazo PD, et al. The development of executive function in early childhood. Monographs of the Society for Research in Child Development. 2003;68(3) doi: 10.1111/j.0037-976x.2003.00260.x. Serial No. 274. [DOI] [PubMed] [Google Scholar]
- 41.Halford GS. Information processing models of cognitive development. In: Goswami U, editor. Blackwell Handbook of Childhood Cognitive Development. Blackwell; 2002. pp. 555–574. [Google Scholar]
- 42.Andrews G, Halford GS. A complexity metric applied to cognitive development. Cognitive Psychology. 2002;45:153–219. doi: 10.1016/s0010-0285(02)00002-6. [DOI] [PubMed] [Google Scholar]
- 43.Andrews G, et al. Theory of mind and relational complexity. Child Development. 2003;74:1476–1499. doi: 10.1111/1467-8624.00618. [DOI] [PubMed] [Google Scholar]
- 44.Halford GS, et al. Integration of category induction and hierarchical classification: One paradigm at two levels of complexity. Journal of Cognition and Development. 2002;3:143–177. [Google Scholar]
- 45.Davis G, Welch VL, Holmes A, Shepherd A. Can attention select only a fixed number of objects at a time? Perception. 2001;30:1227–1248. doi: 10.1068/p3133. [DOI] [PubMed] [Google Scholar]
- 46.Chalmers KA, Halford GS. Young children’s understanding of oddity: Reducing complexity by simple oddity and “most different” strategies. Cognitive Development. 2003;18:1–23. [Google Scholar]
- 47.Birney DP, Halford GS. Cognitive complexity of suppositional reasoning: An application of the relational complexity metric to the knight-knave task. Thinking and Reasoning. 2002;8:109–134. [Google Scholar]
- 48.Acuna BD, et al. Frontal and parietal lobe activation during transitive inference in humans. Cerebral Cortex. 2002;12:1312–1321. doi: 10.1093/cercor/12.12.1312. [DOI] [PubMed] [Google Scholar]
- 49.Waltz JA, et al. A system for relational reasoning in human prefrontal cortex. Psychological Science. 1999;10:119–125. [Google Scholar]
- 50.Christoff K, Prabhakaran V, Dorfman J, Zhao Z, Kroger J, Holyoak KJ, Gabrieli JDE. Rostrolateral prefrontal cortex involvement in relational integration during reasoning. NeuroImage. 2001;14:1136–1149. doi: 10.1006/nimg.2001.0922. [DOI] [PubMed] [Google Scholar]
- 51.Bunge SA, Wendelken C, Badre D, Wagner AD. Analogical reasoning and prefrontal cortex: Evidence for separable retrieval and integration mechanisms. Cerebral Cortex. 2005;15:239–249. doi: 10.1093/cercor/bhh126. [DOI] [PubMed] [Google Scholar]
- 52.Christoff K, Owen AM. Improving reverse neuroimaging inference: cognitive domain versus cognitive complexity. Trends in Cognitive Sciences. 2006;10:352–353. doi: 10.1016/j.tics.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 53.Kroger JK, Sabb FW, et al. Recruitment of anterior dorsolateral prefrontal cortex in human reasoning: A parametric study of relational complexity. Cerebral Cortex. 2002;12(5):477–485. doi: 10.1093/cercor/12.5.477. [DOI] [PubMed] [Google Scholar]
- 54.Halford GS, et al. Problem Decomposability as a Factor in Complexity of the Dimensional Change Card Sort Task. Cognitive Development. (in press) [Google Scholar]
- 55.Lewandowsky S, Duncan M, Brown GDA. Time does not cause forgetting in short-term serial recall. Psychonomic Bulletin & Review. 2004;11:771–790. doi: 10.3758/bf03196705. [DOI] [PubMed] [Google Scholar]
- 56.Halford GS, Andrews G. Reasoning and problem solving. In: Kuhn D, Siegler R, editors. Handbook of Child Psychology: Volume 2, Cognitive, Language and Perceptual Development. 2006. pp. 557–608. [Google Scholar]
- 57.Hirst W, et al. Dividing attention without alternation or automaticity. Journal of Experimental Psychology: General. 1980;109:98–117. [Google Scholar]
- 58.Chi MTH. Knowledge structures and memory development. In: Siegler RS, editor. Children’s thinking: What develops? Erlbaum; 1978. pp. 73–96. [Google Scholar]
- 59.Halford GS, Andrews G. The Development of Deductive Reasoning: How Important is Complexity? Thinking and Reasoning. 2004;10:123–145. [Google Scholar]
- 60.Nosofsky RM, Stanton RD, Zaki SR. Procedural interference in perceptual classification: Implicit learning or cognitive complexity? Memory & Cognition. 2005;33:1256–1271. doi: 10.3758/bf03193227. [DOI] [PubMed] [Google Scholar]