Abstract
Neurons have long held the spotlight as the central players of the nervous system, but we must remember that we have equal numbers of astrocytes and neurons in the brain. Are these cells only filling up the space and passively nurturing the neurons, or do they also contribute to information transfer and processing? After several years of intense research since the pioneer discovery of astrocytic calcium waves and glutamate release onto neurons in vitro, the neuronal-glial studies have answered many questions thanks to technological advances. However, the definitive in vivo role of astrocytes remains to be addressed. In addition, it is becoming clear that diverse populations of astrocytes coexist with different molecular identities and specialized functions adjusted to their microenvironment, but do they all belong to the umbrella family of astrocytes? One population of astrocytes takes on a new function by displaying both support cell and stem cell characteristics in the neurogenic niches. Here, we define characteristics that classify a cell as an astrocyte under physiological conditions. We will also discuss the well-established and emerging functions of astrocytes with an emphasis on their roles on neuronal activity and as neural stem cells in adult neurogenic zones.
1. Introduction
In the late 1800’s, neuroglia were recognized as distinct cellular elements that included all supporting cells in the central nervous system (CNS). Neuroglial cells are subdivided into different classes: astrocytes, oligodendrocytes, and, more recently, NG2 cells (i.e. oligodendrocyte precursor cells). Today, the term glia is commonly used to refer to neuroglia, Schwann cells, and microglia. Occasionally, ependymal cells (also called ependymoglia) are included in the term glia since they are derived from radial glia (Spassky et al., 2005) and share astrocytic properties (Reichenbach and Robinson, 1995; Liu et al., 2006). This review focuses on astrocytes under physiological conditions and will not discuss the reactive astrocytes that contribute to gliosis under pathological conditions.
We divided the review into four main sections encompassing three themes: 1) the definition of an astrocyte, 2) the functions of astrocytes sub-divided into two groups: their well-established and emerging functions, and 3) the novel progenitor function of a sub-group of astrocytes in neurogenic zones. We encourage readers to refer to two recent reviews by Dr. Kimelberg that discusses the identity of astrocytes as well as their supportive and instructional functions (Kimelberg, 2004; 2007). Constructive criticisms of the recent literature as well as historical perspectives are provided in these reviews. Regarding the first theme, we propose that the term astrocyte encompasses a family of cells with shared properties and functions that nevertheless exhibit heterogeneity as a result of their different microenvironments. We discuss the anatomical, antigenic, and electrophysiological features that help define a cell as an astrocyte, as well as recent advances in identifying new astrocyte markers using new transcriptome analysis (Cahoy et al., 2008). Second, we discuss many of the well-established and emerging functions of astrocytes with a special emphasis on their roles on neuronal activity.
One of the accepted roles for astrocytes is their house-keeping functions maintaining a viable nervous system environment for neurons. This includes buffering excess potassium and neurotransmitters, providing nutrients and structural support around synapses, and contributing to the integrity of the blood brain barrier (BBB). Astrocytes are also known to release molecules important for neuronal survival and neurite formation. Some of the emerging functions of astrocytes have been clearly demonstrated, others remain speculative and controversial, as will be discussed in this review. Changes in intracellular calcium (Ca2+) dynamics upon neuronal activity provide a mode of excitability to astrocytes. One recent study reported that Ca2+ transients in individual astrocytes are functionally coupled to neuronal activity with remarkable spatial specificity in the ferret visual cortex in vivo (Schummers et al., 2008). In addition, they showed an unambiguous coupling between the astrocyte response to visual stimuli and local blood flow. However, intercellar Ca2+ waves allowing astrocyte-to-astrocyte communication have not been observed in acute slices or in vivo. The occurrence of intercellular Ca2+ waves may be more expected in pathological situations as proposed in a recent review (Scemes and Giaume, 2006). Novel time-lapse imaging studies clearly revealed that astrocytes in acute slices shape the structural plasticity of synapses. However, their instructive role at synapses, in particular their fast release of gliotransmitters controlling synaptic activity, remains controversial. In particular, issues will be raised regarding the methodologies used to stimulate astrocytes.
Finally, cells expressing the astrocytic marker glial fibrillary acidic protein (GFAP) (Eng et al., 1971; Eng, 1985) display neural stem cell characteristics in the adult neurogenic zones, the subventricular zone (SVZ), and subgranular zone (SGZ) of the hippocampal dentate gyrus. This finding triggered a lot of confusion regarding the identity of GFAP-expressing cells and whether these neural stem cells should be considered astrocytes. This finding also questioned the ability of mature astrocytes to revert into a more immature phenotype to regain their stem cell characteristics. We discuss evidence here that these GFAP-expressing stem cells display characteristics of astrocytes and thus may be part of the astrocyte family. We will also discuss the function of these SVZ astrocytes as neuronal stem cells and critical elements of the stem cell niche that are necessary for maintaining neurogenesis.
2. Defining an astrocyte
The term neuroglia or Nervenkitt (i.e. nerve-putty) was first introduced by Rudolf Virchow, a celebrated pathologist in the 1850’s (please see the review by Somjen (1988) for further details and references). Virchow pictured neuroglia as small round-shaped cells that filled up the extracellular space and were part of the connective tissue. Although the term neuroglia survives, our knowledge on the diversity and properties of neuroglial cells, and in particular astrocytes, has dramatically changed. Astrocytes have been viewed as a homogeneous cell population that have a star-shaped morphology, extend numerous processes surrounding neighboring neurons and blood vessels, and contain intermediate filaments (glial fibrils). While astrocytes are classically defined by their morphology and expression of glial fibrils, defining a cell as an astrocyte is not a simple task as discussed in a recent review (Kimelberg, 2004). With the development of electrophysiological, molecular, and genetic tools, it is now well-accepted that astrocytes represent a diverse population of cells with numerous functions. In addition, the finding that a subpopulation of GFAP-expressing cells displays neural progenitor or stem cell features and that astrocytes possess neuronal properties (e.g. glutamate vesicular release) further confuses the definition of an astrocyte. Below we summarize the characteristics that collectively would help define a cell as an astrocyte (see also Kimerlberg (2004) for additional comments).
2.1. Lineages of astrocytes
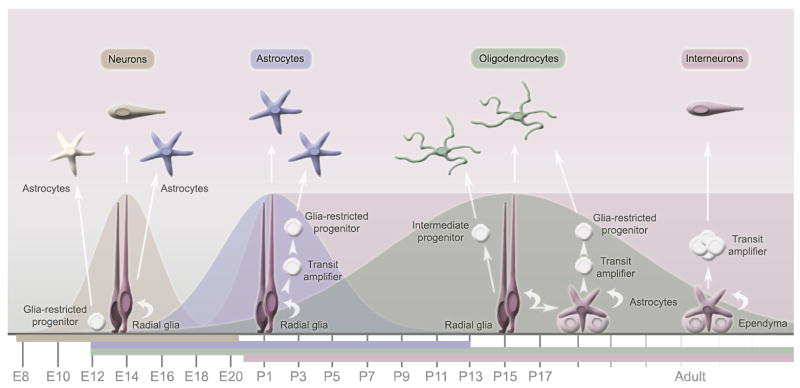
In mammals, gliogenesis, which corresponds to the generation of astrocytes and oligodendrocytes, begins late in embryonic development and continues during the neonatal and postnatal period (Fig. 1). In the cerebral cortex, one of the best-studied regions for gliogenesis, astrocytes are generated from three different sources (Goldman, 2007): radial glia residing in the embryonic ventricular zone (VZ), progenitors in the postnatal SVZ, and a possible third lineage coming from glial-restricted precursors as illustrated in Figure 1. Radial glia, which may also be referred to as radial neuroglia, originate from the early transformation of neuroepithelial cells in the VZ and behave as neural progenitors for both neurons and astrocytes during development (Malatesta et al., 2000; Noctor et al., 2001). After the period of neuronal migration along their radial fibers, radial glia in most regions of the CNS retract their processes and transform into star-shaped astrocytes during the perinatal period (Schmechel and Rakic, 1979). They can also transform into specialized astrocytes such as Bergmann glia in the cerebellum (for reviews see Rakic, 2003; Fishell and Kriegstein, 2003). In the neonatal SVZ, progenitors that display radial glia characteristics before transforming into SVZ astrocytes (see section 2.4) can generate intermediate progenitors. These glial intermediate progenitors migrate into the cortex where they differentiate and become mature astrocytes and oligodendrocytes (Levison and Goldman, 1993; Ganat et al., 2006). Pioneer studies by Dr. James Goldman’s group in the 1990’s using retroviral-mediated gene transfer in vivo selectively infected SVZ cells at postnatal (P) days 1-3. By staining infected cells for glial markers several days post-infection, they found that SVZ cells generate both gray and white matter astrocytes as well as oligodendrocytes (Levison et al., 1993; Levison and Goldman, 1993; Levison and Goldman, 1997). More recent studies suggest the presence of multipotent, bipotential progenitors (Levison and Goldman, 1997; Aguirre et al., 2004; Aguirre and Gallo, 2004), and perhaps astrocyte-restricted progenitors in the neonatal SVZ (Levison and Goldman, 1997). In contrast to the embryonic VZ and neonatal SVZ glial progenitors, the identity and genesis of the glial-restricted precursors is not as clear-cut. These progenitors include bipotential glial progenitors (e.g. O2A progenitors) and progenitors restricted to an astrocytic fate that are thought to exist during embryonic development (Fig. 1, for review see Liu and Rao, 2004; Carmen et al., 2007). These progenitors are hypothesized to be generated directly from neuroepithelial cells and thus bypass the radial glia stage (Liu et al., 2002; Cai et al., 2007). The identity of the different classes of intermediate progenitors needs to be further elucidated to obtain a clear antigenic signature of the lineage (Bryder et al., 2006).
Figure 1. Diagram summarizing the sequence of neuron and glia development.
The generation of the different neuronal and glial cell occurs in a temporally distinct yet overlapping pattern. In rodents, neurogenesis (e.g. generation of projection neurons) peaks at embryonic day 14, astrocytogenesis at postnatal day (P) 2, and oligodendrocytogenesis at P14. The generation of interneurons starts during embryonic life and continues postnatally. However, postnatal interneuron generation is essentially restricted to the olfactory bulb and the dentate gyrus. Astrocytes can be generated from several sources: radial glia during the perinatal life, glia restricted progenitors in the ventricular zone during embryonic life, and from glia restricted progenitors generated from transit amplifier during postnatal and adult life.
These different astrocyte lineages suggest that astrocytes are not created in the same manner, which may provide a developmental explanation for astrocyte diversity in the same brain region. For example, gray matter astrocytes, also called protoplasmic astrocytes (based on their morphology, see below), are generated from embryonic radial glia and, to a lesser extent, intermediate progenitors migrating from the neonatal SVZ. Clearly, these two waves of astrocytic development will generate astrocytes with different patterns of gene expression and possibly functions. This also holds true for white matter astrocytes, called fibrous astrocytes, which are predominantly generated from neonatal SVZ progenitors. Astrocyte diversity can also be obtained from regional differences in radial glia fate (Malatesta et al., 2003). These progressive changes in the glial progenitor environment (e.g. growth factors levels) lead to differential regulation of transcription factors and gene expression (for review see Sauvageot and Stiles, 2002). An example of the genetic diversity of astrocytes is provided by an elegant study on the transcription factor Olig2 using transgenic approaches (Cai et al., 2007). Briefly, in the absence of Olig2, astrocyte formation is severely compromised in the white matter, whereas astrocytes in the cortical gray matter displayed up-regulation of GFAP (Cai et al., 2007). These data strongly suggest that gray and white matter astrocytes differ not only in their spatial locations and morphologies, but also in their transcriptional regulation of gene expression. Another recent study reported a remarkable diversity of astrocytes in the spinal cord, which is likely applicable to other brain regions (Hochstim et al., 2008). The authors identified several positionally distinct subtypes of astrocytes that can be distinguished by their expression of different axon guidance molecules. The positional identity of these astrocytes was found to be specified by a combinatorial homeodomain transcriptional code, resembling the one used to specify neuronal subtypes during development.
2.2 Anatomical, Molecular, and Electrophysiological Characteristics
Technological advances over the past decades have given us many novel tools to study astrocytes. From the early Golgi stains to immunostaining for glial fibrils, dye-filling techniques (e.g. sharp electrode, patch clamp recordings, and now single cell electroporation), and transgenic approaches to visualize fluorescent astrocytes, our understanding of astrocyte characteristics has dramatically evolved over the past decades.
2.2.1. Anatomy
Astrocytes have a highly complex and heterogeneous morphology, as well as an unexpected non-overlapping spatial distribution. First, astrocytes are and remain defined as process-bearing cells distributed throughout the nervous system that lack axons and dendrites. They were originally named for their stellate or star-shaped morphology as shown in Figure 2A. However, they are heterogeneous in their morphologies as illustrated by the following examples: the cerebellar Bergmann glia (previously called Golgi epithelial cells) (Palay and Chan-Palay, 1974), the retinal Müller cells (Newman and Reichenbach, 1996), the pituitary astrocytes pituicyte (Hatton, 1988), those forming the blood-brain-barrier conferring some polarity in their morphology (Fig. 2B), and the SVZ astrocytes (Liu et al., 2005; Liu et al., 2006) (see Fig. 5).
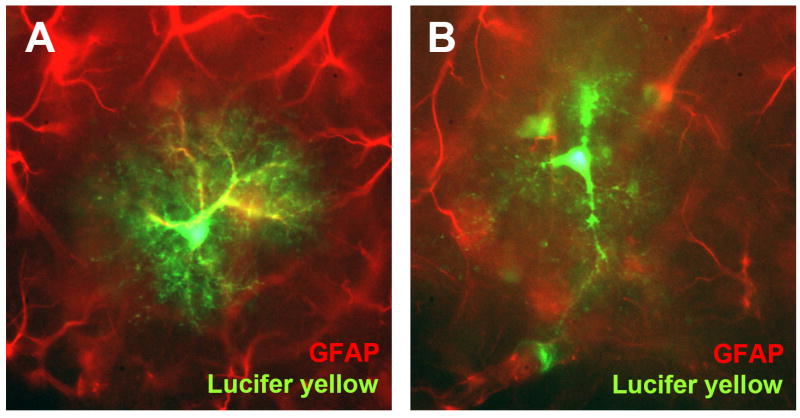
Figure 2. Morphology of astrocytes.
(A and B) Photographs of astrocytes recorded in the hippocampus and filled with lucifer yellow during patch clamp recording. Immunostaining for GFAP was overlaid with the lucifer yellow fill. The cell in (A) displays a typical stellate morphology and strongly stains for GFAP, but does not display dye coupling. The cell in (B) displays dye coupling to other cells and send a process ensheathing a blood vessel typical of an astrocyte, but does not stain for GFAP.
Figure 5. Astrocyte interactions with synapses.
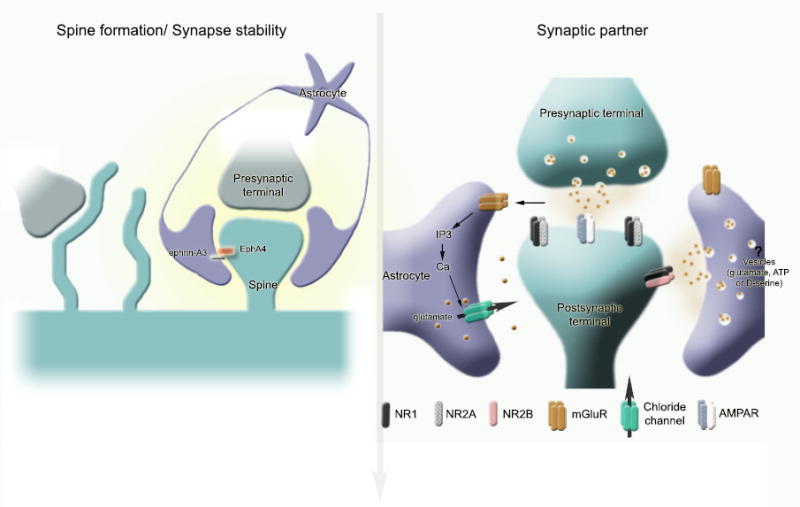
(A) Astrocytes encapsulate synapses including spines. This ensheathment allows spines to remain stable. At the molecular level, the ephrin-A3/EphA4 receptor signaling between astrocytic processes and spines has been shown to regulate the morphology of dendritic spines. In the absence of astrocytic processes, motile filopodia extend from dendrites. The astrocytic contact allows the filopodia to transform into a mature spine. (B) Astrocytes are an active synaptic partner. Not only they take up glutamate via high affinity transporters (not shown here), but they sense glutamate escaping synaptic clefts and release neuroactive substances upon glutamate receptor activation and intracellular Ca2+ elevation. Neuroactive substances include glutamate that is released via either vesicles or via Ca2+-dependent chloride channels, or both. Glial glutamate activates extrasynaptic receptors, including NR2B, on neurons leading to changes in synaptic integration.
The notion of diversity was previously established by dividing astrocytes into two subpopulations based on their location and morphology: the fibrous and protoplasmic astrocytes in the white and gray matter, respectively (Miller and Raff, 1984; Privat and Rataboul, 2007). Protoplasmic astrocytes have many branching processes, which envelop synapses and whose endfeet cover blood vessels. Fibrous astrocytes have long, thin, unbranched processes whose endfeet envelop nodes of Ranvier. This nomenclature (fibrous and protoplasmic) is outdated in light of the great anatomical diversity. Astrocytic plasma membrane is thrown into folds in the form of lamellae and fibers, which infiltrate among the intricate network of neural processes including synaptic terminals, dendrites, and dendritic spines. The degree of synaptic ensheathment by astrocytic processes displays regional variability. In the hippocampus, only 57% of the synapses have astrocytic processes apposed to them. Of these, the astrocytic processes surround less than half (around 43%) of the synaptic interface (Ventura and Harris, 1999). This arrangement may favor neurotransmitter spillover (i.e. diffusion) between synapses. In the cerebellum, Purkinje cells receive two types of excitatory inputs, parallel and climbing fibers, which exhibit differences in their degree of astrocytic ensheathment (67% versus 94%, respectively) (Spacek, 1985; Xu-Friedman et al., 2001). The astrocytic membrane is so convoluted that glial microdomains, i.e. spatially exclusive regions through the fine astrocytic processes, have been observed in Bergmann glia and hippocampal astrocytes using electron microscopy (Grosche et al., 1999; Bushong et al., 2002; Hama et al., 2004). These microdomains can limit the spread of astrocytic Ca2+ increases in response to neuronal stimulation (Grosche et al, 1999). These subcellular compartments have a complex surface consisting of thin membrane sheets and mitochondria. This suggests that an astrocyte may consist of hundreds of independent compartments, each capable of autonomously interacting with the synapses that it ensheathes. Interestingly, recent studies show that adult astrocytes are organized in non-overlapping domains (Bushong et al., 2002; Halassa et al., 2007) and that one hippocampal astrocyte can contact in excess of 100,000 synapses (Bushong et al., 2002). In addition, this compartmentalization of labor by individual astrocytes allows a one-to-one astrocyte-to-synaptic unit communication without information (or noise) mismatch from a second astrocyte receiving different inputs and in a different state of activity. The work of Bushong et al (2002) also led to a re-evaluation of the morphological definition of an astrocyte as being more spongiform rather than star-shaped. The term spongiform indicates that astrocytes possess very dense ramifications of fine processes extending 2-10 μm from the main branches. These anatomical features suggest that one astrocyte can modulate neurotransmission across many synapses (see section 4.5).
2.2.2. Antigenic markers and fluorescent labeling in transgenic mice
Astrocytes are commonly identified by the presence of intermediate filaments (glial fibrils), which are more prominent in white matter than gray matter astrocytes (Privat and Rataboul, 2007). The major component of glial fibrils, glial fibrillary acidic protein (GFAP), is thought to be specific for astrocytes in the CNS (Eng et al., 1971; Bignami et al., 1972). However, the low expression of GFAP may not be readily detected by immunohistochemistry, leading to confusing results regarding the identity of astrocytes. As an example, the cell filled with the fluorescent dye lucifer yellow in Figure 2B was GFAP-immunonegative, but had endfeet covering a blood vessel, suggesting that it may be an astrocyte. It is possible that whole cell patch clamp recordings render the GFAP antigen inaccessible for the anti-GFAP antibody. Other defining characteristics or ways to visualize filaments (such as by electron microscopy) are thus required to define a cell as an astrocyte. In addition, GFAP is expressed by other cell types in the CNS (i.e. ependymal cells, which are also derived from radial glia) that share similar properties with astrocytes (Liu et al., 2006), but are not normally part of the astrocyte family (Spassky et al., 2005). Ependymal cells form an epithelial layer lining the walls of the cerebral ventricles and display morphological features different from astrocytes such as motile cilia. They function as a barrier between the brain parenchyma and cerebrospinal fluid (CSF) and play a role in cerebral fluid balance, toxin metabolism and secretion into the CSF. They thus have specialized functions that distinguish them from astrocytes (Del Bigio, 1995). Amazingly, GFAP has also been located in rat kidney glomeruli and peritubular fibroblasts (Buniatian et al., 2002), leydig cells of the testis (Davidoff et al., 2002), skin keratinocytes (Danielyan et al., 2007), osteocytes of bones, chondrocytes of epiglottis, bronchus (Kasantikul and Shuangshoti, 1989), and stellate-shaped cells of the pancreas and liver (Apte et al., 1998). Another commonly used astrocytic marker is S100B, which belongs to the S100 family of EF-band calcium binding proteins (Baudier et al., 1986). However, S100B is only expressed by a subtype of mature astrocytes that ensheath blood vessels and by NG2-expressing cells (Deloulme et al., 2004; Hachem et al., 2005). NG2-expressing cells are found in both the developing and adult mammalian CNS, and until recently, were referred to as smooth protoplasmic astrocytes because of their astrocytic appearance with less branched processes and a paucity of intracellular filaments (Levine and Card, 1987). However, they are GFAP-immunonegative and have been placed in the oligodendrocyte lineage, and now they are commonly referred to as oligodendrocyte precursor cells (OPCs) or NG2 cells (Nishiyama et al., 1999). Other markers thought to be exclusive to astrocytes include, but are not limited to, the glutamate transporters GLT-1 (or human EAAT2, Rothstein et al., 1994; Danbolt et al., 1998b), glycogen granules, and glutamine synthase (GS), which is an enzyme that catalyzes the conversion of ammonia and glutamate to glutamine (Schousboe et al., 1977; Martinez-Hernandez et al., 1977). However, GS is also found in white and gray matter oligodendrocytes (Cammer, 1990; D’Amelio et al., 1990). Other markers include inwardly rectifying K+ channels like Kir4.1 (Takumi et al., 1995; Higashi et al., 2001) and aquaeporin 4 channels (Nielsen et al., 1997). Additional markers have been found through microarray analysis of astrocytes in vitro (Bachoo et al., 2004). While some of these molecules may be expressed by all astrocytes (e.g., GLT-1), others (e.g. Kir4.1) are only expressed by a subset of astrocytes (Higashi et al., 2001). In addition, some markers provide only punctuate staining (GLT-1) or localize to only some parts of the astrocytic processes (i.e. aquaeporin) rendering identification of the whole cell difficult to interpret. Despite these limitations, new technology can expand our repertoire of astrocytic markers. In a new study, Cahoy and colleagues used a transcriptome database approach from FACS-sorted S100B-GFP-expressing P30 astrocytes and identified a new astrocyte-specific marker, AldhL1 (aldehyde dehydrogenase 1 family, member L1) (Cahoy et al., 2008). This useful genomic approach can provide additional clues for astrocyte development and function.
Investigators have thus taken advantage of transgenic mice where a fluorescent reporter protein is expressed under promoters expressed in astrocytes, including GFAP (Zhuo et al., 1997; Nolte et al., 2001), GLT-1 and GLAST (Regan et al., 2007), S100B (Vives et al., 2003), and BLBP (Schmid et al., 2006). The most commonly used transgenic mice for studying astrocytes are mice expressing green fluorescent protein (GFP) or enhanced GFP under the human GFAP promoter (hGFAP-GFP mice). These mice as well as GLT-1-GFP and BLBP-dsRed2 mice may confer the best selectivity to identify astrocytes compared to the other transgenic mice because they were reported to be specific for astrocytes. However, these transgenic lines may label non-astrocytes as well. GLAST-GFP is essentially expressed in radial glia and immature astrocytes, but is also expressed in oligodendrocytes (Regan et al., 2007). S100B-GFP is expressed in both neurons and astrocytes (Vives et al., 2003). It is also important to acknowledge that not all astrocytes may carry the reporter proteins. This was shown for different lines of GFAP-LacZ mice generated using different elements of the GFAP promoter. LacZ expression showed different distributions, and some were found in neurons (Lee et al., 2008) because the element of the promoter used did not include the sequence necessary to silence GFAP expression in neurons. Despite these common problems with transgenic animals, the hGFAP-GFP mice have allowed investigations of astrocytes’ biophysical properties, behavior, responses to injury, and intracellular Ca2+ activity in acute slices and in vivo. However, one elegant study in particular has raised controversy regarding the specificity of this transgenic line because EGFP expression was found in cells displaying characteristics of NG2 cells (i.e. oligodendrocytes precursor cells) and expressing NG2 transcripts (Matthias et al., 2003). However, because the studies were performed in relatively young mice (P6-P20), some of these cells may have been immature astrocytes generated during the neonatal period via NG2-expressing intermediate progenitor cells as previously addressed (Nolte et al., 2001; Houades et al., 2006). Collectively, transgenic mice containing fluorescent proteins in astrocytes are invaluable and necessary tools, but it is nevertheless important to verify that fluorescept reporter expression coincides with GFAP expression using immunohistochemistry or electron microscopy.
2.2.3. Biophysical properties
Despite the diverse anatomical and antigenic characteristics of astrocytes, astrocytes do share a common set of biophysical properties. We can safely say that in acute slices, astrocytes do not generate action potentials under physiological conditions, have hyperpolarized resting membrane potentials (around -80 or -90 mV), and display large voltage-independent K+ currents, which does not preclude the presence of voltage-dependent K+ currents (Bordey and Sontheimer, 2000). The presence of these biophysical characteristics led investigators to call astrocytes as “passive” cells (Steinhauser et al., 1992), a term that is not used anymore because of its negative connotation. The molecular nature of the channels responsible for large voltage-independent K+ currents remains to be determined. Despite this, the background K+ channels (e.g. TASK-1, TASK-3 and TREK-2), which contain four transmembrane segments and two pore-forming domains, were found to be expressed in cultured astrocytes and may be good candidates for generating voltage-independent K+ currents (Gnatenco et al., 2002). These channels may also underline another degree of variability among populations of astrocytes. It is also well-known that astrocytes in cultures and in acute slices are also coupled by gap junctions, creating a large syncitium of connected cells. However, the current generated through electrical coupling does not appear to significantly contribute to the whole-cell currents in mature astrocytes as tested by acutely dissociating astrocytes (Schools et al., 2006) (an exception are the SVZ astrocytes, Liu et al., 2006). A controversy persists regarding the biophysical properties of astrocytes (Walz, 2000). It remains unclear whether astrocytes with primarily voltage-dependent currents exist or whether they represent a distinct population of glial cells, including NG2 cells (Zhou and Kimelberg, 2001; Matthias et al., 2003; Zhou et al., 2006). However, the age of the mice studied may explain some discrepancies among the studies. Indeed, some recorded glial cells in acute slices may be immature or in an intermediate stage between NG2 cells and fully differentiated GFAP-expressing astrocytes (Zhou et al., 2006). Therefore, membrane physiology in combination with the expression of GFAP and other markers should determine whether these cells belong to the family of astrocytes.
2.2.4. Receptor expression
Astrocytes express a large repertoire of receptors, including G-protein coupled receptors and ionotropic receptors that have been extensively reviewed (for review see Salm and McCarthy, 1992; Hosli and Hosli, 1993; Magistretti et al., 1993; Whitaker-Azmitia and Azmitia, 1994; Kimelberg, 1995; Vernadakis, 1996; Fraser et al., 1997; Porter and McCarthy, 1997; Baba, 1998; Nedergaard et al., 2002; Neary et al., 2004; Abbracchio and Verderio, 2006; Furuta et al., 2007; Fernandez-Ruiz et al., 2007). Astrocytes also express receptors for growth factors, chemokines, steroids, and receptors involved in innate immunity (e.g. Toll-like receptors) that participate in regulating astrocyte development and response to neurons and injury (Owens et al., 2005; Mong and Blutstein, 2006; Vaccarino et al., 2007; Farina et al., 2007; Liu and Neufeld, 2007a). Here, we make three remarks. First, cultured astrocytes express almost every receptor present in neurons while astrocytes in acute slices or in vivo lack some “neuronal” receptors. Typically, mature astrocytes do not express AMPA-type and NMDA-type glutamate receptors. Nevertheless, there are a few exceptions. For example, in acute slices Bergmann glia express calcium-permeable AMPA receptors (Geiger et al., 1995) and cortical astrocytes were recently shown to express NMDA-type glutamate receptors (Lalo et al., 2006). However, these latter receptors in astrocytes are not typical because they are insensitive to magnesium block. Second, astrocytes display heterogeneity in their pattern of receptor expression, as shown by the examples above. In addition, astrocytes will adjust their receptor expression according to their surrounding environment. For instance, in response to brain injury, astrocytes up-regulate the expression of epidermal growth factor receptors (EGFRs), which trigger quiescent astrocytes to become reactive astrocytes (Liu and Neufeld, 2007b). Third, CA1 hippocampal astrocytes do not express P2X7-type ionotropic ATP receptors (Jabs et al., 2007). Although this recent finding needs to be tested in other brain regions, it questions the existing dogma, which originally postulated that astrocytes expressed P2X7, though these data were not convincing due to the lack of selective pharmacology and poor antibodies.
2.3. Functional definitions of astrocytes
All of these diverse structural, biochemical, and biophysical characteristics of astrocytes are tightly related to their functions summarized and discussed in the next sections. The functions of astrocytes can be divided into three groups: those that provide housekeeping functions necessary to maintain neuronal function, those that actively shape synaptic function, and those that act as neural precursors in adult neurogenic regions. Functionally, astrocytes are still defined as cells that do not generate action potentials and do not myelinate axons, but clean up the extracellular space and provide substrates necessary for neuronal function. However, more recent evidence shows that astrocytes can actively contribute to synaptic plasticity and activity by releasing neurotransmitters and affecting blood flow (see below). These emerging functions suggest that astrocytes are active participants in brain activity rather than passive elements in maintaining the extracellular space.
One important note is that neurons and astrocytes share a lot of similar functions, e.g. buffering K+, neurotransmitter, and glucose in the extracellular space. However, astrocytes are often better positioned and possess more efficient mechanisms to perform these functions. For example, some but not all astrocytes contact blood vessels and are thus well-poised to take up glucose and other nutrients. In addition, astrocytes may have specialized functions based on their microenvironment. A clear example is the difference between gray and white matter astrocytes. White matter astrocytes contact the node of Ranvier where they may regulate spike propagation while gray matter astrocytes ensheath synaptic terminals where they influence synaptic transmission.
2.4. The astrocyte-like neural progenitors of the neurogenic zones
A fascinating characteristic of astrocytes that came into light in the last decade is that GFAP-expressing cells can contribute to cell genesis both as stem cells and as important cellular elements of the neurogenic microenvironment (also called niche). In the adult SVZ and subgranular zone (SGZ), the multipotent neural stem cells (interchangeably called neural progenitors) express GFAP (Cameron et al., 1993; Doetsch et al., 1999; Seri et al., 2001). These GFAP-expressing cells in the SVZ give rise to neuroblasts that migrate to the olfactory bulb where they become synaptically integrated olfactory interneurons (Altman, 1969; Luskin, 1993; Doetsch et al., 1999). Another population of neurogenic GFAP-expressing cells has been found in the SGZ, where GFAP-expressing cells can generate newborn granule neurons (Altman and Das, 1965; Kaplan and Hinds, 1977; Cameron et al., 1993).
While these adult stem cells express GFAP, other cell types also express GFAP (see section 2.2.2.), but are not considered astrocytes. It is questionable whether these adult stem cells belong to the astrocyte family. First of all, these GFAP-expressing stem cells express nestin, an intermediate filament marker for embryonic precursor cells that is not present in mature astrocytes (Hockfield and McKay, 1985). In the adult SVZ, nestin is also expressed in neuroblasts and intermediate progenitors (Doetsch et al., 1997). Interestingly, nestin is not expressed in the neonatal SVZ, but is present in immature cortical astrocytes at an early stage of differentiation (Zerlin et al., 1995) and in reactive astrocytes (Clarke et al., 1994), suggesting that nestin expression is not mutually exclusive with an astrocytic identity. GFAP-expressing stem cells do not express S100B, which is expressed in a minority of cells in the SVZ (Platel et al., 2008). Considering that not all mature astrocytes express S100B, this finding again does not contradict with an astrocytic identity.
The following properties of GFAP-expressing stem cells suggest that they belong to the astrocyte family. In the SVZ, GFAP-expressing stem cells are derived from radial glia (Merkle et al., 2004), which are neural progenitors during embryonic development (Malatesta et al., 2000; Noctor et al., 2001), for review see (Campbell and Gotz, 2002; Rakic, 2003; Fishell and Kriegstein, 2003). GFAP-expressing cells in the SVZ and SGZ have anatomical features in common with astrocytes. For instance, they have long processes that envelop and contact blood vessels and neuroblasts (Doetsch et al., 1997; Seri et al., 2004). In addition, just like mature astrocytes, they contain glycogen granules (Sturrock and Smart, 1980; Peretto et al., 1999) and express astrocytic glutamate and GABA transporters (i.e. GLAST and GLT-1, and GAT-3, respectively) (Braun et al., 2003; Bolteus and Bordey, 2004; Liu et al., 2006). Functionally, GFAP-expressing cells of the SVZ studied in acute slices share properties with radial glia and astrocytes. They have K+ conductance at rest, express connexin 43 gap junctions and hemichannels, have functional glutamate transporters and GABAA receptors, but lack AMPA-type glutamate receptors (Filippov et al., 2003; Wang et al., 2005; Liu et al., 2006), which are absent in most mature astrocytes (Matthias et al., 2003) (with the exception of the specialized Bergmann glia) (Muller et al., 1996). Nevertheless, GFAP-expressing cells of the SVZ lack barium-sensitive inwardly rectifying K+ currents (KIR), a hallmark of astrocytic differentiation and cell cycle exit (Bordey and Sontheimer, 1997; Macfarlane and Sontheimer, 2000). Together, these studies suggest that the GFAP-expressing stem cells have characteristics of embryonic radial glia and mature astrocytes, but display subtle differences and retain properties of neural progenitors. Perhaps these cells are retained in a transitional stage between radial glia and astrocytes, due to the persistence of embryonic extracellular matrix molecules. This permissive environment in the neurogenic niche allows the retention of intrinsic genetic programs to maintain “stemness” (Gates et al., 1995) (see additional discussion in section 5.3). In light of these data, GFAP-expressing cells of the SVZ have been termed SVZ astrocytes or astrocyte-like cells. The previous findings strongly suggest that SVZ astrocytes belong to the large family of astrocytes, but they require a sub-branch to distinguish them from mature astrocytes.
Inside the neurogenic niche, it remains unclear whether every GFAP-expressing cell has the potential to behave as a neural stem cell. Alternatively, it is conceivable that two types of GFAP-expressing cells, those that are stem cells or those that play instructive roles on neurogenesis (see section 5), co-exist in addition to the S100B-expressing cells that do not co-express GFAP (Platel et al., 2008). Such a finding would further complicate the nomenclature to properly distinguish these two types of cells. Considering that astrocytes display an incredible degree of plasticity, it is likely that the same cell can both behave as a stem cell and still direct neurogenesis. One option to address this issue would be to determine the genetic profile of proliferative versus non-proliferative SVZ astrocytes. Such an approach could also help to determine the differences between SVZ and mature astrocytes. Future transplant studies into the SVZ of genetically modified mature astrocytes could determine whether they can be reverted to a stem cell phenotype.
3. The well-accepted functions
Because astrocytes lack axons and the ability to form action potentials, astrocytes were traditionally thought to be mere “brain glue” that support neuronal activity. However, astrocytes have several critical functions including promoting neuronal maturation, synapse formation, neuronal survival during development, regulating angiogenesis, and maintaining a viable microenvironment for neurons. Although these functions are well-accepted, they should not be overlooked because much remains to be learned. This is especially true for astrocytes’ contribution to synaptogenesis and angiogenesis for which trophic molecules and their mechanisms of action remain to be evaluated. In addition, a lot can be learned as the contribution of molecularly distinct channels (e.g. Kir4.1) for K+ buffering and neuronal excitability have just begun to be explored. Furthermore, dysfunction in astrocytes can lead to disease progression such as in familial forms of Amyotrophic Lateral Sclerosis (which will not be discussed in this review). Below is a brief description of established astrocytic functions summarized in Figure 3 (for further information see Kimelberg, 2007) that summarizes the supportive functions of astrocytes.
Figure 3. The well-established functions.
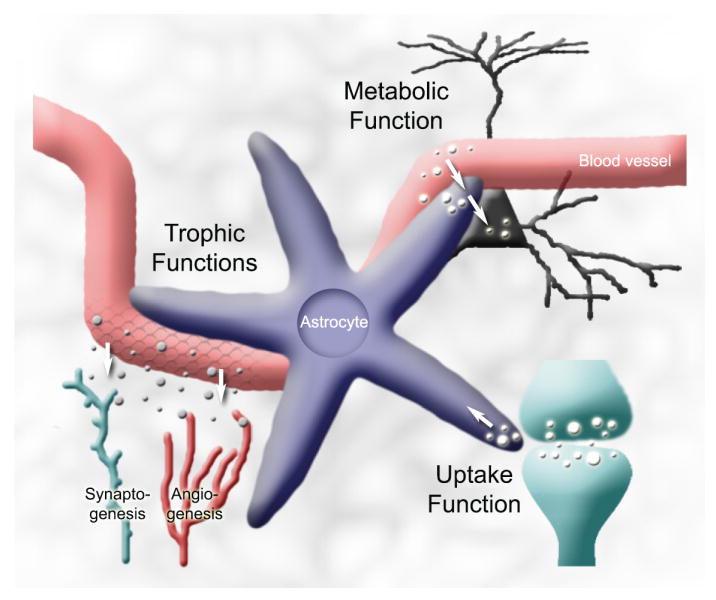
Astrocytes have several homeostatic functions maintaining a viable nervous system environment for neurons. These functions include: (1) providing metabolic support for neurons (section 3.5), (2) taking up K+ and neurotransmitters (sections 3.3 and 3.4, respectively), (3) Synaptogenesis (section 3.1), angiogenesis (sections 3.2.1), and BBB maintenance (section 3.3.2).
3.1. Synthesis of extracellular matrix proteins, adhesion molecules, and trophic factors controlling neuronal maturation and synaptogenesis
Astrocytes are the major source of extracellular matrix (ECM) proteins and adhesion molecules in the CNS. Astrocytes in cultures can either promote or inhibit neurite outgrowth depending on the balance of ECM and adhesion molecules with guidance cues navigating neurites during development or in response to injury. Growth-promoting molecules include (but are not limited to) laminin (Liesi et al., 1983; Liesi, 1985; Liesi and Silver, 1988; Chiu et al., 1991; Shea et al., 1992), N-cadherin (Neugebauer et al., 1988; Tomaselli et al., 1988), neural cell adhesion molecule (NCAM) (Neugebauer et al., 1988; Smith et al., 1990), and fibronectin (Price and Hynes, 1985; Liesi et al., 1986; Matthiessen et al., 1989). More recently, spontaneous calcium oscillations in cultured astrocytes have been shown to regulate neurite growth by maintaining the expression of specific growth-enhancing proteins on astrocytic surface, such as N-cadherin (Kanemaru et al., 2007). On the contrary, inhibitory proteoglycans associated with glial boundaries during development provide guidance cues (Snow et al., 1990; Gonzalez et al., 1993; Steindler, 1993). Astrocytes also synthesize and secrete proteolytic enzymes, in particular the matrix metalloproteinases (MMPs, Wells et al., 1996; Muir et al., 2002), which play a pivotal role in ECM degradation and remodeling (for review see (Shapiro, 1998; Yong et al., 1998). For example astrocytes synthesize MMPs of the gelatinase subfamily, MMP-2 and -9, which contribute to extracellular amyloid-β peptide degradation and clearance (Yin et al., 2006).
Astrocytes are well-known to release growth factors in vitro, including nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3) (Rudge et al., 1992), and fibroblast growth factor (FGF) (Vaca and Wendt, 1992). These molecules control neuronal maturation and survival (for review see Ojeda et al., 2000). Astrocytes have also been shown to control neuronal differentiation in vitro via activity-dependent neurotrophic factor release (Blondel et al., 2000). More specifically, ciliary neurotrophic factor (CNTF) and FGF can be released from cultured astrocytes possibly via a Ca2+-dependent pathway and enhance neuronal survival and induce neuronal growth as well as differentiation (Vaca and Wendt, 1992). Other molecules such as S100B, which stimulates neurite outgrowth as well as astrocytic glutamate uptake, can be released by astrocytes to protect neurons against glutamate excitoxicity (for review see (Donato, 2001). These examples offer only a snapshot of the large repertoire of astrocytic molecules that regulate neuronal maturation and survival under physiological conditions and following injury (see reviews by Emsley et al., 2004; Pehar et al., 2005; Endo, 2005; Mahesh et al., 2006 for additional examples).
More recently, astrocytes were shown to promote synaptogenesis between CNS neurons in vitro and in vivo during development. Interestingly, synaptogenesis coincides with the generation of astrocytes. Retinal ganglion cells cultured in the absence of astrocytes exhibited very little spontaneous synaptic activity but displayed robust postsynaptic excitatory activity when grown on a layer of feeder astrocytes (Pfrieger and Barres, 1997). In fact, astrocytes were found to induce a 7-fold increase in synapse number between retinal ganglionic cells and a nearly 100-fold increase in synaptic activity (Rudge et al., 1992; Seil et al., 1992). Some of the trophic factors secreted by astrocytes include cholesterol (Mauch et al., 2001) and thrombospondins (Christopherson et al., 2005). The latter has been shown to be secreted by immature astrocytes in vivo to induce the formation of ultrastructurally normal synapses (Christopherson et al., 2005). It is likely that future studies will reveal many other molecules that can contribute to synaptogenesis in a temporal and regional-specific manner. It will be exciting to watch the expansion of this emerging field.
3.2. Angiogenesis, Blood-Brain Barrier (BBB) induction and maintenance
Glial-vascular interactions serve a number of important functions that span multiple time scales. In this section, we will discuss long lasting (hours to days) interactions. Later we will elaborate on the fast (seconds to minutes) interactions in the vascular unit, such as transient opening of the blood-brain barrier (BBB) and blood flow regulation. A better understanding of angiogenesis as well as the dynamic interactions between astrocytes and endothelial cells to regulate BBB stability and permeability is critical for understanding the process of tumorigenesis (Stiver, 2004) and neurogenesis. Indeed, adult neural stem cells, i.e. a specialized astrocyte, preferentially reside close to blood vessels and send processes around endothelial cells (Palmer et al., 2000 and see section 5.2). It is unclear which interactions are taking place between endothelial cells and neural stem cells, but there is likely a crosstalk between the cells types to create a neurogenic niche that coordinates growth and response to injury.
3.2.1. Angiogenesis
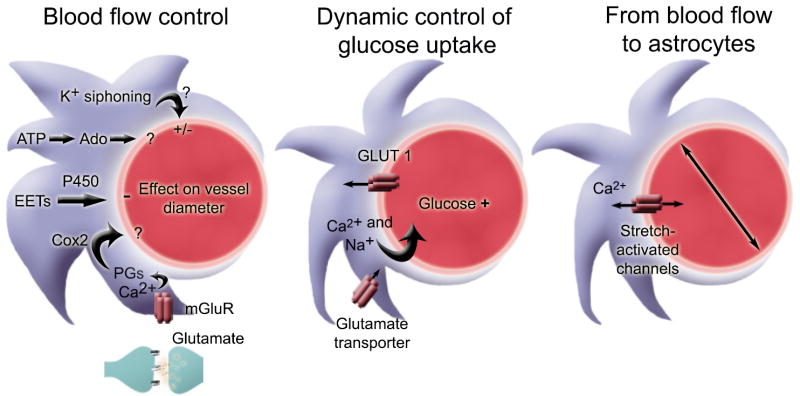
Angiogenesis, the formation of blood vessels, involves several steps including basement membrane degradation, endothelial cell proliferation and recruitment, tube formation, and maturation including reconstitution of the basement membrane. When co-cultured with astrocytes, endothelial cells form capillary-like structures (Laterra et al., 1990; Laterra and Goldstein, 1991; Jiang et al., 1995). Epoxyeicosatrienoic acid (EET), which is the product of cytochrome P450 epoxygenation of arachidonic acid (Zhang and Harder, 2002), is one of the molecules released from astrocytes that can act as a mitogen and morphogen for endothelial cells. In addition, astrocytes synthesize laminin to form the astrocyte-endothelial cell interface resembling the basement membrane (Laterra et al., 1990). An optimal system to study angiogenesis is the retina, which contains regular stellate astrocytes and a specialized astrocytic type, called the Muller cell (Newman and Reichenbach, 1996). In the retina, angiogenesis is controlled by a tight cooperation between retinal neurons, astrocytes, and endothelial cells. In particular, retinal neurons release platelet-derived growth factor (PDGF) to stimulate proliferation of astrocytes, which in turn stimulate blood vessel growth by secreting vascular endothelial cell growth factor (VEGF) (Stone et al., 1995; Fruttiger et al., 1996; Provis et al., 1997). In addition, developing vessels provide feedback signals that trigger astrocyte differentiation, including cessation of cell division and upregulation of GFAP (West et al., 2005). In addition, the final arrangement of retinal blood vessels depends critically on the pre-patterning of astrocytes that directs the extension of filopodia budding off the vascular plexus via VEGF receptor activation (Jiang et al., 1995; Fruttiger et al., 1996; Zhang and Stone, 1997; Gerhardt et al., 2003). Such cooperation may also occur in the CNS, but this remains to be examined.
3.2.2. BBB induction and maintenance
The BBB is the specialized system of brain microvascular endothelial cells that protects the brain from toxic substances in the blood, supplies the CNS with nutrients, and filters excess and toxic molecules from the brain to the bloodstream (for excellent reviews on BBB see Pardridge, 1999; Engelhardt, 2003; Hawkins and Davis, 2005; Zlokovic, 2008). The brain capillaries are 50–100 times tighter than peripheral microvessels as a result of complex tight junctions (zonula occludens) between endothelial cells that limit paracellular diffusion of hydrophilic solutes. As a result, entry across the brain endothelium is effectively confined to transcellular mechanisms using various transport mechanisms (e.g. GLUT1 glucose transporters, L-system carrier L1 for neutral amino acids, glutamate transporters, the efflux carrier P-glycoprotein, for review see (Zlokovic, 2008) and for the original idea see (Davson and Oldendorf, 1967)). Early studies with markers injected into the cerebral spinal fluid and electron microscopy demonstrate that astrocytes do not structurally contribute to the BBB (Brightman and Reese, 1969). Nevertheless, astrocytes send specialized processes, called endfeet, which ensheath the brain vasculature onto which they form rosette-like structures (Kacem et al., 1998). In addition, astrocytes are beleived to regulate the induction of the BBB, i.e. tight junction formation and expression of transport systems (see (Haseloff et al., 2005; Abbott et al., 2006) for review).
During development, astrocytes are thought to contribute to BBB tightening and up-regulation of the different transport mechanisms. Freshly isolated brain endothelial cells in culture retain certain aspects of a BBB phenotype, but with some loss of full barrier characteristics (e.g. leakier tight junctions, down-regulation of enzyme and transport systems) (for review on culture model see Reichel et al., 2003). However, tight junction proteins in the BBB are up-regulated by co-culturing with astrocytes (Rubin et al., 1991; Dehouck et al., 1994; Rist et al., 1997; Sobue et al., 1999). Some of the specific transport systems up-regulated in brain endothelial cells exposed to astrocytes include GLUT1, the L-system and A-system amino acid carriers, and P-glycoprotein (El Hafny et al., 1997) (for review see Bauer and Bauer, 2000). The regulations exerted by cultured astrocytes on BBB induction depend both on cell-cell contact and the diffusion of molecules. Several astrocytic signals regulating different aspects of BBB properties have been identified and include TGF-β (Tran et al., 1999), GDNF (Igarashi et al., 1999), basic FGF (Sobue et al., 1999), IL-6 and hydrocortisone (Hoheisel et al., 1998). The src-suppressed C-kinase substrate (SSeCKS) in astrocytes is responsible for the decreased expression of VEGF and increased release of the anti-permeability factor angiopotein-1 (Lee et al., 2003). Conditioned media from astrocytes over-expressing SSeCKS blocked angiogenesis in vivo and in vitro. In addition, the conditioned media increased tight junction proteins in endothelial cells, consequently decreasing sucrose permeability. It is thought that astrocytes also maintain tight junction and microvascular permeability in the adult brain. However, short-term regulation of permeability in vivo has been difficult to assess and will be discussed later in the emerging functions section.
Finally, it is interesting to point out that endothelial cells have a reciprocal influence on cultured astrocytes by regulating their growth (Estrada et al., 1990), glutamate synthetase activity (Spoerri et al., 1997), laminin production (Wagner and Gardner, 2000), and up-regulation of antioxidant enzymes (Schroeter et al., 1999). One such signaling molecule is the leukaemia inhibitory factor (LIF), released by endothelial cells of the optic nerve, which induces astrocytic differentiation in vitro (Mi et al., 2001).
3.3. Extracellular ion buffering: a focus on K+ buffering
The idea that astrocytes regulate the ionic content of the extracellular space was first proposed by Gerschenfeld et al. in 1959 (Gerschenfeld et al., 1959) (see for review Kimelberg, 2007). During normal neuronal activity, neurotransmission leads to the build up of K+ into the extracellular space, and if not corrected, results in neuronal depolarization, hyperexcitability, and seizures. The pioneering work by Kuffler’s group showed that nerve impulses cause slow depolarization of glia attributable to K+ influx in the amphibian optic nerve (Orkand et al., 1966). This group then proposed the K+ spatial buffering hypothesis, which states that astrocytes take up excess extracellular K+ ions, distribute them through the gap junction-coupled astrocytic syncytium, and extrude the ions at sites of low extracellular K+ level (Kuffler and Nicholls, 1966). This homeostatic function has been confirmed in astrocytes in rat neonortical slices (Holthoff and Witte, 2000). Astrocytes also release K+ directly into the blood stream by direct discharge into capillaries by their end-feet connections. This process, termed spatial siphoning, was first shown in the retina (Newman, 1986).
To achieve spatial K+ buffering, astrocytes are poised with passive uptake (via channels and cotransporters) and active uptake (via Na+/K+ ATPases) capabilities. Passive transport of K+ into astrocytes is achieved predominantly by inwardly rectifying K+ channels (Kir) and to a lesser extent, Na+/K+ or K+/Cl− antiporters, based on pharmacological studies (Ballanyi et al., 1987; Karwoski et al., 1989). In addition, Kir channels are distributed in a highly non-uniform manner, exhibiting high expressions in endfeet (Newman, 1986; Poopalasundaram et al., 2000; Higashi et al., 2001).
Although understanding the mechanisms of astrocytic K+ buffering has been extensively studied over the years, here are two limitations to our knowledge. First, the consequences of impaired astrocytic K+ uptake have remained unclear. Studies using extracellular cesium to block Kir channels induced epileptiform, interictal-like bursting and prevention of long-term depression in the hippocampus (Janigro et al., 1997). However, cesium can have effects independent of Kir channels. To circumvent this problem, Ken McCarthy’s group generated a conditional astrocyte-specific knock-out of Kir4.1 via the human GFAP promoter (Djukic et al., 2007). Kir4.1 was the first astrocytic Kir channels identified (Takumi et al., 1995) and was thought to play an important role in K+ buffering. Conditional knock-out of Kir4.1 resulted in severe depolarization of astrocytes, impairment of astrocyte K+ and glutamate uptake, enhanced short-term synaptic potentiation, and pronounced behavioral abnormalities, including ataxia and seizures. Another limitation with studying astrocytic K+ buffering owes to the fact that astrocytes are heterogeneous and all may not buffer extracellular K+ using the same mechanism. For example, only 50% of astrocytes express Kir4.1 (Higashi et al., 2001), and, therefore, those that lack expression must use alternate processes for K+ buffering. Finally, some of the functions of K+ buffering in astrocytes have gained widespread recognition without being tested. As an example, it was thought that K+ siphoning resulting in increased extracellular K+ around vessels resulting in localized changes in blood flow, a process called neurovascular coupling. However, in the retina, glial K+ siphoning does not seem to contribute to neurovascular coupling (Metea et al., 2007).
3.4. Glutamate and GABA uptake
Astrocytes have been long known to take up and metabolize GABA and glutamate (Schousboe et al., 1992) as well as other transmitters not discussed here (but see (Schousboe and Westergaard, 1995) for review). Among the cloned GABA transporters (GAT1 to GAT4 in mice, GAT-1, BGT-1, GAT-2, and GAT-3 in rats for review see Borden, 1996), astrocytes express a high density of high affinity GABA transporters (GAT-1 and GAT-3, i.e. GAT1 and GAT4) (Brecha and Weigmann, 1994; Morara et al., 1996; Yan and Ribak, 1998; Conti et al., 1999; Barakat and Bordey, 2002) depending on the region, for review see Jursky et al., 1994; Borden, 1996; Palacin et al., 1998). It was reported that GABA transporters in neocortical astrocytes from acute slices are activated by synaptically-released GABA (Kinney and Spain, 2002), suggesting that astrocytes may limit spillover and extrasynaptic GABA receptor activation. Pharmacological blockade of GAT-2/3 in neocortical slices altered synaptic inhibition, suggesting that there is a tonic GAT-3-mediated GABA release (Kinney, 2005). However, another study suggested that synaptic GABA levels in neocortical neurons are controlled primarily by GAT-1, and that GAT-1 and GAT-2/3 work together extrasynaptically to limit tonic currents (for review on tonic current see Farrant and Nusser, 2005). That astrocytic GABA transporters are located near synaptic clefts suggests that astrocytes likely control GABA spillover from the cleft either alone or in cooperation with neuronal transporters. Considering that astrocytes catabolize GABA very quickly, it remains unclear whether GABA transporters directly contribute to synaptic transmission by mediating GABA release (for references see Barakat and Bordey, 2002).
Among the five cloned high affinity glutamate transporters (excitatory amino acid transporter EAAT1 to 5, for review see Palacin et al., 1998; Torres and Amara, 2007), astrocytes express EAAT1 and EAAT2 (also called GLAST and GLT-1, respectively) (Lehre et al., 1995; Chaudhry et al., 1995; Ullensvang et al., 1997; Schmitt et al., 1997; Conti et al., 1998; Minelli et al., 2001). Although astrocytes were thought to express both GLT-1 and GLAST, a recent study using transgenic mice carrying GFP or DsRed under the promoters of GLT-1 and GLAST, respectively, show that GLT-1 promoter activity is almost completely restricted to astrocytes, often in a non-overlapping pattern with GLAST (Regan et al., 2007). The GLAST promoter is active in both radial glia and many astrocytes in the developing CNS but is down-regulated in most astrocytes as the mice mature. Nonetheless, GLAST expression persists in radial-like astrocytes of the SGZ, SVZ, and cerebellum (i.e. Bergmann glia) (Schmitt et al., 1997; Yamada et al., 2000), and is only observed in oligodendrocytes of the white matter. The exciting demonstrations showing that astrocytic glutamate transporter GLT-1 was critical for preventing glutamate build-up during neurotransmission leading to excitotoxicity (Rothstein et al., 1996; Tanaka et al., 1997) led to an avalanche of studies on astrocytic glutamate uptake in both health and disease (for reviews see Gegelashvili and Schousboe, 1997; Furuta et al., 1997; Billups et al., 1998; Danbolt et al., 1998a; Anderson and Swanson, 2000; Danbolt, 2001; Gegelashvili et al., 2001; Gadea and Lopez-Colome, 2001). In particular, a series of elegant studies showed that astrocyte glutamate transporters are activated by synaptically released glutamate in acute slices (Bergles and Jahr, 1997; Bergles et al., 1997; Clark and Barbour, 1997; Bergles and Jahr, 1998; Bordey and Sontheimer, 2003). These findings had important implications for future work as glutamate transporters were then used as sensors to determine the time course of the transient glutamate concentration at synapses (Bergles and Jahr, 1998; Linden, 1998; Mennerick et al., 1999). Thus, transporters were shown to be critical for limiting glutamate spillover from the synaptic cleft (Marcaggi et al., 2003; Huang and Bordey, 2004; Huang et al., 2004). As a result, they may serve to decrease synaptic noise and improve the reliability of synaptic transmission.
3.5. Metabolic support
Before the cloning of the different glutamate transporters, it was well established that glutamate uptake into astrocytes was critical for the glutamate-glutamine cycle (Westergaard et al., 1995; Sonnewald et al., 1997). Briefly, astrocytes take up and metabolize glutamate into glutamine via the glutamine synthetase. Glutamine is then redistributed to neurons for de-novo synthesis of glutamate. Importantly, glutamate transporters are not limited to buffering ambient glutamate; they bridge neuronal activity and astrocytes’ ability to meet this metabolic need (as discussed below).
Astrocytes are uniquely poised to provide a nurturing environment for neurons, a role suggested by Golgi more than 100 years ago. As mentioned previously, astrocyte processes project toward blood vessels that terminate into structures called endfeet, which almost entirely cover the blood vessel walls (Abbott et al., 2006). On the membrane of endfeet facing blood vessels, astrocytes express a specific form of glucose transporters, GLUT1 (Morgello et al., 1995; Yu and Ding, 1998) (for review see Vannucci et al., 1997). It was originally thought that astrocytes take up glucose from the blood making it available to neurons. However, research over the last two decades has generated much controversy over the precise mechanims for how astrocytes metabolically support for neurons. We will review recent evidence regarding two other substrates by which astrocytes can regulate neuronal metabolic responses to activity: glycogen and lactate.
Glycogen serves as the short-term repository for glucose and is found primarily in astrocytes in the brain (Cataldo and Broadwell, 1986; Wender et al., 2000; Kong et al., 2002). Along with the presence of glycogen in astrocytes are the expressions of glycogen synthase and glycogen phosphorylase, enzymes that synthesize and metabolize glycogen, respectively (Pellegri et al., 1996; Pfeiffer-Guglielmi et al., 2003). A possible role for brain glycogen stores has been dismissed due to the negligible concentration of brain glycogen (6-12 μmol) compared with those in the liver (100-500 μmol) and skeletal muscle (300-350 μmol) (see review by (Brown and Ransom, 2007). However, recent evidence has shown that under hypoglycemic conditions and periods of increased tissue energy demand, astrocytic glycogen provides the energy substrate for the brain. Studies in rodent optic nerve (a central white matter tract that is devoid of synapses or neuronal cell bodies) demonstrated that increasing glycogen content by pre-incubation in elevated glucose could prolong axonal function following glucose withdrawal. By contrast, down-regulating glycogen content or inhibiting glycogen metabolism by blocking glycogen phosphorylase function prevented the neuroprotective effects of elevated brain glycogen content (Brown et al., 2003; Brown et al., 2005; Tekkok et al., 2005). The role of glycogen in hypoglycemia has been demonstrated in vivo using a glycogen phosphorylase inhibitor, which increased brain glycogen stores and was able to preserve brain function for up to 90 min during profound systemic hypoglycemia (Suh et al., 2007). Another proposed role for astrocyte glycogen occurs during increased brain activation, when the immediate supply of neuronal glucose is exhausted and unable to meet the high metabolic demand of the tissue. Here, glycogen metabolism can provide a rapid energy source that bypasses the rate-limiting step in glucose metabolism (glucose phosphorylation via hexokinase) (see (Brown and Ransom, 2007) for review). Indeed, in the optic nerve preparation, increased neuronal energy demand induced by high frequency stimulus resulted in a rapid decrease in glycogen content, even with normoglycemic concentrations of bath glucose (Brown et al., 2003). These lines of evidence strongly suggest that astrocyte glycogen provides an important energy source to the brain under physiological conditions of high neuronal activity.
Another important, though controversial, astrocyte metabolic pathway that has emerged in the last two decades is the role of lactate as an oxidative substrate for energy metabolism. It was observed that during the astrocyte glutamate-glutamine cycle for neurotransmitter recycling, astrocytic aerobic glycolysis provides the ATP required for glutamate amidation, which results in lactate production (Martinez-Hernandez et al., 1977; Pellerin and Magistretti, 1994). On the basis of this result, a mechanism of coupling between neuronal activation and glucose utilization was proposed, a model known as the astrocyte-neuron lactate shuttle hypothesis (ANLSH) (Pellerin and Magistretti, 1994). In this model, glucose taken up by astrocytic GLUT1 can be processed in part oxidatively via the tricarboxylic acid (TCA) cycle pathway, while the remaining glucose is converted to lactate and released into the extracellular space via monocarboxylate transporters (MCT1 and MCT4). Lactate released by astrocytes is then transported into neurons via MCT2 and converted to pyruvate for oxidative use in the TCA cycle (for review see Pellerin et al., 2007). A plethora of in vitro and in vivo studies have demonstrated evidence for lactate as a preferential oxidative substrate for neurons (for review see Pellerin, 2003; Pellerin et al., 2007). Here, we will only mention a few key studies. Two independent studies using different quantitative measurements showed that lactate is the predominant oxidative substrate over glucose in cultured neurons (Bouzier-Sore et al., 2003; Itoh et al., 2003; Bouzier-Sore et al., 2006). In addition, it was found that glutamate stimulation caused a reduction of glucose transport in neurons but an increase in astrocytes, implying that under neuronal glutamatergic activation, lactate utilization by neurons is favored (Porras et al., 2004). Finally, in vivo evidence demonstrated that sustained activation of the perforant pathway in the hippocampus led first to a decrease in extracellular lactate concentration, followed by a massive increase, which seem to support the ANLSH (Hu and Wilson, 1997).
There are some arguments against the use of lactate by neurons. First, both glucose and lactate metabolisms require nicotinamide adenine dinucleotide (NAD+), a competition that might favor glycolysis (Cruz et al., 2001) (for review see Chih and Roberts Jr, 2003; Cerdan et al., 2006). Second, the evidence for a net lactate transfer between astrocytes and neurons seems uncertain (Gjedde and Marrett, 2001; Hertz, 2004). Using magnetic resonance spectroscopy, it was shown in vivo that increased neuronal metabolism with higher levels of brain activity is supported by lactate generated within the brain from a nonneuronal source (Serres et al., 2003; Serres et al., 2004; Serres et al., 2005).
Despite all the controversies regarding the brain metabolic pathways, it is important to recognize that these ideas are not mutually exclusive and that under different conditions, neurons may rely upon different sources of substrate (glucose, glycogen, or lactate) to support their function. Based on the emerging role of astrocytes-neuron interactions, it is clear that alternative approaches to improving energy metabolism in neurons (such as enhancing glucose uptake in astrocytes or lactate uptake in neurons) may provide valuable neuroprotection strategies for therapy.
3.6. Others: detoxification and immune functions
One of the most important roles of astrocytes is to protect neurons against excitotoxicity by capturing excess ammonia and glutamate and converting them into glutamine. In addition, astrocytes may also participate in the uptake of some heavy metals, such as lead (Struzynska et al., 2001). Astrocytes contain metal binding proteins such as metallothioneins that endow astrocytes with both neuroprotective and neuroregenerative properties following injury or exposure to toxic metals (Aschner, 1997; Chung et al., 2008).
Astrocytes can serve as a bridge between the CNS and immune system. In particular, astrocytes can phagocytose cells and act as antigen-presenting cells (for references prior 1994 see Montgomery, 1994). For example, cultured astrocytes were shown to present antigens to T lymphocytes in a specific manner which is restricted by the major histocompatibility complex, and in particular they could activate myelin basic protein -specific encephalitogenic T-cell lines (Fontana et al., 1984). Astrocytes can also express class II major histocompatibility complex antigens and costimulatory molecules (B7 and CD40) that are critical for antigen presentation and T-cell activation (for review see Dong and Benveniste, 2001; Farina et al., 2007). Furthermore, astrocytes were found to express receptors involved in innate immunity, including Toll-like receptors, nucleotide-binding oligomerization domains, double-stranded RNA-dependent protein kinase, scavenger receptors, mannose receptor and components of the complement system (Owens, 2005; Farina et al., 2007). Finally, astrocytes produce a wide array of chemokines and cytokines that act as immune mediators in cooperation with those produced by microglia. One intriguing idea is that molecules involved in immune responses may serve additional roles as adhesion molecules between astrocytes and neuronal elements such as dendritic spines. However, this proposed function has not been explored and remains to be elucidated.
4. The emerging and controversial functions
Presumed to be passive elements at synapses, astrocytes were once considered to be mere structural elements that provide anchoring for synapses. However, several recent studies have elucidated the plasticity of astrocytic processes and their participation in synaptic transmission. Like the mobile dendritic spines that respond to changes in activity by altering their structure, astrocytic processes dynamically alter their coupling to neurons in response to environmental cues. Pioneering work performed in the supraoptic nucleus of the rat hypothalamus show that astrocytes are capable of retracting from synapses in a reversible manner during maternal lactation. This retraction reduces coverage of the excitatory synapses and permits greater neurotransmitter diffusion beyond the synapse (Oliet et al., 2001a). These studies open up many avenues for future investigation on the role of astrocytes on neurotransmission. Before discussing the emerging functions of astrocytes, we will discuss how astrocytes sense neuronal activity and their mode of excitability, particularly intracellular calcium dynamics.
4.1. Sensing neuronal activity
It is well established that astrocytes sense neuronal activity through activation of ion channels, transporters, and receptors resulting in fast depolarization and/or intracellular calcium increases (for reviews see Cornell-Bell and Finkbeiner, 1991; Finkbeiner, 1992; Vernadakis, 1996; Parri and Crunelli, 2002; Schipke and Kettenmann, 2004, and more recently (Fiacco and McCarthy, 2006). Astrocytes are endowed with K+ channels and since the 1960’s it has been known that astrocytes are depolarized by neuronal spiking (Kuffler and Nicholls, 1966; Kuffler, 1967). Pioneering work in 1990 showed that glutamate triggers intercellular calcium waves in cultured astrocytes (see next section discussing their existence in vivo) (Cornell-Bell et al., 1990b). Later, others showed that neuronal stimulation triggered calcium waves in hippocampal astrocytes from cultured slices (Dani et al., 1992) and calcium transients in astrocytes in acute slices via metabotropic glutamate receptor activation (Porter and McCarthy, 1996). Calcium transients in astrocytes can be induced following activation of different types of metabotropic receptors (for review see Fiacco and McCarthy, 2006). A series of elegant studies also demonstrated that stimulation of glutamatergic fibers in neuron-astrocyte microislands in vitro trigger glutamate transporter-mediated currents in astrocytes (Mennerick and Zorumski, 1994; Mennerick et al., 1996) and acute slices (Bergles and Jahr, 1997; Clark and Barbour, 1997; Bergles and Jahr, 1998; Bordey and Sontheimer, 2003). Transporter currents provide a sensitive probe to determine the time course and concentration of the neurotransmitter transients at synapses (Luscher et al., 1998; Mennerick et al., 1999). Calcium increases from neuron-to-astrocyte signaling can be confined to subcellular compartments and microdomains, or sometimes the entire cell, as elegantly shown in Bergmann glia in acute slices (Grosche et al., 1999). Interestingly, astrocytic depolarizations following neuronal stimulation display selectivity and short-term plasticity (Linden, 1997; Pasti et al., 1997; Perea and Araque, 2005). These findings suggest that astrocytes have the capacity to process and integrate selective information in response to neuronal activity. Finally, with the development of novel tools, spontaneous Ca2+ transients in astrocytes have been detected in vivo and are regulated by neuronal activity (Hirase et al., 2004; Wang et al., 2006; Winship et al., 2007). In particular, it was shown that whisker stimulation or contralateral hindlimb mechanical stimulation induced a short-latency calcium signal in astrocytes that operated on a time scale similar to neuronal activity (Winship et al., 2007). More recently, an elegant study reported that astrocytes (like neurons) in the ferret visual cortex respond to visual stimuli in vivo and display distinct spatial receptive fields and sharp tuning to visual stimulus features including orientation and spatial frequency (Schummers et al., 2008). Collectively, evoked or spontaneous intracellular calcium dynamics in astrocytes demonstrates a mode of astrocytic excitability since changes in intracellular calcium convey a powerful signal that can travel throughout a cell and activate protein kinases, ion channels, and vesicular release (see section 4.5). However, as discussed below, it is unclear whether intercellular calcium waves can spontaneously arise in normal conditions or following a physiological stimulation in vivo.
Astrocytes express many types of receptors commonly found in neurons. In addition, there is evidence of receptor clustering in astrocytic somata. Indeed, in a recent study, quantal events due to ectopic vesicular release of glutamate was reported in Bergmann glia (Matsui and Jahr, 2003). In a subsequent study, the authors found that the density of functional AMPA-type glutamate receptors at Bergmann glia somata is ~17-fold higher than that at the Purkinje neuron somata (the principal neuronal type of the cerebellum) (Matsui et al., 2005). However, compared with the neuronal postsynaptic site, the clustering of receptors in astrocytes is much lower than that of the neuronal postsynaptic site. For example, the Purkinje neuron’s postsynaptic density (1000 receptors/μm2) is much greater than the AMPA receptor density in Bergmann glia (Momiyama et al., 2003; Tanaka et al., 2005). Nevertheless, these studies provide evidence that astrocytes are poised to sense neuronal activity by expressing the machinery to respond to neurons.
4.2. Calcium dynamics: do intercellular calcium waves exit?
1990 was the year when a paradigm shift took place in the astrocytic field. The existence of glutamate-induced intercellular calcium waves provided a mode of excitability and long distance communication between astrocytes that received much attention (Cornell-Bell et al., 1990b) (for review see Cornell-Bell and Finkbeiner, 1991; Parri and Crunelli, 2002; Fiacco and McCarthy, 2006, and for mechanism based on ATP release and receptor activation see Finkbeiner, 1992; Dupont et al., 2007). This led to several elegant hypotheses related to the role of long-range astrocytic calcium signals on neuronal transmission (Smith, 1992). However, it still remains unclear whether calcium waves between mature astrocytes occur in acute slices or in vivo. It is clear that intracellular calcium transient increases and calcium waves within a cell occur in response to neuronal stimulation both in acute slices (as supposed to cultured slices (Dani et al., 1992)} and in vivo (Hirase et al., 2004; Wang et al., 2006; Winship et al., 2007; Bekar et al., 2008) (for an excellent recent review see Fiacco and McCarthy, 2006). In vivo calcium transients were found to be coordinated (Hirase et al., 2004), but no intercellular waves have been observed in a mature astrocytic network besides calcium waves in retinal Muller cells (Newman and Zahs, 1998). Nevertheless, intercellular calcium waves have been observed in young astrocytes in acute slices from 5-14 days old mice and 5-17-days old rats (Parri et al., 2001; Schipke et al., 2002; Haas et al., 2006). In neonatal thalamic slices, calcium waves occurred spontaneously and resulted in NMDA-type glutamate receptor-activation in neighboring neurons (Parri et al., 2001). In addition, Ca2+ waves could be induced in cortical astrocytes in vivo upon pressure application of a noradrenergic agonist (Bekar et al., 2008). In the recent study in the visual cortext of ferret, it was reported that in vivo, astrocytes behave relatively independent of each other (Schummers et al., 2008). Each astrocyte was reported to be sharply tuned for orientation, and two astrocytes separated by only tens of microns can respond to different, orthogonal stimulus orientations.
Intracellular calcium dynamics represent the mode of excitability in astrocytes and some of its functions on the vasculature and synaptic activity are illustrated in the next sections. Intercellular calcium waves remain to be shown in acute slices and in vivo. Alternatively, other ions may provide long-range communication, as sodium waves have been shown in cultured astrocytes (Bernardinelli et al., 2004). Future studies on intercellular calcium and sodium waves in vivo would elucidate mechanisms of intercellular communication in astrocytes.
4.3. The neurovascular unit: control of BBB permeability, metabolism and vascular tone
4.3.1. BBB permeability
A number of molecules modulate BBB permeability, including neurotransmitters, neuromodulators, arachidonic acid and prostanoids, cytokines, macrophage inflammatory proteins (MIP) and nitric oxide (for reviews see Abbott and Revest, 1991; Abbott, 2000; Hawkins and Davis, 2005; Persidsky et al., 2006). Some of these molecules, including ATP, endothelin-1, glutamate, IL-6 and TNF-a, MIP-2 and nitric oxide, are released from astrocytes. These molecules result in opening of the paracellular pathway by increasing permeability at the tight junctions. However, no study in vivo or in acute slices has directly reported a function of astrocytes on BBB permeability on a second-to-minute time-scale. Nevertheless, astrocyte-endothelial cell interactions may depend on calcium and ATP-receptor-mediated signaling from astrocytes to endothelial cells (Paemeleire and Leybaert, 2000a). Considering that endothelial cytoplasmic calcium is an important factor in the regulation of blood-brain barrier permeability, astrocytes may dynamically regulate BBB permeability. Such a dynamic regulation and transient increase in BBB permeability in the adult brain could be beneficial for allowing plasma molecules (e.g. growth factors) to enter the CNS and promote brain repair.
4.3.2. Metabolism
According to a newly revised model of energy metabolism in the brain, 75% of the glucose that enters the parenchyma may be phosphorylated by astrocytes (Hyder et al., 2006). Glucose enters astrocytes through GLUT1, the major isoform of the facilitative glucose transporter, which is strategically concentrated in astrocyte endfeet, the thin processes that surround capillaries (Morgello et al., 1995; Yu and Ding, 1998; Kacem et al., 1998). It was recently shown in cultured astrocytes that coordinated Na+ and Ca2+ increases in astrocytic endfeet induced dynamic changes in glucose uptake (Porras et al., 2008). Ca2+ or Na+ increases alone were not sufficient to modulate GLUT1 transport activity, but changes in both intracellular ions were required for an increase in glucose uptake. Na+ increases were due to influx through the Na+-glutamate cotransporter and the Na+/K+-ATPase pump. This remarkable finding illustrates a critical function of coordinated Na+ and Ca2+ movement in astrocytes to stimulate glucose uptake. Coordinated Na+ and Ca2+ waves in astrocytes that drive glucose uptake (Mahesh et al., 2006) have also been reported in vitro but not in slices (Paemeleire and Leybaert, 2000b). Manipulations that block Ca2+ waves also inhibited Na+ waves. However, inhibition of glutamate uptake or enzymatic degradation of extracellular glutamate selectively inhibited Na+ waves. These data suggest that glutamate released by a Ca2+-dependent mechanism was taken up by the Na+/glutamate cotransporters into cultured astrocytes, resulting in a regenerative propagation of cytosolic Na+ increases (Bernardinelli et al., 2004). Intracellular Na+ in astrocytes may thus be a powerful intracellular messenger, which couples neuronal activity to glucose uptake and glycolysis (see section 3.5) in astrocytes, thus matching the excitability of a neuroglial network to the metabolic supply.
4.3.3. Control of vascular tone
It has been known for more than a century than brain activity can induce localized changes in cerebral blood flow (CBF) (Mosso, 1880). More specifically, when the activity of a brain region increases, blood flow to that region also increases to provide adequate oxygenation, energy supply, and removal of toxic byproducts of metabolism (for review see Iadecola, 2004). This mechanism, termed functional hyperaemia, is thought to be the basis for functional brain imaging, which maps changes in CBF induced by neural activity (Raichle and Mintun, 2006). Astrocytes, due to their proximity and extensive ensheathment of blood vessels, as well as their ability to sense neuronal activity (e.g. via metabotropic glutamate receptor activation), were suspected to be part of CBF regulation (Nedergaard et al., 2003). However, it was only in the early 2000’s that two research groups discovered that astrocytes participated in neurovascular coupling by matching neuronal activity to vascular tone in acute slices (Zonta et al., 2003; Mulligan and MacVicar, 2004) (see summary diagram in Fig. 4). Stimulation of astrocytes led to intracellular calcium increases and wave propagation to endfeet, which resulted in either vasodilation or vasoconstriction. In one study, uncaging Ca2+ in astrocytes or applying the vasoactive molecule noradrenaline induced Ca2+ rise in endfeet and vasoconstriction of vessels (Mulligan and MacVicar, 2004). The study also found that vasoconstriction was due to activation of the phospholipase A2-arachidonic acid pathway and 20-hydroxyeicosatetraenoic acid production from astrocytic endfeet. Another study showed in rat cortical slices that neuronal activity triggered Ca2+ oscillations in astrocytes resulting in vasodilation (Zonta et al., 2003). Selective activation of single astrocytes (via a patch pipette) contacting an arteriole also caused vessel relaxation. Importantly, the authors showed that in vivo blockade of glutamate-mediated Ca2+ increases in astrocytes reduced the blood flow increase in the somatosensory cortex during contralateral forepaw stimulation. A very recent study also showed the loss of blood flow regulation following visual stimulation in vivo when astrocytic responses to visual stimulation were attenuated (Schummers et al., 2008). The mechanisms of vascular tone regulation is not detailed here, but has been the subject of several excellent and recent reviews (Anderson and Nedergaard, 2003; Ballabh et al., 2004; Straub and Nelson, 2007; Gordon et al., 2007; Iadecola and Nedergaard, 2007).
Figure 4. Astrocyte interactions with the vasculature.
(A) Astrocytic processes ensheath blood vessels, including arterioles, which are composed of endothelial cells and smooth muscles cells (note shown on the diagram). The smooth muscle cells allow the vessels to contract or dilate. Neuronal activity induces mGluR activation in astrocytes leading to the synthesis of arachidonic acid (via PLA2, not shown) and the formation of downstream messengers, including prostaglandins (PGs) and epoxyeicosatrienoic acid (EETs) via Cox2 and P450, respectively. PGs (in particular PGE2) and EETs induce vessel dilation. AA can also pass from astrocytes to smooth muscle cells where its downstream product 20-hydroxyeicosatetraenoic acid (20-HETE) induces vasoconstriction. In addition the activation of Ca2+ activated K+ channels in astrocyte endfeet and the efflux of K+ from astrocytes and subsequently from smooth muscle cells has been suggested to modify vascular tone by hyperpolarization and relaxation of smooth muscle cells, but this does not occur in the retina (See Metea et al, 2007). (B) Glutamate uptake into astrocytes is accompanied by Na+ entry leading to Ca2+ elevation as a result of Na+/Ca2+ exchange. Together Na+ and Ca2+ can stimulate glucose uptake from the blood into astrocytes via GLUT1. (C) One newer hypothesis is whether changes in blood vessel diameter can affect the biology of astrocytes via activation of stretch-activated channels that can be permeable to Ca2+ or other ions.
These exciting findings were followed by a series of elegant studies showing similar coupling between astrocyte activation and blood-flow regulation in the retina (Metea and Newman, 2006) and in the cortex in vivo (Takano et al., 2006; Winship et al., 2007). In the retina, both light and Muller cell stimulation evoked dilation or constriction of arterioles. Vasodilation was blocked by inhibitors of cytochrome P450 epoxygenase, the synthetic enzyme for epoxyeicosatrienoic acids, while vasoconstriction was blocked by an inhibitor of ω-hydroxylase, which synthesizes 20-hydroxyeicosatetraenoicacid. Nitric oxide levels were key players in determining whether vasodilations or vasoconstrictions occur in response to light and astrocyte stimulation. It was also thought that another astrocyte function, i.e. K+ siphoning, contributed to neurovascular coupling. However, a recent study by Metea and colleagues (2007) convincingly showed that although retinal glial cells undoubtedly control neurovascular coupling, the glial K+ siphoning does not appear to be a major contributor (Metea et al., 2007).
It is clear that in vivo studies are more accurate than slice preparations to study blood flow regulation by astrocytes and neurons, as well as blood flow autoregulation in response to physiological fluctuations in blood pressure (for review see Iadecola, 2004). It is also possible to monitor Ca2+ activity in astrocytes and the hemodynamic response using intrinsic signal imaging in vivo (Winship et al., 2007). However, the slice preparation has the advantage of allowing for greater spatial resolution and improved access for pharmacological manipulations and cell stimulation. All of the above studies were performed on arterioles, which are surrounded by a layer of smooth muscle cells that either constrict or dilate upon astrocytic activation. Arterioles branch into capillaries that do not have smooth muscle cells, but these capillaries are lined by pericytes at discrete locations. Pericytes were recently shown to induce the constriction of capillaries, serving an analogous function to arteriolar smooth muscle cells (Peppiatt et al., 2006). Considering that astrocytic endfeet terminate on pericytes as well as smooth muscle cells, it is possible that astrocytes contribute to regulation of capillary tone via pericyte stimulation. Although the concept of astrocytes providing neurons–to-blood vessels coupling and blood flow regulation seems universal, some neurons bypass astrocytes by directly projecting onto blood vessels (for review see Iadecola, 2004). These neurons, some of them being nitridergic, have the ability to directly regulate changes in vascular tone. Finally, most of the research on neurovascular coupling has been unidirectional, i.e. studying signals from neurons to astrocytes and smooth muscle cells. Interestingly, there is an emerging concept of signaling from the blood vessel to astrocytes and then neurons via stretch-activated channels (for hypothesis see Moore and Cao, 2008). Together, the signals among these key players may provide the crosstalk to match neuronal activity and metabolic supply.
4.4. Dynamic control of synaptic structure: from spine to synapse
At the electron microscopy level, 57% of synapses in the mature hippocampus are in direct contact with astrocytes (Ventura and Harris, 1999). But is this ensheathment static or dynamic at the second to minute time-scale? In cultures, it was shown that glutamate induces the formation of filopodia in hippcampal astrocytes (Cornell-Bell et al., 1990a). It was also recently shown that astrocytic processes contacting neuronal somata and wrapping active synaptic terminals (visualized with FM1-43 staining) display a high degree of dynamic morphological changes in acute slices from GFAP-eGFP mice (Hirrlinger et al., 2004). There are two defined modes of spontaneous motility: thin lamellipodia-like membrane protrusions gliding along neuronal surfaces and transient extensions of filopodia-like processes (Hirrlinger et al., 2004). This high motility was proposed to be important for correct placement of gliotransmitter release sites and shaping the neuroblast environment in addition to properly positioning processes containing glutamate transporters. This study also highlighted the need to generate novel transgenic mouse lines with fluorescently tagged signaling molecules on astrocytic processes as well as on neuronal terminals for examining plasticity at synapses. A more recent study analyzed the coordinated motility of both astrocytic processes and spines (Haber et al., 2006). Using time-lapse confocal imaging in acute slices, they found that astrocytes could rapidly extend and retract fine processes to engage and disengage from motile postsynaptic dendritic spines. Surprisingly, astrocytic motility is, on average, higher than its dendritic spine motility. Collectively, these findings and the examples presented below suggest that such a motility of astrocytic processes around synapses is accompanied by changes in synaptic activity.
4.4.1. Structural synapse plasticity
Astrocytes have long been thought to be highly plastic. GFAP expression is itself very dynamic and can be up-regulated by physiological conditions (i.e. salt loading; Gary and Chronwall, 1995) and second messengers within minutes (Neary et al., 1994; Hunter and Hatten, 1995; Kahn et al., 1997; Rajan and McKay, 1998). One striking example of anatomical remodeling in the adult brain resulting in changes in synaptic activity is in the supraoptic nucleus of the hypothalamus (for review see Theodosis et al., 2004; Oliet and Piet, 2004; Oliet et al., 2006). During lactation or dehydration, astrocytes modify their coverage of magnocellular neurons and their synaptic afferent inputs. These changes occur within a few hours and are completely reversible upon the cessation of stimulation. By comparing synaptic transmission and diffusion properties before and during this neuroglial remodeling, it was found that the astrocytic environment surrounding neurons contributes to the regulation of synaptic and extrasynaptic transmission (Piet et al., 2004). In particular, astrocytes control the level of activation of presynaptic metabotropic glutamate receptors on glutamatergic terminals, thereby regulating synaptic strength at excitatory synapses (Oliet et al., 2001b). Astrocytes also constitute a physical barrier to diffusion, spatially and temporally limiting spillover of neurotransmitters, and thus limit extrasynaptic transmission. Another example of anatomical plasticity is in the Bergmann glia of the cerebellum. In a study by Iino et al (2001), Ca2+-permeable AMPA-type glutamate receptors in Bergmann glia were converted into Ca2+-impermeable receptors by adenoviral-mediated delivery of the GluR2 gene in vivo. This conversion retracted the Bergmann glial processes ensheathing synapses on Purkinje neuron dendritic spines and retarded the removal of synaptically released glutamate. In addition, it caused multiple innervations of Purkinje neurons by the climbing fibers where each Purkinje neuron normally receives only one climbing fiber in adult tissue. These findings showed that Ca2+-permeable AMPA receptors in Bergmann glial processes are critical for proper structural and functional interactions between Bergmann glia and glutamatergic synapses. These studies support the idea that astrocytic processes are highly plastic and can remodel upon synaptic receptor activation. It remains to be shown whether the anatomical remodeling and changes in synaptic transmission can occur on a timescale of seconds following electrical stimulation in acute slices or in vivo. Future studies in this area would highlight the physiological importance and implications of this dynamic interaction between neurons and astrocytes at the synapse.
4.4.2. Spine formation
As mentioned in section 3.1, astrocytes promote synaptogenesis during development by releasing several diffusible molecules. In 1992, it was shown that astrocyte-conditioned medium promotes the proliferation of spines prior to the appearance of axons (Seil et al., 1992), an event that precedes synapse formation. Protrusive dendritic activity is prominent in developing neurons (Dailey and Smith, 1996; Ziv and Smith, 1996; Portera-Cailliau et al., 2003). It is thought that a subset of these motile protrusions are stabilized and transformed into stable spines with synaptic contacts (for review see Yuste and Bonhoeffer, 2004). A recent study using two-photon time-lapse imaging in cultured slices showed that astrocytic motility was essential for stabilization of individual dendritic protrusions and their subsequent maturation into spines (Nishida and Okabe, 2007) (Fig. 5A). In this study, manipulating Rac1-dependent signaling in astrocytes resulted in induction of longer, filopodia-like dendritic protrusions. Rac1 belongs to the family of small GTPases of the Rho family that are important regulators of the actin cytoskeleton (for review see de Curtis, 2008). In addition, in the same study, manipulation of ephrin/Eph-dependent neuron-astrocyte signaling suggested the involvement of this signaling pathway in astrocyte-dependent stabilization of newly generated dendritic protrusions. An earlier study showed that the membrane-bound ligand, ephrin-A3, on astrocytes dynamically regulates spine morphology in the hippocampus via local activation of EphA receptors on spines in acute slices (Murai et al., 2003). Improvements in imaging resolution and molecular tools to label different cell types and manipulate their properties would improve our understanding of the function of astrocytes on spine formation and stability.
4.5. Fast modulation of synaptic transmission
There has been an explosion of papers on this topic both at the level of regular manuscript and review publications. We apologize for not crediting all of the reviews on this topic (Volknandt, 2002; Schousboe, 2003; Newman, 2003b; Hertz and Zielke, 2004; Allen and Barres, 2005; Mongin and Kimelberg, 2005; Benarroch, 2005; Haydon and Carmignoto, 2006; Verkhratsky and Toescu, 2006; Parri and Crunelli, 2007; Bains and Oliet, 2007). In an effort to not repeat these reviews, we will essentially highlight some of the pioneer findings and the recent controversies. We also refer to Dr. Kimelberg’s recent review (2007) that provides a critical assessment of the studies on the instructive role of astrocytes at synapses and discuss the significance of the findings. The overall concept is that neurotransmitters released from presynaptic terminals of neurons can activate receptors on neighboring astrocytes, leading to calcium increases, and perhaps wave propagation in young tissue. These changes in astrocytes, along with the release of glial-derived factors, can signal back to the neurons and modulate synaptic transmission in a millisecond-to-second time-scale. Two pioneering studies in 1994 showed that cultured astrocytes can release glutamate or a diffusible messenger resulting in either neuronal NMDA-type glutamate receptor activation or calcium increases in neurons (Parpura et al., 1994; Nedergaard, 1994). In one of these studies, communication between astrocytes and neurons was proposed to be based on a gap junction-mediated process (Nedergaard, 1994). The elevation of astrocyte intracellular calcium was sufficient to induce the release of glutamate (Parpura et al., 1994) and another unknown diffusible molecule (Nedergaard, 1994). Cultured astrocytes can release ATP and glutamate through various means, including volume-sensitive anion channels, calcium-sensitive anion channels, gap junction hemichannels, and fusion of secretory vesicles (Mongin and Kimelberg, 2005) (for review see Evanko et al., 2004). Astrocytic Ca2+ transients induced by metabotropic agonists, brief touch, or photolysis of caged Ca2+ caused the appearance of an NMDA receptor-dependent slow inward current (SIC) in nearby neurons and an increase in the frequency of synaptic currents in the absence of neuronal spiking (Araque et al., 1998). SIC in neurons is thought to result from glutamate-containing vesicle secretion from astrocytes, as it can be prevented by toxins that cleave SNARE proteins or by overexpression of dominant-negative SNAREs in astrocytes (Araque et al., 2000). Ca2+-dependent exocytosis has been confirmed in astrocytes by monitoring whole-cell capacitance and destaining of FM dyes using total internal reflection fluorescence microscopy (TIRFM, Bezzi et al., 2004). It was also shown that cultured astrocytes release glutamate via two modes of secretion from large vesicles (310 nm in diameter) in response to different types of stimulation (Chen et al., 2005). Interestingly, in response to a physiological stimulation, cultured astrocytes release their vesicular contents via a kiss-and-run mechanism. However, it was recently reported that extracellular ATP or glutamate can induce calcium-dependent exocytosis of lysosomes resulting in ATP release (Zhang et al., 2007; Jaiswal et al., 2007). These studies used time-lapse confocal imaging of FM dye-labeled fluorescent puncta or acridine orange loaded puncta, together with extracellular quenching and TIRFM in cultured astrocytes. The functional significance of lysosomal release, if it holds true in acute slices, is unknown. An interesting study also showed that receptor-induced Ca2+ increase is associated with an increase in astrocytic cell volume, which leads to the activation of volume-sensitive channels mediating glutamate release in vitro and in acute slices (Takano et al., 2005). Thus, the relative contribution of each pathway for astrocytic glutamate and ATP release at synapses remains unclear. In addition, cultured astrocytes may not represent astrocytes in physiological conditions; it is thus important to validate these findings in acute slices and in vivo.
In acute brain slices, Ca2+ increases in astrocytes via stimulation of Gq-coupled metabotropic receptors, photolysis of caged IP3 or caged Ca2+, direct mechanical stimulation, or repetitive depolarization of the astrocyte membrane result in activation of presynaptic (Kang et al., 1998; Brockhaus and Deitmer, 2002; Bordey and Sontheimer, 2003; Fiacco and McCarthy, 2004; Liu et al., 2004; Jourdain et al., 2007) and postsynaptic ionotropic receptors in neurons (Newman and Zahs, 1998; Fellin et al., 2004; Lee et al., 2007). Similarly, astrocyte-derived ATP has been shown to alter synaptic efficacy and neuronal excitability (Zhang et al., 2003; Newman, 2003a; Pascual et al., 2005; Gordon et al., 2005). However, the manipulations used to stimulate astrocytes were not physiological. A recent study also reported that vesicular glutamate release from astrocytes resulted in extrasynaptic receptor activation in neighboring neurons (Jourdain et al., 2007). They provided structural evidence of glutamate-containing synaptic-like microvesicles in the astrocytic processes facing NMDA receptors in neurons. As previously performed by others (Kang et al., 1998), the authors intracellularly perfused astrocytes with a calcium chelator (BAPTA) while recording spontaneous synaptic currents in a neighboring neuron using paired patch clamp recording. Using this approach, Jourdain and colleagues (2007) found that the frequency of spontaneous excitatory postsynaptic currents in the recorded neuron was reduced following calcium buffering in surrounding astrocytes. This latter approach suggests that calcium-dependent release of a transmitter from astrocytes tonically modulates spontaneous synaptic activity in hippocampal neurons. However, as recently pointed out in an earlier review, it is difficult to differentiate between a supportive role and an instructive role, i.e. an active release of a transmitter with this experiment (Kimelberg, 2007). To test for a vesicular release mechanism, they intracellularly perfused astrocytes with the active light-chain of tetanus neurotoxin (TeNTLC) while simultaneously recording a neighboring neuron using the double patch clamp approach. TeNTLC selectively cleaves and inactivates the vesicle-associated membrane protein VAMP2 (also called synaptobrevin 2), which is an indispensable component of the SNARE fusion complex. While depolarization of naïve astrocytes induced an increase in synaptic current frequency, depolarization of TeNTLC-loaded astrocytes did not. One caveat of this experiment is that the astrocytic depolarization was not a physiological stimulus. However, in transgenic mice expressing an inducible dominant negative for VAMP2 in astrocytes, astrocytes were shown to tonically suppress synaptic transmission by releasing ATP in vivo (Pascual et al., 2005). As mentioned above, it is important to acknowledge that other mechanisms could account for calcium-dependent glutamate release, such as through lysosome release (shown in vitro) and increase in astrocytic cell volume leading to the activation of volume sensitive channels (Takano et al., 2005). The impact on synaptic activity of each mode of calcium-dependent transmitter release from astrocytes remains to be elucidated.
While these studies offer exciting new ideas into the role of astrocyte-neuron signaling on synaptic transmission, they remain very controversial. In particular, a recent study by Fiacco and colleagues questions the validity of some of these findings (Fiacco et al., 2007). Utilizing the Gq-coupled receptor MrgA1, which is only expressed in nociceptive peripheral sensory neurons (Dong et al., 2001) and has no known endogenous ligand, Fiacco et al. generated a line of mice in which MrgA1 is selectively expressed in astrocytes of the CNS using a portion of the hGFAP promoter. By crossing hGFAP-tTA mice with tetOMrgA1-GFP mice they selectively expressed MrgA1 in astrocytes (~80% of hippocampal astrocytes). The MrgA1 receptor can be activated by the phe-leu-arg-phe amide (FLRFa) peptide (Han et al., 2002), whose application increased Ca2+ in a majority of astrocytes in acute hippocampal slices. Unexpectedly, application of FLRFa did not induce SICs in CA1 pyramidal neurons in hippocampal slices from MrgA1+ mice, nor did it affect the amplitude or kinetics of miniature excitatory postsynaptic currents (mEPSCs) or induce Ca2+ transients in the dendrites of these neurons. However, the authors were able to replicate their previous findings (Fiacco and McCarthy, 2004) by showing that uncaging of IP3 in astrocytes induced a small increase in the frequency of spontaneous excitatory postsynaptic currents in MrgA1+ mice. Very puzzling was the fact that SICs could not be induced in hippocampal pyramidal neurons of wild-type mice following endothelin application or Ca2+ uncaging in astrocytes, manipulations that produced reliable Ca2+ transients in astrocytes. However, NMDA-dependent SIC-like events were observed in CA1 pyramidal neurons when MrgA1+ hippocampal slices were exposed to hypotonic solution. These events were independent of glutamate-loaded vesicle fusions. Cell swelling has previously been shown to contribute to calcium-dependent glutamate release (Takano et al., 2005). Collectively these findings suggest that some of the previously used stimulation methods to induce glutamate release may not be physiological, such as uncaging of IP3 or calcium, and thus questions the validity of previous findings based on these methods. However, we cannot ignore that expression of a foreign receptor in astrocytes may affect their biology considering that an endogenous ligand may exist. In addition, McCarthy and colleagues reported recently that constitutive expression of a different foreign receptor, one coupled to Gi, led to hydrocephalus, despite the fact that the transgenic animals were never exposed to an agonist for this receptor (Sweger et al., 2007). Given these caveats, it will be important to validate previous findings in newly generated mice with a dominant-negative SNARE in astrocytes (Pascual et al., 2005) or in other new lines of mice where vesicular release or calcium handling can be selectively manipulated.
4.6. Astrocytes and Synaptic plasticity
Astrocytes release many diffusible factors other than ATP or glutamate. In particular, the release of tumor necrosis factor-α (TNFα) from astrocytes contributes to the preservation of synaptic strength at excitatory synapses (Beattie et al., 2002). TNFα was shown to enhance synaptic efficacy by increasing surface expression of AMPA receptors, while preventing the actions of endogenous TNFα had the opposite effects. Thus, the continual presence of TNFα is required for preservation of synaptic strength at excitatory synapses. Another attractive astrocytic molecule is D-serine, an allosteric agonist of NMDA-type glutamate receptors (Mustafa et al., 2004). Interestingly, astrocytes were shown to contribute to hippocampal long-term potentiation through release of D-serine in mixed neuron-glia cultures and acute slices (Yang et al., 2003). A recent study also convincingly demonstrated that astrocytes in the supraoptic nucleus can orchestrate synaptic transmission and plasticity by releasing D-serine (Panatier et al., 2006).
5. The astrocyte of the neurogenic zone
With the discovery of neurogenesis in the adult brain, astrocytes stepped into the spotlight as a source of stem cells in discrete regions of the adult brain. The adult CNS has long been regarded as a dormant environment for neuronal regeneration. It was traditionally believed that the mature brain loses its regenerative capacities, lending the brain vulnerable to injury and degeneration. However, specialized neural stem cells reside in the SVZ of the lateral ventricle and the SGZ of the hippocampal dentate gyrus throughout adulthood (Altman and Das, 1965; Altman, 1969; Reynolds and Weiss, 1992; Richards et al., 1992; Eriksson et al., 1998). The specialized neural stem cells in the SVZ and SGZ exhibit the two fundamental properties: the ability to self-renew and generate multiple cells types of the CNS, including neurons, astrocytes, and oligodendrocytes.
Interestingly, as mentioned in section 2.4, a subset of SVZ astrocytes display stem cell characteristics (i.e. multipotency and self-renewal) in rodents and humans (Doetsch et al., 1999; Sanai et al., 2004). It is thought that SVZ astrocytes generate transit-amplifying progenitors that generate neuroblasts. Neuroblasts migrate either to the olfactory bulb or, to a smaller extent, to the piriform cortex where they differentiate into interneurons (Luskin, 1993; Lois and Alvarez-Buylla, 1994) (for review see Bordey, 2006; Lledo et al., 2006). This finding has challenged the definition of an astrocyte and also its functions. Nevertheless, we believe that these cells belong to the family of astrocytes as discussed in section 2.4. What remains unclear is whether each SVZ astrocyte can at some point in time behave as a neural stem cell or whether only a subset of them has this ability. Similarly, can all SVZ astrocytes instruct neurogenesis? We will present data and arguments suggesting that all SVZ astrocytes including those with stem cell ability act as instructors of neurogenesis. Another issue that has recently been raised is whether every astrocyte in the CNS could regain stem cell features, and this will be briefly discussed.
5.1 Are there different types of SVZ astrocytes?
Doetsch and colleagues suggested the existence of two types of SVZ astrocytes (called type B cells) and tanycytes using electron microscopy and proliferative marker thymidine (Doetsch et al., 1997). Thymidine is incorporated into the DNA during the S phase of the cell cycle. Type B cells had irregular contours that filled the spaces between neighboring cells, irregular nuclei with invaginations, and light cytoplasm with few free ribosomes. They also expressed abundant intermediate filaments. Type B cells were divided into type B1 and B2. Type B1 cells were larger than type B2 cells; the chromatin of type B2 cells was clumped as opposed to dispersed in type B1 cells. Type B1 cells were adjacent to ependymal cells, separating neuroblasts from contacting the ependymal. Type B2 cells are mostly located at the interface with the striatal parenchyma. Tanycytes were infrequent and located between ependymal cells, contacted the ventricle, and contained microvilli on their luminal surface. Their nuclei were irregularly shaped and contained dark chromatin aggregates. The cytoplasm of these cells was electron-dense and rich in organelles containing many mitochondria and lysosomes. Interestingly, 10% of type B cells incorporated thymidine and corresponded to type B2 cells; none of the type B1 cells or tanycytes showed any thymidine incorporation, suggesting that they are good candidates for the quiescent SVZ stem cells. Because type B cells and tanycytes stain strongly for GFAP, studies on GFAP-expressing cells do not distinguish tanycytes from type B cells, and both are included in the term SVZ astrocytes. Collectively, type B1 astrocytes, i.e. SVZ astrocytes wedged between or along ependymal cells, may be the SVZ stem cells. It was also suggested in a review on stem cell niches that ependymal cells are the stem cell partners, analogous to the fly stem cell niche (Ohlstein et al., 2004). Using GFAP immunostaining or hGFAP-GFP mice, SVZ astrocytes resembling type B1 or tanycytes and B2 cells are visible based on their differential location in the lateral SVZ (Fig. 6A and B). However, GFAP-GFP cells can also be found at the border of the SVZ or RMS and send processes into the surrounding parenchyma. It is unclear whether these border astrocytes are part of the type B2 cells or a distinct type of B cells. Finally, very few cells expressing S100B have been identified in the SVZ (1-3 per section) (Platel et al., 2008). Surprisingly, S100B-positive cells do not co-express GFAP (Raponi et al., 2007), but a minority co-express NG2 (Platel et al., 2008). The NG2-negative S100B-positive cells are nevertheless thought to represent a different population of fully differentiated astrocytes (Raponi et al., 2007).
Figure 6. The SVZ astrocyte.
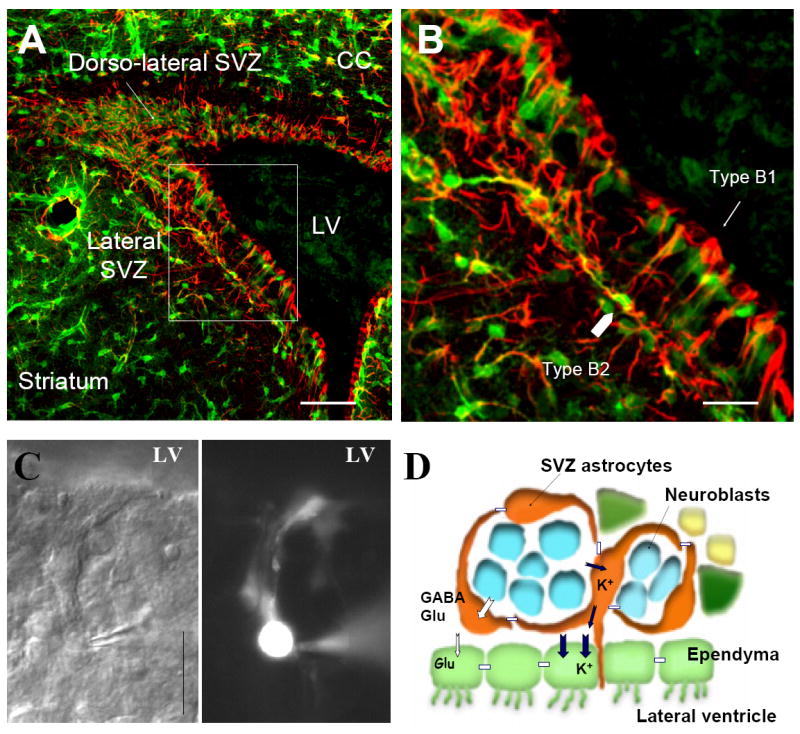
(A and B) Photographs of GFP fluorescence (green) with immunostaining for GFAP (red). The image in the white squares in (A) is shown at higher magnification in (B). Astrocytes in the lateral SVZ have there cell bodies either touching the ventricle or touching the striatum. (C) Photographs of a DIC image and corresponding image of a lucifer yellow-filled astrocyte. The recorded astrocyte has a process projecting toward the lateral ventricle and displays dye coupling. Scale bar: 20 μm. (D) Scheme illustrating the arrangements of the different cell types in the SVZ. SVZ astrocytes (orange) ensheath neuroblasts (blue) and transit amplifying progenitors (green, also called transit amplifiers) are scattered among SVZ cells. Ependymal cells line the lateral ventricle. SVZ astrocytes and ependymal cells display uptake mechanisms for K+, GABA and glutamate.
The search for a neural stem cell marker has been fierce, but unfortunately, an unambiguous marker has not been identified. This is mostly due to limitations in isolating cells and testing self-renewal, which have been based on flow cytometry and neurosphere assays, respectively. Neurospheres may essentially isolate fast cycling cells and thus analyze the behavior of transit amplifiers that retain self-renewal ability although more limited than stem cells. CD15, also called Lewis X antigen (Lex), has been used to isolate cells showing stem cell characteristics, and some of these cells co-express GFAP (Capela and Temple, 2002). Mcm2 has also been used to label SVZ stem cells (Maslov et al., 2004), but it also labels other cycling cells (Platel et al., 2008). It is likely that SVZ neural stem cells will be identified based on staining with several antigenic markers as described for hematopoietic stem cells (Bryder et al., 2006).
Collectively, there are at least two types of astrocytes, S100B-positive/GFAP-negative and GFAP-positive/S100-negative. The GFAP-expressing astrocytes of the SVZ may as well contain two populations although additional antigenic distinctions are required.
5.2. The SVZ astrocyte: neural stem cell, instructor for neurogenesis, or both
It is now well-accepted that SVZ stem cells are GFAP-expressing cells of the SVZ, called here SVZ astrocytes (Fig. 6). However, it remains unclear whether every SVZ astrocyte has the ability to behave as a stem cell or act as an instructor of neurogenesis.
SVZ astrocytes ensheathe migrating neuroblasts and are in close proximity to transit amplifiers (Fig. 6A, B and D). They are thus in a perfect location to control the development of these two cell types. Based on immunostaining data, every SVZ astrocyte expresses glutamate and GABA transporters and has the ability to buffer ambient GABA and glutamate levels (Bolteus and Bordey, 2004; Liu et al., 2006) (Fig. 6D). With the recent findings that GABA exerts a strong influence on both neuroblast and SVZ astrocyte production (Nguyen et al., 2003; Liu et al., 2005), neuroblast migration (Bolteus and Bordey, 2004) as well as neuroblast differentiation (Gascon et al., 2006) (for review see Bordey, 2007), every SVZ astrocyte may thus have the ability to control neurogenesis by regulating GABA levels. Similarly, neuroblasts express AMPA/kainate-type glutamate receptors whose activation leads to intracellular Ca2+ increases (Platel et al., 2007). Thus, regulation of extracellular glutamate levels by uptake into SVZ astrocytes may have considerable influence on neuroblast development. Furthermore, given the novel role of astrocytes in synaptic transmission and control of blow flow, it is appropriate to speculate that SVZ astrocytes have similar functions with regard to neuroblasts and blood vessels (for review see Bordey, 2006). It was shown that neural progenitors preferentially settle close to blood vessels in the SGZ (Palmer et al., 2000), and SVZ astrocytes closely ensheath blood vessels around the SVZ (Mercier et al., 2002). SVZ astrocytes may promote angiogenesis when proliferation is up-regulated or take up glucose necessary for glycolysis to provide metabolic support for proliferating progenitors and neuroblasts. SVZ astrocytes may also guide or stimulate directional neurite outgrowth of migrating neuroblasts as shown for neonatal astrocytes in vitro (Deumens et al., 2004). A highly controlled communication has also been reported from neuroblasts to SVZ astrocytes. For instance, neuroblasts in the SVZ can synthesize and release GABA via a nonsynaptic mechanism, and GABA in turn tonically activates GABAA receptors on the SVZ astrocytes, reducing their proliferation (Liu et al., 2005). This signaling provides a mechanism for the daughter cells to limit stem cell proliferation and thus daughter cell production. This communication is likely bidirectional as SVZ astrocytes, like mature astrocytes, contain glutamate that may signal to neuroblasts via glutamate receptor activation (Platel et al., 2007). In mature astrocytes, Ca2+ and perhaps Na+ dynamics are key to control astrocytes’ functions including glycolysis, neurite outgrowth, and release of glutamate or other diffusible molecules. However, it remains unclear whether SVZ astrocytes display coordinated Ca2+ increases or waves.
Finally, whether each SVZ astrocyte can behave as stem cell at a certain time point during their life is unclear. One possibility to address this question may be to use the recently generated Brainbow mice by Livet and colleagues (Livet et al., 2007). Fluorescent proteins have half-life of 1 to 4 days and persist in the first or second generation of daughter cells. Assuming that each SVZ astrocyte has different color, it may be possible to determine from which SVZ astrocytes lineage using color-coding.
5.3. Implications for parenchymal astrocytes
The presence of these neurogenic astrocytes challenges the traditional definition of astrocytes as described in section 2.4, which are viewed as mature, fully-differentiated cells. In addition, this raises some interesting questions regarding astrocytes: Can all astrocytes act as stem cells if placed in the right environment? Or do mature astrocytes undergo an irreversible genetic change which seals their fate as postmitotic cells? Can astrocytes in the adult brain be reactivated to regain stem cell features and help repair the damaged brain (see (Gotz and Steindler, 2003) for additional discussion).
These questions will likely lead to mixed answers. First, transplantation experiments have underscored the importance of the niche in regulating cell potential. For instance, cultured SGZ progenitors generate granule neurons when transplanted into another SGZ, but not to nonneurogenic brain regions (Gage et al., 1995). The instructive role of the niche is also demonstrated when SGZ progenitors grafted into the rostral migratory stream can adopt the fate of SVZ neural precursors to produce olfactory bulb interneurons (Suhonen et al., 1996). Based on this evidence, we can speculate that the radial glial-derived astrocytic stem cells need the unique components of the niche in order to retain their neurogenic potential. Second, it was recently shown that postnatal astrocytes from P5-7 mice can be reverted into a stem cell when reactivating the proper genetic program in vitro (Berninger et al., 2007). In particular, the proneural genes neurogenin-2 and Mash1 possess the ability to reprogram these young astrocytes to stem cells that can generate neurons. These astrocyte-derived neurons display neuronal excitability and also receive synaptic inputs when co-cultured with embryonic cortical neurons, although they fail to generate a functional presynaptic output within the culturing period. These findings are very exciting and raise hopes that reactive astrocytes that have been known to revert to an immature phenotype could be reprogrammed into neural stem cells and provide newborn neurons for repairing the damaged brain.
6. Conclusion
It is quite remarkable the wealth of information that has been discovered about astrocytes in the last century. The recent advances in our knowledge of astrocytes have raised more questions about their identity and functions. From the evidence discussed in this review, we can appreciate that the morphological and molecular heterogeneity, as well as the diverse origins of astrocytes rival the diversification of neuronal subtypes. However, we can still find a common set of characteristics to define an astrocyte. An astrocyte lacks axons and dendrites, does not spike, expresses GFAP, and has a highly branched and convoluted morphology leading to the ensheathment of neuronal elements (e.g. spines) and encapsulation of blood vessels. While all may be called under the umbrella term astrocytes, more specialized terminology should be applied with regional specification as in the case of SVZ astrocytes. Future studies aimed at better understanding the lineage of astrocytes and their repertoire of transcriptional regulation and molecular markers may be helpful in defining astrocytic populations and functions such as in the study by Cahoy and colleagues (2008).
In defining astrocytes, we should not overlook the well-accepted functions of astrocytes in providing homeostasis for neural networks. Astrocytes synthesize a plethora of extracellular matrix proteins, adhesion molecules, and trophic factors that regulate neuronal maturation and synapse formation. In addition, they form the BBB, regulate extracellular ionic buffering, control neurotransmitter uptake, and provide basic metabolic support for the brain. However, pioneering studies regarding the above trophic and homeostatic functions of astrocytes necessary for proper neural activity were performed in vitro. A major challenge has been to move these findings into an in vivo setting. Advances in molecular biology and virology for gene delivery now provide the means to address astrocytic functions in a physiological setting. Another challenge is mapping the diversity of the astrocyte-associated molecules that are expressed with temporal and spatial specificity. Additional proteomic and gene array analysis of astrocytes acutely isolated from the brain (e.g. with flow cytometry) will help identify new astrocytic molecules and their functions in maintaining normal neuronal functions.
The exciting newly discovered functions of astrocytes have catapulted interest in astrocyte research over the past few decades. The key discovery of calcium waves in astrocytes and the concept of gliotransmission (i.e. neurotransmitter release from astrocytes) brought these cells from the shadows and put them in the spotlight as active participants and modulators of neurotransmission. Intracellular calcium dynamics represent the mode of excitability in astrocytes and some of its in vivo functions on the vasculature and synaptic activity still remain to be elucidated. Another emerging field is the dynamic interaction between astrocytes and synapses. The surprisingly motile astrocytic processes surrounding synapses suggest that they may cause changes in synaptic activity. In addition, the discovery of the fast (millisecond to second time scale) communication between neuronal activity and astrocyte excitability strongly suggests that astrocytes play an important role in modulating synaptic transmission. As we understand more about the mechanisms for these emerging functions of astrocytes, we can eventually lay to rest the controversies surrounding the in vivo roles of astrocytes in neuronal and vascular crosstalk. With the advancements in high-resolution imaging and molecular tools, we may yet uncover new functions of astrocytes.
Perhaps one of the most exciting findings that has hit the astrocyte field is the discovery that astrocytes are the stem cells in the adult neurogenic zone. This finding fundamentally challenges the traditional definition of astrocytes. While some new antigenic markers and physiological properties have been used to define these astrocytic stem cells, we can better define this subclass of astrocytes as we learn about their molecular profile. As discussed above, the stem cells act in concert with resident astrocytes to maintain the neurogenic niche. Future studies will uncover the molecules and mechanisms that allow these specialized germinal zones to retain their regenerative and proliferative capacity in the adult brain. Another key question that has emerged is whether every astrocyte in the CNS has the ability to revert to stem cells given the right environment. Understanding the biology of these adult stem cells has enormous therapeutic potential in treating the diseased and injured brain.
In conclusion, we have just begun to uncover the true nature of astrocytes. With all the enthusiasm surrounding the astrocyte field, this star will undoubtedly create many new odysseys to come.
Acknowledgments
This work was supported by grants from the National Institute of Health (NS048256 and DC007681, A.B.). We are grateful to Dr. Anna Bolteus who designed the diagrams in Figures 1, 3-5.
Abbreviations
- AMPA
alpha-Amino-3-hydroxy-5-Methyl-4-isoxazolePropionic Acid
- ATP
Adenosine Triphosphate
- BBB
Blood Brain Barrier
- BDNF
Brain-Derived Neurotrophic Factor
- BLBP
Brain Lipid Basic Protein
- cKO
conditional Knock-Out
- CNS
Central Nervous System
- CNTF
Ciliary Neurotrophic Factor
- EAAT
Excitatory Amino Acid Transporter
- ECM
ExtraCellular Matrix
- EET
Epoxyeicosatrienoic acids
- FGF
Fibroblast Growth Factor
- GABA
γ Amino-Butyric Acid
- GAT
GABA Transporter
- GFAP
Glial Fibrillary Acidic Protein
- GFP
Green Fluorescent Protein
- GLAST
Glutamate-Aspartate Transporter
- GLUT1
Glucose Transporter 1
- GS
Glutamine Synthetase
- hGFAP
human GFAP
- IL-6
Interleukin-6
- Kir
Inwardly rectifying K+ channels
- LIF
Leukaemia Inhibitory Factor
- MCT1
Monocarboxylate Transporters 1
- MIP
Macrophage Inflammatory Protein
- MMP
Matrix Metalloproteinase
- NCAM
Neural Cell Adhesion Molecule
- NT-3
Neurotrophin-3
- NG2
Neuro-Glial proteoglycan
- NGF
Nerve Growth Factor
- NMDA
N-methyl-D-aspartic acid
- OPC
Oligodendrocyte Progenitor Cells
- P
Postnatal
- PDGF
Platelet-Derived Growth Factor
- SGZ
Subgranular zone
- SIC
Slow Inward Current
- SSeCKS
Src-Suppressed C-Kinase Substrate
- SVZ
Subventricular Zone
- TeNTLC
active Light-Chain of Tetanus Neurotoxin
- TGF-β
Transforming Growth Factor
- TIRFM
Total Internal Reflection Fluorescence Microscopy
- TNFα
Tumor Necrosis Factor-α
- VEGF
Vascular Endothelial Growth Factor
- VZ
Ventricular Zone
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott NJ. Inflammatory mediators and modulation of blood-brain barrier permeability. Cell Mol Neurobiol. 2000;20:131–147. doi: 10.1023/A:1007074420772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott NJ, Revest PA. Control of brain endothelial permeability. Cerebrovasc Brain Metab Rev. 1991;3:39–72. [PubMed] [Google Scholar]
- Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- Abbracchio MP, Verderio C. Pathophysiological roles of P2 receptors in glial cells. Novartis Found Symp. 2006;276:91–103. [PubMed] [Google Scholar]
- Aguirre A, Gallo V. Postnatal neurogenesis and gliogenesis in the olfactory bulb from NG2-expressing progenitors of the subventricular zone. J Neurosci. 2004;24:10530–10541. doi: 10.1523/JNEUROSCI.3572-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre AA, Chittajallu R, Belachew S, Gallo V. NG2-expressing cells in the subventricular zone are type C-like cells and contribute to interneuron generation in the postnatal hippocampus. J Cell Biol. 2004;165:575–589. doi: 10.1083/jcb.200311141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen NJ, Barres BA. Signaling between glia and neurons: focus on synaptic plasticity. Curr Opin Neurobiol. 2005;15:542–548. doi: 10.1016/j.conb.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Altman J. Autoradiographic and histological studies of postnatal neurogenesis. IV. Cell proliferation and migration in the anterior forebrain, with special reference to persisting neurogenesis in the olfactory bulb. J Comp Neurol. 1969;137:433–457. doi: 10.1002/cne.901370404. [DOI] [PubMed] [Google Scholar]
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- Anderson CM, Nedergaard M. Astrocyte-mediated control of cerebral microcirculation. Trends Neurosci. 2003;26:340–344. doi: 10.1016/S0166-2236(03)00141-3. [DOI] [PubMed] [Google Scholar]
- Anderson CM, Swanson RA. Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia. 2000;32:1–14. [PubMed] [Google Scholar]
- Apte MV, Haber PS, Applegate TL, Norton ID, McCaughan GW, Korsten MA, Pirola RC, Wilson JS. Periacinar stellate shaped cells in rat pancreas: identification, isolation, and culture. Gut. 1998;43:128–133. doi: 10.1136/gut.43.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araque A, Li N, Doyle RT, Haydon PG. SNARE protein-dependent glutamate release from astrocytes. J Neurosci. 2000;20:666–673. doi: 10.1523/JNEUROSCI.20-02-00666.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araque A, Sanzgiri RP, Parpura V, Haydon PG. Calcium elevation in astrocytes causes an NMDA receptor-dependent increase in the frequency of miniature synaptic currents in cultured hippocampal neurons. J Neurosci. 1998;18:6822–6829. doi: 10.1523/JNEUROSCI.18-17-06822.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschner M. Astrocyte metallothioneins (MTs) and their neuroprotective role. Ann N Y Acad Sci. 1997;825:334–347. doi: 10.1111/j.1749-6632.1997.tb48445.x. [DOI] [PubMed] [Google Scholar]
- Baba A. Role of endothelin B receptor signals in reactive astrocytes. Life Sci. 1998;62:1711–1715. doi: 10.1016/s0024-3205(98)00133-7. [DOI] [PubMed] [Google Scholar]
- Bachoo RM, Kim RS, Ligon KL, Maher EA, Brennan C, Billings N, Chan S, Li C, Rowitch DH, Wong WH, DePinho RA. Molecular diversity of astrocytes with implications for neurological disorders. Proc Natl Acad Sci U S A. 2004;101:8384–8389. doi: 10.1073/pnas.0402140101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bains JS, Oliet SH. Glia: they make your memories stick! Trends Neurosci. 2007;30:417–424. doi: 10.1016/j.tins.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis. 2004;16:1–13. doi: 10.1016/j.nbd.2003.12.016. [DOI] [PubMed] [Google Scholar]
- Ballanyi K, Grafe P, ten Bruggencate G. Ion activities and potassium uptake mechanisms of glial cells in guinea- pig olfactory cortex slices. J Physiol (Lond) 1987;382:159–174. doi: 10.1113/jphysiol.1987.sp016361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barakat L, Bordey A. GAT-1 and Reversible GABA Transport in Bergmann Glia in Slices. J Neurophysiol. 2002;88:1407–1419. doi: 10.1152/jn.2002.88.3.1407. [DOI] [PubMed] [Google Scholar]
- Baudier J, Glasser N, Gerard D. Ions binding to S100 proteins. I. Calcium- and zinc-binding properties of bovine brain S100 alpha alpha, S100a (alpha beta), and S100b (beta beta) protein: Zn2+ regulates Ca2+ binding on S100b protein. J Biol Chem. 1986;261:8192–8203. [PubMed] [Google Scholar]
- Bauer HC, Bauer H. Neural induction of the blood-brain barrier: still an enigma. Cell Mol Neurobiol. 2000;20:13–28. doi: 10.1023/A:1006939825857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beattie EC, Stellwagen D, Morishita W, Bresnahan JC, Ha BK, Von Zastrow M, Beattie MS, Malenka RC. Control of synaptic strength by glial TNFalpha. Science. 2002;295:2282–2285. doi: 10.1126/science.1067859. [DOI] [PubMed] [Google Scholar]
- Bekar LK, He W, Nedergaard M. Locus Coeruleus {alpha}-Adrenergic-Mediated Activation of Cortical Astrocytes In Vivo. Cereb Cortex. 2008 doi: 10.1093/cercor/bhn040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benarroch EE. Neuron-astrocyte interactions: partnership for normal function and disease in the central nervous system. Mayo Clin Proc. 2005;80:1326–1338. doi: 10.4065/80.10.1326. [DOI] [PubMed] [Google Scholar]
- Bergles DE, Dzubay JA, Jahr CE. Glutamate transporter currents in bergmann glial cells follow the time course of extrasynaptic glutamate. Proc Natl Acad Sci. 1997;94:14821–14825. doi: 10.1073/pnas.94.26.14821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergles DE, Jahr CE. Synaptic activation of glutamate transporters in hippocampal astrocytes. Neuron. 1997;19:1297–1308. doi: 10.1016/s0896-6273(00)80420-1. [DOI] [PubMed] [Google Scholar]
- Bergles DE, Jahr CE. Glial contribution to glutamate uptake at Schaffer collateral- commissural synapses in the hippocampus. J Neurosci. 1998;18:7709–7716. doi: 10.1523/JNEUROSCI.18-19-07709.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardinelli Y, Magistretti PJ, Chatton JY. Astrocytes generate Na+-mediated metabolic waves. Proc Natl Acad Sci U S A. 2004;101:14937–14942. doi: 10.1073/pnas.0405315101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berninger B, Costa MR, Koch U, Schroeder T, Sutor B, Grothe B, Gotz M. Functional properties of neurons derived from in vitro reprogrammed postnatal astroglia. J Neurosci. 2007;27:8654–8664. doi: 10.1523/JNEUROSCI.1615-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezzi P, Gundersen V, Galbete JL, Seifert G, Steinhauser C, Pilati E, Volterra A. Astrocytes contain a vesicular compartment that is competent for regulated exocytosis of glutamate. Nat Neurosci. 2004;7:613–620. doi: 10.1038/nn1246. [DOI] [PubMed] [Google Scholar]
- Bignami A, Eng LF, Dahl D, Uyeda CT. Localization of the glial fibrillary acidic protein in astrocytes by immunofluorescence. Brain Res. 1972;43:429–435. doi: 10.1016/0006-8993(72)90398-8. [DOI] [PubMed] [Google Scholar]
- Billups B, Rossi D, Oshima T, Warr O, Takahashi M, Sarantis M, Szatkowski M, Attwell D. Physiological and pathological operation of glutamate transporters. Prog Brain Res. 1998;116:45–57. doi: 10.1016/s0079-6123(08)60429-x. [DOI] [PubMed] [Google Scholar]
- Blondel O, Collin C, McCarran WJ, Zhu S, Zamostiano R, Gozes I, Brenneman DE, McKay RD. A glia-derived signal regulating neuronal differentiation. J Neurosci. 2000;20:8012–8020. doi: 10.1523/JNEUROSCI.20-21-08012.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolteus AJ, Bordey A. GABA Release and Uptake Regulate Neuronal Precursor Migration in the Postnatal Subventricular Zone. J Neurosci. 2004;24:7623–7631. doi: 10.1523/JNEUROSCI.1999-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borden LA. GABA transporter heterogeneity: pharmacology and cellular localization. Neurochem Int. 1996;29:335–356. doi: 10.1016/0197-0186(95)00158-1. [DOI] [PubMed] [Google Scholar]
- Bordey A. Adult Neurogenesis: Basic Concepts of Signaling. Cell Cycle. 2006;5:722–728. doi: 10.4161/cc.5.7.2614. [DOI] [PubMed] [Google Scholar]
- Bordey A. Enigmatic GABAergic networks in adult neurogenic zones. Brain Res Brain Res Rev. 2007;53:124–134. doi: 10.1016/j.brainresrev.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Bordey A, Sontheimer H. Postnatal development of ionic currents in rat hippocampal astrocytes in situ. J Neurophysiol. 1997;78:461–477. doi: 10.1152/jn.1997.78.1.461. [DOI] [PubMed] [Google Scholar]
- Bordey A, Sontheimer H. Ion channel expression by astrocytes in situ: comparison of different CNS regions. Glia. 2000;30:27–38. doi: 10.1002/(sici)1098-1136(200003)30:1<27::aid-glia4>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Bordey A, Sontheimer H. Modulation of glutamatergic transmission by bergmann glial cells in rat cerebellum in situ. J Neurophysiol. 2003;89:979–988. doi: 10.1152/jn.00904.2002. [DOI] [PubMed] [Google Scholar]
- Bouzier-Sore AK, Voisin P, Bouchaud V, Bezancon E, Franconi JM, Pellerin L. Competition between glucose and lactate as oxidative energy substrates in both neurons and astrocytes: a comparative NMR study. Eur J Neurosci. 2006;24:1687–1694. doi: 10.1111/j.1460-9568.2006.05056.x. [DOI] [PubMed] [Google Scholar]
- Bouzier-Sore AK, Voisin P, Canioni P, Magistretti PJ, Pellerin L. Lactate is a preferential oxidative energy substrate over glucose for neurons in culture. J Cereb Blood Flow Metab. 2003;23:1298–1306. doi: 10.1097/01.WCB.0000091761.61714.25. [DOI] [PubMed] [Google Scholar]
- Braun N, Sevigny J, Mishra SK, Robson SC, Barth SW, Gerstberger R, Hammer K, Zimmermann H. Expression of the ecto-ATPase NTPDase2 in the germinal zones of the developing and adult rat brain. Eur J Neurosci. 2003;17:1355–1364. doi: 10.1046/j.1460-9568.2003.02567.x. [DOI] [PubMed] [Google Scholar]
- Brecha NC, Weigmann C. Expression of GAT-1, a high-affinity gamma-aminobutyric acid plasma membrane transporter in the rat retina. J Comp Neurol. 1994;345:602–611. doi: 10.1002/cne.903450410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brightman MW, Reese TS. Junctions between intimately apposed cell membranes in the vertebrate brain. J Cell Biol. 1969;40:648–677. doi: 10.1083/jcb.40.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockhaus J, Deitmer JW. Long-lasting modulation of synaptic input to Purkinje neurons by Bergmann glia stimulation in rat brain slices. J Physiol. 2002;545:581–593. doi: 10.1113/jphysiol.2002.028423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AM, Ransom BR. Astrocyte glycogen and brain energy metabolism. Glia. 2007;55:1263–1271. doi: 10.1002/glia.20557. [DOI] [PubMed] [Google Scholar]
- Brown AM, Sickmann HM, Fosgerau K, Lund TM, Schousboe A, Waagepetersen HS, Ransom BR. Astrocyte glycogen metabolism is required for neural activity during aglycemia or intense stimulation in mouse white matter. J Neurosci Res. 2005;79:74–80. doi: 10.1002/jnr.20335. [DOI] [PubMed] [Google Scholar]
- Brown AM, Tekkok SB, Ransom BR. Glycogen regulation and functional role in mouse white matter. J Physiol. 2003;549:501–512. doi: 10.1113/jphysiol.2003.042416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryder D, Rossi DJ, Weissman IL. Hematopoietic stem cells: the paradigmatic tissue-specific stem cell. Am J Pathol. 2006;169:338–346. doi: 10.2353/ajpath.2006.060312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buniatian GH, Hartmann HJ, Traub P, Wiesinger H, Albinus M, Nagel W, Shoeman R, Mecke D, Weser U. Glial fibrillary acidic protein-positive cells of the kidney are capable of raising a protective biochemical barrier similar to astrocytes: expression of metallothionein in podocytes. Anat Rec. 2002;267:296–306. doi: 10.1002/ar.10115. [DOI] [PubMed] [Google Scholar]
- Bushong EA, Martone ME, Jones YZ, Ellisman MH. Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J Neurosci. 2002;22:183–192. doi: 10.1523/JNEUROSCI.22-01-00183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, Thompson WJ, Barres BA. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci. 2008;28:264–278. doi: 10.1523/JNEUROSCI.4178-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J, Chen Y, Cai WH, Hurlock EC, Wu H, Kernie SG, Parada LF, Lu QR. A crucial role for Olig2 in white matter astrocyte development. Development. 2007;134:1887–1899. doi: 10.1242/dev.02847. [DOI] [PubMed] [Google Scholar]
- Cameron HA, Woolley CS, McEwen BS, Gould E. Differentiation of newly born neurons and glia in the dentate gyrus of the adult rat. Neuroscience. 1993;56:337–344. doi: 10.1016/0306-4522(93)90335-d. [DOI] [PubMed] [Google Scholar]
- Cammer W. Glutamine synthetase in the central nervous system is not confined to astrocytes. J Neuroimmunol. 1990;26:173–178. doi: 10.1016/0165-5728(90)90088-5. [DOI] [PubMed] [Google Scholar]
- Campbell K, Gotz M. Radial glia: multi-purpose cells for vertebrate brain development. Trends Neurosci. 2002;25:235–238. doi: 10.1016/s0166-2236(02)02156-2. [DOI] [PubMed] [Google Scholar]
- Capela A, Temple S. LeX/ssea-1 is expressed by adult mouse CNS stem cells, identifying them as nonependymal. Neuron. 2002;35:865–875. doi: 10.1016/s0896-6273(02)00835-8. [DOI] [PubMed] [Google Scholar]
- Carmen J, Magnus T, Cassiani-Ingoni R, Sherman L, Rao MS, Mattson MP. Revisiting the astrocyte-oligodendrocyte relationship in the adult CNS. Prog Neurobiol. 2007;82:151–162. doi: 10.1016/j.pneurobio.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Cataldo AM, Broadwell RD. Cytochemical identification of cerebral glycogen and glucose-6-phosphatase activity under normal and experimental conditions. II. Choroid plexus and ependymal epithelia, endothelia and pericytes. J Neurocytol. 1986;15:511–524. doi: 10.1007/BF01611733. [DOI] [PubMed] [Google Scholar]
- Cerdan S, Rodrigues TB, Sierra A, Benito M, Fonseca LL, Fonseca CP, Garcia-Martin ML. The redox switch/redox coupling hypothesis. Neurochem Int. 2006;48:523–530. doi: 10.1016/j.neuint.2005.12.036. [DOI] [PubMed] [Google Scholar]
- Chaudhry FA, Lehre KP, van Lookeren Campagne M, Ottersen OP, Danbolt NC, Storm-Mathisen J. Glutamate transporters in glial plasma membranes: highly differentiated localizations revealed by quantitative ultrastructural immunocytochemistry. Neuron. 1995;15:711–720. doi: 10.1016/0896-6273(95)90158-2. [DOI] [PubMed] [Google Scholar]
- Chen X, Wang L, Zhou Y, Zheng LH, Zhou Z. “Kiss-and-run” glutamate secretion in cultured and freshly isolated rat hippocampal astrocytes. J Neurosci. 2005;25:9236–9243. doi: 10.1523/JNEUROSCI.1640-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chih CP, Roberts EL., Jr Energy substrates for neurons during neural activity: a critical review of the astrocyte-neuron lactate shuttle hypothesis. J Cereb Blood Flow Metab. 2003;23:1263–1281. doi: 10.1097/01.WCB.0000081369.51727.6F. [DOI] [PubMed] [Google Scholar]
- Chiu AY, Espinosa de los MA, Cole RA, Loera S, de Vellis J. Laminin and s-laminin are produced and released by astrocytes, Schwann cells, and schwannomas in culture. Glia. 1991;4:11–24. doi: 10.1002/glia.440040103. [DOI] [PubMed] [Google Scholar]
- Christopherson KS, Ullian EM, Stokes CC, Mullowney CE, Hell JW, Agah A, Lawler J, Mosher DF, Bornstein P, Barres BA. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell. 2005;120:421–433. doi: 10.1016/j.cell.2004.12.020. [DOI] [PubMed] [Google Scholar]
- Chung RS, Hidalgo J, West AK. New insight into the molecular pathways of metallothionein-mediated neuroprotection and regeneration. J Neurochem. 2008;104:14–20. doi: 10.1111/j.1471-4159.2007.05026.x. [DOI] [PubMed] [Google Scholar]
- Clark BA, Barbour B. Currents evoked in Bergmann glial cells by parallel fibre stimulation in rat cerebellar slices. J Physiol (Lond) 1997;502:335–350. doi: 10.1111/j.1469-7793.1997.335bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke SR, Shetty AK, Bradley JL, Turner DA. Reactive astrocytes express the embryonic intermediate neurofilament nestin. Neuroreport. 1994;5:1885–1888. doi: 10.1097/00001756-199410000-00011. [DOI] [PubMed] [Google Scholar]
- Conti F, Debiasi S, Minelli A, Rothstein JD, Melone M. EAAC1, a high-affinity glutamate transporter, is localized to astrocytes and Gabaergic neurons besides pyramidal cells in the rat cerebral cortex. Cerebral cortex. 1998;8:108–116. doi: 10.1093/cercor/8.2.108. [DOI] [PubMed] [Google Scholar]
- Conti F, Zuccarello LV, Barbaresi P, Minelli A, Brecha NC, Melone M. Neuronal, glial, and epithelial localization of gamma-aminobutyric acid transporter 2, a high-affinity gamma-aminobutyric acid plasma membrane transporter, in the cerebral cortex and neighboring structures. J Comp Neurol. 1999;409:482–494. [PubMed] [Google Scholar]
- Cornell-Bell A, Thomas PG, Smith SJ. The excitatory neurotransmitter glutamate causes filopodia formation in cultured hippocampal astrocytes. Glia. 1990a;3:322–334. doi: 10.1002/glia.440030503. [DOI] [PubMed] [Google Scholar]
- Cornell-Bell AH, Finkbeiner SM. Calcium waves in astrocytes. Cell Calcium. 1991;12:185–204. doi: 10.1016/0143-4160(91)90020-f. [DOI] [PubMed] [Google Scholar]
- Cornell-Bell AH, Finkbeiner SM, Cooper MS, Smith SJ. Glutamate induces calcium waves in cultured astrocytes: long-range glial signaling. Science. 1990b;247:470–473. doi: 10.1126/science.1967852. [DOI] [PubMed] [Google Scholar]
- Cruz F, Villalba M, Garcia-Espinosa MA, Ballesteros P, Bogonez E, Satrustegui J, Cerdan S. Intracellular compartmentation of pyruvate in primary cultures of cortical neurons as detected by (13)C NMR spectroscopy with multiple (13)C labels. J Neurosci Res. 2001;66:771–781. doi: 10.1002/jnr.10048. [DOI] [PubMed] [Google Scholar]
- D’Amelio F, Eng LF, Gibbs MA. Glutamine synthetase immunoreactivity is present in oligodendroglia of various regions of the central nervous system. Glia. 1990;3:335–341. doi: 10.1002/glia.440030504. [DOI] [PubMed] [Google Scholar]
- Dailey ME, Smith SJ. The dynamics of dendritic structure in developing hippocampal slices. J Neurosci. 1996;16:2983–2994. doi: 10.1523/JNEUROSCI.16-09-02983.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Danbolt NC, Chaudhry FA, Dehnes Y, Lehre KP, Levy LM, Ullensvang K, Storm-Mathisen J. Properties and localization of glutamate transporters. Prog Brain Res. 1998a;116:23–43. doi: 10.1016/s0079-6123(08)60428-8. [DOI] [PubMed] [Google Scholar]
- Danbolt NC, Lehre KP, Dehnes Y, Chaudhry FA, Levy LM. Localization of transporters using transporter-specific antibodies. Methods Enzymol. 1998b;296:388–407. doi: 10.1016/s0076-6879(98)96028-1. [DOI] [PubMed] [Google Scholar]
- Dani JW, Chernjavsky A, Smith SJ. Neuronal activity triggers calcium waves in hippocampal astrocyte networks. Neuron. 1992;8:429–440. doi: 10.1016/0896-6273(92)90271-e. [DOI] [PubMed] [Google Scholar]
- Danielyan L, Tolstonog G, Traub P, Salvetter J, Gleiter CH, Reisig D, Gebhardt R, Buniatian GH. Colocalization of Glial Fibrillary Acidic Protein, Metallothionein, and MHC II in Human, Rat, NOD/SCID, and Nude Mouse Skin Keratinocytes and Fibroblasts. J Invest Dermatol. 2007;127:555–563. doi: 10.1038/sj.jid.5700575. [DOI] [PubMed] [Google Scholar]
- Davidoff MS, Middendorff R, Kofuncu E, Muller D, Jezek D, Holstein AF. Leydig cells of the human testis possess astrocyte and oligodendrocyte marker molecules. Acta Histochem. 2002;104:39–49. doi: 10.1078/0065-1281-00630. [DOI] [PubMed] [Google Scholar]
- Davson H, Oldendorf WH. Symposium on membrane transport. Transport in the central nervous system. Proc R Soc Med. 1967;60:326–329. doi: 10.1177/003591576706000405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Curtis I. Functions of Rac GTPases during neuronal development. Dev Neurosci. 2008;30:47–58. doi: 10.1159/000109851. [DOI] [PubMed] [Google Scholar]
- Dehouck B, Dehouck MP, Fruchart JC, Cecchelli R. Upregulation of the low density lipoprotein receptor at the blood-brain barrier: intercommunications between brain capillary endothelial cells and astrocytes. J Cell Biol. 1994;126:465–473. doi: 10.1083/jcb.126.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Bigio MR. The ependyma: a protective barrier between brain and cerebrospinal fluid. Glia. 1995;14:1–13. doi: 10.1002/glia.440140102. [DOI] [PubMed] [Google Scholar]
- Deloulme JC, Raponi E, Gentil BJ, Bertacchi N, Marks A, Labourdette G, Baudier J. Nuclear expression of S100B in oligodendrocyte progenitor cells correlates with differentiation toward the oligodendroglial lineage and modulates oligodendrocytes maturation. Mol Cell Neurosci. 2004;27:453–465. doi: 10.1016/j.mcn.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Deumens R, Koopmans GC, Den Bakker CG, Maquet V, Blacher S, Honig WM, Jerome R, Pirard JP, Steinbusch HW, Joosten EA. Alignment of glial cells stimulates directional neurite growth of CNS neurons in vitro. Neuroscience. 2004;125:591–604. doi: 10.1016/j.neuroscience.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Djukic B, Casper KB, Philpot BD, Chin LS, McCarthy KD. Conditional knock-out of Kir4.1 leads to glial membrane depolarization, inhibition of potassium and glutamate uptake, and enhanced short-term synaptic potentiation. J Neurosci. 2007;27:11354–11365. doi: 10.1523/JNEUROSCI.0723-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci. 1997;17:5046–5061. doi: 10.1523/JNEUROSCI.17-13-05046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato R. S100: a multigenic family of calcium-modulated proteins of the EF-hand type with intracellular and extracellular functional roles. Int J Biochem Cell Biol. 2001;33:637–668. doi: 10.1016/s1357-2725(01)00046-2. [DOI] [PubMed] [Google Scholar]
- Dong X, Han S, Zylka MJ, Simon MI, Anderson DJ. A diverse family of GPCRs expressed in specific subsets of nociceptive sensory neurons. Cell. 2001;106:619–632. doi: 10.1016/s0092-8674(01)00483-4. [DOI] [PubMed] [Google Scholar]
- Dong Y, Benveniste EN. Immune function of astrocytes. Glia. 2001;36:180–190. doi: 10.1002/glia.1107. [DOI] [PubMed] [Google Scholar]
- Dupont G, Combettes L, Leybaert L. Calcium dynamics: spatio-temporal organization from the subcellular to the organ level. Int Rev Cytol. 2007;261:193–245. doi: 10.1016/S0074-7696(07)61005-5. [DOI] [PubMed] [Google Scholar]
- El Hafny B, Chappey O, Piciotti M, Debray M, Boval B, Roux F. Modulation of P-glycoprotein activity by glial factors and retinoic acid in an immortalized rat brain microvessel endothelial cell line. Neurosci Lett. 1997;236:107–111. doi: 10.1016/s0304-3940(97)00679-4. [DOI] [PubMed] [Google Scholar]
- Emsley JG, Arlotta P, Macklis JD. Star-cross’d neurons: astroglial effects on neural repair in the adult mammalian CNS. Trends Neurosci. 2004;27:238–240. doi: 10.1016/j.tins.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Endo T. Glycans and glycan-binding proteins in brain: galectin-1-induced expression of neurotrophic factors in astrocytes. Curr Drug Targets. 2005;6:427–436. doi: 10.2174/1389450054021909. [DOI] [PubMed] [Google Scholar]
- Eng LF. Glial fibrillary acidic protein (GFAP): the major protein of glial intermediate filaments in differentiated astrocytes. J Neuroimmunol. 1985;8:203–214. doi: 10.1016/s0165-5728(85)80063-1. [DOI] [PubMed] [Google Scholar]
- Eng LF, Vanderhaeghen JJ, Bignami A, Gerstl B. An acidic protein isolated from fibrous astrocytes. Brain Res. 1971;28:351–354. doi: 10.1016/0006-8993(71)90668-8. [DOI] [PubMed] [Google Scholar]
- Engelhardt B. Development of the blood-brain barrier. Cell Tissue Res. 2003;314:119–129. doi: 10.1007/s00441-003-0751-z. [DOI] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Estrada C, Bready JV, Berliner JA, Pardridge WM, Cancilla PA. Astrocyte growth stimulation by a soluble factor produced by cerebral endothelial cells in vitro. J Neuropathol Exp Neurol. 1990;49:539–549. doi: 10.1097/00005072-199011000-00001. [DOI] [PubMed] [Google Scholar]
- Evanko DS, Zhang Q, Zorec R, Haydon PG. Defining pathways of loss and secretion of chemical messengers from astrocytes. Glia. 2004;47:233–240. doi: 10.1002/glia.20050. [DOI] [PubMed] [Google Scholar]
- Farina C, Aloisi F, Meinl E. Astrocytes are active players in cerebral innate immunity. Trends Immunol. 2007;28:138–145. doi: 10.1016/j.it.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Fellin T, Pascual O, Gobbo S, Pozzan T, Haydon PG, Carmignoto G. Neuronal synchrony mediated by astrocytic glutamate through activation of extrasynaptic NMDA receptors. Neuron. 2004;43:729–743. doi: 10.1016/j.neuron.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Fernandez-Ruiz J, Romero J, Velasco G, Tolon RM, Ramos JA, Guzman M. Cannabinoid CB2 receptor: a new target for controlling neural cell survival? Trends Pharmacol Sci. 2007;28:39–45. doi: 10.1016/j.tips.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Fiacco TA, Agulhon C, Taves SR, Petravicz J, Casper KB, Dong X, Chen J, McCarthy KD. Selective stimulation of astrocyte calcium in situ does not affect neuronal excitatory synaptic activity. Neuron. 2007;54:611–626. doi: 10.1016/j.neuron.2007.04.032. [DOI] [PubMed] [Google Scholar]
- Fiacco TA, McCarthy KD. Intracellular astrocyte calcium waves in situ increase the frequency of spontaneous AMPA receptor currents in CA1 pyramidal neurons. J Neurosci. 2004;24:722–732. doi: 10.1523/JNEUROSCI.2859-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiacco TA, McCarthy KD. Astrocyte calcium elevations: properties, propagation, and effects on brain signaling. Glia. 2006;54:676–690. doi: 10.1002/glia.20396. [DOI] [PubMed] [Google Scholar]
- Filippov V, Kronenberg G, Pivneva T, Reuter K, Steiner B, Wang LP, Yamaguchi M, Kettenmann H, Kempermann G. Subpopulation of nestin-expressing progenitor cells in the adult murine hippocampus shows electrophysiological and morphological characteristics of astrocytes. Mol Cell Neurosci. 2003;23:373–382. doi: 10.1016/s1044-7431(03)00060-5. [DOI] [PubMed] [Google Scholar]
- Finkbeiner S. Calcium waves in astrocytes-filling in the gaps. Neuron. 1992;8:1101–1108. doi: 10.1016/0896-6273(92)90131-v. [DOI] [PubMed] [Google Scholar]
- Fishell G, Kriegstein AR. Neurons from radial glia: the consequences of asymmetric inheritance. Curr Opin Neurobiol. 2003;13:34–41. doi: 10.1016/s0959-4388(03)00013-8. [DOI] [PubMed] [Google Scholar]
- Fontana A, Fierz W, Wekerle H. Astrocytes present myelin basic protein to encephalitogenic T-cell lines. Nature. 1984;307:273–276. doi: 10.1038/307273a0. [DOI] [PubMed] [Google Scholar]
- Fraser DD, Mudrick-Donnon LA, MacVicar BA. Astrocytic GABA receptors. Glia. 1997;11:83–93. doi: 10.1002/glia.440110203. [DOI] [PubMed] [Google Scholar]
- Fruttiger M, Calver AR, Kruger WH, Mudhar HS, Michalovich D, Takakura N, Nishikawa S, Richardson WD. PDGF mediates a neuron-astrocyte interaction in the developing retina. Neuron. 1996;17:1117–1131. doi: 10.1016/s0896-6273(00)80244-5. [DOI] [PubMed] [Google Scholar]
- Furuta A, Rothstein JD, Martin LJ. Glutamate transporter protein subtypes are expressed differentially during rat CNS development. J Neurosci. 1997;17:8363–8375. doi: 10.1523/JNEUROSCI.17-21-08363.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta A, Wada E, Wada K. Function of glial G-protein coupled receptors. Brain Nerve. 2007;59:717–724. [PubMed] [Google Scholar]
- Gadea A, Lopez-Colome AM. Glial transporters for glutamate, glycine and GABA I. Glutamate transporters. J Neurosci Res. 2001;63:453–460. doi: 10.1002/jnr.1039. [DOI] [PubMed] [Google Scholar]
- Gage FH, Coates PW, Palmer TD, Kuhn HG, Fisher LJ, Suhonen JO, Peterson DA, Suhr ST, Ray J. Survival and differentiation of adult neuronal progenitor cells transplanted to the adult brain. Proc Natl Acad Sci U S A. 1995;92:11879–11883. doi: 10.1073/pnas.92.25.11879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganat YM, Silbereis J, Cave C, Ngu H, Anderson GM, Ohkubo Y, Ment LR, Vaccarino FM. Early postnatal astroglial cells produce multilineage precursors and neural stem cells in vivo. J Neurosci. 2006;26:8609–8621. doi: 10.1523/JNEUROSCI.2532-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gary KA, Chronwall BM. Regulation of GFAP expression in glial-like cells of the rat pituitary intermediate lobe by lactation, salt-loading, and adrenalectomy. Glia. 1995;13:272–282. doi: 10.1002/glia.440130404. [DOI] [PubMed] [Google Scholar]
- Gascon E, Dayer AG, Sauvain MO, Potter G, Jenny B, De Roo M, Zgraggen E, Demaurex N, Muller D, Kiss JZ. GABA regulates dendritic growth by stabilizing lamellipodia in newly generated interneurons of the olfactory bulb. J Neurosci. 2006;26:12956–12966. doi: 10.1523/JNEUROSCI.4508-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates MA, Thomas LB, Howard EM, Laywell ED, Sajin B, Faissner A, Gotz B, Silver J, Steindler DA. Cell and molecular analysis of the developing and adult mouse subventricular zone of the cerebral hemispheres. J Comp Neurol. 1995;361:249–266. doi: 10.1002/cne.903610205. [DOI] [PubMed] [Google Scholar]
- Gegelashvili G, Robinson MB, Trotti D, Rauen T. Regulation of glutamate transporters in health and disease. Prog Brain Res. 2001;132:267–286. doi: 10.1016/S0079-6123(01)32082-4. [DOI] [PubMed] [Google Scholar]
- Gegelashvili G, Schousboe A. High affinity glutamate transporters: regulation of expression and activity. Mol Pharmacol. 1997;52:6–15. doi: 10.1124/mol.52.1.6. [DOI] [PubMed] [Google Scholar]
- Geiger JR, Melcher T, Koh DS, Sakmann B, Seeburg PH, Jonas P, Monyer H. Relative abundance of subunit mRNAs determines gating and Ca2+ permeability of AMPA receptors in principal neurons and interneurons in rat CNS. Neuron. 1995;15:193–204. doi: 10.1016/0896-6273(95)90076-4. [DOI] [PubMed] [Google Scholar]
- Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D, Betsholtz C. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol. 2003;161:1163–1177. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerschenfeld HM, Wald F, Zadunaisky JA, De Robertis ED. Function of astroglia in the water-ion metabolism of the central nervous system: an electron microscope study. Neurology. 1959;9:412–425. doi: 10.1212/wnl.9.6.412. [DOI] [PubMed] [Google Scholar]
- Gjedde A, Marrett S. Glycolysis in neurons, not astrocytes, delays oxidative metabolism of human visual cortex during sustained checkerboard stimulation in vivo. J Cereb Blood Flow Metab. 2001;21:1384–1392. doi: 10.1097/00004647-200112000-00002. [DOI] [PubMed] [Google Scholar]
- Gnatenco C, Han J, Snyder AK, Kim D. Functional expression of TREK-2 K+ channel in cultured rat brain astrocytes. Brain Res. 2002;931:56–67. doi: 10.1016/s0006-8993(02)02261-8. [DOI] [PubMed] [Google Scholar]
- Goldman JE. Astrocyte lineage. In: Lazzarini RA, editor. Myelin Biology and disorders. Elsevier Academic Press; New York: 2007. pp. 311–328. [Google Scholar]
- Gonzalez ML, Malemud CJ, Silver J. Role of astroglial extracellular matrix in the formation of rat olfactory bulb glomeruli. Exp Neurol. 1993;123:91–105. doi: 10.1006/exnr.1993.1143. [DOI] [PubMed] [Google Scholar]
- Gordon GR, Baimoukhametova DV, Hewitt SA, Rajapaksha WR, Fisher TE, Bains JS. Norepinephrine triggers release of glial ATP to increase postsynaptic efficacy. Nat Neurosci. 2005;8:1078–1086. doi: 10.1038/nn1498. [DOI] [PubMed] [Google Scholar]
- Gordon GR, Mulligan SJ, MacVicar BA. Astrocyte control of the cerebrovasculature. Glia. 2007;55:1214–1221. doi: 10.1002/glia.20543. [DOI] [PubMed] [Google Scholar]
- Gotz M, Steindler D. To be glial or not-how glial are the precursors of neurons in development and adulthood? Glia. 2003;43:1–3. doi: 10.1002/glia.10251. [DOI] [PubMed] [Google Scholar]
- Grosche J, Matyash V, Moller T, Verkhratsky A, Reichenbach A, Kettenmann H. Microdomains for neuron-glia interaction: parallel fiber signaling to Bergmann glial cells. Nat Neurosci. 1999;2:139–143. doi: 10.1038/5692. [DOI] [PubMed] [Google Scholar]
- Haas B, Schipke CG, Peters O, Sohl G, Willecke K, Kettenmann H. Activity-dependent ATP-waves in the mouse neocortex are independent from astrocytic calcium waves. Cereb Cortex. 2006;16:237–246. doi: 10.1093/cercor/bhi101. [DOI] [PubMed] [Google Scholar]
- Haber M, Zhou L, Murai KK. Cooperative astrocyte and dendritic spine dynamics at hippocampal excitatory synapses. J Neurosci. 2006;26:8881–8891. doi: 10.1523/JNEUROSCI.1302-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachem S, Aguirre A, Vives V, Marks A, Gallo V, Legraverend C. Spatial and temporal expression of S100B in cells of oligodendrocyte lineage. Glia. 2005;51:81–97. doi: 10.1002/glia.20184. [DOI] [PubMed] [Google Scholar]
- Halassa MM, Fellin T, Takano H, Dong JH, Haydon PG. Synaptic islands defined by the territory of a single astrocyte. J Neurosci. 2007;27:6473–6477. doi: 10.1523/JNEUROSCI.1419-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hama K, Arii T, Katayama E, Marton M, Ellisman MH. Tri-dimensional morphometric analysis of astrocytic processes with high voltage electron microscopy of thick Golgi preparations. J Neurocytol. 2004;33:277–285. doi: 10.1023/B:NEUR.0000044189.08240.a2. [DOI] [PubMed] [Google Scholar]
- Han SK, Dong X, Hwang JI, Zylka MJ, Anderson DJ, Simon MI. Orphan G protein-coupled receptors MrgA1 and MrgC11 are distinctively activated by RF-amide-related peptides through the Galpha q/11 pathway. Proc Natl Acad Sci U S A. 2002;99:14740–14745. doi: 10.1073/pnas.192565799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haseloff RF, Blasig IE, Bauer HC, Bauer H. In search of the astrocytic factor(s) modulating blood-brain barrier functions in brain capillary endothelial cells in vitro. Cell Mol Neurobiol. 2005;25:25–39. doi: 10.1007/s10571-004-1375-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton GI. Pituicytes, glia and control of terminal secretion. J Exp Biol. 1988;139:67–79. doi: 10.1242/jeb.139.1.67. [DOI] [PubMed] [Google Scholar]
- Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- Haydon PG, Carmignoto G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol Rev. 2006;86:1009–1031. doi: 10.1152/physrev.00049.2005. [DOI] [PubMed] [Google Scholar]
- Hertz L. The astrocyte-neuron lactate shuttle: a challenge of a challenge. J Cereb Blood Flow Metab. 2004;24:1241–1248. doi: 10.1097/00004647-200411000-00008. [DOI] [PubMed] [Google Scholar]
- Hertz L, Zielke HR. Astrocytic control of glutamatergic activity: astrocytes as stars of the show. Trends Neurosci. 2004;27:735–743. doi: 10.1016/j.tins.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Higashi K, Fujita A, Inanobe A, Tanemoto M, Doi K, Kubo T, Kurachi Y. An inwardly rectifying K(+) channel, Kir4.1, expressed in astrocytes surrounds synapses and blood vessels in brain. Am J Physiol Cell Physiol. 2001;281:C922–C931. doi: 10.1152/ajpcell.2001.281.3.C922. [DOI] [PubMed] [Google Scholar]
- Hirase H, Qian L, Bartho P, Buzsaki G. Calcium dynamics of cortical astrocytic networks in vivo. PLoS Biol. 2004;2:E96. doi: 10.1371/journal.pbio.0020096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirrlinger J, Hulsmann S, Kirchhoff F. Astroglial processes show spontaneous motility at active synaptic terminals in situ. Eur J Neurosci. 2004;20:2235–2239. doi: 10.1111/j.1460-9568.2004.03689.x. [DOI] [PubMed] [Google Scholar]
- Hochstim C, Deneen B, Lukaszewicz A, Zhou Q, Anderson DJ. Identification of Positionally Distinct Astrocyte Subtypes whose Identities Are Specified by a Homeodomain Code. Cell. 2008;133:510–522. doi: 10.1016/j.cell.2008.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockfield S, McKay RD. Identification of major cell classes in the developing mammalian nervous system. J Neurosci. 1985;5:3310–3328. doi: 10.1523/JNEUROSCI.05-12-03310.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoheisel D, Nitz T, Franke H, Wegener J, Hakvoort A, Tilling T, Galla HJ. Hydrocortisone reinforces the blood-brain properties in a serum free cell culture system. Biochem Biophys Res Commun. 1998;247:312–315. [PubMed] [Google Scholar]
- Holthoff K, Witte OW. Directed spatial potassium redistribution in rat neocortex. Glia. 2000;29:288–292. doi: 10.1002/(sici)1098-1136(20000201)29:3<288::aid-glia10>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Hosli E, Hosli L. Receptors for neurotransmitters on astrocytes in the mammalian central nervous system. Prog Neurobiol. 1993;40:477–506. doi: 10.1016/0301-0082(93)90019-o. [DOI] [PubMed] [Google Scholar]
- Houades V, Rouach N, Ezan P, Kirchhoff F, Koulakoff A, Giaume C. Shapes of astrocyte networks in the juvenile brain. Neuron Glia Biol. 2006;2:3–14. doi: 10.1017/S1740925X06000081. [DOI] [PubMed] [Google Scholar]
- Hu Y, Wilson GS. A temporary local energy pool coupled to neuronal activity: fluctuations of extracellular lactate levels in rat brain monitored with rapid-response enzyme-based sensor. J Neurochem. 1997;69:1484–1490. doi: 10.1046/j.1471-4159.1997.69041484.x. [DOI] [PubMed] [Google Scholar]
- Huang H, Bordey A. Glial glutamate transporters limit spillover activation of presynaptic NMDA receptors and influence synaptic inhibition of Purkinje neurons. J Neurosci. 2004;24:5659–5669. doi: 10.1523/JNEUROSCI.1338-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YH, Sinha SR, Tanaka K, Rothstein JD, Bergles DE. Astrocyte glutamate transporters regulate metabotropic glutamate receptor-mediated excitation of hippocampal interneurons. J Neurosci. 2004;24:4551–4559. doi: 10.1523/JNEUROSCI.5217-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter KE, Hatten ME. Radial glial cell transformation to astrocytes is bidirectional: regulation by a diffusible factor in embryonic forebrain. Proc Natl Acad Sci U S A. 1995;92:2061–2065. doi: 10.1073/pnas.92.6.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyder F, Patel AB, Gjedde A, Rothman DL, Behar KL, Shulman RG. Neuronal-glial glucose oxidation and glutamatergic-GABAergic function. J Cereb Blood Flow Metab. 2006;26:865–877. doi: 10.1038/sj.jcbfm.9600263. [DOI] [PubMed] [Google Scholar]
- Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat Rev Neurosci. 2004;5:347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nat Neurosci. 2007;10:1369–1376. doi: 10.1038/nn2003. [DOI] [PubMed] [Google Scholar]
- Igarashi Y, Utsumi H, Chiba H, Yamada-Sasamori Y, Tobioka H, Kamimura Y, Furuuchi K, Kokai Y, Nakagawa T, Mori M, Sawada N. Glial cell line-derived neurotrophic factor induces barrier function of endothelial cells forming the blood-brain barrier. Biochem Biophys Res Commun. 1999;261:108–112. doi: 10.1006/bbrc.1999.0992. [DOI] [PubMed] [Google Scholar]
- Iino M, Goto K, Kakegawa W, Okado H, Sudo M, Ishiuchi S, Miwa A, Takayasu Y, Saito I, Tsuzuki K, Ozawa S. Glia-synapse interaction through Ca2+-permeable AMPA receptors in Bergmann glia. Science. 2001;292:926–929. doi: 10.1126/science.1058827. [DOI] [PubMed] [Google Scholar]
- Itoh Y, Esaki T, Shimoji K, Cook M, Law MJ, Kaufman E, Sokoloff L. Dichloroacetate effects on glucose and lactate oxidation by neurons and astroglia in vitro and on glucose utilization by brain in vivo. Proc Natl Acad Sci U S A. 2003;100:4879–4884. doi: 10.1073/pnas.0831078100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabs R, Matthias K, Grote A, Grauer M, Seifert G, Steinhauser C. Lack of P2X receptor mediated currents in astrocytes and GluR type glial cells of the hippocampal CA1 region. Glia. 2007;55:1648–1655. doi: 10.1002/glia.20580. [DOI] [PubMed] [Google Scholar]
- Jaiswal JK, Fix M, Takano T, Nedergaard M, Simon SM. Resolving vesicle fusion from lysis to monitor calcium-triggered lysosomal exocytosis in astrocytes. Proc Natl Acad Sci U S A. 2007;104:14151–14156. doi: 10.1073/pnas.0704935104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janigro D, Gasparini S, D’Ambrosio R, McKhann G, II, DiFrancesco D. Reduction of K+ uptake in glia prevents long-term depression maintenance and causes epileptiform activity. J Neurosci. 1997;17:2813–2824. doi: 10.1523/JNEUROSCI.17-08-02813.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang B, Bezhadian MA, Caldwell RB. Astrocytes modulate retinal vasculogenesis: effects on endothelial cell differentiation. Glia. 1995;15:1–10. doi: 10.1002/glia.440150102. [DOI] [PubMed] [Google Scholar]
- Jourdain P, Bergersen LH, Bhaukaurally K, Bezzi P, Santello M, Domercq M, Matute C, Tonello F, Gundersen V, Volterra A. Glutamate exocytosis from astrocytes controls synaptic strength. Nat Neurosci. 2007;10:331–339. doi: 10.1038/nn1849. [DOI] [PubMed] [Google Scholar]
- Jursky F, Tamura S, Tamura A, Mandiyan S, Nelson H, Nelson N. Structure, function and brain localization of neurotransmitter transporters. J Exp Biol. 1994;196:283–295. doi: 10.1242/jeb.196.1.283. [DOI] [PubMed] [Google Scholar]
- Kacem K, Lacombe P, Seylaz J, Bonvento G. Structural organization of the perivascular astrocyte endfeet and their relationship with the endothelial glucose transporter: a confocal microscopy study. Glia. 1998;23:1–10. [PubMed] [Google Scholar]
- Kahn MA, Huang CJ, Caruso A, Barresi V, Nazarian R, Condorelli DF, de Vellis J. Ciliary neurotrophic factor activates JAK/Stat signal transduction cascade and induces transcriptional expression of glial fibrillary acidic protein in glial cells. J Neurochem. 1997;68:1413–1423. doi: 10.1046/j.1471-4159.1997.68041413.x. [DOI] [PubMed] [Google Scholar]
- Kanemaru K, Okubo Y, Hirose K, Iino M. Regulation of neurite growth by spontaneous Ca2+ oscillations in astrocytes. J Neurosci. 2007;27:8957–8966. doi: 10.1523/JNEUROSCI.2276-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J, Jiang L, Goldman SA, Nedergaard M. Astrocyte-mediated potentiation of inhibitory synaptic transmission. Nat Neurosci. 1998;1:683–692. doi: 10.1038/3684. [DOI] [PubMed] [Google Scholar]
- Kaplan MS, Hinds JW. Neurogenesis in the adult rat: electron microscopic analysis of light radioautographs. Science. 1977;197:1092–1094. doi: 10.1126/science.887941. [DOI] [PubMed] [Google Scholar]
- Karwoski CJ, Lu HK, Newman EA. Spatial buffering of light-evoked potassium increases by retinal Muller (glial) cells. Science. 1989;244:578–580. doi: 10.1126/science.2785716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasantikul V, Shuangshoti S. Positivity to glial fibrillary acidic protein in bone, cartilage, and chordoma. J Surg Oncol. 1989;41:22–26. doi: 10.1002/jso.2930410109. [DOI] [PubMed] [Google Scholar]
- Kimelberg HK. Receptors on astrocytes-what possible functions? Neurochem Int. 1995;26:27–40. doi: 10.1016/0197-0186(94)00118-e. [DOI] [PubMed] [Google Scholar]
- Kimelberg HK. The problem of astrocyte identity. Neurochem Int. 2004;45:191–202. doi: 10.1016/j.neuint.2003.08.015. [DOI] [PubMed] [Google Scholar]
- Kimelberg HK. Supportive or information-processing functions of the mature protoplasmic astrocyte in the mammalian CNS? A critical appraisal. Neuron Glia Biol. 2007;3:181–189. doi: 10.1017/S1740925X08000094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney GA. GAT-3 transporters regulate inhibition in the neocortex. J Neurophysiol. 2005;94:4533–4537. doi: 10.1152/jn.00420.2005. [DOI] [PubMed] [Google Scholar]
- Kinney GA, Spain WJ. Synaptically evoked GABA transporter currents in neocortical glia. J Neurophysiol. 2002;88:2899–2908. doi: 10.1152/jn.00037.2002. [DOI] [PubMed] [Google Scholar]
- Kong J, Shepel PN, Holden CP, Mackiewicz M, Pack AI, Geiger JD. Brain glycogen decreases with increased periods of wakefulness: implications for homeostatic drive to sleep. J Neurosci. 2002;22:5581–5587. doi: 10.1523/JNEUROSCI.22-13-05581.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuffler SW. Neuroglial cells: physiological properties and a potassium mediated effect of neuronal activity on the glial membrane potential. Proc R Soc Lond B Biol Sci. 1967;168:1–21. doi: 10.1098/rspb.1967.0047. [DOI] [PubMed] [Google Scholar]
- Kuffler SW, Nicholls JG. The physiology of neuroglial cells. Ergeb Physiol. 1966;57:1–90. [PubMed] [Google Scholar]
- Lalo U, Pankratov Y, Kirchhoff F, North RA, Verkhratsky A. NMDA receptors mediate neuron-to-glia signaling in mouse cortical astrocytes. J Neurosci. 2006;26:2673–2683. doi: 10.1523/JNEUROSCI.4689-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laterra J, Goldstein GW. Astroglial-induced in vitro angiogenesis: requirements for RNA and protein synthesis. J Neurochem. 1991;57:1231–1239. doi: 10.1111/j.1471-4159.1991.tb08284.x. [DOI] [PubMed] [Google Scholar]
- Laterra J, Guerin C, Goldstein GW. Astrocytes induce neural microvascular endothelial cells to form capillary-like structures in vitro. J Cell Physiol. 1990;144:204–215. doi: 10.1002/jcp.1041440205. [DOI] [PubMed] [Google Scholar]
- Lee CJ, Mannaioni G, Yuan H, Woo DH, Gingrich MB, Traynelis SF. Astrocytic control of synaptic NMDA receptors. J Physiol. 2007;581:1057–1081. doi: 10.1113/jphysiol.2007.130377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SW, Kim WJ, Choi YK, Song HS, Son MJ, Gelman IH, Kim YJ, Kim KW. SSeCKS regulates angiogenesis and tight junction formation in blood-brain barrier. Nat Med. 2003;9:900–906. doi: 10.1038/nm889. [DOI] [PubMed] [Google Scholar]
- Lee Y, Messing A, Su M, Brenner M. GFAP promoter elements required for region-specific and astrocyte-specific expression. Glia. 2008;56:481–493. doi: 10.1002/glia.20622. [DOI] [PubMed] [Google Scholar]
- Lehre KP, Levy LM, Ottersen OP, Storm-Mathisen J, Danbolt NC. Differential expression of two glial glutamate transporters in the rat brain: quantitative and immunocytochemical observations. J Neurosci. 1995;15:1835–1853. doi: 10.1523/JNEUROSCI.15-03-01835.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine JM, Card JP. Light and electron microscopic localization of a cell surface antigen (NG2) in the rat cerebellum: association with smooth protoplasmic astrocytes. J Neurosci. 1987;7:2711–2720. doi: 10.1523/JNEUROSCI.07-09-02711.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levison SW, Chuang C, Abramson BJ, Goldman JE. The migrational patterns and developmental fates of glial precursors in the rat subventricular zone are temporally regulated. Development. 1993;119:611–622. doi: 10.1242/dev.119.3.611. [DOI] [PubMed] [Google Scholar]
- Levison SW, Goldman JE. Both oligodendrocytes and astrocytes develop from progenitors in the subventricular zone of postnatal rat forebrain. Neuron. 1993;10:201–212. doi: 10.1016/0896-6273(93)90311-e. [DOI] [PubMed] [Google Scholar]
- Levison SW, Goldman JE. Multipotential and lineage restricted precursors coexist in the mammalian perinatal subventricular zone. J Neurosci Res. 1997;48:83–94. [PubMed] [Google Scholar]
- Liesi P. Laminin-immunoreactive glia distinguish regenerative adult CNS systems from non-regenerative ones. EMBO J. 1985;4:2505–2511. doi: 10.1002/j.1460-2075.1985.tb03963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesi P, Dahl D, Vaheri A. Laminin is produced by early rat astrocytes in primary culture. J Cell Biol. 1983;96:920–924. doi: 10.1083/jcb.96.3.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesi P, Kirkwood T, Vaheri A. Fibronectin is expressed by astrocytes cultured from embryonic and early postnatal rat brain. Exp Cell Res. 1986;163:175–185. doi: 10.1016/0014-4827(86)90570-7. [DOI] [PubMed] [Google Scholar]
- Liesi P, Silver J. Is astrocyte laminin involved in axon guidance in the mammalian CNS? Dev Biol. 1988;130:774–785. doi: 10.1016/0012-1606(88)90366-1. [DOI] [PubMed] [Google Scholar]
- Linden DJ. Long-term potentiation of glial synaptic currents in cerebellar culture. Neuron. 1997;18:983–994. doi: 10.1016/s0896-6273(00)80337-2. [DOI] [PubMed] [Google Scholar]
- Linden DJ. Synaptically evoked glutamate transport currents may be used to detect the expression of long-term potentiation in cerebellar culture. J Neurophysiol. 1998;79:3151–3156. doi: 10.1152/jn.1998.79.6.3151. [DOI] [PubMed] [Google Scholar]
- Liu B, Neufeld AH. Activation of epidermal growth factor receptors in astrocytes: from development to neural injury. J Neurosci Res. 2007a;85:3523–3529. doi: 10.1002/jnr.21364. [DOI] [PubMed] [Google Scholar]
- Liu B, Neufeld AH. Activation of epidermal growth factor receptors in astrocytes: from development to neural injury. J Neurosci Res. 2007b;85:3523–3529. doi: 10.1002/jnr.21364. [DOI] [PubMed] [Google Scholar]
- Liu QS, Xu Q, Arcuino G, Kang J, Nedergaard M. Astrocyte-mediated activation of neuronal kainate receptors. Proc Natl Acad Sci U S A. 2004;101:3172–3177. doi: 10.1073/pnas.0306731101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Bolteus AJ, Balkin DM, Henschel O, Bordey A. GFAP-expressing cells in the postnatal subventricular zone display a unique glial phenotype intermediate between radial glia and astrocytes. Glia. 2006;54:394–410. doi: 10.1002/glia.20392. [DOI] [PubMed] [Google Scholar]
- Liu X, Wang Q, Haydar TF, Bordey A. Nonsynaptic GABA signaling in postnatal subventricular zone controls proliferation of GFAP-expressing progenitors. Nat Neurosci. 2005;8:1179–1187. doi: 10.1038/nn1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Rao MS. Glial progenitors in the CNS and possible lineage relationships among them. Biol Cell. 2004;96:279–290. doi: 10.1016/j.biolcel.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wu Y, Lee JC, Xue H, Pevny LH, Kaprielian Z, Rao MS. Oligodendrocyte and astrocyte development in rodents: an in situ and immunohistological analysis during embryonic development. Glia. 2002;40:25–43. doi: 10.1002/glia.10111. [DOI] [PubMed] [Google Scholar]
- Livet J, Weissman TA, Kang H, Draft RW, Lu J, Bennis RA, Sanes JR, Lichtman JW. Transgenic strategies for combinatorial expression of fluorescent proteins in the nervous system. Nature. 2007;450:56–62. doi: 10.1038/nature06293. [DOI] [PubMed] [Google Scholar]
- Lledo PM, Alonso M, Grubb MS. Adult neurogenesis and functional plasticity in neuronal circuits. Nat Rev Neurosci. 2006;7:179–193. doi: 10.1038/nrn1867. [DOI] [PubMed] [Google Scholar]
- Lois C, Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science. 1994;264:1145–1148. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- Luscher C, Malenka RC, Nicoll RA. Monitoring glutamate release during LTP with glial transporter currents. Neuron. 1998;21:435–441. doi: 10.1016/s0896-6273(00)80552-8. [DOI] [PubMed] [Google Scholar]
- Luskin MB. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron. 1993;11:173–189. doi: 10.1016/0896-6273(93)90281-u. [DOI] [PubMed] [Google Scholar]
- Macfarlane SN, Sontheimer H. Changes in ion channel expression accompany cell cycle progression of spinal cord astrocytes. Glia. 2000;30:39–48. doi: 10.1002/(sici)1098-1136(200003)30:1<39::aid-glia5>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Magistretti PJ, Sorg O, Yu N, Martin JL, Pellerin L. Neurotransmitters regulate energy metabolism in astrocytes: implications for the metabolic trafficking between neural cells. Dev Neurosci. 1993;15:306–312. doi: 10.1159/000111349. [DOI] [PubMed] [Google Scholar]
- Mahesh VB, Dhandapani KM, Brann DW. Role of astrocytes in reproduction and neuroprotection. Mol Cell Endocrinol. 2006;246:1–9. doi: 10.1016/j.mce.2005.11.017. [DOI] [PubMed] [Google Scholar]
- Malatesta P, Hack MA, Hartfuss E, Kettenmann H, Klinkert W, Kirchhoff F, Gotz M. Neuronal or glial progeny: regional differences in radial glia fate. Neuron. 2003;37:751–764. doi: 10.1016/s0896-6273(03)00116-8. [DOI] [PubMed] [Google Scholar]
- Malatesta P, Hartfuss E, Gotz M. Isolation of radial glial cells by fluorescent-activated cell sorting reveals a neuronal lineage. Development. 2000;127:5253–5263. doi: 10.1242/dev.127.24.5253. [DOI] [PubMed] [Google Scholar]
- Marcaggi P, Billups D, Attwell D. The role of glial glutamate transporters in maintaining the independent operation of juvenile mouse cerebellar parallel fibre synapses. J Physiol. 2003;552:89–107. doi: 10.1113/jphysiol.2003.044263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Hernandez A, Bell KP, Norenberg MD. Glutamine synthetase: glial localization in brain. Science. 1977;195:1356–1358. doi: 10.1126/science.14400. [DOI] [PubMed] [Google Scholar]
- Maslov AY, Barone TA, Plunkett RJ, Pruitt SC. Neural stem cell detection, characterization, and age-related changes in the subventricular zone of mice. J Neurosci. 2004;24:1726–1733. doi: 10.1523/JNEUROSCI.4608-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui K, Jahr CE. Ectopic release of synaptic vesicles. Neuron. 2003;40:1173–1183. doi: 10.1016/s0896-6273(03)00788-8. [DOI] [PubMed] [Google Scholar]
- Matsui K, Jahr CE, Rubio ME. High-concentration rapid transients of glutamate mediate neural-glial communication via ectopic release. J Neurosci. 2005;25:7538–7547. doi: 10.1523/JNEUROSCI.1927-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthias K, Kirchhoff F, Seifert G, Huttmann K, Matyash M, Kettenmann H, Steinhauser C. Segregated expression of AMPA-type glutamate receptors and glutamate transporters defines distinct astrocyte populations in the mouse hippocampus. J Neurosci. 2003;23:1750–1758. doi: 10.1523/JNEUROSCI.23-05-01750.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthiessen HP, Schmalenbach C, Muller HW. Astroglia-released neurite growth-inducing activity for embryonic hippocampal neurons is associated with laminin bound in a sulfated complex and free fibronectin. Glia. 1989;2:177–188. doi: 10.1002/glia.440020307. [DOI] [PubMed] [Google Scholar]
- Mauch DH, Nagler K, Schumacher S, Goritz C, Muller EC, Otto A, Pfrieger FW. CNS synaptogenesis promoted by glia-derived cholesterol. Science. 2001;294:1354–1357. doi: 10.1126/science.294.5545.1354. [DOI] [PubMed] [Google Scholar]
- Mennerick S, Benz A, Zorumski CF. Components of glial responses to exogeneous and synaptic glutamate in rat hippocampal microcultures. J Neurosci. 1996;16:55–64. doi: 10.1523/JNEUROSCI.16-01-00055.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennerick S, Shen W, Xu W, Benz A, Tanaka K, Shimamoto K, Isenberg KE, Krause JE, Zorumski CF. Substrate turnover by transporters curtails synaptic glutamate transients. J Neurosci. 1999;19:9242–9251. doi: 10.1523/JNEUROSCI.19-21-09242.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennerick S, Zorumski CF. Glial contributions to excitatory neurotransmission in cultured hippocampal cells. Nature. 1994;368:59–62. doi: 10.1038/368059a0. [DOI] [PubMed] [Google Scholar]
- Mercier F, Kitasako JT, Hatton GI. Anatomy of the brain neurogenic zones revisited: fractones and the fibroblast/macrophage network. J Comp Neurol. 2002;451:170–188. doi: 10.1002/cne.10342. [DOI] [PubMed] [Google Scholar]
- Merkle FT, Tramontin AD, Garcia-Verdugo JM, Alvarez-Buylla A. Radial glia give rise to adult neural stem cells in the subventricular zone. Proc Natl Acad Sci U S A. 2004;101:17528–17532. doi: 10.1073/pnas.0407893101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metea MR, Newman EA. Glial cells dilate and constrict blood vessels: a mechanism of neurovascular coupling. J Neurosci. 2006;26:2862–2870. doi: 10.1523/JNEUROSCI.4048-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metea MR, Kofuji P, Newman EA. Neurovascular coupling is not mediated by potassium siphoning from glial cells. J Neurosci. 2007;27:2468–2471. doi: 10.1523/JNEUROSCI.3204-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi H, Haeberle H, Barres BA. Induction of astrocyte differentiation by endothelial cells. J Neurosci. 2001;21:1538–1547. doi: 10.1523/JNEUROSCI.21-05-01538.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RH, Raff MC. Fibrous and protoplasmic astrocytes are biochemically and developmentally distinct. J Neurosci. 1984;4:585–592. doi: 10.1523/JNEUROSCI.04-02-00585.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minelli A, Barbaresi P, Reimer RJ, Edwards RH, Conti F. The glial glutamate transporter GLT-1 is localized both in the vicinity of and at distance from axon terminals in the rat cerebral cortex. Neuroscience. 2001;108:51–59. doi: 10.1016/s0306-4522(01)00375-x. [DOI] [PubMed] [Google Scholar]
- Momiyama A, Silver RA, Hausser M, Notomi T, Wu Y, Shigemoto R, Cull-Candy SG. The density of AMPA receptors activated by a transmitter quantum at the climbing fibre-Purkinje cell synapse in immature rats. J Physiol. 2003;549:75–92. doi: 10.1113/jphysiol.2002.033472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mong JA, Blutstein T. Estradiol modulation of astrocytic form and function: implications for hormonal control of synaptic communication. Neuroscience. 2006;138:967–975. doi: 10.1016/j.neuroscience.2005.10.017. [DOI] [PubMed] [Google Scholar]
- Mongin AA, Kimelberg HK. ATP regulates anion channel-mediated organic osmolyte release from cultured rat astrocytes via multiple Ca2+-sensitive mechanisms. Am J Physiol Cell Physiol. 2005;288:C204–C213. doi: 10.1152/ajpcell.00330.2004. [DOI] [PubMed] [Google Scholar]
- Montgomery DL. Astrocytes : forms, functions, and roles in disease. Vet Pathol. 1994;31:145–167. doi: 10.1177/030098589403100201. [DOI] [PubMed] [Google Scholar]
- Moore CI, Cao R. The Hemo-Neural Hypothesis: On The Role of Blood Flow in Information Processing. J Neurophysiol. 2008;99:2035–2047. doi: 10.1152/jn.01366.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morara S, Brecha NC, Marcotti W, Provini L, Rosina A. Neuronal and glial localization of the GABA transporter GAT-1 in the cerebellar cortex. Neuroreport. 1996;7:2993–2996. doi: 10.1097/00001756-199611250-00039. [DOI] [PubMed] [Google Scholar]
- Morgello S, Uson RR, Schwartz EJ, Haber RS. The human blood-brain barrier glucose transporter (GLUT1 is a glucose transporter of gray matter astrocytes. Glia. 1995;14:43–54. doi: 10.1002/glia.440140107. [DOI] [PubMed] [Google Scholar]
- Mosso A. Sulla circolazione del cervello dell’uomo. Atti R Accad Lincei. 1880;5:237–358. [Google Scholar]
- Muir EM, Adcock KH, Morgenstern DA, Clayton R, von Stillfried N, Rhodes K, Ellis C, Fawcett JW, Rogers JH. Matrix metalloproteases and their inhibitors are produced by overlapping populations of activated astrocytes. Brain Res Mol Brain Res. 2002;100:103–117. doi: 10.1016/s0169-328x(02)00132-8. [DOI] [PubMed] [Google Scholar]
- Muller T, Moller T, Neuhaus J, Kettenmann H. Electrical coupling among Bergmann glial cells and its modulation by glutamate receptor activation. Glia. 1996;17:274–284. doi: 10.1002/(SICI)1098-1136(199608)17:4<274::AID-GLIA2>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Mulligan SJ, MacVicar BA. Calcium transients in astrocyte endfeet cause cerebrovascular constrictions. Nature. 2004;431:195–199. doi: 10.1038/nature02827. [DOI] [PubMed] [Google Scholar]
- Murai KK, Nguyen LN, Irie F, Yamaguchi Y, Pasquale EB. Control of hippocampal dendritic spine morphology through ephrin-A3/EphA4 signaling. Nat Neurosci. 2003;6:153–160. doi: 10.1038/nn994. [DOI] [PubMed] [Google Scholar]
- Mustafa AK, Kim PM, Snyder SH. D-Serine as a putative glial neurotransmitter. Neuron Glia Biol. 2004;1:275–281. doi: 10.1017/S1740925X05000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neary JT, Baker L, Jorgensen SL, Norenberg MD. Extracellular ATP induces stellation and increases glial fibrillary acidic protein content and DNA synthesis in primary astrocyte cultures. Acta Neuropathol. 1994;87:8–13. doi: 10.1007/BF00386249. [DOI] [PubMed] [Google Scholar]
- Neary JT, Kang Y, Shi YF. Signaling from nucleotide receptors to protein kinase cascades in astrocytes. Neurochem Res. 2004;29:2037–2042. doi: 10.1007/s11064-004-6876-y. [DOI] [PubMed] [Google Scholar]
- Nedergaard M. Direct signaling from astrocytes to neurons in cultures of mammalian brain cells. Science. 1994;263:1768–1771. doi: 10.1126/science.8134839. [DOI] [PubMed] [Google Scholar]
- Nedergaard M, Ransom B, Goldman SA. New roles for astrocytes: redefining the functional architecture of the brain. Trends Neurosci. 2003;26:523–530. doi: 10.1016/j.tins.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Nedergaard M, Takano T, Hansen AJ. Beyond the role of glutamate as a neurotransmitter. Nat Rev Neurosci. 2002;3:748–755. doi: 10.1038/nrn916. [DOI] [PubMed] [Google Scholar]
- Neugebauer KM, Tomaselli KJ, Lilien J, Reichardt LF. N-cadherin, NCAM, and integrins promote retinal neurite outgrowth on astrocytes in vitro. J Cell Biol. 1988;107:1177–1187. doi: 10.1083/jcb.107.3.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman EA. High potassium conductance in astrocyte endfeet. Science. 1986;233:453–454. doi: 10.1126/science.3726539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman EA. Glial cell inhibition of neurons by release of ATP. J Neurosci. 2003a;23:1659–1666. doi: 10.1523/JNEUROSCI.23-05-01659.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman EA. New roles for astrocytes: regulation of synaptic transmission. Trends Neurosci. 2003b;26:536–542. doi: 10.1016/S0166-2236(03)00237-6. [DOI] [PubMed] [Google Scholar]
- Newman EA, Reichenbach A. The Muller cell: a functional element of the retina. Trends Neurosci. 1996;19:307–312. doi: 10.1016/0166-2236(96)10040-0. [DOI] [PubMed] [Google Scholar]
- Newman EA, Zahs KR. Modulation of neuronal activity by glial cells in the retina. J Neurosci. 1998;18:4022–4028. doi: 10.1523/JNEUROSCI.18-11-04022.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen L, Malgrange B, Breuskin I, Bettendorff L, Moonen G, Belachew S, Rigo JM. Autocrine/paracrine activation of the GABA(A) receptor inhibits the proliferation of neurogenic polysialylated neural cell adhesion molecule-positive (PSA-NCAM+) precursor cells from postnatal striatum. J Neurosci. 2003;23:3278–3294. doi: 10.1523/JNEUROSCI.23-08-03278.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen S, Nagelhus EA, Amiry-Moghaddam M, Bourque C, Agre P, Ottersen OP. Specialized membrane domains for water transport in glial cells: high-resolution immunogold cytochemistry of aquaporin-4 in rat brain. J Neurosci. 1997;17:171–180. doi: 10.1523/JNEUROSCI.17-01-00171.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida H, Okabe S. Direct astrocytic contacts regulate local maturation of dendritic spines. J Neurosci. 2007;27:331–340. doi: 10.1523/JNEUROSCI.4466-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama A, Chang A, Trapp BD. NG2+ glial cells: a novel glial cell population in the adult brain. J Neuropathol Exp Neurol. 1999;58:1113–1124. doi: 10.1097/00005072-199911000-00001. [DOI] [PubMed] [Google Scholar]
- Noctor SC, Flint AC, Weissman TA, Dammerman RS, Kriegstein AR. Neurons derived from radial glial cells establish radial units in neocortex. Nature. 2001;409:714–720. doi: 10.1038/35055553. [DOI] [PubMed] [Google Scholar]
- Nolte C, Matyash M, Pivneva T, Schipke CG, Ohlemeyer C, Hanisch UK, Kirchhoff F, Kettenmann H. GFAP promoter-controlled EGFP-expressing transgenic mice: a tool to visualize astrocytes and astrogliosis in living brain tissue. Glia. 2001;33:72–86. [PubMed] [Google Scholar]
- Ohlstein B, Kai T, Decotto E, Spradling A. The stem cell niche: theme and variations. Curr Opin Cell Biol. 2004;16:693–699. doi: 10.1016/j.ceb.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Ojeda SR, Ma YJ, Lee BJ, Prevot V. Glia-to-neuron signaling and the neuroendocrine control of female puberty. Recent Prog Horm Res. 2000;55:197–223. [PubMed] [Google Scholar]
- Oliet SH, Panatier A, Piet R. Functional neuronal-glial anatomical remodelling in the hypothalamus. Novartis Found Symp. 2006;276:238–248. [PubMed] [Google Scholar]
- Oliet SH, Piet R. Anatomical remodelling of the supraoptic nucleus: changes in synaptic and extrasynaptic transmission. J Neuroendocrinol. 2004;16:303–307. doi: 10.1111/j.0953-8194.2004.01159.x. [DOI] [PubMed] [Google Scholar]
- Oliet SH, Piet R, Poulain DA. Control of glutamate clearance and synaptic efficacy by glial coverage of neurons. Science. 2001b;292:923–926. doi: 10.1126/science.1059162. [DOI] [PubMed] [Google Scholar]
- Oliet SH, Piet R, Poulain DA. Control of glutamate clearance and synaptic efficacy by glial coverage of neurons. Science. 2001a;292:923–926. doi: 10.1126/science.1059162. [DOI] [PubMed] [Google Scholar]
- Orkand RK, Nicholls JG, Kuffler SW. Effect of nerve impulses on the membrane potential of glial cells in the central nervous system of amphibia. J Neurophysiol. 1966;29:788–806. doi: 10.1152/jn.1966.29.4.788. [DOI] [PubMed] [Google Scholar]
- Owens T. Toll-like receptors on astrocytes: patterning for immunity. J Neuroimmunol. 2005;159:1–2. doi: 10.1016/j.jneuroim.2004.10.015. [DOI] [PubMed] [Google Scholar]
- Owens T, Babcock AA, Millward JM, Toft-Hansen H. Cytokine and chemokine inter-regulation in the inflamed or injured CNS. Brain Res Brain Res Rev. 2005;48:178–184. doi: 10.1016/j.brainresrev.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Paemeleire K, Leybaert L. ATP-dependent astrocyte-endothelial calcium signaling following mechanical damage to a single astrocyte in astrocyte-endothelial co-cultures. J Neurotrauma. 2000a;17:345–358. doi: 10.1089/neu.2000.17.345. [DOI] [PubMed] [Google Scholar]
- Paemeleire K, Leybaert L. Ionic changes accompanying astrocytic intercellular calcium waves triggered by mechanical cell damaging stimulation. Brain Res. 2000b;857:235–245. doi: 10.1016/s0006-8993(99)02436-1. [DOI] [PubMed] [Google Scholar]
- Palacin M, Estevez R, Bertran J, Zorzano A. Molecular biology of mammalian plasma membrane amino acid transporters. Physiol Rev. 1998;78:969–1054. doi: 10.1152/physrev.1998.78.4.969. [DOI] [PubMed] [Google Scholar]
- Palay SL, Chan-Palay V. Cerebellar Cortex, cytology and Organization. Springer-Verlag; New York: 1974. p. 236. [Google Scholar]
- Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425:479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Panatier A, Theodosis DT, Mothet JP, Touquet B, Pollegioni L, Poulain DA, Oliet SH. Glia-derived D-serine controls NMDA receptor activity and synaptic memory. Cell. 2006;125:775–784. doi: 10.1016/j.cell.2006.02.051. [DOI] [PubMed] [Google Scholar]
- Pardridge WM. Blood-brain barrier biology and methodology. J Neurovirol. 1999;5:556–569. doi: 10.3109/13550289909021285. [DOI] [PubMed] [Google Scholar]
- Parpura V, Basarsky TA, Liu F, Jeftinija K, Jeftinija S, Haydon PG. Glutamate-mediated astrocyte-neuron signalling. Nature. 1994;369:744–747. doi: 10.1038/369744a0. [DOI] [PubMed] [Google Scholar]
- Parri HR, Crunelli V. Astrocytes, spontaneity, and the developing thalamus. J Physiol Paris. 2002;96:221–230. doi: 10.1016/s0928-4257(02)00009-8. [DOI] [PubMed] [Google Scholar]
- Parri HR, Gould TM, Crunelli V. Spontaneous astrocytic Ca2+ oscillations in situ drive NMDAR-mediated neuronal excitation. Nat Neurosci. 2001;4:803–812. doi: 10.1038/90507. [DOI] [PubMed] [Google Scholar]
- Parri R, Crunelli V. Astrocytes target presynaptic NMDA receptors to give synapses a boost. Nat Neurosci. 2007;10:271–273. doi: 10.1038/nn0307-271. [DOI] [PubMed] [Google Scholar]
- Pascual O, Casper KB, Kubera C, Zhang J, Revilla-Sanchez R, Sul JY, Takano H, Moss SJ, McCarthy K, Haydon PG. Astrocytic purinergic signaling coordinates synaptic networks. Science. 2005;310:113–116. doi: 10.1126/science.1116916. [DOI] [PubMed] [Google Scholar]
- Pasti L, Volterra A, Pozzan T, Carmignoto G. Intracellular calcium oscillations in astrocytes: a highly plastic, bidirectional form of communication between neurons and astrocytes in situ. J Neurosci. 1997;17:7817–7830. doi: 10.1523/JNEUROSCI.17-20-07817.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pehar M, Vargas MR, Cassina P, Barbeito AG, Beckman JS, Barbeito L. Complexity of astrocyte-motor neuron interactions in amyotrophic lateral sclerosis. Neurodegener Dis. 2005;2:139–146. doi: 10.1159/000089619. [DOI] [PubMed] [Google Scholar]
- Pellegri G, Rossier C, Magistretti PJ, Martin JL. Cloning, localization and induction of mouse brain glycogen synthase. Brain Res Mol Brain Res. 1996;38:191–199. doi: 10.1016/0169-328x(95)00305-c. [DOI] [PubMed] [Google Scholar]
- Pellerin L. Lactate as a pivotal element in neuron-glia metabolic cooperation. Neurochem Int. 2003;43:331–338. doi: 10.1016/s0197-0186(03)00020-2. [DOI] [PubMed] [Google Scholar]
- Pellerin L, Bouzier-Sore AK, Aubert A, Serres S, Merle M, Costalat R, Magistretti PJ. Activity-dependent regulation of energy metabolism by astrocytes: an update. Glia. 2007;55:1251–1262. doi: 10.1002/glia.20528. [DOI] [PubMed] [Google Scholar]
- Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci U S A. 1994;91:10625–10629. doi: 10.1073/pnas.91.22.10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peppiatt CM, Howarth C, Mobbs P, Attwell D. Bidirectional control of CNS capillary diameter by pericytes. Nature. 2006;443:700–704. doi: 10.1038/nature05193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perea G, Araque A. Properties of synaptically evoked astrocyte calcium signal reveal synaptic information processing by astrocytes. J Neurosci. 2005;25:2192–2203. doi: 10.1523/JNEUROSCI.3965-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peretto P, Merighi A, Fasolo A, Bonfanti L. The subependymal layer in rodents: a site of structural plasticity and cell migration in the adult mammalian brain. Brain Res Bull. 1999;49:221–243. doi: 10.1016/s0361-9230(99)00037-4. [DOI] [PubMed] [Google Scholar]
- Persidsky Y, Ramirez SH, Haorah J, Kanmogne GD. Blood-brain barrier: structural components and function under physiologic and pathologic conditions. J Neuroimmune Pharmacol. 2006;1:223–236. doi: 10.1007/s11481-006-9025-3. [DOI] [PubMed] [Google Scholar]
- Pfeiffer-Guglielmi B, Fleckenstein B, Jung G, Hamprecht B. Immunocytochemical localization of glycogen phosphorylase isozymes in rat nervous tissues by using isozyme-specific antibodies. J Neurochem. 2003;85:73–81. doi: 10.1046/j.1471-4159.2003.01644.x. [DOI] [PubMed] [Google Scholar]
- Pfrieger FW, Barres BA. Synaptic efficacy enhanced by glial cells in vitro. Science. 1997;277:1684–1687. doi: 10.1126/science.277.5332.1684. [DOI] [PubMed] [Google Scholar]
- Piet R, Vargova L, Sykova E, Poulain DA, Oliet SH. Physiological contribution of the astrocytic environment of neurons to intersynaptic crosstalk. Proc Natl Acad Sci U S A. 2004;101:2151–2155. doi: 10.1073/pnas.0308408100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platel JC, Gordon V, Heintz T, Bordey A. GFAP-GFP neural progenitors are antigenically homogeneous and anchored in their enclosed mosaic niche. 2008 doi: 10.1002/glia.20735. Submitted. [DOI] [PubMed] [Google Scholar]
- Platel JC, Lacar B, Bordey A. GABA and glutamate signaling: homeostatic control of adult forebrain neurogenesis. J Mol Histol. 2007;38:602–610. doi: 10.1007/s10735-007-9153-y. [DOI] [PubMed] [Google Scholar]
- Poopalasundaram S, Knott C, Shamotienko OG, Foran PG, Dolly JO, Ghiani CA, Gallo V, Wilkin GP. Glial heterogeneity in expression of the inwardly rectifying K(+) channel, Kir4.1, in adult rat CNS. Glia. 2000;30:362–372. doi: 10.1002/(sici)1098-1136(200006)30:4<362::aid-glia50>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Porras OH, Loaiza A, Barros LF. Glutamate mediates acute glucose transport inhibition in hippocampal neurons. J Neurosci. 2004;24:9669–9673. doi: 10.1523/JNEUROSCI.1882-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porras OH, Ruminot I, Loaiza A, Barros LF. Na(+)-Ca(2+) cosignaling in the stimulation of the glucose transporter GLUT1 in cultured astrocytes. Glia. 2008;56:59–68. doi: 10.1002/glia.20589. [DOI] [PubMed] [Google Scholar]
- Porter JT, McCarthy KD. Hippocampal astrocytes in situ respond to glutamate released from synaptic terminals. J Neurosci. 1996;16:5073–5081. doi: 10.1523/JNEUROSCI.16-16-05073.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter JT, McCarthy KD. Astrocytic neurotransmitter receptors in situ and in vivo. Progr Neurobiol. 1997;51:439–455. doi: 10.1016/s0301-0082(96)00068-8. [DOI] [PubMed] [Google Scholar]
- Portera-Cailliau C, Pan DT, Yuste R. Activity-regulated dynamic behavior of early dendritic protrusions: evidence for different types of dendritic filopodia. J Neurosci. 2003;23:7129–7142. doi: 10.1523/JNEUROSCI.23-18-07129.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price J, Hynes RO. Astrocytes in culture synthesize and secrete a variant form of fibronectin. J Neurosci. 1985;5:2205–2211. doi: 10.1523/JNEUROSCI.05-08-02205.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Privat A, Rataboul P. Fibrous and protoplasmic astrocytes. In: Federoff S, Vernadakis A, editors. Astrocytes Development, morphology, and regional specialization of astrocytes. Academic PRess; Orlando, Fl: 2007. pp. 105–129. [Google Scholar]
- Provis JM, Leech J, Diaz CM, Penfold PL, Stone J, Keshet E. Development of the human retinal vasculature: cellular relations and VEGF expression. Exp Eye Res. 1997;65:555–568. doi: 10.1006/exer.1997.0365. [DOI] [PubMed] [Google Scholar]
- Raichle ME, Mintun MA. Brain work and brain imaging. Annu Rev Neurosci. 2006;29:449–476. doi: 10.1146/annurev.neuro.29.051605.112819. [DOI] [PubMed] [Google Scholar]
- Rajan P, McKay RD. Multiple routes to astrocytic differentiation in the CNS. J Neurosci. 1998;18:3620–3629. doi: 10.1523/JNEUROSCI.18-10-03620.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. Elusive radial glial cells: historical and evolutionary perspective. Glia. 2003;43:19–32. doi: 10.1002/glia.10244. [DOI] [PubMed] [Google Scholar]
- Raponi E, Agenes F, Delphin C, Assard N, Baudier J, Legraverend C, Deloulme JC. S100B expression defines a state in which GFAP-expressing cells lose their neural stem cell potential and acquire a more mature developmental stage. Glia. 2007;55:165–177. doi: 10.1002/glia.20445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan MR, Huang YH, Kim YS, Dykes-Hoberg MI, Jin L, Watkins AM, Bergles DE, Rothstein JD. Variations in promoter activity reveal a differential expression and physiology of glutamate transporters by glia in the developing and mature CNS. J Neurosci. 2007;27:6607–6619. doi: 10.1523/JNEUROSCI.0790-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel A, Begley DJ, Abbott NJ. An overview of in vitro techniques for blood-brain barrier studies. Methods Mol Med. 2003;89:307–324. doi: 10.1385/1-59259-419-0:307. [DOI] [PubMed] [Google Scholar]
- Reichenbach A, Robinson SR. Ependymoglia and ependymoglia-like cells. In: Kettenmann H, Ransom BR, editors. Neuroglia. Oxford University Press; New York: 1995. pp. 58–84. [Google Scholar]
- Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- Richards LJ, Kilpatrick TJ, Bartlett PF. De novo generation of neuronal cells from the adult mouse brain. Proc Natl Acad Sci U S A. 1992;89:8591–8595. doi: 10.1073/pnas.89.18.8591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rist RJ, Romero IA, Chan MW, Couraud PO, Roux F, Abbott NJ. F-actin cytoskeleton and sucrose permeability of immortalised rat brain microvascular endothelial cell monolayers: effects of cyclic AMP and astrocytic factors. Brain Res. 1997;768:10–18. doi: 10.1016/s0006-8993(97)00586-6. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Jin L, Kuncl RW, Kanai Y, Hediger MA, Wang Y, Schielke JP, Welty DF. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16:675–686. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Martin L, Levey AI, Dykes-Hoberg M, Jin L, Wu D, Nash N, Kuncl RW. Localization of neuronal and glial glutamate transporters. Neuron. 1994;13:713–725. doi: 10.1016/0896-6273(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Rubin LL, Hall DE, Porter S, Barbu K, Cannon C, Horner HC, Janatpour M, Liaw CW, Manning K, Morales J. A cell culture model of the blood-brain barrier. J Cell Biol. 1991;115:1725–1735. doi: 10.1083/jcb.115.6.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudge JS, Alderson RF, Pasnikowski E, McClain J, Ip NY, Lindsay RM. Expression of Ciliary Neurotrophic Factor and the Neurotrophins-Nerve Growth Factor, Brain-Derived Neurotrophic Factor and Neurotrophin 3-in Cultured Rat Hippocampal Astrocytes. Eur J Neurosci. 1992;4:459–471. doi: 10.1111/j.1460-9568.1992.tb00896.x. [DOI] [PubMed] [Google Scholar]
- Salm AK, McCarthy KD. The evidence for astrocytes as a target for central noradrenergic activity: expression of adrenergic receptors. Brain Res Bull. 1992;29:265–275. doi: 10.1016/0361-9230(92)90056-4. [DOI] [PubMed] [Google Scholar]
- Sanai N, Tramontin AD, Quinones-Hinojosa A, Barbaro NM, Gupta N, Kunwar S, Lawton MT, McDermott MW, Parsa AT, Manuel-Garcia VJ, Berger MS, Alvarez-Buylla A. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature. 2004;427:740–744. doi: 10.1038/nature02301. [DOI] [PubMed] [Google Scholar]
- Sauvageot CM, Stiles CD. Molecular mechanisms controlling cortical gliogenesis. Curr Opin Neurobiol. 2002;12:244–249. doi: 10.1016/s0959-4388(02)00322-7. [DOI] [PubMed] [Google Scholar]
- Scemes E, Giaume C. Astrocyte calcium waves: what they are and what they do. Glia. 2006;54:716–725. doi: 10.1002/glia.20374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schipke CG, Boucsein C, Ohlemeyer C, Kirchhoff F, Kettenmann H. Astrocyte Ca2+ waves trigger responses in microglial cells in brain slices. FASEB J. 2002;16:255–257. doi: 10.1096/fj.01-0514fje. [DOI] [PubMed] [Google Scholar]
- Schipke CG, Kettenmann H. Astrocyte responses to neuronal activity. Glia. 2004;47:226–232. doi: 10.1002/glia.20029. [DOI] [PubMed] [Google Scholar]
- Schmechel DE, Rakic P. A Golgi study of radial glial cells in developing monkey telencephalon: morphogenesis and transformation into astrocytes. Anat Embryol (Berl) 1979;156:115–152. doi: 10.1007/BF00300010. [DOI] [PubMed] [Google Scholar]
- Schmid RS, Yokota Y, Anton ES. Generation and characterization of brain lipid-binding protein promoter-based transgenic mouse models for the study of radial glia. Glia. 2006;53:345–351. doi: 10.1002/glia.20274. [DOI] [PubMed] [Google Scholar]
- Schmitt A, Asan E, Puschel B, Kugler P. Cellular and regional distribution of the glutamate transporter GLAST in the CNS of rats: nonradioactive in situ hybridization and comparative immunocytochemistry. J Neurosci. 1997;17:1–10. doi: 10.1523/JNEUROSCI.17-01-00001.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schools GP, Zhou M, Kimelberg HK. Development of gap junctions in hippocampal astrocytes: evidence that whole cell electrophysiological phenotype is an intrinsic property of the individual cell. J Neurophysiol. 2006;96:1383–1392. doi: 10.1152/jn.00449.2006. [DOI] [PubMed] [Google Scholar]
- Schousboe A. Role of astrocytes in the maintenance and modulation of glutamatergic and GABAergic neurotransmission. Neurochem Res. 2003;28:347–352. doi: 10.1023/a:1022397704922. [DOI] [PubMed] [Google Scholar]
- Schousboe A, Svenneby G, Hertz L. Uptake and metabolism of glutamate in astrocytes cultured from dissociated mouse brain hemispheres. J Neurochem. 1977;29:999–1005. doi: 10.1111/j.1471-4159.1977.tb06503.x. [DOI] [PubMed] [Google Scholar]
- Schousboe A, Westergaard N. Transport of neuroactive amino acids in astrocytes. In: Kettenmann H, Ransom BR, editors. Neuroglia. Oxford University; New York: 1995. pp. 246–258. [Google Scholar]
- Schousboe A, Westergaard N, Sonnewald U, Petersen SB, Yu AC, Hertz L. Regulatory role of astrocytes for neuronal biosynthesis and homeostasis of glutamate and GABA. Prog Brain Res. 1992;94:199–211. doi: 10.1016/s0079-6123(08)61751-3. [DOI] [PubMed] [Google Scholar]
- Schroeter ML, Mertsch K, Giese H, Muller S, Sporbert A, Hickel B, Blasig IE. Astrocytes enhance radical defence in capillary endothelial cells constituting the blood-brain barrier. FEBS Lett. 1999;449:241–244. doi: 10.1016/s0014-5793(99)00451-2. [DOI] [PubMed] [Google Scholar]
- Schummers J, Yu H, Sur M. Tuned responses of astrocytes and their influence on hemodynamic signals in the visual cortex. Science. 2008;320:1638–1643. doi: 10.1126/science.1156120. [DOI] [PubMed] [Google Scholar]
- Seil FJ, Eckenstein FP, Reier PJ. Induction of dendritic spine proliferation by an astrocyte secreted factor. Exp Neurol. 1992;117:85–89. doi: 10.1016/0014-4886(92)90114-6. [DOI] [PubMed] [Google Scholar]
- Seri B, Garcia-Verdugo JM, Collado-Morente L, McEwen BS, Alvarez-Buylla A. Cell types, lineage, and architecture of the germinal zone in the adult dentate gyrus. J Comp Neurol. 2004;478:359–378. doi: 10.1002/cne.20288. [DOI] [PubMed] [Google Scholar]
- Seri B, Garcia-Verdugo JM, McEwen BS, Alvarez-Buylla A. Astrocytes give rise to new neurons in the adult mammalian hippocampus. J Neurosci. 2001;21:7153–7160. doi: 10.1523/JNEUROSCI.21-18-07153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serres S, Bezancon E, Franconi JM, Merle M. Ex vivo analysis of lactate and glucose metabolism in the rat brain under different states of depressed activity. J Biol Chem. 2004;279:47881–47889. doi: 10.1074/jbc.M409429200. [DOI] [PubMed] [Google Scholar]
- Serres S, Bezancon E, Franconi JM, Merle M. Ex vivo NMR study of lactate metabolism in rat brain under various depressed states. J Neurosci Res. 2005;79:19–25. doi: 10.1002/jnr.20277. [DOI] [PubMed] [Google Scholar]
- Serres S, Bouyer JJ, Bezancon E, Canioni P, Merle M. Involvement of brain lactate in neuronal metabolism. NMR Biomed. 2003;16:430–439. doi: 10.1002/nbm.838. [DOI] [PubMed] [Google Scholar]
- Shapiro SD. Matrix metalloproteinase degradation of extracellular matrix: biological consequences. Curr Opin Cell Biol. 1998;10:602–608. doi: 10.1016/s0955-0674(98)80035-5. [DOI] [PubMed] [Google Scholar]
- Shea TB, Beermann ML, Nixon RA. Sequential effects of astroglial-derived factors on neurite outgrowth: initiation by protease inhibitors and potentiation by extracellular matrix components. J Neurosci Res. 1992;31:309–317. doi: 10.1002/jnr.490310212. [DOI] [PubMed] [Google Scholar]
- Smith GM, Rutishauser U, Silver J, Miller RH. Maturation of astrocytes in vitro alters the extent and molecular basis of neurite outgrowth. Dev Biol. 1990;138:377–390. doi: 10.1016/0012-1606(90)90204-v. [DOI] [PubMed] [Google Scholar]
- Smith SJ. Do astrocytes process neural information? Prog Brain Res. 1992;94:119–136. doi: 10.1016/s0079-6123(08)61744-6. [DOI] [PubMed] [Google Scholar]
- Snow DM, Lemmon V, Carrino DA, Caplan AI, Silver J. Sulfated proteoglycans in astroglial barriers inhibit neurite outgrowth in vitro. Exp Neurol. 1990;109:111–130. doi: 10.1016/s0014-4886(05)80013-5. [DOI] [PubMed] [Google Scholar]
- Sobue K, Yamamoto N, Yoneda K, Hodgson ME, Yamashiro K, Tsuruoka N, Tsuda T, Katsuya H, Miura Y, Asai K, Kato T. Induction of blood-brain barrier properties in immortalized bovine brain endothelial cells by astrocytic factors. Neurosci Res. 1999;35:155–164. doi: 10.1016/s0168-0102(99)00079-6. [DOI] [PubMed] [Google Scholar]
- Somjen GG. Nervenkitt: notes on the history of the concept of neuroglia. Glia. 1988;1:2–9. doi: 10.1002/glia.440010103. [DOI] [PubMed] [Google Scholar]
- Sonnewald U, Westergaard N, Schousboe A. Glutamate transport and metabolism in astrocytes. Glia. 1997;21:56–63. doi: 10.1002/(sici)1098-1136(199709)21:1<56::aid-glia6>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Spacek J. Three-dimensional analysis of dendritic spines. III. Glial sheath. Anat Embryol (Berl) 1985;171:245–252. doi: 10.1007/BF00341419. [DOI] [PubMed] [Google Scholar]
- Spassky N, Merkle FT, Flames N, Tramontin AD, Garcia-Verdugo JM, Alvarez-Buylla A. Adult ependymal cells are postmitotic and are derived from radial glial cells during embryogenesis. J Neurosci. 2005;25:10–18. doi: 10.1523/JNEUROSCI.1108-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoerri PE, Grant MB, Gomez J, Vernadakis A. Endothelial cell conditioned media mediated regulation of glutamine synthetase activity in glial cells. Brain Res Dev Brain Res. 1997;104:205–208. doi: 10.1016/s0165-3806(97)00173-9. [DOI] [PubMed] [Google Scholar]
- Steindler DA. Glial boundaries in the developing nervous system. Annu Rev Neurosci. 1993;16:445–470. doi: 10.1146/annurev.ne.16.030193.002305. [DOI] [PubMed] [Google Scholar]
- Steinhauser C, Berger T, Frotschner M, Kettenmann H. Heterogeneity in the membrane current pattern of identified glial cells in the hippocampal slice. Eur J Neurosci. 1992;4:472–484. doi: 10.1111/j.1460-9568.1992.tb00897.x. [DOI] [PubMed] [Google Scholar]
- Stiver SI. Angiogenesis and its role in the behavior of astrocytic brain tumors. Front Biosci. 2004;9:3105–3123. doi: 10.2741/1463. [DOI] [PubMed] [Google Scholar]
- Stone J, Itin A, Alon T, Pe’er J, Gnessin H, Chan-Ling T, Keshet E. Development of retinal vasculature is mediated by hypoxia-induced vascular endothelial growth factor (VEGF) expression by neuroglia. J Neurosci. 1995;15:4738–4747. doi: 10.1523/JNEUROSCI.15-07-04738.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub SV, Nelson MT. Astrocytic calcium signaling: the information currency coupling neuronal activity to the cerebral microcirculation. Trends Cardiovasc Med. 2007;17:183–190. doi: 10.1016/j.tcm.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struzynska L, Bubko I, Walski M, Rafalowska U. Astroglial reaction during the early phase of acute lead toxicity in the adult rat brain. Toxicology. 2001;165:121–131. doi: 10.1016/s0300-483x(01)00415-2. [DOI] [PubMed] [Google Scholar]
- Sturrock RR, Smart IH. A morphological study of the mouse subependymal layer from embryonic life to old age. J Anat. 1980;130:391–415. [PMC free article] [PubMed] [Google Scholar]
- Suh SW, Bergher JP, Anderson CM, Treadway JL, Fosgerau K, Swanson RA. Astrocyte glycogen sustains neuronal activity during hypoglycemia: studies with the glycogen phosphorylase inhibitor CP-316,819 ([R-R*,S*]-5-chloro-N-[2-hydroxy-3-(methoxymethylamino)-3-oxo-1-(phenylmet hyl)propyl]-1H-indole-2-carboxamide) J Pharmacol Exp Ther. 2007;321:45–50. doi: 10.1124/jpet.106.115550. [DOI] [PubMed] [Google Scholar]
- Suhonen JO, Peterson DA, Ray J, Gage FH. Differentiation of adult hippocampus-derived progenitors into olfactory neurons in vivo. Nature. 1996;383:624–627. doi: 10.1038/383624a0. [DOI] [PubMed] [Google Scholar]
- Sweger EJ, Casper KB, Scearce-Levie K, Conklin BR, McCarthy KD. Development of hydrocephalus in mice expressing the G(i)-coupled GPCR Ro1 RASSL receptor in astrocytes. J Neurosci. 2007;27:2309–2317. doi: 10.1523/JNEUROSCI.4565-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano T, Kang J, Jaiswal JK, Simon SM, Lin JH, Yu Y, Li Y, Yang J, Dienel G, Zielke HR, Nedergaard M. Receptor-mediated glutamate release from volume sensitive channels in astrocytes. Proc Natl Acad Sci U S A. 2005;102:16466–16471. doi: 10.1073/pnas.0506382102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano T, Tian GF, Peng W, Lou N, Libionka W, Han X, Nedergaard M. Astrocyte-mediated control of cerebral blood flow. Nat Neurosci. 2006;9:260–267. doi: 10.1038/nn1623. [DOI] [PubMed] [Google Scholar]
- Takumi T, Ishii T, Horio Y, Morishige K, Takahashi N, Yamada M, Yamashita T, Kiyama H, Sohmiya K, Nakanishi S. A novel ATP-dependent inward rectifier potassium channel expressed predominantly in glial cells. J Biol Chem. 1995;270:16339–16346. doi: 10.1074/jbc.270.27.16339. [DOI] [PubMed] [Google Scholar]
- Tanaka J, Matsuzaki M, Tarusawa E, Momiyama A, Molnar E, Kasai H, Shigemoto R. Number and density of AMPA receptors in single synapses in immature cerebellum. J Neurosci. 2005;25:799–807. doi: 10.1523/JNEUROSCI.4256-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Watase K, Manabe T, Yamada K, Watanabe M, Takahashi K, Iwama H, Nishikawa T, Ichihara N, Kikuchi T, Okuyama S, Kawashima N, Hori S, Takimoto M, Wada K. Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science. 1997;276:1699–1702. doi: 10.1126/science.276.5319.1699. [DOI] [PubMed] [Google Scholar]
- Tekkok SB, Brown AM, Westenbroek R, Pellerin L, Ransom BR. Transfer of glycogen-derived lactate from astrocytes to axons via specific monocarboxylate transporters supports mouse optic nerve activity. J Neurosci Res. 2005;81:644–652. doi: 10.1002/jnr.20573. [DOI] [PubMed] [Google Scholar]
- Theodosis DT, Piet R, Poulain DA, Oliet SH. Neuronal, glial and synaptic remodeling in the adult hypothalamus: functional consequences and role of cell surface and extracellular matrix adhesion molecules. Neurochem Int. 2004;45:491–501. doi: 10.1016/j.neuint.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Tomaselli KJ, Neugebauer KM, Bixby JL, Lilien J, Reichardt LF. N-cadherin and integrins: two receptor systems that mediate neuronal process outgrowth on astrocyte surfaces. Neuron. 1988;1:33–43. doi: 10.1016/0896-6273(88)90207-3. [DOI] [PubMed] [Google Scholar]
- Torres GE, Amara SG. Glutamate and monoamine transporters: new visions of form and function. Curr Opin Neurobiol. 2007;17:304–312. doi: 10.1016/j.conb.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Tran ND, Correale J, Schreiber SS, Fisher M. Transforming growth factor-beta mediates astrocyte-specific regulation of brain endothelial anticoagulant factors. Stroke. 1999;30:1671–1678. doi: 10.1161/01.str.30.8.1671. [DOI] [PubMed] [Google Scholar]
- Ullensvang K, Lehre KP, Storm-Mathisen J, Danbolt NC. Differential developmental expression of the two rat brain glutamate transporter proteins GLAST and GLT. Eur J Neurosci. 1997;9:1646–1655. doi: 10.1111/j.1460-9568.1997.tb01522.x. [DOI] [PubMed] [Google Scholar]
- Vaca K, Wendt E. Divergent effects of astroglial and microglial secretions on neuron growth and survival. Exp Neurol. 1992;118:62–72. doi: 10.1016/0014-4886(92)90023-j. [DOI] [PubMed] [Google Scholar]
- Vaccarino FM, Fagel DM, Ganat Y, Maragnoli ME, Ment LR, Ohkubo Y, Schwartz ML, Silbereis J, Smith KM. Astroglial cells in development, regeneration, and repair. Neuroscientist. 2007;13:173–185. doi: 10.1177/1073858406298336. [DOI] [PubMed] [Google Scholar]
- Vannucci SJ, Maher F, Simpson IA. Glucose transporter proteins in brain: delivery of glucose to neurons and glia. Glia. 1997;21:2–21. doi: 10.1002/(sici)1098-1136(199709)21:1<2::aid-glia2>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Ventura R, Harris KM. Three-dimensional relationships between hippocampal synapses and astrocytes. J Neurosci. 1999;19:6897–6906. doi: 10.1523/JNEUROSCI.19-16-06897.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky A, Toescu EC. Neuronal-glial networks as substrate for CNS integration. J Cell Mol Med. 2006;10:826–836. doi: 10.1111/j.1582-4934.2006.tb00527.x. [DOI] [PubMed] [Google Scholar]
- Vernadakis A. Glia-neuron intercommunications and synaptic plasticity. Prog Neurobiol. 1996;49:185–214. doi: 10.1016/s0301-0082(96)00012-3. [DOI] [PubMed] [Google Scholar]
- Vives V, Alonso G, Solal AC, Joubert D, Legraverend C. Visualization of S100B-positive neurons and glia in the central nervous system of EGFP transgenic mice. J Comp Neurol. 2003;457:404–419. doi: 10.1002/cne.10552. [DOI] [PubMed] [Google Scholar]
- Volknandt W. Vesicular release mechanisms in astrocytic signalling. Neurochem Int. 2002;41:301–306. doi: 10.1016/s0197-0186(02)00045-1. [DOI] [PubMed] [Google Scholar]
- Wagner S, Gardner H. Modes of regulation of laminin-5 production by rat astrocytes. Neurosci Lett. 2000;284:105–108. doi: 10.1016/s0304-3940(00)00987-3. [DOI] [PubMed] [Google Scholar]
- Walz W. Controversy surrounding the existence of discrete functional classes of astrocytes in adult gray matter. Glia. 2000;31:95–103. doi: 10.1002/1098-1136(200008)31:2<95::aid-glia10>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Wang LP, Kempermann G, Kettenmann H. A subpopulation of precursor cells in the mouse dentate gyrus receives synaptic GABAergic input. Mol Cell Neurosci. 2005;29:181–189. doi: 10.1016/j.mcn.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Wang X, Lou N, Xu Q, Tian GF, Peng WG, Han X, Kang J, Takano T, Nedergaard M. Astrocytic Ca2+ signaling evoked by sensory stimulation in vivo. Nat Neurosci. 2006;9:816–823. doi: 10.1038/nn1703. [DOI] [PubMed] [Google Scholar]
- Wells GM, Catlin G, Cossins JA, Mangan M, Ward GA, Miller KM, Clements JM. Quantitation of matrix metalloproteinases in cultured rat astrocytes using the polymerase chain reaction with a multi-competitor cDNA standard. Glia. 1996;18:332–340. [PubMed] [Google Scholar]
- Wender R, Brown AM, Fern R, Swanson RA, Farrell K, Ransom BR. Astrocytic glycogen influences axon function and survival during glucose deprivation in central white matter. J Neurosci. 2000;20:6804–6810. doi: 10.1523/JNEUROSCI.20-18-06804.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West H, Richardson WD, Fruttiger M. Stabilization of the retinal vascular network by reciprocal feedback between blood vessels and astrocytes. Development. 2005;132:1855–1862. doi: 10.1242/dev.01732. [DOI] [PubMed] [Google Scholar]
- Westergaard N, Sonnewald U, Schousboe A. Metabolic trafficking between neurons and astrocytes: the glutamate/glutamine cycle revisited. Developmental Neuroscience. 1995;17:203–211. doi: 10.1159/000111288. [DOI] [PubMed] [Google Scholar]
- Whitaker-Azmitia PM, Azmitia EC. Astroglial 5-HT1a receptors and S-100 beta in development and plasticity. Perspect Dev Neurobiol. 1994;2:233–238. [PubMed] [Google Scholar]
- Winship IR, Plaa N, Murphy TH. Rapid astrocyte calcium signals correlate with neuronal activity and onset of the hemodynamic response in vivo. J Neurosci. 2007;27:6268–6272. doi: 10.1523/JNEUROSCI.4801-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu-Friedman MA, Harris KM, Regehr WG. Three-dimensional comparison of ultrastructural characteristics at depressing and facilitating synapses onto cerebellar Purkinje cells. J Neurosci. 2001;21:6666–6672. doi: 10.1523/JNEUROSCI.21-17-06666.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K, Fukaya M, Shibata T, Kurihara H, Tanaka K, Inoue Y, Watanabe M. Dynamic transformation of Bergmann glial fibers proceeds in correlation with dendritic outgrowth and synapse formation of cerebellar Purkinje cells. J Comp Neurol. 2000;418:106–120. [PubMed] [Google Scholar]
- Yan XX, Ribak CE. Developmental expression of gamma-aminobutyric acid transporters (GAT-1 and GAT-3) in the rat cerebellum: evidence for a transient presence of GAT-1 in Purkinje cells. Brain Res Dev Brain Res. 1998;111:253–269. doi: 10.1016/s0165-3806(98)00144-8. [DOI] [PubMed] [Google Scholar]
- Yang Y, Ge W, Chen Y, Zhang Z, Shen W, Wu C, Poo M, Duan S. Contribution of astrocytes to hippocampal long-term potentiation through release of D-serine. Proc Natl Acad Sci U S A. 2003;100:15194–15199. doi: 10.1073/pnas.2431073100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin KJ, Cirrito JR, Yan P, Hu X, Xiao Q, Pan X, Bateman R, Song H, Hsu FF, Turk J, Xu J, Hsu CY, Mills JC, Holtzman DM, Lee JM. Matrix metalloproteinases expressed by astrocytes mediate extracellular amyloid-beta peptide catabolism. J Neurosci. 2006;26:10939–10948. doi: 10.1523/JNEUROSCI.2085-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong VW, Krekoski CA, Forsyth PA, Bell R, Edwards DR. Matrix metalloproteinases and diseases of the CNS. Trends Neurosci. 1998;21:75–80. doi: 10.1016/s0166-2236(97)01169-7. [DOI] [PubMed] [Google Scholar]
- Yu S, Ding WG. The 45 kDa form of glucose transporter 1 (GLUT1) is localized in oligodendrocyte and astrocyte but not in microglia in the rat brain. Brain Res. 1998;797:65–72. doi: 10.1016/s0006-8993(98)00372-2. [DOI] [PubMed] [Google Scholar]
- Yuste R, Bonhoeffer T. Genesis of dendritic spines: insights from ultrastructural and imaging studies. Nat Rev Neurosci. 2004;5:24–34. doi: 10.1038/nrn1300. [DOI] [PubMed] [Google Scholar]
- Zerlin M, Levison SW, Goldman JE. Early patterns of migration, morphogenesis, and intermediate filament expression of subventricular zone cells in the postnatal rat forebrain. J Neurosci. 1995;15:7238–7249. doi: 10.1523/JNEUROSCI.15-11-07238.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Harder DR. Cerebral capillary endothelial cell mitogenesis and morphogenesis induced by astrocytic epoxyeicosatrienoic Acid. Stroke. 2002;33:2957–2964. doi: 10.1161/01.str.0000037787.07479.9a. [DOI] [PubMed] [Google Scholar]
- Zhang JM, Wang HK, Ye CQ, Ge W, Chen Y, Jiang ZL, Wu CP, Poo MM, Duan S. ATP released by astrocytes mediates glutamatergic activity-dependent heterosynaptic suppression. Neuron. 2003;40:971–982. doi: 10.1016/s0896-6273(03)00717-7. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Stone J. Role of astrocytes in the control of developing retinal vessels. Invest Ophthalmol Vis Sci. 1997;38:1653–1666. [PubMed] [Google Scholar]
- Zhang Z, Chen G, Zhou W, Song A, Xu T, Luo Q, Wang W, Gu XS, Duan S. Regulated ATP release from astrocytes through lysosome exocytosis. Nat Cell Biol. 2007;9:945–953. doi: 10.1038/ncb1620. [DOI] [PubMed] [Google Scholar]
- Zhou M, Kimelberg HK. Freshly isolated hippocampal CA1 astrocytes comprise two populations differing in glutamate transporter and AMPA receptor expression. J Neurosci. 2001;21:7901–7908. doi: 10.1523/JNEUROSCI.21-20-07901.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Schools GP, Kimelberg HK. Development of GLAST(+) astrocytes and NG2(+) glia in rat hippocampus CA1: mature astrocytes are electrophysiologically passive. J Neurophysiol. 2006;95:134–143. doi: 10.1152/jn.00570.2005. [DOI] [PubMed] [Google Scholar]
- Zhuo L, Sun B, Zhang CL, Fine A, Chiu SY, Messing A. Live astrocytes visualized by green fluorescent protein in transgenic mice. Dev Biol. 1997;187:36–42. doi: 10.1006/dbio.1997.8601. [DOI] [PubMed] [Google Scholar]
- Ziv NE, Smith SJ. Evidence for a role of dendritic filopodia in synaptogenesis and spine formation. Neuron. 1996;17:91–102. doi: 10.1016/s0896-6273(00)80283-4. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Zonta M, Angulo MC, Gobbo S, Rosengarten B, Hossmann KA, Pozzan T, Carmignoto G. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci. 2003;6:43–50. doi: 10.1038/nn980. [DOI] [PubMed] [Google Scholar]