Abstract
We previously demonstrated that telomere length was markedly reduced in peripheral blood lymphocytes from individuals with schizophrenia. Since reduced telomere length can be caused by decreased telomerase activity, we quantitated basal telomerase activity in peripheral blood lymphocytes derived from individuals with schizophrenia (n=53), unaffected relatives (n=31) and unrelated controls (n=59). Telomerase activity varied greatly among individuals, suggesting that this enzymatic activity is affected by various factors. We observed a nominally significant decrease in telomerase activity among individuals with schizophrenia compared to unaffected individuals (unaffected relatives and unrelated controls). Further studies are needed to investigate the role of telomerase in schizophrenia.
Keywords: lymphocyte, immune, telomere length, premature aging
1. Introduction
In somatic cells, the ends of chromosomes, known as telomeres, progressively shorten with each cell division, a phenomenon resulting from incomplete replication of linear chromosomes by DNA polymerases. Telomerase is a specialized cellular ribonucleoprotein reverse transcriptase that extends telomeres, thereby protecting them and maintaining chromosomal integrity (Blackburn, 2005). In most somatic cells, telomerase is expressed at insufficient levels to completely restore telomeres, and telomeres progressively shorten with each cell division. Telomerase plays a critical role in cellular aging, since maintaining the length of telomeres extends the lifetime of cells (Blackburn, 2005). Thus, telomere length reflects the proliferative history of cells and serves as a mitotic clock of physical aging in many organisms (Blasco, 2005; von Zglinicki and Martin-Ruiz, 2005; Blackburn, 2005).
Abnormal telomere functioning has been implicated in many human diseases associated with aging, including cardiovascular disease (Brouilette et al., 2003; Demissie et al., 2006), neurodegenerative disorders (von Zglinicki et al., 2000; Martin-Ruiz et al., 2006), diabetes (Mitchell and Malone, 2006; Demissie et al., 2006; Jeanclos et al., 1998), and cancer (Blasco, 2005; von Zglinicki and Martin-Ruiz, 2005; Cawthon et al., 2003). These investigations utilized telomere length in peripheral blood cells as a marker for the progression of aging in the whole organism (von Zglinicki and Martin-Ruiz, 2005). Recently, telomere dysfunction in peripheral leukocytes has also been described in psychiatric conditions. Accelerated telomere shortening and decreased telomerase activity has been reported in chronically stressed individuals (Epel et al., 2004) and accelerated telomere shortening has been reported in mood disorders (Simon et al., 2006) and schizophrenia (Kao et al., 2007).
Schizophrenia is not generally conceptualized as an aging disorder since the age of onset usually occurs during adolescence. However, pathological aging may be a component of this disorder, since there are structural brain abnormalities (Buchsbaum and Hazlett, 1997; DeLisi, 1997) and brain hypometabolic patterns (Buchsbaum and Hazlett, 1997; Pettegrew et al., 1993) that mimic findings seen in the elderly. Moreover, individuals with schizophrenia are prone to diseases associated with aging, including diabetes and cardiovascular complications (Hennekens et al., 2005; Mitchell and Malone, 2006), as well as a shorter natural lifespan than the general population (Tsuang and Woolson, 1978). Our recent observation that telomere lengths of peripheral blood lymphocytes is markedly reduced in schizophrenia is consistent with the notion that abnormal aging is present in this disorder (Kao et al., 2007).
Since one mechanism by which telomeres shorten rapidly in cells is reduced telomerase activity, the goal of this study was to quantitate telomerase levels in individuals with schizophrenia and unaffected controls.
2. Materials and Methods
2.1. Subjects
Subjects were recruited from two departments at the Nathan Kline Institute: cohort A was recruited from the Outpatient Research Department (under the direction of W.M.G.), and cohort B and their unaffected first degree family members were recruited as part of a family study within the Center for Advanced Brain Imaging (under the direction of L.E.D.). Unaffected controls were recruited from both departments at the same institute.
Diagnoses were made using DSM-IV criteria. For cohort A, the SCID interview (First et al., 2002; First et al., 2002) was used to establish psychiatric diagnoses. For cohort B, diagnoses were based on a combination of a structured modified SADS interview (Schedule for Affective Disorder and Schizophrenia) (Spitzer and Endicott, 1978) combined with the SIDP-R (Structured Interview for DSM-III-R Personality Disorders, Revised) (Pfohl et al., 1990) or the DIGS (Diagnostic Interview for Genetic Studies) (Nurnberger et al., 1994), a structured interview administered to the participants, and medical records as indicated. The professionals performing clinical evaluations were trained in these procedures by L.E.D. or W.M.G., and all performed periodic diagnostic reliability exercises to maintain consistency.
2.2. Lymphocyte isolation, cell culture, and antipsychotic treatment
Lymphocytes were purified using a ficoll-hypaque gradient (GE Healthcare Biosciences) according to the manufacturer’s specifications. Lymphocytes were washed twice in phosphate buffered saline (PBS) and either stored in aliquots at −80°C, or seeded at a density of 5×105 cells/mL in RPMI/10% FCS in 6-well culture dishes.
Cultured lymphocytes were incubated with varying concentrations of a specific antipsychotic drug for a period of 5 days prior to harvesting for telomerase levels. A range of clozapine and haloperidol doses encompassing previously reported plasma therapeutic ranges of 350–420 ng/mL for clozapine (Mauri et al., 2007) and 4–26 ng/mL for haloperidol (de Oliveira et al., 1996) were used to treat cultured lymphocytes. Stock solutions of clozapine and haloperidol were prepared in methanol.
2.3 Telomerase assays
Telomerase activity was measured using a quantitative telomerase detection kit (Allied Biotech, Inc), which is based on a PCR-designed telomeric repeat amplification protocol (TRAP). Extracts from isolated peripheral blood lymphocytes were frozen at −80°C until used for telomerase assays. A measure of the number of cells used to generate the extract was determined with the CyQuant Cell Proliferation Assay Kit (Invitrogen), which uses a fluorescent dye to quantitate the amount of nucleic acid in the extract. Cell extracts were quantitated based on the amount of nucleic acid (ng) present in the extract. The telomerase assay was conducted essentially as described by the manufacturer, with data collected on an ABI 7700HT DNA Detection System (Applied Biosystems). The number of PCR cycles required for fluorescence to exceed a designated threshold, Ct, serves as a measure of total telomerase activity. Since the Ct value is inversely related to telomerase activity, we used the value 35-Ct as a relative measure of telomerase level (TL), that is, TL=35-Ct. There was a linear relationship between TL and the logarithm of the number of cells, consistent with previous analyses using quantitative PCR (qPCR) to show that Ct is proportional to the logarithm of the number of molecules quantitated in the reaction (Applied Biosystems, 2001). All TL values were normalized to one ng of nucleic acid in the extract, and thus all TL values were normalized to an equivalent number of cells. Between 2–5 independent determinations of TL were made, and the average value used in the final analyses.
2.4. Statistical analyses
A univariate Analysis of Covariance (ANCOVA), utilizing a general linear model, was conducted with telomerase level as the dependent variable, diagnostic group as the independent variable. The effects of age and sex on telomerase were tested in the model as covariates. Only significant covariates were included in the model and reported in the Results section. Pairwise comparisons were conducted on telomerase level in different diagnostic groups, and Tukey-Kramer multiple comparison adjustments were applied to the p-values reported. The assumption of normal distribution was evaluated on the dependent variable measurements in each group, and outliers were removed when necessary. The paired t-test was applied to test the difference of telomerase activity between the antipsychotics treated and untreated cells. All statistical analyses were performing using SAS software (version 9.1) (www.sas.com).
3. Results
3.1. Real-time quantification of telomerase activity
Many assays for telomerase activity are semi-quantitative, and antibody-based assays are not available. Here, we utilized a commercial real-time PCR-based assay for the sensitive quantification of telomerase activity (Allied Biotech, Inc.). The assay involves incubating cell extract with an artificial DNA substrate for telomerase (telomerase reaction), followed by real-time PCR quantification of telomeres added to the substrate (Wege et al., 2003). qPCR was monitored with Sybr Green, an indicator dye that robustly increases fluorescence in the presence of double-stranded DNA (Becker et al., 1996).
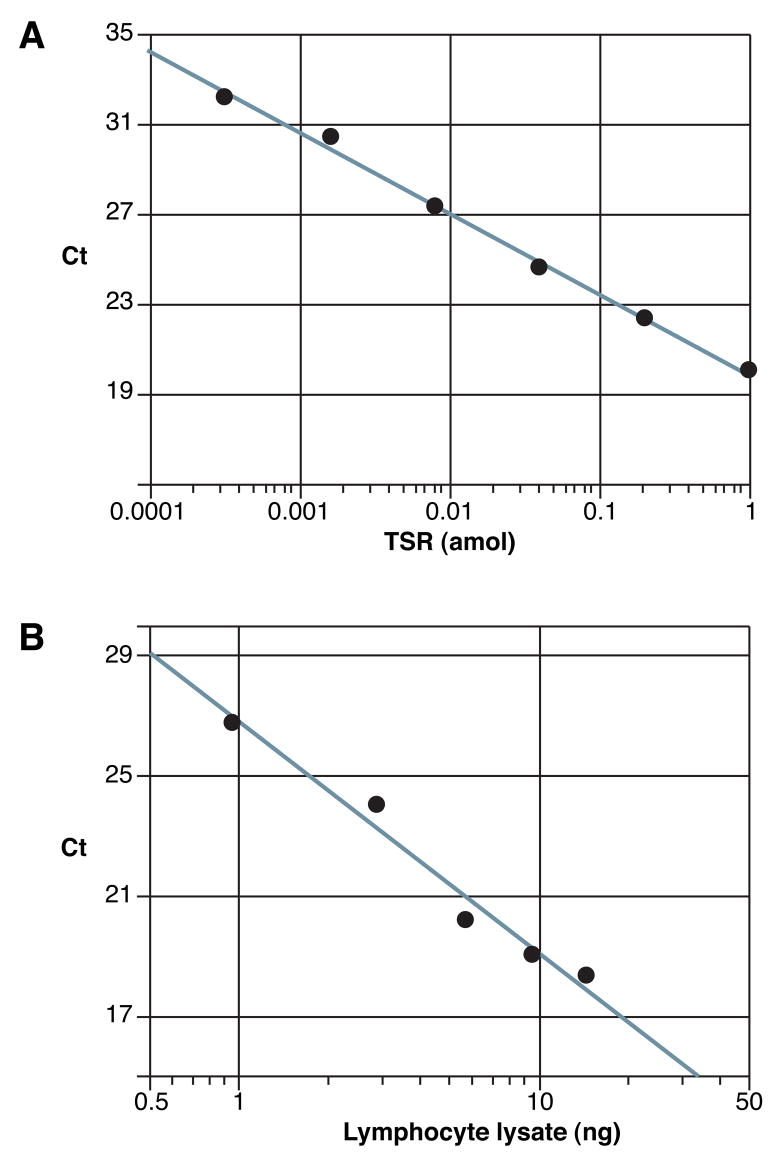
To validate the qPCR method for measuring the number of telomere repeats, increasing amounts of telomere synthetic repeat (TSR) DNA were added to the reaction (Figure 1A). As expected, a linear relationship was observed between the logarithm of the amount of input telomeres (TSR in attomoles) and Ct, number of PCR cycles required for fluorescence to exceed a designated threshold. To determine if varying the amount of cellular extract affects Ct in a quantitative manner, different quantities of lymphocyte extract were added to the assay (Figure 1B). Again, a linear relationship was observed between the logarithm of the amount of extract added and Ct (Figure 1B). These quantitative relationships are consistent with equations used to demonstrate the relationship between DNA quantity and Ct (Applied Biosystems, 2001). All telomerase measurements in each 96-well plate were conducted with TSR DNA and lymphocyte lysate standards, so that measurements could be compared from plate to plate.
Figure 1. Validation of the telomerase qPCR assay.
Quantitation using the qPCR-based telomerase assay was evaluated by adding varying amounts of telomere synthetic repeat (TSR) DNA or varying amounts of lymphocyte lysate to the real-time reaction. A. Typical plot depicting relationship between Ct and amount of telomere synthetic repeat (TSR) DNA. To determine if the assay detects telomere DNA in a quantitative manner, increasing amounts of synthetic telomeres (TSR in attomoles) were added to a 25 μL reaction. A linear relationship was observed between Ct and the base 10 logarithm of input telomeres (R2 = 0.995, p<0.0001). B. Typical plot depicting relationship between Ct and the amount of cellular extract derived from lymphocytes. To determine if the assay detects telomerase activity in a quantitative manner, varying amounts of lymphocyte lysate were added to a 25 μL real-time reaction. The units of cellular lysate (ng) refer to the quantity of nucleic acid in the extract, which is proportional to cell number. A linear relationship was observed between Ct and the base 10 logarithm of the amount of lymphocyte lysate (R2 = 0.973, p=0.0019).
3.2. Effect of antipsychotics on lymphocyte telomerase activity in vitro
Both typical and atypical antipsychotics affect lymphocyte biology (Leykin et al., 1997; Mazzarello et al., 2004; McAllister et al., 1989; Torrey et al., 1989), and could therefore affect telomerase activity, which is stimulated by lymphocyte proliferation (Buchkovich and Greider, 1996). To evaluate the effects of antipsychotics on telomerase activity we treated peripheral blood lymphocytes obtained from healthy volunteers with typical and/or atypical antipsychotics.
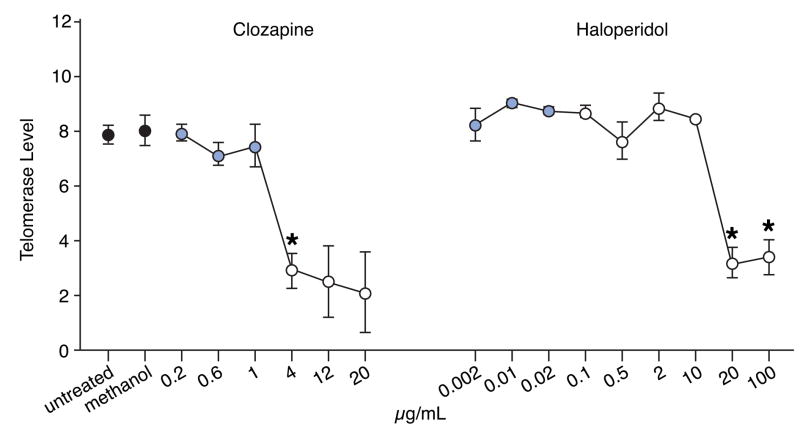
We measured basal telomerase activity (Figure 2) of lymphocytes maintained in culture for 5 days in the presence of varying concentrations of two antipsychotics: clozapine, an atypical antipsychotic, and haloperidol, a typical antipsychotic. Within the therapeutic range normally administered to patients, no effect of telomerase activity was observed for either drug. However, at doses exceeding the therapeutic range, a significant decline in telomerase activity was observed for both drugs. In the case of clozapine, a well-established immunosuppressant (Leykin et al., 1997), the decline in telomerase activity was observed with doses just exceeding the therapeutic range. However, with haloperidol, the dose was at least 1000 fold greater than the maximal therapeutic level before a decline in telomerase activity was observed. No change in cell number was observed at any dose of either drug (data not shown).
Figure 2. Clozapine or haloperidol within the therapeutic range does not affect basal telomerase levels.
Peripheral blood lymphocytes were isolated from three different individuals and seeded onto 6-well culture dishes. Varying quantities of clozapine, an atypical antipsychotic, or haloperidol, a typical antipsychotic, were added to the medium. After 5 days in culture, lymphocytes were harvested and telomerase levels were determined (Materials and Methods). Black-filled circles represent controls (untreated or treated with methanol, which was used to dissolve the drugs). Gray-filled circles represent values obtained with antipsychotics within the therapeutic range (0.2–1.0 μg/mL for clozapine, and 0.002 to 0.02 μg/mL for haloperidol). Open circles represent values obtained with antipsychotics outside the therapeutic range. * represents values that differ significantly from the untreated group (p≤0.05). p-values are corrected for multiple comparisons using Bonferroni’s method. Error bars=standard error of the mean.
3.3. Lymphocyte telomerase activity in schizophrenia
Telomerase levels were measured in a total of 53 individuals with schizophrenia, 59 unaffected, unrelated individuals, and 31 unaffected family members. Peripheral blood lymphocytes were isolated, and telomerase levels were measured using real-time PCR (Table 1). We found considerable variation in lymphocyte telomerase levels among the diagnostic groups examined, suggesting that this enzymatic activity is subject to physiological fluctuation in humans. We found no correlation between telomerase activity and age (R2=0.0025, p=0.57), nor association between telomerase activity and sex (F=0.01, df=1, p=0.91).
Table 1. Reduced telomerase levels in schizophrenia.
Telomerase levels were quantitated from peripheral blood lymphocytes. The subjects were evaluated in two different ways: First, they were categorized into four groups – unaffected controls (Control), unaffected relatives (Family), and two schizophrenia cohorts, A and B; Second, they were categorized into two groups – all unaffected individuals were combined into a single control group [Control+Family], while schizophrenia cohorts A and B were combined into a single schizophrenia group [Schizophrenia (A+B)]. In each evaluation, one-way ANOVA was conducted with telomerase level as the dependent variable and diagnostic group as the independent variable; pairwise comparisons were conducted on means of telomerase levels as well as age in the different diagnostic groups, and Tukey-Kramer multiple comparison adjustments were applied to the p-values reported below. There was a significant association between diagnostic group and telomerase level in both the 4-group data and 2-group data sets (F=3.89, df=3, p=0.01; and F=6.77, df=1, p=0.01, respectively). There were age differences between some of the diagnostic groups, but no effect of age or sex was observed on telomerase levels. The only significant finding in the 4-group data analyses was between unaffected relatives (Family) and schizophrenia cohort A (Schizophrenia A). In the 2-group analyses, a nominally significant difference was observed between all controls [Control+Family] and all individuals with schizophrenia [Schizophrenia (A+B)].
| Diagnostic Group | n (age) | Age (Mean ± SD) | n (TL) | TL (Mean ± SD) | |
|---|---|---|---|---|---|
| 1 | Control | 59 | 27.33 ± 8.14 | 59 | 11.44 ± 1.90 |
| 2 | Family | 25 | 30.97 ± 14.05 | 31 | 12.24 ± 1.03 |
| 3 | Schizophrenia A | 30 | 37.37 ± 9.00 a | 30 | 10.55 ± 2.56 e |
| 4 | Schizophrenia B | 23 | 39.00 ± 10.91 b,c | 23 | 11.18 ± 2.17 |
| 1+2 | Control+Family | 84 | 28.41 ± 10.30 | 90 | 11.72 ± 1.69 |
| 3+4 | Schizophrenia (A+B) | 53 | 38.07 ± 9.81d | 53 | 10.82 ± 2.40 f |
age was significant at p<0.05: a=1 vs 3, p=0.0001; b=1 vs 4, p=0.0001; c=2 vs 4, p=0.03; d=(1+2) vs (3+4), p<0.0001. Note that there are some missing values for age, as shown in the column “n (age)”.
Mean telomerase level was significant at p<0.05: e=2 vs.3, p=0.005.
Mean telomerase level was significant at p<0.05: f=(1+2) vs.(3+4), p=0.01.
The highest mean telomerase levels were found in unaffected relatives of individuals with schizophrenia (Family; Table 1), but these levels did not differ significantly from related family members with schizophrenia (cohort B). The lowest telomerase levels were found in individuals with schizophrenia from cohort A, who were all male and not related to any of the unaffected individuals. When all unaffected individuals as a group (Control+Family) were compared to all individuals with schizophrenia, a nominally significant reduction in telomerase activity was observed in schizophrenia (Table 1).
4. Discussion
Our recent studies demonstrate that significant telomere shortening occurs in individuals with schizophrenia (Kao et al 2008). To gain a better understanding of telomere biology in this disorder, we examined telomerase levels in the same individuals afflicted with schizophrenia. We observed that telomerase activity was highly variable among individuals. Moreover, no correlation between telomerase activity and telomere length was observed (data not shown). Indeed, previous studies have also shown high variability among individuals and little or no correlation between telomerase activity and telomere length in immune cells (Pan et al., 1997; Iwama et al., 1998). Although lymphocyte telomerase levels decline with age, most of the decline occurs between birth and age 40, and thereafter telomerase levels remain relatively constant (Iwama et al., 1998). Lymphocyte telomere lengths decline precipitously before the age of 5, stabilize till young adulthood, and then declines proportionately after the age of 25 (Frenck Jr. et al., 1998). The difference in the rate of decline between telomere length and telomerase levels, particularly after the age of 40, most likely explains the lack of correlation between these two measurements (Iwama et al., 1998). We also did not observe any relationship between telomerase activity and age or gender. Again, this is consistent with previous findings that show constant basal lymphocyte telomerase after the age of 40 (Iwama et al., 1998), and no gender differences in human subjects (Son et al., 2000). Nonetheless, our preliminary results suggest that lymphocyte telomerase activity may be reduced in schizophrenia.
Previous studies examining telomerase levels in psychologically stressed individuals have produced conflicting results (Damjanovic et al., 2007; Epel et al., 2004). Although both groups demonstrate that telomere lengths are decreased in psychological stressed individuals, one group reported a decline of telomerase levels in stressed individuals (Epel et al., 2004) while another group reported a compensatory increase (Damjanovic et al., 2007). As telomerase is an important mechanism for regulating telomere length, further studies are needed to determine the role of this enzyme in psychological stress and other mental disorders.
Two different cohorts of patients were used, A and B. Although diagnostically identical, the cohorts differ in terms of the patient’s functional level and psychiatric/medical status. The individuals in cohort A were more severely and chronically ill than individuals in cohort B, and those in cohort A had lower telomerase levels than in cohort B. Lymphocyte telomerase activity is largely inherited (Kosciolek and Rowley, 1998), and telomerase levels in cohort B did not differ significantly from unaffected relatives (Schizophrenia B vs Family, Table 1). In addition, no difference in telomerase levels was observed between the controls used for both cohorts (Controls vs Family, Table 1). These results suggest that the severity and chronicity of illness may be associated with a decrease in telomerase levels.
Since all of the affected subjects were treated with antipsychotics, we asked whether these drugs affect telomerase activity. We did not observe significant changes in telomerase activity of lymphocytes treated in vitro with antipsychotics within the therapeutic range. However, these studies were performed acutely on lymphocytes in vitro, and we cannot rule out the chronic effects of antipsychotics on telomerase function.
Since telomerase is an enzyme, one would expect its activity to be subject to various forms of regulation. It has been well documented that telomerase levels rise during cell proliferation (Holt and Shay, 1999). Lymphocytes respond to potentially infectious agents by cell proliferation, and any infections harbored by experimental subjects – even subclinical – could affect the results. Moreover, lymphocyte proliferation exhibits diurnal variation (Hiemke et al., 1995). The subjects in our study were recruited for blood draws at various times during the day and were not screened for infections. These factors could contribute to the variability we observed in telomerase levels.
Telomerase has important implications for two fundamental processes in mammals: aging and cancer. If a cell lacks telomerase, telomeres progressively shorten with each round of cell division, eventually eroding the telomeric DNA. Such dramatic telomere erosion leads to structural abnormalities, such as chromosomal fusions, which eventually results in cell death. On the other hand, activation of telomerase expression in a somatic cell can lead to unregulated growth, eventually resulting in cancer.
The telomere biology of lymphocytes may also have implications for understanding the pathophysiology of schizophrenia. There are numerous reports suggesting that immune abnormalities occur in schizophrenia (for review see Strous and Shoenfeld, 2005). Some reports suggest that there is reduced proliferative capability of lymphocytes in a subset of schizophrenia (Chengappa et al., 1995; Craddock et al., 2007). If verified, such a finding would be consistent with decreased telomere length and preliminary evidence of reduced telomerase activity found in these cells.
In summary, we quantitated telomerase activity in schizophrenia, to determine if this could in part account for the marked reduction in lymphocyte telomere length that was previously observed in this disorder (Kao et al 2007). We found preliminary evidence for possible reduction of peripheral blood lymphocyte telomerase activity in schizophrenia. Since basal telomerase activity varies considerably between individuals, future investigation of telomerase activity should control for various factors, including diurnal variation and infections.
Acknowledgments
This research was supported by the US National Institutes of Health (R01 MH070898 to B.P.; R01 NS047209 to H.-T.K.; R21 MH071720 to L.E.D.; R01 MH044292 to Jurg Ott), the Stanley Research Medical Foundation (to B.P.), and the National Alliance for Research on Schizophrenia and Depression (NARSAD). We thank Laura Panek, Alexis Moreno, Kyle Brown, and Laurie Nash for assistance in data collection, recruitment of subjects, diagnostic interviewing, and blood collection. We also thank Shelby Sturgis and Daniel Kolbin for technical assistance in the isolation of peripheral blood lymphocytes.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Applied Biosystems. User Bulletin #2, ABI PRISM 7700 Sequence detection system. P/N 4303859B. Applied Biosystems, Inc; Foster City, CA: 2001. [Google Scholar]
- Becker A, Reith A, Napiwotzki J, Kadenbach B. A quantitative method of determining initial amounts of DNA by polymerase chain reaction cycle titration using digital imaging and a novel DNA stain. Anal Biochem. 1996;237:204–207. doi: 10.1006/abio.1996.0230. [DOI] [PubMed] [Google Scholar]
- Blackburn EH. Telomeres and telomerase: their mechanisms of action and the effects of altering their functions. FEBS Lett. 2005;579:859–862. doi: 10.1016/j.febslet.2004.11.036. [DOI] [PubMed] [Google Scholar]
- Blasco MA. Telomeres and human disease: ageing, cancer and beyond. Nature Rev Genetics. 2005;6:611–622. doi: 10.1038/nrg1656. [DOI] [PubMed] [Google Scholar]
- Brouilette S, Singh RK, Thompson JR, Goodall AH, Samani NJ. White cell telomere length and risk of premature myocardial infarction. Arterioscler Thromb Vasc Biol. 2003;23:842–846. doi: 10.1161/01.ATV.0000067426.96344.32. [DOI] [PubMed] [Google Scholar]
- Buchkovich KJ, Greider CW. Telomerase regulation during entry into the cell cycle in normal human T cells. Mol Biol Cell. 1996;7:1443–1454. doi: 10.1091/mbc.7.9.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchsbaum MS, Hazlett EA. Functional brain imaging and aging in schizophrenia. Schizophr Res. 1997;27:129–141. doi: 10.1016/S0920-9964(97)00076-5. [DOI] [PubMed] [Google Scholar]
- Cawthon RM, Smith KR, O’Brien E, Sivatchenko A, Kerber RA. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet. 2003;361:393–395. doi: 10.1016/S0140-6736(03)12384-7. [DOI] [PubMed] [Google Scholar]
- Chengappa KN, Ganguli R, Yang ZW, Shurin G, Brar JS, Rabin BS. Impaired mitogen (PHA) responsiveness and increased autoantibodies in Caucasian schizophrenic patients with the HLA B8/DR3 phenotype. Biol Psych. 1995;37:546–549. doi: 10.1016/0006-3223(94)00363-8. [DOI] [PubMed] [Google Scholar]
- Craddock RM, Lockstone HE, Rider DA, Wayland MT, Harris LJ, McKenna PJ, Bahn S. Altered T-cell function in schizophrenia: a cellular model to investigate molecular disease mechanisms. PLoS ONE. 2007;2:e692. doi: 10.1371/journal.pone.0000692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damjanovic AK, Yang Y, Glaser R, Kiecolt-Glaser JK, Nguyen H, Laskowski B, Zou Y, Beversdorf DQ, Weng NP. Accelerated telomere erosion is associated with a declining immune function of caregivers of Alzheimer’s disease patients. J Immunol. 2007;179:4249–4254. doi: 10.4049/jimmunol.179.6.4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira IR, de Sena EP, Pereira EL, Miranda AM, de Oliveira NF, Ribeiro MG, de Castro-e-Silva E, Dardennes RM, Samuel-Lajeunesse B, Marcilio C. Haloperidol blood levels and clinical outcome: a meta-analysis of studies relevant to testing the therapeutic window hypothesis. J Clin Pharm Ther. 1996;21:229–236. doi: 10.1111/j.1365-2710.1996.tb01143.x. [DOI] [PubMed] [Google Scholar]
- DeLisi LE. Is schizophrenia a lifetime disorder of brain plasticity, growth and aging? Schizophr Res. 1997;23:119–129. doi: 10.1016/S0920-9964(96)00079-5. [DOI] [PubMed] [Google Scholar]
- Demissie S, Levy D, Benjamin EJ, Cupples LA, Gardner JP, Herbert A, Kimura M, Larson MG, Meigs JB, Keaney JF, Aviv A. Insulin resistance, oxidative stress, hypertension, and leukocyte telomere length in men from the Framingham Heart Study. Aging Cell. 2006;5:325–330. doi: 10.1111/j.1474-9726.2006.00224.x. [DOI] [PubMed] [Google Scholar]
- Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, Cawthon RM. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci USA. 2004;101:17312–17315. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition. (SCID-I/NP) Biometrics Research, New York State Psychiatric Institute; New York: 2002. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. (SCID-I/P) Biometrics Research, New York State Psychiatric Institute; New York: 2002. [Google Scholar]
- Frenck RW, Jr, Blackburn EH, Shannon KM. The rate of telomere sequence loss in human leukocytes varies with age. Proc Natl Acad Sci USA. 1998;95:5607–5610. doi: 10.1073/pnas.95.10.5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennekens CH, Hennekens AR, Hollar D, Casey DE. Schizophrenia and increased risks of cardiovascular disease. Am Heart J. 2005;150:1115–1121. doi: 10.1016/j.ahj.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Hiemke C, Brunner R, Hammes E, Muller H, Meyer zum Buschenfelde KH, Lohse AW. Circadian variations in antigen-specific proliferation of human T lymphocytes and correlation to cortisol production. Psychoneuroendocrinology. 1995;20:335–342. doi: 10.1016/0306-4530(94)00064-h. [DOI] [PubMed] [Google Scholar]
- Holt SE, Shay JW. Role of telomerase in cellular proliferation and cancer. J Cell Physiol. 1999;180:10–18. doi: 10.1002/(SICI)1097-4652(199907)180:1<10::AID-JCP2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Iwama H, Ohyashiki K, Ohyashiki JH, Hayashi S, Yahata N, Ando K, Toyama K, Hoshika A, Takasaki M, Mori M, Shay JW. Telomeric length and telomerase activity vary with age in peripheral blood cells obtained from normal individuals. Hum Genet. 1998;102:397–402. doi: 10.1007/s004390050711. [DOI] [PubMed] [Google Scholar]
- Jeanclos E, Krolewski A, Skurnick J, Kimura M, Aviv H, Warram J, Aviv A. Shortened telomere length in white blood cells of patients with IDDM. Diabetes. 1998;47:482–486. doi: 10.2337/diabetes.47.3.482. [DOI] [PubMed] [Google Scholar]
- Kao H-T, Cawthon RM, DeLisi LE, Bertisch HC, Ji F, Gordon D, Benedict MM, Greenberg WM, Porton B. Rapid telomere erosion in schizophrenia. Mol Psychiatry. 2007 doi: 10.1038/sj.mp.4002105. in press. [DOI] [PubMed] [Google Scholar]
- Kosciolek BA, Rowley PT. Human lymphocyte telomerase is genetically regulated. Genes Chromosomes Cancer. 1998;21:124–130. doi: 10.1002/(sici)1098-2264(199802)21:2<124::aid-gcc8>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Leykin I, Mayer R, Shinitzky M. Short and long-term immunosuppressive effects of clozapine and haloperidol. Immunopharmacology. 1997;37:75–86. doi: 10.1016/s0162-3109(97)00037-4. [DOI] [PubMed] [Google Scholar]
- Martin-Ruiz C, Dickinson HO, Keys B, Rowan E, Kenny RA, von Zglinicki T. Telomere length predicts poststroke mortality, dementia, and cognitive decline. Ann Neurol. 2006;60:174–180. doi: 10.1002/ana.20869. [DOI] [PubMed] [Google Scholar]
- Mauri MC, Volonteri LS, Colasanti A, Fiorentini A, De Gaspari IF, Bareggi SR. Clinical pharmacokinetics of atypical antipsychotics: a critical review of the relationship between plasma concentrations and clinical response. Clin Pharmacokinet. 2007;46:359–388. doi: 10.2165/00003088-200746050-00001. [DOI] [PubMed] [Google Scholar]
- Mazzarello V, Cecchini A, Fenu G, Rassu M, Dessy LA, Lorettu L, Montella A. Lymphocytes in schizophrenic patients under therapy: serological, morphological and cell subset findings. Ital J Anat Embryol. 2004;109:177–188. [PubMed] [Google Scholar]
- McAllister CG, Rapaport MH, Pickar D, Paul SM. Effects of short-term administration of antipsychotic drugs on lymphocyte subsets in schizophrenic patients. Arch Gen Psych. 1989;46:956–957. doi: 10.1001/archpsyc.1989.01810100098019. [DOI] [PubMed] [Google Scholar]
- Mitchell AJ, Malone D. Physical health and schizophrenia. Curr Opin Psychiatry. 2006;19:432–437. doi: 10.1097/01.yco.0000228767.71473.9e. [DOI] [PubMed] [Google Scholar]
- Nurnberger JI, Blehar MC, Kaufman CA, York-Cooler C, Simpson SG, Harkavy-Friedman J, Severe JB, Malaspina D, Reich T. Diagnostic interview for genetic studies: Rationale, unique features and training. Arch Gen Psych. 1994;51:849–862. doi: 10.1001/archpsyc.1994.03950110009002. [DOI] [PubMed] [Google Scholar]
- Pan C, Xue BH, Ellis TM, Peace DJ, Diaz MO. Changes in telomerase activity and telomere length during human T lymphocyte senescence. Exp Cell Res. 1997;231:346–353. doi: 10.1006/excr.1997.3475. [DOI] [PubMed] [Google Scholar]
- Pettegrew JW, Keshavan MS, Minshew NJ. 31P nuclear magnetic resonance spectroscopy: neurodevelopment and schizophrenia. Schizophr Bull. 1993;19:35–53. doi: 10.1093/schbul/19.1.35. [DOI] [PubMed] [Google Scholar]
- Pfohl B, Blum N, Zimmerman M, Stangl D. Structured interview for DSM-III-R Personality Disorders-Revised (SIDP-R) Department of Psychiatry; University of Iowa: 1990. [Google Scholar]
- Simon NM, Smoller JW, McNamara KL, Maser RS, Zalta AK, Pollack MH, Nierenberg AA, Fava M, Wong KK. Telomere shortening and mood disorders: preliminary support for a chronic stress model of accelerated aging. Biol Psych. 2006;60:432–435. doi: 10.1016/j.biopsych.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Son NH, Murray S, Yanovski J, Hodes RJ, Weng N-p. Lineage-specific telomere shortening and unaltered capacity for telomerase expression in human T and B lymphocytes with age. J Immunol. 2000;165:1191–1196. doi: 10.4049/jimmunol.165.3.1191. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Endicott J. Schedule for Affective Disorders and Schizophrenia (SADS) New York: Biometrics Research Division, New York State Psychiatric Institute; 1978. [Google Scholar]
- Torrey EF, Upshaw YD, Suddath R. Medication effect on lymphocyte morphology in schizophrenia. Schizophr Res. 1989;2:385–390. doi: 10.1016/0920-9964(89)90031-5. [DOI] [PubMed] [Google Scholar]
- Tsuang MT, Woolson RF. Excess mortality in schizophrenia and affective disorders. Do suicides and accidental deaths solely account for this excess? Arch Gen Psych. 1978;35:1181–1185. doi: 10.1001/archpsyc.1978.01770340031002. [DOI] [PubMed] [Google Scholar]
- von Zglinicki T, Martin-Ruiz CM. Telomeres as biomarkers for ageing and age-related diseases. Curr Mol Med. 2005;5:197–203. doi: 10.2174/1566524053586545. [DOI] [PubMed] [Google Scholar]
- von Zglinicki T, Serra V, Lorenz M, Saretzki G, Lenzen-Grossimlighaus R, Gessner R, Risch A, Steinhagen-Thiessen E. Short telomeres in patients with vascular dementia: an indicator of low antioxidative capacity and a possible risk factor? Lab Invest. 2000;80:1739–1747. doi: 10.1038/labinvest.3780184. [DOI] [PubMed] [Google Scholar]
- Wege H, Chui MS, Le HT, Tran JM, Zern MA. SYBR Green real-time telomeric repeat amplification protocol for the rapid quantification of telomerase activity. Nuc Acids Res. 2003;31:e3. doi: 10.1093/nar/gng003. [DOI] [PMC free article] [PubMed] [Google Scholar]