Inflammatory breast cancer (IBC) is a rare form of breast malignancy with unknown etiology, only accounting for 1-5% of all breast cancers.1,2 As compared with non-inflammatory locally advanced breast cancer, IBC had significantly higher incidence of loco-regional recurrence and distant soft-tissue and bone metastatic disease, resulting in worse prognosis and poorer survival.3-6 Current multimodality therapy regimens combining chemotherapy, mastectomy, and radiation have improved patient survival substantially.1,4,7,8 Recently developed novel targeted therapies also greatly benefit the management of IBC.8 Since IBC presents commonly as diffuse disease in a large region of the breast, the conventional imaging (mammogram and ultrasound), also the clinical examination, is inadequate in evaluating the primary breast lesion or the disease extent.9,10 Breast imaging, especially MRI, is identified as one of the most important research area. MRI is a potentially useful imaging modality for monitoring response of IBC during treatment, and warrants further investigation.
Breast cancer patients achieving pathological complete response (pCR) after neoadjuvant chemotherapy (NAC) were found to have longer disease-free survival and overall survival.11-14 The current NAC protocol for inflammatory breast cancer is anthracycline-based regimen,15,16 with the reported pCR rate below 15%.6,17 The pCR rate of IBC after anthracycline and taxane treatment is reported to be between 12-33%.18 The objective of this study is to investigate the role of breast MRI in the evaluation of NAC treatment in IBC patients, by correlating MR imaging findings with histopathological results after surgery. Considering the trend of breast conservation surgery, an accurate post-NAC disease staging by MRI may facilitate surgical planning.
In a retrospective review of breast cancer patients in a single institution and enrolled into a breast MRI study from year 2003 to year 2007, 24 patients (age 29-77, mean 47) with IBC (stage IIIB-IV) were identified. The tumor size ranged from 2.5-16 cm (6.6 ± 3.2 cm). All of them had complete MRI follow-up studies, and had received post-NAC surgery with available pathological results. Of the 24 patients, 19 had invasive ductal cancer, 3 had metaplastic cancer, and 2 had mixed ductal/lobular cancer. This study was approved by the Institutional Review Board and was HIPAA-compliant. All patients gave written informed consent for receiving the NAC treatment protocol, and participating in the MRI study.
All IBC received the same treatment protocol. Bi-weekly dose-dense Doxorubicin and Cyclophosphamide (AC) was given as the first-line regimen. After the first two cycles of treatment, the patient’s response was evaluated by the oncologist using all information available, including clinical examination, sonography, mammography, or MRI. If the patient responded well, she continued to receive 2 additional cycles of AC, otherwise she was switched to a taxane-based regimen. After two cycles of AC, 6 patients showed marked reduction of tumor size (> 30%), and 16 patients showed tumor reduction less than 30%. Ten of these 16 patients were switched to taxane-based regimen after 2 cycles of AC due to un-remarkable treatment effect and very limited tumor size reduction. The second-line taxane based regimen consisted of Paclitaxel or Nab-paclitaxel combined with Carboplatin. Fifteen of 24 IBC patients were HER-2 positive, and they also received trastuzumab with taxane. Nine HER-2 negative patients also received bevacizumab. The basic clinical, imaging, and pathological information of these 24 patients was given in Table 1.
Table 1.
Clinical. imaging, and pathological characteristics of 24 IBC patients
| Characteristics | Case Number (N) | Percentage (%) |
|---|---|---|
| Tumor types | ||
| IDC | 19 | 79 % |
| IDC with metaplastic tumor | 3 | 13 % |
| Mixed IDC/ILC | 2 | 8 % |
| MRI morphology | ||
| Mass type | 11 | 46 % |
| Non-mass type | 13 | 54 % |
| Biomarker status | ||
| HER-2 + / HER-2 - | 15/9 | 63% / 37% |
| ER + / ER - | 7/17 | 29% / 71% |
| PR + / PR - | 7/17 | 29% / 71% |
| Final surgery | ||
| Mastectomy | 17 | 71% |
| Lumpectomy | 7 | 29% |
| Final pathology | ||
| pCR | 12 | 50 % |
| Residual cancer | 12 | 50 % |
All patients had at least 4 breast MRI studies: a baseline breast MRI examination prior to NAC, at least 2 follow-up exams during the course of therapy, and a final exam after completing the therapy protocol. The first follow-up MRI during the therapy was performed after 2 cycles of AC to determine early response. The second follow-up was performed after 4 cycles of AC or after 2 cycles of AC plus 2 cycles of taxane-based therapy to determine if the patient responded well during the mid-course of therapy. The final MRI after completeness of all NAC was performed for correlation with pathology to decide the accuracy of MRI in defining residual tumor size and diagnosing pCR. Since the purpose of this study was to explore the feasibility of MRI for diagnosing residual tumor and pCR, only the final MRI was used for correlation. The MRI was done on a 1.5 T MR scanner (Philips Medical Systems, Cleveland, Ohio). The contrast enhanced dynamic imaging was performed using a 3D SPGR pulse sequence with 16 frames, 4 pre-contrast and 12 post-contrast. The detailed procedures for evaluating the response of lesions in MRI to chemotherapy have been described before.19 One radiologist (JHC) with three years experience in reading breast MRI performed the tumor size measurement. The tumor size of each patient in all serial MRI studies (pre-treatment and all follow-up) was analyzed in one sitting by the radiologist to ensure consistent determination of the tumor boundary. The radiologist was blinded to the pathology results. If the 1-dimenional size reduction after completing NAC was less than 30% compared to their pre-treatment size, the case was classified as a non-responder (NR). When residual tumor was present with greater than 30% size reduction, the case was classified as a partial response (PR). Cases in which no enhanced tissues were visible, or with minimal enhancement magnitude equivalent to or lower than the normal breast tissue, were classified as clinical complete response (CCR). A breast MR imaging scientist (MYS) with eight years experience in reading breast MRI also participated in interpreting final breast MRI separately after the completeness of NAC to determine if clinical complete response has been achieved. Final consensus of both readers was used as the results in this study.
After completion of NAC, a definitive surgery was performed. The type of surgery was decided based upon the surgeon’s recommendation and the patient’s personal choice. Seventeen patients received mastectomy and the other 7 patients received lumpectomy. The detailed pathological examination procedures were described previously.19 The residual disease was recorded into one of three categories: 1) No residual cancer cells; 2) No residual invasive cancer, but with DCIS present; 3) Residual invasive cancer. In this study the pCR was defined as no invasive cancer. This definition was used at MD Anderson,20 also was the consensus used at the “Preoperative Therapy in Invasive Breast Cancer” conference organized by NCI in March, 2007.21 In cases with evidence of residual invasive cancer, the pathological size was determined as the longest dimension, either the longest dimension on one H&E stained slide or from the number of blocks (each 5 mm) where the malignant invasive tumor was detected, whichever was greater. Pearson’s correlation was used for comparing MRI determined residual tumor size and pathological size. P < 0.05 was considered significant. Fisher’s Exact test was used to determine the significant difference of MRI for diagnosing pCR between HER-2 positive and HER-2 negative breast cancers.
12 IBC patients (12/24, 50%) achieved pCR. The MRI-evaluated clinical complete response (cCR) was diagnosed in 67% (16/24) of IBC. Partial response was found in 29% (7/24), and one IBC patient was diagnosed as a non-responder, showing less than 30% 1-dimensional size reduction. The true negative rate was 46%. The true positive rate was 29% (7/24), and the false negative rate was 21% (5/24). Overall, the sensitivity of MRI was 58% (7/12), and the specificity was 92% (11/12). The accuracy of MRI-diagnosed cCR in predicting pCR was 69% (11/16) (Figure 1). 13 IBC with residual cancer either on MRI or pathological examination were included for the size correlation. The Pearson’s correlation coefficient was r = 0.61. Nine of 13 patients had discrepancy greater than 10 mm (up to 48 mm) (Figure 2). Among the three patients with the highest discrepancy (41mm, 47mm, and 48mm), two presented non-mass type diffuse enhancements, and one presented multiple mass lesions. The diagnostic performance of MRI for pCR in these 24 patients and further comparison between HER-2 positive and negative patients was listed in Table 2. No significant difference was noted between the two groups.
Figure 1.
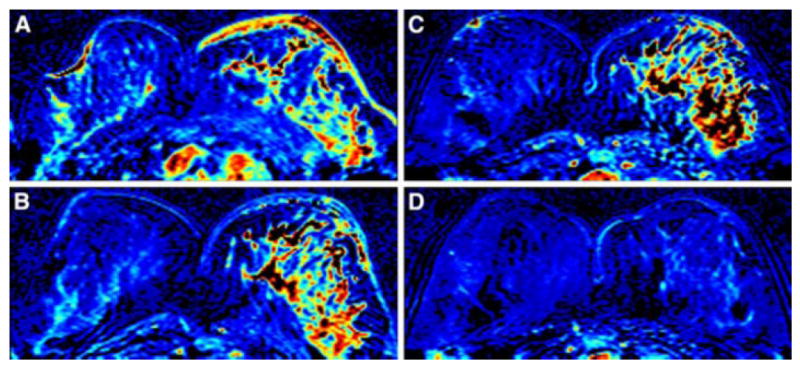
A 43 year-old woman with IBC. (A) Baseline breast MRI prior to the NAC shows a diffusely enhanced tumor in the left breast with diffuse enhancement and thickening of the skin. (B) After 2 cycles of AC, the skin enhancement is subsided but the primary tumor in the left breast remains without improvement. (C) After 4 cycles of AC, the tumor response is still not obvious compared to the image after 2 cycles of AC. (D) After completion of NAC, combining AC plus TCH, both the main tumor and the skin lesion disappear. CCR was hence diagnosed. Final pathology after surgery showed pCR.
Figure 2.
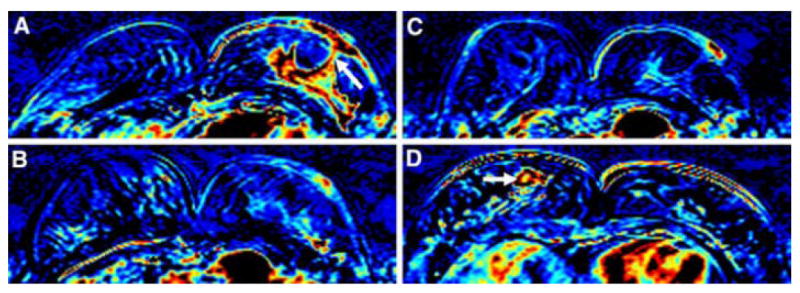
A 47 year-old woman with IBC. (A) Baseline breast MRI prior to the NAC shows a big lobulated enhanced tumor in the left breast with skin invasion through the nipple (arrow). (B) After 1 cycle of AC, both the primary tumor and the skin enhancement subsided remarkably. (C) After 4 cycles of AC, the tumor response is even more obvious compared to the image after 2 cycles of AC. (D) After completion of NAC, combining AC plus TCH, both the main tumor and the skin lesion disappear. CCR was hence diagnosed. Note an enhanced lesion, diagnosed as mastitis, in the right breast (arrow). Final pathology after surgery, however, showed small islands of residual invasive cancer cells distributed in an area of 4.7cm in the left breast.
Table 2.
Overall Diagnostic Performance of MRI
| True Negative | True Positive | False Negative | False Positive | Sensitivity | Specificity | MR accuracy | |
|---|---|---|---|---|---|---|---|
| Inflammatory Cancer (N=24) | 11/24 (46%) | 7/24 (29%) | 5/24 (21%) | 1/24 (4%) | 7/12 (58%) | 11/12 (92%) | 11/16 (69%) |
| HER-2 positive (N=15) | 7/15 (46%) | 4/15 (27%) | 3/15 (20%) | 1/15 (7%) | 4/7 (57%) | 7/8 (88%) | 7/10 (70%) |
| HER-2 negative (N=9) | 4/9 (45%) | 3/9 (33%) | 2/9 (22%) | 0/9 (0%) | 3/5 (60%) | 4/4 (100%) | 4/6 (67%) |
The diagnostic accuracy of MRI for pCR was defined as the ratio between (true negative) / (true negative + false negative), which was not different between HER-2 positive and HER-2 negative patients.
Sensitivity, defined as the ratio between (true positive) / (true positive + false negative), and specificity, defined as the ratio between (true negative) / (true negative + false positive), also did not show significant differences between both groups of HER-2 patients.
Several studies have shown that pCR was the strongest prognostic factor for locally advanced breast cancer,11-14 as well as for IBC.22,23 Using our chemotherapy protocol, IBC achieved a pCR rate of 50%. As compared with other studies, our chemotherapy protocol seemed more effective for IBC to achieve the pCR. In a recent large series study, pCR was only achieved in 13.9% of IBC patients.6 In another study, 120 IBC patients who were randomized to receive high-dose fluorouracil, epirubicin, and cyclophosphamide, the pCR rate was only achieved in 14.7% patients.17 When combining weekly doxorubicin and daily oral cyclophosphamide plus G-CSF followed by weekly paclitaxel as neoadjuvant therapy, the pCR rate of IBC could achieve 33%.18
Research on the accuracy of MR imaging in predicting pCR has been sporadically reported before,24-27 not focusing on specific cancer types. In a recent study by Chen et al.,19 MRI was shown to be superior in correctly predicting pCR for HER-2 positive (18/19, 95%) than HER-2 negative (8/16, 50%) breast cancer, and concluded that the interpretation of residual disease on MRI may need to be more conservative for HER-2 negative patients. In this study, the accuracy of cCR to predict pCR was 69% (11/16) in IBC. The false negative rate was high (5/24, 21%), suggesting interpretation of the complete response in IBC using MRI should be more conservative. This is especially true for non-mass type lesions which accounted for 4 of these 5 false negative cases.
Regarding prediction of residual pathological tumor size by MRI, In 9 of 13 IBC patients, MRI under- or over-estimated the tumor size by > 1 cm. In 3 extreme cases with size discrepancy > 4 cm, the pathological findings showed multiple islands of small residual invasive cancers distributed in a large area. This was also the major reason leading to false negative finding in our previous study.19 The results suggest that MRI findings may need to be interpreted cautiously when planning for conservation surgery regardless of the lesion type.
Although breast conserving surgery is traditionally considered contraindicated in IBC,28 our current chemotherapy protocol has resulted in 7 IBC patients who elected to receive lumpectomy after NAC. Further follow-up studies are needed to determine whether a great NAC response in IBC, along with the lesion types and initial presentations, may be used to select some IBC patients as good candidates for breast conservation surgery.
Several limitations existed in this study. Although the subjects were collected in a period of 4 years, they came from a single institution and the study number was small, making it difficult to draw a clear conclusion on our results. The diagnosis of IBC was based on clinical presentations rather than pathological findings. However, a study of 71 diffuse IBC patients has shown that dermal lymphatic emboli were only noted in 45% patients.29 Therefore, pathological evaluation of skin specimen for diagnosis of IBC with skin involvement might not be necessary, and was not performed in our study. The spatial resolution of the MRI images used in this study was suboptimal, which might affect the ability to detect small tumor foci. Our current protocol at 3 T MR system has improved spatial resolution as well as fat-suppression. Post-contrast enhanced images can now be directly evaluated without subtraction, and are thus less prone to motion problems.
Although with the small number of subjects in our study a definite conclusion could not be drawn, from our results it was indicated that overall MRI is still an appropriate imaging modality in the evaluation of IBC tumor response following NAC. Breast MRI provides clear imaging information for the change of tumor size during the therapy, which is proper to demonstrate responder and non-responder, and allows oncologists to decide whether to continue the therapy or switch to another chemo-regimen. However, MRI showed high false negative diagnosis of pCR for non-mass type of tumor morphology, indicating using MRI for diagnosing pCR in non-mass type IBC should be more conservative. MRI also did not provide high accuracy in determining the final residual tumor size of IBC after NAC, causing obvious discrepancy of tumor size measurement between MR imaging and final pathology. This problem might be due to the intrinsic limitations of MRI in detecting small scattered tumors and faintly enhanced residual tumor. The limitation should be considered when planning for breast conservation surgery after NAC. A more aggressive surgical procedure might be needed for IBC.
Acknowledgments
This study was supported in part by NIH NCI R01 CA90437, CA127927, and CBCRP 9WB-0020.
References
- 1.Cariati M, Bennett-Britton TM, Pinder SE, Purushotham AD. “Inflammatory” breast cancer. Surg Oncol. 2005;14(3):133–43. doi: 10.1016/j.suronc.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 2.Walshe JM, Swain SM. Clinical aspects of inflammatory breast cancer. Breast Dis. 20052006;22:35–44. doi: 10.3233/bd-2006-22105. [DOI] [PubMed] [Google Scholar]
- 3.Radunsky GS, van Golen KL. The current understanding of the molecular determinants of inflammatory breast cancer metastasis. Clin Exp Metastasis. 2005;22(8):615–20. doi: 10.1007/s10585-006-9000-7. [DOI] [PubMed] [Google Scholar]
- 4.Wu M, Merajver SD. Molecular biology of inflammatory breast cancer: applications to diagnosis, prognosis, and therapy. Breast Dis. 20052006;22:25–34. doi: 10.3233/bd-2006-22104. [DOI] [PubMed] [Google Scholar]
- 5.Hance KW, Anderson WF, Devesa SS, Young HA, Levine PH. Trends in inflammatory breast carcinoma incidence and survival: the surveillance, epidemiology, and end results program at the National Cancer Institute. J Natl Cancer Inst. 2005;97(13):966–75. doi: 10.1093/jnci/dji172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cristofanilli M, Valero V, Buzdar AU, et al. Inflammatory breast cancer (IBC) and patterns of recurrence: understanding the biology of a unique disease. Cancer. 2007;110(7):1436–44. doi: 10.1002/cncr.22927. [DOI] [PubMed] [Google Scholar]
- 7.Dawood S, Cristofanilli M. What progress have we made in managing inflammatory breast cancer? Oncology (Williston Park) 2007;21(6):673–9. [PubMed] [Google Scholar]
- 8.Yang CH, Cristofanilli M. Systemic treatments for inflammatory breast cancer. Breast Dis. 20052006;22:55–65. doi: 10.3233/bd-2006-22107. [DOI] [PubMed] [Google Scholar]
- 9.Yasumura K, Ogawa K, Ishikawa H, Takeshita T, Nakagawa Y, Osamura RY. Inflammatory Carcinoma of the Breast: Characteristic Findings of MR Imaging. Breast Cancer. 1997;4(3):161–9. doi: 10.1007/BF02967070. [DOI] [PubMed] [Google Scholar]
- 10.Yang WT, Le-Petross HT, Macapinlac H, et al. Inflammatory breast cancer: PET/CT, MRI, mammography, and sonography findings. Breast Cancer Res Treat. 2008;109(3):417–26. doi: 10.1007/s10549-007-9671-z. [DOI] [PubMed] [Google Scholar]
- 11.Chaturvedi S, McLaren C, Schofield AC, et al. Patterns of local and distant disease relapse in patients with breast cancer treated with primary chemotherapy: do patients with a complete pathological response differ from those with residual tumour in the breast? Breast Cancer Res Treat. 2005;93(2):151–8. doi: 10.1007/s10549-005-4615-y. [DOI] [PubMed] [Google Scholar]
- 12.Ogston KN, Miller ID, Payne S, et al. A new histological grading system to assess response of breast cancers to primary chemotherapy: prognostic significance and survival. Breast. 2003;12:320–7. doi: 10.1016/s0960-9776(03)00106-1. [DOI] [PubMed] [Google Scholar]
- 13.Cleator S, Parton M, Dowsett M. The biology of neoadjuvant chemotherapy for breast cancer. Endocr Relat Cancer. 2002;9:183–95. doi: 10.1677/erc.0.0090183. [DOI] [PubMed] [Google Scholar]
- 14.Honkoop AH, van Diest PJ, de Jong JS, et al. Prognostic role of clinical, pathological and biological characteristics in patients with locally advanced breast cancer. Br J Cancer. 1998;77:621–6. doi: 10.1038/bjc.1998.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ozmen V, Cabioglu N, Igci A, et al. Inflammatory breast cancer: results of antracycline-based neoadjuvant chemotherapy. Breast J. 2003;9(2):79–85. doi: 10.1046/j.1524-4741.2003.09204.x. [DOI] [PubMed] [Google Scholar]
- 16.Bonnefoi H, Diebold-Berger S, Therasse P, et al. Locally advanced/inflammatory breast cancers treated with intensive epirubicin-based neoadjuvant chemotherapy: are there molecular markers in the primary tumour that predict for 5-year clinical outcome? Ann Oncol. 2003;14(3):406–13. doi: 10.1093/annonc/mdg108. [DOI] [PubMed] [Google Scholar]
- 17.Veyret C, Levy C, Chollet P, et al. Inflammatory breast cancer outcome with epirubicin-based induction and maintenance chemotherapy: ten-year results from the French Adjuvant Study Group GETIS 02 Trial. Cancer. 2006;107(11):2535–44. doi: 10.1002/cncr.22227. [DOI] [PubMed] [Google Scholar]
- 18.Ellis GK, Green SJ, Russell CA, Royce ME, Perez EA, Livingston RB. SWOG 0012, a randomized phase III comparison of standard doxorubicin (A) and cyclophosphamide (C) followed by weekly paclitaxel (T) versus weekly doxorubicin and daily oral cyclophosphamide plus G-CSF (G) followed by weekly paclitaxel as neoadjuvant therapy for inflammatory and locally advanced breast cancer. J Clin Oncol; 2006 ASCO Annual Meeting Proceedings Part I; 2006. p. LBA537. [Google Scholar]
- 19.Chen JH, Feig B, Agrawal G, Yu H, Carpenter PM, Mehta PS, Nalcioglu O, Su MY. MRI evaluation of pathologically complete response and residual tumors in breast cancer after neoadjuvant chemotherapy. Cancer. 2008;112(1):17–26. doi: 10.1002/cncr.23130. [DOI] [PubMed] [Google Scholar]
- 20.Mazouni C, Peintinger F, Wan-Kau S, et al. Residual ductal carcinoma in situ in patients with complete eradication of invasive breast cancer after neoadjuvant chemotherapy does not adversely affect patient outcome. J Clin Oncol. 2007;25(19):2650–5. doi: 10.1200/JCO.2006.08.2271. [DOI] [PubMed] [Google Scholar]
- 21.Gralow JR, Burstein HJ, Wood W, et al. Preoperative therapy in invasive breast cancer: pathologic assessment and systemic therapy issues in operable disease. J Clin Oncol. 2008 Feb 10;26(5):814–9. doi: 10.1200/JCO.2007.15.3510. [DOI] [PubMed] [Google Scholar]
- 22.Kuerer HM, Newman LA, Smith TL, et al. Clinical course of breast cancer patients with complete pathologic primary tumor and axillary lymph node response to doxorubicin-based neoadjuvant chemotherapy. J Clin Oncol. 1999;17:460–9. doi: 10.1200/JCO.1999.17.2.460. [DOI] [PubMed] [Google Scholar]
- 23.Buchholz TA, Katz A, Strom EA, et al. Pathologic tumor size and lymph node status predict for different rates of locoregional recurrence after mastectomy for breast cancer patients treated with neoadjuvant versus adjuvant chemotherapy. Int J Radiat Oncol Biol Phys. 2002;53:880–8. doi: 10.1016/s0360-3016(02)02850-x. [DOI] [PubMed] [Google Scholar]
- 24.Martincich L, Montemurro F, Rosa GD, et al. Monitoring response to primary chemotherapy in breast cancer using dynamic contrast-enhanced magnetic resonance imaging. Breast Cancer Res Treat. 2004;83:67–76. doi: 10.1023/B:BREA.0000010700.11092.f4. [DOI] [PubMed] [Google Scholar]
- 25.Martincich L, Montemurro F, Cirillo S, et al. Role of Magnetic Resonance Imaging in the prediction of tumor response in patients with locally advanced breast cancer receiving neoadjuvant chemo-therapy. Radiol Med (Torino) 2003;106:51–8. [PubMed] [Google Scholar]
- 26.Rieber A, Brambs HJ, Gabelmann A, Heilmann V, Kreienberg R, Kühn T. Breast MRI for monitoring response of primary breast cancer to neo-adjuvant chemotherapy. Eur Radiol. 2002;12:1711–9. doi: 10.1007/s00330-001-1233-x. [DOI] [PubMed] [Google Scholar]
- 27.Warren RM, Bobrow LG, Earl HM, et al. Can breast MRI help in the management of women with breast cancer treated by neoadjuvant chemotherapy? Br J Cancer. 2004;90:1349–60. doi: 10.1038/sj.bjc.6601710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kell MR, Morrow M. Surgical aspects of inflammatory breast cancer. Breast Dis. 20052006;22:67–73. doi: 10.3233/bd-2006-22108. [DOI] [PubMed] [Google Scholar]
- 29.Lê MG, Arriagada R, Contesso G, et al. Dermal lymphatic emboli in inflammatory and non-inflammatory breast cancer: a French-Tunisian joint study in 337 patients. Clin Breast Cancer. 2005 Dec;6(5):439–45. doi: 10.3816/CBC.2005.n.049. [DOI] [PubMed] [Google Scholar]


