Abstract
A growing number of proteins devoid of signal peptides have been demonstrated to be released through the non-classical pathways independent of endoplasmic reticulum and Golgi. Among them are two potent proangiogenic cytokines FGF1 and IL1α. Stress-induced transmembrane translocation of these proteins requires the assembly of copper-dependent multiprotein release complexes. It involves the interaction of exported proteins with the acidic phospholipids of the inner leaflet of the cell membrane and membrane destabilization. Not only stress, but also thrombin treatment and inhibition of Notch signaling stimulate the export of FGF1. Non-classical release of FGF1 and IL1α presents a promising target for treatment of cardiovascular, oncologic, and inflammatory disorders.
Keywords: non-classical secretion, FGF1, IL1α
VARIETY OF NON-CLASSICALLY RELEASED PROTEINS
The familiar textbook scheme of protein secretion starts with the cotranslational protein translocation into the endoplasmic reticulum (ER). This translocation requires a molecular exit visa, a hydrophobic signal peptide located usually at the N-terminus of a secretable protein [Blobel, 1995]. After the protein is translocated through the ER membrane, its folding, transport, sorting, and covalent modifications occur in the ER and Golgi. Finally, the protein is released from the cell as a result of the fusion of an exocytotic vesicle with the cell membrane.
Although prevalent, the classical ER-Golgi dependent pathway of protein export is not exclusive. An increasing number of secreted proteins devoid of a signal peptide have been reported to be exported without the help of ER-Golgi. The non-classical protein release is resistant to brefeldin A, the specific inhibitor of classical secretion which inhibits the protein transport from ER to Golgi [Misumi et al., 1986]. Non-classical secretion is not limited to a specific family of proteins. Indeed, proteins belonging to the following functional groups were demonstrated to be released independently of ER-Golgi:
Cytokines: interleukin (IL)1α [Tarantini et al., 2001] and IL1β [Rubartelli et al., 1990; Andrei et al., 1999], fibroblast growth factor (FGF)1 [Jackson et al., 1992], and FGF2 [Mignatti et al., 1992; Florkiewicz et al., 1998; Nickel, 2005], macrophage migration inhibitory factor (MIF) [Lue et al., 2002].
Enzymes: secretory transglutaminase [Aumuller et al., 1999] and sphingosine kinase 1 [Ancellin et al., 2002].
Annexins (Anx): Anx I [Chapman et al., 2003] and Anx II [Kim and Hajjar, 2002; Peterson et al., 2003].
Galectins: galectin 1 [Sango et al., 2004], galectin 3 [Mehul and Hughes, 1997], galectin 9 [Keryer-Bibens et al., 2006].
Heat shock proteins (HSP): HSP70 [Hunter-Lavin et al., 2004; Mambula and Calderwood, 2006] and HSP90 [Yu et al., 2006].
Viral proteins expressed in mammalian cells: HIV Tat [Chang et al., 1997], Herpes VP22 protein [Elliott and O’Hare, 1997], foamy virus Bet protein [Lecellier et al., 2002].
Chromatin associated proteins: High Mobility Group Box 1 (HMBG) [Gardella et al., 2002; Bonaldi et al., 2003], Engrailed 2 [Joliot et al., 1998; Maizel et al., 1999, 2002].
S100 proteins: S100A13 [Landriscina et al., 2001b], S100B [Davey et al., 2001], S100A4 [Flatmark et al., 2004].
Thioredoxin [Ericson et al., 1992; Rubartelli et al., 1992; Rubartelli et al., 1995; Angelini et al., 2002; Tanudji et al., 2003].
Proteins involved in exocytosis: p40 form of synaptotagmin 1 [LaVallee et al., 1998] and syntaxin 2 (also known as epimorphin) [Hirai et al., 2007].
Surface lectin of a unicellular parasite: HASPB protein of Leishmania [Denny et al., 2000; Stegmayer et al., 2005].
Most of non-classically exported proteins exhibit spontaneous release from the cells. However, a group of them, including FGF1, IL1α, IL1β, syntaxin 2 (epimorphin), usually require cell stress to be exported [Jackson et al., 1992; Andrei et al., 1999; Tarantini et al., 2001; Hirai et al., 2007]. Thrombin induces the release of annexin II [Peterson et al., 2003]. Gluco-corticoid treatment stimulates the export of annexin I [Chapman et al., 2003]. Thioredoxin release from dendritic cells is induced by TNFα and lipopolysaccharide [Angelini et al., 2002].
Four general mechanisms are employed by non-classically released proteins to exit the cell: (i) Membrane blebbing: Secretory transglutaminase concentrates in the vicinity of the cell membrane where membrane blebs are formed [Aumuller et al., 1999]. The blebs detach from the cell surface. Apparently, the release of the protein occurs as a result of rupture of detached blebs. (ii) Endolysosomal pathway: IL1β [Andrei et al., 1999], HMBG1 [Gardella et al., 2002], and HSP70 [Mambula and Calderwood, 2006] translocate from the cytosol into endolysosomes which then fuse with the cell membrane and release their content into the extracellular milieu. The transport of non-classically released cytosolic proteins into the endolysosomes is blocked by glibenclamide, the inhibitor of the ATP-binding cassette translocator ABC1 [Andrei et al., 1999; Mambula and Calderwood, 2006]. (iii) Exosome-mediated secretion: HSP90 is exported through exosomes [Yu et al., 2006]. The secretion starts with the inward budding of the limiting membrane into the lumen of endosomes. As a result, cytosolic components are engulfed into the vesicles located inside the enlarged endosomes. Endosome-derived multivesicular bodies fuse with the cell membrane and release vesicles (exosomes) containing sequestered cytosolic components. The ultimate secretion of exported proteins occurs as a result of exosome rupture in the extracellular milieu. (iv) Translocation through the plasma membrane: The export of FGF1, FGF2, IL1α HASPB, galectin 1, and syntaxin 2 (epimorphin) [Jackson et al., 1992; Tarantini et al., 2001; Schafer et al., 2004; Seelenmeyer et al., 2005; Stegmayer et al., 2005; Hirai et al., 2007] proceeds as a result of the translocation of these proteins from the cytosol through the cell membrane to the extracellular compartment.
This review will focus on the export FGF1 and IL1α, two proteins involved in oncologic, cardiovascular, and neurodegenerative diseases that are released through similar export mechanisms.
EXPORT OF FGF1: GROWING LIST OF PARTICIPANT PROTEINS
FGF1 and FGF2 are the prototype members of the FGF family, which is represented by at least 23 proteins in mammals [Coulier et al., 1997; Friesel and Maciag, 1999; Itoh and Ornitz, 2004]. While most FGFs display cleavable or uncleavable [Miyakawa et al., 1999; Miyakawa and Imamura, 2003] hydrophobic signal peptides in their structure and are exported through the ER-Golgi, FGF1 and FGF2 are signal peptide-less proteins [Coulier et al., 1997; Friesel and Maciag, 1999; Itoh and Ornitz, 2004]. Also, unlike most other FGFs the expression of FGF1 and FGF2 in the organism is widespread, and FGF1 binds to all four known types of FGF receptors [Friesel and Maciag, 1999]. Interestingly, FGFs of Drosophila and Caenorhabditis elegans have signal peptides [Coulier et al., 1997]. The artificial addition of a signal peptide to FGF1 transforms it to a potent oncogene [Forough et al., 1993]. We hypothesized that the signal peptide of FGF1 was lost in the course of evolution, to prevent the potentially deleterious effects of the ubiquitous expression of the strong mitogen released through the unregulated classical export pathway [Prudovsky et al., 2003].
Experiments with cells transfected with FGF1 showed that under normal conditions this protein is not released into the medium. However, different types of stress, such as heat shock [Jackson et al., 1992], hypoxia [Mouta Carreira et al., 2001], and low serum [Shin et al., 1996; Matsunaga and Ueda, 2006] induced its export. Stress-induced release of FGF1 was not simply a side effect of loss of cell membrane integrity, since stress conditions did not result in cell lysis as judged by testing the release of the cytosolic enzyme lactate dehydrogenase into the medium. Interestingly, in contrast to the release of FGF1, FGF2 does not require stress for its release, which in most cases occurs spontaneously [Mignatti et al., 1992; Florkiewicz et al., 1998; Nickel, 2005].
FGF1 is released from the cells as a covalent homodimer. The dimerization of FGF1 is the result of the formation of a disulfide bond between cysteine (Cys) residues in position 30 of two interacting monomers [Engleka and Maciag, 1992; Jackson et al., 1995; Tarantini et al., 1995]. Point mutation of Cys 30 inhibits FGF1 export [Tarantini et al., 1995]. NMR studies revealed that amlexanox, an antiinflammatory drug that blocks FGF1 export, binds at close spatial proximity to Cys 30 in FGF1 and thus inhibits its dimerization [Rajalingam et al., 2005a]. These experiments demonstrate the importance of FGF1 dimerization for its export. However, the FGF1 homodimer is only a part of a larger multi-protein complex which is required for its release.
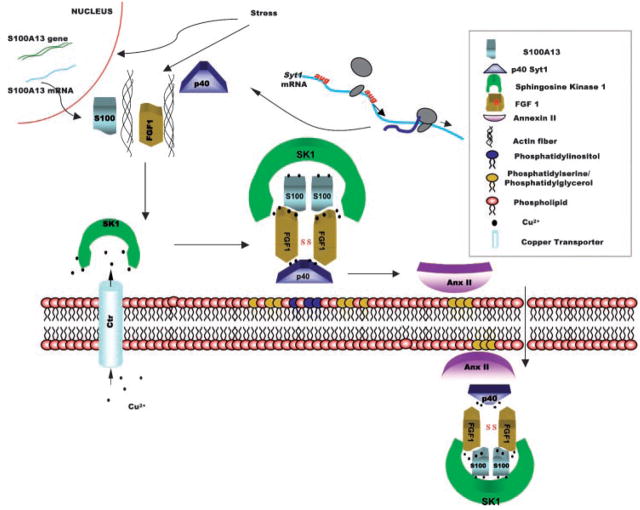
The discovery of FGF1 release muliprotein complex started with the analysis of FGF1-containing brain fractions. The brain is one of the richest sources of FGF1 in the organism. FGF1 was isolated from the brain as a component of a large heparin-binding multiprotein complex. Chromatography studies revealed that this complex also contained a 40 kDa (p40) form of the docking protein synaptotagmin 1 (Syt1), the small calcium binding protein S100A13 and annexin II [Mouta Carreira et al., 1998; Prudovsky et al., 2003]. Several studies revealed the importance of these proteins for FGF1 export. Using an in vitro model of secretion, it was demonstrated that FGF1 is exported as a non-covalent complex containing S100A13 and p40 Syt1 [LaVallee et al., 1998; Mouta Carreira et al., 1998]. Moreover, the expression of dominant negative deletion mutants of Syt1 and S100A13 efficiently blocked FGF1 export [LaVallee et al., 1998; Landriscina et al., 2001b].
RT-PCR analysis showed that heat shock enhanced S100A13 transcription (O. Sideleva and I. Prudovsky, unpublished work). Recently, using the fluorescence resonance energy transfer (FRET) method, Matsunaga and Ueda [2006] observed that in astrocytes the binding of FGF1 to S100A13 is stimulated by cell stress (growth factor starvation), and this effect is mediated by the increase of the intracellular Ca2+ concentration. The three-dimensional solution structures of human and rat S100A13 have been recently determined [Arnesano et al., 2005; Sivaraja et al., 2005; Li et al., 2007]. S100A13 is a globular homodimer with an approximate C2 symmetry [Sivaraja et al., 2005]. The structure of S100A13 consists of four helices in each monomeric unit [Arnesano et al., 2005; Sivaraja et al., 2005; Li et al., 2007]. The four helices are arranged as two calcium binding EF hands (helix-loop-helix motifs) one in the N-terminal and one in the C-terminal portions of the S100A13 monomer [Arnesano et al., 2005; Sivaraja et al., 2005; Li et al., 2007]. Contacts between two monomers of S100A13 occurs largely through hydrophobic interactions provided by helix H4 of one monomer with H1′ and H4′helices of the other. The dimeric interface in S100A13 is laden with aromatic amino acids. In addition, the dimeric structure of S100A13 is stabilized by pairs of hydrophobic interactions among residues in helices H1 and H4 [Arnesano et al., 2005; Sivaraja et al., 2005; Li et al., 2007]. Most of the hydrophobic residues at the dimer interface are highly conserved among the members of the S100 family.
Unlike other members of the S100 protein family, S100A13 shows high affinity binding to hydrophobic dyes such as 1-anilinonapthalene sulfonate [Sivaraja et al., 2005]. The affinity to hydrophobic dyes decreases significantly upon binding of S100A13 to calcium. The three-dimensional structure of S100A13 is characterized by a large patch of negatively charged residues flanked by dense cationic clusters contributed mostly by positively charged residues located at the C-terminal end. It appears that S100A13 recognizes and binds to target protein partners through this unique charged domain [Sivaraja et al., 2005]. Interestingly, deletion of the positively charged C-terminal segment in S100A13 results in complete loss of binding to FGF1 [Sivaraja et al., 2005].
Full length (65 kDa) Syt1 is a transmembrane protein spanning the membrane of exocytotic vesicles, and it is responsible for their docking to the cell membrane [Sudhoff and Rizo, 1996; Marqueze et al., 2000]. p40 Syt1 corresponds to the extravesicular domain of p65 Syt1. In its structure, it displays two acidic phospholipid-and Ca2+-binding C2 domains. p40 Syt1 is the product of the alternative initiation of p65 Syt1 mRNA translation from one of two closely positioned alternative start codons located downstream of and in frame with the main start codon [Bagala et al., 2003]. Thus, the same mRNA can code for a protein participating in classical protein export and another polypeptide involved in non-classical secretion.
The primary structures of p40 Syt1 and S100A13 are devoid of a signal peptide. Both proteins can be released as individual molecules under non-stress conditions [LaVallee et al., 1998; Landriscina et al., 2001b]. The extracellular functions of p40 Syt1 and S100A13 are unknown. In a wide range of concentrations, recombinant p40 Syt1 and S100A13 do not modulate either proliferation or migration of fibroblastoid cells in culture (O. Sideleva and I. Prudovsky, unpublished work). However, it should be noted that some other members of the S100 family which are also exported despite the absence of signal peptides exhibit biological activities [Donato, 2003].
Hla and coworkers [Ancellin et al., 2002] reported that the signal peptide-less enzyme SK1, responsible for phosphorylation of sphingosine and production of a proangiogenic mediator sphingosine 1-phosphate (S1P) is spontaneously released from the cells. Moreover, exported SK1 exhibits enzymatic activity in the extracellular compartment and apparently contributes to the formation of extracellular S1P gradients [Venkataraman et al., 2006] that regulate angiogenesis. Because SK1 is a proangiogenic protein, and FGF1 is one of the most proangiogenic factors ever described, we hypothesized that the secretion of two polypeptides may be coordinated. Coexpression of SK1 with FGF1 in NIH 3T3 cells, resulted in the blockage of spontaneous SK1 release under non-stress conditions. However, at 42°C, both proteins were released in association with each other [Soldi et al., 2007]. Moreover, SK1 knockout cells failed to exhibit stress-induced FGF1 export. Thus, SK1 is a new indispensable member of the FGF1 release complex.
Annexin II also appears to be an element of the FGF1 export pathway. As mentioned above, it was detected in the brain-derived FGF1 containing complex. This protein forms a heterodimer that includes two molecules of Anx II and two molecules of p11 (a member of the S100 protein family) [Kim and Hajjar, 2002]. Anx II is externalized under stress and after thrombin treatment [Peterson et al., 2003; Deora et al., 2004]. Externalized Anx II can serve as a receptor for plasminogen and plasminogen activator [Hajjar and Krishnan, 1999].
COPPER AND RELEASE OF FGF1
Copper ions exhibit strong proangiogenic effects both in vitro and in vivo [Hu, 1998; Parke et al., 1988]. Interestingly, in a cell free system, Cu2+ was found to stimulate the formation of covalent FGF1 dimers [Engleka and Maciag, 1992] and non-covalent complexes including FGF1, p40 Syt1, and S100A13 [Landriscina et al., 2001a]. Cell treatment with the cell-permeable copper chelator tetrathiomolybdate (TM) blocked the stress-dependent FGF1 export [Landriscina et al., 2001a]. These data suggest that the formation of the FGF1 export complex is copper dependent.
Isothermal calorimetry (ITC) studies demonstrated that four copper ions bind to the S100A13 dimer [Sivaraja et al., 2006]. Unlike calcium, binding of copper does not involve a conformational change in S100A13: binding of copper and calcium to S100A13 is not mutually exclusive [Sivaraja et al., 2006]. The copper binding sites are located spatially close to the calcium binding sites. The binding of copper and calcium have opposite effects on the thermodynamic stability of S100A13 [Sivaraja et al., 2006]. Binding of calcium stabilizes the structure of S100A13 quite significantly, but the protein destabilizes appreciably upon binding to copper.
The C2A domain of p40 Syt1 has been shown to bind to copper with an affinity in the nanomolar range [Rajalingam et al., 2005b]. ITC data reveal that four copper ions bind per molecule of the C2A domain [Rajalingam et al., 2005b]. Using paramagnetic effects in multidimensional NMR experiments, Rajalingam et al. [2005b] have shown that three of the four copper ions bind to the calcium binding sites in the loops located at the apex of the structure of the C2A domain. Results of competition experiments, monitored by FRET, show that three copper ions bound at the calcium binding site, can be replaced by calcium [Rajalingam et al., 2005b]. However, the fourth copper ion bound at the unique site (contributed by Gly253 and His254) cannot be displaced by calcium.
SK1 exhibits a very high copper affinity [Soldi et al., 2007]. It is released from copper columns at 0.5 M imidazole, whereas the release of FGF1, p40 Syt1, and S100A13 occurs at 0.05 M imidazole. The overexpression of SK1 rescues the release of FGF1 in the presence of TM [Soldi et al., 2007]. Moreover, in a cell free system, SK1 forms high molecular weight complexes with FGF1 even in the absence of exogenously added copper ions [Soldi et al., 2007]. These results suggest that SK1 is not only part of the FGF1 release complex, but also serves as a donor of copper ions needed for complex assembly.
EXPORT OF IL1α SIMILARITIES WITH FGF1
IL1α is a prototype member of the IL1 family consisting of 10 secreted proteins [Dinarello, 1998; Stylianou and Saklatvala, 1998]. Their activity is mediated by specific IL1 receptors, particularly IL1R1. IL1α and another prototype member of the IL1 family, IL1β, are expressed in many cell types; however the highest levels of their expression are observed in monocytes/macrophages [Dinarello, 1998; Stylianou and Saklatvala, 1998]. All of the members of IL1 family, except the IL1 receptor antagonist (IL1ra) are devoid of the signal peptide.
IL1α and IL1β are potent proinflammatory proteins [Dinarello, 1998]. The injection of IL1α and IL1β into the bloodstream induces a rise of body temperature [Davidson et al., 1990]. The absence of a signal peptide in IL1 proteins might have evolved as a device to protect the organism from their uncontrolled secretion. IL1α and IL1β are produced as larger molecular weight precursors (p) [Dinarello, 1998]. The proteolytic cleavage of pIL1 results in the production of the mature (m) IL1 that is secreted. Although FGF1 and FGF2 present a very low level of amino acid sequence homology with mIL1α and mIL1β all four proteins have similar molecular weights around 17–18 kDa, and display a typical beta-barrel three-dimensional structure composed of 12 β-sheet domains [Graves et al., 1990; Zhu et al., 1991; Venkataraman et al., 1999]. This beta-barrel structure is present in many types of membrane proteins [Minetti and Remeta, 2006]. The non-classical secretion of mIL1α bears striking similarity to the export of FGF1. They share common traits such as stress-dependence [Tarantini et al., 2001], formation of a release complex which includes S100A13 [Mandinova et al., 2003], and Cu2+-dependence [Mandinova et al., 2003]. In addition, similarly to FGF1, Coexpression of mIL1α and SK1 results in the limitation of SK1 release to stress conditions (R. Soldi and I. Prudovsky, unpublished work), indicating that SK1 may participate in IL1α release. Additionally the convergence of FGF1 and mIL1α export pathways is strengthened by the ability of pIL1α coexpression to block stress-induced FGF1 export [Tarantini et al., 2001]. In contrast to FGF1, the export of IL1α does not require p40 Syt1 [Mandinova et al., 2003]. We hypothesized that p40 Syt1 may be substituted in the IL1α release complex by another, still undefined, cytosolic protein which contains C2 domains [Prudovsky et al., 2003].
Recently it was found that the stress-induced export of syntaxin 2 (epimorphin) involves p40 Syt1 and annexin II [Hirai et al., 2007]. It remains to be elucidated whether S100A13, p40 Syt1, SK1, annexin II, and Cu2+ participate in the export of IL1β and FGF2.
TRANSLOCATION THROUGH THE CELL MEMBRANE: KEY PROBLEM OF THE NON-CLASSICAL PROTEIN EXPORT
Under stress, FGF1, S100A13, p40 Syt1, and mIL1α (all exhibiting diffuse cytoplasmic distribution under normal conditions) migrate to the cell membrane [Prudovsky et al., 2002; Mandinova et al., 2003], and this migration depends on the actin cytoskeleton [Prudovsky et al., 2002] (Fig. 1). Significantly, although the expression of dominant negative mutants of S100A13 and p40 Syt1 and copper chelation prevent the release of FGF1 from the stressed cells, they do not interfere with the peripheral translocation of the members of FGF1 export complex [Prudovsky et al., 2002]. These results suggest that the assembly of the complex occurs in the vicinity of the cell membrane [Prudovsky et al., 2003]. Indeed, FRET landscape of cells cotransfected with fluorescently tagged FGF1 and S100A13 indicate that at stress, these proteins associate at the cell membrane (H. Glick and I. Prudovsky, unpublished work).
Fig. 1.
Current working model of stress-induced FGF1 release. Stress enhances the expression of S100A13 and induces the actin cytoskeleton-dependent translocation of FGF1, S100A13, and p40 Syt1 to the inner leaflet of the cell membrane where they bind to acidic phospholipids. SK1 located on the inner leaflet of the cell membrane serves as a source of copper ions (which are provided to SK1 by the transmembrane copper transporters). Copper delivered by SK1 is required for the formation of the release complex. This complex includes the covalent FGF1 dimer, non-covalent S100A13 dimer, p40 Syt1 and SK1. The release complex binds to Anx II, which is also located on the inner leaflet of the cell membrane. Transmembrane flipping of acidic phospholipids results in the export of the FGF1 release complex and Anx II.
Cell membrane is asymmetric: acidic phospholipids, such as phosphatidylglycerol, phosphatidylserine, and phosphatidylinositol are present in the inner but not in the outer leaflet of the cell membrane [Pomorski et al., 2001]. FGF1, mIL1α, and the members of their release pathway specifically bind acidic phospholipids [Tarantini et al., 1995; Marqueze et al., 2000; Heizmann et al., 2002; Mandinova et al., 2003]. Moreover, FGF1 has been demonstrated to destabilize liposomes composed of acidic phospholipids [Mach and Middaugh, 1995]. We confirmed the latter result and also found that S100A13, p40 Syt1, and mIL1α destabilize acidic liposomes but not liposomes composed of the zwitterionic phospholipid phosphatidylcholine [Graziani et al., 2006; Mandinova et al., 2003]. Moreover, a novel and highly sensitive method of Sum Frequency Spectroscopy demonstrated that FGF1 in the nanomolar range of concentrations destabilizes the immobilized planar bilayer of phosphatidylglycerol [Doyle et al., 2004]. Interestingly, although S100A13 and FGF1 permeabilize phosphatidylglycerol, phosphatidylserine, and phosphatidylinositol liposomes, p40 Syt1 destabilizes only phosphatidylinositol liposomes [Graziani et al., 2006]. Conversely, mIL1α permeabilizes phosphatidylserine and phosphatidylglycerol but not phosphatidylinositol liposomes (Graziani and Prudovsky, unpublished work). We suggest that in the inner leaflet of the cell membrane “mosaics” of acidic phospholipids exist that are platforms for the assembly of release complexes from individual components that preferentially bind different lipid elements of the mosaic. Additionally, the combination of proteins with different membrane destabilizing characteristics may enhance the efficiency of complex translocation through the cell membrane.
Mutations of specific lysine clusters in the C2B domain of p40 Syt1 (Lys326,327,331) and in the C-terminal domain of FGF1 (Lys114,115 and Lys126,127) drastically decreased both the export of these proteins and their ability to destabilize liposomes [Graziani et al., 2006]. These results demonstrate that the membrane destabilizing properties of these polypeptides are indeed related to the non-classical export of FGF1.
Many types of extracellular stimuli induce acidic phospholipid flipping to the cell surface [Bevers et al., 1999] where phosphatidylserine is detected using fluorescently tagged recombinant Annexin V. Externalization of acidic phospholipids can be reversible [Fischer et al., 2006]. Heat-shocked NIH 3T3 cells display limited phosphatidylserine-positive membrane domains, but these domains rapidly disappear after the cells are returned to normal temperature (A. Kirov and I. Prudovsky, unpublished work). We suggest that when cell stress stimulates acidic phospholipid flipping, it occurs with higher probability in the areas where the membrane interacts with non-classical protein release complexes containing membrane destabilizing proteins. As a result, the release complexes translocate together with the acidic phospholipids and are exported to the extracellular compartment.
The translocation of proteins through the hydrophobic core of the membrane presents a difficult physical problem. Wesche et al. [2000] using FGF1 mutants with introduced intra-molecular disulfide bonds demonstrated that the escape of internalized extracellular FGF1 through the endosome membrane to the cytosol does not require its extensive unfolding. Mach and Middaugh [1995] showed that FGF1 at neutral pH, ambient temperature and low ionic strength, exists in a partially structured state capable of strong interaction with membrane vesicles. Sanz and Gimenez-Gallego [1997] demonstrated that FGF1 exhibits high lipid binding affinity at acidic pHs. Interestingly, Bychkova et al. [1988] investigating the structure of cytochrome c under acidic conditions, suggested that the negative membrane potential creates an acidic microenvironment which causes a partial denaturation of the proteins. The partially structured states generated at the membrane surface are believed to be highly competent to traverse across membrane bilayers because the partial unfolding results in the exposure of normally hidden hydrophobic amino acid residues [Bychkova et al., 1988; Ptitsyn, 1995]. These states are involved in protein recognition by chaperones [van der Vies et al., 1992], penetration of proteins across biomembranes [van der Goot et al., 1992], and release of ligands bound to proteins [Bychkova et al., 1990]. Recent studies indicate that both FGF1 and the C2A domain of p40 Syt1 interact with small unilamellar vesicles of phosphatidylserine [Rajalingam et al., 2007]. The lipid binding affinities of both FGF1 and the C2A domain of p40 Syt1 are significantly higher in their partially structured states than in their native states. Interestingly, the binding affinity between FGF1 and the C2A of p40 Syt1 domain is also significantly enhanced in their partially structured states (Rajalingam et al., 2007).
Our current working hypothesis is that the copper-dependent FGF1 and mIL1α release complexes assembled at the inner leaflet of the cell membrane flip through the membrane, and this flipping is ensured by the membrane destabilizing properties of the complex components and their partially unfolded structures (Fig. 1).
As mentioned above, SK1, S100A13, and p40 Syt1 can be exported from the cells independently of FGF1. Whereas SK1 appears to be the donor of copper ions needed for complex formation, S100A13 and p40 Syt1 can play a role of chaperones which stabilize FGF1 in the partially unfolded conformation. Indeed, the importance of chaperones for the post-translational membrane translocation of proteins into mitochondria and chloroplasts is well documented [Jackson-Constan et al., 2001].
Interestingly, the laboratory of Walter Nickel reported that the stress-independent export of FGF2 does not require the total unfolding of this protein [Backhaus et al., 2004]. In this experiment, FGF2 was tagged with the DHFR moiety, which can be prevented from total unfolding by DHFR inhibitor aminopterin. The authors demonstrated that aminopterin did not inhibit the export of FGF2:DHFR chimera. Thus, full unfolding is not a prerequisite of FGF2 export. However, this process may still require a partially unfolded state. Another highly interesting publication of the same laboratory reports that in a cell-free system, FGF2 can be transported into inverted (inner side out) vesicles derived from the cell membrane [Schafer et al., 2004]. Most surprisingly, this process did not require ATP hydrolysis. We are currently exploring the ability of FGF1 and other members of its export complex to exhibit membrane translocation in a similar cell free system.
More recently, Nickel and coworkers found that FGF2 and galectin 1 export requires the presence of heparan sulfate proteoglycans (HSPG) on the cell membrane [Seelenmeyer et al., 2005; Zehe et al., 2006]. FGF2 and galectin 1 are both lectins with a high affinity for HSPG. The hypothesis of the authors is that HSPG can serve as “traps” that prevent the reimport of FGF2 and galectin 1 into the cells. This hypothesis is also attractive and plausible for FGF1, which avidly binds heparin [Maciag et al., 1984]. However, most probably it does not apply to S100A13 and SK1 which do not exhibit heparin binding [Landriscina et al., 2001b] [Soldi et al., 2007].
SIGNALING PATHWAYS REGULATING NON-CLASSICAL EXPORT
The export of signal peptide-less proteins appears to be regulated by a variety of cell signaling pathways. FGF1 export can be induced under non-stress conditions by the inhibition of Notch signaling [Small et al., 2003] or by the stimulation of the thrombin receptor PAR1 [Duarte et al., 2006].
Considering the ubiquitous roles of Notch and FGF signaling systems in embryonic and postnatal development and their important role in angiogenesis [Ornitz and Itoh, 2001; Sahlgren and Lendahl, 2006], it would be surprising if these two pathways were not coordinated. The finding that the expression of Notch ligand Jagged 1 is induced in endothelial cells (ECs) during FGF-dependent angiogenesis in vitro [Zimrin et al., 1996] prompted us to explore the cell phenotype induced by expression of the soluble form of Jagged 1 (sJg1) corresponding to the extracellular domain (ecd) of the transmembrane molecule of Jagged 1. NIH 3T3 cells stably transfected with sJg1 assume a phenotype resembling ECs in the process of angiogenesis [Wong et al., 2000]. The most striking characteristic of this phenotype is the formation of multicellular cords on solid substrates. The injection of sJg1 transfectant cells into nude mice results in the formation of highly angiogenic non-metastatic tumors that exhibit very abundant blood vessels and sinusoids [Wong et al., 2000]. Interestingly, the transfection of NIH 3T3 cells with the soluble ecd of Notch 1 (sN1) and Notch 2 (sN2) results in a phenotype similar to sJg1, although not as strongly pronounced. The luciferase reporter analysis demonstrated a strong reduction of Notch-regulated CBF-1-dependent transcriptional activity in sJg1, sN1, and sN2 transfectant cells [Small et al., 2001]. Thus, soluble ecds of Jagged 1, Notch 1 and Notch 2 act as inhibitors of Notch signaling probably by interfering with the binding of transmembrane Notch ligands to their receptors. Later, it was found that the expression of the ecd of another Notch ligand, Delta 1 (sDl1), results in phenotypic changes similar to sJg1 [Trifonova et al., 2004].
sJg1 expression in NIH 3T3 cells induced a tyrosine phosphorylation pattern strikingly similar to that induced by FGF stimulation [Small et al., 2001]. When we sought to determine whether these characteristics were due to the induction of FGF release and/or expression, we found that unlike the vector-transfected NIH 3T3 cells, sJg1 transfectants constitutively released FGF1. The same results were obtained with sN1, sN2, and sDl1 transfectants [Small et al., 2003; Trifonova et al., 2004]. Moreover, sJg1 expression induced the transcription of FGF1, FGF4, and FGF5, which were not expressed in control cells. Transfection of sJg1 cells with constitutively active Notch 1 (caN1) completely abrogated the spontaneous release of FGF1, although it did not interfere with heat shock-induced FGF1 export. Consistent with their tumorigenic ability, sJg1 cells form colonies in soft agar. This colony formation was blocked by caN1 and by a specific chemical inhibitor of FGFR. Thus, the transformed phenotype of sJg1 cells is dependent on the inhibition of Notch signaling and activation of FGF signaling, which involves the derepression of FGF1, FGF4, and FGF5 transcription, and the release of FGF1. In conclusion, Notch seems to interfere with the FGF pathway at the levels of FGF expression and release. The cross-talk between the two pathways proceeds in both directions. Indeed, FGF1 induces the expression of Jagged 1 in NIH 3T3 cells [Small et al., 2003]. Moreover, FGF1 stimulation results in dose-dependent inhibition of both endogenous and caN1-dependent CBF-1-activated transcription [Small et al., 2003].
Serine protease thrombin, generated from prothrombin at sites of vascular injury elicits blood coagulation by cleaving fibrinogen and producing fibrin [Fenton, 1986]. Besides its function as a key enzyme of the coagulation cascade, thrombin is also a mitogen and regulator of angiogenesis [Coughlin, 2001]. Thrombin-induced signaling in the endothelium results in multiple phenotypic changes including: alterations in cell shape, endothelial monolayer permeability [Rabiet et al., 1996], mobilization of adhesive molecules to the endothelial surface [Kaplanski et al., 1998], DNA synthesis [Herbert et al., 1994], and cell migration [Pankonin and Teuscher, 1991]. Thrombin also induces proliferation of vascular smooth muscle cells [Berk et al., 1991]. The expression of several growth factors are induced in response to thrombin including FGF2 [Cucina et al., 2002; Cao et al., 2006], platelet-derived growth factor [Daniel et al., 1986; Harlan et al., 1986], and vascular endothelial growth factor [Bassus et al., 2001]. Thrombin also upregulates the insulin-like growth factor receptor 1 [Delafontaine et al., 1996], and induces the activation of fibroblast growth factor receptor 1 (FGFR1) [Rauch et al., 2004]. It was reported that thrombin treatment induces the appearance of FGF2 in the extracellular matrix [Rauch et al., 2004] and that cholesterol enhances the thrombin-induced release of FGF2 [Rauch et al., 2007].
We recently found that thrombin induces the transcription and non-classical release of FGF1 [Duarte et al., 2006]. Apparently, tissue damage accompanied by thrombin production and blood coagulation may induce local production and export of FGF1, which stimulates wound healing and angiogenesis. Thrombin-induced transcription and release of FGF1 are dependent on activation of thrombin receptor PAR1 [Duarte et al., 2006].
Considering the importance of Notch, FGF and thrombin signaling for many aspects of organism development and homeostasis, particularly for the process of angiogenesis, the study of molecular mechanisms of interaction between these three pathways is clearly an important task with potential translational outcomes in cardiology, oncology, wound healing, and stem cell technologies. It remains to be elucidated whether the multiprotein release complex formation underlying the stress-induced release is also required for FGF1 export stimulated by thrombin and inhibition of Notch signaling.
Little is known about the signaling pathways which regulate stress-induced FGF1 export. This process depends on de novo protein synthesis [Jackson et al., 1992]. However, using cells with knockouts of a variety of heat shock proteins and heat transcription factors, we have so far been unable to demonstrate that any of them are critical for the heat shock-induced FGF1 export (O. Sideleva and I. Prudovsky, unpublished work). Recently Matsunaga and Ueda found that in astrocytes, stress-induced FGF1 export requires an increase of intracellular calcium concentration that is important for the interaction of FGF1 and S100A13. Inhibitor analysis showed that the FGF1 export from astrocytes is regulated by voltage-dependent N-type Ca2+ channel activity [Matsunaga and Ueda, 2006]. Interestingly, stress-induced FGF1 export is also blocked by kynurenic acid, a specific antagonist of glutamate receptors of NMDA type [Di Serio et al., 2005].
In recent years, substantial progress has been achieved in the elucidation of mechanisms involved in the non-classical release of other signal peptide-less proteins. For example, Levine and colleagues demonstrated the involvement of p53 protein in the exosome-mediated secretion of HSP90 [Yu et al., 2006]. The results of Baptiste et al. [2007] indicate that mechano-transduction mediates both the secretion and uptake of galectin 3. Rubartelli and coauthors [Carta et al., 2006] found that microtubules are involved in the export of IL1β mediated by lysosome exocytosis. We can expect that better understanding of the regulation of non-classical release will allow new therapeutic approaches to pathologies involving signal peptide-less proteins.
BIOMEDICAL SIGNIFICANCE OF NON-CLASSICAL FGF1 AND IL1α RELEASE
Historically, FGF1 was discovered to be a potent stimulator of the proliferation of various specialized cells including endothelial and smooth muscle cells [Maciag et al., 1979; Winkles et al., 1987]. Further, FGF1 was demonstrated to stimulate a range of morphogenetic processes including angiogenesis [Thompson et al., 1988], neurite outgrowth [Renaud et al., 1996], and growth of the ureteric bud [Qiao et al., 2001]. Significantly, FGF1 and VEGF exhibit synergistic effects upon angiogenesis in vitro [Xue and Greisler, 2002]. The delivery or expression of FGF1 in vivo enhances vascularization of the myocardium, stimulates repair of ischemic lesions [Sellke et al., 1996; Schumacher et al., 1998; Buehler et al., 2002], reduces intestinal mucosal damages caused by ischemia [Chen et al., 2005], enhances hindlimb collaterization [Hershey et al., 2003], and protects the brain against hypoxic-ischemic injury [Russell et al., 2006]. FGF1 exhibits a stress-protective effect upon different cell types such as neurons [Hashimoto et al., 2002] and cardiomyocytes [Cuevas et al., 1997]. Transgenic mice expressing FGF1 in cardiomyocytes demonstrate a significant attenuation of infarct development in response to coronary artery occlusion [Buehler et al., 2002].
FGF1 is also involved in a variety of pathological processes. McKeehan and coworkers [Yu et al., 2003] demonstrated that FGF1/FGF2 deficient mice exhibit attenuation of carbon tetrachloride-induced hepatic matrix deposition and fibrogenesis. Apparently the release of FGF prototypes may present an interesting target for the prevention of liver fibrosis. The aggressive growth of several tumor types correlates with FGF1 expression [Woolley et al., 2000; Okunieff et al., 2003; Kwabi-Addo et al., 2004]. The expression of wild-type signal peptide-less FGF1 in hypoxic cells results in stress-induced FGF1 release [Mouta Carreira et al., 2001], and the core of solid tumors is characterized by hypoxic conditions [Moeller et al., 2004]. Thus, hypoxia-induced FGF1 release may stimulate tumor angiogenesis and enhance tumor growth.
IL1α a potent proinflammatory cytokine is also a regulator of the EC phenotype in vitro [Ristimaki et al., 1994; Fitzgerald and O’Neill, 1999; Nyberg et al., 2000; Dube et al., 2001]. In particular, IL1α has been reported to exert an effect on EC cultures, which is opposite to the effect of FGF1, by inhibiting EC proliferation [Gerol et al., 1998]. In contrast, IL1α promotes angiogenesis in experimental in vivo systems [Norrby, 1997]. This effect of IL1α may be mediated by induction of the expression of FGF1, FGF2, and VEGF [Ko et al., 1999; Samaniego et al., 1995], particularly in tumor cells [Torisu et al., 2000]. Experiments on IL1α and IL1β knockout mice demonstrated that these cytokines enhance tumor growth apparently through the stimulation of tumor angiogenesis [Voronov et al., 2003]. Local expression of IL1ra impedes restenosis observed after balloon injury to the carotid arteries in rats [Mandinov et al., 2003]. Collectively, these data indicate that, similarly to FGF1, IL1α is an important regulator of blood vessel remodeling.
Clinical studies by Merajver and Brewer [Brewer et al., 2000; Cox et al., 2001] demonstrated that TM represses the growth of stage IV solid tumors in humans. Also, TM attenuates mammary gland tumor formation in Her transgenic mice [Pan et al., 2002]. The evidence that TM inhibits non-classical export of FGF1 and IL1α [Landriscina et al., 2001a; Mandinova et al., 2003] indicate that the tumor-suppressing effect of TM may be due to the blockage of the export of these two proteins. It is also interesting to note that high grade gliomas are characterized by the enhanced expression of S100A13, a critical member of FGF1 and IL1α release complexes, and this correlates with a more angiogenic phenotype of the tumors [Landriscina et al., 2006]. Macrophages are a rich source of FGF1 [Sano et al., 1990; Brogi et al., 1993], and extracellular IL1α is a potent chemoattractant for macrophages [Dinarello, 1996]. It is hypothesized that when TM inhibits the release of IL1α in hypoxic tumor sites, the recruitment of macrophages is limited, and macrophages present in tumors cannot release FGF1 due to Cu2+ depletion. A similar mechanism may be involved in the regulation of restenosis of large vessels after balloon-mediated removal of atherosclerotic plaques [Mandinov et al., 2003]. Balloon injury may induce the release of IL1α and attract FGF1-laden macrophages to the damaged area. Local stress conditions may then stimulate the release of FGF1 from macrophages and, as a result, FGF1-dependent proliferation of smooth muscle cells and the subsequent intimal thickening of the vessel wall. Long-term administration of TM significantly reduces balloon injury-induced restenosis in carotid arteries [Mandinov et al., 2003]. Both intima thickening and formation of the vasa vasorum are drastically inhibited. Additionally, TM downregulates the number of macrophages, as well as FGF1, S100A13, and IL1α levels in carotid artery walls. It was also found that TM inhibits in-stent restenosis in porcine coronary arteries [Mandinov et al., 2006], These data suggest that inhibition of the non-classical release of FGF1 and IL1α by Cu2+ chelation may represent a promising approach to the management of restenosis. More recently, it was demonstrated that in rats TM blocks the development of antigen-induced arthritis, a pathology that involves IL1 [Omoto et al., 2005].
FUTURE DIRECTIONS
Export of signal peptide-less growth factors and cytokines is a dynamically developing research field. The most important aspects of the non-classical release to be addressed are: (1) molecular mechanisms of transmembrane translocation of non-classically released proteins; (2) signaling networks which regulate the non-classical export; (3) role of non-classical protein export during the normal organism development and in pathological conditions. Successful approaches to these problems requires the collaboration of cell and molecular biologists, biophysicists, developmental biologists, and clinical specialists.
Acknowledgments
The authors acknowledge the following grant support: IP—NIH grants HL32348, HL35627, and RR15555 (project 4); ML—PRIN grant # 2004054004-002; DS—NIH grant R15DK070599 and USDA grant 11h93; TKSK—NIH grant RR15569 (project 1), Department of Energy grant DE-FGF02-01ER15161 and the grant of Arkansas Bioscience.
Grant sponsor: NIH; Grant numbers: HL32348, HL35627, RR15555, R15DK070599, RR15569; Grant sponsor: PRIN; Grant number: 2004054004-002; Grant sponsor: USDA; Grant number: 11h93; Grant sponsor: Department of Energy; Grant number: DE-FGF02-01ER15161; Grant sponsor: Arkansas Bioscience.
Footnotes
The review is dedicated to the memory of Tom Maciag, who made seminal contributions to the study of non-classical protein export.
References
- Ancellin N, Colmont C, Su J, Li Q, Mittereder N, Chae SS, Stefansson S, Liau G, Hla T. Extracellular export of sphingosine kinase-1 enzyme. Sphingosine 1-phosphate generation and the induction of angiogenic vascular maturation. J Biol Chem. 2002;277:6667–6675. doi: 10.1074/jbc.M102841200. [DOI] [PubMed] [Google Scholar]
- Andrei C, Dazzi C, Lotti L, Torrisi MR, Chimini G, Rubartelli A. The secretory route of the leaderless protein interleukin 1beta involves exocytosis of endolysosome-related vesicles. Mol Biol Cell. 1999;10:1463–1475. doi: 10.1091/mbc.10.5.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelini G, Gardella S, Ardy M, Ciriolo MR, Filomeni G, Di Trapani G, Clarke F, Sitia R, Rubartelli A. Antigen-presenting dendritic cells provide the reducing extracellular microenvironment required for T lymphocyte activation. Proc Natl Acad Sci USA. 2002;99:1491–1496. doi: 10.1073/pnas.022630299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnesano F, Banci L, Bertini I, Fantoni A, Tenori L, Viezzoli MS. Structural interplay between calcium(II) and copper(II) binding to S100A13 protein. Angew Chem Int Ed Engl. 2005;44:6341–6344. doi: 10.1002/anie.200500540. [DOI] [PubMed] [Google Scholar]
- Aumuller G, Wilhelm B, Seitz J. Apocrine secretion—fact or artifact? Anat Anz. 1999;181:437–446. doi: 10.1016/S0940-9602(99)80020-X. [DOI] [PubMed] [Google Scholar]
- Backhaus R, Zehe C, Wegehingel S, Kehlenbach A, Schwappach B, Nickel W. Unconventional protein secretion: Membrane translocation of FGF-2 does not require protein unfolding. J Cell Sci. 2004;117:1727–1736. doi: 10.1242/jcs.01027. [DOI] [PubMed] [Google Scholar]
- Bagala C, Kolev V, Mandinova A, Soldi R, Mouta C, Graziani I, Prudovsky I, Maciag T. The alternative translation of synaptotagmin 1 mediates the non-classical release of F GF1. Biochem Biophys Res Commun. 2003;310:1041–1047. doi: 10.1016/j.bbrc.2003.09.119. [DOI] [PubMed] [Google Scholar]
- Baptiste TA, James A, Saria M, Ochieng J. Mechano-transduction mediated secretion and uptake of galectin-3 in breast carcinoma cells: Implications in the extracellular functions of the lectin. Exp Cell Res. 2007;313:652–664. doi: 10.1016/j.yexcr.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassus S, Herkert O, Kronemann N, Gorlach A, Bremerich D, Kirchmaier CM, Busse R, Schini-Kerth VB. Thrombin causes vascular endothelial growth factor expression in vascular smooth muscle cells: Role of reactive oxygen species. Arterioscler Thromb Vasc Biol. 2001;21:1550–1555. doi: 10.1161/hq0901.095148. [DOI] [PubMed] [Google Scholar]
- Berk BC, Taubman MB, Griendling KK, Cragoe EJ, Jr, Fenton JW, Brock TA. Thrombin-stimulated events in cultured vascular smooth-muscle cells. Biochem J. 1991;274(Pt 3):799–805. doi: 10.1042/bj2740799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevers EM, Comfurius P, Dekkers DW, Zwaal RF. Lipid translocation across the plasma membrane of mammalian cells. Biochim Biophys Acta. 1999;1439:317–330. doi: 10.1016/s1388-1981(99)00110-9. [DOI] [PubMed] [Google Scholar]
- Blobel G. Unidirectional and bidirectional protein traffic across membranes. Cold Spring Harb Symp Quant Biol. 1995;60:1–10. doi: 10.1101/sqb.1995.060.01.003. [DOI] [PubMed] [Google Scholar]
- Bonaldi T, Talamo F, Scaffidi P, Ferrera D, Porto A, Bachi A, Rubartelli A, Agresti A, Bianchi ME. Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect it towards secretion. EMBO J. 2003;22:5551–5560. doi: 10.1093/emboj/cdg516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer GJ, Dick RD, Grover DK, LeClaire V, Tseng M, Wicha M, Pienta K, Redman BG, Jahan T, Sondak VK, Strawderman M, LeCarpentier G, Merajver SD. Treatment of metastatic cancer with tetrathiomolybdate, an anticopper, antiangiogenic agent: Phase I study. Clin Cancer Res. 2000;6:1–10. [PubMed] [Google Scholar]
- Brogi E, Winkles JA, Underwood R, Clinton SK, Alberts GF, Libby P. Distinct patterns of expression of fibroblast growth factors and their receptors in human atheroma and nonatherosclerotic arteries. Association of acidic FGF with plaque microvessels and macrophages. J Clin Invest. 1993;92:2408–2418. doi: 10.1172/JCI116847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buehler A, Martire A, Strohm C, Wolfram S, Fernandez B, Palmen M, Wehrens XH, Doevendans PA, Franz WM, Schaper W, Zimmermann R. Angiogenesis-independent cardioprotection in FGF-1 transgenic mice. Cardiovasc Res. 2002;55:768–777. doi: 10.1016/s0008-6363(02)00494-7. [DOI] [PubMed] [Google Scholar]
- Bychkova VE, Pain RH, Ptitsyn OB. The ’molten globule’ state is involved in the translocation of proteins across membranes? FEBS Lett. 1988;238:231–234. doi: 10.1016/0014-5793(88)80485-x. [DOI] [PubMed] [Google Scholar]
- Bychkova VE, Bartoshevich SF, Klenin SI. [Comparative study of diffusion coefficients of alpha-lactalbumins and lysozyme using polarization interferometer] Biofizika. 1990;35:242–248. [PubMed] [Google Scholar]
- Cao H, Dronadula N, Rao GN. Thrombin induces expression of FGF-2 via activation of PI3K-Akt-Fra-1 signaling axis leading to DNA synthesis and motility in vascular smooth muscle cells. Am J Physiol Cell Physiol. 2006;290:C172–C182. doi: 10.1152/ajpcell.00284.2005. [DOI] [PubMed] [Google Scholar]
- Carta S, Tassi S, Semino C, Fossati G, Mascagni P, Dinarello CA, Rubartelli A. Histone deacetylase inhibitors prevent exocytosis of interleukin-1beta-containing secretory lysosomes: Role of microtubules. Blood. 2006;108:1618–1626. doi: 10.1182/blood-2006-03-014126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HC, Samaniego F, Nair BC, Buonaguro L, Ensoli B. HIV-1 Tat protein exits from cells via a leaderless secretory pathway and binds to extracellular matrix-associated heparan sulfate proteoglycans through its basic region. Aids. 1997;11:1421–1431. doi: 10.1097/00002030-199712000-00006. [DOI] [PubMed] [Google Scholar]
- Chapman LP, Epton MJ, Buckingham JC, Morris JF, Christian HC. Evidence for a role of the adenosine 5′-triphosphate-binding cassette transporter A1 in the externalization of annexin I from pituitary folliculo-stellate cells. Endocrinology. 2003;144:1062–1073. doi: 10.1210/en.2002-220650. [DOI] [PubMed] [Google Scholar]
- Chen W, Fu XB, Ge SL, Sun TZ, Li WJ, Sheng ZY. Acid fibroblast growth factor reduces rat intestinal mucosal damage caused by ischemia-reperfusion insult. World J Gastroenterol. 2005;11:6477–6482. doi: 10.3748/wjg.v11.i41.6477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlin SR. Protease-activated receptors in vascular biology. Thromb Haemost. 2001;86:298–307. [PubMed] [Google Scholar]
- Coulier F, Pontarotti P, Roubin R, Hartung H, Goldfarb M, Birnbaum D. Of worms and men: An evolutionary perspective on the fibroblast growth factor (FGF) and FGF receptor families. J Mol Evol. 1997;44:43–56. doi: 10.1007/pl00006120. [DOI] [PubMed] [Google Scholar]
- Cox C, Teknos TN, Barrios M, Brewer GJ, Dick RD, Merajver SD. The role of copper suppression as an antiangiogenic strategy in head and neck squamous cell carcinoma. Laryngoscope. 2001;111:696–701. doi: 10.1097/00005537-200104000-00024. [DOI] [PubMed] [Google Scholar]
- Cucina A, Borrelli V, Lucarelli M, Sterpetti AV, Cavallaro A, Strom R, Santoro-D’Angelo L, Scarpa S. Autocrine production of basic fibroblast growth factor translated from novel synthesized mRNA mediates thrombin-induced mitogenesis in smooth muscle cells. Cell Biochem Funct. 2002;20:39–46. doi: 10.1002/cbf.938. [DOI] [PubMed] [Google Scholar]
- Cuevas P, Carceller F, Lozano RM, Crespo A, Zazo M, Gimenez-Gallego G. Protection of rat myocardium by mitogenic and non-mitogenic fibroblast growth factor during post-ischemic reperfusion. Growth Factors. 1997;15:29–40. doi: 10.3109/08977199709002110. [DOI] [PubMed] [Google Scholar]
- Daniel TO, Gibbs VC, Milfay DF, Garovoy MR, Williams LT. Thrombin stimulates c-sis gene expression in microvascular endothelial cells. J Biol Chem. 1986;261:9579–9582. [PubMed] [Google Scholar]
- Davey GE, Murmann P, Heizmann CW. Intracellular Ca2+ and Zn2+ levels regulate the alternative cell density-dependent secretion of S100B in human glioblastoma cells. J Biol Chem. 2001;276:30819–30826. doi: 10.1074/jbc.M103541200. [DOI] [PubMed] [Google Scholar]
- Davidson J, Milton AS, Rotondo D. A study of the pyrogenic actions of interleukin-1 alpha and interleukin-1 beta: Interactions with a steroidal and a non-steroidal anti-inflammatory agent. Br J Pharmacol. 1990;100:542–546. doi: 10.1111/j.1476-5381.1990.tb15843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delafontaine P, Anwar A, Lou H, Ku L. G-protein coupled and tyrosine kinase receptors: Evidence that activation of the insulin-like growth factor I receptor is required for thrombin-induced mitogenesis of rat aortic smooth muscle cells. J Clin Invest. 1996;97:139–145. doi: 10.1172/JCI118381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny PW, Gokool S, Russell DG, Field MC, Smith DF. Acylation-dependent protein export in Leishmania. J Biol Chem. 2000;275:11017–11025. doi: 10.1074/jbc.275.15.11017. [DOI] [PubMed] [Google Scholar]
- Deora AB, Kreitzer G, Jacovina AT, Hajjar KA. An annexin 2 phosphorylation switch mediates p11-dependent translocation of annexin 2 to the cell surface. J Biol Chem. 2004;279:43411–43418. doi: 10.1074/jbc.M408078200. [DOI] [PubMed] [Google Scholar]
- Di Serio C, Cozzi A, Angeli I, Doria L, Micucci I, Pellerito S, Mirone P, Masotti G, Moroni F, Tarantini F. Kynurenic acid inhibits the release of the neurotrophic fibroblast growth factor (FGF)-1 and enhances proliferation of glia cells, in vitro. Cell Mol Neurobiol. 2005;25:981–993. doi: 10.1007/s10571-005-8469-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- Dinarello CA. Interleukin-1, interleukin-1 receptors and interleukin-1 receptor antagonist. Int Rev Immunol. 1998;16:457–499. doi: 10.3109/08830189809043005. [DOI] [PubMed] [Google Scholar]
- Donato R. Intracellular and extracellular roles of S100 proteins. Microsc Res Tech. 2003;60:540–551. doi: 10.1002/jemt.10296. [DOI] [PubMed] [Google Scholar]
- Doyle AW, Fick J, Himmelhaus M, Eck W, Graziani I, Prudovsky I, Grunze M, Maciag T, Neivandt D. Protein deformation of lipid hybrid bilayer membranes studied by Sum Frequency Generation Vibrational Spectroscopy (SFS) Langmuir. 2004;20:8961–8965. doi: 10.1021/la0484220. [DOI] [PubMed] [Google Scholar]
- Duarte M, Kolev V, Soldi R, Kirov A, Graziani I, Oliveira SM, Kacer D, Friesel R, Maciag T, Prudovsky I. Thrombin induces rapid PAR1-mediated non-classical FGF1 release. Biochem Biophys Res Commun. 2006;350:604–609. doi: 10.1016/j.bbrc.2006.09.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube PH, Revell PA, Chaplin DD, Lorenz RG, Miller VL. A role for IL-1 alpha in inducing pathologic inflammation during bacterial infection. Proc Natl Acad Sci USA. 2001;98:10880–10885. doi: 10.1073/pnas.191214498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott G, O’Hare P. Intercellular trafficking and protein delivery by a herpesvirus structural protein. Cell. 1997;88:223–233. doi: 10.1016/s0092-8674(00)81843-7. [DOI] [PubMed] [Google Scholar]
- Engleka KA, Maciag T. Inactivation of human fibroblast growth factor-1 (FGF-1) activity by interaction with copper ions involves FGF-1 dimer formation induced by copper-catalyzed oxidation. J Biol Chem. 1992;267:11307–11315. [PubMed] [Google Scholar]
- Ericson ML, Horling J, Wendel-Hansen V, Holmgren A, Rosen A. Secretion of thioredoxin after in vitro activation of human B cells. Lymphokine Cytokine Res. 1992;11:201–207. [PubMed] [Google Scholar]
- Fenton JW., II Thrombin. Ann NY Acad Sci. 1986;485:5–15. doi: 10.1111/j.1749-6632.1986.tb34563.x. [DOI] [PubMed] [Google Scholar]
- Fischer K, Voelkl S, Berger J, Andreesen R, Pomorski T, Mackensen A. Antigen recognition induces phosphatidylserine exposure on the cell surface of human CD8+ T cells. Blood. 2006;108:4094–4101. doi: 10.1182/blood-2006-03-011742. [DOI] [PubMed] [Google Scholar]
- Fitzgerald KA, O’Neill LA. Characterization of CD44 induction by IL-1: A critical role for Egr-1. J Immunol. 1999;162:4920–4927. [PubMed] [Google Scholar]
- Flatmark K, Maelandsmo GM, Mikalsen SO, Nustad K, Varaas T, Rasmussen H, Meling GI, Fodstad O, Paus E. Immunofluorometric assay for the metastasis-related protein S100 A4: Release of S100A4 from normal blood cells prohibits the use of S100A4 as a tumor marker in plasma and serum. Tumour Biol. 2004;25:31–40. doi: 10.1159/000077721. [DOI] [PubMed] [Google Scholar]
- Florkiewicz RZ, Anchin J, Baird A. The inhibition of fibroblast growth factor-2 export by cardenolides implies a novel function for the catalytic subunit of Na+, K+ATPase. J Biol Chem. 1998;273:544–551. doi: 10.1074/jbc.273.1.544. [DOI] [PubMed] [Google Scholar]
- Forough R, Xi Z, MacPhee M, Friedman S, Engleka KA, Sayers T, Wiltrout RH, Maciag T. Differential transforming abilities of non-secreted and secreted forms of human fibroblast growth factor-1. J Biol Chem. 1993;268:2960–2968. [PubMed] [Google Scholar]
- Friesel R, Maciag T. Fibroblast growth factor prototype release and fibroblast growth factor receptor signaling. Thromb Haemost. 1999;82:748–754. [PubMed] [Google Scholar]
- Gardella S, Andrei C, Ferrera D, Lotti LV, Torrisi MR, Bianchi ME, Rubartelli A. The nuclear protein HMGB1 is secreted by monocytes via a non-classical, vesicle-mediated secretory pathway. EMBO Rep. 2002;3:995–1001. doi: 10.1093/embo-reports/kvf198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerol M, Curry L, McCarroll L, Doctrow S, RayChaudhury A. Growth regulation of cultured endothelial cells by inflammatory cytokines: Mitogenic, anti-proliferative and cytotoxic effects. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1998;120:397–404. doi: 10.1016/s0742-8413(98)10064-6. [DOI] [PubMed] [Google Scholar]
- Graves BJ, Hatada MH, Hendrickson WA, Miller JK, Madison VS, Satow Y. Structure of interleukin 1 alpha at 2.7-A resolution. Biochemistry. 1990;29:2679–2684. doi: 10.1021/bi00463a009. [DOI] [PubMed] [Google Scholar]
- Graziani I, Bagala C, Duarte M, Soldi R, Kolev V, Tarantini F, Kumar TK, Doyle A, Neivandt D, Yu C, Maciag T, Prudovsky I. Release of FGF1 and p40 synaptotagmin 1 correlates with their membrane destabilizing ability. Biochem Biophys Res Commun. 2006 doi: 10.1016/j.bbrc.2006.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajjar KA, Krishnan S. Annexin II: A mediator of the plasmin/plasminogen activator system. Trends Cardiovasc Med. 1999;9:128–138. doi: 10.1016/s1050-1738(99)00020-1. [DOI] [PubMed] [Google Scholar]
- Harlan JM, Thompson PJ, Ross RR, Bowen-Pope DF. Alpha-thrombin induces release of platelet-derived growth factor-like molecule(s) by cultured human endothelial cells. J Cell Biol. 1986;103:1129–1133. doi: 10.1083/jcb.103.3.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto M, Sagara Y, Langford D, Everall IP, Mallory M, Everson A, Digicaylioglu M, Masliah E. Fibroblast growth factor 1 regulates signaling via the glycogen synthase kinase-3beta pathway. Implications for neuroprotection. J Biol Chem. 2002;277:32985–32991. doi: 10.1074/jbc.M202803200. [DOI] [PubMed] [Google Scholar]
- Heizmann CW, Fritz G, Schafer BW. S100 proteins: Structure, functions and pathology. Front Biosci. 2002;7:d1356–d1368. doi: 10.2741/A846. [DOI] [PubMed] [Google Scholar]
- Herbert JM, Dupuy E, Laplace MC, Zini JM, Bar Shavit R, Tobelem G. Thrombin induces endothelial cell growth via both a proteolytic and a non-proteolytic pathway. Biochem J. 1994;303(Pt 1):227–231. doi: 10.1042/bj3030227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershey JC, Corcoran HA, Baskin EP, Gilberto DB, Mao X, Thomas KA, Cook JJ. Enhanced hindlimb collateralization induced by acidic fibroblast growth factor is dependent upon femoral artery extraction. Cardiovasc Res. 2003;59:997–1005. doi: 10.1016/s0008-6363(03)00522-4. [DOI] [PubMed] [Google Scholar]
- Hirai Y, Nelson CM, Yamazaki K, Takebe K, Przybylo J, Madden B, Radisky DC. Non-classical export of epimorphin and its adhesion to {alpha}vintegrin in regulation of epithelial morphogenesis. J Cell Sci. 2007;120:2032–2043. doi: 10.1242/jcs.006247. [DOI] [PubMed] [Google Scholar]
- Hu GF. Copper stimulates proliferation of human endothelial cells under culture. J Cell Biochem. 1998;69:326–335. doi: 10.1002/(sici)1097-4644(19980601)69:3<326::aid-jcb10>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Hunter-Lavin C, Davies EL, Bacelar MM, Marshall MJ, Andrew SM, Williams JH. Hsp70 release from peripheral blood mononuclear cells. Biochem Biophys Res Commun. 2004;324:511–517. doi: 10.1016/j.bbrc.2004.09.075. [DOI] [PubMed] [Google Scholar]
- Itoh N, Ornitz DM. Evolution of the Fgf and Fgfr gene families. Trends Genet. 2004;20:563–569. doi: 10.1016/j.tig.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Jackson A, Friedman S, Zhan X, Engleka KA, Forough R, Maciag T. Heat shock induces the release of fibroblast growth factor 1 from NIH 3T3 cells. Proc Natl Acad Sci USA. 1992;89:10691–10695. doi: 10.1073/pnas.89.22.10691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson A, Tarantini F, Gamble S, Friedman S, Maciag T. The release of fibroblast growth factor-1 from NIH 3T3 cells in response to temperature involves the function of cysteine residues. J Biol Chem. 1995;270:33–36. doi: 10.1074/jbc.270.1.33. [DOI] [PubMed] [Google Scholar]
- Jackson-Constan D, Akita M, Keegstra K. Molecular chaperones involved in chloroplast protein import. Biochim Biophys Acta. 2001;1541:102–113. doi: 10.1016/s0167-4889(01)00148-3. [DOI] [PubMed] [Google Scholar]
- Joliot A, Maizel A, Rosenberg D, Trembleau A, Dupas S, Volovitch M, Prochiantz A. Identification of a signal sequence necessary for the unconventional secretion of Engrailed homeoprotein. Curr Biol. 1998;8:856–863. doi: 10.1016/s0960-9822(07)00346-6. [DOI] [PubMed] [Google Scholar]
- Kaplanski G, Marin V, Fabrigoule M, Boulay V, Benoliel AM, Bongrand P, Kaplanski S, Farnarier C. Thrombin-activated human endothelial cells support monocyte adhesion in vitro following expression of intercellular adhesion molecule-1 (ICAM-1; CD54) and vascular cell adhesion molecule-1 (VCAM-1; CD106) Blood. 1998;92:1259–1267. [PubMed] [Google Scholar]
- Keryer-Bibens C, Pioche-Durieu C, Villemant C, Souquere S, Nishi N, Hirashima M, Middeldorp J, Busson P. Exosomes released by EBV-infected nasopharyngeal carcinoma cells convey the viral latent membrane protein 1 and the immunomodulatory protein galectin 9. BMC Cancer. 2006;6:283. doi: 10.1186/1471-2407-6-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Hajjar KA. Annexin II: A plasminogen-plasminogen activator co-receptor. Front Biosci. 2002;7:d341–d348. doi: 10.2741/kim. [DOI] [PubMed] [Google Scholar]
- Ko Y, Totzke G, Gouni-Berthold I, Sachinidis A, Vetter H. Cytokine-inducible growth factor gene expression in human umbilical endothelial cells. Mol Cell Probes. 1999;13:203–211. doi: 10.1006/mcpr.1999.0236. [DOI] [PubMed] [Google Scholar]
- Kwabi-Addo B, Ozen M, Ittmann M. The role of fibroblast growth factors and their receptors in prostate cancer. Endocr Relat Cancer. 2004;11:709–724. doi: 10.1677/erc.1.00535. [DOI] [PubMed] [Google Scholar]
- Landriscina M, Bagala C, Mandinova A, Soldi R, Micucci I, Bellum S, Prudovsky I, Maciag T. Copper induces the assembly of a multiprotein aggregate implicated in the release of fibroblast growth factor 1 in response to stress. J Biol Chem. 2001a;276:25549–25557. doi: 10.1074/jbc.M102925200. [DOI] [PubMed] [Google Scholar]
- Landriscina M, Soldi R, Bagala C, Micucci I, Bellum S, Tarantini F, Prudovsky I, Maciag T. S100A13 participates in the release of fibroblast growth factor 1 in response to heat shock in vitro. J Biol Chem. 2001b;276:22544–22552. doi: 10.1074/jbc.M100546200. [DOI] [PubMed] [Google Scholar]
- Landriscina M, Schinzari G, Di Leonardo G, Quirino M, Cassano A, D’Argento E, Lauriola L, Scerrati M, Prudovsky I, Barone C. S100A13, a new marker of angiogenesis in human astrocytic gliomas. J Neuro-oncol. 2006;80:251–259. doi: 10.1007/s11060-006-9189-y. [DOI] [PubMed] [Google Scholar]
- LaVallee TM, Tarantini F, Gamble S, Carreira CM, Jackson A, Maciag T. Synaptotagmin-1 is required for fibroblast growth factor-1 release. J Biol Chem. 1998;273:22217–22223. doi: 10.1074/jbc.273.35.22217. [DOI] [PubMed] [Google Scholar]
- Lecellier CH, Vermeulen W, Bachelerie F, Giron ML, Saib A. Intra- and intercellular trafficking of the foamy virus auxiliary bet protein. J Virol. 2002;76:3388–3394. doi: 10.1128/JVI.76.7.3388-3394.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Zhang PF, Pan XW, Chang WR. Crystal structure study on human S100A13 at 2.0 A resolution. Biochem Biophys Res Commun. 2007;356:616–621. doi: 10.1016/j.bbrc.2007.03.014. [DOI] [PubMed] [Google Scholar]
- Lue H, Kleemann R, Calandra T, Roger T, Bernhagen J. Macrophage migration inhibitory factor (MIF): Mechanisms of action and role in disease. Microbes Infect. 2002;4:449–460. doi: 10.1016/s1286-4579(02)01560-5. [DOI] [PubMed] [Google Scholar]
- Mach H, Middaugh CR. Interaction of partially structured states of acidic fibroblast growth factor with phospholipid membranes. Biochemistry. 1995;34:9913–9920. doi: 10.1021/bi00031a013. [DOI] [PubMed] [Google Scholar]
- Maciag T, Cerundolo J, Ilsley S, Kelley PR, Forand R. An endothelial cell growth factor from bovine hypothalamus: Identification and partial characterization. Proc Natl Acad Sci USA. 1979;76:5674–6578. doi: 10.1073/pnas.76.11.5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciag T, Mehlman T, Friesel R, Schreiber AB. Heparin binds endothelial cell growth factor, the principal endothelial cell mitogen in bovine brain. Science. 1984;225:932–935. doi: 10.1126/science.6382607. [DOI] [PubMed] [Google Scholar]
- Maizel A, Bensaude O, Prochiantz A, Joliot A. A short region of its homeodomain is necessary for engrailed nuclear export and secretion. Development. 1999;126:3183–3190. doi: 10.1242/dev.126.14.3183. [DOI] [PubMed] [Google Scholar]
- Maizel A, Tassetto M, Filhol O, Cochet C, Prochiantz A, Joliot A. Engrailed homeoprotein secretion is a regulated process. Development. 2002;129:3545–3553. doi: 10.1242/dev.129.15.3545. [DOI] [PubMed] [Google Scholar]
- Mambula SS, Calderwood SK. Heat shock protein 70 is secreted from tumor cells by a nonclassical pathway involving lysosomal endosomes. J Immunol. 2006;177:7849–7857. doi: 10.4049/jimmunol.177.11.7849. [DOI] [PubMed] [Google Scholar]
- Mandinov L, Mandinova A, Kyurkchiev S, Kyurkchiev D, Kehayov I, Kolev V, Soldi R, Bagala C, De Muinck ED, Lindner V, Post MJ, Simons M, Bellum S, Prudovsky I, Maciag T. Copper chelation represses the vascular response to injury. Proc Natl Acad Sci USA. 2003;100:6700–6705. doi: 10.1073/pnas.1231994100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandinov L, Moodie KL, Mandinova A, Zhuang Z, Redican F, Baklanov D, Lindner V, Maciag T, Simons M, de Muinck ED. Inhibition of in-stent restenosis by oral copper chelation in porcine coronary arteries. Am J Physiol Heart Circ Physiol. 2006;291:H2692–H2697. doi: 10.1152/ajpheart.00148.2006. [DOI] [PubMed] [Google Scholar]
- Mandinova A, Soldi R, Graziani I, Bagala C, Bellum S, Landriscina M, Tarantini F, Prudovsky I, Maciag T. S100A13 mediates the copper-dependent stress-induced release of IL-1{alpha} from both human U937 and murine NIH 3T3 cells. J Cell Sci. 2003;116:2687–2696. doi: 10.1242/jcs.00471. [DOI] [PubMed] [Google Scholar]
- Marqueze B, Berton F, Seagar M. Synaptotagmins in membrane traffic: Which vesicles do the tagmins tag? Biochimie. 2000;82:409–420. doi: 10.1016/s0300-9084(00)00220-0. [DOI] [PubMed] [Google Scholar]
- Matsunaga H, Ueda H. Voltage-dependent N-type Ca2+ channel activity regulates the interaction between FGF-1 and S100A13 for stress-induced non-vesicular release. Cell Mol Neurobiol. 2006 doi: 10.1007/s10571-006-9016-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehul B, Hughes RC. Plasma membrane targetting, vesicular budding and release of galectin 3 from the cytoplasm of mammalian cells during secretion. J Cell Sci. 1997;110(Pt 10):1169–1178. doi: 10.1242/jcs.110.10.1169. [DOI] [PubMed] [Google Scholar]
- Mignatti P, Morimoto T, Rifkin DB. Basic fibroblast growth factor, a protein devoid of secretory signal sequence, is released by cells via a pathway independent of the endoplasmic reticulum-Golgi complex. J Cell Physiol. 1992;151:81–93. doi: 10.1002/jcp.1041510113. [DOI] [PubMed] [Google Scholar]
- Minetti CA, Remeta DP. Energetics of membrane protein folding and stability. Arch Biochem Biophys. 2006;453:32–53. doi: 10.1016/j.abb.2006.03.023. [DOI] [PubMed] [Google Scholar]
- Misumi Y, Miki K, Takatsuki A, Tamura G, Ikehara Y. Novel blockade by brefeldin A of intracellular transport of secretory proteins in cultured rat hepatocytes. J Biol Chem. 1986;261:11398–11403. [PubMed] [Google Scholar]
- Miyakawa K, Imamura T. Secretion of FGF-16 requires an uncleaved bipartite signal sequence. J Biol Chem. 2003;278:35718–35724. doi: 10.1074/jbc.M300690200. [DOI] [PubMed] [Google Scholar]
- Miyakawa K, Hatsuzawa K, Kurokawa T, Asada M, Kuroiwa T, Imamura T. A hydrophobic region locating at the center of fibroblast growth factor-9 is crucial for its secretion. J Biol Chem. 1999;274:29352–29357. doi: 10.1074/jbc.274.41.29352. [DOI] [PubMed] [Google Scholar]
- Moeller BJ, Cao Y, Vujaskovic Z, Li CY, Haroon ZA, Dewhirst MW. The relationship between hypoxia and angiogenesis. Semin Radiat Oncol. 2004;14:215–221. doi: 10.1016/j.semradonc.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Mouta Carreira C, LaVallee TM, Tarantini F, Jackson A, Lathrop JT, Hampton B, Burgess WH, Maciag T. S100A13 is involved in the regulation of fibroblast growth factor-1 and p40 synaptotagmin-1 release in vitro. J Biol Chem. 1998;273:22224–22231. doi: 10.1074/jbc.273.35.22224. [DOI] [PubMed] [Google Scholar]
- Mouta Carreira C, Landriscina M, Bellum S, Prudovsky I, Maciag T. The comparative release of FGF1 by hypoxia and temperature stress. Growth Factors. 2001;18:277–285. doi: 10.3109/08977190109029116. [DOI] [PubMed] [Google Scholar]
- Nickel W. Unconventional secretory routes: Direct protein export across the plasma membrane of mammalian cells. Traffic. 2005;6:607–614. doi: 10.1111/j.1600-0854.2005.00302.x. [DOI] [PubMed] [Google Scholar]
- Norrby K. Interleukin-1-alpha and de novo mammalian angiogenesis. Microvasc Res. 1997;54:58–64. doi: 10.1006/mvre.1997.2024. [DOI] [PubMed] [Google Scholar]
- Nyberg P, Wikman AL, Nennesmo I, Lundberg I. Increased expression of interleukin 1alpha and MHC class I in muscle tissue of patients with chronic, inactive polymyositis and dermatomyositis. J Rheumatol. 2000;27:940–948. [PubMed] [Google Scholar]
- Okunieff P, Fenton BM, Zhang L, Kern FG, Wu T, Greg JR, Ding I. Fibroblast growth factors (FGFS) increase breast tumor growth rate, metastases, blood flow, and oxygenation without significant change in vascular density. Adv Exp Med Biol. 2003;530:593–601. doi: 10.1007/978-1-4615-0075-9_58. [DOI] [PubMed] [Google Scholar]
- Omoto A, Kawahito Y, Prudovsky I, Tubouchi Y, Kimura M, Ishino H, Wada M, Yoshida M, Kohno M, Yoshimura R, Yoshikawa T, Sano H. Copper chelation with tetrathiomolybdate suppresses adjuvant-induced arthritis and inflammation-associated cachexia in rats. Arthritis Res Ther. 2005;7:R1174–R1182. doi: 10.1186/ar1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornitz DM, Itoh N. Fibroblast growth factors. Genome Biol. 2001;2 doi: 10.1186/gb-2001-2-3-reviews3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Q, Kleer CG, van Golen KL, Irani J, Bottema KM, Bias C, De Carvalho M, Mesri EA, Robins DM, Dick RD, Brewer GJ, Merajver SD. Copper deficiency induced by tetrathiomolybdate suppresses tumor growth and angiogenesis. Cancer Res. 2002;62:4854–4859. [PubMed] [Google Scholar]
- Pankonin G, Teuscher E. Stimulation of endothelial cell migration by thrombin. Biomed Biochim Acta. 1991;50:1073–1078. [PubMed] [Google Scholar]
- Parke A, Bhattacherjee P, Palmer RM, Lazarus NR. Characterization and quantification of copper sulfate-induced vascularization of the rabbit cornea. Am J Pathol. 1988;130:173–178. [PMC free article] [PubMed] [Google Scholar]
- Peterson EA, Sutherland MR, Nesheim ME, Pryzdial EL. Thrombin induces endothelial cell-surface exposure of the plasminogen receptor annexin 2. J Cell Sci. 2003;116:2399–2408. doi: 10.1242/jcs.00434. [DOI] [PubMed] [Google Scholar]
- Pomorski T, Hrafnsdottir S, Devaux P, van Meer G. Lipid distribution and transport across cellular membranes. Cell Dev Biol. 2001:139–148. doi: 10.1006/scdb.2000.0231. [DOI] [PubMed] [Google Scholar]
- Prudovsky I, Bagala C, Tarantini F, Mandinova A, Soldi R, Bellum S, Maciag T. The intracellular translocation of the components of the fibroblast growth factor 1 release complex precedes their assembly prior to export. J Cell Biol. 2002;158:201–208. doi: 10.1083/jcb.200203084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prudovsky I, Mandinova A, Soldi R, Bagala C, Graziani I, Landriscina M, Tarantini F, Duarte M, Bellum S, Doherty H, Maciag T. The non-classical export routes: FGF1 and IL-1alpha point the way. J Cell Sci. 2003;116:4871–4881. doi: 10.1242/jcs.00872. [DOI] [PubMed] [Google Scholar]
- Ptitsyn OB. Molten globule and protein folding. Adv Protein Chem. 1995;47:83–229. doi: 10.1016/s0065-3233(08)60546-x. [DOI] [PubMed] [Google Scholar]
- Qiao J, Bush KT, Steer DL, Stuart RO, Sakurai H, Wachsman W, Nigam SK. Multiple fibroblast growth factors support growth of the ureteric bud but have different effects on branching morphogenesis. Mech Dev. 2001;109:123–135. doi: 10.1016/s0925-4773(01)00592-5. [DOI] [PubMed] [Google Scholar]
- Rabiet MJ, Plantier JL, Rival Y, Genoux Y, Lampugnani MG, Dejana E. Thrombin-induced increase in endothelial permeability is associated with changes in cell-to-cell junction organization. Arterioscler Thromb Vasc Biol. 1996;16:488–496. doi: 10.1161/01.atv.16.3.488. [DOI] [PubMed] [Google Scholar]
- Rajalingam D, Kumar TK, Soldi R, Graziani I, Prudovsky I, Yu C. Molecular mechanism of inhibition of non-classical FGF-1 export. Biochemistry. 2005a;44:15472–15479. doi: 10.1021/bi0516071. [DOI] [PubMed] [Google Scholar]
- Rajalingam D, Kumar TK, Yu C. The C2A domain of synaptotagmin exhibits a high binding affinity for copper: Implications in the formation of the multiprotein FGF release complex. Biochemistry. 2005b;44:14431–14442. doi: 10.1021/bi051387r. [DOI] [PubMed] [Google Scholar]
- Rajalingam D, Graziani I, Prudovsky I, Yu C, Kumar TK. Relevance of partially structured states in the non-classical release of acidic fibroblast growth factor. Biochemistry. 2007 doi: 10.1021/bi7002586. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch BH, Millette E, Kenagy RD, Daum G, Clowes AW. Thrombin- and factor Xa-induced DNA synthesis is mediated by transactivation of fibroblast growth factor receptor-1 in human vascular smooth muscle cells. Circ Res. 2004;94:340–345. doi: 10.1161/01.RES.0000111805.09592.D8. [DOI] [PubMed] [Google Scholar]
- Rauch BH, Scholz GA, Baumgartel-Allekotte D, Censarek P, Fischer JW, Weber AA, Schror K. Cholesterol enhances thrombin-induced release of fibroblast growth factor-2 in human vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2007;27:e20–e25. doi: 10.1161/01.ATV.0000258793.51013.34. [DOI] [PubMed] [Google Scholar]
- Renaud F, Desset S, Oliver L, Gimenez-Gallego G, Van Obberghen E, Courtois Y, Laurent M. The neurotrophic activity of fibroblast growth factor 1 (FGF1) depends on endogenous FGF1 expression and is independent of the mitogen-activated protein kinase cascade pathway. J Biol Chem. 1996;271:2801–2811. doi: 10.1074/jbc.271.5.2801. [DOI] [PubMed] [Google Scholar]
- Ristimaki A, Garfinkel S, Wessendorf J, Maciag T, Hla T. Induction of cyclooxygenase-2 by interleukin-1 alpha. Evidence for post-transcriptional regulation. J Biol Chem. 1994;269:11769–11775. [PubMed] [Google Scholar]
- Rubartelli A, Cozzolino F, Talio M, Sitia R. A novel secretory pathway for interleukin-1 beta, a protein lacking a signal sequence. EMBO J. 1990;9:1503–1510. doi: 10.1002/j.1460-2075.1990.tb08268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubartelli A, Bajetto A, Allavena G, Wollman E, Sitia R. Secretion of thioredoxin by normal and neoplastic cells through a leaderless secretory pathway. J Biol Chem. 1992;267:24161–24164. [PubMed] [Google Scholar]
- Rubartelli A, Bonifaci N, Sitia R. High rates of thioredoxin secretion correlate with growth arrest in hepatoma cells. Cancer Res. 1995;55:675–680. [PubMed] [Google Scholar]
- Russell JC, Szuflita N, Khatri R, Laterra J, Hossain MA. Transgenic expression of human FGF-1 protects against hypoxic-ischemic injury in perinatal brain by intervening at caspase-XIAP signaling cascades. Neurobiol Dis. 2006;22:677–690. doi: 10.1016/j.nbd.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Sahlgren C, Lendahl U. Notch signaling and its integration with other signaling mechanisms. Regen Med. 2006;1:195–205. doi: 10.2217/17460751.1.2.195. [DOI] [PubMed] [Google Scholar]
- Samaniego F, Markham PD, Gallo RC, Ensoli B. Inflammatory cytokines induce AIDS-Kaposi’s sarcoma-derived spindle cells to produce and release basic fibroblast growth factor and enhance Kaposi’s sarcoma-like lesion formation in nude mice. J Immunol. 1995;154:3582–3592. [PubMed] [Google Scholar]
- Sango K, Tokashiki A, Ajiki K, Horie M, Kawano H, Watabe K, Horie H, Kadoya T. Synthesis, localization and externalization of galectin-1 in mature dorsal root ganglion neurons and Schwann cells. Eur J Neurosci. 2004;19:55–64. doi: 10.1046/j.1460-9568.2003.03102.x. [DOI] [PubMed] [Google Scholar]
- Sano H, Forough R, Maier JA, Case JP, Jackson A, Engleka K, Maciag T, Wilder RL. Detection of high levels of heparin binding growth factor-1 (acidic fibroblast growth factor) in inflammatory arthritic joints. J Cell Biol. 1990;110:1417–1426. doi: 10.1083/jcb.110.4.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz JM, Gimenez-Gallego G. A partly folded state of acidic fibroblast growth factor at low pH. Eur J Biochem. 1997;246:328–335. doi: 10.1111/j.1432-1033.1997.00328.x. [DOI] [PubMed] [Google Scholar]
- Schafer T, Zentgraf H, Zehe C, Brugger B, Bernhagen J, Nickel W. Unconventional secretion of fibroblast growth factor 2 is mediated by direct translocation across the plasma membrane of mammalian cells. J Biol Chem. 2004;279:6244–6251. doi: 10.1074/jbc.M310500200. [DOI] [PubMed] [Google Scholar]
- Schumacher B, Pecher P, von Specht BU, Stegmann T. Induction of neoangiogenesis in ischemic myocardium by human growth factors: First clinical results of a new treatment of coronary heart disease. Circulation. 1998;97:645–650. doi: 10.1161/01.cir.97.7.645. [DOI] [PubMed] [Google Scholar]
- Seelenmeyer C, Wegehingel S, Tews I, Kunzler M, Aebi M, Nickel W. Cell surface counter receptors are essential components of the unconventional export machinery of galectin-1. J Cell Biol. 2005;171:373–381. doi: 10.1083/jcb.200506026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellke FW, Li J, Stamler A, Lopez JJ, Thomas KA, Simons M. Angiogenesis induced by acidic fibroblast growth factor as an alternative method of revascularization for chronic myocardial ischemia. Surgery. 1996;120:182–188. doi: 10.1016/s0039-6060(96)80286-8. [DOI] [PubMed] [Google Scholar]
- Shin JT, Opalenik SR, Wehby JN, Mahesh VK, Jackson A, Tarantini F, Maciag T, Thompson JA. Serum-starvation induces the extracellular appearance of FGF-1. Biochim Biophys Acta. 1996;1312:27–38. doi: 10.1016/0167-4889(96)00013-4. [DOI] [PubMed] [Google Scholar]
- Sivaraja V, Kumar TK, Prudovsky I, Yu C. Three-dimensional solution structure of a unique S100 protein. Biochem Biophys Res Commun. 2005;335:1140–1148. doi: 10.1016/j.bbrc.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Sivaraja V, Kumar TK, Rajalingam D, Graziani I, Prudovsky I, Yu C. Copper binding affinity of S100A13, a key component of the FGF-1 nonclassical copper-dependent release complex. Biophys J. 2006;91:1832–1843. doi: 10.1529/biophysj.105.079988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small D, Kovalenko D, Kacer D, Liaw L, Landriscina M, Di Serio C, Prudovsky I, Maciag T. Soluble Jagged 1 represses the function of its transmembrane form to induce the formation of the Src-dependent chord-like phenotype. J Biol Chem. 2001;276:32022–32030. doi: 10.1074/jbc.M100933200. [DOI] [PubMed] [Google Scholar]
- Small D, Kovalenko D, Soldi R, Mandinova A, Kolev V, Trifonova R, Bagala C, Kacer D, Battelli C, Liaw L, Prudovsky I, Maciag T. Notch activation suppresses fibroblast growth factor-dependent cellular transformation. J Biol Chem. 2003;278:16405–16413. doi: 10.1074/jbc.M300464200. [DOI] [PubMed] [Google Scholar]
- Soldi R, Mandinova A, Venkataraman K, Hea T, Vadas M, Pitson S, Duarte M, Graziani I, Kolev V, Kacer D, Kirov A, Maciag T, Prudovsky I. Sphingosine kinase 1 is a critical component of the copper-dependent FGF1 export pathway. Exp cell Res. 2007 doi: 10.1016/j.yexcr.2007.05.031. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegmayer C, Kehlenbach A, Tournaviti S, Wegehingel S, Zehe C, Denny P, Smith DF, Schwappach B, Nickel W. Direct transport across the plasma membrane of mammalian cells of Leishmania HASPB as revealed by a CHO export mutant. J Cell Sci. 2005;118:517–527. doi: 10.1242/jcs.01645. [DOI] [PubMed] [Google Scholar]
- Stylianou E, Saklatvala J. Interleukin-1. Int J Biochem Cell Biol. 1998;30:1075–1079. doi: 10.1016/s1357-2725(98)00081-8. [DOI] [PubMed] [Google Scholar]
- Sudhoff TC, Rizo J. Synaptotagmins: C2-domain proteins that regulate membrane traffic. Neuron. 1996;17:379–388. doi: 10.1016/s0896-6273(00)80171-3. [DOI] [PubMed] [Google Scholar]
- Tanudji M, Hevi S, Chuck SL. The nonclassic secretion of thioredoxin is not sensitive to redox state. Am J Physiol Cell Physiol. 2003;284:C1272–C1279. doi: 10.1152/ajpcell.00521.2002. [DOI] [PubMed] [Google Scholar]
- Tarantini F, Gamble S, Jackson A, Maciag T. The cysteine residue responsible for the release of fibroblast growth factor-1 residues in a domain independent of the domain for phosphatidylserine binding. J Biol Chem. 1995;270:29039–29042. doi: 10.1074/jbc.270.49.29039. [DOI] [PubMed] [Google Scholar]
- Tarantini F, Micucci I, Bellum S, Landriscina M, Garfinkel S, Prudovsky I, Maciag T. The precursor but not the mature form of IL1alpha blocks the release of FGF1 in response to heat shock. J Biol Chem. 2001;276:5147–5151. doi: 10.1074/jbc.C000714200. [DOI] [PubMed] [Google Scholar]
- Thompson JA, Anderson KD, DiPietro JM, Zwiebel JA, Zametta M, Anderson WF, Maciag T. Site-directed neovessel formation in vivo. Science. 1988;241:1349–1352. doi: 10.1126/science.2457952. [DOI] [PubMed] [Google Scholar]
- Torisu H, Ono M, Kiryu H, Furue M, Ohmoto Y, Nakayama J, Nishioka Y, Sone S, Kuwano M. Macrophage infiltration correlates with tumor stage and angiogenesis in human malignant melanoma: Possible involvement of TNFalpha and IL-1alpha. Int J Cancer. 2000;85:182–188. [PubMed] [Google Scholar]
- Trifonova R, Small D, Kacer D, Kovalenko D, Kolev V, Mandinova A, Soldi R, Liaw L, Prudovsky I, Maciag T. The non-transmembrane form of Delta1, but not of Jagged1, induces normal migratory behavior accompanied by fibroblast growth factor receptor 1-dependent transformation. J Biol Chem. 2004;279:13285–13288. doi: 10.1074/jbc.C300564200. [DOI] [PubMed] [Google Scholar]
- van der Goot FG, Lakey JH, Pattus F. The molten globule intermediate for protein insertion or translocation through membranes. Trends Cell Biol. 1992;2:343–348. [PubMed] [Google Scholar]
- van der Vies SM, Viitanen PV, Gatenby AA, Lorimer GH, Jaenicke R. Conformational states of ribulosebi-sphosphate carboxylase and their interaction with chaperonin 60. Biochemistry. 1992;31:3635–3644. doi: 10.1021/bi00129a012. [DOI] [PubMed] [Google Scholar]
- Venkataraman G, Raman R, Sasisekharan V, Sasisekharan R. Molecular characteristics of fibroblast growth factor-fibroblast growth factor receptor-heparin-like glycosaminoglycan complex. Proc Natl Acad Sci USA. 1999;96:3658–3663. doi: 10.1073/pnas.96.7.3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkataraman K, Thangada S, Michaud J, Oo ML, Ai Y, Lee YM, Wu M, Parikh NS, Khan F, Proia RL, Hla T. Extracellular export of sphingosine kinase-1a contributes to the vascular S1P gradient. Biochem J. 2006;397:461–471. doi: 10.1042/BJ20060251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voronov E, Shouval DS, Krelin Y, Cagnano E, Benharroch D, Iwakura Y, Dinarello CA, Apte RN. IL-1 is required for tumor invasiveness and angiogenesis. Proc Natl Acad Sci USA. 2003;100:2645–2650. doi: 10.1073/pnas.0437939100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesche J, Wiedlocha A, Falnes PO, Choe S, Olsnes S. Externally added aFGF mutants do not require extensive unfolding for transport to the cytosol and the nucleus in NIH/3T3 cells. Biochemistry. 2000;39:15091–15100. doi: 10.1021/bi001831k. [DOI] [PubMed] [Google Scholar]
- Winkles JA, Friesel R, Burgess WH, Howk R, Mehlman T, Weinstein R, Maciag T. Human vascular smooth muscle cells both express and respond to heparin-binding growth factor I (endothelial cell growth factor) Proc Natl Acad Sci USA. 1987;84:7124–7128. doi: 10.1073/pnas.84.20.7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong MK, Prudovsky I, Vary C, Booth C, Liaw L, Mousa S, Small D, Maciag T. A non-transmembrane form of Jagged-1 regulates the formation of matrix- dependent chord-like structures. Biochem Biophys Res Commun. 2000;268:853–859. doi: 10.1006/bbrc.2000.2173. [DOI] [PubMed] [Google Scholar]
- Woolley PV, Gollin SM, Riskalla W, Finkelstein S, Stefanik DF, Riskalla L, Swaney WP, Weisenthal L, McKenna RJ., Jr Cytogenetics, immunostaining for fibroblast growth factors, p53 sequencing, and clinical features of two cases of cystosarcoma phyllodes. Mol Diagn. 2000;5:179–190. doi: 10.1054/modi.2000.9405. [DOI] [PubMed] [Google Scholar]
- Xue L, Greisler HP. Angiogenic effect of fibroblast growth factor-1 and vascular endothelial growth factor and their synergism in a novel in vitro quantitative fibrin-based 3-dimensional angiogenesis system. Surgery. 2002;132:259–267. doi: 10.1067/msy.2002.125720. [DOI] [PubMed] [Google Scholar]
- Yu C, Wang F, Jin C, Huang X, Miller DL, Basilico C, McKeehan WL. Role of fibroblast growth factor type 1 and 2 in carbon tetrachloride-induced hepatic injury and fibrogenesis. Am J Pathol. 2003;163:1653–1662. doi: 10.1016/S0002-9440(10)63522-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Harris SL, Levine AJ. The regulation of exosome secretion: A novel function of the p53 protein. Cancer Res. 2006;66:4795–4801. doi: 10.1158/0008-5472.CAN-05-4579. [DOI] [PubMed] [Google Scholar]
- Zehe C, Engling A, Wegehingel S, Schafer T, Nickel W. Cell-surface heparan sulfate proteoglycans are essential components of the unconventional export machinery of FGF-2. Proc Natl Acad Sci USA. 2006;103:15479–15484. doi: 10.1073/pnas.0605997103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Komiya H, Chirino A, Faham S, Fox GM, Arakawa T, Hsu BT, Rees DC. Three-dimensional structures of acidic and basic fibroblast growth factors. Science. 1991;251:90–93. doi: 10.1126/science.1702556. [DOI] [PubMed] [Google Scholar]
- Zimrin AB, Pepper MS, McMahon GA, Nguyen F, Montesano R, Maciag T. An antisense oligonucleotide to the notch ligand jagged enhances fibroblast growth factor-induced angiogenesis in vitro. J Biol Chem. 1996;271:32499–32502. doi: 10.1074/jbc.271.51.32499. [DOI] [PubMed] [Google Scholar]